- 1Department of Surgery, School of Clinical Sciences, Monash University, Clayton, VIC, Australia
- 2Department of Gastroenterology and Hepatology, Monash Health, Melbourne, VIC, Australia
- 3Department of Medical Oncology, Western Health, VIC, Australia
- 4Oncology Department, Peninsula And Southeast Oncology, Frankston, VIC, Australia
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy with a dismal prognosis. Molecular profiling to improve the diagnosis and management of PDAC holds promise for informing more targeted therapies such as Kirsten rat sarcoma viral oncogene homologue (KRAS) G12C inhibition to deliver better outcomes for these patients. In this report, we present two patients with advanced PDAC with KRAS G12C mutations who achieved remarkable disease control and prolonged survival following treatment with the KRAS G12C inhibitor D1553 (garsorasib). Case 1, a 74-year-old woman with recurrent PDAC post-surgical resection and chemotherapy, exhibited a significant biochemical and radiologic response upon initiation of targeted therapy. Case 2, a 75-year-old woman initially treated with Folinic acid, fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) and stereotactic body radiotherapy (SBRT), demonstrated sustained disease stability for over three years on KRAS G12C inhibitor therapy. Both patients maintained excellent performance status with minimal treatment-related toxicity. These cases underscore the potential of KRAS-directed therapies in PDAC and illustrate the importance of molecular profiling in identifying eligible patients. The findings support further investigation into the durability of KRAS G12C inhibition, resistance mechanisms, and combination treatment strategies to optimize patient outcomes.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer, accounting for about 90% of all cases. PDAC ranks as the seventh most common cause of cancer-related mortality worldwide with a five-year survival rate below 10%. However, projections indicate that by 2030, it will rise to become the second leading cause of cancer deaths globally (1). In 2025, an estimated 67,440 Americans are expected to be diagnosed with PDAC, with approximately 51,980 anticipated deaths (2). The incidence rates are approximately four times higher in countries with a higher Human Development Index (HDI) compared to those with a lower HDI with the highest rates of occurrence are observed in Europe, North America, and Australia/New Zealand (1). Currently, over 80% of individuals diagnosed with PDAC have either locally advanced or metastatic disease at the time of presentation. In such cases, systemic chemotherapy is the primary therapeutic approach. Although there have been gradual improvements in treatment strategies, the overall outlook for patients remains bleak, with a median five-year survival rate lingering at a mere 10% (3).
Genetic alterations in KRAS are detected in nearly 90% of pancreatic ductal adenocarcinomas, the most common form of pancreatic malignancy (4). The KRAS G12C variant (characterized by the substitution of glycine with cysteine at codon 12) is identified in roughly 1–2% of affected individuals (5). Sotorasib, a targeted small-molecule inhibitor, selectively and irreversibly binds to KRAS G12C, blocking its oncogenic activity. The U.S. Food and Drug Administration granted expedited approval for sotorasib in the treatment of patients with KRAS G12C mutated non–small-cell lung cancer (NSCLC) who have undergone at least one prior systemic therapy (6). D-1553 (garsorasib), a potent and selective inhibitor of KRAS G12C mutation, represented a promising therapeutic agent for treating NSCLC patients with this mutation (7). Additionally, a single-group phase 1–2 trial indicated that in individuals with advanced PDAC harboring the KRAS G12C mutation who had undergone prior systemic therapy, sotorasib demonstrated therapeutic efficacy against the malignancy while maintaining a tolerable safety profile. The treatment was associated with a median overall survival (OS) of 6.9 months and a progression-free survival (PFS) of 4.0 months (8).
The application of KRAS G12C inhibitors in PDAC is still being evaluated, but early clinical data suggests therapeutic benefits for selecting patients. In this report, we describe two exceptional cases of advanced PDAC patients with KRAS G12C mutations who achieved long-term disease control following treatment with the investigational KRAS G12C inhibitor D1553. These cases highlight the potential of targeted therapy informed by molecular profiling to potentially extend survival and improve quality of life in PDAC. Furthermore, they underscore the importance of continued research into combination therapies and resistance mechanisms to optimize treatment efficacy and patient outcomes.
Methods
Clinical and molecular information for the patients were sourced from the Victorian Pancreatic Cancer Biobank and Endoscopic Ultrasound Molecular Evaluation of Pancreatic Cancer (EU-ME-PC) trial (ACTRN12620000762954) (9), following approval from the local institutional ethics committees (HREC/15/MonH/117 and HREC/61006/MonH-2020-200407). Prior to inclusion, patients gave explicit written consent for the use of anonymized clinical and molecular data, as well as deidentified images, in research and publication. KRAS mutations were initially identified using the KRAS StripAssay (ViennaLab). To further characterize the tumors, comprehensive genomic profiling was conducted using the TruSight™ Oncology 500 (TSO-500) panel, which requires a minimum tumor cellularity of approximately 10-20% to ensure reliable variant detection (although variant allele frequencies as low as 1% were accepted) as part of the EU ME PC trial (9).
