- Department of Clinical Laboratory, Second Affiliated Hospital of Dalian Medical University, Dalian, China
Introduction: CD82, a metastasis suppressor, is known to be palmitoylated, yet the functional significance of this modification in drug response remains unclear. This study investigates the role of CD82 palmitoylation in modulating chemosensitivity in triple-negative breast cancer (TNBC) cells.
Methods: A palmitoylation-deficient CD82 mutant (C5A/C74A/C83A) was generated and expressed in MDA -MB-231 breast cancer cells. Protein palmitoylation was assessed via acyl-biotin exchange assay. Subcellular localization was analyzed by co-immunofluorescence with early endosome (EEA1) and vesicular (VPS4A) markers. Apoptosis was evaluated by measuring levels of apoptotic markers. The cytotoxic effect of the CD82 mutation in combination with gefitinib, doxorubicin, paclitaxel, and camptothecin was quantified using TUNEL and Annexin V/PI assays.
Results: The CD82 palmitoylation-deficient mutant exhibited reduced palmitoylation and increased colocalization with EEA1 and VPS4A. This mutation triggered intrinsic apoptosis, as evidenced by elevated levels of Caspase-3, Cleaved-caspase-3, and Cytochrome C. Strikingly, it synergistically enhanced the cytotoxicity of both gefitinib and doxorubicin, significantly increasing apoptosis in treated cells.
Discussion: Our findings reveal a novel role for CD82 palmitoylation in regulating drug-induced apoptosis. Disruption of palmitoylation potentiates chemotherapy-induced cell death, providing a molecular rationale for targeting CD82 palmitoylation as a combinatorial strategy to overcome therapeutic resistance in TNBC.
Introduction
Tetraspanins, a conserved family of four-transmembrane proteins, orchestrate critical cellular processes, including migration, protein trafficking, and signal transduction, through dynamic membrane network formation (1). CD82 (KAI1), a prominent tetraspanin, functions as a metastasis suppressor by modulating cell adhesion, Wnt signaling, and senescence, while maintaining muscle homeostasis through satellite cell regulation (2).
Emerging evidence implicates CD82 in TLR9 trafficking and AML chemoresistance, where its overexpression activates the PKCα/β1-integrin pathways to confer drug resistance. Tetraspanin trafficking involves Rab GTPase-mediated sorting through early endosomes, with palmitoylation serving as a critical determinant for membrane retention versus lysosomal degradation via ESCRT complexes (3–8).
Tetraspanins broadly influence drug sensitivity-CD9 knockdown reduces chemoresistance in breast cancer, whereas CD151 deletion sensitizes cells to gefitinib and camptothecin (9, 10). In breast cancer therapeutics, CD63-positive cancer-associated fibroblasts (CAFs) significantly influence tumor cell sensitivity to tamoxifen via paracrine signaling mechanisms (11). CD81 has been implicated in chemotherapy resistance in hematological malignancies, where it mediates drug tolerance via Bruton’s tyrosine kinase (BTK) signaling in both AML and ALL (12). Emerging evidence has identified CD82 as a critical chemoresistance regulator in AML, where treatment-induced upregulation promotes drug resistance via PKCα/β1-integrin pathway activation. CD82-targeting antibodies synergize with cytarabine to enhance therapeutic efficacy through pathway modulation (13, 14).
Preliminary data revealed that palmitoylation-deficient CD82 mutants (C5A/C74A/C83A) increased apoptotic susceptibility in breast cancer cells, although the mechanistic link between CD82 palmitoylation and drug sensitivity remains unexplored.
We hypothesized that these mutations disrupt membrane stability, amplify endosomal–lysosomal trafficking, and selectively activate intrinsic apoptosis, thereby potentiating chemosensitivity.
This study investigates the hypothesis that palmitoylation-deficient CD82 mutants sensitize triple-negative breast cancer cells to chemotherapy by disrupting membrane microdomains and amplifying intrinsic apoptosis, thereby offering a novel combinatorial therapeutic strategy.
Materials and methods
Materials
Antibodies
• Anti-CD82 Rabbit IgG (Cat No. ab66400, ab108347), anti-CD82 Mouse IgG (Cat No. ab200843, ab59509) were obtained from Abcam, Cambridge, UK. Anti-PERK Rabbit IgG (Cat No. 24390-1-AP, BS3636), Anti-EEA1 C Rabbit Polyclonal IgG (Cat No. 81245-1-RR, BS6299), Anti-VPS4A Rabbit Monoclonal IgG (Cat No. 14272-1-AP, BS80712) were obtained from Bioworld Biotechnology (Wuhan, China).
