- 1Department of Otolaryngology – Head and Neck Surgery, Thomas Jefferson University Hospital, Philadelphia, PA, United States
- 2Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA, United States
- 3Sidney Kimmel Cancer Center, Thomas Jefferson University Hospital, Philadelphia, PA, United States
Introduction: Phosphodiesterase-5 (PDE5) inhibitors, commonly prescribed for erectile dysfunction, may exhibit immunomodulatory properties by reducing myeloid-derived suppressor cell activity and enhancing T-cell responses. This study evaluated the association between PDE5 inhibitor use and overall survival (OS) in male-predominant solid organ malignancies.
Methods: Male-predominant solid tumors were identified using male-to-female incidence ratio ≥3:1 in SEER database: prostate, bladder, colon, esophageal, hypopharyngeal, laryngeal, tonsillar, and testicular cancers. Using the TriNetX Research Network, we compared male patients prescribed PDE5 inhibitors within 6 months of cancer diagnosis to those without PDE5 exposure. Propensity score matching (PSM) was performed on demographic, clinical, and oncologic variables. OS was assessed in a combined pan-cancer cohort, stratified by overall stage, and subgroups of included cancers. Kaplan-Meier survival analyses were conducted for 1-, 3-, and 5-year OS from diagnosis.
Results: After PSM, 108,630 patients were included in each arm of the pan-cancer analysis. PDE5 inhibitor exposure was associated with significantly improved OS at 1, 3, and 5 years compared with controls (5-year OS 93.8% vs 86.6%; HR, 0.42 [95% CI, 0.41–0.44]). Stratified analyses demonstrated significantly improved OS across all four stages, with hazard ratios ranging from 0.40 to 0.57. Subgroup analyses by tumor site likewise showed statistically significant improvement in OS for every included cancer type, with hazard ratios ranging from 0.35 to 0.70.
Conclusions: PDE5 inhibitor use was consistently associated with improved OS across all male-predominant cancers, including in stage-stratified analyses. These findings support further investigation into the potential role of PDE5 inhibitors in cancer outcomes.
Introduction
The tumor microenvironment (TME) promotes immune evasion and therapeutic resistance through the activity of myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), which impair cytotoxic T-cell function and establish and immunosuppressive microenvironment (1–3). These cells also contribute to metabolic dysregulation via increased polyamine production driven by arginase 1 activity (4–6). Given these mechanisms, pharmacologic strategies that disrupt MDSC and TAM function are of growing interest.
Phosphodiesterase-5 (PDE5) inhibitors, originally developed for pulmonary hypertension and more widely known for treating erectile dysfunction, have been shown to possess unexpected immunomodulatory properties (7, 8). A chance clinical observation in a patient receiving the PDE5 inhibitor tadalafil suggested a significant antimyeloma response, potentially through reduction of MDSC-mediated immunosuppression (9). Preclinical studies subsequently demonstrated that PDE5 inhibitors can downregulate arginase 1 and nitric oxide synthase-2 in tumor-associated myeloid cells, leading to reduced immune suppression and increased CD8+ T-cell infiltration into tumors (10–12).
Several retrospective studies have suggested that PDE5 inhibitor use may be associated with a lower incidence of colorectal cancer (13). Furthermore, experimental models combining PDE5 inhibitors with vaccine-based or checkpoint-based immunotherapies have reported improved antitumor responses in pancreatic ductal adenocarcinoma and hepatocellular carcinoma (14, 15). Despite this growing body of preclinical and early clinical data, large real-world analyses evaluating the impact of PDE5 inhibitors on survival are lacking. In this study, we investigated the association between PDE5 inhibitor exposure and overall survival in male-predominant solid organ malignancies, using propensity score–matched data from a large real-world global database.
Materials and methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The institutional review board of Thomas Jefferson University exempted the study from review and waived informed consent because only deidentified records were used. Data were obtained from the TriNetX Collaborative Network (TriNetX, LLC, Cambridge, MA), a real-time multicenter research platform of health care organizations across the world. It contains deidentified electronic health record (EHR) data from more than 130 million patients across diverse demographic and socioeconomic backgrounds. From this network, we queried patients in the United States. Data from 2000 through 2025 were analyzed. Race information was obtained from institutional EHRs and categorized as Asian, American Indian or Alaska Native, Black or African American, Native Hawaiian or Other Pacific Islander, White, or unknown. Ethnicity was coded as Hispanic or Latino, not Hispanic or Latino, or unknown/not reported, based on standardized EHR data.
Study population
This epidemiologic investigation used data from 103 healthcare organizations in the United States between 2000 and 2025. International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes were used to define cancer type and patient characteristics. Male-predominant solid organ cancers were defined as malignancies with a male-to-female incidence ratio ≥3:1 in the Surveillance, Epidemiology, and End Results (SEER) database and were confirmed to show similar sex distribution patterns within the TriNetX dataset. This threshold was selected to capture cancers with clear male predominance while maintaining sufficient cohort size for a representative pan-cancer analysis. The cancer types included were prostate, bladder, colon, esophageal, hypopharyngeal, laryngeal, tonsillar, and testicular. Eligible patients were male, aged 18 years or older, with a primary diagnosis of one of the abovementioned cancers. We restricted the cohort to adult males to reduce sex- and indication-related confounding and to ensure adequate exposure prevalence, as PDE5 inhibitors are prescribed primarily to men for erectile dysfunction/benign prostatic hyperplasia. The exposure cohort included patients prescribed a PDE5 inhibitor (avanafil, sildenafil, tadalafil, or vardenafil) within 6 months before or after initial cancer diagnosis. The control cohort comprised patients with no record of PDE5 inhibitor prescription. Patients without documented survival follow-up or complete oncologic staging information were excluded.
Outcome variables
The primary outcome was overall survival (OS), defined from the date of first cancer diagnosis and evaluated at 1, 3, and 5 years. To account for potential confounders, OS was analyzed in three ways: (1) in a combined pan-cancer cohort, (2) stratified by overall stage, and (3) subgroups of individual cancer types. Propensity score matching (PSM) was performed to further balance key demographic, clinical, and oncologic variables that could influence survival for all three analyses. Propensity score matching was repeated independently for each stage-stratified and cancer-specific analysis to ensure balanced covariate distributions within these subsets. Finally, to contextualize survival outcomes, the 5-year OS for each cancer type was evaluated in all TriNetX patients.
