- Deqing People’s Hospital (Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University), Zhejiang, Huzhou, China
Gallbladder carcinosarcoma (GBCS) is indeed an exceptionally rare and aggressive malignancy, accounting for less than 1% of all primary gallbladder tumors. This tumor is characterized by the presence of both carcinomatous (epithelial) and sarcomatous (mesenchymal) components, making it a unique and diagnostically challenging entity. Herein, we report a case of a 71-year-old female patient who presented with a one-year history of vague epigastric pain. AFP levels were markedly elevated. Preoperative imaging revealed gallbladder enlargement with heterogeneous contrast enhancement, raising suspicion of malignancy. The patient subsequently underwent radical cholecystectomy, and postoperative histopathological examination confirmed the diagnosis of GBCS with osteosarcomatous differentiation. The patient has since completed her first cycle of chemotherapy. Even with radical resection and adjuvant chemotherapy, GBCS carries a grave prognosis. Heightened clinical suspicion, thorough imaging assessment, and confirmatory histopathological biopsy are essential for accurate preoperative diagnosis. Surgical optimization and personalized treatment strategies remain critical to improving outcomes.
1 Introduction
Gallbladder carcinosarcoma (GBCS) is an exceptionally rare and aggressive malignancy of the digestive tract, representing fewer than 1% of primary gallbladder tumors (1). Histologically, it exhibits biphasic differentiation, combining epithelial and mesenchymal elements, with adenocarcinoma as the most frequent epithelial component and spindle cells predominating in the mesenchymal portion; osteosarcomatous differentiation is particularly uncommon (2). Multidisciplinary approaches, including neoadjuvant therapies and targeted treatments, are being investigated to improve outcomes. Increased awareness and reporting of cases are essential to advance research and develop effective therapeutic protocols (3, 4).
This report details the clinical manifestations, radiological characteristics, and pathological features of GBCS. As current evidence consists largely of sporadic case reports and no formal diagnostic or therapeutic guidelines exist, this study aims to enhance the understanding of this rare malignant tumor and to offer a reference for clinical management in comparable cases.
This case report documents a 71-year-old female patient presenting with abdominal pain who was diagnosed with GBCS after radical cholecystectomy. The tumor displayed mesenchymal differentiation into osteosarcomatous components and was associated with markedly elevated AFP levels, which is exceptionally rare among cases and further underscores the uniqueness of this case (5). The confluence of this rare entity and its unusual clinicopathological characteristics renders this case highly instructive for improving diagnostic and therapeutic approaches to GBCS.
2 Case report
A 71-year-old female farmer with no prior medical history was admitted on June 23, 2025, reporting persistent dull epigastric pain for one year, occasionally accompanied by nausea and vomiting. Physical examination showed abdominal symmetry without distension, with mild epigastric tenderness but no Murphy’s sign.No scleral icterus or cutaneous jaundice was observed.
Laboratory tests indicated hepatic dysfunction, with ALT (205 IU/L), AST (127 IU/L), ALP (374 IU/L), GGT (369 IU/L), and LDH (341 IU/L) all elevated, while bilirubin remained normal. Notably, AFP levels reached 2,786 μg/L.
Ultrasonography detected an 8.3 × 3.6 × 4.5 cm hypoechoic mass with well-defined margins in the gallbladder fossa. Contrast-enhanced abdominal CT revealed gallbladder enlargement with irregular wall enhancement, suggesting adjacent hepatic inflammation, along with suspicious soft tissue thickening in the common bile duct and gallbladder neck. Abdominal MRI further confirmed irregular gallbladder wall enhancement, nodular enhancement at the neck (suspicious for malignancy), and abnormal hepatic perfusion (Figure 1).
