- 1Department of Oral Surgery, Policlinico Riuniti Foggia, Foggia, Italy
- 2Pathological Anatomy Unit, Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
- 3Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
- 4Department of Medicine and Surgery, LUM University, Casamassima, Italy
- 5Neonatology end Neonatal Intensive Care Unit, Policlinico Riuniti Foggia, Foggia, Italy
- 6Legal Medicine Unit, Ascoli Piceno Hospital C-G. Mazzoni, Ascoli Piceno, Italy
- 7Pathology Unit, Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
Congenital granular cell epulis (CGCE) is a rare benign tumor typically presenting at birth and most commonly located on the maxillary alveolar ridge.
Case Presentation: We report the case of a five-day-old female with a smooth, multilobulated mass on the right maxillary alveolar ridge causing feeding difficulties. Surgical excision was performed under sedation. Histological and immunohistochemical analysis confirmed the diagnosis of CGCE. No recurrence was observed at one-month follow-up.
Conclusion: Early diagnosis and surgical treatment of CGCE are essential to avoid functional impairment. The prognosis is excellent following complete excision.
1 Introduction
Congenital granular cell epulis (CGCE) is a rare benign lesion that typically presents at birth or during early infancy, showing a marked female predominance (1, 2). It most frequently arises from the maxillary alveolar ridge and is characterized by the presence of large granular cells. Despite overlapping features with other oral granular cell lesions—such as adult granular cell tumor (GCT)—CGCE exhibits distinct histopathological and immunohistochemical properties, which are essential for its diagnosis (3, 4). Surgical excision is usually curative, and the prognosis is generally excellent (5).
2 Case report
2.1 Patient information
The patient, a five-day-old female neonate born at term after an uneventful pregnancy and delivery, with no associated congenital anomalies or relevant family history, was admitted to the Neonatal Intensive Care Unit due to the presence of a voluminous oral mass observed at birth.
2.2 Clinical findings
On clinical examination, the lesion appeared as a well-defined, smooth-surfaced, pink, and multilobulated mass arising from the right maxillary edentulous alveolar ridge, measuring approximately 1 cm in its greatest dimension. It was firm in consistency and attached by a pedunculated base, allowing partial mobility on palpation. The overlying mucosa was intact, with no evidence of ulceration, bleeding, or local inflammation (Figures 1A,B).
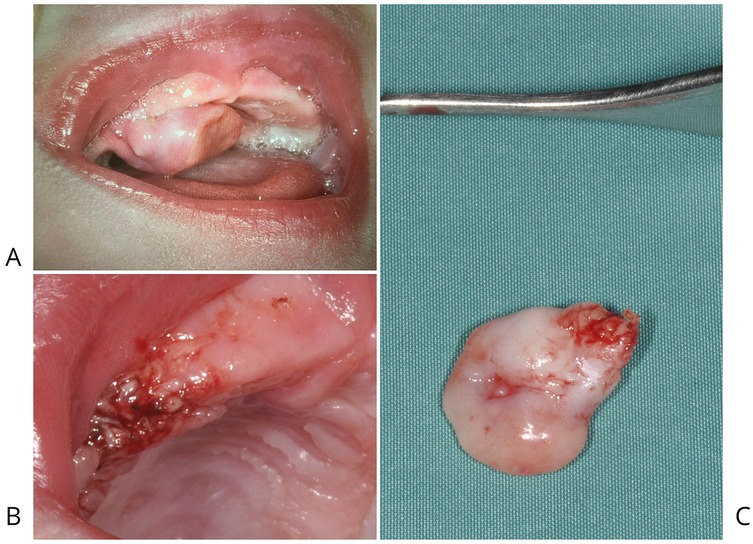
Figure 1. (A) CGCE attached to the right maxillary edentulous alveolar ridge by a pedicle, was responsible for causing significant feeding difficulties in the newborn. (B) Post-surgical image of the patient following electrosurgical excision of CGCE located on the alveolar portion of the right maxilla. (C) Smooth, pink, multilobulated mass located on alveolar portion of the right maxilla.
The mass was readily visible upon intraoral inspection and significantly interfered with normal feeding. During breastfeeding attempts, the lesion mechanically obstructed the infant's ability to latch and suck, causing ineffective feeding and nutritional concern. Despite the lesion's size and protrusion into the oral cavity, the neonate exhibited no signs of respiratory distress at rest, and oxygen saturation remained within normal limits. However, the potential for dynamic airway compromise during feeding or crying could not be excluded.
The lesion's location, clinical features, and age of onset were suggestive of congenital granular cell epulis (CGCE). However, other differential diagnoses considered at this stage included congenital hemangioma, lymphangioma, congenital teratoma, neuroectodermal tumor of infancy, and other rare oral soft tissue tumors of the neonate.
2.3 Diagnostic assessment
2.3.1 Macroscopic examination
The excised lesion measured approximately 1.0 × 0.8 × 0.2 cm and appeared as a smooth, pink, and multilobulated mass (Figure 1C). Gross features were consistent with the clinical presentation of a benign soft tissue tumor arising from the alveolar ridge. The mass was well-circumscribed and exhibited elastic consistency without signs of ulceration or hemorrhage, supporting a preliminary diagnosis of congenital epulis with granular cell morphology.
2.3.2 Histological examination
The specimen was fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 4 μm thickness. Sections were placed on non-coated glass slides and stained with hematoxylin and eosin (H&E) for standard histopathological evaluation (Figure 2). Microscopy revealed a proliferation of large polygonal cells with abundant granular eosinophilic cytoplasm and centrally located small nuclei. These cells were arranged in sheets within a well-vascularized stroma containing a dense capillary network. Scattered histiocytic inflammatory elements, positive for CD68, were observed in the background (Figures 3A,B).