Experimental equipment
• Clean bench (Beijing Medical Equipment Factory), optical microscope (Olympus Corporation of Japan), inverted fluorescence microscope (Leica), upright high-resolution imaging system (Leica), SpectraMax microplate reader (Molecular Devices), Western Blot electrophoresis tank (BIO-RAD), Western Blot transfer electrophoresis instrument (BIO-RAD), −80 °C freezer (Haier, Qingdao), Odyssey fluorescence scanning imaging system (LI-COR), electronic balance (Shenyang Longteng Instrument Factory), manual pipette (Thermo Fisher), microliter injector (Shanghai Gaoge Industry and Trade Co), ultrasonic cell disruptor (USA, Qsonica), benchtop refrigerated incubator shaker (Taicang Huamei Biochemical Instrument Factory), PH meter (OHAUS), and flow cytometer (Agilent).
Construction of CD82 palmitoylation mutant
Design and reverse PCR amplification of mutant sites: Using site-directed mutagenesis based on the wild-type CD82 contracted plasmid, we constructed single-point mutations, double-point mutations and triple mutations of KAI1/CD82 plasma proximal membrane cysteine residues (Cys5, Cys74, and Cys83) of palmitoylation to alanine for protein structure stability. Next, primers were designed for the 5` end connection and 3` end in the opposite direction (Table 1).

Table 1. Sequences of oligonucleotide primers used to construct the site-directed mutations of CD82.
Cell culture and transfection
The breast cancer cell line (MDA-MB-231) was purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Cultured cells were grown in dishes (10 cm) or plates (six wells) in DMEM (HyClone, LA, USA) supplemented with 10% FBS (tBD Bioscience, Bristol, UK), antibiotic-100 U/ml penicillin, antibiotic-streptomycin (100) (Beyotime) in humidified air at 37 °C with 5% CO2. Plasmids overexpressing CD82, CD82C5A/74/83A (GenePharm, Shanghai, China) were transfected into breast cancer cell lines (MDA-MB-231) at 40% confluence. After 48 h, the infected cells were harvested.
Protein extraction and protein immunoblot analysis
Total protein was extracted from cultured cells using RIPA Lysis Buffer (Beyotime, Shanghai, China). Protein concentration was measured using a BCA Protein Assay Kit (Beyotime, Shanghai, China). The protein was immunoblotted with 1:1,000 antibodies against CD82 and other antibodies (Abcam, USA) according to the manufacturer’s instructions. β-actin or GAPDH served as an internal control. The blots were detected using a Bio-Rad Bioimaging system (Bio-Rad, CA, USA). Western blotting was performed as described previously.
Cell growth assay (CCK8)
Cells were seeded at a density of 5,000 cells per well in 100 μL of suspension in a 96-well plate, with 200 μL of PBS in the peripheral wells. Different concentrations of the drug were added to the plate to establish a concentration gradient, with three to six replicates for each concentration. Three blank wells (without cells) were set up and culture medium containing 10% CCK8 was added. After incubation for 48 h (or 24 h), the culture medium was removed and replaced with medium containing 10% CCK8 and incubated for another 2 h–4 h. The absorbance was measured at 450 nm using a microplate reader.
TUNEL experiment
The cells were transfected for 48 h, fixed with 4% paraformaldehyde for 30 min at room temperature, washed three times with PBS, and shaken slowly for 5 min each time. A total of 5% Trition X-100 was fixed for 30 min at room temperature, washed three times with PBS; TdT labeling buffer was prepared before use according to the number of samples to be analyzed, 1 μL of TdT enzyme per well, 10 μL of Equilibration buffer, 5 μL of label Mix Green, 34 μL of dd H2O per well. Add 5 μL of reaction mixture to each sample and incubate at 37° for 60 min; wash three times with PBS for 5 min each time; add anti-burst fluorescent blocker containing DAPI and observe by fluorescence microscope.
Acyl-biotinyl exchange assay
After transfecting with CD82 mutant plasmid, culture until cell count reaches ~1 × 107. The cells were washed three times with pre-cooled PBS, scraped, and centrifuged at room temperature to collect them. Lysis buffer (1% Triton×-100 and NEM) was prepared, and the cells were lysed overnight at 4 °C. The next day, the Protein A/G magnetic beads were incubated with the CD82 primary antibody (IgG was used as a control). After incubation, the beads were washed. The lysate was centrifuged, and the supernatant was used as the input group to quantify the protein. The lysate was incubated with the beads for 3 h. Wash the beads again. The HAM solution was prepared and incubated with beads to cleave thioester bonds and generate free thiols. BMCC solution was then prepared and incubated with the beads to convert free thiols to biotin. Finally, the beads were treated with 1 × SDS, boiled to denature the proteins, and the supernatant was collected for WB analysis of palmitoylation levels.
