- 1Department of Biomaterials and Biomimetics, Division of Biomaterials, College of Dentistry, New York University, New York, NY, United States
- 2Department of Periodontics, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran
- 3Department of Dental Research, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Restorative Dentistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 5Department of Dentistry and Implantology, Institute of Fundamental Medicine and Biology, Kazan (Volga Region) Federal University, Russia
- 6Department of Dental Research, Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
- 7Department of Research, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
- 8Department of Health, Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
- 9Department of Dental Research, Dental Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
- 10Department of Dental Research, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 11Department of Dental Research, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
- 12Department of Prosthodontics, Faculty of Dentistry, Babol University of Medical Sciences, Babol, Iran
- 13Department of Health, School of Health, Iran University of Medical Science, Tehran, Iran
Background/purpose: The present study aimed to evaluate the effect of pomegranate extract on the prevention of dental caries compared to standard care, placebo, and no intervention.
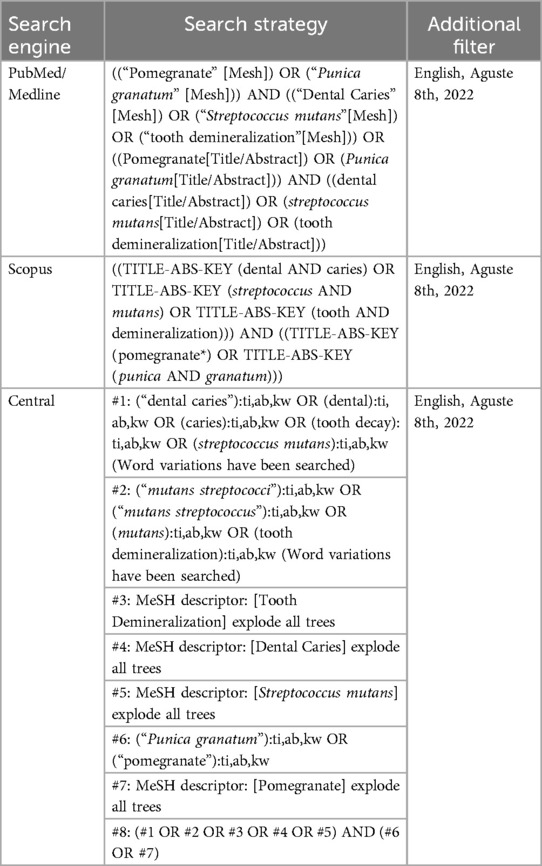
Materials and methods: A bibliographic search in four databases, including PubMed, Scopus, Google Scholar, and CENTRAL, yielded 291 studies until September 8, 2023. The search was performed among the studies written in English using the search terms “Punica granatum” AND (“dental caries” OR “Streptococcus mutans” OR “tooth demineralization”) After screening the titles/abstracts and full texts of these studies, 7 articles were chosen.
Results: In all 7 articles, pomegranate mouthwash was used as the intervention. In 5 studies, the control group used 0.2% chlorhexidine (CHX) mouthwash. Additionally, 4 articles reported a reduction in the mean Streptococcus mutans plaque count in both groups; however, better results were observed in the CHX mouthwash group. In one study, no significant difference was reported between the study and control groups. Finally, one study showed the significant superiority of a hydroalcoholic extract of pomegranate mouthwash over CHX mouthwash.
Conclusion: Overall, the results suggest that pomegranate extract mouthwash is highly effective in reducing caries-causing bacteria. No side effects were reported for pomegranate use in these studies.
Systematic Review Registration: https://osf.io/69gpc/
Introduction
Although the prevalence of dental caries has decreased in recent years, it is still considered among the most common chronic diseases in both adults and children (1, 2). Globally an estimated 3 billion people suffer from dental caries, with 60%–90% of adults and children experiencing it during their lifetime (3). In this regard, Poor oral health has been linked to systemic conditions, including neurovascular and cardiovascular diseases, due to chronic inflammation and bacterial infiltration from carious lesions (4–7).
Many factors have been associated with initiating dental caries, including poor oral hygiene, xerostomia, a high-sugar diet, fluoride deficiency, and inadequate routine dental care (8). Among these, bacterial colonization is considered the primary driver of caries incidence (1, 2). Streptococcus mutans (S. mutans), in particular, plays a key role in caries initiation by adhering to tooth surfaces and forming plaque biofilm (9). Alongside other bacteria, S. mutans ferments dietary carbohydrates, producing acids that demineralize enamel and dentin, leading to mineral loss and cavitation (10–12). Additionally, S. mutans enhances biofilm formation by producing extracellular polysaccharides, which provide adhesion sites and nutrients for other bacteria (13).
Various preventive strategies have been proposed to reduce dental caries, such as limiting sugar intake, water fluoridation, improving oral hygiene, regular dental visits, fissure sealants, and silver diamine fluoride application (3, 14–16). Medicinal plant extracts have also gained attention for their potential to inhibit biofilm formation by either preventing bacterial adhesion or reducing cariogenic bacterial counts (17). However, few natural compounds have been widely adopted due to limitations in taste, cost, efficacy, odor, and stability (18).
Among natural agents, pomegranate (Punica granatum L.) has been extensively studied for its antimicrobial properties. Traditionally used to treat dysentery, diarrhea, and respiratory infections (19, 20). pomegranate extracts have demonstrated efficacy against bacterial and viral pathogens (13, 21–23). Notably, pomegranate aril and peel extracts exhibit antimicrobial activity against Staphylococcus aureus and Escherichia coli (24). The fruit's phytochemicals, particularly ellagic acid and hydrolyzable tannins (e.g., punicalagin), contribute to its antimicrobial effects (25).
Recent studies have demonstrated that pomegranate peel polyphenolic extracts are effective against both planktonic and biofilm-forming oral bacteria such as S. mutans, S. mitis, S. oralis, and R. dentocariosa (1, 26). Synergistic effects with agents like myrtle or honey further enhance biofilm disruption through mechanisms such as adhesion inhibition, interference with matrix synthesis, and membrane destabilization (27, 28).
