- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Radiology, Peking University Cancer Hospital & Institute, Beijing, China
- 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Breast Center, Peking University Cancer Hospital & Institute, Beijing, China
Background: Background parenchymal enhancement (BPE) observed on dynamic contrast-enhanced (DCE) MRI of the contralateral breast is considered to be associated with survival outcomes. However, the prognostic significance of BPE in triple-negative breast cancer (TNBC) is unclear.
Methods: Between March 2017 and June 2019, 76 TNBC patients undergoing neoadjuvant therapy and subsequent surgery were included in the study. All patients underwent DCE MRI before and after neoadjuvant therapy. Radiologists graded BPE as minimum, mild, moderate, and marked. The BPE level was analyzed according to clinicopathological characteristics and MRI findings. Survival analysis was conducted for clinicopathological characteristics and MRI findings according to disease-free survival (DFS).
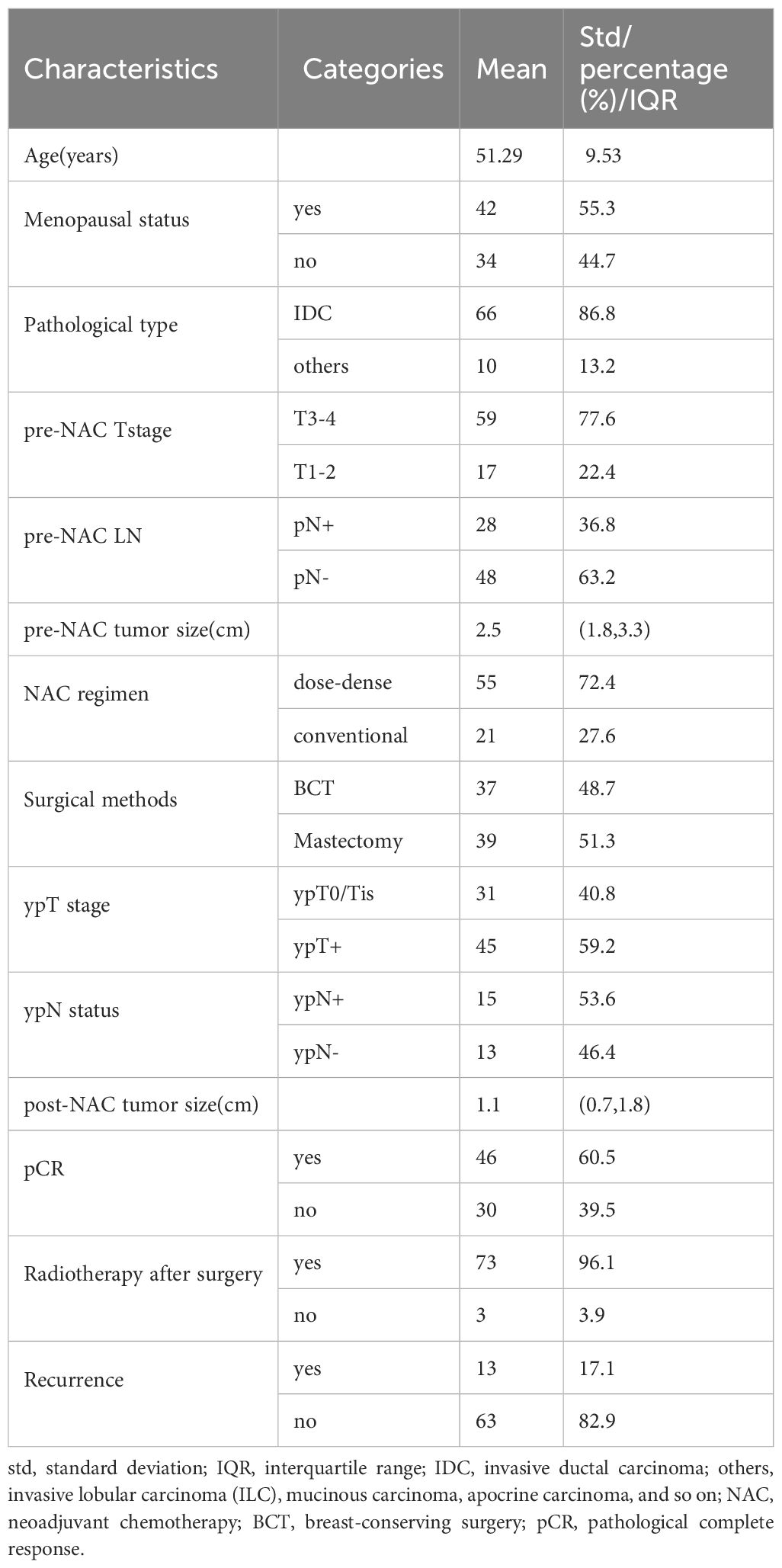
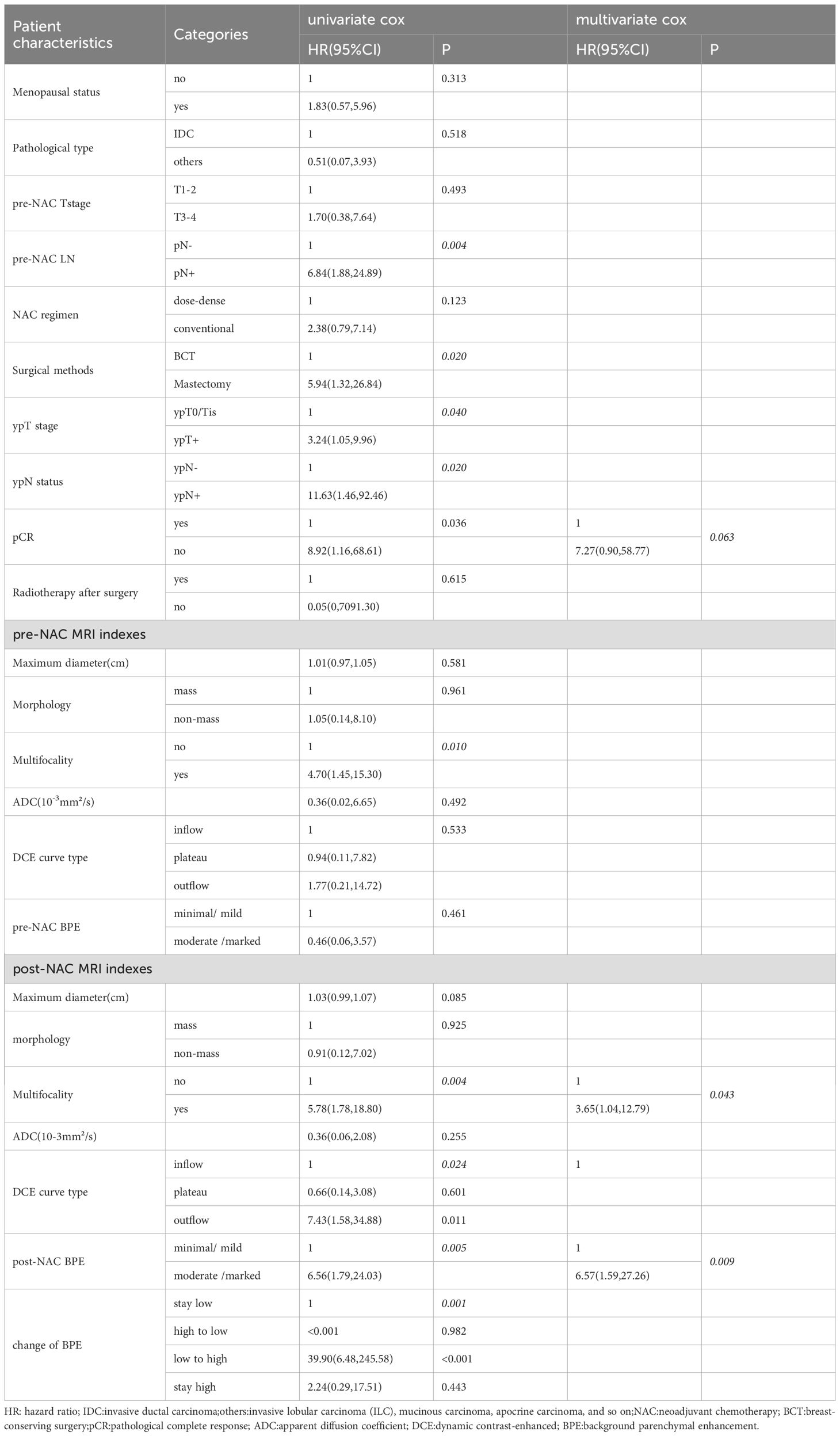
Results: The mean age was 51.29 ± 9.53 years; 46 (60.5%) patients achieved pathological complete response (pCR), and 13 (17.1%) patients developed recurrence, with a median follow-up of 80 months (interquartile range: 64, 90). Dichotomous BPE (minimal/mild vs. moderate/marked) on post-NAC MRI was statistically associated with post-NAC ADC and menopausal status. Patients with BPE changing from high to low level demonstrated statistically lower recurrence rate than patients with BPE changing from low to high (P = 0.022). BPE on post-NAC MRI was in the final multivariate Cox model for DFS (HR = 6.57, minimal/mild: HR = 1), along with multifocality on post-NAC MRI (HR = 3.65, no multifocality: HR = 1) and pCR (HR = 7.27, pCR: HR = 1).
