- 1The Department of Laboratory, Jiangyin People’s Hospital, Jiangyin, Jiangsu, China
- 2The Department of Oncology, Jiangyin People’s Hospital, Jiangyin, Jiangsu, China
- 3The Department of Pathology, Jiangyin People’s Hospital, Jiangyin, Jiangsu, China
- 4The Department of Radiology, Jiangyin People’s Hospital, Jiangyin, Jiangsu, China
Background: Primary splenic angiosarcoma (PSA) is an exceedingly rare and aggressive malignancy with a poor prognosis. This report aims to present a rare case of PSA that progressed to fatal hepatic rupture due to rapid metastatic spread following splenectomy and adjuvant therapy; a review of the relevant literature is also provided to discuss the diagnostic and therapeutic challenges associated with this condition.
Case presentation: A 52-year-old male patient presented with acute abdominal pain and hypovolemic shock. Imaging revealed splenic rupture with hemoperitoneum. An emergency splenectomy was performed, and histopathological examination confirmed the diagnosis of angiosarcoma. The patient received postoperative chemotherapy (liposomal paclitaxel) and subsequent targeted therapy (sunitinib). However, rapid hepatic metastasis occurred, leading to spontaneous hepatic rupture and death 6 months after the initial diagnosis.
Conclusion: The condition poses a diagnostic and therapeutic challenge due to its nonspecific presentation and high aggressiveness. Early splenectomy remains the prevailing standard of care. This case demonstrates the potential inefficacy of sunitinib against PSA-derived hepatic metastases and emphasizes the critical importance of early diagnosis and intervention before splenic rupture occurs. The potential for combination therapies, including immunotherapy, to represent future investigative avenues is a promising area for future research.
Introduction
Primary splenic angiosarcoma (PSA) is an exceptionally rare and aggressive malignant vascular tumor that originates from splenic vascular endothelial cells, particularly those of mesenchymal origin within the splenic sinus network. Its estimated incidence ranges from 0.14 to 0.23 cases per million, with the condition predominantly affecting individuals between the ages of 50 and 79, exhibiting a reported male-to-female ratio of approximately 4:3 (1, 2). Since its initial description by Langhans in 1879, the documented cases in English literature have numbered fewer than 300 (3–5).
This study explores the clinical characteristics of PSA, emphasizing its highly aggressive behavior and frequent diagnosis at advanced stages. We highlight the necessity for enhanced clinical awareness and the development of improved management strategies. Existing literature consists largely of isolated case reports, and there is a paucity of comprehensive clinical guidelines. This case report describes a patient with PSA who presented with spontaneous splenic rupture. Despite undergoing 4.5 cycles of paclitaxel-based chemotherapy and 1 month of sunitinib targeted therapy, the patient ultimately succumbed to acute liver rupture resulting from rapidly progressive metastatic dissemination. The rapid development of hepatic metastasis after splenic rupture, coupled with the lack of response to sunitinib, offers critical insights into the aggressive behavior of PSA and underscores the need for more effective treatment approaches.
Case report
Initial presentation and emergency intervention (August 2023)
A 52-year-old man was admitted on 8 August 2023, with acute left-sided abdominal pain and syncope without any obvious triggers 7 h earlier. The patient presented with hypotensive shock and was managed with blood transfusions and fluid resuscitation. Computed tomography (CT) imaging revealed multiple splenic lesions, substantial abdominal and pelvic blood accumulation, and multiple cysts in the liver (Figure 1). The results of the laboratory tests indicated the presence of severe hemorrhage (Hb: 90 g/L) and critical thrombocytopenia (PLT: 23×109/L). Concurrently, the serum levels of the tumor markers CEA, CA125, CA153, CA199, and CA242 were all found to be within normal ranges. In view of the hemodynamic instability, which was indicative of ongoing hemorrhage, an emergency splenectomy was performed.