Antimicrobial peptides derived from pomegranate have also shown anti-cariogenic effects by blocking S. mutans adhesion without toxicity to human keratinocytes (29). Other studies support pomegranate's broad-spectrum activity against cariogenic bacteria (1, 30–32).
Despite promising evidence, a comprehensive systematic review is needed to unite findings and guide clinical applications. This study aims to evaluate the efficacy of pomegranate extract in preventing dental caries compared to standard care, placebo, or no intervention.
Materials and methods
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (33) to ensure methodological rigor and transparency. A pre-registered protocol titled “Effects of Pomegranate Extract on Preventing Dental Caries: A Systematic Review” was filed with the Open Science Framework (OSF; registration DOI: 10.17605/OSF.IO/69GPC and URL: https://osf.io/69gpc/).
Eligibility criteria
Inclusion criteria
This study included randomized clinical trials (RCTs) involving healthy participants of any age classified as high-risk for dental caries based on validated caries risk assessment tools.
Exclusion criteria
Exclusion criteria comprised individuals currently using mouthwash or who had taken medications that reduce salivary flow (such as antibiotics or anti-inflammatory drugs) within the past month. We also excluded current or former alcohol consumers, smokers, and users of paan or gutka. Medically compromised individuals (e.g., those with diabetes mellitus, renal disease, gastrointestinal disorders, or respiratory diseases) were excluded, as were patients undergoing orthodontic treatment, dental rehabilitation, or those with missing teeth (unless due to reasons unrelated to dental caries). Additional exclusions included individuals with a history of radiation therapy, active oral infections (e.g., abscess, cellulitis), or conditions requiring pulp therapy or extraction, as well as those with known allergies to mouthwash or study-related drugs. Non-randomized studies, animal research, case reports, case series, letters to the editor, and gray literature were also excluded.
Data extraction
Two independent reviewers conducted a systematic search of English-language articles published in peer-reviewed journals between 2006 and 2023. The search was performed from December 2, 2018, to September 8, 2023, across PubMed, Scopus, and Google Scholar. Search terms included “Punica granatum” [MeSH], “dental caries” [MeSH], “Streptococcus mutans” [MeSH], and “tooth demineralization” [MeSH]. The initial search results were compiled into an abstract database focusing on studies examining the effects of Punica granatum on dental caries. The search strategy outcomes are summarized in Table 1.
Quality assessment
fter the initial literature search, abstracts were screened for consistency with the PICOS (Population, Intervention, Comparison, Outcomes, Study design) objectives. Studies that did not address the primary research questions of this systematic review were excluded.
Articles selected for full-text review were entered into an Excel spreadsheet, and the two reviewers assessed agreement on inclusion. The final selection was based on a full-text review, with study quality and risk of bias evaluated using the Cochrane Collaboration's tool.
This study prioritized in vitro and in vivo research using dental models (human or bovine tooth fragments) and interventions involving Punica granatum extract. Additional relevant studies were identified through snowball sampling (citation tracking of reference lists in selected articles) and included if they met the primary and secondary search criteria.
Following the final review, the reviewers summarized the findings, identified key themes, and reached a consensus on the reported outcomes.
Results
Study selection
The results of the evaluated studies are summarized in Table 2. Initial database searches using predefined keywords yielded 83 articles from Scopus, 24 from PubMed, 150 from Google Scholar, and 31 from the CENTRAL (Cochrane Central Register of Controlled Trials) database. After removing duplicates using EndNote X9, 182 articles remained. Two reviewers independently conducted title/abstract and full-text screening. Ultimately, six articles that evaluated the effect of Punica granatum on dental caries met the inclusion criteria (Figure 1). The included studies assessed outcomes such as tooth demineralization, Streptococcus mutans count, and dental caries.
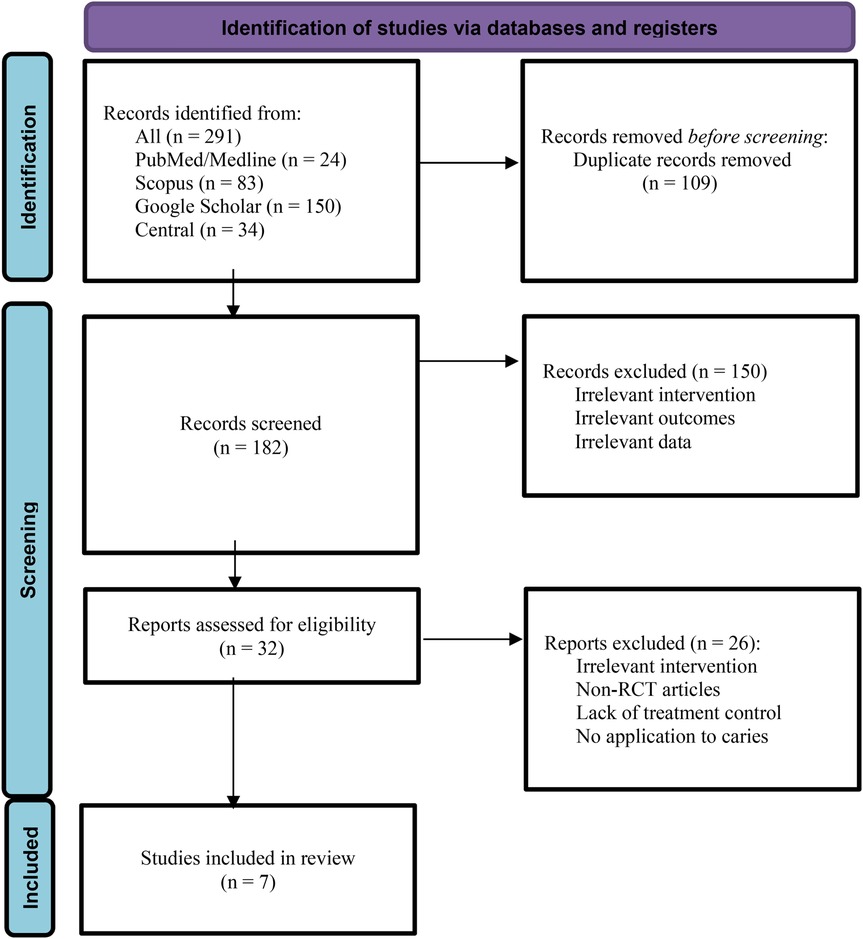
Figure 1. PRISMA flowchart used for detailing the database searches, the number of abstracts screened, and the full texts retrieved.
