- Department of Pharmacy, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Background: The administration of immune checkpoint inhibitors (ICIs) prior to liver transplantation (LT) for hepatocellular carcinoma (HCC) has been reported. Several studies suggest that ICIs may elevate the risk of allograft rejection (AR) and influence other clinical outcomes. This meta-analysis aimed to assess the efficacy and safety of pre-LT ICI treatment in HCC patients.
Methods: A systematic literature search was conducted in PubMed, Embase, Cochrane, and Web of Science for retrospective studies and randomized controlled trials (RCTs) examining pre-LT ICI therapies in HCC patients. Random-effects models were employed to evaluate treatment effects on allograft rejection (AR), complete recovery rate among patients with AR, graft loss, HCC recurrence, and progression-free survival (PFS). Common-effects models were used to assess overall mortality and AR-related mortality. Study quality was evaluated using the JBI critical appraisal tools. The review was registered with PROSPERO (CRD42024616267).
Results: Studies involving HCC patients receiving pre-LT ICIs for downstaging or bridging were included. After screening databases from inception to 31 December 2024, eight studies (n = 229 patients) were included. The studies had diverse designs and were primarily from China and the US. The pooled post-LT AR rate across all eight studies was 19% (95% CI: 12%–30%). The incidence of AR was 24% in the PD-L1 inhibitor group, 18% in the PD-1 inhibitor group, and 20% in the bispecific/combination therapies group. The complete recovery rate among patients with AR was 78% (95% CI: 59%–97%), and graft loss occurred in 4% (95% CI: 1%–7%). The HCC recurrence rate across six studies was 24% (95% CI: 12%–36%). The pooled median recurrence-free survival (RFS) was 17.63 months (95% CI: 11.57–23.69 months). Overall mortality was 8% (95% CI: 4%–12%), and AR-related mortality was 2% (95% CI: 0%–5%). Sensitivity analysis supported the robustness of the results, while funnel plots indicated potential publication bias for several outcomes. This meta-analysis offers a comprehensive synthesis of the impact of pre-LT ICIs on post-transplantation outcomes.
Conclusion: The use of ICIs as bridging or downstaging therapy prior to liver transplantation in HCC patients appears feasible.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024616267.
Introduction
According to global cancer statistics 2022, liver cancer ranks as the sixth most commonly diagnosed cancer and the third leading cause of cancer-related deaths globally (1). Notably, hepatocellular carcinoma (HCC) accounts for 75%–85% of primary liver cancer cases (2). The pathogenesis of HCC is tightly linked to chronic liver injury and inflammation. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are major risk factors for the development of HCC. In addition, exposure to aflatoxin, heavy alcohol consumption, obesity, type 2 diabetes, and smoking are also associated with an increased risk of HCC (2). Clinically, HCC is characterized by “silent progression” in its early stages. Owing to the liver’s robust regenerative capacity, early-stage HCC patients typically lack specific symptoms, with only mild, non-specific complaints (e.g., fatigue, anorexia) in rare cases. This leads to delayed diagnosis: approximately 60%–70% of patients are diagnosed at intermediate or advanced stages, when curative treatment options are limited. Consequently, the overall prognosis of HCC remains poor, with a 5-year survival rate of less than 10% for advanced-stage patients—underscoring the urgent need for improved screening tools and therapeutic strategies (3).
In the current clinical management paradigm, treatment strategies for HCC are stratified by disease stage and liver function. For early-stage HCC patients with well-preserved liver function (Child–Pugh Class A), surgical resection is the first-line curative option. However, for early-stage patients with decompensated liver function (e.g., Child–Pugh Class B/C) or those with small tumors but poor hepatic reserve (e.g., cirrhotic liver with portal hypertension), liver transplantation (LT) is the preferred treatment. LT offers a dual benefit: it removes the tumor and replaces the diseased liver, achieving long-term disease control in eligible patients. Additionally, LT is applicable to patients with locally advanced HCC whose viable tumor burden is reduced to within acceptable transplant criteria [e.g., Milan criteria, University of California San Francisco (UCSF) criteria] following locoregional therapies (LRTs) or systemic therapy (4, 5).
Immunotherapy based on immune checkpoint inhibitors (ICIs) has made significant progress in the treatment of advanced HCC. Currently, the approved ICIs mainly include three categories: programmed cell death protein 1 (PD-1) inhibitors, programmed cell death 1 ligand 1 (PD-L1) inhibitors, and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitors. The National Comprehensive Cancer Network (NCCN) recommends ICIs as a first-line or subsequent-line treatment for advanced HCC (6). In multiple phase 3 clinical studies, ICIs in combination with other compounds have demonstrated meaningful improvements in patients with advanced HCC (7, 8). The IMbrave050 trial demonstrated that atezolizumab plus bevacizumab yielded significantly higher overall survival (OS) and progression-free survival (PFS) than sorafenib alone in patients with advanced or unresectable HCC (7). Currently, several new trials are being carried out to explore the use of ICIs to replace or supplement LRTs in treating patients with unresectable HCC. A recent randomized clinical trial (RCT) reported that, in unresectable HCC amenable to embolization, the combination of durvalumab, bevacizumab, and transarterial chemoembolization (TACE) outperforms TACE alone in terms of objective responses and PFS (9). Small-scale trials have also shown that ICI treatment prior to resection has promising efficacy for patients with resectable HCC (10).
Although the indications for ICI treatment are expected to expand to a broader population of HCC patients, the use of ICI prior to LT still presents potential concerns regarding the increased risk of allograft rejection (AR) (11). The period (the time from the last ICI treatment to LT) and the type of ICIs may be related to AR risk (11, 12). In recent years, an increasing number of retrospective studies and RCTs have been attempting to demonstrate that ICIs are a potential treatment strategy for bridging or downstaging prior to LT (13–16). The PLENTY pilot study is the first RCT to assess the efficacy of ICIs in LT recipients diagnosed with HCC beyond the Milan criteria (MC). This study demonstrated that pembrolizumab plus lenvatinib yielded a favorable objective response and improved recurrence-free survival (RFS) without increasing AR after LT (16). However, a notable limitation of this study is its relatively small sample size. Given the limited sample size of reports, the safety data on the use of ICIs prior to LT remain inadequate. Consequently, we conducted a meta-analysis of relevant retrospective trials and RCTs to determine the impact of using ICIs before LT on post-transplantation outcomes, including AR, HCC recurrence, and mortality.
Methods
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). The protocol of our study was registered in the International Prospective Register of Systematic Reviews (PROSPERO)—CRD42024616267.
Inclusion and exclusion criteria
The inclusion criteria of included studies were as follows: 1) population: patients diagnosed with HCC; 2) intervention: recipients who had undergone pre-LT ICI therapy for downstaging or bridging during their waiting period for LT; 3) study type: retrospective studies and RCTs; 4) outcomes: the clinical outcomes of interest were reported, including AR, the full recovery rate of patients with AR, graft loss, PFS, HCC recurrence, overall mortality, and AR-related mortality; and 5) all studies must be written in English or Chinese. The exclusion criteria were as follows: 1) articles that only mentioned methods or protocols; and 2) reviews, guidelines, case reports, and conference abstracts.
Database search strategy and screening process
Two independent investigators retrieved relevant studies from PubMed, Embase, Cochrane, and Web of Science. The search period was from database inception to 31 December 2024. The search terms (HCC, LT, and ICIs) were based on three key concepts and adapted for each database search. The full search formulas for each database are provided in the Supplementary Appendix.
After the initial literature search was completed, duplicate reports were removed upon identification using EndNote. Two investigators excluded irrelevant records by screening titles and abstracts and further excluded studies that did not meet the inclusion criteria by reading the full text. In the studies of overlapping patient cases, we included the most recent study or the publication with the most complete data. In the event of a disagreement between the investigators, the final determination was made in collaboration with the senior investigator.
Data extraction and risk of bias assessment
Two investigators independently extracted the key characteristics and outcomes from the eligible studies, as well as from the relevant Supplementary Materials, by strictly following a predefined data extraction table. The data extracted included authors, year of publication, country of origin, study design, sample size, patient age, treatment regimens, follow-up, and reported outcomes (AR, HCC recurrence, RFS, mortality, etc.). PFS was defined as the time elapsed until radiological evidence of tumor recurrence after LT. Data extraction was meticulously performed by one investigator and subsequently cross-checked for accuracy by another investigator.
The risk of bias was evaluated by two seasoned investigators to ensure objectivity and reliability of the assessment process. All studies were assessed using the JBI critical appraisal tools (18). For each domain, a determination was made and assigned as either “yes,” “no,” “unclear,” or “not applicable.” Any discrepancies regarding the quality assessment were amicably resolved upon confirmation by a senior investigator.
Statistical analysis
The R software (version 4.1.2) was used for data analysis (http://www.r-project.org/). Heterogeneity was evaluated using the chi-squared test along with the I² statistic. A p-value ≤0.05 was indicative of a statistically significant difference. If significant heterogeneity was present, as determined by a p-value ≤0.05 and I2 >50%, a random-effects model was then applied for the analysis. Otherwise, the common-effects model was used. Moreover, a sensitivity analysis was meticulously conducted to evaluate the stability and reliability of the results. Finally, funnel plots were used to assess the publication bias.
