- 1Department of Urology/Institute of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Urology, People’s Hospital of Deyang City, Chengdu University of Traditional Chinese Medicine, Deyang, China
Background: Bladder cancer (BC) prognosis remains challenging to predict accurately with conventional tools. Systemic immune-inflammation index (SII) has emerged as a promising biomarker reflecting the tumor microenvironment. However, existing studies are limited by small sample sizes, heterogeneous designs, and inconsistent endpoints. This updated meta-analysis aims to comprehensively evaluate the association between high SII and key survival outcomes in BC patients.
Methods: We systematically searched PubMed, Embase, Web of Science, and Cochrane up to August 2025. Cohort studies reporting hazard ratios (HRs) for overall survival (OS), recurrence-free survival (RFS), progression-free survival (PFS), or cancer-specific survival (CSS) comparing high vs. low SII groups in histologically confirmed BC were included. Study quality was assessed using the Newcastle-Ottawa Scale. Pooled HRs with 95% confidence intervals (CIs) were calculated using a random-effects model. Subgroup analyses by pathological type (NMIBC vs. MIBC) and sensitivity analyses were performed. Publication bias was evaluated via funnel plots and Egger’s test.
Results: Sixteen cohort studies involving 2,352 patients were analyzed. Meta-analysis revealed that elevated SII was significantly associated with worse OS (HR=1.66, 95% CI: 1.30–2.12, P < 0.0001) and RFS (HR=1.50, 95% CI: 1.28–1.76, P < 0.00001), with substantial heterogeneity (OS: I² = 81%; RFS: I² = 59%). Subgroup analysis showed significant predictive value of SII for RFS in both NMIBC (HR=1.55, 95% CI: 1.27–1.89, P < 0.0001; heterogeneity reduced to I² = 37%) and MIBC (HR=1.13, 95% CI: 1.01–1.26, P=0.03). However, OS subgroup associations for NMIBC (HR=1.15, P=0.50) and MIBC (HR=1.92, P=0.07) were non-significant. No significant associations were found for PFS (HR=1.55, 95% CI: 0.92–2.60, P=0.10, I² = 68%) or CSS (HR=1.50, 95% CI: 0.95–2.37, P=0.08, I² = 69%), likely due to limited study numbers (4 and 3, respectively). Significant publication bias was detected for OS and RFS.
Conclusion: Elevated SII is significantly associated with poorer overall and recurrence-free survival in bladder cancer patients, particularly highlighting its potential predictive value for recurrence risk in NMIBC. However, significant heterogeneity, publication bias, and retrospective design limitations necessitate caution in interpretation. Future large-scale, prospective studies with standardized SII measurement and dynamic monitoring are crucial to validate its clinical utility and define optimal cut-offs for integration into risk-stratified management strategies.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251145769, Prospero identifier, CRD420251145769.
Introduction
Bladder cancer is the tenth most common malignancy worldwide, and its burden continues to grow (1). In 2020, an estimated 573,000 people were diagnosed with bladder cancer worldwide, equivalent to 5.6 cases per 100,000 people (2). Clinically, non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) differ fundamentally in their biological behavior and treatment strategies. While NMIBC patients enjoy a five-year survival rate exceeding 85%, recurrence rates are as high as 60%-70% (3, 4). Meanwhile, even after radical cystectomy, 50% of MIBC patients will develop metastases within two years (5). Current prognostic assessment relies primarily on traditional indicators such as TNM staging and histological grade, but these static parameters are unable to dynamically reflect the evolving tumor microenvironment. In particular, for patients with intermediate- and high-risk NMIBC undergoing transurethral resection of bladder tumors (TURBT), existing prognostic tools, such as the European Organization for Research and Treatment (EORTC) scoring system, misjudge recurrence risk by over 30% (6). This clinical dilemma has driven the exploration of novel biomarkers, with systemic inflammation, a key area of research, becoming a hot topic due to its central role in tumor progression. Tumor-associated inflammation creates a microenvironment conducive to tumor growth and metastasis by promoting angiogenesis, accelerating DNA damage, and suppressing anti-tumor immunity (7). Basic research has demonstrated that interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) released by neutrophils can activate the STAT3 pathway to induce bladder cancer cell proliferation (8, 9), while platelet-derived microparticles promote epithelial-mesenchymal transition through transforming growth factor-β (TGF-β) signaling (10). These findings provide a theoretical foundation for the application of inflammatory markers in the prognostic assessment of bladder cancer.
The Systemic Inflammatory Index (SII), an emerging comprehensive inflammatory marker, integrates neutrophil, platelet, and lymphocyte counts (calculated as: SII = neutrophil count × platelet count/lymphocyte count), providing a multidimensional quantification of systemic inflammatory status (11, 12). Compared to single indices such as the neutrophil-lymphocyte ratio (NLR) or platelet-lymphocyte ratio (PLR), the SII provides a more comprehensive quantification of the systemic inflammatory state and the balance of pro-tumor and anti-tumor immunity in the tumor microenvironment by integrating neutrophil, platelet and lymphocyte counts into a comprehensive indicator, which may give it a superior prognostic prediction potential (13, 14). In the field of bladder cancer, the SII is particularly valuable in pathophysiology: surgical stress or the tumor itself can induce the release of immature neutrophils from the bone marrow (15). The arginase 1 (ARG1) secreted by these neutrophils depletes arginine in the microenvironment, directly inhibiting CD8+ T cell function (16). Activated platelets, through P-selectin, mediate tumor cell-endothelial cell adhesion, promoting hematogenous metastasis (17). A retrospective cohort study by Salari et al. demonstrated that elevated SII was significantly associated with worse OS, highlighting its potential as a marker of disease aggressiveness (18). However, existing evidence is conflicting: a retrospective study by Demirci et al. of 173 elderly patients with NMIBC demonstrated no significant association between SII and OS (19). This discrepancy may be due to methodological heterogeneity, including differences in the timing of SII testing, cutoff definition, and endpoint criteria.
Although more than a dozen studies have explored the value of SII in bladder cancer prognosis, a systematic evidence synthesis is lacking. Previous studies have three key limitations: first, the sample size is generally insufficient, resulting in weak statistical power; second, the study designs are highly heterogeneous, with patients included at different stages of treatment, such as after TURBT surgery, before radical cystectomy, and with BCG maintenance therapy, and the confounding effects of adjuvant therapy are not adequately controlled; finally, the definitions of prognostic endpoints are inconsistent, including multiple indicators such as overall survival (OS), recurrence-free survival (RFS), and progression-free survival (PFS), making data comparability difficult. This study, the latest and largest comprehensive evidence-based evaluation of the prognostic value of SII in bladder cancer, strictly adheres to the PRISMA 2020 statement. We systematically searched four major databases (PubMed/Embase/Web of Science/Cochrane) up to August 2025. Using a random-effects model, we integrated data from 16 cohort studies totaling 2,352 patients. We focused on analyzing the strength of the association between SII and key survival outcomes (OS, RFS, PFS, and CSS). We also explored the robustness of our results through pathological subgroup analyses and sensitivity testing, ultimately providing evidence-based decision-making for clinical practice.