Annexin V-FITC/PI double staining assay
After inducing apoptosis according to the experimental protocol, the cells were centrifuged at 300×g for 5 min, the supernatant was discarded, the cells were washed once with PBS, and the cells were gently resuspended and counted. Take 1–5 × 105 resuspended cells, centrifuge again at 300×g for 5 min, discard the supernatant, and resuspend the cells in 500 μL of diluted 1×Annexin V Binding Buffer. Then, 5 μL of Annexin V-FITC and 5 μL of PI (50 μg/mL) were added, mixed gently by vortexing, and incubated at room tempThe cells were washed three times with pre-cooled PBS, serature in the dark for 15 min–20 min. After incubation, the cells were immediately detected using a flow cytometer. If immediate detection is not possible, place the sample on ice, avoid light exposure, and complete the detection within 1 h.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde (PFA) for 15 min and 1% NP-40 for 20 min at 25°C, and then washed three times with PBS. Subsequently, 5% normal goat serum was used for blocking at 37°C for 1 h. The cells were incubated with rabbit anti-EEA1 or anti-VPS4A (1:100; Bioworld) primary antibody (1:100; Bioworld) overnight at 4°C, followed by incubation with fluorophore-conjugated secondary antibodies (1:50; ABclonal) for 1 h. The cell nuclei were stained with DAPI (Beyotime), and the cells were mounted on cover glasses with an antifade mounting medium. Imaging was performed using a Leica Tcssp8 laser scanning confocal microscope (Leica, Wetzlar, Germany) or a Leica DM4B positive fluorescence microscope (Leica).
Statistical analyses
All the results were repeated several times (≥3) and consistently similar results were collected for statistical analyses. Image J and GraphPad Prism 6 were used to analyze the data. SPSS software was used for two-way ANOVA to analyze the synergy between drugs and mutations. The results are expressed as mean ± standard error of the mean. Note: “*” indicates that compared with the Blank group, P <0.05, “**” indicates that compared with the Blank group (P <0.01), “***” means compared with the Blank group (P <0.001).
Results
Mutation of CD82C5A/C74A/C83A reduce palmitoylation and membrane stability
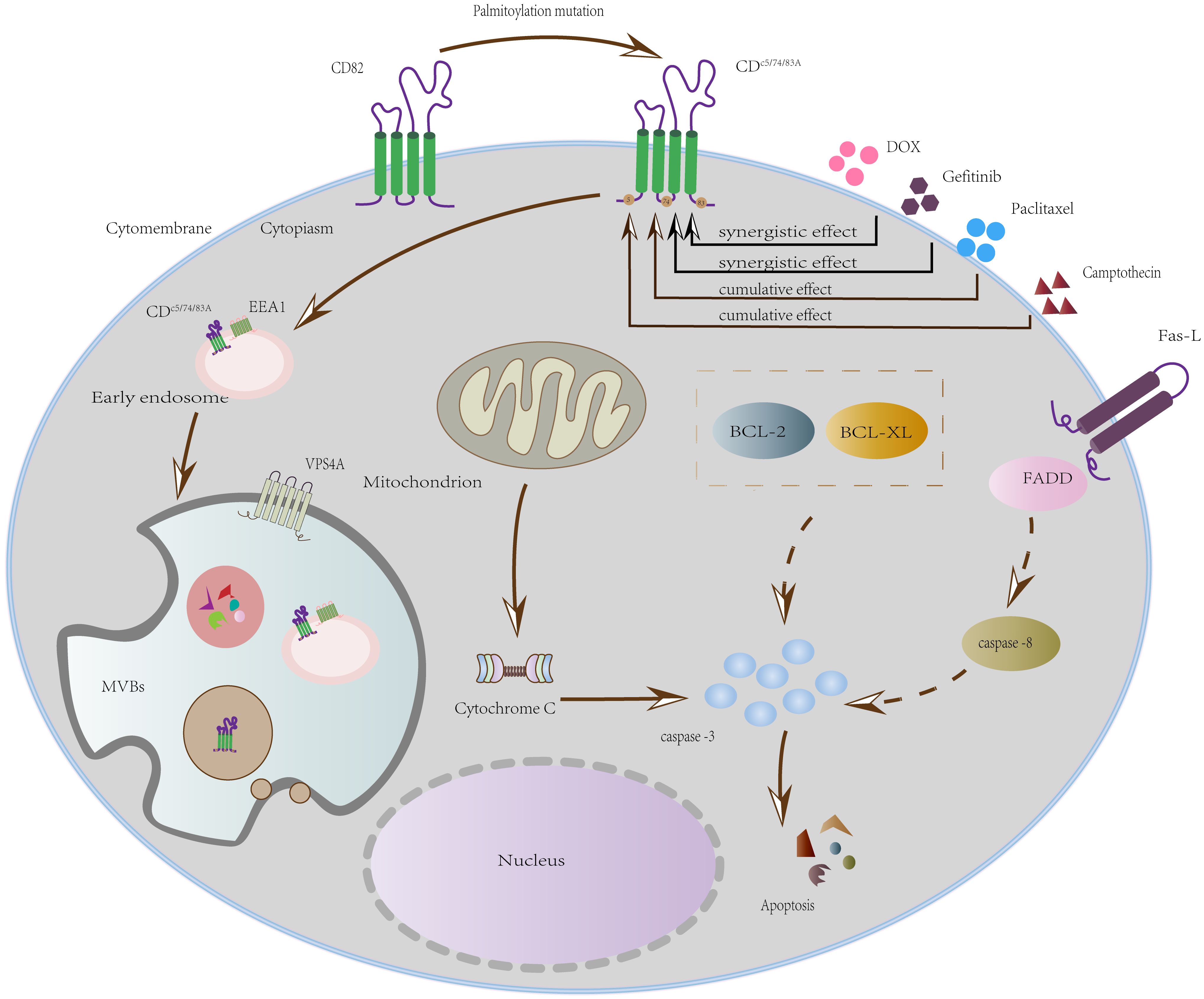