Results
Search results
The initial search yielded a total of 1,039 published relevant studies from four databases (PubMed = 62, Embase = 661, Web of Science = 254, and Cochrane Library = 62). Approximately 37 studies were retained after removing duplicates and screening the titles and abstracts. The remaining full-text articles were carefully assessed, and eight studies (n = 229 patients) were included in the meta-analysis (13–16, 19–22). The number of studies included at each stage of the selection process was outlined in the PRISMA flow diagram (Figure 1).
Characteristics of the included studies
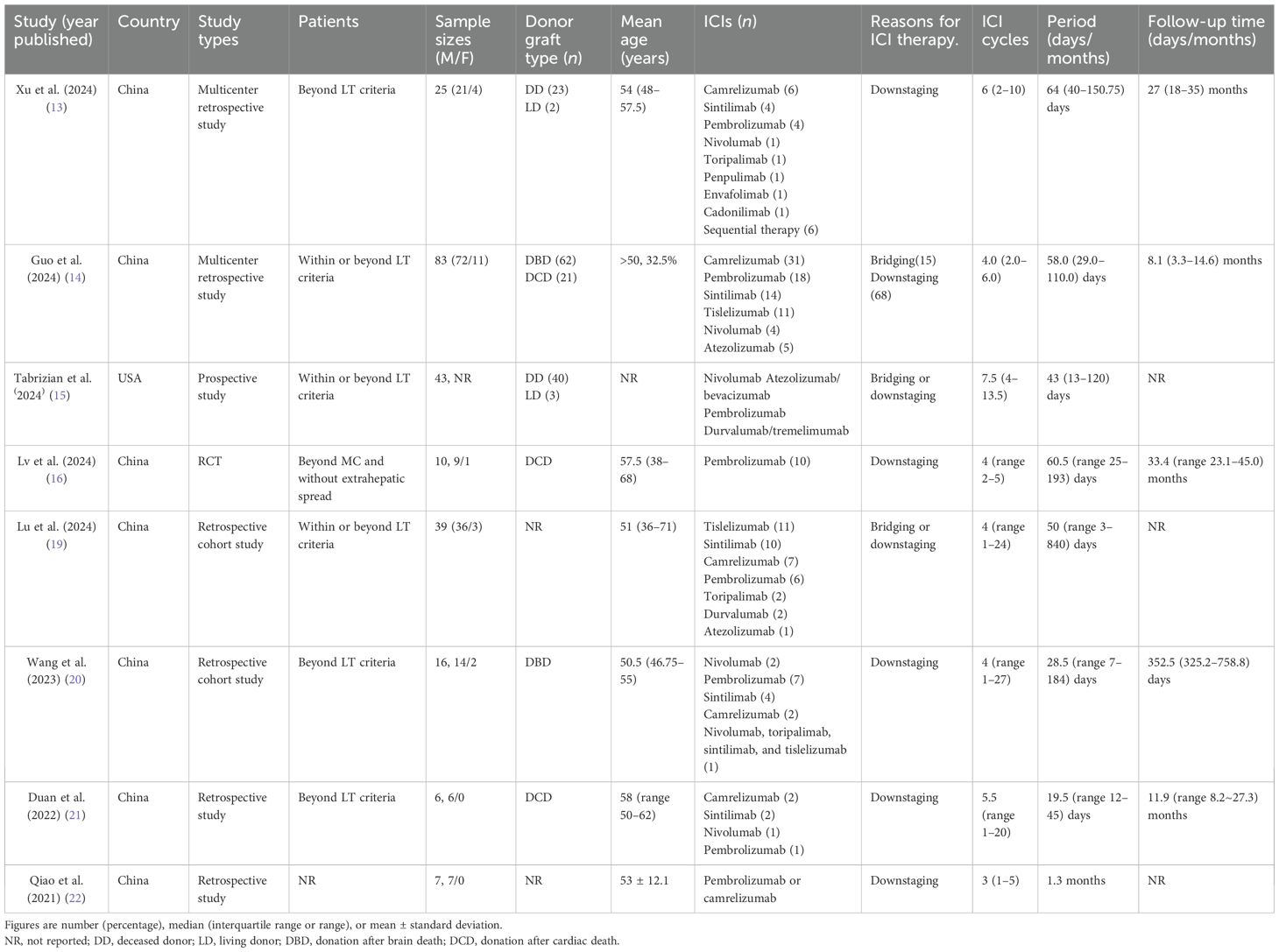
Table 1 describes the characteristics of all studies included in the systematic review. Seven studies (13, 14, 16, 19–22) were conducted in China, and one study (15) was conducted in the United States between 2021 and 2024.
There were one RCT, two retrospective cohort studies, and five retrospective studies. The median age of the patients ranged from 50.5 to 58 years. Approximately 90% of the patients were men. Four studies used only deceased donors, while two used both deceased and living donors. Five studies investigated the use of ICIs for downstaging treatment in patients with HCC beyond the LT criteria (13, 16, 20, 21). Three studies explored the application of ICIs for the downstaging or bridging treatment in patients with HCC who exceeded the LT criteria (14, 15, 19). The median number of ICI cycles prior to LT spanned from 3 to 7.5, with the median period ranging from 19.5 to 64 days. The median follow-up duration ranged from 8.1 to 33.4 months.
The risk of bias of the included studies
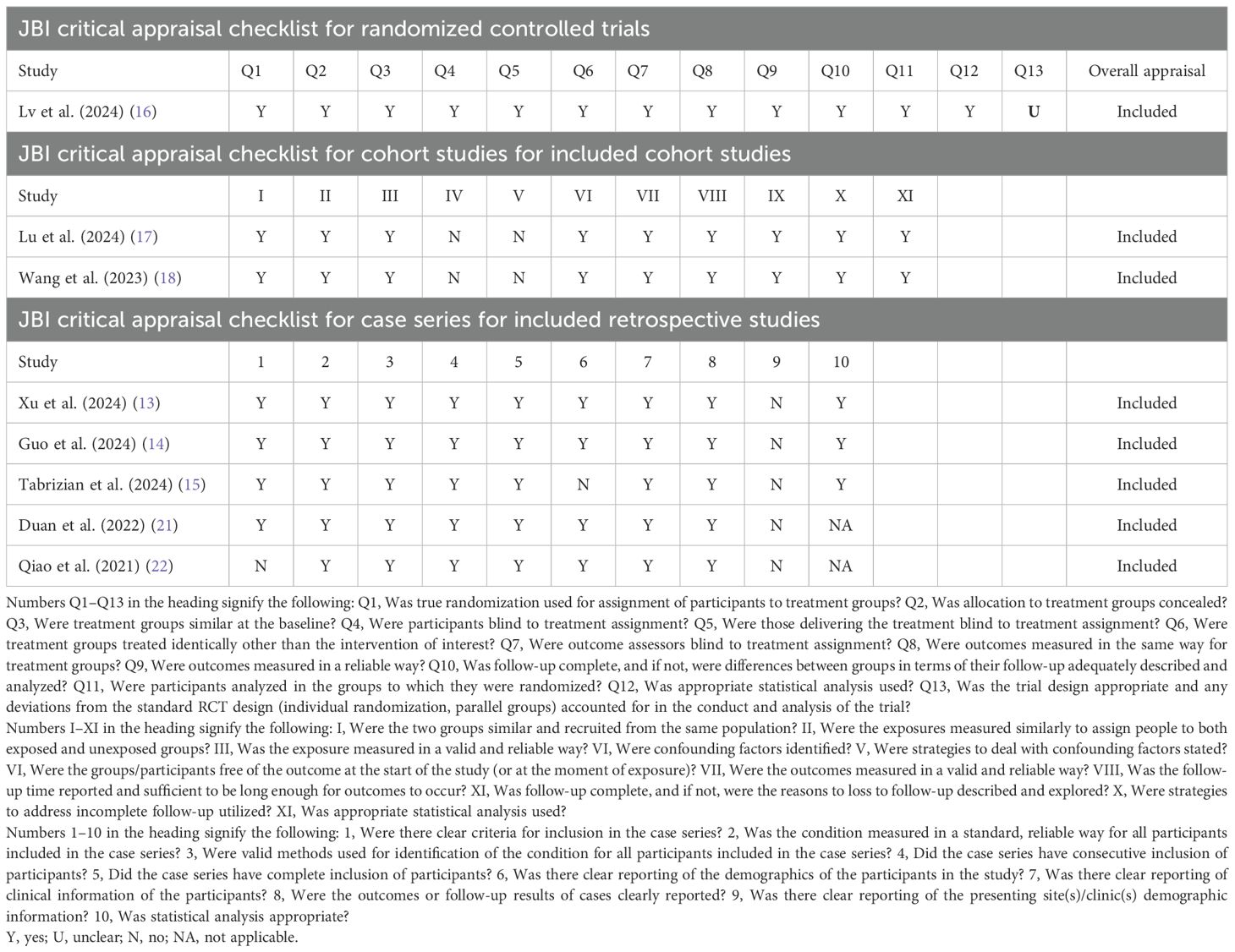
One randomized controlled study was assessed using the JBI Critical Appraisal Checklist for RCTs, which was rated “unclear” for Q13. Two retrospective cohort studies were assessed using the JBI Critical Appraisal Checklist for Cohort Studies, which was rated “no” for IV and V. Five retrospective studies were assessed using the JBI Critical Appraisal Checklist for Case Series, which contains 10 items that assess the quality of case reports based on the selection of cases, disease or health problem evaluation, and presentation of case data. The details of the quality assessment are presented in Table 2.