Conclusion: Contralateral BPE and its change after neoadjuvant chemotherapy may reflect the recurrence risk in triple-negative breast cancer patients.
Introduction
Background parenchyma enhancement (BPE) refers to the enhancement manifestation of the normal breast tissue in breast dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) examination (1). BPE is usually evaluated according to a four-point scale of minimum, mild, moderate, and marked (1). High BPE can reflect an increase in vascular permeability, promote angiogenesis in the tumor microenvironment, and accelerate tumor growth and metastasis (2). Previous studies have proved that increased BPE can serve as a marker for developing breast cancer (3, 4) and may indicate a higher risk for the recurrence of breast cancer (5–7).
BPE is affected by estrogen levels, the menstrual cycle, menopausal status, and hormone replacement therapy (8, 9). Therefore, high BPE may indicate a poor response to endocrine therapy, potentially linked to the hormone-dependent proliferation pathway. This suggests that the prognostic value of BPE may be more significant in hormone receptor-positive breast cancer. In triple-negative breast cancer (TNBC), studies provided inconsistent evidence, whether high BPE was associated with a worse prognosis (10, 11), which may be related to the heterogeneity of TNBC and its relatively low dependence on the hormonal microenvironment. In addition, there are relatively few studies focusing on the dynamic changes of BPE after treatment according to survival outcomes in TNBC.
In summary, although BPE is a potential prognostic factor for breast cancer, its characteristics in TNBC and whether it may serve as a non-invasive imaging biomarker for risk stratification remain unknown at present. Therefore, this study proposed a retrospective analysis to evaluate whether BPE of the contralateral breast on breast MRI before and after neoadjuvant chemotherapy (NAC) and its dynamic changes are associated with primary breast cancer features and disease-free survival outcomes in TNBC patients.
Materials and methods
Patients
This study was approved by our institutional review board, and the patient informed consent was waived due to the retrospective design. Data from patients with operable TNBC confirmed by histology were retrospectively collected between March 2017 and June 2019 from the database of Peking University Cancer Hospital. The inclusion criteria include the following: age ≥18 years, received NAC before surgery, and received DCE MRI before and after NAC. The exclusion criteria were as follows: bilateral breast cancer, quality of MRI not meeting the requirements for evaluation, incomplete clinicopathological data, and lost to follow-up after surgery.
All tumors were evaluated by immunohistochemistry (IHC) for estrogen (ER) and progesterone (PR) receptors as well as for Her-2/neu by IHC and/or fluorescence in situ hybridization (FISH). TNBC was defined by a finding of ER and PR <1% and Her-2 (0, 1+, or 2+). If Her-2 was expressed at 2+, then the follow-up FISH should be negative. Clinicopathologic data included age, NAC regimen, pathological type, original state of axillary lymph node (LN), and pathological complete response (pCR) status. Axillary LN status was defined by needle biopsy as pN+ and pN− before NAC. Pathological complete response was defined as no invasive residual cancer cells in the breast (ypTis/0) from the total samples. LNpCR was defined as no residual cancer cells in the axillary LNs (ypN0) only from the pre-NAC pN+ samples.
MR examination
MR examinations were carried out within 2 weeks before NAC and 2 weeks before surgery for each patient. All breast MRI examinations were performed using a 1.5T system (GE Optima MR360; GE Healthcare,Tianjin,China; GE Healthcare) equipped with an 8-channel breast coil (GE Healthcare, Tianjin,China; GE Healthcare) with patients in the prone position.
Firstly, axial T2-weighted, fat-suppressed, short inversion time inversion recovery sequences were performed (TR = 5,000–5,800 ms, TE = 63.49 ms, TI = 160 ms, slice thickness = 4 mm, no interlayer gap, matrix size = 256 × 256, field of view = 28~36 cm, NEX = 2). Secondly, axial DWI examinations were performed using a diffusion-weighted echo planar imaging sequence with b-values of 0 and 1,000 s/mm (TR = 8,000 ms, TE = 79.7 ms, field of view = 32 × 18 cm, matrix = 150 × 80, slice thickness = 4.0 mm). Diffusion gradients were applied in three orthogonal directions.
Then, a dynamic enhanced axial three-dimensional vibrant SPGR sequence (TR = 6.4 ms, TE = 3.0 ms, TI = 7.0 ms, flip angle = 15°, slice thickness = 2.2 mm, with 50% overlap, matrix size = 320 × 320, field of vision = 28–36 cm, sequential K space filling, scan time per acquisition = 60 s). The sequence was repeated six times, with the first phase acquired before contrast enhancement and the other five phases acquired after contrast enhancement. The contrast agent (Gd-DTPA) was injected into the anterior elbow vein by a power syringe at a speed of 2.0 mL/s based on the patient’s weight (0.2 mmol/kg) and flushed with 20 mL of saline. The injection of the contrast agent and the second phase started at the same time.