The CD82C5A/C74A/C83A overexpression plasmid significantly increased CD82 expression in MDA-MB-231 cells after 48-hour transfection (Figure 1A).The ABE assay showed high palmitoylation levels in CD82WT cells but a significant reduction in the CD82C5A/C74A/C83A mutant after transfection of MDA-MB-231 cells (Figure 1B). The CD82C5A/C74A/C83A mutation reduces palmitoylation, potentially destabilizing membranes and enhancing recycling via the early endosomal vesicle pathway. Immunofluorescence revealed CD82 co-localization with EEA1 and VPS4A in mutant but not in wild-type cells. This suggests that impaired palmitoylation promotes CD82 trafficking via the recycling pathway, leading to increased apoptosis (Figures 1C, D).

Figure 1. Mutation of CD82 at Cys5, Cys74, and Cys83 reduce palmitoylation levels and membrane stability in breast cancer cells. (A) Plasmid transfection induced overexpression of the CD82C5A/C74A/C83A mutant. (B) The acyl-biotin switch assay (ABE) was used to assess the palmitoylation levels of CD82 following the CD82C5A/C74A/C83A mutation. (C, D) Immunofluorescence assays examined the fluorescent colocalization of CD82 with the early endosome marker EEA1 and the intracellular vesicle marker VPS4A in MDA-MB-231 breast cancer cells after the CD82C5A/C74A/C83A mutation.
Mutation of CD82C5A/C74A/C83A promotes apoptosis through the mitochondrial pathway
CD82C5A/C74A/C83A and staurosporine (positive control) significantly increased apoptosis in MDA-MB-231 cells compared to controls (DMSO, transfection reagent, empty vector, and CD82WT), as shown by Annexin V-FITC/PI (Figure 2B) and TUNEL assays (Figure 2C). These results suggest that CD82C5A/C74A/C83A promotes apoptosis in breast cancer cells.

Figure 2. Mutation of CD82 at Cys5, Cys74, and Cys83 exhibit increased apoptosis in breast cancer cells. (A) Mitochondrial pathway related apoptotic proteins: Caspase-3, Cleaved-caspase-3, and Cytochrome C were detected. ER pathway related apoptotic proteins: PERK, IRE1, and ATF6 were analyzed. Death receptor pathway related apoptotic proteins: Caspase-8 and FasL were examined. (B) An apoptosis assay using AnexinV-FITC/PI double staining was performed. (C) The TUNEL assay was also conducted to assess apoptosis. Images were captured at ×100 magnification. Scale bar = 10 μm.