Statistical analysis
Descriptive statistics were used to summarize baseline demographic and clinical characteristics. Continuous variables were reported as mean (range), and categorical variables as frequency (percentage). PSM was conducted in a 1:1 ratio using nearest-neighbor matching without replacement and a caliper size of 0.1 pooled standard deviations. Patients were matched based on ICD‐10 codes for age at diagnosis, race, ethnicity, cardiovascular, circulatory and respiratory diseases, type 2 diabetes, tobacco use, alcohol use disorders, and included cancers (Tables 1, 2). Covariate balance after matching was assessed using standardized differences. Kaplan–Meier survival analyses were performed to estimate OS, and survival curves were compared using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression models. All analyses were performed using the TriNetX Analytics platform.
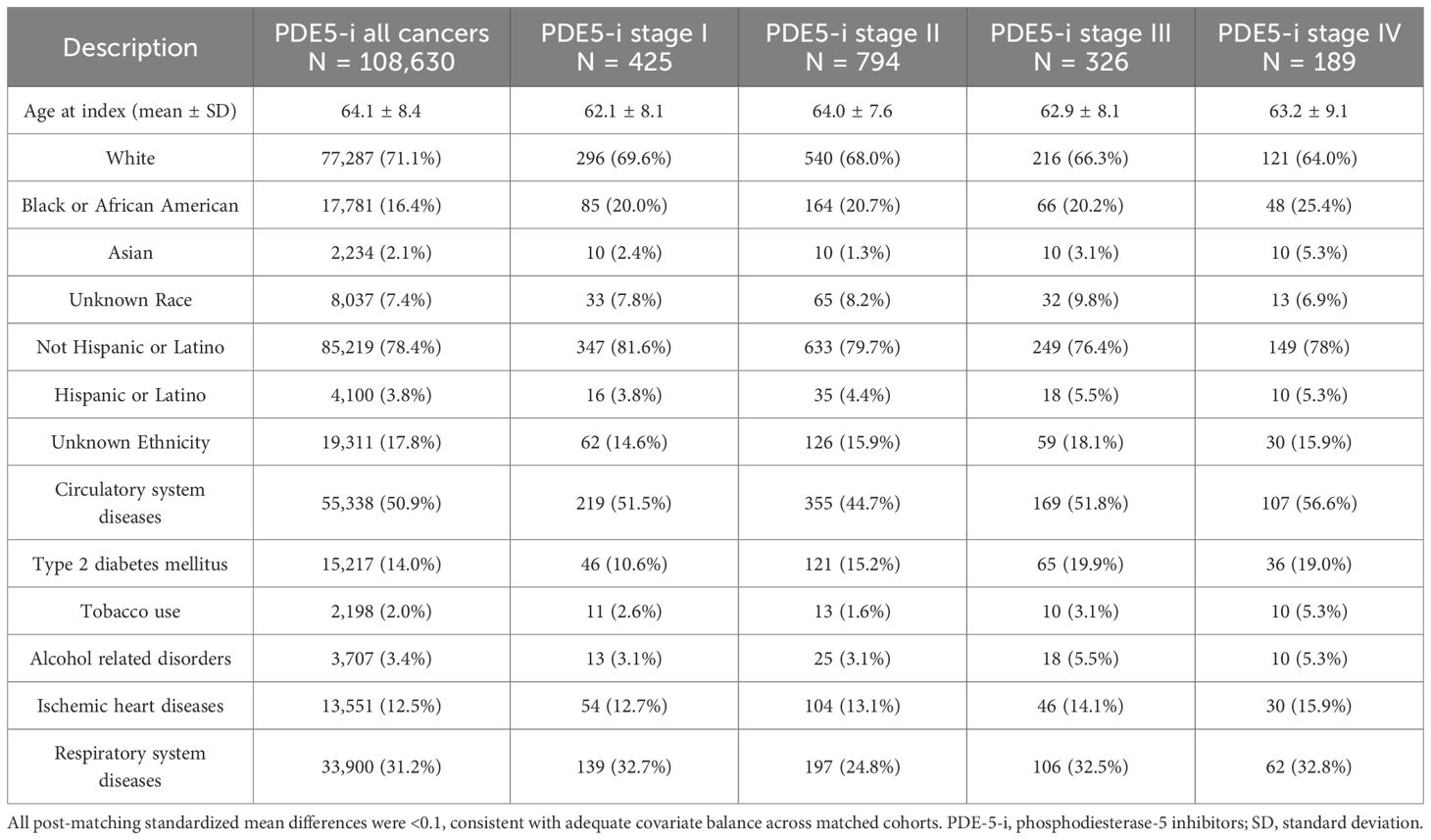
Table 1. Phosphodiesterase-5 inhibitors exposed cohort baseline characteristics for all cancers combined and stratified by overall stage, after propensity score matching (PSM).
