- 1Department of Urology, West China School of Medicine, Sichuan University, Sichuan University Affiliated Chengdu Second People’s Hospital, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
- 2Department of Internal Medicine, Chengdu Shuangliu Hospital of Traditional Chinese Medicine, Chengdu, Sichuan, China
Retroperitoneal dedifferentiated liposarcoma (RP DDLS) is considered highly malignant, with a high recurrence rate and poor prognosis, and is usually asymptomatic until the advanced stages. Retroperitoneal liposarcoma with colon invasion is extremely rare, with only a few cases reported in the literature. This study reported the case of a 75-year-old male patient with a retroperitoneal space-occupying lesion without any clinical symptoms. Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) scans suggested a neoplastic lesion. After adequate preoperative preparation, the patient underwent laparoscopic tumor resection and right hemicolectomy. Pathological and immunohistochemical examinations of the resected retroperitoneal mass and right hemicolon revealed dedifferentiated liposarcoma (DDLPS). Dedifferentiated liposarcomas are characterized by local invasiveness and a high recurrence rate. The association between dedifferentiated liposarcoma and invasive behavior observed in this patient underscores the importance of multidisciplinary collaboration as the primary treatment strategy. Nevertheless, complete tumor resection remains crucial for improving the prognosis and reducing the high local recurrence rate.
Introduction
Retroperitoneal liposarcoma is a rare and complex tumor originating from mesenchymal tissue, accounting for approximately 0.07% to 0.2% of all malignant tumors and approximately 15% of soft tissue sarcomas (1). These masses are usually detected incidentally or present at an advanced stage owing to their anatomical location and slow growth. Despite their large size, histological invasion of adjacent organs is uncommon; they typically exhibit compressive rather than infiltrative behavior (2). Retroperitoneal liposarcomas are considered highly malignant and have a high recurrence rate, with a 5-year survival rate of approximately 42.6% for dedifferentiated tumors (3). CT and ultrasound-guided aspiration biopsy are helpful for the preoperative diagnosis and evaluation of patients. Surgical resection of negative margins is the preferred treatment option. Retroperitoneal liposarcoma with colonic infiltration is extremely rare. Herein, we present a case of a rare dedifferentiated retroperitoneal liposarcoma with colon infiltration, which is helpful for clinicians to further understand the clinical characteristics, treatment strategies of dedifferentiated liposarcoma, and the mechanism of colonic invasion.
Case presentation
A 75-year-old male patient was admitted to the hospital for the detection of a retroperitoneal space-occupying lesion during physical examination. During the disease course, the patient occasionally experienced dull pain in the right waist, without abdominal distension, abdominal pain, diarrhea, hematochezia, dizziness, or palpitations. The patient had not received any specific treatment previously and had no family history of the disease or similar diseases. Three months ago, the patient underwent gastroscopy and colonoscopy due to upper abdominal pain, which revealed a neoplasm of approximately 3mm in size in the transverse colon. The patient underwent endoscopic resection of the transverse colonic lesion, and the abdominal pain improved after the operation. The pathological diagnosis of the resected lesion was hyperplastic polyp. The patient’s vital signs were normal. There was no swelling, tenderness or pain induced by tapping over either kidney area. Blood test indicators were within the normal range. Abdominal CT revealed a nodular slightly hyperdense shadow in the perirenal space on the right side, measuring approximately 3.3 cm × 2.7 cm, with ill-defined borders, compressing the right kidney. The solid component showed enhancement, and a neoplastic lesion with possible hemorrhage was suspected (Figure 1). Abdominal MRI revealed a neoplastic lesion (Figure 2). To further confirm the diagnosis and provide treatment, we planned to perform laparoscopic tumor resection. The patient was regularly administered phenoxybenzamine 10 mg p.o. tid for 1 week. After adequate preoperative preparation, the patient was scheduled to undergo laparoscopic resection of the right retroperitoneal tumor. During the operation, a retroperitoneal tumor approximately 4.0 cm × 3.0 cm in size was identified. The tumor involved the hepatic flexure of the colon, ascending colon, and mesocolon, which made dissection difficult. After obtaining consent from the patient’s family, the patient also underwent laparoscopic right hemicolectomy. Pathological examination of the resected retroperitoneal mass and right hemicolon revealed a spindle-cell tumor (Figure 3). Immunohistochemistry showed that the tumor cells were positive for the expression of CD34, S100, SMA, Desmin, P16, CDK4, MDM2, RB1, and MyoD1, with a Ki-67 index of 30%-40%, and negative for EMA, PCK, CD117, Dog-1, MUC4, Myogenin, and HMB45 (Figure 4). Immunofluorescence staining revealed MDM2 gene amplification. Pathological and immunohistochemical analyses confirmed a dedifferentiated liposarcoma. The patient was discharged after surgery. During the 12-month follow-up period after surgery, no tumor recurrence was observed. However, the patient was advised to undergo regular reexamination and lifelong follow-up.