Risk of bias assessment
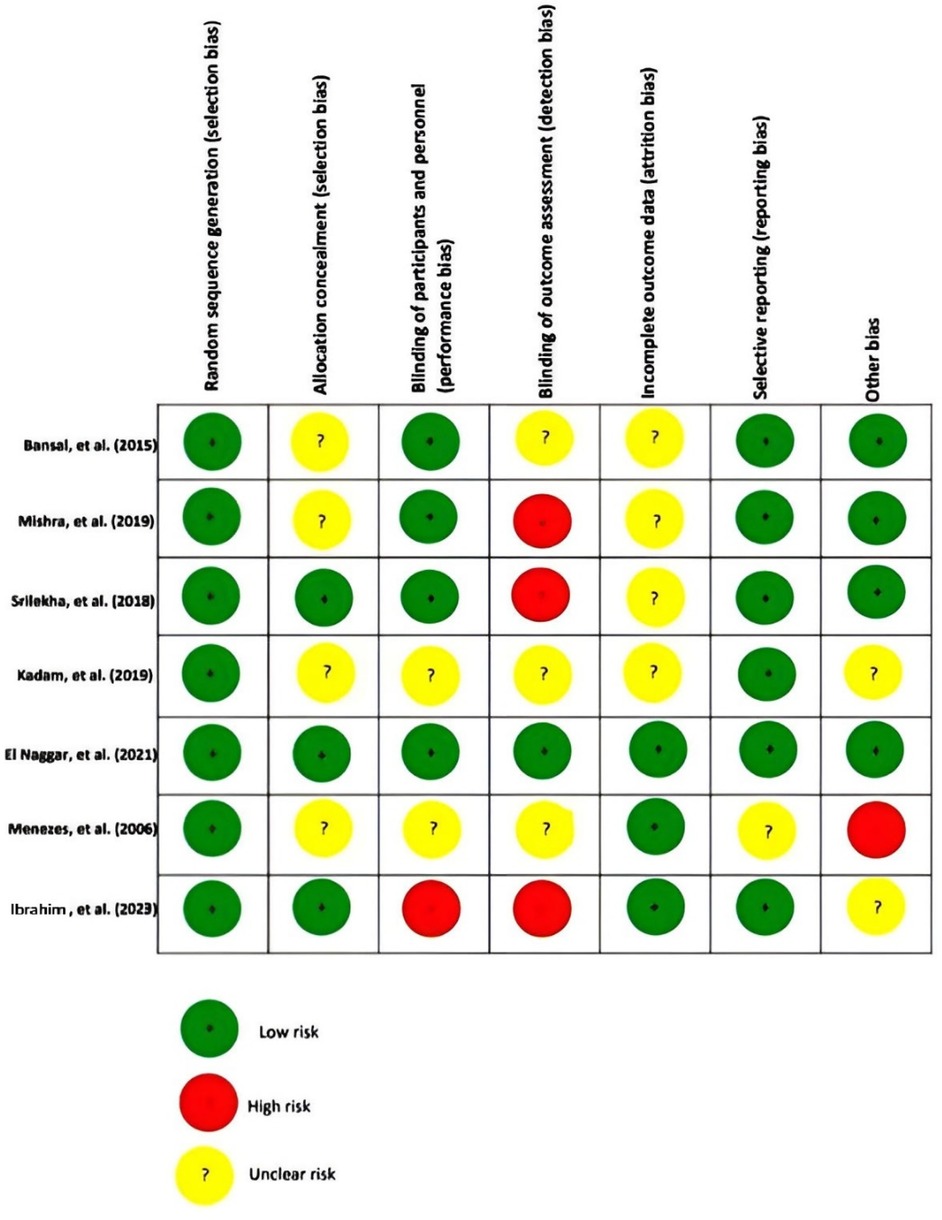
The risk of bias in this review article was examined using the Cochrane Risk of Bias assessment tool. Three of these articles had a high-risk bias for criteria of detection bias (34–36), and 3 had an unclear risk for selection bias (35, 37, 38). Four of them had an unclear risk for attrition bias (34, 35, 37, 38), and two had an unclear risk for performance bias (36, 37). Figure 2 summarizes the evaluation bias risk of the included articles.