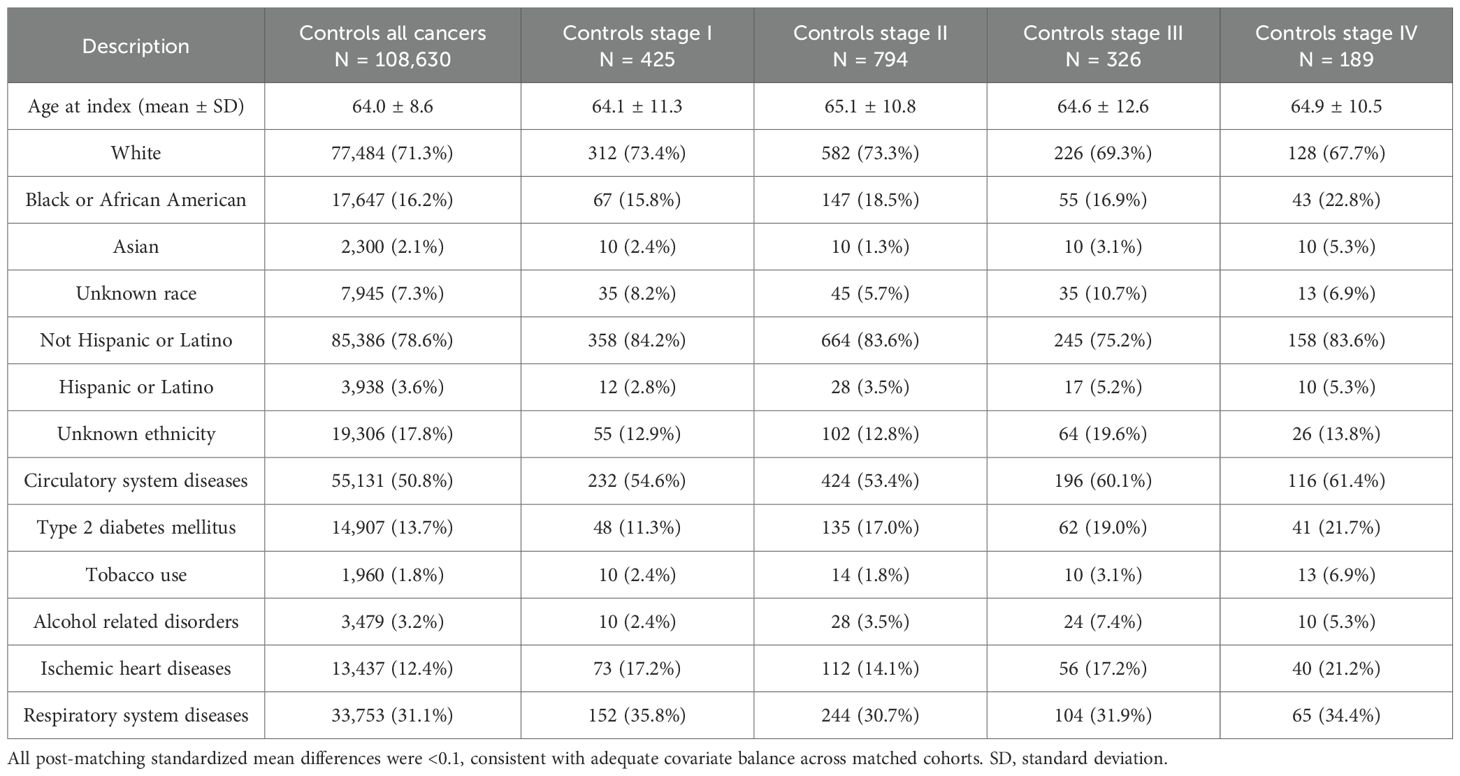
Table 2. Control cohort baseline characteristics for all cancers combined and stratified by overall stage, after propensity score matching (PSM).
Results
All cancers
Before propensity score matching (PSM), the PDE5 inhibitors exposed cohort included 109,830 patients, and the control cohort included 1,393,522 patients. After PSM, 108,630 patients were included in each cohort. No significant differences in age, demographics, comorbid conditions and distribution of included cancers were observed between the two groups after matching (Tables 1, 2). In the overall pan-cancer analysis of male‐predominant solid organ malignancies, PDE5 inhibitor exposure was associated with significantly improved OS at all measured timepoints compared with controls (1‐year OS 98.6% vs 95.7%, p<0.001; 3‐year OS 96.1% vs 90.7%, p<0.001; 5‐year OS 93.8% vs 86.6%, p<0.001; HR, 0.42 [95% CI, 0.41–0.44]; Figure 1, Table 3).

Figure 1. Kaplan–Meier curves for overall survival (OS) up to 5 years in male patients with male-predominant cancers, displaying all cancers combined and stratified by overall stage. OS is shown for phosphodiesterase-5 inhibitor exposed (PDE5-i) group versus controls, after propensity score matching (PSM). Hazard ratios (HR) and 95% confidence intervals (CI) are provided for each cancer type.
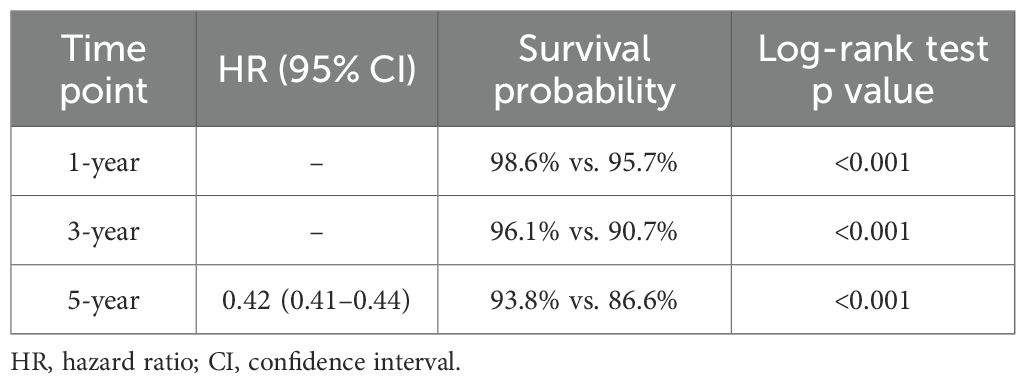
Table 3. Overall survival in patients prescribed PDE5 inhibitors compared with controls for all male-predominant cancers.
All cancers stratified by overall stage
Staging data was available for 2,626 patients in the PDE5 inhibitor cohort and 23,983 patients in the control cohort. Post‐PSM, each cohort included 429, 794, 326 and 189 patients for Stage I, II, III and IV, respectively (Tables 1, 2). For Stage I cancers, PDE5 inhibitor exposure was associated with significantly improved 3- and 5-year OS compared with controls (1-year OS not measurable; 3‐year OS 96.1% vs 92.6%, p=0.03; 5‐year OS 92.1% vs 87.6%, p=0.02; HR, 0.57 [95% CI, 0.35–0.93]; Figure 1). For Stage II cancers, PDE5 inhibitor exposure was also associated with significantly improved 3- and 5-year OS compared with controls (1-year OS not measurable; 3‐year OS 97.0% vs 90.5%, p<0.001; 5‐year OS 93.3% vs 85.5%, p<0.001; HR, 0.41 [95% CI, 0.28–0.59]; Figure 1). For Stage III cancers, PDE5 inhibitor exposure was associated with significantly improved OS at all timepoints compared with controls (1-year OS 96.4% vs 92.9%, p=0.05; 3‐year OS 91.7% vs 82.0%, p=0.001; 5‐year OS 88.3% vs 71.4%, p<0.001; HR, 0.40 [95% CI, 0.26–0.60]; Figure 1). For Stage IV cancers, PDE5 inhibitor exposure was also associated with significantly improved OS at all timepoints compared with controls (1-year OS 92.8% vs 82.4%, p=0.004; 3‐year OS 75.1% vs 56.5%, p<0.001; 5‐year OS 65.6% vs 47.2%, p<0.001; HR, 0.52 [95% CI, 0.37–0.74]; Figure 1).