Western blot analysis demonstrated a marked upregulation of Caspase-3, Cleaved-caspase-3, and Cytochrome C in CD82C5A/C74A/C83A mutants, strongly suggesting the activation of mitochondrial-mediated apoptotic pathways. No significant changes were observed in endoplasmic reticulum stress markers (PERK, IRE1, and ATF6) or death receptor pathway components(Caspase-8,FasL) (Figure 2A).These findings suggest that CD82C5A/C74A/C83A-induced apoptosis is independent of the endoplasmic reticulum or death receptor pathways. Collectively, these results demonstrate that CD82C5A/C74A/C83A specifically activates the intrinsic mitochondrial apoptosis pathway.
The combination of the mutation of CD82C5A/C74A/C83A with chemotherapeutic agents (camptothecin, gefitinib, paclitaxel, doxorubicin) was found to enhance apoptosis in breast cancer cells
TUNEL assay revealed minimal apoptotic activity in CD82WT cells, whereas chemotherapeutic treatment (camptothecin, gefitinib, paclitaxel, and doxorubicin) induced significant apoptosis. Notably, CD82C5A/C74A/C83A cells exhibited substantial baseline apoptosis, which was further enhanced upon drug exposure compared to wild type cells. Strikingly, drug-treated CD82C5A/C74A/C83A cells demonstrated significantly higher apoptotic rates than drug-treated CD82WT cells, suggesting synergistic effects between the CD82 mutation and chemotherapy (Figures 3A, B).

Figure 3. The combination of the mutation of CD82 at Cys5, Cys74, and Cys83 with chemotherapeutic agents (camptothecin, gefitinib, paclitaxel, doxorubicin) was found to enhance apoptosis in breast cancer cells. (A) Apoptosis was detected by TUNEL assay in CD82 WT cells and CD82 WT cells treated with camptothecin, gefitinib, paclitaxel, or doxorubicin. Scale bar = 10 μm. (B) apoptosis was assessed by TUNEL assay in CD82C5A/C74A/C83A mutant cells and mutant cells treated with the same drugs. Scale bar = 10 μm.
Mutation of CD82C5A/C74A/C83A showed an additive effect with camptothecin and paclitaxel in promoting apoptosis in breast cancer cells
To study whether CD82C5A/C74A/C83A enhances tumor drug sensitivity, we determined the half inhibitory concentrations (IC50) of camptothecin in MDA-MB-231 cells using the CCK8 assay. (Figure 4C). We investigated the impact of the CD82C5A/C74A/C83A mutation in combination with drugs on apoptosis. CD82C5A/C74A/C83A enhanced apoptosis in combination with camptothecin; however, this effect was strictly additive rather than synergistic (Figure 4A).

Figure 4. Mutation of CD82 at Cys5, Cys74, and Cys83 showed an additive effect with camptothecin and a synergistic effect with gefitinib in inducing breast cancer cell apoptosis. (A, B) Annexin V-FITC/PI double-staining flow cytometry detected apoptosis rates in four groups: CD82 WT, CD82C5A/C74A/C83A, CD82WT with 1.5μM Camptothecin, and CD82C5A/C74A/C83A with 10 μM gefitinib. Statistical analysis covered three biological replicates. *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001 vs. the control group (statistically significant differences). (C) The CCK8 assay measured the half-inhibitory concentrations of camptothecin and gefitinib in MDA-MB-231 cells.

Figure 5. Mutation of CD82 at Cys5, Cys74, and Cys83 exhibits a synergistic effect with doxorubicin in promoting apoptosis in breast cancer cells. (A) Annexin V-FITC/PI double - staining flow cytometry was used to assess apoptosis. (B) The half-inhibitory concentration of doxorubicin in MDA-MB-231 cells was determined by CCK8 assay. **p < 0.01, ***p < 0.001, ****p < 0.0001.
To explore whether the CD82C5A/C74A/C83A mutation enhances tumor drug sensitivity, we determined the IC50 of paclitaxel in MDA-MB-231 cells using the CCK8 assay (Figure 6B). The CD82C5A/C74A/C83A mutation combined with paclitaxel significantly enhanced apoptosis compared to either treatment alone (Figure 6A), although their interaction remained additive rather than synergistic. These findings suggest that although the CD82C5A/C74A/C83A mutation potentiates paclitaxel-induced cell death, it does not engage cooperative mechanisms to achieve true synergy.