Study locations and design
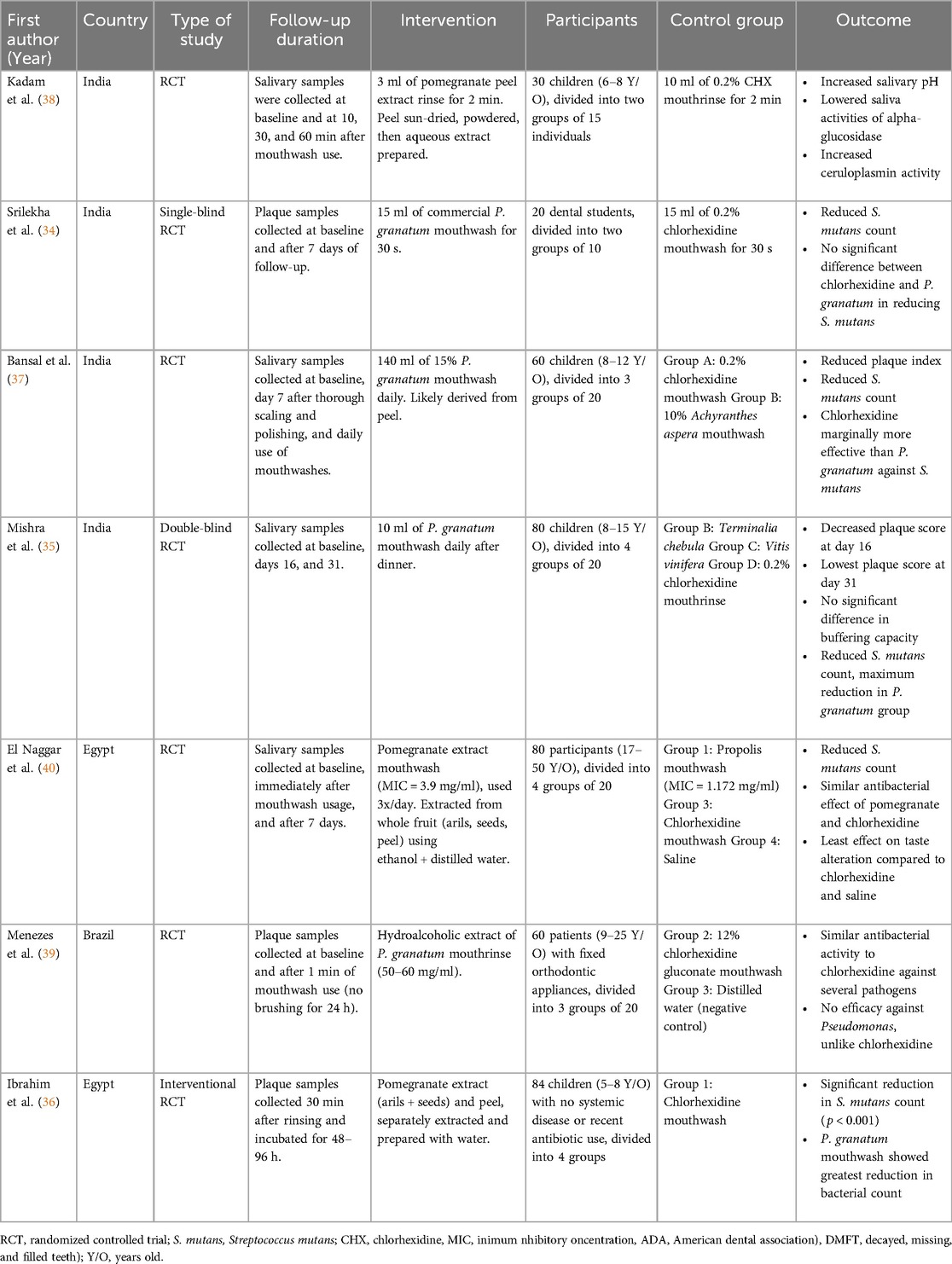
Among seven articles, 4 were performed in India (34, 35, 37, 38), 1 in Brazil (39), and 2 in Egypt (36, 40). All of the studies were randomized clinical trials (34–40).
Study participants
In four of them, participants were children (35–37), and in 2 articles, adults were evaluated (34, 35, 37–40). Finally, 1 article included participants with a wide age range from 9 to 50 years (39).
Preparation methods of pomegranate extracts
All seven studies used pomegranate mouthwash as the intervention. Preparation methods varied by plant part (peel, juice, or whole fruit), solvent, and technique. Peel extracts were most common. Kadam et al. (38) sun- and oven-dried the peel, powdered it, and used aqueous Soxhlet extraction. Bansal et al. (37) and Mishra et al. (35) also used peel extracts but did not describe methods. Whole fruit extracts were used by El Naggar et al. (40) and Menezes et al. (39) Juice and peel extracts were used by Ibrahim et al. (36), juice was hand-extracted and concentrated by heating; peel was air-dried, pulverized, mixed with water, and filtered. Srilekha et al. (34) used a commercial product with unspecified extraction details.
Control interventions
In six studies, control groups used 0.2% chlorhexidine (CHX) mouthwash (34–38, 40). Among these, three also included other mouthwashes in their experimental groups: Mishra et al. (35) used Terminalia chebula and Vitis vinifera extracts; Bansal et al. (37) included 10% Achyranthes aspera; and El Naggar et al. (40) evaluated saline alongside CHX. Only one study did not use CHX; instead, the control group received distilled water (39).
Antibacterial and clinical outcomes
Seven studies evaluated the effects of Punica granatum (pomegranate) mouthwash on oral health outcomes, including antibacterial efficacy, salivary pH, enzymatic activity, and plaque index. Five studies (34–37, 39) reported a reduction in S. mutans count following pomegranate mouthwash use. In Bansal et al. (37), P. granatum, CHX, and A. aspera mouthwashes significantly reduced both plaque index and S. mutans count after seven days, though CHX showed the greatest effect. Mishra et al. (35) found pomegranate mouthwash produced the largest decrease in S. mutans compared to T. chebula, V. vinifera, and CHX, although pH and buffering capacity did not differ significantly between groups. Their findings suggest P. granatum's preventive effect may involve inhibiting hydrolytic enzymes or interfering with bacterial adhesion to teeth.
El Naggar et al. (40) compared the effects of saline (control), CHX, pomegranate, and propolis mouthwashes. All groups showed an immediate rise in salivary pH post-use, with no significant differences between groups. After seven days, pH significantly declined in the pomegranate and propolis groups, while no change was observed in the CHX and saline groups. Bacterial counts were similar across all groups. This study noted that pomegranate mouthwash reduced alpha-glucosidase activity and increased ceruloplasmin, an antioxidant enzyme.
Menezes et al. (39) demonstrated that a hydroalcoholic pomegranate extract exhibited strong antibacterial activity against dental plaque microorganisms. It reduced CFU/ml by 84%, outperforming CHX (79%) and distilled water (11%). Similarly, Ibrahim et al. (36) found that pomegranate mouthwash led to a significant reduction in S. mutans count in children, with the greatest antibacterial effect observed in the pomegranate group compared to other treatments, such as CHX.
Finally, Srilekha et al. (34) assessed S. mutans counts after immediate and seven-day use of CHX and pomegranate mouthwash. Both groups showed significant reductions at day seven compared to baseline, with no significant difference between them. They also reported that pomegranate's effects were linked to reduced alpha-glucosidase activity and enhanced ceruloplasmin levels. Participants in this study had fair-to-good plaque index scores. Kadam et al. (38) also reported that pomegranate peel extract mouthwash significantly increased salivary pH and decreased salivary alpha-glucosidase activity, an enzyme that breaks down sucrose, while also enhancing ceruloplasmin activity, an antioxidant enzyme. The authors concluded that pomegranate mouthwash may have protective effects against dental caries by influencing these enzymatic activities.