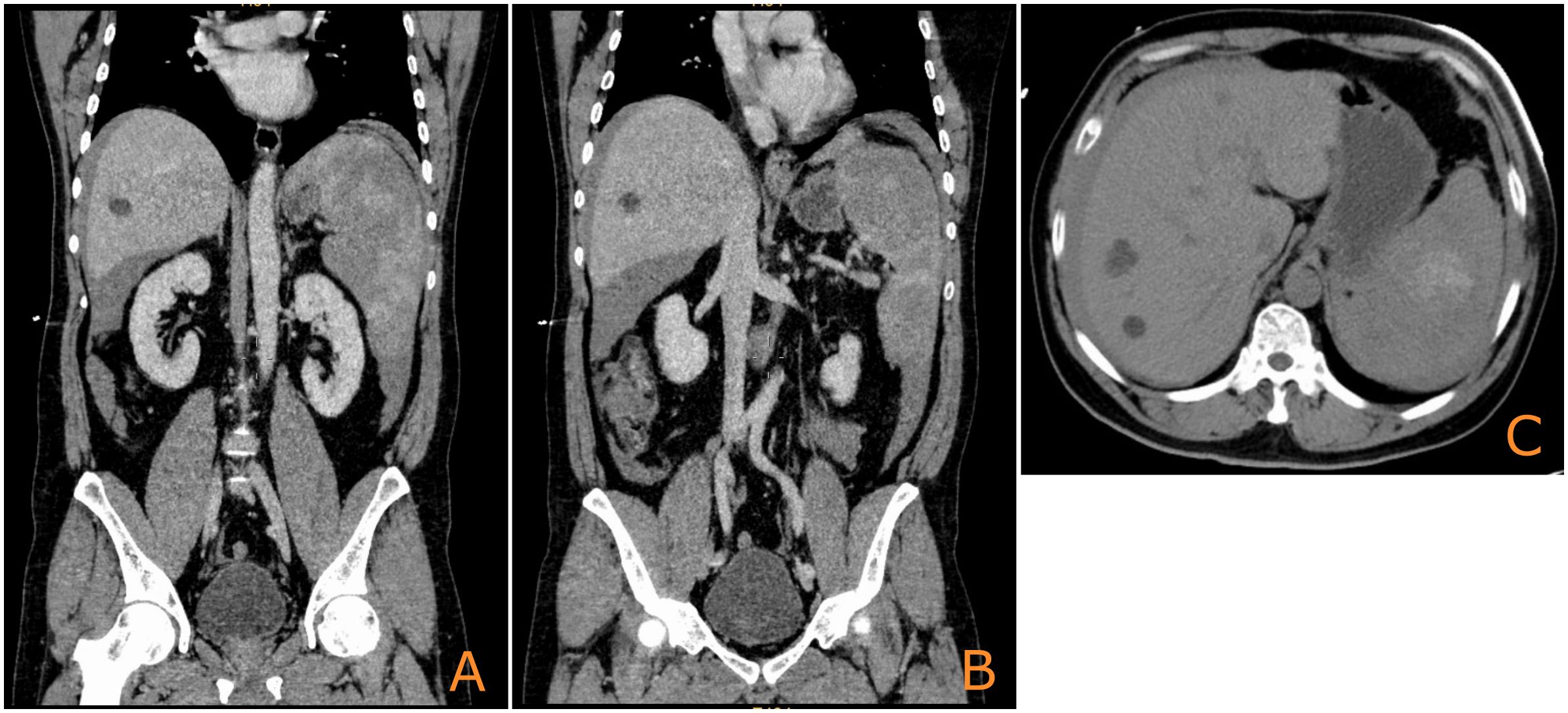
Figure 1. Initial CT image of the spleen upon admission showing multiple nodular occupations [(A, B): coronal plane; (C): axial plane].
Surgical findings and pathological diagnosis
The surgery revealed approximately 2,000 mL of hemoperitoneum. The spleen was observed to be enlarged, with a nodular, dark red-gray cut surface and two rupture sites. Its length was measured to be approximately 6 cm. A subsequent histopathological examination of the resected spleen revealed a malignant vascular tumor (Figure 2). Immunohistochemical analysis showed positive staining for CD31, CD34, and ERG, a high Ki-67 proliferation index (>60%), and retained INI-1 expression, supporting a diagnosis of angiosarcoma (Figure 3).