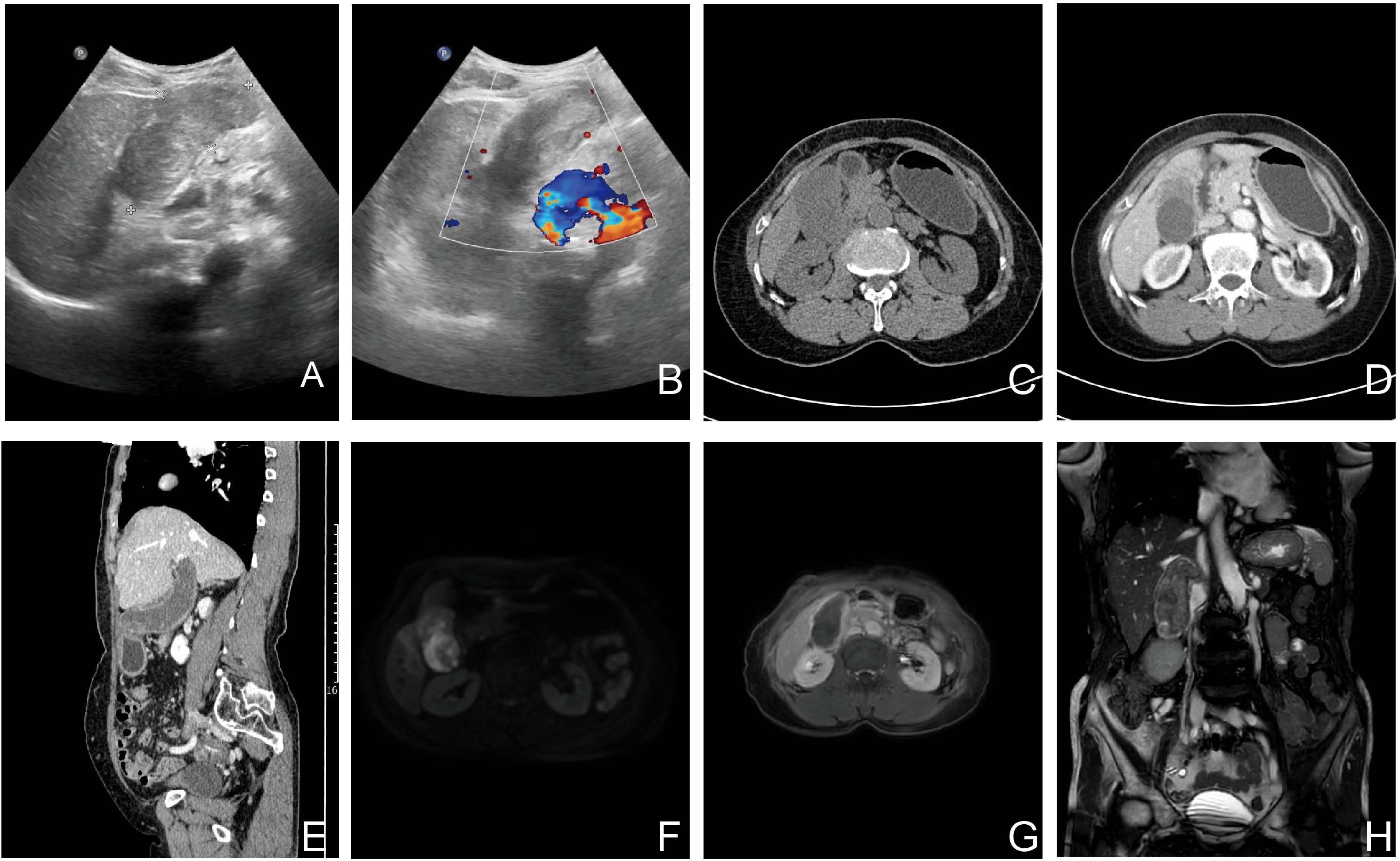
Figure 1. Preoperative imaging of the GBCS (Ultrasound, CT and MRI). Color doppler ultrasound images: (A, B) The images show a hypoechoic mass with well-defined margins. The Doppler signals (red and blue) demonstrate the vascular distribution around the lesion. These findings have some value in the preoperative differentiation between benign and malignant lesions but are non-specific. (C-E) Unenhanced and contrast-enhanced CT demonstrate an enlarged gallbladder with diffuse wall thickening and enhancement upon contrast administration. (F-H) Diffusion-weighted imaging (DWI), contrast-enhanced T1-weighted, and T2-weighted coronal images show gallbladder wall thickening accompanied by enhancement and restricted diffusion. These findings are highly suggestive of malignancy.