Allograft rejection
All eight included studies documented the AR rate after LT. The immunosuppression regimens (Table 3) were reported in seven studies: the immunosuppressive maintenance regimens were all based on CNIs, whereas basiliximab was used for induction therapy in four of these studies (13, 14, 19, 20). Five studies (13–15, 19, 20) proposed their own definitions of AR, and three studies (14, 15, 22) further specified their classification of AR according to the updated Banff classification (23). Five studies (13, 14, 19, 20, 22) further recorded the severity of AR according to the rejection activity index (RAI, Supplementary Table S1).
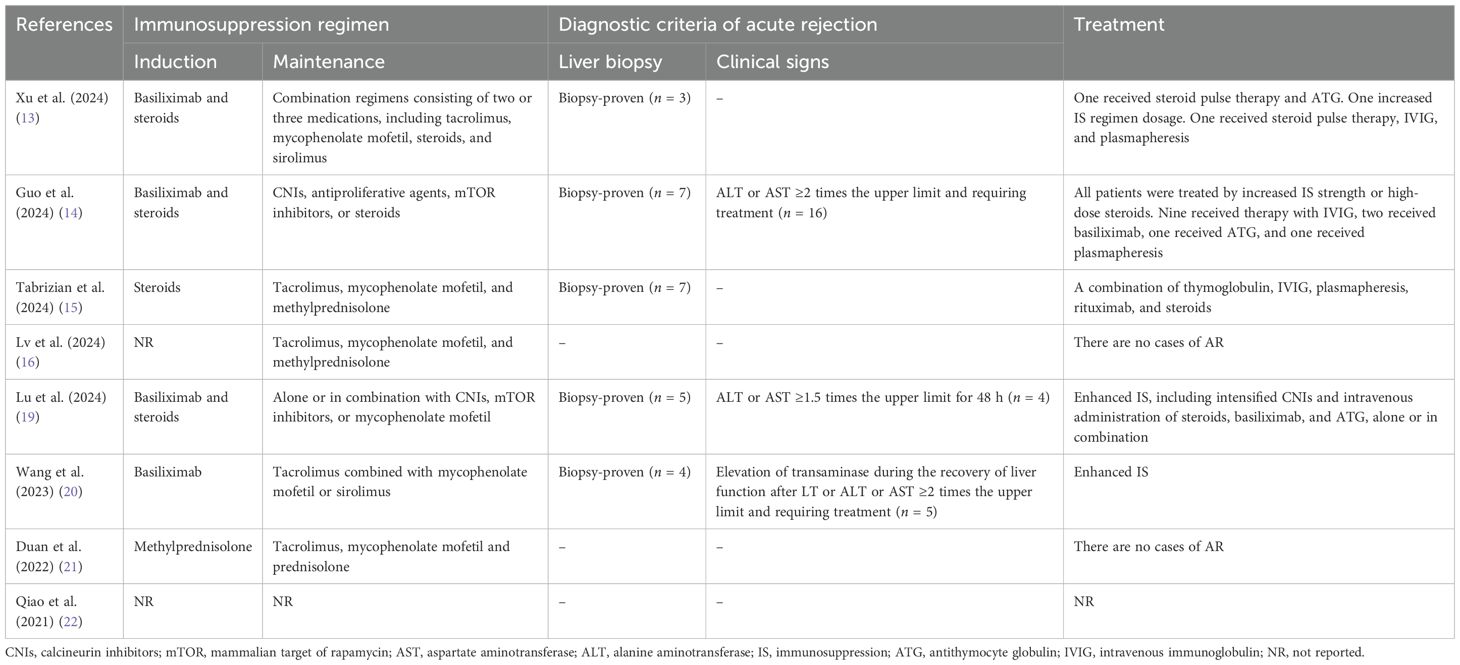
Table 3. Overview of the immunosuppression regimen, diagnostic criteria, and treatment of allograft rejection.
Rejection occurred within 4–150 days post-transplant (Supplementary Table S1). The AR rates across the studies varied from 0% to 56%. The random-effects model was used because of the significant heterogeneity (I2 = 73.7%, p = 0.0004, Figure 2). Due to the substantial heterogeneity observed, a subgroup analysis was conducted by categorizing studies according to the type of ICIs used: PD-1 inhibitors, PD-L1 inhibitors, and bispecific/combination therapies. This stratification resulted in a remarkable reduction in heterogeneity, with the overall I² decreasing from 73.7% to 0% (Figures 2, 3). This indicates that the considerable variation between studies was primarily attributable to differences in the type of therapeutic agents used. The pooled incidence of AR was 18% [95% confidence interval (95% CI): 9%–33%] in the PD-1 inhibitor group, with moderate heterogeneity (I² = 40.2%, p = 0.11). In the PD-L1 inhibitor group, the AR incidence was 24% (95% CI: 9%–49%), with no detectable heterogeneity (I² = 0%). The bispecific/combination therapy group exhibited an AR incidence of 20% (95% CI: 3%–69%). Although the point estimates of AR incidence varied slightly across the three subgroups (18%, 24%, and 20%, respectively), statistical testing indicated that these differences were not significant (p = 0.8982). This suggests that there may be no substantial difference in the incidence of AR between the different types of ICIs. The total AR rate was 19% (95% CI: 12%–30%).