Case description
Case 1
A 74-year-old woman presented with progressive abdominal and back pain, along with significant unintentional weight loss over several months in July 2018. Her past medical history included osteoarthritis (OA) with bilateral hip replacements.
A CT scan revealed an 8.7 × 4.5 × 5.3 cm pancreatic mass. A comparative analysis of prior imaging (Figure 1A) and the most recent scan (Figure 1B) showed significant morphological changes over an 8-year period. Notably, serum CA 19–9 was significantly elevated at 5542 U/m, but liver function tests were normal.
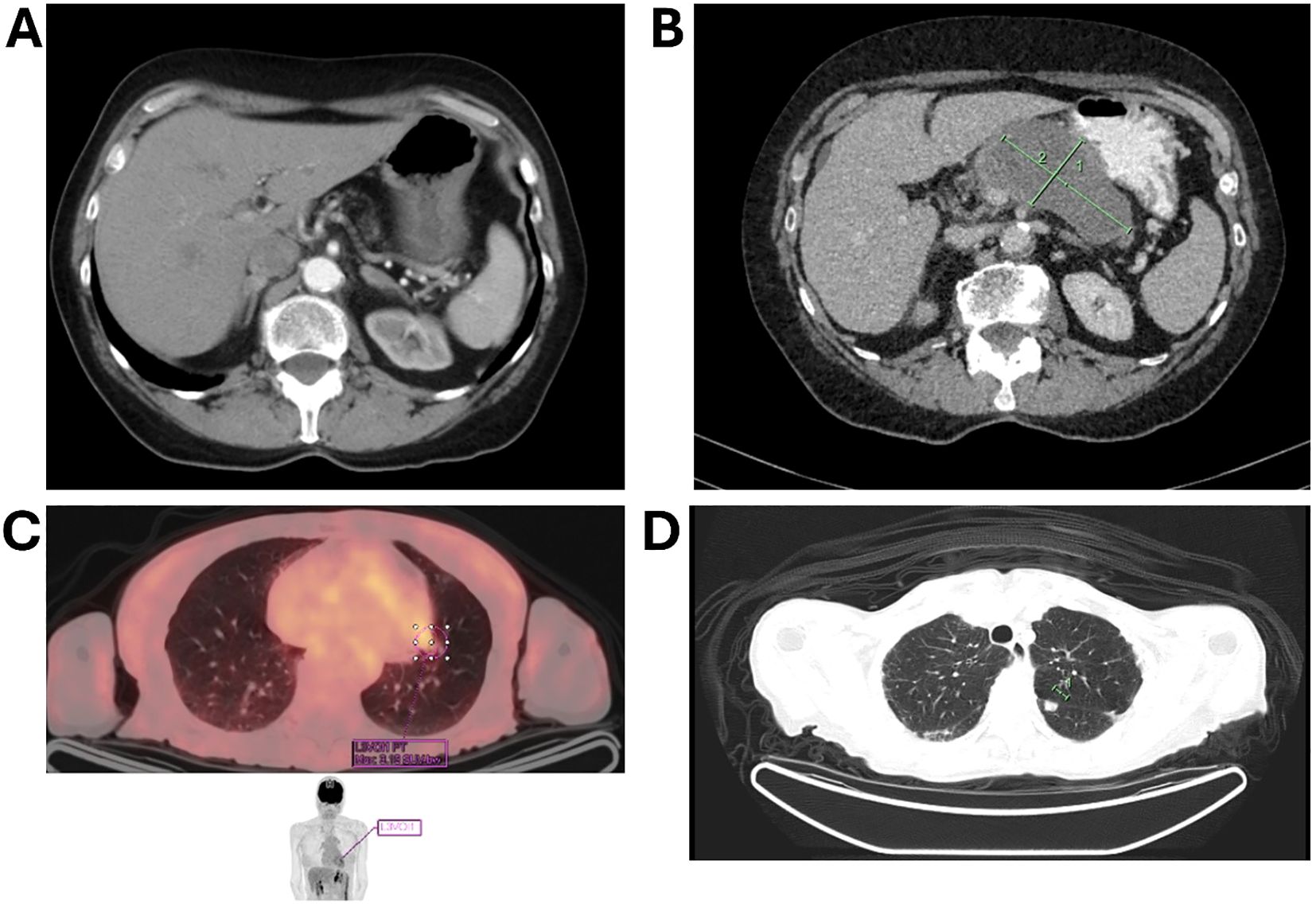
Figure 1. CT imaging comparison demonstrating an 8.7 × 4.5 × 5.3 cm pancreatic mass. (A) earlier scan; (B) a scan after 8 years, illustrating significant morphological changes over 8 years; (C) imaging of a solitary lung lesion indicative of early metastatic spread; (D) CT scan demonstrating a second lung lesion treated with radiotherapy.
