- 1Nursing School, Shandong Second Medical University, Weifang, Shandong, China
- 2Department of Oncology, Sunshine Union Hospital, Weifang, Shandong, China
- 3Department of Anesthesiology, Rocket Force Characteristic Medical Center, Beijing, China
- 4Department of Emergency Medicine, Rocket Force Characteristic Medical Center, Beijing, China
- 5Nursing Department, Sunshine Union Hospital, Weifang, Shandong, China
- 6Department of Respiratory Medicine, Rocket Force Characteristic Medical Center, Beijing, China
Background: Steroid-refractory acute graft-versus-host disease (aGVHD) remains a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT), with limited effective treatment options. Mesenchymal stem cells (MSCs) have emerged as a promising therapeutic approach due to their immunomodulatory and tissue-repair properties. However, inconsistent results existed.
Methods: A systematic literature search was conducted in PubMed, Embase, and the Cochrane Central Register of Controlled Trials up to May 2025. Randomized controlled trials (RCTs) evaluating MSCs plus second-line therapy versus second-line therapy alone in patients with steroid-refractory aGVHD were included. Meta-analysis was performed using random-effects models to pool risk ratios (RR) or hazard ratios (HR) with 95% confidence intervals (CIs).
Results: Four RCTs comprising 650 patients were included. MSC administration significantly improved overall response (RR: 1.13, 95% CI: 1.03-1.23, P = 0.007) and complete response rates (RR: 1.43, 95% CI: 1.19-1.70, P < 0.001). Subgroup analysis showed consistent benefits in patients with skin or gut involvement, multiorgan disease, and adults. MSC treatment also reduced the incidence of chronic GVHD (HR: 0.60, 95% CI: 0.42-0.86, P = 0.005) and improved failure-free survival (HR: 0.72, 95% CI: 0.54-0.95, P = 0.022), although no significant overall survival benefit was observed. The safety profile was comparable with controls.
Conclusions: The addition of MSCs to second-line therapy significantly improves treatment response, reduces chronic GVHD incidence, and prolongs failure-free survival in patients with steroid-refractory aGVHD, with a favorable safety profile. These findings support MSC-based therapy as a promising strategy for this high-risk population.
1 Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment for most hematological malignancies (1). However, 40% to 60% of recipients develop moderate to severe acute graft-versus-host disease (aGVHD) (2, 3), which remains one of the most common complications and a leading cause of mortality following allo-HSCT (4).
Corticosteroids represent the standard first-line therapy for aGVHD (5, 6), yet complete response is achieved in only approximately 50% of patients (7, 8). Response rates are particularly poor among those with visceral and/or multiorgan involvement (9). To date, no optimal second-line treatment has been established for steroid-refractory aGVHD, and the prognosis for these patients remains dismal (4). Hence, there is an urgent need for more effective therapeutic strategies.
Mesenchymal stem cells (MSCs) exhibit broad immunomodulatory capacity through their ability to regulate key immune populations—including T and B lymphocytes, natural killer cells, and dendritic cells—by influencing their proliferation, activation, and maturation (10–13). Furthermore, MSCs promote the expansion of regulatory T cells and help rebalance the Th1/Th2 ratio (14). Coupled with their multipotent differentiation potential toward mesodermal lineages, these immunoregulatory and regenerative properties provide a strong mechanistic rationale for exploring MSC-based therapies in aGVHD.
Several clinical studies have explored MSCs specifically for steroid-refractory aGVHD (15–18). However, reported outcomes regarding their efficacy remain inconsistent, and their definitive role in managing this condition continues to be debated. Given that individual trials are often limited by small sample sizes and inadequate statistical power to definitively establish clinical benefits, we performed a meta-analysis of randomized controlled trials (RCTs) to comprehensively evaluate the efficacy and safety of MSCs combined with second-line therapy in patients with steroid-refractory aGVHD after allo-HSCT.
2 Materials and methods
2.1 Search strategy
A comprehensive systematic search was performed in PubMed, Embase (Excerpta Medica Database), and the Cochrane Central Register of Controlled Trials (CENTRAL) using keywords and Medical Subject Headings (MeSH) terms related to “mesenchymal stem cells,” “mesenchymal stromal cells,” “MSC,” “graft versus host disease,” “GVHD,” and “graft vs. host disease”. The search aimed to identify RCTs evaluating the efficacy and safety of MSCs in patients with steroid-refractory acute GVHD following allo-HSCT for hematological diseases.
The search included all publications available up to May 2025 without language restrictions. Detailed search strategies for each database are provided in Supplementary Tables 1-3. Additionally, the reference lists of all included studies were manually screened to identify further relevant publications. This meta-analysis was designed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (19).
2.2 Selection criteria
Two investigators independently assessed all potentially eligible studies. RCTs evaluating the efficacy and safety of MSCs in patients with steroid-refractory aGVHD following allo-HSCT for hematological diseases were included. Eligibility was independent of patient demographics, donor-recipient human leukocyte antigen (HLA) matching, graft source, conditioning regimens, GVHD prophylaxis, and MSC origin, dosage, or timing of administration.
Steroid-refractory aGVHD was defined as progression within 3 days, no improvement after 7 days, failure to achieve complete response by day 14 of steroid therapy, or recurrence during steroid taper.
The primary endpoint was the overall response (OR) rate at day 28, comprising complete response (CR) or partial response (PR). CR was defined as the complete resolution of manifestations in all affected organs. PR referred to improvement of at least one stage in every initially involved organ, without progression in any other organ. Secondary efficacy endpoints included cumulative incidence of chronic GVHD, failure-free survival (FFS), and overall survival (OS). FFS was defined as the time from randomization to the earliest event among relapse or progression of the underlying disease, non-relapse mortality, or initiation of new systemic aGVHD therapy. OS referred to the time from randomization to death from any cause. Safety endpoints encompassed adverse events (AEs) and severe AEs graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), as well as rates of disease relapse and infections.
Any discrepancies between the two investigators were resolved through discussion or by consulting a third specialist.
