Abstract
Background:
The relationship between autoantibody profiles and immune checkpoint inhibitor (ICI) therapy in cancer patients remains incompletely characterized. This cross-sectional study investigated serum autoantibody (AAb) prevalence and profiles across multiple tumor types before and after ICI therapy.
Methods:
This study analyzed serum autoantibodies in 808 participants: 358 treatment-naïve cancer patients (ICI- group), 250 cancer patients following ≥6 months of ICI therapy (ICI+ group), and 200 healthy controls (HC). The cancer cohort comprised 10 solid tumor types. Serum samples were analyzed for antinuclear antibodies (ANA), antiphospholipid antibodies (aPL), anti-neutrophil cytoplasmic antibodies (ANCA), and anti-thyroid antibodies (ATA) using automated quantitative immunoassays.
Results:
Cancer patients demonstrated significantly elevated AAb prevalence compared to healthy controls. The ICI− group showed positivity rates of 20.1% for ANA, 11.5% for aPL, 1.7% for ANCA, and 17.3% for ATA, compared to 10.5%, 4.5%, 1.5%, and 9.5% in healthy controls, respectively. After ICI therapy, ANA positivity increased to 33.6%. CTLA-4 inhibitor recipients demonstrated higher ANA frequencies than PD-1/PD-L1 monotherapy recipients (57.1% vs 27.1%),. Tumor stage did not significantly influence AAb prevalence. Colorectal, hepatocellular, and renal cancers showed significant ANA increases after ICI treatment. Anti-Scl-70, anti-SSA-60, and anti-RNP were the most frequently elevated ANA subtypes. Anti-thyroglobulin was the most responsive ATA subtype following ICI therapy.
Conclusions:
ANA profiles vary across tumor types and differ between treatment-naïve and ICI-treated patients. CTLA-4-treated patients exhibit higher ANA frequency. Different tumors exhibit distinct preferences for AAb expression patterns. Serum AAb profiling may serve as a valuable tool for immunotherapy monitoring and risk stratification for immune-related adverse events.
1 Introduction
Serum autoantibodies (AAb) are pivotal diagnostic markers for autoimmune disorders. Over recent decades, the complex relationship between autoimmunity and malignancy has gained attention, as cancer patients frequently develop humoral immune responses against self-antigens (1, 2). Studies demonstrate significantly elevated AAb levels, particularly antinuclear antibodies (ANA), in cancer patients, although the underlying mechanisms remain incompletely understood (3–5).
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy and are now guideline-recommended first-line treatments for multiple malignancies (6, 7). ICIs are a class of anticancer drugs that work by releasing the “brakes” on the immune system through interaction with co-inhibitory and co-stimulatory molecules to regulate T cell activation. PD-1/PD-L1 blockade primarily affects peripheral tolerance, while CTLA-4 inhibition disrupts T-cell priming in lymph nodes (8–10). However, this immune activation can trigger immune-related adverse events (irAEs) through nonspecific immune system stimulation affecting virtually all organ systems. Previous studies suggested that AAb may serve as prognostic factors for non-small-cell lung cancer patients initiating immuno-therapy (11), with AAb occurrence associated with favorable prognosis and irAE prediction in certain cancers (12–15).
Despite these observations, the specific roles of different AAb subtypes in tumor development, progression, and immunotherapy response across various cancer types remain poorly defined. Available data on baseline AAb prevalence and immunotherapy-associated changes are incomplete or sometimes contradictory (12, 16, 17). While indirect immunofluorescence assay (IFA) using HEp-2 cells remains the American College of Rheumatology-recommended method for ANA screening, the interpretation of semi-quantitative AAb results is often complicated by interobserver variability and different cutoff values in clinical applications (18). Systematic research on baseline and post-immunotherapy AAb prevalence across multiple tumor types is lacking.
This cross-sectional study aimed to comprehensively evaluate AAb types and positivity rates in patients with different solid tumors by comparing treatment-naïve patients with those following immunotherapy using automated quantitative methods. Additionally, we explored potential associations between AAb profiles and clinical-pathological characteristics to provide essential data for efficacy monitoring and irAEs risk stratification in cancer immunotherapy.
2 Materials and methods
2.1 Study design and participants
This cross-sectional study examined AAb types and levels in healthy individuals, treatment-naïve cancer patients, and cancer patients following at least 6 months of immunotherapy. The study was conducted from March to April 2023 at Peking University Cancer Hospital. The control population comprised 200 healthy individuals (91 males, 109 females; median age 49 years, range 23-71) undergoing routine physical examinations without evidence of malignancy or autoimmune disease. The treatment-naïve cohort included 358 hospitalized cancer patients (160 males, 198 females; age ranges 18–88 and 16–82 years, respectively) without prior therapy. The post-immunotherapy cohort comprised 250 hospitalized cancer patients who had received ICI therapy for at least 6 months or completed a minimum of 6 treatment cycles, whichever occurred first. This dual criterion accommodates different dosing schedules. The cancer types included: gastric, colorectal, esophageal, hepatocellular, lung, breast, cervical, renal, lymphoma, and melanoma. Inclusion criteria were: (1) histologically confirmed cancer diagnosis; (2) for the ICI+ group, completion of at least 6 months or 6 cycles of ICI therapy. Exclusion criteria included: (1) pre-existing autoimmune diseases or conditions affecting AAb levels; (2) pregnancy, HIV positivity, systemic infection, or blood transfusion within 60 days. Due to the cross-sectional design, pre- and post-immunotherapy cohorts were independent groups sampled contemporaneously rather than matched pairs.
All patients’ sera were examined for antinuclear antibodies (ANA), antiphospholipid antibodies (aPL), anti-neutrophil cytoplasmic antibodies (ANCA), and anti-thyroid antibodies (ATA). Sera were collected from the Department of Clinical Laboratory (Peking University Cancer Hospital) with informed consent. Blood samples were collected in serum tubes from antecubital veins and frozen at −20 °C before use. Sera underwent no more than two freeze-thaw cycles.
Clinical and laboratory data were retrospectively collected from electronic medical records, including age, sex, underlying conditions, histological type, and TNM classification.
2.2 Serum autoantibodies analysis
Sixteen ANA subtypes were measured using a Luminex-based multiplex assay (Tesmi-F4000; Tellgen®, Shanghai, China): anti-SSB, SSA, Ro-52(SSA-52), Sm, Scl-70, RNP, Ribosomal P, PM/Scl, PCNA, Nucleosome, AMA-M2, Jo-1, Histone, dsDNA, Centromere B, and C1q. Antigens were covalently cross-linked to fluorescence-coded microspheres. Goat anti-human IgG antibody-labeled phycoerythrin was added, then microspheres were then resuspended in sheath fluid. The manufacturer’s recommended cut-off for positivity was >20 arbitrary units (AU) for all antibodies except anti-dsDNA (>20 IU/mL) and C1q (>10 U/mL).
IgG, IgM, and IgA antibodies against cardiolipin (CL) and β2-glycoprotein I were measured using fully automated chemiluminescence immunoassay (iFlash 3000, YHLO Biotech, Shenzhen, China). Anti-myeloperoxidase (MPO IgG), anti-proteinase 3 (PR3 IgG), and anti-glomerular basement membrane (GBM IgG) were measured using the same platform with manufacturer-recommended cutoffs.