Prostate cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 99,010 patients with prostate cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with significantly improved OS at all timepoints compared with controls (1‐year OS 99.2% vs 96.7%, p<0.001; 3‐year OS 97.3% vs 92.0%, p<0.001; 5‐year OS 95.3% vs 88.1%, p<0.001; HR, 0.35 [95% CI, 0.34–0.37]; Figure 2). The 5-year OS of the entire TriNetX prostate cancer cohort is 85.5% (N = 1,074,022).
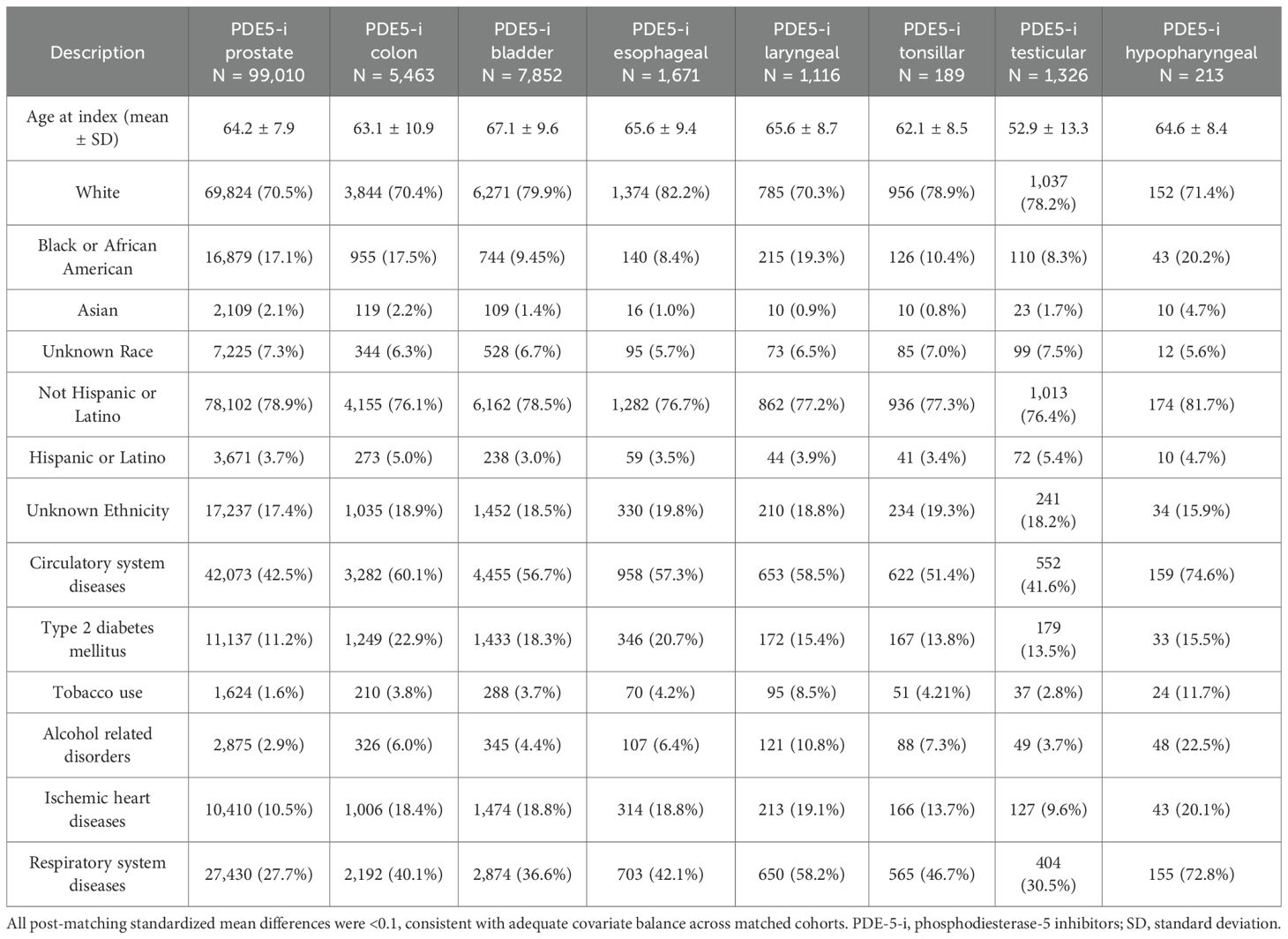
Table 4. Phosphodiesterase-5 inhibitors exposed cohort baseline characteristics for each cancer type, after propensity score matching (PSM).
