- 1Central Asian Institute for Medical Research, Astana, Kazakhstan
- 2Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan
- 3Research Institute of Life and Health Sciences, Higher School of Medicine, Kokshetau University named after Sh. Ualikhanov, Kokshetau, Kazakhstan
- 4Asian Pacific Organization for Cancer Prevention, Bishkek, Kyrgyzstan
- 5Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan
- 6Academy of Public Administration under the President of the Republic of Kazakhstan, Astana, Kazakhstan
- 7Astana Medical University, Astana, Kazakhstan
- 8Kokshetau Higher Medical College, Kokshetau, Kazakhstan
- 9National Scientific Center of Traumatology and Orthopedics named after Academician Batpenov N.D. of the Ministry of Health of the Republic of Kazakhstan, Astana, Kazakhstan
- 10Research Center for Social Determinants of Health, Research Institute for Metabolic and Obesity Disorders, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Prostate cancer is a leading malignancy among men globally and continues to be a growing concern in Kazakhstan, where evidence regarding its long-term epidemiological trends is limited.
Objectives: This study seeks to assess national and regional trends in prostate cancer incidence, stage distribution, and morphological verification in Kazakhstan from 2005 to 2024.
Methods: A nationwide observational study utilizing registry data from the Unified Nationwide Electronic Health System was performed. Incident cases (ICD-10 code C61) were examined according to age, geographical region, and stage at diagnosis. The incidence rates were adjusted to the WHO World Standard Population (2000–2025) by age. Joinpoint regression was used to look at temporal trends, and it showed the annual percent change (APC) and the average annual percent change (AAPC) with 95% confidence intervals.
Results: From 2005–2024, 21,756 prostate cancer cases were recorded, with a mean age at diagnosis of 69.8 years. The age-standardized incidence rate (ASR) increased from 11.9 to 20.7 per 100,000 men (APC = +2.6%, p = 0.002). Four distinct periods were identified: an early decline (2005–2008), a sharp rise (2008–2016), a downturn (2016–2020), and a renewed increase (2020–2024). Age-specific incidence was negligible below 50 years, peaking at 75–79 years (228.6 per 100,000). Regional analyses revealed heterogeneous trends: monotonic increases in Atyrau, Aktobe, and Almaty (region), contrasted by boom–dip–rebound profiles in Karaganda, Pavlodar, and Almaty City. The proportion of early-stage (I–II) cases rose from 32.8% to 56.9%, while stage III declined from 49.7% to 22.9%; stage IV increased slightly (17.3% → 20.2%). Morphological verification improved nationally (mean ≈ 92%) and plateaued after 2015.
Conclusions: Kazakhstan shows an increase in prostate cancer cases, with more cases being diagnosed at an earlier stage but still a lot of cases being diagnosed at a later stage. This is probably due to the PSA screening program from 2013 to 2017, changes in policy since then, and problems with diagnosis during the pandemic. To get better results, we need to improve early detection, timely biopsy pathways, and connections to mortality and survival data.
1 Introduction
Prostate cancer remains a major global oncology challenge: according to GLOBOCAN 2022, it is the most commonly diagnosed cancer in men in most countries and ranks among the leading causes of cancer death (1). Accounting for population ageing and continuation of current incidence trends, The Lancet Commission projects a doubling of the global prostate cancer burden—from ~1.4 million new cases in 2020 to ~2.9 million by 2040 (2). Despite its frequency and impact on mortality, few lifestyle or environmental determinants have been established; advancing age, family history, and specific genetic alterations remain the only consistently validated risk factors, while proposed roles for smoking, excess adiposity, and selected dietary factors are suggestive but inconclusive (3).
Within Kazakhstan, national and regional analyses by local investigators documented rising incidence during 2007–2016—a period that overlaps temporally with the introduction of prostate-specific antigen (PSA)–based screening—while the largest cities (Almaty and Astana) consistently exhibited higher burdens than other regions (4–6). The 2013–2017 pilot population screening program demonstrated operational feasibility (hundreds of thousands of PSA tests) but also exposed critical limitations—from pre-analytical issues to the modest diagnostic specificity of PSA and the need for more judicious use of reflex indices/biopsy (7, 8). Local economic–epidemiological assessments (e.g., Pavlodar region) further underscored the sensitivity of outcomes to system capacity and patient routing (9). Against a background of limited public awareness, these factors can generate a "prevalence surge" at screening initiation and attenuating oscillations following program scale-down.
This highlights the pressing need for further study into the regional and temporal patterns of prostate cancer incidence and stage-specific trends. By addressing these gaps and study the effect of covid-19 pandemic on prostate cancer incidence in Kazakhstan, this study aims to provide critical data that can inform more targeted, evidence-based interventions, improving early detection and ultimately reducing the burden of advanced prostate cancer in Kazakhstan.
We undertook a comprehensive assessment of prostate cancer epidemiology in Kazakhstan over 2005–2024 with three emphases: a) national and regional incidence levels (age-standardized rates) and their age-specific profiles; b) stage distribution and temporal trends; and c) morphological verification as a key marker of data validity.
2 Materials and methods
2.1 Study design and setting
We conducted a nationwide, population-based, observational time-trend study of prostate cancer in Kazakhstan for calendar years 2005-2024. The unit of analysis was the male resident population at national and regional levels. Regions were defined according to the administrative structure used by the registry for longitudinal consistency (including the two cities of republican significance).
2.2 Data sources and case definition
Incident prostate cancer cases were extracted from the Unified Nationwide Electronic Health System using ICD-10 code C61 as the primary site. Duplicate records, non-resident cases, and records with unknown sex were excluded. Stage at diagnosis followed registry TNM conventions; for analysis we collapsed stage into I–II, III, IV, and unknown/unstaged. For morphological verification (MV) we used the registry variable indicating microscopic confirmation (histology/cytology) and calculated the proportion MV among all incident cases each year.
2.3 Population denominators
Annual male mid-year population estimates by 5-year age group and by region, including administrative details, were sourced from the Bureau of National Statistics of the Agency for Strategic Planning and Reforms of the Republic of Kazakhstan (10). These denominators were used to compute crude, age-specific, and age-standardized rates.
2.4 Statistical analysis
All incidence rates were calculated per 100,000 men and directly standardized to the WHO World Standard Population (2000–2025) using 5-year age groups (11). To eliminate the influence of the age structure of the male population in Kazakhstan on the incidence rate, age standardization was performed using the WHO World Standard Population. This approach adjusted for the comparatively younger demographic profile of the country, thereby ensuring that observed differences were not driven by population age composition. As a result, the age-standardized rate was higher than the crude incidence rate, which reflects the larger proportion of older age groups in the world standard population. This adjustment increases the calculated incidence value and allows for valid and comparable interpretation of the results in relation to global data.
For Joinpoint modelling, we worked on the log-rate scale and selected a log-linear model, assuming that the natural logarithm of the age-standardized rate changes linearly over time; the standard error (SE) of natural logarithm of the age-standardized incidence rate was calculated using the usual Poisson approximation based on case counts. For morphological verification (MV) %, standard errors were computed from binomial variance using the Wilson score formulation; these SEs were exported to Joinpoint when modelling MV as a proportion.
Temporal trends in overall and stage-specific incidence rates were estimated using the Joinpoint Regression Program (version 5.4.0, National Cancer Institute, Bethesda, MD, USA) (12). The primary model was chosen using the data-driven Weighted BIC among candidates with 0–3 joinpoints. To assess robustness, we also applied the Monte Carlo permutation test implemented in the Joinpoint Regression Program with an overall significance level α=0.05 and a maximum of 4,499 permutations (13). P-values for additional joinpoints were obtained from the empirical permutation distribution. To control the family-wise type-I error across multiple joinpoint tests, the software's built-in Bonferroni correction was used. The permutation-based selection yielded the same (or highly similar) number and location of joinpoints and comparable Annual Percent Change (APC)/Average Annual Percent Change (AAPC) estimates; therefore, the study conclusions were unchanged. 95% confidence intervals for APC/AAPC were computed via the Empirical Quantile method with 5,001 resamples. For MV (%), the same framework was applied to proportions with their SEs.