Figure 1. Abdominal CT. This image showed flocculent, nodular, and cord-like mixed-density lesions in the right perirenal space. The largest nodule measured approximately 3.3 cm × 2.7 cm with ill-defined borders, causing slight compression of the right kidney, and the solid components of the lesions showed enhancement. Additionally, the right lateral conical fascia and prerenal fascia were thickened, with obvious enhancement of the nodular shadows within them [(A–C), red arrow].

Figure 2. Abdominal MRI: This image showed flocculent, nodular, and cord-like mixed-signal lesions in the right perirenal space. The largest nodule measured approximately 3.0 cm × 2.4 cm with ill-defined borders, causing slight compression of the right kidney, and the solid components of the lesions exhibited enhancement. In addition, the right lateral conical fascia and prerenal fascia were thickened, with clearly enhancing nodular shadows observed within them [(A–C), red arrow].

Figure 3. Pathology. This image showed the tumor cells were spindle-shaped and pleomorphic; In some areas, the stroma was myxoid, which contains a small amount of well-differentiated adipocytic components and heterologous differentiation components [H&E, 100x].
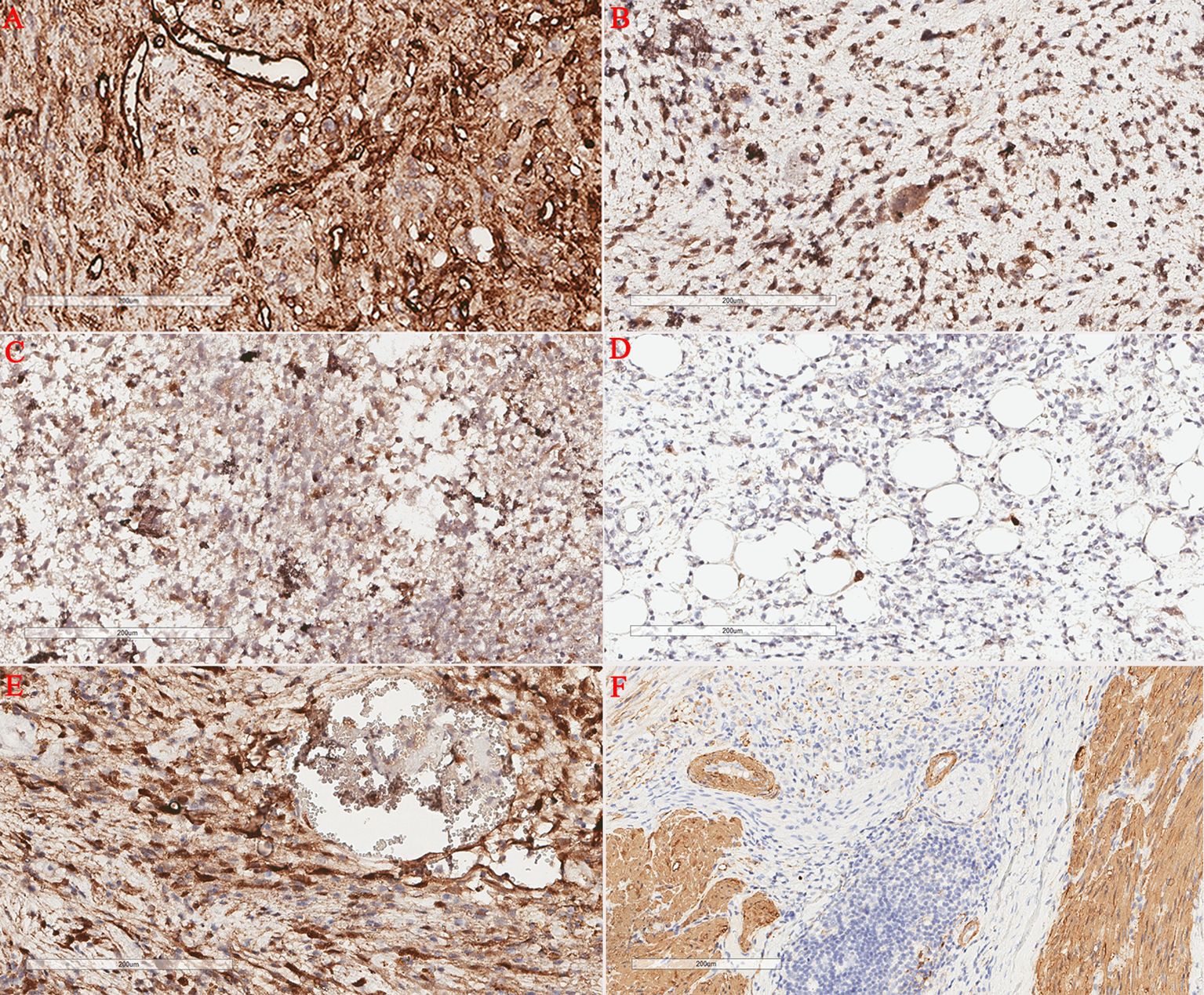
Figure 4. Immunohistochemical staining. Retroperitoneal tumor cells showed positive CD34 expression (A); Retroperitoneal tumor cells showed positive CDK4 expression (B); Retroperitoneal tumor cells showed positive P16 expression (C); The in situ fluorescence hybridization test revealed MDM2 gene amplification (D). Retroperitoneal tumor cells showed positive S100 expression (E); Retroperitoneal tumor cells showed positive SMA expression (F).
Discussion
Retroperitoneal liposarcomas are rare malignant tumors, accounting for 15%-20% of all soft tissue sarcomas, with an overall incidence of 0.3-0.4 per 100,000 population annually (4, 5). Although retroperitoneal liposarcomas can occur in any age group, their peak incidence is in the sixth to seventh decades of life, with no significant sex or racial predilection (6). The etiology of retroperitoneal liposarcoma remains unclear. However, the European Society for Medical