2.3 Data extraction
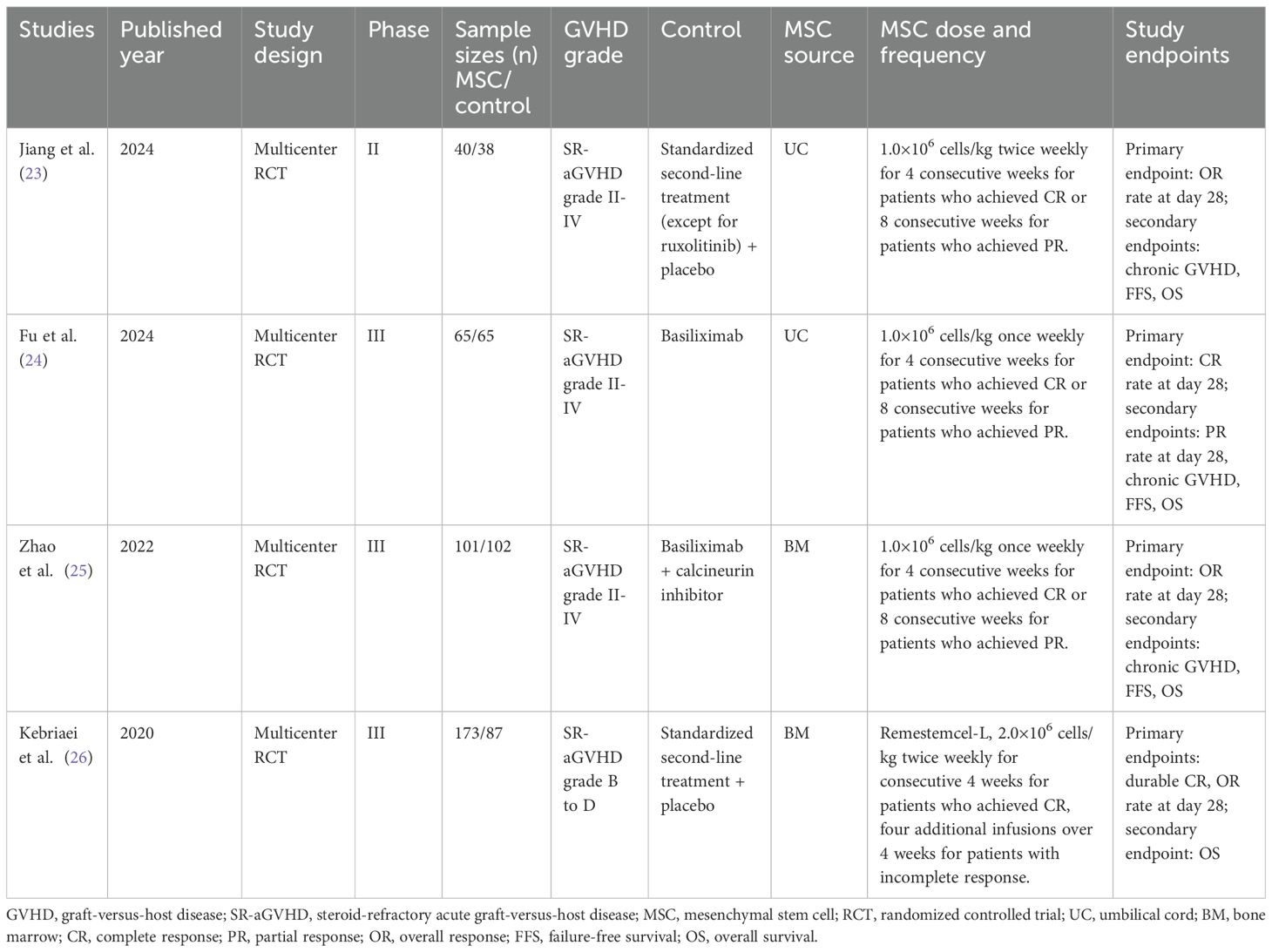
Two investigators independently extracted data from the included studies. The following information was collected: first author, year of publication, patient characteristics (underlying diagnosis and sample size), donor type (related or unrelated), HLA compatibility (matched, mismatched, or haploidentical), source of hematopoietic stem cells (bone marrow, peripheral blood, or umbilical cord blood), conditioning regimen (busulfan-based or total body irradiation-based), GVHD prophylaxis, MSC origin (umbilical cord [UC], bone marrow [BM], or adipose tissue), and MSC dosage, frequency of administration, and all prespecified outcomes.
Any discrepancies between the two investigators were resolved through consensus or by consulting a third expert. When necessary, the corresponding authors of the original studies were contacted to obtain missing or incomplete data.
2.4 Methodological quality evaluation
The methodological quality of the included studies was evaluated using the Cochrane Risk of Bias Tool, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) (20). Two investigators independently assessed each study and assigned ratings of “high,” “low,” or “unclear” risk of bias across the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. Any discrepancies between the investigators were resolved through discussion.
2.5 Statistical analyses
Risk ratios (RR) with 95% confidence intervals (CI) were calculated for dichotomous outcomes. For time-to-event data, hazard ratios (HR) and corresponding 95% CIs were pooled. Heterogeneity among studies was evaluated using the I² statistic, with I² > 50% and a P-value < 0.10 indicating substantial heterogeneity (21). A random-effects model was applied for all meta-analyses to yield more conservative estimates (22), regardless of heterogeneity levels. Predefined subgroup analyses were performed based on the following variables: aGVHD severity (grades II-IV vs. III-IV), organ involvement (e.g., liver, skin, gut), number of organs affected (single vs. multiple), patient age (children vs. adults), and MSC source (UC vs. BM). A P-value < 0.05 was considered statistically significant. All analyses were conducted using Stata version 12.0 (Stata Corporation, College Station, TX, USA).
3 Results
3.1 Study selection and characteristics
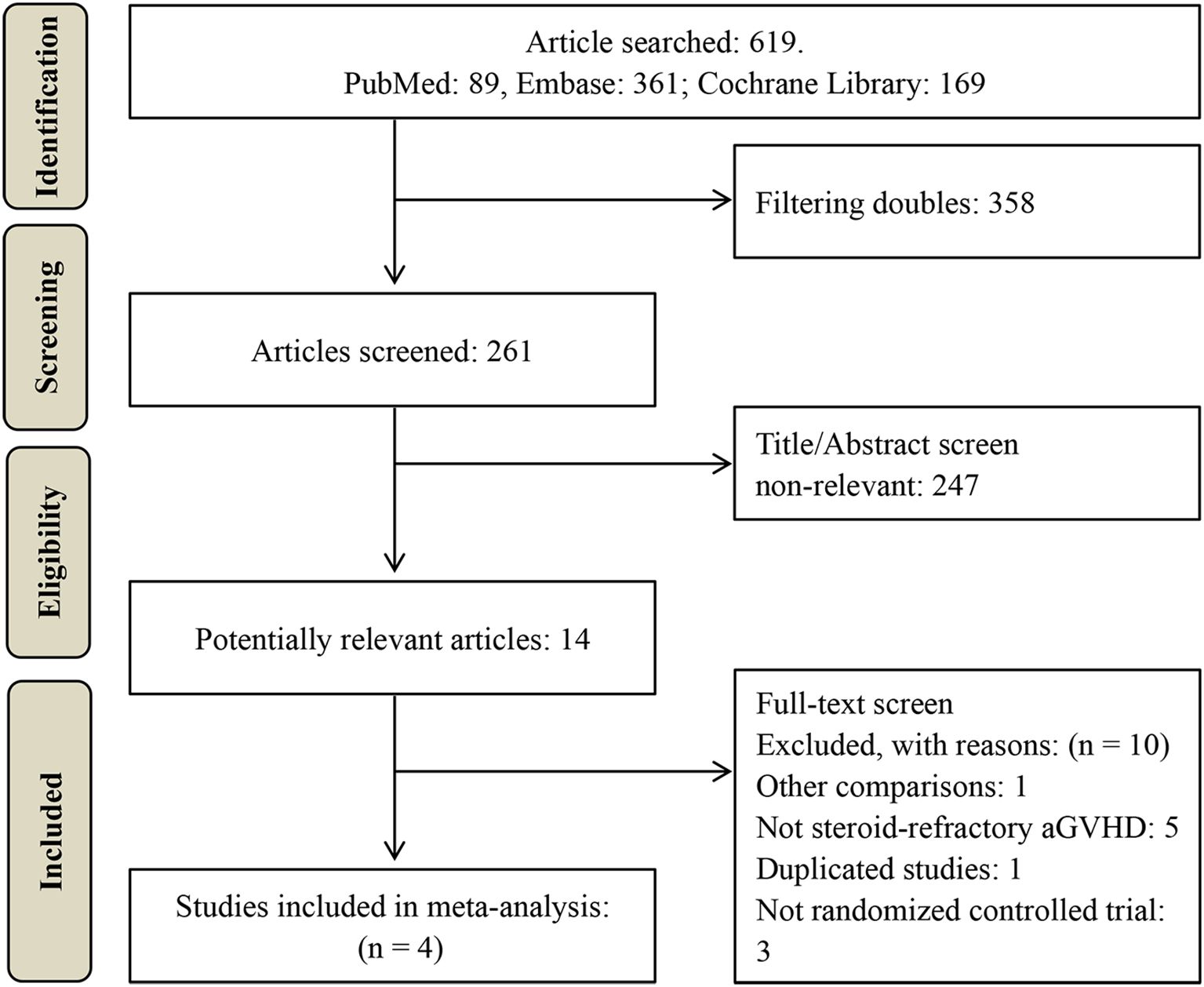
A total of 619 potentially relevant studies were initially identified through the literature search. After removing duplicates, 261 records underwent title and abstract screening, resulting in the exclusion of 247 articles. The remaining 14 studies were subjected to full-text assessment, of which 10 were excluded. Ultimately, four RCTs comprising 650 patients were included in the meta-analysis (23–26), as illustrated in Figure 1. The characteristics of these studies are summarized in Tables 1, 2.