Methods
Literature search
This meta-analysis was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 2020 statement (20) and has been prospectively registered in the PROSPERO (CRD420251145769). The PRISMA checklist was presented in Supplementary Table S1. We conducted a systematic literature search via PubMed, Embase, Web of Science, Cochrane up to August, 2025 for studies that evaluated the role of SII in the prognosis of patients with bladder cancer. We searched the literature through the following terms: “Urinary Bladder Neoplasms”, “systemic immune-inflammation index”, and “SII”. The detailed search strategies in PubMed are as follows: ((systemic immune-inflammation index) OR (SII)) AND ((“Urinary Bladder Neoplasms”[Mesh]) OR ((((((((Urinary Bladder Neoplasm) OR (Bladder Neoplasm)) OR (Bladder Tumor)) OR (Urinary Bladder Cancer)) OR (Bladder Cancer)) OR (Cancer of Bladder)) OR (Malignant Tumor of Urinary Bladder)) OR (Bladder Carcinoma))). Furthermore, we manually screened the bibliography lists of all included studies. Two authors retrieved and assessed eligible articles independently. Any differences in literature retrieval were resolved by discussion. Searching details were shown in Supplementary Table S2.
Inclusion and exclusion criteria
Articles were eligible when meeting the following standards:
P: Patients diagnosed with bladder cancer by pathology.
E: Patients with high SII levels (high and low criteria depend on the cut-off of each study).
C: Patients with low SII levels (high and low criteria depend on the cut-off of each study).
O: at least one survival outcome (OS, PFS, RFS, CSS, etc.) was evaluated.
S: study design was randomized controlled trial, cohort, or case-control.
We excluded study protocols, unpublished studies, non-original studies (including letters, comments, abstracts, correction, and reply), studies without sufficient data, and reviews.
Data abstraction
Data abstraction was conducted by two authors severally. Any differences were settled by another author. We abstracted following information from eligible studies: first author name, published year, study region, study design, research population, sample size, age, gender, TNM stage, SII cut-off, OS, RFS, PFS, CSS. If the research data is insufficient, corresponding authors were contacted for full data if available.
Quality evaluation
The Newcastle-Ottawa Scale (NOS) was applied for assessing the quality of included cohort studies (21), and studies with 7–9 points were considered as high quality (22). Studies with NOS scores below 6 were not included for quantitative analysis. Two authors severally assessed the quality of all included studies, and any disagreement was settled by discussion.
Statistical analysis
Meta-analysis was conducted in Review Manager 5.4.1 edition. HR were used for the synthesis of survival data. Metrics were presented with 95%CIs. The chi-squared (χ2) test (Cochran’s Q) and inconsistency index (I2) were applied for the evaluation of the heterogeneity of each outcome (23). χ2 P value less than 0.1 or I2 more than 50% were regarded as high heterogeneity. The random-effects model was applied to calculate the total HR for each outcome. In addition, we conducted sensitivity analysis to assess the influence of every included study on the total HR. To identify the source of heterogeneity, we performed subgroup analysis according to the pathological type of bladder cancer. Moreover, we assessed the potential publication bias by producing funnel plots through Review Manager 5.4.1 edition as well as through performing Egger’s regression tests (24) through Stata 15.1 edition (Stata Corp, College Station, Texas, USA). P value < 0.05 was considered as statistically significant publication bias.
Results
Literature retrieval, study characteristics, and baseline
Figure 1 shows the flowchart of the literature retrieval and selection process. A total of 213 related studies in PubMed (n = 63), Embase (n = 87), Web of Science (n = 59), Cochrane (n = 4) were identified via systematically literature search. After removing duplicate studies, a total of 131 titles and abstracts were evaluated. Eventually, 16 cohort studies including 2352 patients were included for meta-analysis (18, 19, 25–38). Table 1 presents the characteristics and quality evaluation of each eligible cohort study. Detailed NOS score results are shown in Supplementary Table S3.
OS
Results of OS were synthesized from 10 cohort studies, and meta-analysis revealed a significantly shorter OS in the group with high SII compared with the group with low SII (HR: 1.66; 95% CI: 1.30, 2.12; P<0.0001). A significant heterogeneity was observed (I2 = 81%, P<0.00001) (Figure 2A). Subgroup analysis based on bladder cancer pathological type showed that SII had no effective prognostic value for both NMIBC (HR: 1.15; 95% CI: 0.76, 1,75; P=0.50) and MIBC (HR: 1.92; 95% CI: 0.94, 3.92; P=0.07) (Figure 2B). Furthermore, heterogeneity analysis revealed a significant decrease in heterogeneity in the NMIBC subgroup (I2 = 26%) (Figure 2B).
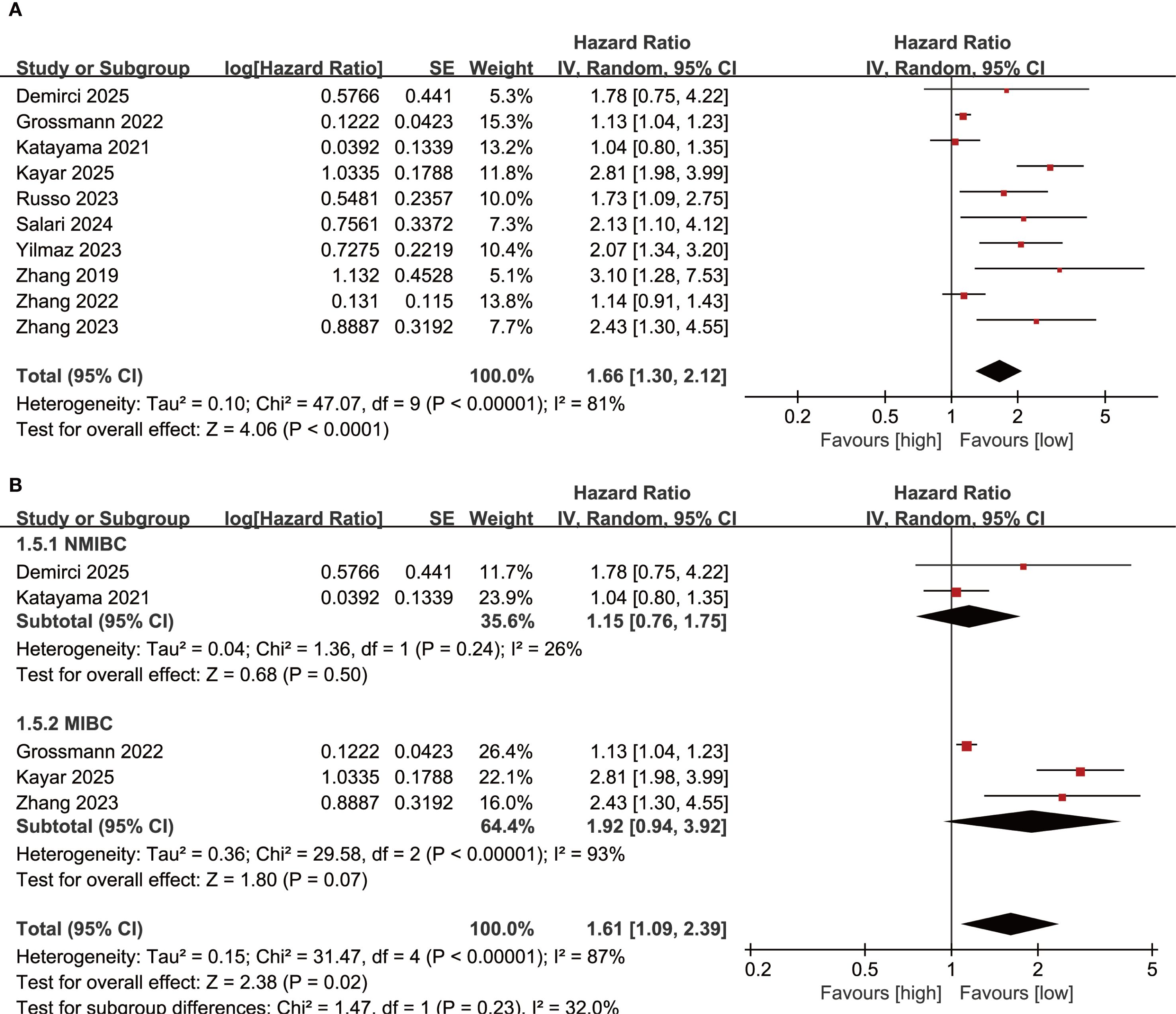
Figure 2. (A) Forest plots of OS; (B) Subgroup analysis of OS based on bladder cancer pathological type.