Image evaluation
All MRI images were retrospectively obtained and assessed by a radiologist (H.B.Z.), who was blinded to the clinicopathologic and follow-up data. Two experienced radiologists (H.B.Z. and X.L.G.) independently conducted the BPE evaluation. The contralateral normal breast was used for image analysis. BPE was qualitatively assessed based on the intensity and volume of enhancement of normal fibroglandular tissues, using four categories defined by the Breast Imaging Reporting and Data System (BI-RADS) atlas: minimal, mild, moderate, or marked (1). In this study, BPE was assessed at the early arterial phase (first enhancement phase, in which the central K space time was approximately 30 s after contrast agent administration) in accordance with Breast Imaging Reporting and Data System (BI-RADS) Magnetic Resonance Imaging (MRI) lexicon (12). A third senior radiologist (Y.S.S.) was consulted to resolve discrepancies. DCE curve type (outflow/plateau/inflow), apparent diffusion coefficient (ADC value calculated from diffusion-weighted imaging with b-values of 0 and 1,000 s/mm), multifocality (yes/no), morphology (mass/non-mass), and maximum diameter (cm) were evaluated by the third radiologist.
Treatment and follow-up data
Two NAC regimens were used. The dose-dense (ddEC-wP) involved 4 cycles of epirubicin and cyclophosphamide every 2 weeks and then 12 weeks of paclitaxel weekly, with prophylactic pegfilgrastim or rhG-CSF. The conventional (EC-wP) had the same drugs/doses but at 3-week intervals for 4 cycles, with no prophylaxis, and then 12 weeks of paclitaxel weekly.
The surgical method depended on the patient’s will and the medical evaluation after NAC. Axillary LN dissection (ALND) was conducted for those with positive LN. Radiotherapy was conducted for patients undergoing breast-conserving surgery (BCT) or positive LN after NAC.
The primary endpoint was disease-free survival (DFS). DFS was calculated from the data of neoadjuvant treatment to the earliest occurrence of local recurrence, distant relapse, or death without prior relapse. In cases where the disease spread to the contralateral breast simultaneously with local and/or other distant site recurrences, it was regarded as a relapse. However, if the disease emerged solely in the contralateral breast without any local or distant recurrence, it was classified as a second primary cancer and not counted as a DFS failure. The follow-up time was censored at the last follow-up date for patients without follow-up events. All patients were followed according to a uniform institutional protocol. During the first 2 years postoperatively, follow-up was conducted every 3 months. From year 2 to year 5, follow-up was performed every 6 months. Beyond 5 years, annual follow-up was carried out. At each visit, surveillance included physical examination, breast/chest wall/axillary ultrasonography, imaging of the chest and abdomen (via CT or ultrasonography), and serum tumor marker assessment.
Statistical analysis
Normally distributed continuous variables were represented by means and standard deviation, while non-normally distributed continuous variables were represented by median and interquartile range (IQR). Categorical variables were represented by numbers. The independent sample t-test or Mann–Whitney test was used for comparisons of continuous variables between groups. Comparisons of categorical variables were conducted using the chi-square test or Fisher’s exact test. Cohen’s weighted kappa index (κ) was used to evaluate interobserver agreement, with 0.0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 indicating poor, fair, moderate, substantial, and excellent agreement, respectively (13). Univariate and multivariate Cox regression analyses were conducted to detect the prognostic effect of patient characteristics and MRI assessments according to DFS. Before the multivariate Cox regression was conducted, multicollinearity was detected. If the variance inflation factor (VIF) ≥10, strong multicollinearity was considered to exist, and then the variable with a lower P-value in the univariate analysis was retained for further multivariate analysis. Hazard ratios (HRs) with 95% confidence intervals were calculated. Kaplan–Meier curves with log-rank estimates were applied for the BPE groups. Two-sided P-values less than 0.05 were considered statistically significant. All statistical analysis was conducted using R4.4.2(R Core Team,Vienna, Austria).
Results
Patient characteristics and disease recurrences
Between March 2017 and June 2019, 112 TNBC patients, 18 to 75 years of age, who received NAC before surgery, were candidates for this study. Thirty-four candidates were excluded due to a lack of MRI examination either before or after neoadjuvant therapy, and two candidates were excluded due to loss to follow-up after surgery (Figure 1). Finally, a total of 76 patients who met the criteria were included in the analysis, with 51.29 ± 9.53 years of age. Among them, 55 (72.4%) patients received dose-dense NAC. Finally, 46 (69.5%) patients achieved pCR and 30 (39.5%) patients did not. The median follow-up was 80 months (IQR: 64, 90) after surgery, and 13 (17.1%) patients developed recurrence. The patients’ characteristics are listed in Table 1.
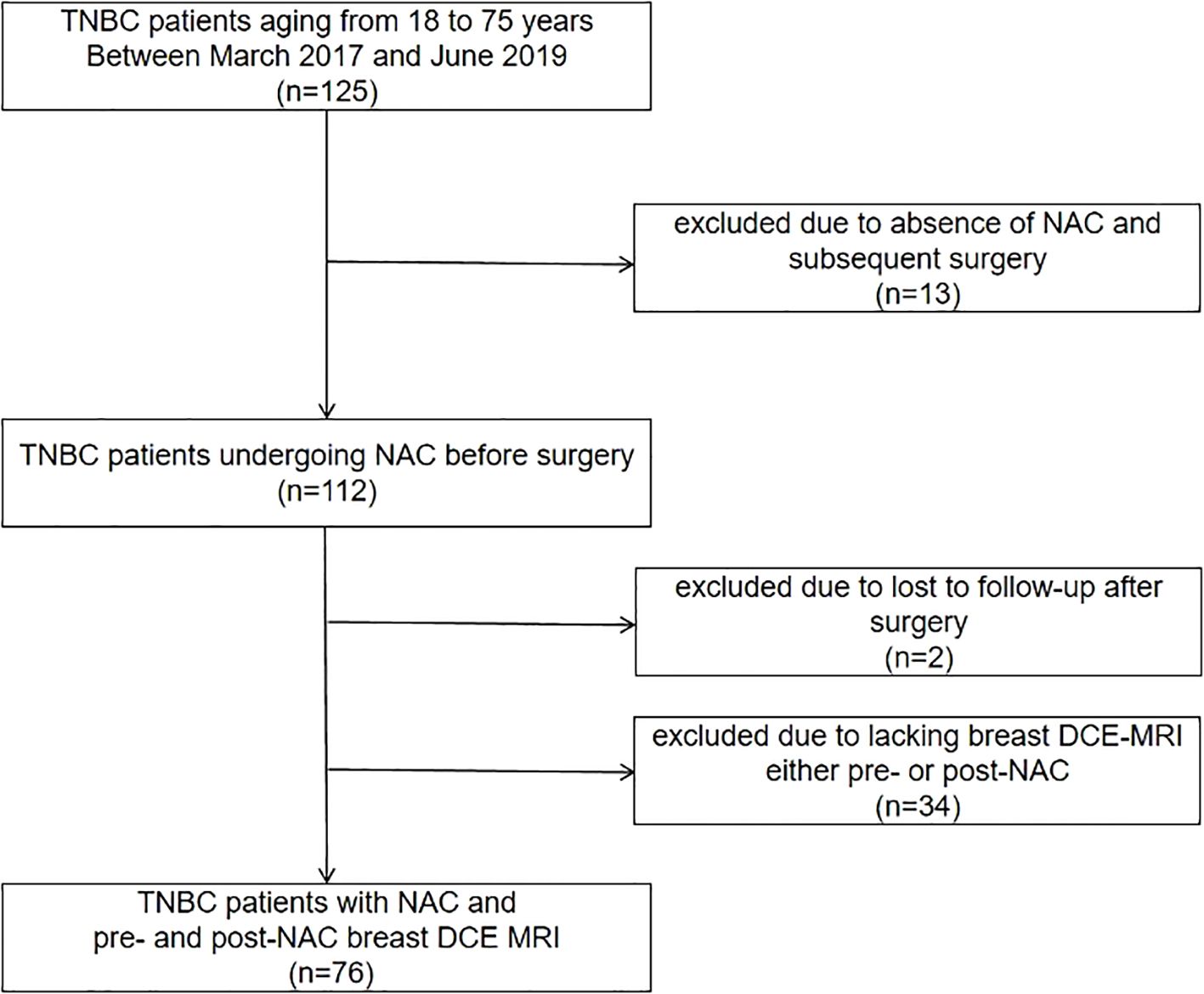
Figure 1. Flowchart for patient inclusion. TNBC, triple-negative breast cancer; NAC, neoadjuvant chemotherapy; DCE MRI, dynamic contrast-enhanced magnetic resonance imaging.