Figure 6. Mutation of CD82 at Cys5, Cys74, and Cys83 exhibits an additive effect with paclitaxel in promoting apoptosis in breast cancer cells. (A) Apoptosis rates were assessed by Annexin V-FITC/PI double-staining flow cytometry in four groups: CD82WT, CD82C5A/C74A/C83A. (B) The half-inhibitory concentration of paclitaxel in MDA-MB-231 cells was measured by CCK8 assay.
Mutation of CD82C5A/C74A/C83A exhibits a synergistic effect with gefitinib and doxorubicin in promoting apoptosis in breast cancer cells
To explore whether the CD82C5A/C74A/C83A mutation enhances tumor drug sensitivity, we used the CCK8 assay to determine the half inhibitory concentration (IC50) of gefitinib and doxorubicin using the CCK8 assay in MDA-MB-231 cells (Figures 4C, 5B).
In gefitinib-treated cells, CD82C5A/C74A/C83A potentiated apoptosis induction beyond additive expectations, revealing a synergistic interaction between the mutation and the tyrosine kinase inhibitor (Figure 4B). This indicates that the CD82C5A/C74A/C83A mutation increases the sensitivity of MDA-MB-231 cells to gefitinib.
The CD82C5A/C74A/C83A mutation synergistically enhanced doxorubicin-induced apoptosis in MDA-MB-231 cells, with the combination treatment demonstrating significantly greater cytotoxicity than either treatment alone (Figure 5A).
These synergistic interactions suggest that the mutation may sensitize triple-negative breast cancer cells to anthracycline-based chemotherapy, potentially offering a therapeutic strategy for treatment-resistant cases.
Discussion
CD82 (KAI1) is a metastasis suppressor that inhibits tumor progression through multiple mechanisms, including the attenuation of cell motility, maintenance of cellular polarity, and induction of apoptosis in response to extracellular stimuli, while also regulating protein trafficking and membrane dynamics (15, 16). As a tetraspanin, CD82 critically maintains membrane stability and cell migration, with its palmitoylation sites being essential for these functions, as their mutation disrupts membrane homeostasis. Following internalization, CD82 undergoes endosomal sorting via recycling pathways, a process essential for cellular homeostasis, and its dysregulation contributes to disease pathogenesis (17).
S-palmitoylation, a reversible post-translational modification mediated by ZDHHC enzymes and APTs, regulates diverse cellular processes including signal transduction and membrane trafficking, with dysregulation implicated in cancer pathogenesis through modulation of oncoproteins and tumor suppressors (18–20). The dynamic regulation of protein palmitoylation in cancer cells through altered expression of ZDHHCs/APTs represents a promising therapeutic target for modulating drug sensitivity and overcoming chemoresistance in multiple signaling pathways (21).
Our ABE assays confirmed that the C5A/C74A/C83A mutations effectively reduced CD82 palmitoylation, aligning with prior reports that these residues critically maintain tetraspanin web integrity and providing mechanistic insight into the apoptotic phenotype. The enhanced drug sensitivity resembles AML models, where CD82 targeting improved cytarabine efficacy, but differs mechanistically by exploiting intrinsic apoptosis rather than PKCα/β1-integrin modulation, highlighting tissue-specific pathway engagement. These observations may arise via several non-exclusive mechanisms, including palmitoylation loss disrupting CD82-Rab11a/FIP2 complexes and their survival signal recycling function, mutant accumulation in endosomes overloading vesicular quality control, or altered membrane fluidity indirectly activating BAX/BAK (22, 23).
While tetraspanins are known to modulate drug resistance, this study is the first status to directly link to chemosensitivity, demonstrating synergistic effects with gefitinib and doxorubicin. The mitochondrial apoptosis preference contrasts with CD82’s established roles in membrane microdomain organization, suggesting that palmitoylation loss may trigger distinct downstream effects. Wild-type CD82 supports metastasis suppression through adhesion modulation, whereas the mutants induce cytotoxicity via organelle stress pathways. Emerging evidence reveals that protein palmitoylation critically modulates drug sensitivity, with PD-L1 hyperpalmitoylation driving cisplatin resistance in bladder cancer by stabilizing immune checkpoint signaling, while ZDHHC22-mediated palmitoylation overcomes endocrine resistance in ER-negative breast cancer via mTOR destabilization and AKT pathway suppression (24), establishing palmitoylation as both a predictive biomarker and therapeutic target for treatment-resistant malignancies.