2.5 Ethics approval
This study utilized publicly available administrative data, and thus did not require direct interaction with individuals. Ethical approval was granted by the Local Ethics Commission of the Central Asian Institute for Medical Research.
3 Results
3.1 Incidence of prostate cancer in Kazakhstan (2005–2024)
During the period 2005–2024, 21,756 cases of prostate cancer were identified, representing an average annual number of 1209. The crude and standardized incidence rates were respectively of 12.6/100,000 men and 17.5/100,000 men (Table 1). The mean age at diagnostic was of 69.8 ± 0.2 years and the age specific incidence rate (ASIR) increased regularly with age.
3.2 Trends in incidence of prostate cancer in Kazakhstan (2005–2024)
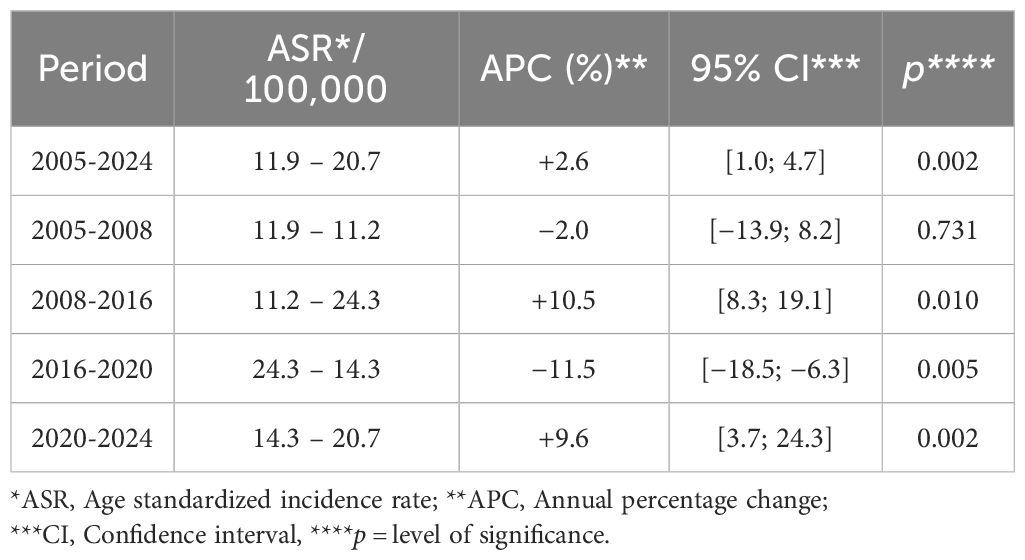
The ASR increased significantly from 11.9 in 2005 to 20.7 in 2024 with an APC of +2.6% (p = 0.002) (Table 2; Figure 1). Trends analysis has also described four periods: 2005–2008 APC of −2.0% (p = 0.731; not significant); 2008–2016 APC of +10.5% (p = 0.010); 2016–2020 APC of −11.5% (p = 0.005); and 2020–2024 APC of +9.6% (p = 0.002) (Table 2; Figure 1).

Figure 1. Trends in the age standardized incidence rate of prostate cancer for the period 2005-2024.
3.3 Age-specific pattern
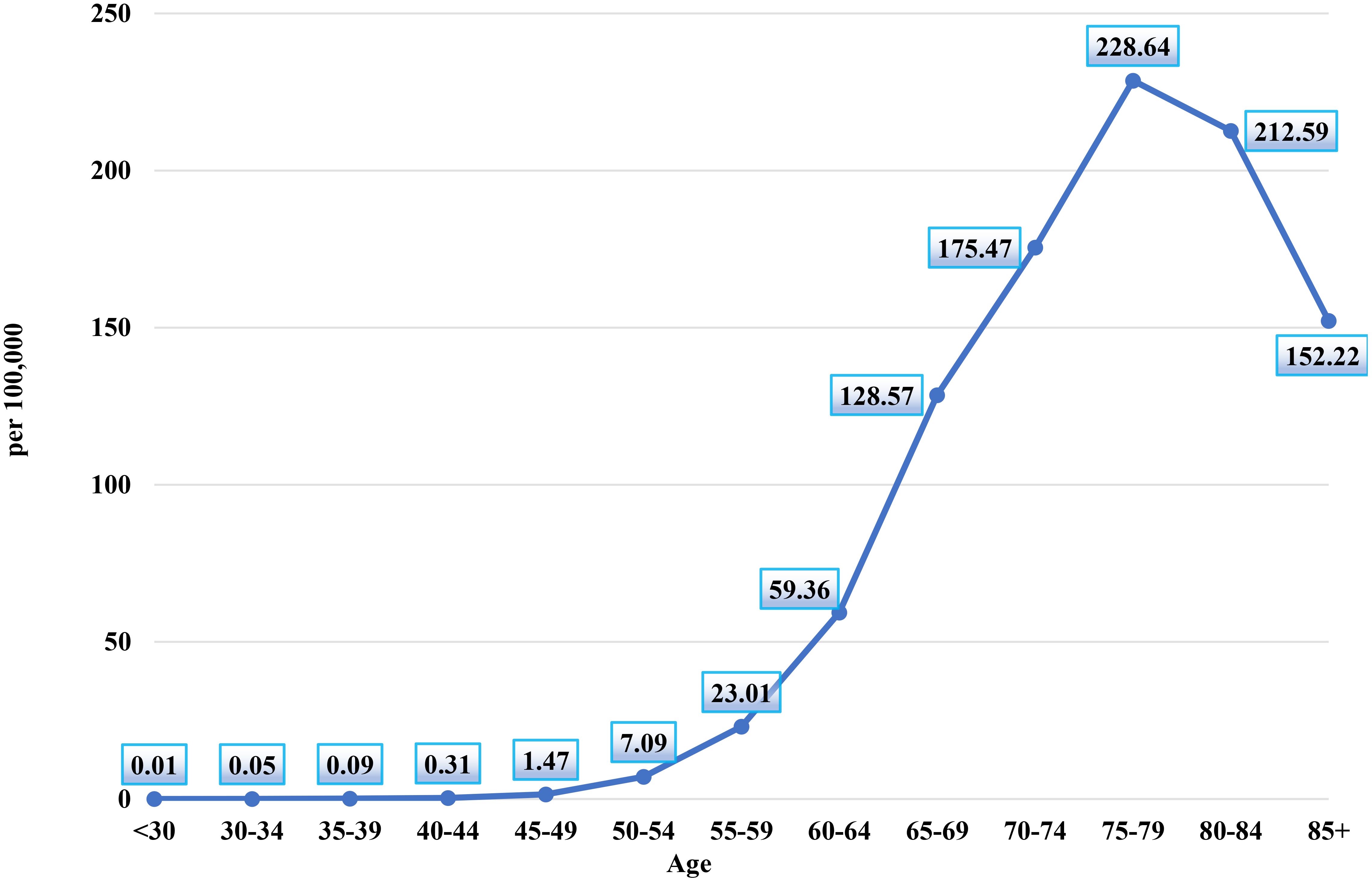
Figure 2 illustrates the age-specific incidence rates of prostate cancer per 100,000 men in Kazakhstan between 2005 and 2024. The chart reveals a clear upward trend in prostate cancer incidence with advancing age, underscoring the disease's increasing prevalence among older male populations.