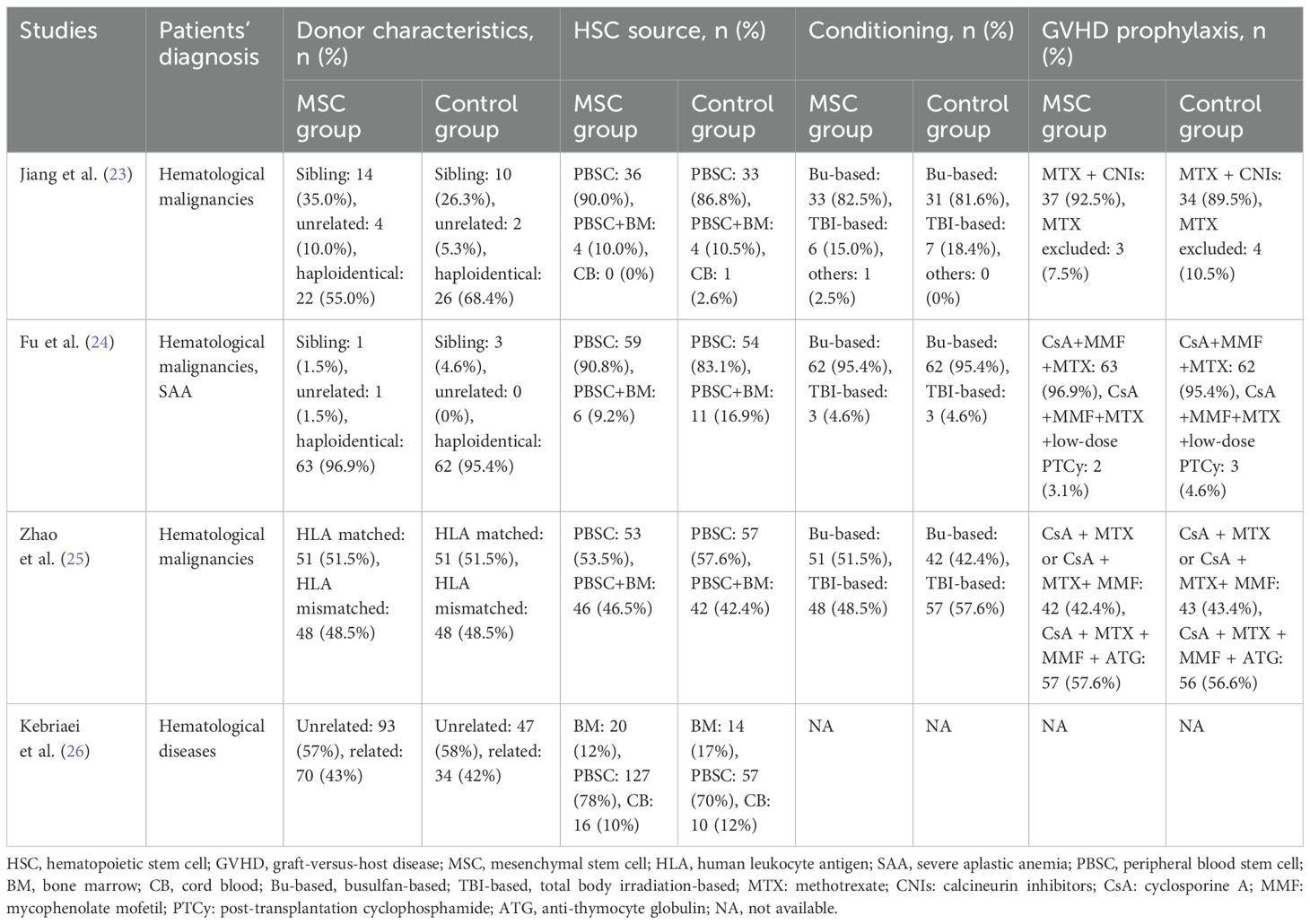
Table 2. Summary of the transplantation-related characteristics of the included randomized controlled trials.
All included studies were multicenter RCTs, including one phase II trial (23) and three phase III trials (24–26). Sample sizes ranged from 78 to 260 participants. All patients exhibited steroid-refractory aGVHD of grade II or above. Control groups received institutional standard second-line therapy for steroid-refractory aGVHD, whereas the intervention groups received additional MSC treatment. The source of MSCs was BM in two studies (25, 26) and UC in the other two (23, 24). Dosing regimens varied: two studies administered MSCs at 1×106 cells/kg once weekly for 4 weeks in patients achieving CR or 8 weeks in those with PR (24, 25); one study used a similar dose but administered twice weekly (23); and the remaining study administered 2×106 cells/kg twice weekly (26). One study enrolled exclusively adult patients (24), whereas the other three included both pediatric and adult populations (23, 25, 26).
3.2 Methodological quality evaluation
All four included RCTs described the process of randomization and provided detailed methods for random sequence generation (23–26). Two of the trials were conducted in an open-label manner (24, 25), whereas the other two were double-blinded (23, 26). Blinding of outcome assessors was implemented in all studies (23–26). With regard to incomplete outcome data, selective reporting, and other potential sources of bias, all trials were judged to be at low risk. A summary of the methodological quality assessment is presented in Supplementary Figure 1.
3.3 Overall response, complete response, and subgroup analysis
All four studies reported OR rates. Pooled analysis demonstrated a significantly higher OR rate in the MSC group compared with the control group (RR: 1.13, 95% CI: 1.03-1.23, P = 0.007) (Figure 2A). CR rates were reported in three studies, with pooled results also showing a significant improvement in the MSC group (RR: 1.43, 95% CI: 1.19-1.70, P < 0.001) (Figure 2B).