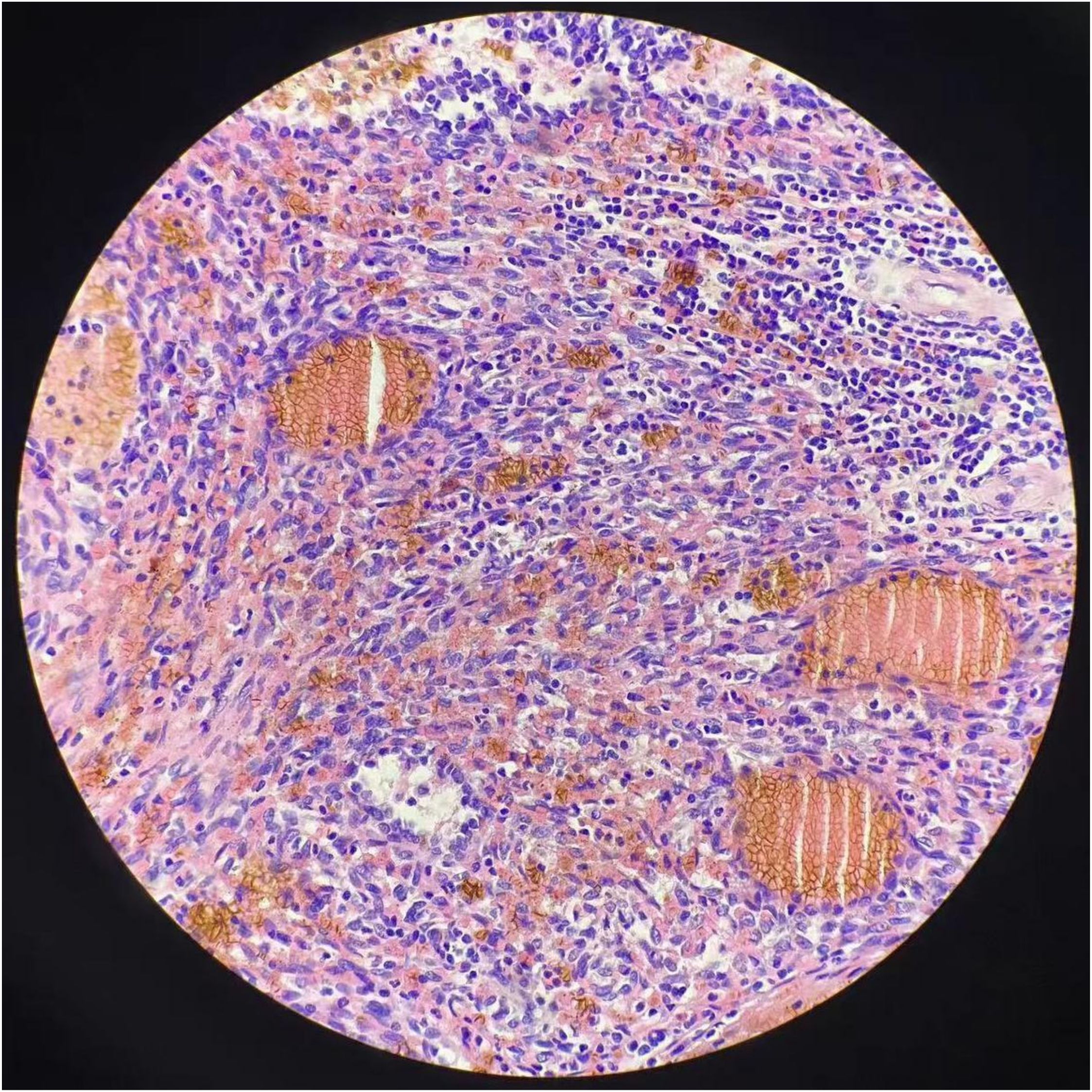
Figure 2. Hematoxylin and eosin (H&E) staining reveals regular nodules consisted of hyperplastic, dilated blood vessels with lumens of varying sizes, some with hemorrhage and necrosis.