The chart shows that the incidence of prostate cancer is exceptionally low in younger age groups, with the rate in men under 30 years at 0.01 per 100,000. The incidence remains minimal in subsequent age groups, with the 30–34 years group at 0.05 per 100,000 and the 35–39 years group at 0.09 per 100,000, indicating that prostate cancer is rare in these early adult years.
A notable increase in incidence is observed beginning in the 40–44 years group, where the rate rises to 0.31 per 100,000, continuing to grow gradually through the 45–49 years (1.47 per 100,000) and 50–54 years (7.09 per 100,000) age groups.
The rate of increase accelerates in the older age brackets. The incidence reaches 23.01 per 100,000 men in the 55–59 years group, and further climbs to 59.36 per 100,000 in the 60–64 years group. The sharpest increase occurs between the 65–69 years and 70–74 years groups, with the incidence rising from 128.57 per 100,000 to 175.47 per 100,000.
The peak incidence rate is observed in the 75–79 years age group, where the rate reaches 228.64 per 100,000, marking the highest point on the chart. This indicates that prostate cancer incidence is most concentrated in men aged 75–79. Following this peak, the rate slightly decreases in the 80–84 years group (212.59 per 100,000) and again in the 85+ years group (152.22 per 100,000), suggesting a slight decline in incidence among the very elderly, though the rates remain high.
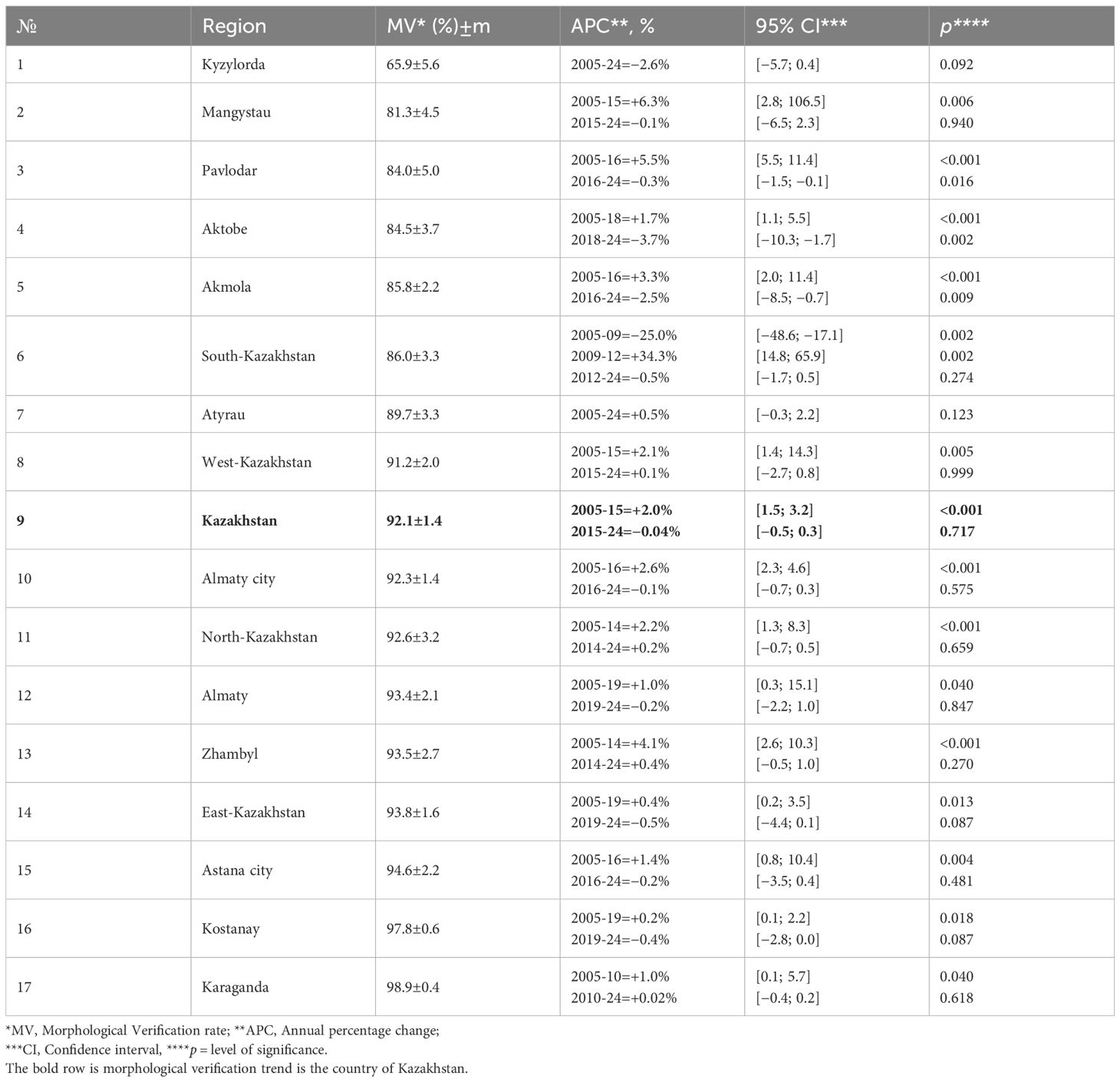
Across 2005–2024, age-specific incidence remained negligible in men <50 years (APC −2.0%/year; p=0.214). In 50–64 years the pattern was cyclical: a significant rise up to the mid-2010s (50–54: +7.9%/year; 55–59: +6.9%/year; 60–64: +11.0%/year), followed by a sharp downturn until 2019-2020 (−13.4%, −28.3%, and −21.7%/year, respectively) and a partial rebound thereafter. For 65–69 years, rates surged in 2009–2016 (+17.5%/year; p=0.004), dropped in 2016–2020 (−19.8%/year; p=0.001), and rose again in 2020–2024 (+11.0%/year; p=0.002) (Figure 3A; Table 3).