Antithyroid antibodies (ATA), including Anti-TSH receptor (A-TSHR), anti-thyroglobulin (A-TG), and anti-thyroid peroxidase (A-TPO), were measured using electrochemiluminescence immunoassay (Elecsys and Cobas e 602 analyzers, Roche Diagnostics GmbH, Germany). Positivity thresholds were: A-TSHR >1.22 IU/L, A-TPO >34 IU/mL, and A-TG >115 IU/mL. All AAb were measured at the clinical laboratory of medicine in Peking University Cancer Hospital.
All autoantibody assays underwent rigorous quality control procedures. For the Luminex-based multiplex ANA panel, we analyzed quality control materials provided by the manufacturer at the beginning of each testing batch. For chemiluminescence assays (aPL and ANCA), we used commercial quality control materials with established target ranges. Inter-assay CV was <5% for all analytes. For anti-thyroid antibodies measured by electrochemiluminescence, quality control samples were tested daily, with acceptance criteria of ±8% of target values. All quality control results were within acceptable ranges throughout the study period.
2.3 Statistical analysis
Logistic regression analysis was performed to adjust for age, sex, and clinical tumor staging. Qualitative variables were expressed as numbers and percentages. Quantitative variables were presented as means (± standard deviation) for normal distributions or medians (interquartile ranges) for non-normal distributions. Group comparisons utilized Student’s t-test or Mann-Whitney U test as appropriate. Frequencies were compared using Fisher’s exact test or Chi-square test. To account for multiple comparisons, we applied Bonferroni correction in comparing four AAb categories (ANA, aPL, ANCA, ATA) between three groups (HC, ICI-, ICI+). Statistical analyses were performed using SPSS software (version 26.0, IBM Corp., Armonk, NY, USA), with figures created using GraphPad Prism (version 9.0, La Jolla, CA, USA). Two-tailed P values <0.05 were considered statistically significant.
3 Results
3.1 Patient characteristics
We enrolled 358 treatment-naïve cancer patients (ICI- patients) and 250 cancer patients who had received at least 6 months of ICI therapy (ICI+ patients). Additionally, we collected serum samples from 200 healthy individuals undergoing routine physical examinations during the same period as controls. The two cancer patient groups showed no significant differences in gender distribution or age (P>0.05). The cancer types and their respective frequencies in both patient groups are presented in Table 1.
Table 1
| Characteristics | ICI- patients | ICI+ patients | P value |
|---|---|---|---|
| Males/Females | 160/198 | 101/149 | 0.293 |
| Age (yr)* | 58.4 ± 12.9 (16-88) | 60.2 ± 11.6 (18-89) | 0.119 |
| Cancer involving organs | Number of patients (%) | ||
| Stomach | 50 (14.0) | 33 (13.2) | |
| Colorectal | 50 (14.0) | 31 (12.4) | |
| Esophageal | 30 (8.4) | 22 (8.8) | |
| Liver | 30 (8.4) | 19 (7.6) | |
| Lung | 50 (14.0) | 31 (12.4) | |
| Breast | 23 (6.4) | 14 (5.6) | |
| Cervic | 19 (5.3) | 18 (7.2) | |
| Kidney | 28 (7.8) | 13 (5.2) | |
| Lymphoma | 38 (10.6) | 32 (12.8) | |
| Melanoma | 40 (11.2) | 37 (14.8) | |
| Cancer stages | |||
| Early stage (I/II) | 104 (29.0) | 18 (7.2) | <0.001 |
| Advanced | 233 (65.1) | 220 (88) | |
| Unknown | 21 (5.9) | 12 (4.8) | |
Clinical and laboratory characteristics of the 358 cancer patients (ICI-) and 250 cancer patients after immunotherapy (ICI+).
*mean ± s.d. (range).
3.2 Relationship between AAb prevalence, tumor stage, and ICI type
We investigated the AAb profiles in patients with tumors at different stages receiving ICIs. As shown in Table 2, although no statistical significance was observed, patients with advanced-stage tumors (stages III/IV) exhibited increased ANA positivity rates (34.1%) and slightly decreased ANCA positivity rates (2.7%) compared to those with early-stage tumors (stages I/II), while aPL and ATA showed no notable changes. These findings suggest that tumor stage may not critically influence AAb formation.
Table 2
| AAb types | I/II (n = 18) | III/IV (n = 220) | P value |
|---|---|---|---|
| ANA | 4 (22.2) | 75 (34.1) | 0.304 |
| aPL | 2 (11.1) | 22 (10.0) | 1.0 |
| ANCA | 1 (5.6) | 6 (2.7) | 0.428 |
| ATA | 4 (22.2) | 47 (21.4) | 1.0 |
The prevalence of autoantibodies in immunotherapy patients across different cancer stages n (%).
We examined AAb profiles according to ICI types (Table 3). ANA positivity was significantly higher in patients receiving CTLA-4 monotherapy (57.1%) or combination therapy (51.4%) compared to PD-1/PD-L1 monotherapy (27.6%; P = 0.005 and P = 0.002, respectively). No significant differences were observed in aPL, ANCA, or ATA positivity across treatment groups.
Table 3
| AAb types | CTLA-4 (n = 21) | PD-1/PD-L1+CTLA4 (n = 37) | PD-1/PD-L1 (n = 192) | ||
|---|---|---|---|---|---|
| Pos. | P value | Pos. | P value | Pos. | |
| ANA | 12 (57.1) | 0.005 | 20 (54.1) | 0.002 | 52 (27.1) |
| aPL | 2 (9.5) | 1.0 | 3 (8.1) | 1.0 | 21 (10.9) |
| ANCA | 0 (0) | 1.0 | 1 (2.7) | 1.0 | 5 (2.6) |
| ATA | 5 (23.8) | 0.795 | 8 (21.6) | 0.971 | 40 (20.8) |
The prevalence of autoantibodies in cancer patients treated with different immunotherapy drugs n (%).
P < 0.05, CTLA-4 vs PD-1/PD-L1, and PD-1/PD-L1+CTLA4 vs PD-1/PD-L1, respectively.
3.3 AAb Prevalence in ICI−, ICI+, and control groups
In healthy controls (HC), baseline positivity rates were 10.5% for ANA, 4.5% for aPL, 1.5% for ANCA, and 9.5% for ATA, which are comparable to published data and confirm that our detection system accurately assessed serum AAb (Table 4) (19–22). Treatment-naïve cancer patients showed significantly elevated rates: 20.1% for ANA (P = 0.003), 11.5% for aPL (P = 0.006), 1.7% for ANCA (P = 1.0), and 17.3% for ATA (P = 0.012), suggesting tumor-associated B-cell activation or autoimmune dysregulation. In ICI+ group, ANA positivity increased to 33.6% (P < 0.001 vs ICI- group), while aPL, ANCA, and ATA showed no significant changes. Among ANA subtypes, the five most prevalent in cancer patients were anti-SSA-60, anti-Scl-70, anti-RNP, anti-Nucleosome, and anti-AMA-M2. Anti-Scl-70 showed significant post-immunotherapy elevation (8.8% vs 4.5%, P = 0.03).