Subsequent MRI revealed a heterogeneous mass within the pancreatic body and tail without notable vascularity or enhancement associated with portal venous stenosis with splenic vein occlusion. Endoscopic ultrasound showed a 15 cm hypodense mass; FNA cytology was inconclusive due to extensive necrosis. A separate pass was taken and frozen for biobanking with the plan for subsequent genomic profiling.
The patient was diagnosed with borderline resectable pancreatic cancer and was referred for pseudoneoadjuvant chemotherapy. Treatment with gemcitabine and nab-paclitaxel resulted in a marked reduction in tumor size and CA 19–9 level, which fell to 58 IU/mL (Figure 1C). However, chemotherapy was discontinued after 2.5 cycles due to severe skin toxicity.
The patient then underwent surgical resection with en-bloc distal pancreatectomy, splenectomy, and near-total gastrectomy, along with a circumferential portal vein resection. The final pathology report confirmed pT3N0 disease with invasion into the gastric muscularis propria. All surgical margins were negative, and no lymphovascular or perineural invasion was detected. A KRAS StripAssay revealed a KRAS G12C mutation.
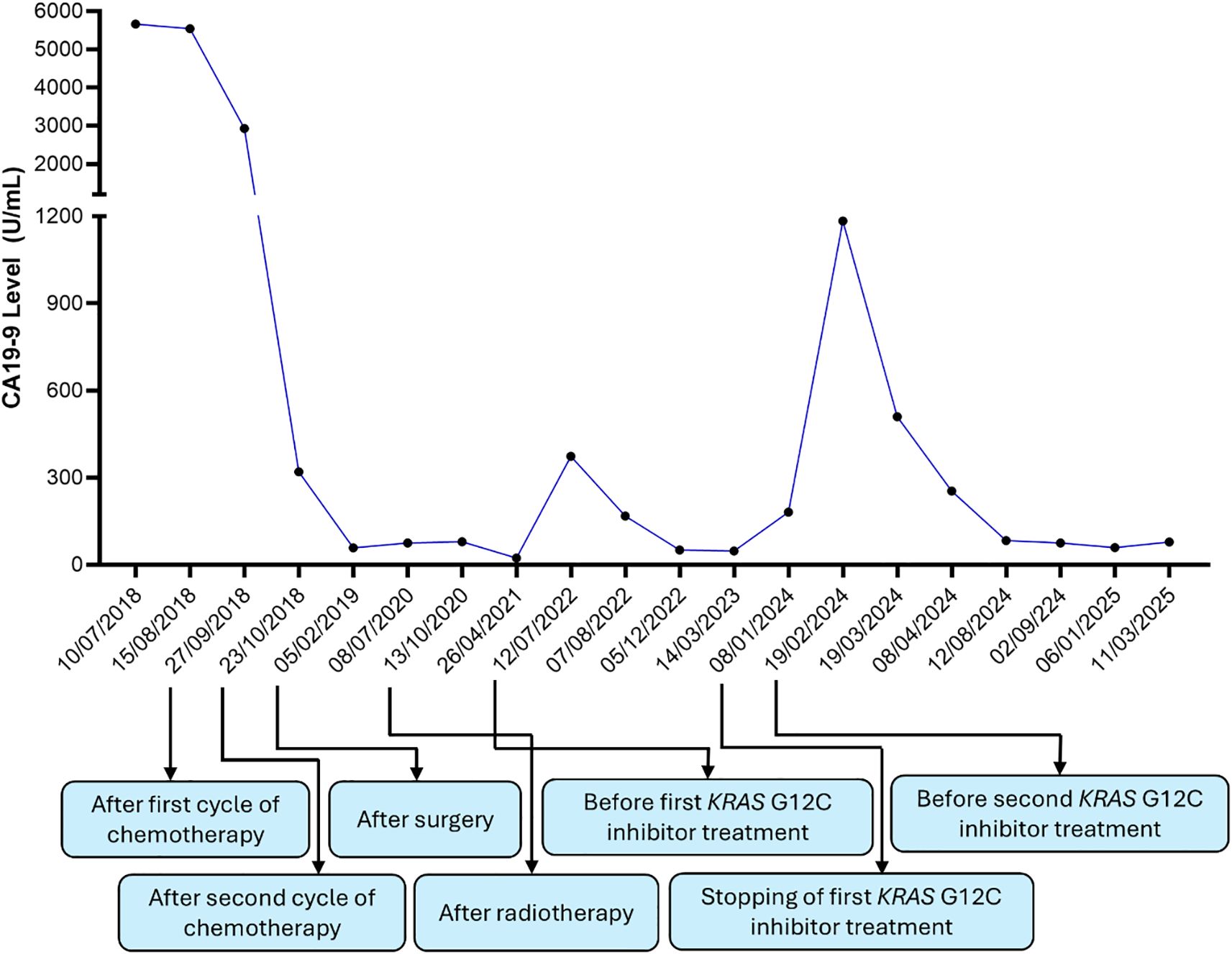
During routine follow-up, approximately 12 months after surgery (around 18 months from initial diagnosis), serial imaging and biomarker assessments showed disease progression. A solitary lung lesion was identified and successfully resected which was confirmed to be metastatic pancreatic cancer (Figure 1C). Later, a second pulmonary lesion was detected and treated with radiotherapy (Figure 1D), with subsequent imaging demonstrating a positive therapeutic response (Figure 2A). Despite these interventions, recurrence of the initial lung lesion occurred (Figure 2B), necessitating repeat radiotherapy, which again yielded a favorable response (Figure 2C). Eventually, new PET-avid pulmonary lesions were detected, coinciding with a rise in CA 19–9 levels, suggesting further disease progression.
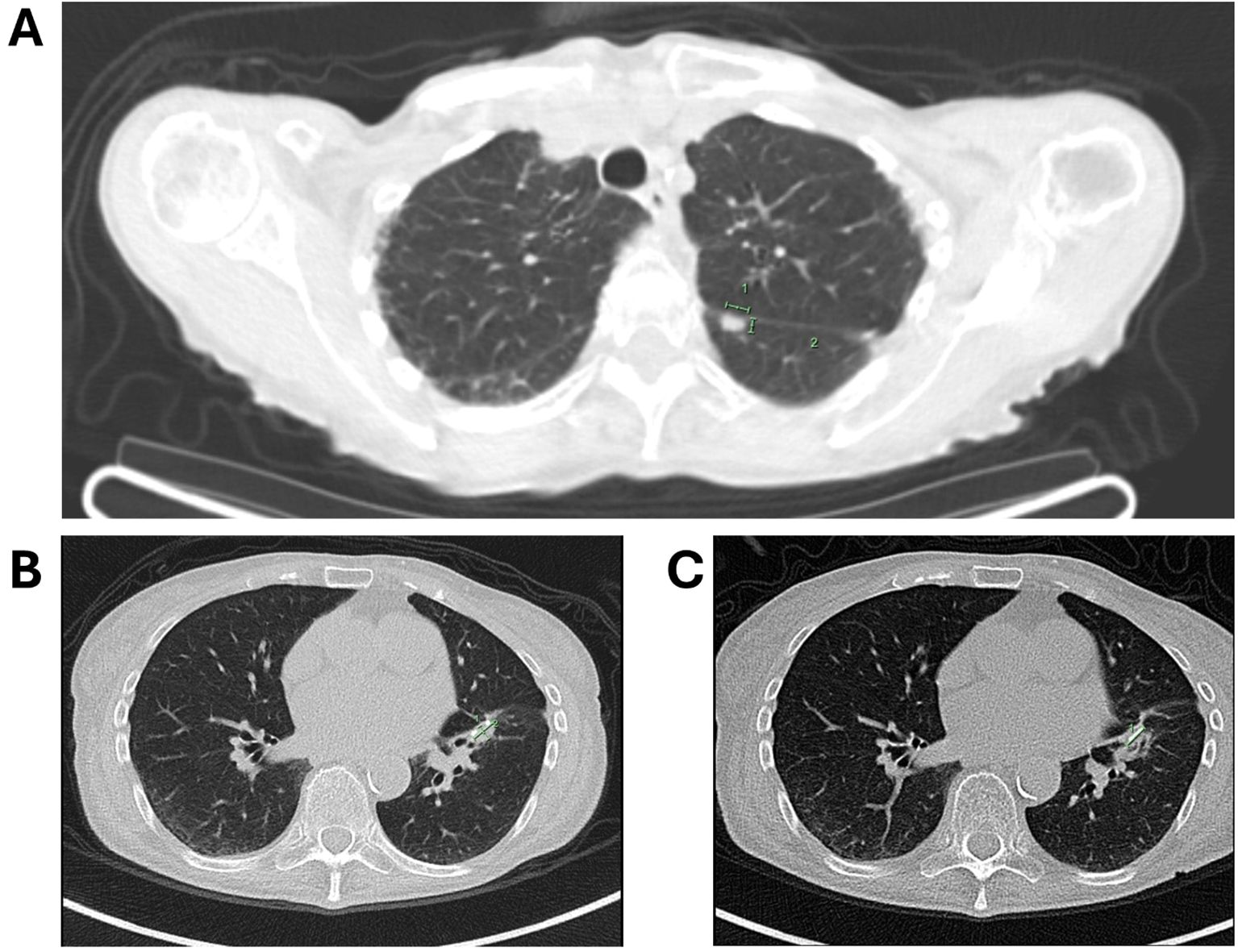
Figure 2. (A) Follow-up imaging showing a good response to radiotherapy for the second lung lesion; (B) imaging depicting the recurrence of the first lung lesion; (C) subsequent imaging showing recurrence of the first lung lesion with a favorable response to radiotherapy.