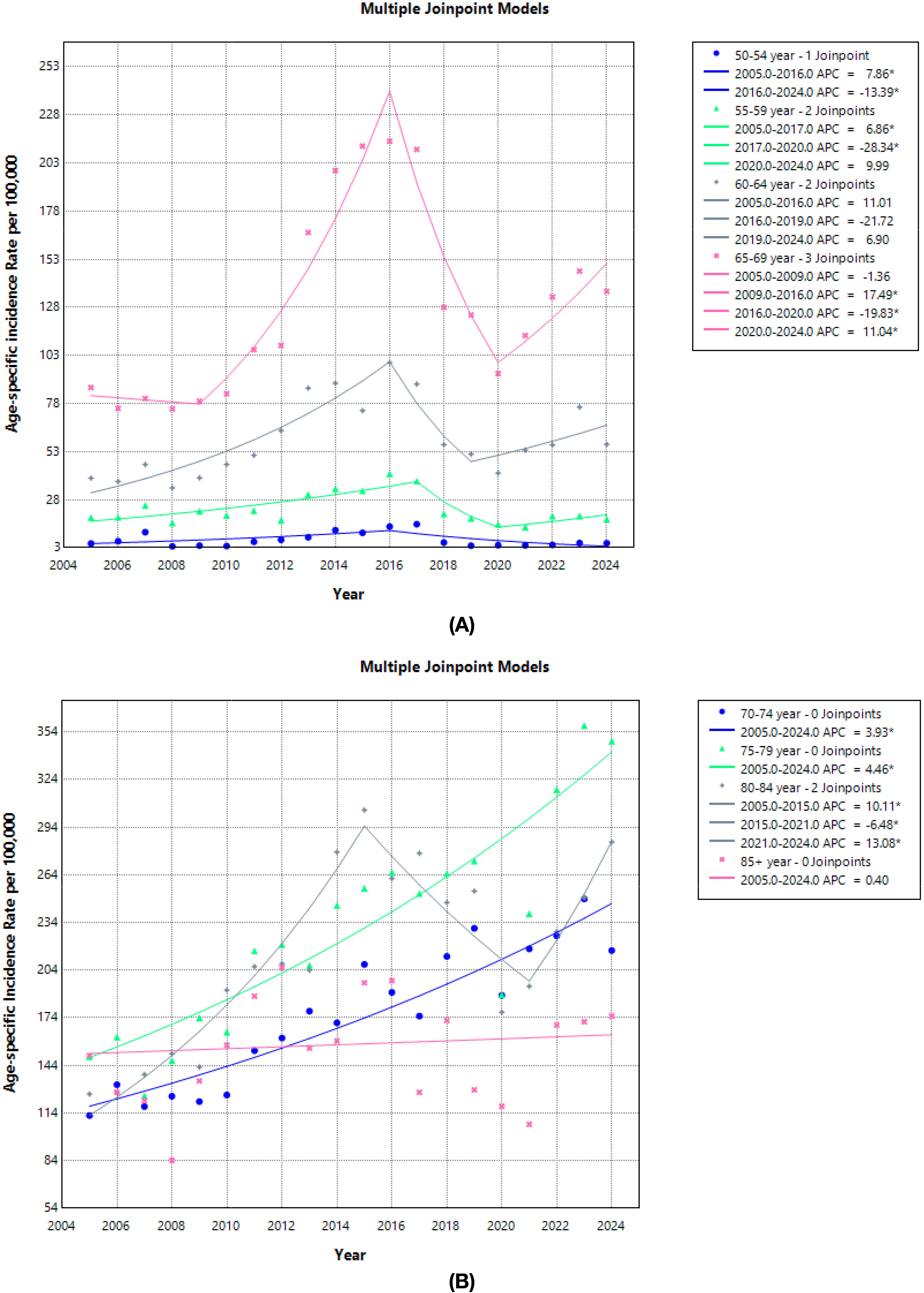
Figure 3. Trends in the age group specific incidence rate of prostate cancer cases for the period 2005-2024. (A) Trends in the age group (50-54, 55-59, 60-64, and 65-69) specific incidence rate of prostate cancer cases for the period 2005-2024. (B) Trends in the age group (70-74, 75-79, 80-44, and 85< year) specific incidence rate of prostate cancer cases for the period 2005-2024.
In older ages the burden increased more steadily. In 70–74 and 75–79 years the whole period showed significant growth (APC + 3.9% and +4.5%/year, respectively; both p<0.001). Among 80–84 years, a long increase in 2005–2015 (+10.1%/year; p=0.001) was followed by a mid-period dip (−6.5%/year; p=0.001) and renewed growth in 2021–2024 (+13.1%/year; p=0.001). The 85+ group was essentially stable (APC + 0.4%/year; p=0.489) (Figure 3B; Table 3).
3.4 Regional incidence rates and trends
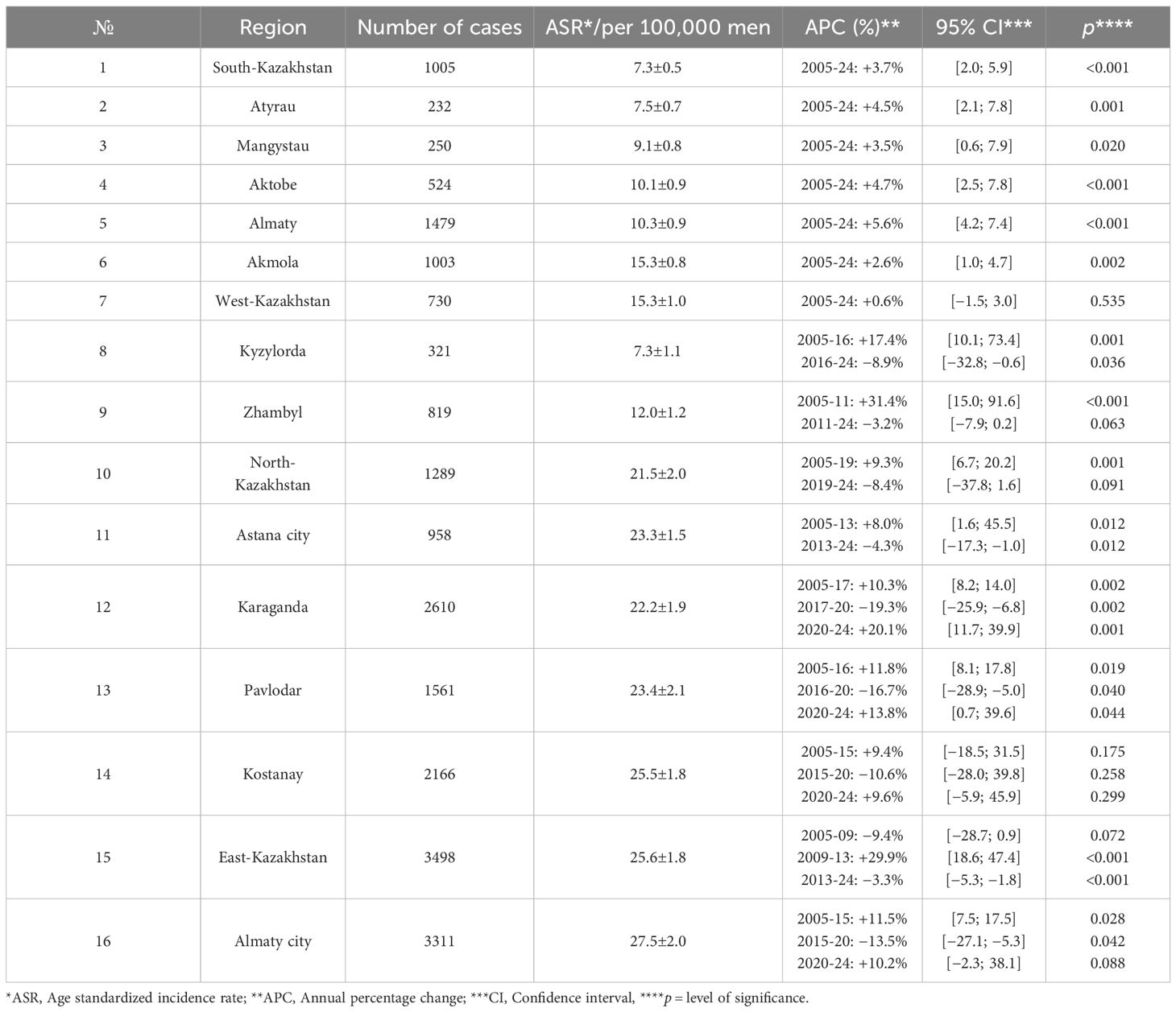
Monotonic, statistically significant increases were observed in South-Kazakhstan, Atyrau, Mangystau, Aktobe, Almaty (region), and Akmola (single-segment APCs ≈2.6 – 5.6%/year; p<0.05). West-Kazakhstan showed no significant long-term change (Figure 4A; Table 4).

Figure 4. Age standardized incidence rate trends of prostate cancer by region, 2005-2024. (A) Age standardized incidence rate trends of prostate cancer by region (South-Kazakhstan, Atyrau, Mangystau, Aktobe, Almaty, Akmola, and West-Kazakhstan), 2005-2024. (B) Age standardized incidence rate trends of prostate cancer by region (Kyzylorda, Zhambyl, North-Kazakhstan, Astana City), 2005-2024. (C) Age standardized incidence rate trends of prostate cancer by region (Karaganda, Pavlodar, Kostanay, East-Kazakhstan, Almaty City), 2005-2024.