RFS
Results of RFS were synthesized from 11 cohort studies, and meta-analysis revealed a significantly shorter RFS in the group with high SII compared with the group with low SII (HR: 1.50; 95% CI: 1.28, 1.76; P<0.00001). A significant heterogeneity was observed (I2 = 59%, P=0.006) (Figure 3A). Subgroup analysis based on bladder cancer pathological type revealed that SII had significant prognostic value for both NMIBC (HR: 1.55; 95% CI: 1.27, 1.89; P<0.0001) and MIBC (HR: 1.13; 95% CI: 1.01, 1.26; P=0.03). Furthermore, heterogeneity analysis revealed a significant decrease in heterogeneity in the NMIBC subgroup (I2 = 37%) (Figure 3B).
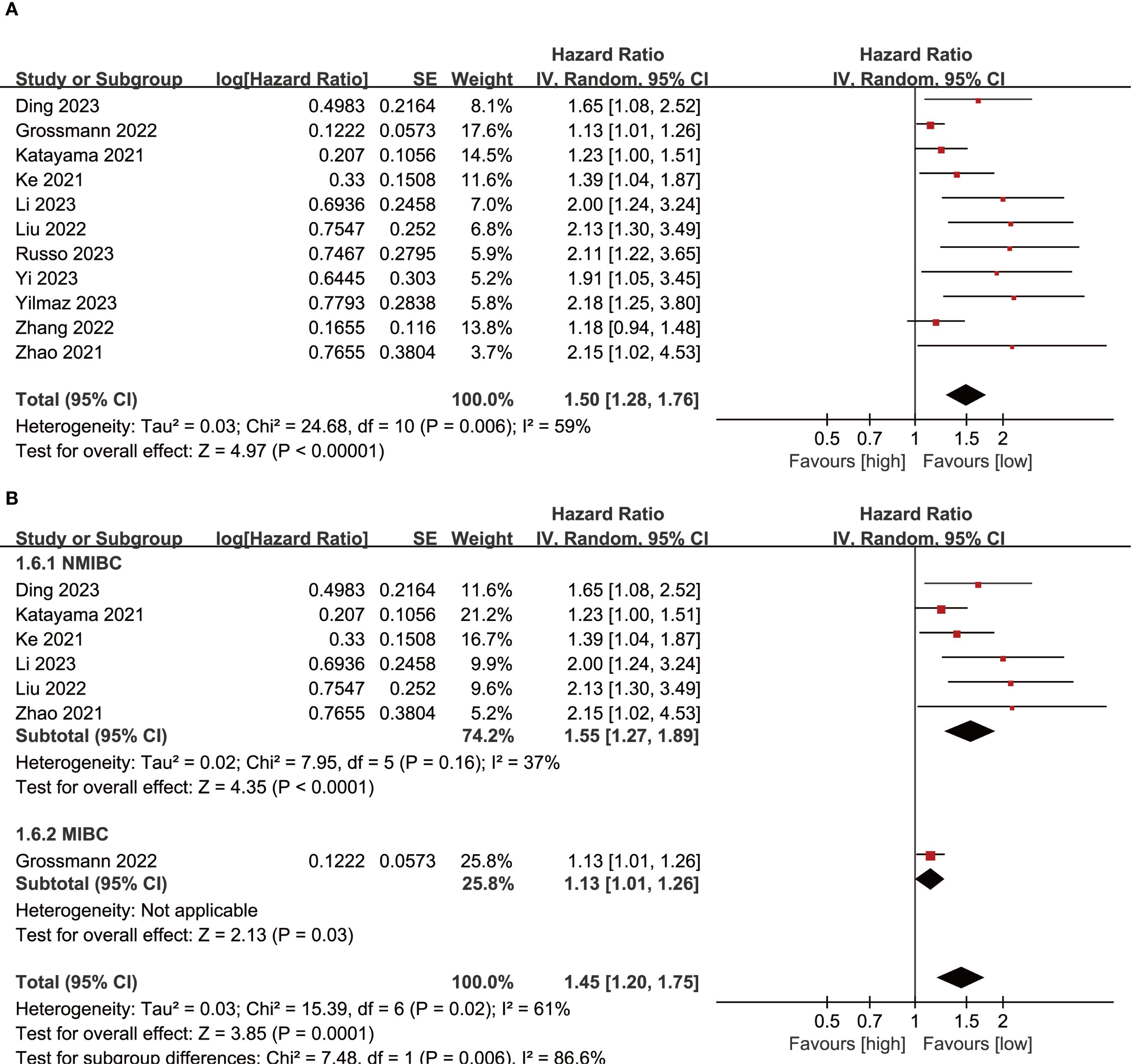
Figure 3. (A) Forest plots of RFS; (B) Subgroup analysis of RFS based on bladder cancer pathological type.
PFS
Results of PFS were synthesized from 4 cohort studies, and meta-analysis found no significant correlation between SII and PFS in bladder cancer patients (HR: 1.55; 95% CI: 0.92, 2.60; P=0.10). A significant heterogeneity was observed (I2 = 68%, P=0.02) (Figure 4A).
CSS
Results of CSS were synthesized from 3 cohort studies, meta-analysis found no significant correlation between SII and CSS in bladder cancer patients (HR: 1.50; 95% CI: 0.95, 2.37; P=0.08). A significant heterogeneity was observed (I2=69%, P=0.04) (Figure 4B).