![Forest plot showing three study groups: PD-1 Inhibitor, PD-L1 Inhibitor, and Bispecific/Combination. Each group includes individual studies with event data, totals, proportions, confidence intervals, and heterogeneity metrics. Summary effect sizes with confidence intervals are indicated by black diamonds. Red squares with horizontal lines represent individual study estimates and confidence intervals on the plot. Total sample size is 229, with a combined effect estimate of 0.19, 95% CI [0.12, 0.30], and no significant heterogeneity.](https://www.frontiersin.org/files/Articles/1689820/fonc-15-1689820-HTML/image_m/fonc-15-1689820-g003.jpg)
Figure 3. Forest plot showing changes in AR rate after liver transplantation by drug type. AR, allograft rejection.
All of the included studies provided data on the treatment of AR (Table 3). All recipients with AR were treated by enhanced immunosuppression, including intensified oral regimens and intravenous administration of steroids, basiliximab, antithymocyte globulin (ATG), intravenous immunoglobulin (IVIG), plasmapheresis, and rituximab, alone or in combination (Table 3).
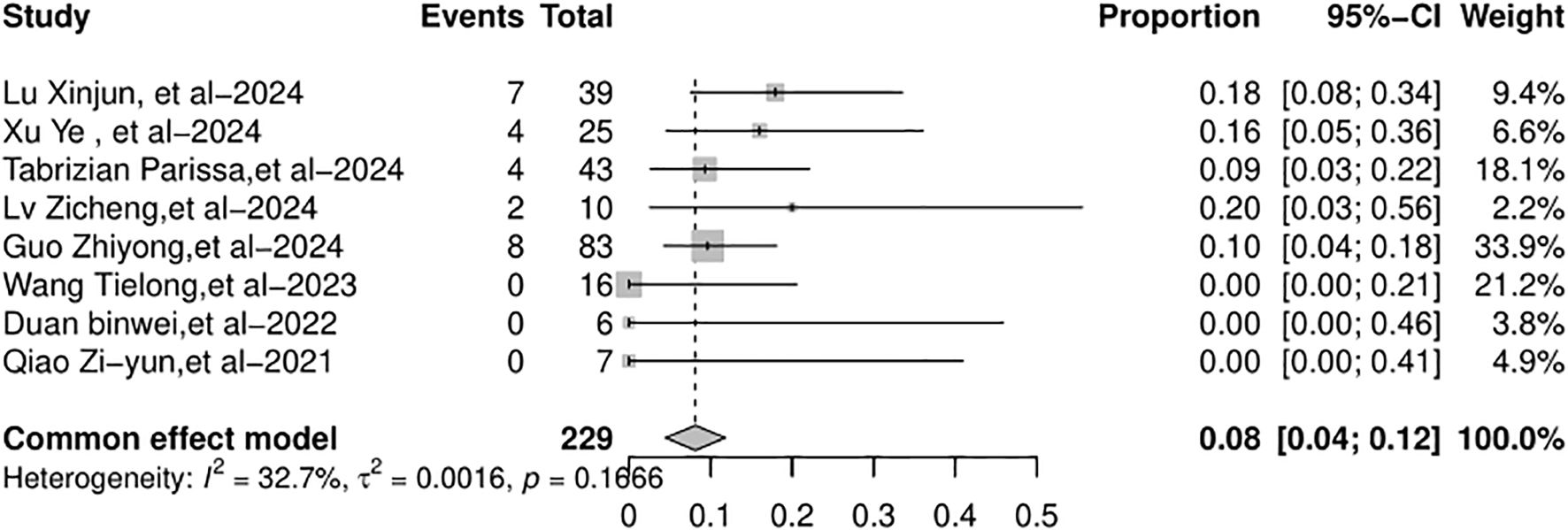
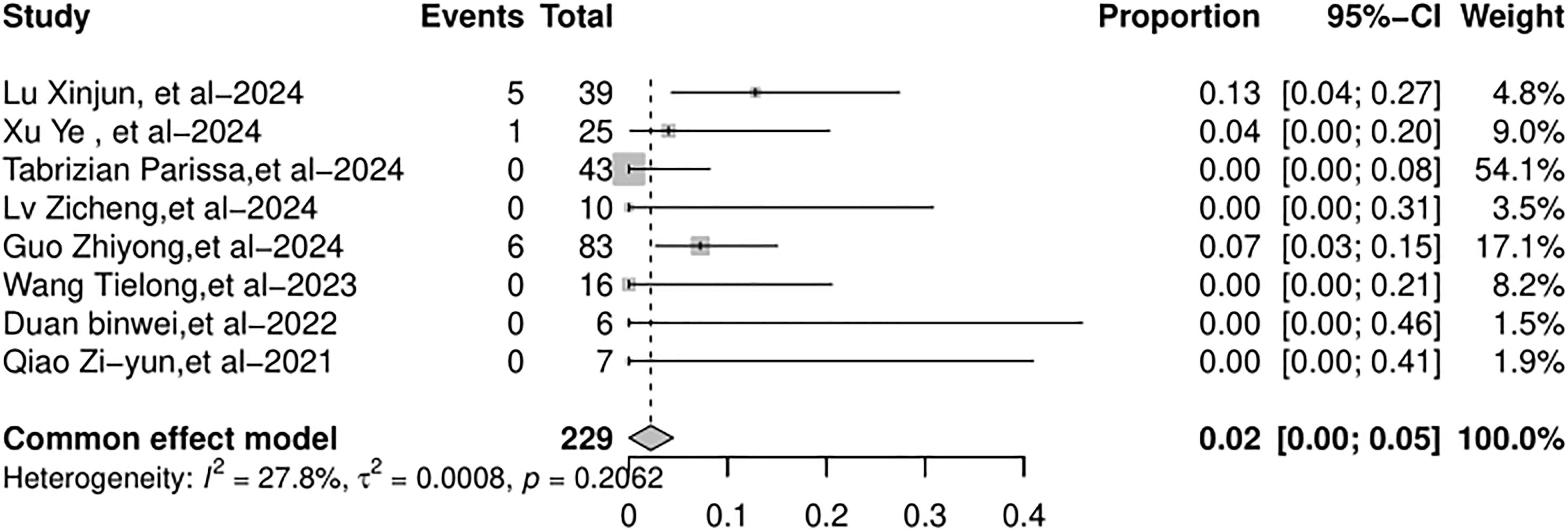
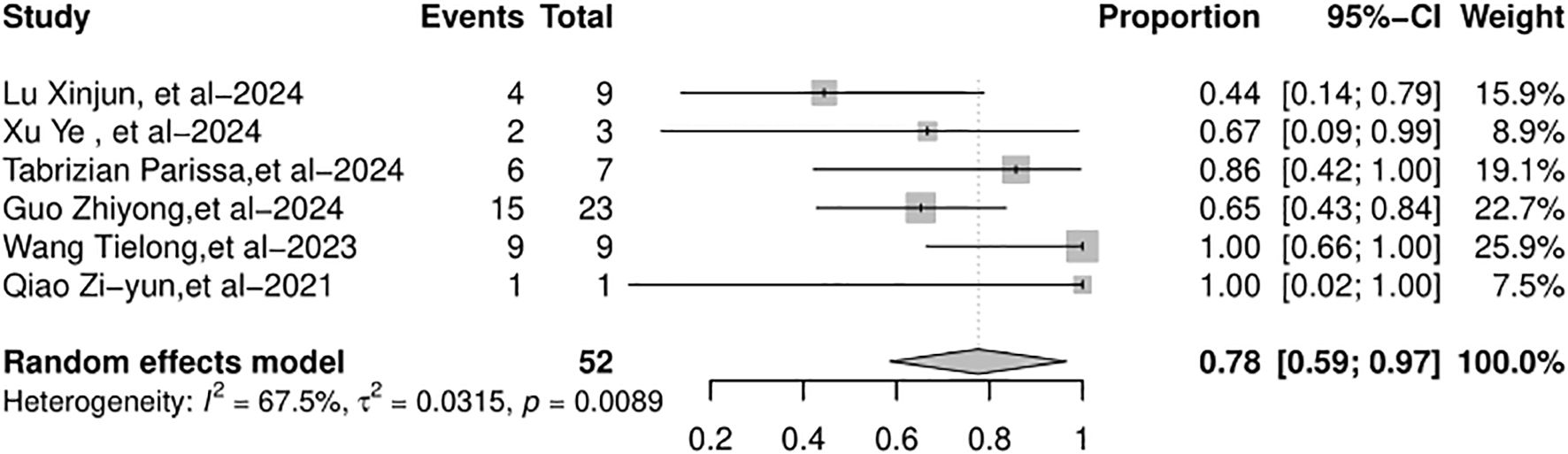
The full recovery rate across the studies varied from 44% to 100%. The random-effects model was used because of the significant heterogeneity (I2 = 67.5%, p = 0.0089). The full recovery rate of the patients with AR (Figure 4) was 78% (95% CI: 59%–97%). The graft loss varied from 0% to 10%. The common-effects models were used and the graft loss (Figure 5) was 4% (95% CI: 1%–7%).
Hepatocellular carcinoma recurrence
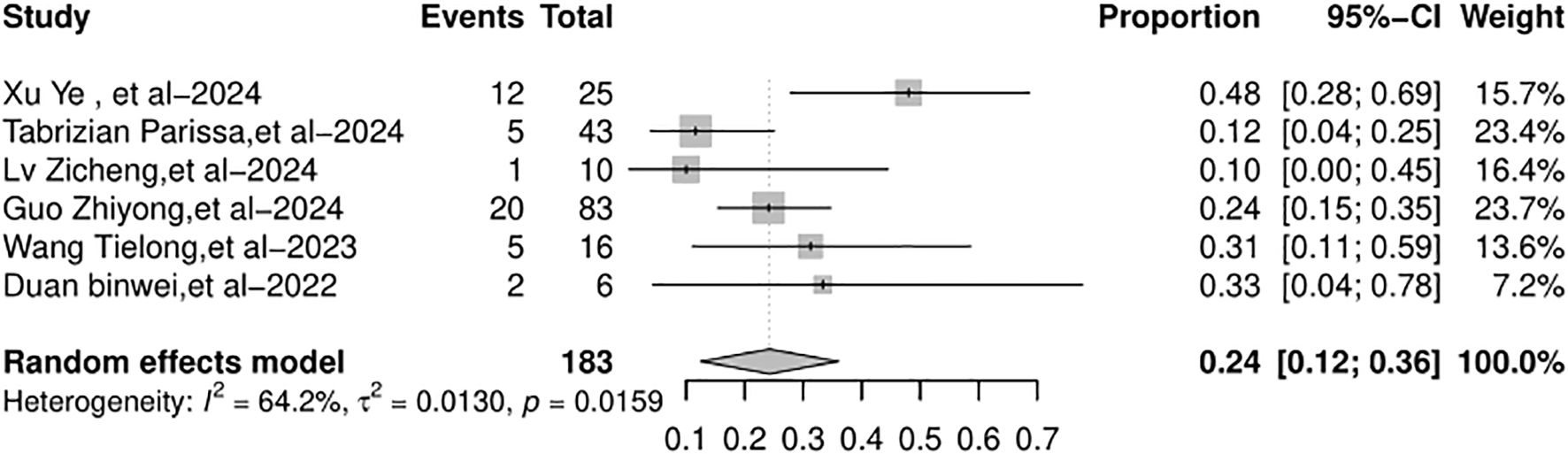
Six studies included in the meta-analysis reported the HCC recurrence rate after LT. The HCC recurrence rate across the studies varied from 10% to 48%. The random-effects model was used because of the significant heterogeneity (I2 = 64.2%, p = 0.0159). The overall HCC recurrence rate (Figure 6) was 24% (95% CI: 12%–36%).
Survival
RFS data were available in four out of eight studies, although the median RFS was statistically reached in only three of them. When the data were pooled, there was high heterogeneity and the funnel plot appeared symmetric. In the random-effects model (I2 = 75.3%, p = 0.0176), the pooled median RFS was 17.63 months (95% CI: 11.57–23.69 months), as shown in Figure 7.
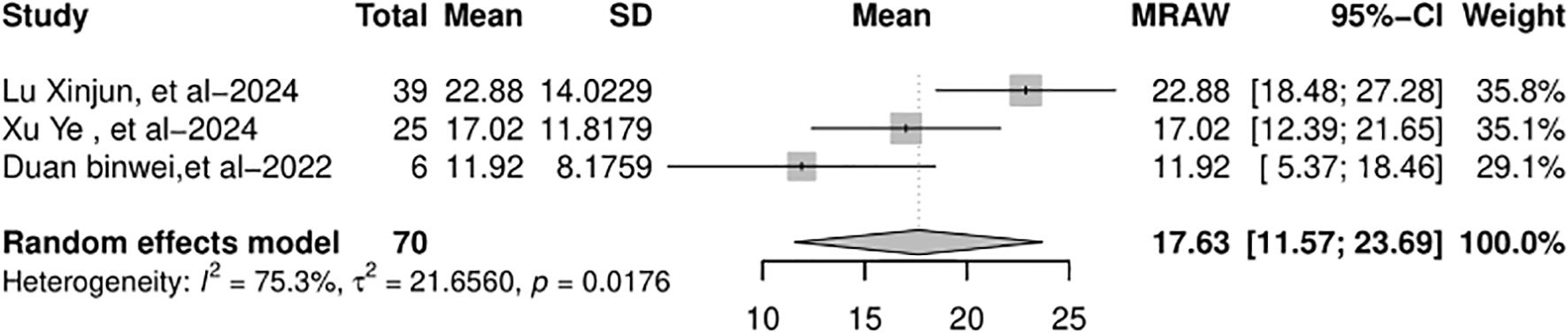
Figure 7. Forest plot of the pooled results of recurrence-free survival. RFS, recurrence-free survival.
