- 1Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 2Department of Radiation Oncology, Shenzhen Luohu People’s Hospital, Shenzhen, China
Objective: To explore the efficacy and safety of toripalimab combined with gemcitabine and cisplatin (GP) induction chemotherapy and sequential concurrent chemoradiotherapy in LANPC treatment.
Methods: This was a retrospective analysis of 105 patients with LANPC from December 2019 to December 2022. In total, 50 patients received two or three cycles of GP induction chemotherapy and 55 patients received toripalimab plus GP. Toripalimab (240 mg) was given intravenously on the first day of each cycle of induction chemotherapy. All patients received radiotherapy or concurrent chemoradiotherapy with cisplatin.
Results: After induction therapy, 17 (30.9%) patients in the GP plus toripalimab group and 6 (12.0%) in the GP alone group achieved complete response (CR) (p=0.019). After a median follow-up of 38.6 months, 16.0% (8/50) of the patients in the GP group and 3.6% (2/55) of the patients in the toripalimab plus GP group experienced recurrence or metastasis. There were 2 deaths in the GP group and no deaths in the toripalimab plus GP group. The 2-year event-free survival (EFS) rate was higher in the toripalimab plus GP group than in the GP group (98.1% vs. 85.4% (HR, 0.28; 95% confidence interval [CI], 0.08–0.97; p=0.024)). The 2-year overall survival, locoregional relapse-free survival and distant metastasis-free survival rates for toripalimab plus GP vs. GP alone were 100.0% vs. 100.0% (p=1.00), 98.1% vs. 89.5% (p=0.086), and 100.0% vs. 95.9% (p=0.15), respectively. Grade 3–4 adverse events (AEs) occurred in 26 (47.3%) and 29 (58.0%) patients in the toripalimab plus GP and GP alone arms, respectively. The most common grade 3–4 AEs were neutropenia (20 [36.4%] vs. 21 [42.0%]), leukopenia (18 [32.7%] vs. 17 [34.0%]), and vomiting (15 [27.3%] vs. 12 [24.0%]) in the toripalimab plus GP arm compared with the GP alone arm. Immune-related AEs of grade 3–4 occurred in three (5.5%) patients in the toripalimab plus GP arm.
Conclusions: The addition of toripalimab to GP induction chemotherapy significantly improves EFS without increasing toxicity in LANPC.
Introduction
Nasopharyngeal carcinoma is a malignant tumor that occurs in the nasopharyngeal epithelium. The disease has a special regional distribution and is common in South China, Southeast Asia, and North Africa (1). More than 70% of patients have locoregionally advanced disease at the time of diagnosis.
Nasopharyngeal carcinoma is highly sensitive to ionizing radiation. Radiotherapy or the combination of intensity-modulated radiotherapy (IMRT) and platinum-based chemotherapy is the backbone of treatment for NPC (2, 3). However, for locally advanced NPC (LANPC), distant metastasis and local recurrence are the main failure patterns of therapy (2, 4, 5). Identifying a way to address this issue is a hot topic in clinical research. Studies have shown that the addition of induction chemotherapy (IC) to chemoradiotherapy significantly improves the recurrence-free survival and overall survival of patients with LANPC (6–9).
In recent years, immune checkpoint inhibitors have attracted great interest among researchers. Previous studies, including CAPTAIN-1st, JUPITER-02 and RATIONALE-309, have shown that immune checkpoint inhibitors improve the survival of patients with recurrent or metastatic nasopharyngeal carcinoma (10–15). Nevertheless, in the context of LANPC, the efficacy and safety of gemcitabine and cisplatin induction chemotherapy plus toripalimab are unclear. Hence, we conducted this retrospective study to evaluate the efficacy and safety of adding toripalimab to gemcitabine and cisplatin induction chemotherapy in patients with LANPC.
Materials and methods
Patients
This was a retrospective study of the clinical data of patients with locally advanced NPC who received induction therapy at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital between December 2019 and December 2022. The inclusion criteria for this study were as follows: (i) age ≥18 years; (ii) pathologically diagnosed with NPC; (iii) stage III/IVa in accordance with the 8th Edition of the AJCC; (iv) Karnofsky performance score (KPS) ≥70; (v) receiving IC or IC plus toripalimab followed by definitive CCRT; (vi) receiving concurrent chemotherapy with cisplatin; and (vii) receiving IMRT. The exclusion criteria were as follows: (i) received concurrent immunotherapy; (ii) received adjuvant chemotherapy or adjuvant immunotherapy after CCRT; (iii) underwent surgery before IC; or (iv) had a second malignancy.
Treatment
All patients received radical IMRT at our hospital as previously described (16). The radiation used in radiotherapy is 6MV-X rays. Briefly, the radiation doses used were as follows: 69.96 Gy at 2.12 Gy/fraction to the planning target volume (PTV) of the nasopharyngeal gross tumor volume (GTV), 69.96 Gy to the PTV of the GTV of the metastatic lymph nodes, 60.06 Gy to the PTV of the high-risk clinical target volume, and 54.45 Gy to the PTV of the low-risk clinical target volume. If the patient received concurrent nimotuzumab therapy, nimotuzumab (200 mg/week) was given intravenously on the first day of radiotherapy.