Regions with a rise followed by a significant decline included Kyzylorda (2005–2016 +17.4%/year; 2016–2024 −8.9%/year) and Astana city (2005–2013 +8.0%/year; 2013–2024 −4.3%/year). In Zhambyl and North-Kazakhstan the significant early increase (2005–2010 +31.4%/year and 2005–2019 +9.3%/year) was followed by a non-significant decrease (2011–2024 −3.2%/year and 2019–2024 −8.4%/year) (Figure 4B; Table 4).
"Boom–dip–rebound" profiles were evident in Karaganda (2005–2017 +10.3%/year; 2017–2020 −19.3%/year; 2020–2024 +20.1%/year), Pavlodar (2005–2016 +11.8%/year; 2016–2020 −16.7%/year; 2020–2024 +13.8%/year) and Almaty city (2005–2015 +11.5%/year; 2015–2020 −13.5%/year; 2020–2024 +10.2%/year). Kostanay showed non-significant changes across all segments. East Kazakhstan showed decline in 2005–2009, followed by increase in 2009–2013 (APC + 29.9%/year; p<0.001) and decrease in 2013–2024 (APC −3.3%/year; p<0.001) (Figure 4C; Table 4).
3.5 Trend of prostate cancer cases according to the extension stage
From 2005 to 2024, the stage distribution shifted toward earlier diagnosis. The proportion at stage I–II rose from 32.8% to 56.9% (+24.1 percentage points), while stage III declined from 49.7% to 22.9% (−26.8 points); stage IV changed little overall (17.3% → 20.2%, +2.9 points). The share of stage I–II first surpassed stage III in 2012 and remained higher thereafter. Cases with unspecified stage fell from 0.17% to 0%. Across the entire period, the mean proportions were 53.0% for stage I–II (95% CI 49.0-56.9), 29.3% for stage III (95% CI 25.8-32.9), and 17.6% for stage IV (95% CI 15.5-19.7). Linear trends indicate strong trends for stage I–II (R²=0.72) and stage III (R²=0.92), with no meaningful linear trend for stage IV (R²=0.02) (Figure 5).
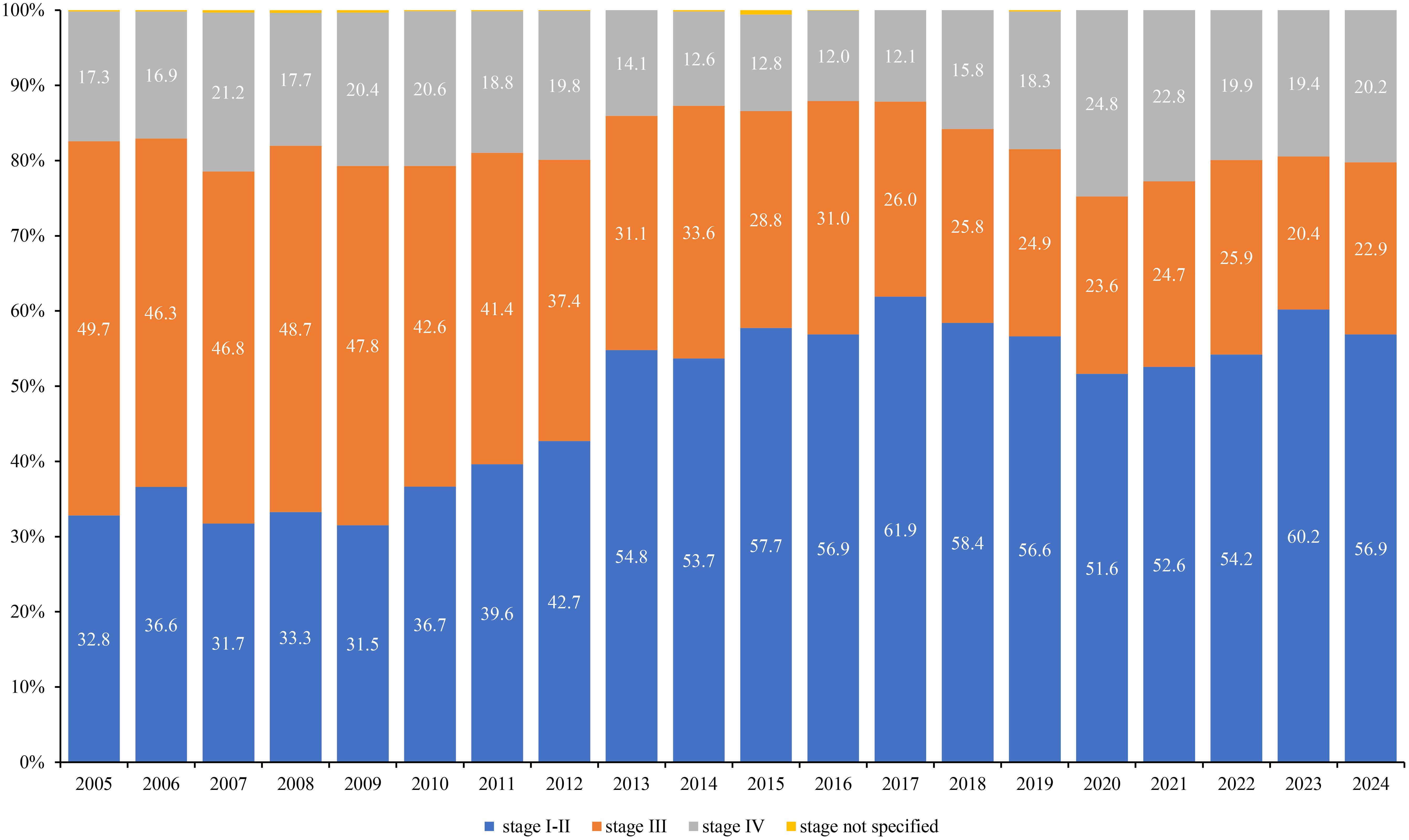
Figure 5. Dynamics of indicators of early diagnosis (stage I–II) and neglect (stage III and IV) of prostate cancer in Kazakhstan in 2005-2024.
It is important to note that the concurrent rise in early-stage cases (stage I–II) alongside the relative stability of late-stage cases (stage IV) requires further considerations. Several factors could be influencing this trend, including potential lead-time bias, overdiagnosis, and diagnostic delays. Lead-time bias could cause an apparent increase in early-stage diagnoses due to the earlier detection of cases that would have previously presented at a later stage. Overdiagnosis might contribute to the rise in stage I–II diagnoses, particularly as more prostate cancers are detected through widespread screening that may not have otherwise caused symptoms or death. Additionally, diagnostic delays for more advanced cases might partially explain why the proportion of stage IV diagnoses has remained relatively constant, despite overall improvements in early detection.