All eight studies included in the meta-analysis documented the overall mortality and AR-related mortality. Common-effects models were used. The overall mortality rate was 8% (95% CI: 4%–12%), as shown in Figure 8. The AR-related mortality was 2% (95% CI: 0%–5%), as shown in Figure 9.
Sensitivity analysis
Sensitivity analysis was performed by sequentially excluding one study at a time to evaluate the impact of each individual study on the pooled outcomes. The findings of this analysis clearly demonstrated that none of the pooled results with 95% CIs were substantially affected by any individual study. This indicated that the results of this meta-analysis were relatively reliable. The results of the sensitivity analysis are presented in Supplementary Figure S1.
Publication bias
To ensure the validity of the meta-analysis results, funnel plots (Supplementary Figure S2) were used to estimate the publication bias. We considered that the publication bias exists for the AR rate, the full recovery rate of AR, graft loss, HCC recurrence rate, RFS, mortality, and AR-related mortality.
Discussion
As the global prevalence and mortality rates of HCC have been increasing significantly, there is an urgent need for further advancements in treatment and management approaches. In recent years, ICI therapy has shown great promise for the treatment of HCC by reducing the mortality associated with the disease. In particular, the use of ICIs prior to LT is of great concern. For patients with HCC within the MC who respond to LRTs and undergo prompt LT, the probability of post-LT recurrence is so low that the consideration of immunotherapy lacks justification (5). For HCC patients within the MC, if they do not respond to LRT, need repeated LRT due to extended waiting times, or do not meet the LRT eligibility criteria, ICIs as a bridging treatment emerge as an appealing alternative (5, 15). For patients with HCC beyond the LT criterion, ICIs as a downstaging treatment are highly attractive, hoping to increase the proportion of patients who can receive LT and prolong patient survival (5, 15). The successful incorporation of ICIs into pre-LT downstaging or bridging therapy is supported by effective tumor size reduction within the LT criteria in a previous study (15). Complementing this, there is an impressive 3-year intention-to-treat survival rate of 71.1%, a post-LT survival rate of 85%, and a lack of high-grade adverse events during the waitlist period (15). Although the use of ICIs as downstaging or bridging therapies for LT in HCC patients is rapidly increasing, evidence regarding the feasibility and safety of ICI treatment prior to LT remains limited and controversial. In recent years, several new retrospective studies and RCT on the use of ICIs prior to LT have been published. We conducted this meta-analysis to comprehensively investigate the feasibility and safety of ICI treatment before LT.
Currently, post-LT AR is the main concern when ICIs are used for pre-LT treatment. It has been reported that the use of ICIs prior to LT may result in severe AR and subsequent graft loss (24, 25). The incidence of AR among patients receiving pre-LT ICI therapy varies across different studies, ranging from 0% to 56% (13–16, 19–22), whereas the incidence of AR is 10% to 30% in recipients not receiving ICIs (26, 27). The PLENTY pilot study showed that neither group experienced AR after LT, which is the only RCT that evaluated pre-LT ICI treatment in recipients diagnosed with HCC beyond MC (16). To our knowledge, our systematic review included the largest number of patients treated with ICIs prior to LT. The incidence of AR in our study was 19%. The full recovery rate of patients with AR was 78% and graft loss was 4%. Graft failure was reported in 6.3% at 6 months and 7.9% at 1 year for adult LT recipients not receiving ICIs in 2022 (27). The overall mortality rate was 8% and the AR-related mortality rate was 2% in our study. The overall mortality rate was reported to be 5.0% at 6 months and 6.5% at 1 year among adult LT recipients who did not receive ICIs by 2022 (27). From the above data, it appears that the incidence of AR and mortality among patients receiving ICIs is not higher than that among those who do not receive ICIs. However, it should be noted that the studies on the use of ICIs for pre-LT treatment have a limited number of participants, and the results have certain limitations. Regarding the impact of pre-LT use of ICIs on rejection, Tabrizian et al. mentioned that even when ICIs are used, there are many unique factors in the liver and during LT that can reduce the risk of AR (28): 1) Liver transplantation surgery usually involves significant blood loss, which, to a certain extent, clears the circulating ICIs. 2) Most significantly, when the liver is reperfused, extensive immunosuppression is initiated, which halts T-cell responses. 3) The liver possesses remarkable regenerative capacity, enabling it to recover from injuries. 4) The expression of major histocompatibility complex (MHC) class II antigens is rather feeble in the liver (5, 28).
The washout period of ICIs may be related to AR risk (11, 12). The importance of the washout period prior to LT and how long this washout period should last remain unclear. Additionally, the timing of LT is uncertain, making it extremely difficult to specify the exact washout period. Most studies have suggested that a time interval of 1 to 3 months is relatively safe (12, 15, 29). As a pilot study for a randomized controlled trial, PLENTY specified a 6-week washout period. No cases of rejection were observed among the enrolled patients. Wang et al. revealed a significant difference in the washout period between the rejection group and the non-rejection group (20). The median washout period in the rejection group was 21 days (15.5–27.5), while in the non-rejection group, it was 60 days (24-167) (20). Guo et al. demonstrated that the washout period of ICIs longer than 30 days was an independent protective factor against allograft rejection (14). An individual patient data meta-analysis revealed that the median washout period for patients with a ≤20% probability of allograft rejection was 94 (196) days (11). Kuo et al. discovered that a 1.5-fold half-life was the shortest safe washout period correlated with significant rejection-free survival (30). Although the appropriate length remains to be determined, a washout period prior to LT may be necessary. Different ICIs have varying half-lives and receptor occupancy levels, and the required safe washout periods before LT may also differ. ICIs are monoclonal antibodies that persist in the body for a long time after administration. The shortest half-life was 5 days (camrelizumab), whereas the longest extended beyond 20 days (pembrolizumab) (31–34). All ICIs bind to their targets with high affinity. In patients with advanced solid tumors, the occupancy of camrelizumab on PD-1 remained durable for at least 28 days following a single infusion at doses of 200 and 400 mg (35). The PD-1 occupancy of nivolumab only begins to decay 85 days after administration at a dose of 10 mg/kg (36). Considering the pharmacokinetic properties and clinical experience of different ICIs, it is advisable to develop more precise and individualized guidelines for the washout period prior to transplantation. For certain ICIs with a shorter half-life, a relatively brief washout period may suffice, whereas those with a longer half-life are likely to require an extended washout duration. Currently, there is no available pharmacokinetic data before and after LT. Blood loss and resuscitation during surgery may result in a drastic reduction in serum levels. Further research is still needed to address this aspect.
Apart from the period, some researchers have mentioned that different ICI therapies prior to LT also vary in terms of the risk of post-transplantation rejection (5). The immune targets of ICIs include the PD-1 and its ligand, PD-L1, and CTLA-4. The commonly used PD-1 inhibitors include camrelizumab, sintilimab, pembrolizumab, nivolumab, toripalimab, tislelizumab, and penpulimab. Envafolimab, atezolizumab, and durvalumab are PD-L1 inhibitors, while ipilimumab is a CTLA-4 inhibitor. The PD-1/PD-L1 interaction, which promotes Treg development and maintenance, suppresses T-cell activation, and causes T-cell exhaustion, is crucial for inducing and maintaining solid organ tolerance (37). In contrast to control wild-type mice, PD-1−/− or PD-L1−/− recipient mice rejected cardiac allograft transplantation, even when immunosuppressive treatment was administered (38). Similarly, in a mouse model of LT, blocking the PD-1/PD-L1 pathway with anti-PD-L1 antibodies or using PD-L1 knockout mice as donors resulted in graft rejection (39). These experiments indicate that the expression of PD-L1 on both the recipient’s cells and graft cells is crucial for maintaining graft acceptance. Although PD-1 and PD-L1 inhibitors have often been used interchangeably, evidence indicates that alloimmune responses (i.e., rejection) are more likely to occur with anti-PD-L1 agents than with anti-PD-1 agents (5). This is because PD-L1, aside from being the ligand for PD-1, also serves as a ligand for the B7-1 (CD 80) checkpoint (12). Compared with PD-1/PD-L1, CTLA-4 has less impact on allograft acceptance (40). Our analysis revealed that the incidence of AR was 24% in the PD-L1 inhibitor group, 18% in the PD-1 inhibitor group, and 20% in the bispecific/combination therapies group. Although numerically higher in the PD-L1 group compared to the PD-1 group, this difference did not reach statistical significance. None of the studies included in our analysis involved patients treated with CTLA-4 inhibitors alone. Among the five patients in the bispecific/combination therapies group, one received cadonilimab (a PD-1/CTLA-4-bispecific antibody), three were treated with a combination of PD-1 and PD-L1 inhibitors, and one received a combined PD-L1 and CTLA-4 inhibitor. The impact of pre-LT application of different ICIs on post-transplantation rejection still requires further in-depth exploration. Currently, there is only one case series regarding the pre-LT use of PD-L1 inhibitors (atezolizumab–bevacizumab) in patients with HCC. In this series, none of the five patients experienced recurrence or rejection after the surgery (41). There are four case series regarding pre-LT use of PD-1 inhibitors (nivolumab) in HCC patients (Supplementary Table S2) (42–45). In one study, none of the five patients experienced rejection (43). In the remaining three studies, cases of rejection were reported, and the washout periods of ICIs in all these cases were less than 90 days (41–43). Future studies should focus on comparing the efficacy and safety profiles of different ICIs—including PD-1 inhibitors, PD-L1 inhibitors, and CTLA-4 inhibitors—in the neoadjuvant setting prior to liver transplantation. Such head-to-head comparative studies are of great significance, as they can provide definitive evidence for identifying the optimal immunotherapeutic strategy, thereby balancing the potential antitumor efficacy against the risk of adverse events, including allograft rejection.