Table 4
| AAb types | ICI- (n = 358) | ICI+ (n = 250) | HC (n = 200) | ||
|---|---|---|---|---|---|
| Pos. | P value | Pos. | P value | Pos. | |
| ANAs | 72 (20.1) | 0.003 | 84 (33.6) | <0.001 | 21 (10.5) |
| SSB | 2 (0.6) | 0.539 | 4 (1.6) | 0.389 | 0 (0) |
| SSA-60 | 14 (3.9) | 0.048 | 18 (7.2) | 0.074 | 2 (1.0) |
| Ro-52 | 7 (2.0) | 0.955 | 4 (1.6) | 0.989 | 3 (1.5) |
| Sm | 0 (0) | 0.358 | 1 (0.4) | 0.411 | 1 (0.5) |
| Scl-70 | 16 (4.5) | 0.132 | 22 (8.8) | 0.03 | 4 (2) |
| RNP | 18 (5.0) | 0.036 | 16 (6.4) | 0.469 | 3 (1.5) |
| Ribosomal P | 3 (0.8) | 0.487 | 3 (1.2) | 0.978 | 0 (0) |
| PM/Scl | 3 (0.8) | 0.487 | 7 (2.8) | 0.122 | 0 (0) |
| PCNA | 0 (0) | — | 0 (0) | — | 0 (0) |
| Nucleosome | 12 (3.4) | 0.359 | 13 (5.2) | 0.259 | 4 (2.0) |
| AMA-M2 | 11 (3.1) | 0.453 | 8 (3.2) | 0.929 | 4 (2.0) |
| Jo-1 | 1 (0.3) | 1.0 | 2 (0.8) | 0.754 | 0 (0) |
| Histone | 0 (0) | 0.011 | 2 (0.8) | 0.169 | 5 (2.5) |
| dsDNA Centromere B |
4 (1.1) 2 (0.6) |
1.0 0.539 |
2 (0.8) 1 (0.4) |
1.0 1.0 |
2 (1.0) 0 (0) |
| C1q | 5 (1.4) | 0.096 | 3 (1.2) | 1.0 | 8 (4.0) |
| aPL | 41 (11.5) | 0.006 | 26 (10.4) | 0.683 | 9 (4.5) |
| CL IgG | 3 (0.8) | 1.0 | 2 (0.8) | 1.0 | 2 (1.0) |
| CL IgM | 24 (6.7) | 0.032 | 15 (6.0) | 0.727 | 5 (2.5) |
| CL IgA | 1 (0.3) | 1.0 | 0 (0) | 1.0 | 0 (0) |
| β2-GPI IgG | 0 (0) | — | 1 (0.4) | 0.411 | 0 (0) |
| β2-GPI IgM | 38 (10.6) | 0.003 | 18 (7.2) | 0.152 | 7 (3.5) |
| β2-GPI IgA | 0 (0) | — | 0 (0) | — | 0 (0) |
| ANCA | 6 (1.7) | 1.0 | 6 (2.4) | 0.737 | 3 (1.5) |
| MPO IgG | 4 (1.1) | 1.0 | 3 (1.2) | 1.0 | 2 (1.0) |
| PR3 IgG | 1 (0.3) | 1.0 | 2 (0.8) | 0.754 | 1 (0.5) |
| GBM IgG | 1 (0.3) | 1.0 | 2 (0.8) | 0.754 | 0 (0) |
| ATA | 62 (17.3) | 0.012 | 53 (21.2) | 0.229 | 19 (9.5) |
| A-TG | 32 (8.9) | 0.425 | 36 (14.4) | 0.036 | 14 (7.0) |
| A-TPO | 28 (7.8) | 0.131 | 20 (8.0) | 0.936 | 9 (4.5) |
| A-TSHR | 16 (4.5) | 0.026 | 14 (5.6) | 0.526 | 2 (1.0) |
Prevalence of the autoantibodies in cancer patients without any treatment (ICI-), cancer patients after at least 6-month immunotherapy (ICI+), and healthy controls (HC) n (%).
P < 0.05, ICI-vs HC, and ICI+ vs ICI-, respectively.
— too few case numbers to calculate.
Bold values indicated the total positive rates and P values of the four types of AAb.
Within the aPL profile, CL IgM (6.7%) and β2-GPI IgM (10.6%) showed the highest positivity rates in ICI- patients, significantly exceeding healthy controls (P = 0.032 and P = 0.003, respectively). Only one patient tested positive for CL IgA, while all β2-GPI IgA tests were negative. No significant differences in aPL profiles were observed between ICI- and ICI+ groups.
ANCA positivity in ICI− patients was low (1.7%), showing no statistical difference from controls or ICI+ patients. ATA positivity reached 17.3% in ICI− patients, higher than controls (9.5%, P = 0.012). Total ATA positivity showed an upward trend after immunotherapy (21.2%), with only A-TG reaching statistical significance (14.4%, P = 0.036), while A-TPO and A-TSHR remained unchanged.
3.4 Prevalence of AAb in patients with different types of cancers
We investigated whether different tumor types exhibit distinct antibody distribution patterns by comparing AAb prevalence between ICI− patients and healthy volunteers. Nearly all tumor types showed increased AAb generation. Patients with melanoma, hepatocellular carcinoma, gastric cancer, esophageal cancer, and lymphoma demonstrated significantly higher ANA positivity (P < 0.05). Notably, lymphoma exhibited an ANA positivity rate of 36.8%, substantially higher than other tumors, suggesting increased lymphocyte antigen epitope activation or autoimmune phenomena (Figure 1A). For aPL, lymphoma (15.8%), colorectal cancer (18%), and hepatocellular carcinoma (20%) showed significantly elevated rates compared to healthy controls (Figure 1B). ANCA positivity was generally low, with marginal elevations only in colorectal cancer, lung cancer, lymphoma, and gastric cancer (Figure 1C). ATA positivity in ICI− patients was elevated compared to controls, though not significantly (Figure 1D).