Oncology (ESMO) has identified several predisposing factors for retroperitoneal liposarcoma, such as genetic abnormalities (neurofibromatosis type 1, Li-Fraumeni syndrome, FAP/Gardner syndrome), ionizing radiation exposure (especially in children who have undergone multiple computed tomography scans), and diabetes mellitus (7, 8).
Patients with retroperitoneal liposarcoma often present with symptoms such as abdominal distension, pain, nausea, and vomiting due to compression of the abdominal organs. Diagnosis and treatment are challenging because of the large tumor size, complex anatomical relationships, and abundant blood supply. Liposarcomas are classified as well-differentiated, myxoid, dedifferentiated, pleomorphic, and undifferentiated (9). The well-differentiated subtype is the most common sarcoma occurring in the retroperitoneum, with a favorable prognosis and 5-year survival rate of 90% (10). The annual incidence of dedifferentiated liposarcoma is less than 0.1 cases per million people (11), which makes our case even rarer. Dedifferentiated tumors have a high tendency for local recurrence, metastasis, and poor prognosis (12).
As a histological classification system, the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) has been widely used to assess the aggressiveness of soft tissue sarcomas. This system classifies tumors into three grades (grade 1, 2, and 3) based on differentiation, mitotic index, and tumor necrosis (13). It has been reported that grade 1 and grade 2 liposarcomas receiving early intervention show significant local benefits, while grade 3 tumors are significantly associated with an increased likelihood of distant metastasis (4, 14). Tumor differentiation grade has also been identified as a key prognostic factor after surgery. The main reason for this may be attributed to the unique biological characteristics of poorly differentiated tumors. Low-grade tumor cells typically exhibit strong proliferative and invasive capacities. On the other hand, poorly differentiated tumors often show more extensive vascular and perineural invasion, which may lead to residual micrometastases that are difficult to completely eradicate during surgery (14).