The management of post-LT immunosuppression differs across various centers (13–16, 19–22). The use of high-dose steroids for immune induction in LT widely suppresses the immune response. Specifically, the proliferation of T cells and T-cell apoptosis almost cease immediately. Given the benefits of reducing steroid use in LT for HCC, the steroid dosages in the immune induction regimens for LT currently vary across different centers. High-dose steroids actually serve as the treatment for severe ICI-related immune reactions (46, 47). It is possible that the variability in the intensity of early-stage steroid dosing explains some of the differences in the reported incidence of AR among patients who received ICIs prior to LT. The induction of immunosuppression using T-cell-depleting agents, such as ATG, leads to a substantial depletion of T cells. This could be an efficient means to prevent ICI-related rejection. Given the use of ICIs prior to LT, research on immunosuppression management, especially in terms of immune induction schemes, is limited, and there is currently no consensus on the optimal plan. However, it is clear that a stronger immunosuppression regimen should be used in patients with pre-LT use of ICIs to guard against allograft rejection.
Emerging evidence suggests that biomarkers could enhance the prediction of rejection risk in patients receiving ICIs prior to LT. Tabrizian et al. mentioned that a possible explanation for the sporadic cases of severe rejection observed could be the presence of immunological memory against the alloantigens presented in the liver graft (5). This is supported by observations that ICI-responsive cancer patients often harbor pre-existing memory T cells targeting tumor antigens (48). The enzyme-linked immunospot (ELISpot) assay could be employed to detect donor-reactive memory T cells through interferon-γ (IFN-γ) secretion, providing a functional measure of alloreactive potential (49). If such pre-existing memory T-cell responses are conclusively linked to post-transplant rejection in ICI-treated patients, IFN-γ-based assays may offer a practical tool for risk stratification. Furthermore, Qiao et al. reported that patients who experienced acute rejection after pre-LT ICI exposure showed rapid increases in the CD4/CD8 ratio and CD8+CD3+ T-cell counts within 5 days post-transplant (22), suggesting that early immune monitoring could aid rejection prediction. Additionally, existing research evidence has demonstrated a positive correlation between the expression levels of PD-L1 and PD-1 and the severity of post-transplant rejection in the fields of heart and corneal transplantation. This suggests that the expression levels of PD-L1 and PD-1 in the graft hold potential value as predictive indicators for acute rejection. However, clinical studies investigating this correlation in the context of liver transplantation are currently limited by small sample sizes (50, 51). Therefore, it is proposed that future multicenter, large-cohort studies are needed to further validate the predictive efficacy of graft PD-L1 and PD-1 expression levels for acute rejection in liver transplantation. In summary, these findings highlight the potential utility of integrated biomarker platforms—which incorporate cellular, soluble molecular, and transcriptional profiling—in refining pre- and post-transplant risk assessment, enhancing the identification and management of high-risk patients, and supporting the personalized management of ICI-related rejection.
Besides rejection, long-term oncologic outcomes represent a particular concern for patients who are undergoing ICI treatment prior to LT. Our study showed that the HCC recurrence rate was 24% and the pooled median RFS was 17.63 months. In a large-scale multicenter study, the recurrence rate of HCC after LT was 10% among patients within the MC (4). For patients who met the MC criteria after successful downstaging, the HCC recurrence rate after LT reached 15.8%. In contrast, for patients who exceeded the MC criteria, the recurrence rate was as high as 35.2% (4). In the patients included in our study, ICIs were mainly used for downstaging treatment. At the time of LT, some patients met the LT criteria, while others still exceeded them. Therefore, the recurrence rate in our study was higher than that of patients who have successfully undergone downstaging treatment. Additionally, Rezaee-Zavareh et al. mentioned that the number of ICI cycles and tumor burden are likely to exert an impact on the recurrence risk (11). Fewer ICI cycles and a high tumor burden are related to a higher risk of recurrence (11). During the short follow-up period, the rates of post-LT HCC recurrence among patients with complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were 0%, 19.5%, 37.0%, and 33.3%, respectively (14). Therefore, Guo et al. reported that transplantation can be performed in patients with CR or PR after undergoing downstaging treatment with ICIs prior to LT. Nevertheless, caution should be exercised when considering LT in patients with PD or SD (14).
ICIs may cause various systemic adverse events during antitumor treatment, and patients awaiting liver transplantation often have underlying conditions like abnormal liver function and immune dysregulation. Thus, this study recommends the following: strengthening monitoring for major adverse events (e.g., hypersensitivity reactions, infections, bleeding) and developing targeted management strategies to enhance treatment safety in future clinical practice and research. 1) Monitoring system construction a) core mechanism: Establish a multidimensional, full-cycle assessment framework. b) Pre-ICI screening: Comprehensively evaluate patients’ underlying diseases (e.g., allergic disease history, chronic infections, coagulation disorders) and review past medical history to predict ICI-related risks (52, 53). Conduct baseline tests (blood routine, liver/kidney function, inflammatory markers, infectious disease screening, coagulation function) to exclude high-risk ineligible patients. c) During ICI monitoring: Shorten monitoring intervals (repeat lab tests every 2 weeks) and combine regular physical exams, imaging (chest CT for infections, abdominal imaging for bleeding risks), and patient self-reports (e.g., rash, fever, dyspnea) to detect early adverse event signs. Prioritize hypersensitivity reactions: acute reactions (anaphylactic shock, laryngeal edema, severe rash) within minutes to hours post-administration and delayed reactions (maculopapular rash, pruritus with mucosal involvement) 1–2 weeks later (43). Cover both common bacterial infections (e.g., pneumonia, urinary tract infection) and opportunistic infections (e.g., cytomegalovirus pneumonia, fungal infection) for infection monitoring. Focus on gastrointestinal bleeding (melena, hematemesis), intra-abdominal bleeding, and puncture-site bleeding for bleeding monitoring; regularly test prothrombin time, INR, and platelet count; and assess risk factors (active ulcers, portal hypertension). 2) Management strategy formulation a) guiding principle: Follow “stratified management and precision intervention”; adopt a stepped protocol based on adverse event severity (per CTCAE criteria). b) Stratified responses: grade 1–2 mild events (e.g., mild rash, low-grade fever): Continue ICI treatment under close monitoring with symptomatic care. Grade 3–4 severe events (e.g., anaphylactic shock, severe pneumonia, massive gastrointestinal bleeding): Immediately discontinue ICIs and initiate emergency treatment. c) Multidisciplinary collaboration: Establish an adverse event emergency team (integrating hepatology, transplant surgery, infectious diseases, and allergy/immunology). This enables prompt multidisciplinary consultations, individualized plans, minimized impact on patient prognosis, and enhanced safety of pre-liver transplantation ICI therapy.
There are still numerous issues to be resolved regarding pre-LT ICI therapy for HCC: 1) Patient selection: patients who exceed LT criteria and have achieved a favorable response after ICI treatment may gain more benefits after LT; 2) timing: maintaining a washout period of 1 to 3 months may be relatively safe. The washout periods required for different ICIs may vary, as they differ in terms of response rates, half-lives, and target occupancy times; 3) selection of ICIs: the type, dosage, and number of ICI cycles, along with whether they are used singly or in combination for bridging or downstaging, can also impact outcomes; 4) development of biomarkers: develop biomarkers such as graft PD-L1 expression, tumor mutation burden, tumor-infiltrating lymphocytes, T-cell ratio, and IL-6 as predictive factors for ICI response and allograft rejection, and integrate these markers with artificial intelligence models (54, 55); 5) the multidisciplinary team: in pre-LT ICI treatment for HCC patients, the multidisciplinary team (MDT) should be involved throughout the process, including recipient assessment, medication regimen formulation, efficacy evaluation, and monitoring, prevention, and treatment of adverse reactions. The MDT management model can provide recipients with a more scientific, reasonable, and comprehensive treatment plan, thereby increasing the treatment benefits for recipients.