The patient’s medical history and diagnostic evaluations led the Hepatobiliary Surgery team to suspect a gallbladder tumor preoperatively. On June 27, 2025, surgeons performed laparoscopic cholecystectomy, revealing marked gallbladder wall edema (12×6×4 cm) and a 4×4 cm fundal mass. The resected specimen underwent pathological assessment, with frozen section analysis confirming malignancy. After consulting the patient’s family, the surgical team proceeded with open radical cholecystectomy to address the gallbladder cancer.
Gross examination showed a gallbladder measuring 12.0 cm × 5.5 cm × 4.5 cm with diffusely thickened, indurated walls (approximately 1.5 cm). The cut surface appeared gray-white with a roughened mucosa, while the serosal surface adhered to hepatic tissue (5 cm × 4.5 cm × 3.0 cm), displaying a gray-yellow cut surface interspersed with focal gray-white areas. Histopathological examination revealed a tumor composed of epithelial carcinoma and mesenchymal elements. The carcinoma component exhibited atypical glandular structures, cords, and nests with marked cellular pleomorphism, nuclear atypia, and frequent pathological mitoses, consistent with adenocarcinoma by immunohistochemistry. The mesenchymal component alternated between densely packed blue-stained cellular zones and loosely arranged pink-stained regions. The dense areas contained spindle cells arranged in fascicles or sheets, whereas the loose areas featured round to polygonal cells embedded in abundant pink osteoid-like matrix. Transitional zones between these components were present, with morphology and immunoprofile (vimentin-positive, CK-negative) supporting osteosarcoma. Histopathological examination (HPE) identified a diffusely infiltrating malignant gallbladder tumor exhibiting morphological and immunohistochemical characteristics of carcinosarcoma, with both poorly differentiated adenocarcinoma and osteosarcoma components. The tumor breached the serosal layer and extended into the adjacent hepatic parenchyma, with lymph node metastasis detected in 1 of 6 examined lymph nodes (Station 13: 1/1; Stations 8 and 12: 0/5) (Figure 2). Immunohistochemical (IHC) analysis yielded the following results: The adenocarcinoma component expressed CK, CK7, CK19, and CEA, with focal GPC3 positivity. The osteosarcoma component showed diffuse vimentin expression and focal S-100 reactivity. No immunoreactivity was observed for myogenin, CD117, or DOG1. CD34 and D2–40 selectively labeled vascular and lymphatic structures, respectively. The Ki67 proliferation index peaked at 80% in hotspot areas (Figure 3).
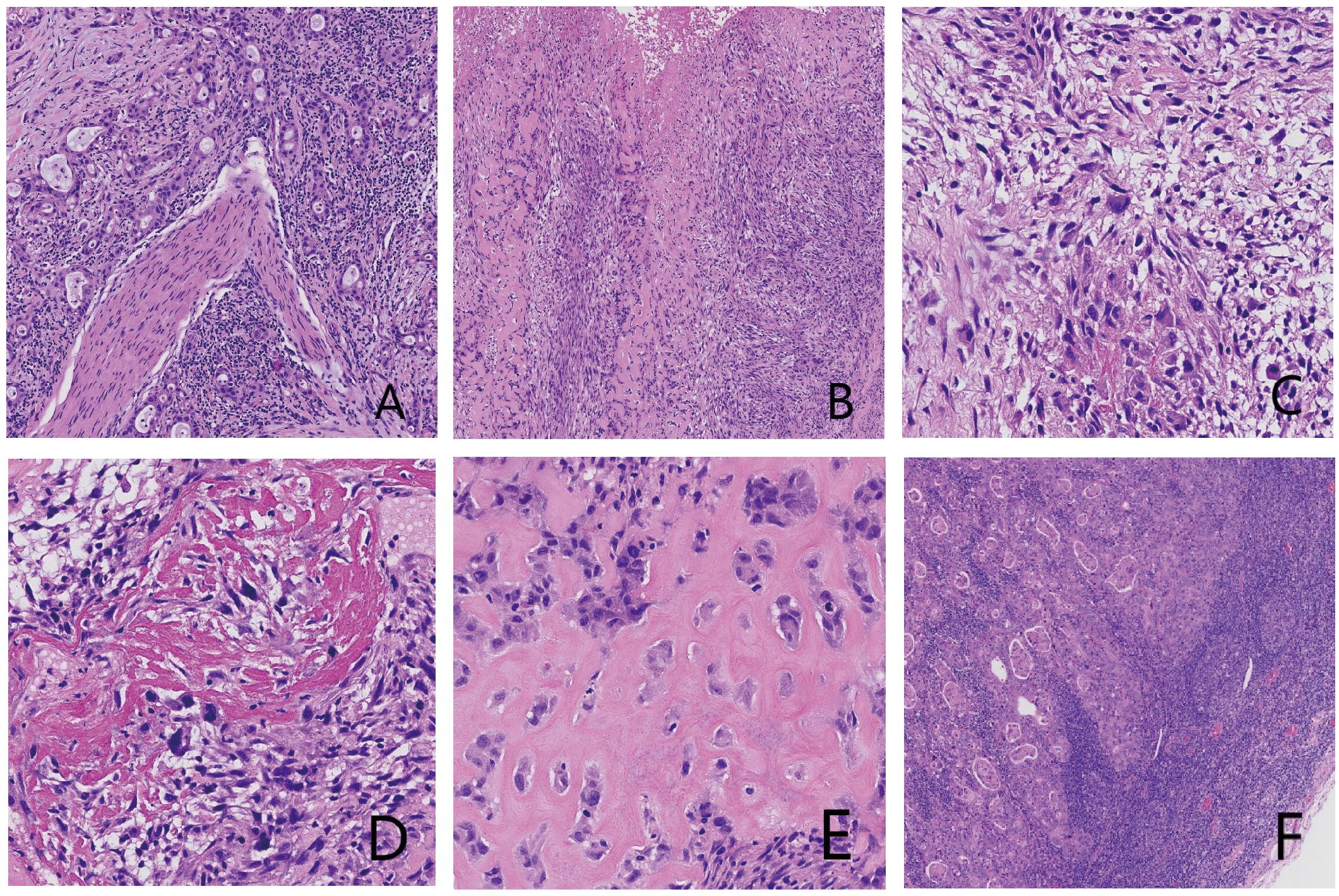
Figure 2. Histopathological micrographs of tumor tissue with hematoxylin and eosin (H&E) staining: (A) Adenocarcinoma component. Central adenocarcinoma tissue with perineural invasion is observed. (B) Mesenchymal component, showing cellular dense and loose areas. (C) Lower magnification of the mesenchymal component. Spindle-shaped cells with eosinophilic and pale cytoplasm are present; a multinucleated giant cell is visible in the center. (D) Ossified area. (E) Eosinophilic osteoid matrix with polygonal cells exhibiting hyperchromatic nuclei. (F) Lymph node metastasis composed of adenocarcinoma.