Publication bias and sensitivity analysis
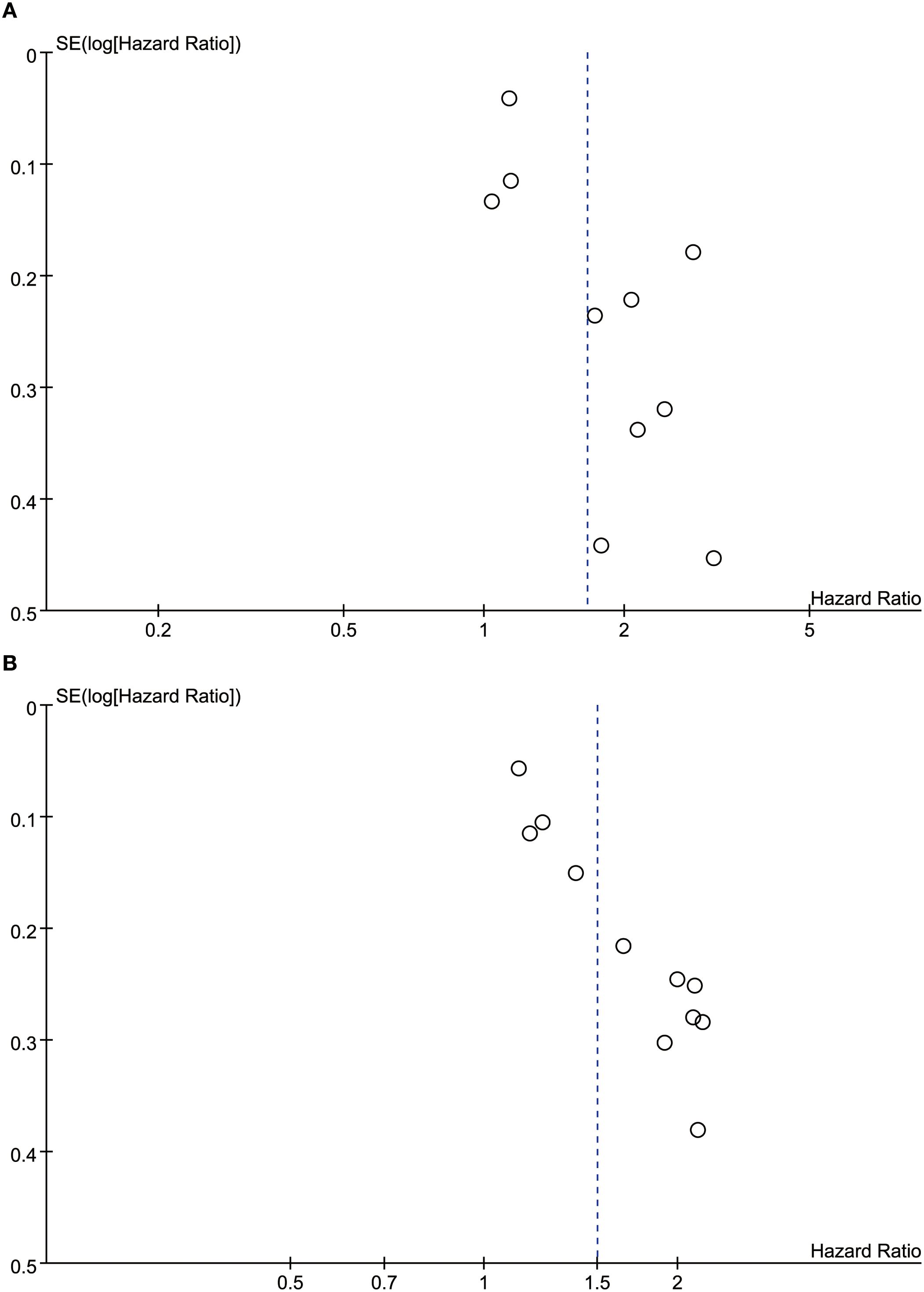
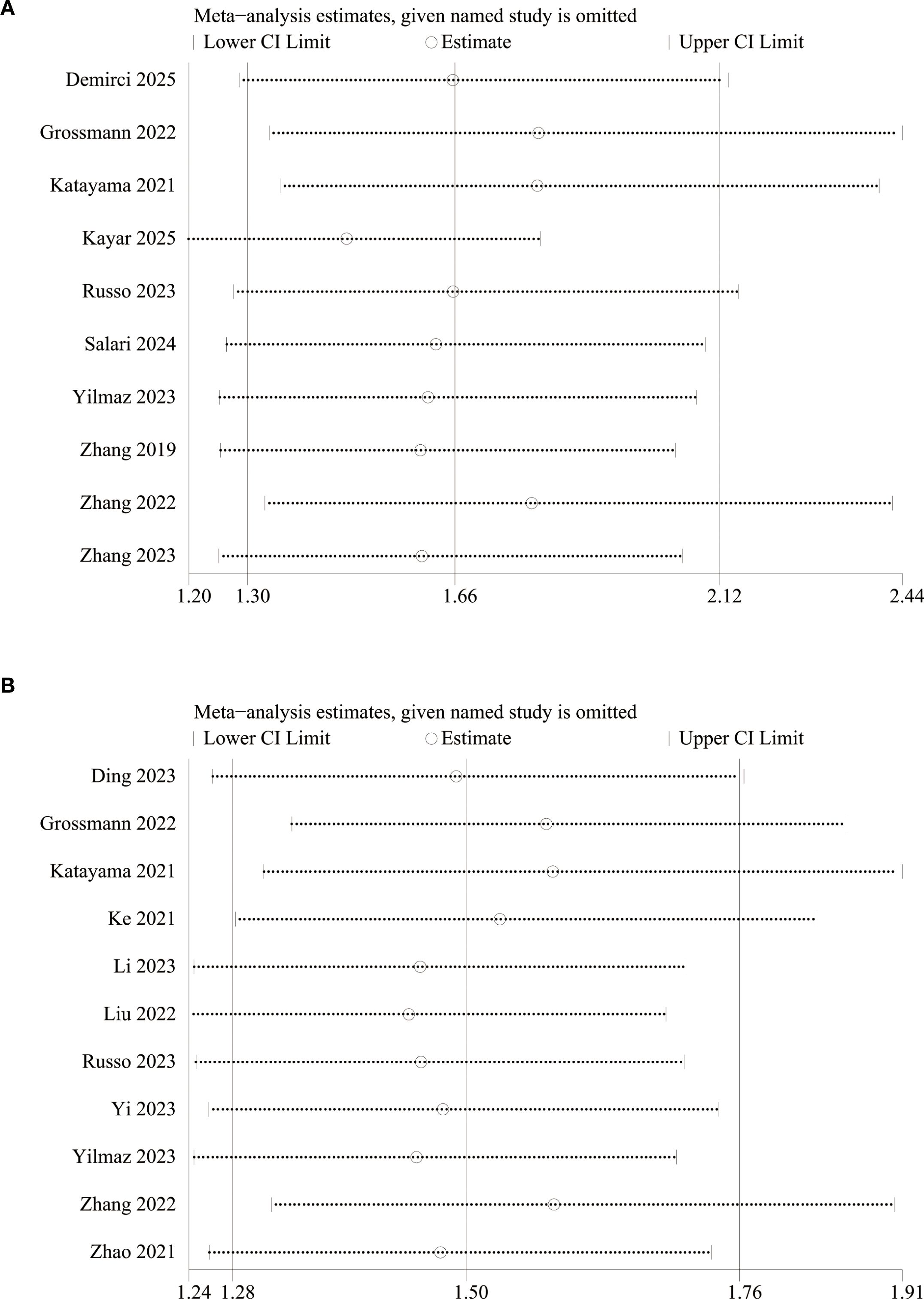
We assessed the potential publication bias through funnel plots and Egger’s regression tests for OS and RFS. Funnel plots and Egger’s test both showed significant publication bias in OS (Figure 5A, P=0.011) and RFS (Figure 5B, P=0.001). In addition, we performed sensitivity analysis for the results of OS and RFS to assess the effect of each cohort study on the total HR via excluding eligible cohort studies one by one. Sensitivity analysis found that the new total HR kept stable after removing of each cohort study for OS (Figure 6A) and RFS (Figure 6B).