The induction chemotherapy regimens used were gemcitabine (1000 mg/m2 on days 1 and 8) and cisplatin (80 mg/m2 on day 1) every 3 weeks for 2–3 cycles. Concurrent chemotherapy consisted of 100 mg of cisplatin per square meter. Toripalimab (240 mg) was given intravenously on the first day of each cycle of IC.
Clinical endpoints
The endpoints included event-free survival (EFS, the time from the start of treatment to disease progression or death from any cause), overall survival (OS, the time from the start of treatment to death from any cause), distant metastasis-free survival (DMFS, the time from the start of treatment to distant metastasis) and locoregional recurrence-free survival (LRFS, the time from the start of treatment to locoregional recurrence). Efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECISTv1.1). Acute toxicities during treatment were graded according to the Common Terminology Criteria for Adverse Events (version 4.0), and late toxicities related to radiotherapy were evaluated on the basis of the Late Radiation Morbidity Scoring Scheme of the Radiation Therapy Oncology Group.
Statistical analysis
The chi-squared test or Fisher’s Freeman–Halton test was used for the comparison of categorical variables. One-way analysis of variance (ANOVA) was used for the comparison of differences in the numerical variables among groups. Two-group comparisons of the survival data via Kaplan–Meier curves and analyzed by mean of log-rank tests. A multivariate Cox proportional hazards model was used to calculate hazard ratios (HRs), 95% confidence intervals (CIs) and independent prognostic factors. p<0.05 was considered statistically significant.
Results
Patient characteristics
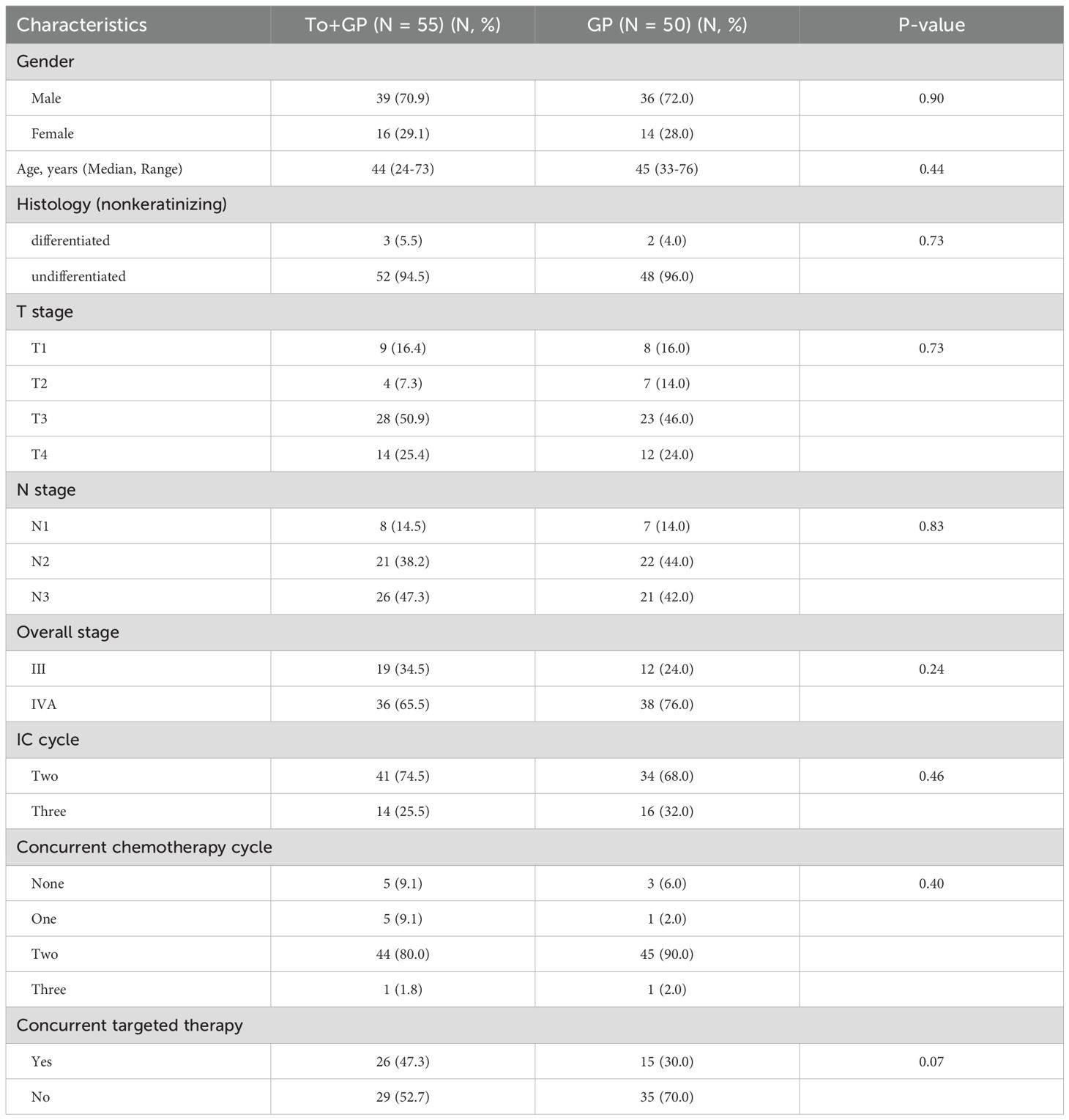
In total, 105 patients, including 50 (47.6%) patients with GP alone and 55 (52.4%) patients with GP combined with toripalimab, were eligible for this study. The characteristics of the patients at baseline are summarized in Table 1. Most patients had N2 or N3 disease of the cervical lymph nodes or bulky primary tumors (T3 or T4). According to the 8th edition of the AJCC, 31 (29.5%) patients were in stage III, and 64 (61.0%) were in stage IVA. There were no significant differences in age, sex, T stage, N stage, TNM stage, pathological characteristics, cycles of IC, cycles of concurrent chemotherapy, or concurrent nimotuzumab between the two groups.