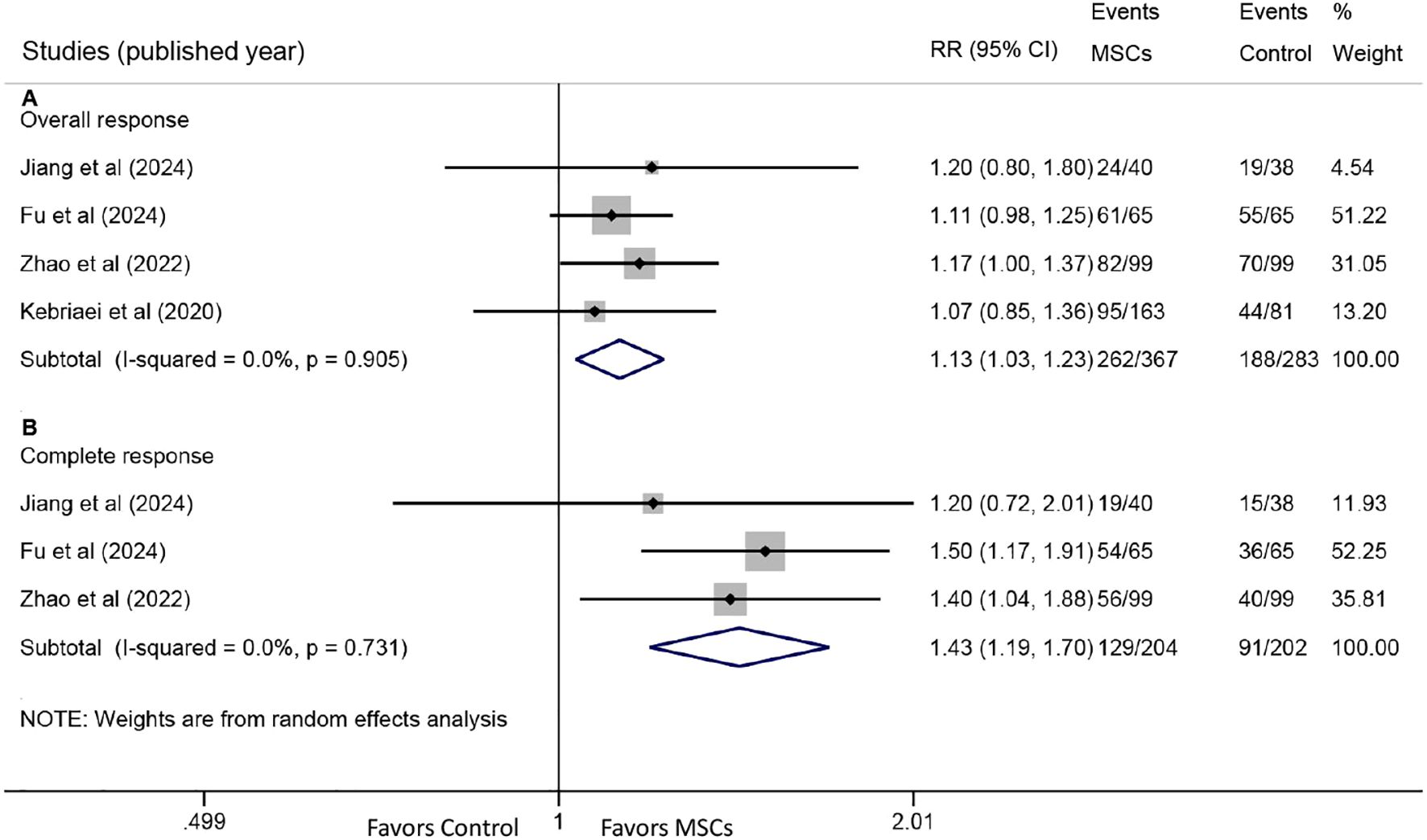
Figure 2. Forest plots for overall response (OR) and complete response (CR). (A) Forest plot comparing the OR rate at day 28 between the MSC group and the control group. (B) Forest plot comparing the CR rate at day 28 between the MSC group and the control group.
Prespecified subgroup analyses based on OR data revealed consistent benefits across several subgroups. Improved OR was observed in both grade II-IV (RR: 1.13, 95% CI: 1.03-1.23, P = 0.007) and grade III-IV aGVHD (RR: 1.22, 95% CI: 1.05-1.41, P = 0.008) (Figure 3A). Significant OR enhancement was identified in patients with skin involvement (RR: 1.10, 95% CI: 1.00-1.21, P = 0.044) and gut involvement (RR: 1.18, 95% CI: 1.05-1.33, P = 0.006), but not in those with liver involvement (RR: 1.16, 95% CI: 0.94-1.44, P = 0.167) (Figure 3B).
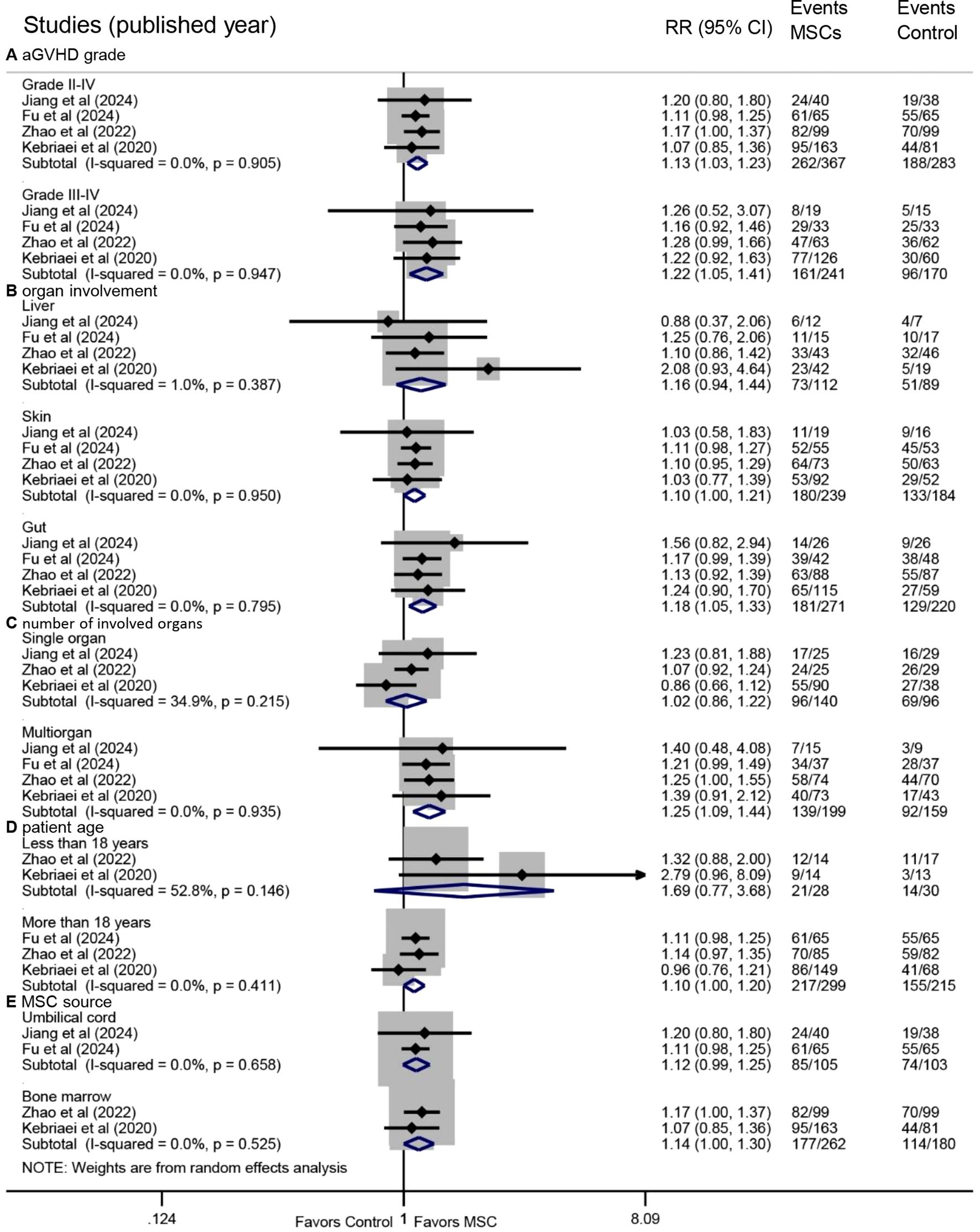
Figure 3. Forest plots of subgroup analyses for overall response. Subgroup analyses were performed based on (A) aGVHD grade, (B) organ involvement, (C) number of involved organs, (D) patient age, and (E) MSC source. aGVHD, acute graft-versus-host disease.