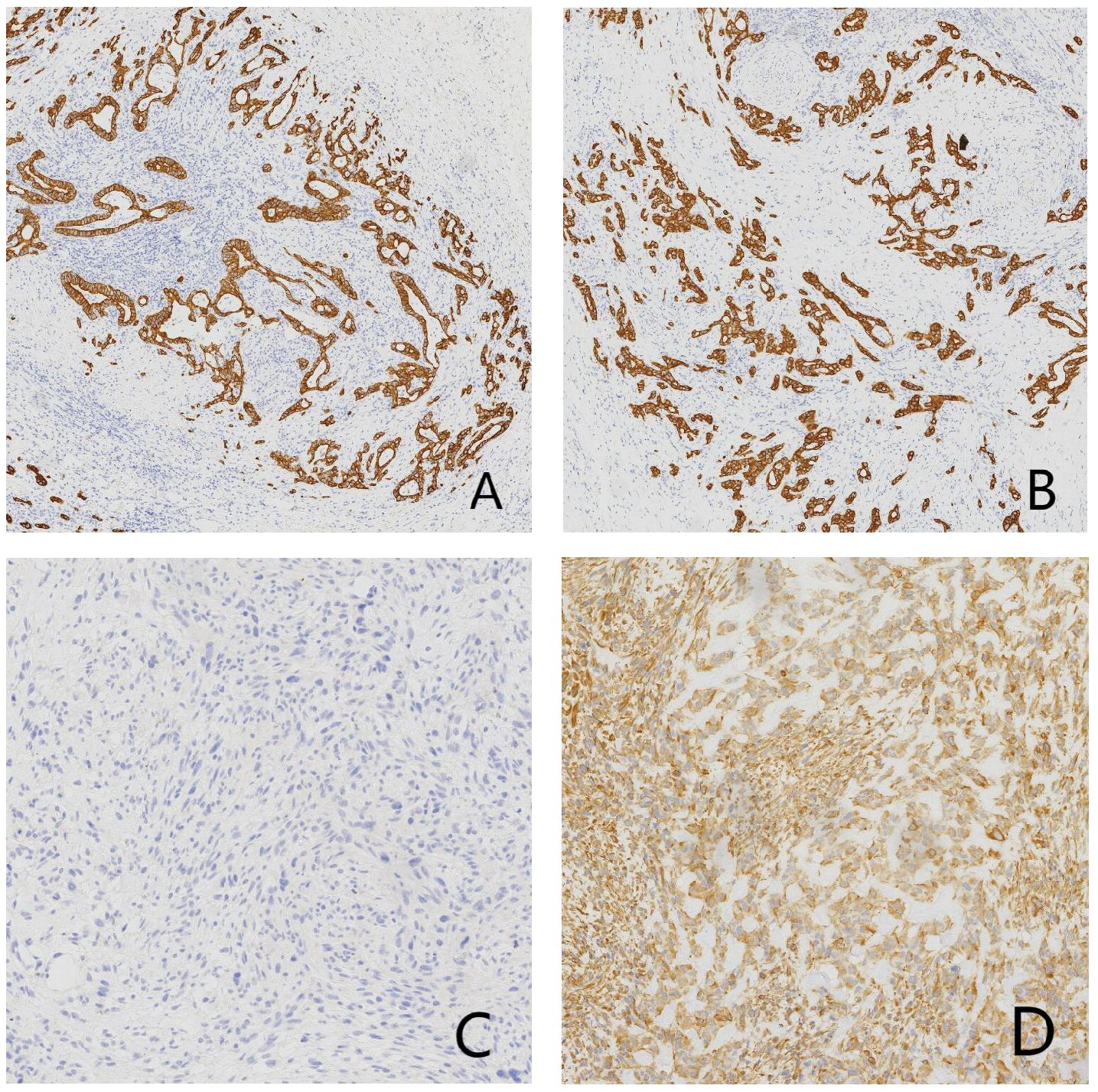
Figure 3. Immunohistochemical (IHC) micrographs of tumor tissue: (A) Positive expression of CK in the carcinomatous component. (B) Positive expression of CK19 in the adenocarcinoma component. (C) Negative expression of CK in the mesenchymal component. (D) Positive expression of vimentin in the mesenchymal component.
The patient’s postoperative symptoms improved significantly, with epigastric distension and pain substantially alleviated, and no vomiting, nausea, or palpitation observed. The patient was discharged on July 13, 2025. The first cycle of chemotherapy was completed on July 31, 2025, followed by discharge(specific regimen: gemcitabine 1000 mg D1, 8 + carboplatin 300 mg D1). During follow-up to date, the patient has maintained good general condition with no evidence of metastatic lesions. Further follow-up is required for ongoing monitoring.
3 Discussion
GBCS represents an exceptionally rare malignancy, with limited clinical experience and poorly characterized pathophysiology. This aggressive tumor predominantly occurs in middle-aged and elderly patients, showing a female predominance and frequent association with cholelithiasis (1, 6). Clinical presentation typically involves nonspecific right upper quadrant pain and abdominal masses, though some patients develop fever, jaundice, or gastrointestinal symptoms (7, 8). Advanced cases often demonstrate direct organ invasion or distant metastasis through lymphatic and hematogenous routes (3, 9). The current case involved a 71-year-old woman whose mild epigastric pain represented the sole clinical manifestation, without accompanying cholelithiasis or jaundice—factors that likely contributed to diagnostic delay.