There are several limitations in our study that should be acknowledged. First, the majority of included studies were retrospective in design, with relatively small sample sizes and a lack of control groups, which limits the ability to draw definitive conclusions regarding efficacy and risk assessments. Second, the potential for publication bias must be considered, as studies with positive outcomes are more likely to be published, while negative results may be underrepresented. Third, significant heterogeneity was observed across the studies, which may stem from variations in treatment intent (e.g., downstaging vs. bridging), the timing of ICI administration relative to liver transplantation, and the use of concurrent or prior locoregional therapies. Although planned subgroup analyses were conducted to explore sources of heterogeneity, the limited number of studies constrained a thorough investigation. Additionally, approximately half of the reported rejection cases were not biopsy-confirmed, making it difficult to definitively attribute these events to ICI use. The follow-up duration in most studies was relatively short, and data on long-term outcomes such as overall survival were often unavailable. Furthermore, as most patients received additional treatments prior to transplantation (e.g., tyrosine kinase inhibitors), it remains challenging to isolate the specific contribution of ICIs to post-transplant outcomes. Although locoregional therapy did not significantly influence the primary outcomes such as graft rejection, its interplay with ICIs warrants further investigation.
Conclusion
The results of the current study indicate that the use of ICIs for bridging or downstaging in the treatment of HCC prior to liver transplantation is feasible. To mitigate the risk of allograft rejection effectively, a washout period spanning 1–3 months is considered reasonable. This meta-analysis calls for well-designed prospective RCTs to evaluate the efficacy and safety of different ICIs in the bridging or downstaging treatment of HCC prior to LT.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DL: Formal Analysis, Conceptualization, Writing – original draft. XW: Formal Analysis, Methodology, Writing – review & editing. XL: Data curation, Formal Analysis, Methodology, Writing – original draft. JL: Formal Analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Scientific Research Project of Wu Jieping Medical Foundation (No. 320.6750.2024-06-41), PRP/SDLPA2101-2025, awarded by the Shandong Pharmacists Association for the project “Hospital Pharmacy Optimization and Development”, and the Clinical Research Fund for Hospital Pharmacy Optimization and Development of Shandong Pharmacists Association.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1689820/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, et al. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. (2022) 161:108–18. doi: 10.1016/j.ejca.2021.11.023
3. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
4. Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian V, Yao F, et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg. (2022) 157:779–88. doi: 10.1001/jamasurg.2022.2804
5. Tabrizian P, Abdelrahim M, and Schwartz M. Immunotherapy and transplantation for hepatocellular carcinoma. J Hepatol. (2024) 80:822–5. doi: 10.1016/j.jhep.2024.01.007
6. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
7. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
8. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2022) 23:77–90. doi: 10.1016/S1470-2045(21)00604-5
9. Sangro B, Kudo M, Erinjeri JP, Galle PR, Chan SL, Knox JJ, et al. Durvalumab with or without bevacizumab with transarterial chemoembolisation in hepatocellular carcinoma (EMERALD-1): a multiregional, randomised, double-blind, placebo-controlled, phase 3 study. Lancet. (2025) 405:216–32. doi: 10.1016/S0140-6736(24)01870-1
10. Marron TU, Fiel MI, Hamon P, Fiaschi N, Kim E, Ward SC, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. (2022) 7:219–29. doi: 10.1016/S2468-1253(21)00385-X
11. Rezaee-Zavareh MS, Yeo YH, Wang T, Huang DQ, Tabrizian P, Thrift AP, et al. Impact of pre-transplant immune checkpoint inhibitor use on post-transplant outcomes in HCC: a systematic review and individual patient data meta-analysis. J Hepatol. (2025) 82:107–19. doi: 10.1016/j.jhep.2024.09.015
12. Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses. vivo. J Immunol. (2005) 174:3408–15. doi: 10.4049/jimmunol.174.6.3408
13. Xu Y, Yan Y, Liu D, Zhang C, Li Q, Shen Y, et al. Risk of transplant rejection associated with ICIs prior to liver transplantation in HCC: a multicenter retrospective study. Int Immunopharmacol. (2024) 143:113400. doi: 10.1016/j.intimp.2024.113400
14. Guo Z, Liu Y, Ling Q, Xu X, Xu J, Xu F, et al. Pretransplant use of immune checkpoint inhibitors for hepatocellular carcinoma: a multicenter, retrospective cohort study. Am J Transplant. (2024) 24:1837–56. doi: 10.1016/j.ajt.2024.05.009
15. Tabrizian P, Holzner ML, Ajmera V, Florman S, Schwartz M, Hoteit M, et al. Intention-to-treat outcomes of patients with hepatocellular carcinoma receiving immunotherapy before liver transplant: the multicenter VITALITY study. J Hepatol. (2025) 82:512–22. doi: 10.1016/j.jhep.2024.10.012
16. Lv Z, Xiang X, Yong JK, Chen Z, Wang W, Wu H, et al. Pembrolizumab in combination with LEnvatinib in participants with hepatocellular carcinoma before liver transplant as Neoadjuvant TherapY-PLENTY pilot study. Int J Surg. (2024) 110:6647–57. doi: 10.1097/JS9.0000000000001567
17. Moher D, Liberati A, Tetzlaff J, and Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. The Joanna Briggs Institute. Critical appraisal tools for use in JBI systematic reviews (2025). Available online at: https://jbi.global/critical-appraisal-tools (Accessed January 21, 2025).
19. Lu X, Zhu Q, Cai J, Wei T, Dong Z, Chen J, et al. Pretransplant immunotherapy increases acute rejection yet improves survival outcome of HCC patients with MVI post-liver transplantation. Cancer Immunol Immunother. (2024) 74:18. doi: 10.1007/s00262-023-03578-1
20. Wang T, Chen Z, Liu Y, Li M, Zhang Y, Fan J, et al. Neoadjuvant programmed cell death 1 inhibitor before liver transplantation for HCC is not associated with increased graft loss. Liver Transpl. (2023) 29:598–606. doi: 10.1097/LVT.0000000000000123
21. Duan BW, Li WL, Cao JN, Wang ML, Wang GS, and Liu JF. Immune checkpoint inhibitors combined with TKIs as a bridge therapy for advanced HCC before liver transplantation. Chin J Hepatobiliary Surg. (2022) 28:28–32. doi: 10.3760/cma.j.cn113884-20210806-00254
22. Qiao ZY, Zhang ZJ, Lv ZC, Chen Z, Li ZW, Wang GS, et al. Neoadjuvant programmed cell death 1 (PD-1) inhibitor treatment in patients with hepatocellular carcinoma before liver transplant: a cohort study and literature review. Front Immunol. (2021) 12:653437. doi: 10.3389/fimmu.2021.653437
23. Demetris AJ, Bellamy C, Hübscher SG, O’Leary J, Randhawa PS, Feng S, et al. 2016 comprehensive update of the Banff working group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. (2016) 16:2816–35. doi: 10.1111/ajt.13909