Multiorgan involvement was associated with a significantly higher OR following MSC treatment (RR: 1.25, 95% CI: 1.09-1.44, P = 0.002), whereas no significant benefit was observed in single-organ involvement (RR: 1.02, 95% CI: 0.86-1.22, P = 0.803) (Figure 3C). A statistically significant improvement was seen in adult patients (RR: 1.10, 95% CI: 1.00-1.20, P = 0.048), but not in pediatric patients (RR: 1.69, 95% CI: 0.77-3.68, P = 0.188) (Figure 3D). Furthermore, MSC administration derived from BM was associated with a significant improvement in OR (RR: 1.14, 95% CI: 1.00-1.30, P = 0.047), whereas those from UC showed a trend without reaching statistical significance (RR: 1.12, 95% CI: 0.99-1.25, P = 0.063) (Figure 3E).
3.4 Chronic GVHD, failure-free survival, and overall survival
Two studies provided HR data for chronic GVHD. Pooled analysis demonstrated a significantly lower cumulative incidence of chronic GVHD in the MSC group compared with the control group (HR: 0.60, 95% CI: 0.42-0.86, P = 0.005) (Figure 4A). Additionally, the incidence of moderate/severe chronic GVHD was also significantly reduced in the MSC group (HR: 0.40, 95% CI: 0.22-0.76, P = 0.005) (Figure 4B).
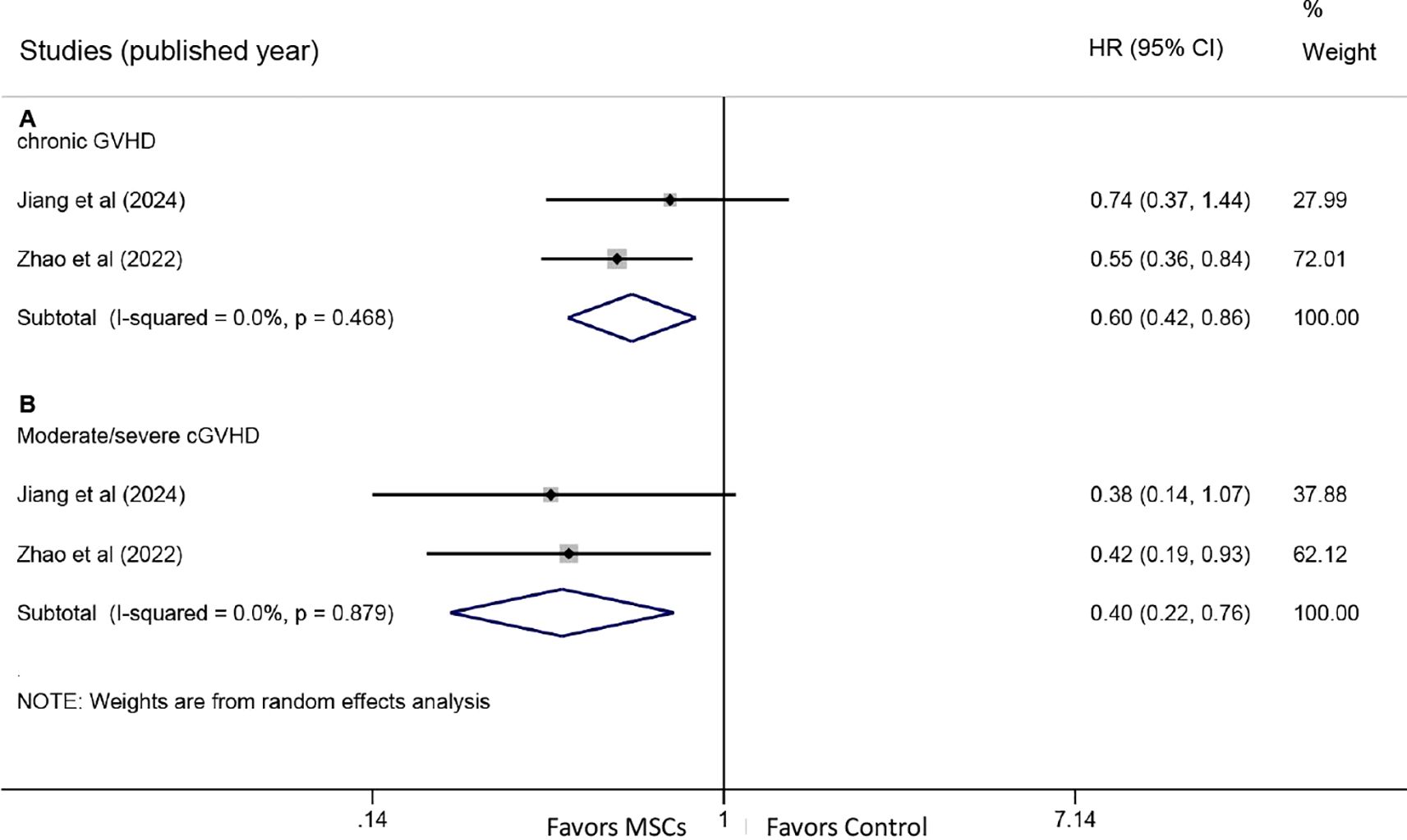
Figure 4. Forest plots for chronic graft-versus-host disease. (A) Forest plot comparing the cumulative incidence of all-grade chronic GVHD between the MSC group and the control group. (B) Forest plot comparing the incidence of moderate/severe chronic GVHD between the MSC group and the control group.