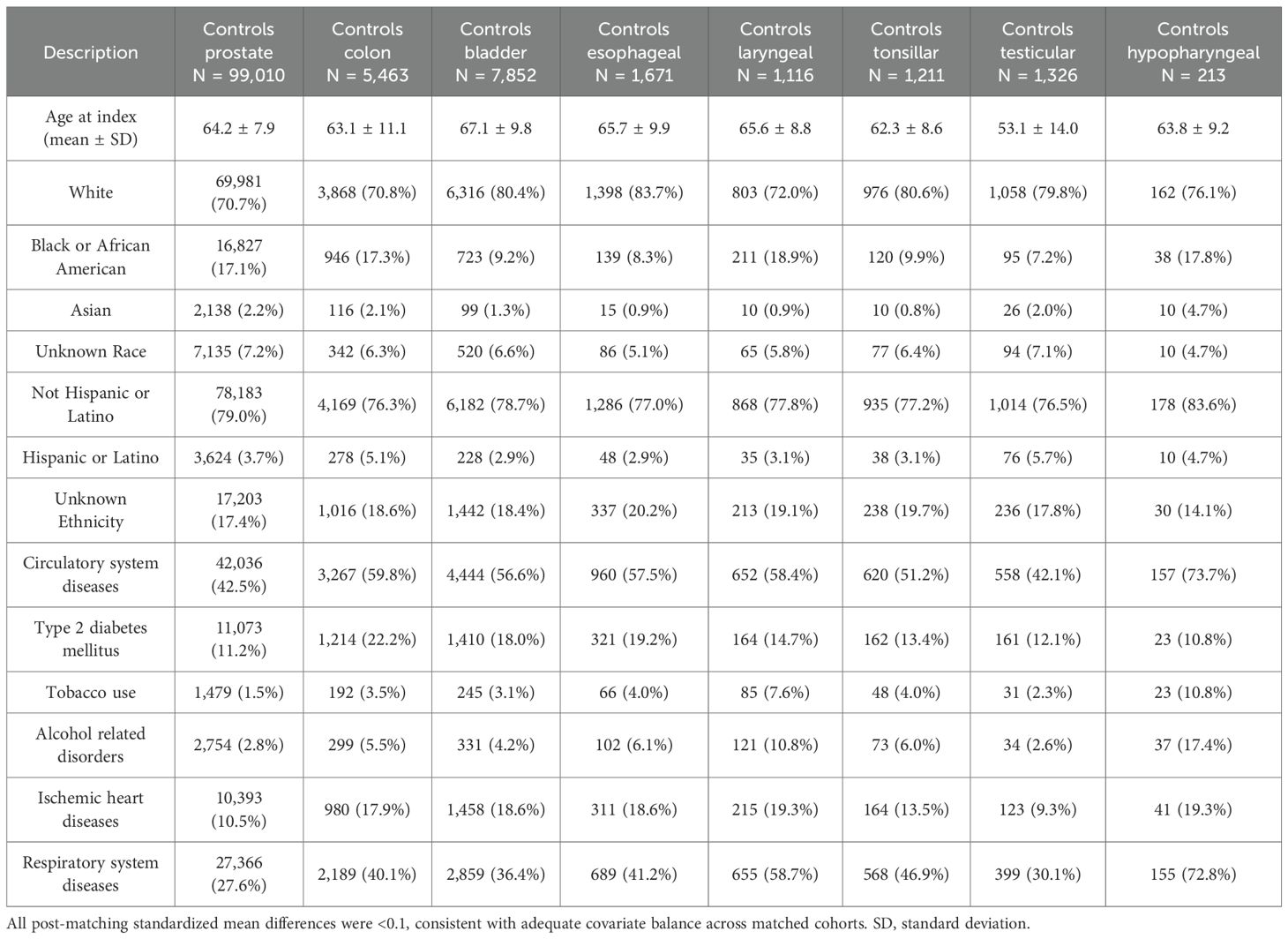
Table 5. Control cohort baseline characteristics for each cancer type, after propensity score matching (PSM).
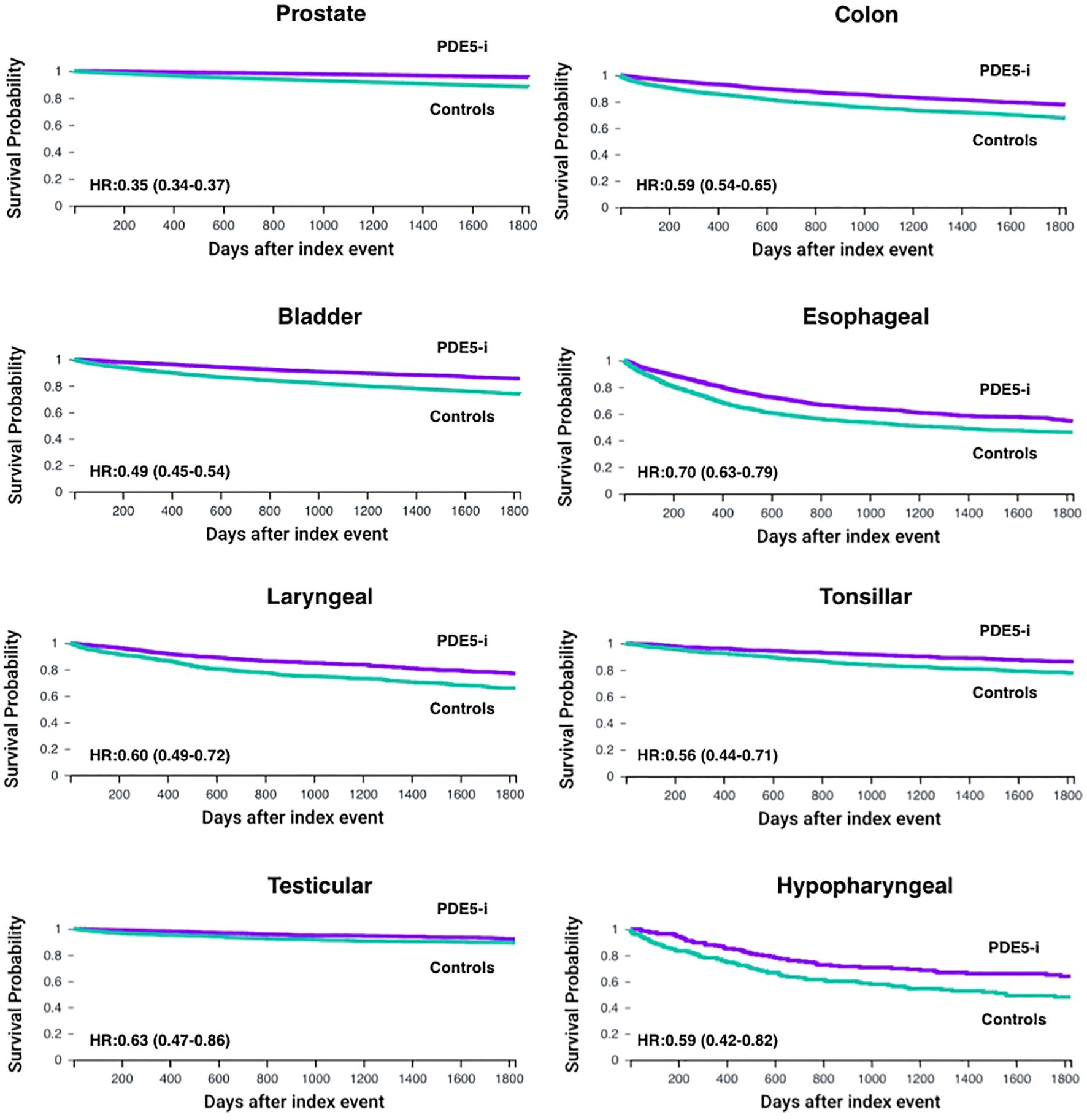
Figure 2. Kaplan–Meier curves for overall survival (OS) up to 5 years in male patients with male-predominant cancers, displaying individual cancer types. OS is shown for phosphodiesterase-5 inhibitor exposed (PDE5-i) group versus controls, after propensity score matching (PSM) for age, demographics and potential clinical confounders. Hazard ratios (HR) and 95% confidence intervals (CI) are provided for each cancer type.