Discussion
This is the first systematic review to comprehensively examine the effects of Punica granatum mouthwash on salivary pH, S. mutans levels in saliva and dental plaque, and plaque index.
Adverse effects of CHX vs. Punica granatum mouthwash
CHX mouthwash is commonly prescribed as the gold standard for preventing plaque formation and gingivitis. However, long-term use of CHX is associated with several side effects. Commonly reported effects include taste alteration, oral numbness, dry mouth (xerostomia), and discoloration of the tongue and teeth, with tooth staining being the most frequent reason for discontinuation (41). Other long-term effects may include calculus accumulation, oral paraesthesia, mucosal desquamation, and, in rare cases, parotid gland swelling (42). Additionally, concerns have arisen regarding antimicrobial resistance, particularly with ESKAPE pathogens, following repeated low-level exposure to CHX (43).
Punica granatum mouthwash did not cause taste changes compared to CHX (40). None of the studies reported adverse events associated with Punica granatum mouthwash. A potential limitation in the reviewed studies is the inadequate reporting of adverse events. Most trials did not specify whether adverse effects were actively monitored, and none used standardized tools for adverse event assessment. Short follow-up durations, especially in studies with outcomes measured within minutes to a few days, may have further limited the detection of delayed or mild side effects.
Polyphenolic components and their antimicrobial action in Punica granatum extract
The antimicrobial effect of P. granatum is primarily attributed to its polyphenols. These polyphenols may affect bacterial cell walls, discourage microbial coaggregation, and inhibit bacterial enzymes through oxidation (44). The bioactivity of pomegranate extract is largely due to its high content of ellagitannins, particularly punicalagin. Punicalagin modulates oxidative stress by scavenging free radicals and inducing cellular antioxidant defenses, such as upregulation of superoxide dismutase and glutathione peroxidase, while suppressing pro-inflammatory cytokines like IL-6 and TNF-α (45, 46). A study by Scaglione et al. (47) found that pomegranate peel extract (PPE) exhibited antimicrobial activities without inducing ROS production or cytotoxicity in human cell lines after 30 h, supporting its selective bioactivity and low toxicity in primary cells and macrophage models (48). Furthermore, the depletion of punicalagin and related compounds during bacterial incubation, as observed by Peppoloni et al. (49) suggests active interaction with microbial targets, likely through membrane disruption, enzyme inhibition, and interference with quorum sensing, as proposed in studies of polyphenol–pathogen dynamics (50).
Microbial composition of dental plaque and effects of Punica granatum on Non-S. mutans Species
In addition to S. mutans, dental plaque harbors over 400 distinct bacterial species that digest carbohydrates, produce acid, and contribute to the development of dental caries. These microorganisms can be broadly categorized based on oxygen tolerance, Gram staining characteristics, and morphology (51):
• Gram-positive aerobes: S. sanguis, S. sobrinus, S. salivarius, Actinomyces viscosus
• Gram-negative facultatives: Actinobacillus, Capnocytophaga, Eikenella corrodens
• Gram-negative anaerobes: Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, Bacteroides forsythus, Campylobacter rectus
• Spirochetes: Treponema denticola
Among the reviewed studies, only one evaluated the antimicrobial activity of Punica granatum mouthwash and CHX against non-Streptococcus mutans species in dental plaque. Both agents demonstrated efficacy against opportunistic pathogens, including Staphylococcus spp., Escherichia coli, Klebsiella spp., and Proteus spp. Notably, Staphylococcus epidermidis was found to be susceptible to the hydroalcoholic extract (HAE) of Punica granatum but resistant to CHX (39).
Effectiveness of Punica granatum mouthwash in modifying salivary pH
Saliva's pH and buffering capacity are critical in determining the risk of dental caries. Acidic pH promotes enamel demineralization, increasing the risk of caries (52). This review found that Punica granatum mouthwash increases salivary pH.
However, the comparative effectiveness of Punica granatum relative to chlorhexidine (CHX) remains inconclusive. In a study by El Naggar et al. (40), CHX use over 7 days resulted in a significantly greater increase in salivary pH compared to Punica granatum mouthwash, indicating superior efficacy. In contrast, findings from Mishra et al. (35) and Kadam et al. (38) showed no statistically significant difference between the two interventions, suggesting comparable outcomes.
The time frame in which Punica granatum mouthwash affects salivary pH varies widely across studies, ranging from immediate effects to 15 days. Even a single use of Punica granatum mouthwash has been shown to significantly increase salivary pH (40). However, comparisons between studies are difficult due to differences in factors such as mouthwash concentration, formulation type (aqueous or hydroalcoholic), and frequency of use. Therefore, while Punica granatum shows promise in increasing salivary pH, further studies are needed to determine whether it consistently matches the effectiveness of CHX.
Effect of Punica granatum mouthwash on plaque formation and bacterial reduction
Furthermore, only two studies explicitly showed the Punica granatum mouthwash decreased the plaque index with the same efficacy as CHX (35, 37). In terms of S. mutans reduction in dental plaque, theree of the included studies reported similar antimicrobial efficacy between P. granatum and CHX (34, 39, 40) although the aqueous extract used in Menezes et al. (39), was less effective than the hydroalcoholic version and CHX. One study found CHX to be marginally more effective than P. granatum (37), while Ibrahim et al. (36) reported greater bacterial reduction with P. granatum than CHX.
It has been suggested that P. granatum reduces dental plaque by inhibiting the bacteria responsible for its formation. Tannins such as punicalin and punicafolin, found in pomegranate peel, inhibit human salivary alpha-amylase, an enzyme that facilitates plaque formation by providing substrates for cariogenic bacteria. Alpha-amylase catalyzes the hydrolysis of starch to produce oligosaccharides, which bind to both enamel and cariogenic bacteria, promoting plaque formation. By preventing bacterial adhesion to enamel, P. granatum helps reduce plaque formation (53, 54).
Demographics and sample characteristics of included studies
Most of the reviewed studies involved healthy children and young adults aged 6–15 years. Only two studies assessed the efficacy of Punica granatum mouthwash in individuals at high caries risk: one focused on 9–25-year-olds using fixed orthodontic appliances (39), and the other included 17–50-year-olds with high caries risk based on the ADA caries risk assessment (40).