She was enrolled in the EU ME PC study and TSO-500 panel identified the previously known KRAS G12C mutation. Additional findings included a low Tumour Mutation Burden (TMB) at 6.3 mutations per megabase (mut/Mb), and the absence of Microsatellite Instability (MSI) with only 0.9% unstable sites. The KRAS G12 Variant Allele Frequency (VAF) was 12.6%.
Given these findings, the patient was enrolled in a clinical trial investigating a KRAS G12C inhibitor, D1553 on 8 June 2022 (NCT04585035). Following the initiation of KRAS G12C inhibitor therapy, the patient exhibited a significant biochemical response, with a fall in CA 19–9 levels and a complete metabolic response on PET scan. The treatment was associated with moderate diarrhea (intermittent, grade 1-2) which was controlled with loperamide. The response was durable, lasting almost 16 months before a gradual rise in CA 19-9, suggesting resistance. She then commenced on a second KRAS G12C inhibitor, RMC-6291, also known as elironrasib as part of a clinical trial (NCT05462717), with a further complete metabolic response and return of CA 19–9 to near normal (Figure 3). The patient remains well for 7 years following the initial diagnosis.
Case 2
A 75‐year‐old (original age of patient at start) female who had no significant past medical history and first presented with vague abdominal pain and nausea in late 2019. Imaging revealed a locally advanced pancreatic mass in the uncinate process with arterial and venous encasement but no distant metastases. EUS-FNA confirmed PDAC. She received eight cycles of modified FOLFIRINOX with excellent radiological and biochemical response, followed by stereotactic body radiotherapy (SBRT).
Molecular testing of the formalin-fixed EUS-FNA biopsy using a KRAS StripAssay previously identified a KRAS G12C mutation. Comprehensive molecular profiling was then attempted on the formalin-fixed EUS-FNB specimen with the TSO-500 panel; however, this was unsuccessful. Her CA19–9 level subsequently rose to 103, and repeat CT and PET imaging in May 2022 demonstrated persistent avidity in the uncinate process tumor without evidence of metastases. She was enrolled in the trial investigating D1553–101 as described above (NCT04585035). Over more than three years on therapy, she has maintained sustained disease control, with serial imaging showing partial response (primary lesion reduced from ~15 mm to 10 mm) and a persistent normalization of her CA19–9 level. Regular assessments by the oncology team have confirmed that her disease remains controlled, reflecting a favorable biological response to targeted therapy and she has maintained an excellent performance status throughout her treatment. The targeted therapy has been well tolerated overall, with only minor adverse effects reported. She experiences mild gastrointestinal symptoms (characterized by fluctuating bowel habits and low-grade abdominal cramping) which have been managed effectively with supportive measures such as pancreatic enzyme supplementation (Creon) and, when necessary, dose adjustments of D1553-101 (for instance, a temporary reduction to 400 mg twice daily was implemented to alleviate symptoms).
Just over four years after her PDAC diagnosis, she developed right-sided colon cancer [T3N0, mismatch repair (MMR) deficient B-Raf proto-oncogene, serine/threonine kinase (BRAF) positive tumor consistent with a colorectal primary] treated with laparoscopic right hemicolectomy without adjuvant therapy. She is now almost 6 years from her initial PDAC diagnosis, with high overall quality of life and normal daily activity.