Efficacy
After induction therapy, 17 (30.9%), 33 (60.0%) and 5 (9.1%) patients in the GP plus toripalimab group and 6 (12.0%), 41 (82.0%) and 3 (6.0%) patients in the GP alone group achieved complete response (CR), partial response (PR) and stable disease (SD), respectively (p=0.041). There were no patients who had progressive disease (PD) in either group.
At the last follow-up on June 30, 2024, the median follow-up time was 38.6 months (range, 7.7-53.3). By the cutoff time, 93.3% (98/105) of the patients had been followed for at least 24 months. There were 10 events of disease progression or death (9.5% of patients in the entire population), including 8 of 50 patients (16.0%) in the GP alone group and 2 of 55 (3.6%) in the GP plus toripalimab group. We recorded a total of 2 events of death (1 death 29.5 months after diagnosis and 1 death 34.4 months after diagnosis) in the GP alone group and none in the GP plus toripalimab group. The 2-year EFS was 98.1% in the GP plus toripalimab group and 85.4% in the GP alone group (HR, 0.28; 95% CI, 0.08–0.97; p=0.024) (Figure 1). The 2-year OS, LRFS and DMFS rates for the GP plus toripalimab group and the GP alone group were 100.0% and 100.0% (p=1), 98.1% and 89.5% (p=0.086), and 100.0% and 95.9% (p=0.15), respectively (Figure 1).
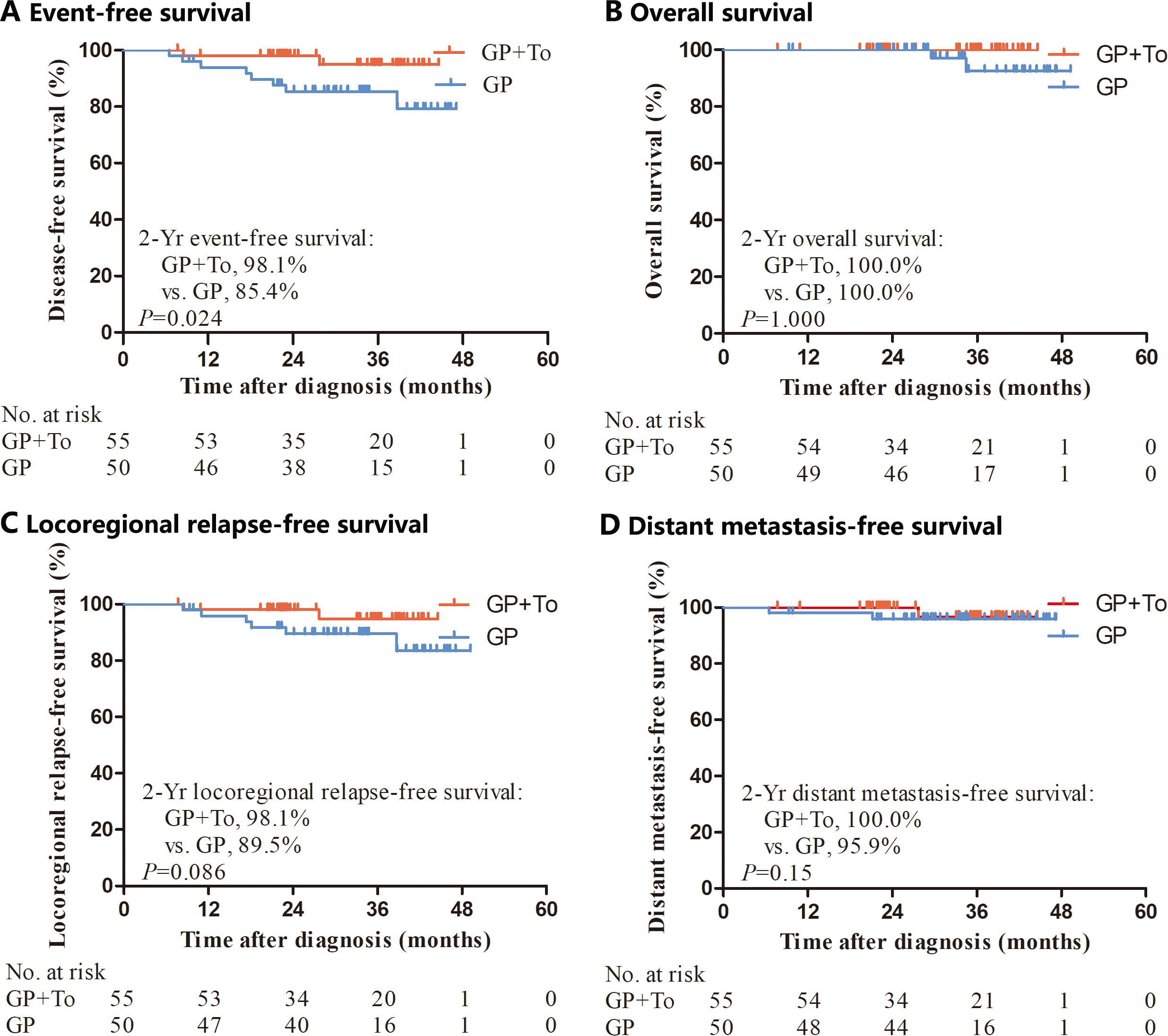