The studies used various parts of Punica granatum, including seeds (38), fruit (34), peel (37), arils, exocarp, and mesocarp (40). Only one study extracted a hydroalcoholic solution directly from Punica granatum and evaluated its effect on S. mutans levels in dental plaque (39). Therefore, it remains unclear which specific component of Punica granatum is responsible for its antibacterial effects against S. mutans.
Summary and limitations of Punica granatum mouthwash in oral health
Compared to a previous systematic review evaluating the efficacy of pomegranate-based products in oral health, the systematic review and meta-analysis by Javan et al. (55) examined the effects of pomegranate vs. CHX on periodontal indices such as plaque index, gingival index (GI), and bleeding index (BI). Their results showed that while CHX had a significantly greater impact on plaque reduction at 14–15 days, no significant differences were found in shorter durations or for GI and BI, suggesting comparable anti-inflammatory effects between Punica granatum and CHX in periodontal contexts. similarly in our review, while most included studies also reported CHX to be more effective, at least one study demonstrated superior efficacy of hydroalcoholic pomegranate extract, and none reported adverse effects with Punica granatum use. Thus, both reviews highlight the antimicrobial and anti-inflammatory potential of pomegranate. In conclusion, our findings from the reviewed studies showed that Punica granatum mouthwash has antimicrobial potential compared to CHX mouthwash without any reported side effects. This mouthwash might be beneficial in preventing dental caries by decreasing the plaque index, increasing salivary pH, and reducing the levels of S. mutans in saliva and dental plaques. Such mouthwash would be less costly and well tolerated by patients.
Nevertheless, this systematic review has several limitations that should be taken into account when interpreting the results. First, the number of available studies on Punica granatum mouthwash was limited, and most had relatively small sample sizes. There was also considerable variation among the studies in terms of the type of formulation used (aqueous vs. hydroalcoholic), the concentration of P. granatum extract, the duration and frequency of use, and the specific plant parts used (peel, seeds, fruit, or whole fruit components). Additionally, only a few studies investigated the antimicrobial activity of P. granatum against a broader range of oral bacteria beyond S. mutans, despite the fact that dental plaque contains a diverse bacterial population. The follow-up periods were generally short—ranging from a single application to just a few days—which limits the understanding of the mouthwash's long-term effectiveness and safety. While no adverse effects were reported, most studies did not clearly describe how adverse events were monitored, raising the possibility of underreporting. These limitations highlight the need for larger, well-designed clinical trials using standardized mouthwash formulations, longer follow-up durations, broader microbial assessments, and more rigorous safety reporting.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
NR: Writing – original draft, Writing – review & editing. YS: Writing – original draft, Writing – review & editing. ZA: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing. HT: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. FK: Writing – original draft, Writing – review & editing. GA: Writing – original draft, Writing – review & editing. NA: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing. MV: Writing – original draft, Writing – review & editing. FA: Writing – original draft, Writing – review & editing. NS: Writing – original draft, Writing – review & editing. MQ: Writing – original draft, Writing – review & editing. MN: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferrazzano GF, Scioscia E, Sateriale D, Pastore G, Colicchio R, Pagliuca C, et al. In vitro antibacterial activity of pomegranate juice and peel extracts on cariogenic bacteria. BioMed Res Int. (2017) 2017. doi: 10.1155/2017/2152749
2. Mosaddad SA, Hussain A, Tebyaniyan H. Green alternatives as antimicrobial agents in mitigating periodontal diseases: a narrative review. Microorganisms. (2023) 11(5):1269. doi: 10.3390/microorganisms11051269
3. Delimont NM, Carlson BN. Prevention of dental caries by grape seed extract supplementation: a systematic review. Nutr Health. (2020) 26(1):43–52. doi: 10.1177/0260106019887890
4. Dietrich T, Webb I, Stenhouse L, Pattni A, Ready D, Wanyonyi K, et al. The relationship between oral health and cardiovascular disease: a rapid review. Br Dent J. (2017) 222:381–5. doi: 10.1038/sj.bdj.2017.224
5. Jepsen S, Stadlinger B, Terheyden H, Sanz M. Guest editorial science transfer: oral health and general health-the links between periodontitis, atherosclerosis, and diabetes. J Clin Periodontol. (2016) 42(12):1071–3. doi: 10.1111/jcpe.12484
7. Sheiham A, James W. Diet and dental caries: the pivotal role of free sugars reemphasized. J Dent Res. (2015) 94(10):1341–7. doi: 10.1177/0022034515590377
8. Dentistry. AAoP. Guideline on periodicity of examination, preventive dental services, anticipatory guidance/counseling, and oral treatment for infants, children, and adolescents. Pediatr Dent. (2013) 35(5):E148–56.24290543
9. Hamad AA, Alhumaidi MS, Manayi A. Evaluation of the impact of some plant extracts against spp. Isolated from dental decay infection. Open Microbiol J. (2023) 17(1). doi: 10.2174/18742858-v17-e230418-2022-25
10. Bowen W, Koo H. Biology of streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. (2011) 45(1):69–86. doi: 10.1159/000324598
11. Nishikawara F, Nomura Y, Imai S, Senda A, Hanada N. Evaluation of cariogenic bacteria. Eur J Dent. (2007) 1(1):31–9. doi: 10.1055/s-0039-1698309
12. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. (2007) 369(9555):51–9. doi: 10.1016/S0140-6736(07)60031-2
13. Gulube Z, Patel M. Effect of Punica granatum on the virulence factors of cariogenic bacteria Streptococcus mutans. Microb Pathog. (2016) 98:45–9. doi: 10.1016/j.micpath.2016.06.027