Discussion
The cases described here exemplify the transformative potential of targeted therapy in PDAC, particularly in the subset of patients harboring the KRAS G12C mutation. Historically, PDAC has been associated with poor prognosis, limited treatment options, and resistance to conventional chemotherapy (10). Both of these patients had KRAS testing as part of screening for potential inclusion in a trial of anti- Epidermal growth factor receptor (EGFR) monoclonal antibody, panitumumab, in KRAS wild-type pancreatic cancer (11). Although they were not candidates for that trial with the advent of KRAS G12C inhibitors, they became eligible for an exciting new therapeutic approach for a mutation that long considered undruggable (12). However, there is still only a handful of clinical trials reporting the efficacy of KRAS G12C inhibitors in improving survival in PDAC (8, 13–16).
KRAS mutations occur in over 90% of pancreatic ductal adenocarcinomas (PDAC), with G12D being the most common variant. KRAS G12C, while less frequent, offers the opportunity for targeted intervention due to the development of covalent inhibitors that irreversibly bind to the mutant cysteine residue, disrupting oncogenic signaling (17). There is limited data on the efficacy of KRAS G12C inhibitors in improving OS in PDAC subjects. A recent study revealed that the median PFS was 4.0 months (95% CI, 2.8 to 5.6), and the median OS was 6.9 months (95% CI, 5.0 to 9.1) in PDAC patients receiving Sotorasib (8). Since our cases demonstrated nearly 40 months of survival (as of July 2025) after treatment with D1553, they are considered exceptional. D1553, the investigational KRAS G12C inhibitor administered to both patients in this study, provided sustained clinical benefit, highlighting the therapeutic potential of targeting this pathway. It should be noted that a trial (NCT04585035) on the efficacy of D1553 on KRAS G12C harboring PDAC subjects is underway and will add to the potential therapeutic arsenal available for these patients.
Case 1 exhibited progressive disease despite multimodal interventions, including chemotherapy, extensive surgical resection, and metastasectomy and radiotherapy for pulmonary recurrences. Her genomic profile revealed a low TMB and stable MSI, suggesting limited responsiveness to immunotherapy (18). The identification of KRAS G12C allowed enrollment in a clinical trial, providing a molecularly guided treatment strategy. Within months of initiating therapy, a marked biochemical response was observed, as indicated by declining CA 19–9 levels. The durable disease control seen in the patient, who was previously refractory to a combination of local and systemic therapies, highlights the efficacy of KRAS G12C inhibition in a heavily pretreated patient.
Similarly, case 2 demonstrated an exceptional response following treatment with D1553. Her initial management with FOLFIRINOX and SBRT provided significant tumor reduction; however, the sustained benefit over a three-year period while on KRAS G12C inhibition is remarkable. The stable disease status, maintained tumor shrinkage, and minimal toxicity emphasize the potential of targeted therapies to not only improve survival but also maintain quality of life in patients with advanced PDAC. Her case further validates the role of KRAS-directed therapy as an effective long-term treatment strategy.
Unlike conventional chemotherapy, which often results in significant toxicity limiting long-term use, KRAS G12C inhibitors offer a more tolerable safety profile (8). Both patients remained active and independent, experiencing only mild gastrointestinal symptoms that were effectively managed with pancreatic enzyme supplementation (Creon) and dose adjustments. This is a critical consideration in PDAC management, where treatment-induced morbidity frequently diminishes quality of life.
In case 2, ongoing monitoring confirmed disease stability with no new metastatic lesions - a notable outcome given the historical progression pattern of PDAC. Case 1, despite prior pulmonary metastases, achieved prolonged disease control, further reinforcing the durability of KRAS-targeted inhibition. The ability to maintain stable disease over extended periods is particularly relevant given the limited options traditionally available for patients who progress on standard chemotherapy.
While the results observed in these cases are encouraging, several challenges remain in the broader application of KRAS G12C inhibitors. One of the primary concerns is the potential emergence of resistance mechanisms, which have been reported in other malignancies treated with KRAS-targeted agents (19). In our case, repeat biopsy was not performed at the time of resistance after 16 months on D1553 therapy; therefore, we were unable to directly characterize the molecular mechanisms underlying resistance. However, prior studies have identified several mechanisms of acquired resistance to KRAS G12C inhibitors. These include secondary mutations within KRAS itself (such as Y96D, R68S, and H95 substitutions) that impair drug binding, KRAS amplification leading to sustained signaling, and alterations in parallel or downstream pathways including RTK, PI3K, SHP2, and MAPK signaling (12). While our patient was subsequently enrolled in a second KRAS G12C inhibitor trial (RMC-6291), the precise resistance mechanism remains unknown. This highlights the importance of repeat biopsy and molecular profiling at progression to inform next-line therapeutic strategies.