Figure 1. Kaplan–Meier analysis of event-free survival, overall survival, locoregional relapse-free survival and distant metastasis-free survival. GP, Gemcitabine and cisplatin; GP+To, Gemcitabine and cisplatin and toripalimab.
Adverse events
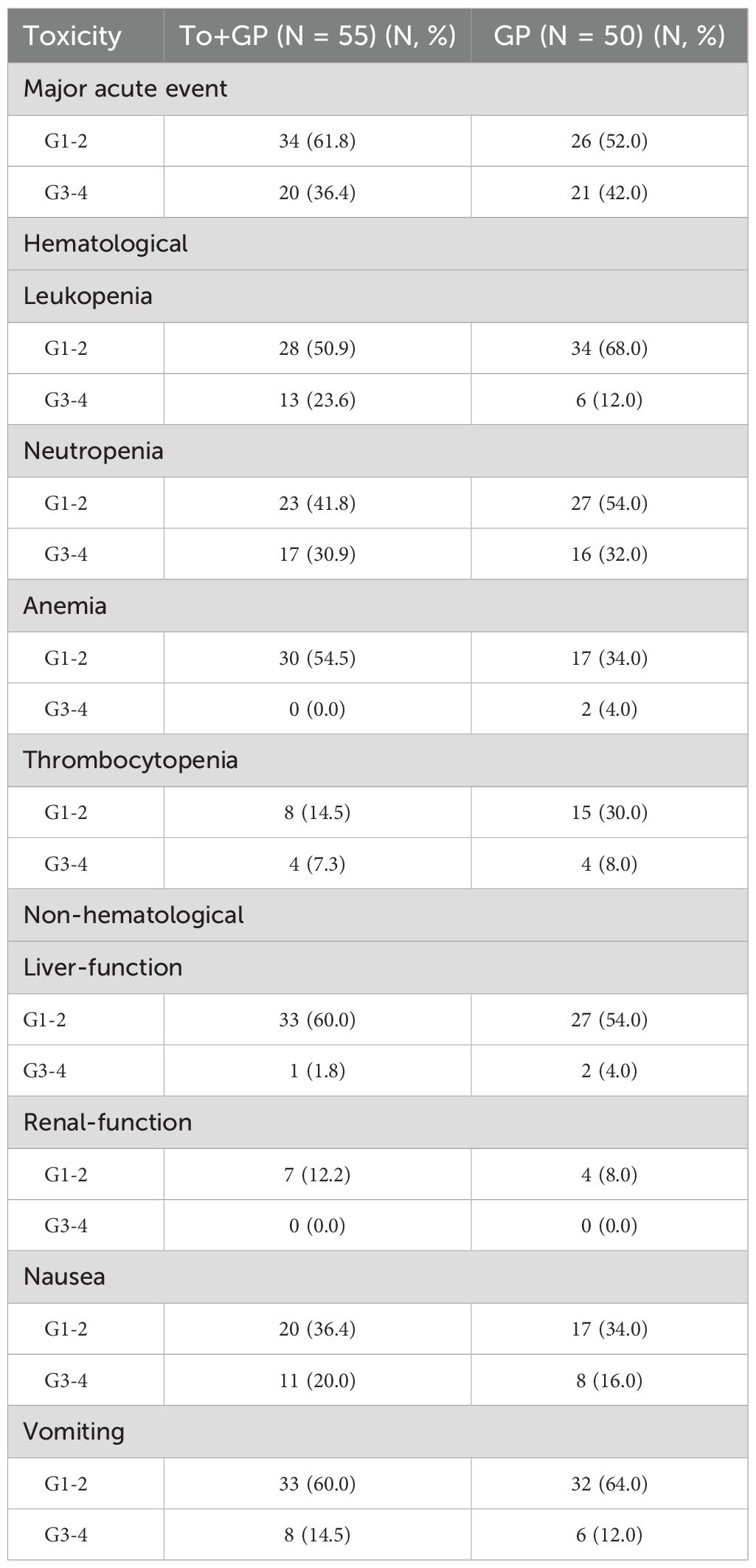
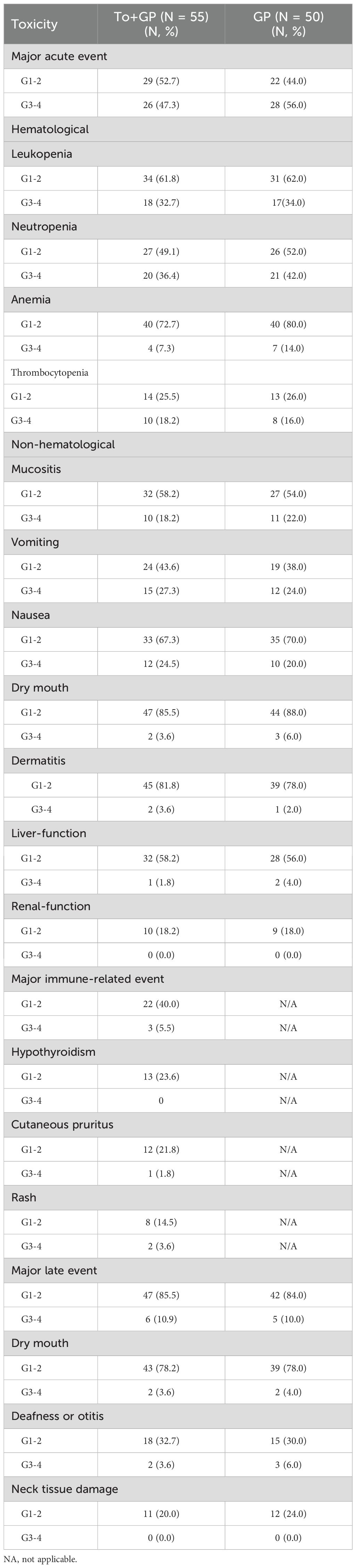
During induction therapy, acute Grade 3 or 4 adverse events occurred in 20 patients (36.4%) in the GP plus toripalimab group and 21 patients (42.0%) in the GP group (p=0.69). Neutropenia was the most common event (17 patients [30.9%] in the GP plus toripalimab group and 16 patients [32.0%] in the GP group), followed by leukopenia (13 [23.6%] vs. 6 [12.0%]) and nausea (11 [20.0%] vs. 8 [16.0%]) (Table 2). During the entire treatment period, 26 patients (47.3%) in the GP plus toripalimab group and 28 (56.0%) in the GP group experienced Grade 3 or 4 adverse events (Table 3). Neutropenia was still the most common grade 3 or 4 toxicity (20 patients [36.4%] in the GP plus toripalimab group and 21 [42.0%] in the GP group). Immune-related AEs of grade 3–4 occurred in 3 [5.5%] patients in the toripalimab plus GP arm. The most common immune-related AEs of grade 3–4 were hypothyroidism, cutaneous pruritus, and rash. There were no treatment-related deaths in the two groups.
Discussion
Nasopharyngeal carcinoma is an epithelial malignancy located in the nasopharynx and is closely related to Epstein–Barr virus (EBV) infection. Although NPC is sensitive to radiotherapy and although radiotherapy alone can cure approximately 90% of patients in the early stage, most patients have locoregionally advanced disease at diagnosis.
Radiotherapy combined with chemotherapy is the main treatment strategy for locally advanced nasopharyngeal carcinoma. Induction chemotherapy, which is used before radiotherapy, reduces the tumor burden and is well tolerated by patients. Interestingly, a multicenter, randomized phase III trial revealed that induction chemotherapy with gemcitabine and cisplatin before concurrent chemoradiotherapy significantly improved overall survival in patients with locally advanced nasopharyngeal carcinoma, and there was no increase in late toxicity (9).
Epstein–Barr virus (EBV) is strongly associated with NPC progression and is considered a risk factor for prognosis. EBV−infected epithelial cells often express EBV antigens, which promote the transformation of normal cells into NPC cells (17). Moreover, EBV antigens are the main targets of T cells (18, 19). In addition, tumor tissue is characterized by many immune infiltrates, such as T cells, B cells, dendritic cells (DCs), monocytes and eosinophils (20). In light of these factors, NPC is primarily suitable for immunotherapy.