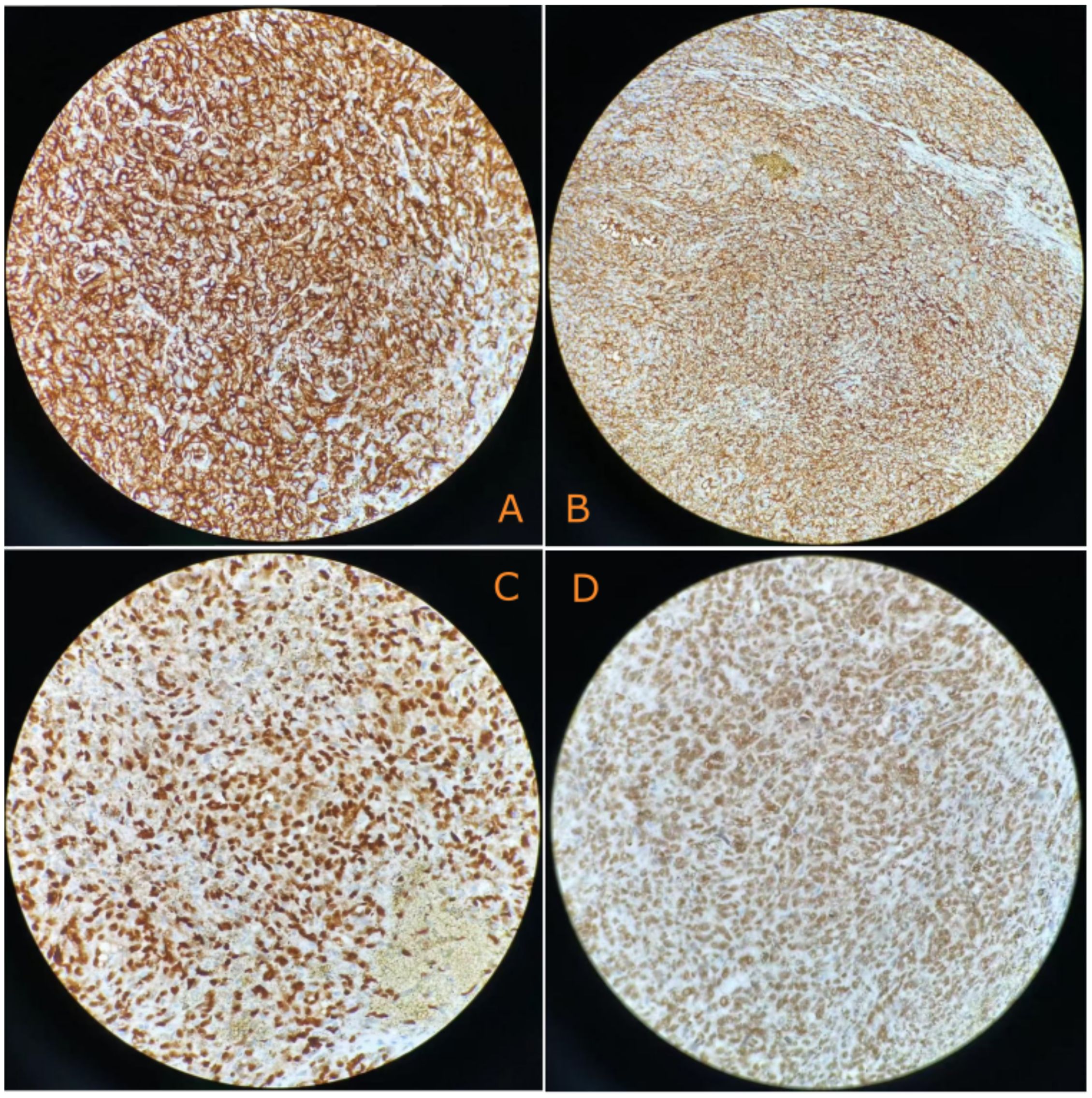
Figure 3. Immunohistochemical characterization reveals the following: (A) Tumor cells diffusely expressed CD31(+) (400×). (B) Tumor cells diffusely expressed CD34(+) (400×). (C) Tumor cells diffusely expressed EGR(+) (400×). (D) Tumor cells diffusely expressed INI-1(+) (400×).
Postoperative course and adjuvant therapy
The patient recovered well and was discharged after 10 days. Subsequently, he commenced adjuvant chemotherapy with liposomal paclitaxel (150 mg, d1, d8) in late September 2023 and was completing 3.5 cycles. A surveillance CT on 27 November 2023 showed no evidence of liver metastasis. Throughout the entire chemotherapy process, the patient’s platelet count remained normal. The patient reported mild fatigue but no other discomfort and therefore continued working.
Disease progression and outcome
A follow-up CT on 14 January 2024 revealed new multiple liver metastases. After reviewing the literature (6), the clinician initiated an experimental targeted therapy based on sunitinib (50 mg daily). Despite this, the patient presented with acute upper abdominal pain on 10 February 2024. CT confirmed hepatic rupture with hemorrhage. The patient opted for conservative management and succumbed shortly afterwards.
A timeline with relevant data
The patient’s clinical course, detailed in Table 1, was marked by the following: subsequent to the initial discovery of a substantial splenic mass, the patient did not accord it sufficient attention and did not undergo any subsequent follow-up examinations during the ensuing year. One year later, the patient was readmitted to hospital urgently due to spontaneous rupture of the spleen. Despite undergoing a splenectomy, followed by postoperative chemotherapy and targeted therapy, the patient’s survival was limited to a mere 6 months.