Figure 1
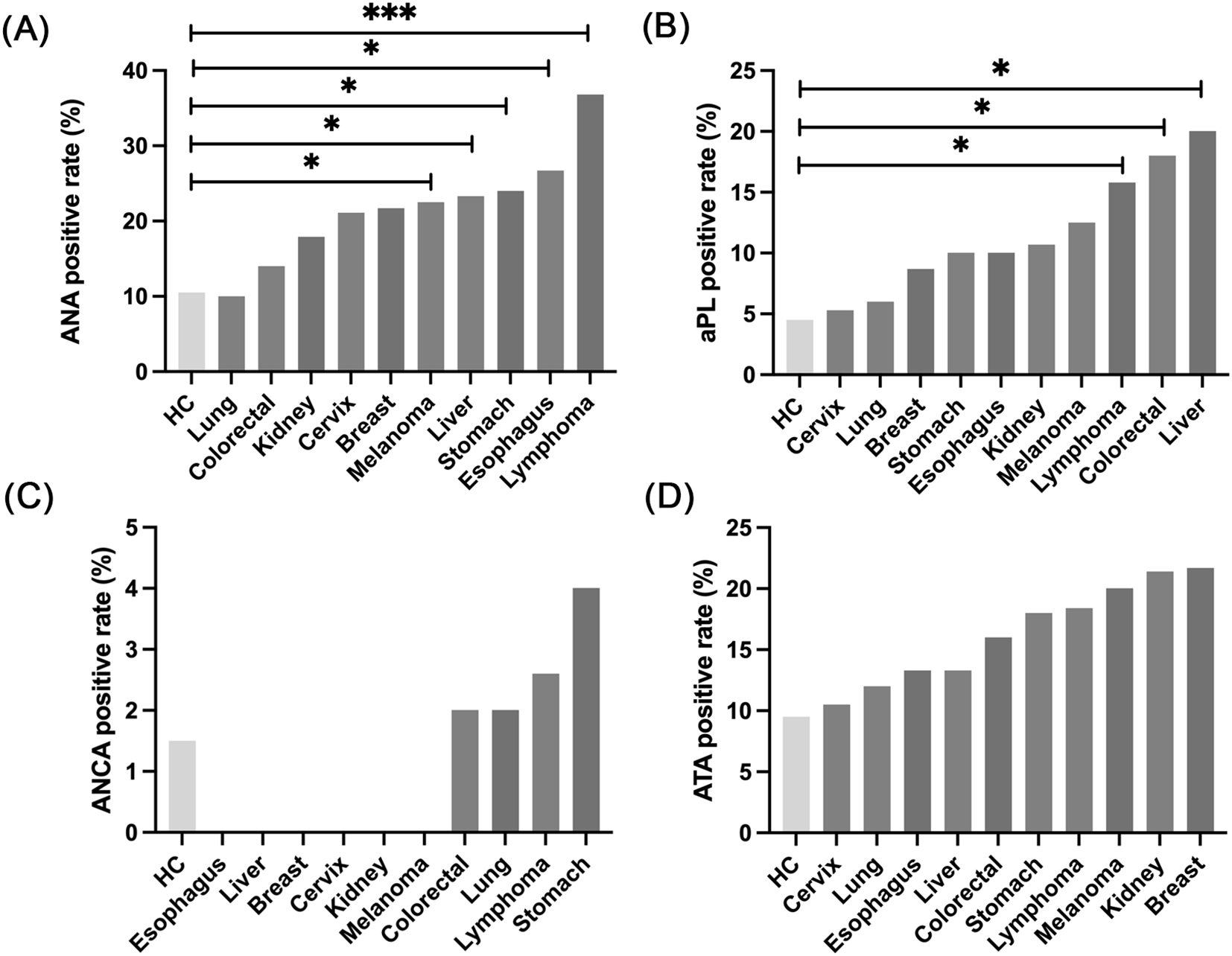
Prevalence of autoantibodies among treatment-naïve cancer patients. (A) Comparison of ANA positivity rates between patients with different cancer types and healthy controls. Significant higher positivity rates were observed in patients with melanoma, hepatocellular carcinoma, gastric cancer, esophageal cancer, and lymphoma compared to healthy controls. (B) Comparison of aPL positivity rates between patients with different cancer types and healthy controls. Patients with lymphoma, colorectal cancer, and hepatocellular carcinoma exhibited significantly higher positivity rates compared to healthy controls. (C) Comparison of ANCA positivity rates between patients with different cancer types and healthy controls. Patients with colorectal cancer, lung cancer, lymphoma, and gastric cancer demonstrated significantly higher positivity rates compared to healthy controls. ANCA positivity was not detected in patients with esophageal cancer, hepatocellular carcinoma, breast cancer, cervical cancer, renal cell carcinoma, or melanoma. (D) Comparison of ATA positivity rates between patients with different cancer types and healthy controls. All cancer types exhibited significantly higher ATA positivity rates compared to healthy controls. *P < 0.05; ***P < 0.001.
Comparison between ICI- and ICI+ groups revealed significant ANA increases in colorectal cancer (32.3%, P = 0.049), hepatocellular carcinoma (52.6%, P = 0.036), and renal cell carcinoma (53.8%, P = 0.047) compared with the ICI- group (Table 5). All tumor types except gastric cancer showed increased ANA positivity following immunotherapy. aPL and ANCA profiles remained largely unchanged, while ATA positivity increased in 9 of 10 tumor types (excluding melanoma), though without statistical significance.
Table 5
| Cancer | ANAs | aPL | ANCA | ATA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICI- | ICI+ | P | ICI- | ICI+ | P | ICI- | ICI+ | P | ICI- | ICI+ | P | |
| Stomach | 12 (24) | 7 (21.2) | 0.767 | 5 (10) | 4 (12.1) | 1.0 | 2 (4) | 2 (6.1) | 1.0 | 9 (18) | 7 (21.2) | 0.717 |
| Colorectal | 7 (14) | 10 (32.3) | 0.049 | 9 (18) | 2 (6.5) | 0.254 | 1 (2) | 0 (0) | 1.0 | 8 (16) | 7 (22.6) | 0.459 |
| Esophagus | 8 (26.7) | 6 (27.3) | 0.961 | 3 (10) | 3 (13.6) | 1.0 | 0 (0) | 1 (4.5) | 0.423 | 4 (13.3) | 5 (22.7) | 0.607 |
| liver | 7 (23.3) | 10 (52.6) | 0.036 | 6 (20) | 4 (21.1) | 1.0 | 0 (0) | 1 (5.3) | 0.388 | 4 (13.3) | 5 (26.3) | 0.444 |
| Lung | 5 (10) | 8 (25.8) | 0.116 | 3 (6) | 3 (9.7) | 0.859 | 1 (2) | 1 (3.2) | 1.0 | 6 (12) | 6 (19.4) | 0.559 |
| Breast | 5 (21.7) | 5 (35.7) | 0.454 | 2 (8.7) | 1 (7.1) | 1.0 | 0 (0) | 0 (0) | — | 5 (21.7) | 5 (35.7) | 0.454 |
| Cervix | 4 (21.1) | 5 (27.8) | 0.714 | 1 (5.3) | 0 (0) | 1.0 | 0 (0) | 0 (0) | — | 2 (10.5) | 3 (16.7) | 0.660 |
| Kidney | 5 (17.9) | 7 (53.8) | 0.047 | 3 (10.7) | 1 (7.7) | 1.0 | 0 (0) | 0 (0) | — | 6 (21.4) | 3 (23.1) | 1.0 |
| Lymphoma | 14 (36.8) | 13 (40.6) | 0.746 | 6 (15.8) | 8 (25.0) | 0.337 | 1 (2.6) | 0 (0) | 1.0 | 7 (18.4) | 6 (18.8) | 0.972 |
| Melanoma | 9 (22.5) | 12 (32.4) | 0.328 | 5 (12.5) | 3 (8.1) | 0.797 | 0 (0) | 1 (2.7) | 0.481 | 8 (20.0) | 6 (16.2) | 0.667 |
The impact of immunotherapy on autoantibody positivity rates across different tumor types n (%).
— too few case numbers to calculate.
Interestingly, analysis of the five most prevalent ANA subtypes across 10 tumors revealed tumor-specific expression patterns. Anti-SSA-60 showed the highest positivity in renal cell carcinoma, lymphoma, and lung cancer. Anti-Scl-70, while broadly expressed, predominated in lung cancer, renal cell carcinoma, and melanoma (approximately 30% of ANA-positive cases). Anti-RNP showed highest rates in breast and cervical cancers (approximately 33-40%). Anti-Nucleosome preferentially occurred in gastrointestinal tumors, esophageal cancer, and hepatocellular carcinoma. High anti-AMA-M2 positivity (approximately 36-38%) in hepatocellular carcinoma suggests hepatic tissue destruction may induce hepatic autoimmunity (Figure 2A).