Clinically, these findings suggest that the CD82 palmitoylation status could be used to stratify patients for combination therapies, particularly in TNBC, where mitochondrial apoptosis pathways remain targetable and current treatments lack precision. Limitations include uncharacterized effects on other tetraspanin network members that may contribute to the phenotype and the need for in vivo validation of the chemosensitization effect. Future studies should map the precise molecular cascade linking palmitoylation-deficient CD82 to mitochondrial outer membrane permeabilization and explore whether this mechanism extends to other therapy-resistant cancers.
The schematic (Figure 7) delineates how DOX and gefitinib induce mitochondrial apoptosis by mediating MOMP, cytochrome c release, and caspase activation. We propose that the internalization of CD82 (via triple-palmitoylation-site mutation) functions as a cellular sensitization mechanism for this intrinsic pathway, thereby explaining the synergistic effect of this mutant with doxorubicin (DOX) and gefitinib.
While this study establishes a mechanistic rationale for tetraspanin-targeted combination therapies, the precise metabolic rewiring and upstream regulators governing palmitoylation-deficient tetraspanin-induced apoptosis remain to be fully characterized. Future studies should delineate how tetraspanin palmitoylation status integrates with oncogenic signaling networks to advance both fundamental understanding and therapeutic translation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
LL: Investigation, Writing – original draft, Conceptualization, Data curation. YY: Conceptualization, Writing – original draft, Data curation, Investigation. SC: Writing – original draft, Investigation. YHZ: Writing – original draft. CZ: Writing – original draft. XY: Writing – original draft. HX: Writing – original draft. SW: Writing – original draft. XG: Writing – original draft. WH: Writing – original draft, Data curation, Conceptualization, Methodology. YZ: Methodology, Project administration, Conceptualization, Data curation, Writing – original draft. YL: Funding acquisition, Writing – original draft, Writing – review & editing, Resources, Project administration, Conceptualization, Methodology, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the grants from the Key Disciplines of the Second Affiliated Hospital of Dalian Medical University (No. dy2yxkgg202004) and Liaoning Provincial Department of Education Basic Research Project (No. JYTMS20230562) and Horizontal Research Project (No. HX20220017) and (HXLX20230136).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1699420/full#supplementary-material
References
1. Susa K, Kruse A, and Blacklow S. Tetraspanins: structure, dynamics, and principles of partner-protein recognition. Trends Cell Biol. (2024) 34(6):509–22. doi: 10.1016/j.tcb.2023.09.003
2. Hall A, Fontelonga T, Wright A, Bugda Gwilt K, Widrick J, Pasut A, et al. Tetraspanin CD82 is necessary for muscle stem cell activation and supports dystrophic muscle function. Skelet Muscle. (2020) 10:34. doi: 10.1186/s13395-020-00252-3
3. Khan NS, Lukason DP, Feliu M, Ward RA, Lord AK, Reedy JL, et al. CD82 controls CpG-dependent TLR9 signaling. FASEB J. (2019) 33:12500–14. doi: 10.1096/fj.201901547R
4. Chigita S, Sugiura T, Abe M, Kobayashi Y, Shimoda M, Onoda M, et al. CD82 inhibits canonical Wnt signalling by controlling the cellular distribution of beta-catenin in carcinoma cells. Int J Oncol. (2012) 41:2021–8. doi: 10.3892/ijo.2012.1671
5. Tonoli H and Barrett JC. CD82 metastasis suppressor gene: a potential target for new therapeutics? Trends Mol Med. (2005) 11:563–70.
6. Feng J, Huang C, Wren JD, Wang DW, Yan J, Zhang J, et al. Tetraspanin CD82: a suppressor of solid tumors and a modulator of membrane heterogeneity. Cancer Metastasis Rev. (2015) 34:619–33. doi: 10.1007/s10555-015-9585-x
7. Gallon M and Cullen PJ. Retromer and sorting nexins in endosomal sorting. Biochem Soc Trans. (2015) 43:33–47. doi: 10.1042/BST20140290
8. Jovic M, Sharma M, Rahajeng J, and Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol histopathology. (2010) 25:99–112.