Discussion
This study, conducted through a systematic meta-analysis of 16 cohort studies (enrolling a total of 2,352 bladder cancer patients) through August 2025, comprehensively evaluated the association between SII and bladder cancer prognosis. The results showed that elevated SII was significantly associated with worse OS, with a pooled HR of 1.66 (95% CI: 1.30–2.12, P < 0.0001). High SII levels also predicted shorter RFS, with a pooled HR of 1.50 (95% CI: 1.28–1.76, P < 0.00001). Subgroup analyses revealed that SII had a significant predictive value for recurrence risk in patients with NMIBC (HR = 1.55). This association was also observed in patients with MIBC, but to a lesser extent (HR = 1.13). However, the predictive value of SII for PFS and CSS did not reach statistical significance. It should be pointed out that this study has obvious heterogeneity and potential publication bias, and all evidence comes from retrospective studies. These factors need to be fully considered when interpreting the results.
This study, through a meta-analysis, confirmed that elevated SII is significantly associated with poor prognosis in bladder cancer patients. This finding has profound implications for tumor immunobiology. As a systemic inflammatory marker, SII integrates information from three immune cell types: neutrophils, platelets, and lymphocytes (39). Its elevation reflects the formation of a pro-tumor immune microenvironment. Neutrophils promote angiogenesis and extracellular matrix degradation by secreting factors such as interleukin-8 (IL-8) and matrix metalloproteinase-9 (MMP-9), accelerating tumor invasion and metastasis (40). Platelets, through the release of transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF), induce epithelial-mesenchymal transition (EMT) and inhibit cytotoxic T lymphocyte function (41). Depletion of lymphocytes, particularly CD8+ T cells, leads to impaired immune surveillance (42). This triple imbalance in the bladder cancer microenvironment contributes to an immunosuppressive state, promoting tumor cell evasion of immune clearance. This study observed a strong association between elevated SII and significantly shortened OS (HR=1.66, P<0.0001) and RFS (HR=1.50, P<0.00001), providing clinical evidence for the “inflammation-tumor progression” theory.
This study found significant differences in the prognostic value of SII for different clinical endpoints and bladder cancer subtypes, a stratification effect with important clinical implications. Regarding overall survival, a high SII level was associated with a 66% increased risk of death. However, subgroup analysis revealed that this association did not reach statistical significance in NMIBC (HR=1.15, P=0.50) or MIBC (HR=1.92, P=0.07), reflecting the confounding of multiple factors in OS prognosis. In contrast, SII demonstrated significant predictive value for recurrence risk in NMIBC (HR=1.55, P < 0.0001) but was less effective in MIBC (HR=1.13, P=0.03). This discrepancy may be due to the distinct biological nature of the two bladder cancer types: NMIBC recurrence is often driven by a dysregulated in situ immune microenvironment, while MIBC progression is more likely to involve systemic metastatic mechanisms (43, 44). Despite pooled HRs of 1.55 and 1.50 for PFS and CSS, respectively, no significant associations were established due to insufficient inclusion of studies (only four and three) and high heterogeneity (I² = 68%-69%). From a clinical perspective, the core value of the SII lies in its convenience: as a derivative of routine blood tests, it is low-cost and results are available within 24 hours, making it particularly suitable for primary care settings. Based on this evidence, a tiered approach is recommended for clinical practice: routine postoperative SII testing for NMIBC patients. When the SII exceeds the optimal cutoff, an intensive follow-up program (such as cystoscopy and urine cytology every three months) should be initiated; for MIBC patients, a comprehensive evaluation combining imaging and molecular markers is required (45). The value of dynamic SII monitoring is even more noteworthy: changes in SII before and after surgery may better reflect treatment response than single-measurement values. However, this study was limited by its retrospective design and was unable to analyze this dimension.
The high degree of heterogeneity across studies (pooled I² = 81% for OS and 59% for RFS) is a key point of contention in these results. Subgroup analyses provide key insights into the sources of heterogeneity. After stratification by pathology type, OS heterogeneity in the NMIBC subgroup was significantly reduced (I² decreased from 81% to 26%), suggesting that differences in study populations were the primary confounding factor. A thorough analysis of the primary study characteristics revealed three sources of heterogeneity: first, lack of standardization of SII testing, with significant variation in cutoff values across studies and lack of standardized testing timing; second, variability in endpoint definitions, including RFS criteria (cytology-positive vs. pathologically confirmed recurrence vs. radiographic progression); and most importantly, confounding by treatment modalities: included studies included a variety of interventions, including TURBT, radical cystectomy, and intravenous BCG therapy, without adjustment for the impact of different treatments on inflammatory status. The unique performance of the MIBC subgroup is particularly noteworthy: although the pooled HR for OS reached 1.92, the wide confidence interval (0.94-3.92) and limited sample size (only three studies) significantly underpowered the statistical power. This reflects methodological flaws in MIBC research: large studies have focused on postoperative survival analysis but have not excluded patients receiving neoadjuvant chemotherapy. Furthermore, racial differences have not been adequately assessed: studies in Asian populations have generally reported higher SII effect sizes than those in European and American populations, possibly due to differences in genetic background. These confounding factors have led to conflicting conclusions in some studies: Ke et al. (28) reported significant predictive value of SII for NMIBC recurrence, while Zhang et al. (31) found negative results.