Figure 2
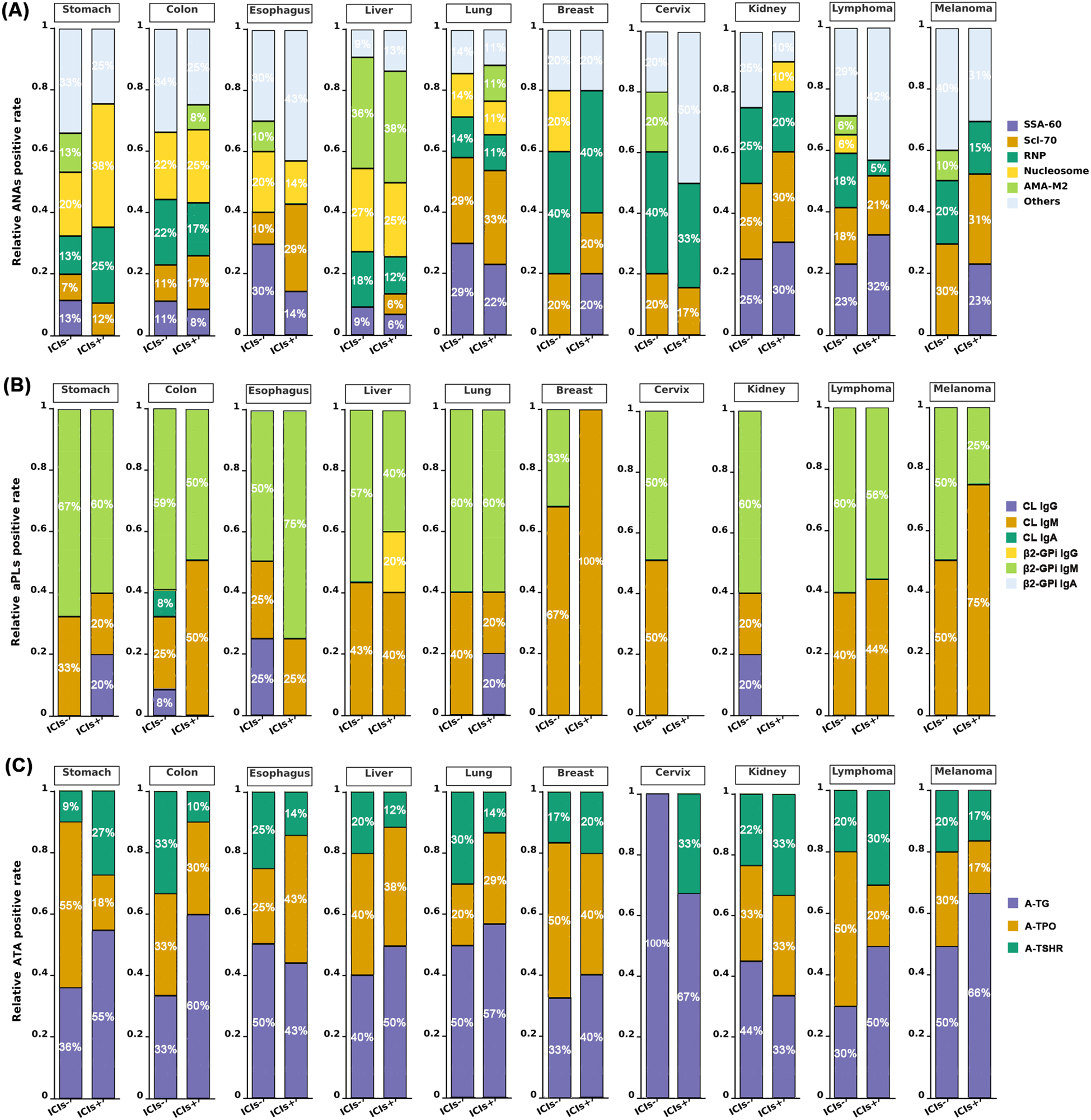
Effects of immunotherapy on autoantibody subtype positivity patterns in cancer patients. (A) The five most frequently detected autoantibodies from the ANA panel in cancer patients were selected to analyze ANA positivity patterns across different tumor types before and after immunotherapy. Remaining ANA specificities were categorized as “others”. (B) Analysis of aPL positivity patterns across different tumor types before and after immunotherapy demonstrated that IgM was the predominant isotype for both anti-cardiolipin and anti-β2-glycoprotein I antibodies. (C) Analysis of ATA positivity patterns across different tumor types before and after immunotherapy showed that anti-TG and anti-TPO exhibited higher positivity rates, with increased anti-TG positivity observed following immunotherapy in most tumor types.
AAb for Cardiolipin and β2-Glycoprotein I in various tumor types were predominantly IgM subtype (approximately 20%-100%), followed by IgG (8-25%). IgA was least frequent, with only one CL IgA-positive case in an untreated colorectal cancer patient. aPL distribution varied considerably among tumor types, with no positive cases in immunotherapy-treated cervical and renal cancer patients (Figure 2B).
Among thyroid-associated AAb, A-TG demonstrated the most significant treatment-related changes. A-TG positivity was significantly elevated in ICI+ patients compared to untreated controls across all tumor types. Although this difference was less pronounced when stratified by individual tumor types, A-TG maintained the highest positive expression rate across most malignancies. Following immunotherapy, A-TG positivity increased in all tumor types except esophageal, cervical, and renal cancers. Conversely, A-TPO prevalence decreased in multiple tumor types receiving immuno-therapy, including gastrointestinal tumors, hepatocellular carcinoma, breast cancer, lymphoma, and melanoma. This inverse relationship between A-TG and A-TPO suggests that A-TG may represent the most clinically relevant thyroid AAb requiring post-immunotherapy monitoring. A-TSHR positivity was significantly higher in cancer patients compared to healthy controls (P = 0.026). However, among immunotherapy-treated patients, A-TSHR did not exhibit consistent patterns, with approximately half of the tumor types showing increased expression (Figure 2C). ANCA showed minimal positivity across cancer patients and was excluded from detailed analysis due to limited sample size and potential analytical bias.
4 Discussion
Elevated AAb prevalence in cancer patients has been well-documented over recent decades (23–25), reflecting profound immune system remodeling and potentially predicting treatment response and irAEs (13, 26, 27). However, the dynamic serum AAb patterns during ICI therapy remain complex and controversial, possibly due to differential clinical significance across cancer types and methodological variations. We employed automated Luminex multiplex and chemiluminescent immunoassays to systematically evaluate AAb profiles before and after immunotherapy across multiple solid tumors. To our knowledge, this is the first study to investigate AAb prevalence changes following immunotherapy across multiple cancer types.
The mechanisms underlying elevated baseline AAb positivity in cancer patients are multifactorial. Tumor-associated antigens (TAAs) constitute the primary drivers, as cancer cells express aberrant proteins through oncogene activation, tumor suppressor inactivation, and abnormal post-translational modifications (28). These altered proteins, including overexpressed nucleophosmin, nucleolin, and mutant p53, become immunogenic targets that cross-react with normal cellular components. Additionally, the tumor microenvironment promotes autoimmunity through chronic inflammation and extensive cell death. Apoptotic and necrotic tumor cells release sequestered nuclear antigens, breaking immunological tolerance and exposing cryptic self-antigens (29). Inflammatory cytokines (TNF-α, IL-1, IL-6) at tumor sites create a pro-autoimmune milieu where the immune system generates AAb against tumor-associated targets, potentially increasing autoimmune risk (30). Dual BCR/TLR7 signaling promotes autoantibody-producing plasma cell differentiation, which correlates with antitumor responses and may explain the frequent association between immunotherapy efficacy and autoimmune toxicity (13, 31).