GBCS lacks reliable serum biomarkers or specific radiological characteristics, complicating preoperative diagnosis (10). Most reported cases indicate that CEA levels typically remain normal or mildly elevated, while CA19–9 is often moderately elevated, generally below 100 IU/mL (11, 12). In contrast, our patient exhibited a normal CA19–9 level (28.2 IU/L) but a significantly elevated AFP level. A previous case reported an AFP-producing gallbladder carcinosarcoma with a level of 1,495 ng/mL, while the level in our case was even higher (2,786 μg/L), potentially associated with hepatic invasion by the gallbladder tumor. Follow-up revealed a rapid decline in serum AFP levels after resection, with the current level at 8.51 μg/L, approaching the normal range (<7 μg/L)(Supplementary Figure S1). Some scholars suggest that AFP-producing gallbladder carcinomas metastasize more frequently to the liver and have a poorer prognosis compared to those not producing AFP (13).
Radiologically, the gallbladder often exhibits mild-to-moderate heterogeneous persistent enhancement, most commonly localized at the fundus of the gallbladder, followed by diffuse involvement of the entire organ (14). Due to the paucity of early clinical symptoms and nonspecific radiological characteristics, early diagnosis of gallbladder carcinosarcoma is challenging, and definitive diagnosis largely relies on postoperative pathological examination. Initial laparoscopic cholecystectomy plans were revised to radical resection after intraoperative frozen section revealed malignancy, underscoring pathology’s decisive role in surgical management. Histopathological examination identified an osteosarcomatous component within the sarcomatous tissue, representing the rarest subtype.
In pathological diagnosis, GBCS must be differentiated from benign lesions and sarcomatoid carcinoma. The distinction from benign lesions is relatively straightforward based on the presence of cellular atypia. Sarcomatoid carcinoma exhibits two morphological patterns: spindle cell type and giant cell type. In the present case, the densely cellular areas demonstrated spindle-shaped morphology with frequent giant cells, necessitating differential diagnosis. GBCS is characterized by two distinct components—carcinoma and sarcoma—without intermingling or poorly defined tissue boundaries. In contrast, sarcomatoid carcinoma shows indistinct demarcation between carcinomatous and sarcomatoid components (15, 16). Immunohistochemical markers for epithelial and mesenchymal differentiation further aid in differentiation. Sarcomatoid carcinoma, being epithelial in origin, expresses epithelial markers such as cytokeratin (CK) in all components. Conversely, carcinosarcoma demonstrates both epithelial and mesenchymal differentiation, with the mesenchymal component expressing markers like vimentin while lacking epithelial marker expression (17–19). Additionally, the prominent glandular differentiation and positive CK7/CK19 expression in this case excluded the diagnosis of undifferentiated carcinoma. Microscopically, the marked cellular atypia facilitated differentiation from gallbladder adenomyomatosis. Moreover, the clear demarcation between carcinomatous and sarcomatous components, absence of transitional zones suggestive of metastasis, and corroborative immunohistochemical findings confirmed the definitive diagnosis of GBCS.
With a median survival of 5.5 months, GBCS carries a dismal prognosis, though radical resection improves 5-year survival to 31%, particularly for early-stage disease (7, 20). While adjuvant chemotherapy is routinely recommended, no standardized regimen exists, and treatment efficacy remains unproven. Targeted treatments are being investigated to improve outcomes (21, 22).The patient received gemcitabine-carboplatin therapy postoperatively (23), tolerating the first cycle well, with ongoing follow-up.