BPE on baseline breast MRI according to patient characteristics and MRI indexes
Excellent interobserver agreement was obtained for BPE evaluation with a weighted kappa coefficient of 0.904. The dichotomous BPE (minimal/mild vs. moderate/marked) on pre-NAC MRI and post-NAC MRI were both statistically associated with menopausal status (P = 0.045 and 0.015, respectively). The dichotomous BPE (minimal/mild vs. moderate/marked) on post-NAC MRI was also associated with ADC values, with minimal/mild BPE showing higher ADC values (P = 0.004). While BPE was dichotomized as minimal BPE and mild/moderate/marked BPE, those showing minimal BPE on post-NAC MRI had statistically less multifocality than those with mild/moderate/marked on post-NAC MRI, with P-values of 0.007 for multifocality on pre-NAC MRI and 0.011 for multifocality on post-NAC MRI. The BPE on pre-NAC MRI did not demonstrate a significant association with either clinicopathological or other MRI characteristics. The results are shown in Tables 2, 3.
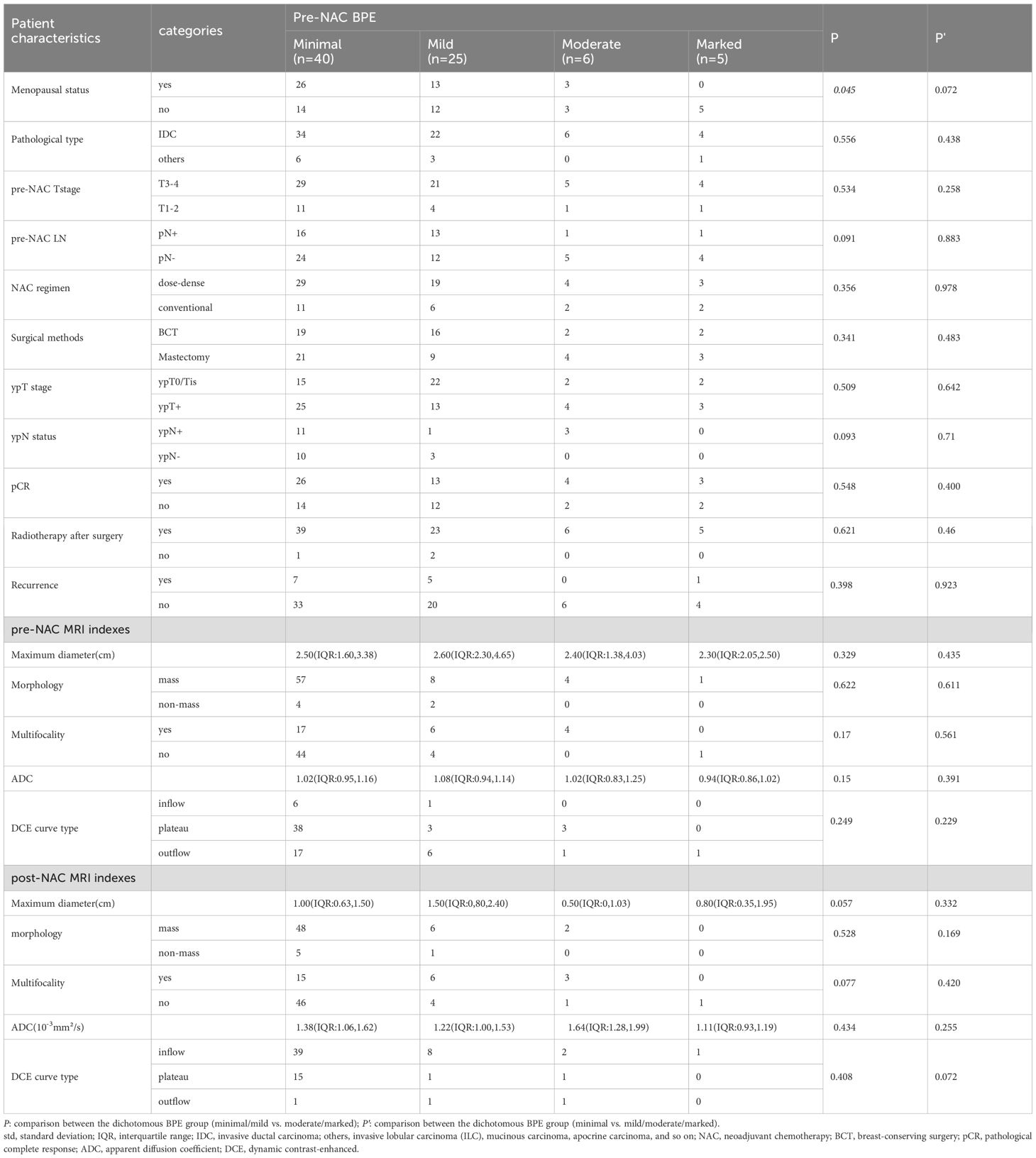
Table 2. Background parenchyma enhancement (BPE) on pre-NAC breast MRI according to patient characteristics and MRI indexes.