Our finding that AAb frequency remained independent of tumor staging suggests that tumor burden may not critically influence AAb formation, consistent with previous lymphoma studies (32). However, the type of ICI significantly influenced ANA formation. Patients receiving CTLA-4 inhibitors, alone or combined with PD-1/PD-L1 inhibitors, demonstrated significantly higher ANA frequencies compared to PD-1/PD-L1 mono-therapy. This differential effect reflects distinct immunological mechanisms. CTLA-4 blockade disrupts early T-cell activation checkpoints, causing broad immune activation and regulatory T-cell depletion, which removes peripheral tolerance mechanisms. This leads to enhanced T-B cell interactions, increased germinal center formation, and direct B-cell activation (31, 33, 34). Moreover, ipilimumab’s IgG1 structure enables complement activation and antibody-dependent cellular cytotoxicity, potentially causing tissue damage that releases autoantigens (35). Conversely, PD-1/PD-L1 inhibition primarily reverses late-stage T-cell exhaustion in peripheral tissues, producing more localized autoimmune reactions. The IgG4 structure of these antibodies results in reduced complement activation (36, 37).
The significantly elevated ANA prevalence in cancer patients likely represents a secondary phenomenon resulting from exposure to aberrant tumor antigens, rather than playing a direct role in tumorigenesis, though these antibodies may serve as valuable biomarkers (25). We identified five ANA subtypes with frequencies exceeding 3%: anti-AMA-M2, anti-Nucleosome, anti-SSA-60, anti-Scl-70, and anti-RNP, with anti-SSA-60 and anti-RNP significantly elevated compared to healthy controls. Anti-SSA-60 targets the cytoplasmic Ro60 protein involved in RNA metabolism, which undergoes dysregulation during tumorigenesis, resulting in enhanced immunogenicity (38). Anti-RNP antibodies target small nuclear ribonucleoproteins critical for pre-mRNA splicing, potentially released through tumor cell necrosis and apoptosis (39). Anti-Nucleosome antibody elevation correlates with massive nucleosome release during tumor cell apoptosis and necrosis processes (3, 40).
ICI+ patients demonstrated higher ANA detection frequency (33.6%) compared to ICI- patients (20.1%), with anti-Scl-70 showing significant elevation. Tumor-specific patterns emerged: melanoma, hepatocellular carcinoma, gastric cancer, esophageal cancer, and lymphoma demonstrated high baseline ANA frequencies, while colorectal, hepatocellular, and renal cancers showed significant post-immunotherapy increases. Distinct antibody expression patterns characterized different cancers; anti-AMA-M2 showed elevated frequencies in hepatocellular carcinoma both before and after immunotherapy, while anti-Nucleosome pre-dominated in gastrointestinal tumors. These findings suggest tumor-specific AAb panels may serve as potential biomarkers for monitoring therapeutic efficacy or predicting irAEs. Sample size limitations should be considered when interpreting tumor-specific findings. Several cancer types had relatively small sample sizes in the ICI+ group, including renal cell carcinoma (n=13) and breast cancer (n=14). While we observed statistically significant ANA increases in colorectal, hepatocellular, and renal cancers following ICI therapy, the limited sample sizes increase the risk of Type I errors and reduce statistical power to detect true differences. The observed effects may not be generalizable and require validation in larger cohorts.
aPL are primarily associated with thromboembolic events, a major cause of cancer-related mortality (22). Our data revealed that the prevalence of aPL in cancer patients was consistently elevated compared to healthy individuals. Specifically, patients with lymphoma, colorectal cancer, and hepatocellular carcinoma demonstrated significantly higher frequencies of aPL than healthy controls, with IgM aCL and IgM anti-β2-GPI showing highest prevalence. aPL development in cancer patients results from two synergistic mechanisms. First, tumor-mediated chronic inflammation produces AAb against phospholipid-binding proteins, particularly β2-glycoprotein I. Second, cancer cells present novel antigens that trigger cross-reactive antibody responses, with certain malignancies directly secreting aCL (41). Clonal B-cells lacking normal regulatory mechanisms undergo expansion, producing monoclonal immunoglobulins with aPL activity (42). Alcoholic liver disease and hepatitis B/C virus infections associated with liver cancer have also been shown to induce aPL formation (43–45). The coexistence of tumors and antiphospholipid syndrome (APS) should also be considered, as multiple studies have reported the association between advanced tumors and APS (46). Notably, aPL levels showed minimal variation between pre- and post-immunotherapy timepoints across tumor types, suggesting anti-tumor immune response reactivation may not significantly influence aPL expression. The IgA aCL and IgA β2-GPI occurred at relatively low frequencies in our cancer patient group, limiting our ability to assess their potential roles in tumorigenesis and immunotherapeutic responses.
ANCA prevalence in healthy individuals is relatively low, ranging from approximately 0% to 5.1% (47). Currently, large-scale studies directly comparing ANCA prevalence between cancer patients and healthy populations remain scarce. In this study, we observed slightly elevated ANCA frequency in cancer patients both before and after immunotherapy. Due to limited positive cases, the clinical significance of ANCA and its potential role in guiding immunotherapy decisions remain to be elucidated through larger-scale investigations.
ATA showed significantly elevated prevalence across nearly all tumor types compared to healthy controls, consistent with previous reports (27, 48, 49). Post-immunotherapy ATA frequency increased overall, with only A-TG reaching statistical significance. This reflects non-specific immune activation by immunotherapy, inducing autoimmune responses against vulnerable endocrine organs (50). The differential response between A-TG and A-TPO contrasts with some reports suggesting A-TPO as a potential irAEs biomarker, possibly due to variations in tumor types and therapeutic agents studied (51, 52).
Our findings raise important clinical questions regarding the role of AAb screening in ICI therapy management. Current evidence does not support routine pre-treatment AAb screening or treatment modification based solely on AAb elevations. Several studies demonstrate that patients with pre-existing autoimmune conditions or positive AAb can safely receive ICIs with appropriate monitoring (5, 12). However, AAb profiling may serve two potential clinical roles: risk stratification for immune-related adverse events (irAEs), with some evidence suggesting AAb-positive patients may have higher irAE risk (13–15, 27). Biomarker development for treatment response, as AAb development during therapy may correlate with anti-tumor efficacy (11, 12). Prospective studies are needed to establish whether AAb monitoring can guide personalized immunotherapy approaches, such as heightened irAE surveillance in high-risk patients or treatment optimization in AAb-positive responders. Until such evidence emerges, we recommend that ICI treatment decisions prioritize established clinical factors rather than AAb status alone, while remaining vigilant for clinical manifestations of autoimmunity.
Several limitations of our cross-sectional design warrant consideration. First, the observed differences in AAb prevalence between ICI- and ICI+ groups represent associations rather than causal relationships. Our study cannot definitively establish that ICIs directly cause AAb elevation, as alternative explanations exist: (1) patients who develop AAb may have differential treatment outcomes affecting their representation in the post-ICI cohort; (2) disease progression itself may influence AAb profiles independent of treatment; (3) time-dependent changes in immune status may confound comparisons between independently sampled cohorts. Prospective longitudinal studies following individual patients from treatment initiation through therapy would provide stronger evidence for causal relationships between ICI exposure and AAb changes. Second, we cannot exclude potential confounding effects from concurrent radiotherapy, chemotherapy, or other treatment modalities. Future investigations should establish multi-parameter risk assessment models based on continuous AAb profile dynamics. This approach could enhance prediction accuracy and facilitate high-risk patient identification and immunotherapy optimization.