In the last few years, immune checkpoint inhibitors, as part of immunotherapy, have developed rapidly in the clinical treatment of tumors. Immune checkpoint inhibitors suppress the binding of immunosuppressive signals to the corresponding ligands (e.g., PD−1/PD−L1), ultimately attenuating immunosuppressive regulation, reducing the T-cell suppression state and preventing immune escape. Toripalimab, an immune checkpoint inhibitor, has been shown to have promising efficacy in NPC treatment. The POLARIS−02 trial was a phase II study investigating the efficacy and safety of toripalimab in standard chemotherapy−refractory recurrence or metastasis (R/M) NPC. The results demonstrated that 20.5% of patients achieved an objective response, and the median DoR and OS were 12.8 months and 17.4 months, respectively. Twenty-seven patients (14.2%, 27/190) reported grade 3–5 adverse events (21). Researchers have also evaluated the role of the addition of toripalimab to gemcitabine plus cisplatin chemotherapy as a first-line treatment for RM-NPC. JUPITER−02, a multicenter randomized phase III study enrolling 289 patients with RM-NPC, explored the antitumor activity and toxicity of toripalimab or placebo plus GP as first−line care for patients. The data revealed that the PFS in the toripalimab group was markedly prolonged compared with that in the placebo group (11.7 versus 8.0 months, p=0.0003). Compared with the placebo group, the toripalimab group had a higher objective response rate (77.4% vs. 66.4%, P = 0.0335). There was no significant difference in adverse reactions ≥ grade 3 between the two groups (89.0% vs. 89.5%) (14). In accordance with the JUPITER−02 trial, toripalimab alone or in combination with chemotherapy was approved as the first−line treatment for patients with R/M NPC in China.
More and more studies reported that adding immune checkpoint inhibitors into the primary treatment for LANPC increased the progression-free survival, with manageable toxicity. However, the type, dosage, and timing of integration (induction phase, concurrent phase, and adjuvant phase) of immune checkpoint inhibitors into standard primary treatment of LANPC varies among these studies (22–24). Interestingly, a randomised, double-blind, phase 2 trial demonstrated that a so-called sandwich approach involving toripalimab (in the neoadjuvant and adjuvant phases) combined with concurrent chemoradiotherapy could be a highly promising therapy for the treatment of LANPC (25). During the induction period, chemotherapy was not administered and only immunotherapy was employed in the trial (25). In addition, a recent study has shown toripalimab combination therapy without concurrent cisplatin was a feasible therapy with high efficacy in failure-free survival and low toxicity in LANPC (26). Several studies have also shown induction immunochemotherapy combined with concurrent chemoradiotherapy has promising antitumor activity with a manageable safety profile in patients with LANPC (27–30). However, the role of gemcitabine and cisplatin induction chemotherapy plus toripalimab in LANPC was unclear.
In this retrospective study, we explored the efficacy and toxicity of the addition of toripalimab to GP induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma patients. The majority of patients with poor prognostic factors had N2 or N3 disease or T3 or T4 nasopharyngeal lesions. Our data revealed that the complete response rate after induction therapy was 30.9% (17/55) in the toripalimab plus GP group, which was obviously higher than that (12.0%, 6/50) in the GP group. The 2-year event-free survival rate was 98.1% in the toripalimab plus GP group and 85.4% in the GP group, which was significant (p=0.024). Grade 3–4 acute AEs during induction treatment occurred in 20 (36.4%) patients in the toripalimab plus GP group versus 21 (42.0%) in the GP group. The most common grade 3–4 acute AEs were neutropenia (17 [30.9%] vs. 16 [32.0%]) and leukopenia (13 [23.6%] vs. 6 [12.0%]) between the toripalimab group and the GP alone group. No patient died in either group 2 years after treatment. In addition, There were 3 [5.5%] patients having immune-related AEs of grade 3–4 in the toripalimab group. Liu X et al. reported that grade 3–4 immune-related AEs occurred in 10% (20/200) of patients after the addition of sintilimab (a PD-1 inhibitor) to standard therapy (GP induction chemotherapy followed by concurrent cisplatin radiotherapy) for 12 cycles (3 induction, 3 concurrent, and 6 adjuvant cycles) in high-risk LANPC patients (31). There may be two reasons for the difference in immune-related AEs between the two studies. First, the internal structures of toripalimab and sintilimab are different. Second, the total dose of toripalimab (240 mg once every 3 weeks for 2 or 3 cycles) used in the induction phase was less than that of sintilimab (200 mg once every 3 weeks for 12 cycles) used in the whole treatment phase. In our study, we excluded patients who received adjuvant immunotherapy because we wanted only to explore the role of induction immunotherapy in LANPC, and the number of patients receiving adjuvant immunotherapy was very small due to COVID-19.
However, this study had several limitations. First, this was a retrospective study, and the sample size was small, resulting in potential biases. Second, the follow-up duration may have been insufficient. Third, for a period of time, due to the EBV-DNA testing method, the positive rate of EBV-DNA was low. After the improvement of testing method, the positive rate became more accurate. The testing methods were inconsistent before and after, so this data is not included in this study.