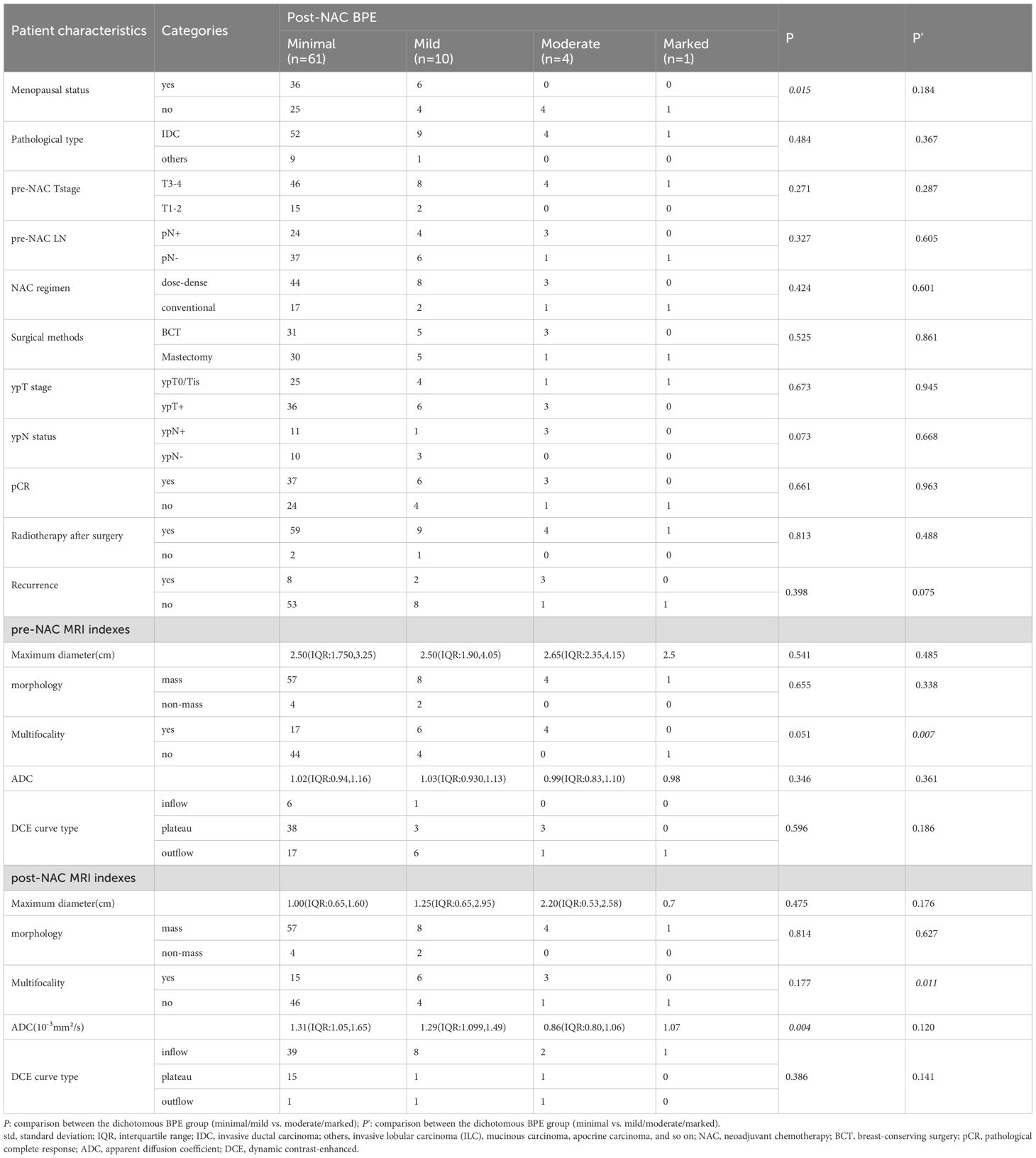
Table 3. Background parenchyma enhancement (BPE) on post-NAC breast MRI according to patient characteristics and MRI indexes.
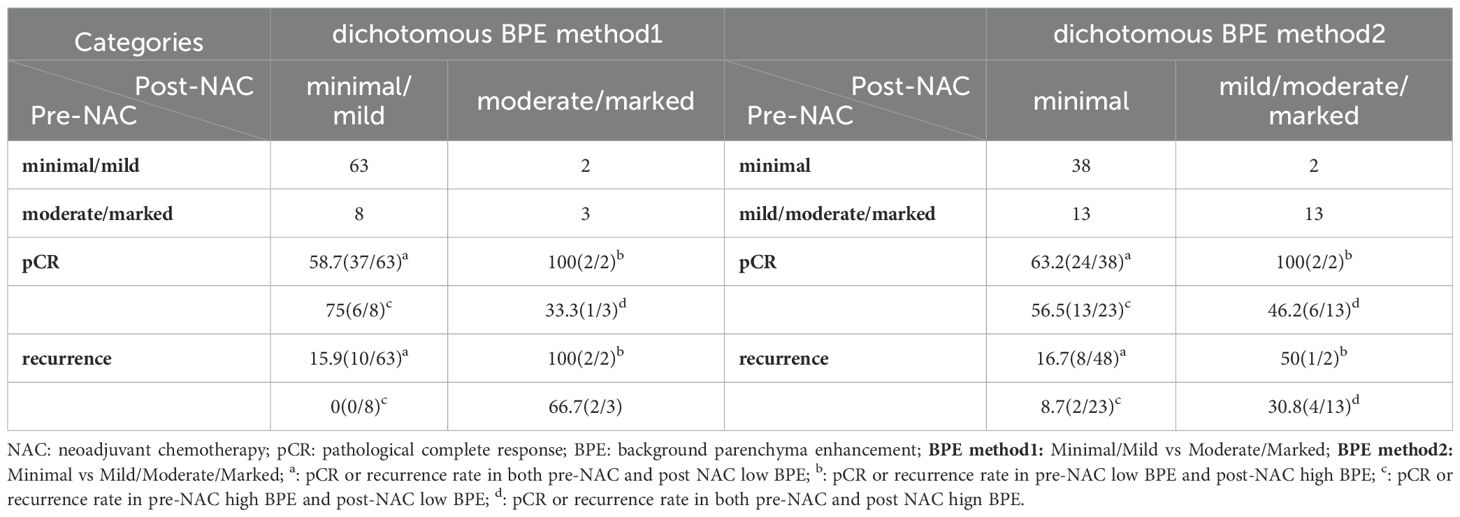
The change of BPE after neoadjuvant therapy was analyzed according to pCR and recurrence (Table 4). The results showed that when a high BPE on pre-NAC MRI changed into low BPE on post-NAC MRI, no patient suffered recurrence (dichotomous BPE method 1), while if a low BPE on pre-NAC MRI changed into high BPE on post-NAC MRI, patients presented a high risk for recurrence (recurrence rate: 100%, dichotomous BPE method 1), and the P-value was 0.022. In dichotomous BPE method 2, similar results were observed (recurrence of BPE from high to low vs. BPE from low to high: 8.7% vs. 50%), but no statistically significant difference was observed (P = 0.23). However, BPE changes were not associated with pCR, neither in dichotomous BPE method 1 nor in dichotomous BPE method 2 (P = 0.406 and 0.611, respectively). Examples of BPE changes are shown in Figure 2.