14. Bridge G, Martel A-S, Lomazzi M. Silver diamine fluoride: transforming community dental caries program. Int Dent J. (2021) 71(6):458–61. doi: 10.1016/j.identj.2020.12.017
15. Lam PP, Sardana D, Lo EC, Yiu CK. Fissure sealant in a nutshell. Evidence-based meta-evaluation of sealants’effectiveness in caries prevention and arrest. J Evid Based Dent Pract. (2021) 21(3):101587. doi: 10.1016/j.jebdp.2021.101587
16. Yu OY, Lam WY-H, Wong AW-Y, Duangthip D, Chu C-H. Nonrestorative management of dental caries. Dent J. (2021) 9(10):121. doi: 10.3390/dj9100121
17. Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-Based Complementary Altern Med. (2011) 2011. doi: 10.1093/ecam/nep067
18. Ferrazzano GF, Roberto L, Catania MR, Chiaviello A, De Natale A, Roscetto E, et al. Screening and scoring of antimicrobial and biological activities of Italian vulnerary plants against major oral pathogenic bacteria. Evidence-Based Complementary Altern Med. (2013) 2013. doi: 10.1155/2013/316280
19. Dey D, Ray R, Hazra B. Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm Biol. (2015) 53(10):1474–80. doi: 10.3109/13880209.2014.986687
20. Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol. (2012) 143(2):397–405. doi: 10.1016/j.jep.2012.07.004
21. Acquadro S, Civra A, Cagliero C, Marengo A, Rittà M, Francese R, et al. Punica granatum leaf ethanolic extract and ellagic acid as inhibitors of Zika virus infection. Planta Med. (2020) 86(18):1363–74. doi: 10.1055/a-1232-5705
22. de Lima LB, da Silva WAV, Santos D, Machado ECF, Procópio JCB, F T, et al. Evaluation of antioxidant, antibacterial and enhancement of antibiotic action by Punica granatum leaves crude extract and enriched fraction against multidrug-resistant Bacteria. Chem Biodiversity. (2021) 18(12):e2100538. doi: 10.1002/cbdv.202100538
23. Jam N, Hajimohammadi R, Gharbani P, Mehrizad A. Antibacterial activity of Punica granatum L. And Areca nut (PA) combined extracts against some food born pathogenic bacteria. Saudi J Biol Sci. (2022) 29(3):1730–6. doi: 10.1016/j.sjbs.2021.10.057
24. Pagliarulo C, De Vito V, Picariello G, Colicchio R, Pastore G, Salvatore P, et al. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. (2016) 190:824–31. doi: 10.1016/j.foodchem.2015.06.028
25. Howell AB, D’Souza DH. The pomegranate: effects on bacteria and viruses that influence human health. Evidence-Based Complementary Altern Med. (2013) 2013. doi: 10.1155/2013/606212
26. Vahid-Dastjerdi E, Monadi E, Khalighi HR, Torshabi M. Down-regulation of glycosyl transferase genes in Streptococcus mutans by Punica granatum L. flower and Rhus coriaria L. fruit water extracts. Iran J Pharm Res. (2016) 15(2):513.27642322
27. Sateriale D, Facchiano S, Colicchio R, Pagliuca C, Varricchio E, Paolucci M, et al. In vitro synergy of polyphenolic extracts from honey, myrtle and pomegranate against oral pathogens. S. mutans and R. dentocariosa. Front Microbiol. (2020) 11:1465. doi: 10.3389/fmicb.2020.01465
28. Sateriale D, Imperatore R, Colicchio R, Pagliuca C, Varricchio E, Volpe MG, et al. Phytocompounds vs. Dental plaque bacteria: in vitro effects of myrtle and pomegranate polyphenolic extracts against single-species and multispecies oral biofilms. Front Microbiol. (2020) 11:592265. doi: 10.3389/fmicb.2020.592265
29. Kokilakanit P, Koontongkaew S, Roytrakul S, Utispan K. A novel non-cytotoxic synthetic peptide, pug-1, exhibited an antibiofilm effect on Streptococcus mutans adhesion. Lett Appl Microbiol. (2020) 70(3):151–8. doi: 10.1111/lam.13265
30. Jacob B, Malli Sureshbabu N, Ranjan M, Ranganath A, Siddique R. The antimicrobial effect of pomegranate peel extract versus chlorhexidine in high caries risk individuals using quantitative real-time polymerase chain reaction: a randomized triple-blind controlled clinical trial. Int J Dent. (2021) 2021. doi: 10.1155/2021/5563945
31. Mehta VV, Rajesh G, Rao A, Shenoy R, BH MP. Antimicrobial efficacy of Punica granatum mesocarp, nelumbo nucifera leaf, psidium guajava leaf and coffea canephora extract on common oral pathogens: an in vitro study. J Clin Diagn Res. (2014) 8(7):ZC65.25177642
32. Gallas JA, Pelozo LL, Oliveira WP, Salvador SL, Corona SM, Souza-Gabriel AE, et al. Characterization, antimicrobial activity, and antioxidant efficacy of a pomegranate peel solution against persistent root canal pathogens. Cureus. (2023) 15(8).37692706
33. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71.
34. Srilekha M, Jayashri P. Comparing the antimicrobial effectiveness of Punica granatum and chlorhexidine-containing mouthwash: a single-blind randomized clinical trial. Drug Invent Today. (2018) 10(8).
35. Mishra P, Marwah N, Agarwal N, Chaturvedi Y, Suohu T. Comparison of Punica granatum, Terminalia chebula, and Vitis vinifera seed extracts used as mouthrinse on salivary Streptococcus mutans levels in children. J Contemp Dent Pract. (2019) 20(8):920–7. doi: 10.5005/jp-journals-10024-2617
36. Ibrahim OS, Elghazawy RK, Ahmed NM, Abd Elaziz AM. Evaluation of antimicrobial efficacy of green tea, garlic with lime, pomegranate extract and chlorhexidine mouth rinses in a group of Egyptian children (A randomized clinical trial). Adv Dent J. (2023) 5(4):831–9. doi: 10.21608/adjc.2023.208790.1303
37. Bansal A, Marwah N, Nigam AG, Goenka P, Goel D. Effect of achyranthes aspera, 0.2% aqueous chlorhexidine gluconate and Punica granatum oral rinse on the levels of salivary Streptococcus mutans in 8 to 12 years old children. J Contemp Dent Pract. (2015) 16(11):903–9. doi: 10.5005/jp-journals-10024-1779
38. Kadam NS, Kunte SS, Patel AR, Shah PP, Lodaya RR, Lakade LS. Comparative evaluation of the effect of pomegranate peel extract and chlorhexidine 0.2% mouthwash on salivary pH in children between 6 and 8 years of age: an in vivo study. J Int Oral Health. (2019) 11(1):40. doi: 10.4103/jioh.jioh_207_18
39. Menezes SM, Cordeiro LN, Viana GS. Punica granatum (pomegranate) extract is active against dental plaque. J Herb Pharmacother. (2006) 6(2):79–92. doi: 10.1080/J157v06n02_07
40. El Naggar RA, Ibrahim SH, Mosallam RS, Khairy MA. Effect of propolis and pomegranate extract mouthwashes on taste alteration, salivary pH and antibacterial activity in high caries risk patients: a randomized control trial. Indian J Public Health Res Dev. (2021) 12(3).