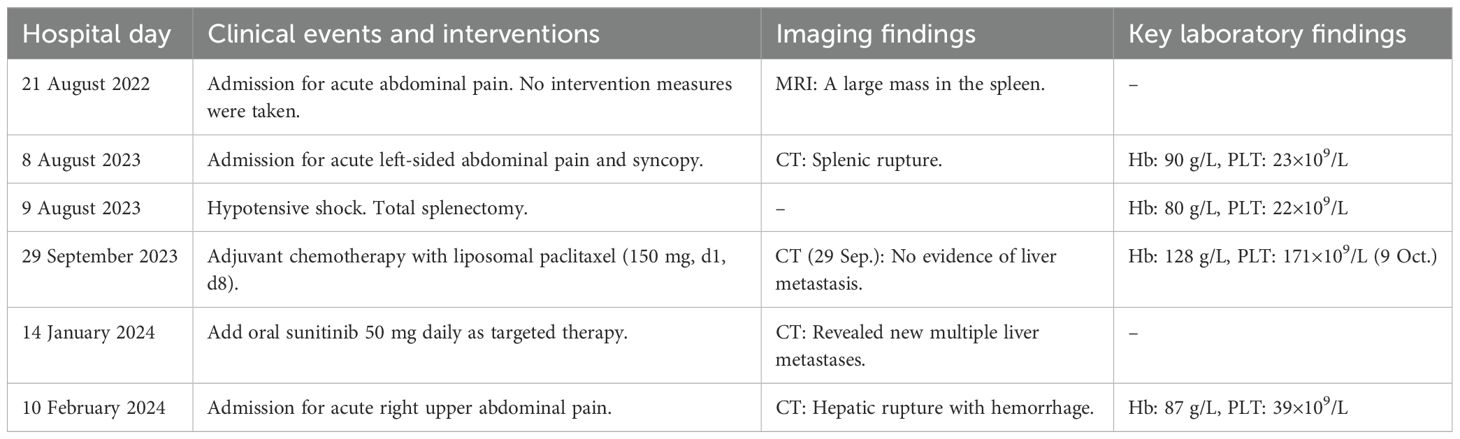
Table 1. Timeline of diagnosis, treatment, and supporting investigations for patients with liver metastases following PSA surgery.
Discussion
Diagnostic challenges and pathological features
PSA is notoriously difficult to diagnose preoperatively. The definitive diagnosis of PSA depends on biopsy or pathological examination. However, splenic biopsy is considered for use with caution owing to the high risks of procedure-induced rupture and metastasis, as well as its limited diagnostic utility (7). As seen in our case and others, spontaneous rupture is often the presenting symptom. Hemorrhage represents the primary laboratory abnormality in PSA, accompanied by other findings such as leukopenia, thrombocytopenia, and an elevated erythrocyte sedimentation rate (8). The definitive diagnosis relies on pathology and IHC, as demonstrated by the positive staining of vascular markers observed in our patient.
Treatment strategies and literature comparison
Splenectomy is the primary and potentially curative treatment, especially if performed prior to rupture or metastasis (9). The role of adjuvant therapy remains poorly defined, as most chemotherapeutic regimens are empirically derived and lack support from large-scale clinical evidence. According to reports, treatment regimens based on paclitaxel or anthracycline chemotherapy are commonly used (10, 11). A retrospective analysis of 28 patients with soft tissue sarcoma demonstrated a clinical benefit rate of 50% in evaluable patients receiving at least four cycles of nivolumab combined with pazopanib (12). In a multicenter phase II trial, sorafenib exhibited activity in metastatic angiosarcoma, with tumor recurrence following treatment cessation—a pattern consistent with other targeted therapies in oncology (13). Another open-label, multicenter phase II study indicated that bevacizumab has promising efficacy in angiosarcoma, achieving stable disease in 11 patients with a favorable safety profile (14). In 2022, Pan et al. reported a case of hepatic metastasis from PSA that was managed with splenectomy followed by adjuvant therapy combining sorafenib and camrelizumab, resulting in no recurrence or metastasis after 15 months of follow-up (15). These reports suggest potential for targeted and immunotherapy combinations.