In summary, the addition of toripalimab to GP induction chemotherapy significantly improves the EFS of LANPC patients in the era of IMRT, and toxicity is tolerable. Nevertheless, these findings need to be validated in prospective clinical trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Cancer Hospital&Shenzhen Hospital Chinese Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RL: Data curation, Methodology, Writing – original draft, Writing – review & editing. LL: Methodology, Writing – original draft, Writing – review & editing. XL: Methodology, Writing – original draft, Writing – review & editing. FL: Methodology, Writing – original draft, Writing – review & editing. FF: Methodology, Writing – original draft, Writing – review & editing. TZ: Methodology, Writing – original draft, Writing – review & editing. LM: Data curation, Writing – original draft, Writing – review & editing. PC: Data curation, Writing – original draft, Writing – review & editing. ZW: Investigation, Writing – original draft, Writing – review & editing. JJ: Supervision, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Shenzhen Science and Technology Program (grant no. KCXFZ20211020172542002); the Shenzhen Science and Technology Program (grant no. JCYJ20220530153801004); the Sanming Project of Medicine in Shenzhen (grant nos. SZXK013, SZSM201612063, SZSM202211030); the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen (grant no. E010322023); the Shenzhen High-level Hospital Construction Fund (No. grant number); National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen E010222004; National High Level Hospital Clinical Research Funding, Cooperation Fund of CHCAMS Beijing & Langfang & SZCH CFA202502020.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LANPC, locoregionally advanced nasopharyngeal carcinoma; GP, gemcitabine and cisplatin; CR, complete response; EFS, event-free survival; CI, confidence interval; AEs, adverse events; IMRT, intensity-modulated radiotherapy; IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy; PTV: planning target volume; GTV, gross tumor volume; OS, overall survival; DMFS, distant metastasis-free survival; LRFS, locoregional recurrence-free survival; ANOVA, One-way analysis of variance; HRs, hazard ratios; PR, partial response; SD, stable disease; PD, progressive disease; EBV, Epstein–Barr virus; DCs, dendritic cells; R/M, recurrence or metastasis.
References
1. Chen YP, Chan A, Le QT, Blanchard P, Sun Y, and Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
2. Lee AW, Ng WT, Chan LL, Hung WM, Chan CCC, Sze HCK, et al. Evolution of treatment for nasopharyngeal cancer–success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. (2014) 110:377–84. doi: 10.1016/j.radonc.2014.02.003
3. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
4. Blanchard P, Lee A, Marguet S, Leclercq JL, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. (2015) 16:645–55. doi: 10.1016/S1470-2045(15)70126-9
5. Chen L, Zhang Y, Lai SZ, Li WF, Hu WH, Sun R, et al. 10-year results of therapeutic ratio by intensity-modulated radiotherapy versus two-dimensional radiotherapy in patients with nasopharyngeal carcinoma. Oncologist. (2019) 24:e38–45. doi: 10.1634/theoncologist.2017-0577
6. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
7. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int J Cancer. (2019) 145:295–305. doi: 10.1002/ijc.32099
8. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Final overall survival analysis of gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma: A multicenter, randomized phase III trial. J Clin Oncol. (2022) 40:2420–5. doi: 10.1200/JCO.22.00327
9. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
10. Fang W, Yang Y, Ma Y, Hong SD, Lin LZ, He XH, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. (2018) 19:1338–50. doi: 10.1016/S1470-2045(18)30495-9
11. Ma Y, Yang Y, Ma Y, Yang YP, Hong SD, Zhao YY, et al. A phase I/II open-label study of nivolumab in previously treated advanced or recurrent nasopharyngeal carcinoma and other solid tumors. Oncologist. (2019) 24:891–e431. doi: 10.1634/theoncologist.2019-0284
12. Shen L, Guo J, Zhang Q, Pan HM, Yuan Y, Bai YX, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. (2020) 8:e000437. doi: 10.1136/jitc-2019-000437
13. Yang Y, Qu S, Li J, Hu CS, Xu MJ, Li WD, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2021) 22:1162–74. doi: 10.1016/S1470-2045(21)00302-8
14. Mai HQ, Chen QY, Chen D, Hu CS, Yang KY, Wen JY, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. (2021) 27:1536–43. doi: 10.1038/s41591-021-01444-0
15. Yang YP, Pan JJ, Wang H, Zhao YY, Qu SH, Chen NY, et al. Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: A multicenter phase 3 trial (RATIONALE-309). Cancer Cell. (2023) 41:1061–72. doi: 10.1016/j.ccell.2023.04.014
16. Xiang X, Chen P, Lan F, Ma L, Jin J, and Zhang Y. The short-term efficacy and safety of induction chemotherapy combined with PD-1 inhibitor or anti-EGFR in locoregionally advanced nasopharyngeal carcinoma. Front Oncol. (2023) 13:1110281. doi: 10.3389/fonc.2023.1110281
17. Wang L, Tian WD, Xu X, Nie B, Lu J, Liu X, et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer. (2014) 120:363–72. doi: 10.1002/cncr.28418
18. Fu T, Voo KS, and Wang RF. Critical role of EBNA1-specific CD4+ T cells in the control of mouse Burkitt lymphoma in vivo. J Clin Invest. (2004) 114:542–50. doi: 10.1172/JCI22053
19. Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF, et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. (2002) 62:6952–8.