41. Deus FP, Ouanounou A. Chlorhexidine in dentistry: pharmacology, uses, and adverse effects. Int Dent J. (2022) 72(3):269–77. doi: 10.1016/j.identj.2022.01.005
42. Brookes ZLS, Bescos R, Belfield LA, Ali K, Roberts A. Current uses of chlorhexidine for management of oral disease: a narrative review. J Dent. (2020) 103:103497. doi: 10.1016/j.jdent.2020.103497
43. Cieplik F, Jakubovics NS, Buchalla W, Maisch T, Hellwig E, Al-Ahmad A. Resistance toward chlorhexidine in oral bacteria–is there cause for concern? Front Microbiol. (2019) 10:587. doi: 10.3389/fmicb.2019.00587
44. Abdollahzadeh S, Mashouf R, Mortazavi H, Moghaddam M, Roozbahani N, Vahedi M. Antibacterial and antifungal activities of Punica granatum peel extracts against oral pathogens. J Dent. (2011) 8(1):1.
45. Benchagra L, Berrougui H, Islam MO, Ramchoun M, Boulbaroud S, Hajjaji A, et al. Antioxidant effect of Moroccan pomegranate (Punica granatum L. sefri variety) extracts rich in punicalagin against the oxidative stress process. Foods. (2021) 10(9):2219. doi: 10.3390/foods10092219
46. Du L, Li J, Zhang X, Wang L, Zhang W, Yang M, et al. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr Res. (2019) 63.
47. Scaglione E, Sateriale D, Mantova G, Di Rosario M, Continisio L, Vitiello M, et al. Antimicrobial efficacy of Punica granatum Lythraceae peel extract against pathogens belonging to the ESKAPE group. Front Microbiol. (2024) 15:1383027. doi: 10.3389/fmicb.2024.1383027
48. Fernandes GL, Vieira APM, Danelon M, Emerenciano NG, Berretta AA, Buszinski AFM, et al. Pomegranate extract potentiates the anti-demineralizing, anti-biofilm, and anti-inflammatory actions of non-alcoholic mouthwash when associated with sodium-fluoride trimetaphosphate. Antibiotics. (2022) 11(11):1477. doi: 10.3390/antibiotics11111477
49. Peppoloni S, Colombari B, Tagliazucchi D, Odorici A, Ventrucci C, Meto A, et al. Attenuation of Pseudomonas aeruginosa virulence by pomegranate peel extract. Microorganisms. (2022) 10(12):2500. doi: 10.3390/microorganisms10122500
50. Mandal MK, Domb AJ. Antimicrobial activities of natural bioactive polyphenols. Pharmaceutics. (2024) 16(6):718. doi: 10.3390/pharmaceutics16060718
51. Peterson SN, Snesrud E, Liu J, Ong AC, Kilian M, Schork NJ, et al. The dental plaque microbiome in health and disease. PLoS One. (2013) 8(3):e58487. doi: 10.1371/journal.pone.0058487
52. Rajesh K, Hegde S, Kumar MA. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp Clin Dent. (2015) 6(4):461. doi: 10.4103/0976-237X.169846
53. Umar D, Dilshad B, Farhan M, Ali A, Baroudi K. The effect of pomegranate mouthrinse on Streptococcus mutans count and salivary pH: an in vivo study. J Adv Pharm Technol Res. (2016) 7(1):13. doi: 10.4103/2231-4040.173266
54. Karkare RS, Shamim SF. Role of pomegranate in preventive dentistry. Int J Res Ayurveda Pharm. (2012) 3(5). doi: 10.7897/2277-4343.03513
Keywords: dental caries, pomegranate, Punica granatum, Streptococcus mutans, tooth demineralization
Citation: Rafeie N, Salimi Y, Aghamir ZS, Amini A, Taheri H, Sadreddini S, Kamali F, Akbarian G, Azizi N, Bagherianlemraski M, Valizadeh M, Alimohammadi F, Sedighnia N, Qadirifard M and Naziri M (2025) Effects of pomegranate extract on preventing dental caries: a systematic review. Front. Oral Health 6:1484364. doi: 10.3389/froh.2025.1484364
Received: 19 October 2024; Accepted: 6 May 2025;
Published: 27 May 2025.
Edited by:
Caterina Pagliarulo, University of Sannio, ItalyReviewed by:
Daniela Sateriale, University of Sannio, ItalyShradha Devi Dwivedi, Pandit Ravishankar Shukla University, India
Baburao Chandakavathe, DSTS Mandal’s College of pharmacy Solapur, India
Copyright: © 2025 Rafeie, Salimi, Aghamir, Amini, Taheri, Sadreddini, Kamali, Akbarian, Azizi, Bagherianlemraski, Valizadeh, Alimohammadi, Sedighnia, Qadirifard and Naziri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Qadirifard, bW9oYW1tYWQucWFkaXJpZmFyZEBzaGFoZWQuYWMuaXI=; Mahdyieh Naziri, bmF6aXJpbWFoZHlpZWhAeWFob28uY29t
†These authors have contributed equally to this work
 Niyousha Rafeie
Niyousha Rafeie Yasaman Salimi
Yasaman Salimi Zahra Sadat Aghamir
Zahra Sadat Aghamir Amirhesam Amini4
Amirhesam Amini4 Sarvin Sadreddini
Sarvin Sadreddini Mobina Bagherianlemraski
Mobina Bagherianlemraski Farnoosh Alimohammadi
Farnoosh Alimohammadi Mahdyieh Naziri
Mahdyieh Naziri