Contrast-enhanced abdominal computed tomography is the preferred method for evaluating retroperitoneal liposarcoma (15). These imaging techniques not only provide detailed information about the tumor’s anatomical location, size, and adjacent organs but also help assess the relationship between the tumor and adjacent visceral and neurovascular structures, which is crucial for formulating our surgical plan. On CT, retroperitoneal dedifferentiated liposarcoma presents as soft tissue density or mixed density. Among these findings, ground-glass density foci suggest the possible presence of myxoid components, and calcification or ossification can be seen in some cases. On contrast-enhanced CT and MRI, the dedifferentiated components typically exhibit marked heterogeneous enhancement, with central cystic change and necrosis (15–17). Although needle biopsy is the gold standard for diagnosing liposarcoma, it is not recommended unless the patient is unfit for surgery due to physical condition or requires preoperative chemotherapy or radiotherapy (18). MDM2 amplification is detected in nearly all atypical lipomatous tumors (ALT), well-differentiated liposarcomas (WDL), and dedifferentiated liposarcomas, and is regarded as the driver gene of these tumors (19, 20). Therefore, fluorescence in situ hybridization (FISH) for detecting MDM2 gene amplification at the DNA level and immunohistochemistry for assessing MDM2 protein overexpression are effective auxiliary diagnostic tools (19–22). In particular, FISH-based detection of MDM2 gene amplification status has been universally recognized by the medical community as the “gold standard” for the diagnosis of ALT, WDL, and DDL.
Surgical resection is the preferred treatment for liposarcoma and serves as the primary prognostic factor for recurrence and survival. Complete tumor resection remains the main therapeutic goal for de novo tumors or recurrent tumors. However, achieving negative margins can be challenging owing to the proximity of neurovascular structures, such as the aorta, vena cava, and spine, as well as the difficulty in distinguishing between normal adipose tissue and well-differentiated tumor tissue. Nevertheless, adjacent organ involvement often occurs because of tumor growth or compression. Complete surgical resection of the tumor is the most critical part of treatment for these patients. Even if combined organ resection is required, the tumor must be resected as completely as possible, which can reduce the risk of tumor recurrence and improve the patient’s chance of survival. However, even with tumor resection achieving negative margins, it remains difficult to completely prevent recurrence (23), which may be associated with the biological behavior of dedifferentiated tumors. Retroperitoneal liposarcoma has a high recurrence rate; specifically, the recurrence rate of dedifferentiated liposarcoma is as high as 80% with progressively shortened recurrence intervals (24, 25). Most patients experience multiple recurrences and require multiple surgery procedures. Most patients die of repeated recurrences. The high recurrence rate remains a major challenge in the current treatment of retroperitoneal liposarcoma. Therefore, relying solely on surgery is insufficient to fully address the high recurrence rate of DDLPS and comprehensive treatment approaches are required to improve patient prognosis.
Adjuvant therapy has been proposed as a salvage approach in cases of surgical resection with positive margins; however, its efficacy has been limited (26). A meta-analysis by Li et al. analyzed more than 30,000 patients and compared surgical treatment with and without adjuvant therapy (radiotherapy or chemotherapy). The conclusion was that patients who received adjuvant radiotherapy had a 20% reduction in the risk of death compared to those who received surgery alone (27). However, chemotherapy did not improve survival; instead, it increased the risk of death by 11% (27). For patients with localized and resectable recurrence, repeated resection remains the treatment with the greatest impact on survival, with a median overall survival of up to 5 years (28, 29).
The treatment of retroperitoneal liposarcomas requires multidisciplinary collaboration. In cases in which complete resection is not feasible or for managing high-grade sarcomas, preoperative radiotherapy and chemotherapy may be considered. However, preoperative radiotherapy is not currently recommended for resectable retroperitoneal liposarcomas (5). The prognosis of patients with unresectable local and/or metastatic dedifferentiated liposarcoma is poor. Anthracycline-based therapy is regarded as the standard initial treatment for advanced dedifferentiated liposarcoma, whereas eribulin is currently approved for the treatment of patients with unresectable or metastatic liposarcoma who have previously received anthracycline-based therapy (30, 31). However, their efficacy is limited. A recent in vitro study using human tissues as research subjects has shown that trabectedin, as a second-line chemotherapy drug, has promising efficacy in treating patients with refractory or recurrent liposarcoma by regulating the tumor microenvironment and extracellular matrix (32). However, the efficacy of immunotherapy, radiofrequency ablation, CAR-T cell therapy, and TCR-T cell therapy remains controversial, and these novel treatment approaches are currently in the research stage.