24. Yin J, Wen M, Cheng J, Wang Y, Yi S, Xie Y, et al. A patient with failed liver transplantation after the use of PD-1 blockade combined with lenvaxen. Front Med (Lausanne). (2022) 9:712466. doi: 10.3389/fmed.2022.712466
25. Nordness MF, Hamel S, Godfrey CM, Shi C, Johnson DB, Goff LW, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant. (2020) 20:879–83. doi: 10.1111/ajt.1570
26. Levitsky J, Goldberg D, Smith AR, Mansfield SA, Shetty K, Hoteit M, et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenterol Hepatol. (2017) 15:584–593.e2. doi: 10.1016/j.cgh.2016.07.028
27. Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2023 annual data report: liver. Am J Transplant. (2025) 25:S193–287. doi: 10.1016/j.ajt.2024.11.008
28. Ronca V, Wootton G, Milani C, and Cain O. The immunological basis of liver allograft rejection. Front Immunol. (2020) 11:2155. doi: 10.3389/fimmu.2020.02155
29. Wassmer CH, El Hajji S, Papazarkadas X, Poletti PA, and Toso C. Immunotherapy and liver transplantation: a narrative review of basic and clinical data. Cancers (Basel). (2023) 15:4574. doi: 10.3390/cancers15184574
30. Kuo FC, Chen CY, Lin NC, Liu C, Hsia CY, and Loong CC. Optimizing the safe washout period for liver transplantation following immune checkpoint inhibitors with atezolizumab, nivolumab, or pembrolizumab. Transplant Proc. (2023) 55:878–83. doi: 10.1016/j.transproceed.2023.04.006
31. Lickliter JD, Gan HK, Voskoboynik M, Arkenau HT, Moreno V, Fischer T, et al. A first-in-human dose finding study of camrelizumab in patients with advanced or metastatic cancer in Australia. Drug Des Devel Ther. (2020) 14:1177–89. doi: 10.2147/DDDT.SS237987
32. Lee KW, Lee DH, Kang JH, Kim DW, Kim MR, Oh DY, et al. Phase I pharmacokinetic study of nivolumab in Korean patients with advanced solid tumors. Oncologist. (2018) 23:155–e17. doi: 10.1634/theoncologist.2017-0244
33. Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. (2015) 21:4286–93. doi: 10.1158/1078-0432.CCR-14-2607
34. Tang B, Yan X, Sheng X, Si L, Cui C, Kong Y, et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol. (2019) 12:7. doi: 10.1186/s13045-018-0693-2
35. Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. (2018) 119:538–45. doi: 10.1038/s41416-018-0100-3
36. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. (2010) 28:3167–75. doi: 10.1200/JCO.2009.26.7609
37. Dong Y, Sun Q, and Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. (2017) 8:2171–86. doi: 10.18632/oncotarget.13856
38. Wang L, Han R, and Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. (2007) 37:2983–90. doi: 10.1002/eji.200737600
39. Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. (2010) 10:40–6. doi: 10.1111/j.1600-6143.2009.02862.x
40. Li W, Zheng XX, Kuhr CS, and Perkins JD. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant. (2005) 5:978–86. doi: 10.1111/j.1600-6143.2005.00792.x
41. Kulkarni AV, Kumaraswamy P, Menon B, Goel A, Thomas SS, Duseja A, et al. Downstaging with atezolizumab-bevacizumab: a case series. J Liver Cancer. (2024) 24:224–33. doi: 10.17998/jlc.2024.05.13
42. Tabrizian P, Florman SS, and Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. (2021) 21:1979–80. doi: 10.1111/ajt.1649
43. Schnickel GT, Fabbri K, Hosseini M, Berumen J, Cao S, David R, et al. Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am J Transplant. (2022) 22:1699–704. doi: 10.1111/ajt.16977
44. Dave S, Yang K, Schnickel GT, Hosseini M, Ross DJ, Sadeghi N, et al. The impact of treatment of hepatocellular carcinoma with immune checkpoint inhibitors on pre- and post-liver transplant outcomes. Transplantation. (2022) 106:e308–9. doi: 10.1097/TP.0000000000004089
45. Chen Z, Hong X, Wang T, Li M, Zhang Y, Fan J, et al. Prognosis after liver transplantation in patients treated with anti-PD-1 immunotherapy for advanced hepatocellular carcinoma: case series. Ann Palliat Med. (2021) 10:9354–61. doi: 10.21037/apm-21-1863
46. Reynolds KL and Guidon AC. Diagnosis and management of immune checkpoint inhibitor-associated neurologic toxicity: illustrative case and review of the literature. Oncologist. (2019) 24:435–43. doi: 10.1634/theoncologist.2018-0359
47. Waqas A, Zaffar J, Jalil A, and Butt S. Nivolumab-induced isolated neutropenia. Cureus. (2023) 15:e45675. doi: 10.7759/cureus.45675
48. Luoma AM, Suo S, Wang Y, Gunasti L, Porter CBM, Nabilsi N, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. (2022) 185:2918–2935.e29. doi: 10.1016/j.cell.2022.06.018
49. Gebauer BS, Hricik DE, Atallah A, Bryan C, Knapp C, Gozdziak P, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant. (2002) 2:857–66. doi: 10.1034/j.1600-6143.2002.20912.x
50. Yi G, Yi R, Chen X, Peng L, Huang G, Fu M, et al. The role of soluble programmed death protein-1 (sPD-1) and soluble programmed death ligand-1 (sPD-L1) in rat corneal transplantation rejection. Adv Clin Exp Med. (2021) 30:93–100. doi: 10.17219/acem/89803
51. Choudhary A, Meijers W, Besharati S, Zhu Q, Lindenfeld J, Brinkley M, et al. Role of PD-L1 in heart transplant rejection. Eur Heart J. (2020) 41:ehaa946.1106. doi: 10.1093/ehjci/ehaa946.1106
52. Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, and Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: A dermatology perspective on management. J Cutan Med Surg. (2021) 25:59–76. doi: 10.1177/1203475420943260
53. Sharma P, Hu-Lieskovan S, Wargo JA, and Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
54. Li JT, Gu A, Tang NN, Zengin G, Li MY, and Liu Y. Patient-derived xenograft models inpan-cancer: From bench to clinic. Interdiscip. Med. (2025) 3:e20250016. doi: 10.1002/INMD.20250016
Keywords: hepatocellular carcinoma, immune checkpoint inhibitors, liver transplant, allograft rejection, meta-analysis
Citation: Liu D, Wang X, Liu X and Li J (2025) Navigating the risks: a systematic review of immune checkpoint inhibitor therapy before liver transplant for hepatocellular carcinoma and its impact on allograft rejection and survival outcomes. Front. Oncol. 15:1689820. doi: 10.3389/fonc.2025.1689820
Received: 20 August 2025; Accepted: 10 October 2025;
Published: 29 October 2025.
Edited by:
Yan Yan, Mayo Clinic Florida, United StatesReviewed by:
Meng-Yao Li, Shanghai Jiao Tong University, ChinaWenya Tian, IQVIA Laboratories, United States
Copyright © 2025 Liu, Wang, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqaW5nMTk3MjEyQDEyNi5jb20=
 Donghua Liu
Donghua Liu Jing Li
Jing Li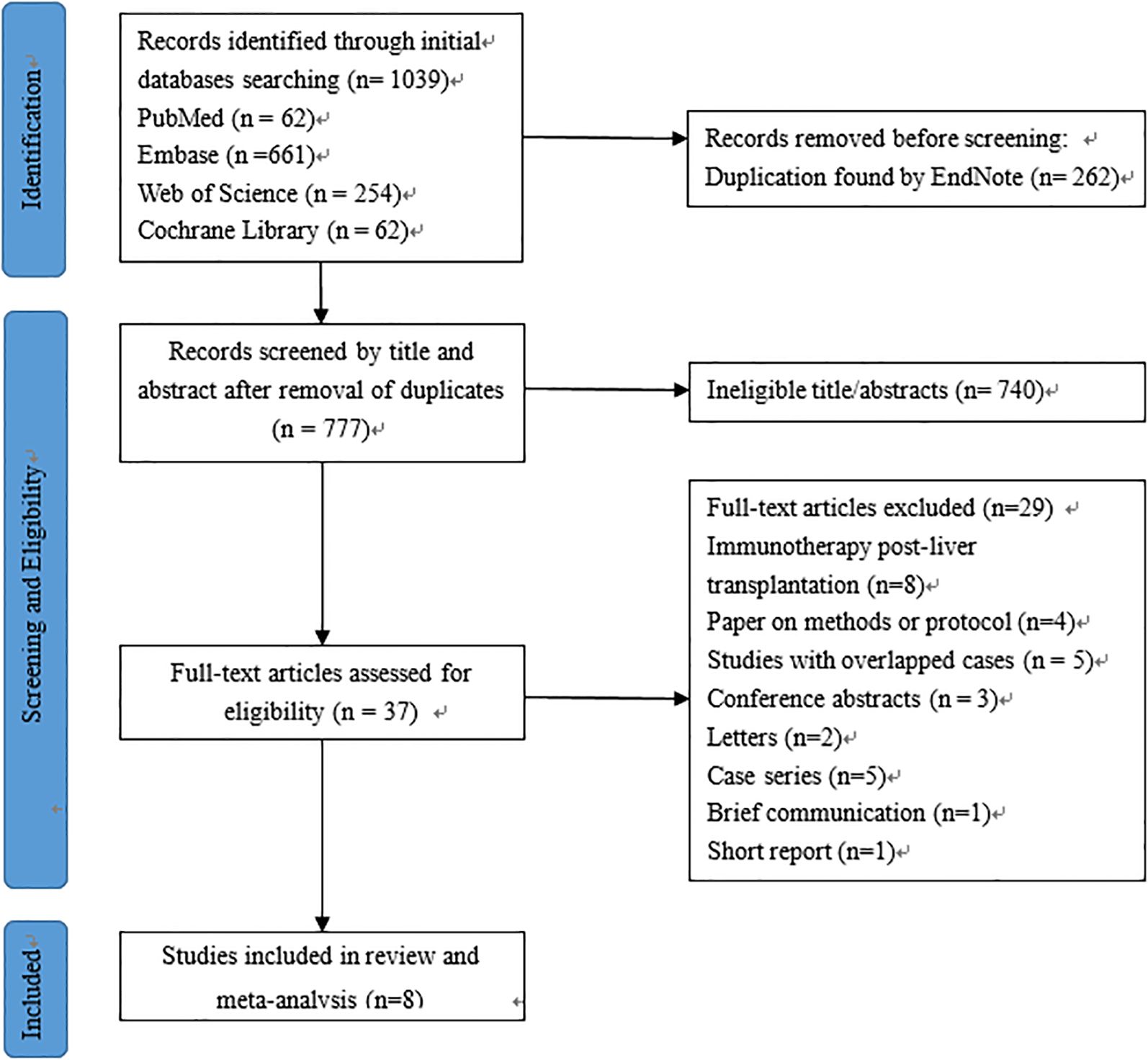
![Forest plot showing the results of eight studies, listing authors, years, events, totals, proportions, confidence intervals (CI), and weights. The random effects model shows a proportion of 0.18 with a 95% CI of [0.07, 0.28]. Total heterogeneity is 73.7%, with a p-value of 0.0004.](https://www.frontiersin.org/files/Articles/1689820/fonc-15-1689820-HTML/image_m/fonc-15-1689820-g002.jpg)
![Forest plot displaying the results of eight studies. Each study is represented with event and total counts, a proportion with a 95% confidence interval, and weight. Studies listed are from 2021 to 2024. Proportions range from 0.00 to 0.10. The common effect model shows a proportion of 0.04 with a 95% confidence interval of [0.01; 0.07], and total weight of 100.0%. Heterogeneity is zero with a p-value of 0.6343.](https://www.frontiersin.org/files/Articles/1689820/fonc-15-1689820-HTML/image_m/fonc-15-1689820-g005.jpg)