20. Le QT, Colevas AD, O’Sullivan B, Lee AWM, Lee N, Ma B, et al. Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J Natl Cancer Inst. (2019) 111:655–63. doi: 10.1093/jnci/djz044
21. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XU, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: A phase II clinical trial (POLARIS-02). J Clin Oncol. (2021) 39:704–12. doi: 10.1200/JCO.20.02712
22. Cai MY, Wang YF, Ma HR, Yang L, and Xu ZY. Advances and challenges in immunotherapy for locally advanced nasopharyngeal carcinoma. Cancer Treat Rev. (2024) 131:102840. doi: 10.1016/j.ctrv.2024.102840
23. Jiang W, Lv JW, Tang LL, Sun Y, Chen YP, and Ma J. Enhancing efficacy and reducing toxicity: Therapeutic optimization in locoregionally advanced nasopharyngeal carcinoma. Cell Rep Med. (2024) 5:101594. doi: 10.1016/j.xcrm.2024.101594
24. Liang YL, Liu X, Shen LF, Hu GY, Zou GR, Zhang N, et al. Adjuvant PD-1 blockade with camrelizumab for nasopharyngeal carcinoma: the DIPPER randomized clinical trial. JAMA. (2025) 333:1589–98. doi: 10.1001/jama.2025.1132
25. Liu SL, Li XY, Yang JH, Wen DX, Guo SS, Liu LT, et al. Neoadjuvant and adjuvant toripalimab for locoregionally advanced nasopharyngeal carcinoma: a randomised, single-centre, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. (2024) 25:1563–75. doi: 10.1016/S1470-2045(24)00504-7
26. Xu C, Liang XY, Huang XQ, Jin F, Yang KY, Hu GY, et al. Toripalimab combination therapy without concurrent cisplatin for nasopharyngeal carcinoma: the DIAMOND randomized clinical trial. JAMA. (2025) 334:973–83. doi: 10.1001/jama.2025.13205
27. Wu KP, Luo XQ, Li QQ, Yang HC, Ji MC, Zhu X, et al. Efficacy and safety of induction immunotherapy plus chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a meta-analysis. Br J Cancer. (2025). doi: 10.1038/s41416-025-03169-0
28. He JQ, Luo GQ, Liu S, Chen LL, Chen ZH, Zhang B, et al. Tislelizumab plus neoadjuvant chemotherapy and concurrent chemoradiotherapy versus neoadjuvant chemotherapy and concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma: A retrospective study. Transl Oncol. (2024) 48:102058. doi: 10.1016/j.tranon.2024.102058
29. Lai MF, Li S, Jin ZW, Chang MZ, Li FM, Yang PX, et al. Tislelizumab and GP regimen neoadjuvant therapy followed by concurrent chemoradiotherapy with nimotuzumab in patients with stage IVA nasopharyngeal carcinoma: a retrospective study. Cancer Immunol Immunother. (2025) 74:241. doi: 10.1007/s00262-025-04100-5
30. Wang ZQ, Sun Y, Wang QX, Chai YL, Sun J, Zhang XM, et al. Induction chemotherapy plus camrelizumab combined with concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma in non-endemic areas: a phase 2 clinical trial in North China. BMC Med. (2025) 23:126. doi: 10.1186/s12916-025-03964-9
31. Liu X, Zhang Y, Yang KY, Zhang N, Jin F, Zou GR, et al. Induction-concurrent chemoradiotherapy with or without sintilimab in patients with locoregionally advanced nasopharyngeal carcinoma in China (CONTINUUM): a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. (2024) 403:2720–31. doi: 10.1016/S0140-6736(24)00594-4
Keywords: toripalimab, induction chemotherapy, nasopharyngeal carcinoma, immunotherapy, gemcitabine and cisplatin
Citation: Liang R, Lei L, Li X, Lan F, Fu F, Zou T, Ma L, Chen P, Wang Z, Jin J and Zhang J (2025) Toripalimab plus gemcitabine and cisplatin induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: a retrospective study. Front. Oncol. 15:1704442. doi: 10.3389/fonc.2025.1704442
Received: 13 September 2025; Accepted: 31 October 2025;
Published: 24 November 2025.
Edited by:
Francesco Sabbatino, University of Salerno, ItalyReviewed by:
Ana Varges Gomes, Centro Hospitalar Universitário do Algarve, PortugalHong Sun, Fudan University, China
Copyright © 2025 Liang, Lei, Li, Lan, Fu, Zou, Ma, Chen, Wang, Jin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Jin, amluamluZ0Bjc2NvLm9yZy5jbg==; Jianghu Zhang, emhhbmdqaWFuZ2h1Z2RAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Renba Liang
Renba Liang Ling Lei1†
Ling Lei1† Xinxiao Li
Xinxiao Li Jianghu Zhang
Jianghu Zhang