Two studies reported HRs for FFS. The meta-analysis revealed a significant improvement in FFS with MSC treatment (HR: 0.72, 95% CI: 0.54-0.95, P = 0.022) (Figure 5A). Similarly, two studies provided HR data for OS. Pooled results indicated no statistically significant difference in OS between the MSC and control groups (HR: 0.84, 95% CI: 0.48-1.47, P = 0.542) (Figure 5B).
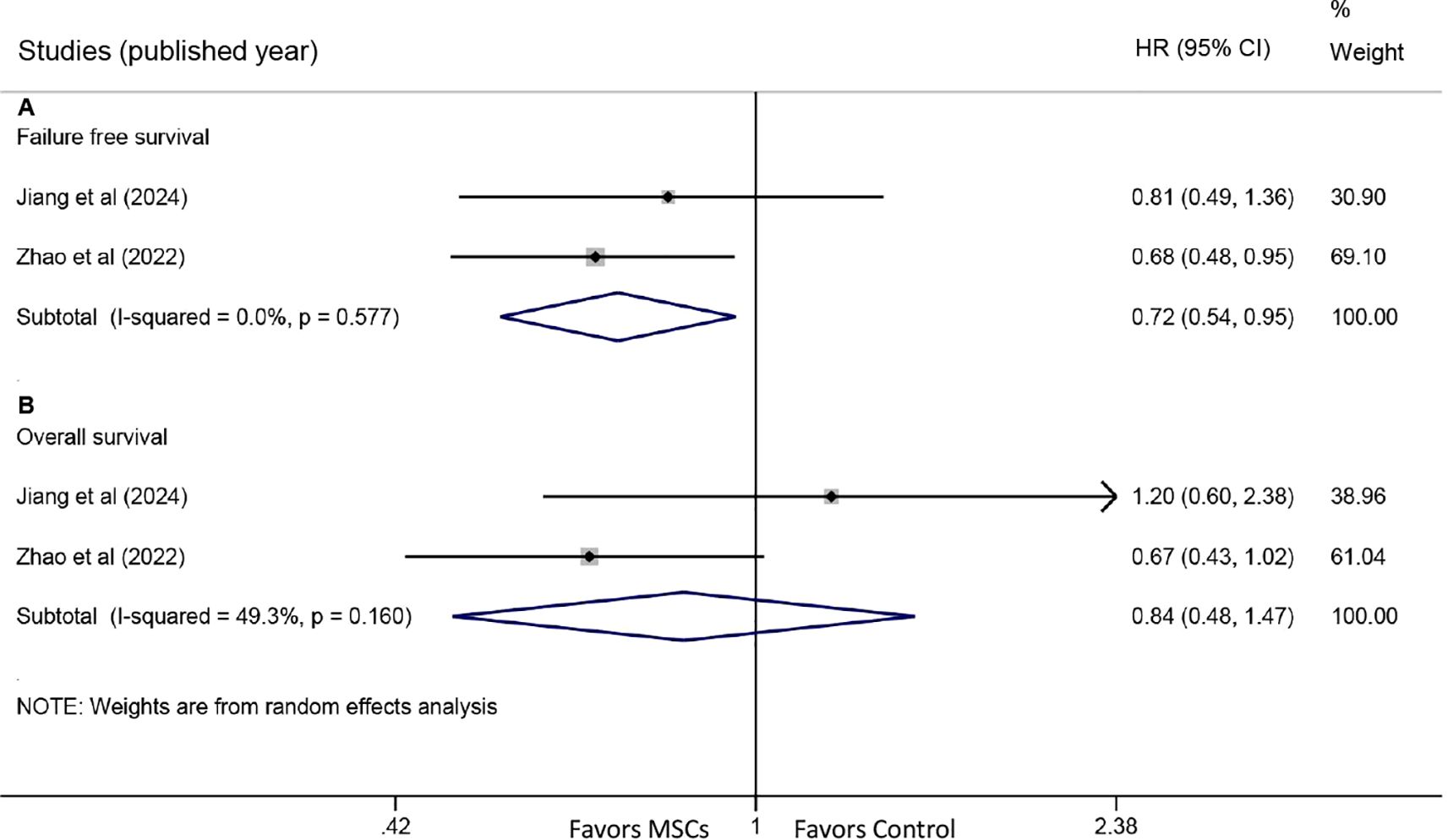
Figure 5. Forest plots for failure-free survival (FFS) and overall survival (OS). (A) Forest plot comparing FFS between the MSC group and the control group. (B) Forest plot comparing OS between the MSC group and the control group.
3.5 Safety profile
Three studies reported data on any AEs. The pooled analysis showed that the incidence of any AEs was comparable between the MSC group and the control group (RR: 1.00, 95% CI: 0.95-1.05, P = 0.961) (Figure 6A). Similarly, three studies provided data on severe AEs, with meta-analysis indicating no significant difference between the groups (RR: 1.01, 95% CI: 0.92-1.11, P = 0.795) (Figure 6B).
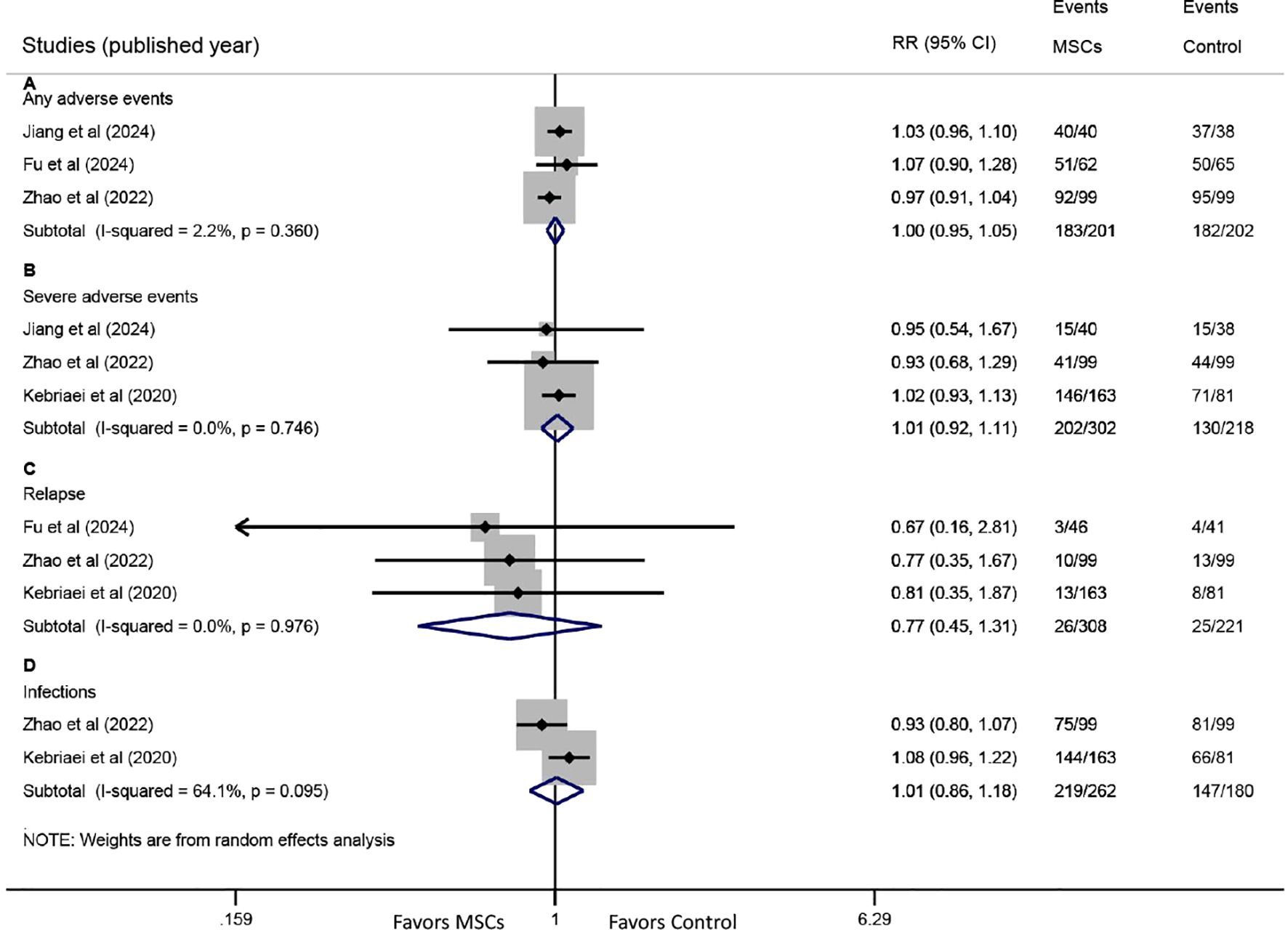
Figure 6. Forest plots for safety endpoints. Forest plots comparing the incidence of (A) any adverse events (AEs), (B) severe AEs, (C) primary disease relapse, and (D) infections between the MSC group and the control group.