4 Conclusion
GBCS with osteosarcomatous differentiation is an exceptionally rare malignancy whose pathophysiology remains poorly understood. The nonspecific clinical, laboratory, and imaging features frequently result in misdiagnosis before surgery. While optimal treatment strategies remain undefined, early-stage cases demonstrate the most favorable outcomes following surgical resection. Further case documentation would advance our knowledge of cancer epidemiology to improve diagnosis, therapeutics, and management strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Deqing People’s Hospital Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
JS: Validation, Project administration, Writing – review & editing, Supervision, Formal Analysis, Writing – original draft, Conceptualization. MS: Writing – review & editing, Formal Analysis, Validation, Data curation. JY: Formal Analysis, Writing – review & editing, Validation. XS: Validation, Writing – review & editing, Formal Analysis. ZS: Conceptualization, Investigation, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1692825/full#supplementary-material
References
1. Subhi M, Elena D, Valerya A, and Safi K. Carcinosarcoma of the gallbladder: A rare tumor. World J Oncol. (2022) 13:103–106. doi: 10.14740/wjon1495
2. Pallavi P, Jyoti V, Rajneesh KS, and Riti Y. Carcinosarcoma of gallbladder with osteosarcomatous differentiation - a case report with review of literature. Indian J Surg Oncol. (2023) 13:731–740. doi: 10.1007/s13193-022-01552-4
3. Toru K, Yasutoshi K, Tomohiro K, Ayako M, Tadashi H, and Ichiro T. Two cases of resected gallbladder carcinosarcoma with a contrasting course. Int J Surg Case Rep. (2022) 92:106915. doi: 10.1016/j.ijscr.2022.106915
4. Juan C R, Patricia G, Vinay K K, Shishir K M, Milind J, and Jill K. Gallbladder cancer. Nat Rev Dis Primers. (2022) 8:69. doi: 10.1038/s41572-022-00398-y
5. Kazunori S, Kazuhiro I, Toyokazu A, Sumio N, Shin-ichi T, Makoto F, et al. Carcinosarcoma of the gallbladder producing alpha-fetoprotein and manifesting as leukocytosis with elevated serum granulocyte colony-stimulating factor: report of a case. Surg Today. (2009) 39:241–6. doi: 10.1007/s00595-008-3833-4
6. Thomas Zheng Jie T, Branden Qi Yu C, and Vishal G S. Carcinosarcoma of gallbladder: A world review. World J Clin Oncol. (2022) 12:1244–1263. doi: 10.5306/wjco.v12.i12.1244
7. Yi D, Min M, Qi-Zhi L, Yuan-Jun L, Fan X, and Chun-Hua W. Gallbladder carcinosarcoma with a poor prognosis: A case report. World J Clin cases. (2024) 12:1817–1823. doi: 10.12998/wjcc.v12.i10.1817
8. Ruhaid K, Rahul K, Raees L, Daniel N, Luka O, and Khurram C. Gallbladder carcinosarcoma masquerading as a hepatic abscess. Radiol Case Rep. (2020) 16:152–156. doi: 10.1016/j.radcr.2020.10.058
9. Aashray S, George J H, Steven M C, Carol G, and Olivia A. Orbital metastasis in advanced gallbladder and biliary tract Malignancy. BMJ Case Rep. (2025) 18:e265765. doi: 10.1136/bcr-2025-265765
10. Aqdas A AO, Abdullah M A, Abdullah Saleh A, and Abdulwahab A A. Gallbladder cancer of two histological origins: A case report and review of literature. Int J Surg Case Rep. (2021) 81:105704. doi: 10.1016/j.ijscr.2021.105704
11. Hiroyuki M, Toru M, Teichi S, Rie M, Junichi K, Junya S, et al. A large carcinosarcoma of the gallbladder accompanied by pancreaticobiliary maljunction: A case with a six-year survival. Intern Med. (2019) 58:2809–2817. doi: 10.2169/internalmedicine.2783-19