Limitations of this study profoundly impact the robustness and clinical applicability of the conclusions. First, all included studies in this meta-analysis were retrospective in design, subject to unavoidable selection bias. Most studies included only single-center samples and excluded patients with comorbid autoimmune diseases or infections, resulting in insufficient representation of the real world. Furthermore, sample size imbalance was significant. Although the RFS analysis included 11 studies, approximately 60% of the samples were concentrated in four large studies, while the CSS analysis included only three studies, resulting in significant statistical power deficiency. Second, the lack of standardization in SII measurement poses a significant challenge. Blood specimen processing time (from collection to detection) affects neutrophil activity, differences in detection systems between laboratories lead to high counting errors, and the influence of circadian rhythms on inflammatory markers was not considered. In addition, possible racial or regional differences in the prognostic value of SII need to be carefully considered. For example, studies on Asian populations included in this analysis often show higher effect sizes than those in European and American studies. This may be due to differences in genetic background, lifestyle, or medical system. Therefore, we recommend that future studies conduct stratified analyses by population to verify the universality of the conclusions of this study. Furthermore, key prognostic covariates were inadequately controlled: only five studies adjusted for TNM stage, and only two adjusted for molecular subtypes. Immunotherapy-related markers such as PD-L1 expression were not included in the analysis. At the same time, the lack of dynamic monitoring of treatment response is also a problem that cannot be ignored. All studies used baseline or postoperative SII values and failed to evaluate the impact of SII changes during neoadjuvant chemotherapy or immunotherapy on prognosis. Finally, the problem of publication bias is even more serious. The Egger test showed that there was a significant publication bias in both OS (P=0.011) and RFS (P=0.001), and the asymmetry of the funnel plot suggested that studies with negative results may not have been published. These limitations together make it difficult for the current level of evidence to support SII as an independent prognostic indicator in clinical guidelines, especially for the prognostic judgment of MIBC patients, which requires caution.
To overcome the current evidence bottleneck, the following targeted research is urgently needed. The primary task is to establish a globally unified standard operating procedure (SOP) for SII testing. Core components include: clearly defined testing timing; standardized anticoagulation protocols; standardized specimen processing procedures; and a cross-platform calibration system. Regarding cohort design, a multicenter, prospective registry of inflammatory markers in bladder cancer should be initiated, with predefined key subgroups: stratified by pathological type (NMIBC vs. MIBC), treatment modality (surgery vs. chemoradiotherapy vs. immunotherapy), and molecular subtype (basal vs. luminal), using standardized endpoints. Furthermore, mechanistic insights are needed. Future studies could elucidate the spatial relationship between SII components and the tumor immune microenvironment using multicolor flow cytometry, establish a characteristic immunogenomic profile of patients with high SII using single-cell sequencing, and conduct organoid co-culture experiments to validate the effects of inflammatory mediators on bladder cancer cell stemness. Of greatest translational value are interventional studies, which would randomly assign patients with NMIBC and high SII to standard BCG instillation versus combined anti-inflammatory therapy for recurrence prevention, while also monitoring the correlation between dynamic changes in SII during treatment and remodeling of the tumor immune microenvironment.
Conclusion
High SII was associated with shorter OS and RFS in patients with bladder cancer. Due to the simplicity and low cost of routine blood test, SII can be widely used to evaluate prognosis and construct risk prediction model for bladder cancer. Given the limitations of retrospective studies, population selection bias, and potential heterogeneity, more large-scale, multi-center, prospective clinical studies are needed to further validate the relationship between SII and the prognosis of patients with bladder cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JB: Formal Analysis, Software, Writing – original draft. YH: Formal Analysis, Software, Writing – original draft. BC: Data curation, Software, Writing – original draft. BR: Data curation, Software, Writing – original draft. SJ: Conceptualization, Formal Analysis, Writing – original draft. JL: Formal Analysis, Software, Writing – original draft. JC: Formal Analysis, Software, Writing – original draft. QW: Funding acquisition, Supervision, Writing – review & editing. DC: Project administration, Supervision, Writing – review & editing. LL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by National Key Research and Development Program of China (2022YFC3602905) and the National Natural Science Foundation of China (Grant No.82170784,82370775). The funders played no role in the study design, data collection, analysis, or interpretation; report writing; or the decision to submit the article for publication.
Acknowledgments
We are very grateful to the funders and the Sichuan University Library for their support in the literature search of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1707657/full#supplementary-material
References
1. Zi H, Liu M-Y, Luo L-S, Huang Q, Luo P-C, Luan H-H, et al. Global burden of benign prostatic hyperplasia, urinary tract infections, urolithiasis, bladder cancer, kidney cancer, and prostate cancer from 1990 to 2021. Military Med Res. (2024) 11:64. doi: 10.1186/s40779-024-00569-w
2. Zhang Y, Rumgay H, Li M, Yu H, Pan H, and Ni J. The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. J Global Health. (2023) 13:04109. doi: 10.7189/jogh.13.04109
3. McNall S, Hooper K, Sullivan T, Rieger-Christ K, and Clements M. Treatment modalities for non-muscle invasive bladder cancer: an updated review. Cancers. (2024) 16:1843. doi: 10.3390/cancers16101843
4. Jaromin M, Konecki T, and Kutwin P. Revolutionizing treatment: breakthrough approaches for BCG-unresponsive non-muscle-invasive bladder cancer. Cancers. (2024) 16:1366. doi: 10.3390/cancers16071366
5. Marcq G, Afferi L, Neuzillet Y, Nykopp T, Voskuilen CS, Furrer MA, et al. Oncological outcomes for patients harboring positive surgical margins following radical cystectomy for muscle-invasive bladder cancer: a retrospective multicentric study on behalf of the YAU urothelial group. Cancers. (2022) 14:5740. doi: 10.3390/cancers14235740
6. Krajewski W, Aumatell J, Subiela JD, Nowak Ł, Tukiendorf A, Moschini M, et al. Accuracy of the CUETO, EORTC 2016 and EAU 2021 scoring models and risk stratification tables to predict outcomes in high–grade non-muscle-invasive urothelial bladder cancer. Urol Oncol. (2022) 40:491–e11. doi: 10.1016/j.urolonc.2022.06.008
7. Yang Y, Li S, To KKW, Zhu S, Wang F, and Fu L. Tumor-associated macrophages remodel the suppressive tumor immune microenvironment and targeted therapy for immunotherapy. J Exp Clin Cancer Res. (2025) 44:145. doi: 10.1186/s13046-025-03377-9
8. Martins-Lima C, Chianese U, Benedetti R, Altucci L, Jerónimo C, and Correia MP. Tumor microenvironment and epithelial-mesenchymal transition in bladder cancer: Cytokines in the game? Front Mol Biosci. (2023) 9:1070383. doi: 10.3389/fmolb.2022.1070383
9. Catalano M, Roviello G, Santi R, Villari D, Spatafora P, Galli IC, et al. Inflammation in urological Malignancies: the silent killer. Int J Mol Sci. (2023) 24:866. doi: 10.3390/ijms24010866
10. Takemoto A, Okitaka M, Takagi S, Takami M, Sato S, Nishio M, et al. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci Rep. (2017) 7:42186. doi: 10.1038/srep42186
11. Hu B, Wang L, Qu S, and Zhang T. Construction of a column-line graphical model of poor outcome of neoadjuvant regimens for muscle-invasive bladder cancer based on NLR, dNLR and SII indicators. World J Surg Oncol. (2025) 23:274. doi: 10.1186/s12957-025-03903-1
12. Bizzarri FP, Campetella M, Russo P, Palermo G, Moosavi SK, Rossi F, et al. Prognostic value of PLR, SIRI, PIV, SII, and NLR in non-Muscle invasive bladder cancer: can inflammatory factors influence pathogenesis and outcomes? Cancers. (2025) 17:2189. doi: 10.3390/cancers17132189
13. Ozcan L, Polat EC, Baran C, Boylu A, Erkoc M, and Otunctemur A. Systemic inflammatory index: A promising non-invasive marker for the prediction of response to neoadjuvant chemotherapy prior to cystectomy. Urol Int. (2024) 108:226–33. doi: 10.1159/000537894
14. Dionese M, Bimbatti D, Pierantoni F, Lai E, Erbetta E, Cavasin N, et al. Systemic inflammation indexes and risk of immune-related adverse events in patients with metastatic urothelial carcinoma treated with immunotherapy. Anticancer Res. (2024) 44:4379–86. doi: 10.21873/anticanres.17267
15. Onuma AE, Zhang H, Gil L, Huang H, and Tsung A. Surgical stress promotes tumor progression: a focus on the impact of the immune response. J Clin Med. (2020) 9:4096. doi: 10.3390/jcm9124096
16. Canè S, Geiger R, and Bronte V. The roles of arginases and arginine in immunity. Nat Rev Immunol. (2025) 25:266–84. doi: 10.1038/s41577-024-01098-2
17. Wang X, Zhao S, Wang Z, and Gao T. Platelets involved tumor cell EMT during circulation: communications and interventions. Cell Communication Signaling. (2022) 20:82. doi: 10.1186/s12964-022-00887-3
18. Salari A, Ghahari M, Bitaraf M, Fard ES, Haddad M, Momeni SA, et al. Prognostic value of NLR, PLR, SII, and dNLR in urothelial bladder cancer following radical cystectomy. Clin Genitourin Cancer. (2024) 22:102144. doi: 10.1016/j.clgc.2024.102144
19. Demirci A, Uzel T, Bolat A, and Başar H. Do the ınflammatory markers have a prognostic role in an elderly patient population diagnosed with non-muscle ınvasive bladder cancer? Cirugia y cirujanos. (2025) 93:202–10. doi: 10.24875/CIRUE.M23000834
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.). (2021) 372:n71. doi: 10.1136/bmj.n71
21. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2011).
22. Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose-response meta-analysis. Nutrients. (2019) 11. doi: 10.3390/nu11040826
23. Higgins JP and Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
24. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed.). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
25. Grossmann NC, Schuettfort VM, Pradere B, Rajwa P, Quhal F, Mostafaei H, et al. Impact of preoperative systemic immune-inflammation Index on oncologic outcomes in bladder cancer patients treated with radical cystectomy. Urologic Oncol. (2022) 40:106.e11–106.e19. doi: 10.1016/j.urolonc.2021.10.006
26. Russo P, Marino F, Rossi F, Bizzarri FP, Ragonese M, Dibitetto F, et al. Is systemic immune-inflammation index a real non-invasive biomarker to predict oncological outcomes in patients eligible for radical cystectomy? Med (Kaunas Lithuania). (2023) 59:2063. doi: 10.3390/medicina59122063
27. Zhao R, Shan J, Nie L, Yang X, Yuan Z, Xu H, et al. The predictive value of the ratio of the product of neutrophils and hemoglobin to lymphocytes in non-muscular invasive bladder cancer patients with postoperative recurrence. J Clin Lab Anal. (2021) 35:e23883. doi: 10.1002/jcla.23883
28. Ke ZB, Chen H, Chen JY, Cai H, Lin YZ, Sun XL, et al. Preoperative abdominal fat distribution and systemic immune inflammation were associated with response to intravesical Bacillus Calmette-Guerin immunotherapy in patients with non-muscle invasive bladder cancer. Clin Nutr (Edinburgh Scotland). (2021) 40:5792–801. doi: 10.1016/j.clnu.2021.10.019
29. Liu P, Chen S, Gao X, Liang H, Sun D, Shi B, et al. Preoperative sarcopenia and systemic immune-inflammation index can predict response to intravesical Bacillus Calmette-Guerin instillation in patients with non-muscle invasive bladder cancer. Front Immunol. (2022) 13:1032907. doi: 10.3389/fimmu.2022.1032907
30. Ding L, Wang X, Deng X, Xia W, Wang K, Yu X, et al. Preoperative systemic immune-inflammation index as a significant prognostic factor after TURBT in patients with non-muscle-invasive bladder cancer: A retrospective study based on propensity score matching analysis. Cancer Med. (2023) 12:7019–28. doi: 10.1002/cam4.5501
31. Zhang S, Du J, Zhong X, Tan P, Xu H, Zhang J, et al. The prognostic value of the systemic immune-inflammation index for patients with bladder cancer after radical cystectomy. Front Immunol. (2022) 13:1072433. doi: 10.3389/fimmu.2022.1072433
32. Katayama S, Mori K, Pradere B, Laukhtina E, Schuettfort VM, Quhal F, et al. Prognostic value of the systemic immune-inflammation index in non-muscle invasive bladder cancer. World J Urol. (2021) 39:4355–61. doi: 10.1007/s00345-021-03740-3
33. Yi X, Pi J, Liu C, Xiong Y, Liu J, Fu W, et al. The relationship between inflammatory response markers and the prognosis of non-muscle invasive bladder cancer and the development of a nomogram model. Front Oncol. (2023) 13:1189086. doi: 10.3389/fonc.2023.1189086
34. Zhang X and Liu Q. Systemic immune inflammation index and T-staging predict prognosis in patients with muscle-invasive bladder cancer. Archivos espanoles Urol. (2023) 76:511–8. doi: 10.56434/j.arch.esp.urol.20237607.63
35. Deng-Xiong L, Qing-Xin Y, De-Chao F, Fa-Cai Z, Rui-Cheng W, Shi X, et al. Systemic immune-inflammation index (SII) during induction has higher predictive value than preoperative SII in non-muscle-invasive bladder cancer patients receiving intravesical bacillus calmette -guerin. Clin Genitourin Cancer. (2023) 21:e145–52. doi: 10.1016/j.clgc.2022.11.013
36. Zhang W, Wang R, Ma W, Wu Y, Maskey N, Guo Y, et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Trans Med. (2019) 7:431. doi: 10.21037/atm.2019.09.02
37. Yilmaz H, Cinar NB, Avci IE, Telli E, Uslubas AK, Teke K, et al. The systemic inflammation response index: An independent predictive factor for survival outcomes of bladder cancer stronger than other inflammatory markers. Urologic Oncol. (2023) 41:256.e1–8. doi: 10.1016/j.urolonc.2022.11.011
38. Kayar R, Kayar K, Tokuç E, Topaktaş R, Çiçek M, Demir S, et al. Value of preoperative inflammatory burden index in predicting overall survival and progression in nonmetastatic muscle-invasive bladder cancer. Archivos espanoles Urol. (2025) 78:660–70. doi: 10.56434/j.arch.esp.urol.20257806.89
39. Bolat D, Baltaci S, Akgul M, Karabay E, Izol V, Aslan G, et al. Predictive role of the systemic immune inflammation index for intravesical BCG response in intermediate- and high-risk non-muscle-invasive bladder cancer. Urol Int. (2023) 107:617–23. doi: 10.1159/000528740
40. Poto R, Cristinziano L, Modestino L, de Paulis A, Marone G, Loffredo S, et al. Neutrophil extracellular traps, angiogenesis and cancer. Biomedicines. (2022) 10:431. doi: 10.3390/biomedicines10020431
41. Farooqi AA and Siddik ZH. Platelet-derived growth factor (PDGF) signalling in cancer: rapidly emerging signalling landscape. Cell Biochem Funct. (2015) 33:257–65. doi: 10.1002/cbf.3120
42. Kurachi M. CD8+ T cell exhaustion. Semin Immunopathol. (2019) 41:327–37. doi: 10.1007/s00281-019-00744-5
43. Chen Z, Zhang T, Li W, Hu J, Ou Y, Ye F, et al. Single-cell RNA sequencing analysis reveals the dynamic changes in the tumor microenvironment during NMIBC recurrence. Apoptosis. (2025) 30:282–96. doi: 10.1007/s10495-024-02044-2
44. Lee Y-C, Lam H-M, Rosser C, Theodorescu D, Parks WC, and Chan KS. The dynamic roles of the bladder tumour microenvironment. Nat Rev Urol. (2022) 19:515–33. doi: 10.1038/s41585-022-00608-y
Keywords: bladder cancer, systemic immune-inflammation index, prognostic indicators, overall survival, recurrence-free survival
Citation: Bai J, Huang Y, Chen B, Ran B, Jian S, Li J, Chen J, Wei Q, Cao D and Liu L (2025) Prognostic value of the systemic immune-inflammation index in bladder cancer: an update evidence-based analysis. Front. Oncol. 15:1707657. doi: 10.3389/fonc.2025.1707657
Received: 17 September 2025; Accepted: 09 October 2025;
Published: 24 October 2025.