In conclusion, our findings demonstrate that cancer patients exhibit increased ANA, aPL, and ATA frequencies compared to healthy individuals, with tumor-specific AAb preferences that are independent of disease staging. Immunotherapy further enhanced ANA frequency, particularly following CTLA-4 inhibitor administration. Among the four AAb profiles examined, ANA and A-TG demonstrated the most pronounced post-immunotherapy changes, while ANCA monitoring appears to have limited predictive value. Furthermore, different tumor types exhibit distinct ANA expression patterns. These distinct profiles across cancers suggest AAb may serve as potential biomarkers for predicting immunotherapy efficacy or irAEs development.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Peking University Cancer Hospital (No.2023YJZ45). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KC: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. BG: Data curation, Formal Analysis, Investigation, Writing – original draft. RL: Data curation, Formal Analysis, Methodology, Writing – original draft. JW: Formal Analysis, Methodology, Software, Writing – original draft. CZ: Formal Analysis, Methodology, Software, Writing – original draft. GX: Conceptualization, Formal Analysis, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Beijing Natural Science Foundation (grant number 7244293).
Acknowledgments
We thank the company Shanghai Tellgen Corporation and Shenzhen YHLO Biotech Co., Ltd for providing the technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Wasserman J Glas U Blomgren H . Autoantibodies in patients with carcinoma of the breast. Correlation with prognosis. Clin Exp Immunol. (1975) 19:417–22.
2
Syrigos KN Charalambopoulos A Pliarchopoulou K Varsamidakis N Machairas A Mandrekas D . The prognostic significance of autoantibodies against dsdna in patients with colorectal adenocarcinoma. Anticancer Res. (2000) 20:4351–3.
3
Nisihara R Machoski MCC Neppel A Maestri CA Messias-Reason I Skare TL . Anti-nuclear antibodies in patients with breast cancer. Clin Exp Immunol. (2018) 193:178–82. doi: 10.1111/cei.13136
4
Blaes F Klotz M Huwer H Straub U Kalweit G Schimrigk K et al . Antineural and antinuclear autoantibodies are of prognostic relevance in non-small cell lung cancer. Ann Thorac Surg. (2000) 69:254–8. doi: 10.1016/s0003-4975(99)01198-4
5
Xie W Huang H Xiao S Fan Y Deng X Zhang Z . Immune checkpoint inhibitors therapies in patients with cancer and preexisting autoimmune diseases: A meta-analysis of observational studies. Autoimmun Rev. (2020) 19:102687. doi: 10.1016/j.autrev.2020.102687
6
Andre T Shiu KK Kim TW Jensen BV Jensen LH Punt C et al . Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. New Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
7
Reck M Rodriguez-Abreu D Robinson AG Hui R Csoszi T Fulop A et al . Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with pd-L1 tumor proportion score >/= 50. J Clin Oncol. (2021) 39:2339–49. doi: 10.1200/JCO.21.00174
8
Babamohamadi M Mohammadi N Faryadi E Haddadi M Merati A Ghobadinezhad F et al . Anti-ctla-4 nanobody as a promising approach in cancer immunotherapy. Cell Death Dis. (2024) 15:17. doi: 10.1038/s41419-023-06391-x
9
Daban A Gonnin C Phan L Saldmann A Granier C Lillo-Lelouet A et al . Preexisting autoantibodies as predictor of immune related adverse events (Iraes) for advanced solid tumors treated with immune checkpoint inhibitors (Icis). Oncoimmunology. (2023) 12:2204754. doi: 10.1080/2162402X.2023.2204754
10
Zhang A Fan T Liu Y Yu G Li C Jiang Z . Regulatory T cells in immune checkpoint blockade antitumor therapy. Mol Cancer. (2024) 23:251. doi: 10.1186/s12943-024-02156-y
11
Mitchell P Thatcher N Socinski MA Wasilewska-Tesluk E Horwood K Szczesna A et al . Tecemotide in unresectable stage iii non-small-cell lung cancer in the phase iii start study: updated overall survival and biomarker analyses. Ann Oncol. (2015) 26:1134–42. doi: 10.1093/annonc/mdv104
12
Morimoto K Yamada T Nakamura R Katayama Y Tanaka S Takumi C et al . Impact of preexisting antinuclear antibodies on combined immunotherapy and chemotherapy in advanced non-small cell lung cancer patients. Med Oncol. (2020) 37:111. doi: 10.1007/s12032-020-01440-3
13
Ghosh N Postow M Zhu C Jannat-Khah D Li QZ Vitone G et al . Lower baseline autoantibody levels are associated with immune-related adverse events from immune checkpoint inhibition. J Immunother Cancer. (2022) 10:e004008. doi: 10.1136/jitc-2021-004008
14
Ravi P Freeman D Thomas J Ravi A Mantia C McGregor BA et al . Comprehensive multiplexed autoantibody profiling of patients with advanced urothelial cancer. J Immunother Cancer. (2024) 12:e008215. doi: 10.1136/jitc-2023-008215
15
Ceccarelli F Natalucci F Picciariello L Cirillo A Olivieri G Veroli M et al . Antinuclear antibodies may predict the development of immune-related adverse events in asymptomatic patients treated with immune checkpoint inhibitors: results from a single-center cohort. Clin Exp Med. (2024) 24:72. doi: 10.1007/s10238-024-01317-z
16
Tang H Geng R Xu X Wang Y Zhou J Zhang S et al . Safety and efficacy of pd-1/pd-L1 inhibitors in cancer patients with preexisting autoantibodies. Front Immunol. (2022) 13:893179. doi: 10.3389/fimmu.2022.893179
17
Barth DA Stanzer S Spiegelberg J Bauernhofer T Absenger G Posch F et al . Evaluation of autoantibodies as predictors of treatment response and immune-related adverse events during the treatment with immune checkpoint inhibitors: A prospective longitudinal pan-cancer study. Cancer Med. (2022) 11:3074–83. doi: 10.1002/cam4.4675
18
Infantino M Meacci F Grossi V Manfredi M Benucci M Merone M et al . The burden of the variability introduced by the hep-2 assay kit and the cad system in ana indirect immunofluorescence test. Immunol Res. (2017) 65:345–54. doi: 10.1007/s12026-016-8845-3
19
Satoh M Chan EK Ho LA Rose KM Parks CG Cohn RD et al . Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. (2012) 64:2319–27. doi: 10.1002/art.34380
20
Vila P Hernandez MC Lopez-Fernandez MF Batlle J . Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb Hemost. (1994) 72:209–13.
21
Guchelaar NAD Waling MM Adhin AA Van Daele PLA Schreurs MWJ Rombach SM . The value of anti-neutrophil cytoplasmic antibodies (Anca) testing for the diagnosis of anca-associated vasculitis, a systematic review and meta-analysis. Autoimmun Rev. (2021) 20:102716. doi: 10.1016/j.autrev.2020.102716
22
Hollowell JG Staehling NW Flanders WD Hannon WH Gunter EW Spencer CA et al . Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. (2002) 87:489–99. doi: 10.1210/jcem.87.2.8182
23
Armas JB Dantas J Mendonca D Farto R Ribeiro M Herrero-Beaumont G et al . Anticardiolipin and antinuclear antibodies in cancer patients–a case control study. Clin Exp Rheumatol. (2000) 18:227–32.