Colon cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 5,463 patients with colon cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with improved OS at all timepoints compared with controls (1‐year OS 93.4% vs 86.9%, p<0.001; 3‐year OS 84.1% vs 75.3%, p<0.001; 5‐year OS 77.7% vs 67.4%, p<0.001; HR, 0.59 [95% CI, 0.54–0.65]; Figure 2). The 5-year OS of the entire TriNetX colon cancer male cohort is 68.1% (N = 229,821).
Bladder cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 7,852 patients with bladder cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with improved OS at all timepoints compared with controls (1‐year OS 96.3% vs 90.7%, p<0.001; 3‐year OS 90.2% vs 80.8%, p<0.001; 5‐year OS 85.3% vs 73.8%, p<0.001; HR, 0.49 [95% CI, 0.45–0.54]; Figure 2). The 5-year OS of the entire TriNetX bladder cancer male cohort is 72.7% (N = 219,836).
Esophageal cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 1,671 patients with esophageal cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with improved OS at all timepoints compared with controls (1‐year OS 81.5% vs 69.8%, p<0.001; 3‐year OS 62.6% vs 51.2%, p<0.001; 5‐year OS 54.5% vs 46.0%, p<0.001; HR, 0.70 [95% CI, 0.63–0.79]; Figure 2). The 5-year OS of the entire TriNetX esophageal cancer male cohort is 44.6% (N = 83,839).
Laryngeal cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 1,116 patients with laryngeal cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with improved OS at all timepoints compared with controls (1‐year OS 92.5% vs 85.0%, p<0.001; 3‐year OS 84.1% vs 73.3%, p<0.001; 5‐year OS 76.9% vs 65.8%, p<0.001; HR, 0.60 [95% CI, 0.49–0.72]; Figure 2). The 5-year OS of the entire TriNetX laryngeal cancer male cohort is 65.9% (N = 62,015).
Tonsillar cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 1,211 patients with tonsillar cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with significantly improved OS at all timepoints compared with controls (1‐year OS 96.2% vs 90.7%, p<0.001; 3‐year OS 90.7% vs 81.0%, p<0.001; 5‐year OS 86.1% vs 77.5%, p<0.001; HR, 0.56 [95% CI, 0.44–0.71]; Figure 2). The 5-year OS of the entire TriNetX tonsillar cancer male cohort is 76.7% (N = 35,576).
Testicular cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 1,326 patients with testicular cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with significantly improved OS at all timepoints compared with controls (1‐year OS 98.3% vs 94.5%, p<0.001; 3‐year OS 94.9% vs 90.2%, p<0.001; 5‐year OS 92.2% vs 89.2%, p=0.003; HR, 0.63 [95% CI, 0.47–0.86]; Figure 2). The 5-year OS of the entire TriNetX testicular cancer male cohort is 91.3% (N = 50,737).
Hypopharyngeal cancer
Post‐PSM, the PDE5 inhibitor and control cohorts each included 213 patients with hypopharyngeal cancer (Tables 4, 5). PDE5 inhibitor exposure was associated with significantly improved OS at all timepoints compared with controls (1‐year OS 87.4% vs 76.4%, p=0.004; 3‐year OS 69.9% vs 58.2%, p=0.008; 5‐year OS 63.9% vs 48.0%, p=0.002; HR, 0.59 [95% CI, 0.42–0.82]; Figure 2). The 5-year OS of the entire TriNetX hypopharyngeal cancer male cohort is 56.6% (N = 16,042).
Discussion
This study provides population-level, propensity score-matched data evaluating the association between PDE5 inhibitor exposure and OS in male-predominant cancers. In a cohort of 217,260 matched male patients drawn from a large multi-institutional database, PDE5 inhibitor use was associated with an overall survival advantage across all stages and cancer types at all timepoints investigated. This analysis represents, to the best of our knowledge, the first pan-cancer evaluation of PDE5 inhibitors in male-predominant tumors, expanding upon prior site-specific observations in colorectal, prostate, and gastric cancers. By demonstrating a survival advantage not only in genitourinary and gastrointestinal cancers, but also in head and neck subsites where prior work has been limited to immune modulation rather than survival outcomes, this study suggests a potentially generalizable oncologic benefit of PDE5 inhibitors and highlights a widely prescribed, well-tolerated drug class as a candidate for repurposing in oncology. Restricting the analysis to male-predominant cancers was intentional to minimize sex-based confounding and to focus on populations where PDE5 inhibitor exposure is clinically common. These tumor types also share relevant clinical and epidemiologic features that help reduce heterogeneity when assessing post-diagnosis PDE5 inhibitor exposure. While analyses of sex-balanced or female-predominant cancers were beyond the present scope, future studies may explore whether these associations extend to broader oncologic populations.
The survival advantage observed in our analysis extends the emerging body of evidence supporting an oncologic role for PDE5 inhibitors. In a cohort of 161 patients with gastric cancer, Zhang et al. reported lower cancer-specific mortality among PDE5 inhibitor users (adjusted HR 0.66, 95% CI 0.47-0.92). This clinical observation was supported by mechanistic studies in gastric cancer cell lines, tumor organoids, and xenograft models, demonstrating that sildenafil suppresses tumor growth by inhibiting PDE5, destabilizing c-MYC, and downregulating IL-6/JAK/STAT3 signaling (16). Consistent with this evidence, Huang et al. studied a nationwide cohort of 12,465 male patients with stage I-III colorectal cancer and found that post-diagnosis PDE5 inhibitor use was associated with a reduction in cancer-specific mortality and metastatic progression, with the strongest protective effect observed in those treated after open surgery (adjusted HR 0.82, 95% CI 0.67-0.99; adjusted HR 0.85, 95% CI 0.74-0.98, respectively) (17). In addition, in a propensity score-matched analysis of 1,058 patients undergoing robot-assisted radical prostatectomy, Lee et al. reported that PDE5 inhibitor use was associated with a lower mortality risk without an increase in cancer recurrence (18). Taken together, prior studies provide the mechanistic and epidemiologic foundation for an oncologic role of PDE5 inhibitors, which our work extends across additional male-predominant cancer sites including prostate, bladder, laryngeal, and esophageal. Importantly, these mechanistic data provide biological plausibility but do not establish causality in the context of our observational findings. Thus, the associations reported here should be interpreted as hypothesis-generating rather than evidence of a direct therapeutic effect.