9. Ullah M, Akbar A, Ng NN, Concepcion W, and Thakor AS. Mesenchymal stem cells confer chemoresistance in breast cancer via a CD9 dependent mechanism. Oncotarget. (2019) 10. doi: 10.18632/oncotarget.26952
10. Hwang S, Takimoto T, and Hemler ME. Integrin-independent support of cancer drug resistance by tetraspanin CD151. Cell Mol Life Sci. (2019) 76:1595–604. doi: 10.1007/s00018-019-03014-7
11. Song Z, Mao J, Barrero RA, Wang P, Zhang F, and Wang T. Development of a CD63 aptamer for efficient cancer immunochemistry and immunoaffinity-based exosome isolation. Molecules. (2020) 25. doi: 10.3390/molecules25235585
12. Garcia-Mayea Y, Mir C, Carballo L, Castellvi J, Temprana-Salvador J, Lorente J, et al. TSPAN1: A novel protein involved in head and neck squamous cell carcinoma chemoresistance. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12113269
13. Floren M, Restrepo Cruz S, Termini CM, Marjon KD, Lidke KA, and Gillette JM. Tetraspanin CD82 drives acute myeloid leukemia chemoresistance by modulating protein kinase C alpha and beta1 integrin activation. Oncogene. (2020) 39:3910–25. doi: 10.1038/s41388-020-1261-0
14. Nishioka C, Ikezoe T, and Yokoyama A. Blockade of CD82 by a monoclonal antibody potentiates anti-leukemia effects of AraC. vivo. Cancer Med. (2015) 4:1426–31. doi: 10.1002/cam4.482
15. Tsai YC and Weissman AM. Dissecting the diverse functions of the metastasis suppressor CD82/KAI1. FEBS Lett. (2011) 585:3166–73. doi: 10.1016/j.febslet.2011.08.031
16. Yan W, Huang J, Zhang Q, and Zhang J. Role of metastasis suppressor KAI1/CD82 in different cancers. J Oncol. (2021) 2021:9924473. doi: 10.1155/2021/9924473
17. O’Sullivan MJ and Lindsay AJ. The endosomal recycling pathway-at the crossroads of the cell. Int J Mol Sci. (2020) 21.
18. Shahid M, Kim M, Jin P, Zhou B, Wang Y, Yang W, et al. S-palmitoylation as a functional regulator of proteins associated with cisplatin resistance in bladder cancer. Int J Biol Sci. (2020) 16:2490–505. doi: 10.7150/ijbs.45640
19. Zhou B, Liu L, Reddivari M, and Zhang XA. The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res. (2004), 7455–63. doi: 10.1158/0008-5472.CAN-04-1574
20. Ko PJ and Dixon SJ. Protein palmitoylation and cancer. EMBO Rep. (2018) 19. doi: 10.15252/embr.201846666
21. Liu Z, Xiao M, Mo Y, Wang H, Han Y, Zhao X, et al. Emerging roles of protein palmitoylation and its modifying enzymes in cancer cell signal transduction and cancer therapy. Int J Biol Sci. (2022) 18:3447–57. doi: 10.7150/ijbs.72244
22. Bu J, Zhong W, Li M, He S, Zhang M, Zhang Y, et al. CD82 palmitoylation site mutations at Cys5+Cys74 affect EGFR internalization and metabolism through recycling pathway. Acta Biochim Biophys Sin (Shanghai). (2022) 54:400–8. doi: 10.3724/abbs.2022011
23. Tacar O, Sriamornsak P, and Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. (2013) 65:157–70. doi: 10.1111/j.2042-7158.2012.01567.x
Keywords: CD82, palmitoylation, apoptosis, drug sensitivity, breast cancer
Citation: Li L, Yu Y, Chen S, Zhang Y, Zhang C, Yao X, Xu H, Wu S, Gong X, Han W, Zhang Y and Li Y (2025) Mechanistic role of CD82 palmitoylation in augmenting antitumor drug sensitivity via apoptosis regulation. Front. Oncol. 15:1699420. doi: 10.3389/fonc.2025.1699420
Received: 05 September 2025; Accepted: 17 November 2025; Revised: 11 November 2025;
Published: 03 December 2025.
Edited by:
Raivis Zalubovskis, Latvian Institute of Organic Synthesis (LAS), LatviaReviewed by:
Yanfei Zhang, The University of Manchester, United KingdomDian Yuliartha Lestari, University of Muhammadiyah Malang, Indonesia
Copyright © 2025 Li, Yu, Chen, Zhang, Zhang, Yao, Xu, Wu, Gong, Han, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Li, TGl5aW5na2V0aXp1QDE2My5jb20=; Yu Zhang, NzM1ODgxMTUyNkBxcS5jb20=; Weiping Han, aGFud2VpcGluZzIwMDBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Lanyan Li†
Lanyan Li† Ying Li
Ying Li