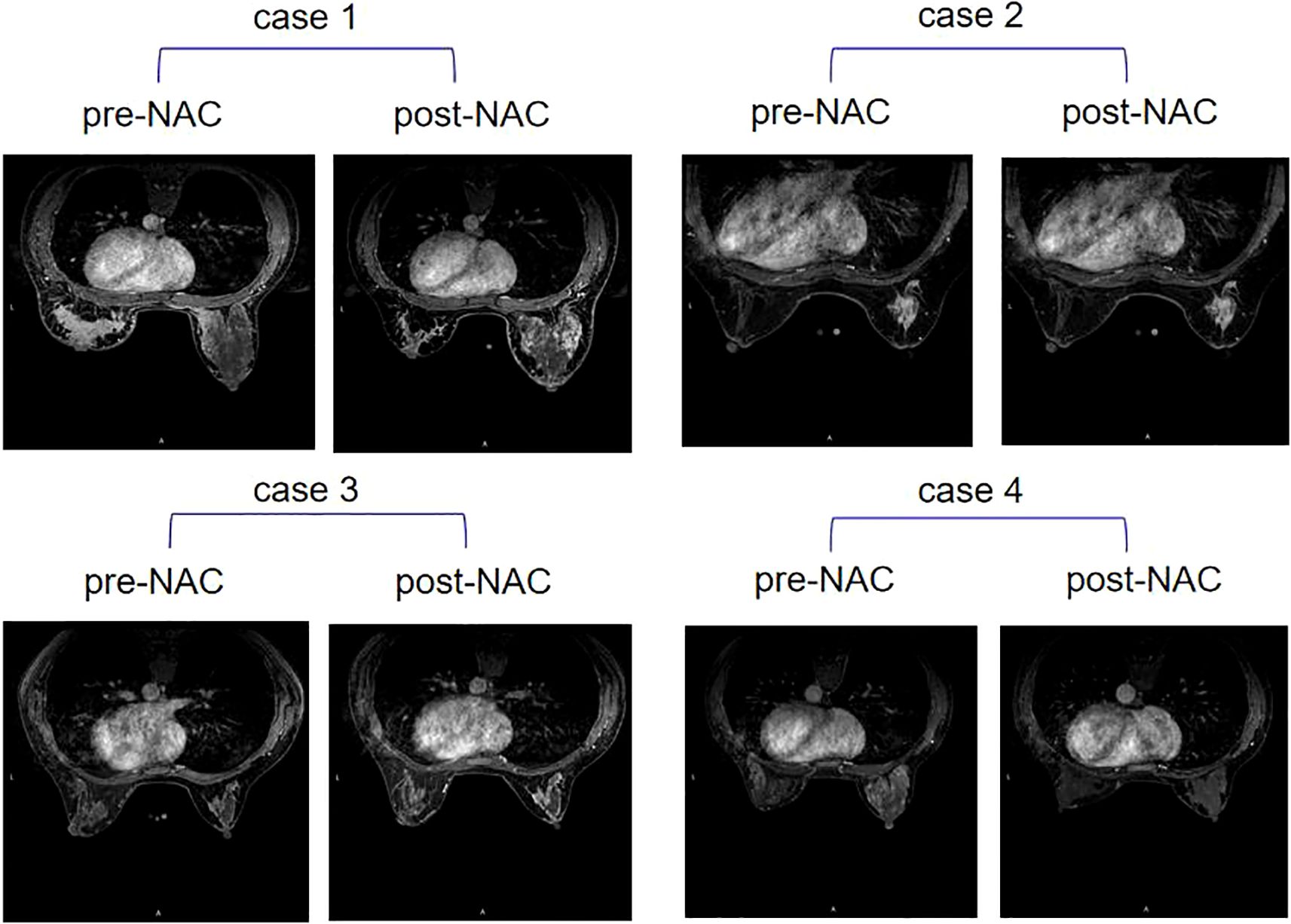
Figure 2. Four examples of contralateral BPE change after NAC. Case 1: BPE stayed high on pre-NAC MRI (moderate) and post-NAC MRI (moderate); case 2: BPE stayed low on pre-NAC MRI (minimum) and post-NAC MRI (minimum); case 3: BPE changed from low on pre-NAC MRI (mild) to high on post-NAC MRI (moderate); case 4: BPE changed from high on pre-NAC MRI (marked) to low on post-NAC MRI (minimum). BPE, background parenchymal enhancement; NAC, neoadjuvant chemotherapy.
Survival analysis
Univariate Cox regression showed pre-NAC LN, surgical methods, ypT stage, ypN status, pCR, multifocality on pre-NAC and post-NAC MRI, DCE curve type, and BPE on post-NAC MRI and the change of BPE were significant factors for disease-free survival (Table 5). According to the multivariate Cox regression analysis, BPE and multifocality on post-NAC MRI, as well as pCR, were included in the final multivariate model, with adjusted HRs of 6.57, 3.65, and 7.27, respectively. Kaplan–Meier curves for BPE on post-NAC MRI and the change of BPE are shown in Figure 3.
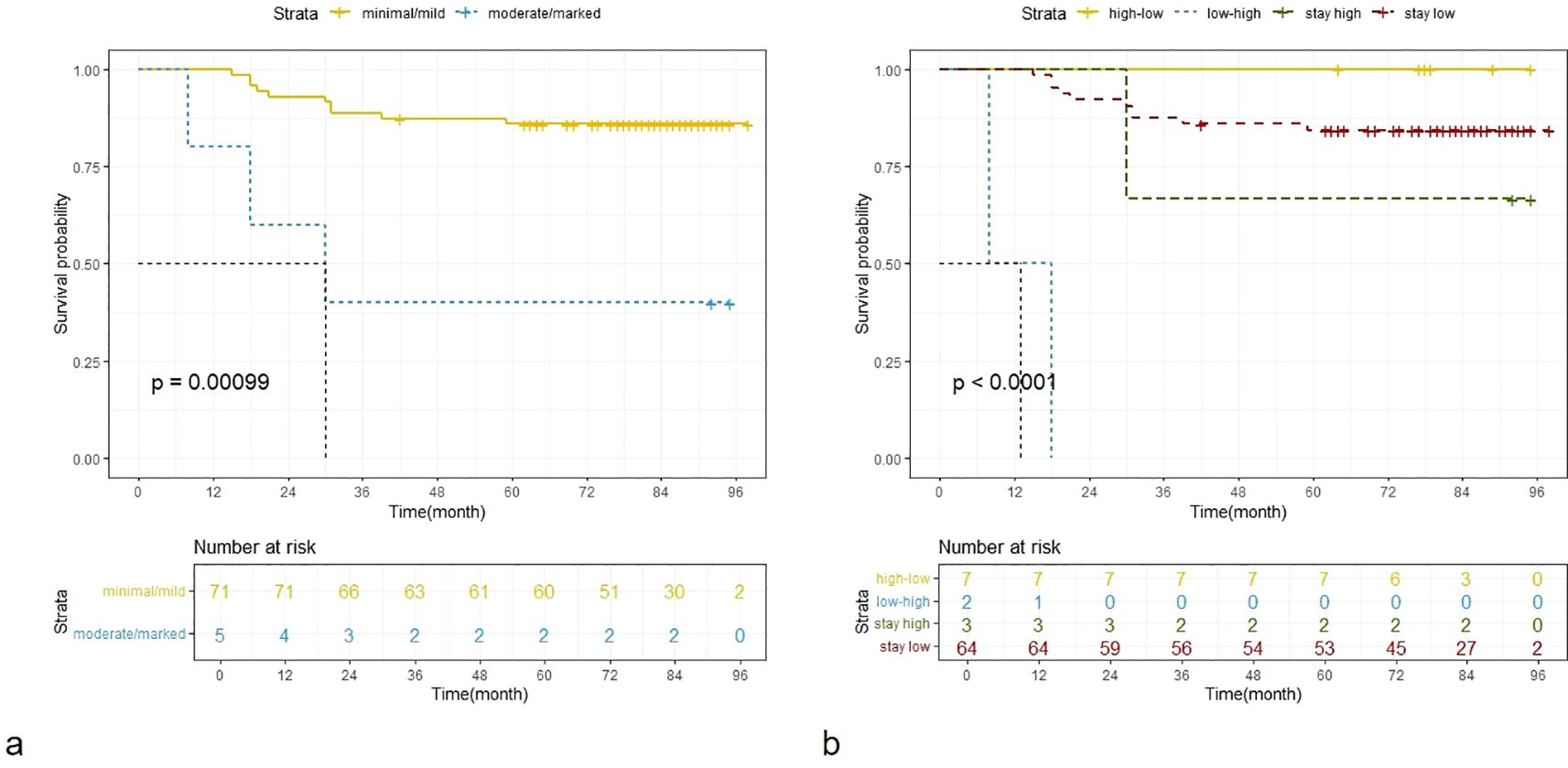
Figure 3. Kaplan–Meier curves for BPE on post-NAC MRI (a) and the change of BPE (b). BPE, dichotomous background parenchymal enhancement; NAC, neoadjuvant chemotherapy.