The association observed in our study is consistent with preclinical evidence demonstrating immunomodulatory effects of PDE5 inhibition within the tumor microenvironment (8, 14). Specifically, PDE5 inhibition downregulates arginase-1 and nitric oxide synthase in myeloid-derived suppressor cells (MDSCs), thereby impairing their suppressive function and facilitating cytotoxic T-cell infiltration (8, 19). In murine tumor models, PDE5 inhibition slows tumor growth and enhances antitumor immune responses (10). Clinical studies in patients with head and neck squamous cell carcinoma have shown that tadalafil reduces circulating MDSCs and increases tumor-specific immune activation (20, 21). Moreover, in multiple preclinical models, including melanoma, pancreatic adenocarcinoma, and hepatocellular carcinoma, PDE5 inhibitors have demonstrated synergy with immune checkpoint blockade, further supporting their potential as immunomodulatory adjuncts in cancer therapy (14, 15, 22).
Clinically, these findings support the rationale for considering PDE5 inhibitors as adjuncts in oncologic therapy. These agents are inexpensive, orally administered, and have well-established safety profiles in men, making them potential candidates for therapeutic repurposing. Nonetheless, PDE5 inhibitors are not without contraindications, particularly in patients with cardiovascular instability or nitrate use, and their safety in cancer populations has not been fully characterized. Observational data also suggest that perioperative PDE5 inhibitor use may reduce the pro-metastatic effects of surgical stress by limiting the expansion of immunosuppressive myeloid cells, further supporting their role alongside other cancer treatments (23). However, given the absence of randomized clinical trials evaluating PDE5 inhibitors for cancer outcomes, these observational associations should not be taken to imply clinical benefit and should be interpreted cautiously.
This study has several limitations. Despite propensity score matching across demographic, oncologic, and comorbidity variables, residual confounding remains possible given the lack of data on performance status, treatment intensity, and socioeconomic context. Due to limited sample sizes, stage-stratified analyses were only feasible in the combined pan-cancer cohort and may not fully capture survival outcomes within each individual cancer type. Reliance on TriNetX data restricted our analyses to overall survival, as standardized progression or recurrence events are not uniformly captured across contributing institutions in TriNetX, precluding a reliable progression-free and disease-free survival analyses. Exposure was defined by any PDE5 inhibitor prescription within 6 months of diagnosis; adherence, dose, duration, and agent-specific effects were not available, and we could not distinguish short-term or perioperative use from sustained therapy. This approach may capture heterogeneous usage patterns, including peri-diagnostic or short-term prescriptions, and may introduce exposure misclassification. Treatment-level data, including surgery, radiation, chemotherapy, and immunotherapy, were variably captured across institutions in TriNetX and were not uniformly available. The platform does not support user-defined multivariable or Cox regression analyses beyond built-in Kaplan–Meier and hazard ratio tools; thus, accounting for these variables was not feasible and remains an acknowledged limitation. Because patients must survive long enough after diagnosis to receive a prescription, some degree of immortal-time bias is possible. The particularly strong association observed in prostate cancer may partly reflect channeling bias, as younger or healthier men are more likely to receive PDE5 inhibitors. Additionally, the TriNetX population may not reflect the exact survival trends of the general population in the examined cancers. To provide context, we reported 5-year overall survival estimates from all TriNetX patients of each included cancer type. These values differ from those published in population-based datasets, reflecting possible differences in participating institutions, referral patterns, patient health-seeking behavior, and data-capture methods (24, 25). Lastly, this analysis was restricted to male patients with cancers showing male predominance to reduce sex-based confounding and reflect the population most likely to receive PDE5 inhibitors. As such, the findings may not generalize to sex-balanced or female-predominant cancers, which warrant dedicated evaluation.
Future research should focus on prospective randomized trials to determine whether PDE5 inhibitors can improve outcomes when added to standard cancer treatments, especially in combination with established immunotherapy regimens. These studies should go beyond survival data and explore biomarkers that reflect immune activation and tumor response. Additionally, such studies could prioritize cancer types with the strongest observed associations (e.g., prostate, colorectal), as well as advanced-stage disease where immunomodulation may be most impactful. It will also be important to clarify when and how these drugs are most effective, whether given around the time of surgery, as ongoing therapy, or alongside immunotherapy.
Conclusion
Using a real-world global database, population-level data, this large cohort study found that PDE5 inhibitor use is associated with improved 1-, 3-, and 5-year overall survival in male patients with male-predominant solid tumors. These findings support further investigation into the immunologic and therapeutic effects of PDE5 inhibitors in oncology.