Colonic invasion is a rare manifestation of liposarcomas. A literature review identified only four cases of colonic invasion, and a summary of these cases yielded four hypotheses to explain the pathogenesis of this clinical manifestation: (1) The most obvious mechanism is histological invasion of the colon, extending to the mucosa; (2) ischemic colitis secondary to vascular compression; (3) invasion of a portion of the digestive tract associated with strong adhesion of the tumor to the colon; (4) Long-term exogenous compression of the colon leads to partial invasion of the histological layers and mucosal atrophy (33–36).
Patient perspective
Since I was diagnosed with a retroperitoneal tumor, it has caused immense psychological stress and severely affected my quality of life. The doctors helped me make the correct diagnosis and completely remove the tumor. My fear and anxiety had significantly improved. Physical and psychological healing was achieved. I think I have been successfully treated.
Conclusion
Retroperitoneal dedifferentiated liposarcomas with colonic invasion are an extremely rare. Retroperitoneal liposarcomas typically exhibit compressive rather than invasive behavior toward adjacent organs. However, dedifferentiated liposarcomas are characterized by local invasiveness and a higher recurrence rate. The association between dedifferentiated liposarcoma and invasive behavior observed in this patient underscores the importance of multidisciplinary collaboration as the primary treatment strategy. Nevertheless, complete tumor resection remains crucial for improving the prognosis and reducing the high local recurrence rate. Although surgical resection can temporarily address the short-term issues of patients with retroperitoneal liposarcoma, more effective treatment regimens still need to be explored to reduce the risk of recurrence or metastasis and improve patient survival rates.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Sichuan University affiliated Chengdu Second People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XT: Supervision, Writing – original draft, Writing – review & editing, Data curation, Conceptualization, Investigation. XZ: Writing – original draft, Formal analysis, Supervision, Data curation. CH: Supervision, Writing – original draft, Data curation, Conceptualization, Methodology.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CT, Computed Tomography; MRI, Magnetic Resonance Imaging; ESMO, European Society for Medical Oncology, RP DDLS, Retroperitoneal dedifferentiated liposarcoma; DDLPS, Dedifferentiated liposarcoma; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer; ALT, Atypical Lipomatous Tumors; WDL, Well-differentiated Liposarcomas; DNA, Deoxyribonucleic Acid; FISH, Fluorescence In Situ Hybridization.
References
1. Vijay A and Ram L. Retroperitoneal liposarcoma: a comprehensive review. Am J Clin Oncol. (2015) 38:213–9. doi: 10.1097/COC.0b013e31829b5667
2. Liles JS, Tzeng CW, Short JJ, Kulesza P, and Heslin MJ. Retroperitoneal and intra-abdominal sarcoma. Curr Probl Surg. (2009) 6:445–503. doi: 10.1067/j.cpsurg.2009.01.004
3. Gootee J, Aurit S, Curtin C, and Silberstein P. Primary anatomical site, adjuvant therapy, and other prognostic variables for dedifferentiated liposarcoma. J Cancer Res Clin Oncol. (2019) 145:181–92. doi: 10.1007/s00432-018-2777-3
4. Italiano A, Delva F, Mathoulin-Pelissier S, Le Cesne A, Bonvalot S, Terrier P, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French sarcoma group database. Ann Oncol. (2010) 21:2436–41. doi: 10.1093/annonc/mdq238
5. Gladdy RA. Precision guidelines for soft tissue and visceral sarcomas: the evidence, expert experience and ensuring optimal care for rare cancers, a 2021 update from ESMO–EURACAN–GENTURIS. Ann Oncol. (2021) 32:1–2. doi: 10.1016/j.annonc.2021.08.2155