Discussion
This study provided evidence that moderate or marked BPE on post-NAC MRI indicated worse DFS, while BPE changing from high level (moderate/marked) to low level (minimal/mild) after NAC indicated better DFS. BPE on post-NAC MRI was an independent prognostic factor along with multifocality on post-NAC MRI and pCR status.
The physiological mechanisms of BPE are mainly related to angiogenesis, increased vascular permeability, and changes in the extracellular space (2). Additionally, fluctuations in hormone levels, particularly estrogen and progesterone, can significantly influence the physiological state of breast tissue in women. During the menstrual cycle, fluctuations in hormone levels will promote angiogenesis and increase vascular permeability within the breast tissue, resulting in obvious BPE on MRI (9). Therefore, breast MRI examinations should be conducted at the time that avoids the menstrual cycle. In this study, we also found that BPE was obviously higher in premenopausal patients. At the same time, extracellular matrix remodeling in the breast stroma and the release of inflammatory factors may also lead to an obvious BPE by enhancing local vascular permeability (14). Moreover, some studies have found that quantitative BPE varies between BRCA mutation carriers and non-carriers, as well as high-risk non-BRCA mutation carriers and non-high-risk non-BRCA mutation carriers (15, 16), which may be related to the abnormal proliferation of the breast stroma caused by DNA repair defects. However, there was one study that concluded a non-significant relation (17).
In recent years, studies have focused on the treatment response based on the BPE level. Many studies suggested a positive relationship between BPE level and residual tumor; obvious decreased BPE after neoadjuvant treatment could indicate higher rates of pCR, while higher BPE after neoadjuvant treatment usually means a poor response (18–20). Although BPE changes for pCR were reported in several articles, there is still controversy over it (21), as the study was carried out in hormone receptor (HR)-negative patients. Onishi et al. (22) found that non-suppressed BPE may be associated with inferior response to NAC in HR-positive patients, while in HR-negative patients, a similar tendency was observed without statistical significance. In our study, we did not detect a statistically significant association between BPE and pCR status (P = 0.548 for pre-NAC BPE and 0.661 for post-NAC BPE), which may be mainly due to the study population of TNBC patients.
From a physiological perspective, increased BPE may be associated with poorer prognosis, which has also been verified in some studies (5, 7, 23, 24). However, some studies have reached different conclusions, finding that contralateral BPE was not significantly associated with survival outcomes (25–27). These studies were usually conducted among patients with HR-positive breast cancer, or they included different molecular subtypes. There are a few related studies on triple-negative breast cancer. Consequently, the findings of this study offer valuable evidence regarding the potential of BPE as a prognostic indicator for TNBC. This research could potentially contribute to improve the prognostic evaluation and treatment of TNBC. If BPE can accurately identify patients with poor prognosis among TNBC patients, it may help manage targeted intensive treatment and timely adjustment of treatment plans. In addition, BPE could also serve as a means to monitor prognosis and recurrence for TNBC patients.
This study has some limitations. Firstly, the sample size is relatively small. The impact of the changes in BPE before and after neoadjuvant treatment on prognosis still needs to be verified in a large sample population. Secondly, BPE evaluation in this study relied on subjective judgment. Although subjective judgment is easy to operate and perfect interobserver agreement has been achieved, it should still be noted that there was a discrepancy in the evaluation of 6 cases out of 76 (7.9%). Thus, it is recommended to apply artificial intelligence-based quantitative measurement tools to eliminate the influence of the raters when used in clinical practice. Thirdly, this study was conducted among TNBC patients. In the future, it is necessary to investigate the impact of BPE changes on the prognosis among patients with hormone receptor-positive breast cancer. Moreover, by combining the physiological mechanisms of BPE, the heterogeneity of BPE changes can be more accurately understood in different molecular subtypes of breast cancer. Additionally, studies have shown that multiparametric MRI sequences can predict tumor proliferative activity and tumor immune microenvironment characteristics (28, 29). Therefore, research on the association between BPE and these biological markers will also help us understand the role of BPE in prognostic assessment.
In conclusion, this study suggested that BPE on post-NAC and its variation after neoadjuvant chemotherapy may be used to indicate the recurrence risk in TNBC patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Beijing Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This was a retrospective study without intervention on participants. The study only used medical records.
Author contributions
X-TL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. XW: Conceptualization, Investigation, Data curation, Writing – original draft. H-TZ: Formal analysis, Investigation, Methodology, Writing – review & editing. NS: Formal Analysis, Investigation, Methodology, Writing – review & editing. H-BZ: Data curation, Investigation, Writing – review & editing, Methodology. LY: Investigation, Writing – review & editing. X-LG: Writing – review & editing, Investigation. YL: Investigation, Writing – review & editing, Data curation. Z-QF: Conceptualization, Resources, Supervision, Writing – review & editing. Y-SS: Writing – review & editing, Conceptualization, Funding acquisition, Resources, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Key R&D Program of China (2023YFC3402805).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BPE, background parenchymal enhancement; DCE, dynamic contrast-enhanced; NAC, neoadjuvant chemotherapy; ADC, apparent diffusion coefficient; pCR, pathological complete response; Std, standard deviation; IQR, interquartile range; IDC, invasive ductal carcinoma; BCT, breast-conserving surgery; DFS, disease-free survival.
References
1. Morris E, Comstock C, Lee C, Lehman C, Ikeda D, and Newstead G. ACR BI-RADS® Magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston, VA, USA (2013).