Efficacy of sunitinib and aggressive tumor behavior
Our case contributes to the limited experience with sunitinib in PSA. The rapid disease progression culminating in hepatic rupture soon after starting sunitinib suggests either innate or rapidly acquired resistance. This resistance is likely multifactorial in nature, primarily driven by the tumor’s exceptionally high proliferative capacity, as evidenced by the Ki-67 index exceeding 60% (8). Such an immense proliferative drive may simply overwhelm the primarily cytostatic effect of a single-agent tyrosine kinase inhibitor (TKI). Moreover, highly aggressive angiosarcomas exhibit considerable genomic plasticity, allowing them to activate alternative signaling pathways (e.g., MET, AXL, and MAS) (13, 16), thereby bypassing VEGFR inhibition by sunitinib. Concurrently, sunitinib may have inadvertently exacerbated the structural abnormality and fragility of the tumor vasculature within the metastases, promoting a hypoxic, acidic, and pro-invasive tumor microenvironment (17, 18).
Lessons on early intervention
Tumor rupture leads to the dissemination of a large number of highly invasive and proliferative tumor cells into the peritoneal cavity. Once infiltrated by substantial tumor cells, the peritoneal cavity becomes a reservoir for subsequent implantation and growth of tumors, particularly in the highly vascularized liver. This offers a plausible explanation for the rapid and extensive liver metastases observed shortly after surgery. Although splenectomy is a cornerstone of radical therapy for PSA, its therapeutic efficacy is significantly compromised when performed under emergency conditions caused by tumor rupture.
Analysis of relevant cases (Table 2) indicated that patients with PSA with liver metastasis who underwent splenectomy prior to rupture exhibited improved survival, with the longest observed survival reaching 48 months. This case highlights a significant missed opportunity for early intervention. One year ago (August 2022), a magnetic resonance imaging (MRI) scan performed for abdominal pain revealed a large mass in the spleen (Figure 4). The patient declined further investigation, thereby missing the chance for early intervention. Had a splenectomy been pursued at that time, the outcome might have been more favorable.
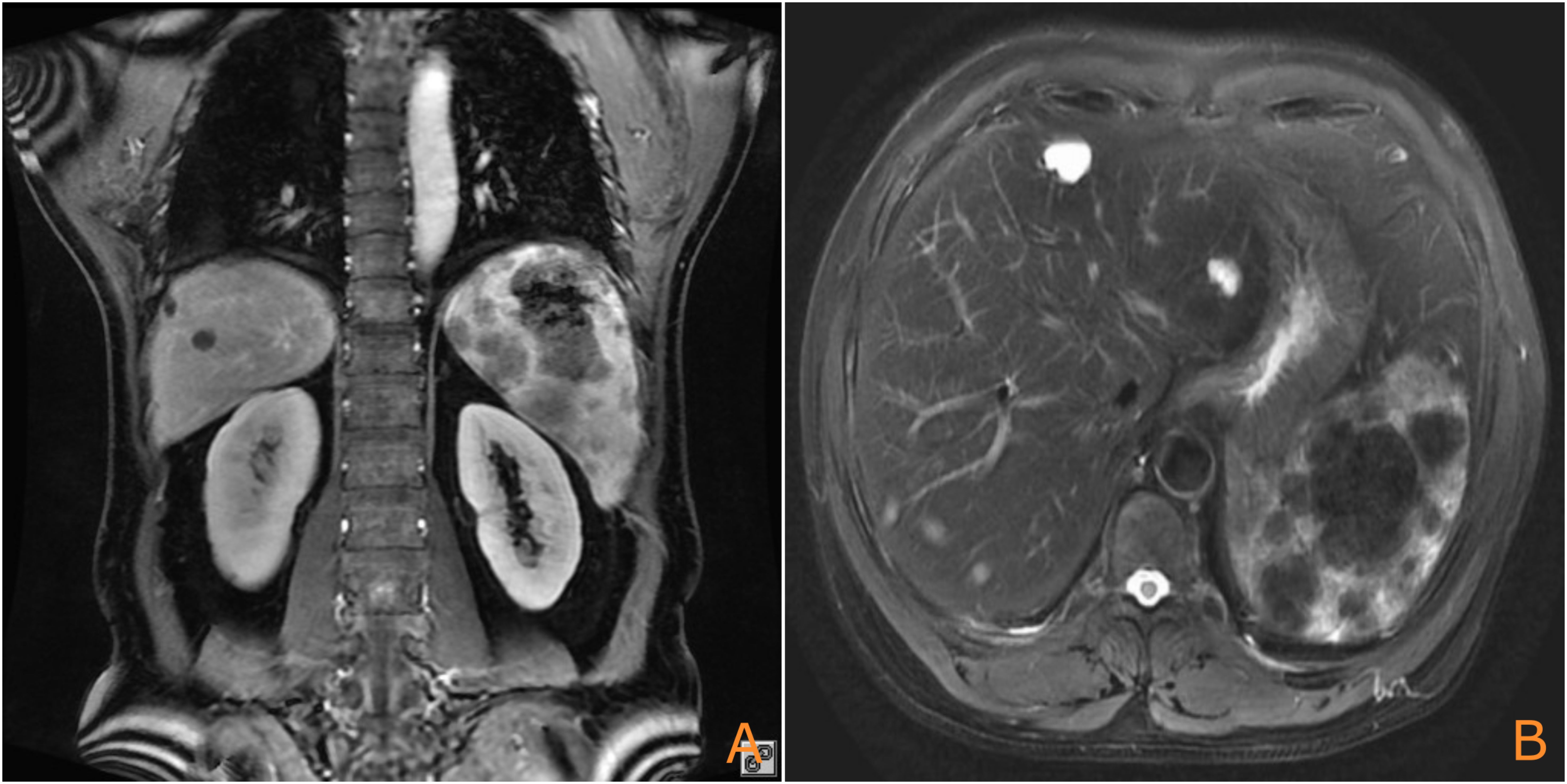
Figure 4. Prior MRI (August 2022) showing the splenic mass (arrow), which was not intervened upon [(A): coronal plane; (B): axial plane].