Three studies reported relapse rates of the primary disease. Pooled results revealed no significant difference between the MSC and control groups (RR: 0.77, 95% CI: 0.45-1.31, P = 0.332) (Figure 6C). Data on infections were available from two studies, and no significant difference was observed between the two groups (RR: 1.01, 95% CI: 0.86-1.18, P = 0.922) (Figure 6D).
4 Discussion
This meta-analysis, based on data from four RCTs, demonstrates that the addition of MSCs to second-line therapy significantly improves both OR and CR rates in patients with steroid-refractory aGVHD following allo-HSCT. Furthermore, MSC treatment was associated with a significantly reduced incidence of chronic GVHD and prolonged failure-free survival, while maintaining a safety profile comparable with that of standard second-line therapy alone.
Unlike some rapid-acting second-line agents, MSCs exhibit a more gradual onset of action (23). This supports the rationale for combining MSCs with other second-line treatments—such as basiliximab or calcineurin inhibitors—to harness both the early benefits of conventional therapies and the sustained immunomodulatory effects of MSCs through complementary mechanisms. In our analysis, the combination of MSCs and second-line therapy achieved an OR rate of 71.4% (262/367) in steroid-refractory aGVHD, a finding consistent with a previous meta-analysis of non-randomized studies that reported an OR rate of 72% in comparable patient populations (27).
Our meta-analysis revealed significantly higher OR rates in patients with skin or gut involvement, as well as in those with multiorgan involvement, but not in patients with liver involvement or single-organ disease. One possible explanation is that multiorgan involvement reflects a more severe inflammatory state, which may respond more favorably to MSC therapy due to its dual mechanisms of immunomodulation and tissue repair (28, 29). It should also be noted that the number of patients with liver involvement included in this analysis was relatively small; therefore, these results should be interpreted with caution.
In contrast to previous studies suggesting that pediatric patients with steroid-refractory aGVHD may respond better to MSCs than adults (16, 30, 31), our meta-analysis showed a significant improvement in OR among adult patients, but not in pediatric subgroups. This discrepancy may be attributable to the limited number of pediatric patients included (49 in the MSC group and 44 in the control group), which likely underpowered the subgroup analysis to detect a significant treatment effect. Thus, the efficacy of MSCs in children warrants further investigation in larger, prospective studies. It is noteworthy that the study by Kurtzberg et al. (32), which supported the US FDA approval of MSC therapy for pediatric steroid-refractory aGVHD, reported an OR rate of 70.4% with Remestemcel-L treatment in children. This finding is consistent with the pooled OR rate of 71.4% observed in our meta-analysis.
MSCs derived from different sources exhibit distinct immunomodulatory properties and clinical benefits in GVHD (33, 34). This meta-analysis found that BM-MSCs were associated with a significantly higher OR, whereas UC-MSCs did not show a statistically significant effect. Two observations warrant attention: First, there was a trend toward improved OR in patients receiving UC-MSCs (P = 0.063); second, the two RCTs utilizing UC-MSCs had relatively small sample sizes compared with those using BM-MSCs (ranging from 78 to 260 patients across studies). This variability is further evidenced by results from pivotal clinical trials of commercially approved MSC products: Temcell (Japan-approved BM-MSCs) reported an OR of 61% (35), Remestemcel-L (US-approved BM-MSCs) achieved 70.4% in its pivotal trial (32), while Amimatoside Injection (China-approved UC-MSCs) demonstrated 71.9% in a phase II RCT (23). Given the heterogeneous outcomes across both investigational and approved products, the optimal MSC source warrants further investigation through well-designed randomized comparative trials.
Steroid-refractory aGVHD is a known risk factor for subsequent chronic GVHD (36). Our analysis suggests that the beneficial effects of MSCs in aGVHD extend to a reduced incidence of chronic GVHD and improved FFS. However, no significant improvement in OS was observed. This lack of OS benefit should be interpreted with caution due to the limited sample size and relatively short follow-up periods in the included studies.
This meta-analysis has several limitations that should be considered when interpreting the findings. First, the inclusion of only four RCTs with relatively small total sample sizes substantially limits the statistical power of our analysis, particularly in subgroup assessments. This constraint necessitates that conclusions regarding specific subpopulations—such as pediatric patients or those with liver involvement—be viewed as preliminary rather than definitive. Second, the clinical heterogeneity across the included studies must be acknowledged. Variations in MSC sources (UC versus BM), dosing regimens, and background second-line therapies likely influenced the outcomes and contributed to the observed inconsistencies, potentially affecting the generalizability of the pooled results. Finally, the efficacy of combining MSCs with newer agents like ruxolitinib (37, 38), which is now a standard therapy for steroid-refractory aGVHD, remains an important unanswered question and warrants investigation in future trials.