24
Hsu SJ Chao YC Lin XH Liu HH Zhang Y Hong WF et al . Antinuclear antibody (Ana) status predicts immune-related adverse events in liver cancer patients undergoing anti-pd-1 therapy. Clin Exp Immunol. (2023) 212:239–48. doi: 10.1093/cei/uxad036
25
Skare TL Neppel A Machoski MCC Maestri CA Messias-Reason I Nisihara R . Antinuclear antibodies in patients with cervical lesions and invasive cervical cancer. Immunol Lett. (2019) 208:8–10. doi: 10.1016/j.imlet.2019.03.002
26
Alserawan L Anguera G Zamora Atenza C Serra Lopez J Martinez-Martinez L Riudavets Melia M et al . Association between changes in the patterns of antinuclear autoantibodies during immune checkpoint inhibition therapy and the development of severe immune related adverse events. Int J Mol Sci. (2022) 23:12641. doi: 10.3390/ijms232012641
27
Borgers JSW Van Wesemael TJ Gelderman KA Rispens T Verdegaal EME Moes D et al . Autoantibody-positivity before and seroconversion during treatment with anti-pd-1 is associated with immune-related adverse events in patients with melanoma. J Immunother Cancer. (2024) 12:e009215. doi: 10.1136/jitc-2024-009215
28
Heo CK Bahk YY Cho EW . Tumor-associated autoantibodies as diagnostic and prognostic biomarkers. BMB Rep. (2012) 45:677–85. doi: 10.5483/bmbrep.2012.45.12.236
29
Perez-Bucio C Behere A Landegren N . Mechanisms of autoimmune-mediated paraneoplastic syndromes: immune tolerance and disease pathogenesis. Front Immunol. (2025) 16:1608934. doi: 10.3389/fimmu.2025.1608934
30
Tan EM Zhang J . Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. (2008) 222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x
31
Buchbinder EI Desai A . Ctla-4 and pd-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. (2016) 39:98–106. doi: 10.1097/COC.0000000000000239
32
Barreno-Rocha SG Guzman-Silahua S Cardona-Munoz EG Zavala-Cerna MG Munoz Gaytan DE Riebeling-Navarro C et al . Frequency of autoantibodies on non-hodgkin lymphoma. Healthcare. (2023) 11:2210. doi: 10.3390/healthcare11152210
33
Topalian SL Hodi FS Brahmer JR Gettinger SN Smith DC McDermott DF et al . Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. New Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
34
Liu W Luo Z Liu Y Sun B . Current landscape and tailored management of immune-related adverse events. Front Pharmacol. (2023) 14:1078338. doi: 10.3389/fphar.2023.1078338
35
Chand D Savitsky DA Krishnan S Mednick G Delepine C Garcia-Broncano P et al . Botensilimab, an fc-enhanced anti-ctla-4 antibody, is effective against tumors poorly responsive to conventional immunotherapy. Cancer Discov. (2024) 14:2407–29. doi: 10.1158/2159-8290.CD-24-0190
36
Brahmer JR Hammers H Lipson EJ . Nivolumab: targeting pd-1 to bolster antitumor immunity. Future Oncol. (2015) 11:1307–26. doi: 10.2217/fon.15.52
37
Chera A Stancu AL Bucur O . Thyroid-related adverse events induced by immune checkpoint inhibitors. Front Endocrinol. (2022) 13:1010279. doi: 10.3389/fendo.2022.1010279
38
Mahla RS Jones EL Dustin LB . Ro60-roles in rna processing, inflammation, and rheumatic autoimmune diseases. Int J Mol Sci. (2024) 25:7705. doi: 10.3390/ijms25147705
39
Maniatis T Reed R . The role of small nuclear ribonucleoprotein particles in pre-mrna splicing. Nature. (1987) 325:673–8. doi: 10.1038/325673a0
40
Hojan K Adamska K Lewandowska A Procyk D Leporowska E Osztynowicz K et al . Neural and onconeural autoantibodies and blood-brain barrier disruption markers in patients undergoing radiotherapy for high-grade primary brain tumor. Diagnostics. (2024) 14:307. doi: 10.3390/diagnostics14030307
41
Gomez-Puerta JA Espinosa G Cervera R . Antiphospholipid antibodies: from general concepts to its relation with Malignancies. Antibodies. (2016) 5:18. doi: 10.3390/antib5030018
42
Kungwankiattichai S Nakkinkun Y Owattanapanich W Ruchutrakool T . High incidence of antiphospholipid antibodies in newly diagnosed patients with lymphoma and a proposed apl predictive score. Clin Appl Thromb Hemost. (2020) 26:1076029620928392. doi: 10.1177/1076029620928392
43
Biron C Andreani H Blanc P Ramos J Ducos J Guigue N et al . Prevalence of antiphospholipid antibodies in patients with chronic liver disease related to alcohol or hepatitis C virus: correlation with liver injury. J Lab Clin Med. (1998) 131:243–50. doi: 10.1016/s0022-2143(98)90096-8
44
Rolla R Vay D Mottaran E Parodi M Vidali M Sartori M et al . Antiphospholipid antibodies associated with alcoholic liver disease specifically recognize oxidized phospholipids. Gut. (2001) 49:852–9. doi: 10.1136/gut.49.6.852
45
Melayah S Kallala O Ben Ahmed M Fodha I Yacoub Jemni S Ghedira I et al . Iga anti-beta-2 glycoprotein I antibodies in chronic hepatitis C. Arab J Gastroenterol. (2022) 23:26–31. doi: 10.1016/j.ajg.2021.12.003
46
Tota V Dagonnier M Wery D Binet L Nagy N Durieux V et al . Antiphospholipid syndrome-induced ischemic stroke following pembrolizumab: case report and systematic review. Lung Cancer. (2021) 160:59–65. doi: 10.1016/j.lungcan.2021.07.021
47
Deshpande P Lucas M Brunt S Lucas A Hollingsworth P Bundell C . Low level autoantibodies can be frequently detected in the general Australian population. Pathology. (2016) 48:483–90. doi: 10.1016/j.pathol.2016.03.014
48
Xiao Y Zhou Q Xu Y Yuan SL Liu QA . Positive thyroid antibodies and risk of thyroid cancer: A systematic review and meta-analysis. Mol Clin Oncol. (2019) 11:234–42. doi: 10.3892/mco.2019.1886
49
Karaviti D Kani ER Karaviti E Gerontiti E Michalopoulou O Stefanaki K et al . Thyroid disorders induced by immune checkpoint inhibitors. Endocrine. (2024) 85:67–79. doi: 10.1007/s12020-024-03718-2
50
Wright JJ Powers AC Johnson DB . Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. (2021) 17:389–99. doi: 10.1038/s41574-021-00484-3
51
Cho YA Kong SY Shin A Lee J Lee EK Lee YJ et al . Biomarkers of thyroid function and autoimmunity for predicting high-risk groups of thyroid cancer: A nested case-control study. BMC Cancer. (2014) 14:873. doi: 10.1186/1471-2407-14-873
52
El Sabbagh R Azar NS Eid AA Azar ST . Thyroid dysfunctions due to immune checkpoint inhibitors: A review. Int J Gen Med. (2020) 13:1003–9. doi: 10.2147/IJGM.S261433
Summary
Keywords
autoantibody, antinuclear antibody, cancers, immune checkpoint inhibitor, immunotherapy
Citation
Chen K, Guo B, Liu R, Wang J, Zhang C and Xu G (2025) Serum autoantibody profiles in cancer patients treated with immune checkpoint inhibitors: a cross-sectional study. Front. Oncol. 15:1716014. doi: 10.3389/fonc.2025.1716014
Received
30 September 2025
Revised
04 November 2025
Accepted
06 November 2025
Published
24 November 2025
Volume
15 - 2025
Edited by
Cuiling Zheng, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Reviewed by
Guanghui Zheng, Capital Medical University, China
Xiaowen Zhu, Capital Medical University, China
Updates
Copyright
© 2025 Chen, Guo, Liu, Wang, Zhang and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guobing Xu, bjzlxgb@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.