Conclusion
In conclusion, PSA is a highly aggressive tumor where early diagnosis and splenectomy offer the best chance for survival. Imaging is a crucial diagnostic tool, but pathology is definitive. For suspected splenic masses, initial contrast-enhanced MRI is recommended to inform the subsequent consideration of prophylactic splenectomy. This case illustrates that even with surgery and standard chemotherapy, the disease may still follow a fulminant course. The failure of sunitinib in this context indicates a need for more effective systemic therapies. Future efforts should focus on molecular profiling of PSA to identify actionable targets and explore novel combination regimens, including immunotherapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Jiangyin People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
ZW: Writing – original draft, Writing – review & editing. XC: Writing – review & editing, Supervision. YYZ: Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Jiangyin Municipal Health Commission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li R, Li M, Zhang LF, Liu XM, Hu TZ, Xia XJ, et al. Clinical characteristics and prognostic factors of primary splenic angiosarcoma: A retrospective clinical analysis from China. Cell Physiol Biochem. (2018) 49:1959–69. doi: 10.1159/000493656
2. Cao L, Hong J, Wang Y, Yu J, Ma R, Li J, et al. A primary splenic angiosarcoma hepatic metastasis after splenectomy and its genomic alteration profile. Med (Baltimore). (2019) 98:e16245. doi: 10.1097/MD.0000000000016245
3. Falk S, Krishnan J, and Meis JM. Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol. (1993) 17:959–70. doi: 10.1097/00000478-199310000-00001
4. Kohutek F, Badik L, and Bystricky B. Primary angiosarcoma of the spleen: rare diagnosis with atypical clinical course. Case Rep Oncol Med. (2016) 2016:4905726. doi: 10.1155/2016/4905726
5. Juin Hsien BL and Shelat VG. Spleen angiosarcoma: a world review. Expert Rev Gastroenterol Hepatol. (2021) 15:1115–41. doi: 10.1080/17474124.2021.1945920
6. Deshpande A, Munoz J, Kelemen K, Dabak V, Hanbali A, and Kurzrock R. Images in immunotherapy and precision oncology: angiosarcoma of the spleen and liver. J Immunother Precis Oncol. (2023) 6:56–8. doi: 10.36401/JIPO-22-22
7. Fiorentino MD, Monteiro JMC, de Siqueira REB, Kim EIM, Curi AP, Ferrreira CR, et al. Primary splenic angiosarcoma: a rare entity often associated with rupture and hemoperitoneum. Autops Case Rep. (2019) 9:e2019100. doi: 10.4322/acr.2019.100
8. Chen X, Li H, Wang F, and Liu H. Early detection and integral resection are keys to extend survival in patients suffered from primary angiosarcoma of the spleen: A care-compliant case report and literature review. Med (Baltimore). (2018) 97:e9718. doi: 10.1097/MD.0000000000009718
9. Duan YF, Jiang Y, Wu CX, and Zhu F. Spontaneous rupture of primary splenic angiosarcoma: a case report and literature review. World J Surg Oncol. (2013) 11:53. doi: 10.1186/1477-7819-11-53
10. Vakkalanka B and Milhem M. Paclitaxel as neoadjuvant therapy for high grade angiosarcoma of the spleen: a brief report and literature review. Clin Med Insights Oncol. (2010) 4:107–10. doi: 10.4137/CMO.S5329
11. de Azevedo OS, do Nascimento Santos B, de Souza Liboni N, da Costa JF, and de Campos OD. Splenic angiosarcoma: A diagnostic splenectomy finding. Case Rep Oncol. (2016) 9:733–7. doi: 10.1159/000452619