12. Seema Rani S, Prem P, Rakesh Kumar S, and Dinesh Kumar S. Assessment of tumor markers CA 19-9, CEA, CA 125, and CA 242 for the early diagnosis and prognosis prediction of gallbladder cancer. World J Gastrointest Surg. (2022) 14:1272–1284. doi: 10.4240/wjgs.v14.i11.1272
13. Nutan D, Shalini T, and Vikas Kumar B. Clear-cell adenocarcinoma of the gallbladder with alpha-fetoprotein production: A case report and review of the literature. Gastrointest Tumors. (2021) 8:52–57. doi: 10.1159/000512955
14. Xiaorong C, Yuwen Z, Xinyao S, Guixia W, and Meng Q. Gallbladder carcinosarcoma: current perspectives and new development. Expert Rev Gastroenterol Hepatol. (2021) 15:1107–1114. doi: 10.1080/17474124.2021.1919509
15. Youpeng L, Li C, Ying C, and Furong W. Carcinosarcoma and sarcomatoid carcinoma of the stomach: Two case reports. Med (Baltimore). (2021) 100:e24697. doi: 10.1097/md.0000000000024697
16. Qi-Bo W, Bo-Kang C, Jian-Ming W, Qiu-Liang W, Ji-Liang Q, and Xiao-Jun L. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg. (2012) 16:1715–26. doi: 10.1007/s11605-012-1946-y
17. Michał K, Anna G, Bartłomiej R, Adam G, Johannes H, Wojciech B, et al. Immunohistochemical evaluation of mismatch repair proteins and p53 expression in extrauterine carcinosarcoma/sarcomatoid carcinoma. Contemp Oncol (Pozn). (2020) 24:1–4. doi: 10.5114/wo.2020.94718
18. Mohan Shankar GP, Pallab S, Arpan DB, Resham B, Geeta R, and Shailendra Kumar DD. Uterine carcinosarcoma: Unraveling the role of epithelial-to-mesenchymal transition in progression and therapeutic potential. FASEB J. (2024) 38:e70132. doi: 10.1096/fj.202401991R
19. Yan H, Junhong G, Shaoling L, Jiafu L, Jianping X, Wei Y, et al. The correlation between histologic, immunophenotypic, and molecular characteristics of pulmonary sarcomatoid carcinoma reveals that sarcomatoid change is potentially derived from epithelial carcinoma cells undergoing epithelial-mesenchymal transition. Appl Immunohistochem Mol Morphol. (2022) 31:17–25. doi: 10.1097/pai.0000000000001060
20. Takehiro O, Zhao-Li S, Robert A M, and Kazuhiro H. Surgical outcome of carcinosarcoma of the gall bladder: a review. World J Gastroenterol. (2009) 15:4877–82. doi: 10.3748/wjg.15.4877
21. Yedan L, Junbo L, Jilan Y, Tingfeng L, Xi W, Dan L, et al. Long-term survival was achieved through multidisciplinary treatment of a patient with gallbladder carcinosarcoma accompanied by KRAS mutation: a case report and literature review. Front Oncol. (2025) 14:1506949. doi: 10.3389/fonc.2024.1506949
22. Qin-Qin L, Hao-Ming L, Hong-Wei H, Cai-Ni Y, Chao L, and Rui Z. Complete response to combined chemotherapy and anti-PD-1 therapy for recurrent gallbladder carcinosarcoma: A case report and literature review. Front Oncol. (2022) 12:803454. doi: 10.3389/fonc.2022.803454
Keywords: gallbladder carcinosarcoma, osteosarcomatous differentiation, AFP, differential diagnosis, epithelial, mesenchymal
Citation: Shi J, Shen M, Yao J, Shen X and Su Z (2025) Case Report: A rare case of gallbladder carcinosarcoma with osteosarcomatous differentiation. Front. Oncol. 15:1692825. doi: 10.3389/fonc.2025.1692825
Received: 26 August 2025; Accepted: 10 November 2025; Revised: 04 November 2025;
Published: 20 November 2025.
Edited by:
Natale Calomino, University of Siena, ItalyReviewed by:
Anisse Tidjane, Oran University 1 Ahmed Ben Bella, AlgeriaGiorgio Micheletti, University of Siena, Italy
Copyright © 2025 Shi, Shen, Yao, Shen and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajun Shi, MTI4MTgyOTg1MUBxcS5jb20=; Miao Shen, NTg0MjEwNDc3QHFxLmNvbQ==
 Jiajun Shi
Jiajun Shi Miao Shen*
Miao Shen*