6. Chouairy CJ, Abdul-Karim FW, and MacLennan GT. Retroperitoneal liposarcoma. J Urol. (2007) 177:1145. doi: 10.1016/j.juro.2006.12.006
7. Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2021) 32:1348–65. doi: 10.1016/j.annonc.2021.07.006
8. Krille L, Zeeb H, Jahnen A, Mildenberger P, Seidenbusch M, Schneider K, et al. Computed tomographies and cancer risk in children: a literature overview of CT practices, risk estimations and an epidemiologic cohort study proposal. Radiat Environ Biophys. (2012) 51:103–11. doi: 10.1007/s00411-012-0405-1
9. Schaefer I and Gronchi A. WHO Pathology: Highlights of the 2020 sarcoma update. Surg Oncol Clin N Am. (2022) 31:321–40. doi: 10.1016/j.soc.2022.03.001
10. Singer S, Antonescu CR, Riedel E, and Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. (2003) 238:358–70. doi: 10.1097/01.sla.0000086542.11899.38
11. Nishio J, Nakayama S, Nabeshima K, and Yamamoto T. Biology and management of dedifferentiated liposarcoma: state of the art and perspectives. J Clin Med. (2021) 10:3230. doi: 10.3390/jcm10153230
12. Nijhuis PH, Sars PR, Plaat BE, Molenaar WM, Sluiter WJ, and Hoekstra HJ. Clinico-pathological data and prognostic factors in completely resected AJCC stage I-III liposarcomas. Ann Surg Oncol. (2000) 7:535–43. doi: 10.1007/s10434-000-0535-6
13. Hettler M, Kitz J, Seif Amir Hosseini A, Guhlich M, Panahi B, Ernst J, et al. Comparing apparent diffusion coefficient and FNCLCC grading to improve pretreatment grading of soft tissue sarcoma-a translational feasibility study on fusion imaging. Cancers (Basel). (2022) 14:4331. doi: 10.3390/cancers14174331
14. Zou B, Chen X, Gao H, Liu S, Li W, and Ye Y. Prognostic factors in retroperitoneal liposarcoma after R0 resection: a study of age, histologic type, tumor stage, and differentiation grade. Am J Transl Res. (2025) 17:5975–86. doi: 10.62347/FQAD9826
15. Lahat G, Madewell JE, Anaya DA, Qiao W, Tuvin D, Benjamin RS, et al. Computed tomography scan-driven selection of treatment for retroperitoneal liposarcoma histologic subtypes. Cancer. (2009) 115:1081–90. doi: 10.1002/cncr.24045
16. Zhang JY, Yu XD, Song Y, Zhang HT, Chen Y, Ouyang H, et al. Comparison of imaging and pathologic findings of retroperitoneal dedifferentiated liposarcoma. Zhonghua Zhong Liu Za Zhi. (2019) 41:223–8. doi: 10.3760/cma.j.issn.0253-3766.2019.03.013
17. Xiao J, Liu J, Chen M, Liu W, and He X. Diagnosis and prognosis of retroperitoneal liposarcoma: A single asian center cohort of 57 cases. J Oncol. (2021) 2021:7594027. doi: 10.1155/2021/7594027
18. Matthyssens LE, Creytens D, and Ceelen WP. Retroperitoneal liposarcoma: current insights in diagnosis and treatment. Front Surg. (2015) 2:4. doi: 10.3389/fsurg.2015.00004
19. Sirvent N, Coindre JM, Maire G, Hostein I, Keslair F, Guillou L, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol. (2007) 31:1476–89. doi: 10.1097/PAS.0b013e3180581fff
20. Zhang H, Erickson-Johnson M, Wang X, Oliveira JL, Nascimento AG, Sim FH, et al. Molecular testing for lipomatous tumors: critical analysis and test recommendations based on the analysis of 405 extremity-based tumors. Am J Surg Pathol. (2010) 34:1304–11. doi: 10.1097/PAS.0b013e3181e92d0b
21. Thway K, Flora R, Shah C, Olmos D, and Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. (2012) 36:462–9. doi: 10.1097/PAS.0b013e3182417330
22. Aleixo PB, Hartmann AA, Menezes IC, Meurer RT, and Oliveira AM. Can MDM2 and CDK4 make the diagnosis of well differentiated/dedifferentiated liposarcoma? An immunohistochemical study on 129 soft tissue tumours. J Clin Pathol. (2009) 62:1127–35. doi: 10.1136/jcp.2009.070201