12. Paoluzzi L, Cacavio A, Ghesani M, Karambelkar A, Rapkiewicz A, Weber J, et al. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. (2016) 6:24. doi: 10.1186/s13569-016-0064-0
13. Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. (2009) 27:3133–40. doi: 10.1200/JCO.2008.20.4495
14. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. (2013) 24:257–63. doi: 10.1093/annonc/mds237
15. Pan D, Li TP, Xiong JH, Wang SB, Chen YX, Li JF, et al. Treatment with sorafenib plus camrelizumab after splenectomy for primary splenic angiosarcoma with liver metastasis: A case report and literature review. World J Clin Cases. (2022) 10:2818–28. doi: 10.12998/wjcc.v10.i9.2818
16. Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. (2016) 35:2687–97. doi: 10.1038/onc.2015.343
17. Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U.S.A. (2012) 109:17561–6. doi: 10.1073/pnas.1215397109
18. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, and Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29
19. Garg A, Pena L, Lauwers GY, Druta M, and Kis B. Radioembolization of primary splenic angiosarcoma complicated by delayed gastric hemorrhage. J Vasc Interventional Radiol. (2025) 36:187–9.e1. doi: 10.1016/j.jvir.2024.09.014
20. Shao M, Qi W, Xu R, Luo Z, Liao F, and Fan S. Application value of (18)F-FDG PET/CT in primary spleen angiosarcoma with liver metastasis: a case report and literature review. Front Oncol. (2024) 14:1366560. doi: 10.3389/fonc.2024.1366560
21. Xu W, Wang K, Gu W, Nie X, Zhang H, Tang C, et al. Case report: complete remission with anti-PD-1 and anti-VEGF combined therapy of a patient with metastatic primary splenic angiosarcoma. Front Oncol. (2022) 12:809068. doi: 10.3389/fonc.2022.809068
22. Takehara M, Miyamoto H, Fujino Y, Tomonari T, Taniguchi T, Kitamura S, et al. Long-Term Survival due to Chemotherapy including Paclitaxel in a Patient with Metastatic Primary Splenic Angiosarcoma. Case Rep Gastroenterol. (2021) 15:910–8. doi: 10.1159/000519211
23. Ozcan B, Cevener M, Kargi AO, Dikici H, Yildiz A, Ozdogan M, et al. Primary splenic angiosarcoma diagnosed after splenectomy for spontaneous rupture. Turk J Surg. (2018) 34:68–70. doi: 10.5152/turkjsurg.2017.3207
24. Cho EA, Choi WY, Kim SH, Hong JY, Jung SH, Kim MJ, et al. Rapidly progressing primary splenic angiosarcoma with fatal hemorrhagic event. J Chemother. (2014) 26:248–52. doi: 10.1179/1973947813Y.0000000146
25. Ferreira BP, Rodler ET, Loggers ET, Pollack SM, and Jones RL. Systemic therapy in primary angiosarcoma of the spleen. Rare Tumors. (2012) 4:e55. doi: 10.4081/rt.2012.e55
Keywords: primary splenic angiosarcoma, liver metastasis, splenic rupture, liver rupture, case report, sunitinib
Citation: Wu Z, Cao X, Zuo Y and Zheng Y (2025) Spontaneous hepatic rupture: A catastrophic complication in a patient with primary splenic angiosarcoma and hepatic metastasis: case report and literature review. Front. Oncol. 15:1708613. doi: 10.3389/fonc.2025.1708613
Received: 19 September 2025; Accepted: 31 October 2025;
Published: 18 November 2025.
Edited by:
Ulrich Ronellenfitsch, Medical Faculty of the Martin-Luther-University Halle-Wittenberg, GermanyReviewed by:
Talar Telvizian, Lankenau Medical Center, United StatesLeizhou Xia, Nanjing Drum Tower Hospital, China
Copyright © 2025 Wu, Cao, Zuo and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhidan Wu, d3V6aGlkYW4uY2hpbmFAZ21haWwuY29t
 Zhidan Wu
Zhidan Wu Xiangming Cao2
Xiangming Cao2