In summary, this meta-analysis indicates that the addition of MSCs to second-line therapy significantly improves overall and complete response rates in patients with steroid-refractory aGVHD and is associated with prolonged FFS and a comparable safety profile. These findings support the use of MSCs as an effective and safe therapeutic option for steroid-refractory aGVHD in patients undergoing allo-HSCT for hematological diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shandong Second Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MX: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing, Validation. YY: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. RZ: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. DW: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. LW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Scientific Research Project of Weifang Municipal Health Commission under Grant number 2023X132984, and Weifang Municipal Science & Technology Development Project under Grant number 2024YX103. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1704963/full#supplementary-material
References
1. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. (2006) 354:1813–26. doi: 10.1056/NEJMra052638
2. Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. (2012) 119:296–307. doi: 10.1182/blood-2011-06-364265
3. Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. (2010) 363:2091–101. doi: 10.1056/NEJMoa1004383
4. Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. (2005) 36:757–69. doi: 10.1038/sj.bmt.1705140
5. Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological Malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. (2024) 11:e147–e59. doi: 10.1016/s2352-3026(23)00342-3
6. Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. (2014) 49:168–73. doi: 10.1038/bmt.2013.107
7. Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, et al. Steroid-refractory acute GVHD: predictors and outcomes. Adv Hematol. (2011) 2011:601953. doi: 10.1155/2011/601953
8. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological Malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. (2020) 7:e157–e67. doi: 10.1016/s2352-3026(19)30256-x
9. MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. (2015) 21:761–7. doi: 10.1016/j.bbmt.2015.01.001
10. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. (2003) 101:3722–9. doi: 10.1182/blood-2002-07-2104
11. Pradier A, Passweg J, Villard J, and Kindler V. Human bone marrow stromal cells and skin fibroblasts inhibit natural killer cell proliferation and cytotoxic activity. Cell Transplant. (2011) 20:681–91. doi: 10.3727/096368910x536545
12. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. (2006) 107:367–72. doi: 10.1182/blood-2005-07-2657
13. Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. (2004) 13:263–71. doi: 10.1089/154732804323099190
14. Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol. (2016) 34:2843–50. doi: 10.1200/jco.2015.65.3642
15. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. (2004) 363:1439–41. doi: 10.1016/s0140-6736(04)16104-7
16. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. (2008) 371:1579–86. doi: 10.1016/s0140-6736(08)60690-x
17. Soder RP, Dawn B, Weiss ML, Dunavin N, Weir S, Mitchell J, et al. A phase I study to evaluate two doses of wharton’s jelly-derived mesenchymal stromal cells for the treatment of de novo high-risk or steroid-refractory acute graft versus host disease. Stem Cell Rev Rep. (2020) 16:979–91. doi: 10.1007/s12015-020-10015-8
18. Muroi K, Miyamura K, Okada M, Yamashita T, Murata M, Ishikawa T, et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol. (2016) 103:243–50. doi: 10.1007/s12185-015-1915-9
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) d598:343. doi: 10.1136/bmj.d5928
21. Higgins JP and Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
22. DerSimonian R and Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
23. Jiang E, Qian K, Wang L, Yang D, Shao Y, Hu L, et al. Efficacy and safety of human umbilical cord-derived mesenchymal stem cells versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease: a multicentre, randomized, double-blind, phase 2 trial. BMC Med. (2024) 22:555. doi: 10.1186/s12916-024-03782-5
24. Fu H, Sun X, Lin R, Wang Y, Xuan L, Yao H, et al. Mesenchymal stromal cells plus basiliximab improve the response of steroid-refractory acute graft-versus-host disease as a second-line therapy: a multicentre, randomized, controlled trial. BMC Med. (2024) 22:85. doi: 10.1186/s12916-024-03275-5
25. Zhao K, Lin R, Fan Z, Chen X, Wang Y, Huang F, et al. Mesenchymal stromal cells plus basiliximab, calcineurin inhibitor as treatment of steroid-resistant acute graft-versus-host disease: a multicenter, randomized, phase 3, open-label trial. J Hematol Oncol. (2022) 15:22. doi: 10.1186/s13045-022-01240-4
26. Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, et al. A phase 3 randomized study of remestemcel-L versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. (2020) 26:835–44. doi: 10.1016/j.bbmt.2019.08.029
27. Hashmi S, Ahmed M, Murad MH, Litzow MR, Adams RH, Ball LM, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol. (2016) 3:e45–52. doi: 10.1016/s2352-3026(15)00224-0
28. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
29. Spees JL, Lee RH, and Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. (2016) 7:125. doi: 10.1186/s13287-016-0363-7
30. Chen X, Wang C, Yin J, Xu J, Wei J, and Zhang Y. Efficacy of mesenchymal stem cell therapy for steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: A systematic review and meta-analysis. PloS One. (2015) 10:e0136991. doi: 10.1371/journal.pone.0136991
31. Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, et al. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. (2014) 20:375–81. doi: 10.1016/j.bbmt.2013.11.033
32. Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, et al. A phase 3, single-arm, prospective study of remestemcel-L, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. (2020) 26:845–54. doi: 10.1016/j.bbmt.2020.01.018
33. Wang L, Zhu CY, Ma DX, Gu ZY, Xu CC, Wang FY, et al. Efficacy and safety of mesenchymal stromal cells for the prophylaxis of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. Ann Hematol. (2018) 97:1941–50. doi: 10.1007/s00277-018-3384-8
34. Grégoire C, Ritacco C, Hannon M, Seidel L, Delens L, Belle L, et al. Comparison of mesenchymal stromal cells from different origins for the treatment of graft-vs. -Host-Dis Human Mouse Model Front Immunol. (2019) 10:619. doi: 10.3389/fimmu.2019.00619
35. Murata M, Terakura S, Wake A, Miyao K, Ikegame K, Uchida N, et al. Off-the-shelf bone marrow-derived mesenchymal stem cell treatment for acute graft-versus-host disease: real-world evidence. Bone Marrow Transplant. (2021) 56:2355–66. doi: 10.1038/s41409-021-01304-y
36. Herzog S, Weisdorf DJ, Shanley R, Rayes A, Holtan SG, Young JA, et al. Chronic GVHD after steroid-sensitive, -dependent, and -refractory acute GVHD: incidence and clinical outcomes. Blood Adv. (2023) 7:3644–50. doi: 10.1182/bloodadvances.2022009505
37. Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. (2020) 382:1800–10. doi: 10.1056/NEJMoa1917635
Keywords: mesenchymal stem cells, steroid-refractory, acute graft-versus-host disease, hematopoietic stem cell transplantation, meta-analysis, randomized controlled trial
Citation: Xiao M, Yan Y, Zhang R, Wang D, Wang L, Zhao Y and Yang J (2025) Mesenchymal stem cells added to second-line therapy improve response and failure-free survival in steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: A meta-analysis of randomized controlled trials. Front. Oncol. 15:1704963. doi: 10.3389/fonc.2025.1704963
Received: 14 September 2025; Accepted: 21 October 2025;
Published: 04 November 2025.
Edited by:
Roberto Crocchiolo, Niguarda Ca’ Granda Hospital, ItalyReviewed by:
Gil Cunha De Santis, Hemocentro Foundation of Ribeirão Preto, BrazilNayoun Kim, Catholic University of Korea, Republic of Korea
Copyright © 2025 Xiao, Yan, Zhang, Wang, Wang, Zhao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wang, d2FuZ2xpcGxhZ2hAaG90bWFpbC5jb20=; Yameng Zhao, OTg3MTk3NTU4QHFxLmNvbQ==; Jinhong Yang, eWFuZ2ppbmhvbmdfcm1AaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Man Xiao1,2†
Man Xiao1,2† Li Wang
Li Wang Jinhong Yang
Jinhong Yang