3.6 Trends in prostate cancer incidence by stage and region
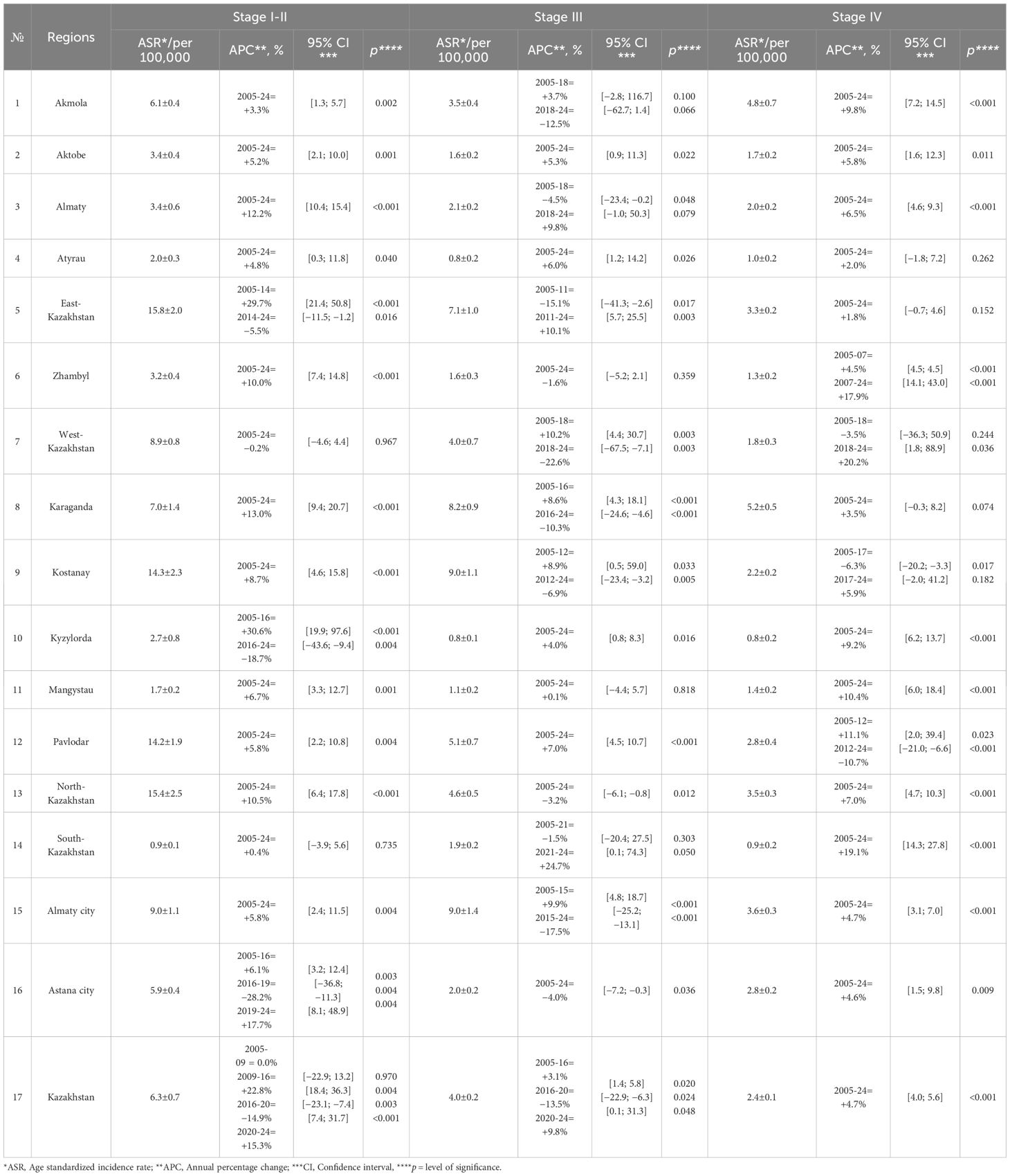
Nationally, the mean stage-specific incidence rates (per 100,000) were 6.3 ± 0.7 for stage I–II, 4.0 ± 0.2 for stage III, and 2.4 ± 0.1 for stage IV. Regionally, early-stage incidence was highest in East-Kazakhstan, North-Kazakhstan, Kostanay, and Pavlodar, and lowest in South-Kazakhstan, Mangystau, Atyrau and Kyzylorda; stage III was highest in Almaty city, Kostanay, Karaganda, and East-Kazakhstan and lowest in Atyrau, Kyzylorda, and Mangystau; stage IV was highest in Karaganda and Akmola and lowest in Kyzylorda, South-Kazakhstan and Atyrau (Table 5).
At the national level, stage I–II incidence increased in 2009–2016, declined in 2016–2020, and increased again in 2020–2024; stage III rose until 2016, declined in 2016–2020, and rose slightly in 2020–2024; stage IV increased throughout 2005–2024. Regionally, stage I–II increased monotonically in Akmola, Aktobe, Almaty (region), Atyrau, Zhambyl, Karaganda, Kostanay, Mangystau, Pavlodar, North-Kazakhstan, and Almaty city, showed no change in West-Kazakhstan and South-Kazakhstan, and followed segmented patterns in East-Kazakhstan (increase to 2014, decrease to 2024) and Astana city (increase to 2016, decrease to 2019, increase to 2024). For stage III, long-term decreases were observed in Zhambyl, North-Kazakhstan, and Astana city; increases in Aktobe, Atyrau, Pavlodar, and Kyzylorda; and no long-term change in Mangystau. Rise-then-fall trajectories occurred in Akmola, West-Kazakhstan, Karaganda, Kostanay, and Almaty city, whereas fall-then-rise patterns were seen in Almaty (region), East-Kazakhstan, and South-Kazakhstan. For stage IV, statistically significant long-term increases were observed in Akmola, Aktobe, Almaty (region), Kyzylorda, Mangystau, North-Kazakhstan, South-Kazakhstan, Almaty city, and Astana city. No significant long-term change was detected in Atyrau, East-Kazakhstan, Karaganda. Zhambyl exhibited two significant increasing segments. West-Kazakhstan had a non-significant decline to 2018 followed by a significant increase in 2018–2024. Pavlodar increased to 2012 and then declined significantly thereafter. Kostanay declined significantly to 2017 and then increased non-significantly in 2017–2024 (Table 5).
3.7 Regional patterns in morphological verification rates
Nationally, morphological verification was high (mean ≈92%) and improved during 2005–2015 (APC + 2.0%/year, p < 0.001), then stabilized with no further change in 2015–2024 (APC −0.04%/year) (Figure 6A; Table 6).
Figure 6. Trends of morphological verification rates by regions. (A) Trends of Morphological Verification Rates in Kazakhstan. (B) Trends of Morphological Verification Rates by Regions (Kyzylorda, Mangystau, Pavlodar, Aktobe, Akmola, South-Kazakhstan, Atyrau, and West-Kazakhstan). (C) Trends of Morphological Verification Rates by Regions (Almaty City, North-Kazakhstan, Almaty, Zhambyl, East-Kazakhstan, Astana City, Kostanay, and Karaganda).
Across regions, five reproducible patterns emerged:
1. Ceiling and stable (mean ≥97%): Karaganda (mean ≈99%) and Kostanay (≈98%) showed only minimal early increases, then flat trends thereafter (Figure 6C; Table 6).
2. High with modest-to-substantial early improvement then plateau (means ~92–95%): early gains were steeper in Almaty city and North-Kazakhstan (APC of +2.6% and +2.2%/year, p < 0.001), modest in East-Kazakhstan, Almaty region and Astana city (APC of +0.4%, +1.0% and 1.4%/year, p < 0.05), and substantial in Zhambyl (+4.1%/year to 2014, p < 0.001), followed by stable trends thereafter (Figure 6C; Table 6).
3. Moderate catch-up then softening (means ~81–91%): Pavlodar, Aktobe, Akmola rose significantly and later showed declines; Mangystau rose steeply to 2015 and then flattened; Atyrau showed no long-term change; West-Kazakhstan increased modestly to 2015 then plateaued (Figure 6B; Table 6).
4. Transitional/oscillatory: South-Kazakhstan declined sharply in 2005–2009, rebounded in 2009–2012, then remained stable (Figure 6B; Table 6).
5. Persistently low outlier: Kyzylorda maintained the lowest morphological verification (mean ≈ 66%) with a non-significant downward tendency (Figure 6B; Table 6).
By the late 2010s, most regions converged to very high morphological verification (≈90–99%), mirroring the national plateau.