2. Sung JS, Corben AD, Brooks JD, Edelweiss M, Keating DM, Lin C, et al. Histopathologic characteristics of background parenchymal enhancement (BPE) on breast MRI. Breast Cancer Res Treat. (2018) 172:487–96. doi: 10.1007/s10549-018-4916-6
3. Thompson CM, Mallawaarachchi I, Dwivedi DK, Ayyappan AP, Shokar NK, Lakshmanaswamy R, et al. The association of background parenchymal enhancement at breast MRI with breast cancer: A systematic review and meta-analysis. Radiology. (2019) 292:552–61. doi: 10.1148/radiol.2019182441
4. Arasu VA, Miglioretti DL, Sprague BL, Alsheik NH, Buist DSM, Henderson LM, et al. Population-based assessment of the association between magnetic resonance imaging background parenchymal enhancement and future primary breast cancer risk. J Clin Oncol. (2019) 37:954–63. doi: 10.1200/JCO.18.00378
5. Wang H, van der Velden BHM, Verburg E, Bakker MF, Pijnappel RM, Veldhuis WB, et al. Assessing quantitative parenchymal features at baseline dynamic contrast-enhanced MRI and cancer occurrence in women with extremely dense breasts. Radiology. (2023) 308:e222841. doi: 10.1148/radiol.222841
6. Lee SH, Jang MJ, Yoen H, Lee Y, Kim YS, Park AR, et al. Background parenchymal enhancement at postoperative surveillance breast MRI: association with future second breast cancer risk. Radiology. (2023) 306:90–9. doi: 10.1148/radiol.220440
7. Zhang M, Sadinski M, Haddad D, Bae MS, Martinez D, Morris EA, et al. Background parenchymal enhancement on breast MRI as a prognostic surrogate: correlation with breast cancer oncotype dx score. Front Oncol. (2021) 10:595820. doi: 10.3389/fonc.2020.595820
8. King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, and Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol. (2012) 22:2641–7. doi: 10.1007/s00330-012-2553-8
9. Heller SL, Young Lin LL, Melsaether AN, Moy L, and Gao Y. Hormonal effects on breast density, fibroglandular tissue, and background parenchymal enhancement. Radiographics. (2018) 38:983–96. doi: 10.1148/rg.2018180035
10. Rella R, Bufi E, Belli P, Scrofani AR, Petta F, Borghetti A, et al. Association between contralateral background parenchymal enhancement on MRI and outcome in patients with unilateral invasive breast cancer receiving neoadjuvant chemotherapy. Diagn Interv Imaging. (2022) 103(10):486–94. doi: 10.1016
11. Mema E, Schnabel F, Chun J, Kaplowitz E, Price A, Goodgal J, et al. The relationship of breast density in mammography and magnetic resonance imaging in women with triple negative breast cancer. Eur J Radiol. (2020) 124:108813. doi: 10.1016/j.ejrad.2020.108813
12. Giess CS, Yeh ED, Raza S, and Birdwell RL. Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics. (2014) 34:234–47. doi: 10.1148/rg.341135034
13. Brennan P and Silman A. Statistical methods for assessing observervariability in clinical measures. BMJ. (1992) 304:1491–4. doi: 10.1136/bmj.304.6840.1491
14. Herrera-Quintana L, Vázquez-Lorente H, and Plaza-Diaz J. Breast cancer: extracellular matrix and microbiome interactions. Int J Mol Sci. (2024) 25:7226. doi: 10.3390/ijms25137226
15. Yan R, Murakami W, Mortazavi S, Yu T, Chu FI, Lee-Felker S, et al. Quantitative assessment of background parenchymal enhancement is associated with lifetime breast cancer risk in screening MRI. Eur Radiol. (2024) 34:6358–68. doi: 10.1007/s00330-024-10758-9
16. Murakami W, Mortazavi S, Yu T, Kathuria-Prakash N, Yan R, Fischer C, et al. Clinical significance of background parenchymal enhancement in breast cancer risk stratification. J Magn Reson Imaging. (2024) 59:1742–57. doi: 10.1002/jmri.29015
17. Goodburn R, Kousi E, Sanders C, Macdonald A, Scurr E, Bunce C, et al. Quantitative background parenchymal enhancement and fibro-glandular density at breast MRI: Association with BRCA status. Eur Radiol. (2023) 33:6204–12. doi: 10.1007/s00330-023-09592-2
18. Ren Z, Pineda FD, Howard FM, Hill E, Szasz T, Safi R, et al. Differences between ipsilateral and contralateral early parenchymal enhancement kinetics predict response of breast cancer to neoadjuvant therapy. Acad Radiol. (2022) 29:1469–79. doi: 10.1016/j.acra.2022.02.008
19. You C, Gu Y, Peng W, Li J, Shen X, Liu G, et al. Decreased background parenchymal enhancement of the contralateral breast after two cycles of neoadjuvant chemotherapy is associated with tumor response in HER2-positive breast cancer. Acta Radiol. (2018) 59:806–12. doi: 10.1177/0284185117738560
20. Virostko J, Kuketz G, Higgins E, Wu C, Sorace AG, DiCarlo JC, et al. The rate of breast fibroglandular enhancement during dynamic contrast-enhanced MRI reflects response to neoadjuvant therapy. Eur J Radiol. (2021) 136:109534. doi: 10.1016/j.ejrad.2021.109534
21. Li X and Yan F. Predictive value of background parenchymal enhancement on breast magnetic resonance imaging for pathological tumor response to neoadjuvant chemotherapy in breast cancers: a systematic review. Cancer Imaging. (2024) 24:35. doi: 10.1186/s40644-024-00672-0
22. Onishi N, Li W, Newitt DC, Harnish RJ, Strand F, Nguyen AA, et al. Breast MRI during neoadjuvant chemotherapy: lack of background parenchymal enhancement suppression and inferior treatment response. Radiology. (2021) 301:295–308. doi: 10.1148/radiol.2021203645
23. Lim Y, Ko ES, Han BK, Ko EY, Choi JS, Lee JE, et al. Background parenchymal enhancement on breast MRI: association with recurrence-free survival in patients with newly diagnosed invasive breast cancer. Breast Cancer Res Treat. (2017) 163:573–86. doi: 10.1007/s10549-017-4217-5
24. Rella R, Bufi E, Belli P, Scrofani AR, Petta F, Borghetti A, et al. Association between contralateral background parenchymal enhancement on MRI and outcome in patients with unilateral invasive breast cancer receiving neoadjuvant chemotherapy. Diagn Interv Imaging. (2022) 103:486–94. doi: 10.1016/j.diii.2022.04.004
25. Shin GW, Zhang Y, Kim MJ, Su MY, Kim EK, Moon HJ, et al. Role of dynamic contrast-enhanced MRI in evaluating the association between contralateral parenchymal enhancement and survival outcome in ER-positive, HER2-negative, node-negative invasive breast cancer. J Magn Reson Imaging. (2018) 48:1678–89. doi: 10.1002/jmri.26176
26. Lo Gullo R, Daimiel I, Rossi Saccarelli C, Bitencourt A, Sevilimedu V, Martinez DF, et al. MRI background parenchymal enhancement, fibroglandular tissue, and mammographic breast density in patients with invasive lobular breast cancer on adjuvant endocrine hormonal treatment: associations with survival. Breast Cancer Res. (2020) 22:93. doi: 10.1186/s13058-020-01329-z
27. Kim JY, Kim JJ, Lee JW, Lee NK, Kim S, Nam KJ, et al. Are background breast parenchymal features on preoperative breast MRI associated with disease-free survival in patients with invasive breast cancer? Radiol Med. (2024) 129:1790–801. doi: 10.1007/s11547-024-01914-8
28. Xu J, Zhang L, Liu Q, and Zhu J. Preoperative multiparameter MRI-based prediction of Ki-67 expression in primary central nervous system lymphoma. Prec Radiat Oncol. (2025) 9:23–34. doi: 10.1002/pro6.70005
Keywords: breast neoplasms, magnetic resonance imaging, background parenchymal enhancement, disease-free survival, neoadjuvant chemotherapy
Citation: Li X-T, Wang X, Zhu H-T, Sun N, Zhu H-B, You L, Gu X-L, Luo Y, Fan Z-Q and Sun Y-S (2025) Background parenchymal enhancement of the contralateral breast on preoperative contrast-enhanced breast MRI as a potential predictive factor for disease-free survival in triple-negative breast cancer patients. Front. Oncol. 15:1700320. doi: 10.3389/fonc.2025.1700320
Received: 06 September 2025; Accepted: 29 September 2025;
Published: 21 October 2025.
Edited by:
Shuai Ren, Affiliated Hospital of Nanjing University of Chinese Medicine, ChinaReviewed by:
Zhen-Hui Li, Yunnan Cancer Hospital, ChinaTong Tong, Fudan University Cancer Hospital, China
Copyright © 2025 Li, Wang, Zhu, Sun, Zhu, You, Gu, Luo, Fan and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Shi Sun, c3lzMjdAMTYzLmNvbQ==; Zhao-Qing Fan, emhxZmFuQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Xiao-Ting Li1†
Xiao-Ting Li1† Hai-Tao Zhu
Hai-Tao Zhu Ying-Shi Sun
Ying-Shi Sun