Adaptive signaling pathways, secondary mutations, and compensatory oncogenic circuits may eventually limit the efficacy of monotherapy approaches. Future research should explore combination strategies, such as dual inhibition of KRAS and other key signaling pathways (such as SHP2, PI3K, or MEK), to enhance and prolong responses. Furthermore, KRAS mutations are not uniform in PDAC, and only a subset of patients harbors the G12C alteration. Expanding the therapeutic reach of targeted agents to include other KRAS variants, such as G12D and G12V, remains an active area of investigation (17). The development of pan-KRAS inhibitors and allele-specific agents will be crucial in broadening treatment applicability.
It should be noted that germline testing was not performed in these cases. Nonetheless, germline alterations in DNA damage repair genes such as BRCA1/2, PALB2, and ATM, as well as MMR deficiency, are clinically relevant in PDAC due to their therapeutic implications (20). Patients harboring BRCA1/2 or PALB2 mutations may benefit from platinum-based chemotherapy and Poly (ADP-ribose) polymerase (PARP) inhibitors (21), whereas those with MMR deficiency may be eligible for immune checkpoint inhibitor therapy (22). Notably, Case 2 later developed an MMR deficient colon cancer, although this appears to have been somatic rather than germline given the positive B-raf immunohistochemistry. Nevertheless, this highlights the importance of incorporating germline testing into the management of PDAC, both to guide targeted therapy and to inform genetic counseling for patients and families.
Conclusion
These two cases signal a paradigm shift in the management of PDAC with the move toward more targeted therapy such as KRAS G12C inhibitors. The remarkable clinical responses, sustained disease control, and improved quality of life underscore the potential of molecularly driven therapies in an otherwise treatment-refractory malignancy. Nonetheless, we acknowledge that these cases may be subject to selection and confounding biases, and the prolonged survival could partly reflect unusually indolent tumor biology rather than solely the therapeutic effect of KRAS G12C inhibition. As case reports inherently highlight exceptional outcomes, the findings should be interpreted with caution until validated in prospective clinical trials. While further research is needed to optimize treatment sequencing, overcome resistance, and expand the spectrum of targetable KRAS mutations, these cases provide compelling evidence supporting the integration of targeted therapy into PDAC management. The success of KRAS G12C inhibition represents a significant step forward in addressing the unmet needs of this challenging disease, offering renewed hope to patients and clinicians alike.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Monash Health, Monash University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SA: Writing – original draft, Investigation, Data curation, Writing – review & editing. OM: Writing – original draft, Data curation, Investigation, Visualization, Writing – review & editing. LL: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. VG: Writing – review & editing, Data curation, Writing – original draft, Methodology, Conceptualization, Investigation. DC: Writing – review & editing, Writing – original draft, Investigation, Formal Analysis, Data curation, Project administration, Validation, Conceptualization, Methodology, Visualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. Ca. (2025) 75:10. doi: 10.3322/caac.21871
3. Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, et al. Chemotherapy for pancreatic cancer. La Presse Médicale. (2019) 48:e159–74. doi: 10.1016/j.lpm.2019.02.025
4. Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. (2016) 531:47–52. doi: 10.1038/nature16965
5. Luo J. KRAS mutation in pancreatic cancer. In: Seminars in oncology. 1600 John F Kennedy Boulevard, Ste 1800, Philadelphia, Usa, Pa, 19103-2899: Elsevier (2021).