4 Discussion
From 2005 to 2024, a total of 21,756 cases of prostate cancer were identified in Kazakhstan. The crude and standardized incidence rates were 12.6 and 17.5 per 100,000 men, respectively. The mean age at diagnosis was 69.8 years, and the age-specific incidence rate (ASIR) showed a consistent increase with age. The ASIR significantly rose from 11.9 in 2005 to 20.7 in 2024, with an annual percent change (APC) of +2.6% (p=0.002). Trends revealed distinct periods: a slight decline from 2005–2008, a sharp increase from 2008–2016, a notable decline from 2016–2020, and a rebound from 2020–2024. Age-specific incidence remained negligible in men under 50 years and showed cyclical patterns in those aged 50–64, with steady increases in older age groups. Geographically, regions like South-Kazakhstan, Atyrau, Mangystau, and Aktobe experienced steady increases, while West-Kazakhstan showed no significant long-term change. The incidence of prostate cancer staged earlier in the study period, with the proportion of stage I–II cases rising significantly from 32.8% to 56.9%, while stage III decreased from 49.7% to 22.9%. The national morphological verification rate remained high, averaging 92%, with regional variations reflecting substantial improvements in most areas.
4.1 Prostate cancer incidence: global context
Globally, prostate cancer incidence has shown substantial variation across regions, with developed countries, particularly those in North America, Northern Europe, and Oceania, experiencing the highest rates (14). Schafer et al. reported that the age-standardized incidence rate (ASIR) for prostate cancer in the world reached 29.4 per 100,000 in 2022 (14).
In the United States, prostate cancer age-adjusted incidence rates decreased from 165.8 per 100,000 in 2007 to 101 per 100,000 in 2014 (APC=-6.51, p<0.05) and then increased to 121.2 per 100,000 in 2021 (APC = 1.87, p< 0.05) (15). Similarly, Gomez et al. found that the incidence of localized prostate cancer increased by 3.7% annually from 2014 to 2017 (16).
In Kazakhstan, the prostate cancer incidence has mirrored global trends, with a notable increase over the past two decades. The data from Kazakhstan's prostate cancer registry show that the age-standardized incidence rate (ASIR) rose from 11.9 per 100,000 in 2005 to 20.7 per 100,000 in 2024, reflecting a 2.6% annual increase. Similar to global trends, this increase can likely be attributed to improvements in healthcare infrastructure, enhanced screening, and greater awareness of the disease. However, Kazakhstan's incidence rate remains lower than that of high-income countries, such as the United States, where the incidence reached 121.2 per 100,000 in 2021 (14).
4.2 Age-specific incidence patterns and trends
Age-specific incidence rates reveal that prostate cancer incidence increases significantly with age, a trend observed both in Kazakhstan and globally. Jacklin et al. noted that older men are more likely to be diagnosed with prostate cancer due to the nature of the disease's progression (17), and Kazakhstan's findings corroborate this, with the highest incidence observed in men aged 75–79 years, where the rate was 228.64 per 100,000. This trend aligns with findings from California, United States, where Van Blarigan et al. (18) reported that 92.1% of prostate cancer cases were among men aged 55 and older. Between 2004 and 2021, California recorded 387,636 prostate cancer cases, including 27,938 at the distant stage, and 58,754 prostate cancer-related deaths. In this cohort, 203,038 cases (52.4%) occurred in men aged 55 to 69, while 153,884 cases (39.7%) were diagnosed in men aged 70 and above (18).
4.3 Regional variations in prostate cancer incidence
Regional disparities in prostate cancer incidence are evident in both Kazakhstan and globally, and they are often driven by differences in healthcare access and socioeconomic factors. Seikkula et al. found that in Finland, urban area have higher incidence rate of prostate cancer than rural area (19).
A similar regional pattern was observed in Kazakhstan. Urban centers like Almaty and Karaganda reported higher prostate cancer incidence, likely due to better access to screening programs and healthcare services. In contrast, rural regions such as South Kazakhstan and Mangystau exhibited lower incidence rates, which could indicate underdiagnosis or delayed diagnoses of prostate cancer.
4.4 Covid-19 pandemic effect on prostate cancer incidence rate
Although Kazakhstan-specific data on PSA screening uptake and diagnostic throughput are not available, the observed decline in incidence between 2016 and 2020, followed by a rebound after 2020, is temporally compatible with contraction and subsequent partial recovery of oncological service activity during and after the COVID-19 pandemic. Similar diagnostic disruptions were widely reported worldwide. According to Soerjomataram et al., 65.6% of population-based cancer registries across 90 countries experienced interruptions in case reporting, data collection, and pathology verification due to pandemic-related restrictions. These disruptions likely resulted in under-registration of new cancer cases and delayed diagnosis globally (20).
More specifically for prostate cancer, Mangone et al. conducted a population-based analysis in Northern Italy and demonstrated a 31% reduction in newly diagnosed prostate cancer cases in 2020 (21). Taken together, these factors suggest that the temporary downturn in prostate cancer incidence in 2016–2020 and its rebound after 2020 may represent a compound effect of (a) reduced diagnostic and screening activity during the pandemic, (b) delayed registration and patient flow disruptions due to national quarantine measures, and (c) gradual restoration of service capacity in 2021–2022. This interpretation aligns with international evidence on COVID-19-induced diagnostic backlogs and supports the inclusion of pandemic-related contextual factors when interpreting prostate cancer incidence trends in Kazakhstan.
4.5 Strengths and limitations
4.5.1 Strengths
This study utilizes a comprehensive nationwide dataset covering a 20-year period (2005–2024), facilitating a thorough evaluation of long-term trends and regional variations in prostate cancer incidence. Age-standardized rates were calculated according to international standards, and age-specific patterns were meticulously delineated. Using Joinpoint segmented regression gives clear estimates of period-specific APCs and AAPCs, which show when incidence "bump-dip-rebound" patterns happen. Stage-specific and morphological-verification analyses enhance the validity of registry signals and augment the study's policy relevance by correlating temporal trends with diagnostic and screening dynamics.
4.5.2 Limitations
The analysis is based on registry data, which makes it ecological. Individual-level factors like PSA testing history, biopsy results, Gleason grade, comorbidities, and treatment are not available, which makes it hard to draw conclusions about the effects of screening and outcomes.
Changes in diagnostic practice, staging, or coding may explain some of the temporal trends, rather than real changes in disease occurrence.
Several joinpoint segments only cover a few years and small counts, so short-term APC estimates should be taken with a grain of salt. Even though morphological verification is high nationally, there is meaningful regional variability (notably lower %MV in some regions), which could lead to different ascertainment across areas. Finally, the lack of linked mortality and survival data makes it impossible to see if earlier-stage detection led to better outcomes; pandemic-era disruptions around 2020–2021 may also make it hard to interpret trends.
5 Conclusion
In sum, Kazakhstan exhibits growing incidence with period inflections, a national shift toward earlier stage at diagnosis, and concurrent increases in stage IV, set against high and converging morphological verification. These features align with global narratives on PSA dynamics and pandemic disruptions, while regional typologies identify where early-detection gains are durable and where late-stage pressure persists. Moving from description to attribution will require formal difference-in-differences/event-study designs and sensitivity analyses that exclude 2020–2021, alongside audits of screening uptake, diagnostic capacity, and time-to-biopsy/treatment to ensure that earlier detection translates into better outcomes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the Local Ethics Commission of the Central Asian Institute for Medical Research. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NI: Funding acquisition, Writing – review & editing, Project administration, Supervision. IK: Funding acquisition, Writing – review & editing, Project administration, Supervision. NY: Data curation, Investigation, Conceptualization, Writing – review & editing. ZT: Writing – review & editing, Conceptualization, Investigation, Data curation. GI: Methodology, Writing – original draft, Validation. DT: Validation, Methodology, Writing – original draft. AJ: Methodology, Validation, Writing – original draft. RM: Formal analysis, Writing – review & editing. GK: Validation, Methodology, Writing – original draft. AK: Formal analysis, Writing – review & editing. SD: Writing – review & editing, Formal analysis. DB: Formal analysis, Writing – review & editing. IS: Writing – review & editing, Formal analysis. KN: Writing – original draft, Validation, Methodology. AB: Formal analysis, Writing – review & editing. NM: Methodology, Validation, Writing – original draft. AH: Formal analysis, Writing – review & editing, Writing – original draft. ZB: Funding acquisition, Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Ministry of Science and Higher Education of the Republic of Kazakhstan (project No. AP23484167: “The burden of prostate cancer in Kazakhstan: assessment of the current situation, the role of determinants and innovative ways to solve it”).