Data availability statement
TriNetX data are available to qualified researchers via institutional agreements with TriNetX and cannot be publicly shared by the authors. SEER data are publicly available. Requests to access these datasets should be directed to https://live.trinetx.com.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
DU: Conceptualization, Writing – original draft, Investigation, Writing – review & editing, Methodology, Formal analysis, Data curation, Visualization. SH: Conceptualization, Investigation, Writing – review & editing, Writing – original draft, Formal analysis, Visualization. HS: Investigation, Writing – original draft, Conceptualization, Data curation, Formal analysis. AW: Investigation, Writing – original draft, Formal analysis, Data curation. MA: Data curation, Visualization, Writing – review & editing, Writing – original draft, Investigation. MM: Writing – original draft. PK: Writing – original draft. AB: Writing – original draft. JJ: Writing – original draft. EM: Project administration, Writing – original draft, Supervision, Writing – review & editing, Methodology. AL: Writing – review & editing, Methodology, Supervision, Writing – original draft, Validation, Conceptualization, Project administration.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, and Umansky V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol. (2024) 21:147–64. doi: 10.1038/s41571-023-00846-y
2. Chen H, Xu Z, and Varner J. Targeting myeloid cells to improve cancer immune therapy. Front Immunol. (2025) 16:1623436. doi: 10.3389/fimmu.2025.1623436
3. Zhao Y, Du J, and Shen X. Targeting myeloid-derived suppressor cells in tumor immunotherapy: Current, future and beyond. Front Immunol. (2023) 14:1157537. doi: 10.3389/fimmu.2023.1157537
4. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
5. Gabrilovich DI and Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. (2009) 9:162–74. doi: 10.1038/nri2506
6. Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, et al. l -arginine consumption by macrophages modulates the expression of CD3ζ Chain in T lymphocytes. J Immunol. (2003) 171:1232–9. doi: 10.4049/jimmunol.171.3.1232
7. Andersson K. PDE5 inhibitors – pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. (2018) 175:2554–65. doi: 10.1111/bph.14205
8. Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. (2006) 203:2691–702. doi: 10.1084/jem.20061104
9. Noonan KA, Ghosh N, Rudraraju L, Bui M, and Borrello I. Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Res. (2014) 2:725–31. doi: 10.1158/2326-6066.CIR-13-0213
10. Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. (2015) 21:30–8. doi: 10.1158/1078-0432.CCR-14-1716
11. Luginbuhl AJ, Johnson JM, Harshyne LA, Linnenbach AJ, Shukla SK, Alnemri A, et al. Tadalafil enhances immune signatures in response to neoadjuvant nivolumab in resectable head and neck squamous cell carcinoma. Clin Cancer Res. (2022) 28:915–27. doi: 10.1158/1078-0432.CCR-21-1816
12. Serafini P, Borrello I, and Bronte V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. (2006) 16:53–65. doi: 10.1016/j.semcancer.2005.07.005
13. Sutton SS, Magagnoli J, Cummings TH, and Hardin JW. The association between phosphodiesterase-5 inhibitors and colorectal cancer in a national cohort of patients. Clin Transl Gastroenterol. (2020) 11:e00173. doi: 10.14309/ctg.0000000000000173
14. Gross NE, Zhang Z, Mitchell JT, Charmsaz S, Hernandez AG, Coyne EM, et al. Phosphodiesterase-5 inhibition collaborates with vaccine-based immunotherapy to reprogram myeloid cells in pancreatic ductal adenocarcinoma. JCI Insight. (2024) 9:e179292. doi: 10.1172/jci.insight.179292
15. Wang X, Zhang Q, Zhou J, Xiao Z, Liu J, Deng S, et al. T cell-mediated targeted delivery of tadalafil regulates immunosuppression and polyamine metabolism to overcome immune checkpoint blockade resistance in hepatocellular carcinoma. J Immunother Cancer. (2023) 11:e006493. doi: 10.1136/jitc-2022-006493
16. Zhang Z, Huang W, Huang D, Xu Z, Xie Q, Tan X, et al. Repurposing of phosphodiesterase-5 inhibitor sildenafil as a therapeutic agent to prevent gastric cancer growth through suppressing c-MYC stability for IL-6 transcription. Commun Biol. (2025) 8:85. doi: 10.1038/s42003-025-07519-9
17. Huang W, Sundquist J, Sundquist K, and Ji J. Phosphodiesterase-5 inhibitors use and risk for mortality and metastases among male patients with colorectal cancer. Nat Commun. (2020) 11:3191. doi: 10.1038/s41467-020-17028-4
18. Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, et al. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res. (2017) 7:41–52.
19. Weed DT, Vella JL, Reis IM, de la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. (2015) 21:39–48. doi: 10.1158/1078-0432.CCR-14-1711
20. Tai LH, Alkayyal AA, Leslie AL, Sahi S, Bennett S, Tanese De Souza C, et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. OncoImmunology. (2018) 7:e1431082. doi: 10.1080/2162402X.2018.1431082
21. Lee J, Kim HR, Heo JE, Jang WS, Lee KS, Kang SK, et al. Phosphodiesterase-5 inhibitor use in robot assisted radical prostatectomy patients is associated with reduced risk of death: A propensity score matched analysis of 1,058 patients. World J Mens Health. (2023) 41:892. doi: 10.5534/wjmh.220063
22. Hassel JC, Jiang H, Bender C, Winkler J, Sevko A, Shevchenko I, et al. Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Ta dalafil in patients with metastatic Melanoma (TaMe). OncoImmunology. (2017) 6:e1326440. doi: 10.1080/2162402X.2017.1326440
23. Pantziarka P, Sukhatme V, Crispino S, Bouche G, Meheus L, and Sukhatme VP. Repurposing drugs in oncology (ReDO)—selective PDE5 inhibitors as. ecancer (2018). Available online at: https://www.ecancer.org/journal/12/full/824-repurposing-drugs-in-oncology-redo-selective-pde5-inhibitors-as-anti-cancer-agents.php (Accessed September 9, 2025).
24. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program . National Cancer Institute. Available online at: https://seer.cancer.gov (Accessed September 19, 2025).
25. National Cancer Institute. National Cancer Care Registry (NCCR) . National Cancer Institute. Available online at: https://nccr.cancer.gov (Accessed September 19, 2025).
Keywords: phosphodiesterase-5 inhibitors, overall survival, male-predominant cancers, solid tumors, large database analysis, immuno-oncology
Citation: Uralov D, Harrington SR, Samarah H, Walia AS, Aweeda M, Mustafa MU, Kumar P Jr., Briskin A, Jackson J, Mastrolonardo EV and Luginbuhl AJ (2025) Improved survival with phosphodiesterase-5 inhibitor use in men with male-predominant cancers: real-world large database study. Front. Oncol. 15:1720304. doi: 10.3389/fonc.2025.1720304
Received: 07 October 2025; Accepted: 25 November 2025; Revised: 16 November 2025;
Published: 10 December 2025.
Edited by:
Zebo Jiang, Zhuhai Hospital of Integrated Traditional Chinese & Western Medicine, ChinaReviewed by:
Won Jin Ho, Johns Hopkins University, United StatesYanyi Sun, Johns Hopkins Medicine, Johns Hopkins University, Baltimore, United States, in collaboration with reviewer WH
Huihui Chen, Independent Researcher, Xiangzhou, China
Han Xie, Third Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2025 Uralov, Harrington, Samarah, Walia, Aweeda, Mustafa, Kumar, Briskin, Jackson, Mastrolonardo and Luginbuhl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Uralov, ZGFuaWVsLnVyYWxvdkBqZWZmZXJzb24uZWR1
 Daniel Uralov
Daniel Uralov Sarah R. Harrington1
Sarah R. Harrington1 Hani Samarah
Hani Samarah Adam J. Luginbuhl
Adam J. Luginbuhl