23. Bachmann R, Eckert F, Gelfert D, Strohäker J, Beltzer C, and Ladurner R. Perioperative strategy and outcome in giant retroperitoneal dedifferentiated liposarcoma-results of a retrospective cohort study. World J Surg Oncol. (2020) 18:296. doi: 10.1186/s12957-020-02069-2
24. Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, and Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. (2009) 250:977–82. doi: 10.1097/sla.0b013e3181b2468b
25. Lu W, Lau J, Xu MD, Zhang Y, Jiang Y, Tong HX, et al. Recurrent abdominal liposarcoma: analysis of 19 cases and prognostic factors. World J Gastroenterol. (2013) 19:4045–52. doi: 10.3748/wjg.v19.i25.4045
26. Nathenson MJ, Barysauskas CM, Nathenson RA, Regine WF, Hanna N, and Sausville E. Surgical resection for recurrent retroperitoneal leiomyosarcoma and liposarcoma. World J Surg Oncol. (2018) 16:203. doi: 10.1186/s12957-018-1505-4
27. Li X, Wu T, Xiao M, Wu S, Min L, and Luo C. Adjuvant therapy for retroperitoneal sarcoma: a meta-analysis. Radiat Oncol. (2021) 16:196. doi: 10.1186/s13014-021-01774-w
28. Gyorki DE and Brennan MF. Management of recurrent retroperitoneal sarcoma. J Surg Oncol. (2014) 109:53–9. doi: 10.1002/jso.23463
29. Siew CCH, Cardona K, and van Houdt WJ. Management of recurrent retroperitoneal sarcomas. Eur J Surg Oncol. (2023) 49:1115–24. doi: 10.1016/j.ejso.2022.06.008
30. Bramwell VH, Anderson D, and Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft-tissue sarcoma: A meta-analysis and clinical practice guideline. Sarcoma. (2000) 4:103–12. doi: 10.1080/13577140020008066
31. Zhang M, Huang J, Zheng X, Huang P, and Yang X. Cost-effectiveness analysis of eribulin versus dacarbazine in patients with advanced liposarcoma. Sci Rep. (2025) 15:2084. doi: 10.1038/s41598-024-84247-w
32. De Vita A, Recine F, Miserocchi G, Pieri F, Spadazzi C, Cocchi C, et al. The potential role of the extracellular matrix in the activity of trabectedin in ups and L-sarcoma: evidences from a patient-derived primary culture case series in tridimensional and zebrafish models. J Exp Clin Cancer Res. (2021) 40:165. doi: 10.1186/s13046-021-01963-1
33. Sato Y, Yamamoto S, and Fujita S. Retroperitoneal liposarcoma with colonic involvement: a case report. Jpn J Clin Oncol. (2014) 44:374–8. doi: 10.1093/jjco/hyu009
34. Wanchick K and Lucha P. Dedifferentiated retroperitoneal liposarcoma presenting as lower gastrointestinal bleeding, a case report and review of the literature. Mil Med. (2009) 174:328–30. doi: 10.7205/milmed-d-01-9608
35. Goh TL, Lastik J, Schmigylski R, and Murray IA. Ischaemic colitis secondary to retroperitoneal liposarcoma. BMJ Case Rep. (2021) 14:e245595. doi: 10.1136/bcr-2021-245595
36. Forero-Escobedo S, Gonzalez-Rodriguez SM, Ramírez-Rincón JA, Garcia-Bernal VA, Polanco-Perdomo S, and Echeverri-Torrents S. Giant retroperitoneal liposarcoma with colonic infiltration as a cause of gastrointestinal bleeding: Case report and literature review. Int J Surg Case Rep. (2025) 129:111142. doi: 10.1016/j.ijscr.2025.111142
Keywords: colonic invasion, dedifferentiated tumor, liposarcoma, oncological surgery, retroperitoneal neoplasia, soft tissue sarcoma
Citation: Tu X, Zhuang X and Huang C (2025) Retroperitoneal dedifferentiated liposarcoma with colon infiltration: a case report and literature review. Front. Oncol. 15:1724071. doi: 10.3389/fonc.2025.1724071
Received: 13 October 2025; Accepted: 27 November 2025; Revised: 26 November 2025;
Published: 10 December 2025.
Edited by:
Zhuang Aobo, Xiamen University, ChinaReviewed by:
Elias Gallardo Navarro, General Surgery, MexicoMari Øines, Nordsjællands Hospital, Denmark
Copyright © 2025 Tu, Zhuang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaoyou Huang, NjM5ODQyMzUxQHFxLmNvbQ==
†These authors have contributed equally to this work
 Xi Tu
Xi Tu Xiyao Zhuang2†
Xiyao Zhuang2†