6. Nakajima EC, Drezner N, Li X, Mishra-Kalyani PS, Liu Y, Zhao H, et al. FDA approval summary: sotorasib for KRAS G12C-mutated metastatic NSCLC. Clin Cancer Res. (2022) 28:1482–6. doi: 10.1158/1078-0432.CCR-21-3074
7. Li Z, Song Z, Zhao Y, Wang P, Jiang L, Gong Y, et al. D-1553 (Garsorasib), a potent and selective inhibitor of KRAS(G12C) in patients with NSCLC: phase 1 study results. J Thorac Oncol. (2023) 18:940–51. doi: 10.1016/j.jtho.2023.03.015
8. Strickler JH, Satake H, George TJ, Yaeger R, Hollebecque A, Garrido-Laguna I, et al. Sotorasib in KRAS p. G12C–mutated advanced pancreatic cancer. New Engl J Med. (2023) 388:33–43. doi: 10.1056/NEJMoa2208470
9. Masoumi-Moghaddam S, Lundy J, Gao H, Rathi V, Swan M, Desmond C, et al. The EUS molecular evaluation of pancreatic cancer: A prospective multicenter cohort trial. Endoscopic ultrasound. (2021) 10:335–43. doi: 10.4103/EUS-D-20-00230
10. Bengtsson A, Andersson R, and Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. (2020) 10:16425. doi: 10.1038/s41598-020-73525-y
11. Lundy J, Harris M, Zalcberg J, Zimet A, Goldstein D, Gebski V, et al. EUS-FNA biopsies to guide precision medicine in pancreatic cancer: results of a pilot study to identify KRAS wild-type tumours for targeted therapy. Front Oncol. (2021) 11:770022. doi: 10.3389/fonc.2021.770022
12. Liu J, Kang R, and Tang D. The KRAS-G12C inhibitor: activity and resistance. Cancer Gene Ther. (2022) 29:875–8. doi: 10.1038/s41417-021-00383-9
13. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. New Engl J Med. (2020) 383:1207–17. doi: 10.1056/NEJMoa1917239
14. Strickler JH, Satake H, Hollebecque A, Sunakawa Y, Tomasini P, Bajor DL, et al. First data for sotorasib in patients with pancreatic cancer with KRAS p. G12C mutation: a phase I/II study evaluating efficacy and safety. Am Soc Clin Oncol. (2022) 40. doi: 10.1200/JCO.2022.40.36_suppl.360490
15. Bekaii-Saab TS, Spira AI, Yaeger R, Buchschacher GL, McRee AJ, Sabari JK, et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation. Am Soc Clin Oncol. (2022) 40. doi: 10.1200/JCO.2022.40.4_suppl.519
16. Bekaii-Saab TS, Yaeger R, Spira AI, Pelster MS, Sabari JK, Hafez N, et al. Adagrasib in advanced solid tumors harboring a KRAS G12C mutation. J Clin Oncol. (2023) 41:4097–106. doi: 10.1200/JCO.23.00434
17. Linehan A, O’Reilly M, McDermott R, and O’Kane GM. Targeting KRAS mutations in pancreatic cancer: opportunities for future strategies. Front Med. (2024) 11:1369136. doi: 10.3389/fmed.2024.1369136
18. Jardim DL, Goodman A, de Melo Gagliato D, and Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. (2021) 39:154–73. doi: 10.1016/j.ccell.2020.10.001
19. Chour A, Toffart AC, Berton E, and Duruisseaux M. Mechanisms of resistance to KRASG12C inhibitors in KRASG12C-mutated non-small cell lung cancer. Front Oncol. (2024) 14:1328728. doi: 10.3389/fonc.2024.1328728
20. Casolino R, Corbo V, Beer P, Hwang CI, Paiella S, Silvestri V, et al. Germline aberrations in pancreatic cancer: implications for clinical care. Cancers. (2022) 14:3239. doi: 10.3390/cancers14133239
21. Principe DR. Precision medicine for BRCA/PALB2-mutated pancreatic cancer and emerging strategies to improve therapeutic responses to PARP inhibition. Cancers. (2022) 14:897. doi: 10.3390/cancers14040897
22. Coston T, Desai A, Babiker H, Sonbol MB, Chakrabarti S, Mahipal A, et al. Efficacy of immune checkpoint inhibition and cytotoxic chemotherapy in mismatch repair-deficient and microsatellite instability-high pancreatic cancer: Mayo Clinic experience. JCO Precis Oncol. (2023) 7:e2200706. doi: 10.1200/PO.22.00706
Keywords: pancreatic ductal adenocarcinoma, KRAS, G12C inhibitor, chemotherapy, case report
Citation: Aslani S, McKay O, Lipton L, Ganju V and Croagh D (2025) Case Report: Two cases of long-term survival in advanced pancreatic cancer patients following treatment with KRAS G12C inhibitors. Front. Oncol. 15:1691760. doi: 10.3389/fonc.2025.1691760
Received: 24 August 2025; Accepted: 10 October 2025;
Published: 28 October 2025.
Edited by:
Gh Rasool Bhat, Sher - i - Kashmir Institute of Medical Sciences, IndiaReviewed by:
Amjad Husain, Cancer Research Institute, IndiaTuan Hoang, Princess Margaret Cancer Centre, Canada
Copyright © 2025 Aslani, McKay, Lipton, Ganju and Croagh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Croagh, ZGFuaWVsLmNyb2FnaEBtb25hc2hoZWFsdGgub3Jn; ZGFuaWVsLmNyb2FnaEBtb25hc2guZWR1
 Saeed Aslani
Saeed Aslani Owen McKay
Owen McKay Lara Lipton3
Lara Lipton3 Daniel Croagh
Daniel Croagh