Acknowledgments
The authors express their sincere gratitude to the Ministry of Healthcare of the Republic of Kazakhstan for providing the data essential for this research. This work was supported by the Ministry of Science and Higher Education of the Republic of Kazakhstan (project No. AP23484167: “The burden of prostate cancer in Kazakhstan: assessment of the current situation, the role of determinants and innovative ways to solve it”). Selected findings from this study form the basis of the Candidate of Medical Sciences dissertation of Nariman Yermek, being conducted within the postgraduate program of the I.K. Akhunbaev Kyrgyz State Medical Academy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used ChatGPT 4o in order to improve language and readability. After using this service, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
APC, Annual Percent Change; AAPC, Average Annual Percent Change; ASIR, Age-Specific Incidence Rate; ASR, Age-Standardized Rate; CI, Confidence Interval; CIR, Crude Incidence Rate; ICD-10, International Classification of Diseases, 10th Revision; MV, Morphological Verification; PSA, Prostate-Specific Antigen; SE, Standard Error; TNM, Tumor–Node–Metastasis classification; WHO, World Health Organization
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. James ND, Tannock I, N’Dow J, Feng F, Gillessen S, Ali SA, et al. The Lancet Commission on prostate cancer: planning for the surge in cases. Lancet. (2024) 403:1683–722. doi: 10.1016/S0140-6736(24)00651-2
3. International WCRF. Prostate cancer(2025). Available online at: https://www.wcrf.org/preventing-cancer/cancer-types/prostate-cancer/ (Accessed September 10, 2025).
4. Smailova D, Ospanov E, Gazaliyeva M, Kaidarova D, Shatkovskaya O, Zamanbekova Z, et al. Epidemiology of prostate cancer in the republic of Kazakhstan. Iranian J Public Health. (2019) 48:2216. doi: 10.18502/ijph.v48i12.3554
5. Kassymbekova F, Glushkova N, Dunenova G, Kaidarova D, Kissimova-Skarbek K, Wengler A, et al. Burden of major cancer types in Almaty, Kazakhstan. Sci Rep. (2024) 14:20536. doi: 10.1038/s41598-024-71449-5
6. Tokanova S, Semenova Y, Bolsynbekova S, and Zhumykbaeva N. Mortality and morbidity from prostate cancer in the Republic of Kazakhstan from 2007 to 2016. Georgian Med News. (2017) 2017(272):17–22.
7. Ishkinin Y, Zhylkaidarova A, Nurgaliyev N, Auyezova E, Oshibayeva A, and Gorbunova N. Population-based prostate cancer screening in Kazakhstan. Iranian J Public Health. (2017) 46:917.
8. Akizhanova M, Iskakova EE, Kim V, Wang X, Kogay R, Turebayeva A, et al. PSA and Prostate Health Index based prostate cancer screening in a hereditary migration complicated population: implications in precision diagnosis. J Cancer. (2017) 8:1223. doi: 10.7150/jca.18012
9. Smailova DS, Fabbro E, Ibrayev SE, Brusati L, Semenova YM, Samarova US, et al. Epidemiological and economic evaluation of a pilot prostate cancer screening program. Prostate Cancer. (2020) 2020:6140623. doi: 10.1155/2020/6140623
10. KZ G. Bureau of National Statistics of the Agency for strategic planning and reforms of the Republic of Kazakhstan. GOV KZ stat gov kz [Google Scholar] (2023).
11. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, and Inoue M. Age standardization of rates: a new WHO standard. Geneva: World Health Organization. (2001) 9:1–14.
12. National Cancer Institute DoCCaPS. Joinpoint trend analysis software(2025). Available online at: https://surveillance.cancer.gov/joinpoint/ (Accessed March 15, 2025).
13. Kim HJ, Fay MP, Feuer EJ, and Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. (2000) 19:335–51. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z
14. Schafer EJ, Laversanne M, Sung H, Soerjomataram I, Briganti A, Dahut W, et al. Recent patterns and trends in global prostate cancer incidence and mortality: an update. Eur Urol. (2025) 87:302–13. doi: 10.1016/j.eururo.2024.11.013
15. Merrill RM. Prostate cancer incidence rates, trends, and treatment related to prostate-specific antigen screening recommendations in the United States. Cancer Epidemiol. (2024) 93:102700. doi: 10.1016/j.canep.2024.102700
16. Gomez SL, Washington SL 3rd, Cheng I, Huang FW, and Cooperberg MR. Monitoring prostate cancer incidence trends: value of multiple imputation and delay adjustment to discern disparities in stage-specific trends. Eur Urol. (2021) 79:42–3. doi: 10.1016/j.eururo.2020.10.022
17. C J, Y P, Sf B, and Rj B. More men die with prostate cancer than because of it” - an old adage that still holds true in the 21st century. Cancer Treat Res Commun. (2021) 26:100225. doi: 10.1016/j.ctarc.2020.100225
18. Van Blarigan EL, McKinley MA, Washington SL 3rd, Cooperberg MR, Kenfield SA, Cheng I, et al. Trends in prostate cancer incidence and mortality rates. JAMA Netw Open. (2025) 8:e2456825. doi: 10.1001/jamanetworkopen.2024.56825
19. Seikkula H, Kaipia A, Boström PJ, Malila N, Pitkäniemi J, and Seppä K. Periodic trends in geographical variation of prostate cancer incidence and mortality in Finland between 1985 and 2019. Acta Oncol. (2022) 61:1209–15. doi: 10.1080/0284186X.2022.2112971
20. Soerjomataram I, Bardot A, Aitken J, Piñeros M, Znaor A, Steliarova-Foucher E, et al. Impact of the COVID-19 pandemic on population-based cancer registry. Int J Cancer. (2022) 150:273–8. doi: 10.1002/ijc.33792
Keywords: prostate cancer, Kazakhstan, epidemiology, incidence trends, PSA screening, joinpoint regression, stage distribution, morphological verification
Citation: Igissinova G, Igissin N, Kudaibergenova I, Yermek N, Telmanova Z, Turebayev D, Jexenova A, Moldagali R, Khairli G, Kazhitaev A, Dyakov S, Baibosynov D, Shishkin I, Nogoibaeva K, Baytelieva A, Mamatov N, Halimi A and Bilyalova Z (2025) Stage-specific and regional trends in prostate cancer incidence in Kazakhstan, 2005–2024. Front. Oncol. 15:1719720. doi: 10.3389/fonc.2025.1719720
Received: 06 October 2025; Accepted: 10 November 2025; Revised: 02 November 2025;
Published: 28 November 2025.
Edited by:
Shaokai Zhang, Henan Provincial Cancer Hospital, ChinaReviewed by:
Cheng Song, Renmin University of China, ChinaXinying Yue, Huazhong University of Science and Technology, China
Copyright © 2025 Igissinova, Igissin, Kudaibergenova, Yermek, Telmanova, Turebayev, Jexenova, Moldagali, Khairli, Kazhitaev, Dyakov, Baibosynov, Shishkin, Nogoibaeva, Baytelieva, Mamatov, Halimi and Bilyalova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zarina Bilyalova, YmlseWFsb3ZhLnBoZEBnbWFpbC5jb20=
 Gulnur Igissinova1,2
Gulnur Igissinova1,2 Nurbek Igissin
Nurbek Igissin Zhansaya Telmanova
Zhansaya Telmanova Altynai Baytelieva
Altynai Baytelieva Aram Halimi
Aram Halimi