Edited by:
Rui Miguel Gil Da Costa, Federal University of Maranhão, BrazilReviewed by:
Lorenzo Mortara, University of Insubria, ItalyAnna Di Spirito, University of Insubria, Varese, Italy, in collaboration with reviewer LM
Francesco Pio Bizzarri, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2025 Bai, Huang, Chen, Ran, Jian, Li, Chen, Wei, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangren Liu, bGl1bGlhbmdyZW5Ac2N1LmVkdS5jbg==; Dehong Cao, aHhjYW9kZWhvbmdAMTYzLmNvbQ==; Qian Wei, d2VpcWlhbmc5MzNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jingxing Bai1†
Jingxing Bai1† Yin Huang
Yin Huang Biao Ran
Biao Ran Jinze Li
Jinze Li Qian Wei
Qian Wei Dehong Cao
Dehong Cao Liangren Liu
Liangren Liu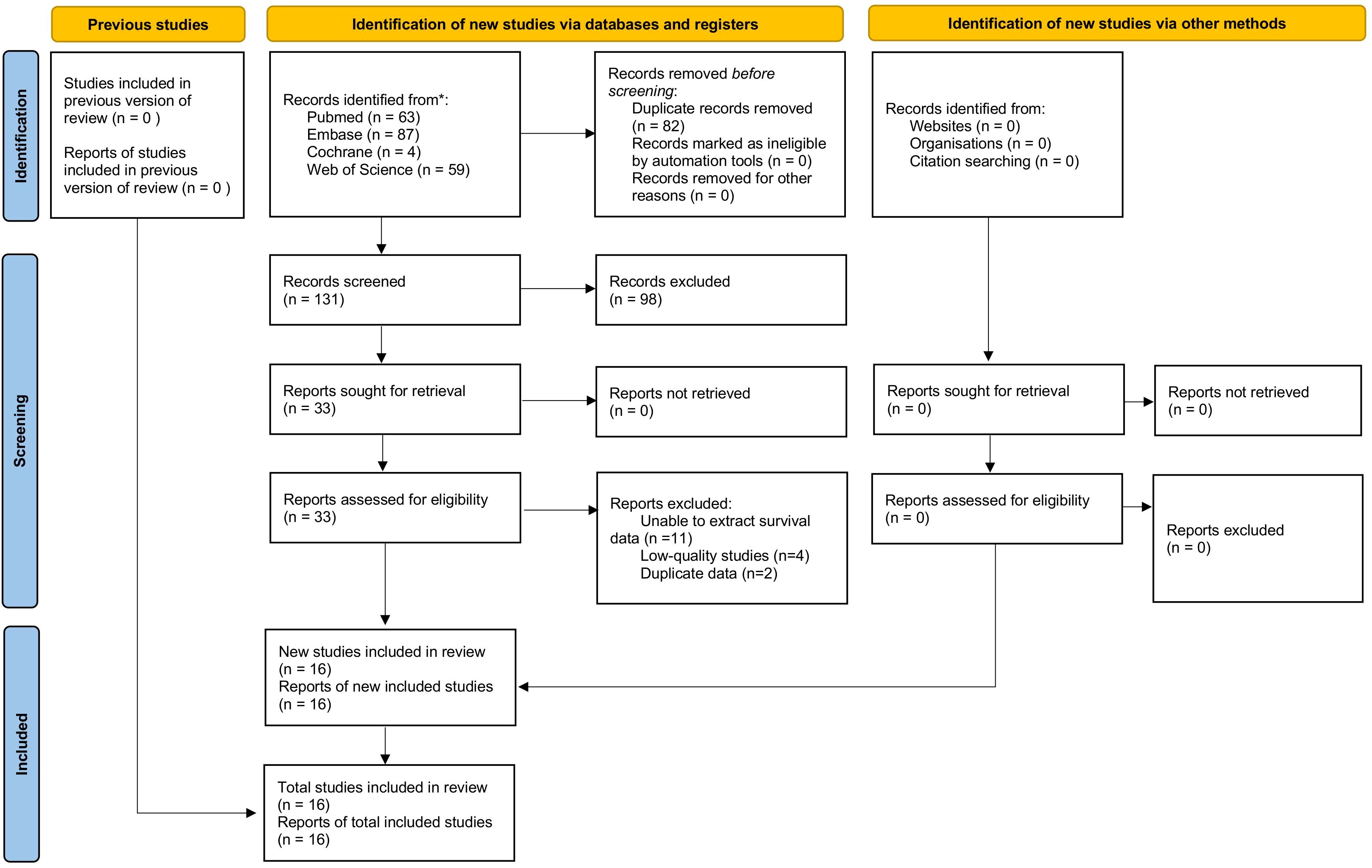
![Two forest plots titled A and B compare studies analyzing hazard ratios with 95% confidence intervals. Plot A includes studies by Demirci 2025, Katayama 2021, Kayar 2025, and Li 2023, showing a total effect of 1.55 [0.92, 2.60]. Plot B includes studies by Grossmann 2022, Katayama 2021, and Russo 2023, with a total effect of 1.50 [0.95, 2.37]. Heterogeneity is indicated by I² values of 68% and 69% respectively.](https://www.frontiersin.org/files/Articles/1707657/fonc-15-1707657-HTML/image_m/fonc-15-1707657-g004.jpg)