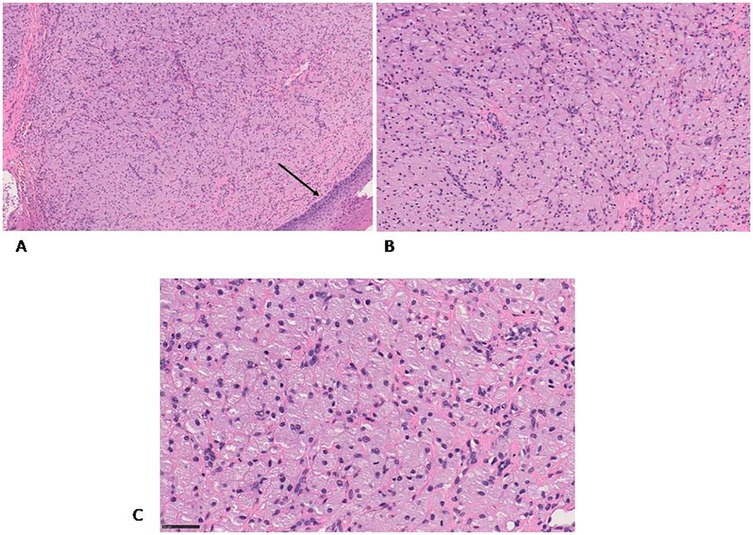
Figure 2. H&E staining (A) the granular cells are covered by flattened stratified squamous epithelium (black arrow) (B,C) the granular cells are well defined and contain an eccentric nucleus, they are arranged in a network of capillaries. Original magnification 10× (A), 20× (B) and 40× (C) NanoZoomer S60 C13210 series.
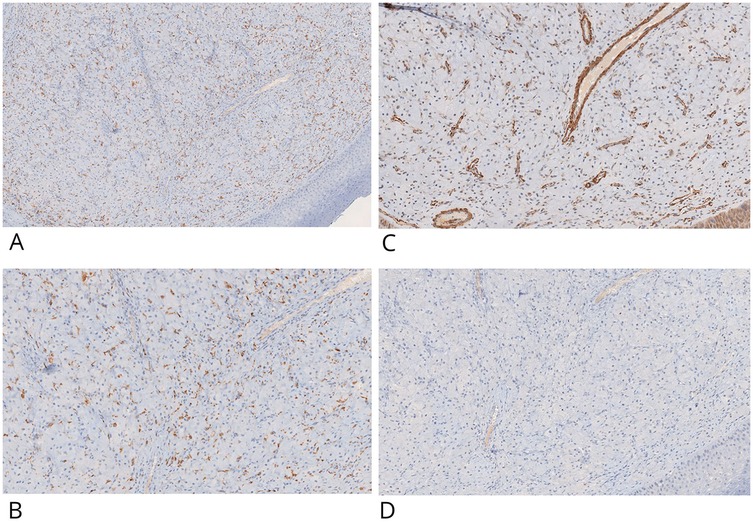
Figure 3. IHC positive staining of CD68 in inflammatory histiocytic elements and negative staining in granular cells (CD68-) (A,B). Granular cells showed no IHC staining potential for actin-smooth muscle (C) and desmin (D) Original magnification 10× (A) and 20× (B–D) ×20 NanoZoomer S60 C13210 series.
2.3.3 Immunohistochemical analysis
Immunohistochemistry (IHC) was performed on 4 μm paraffin-embedded sections mounted on poly-L-lysine-coated glass slides, using the linker streptavidin-biotin horseradish peroxidase (LSAB-HRP) method on the Ventana Benchmark XT autostainer. The following primary antibodies (Ventana/Roche, prediluted) were employed: smooth muscle actin (clone 1A4), desmin (clone DE-R11), vimentin (clone V9), CD68 (clone KP-1), CD1a (clone EP3622) (Figures 3C,D, 4A,B), neuron-specific enolase (NSE, clone MRQ-55) (Figures 4C,D), and S100 (polyclonal). Negative control slides were included to ensure antibody specificity.
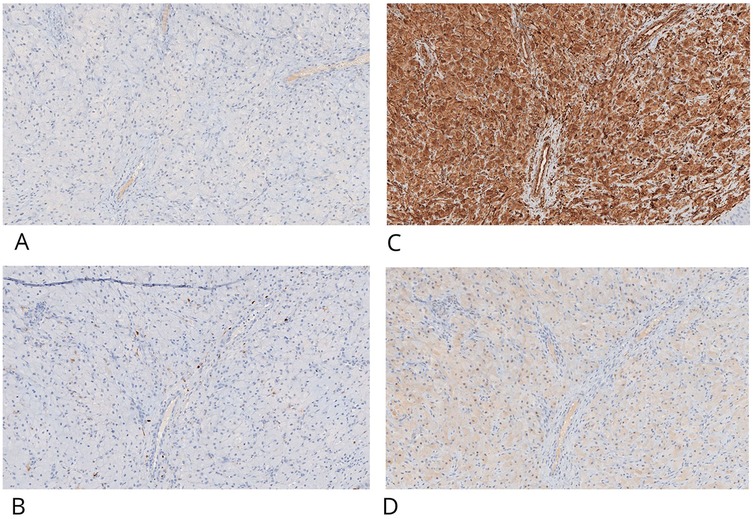
Figure 4. CGCE showed negative IHC staining for CD-1a (A) and S100, light positive is present in interstitial cells (B) granular cells are positive for IHC staining for vimentin (C) and neuron-specific enolase- NSE (D) original magnification ×20 nanoZoomer S60 C13210 series.
Immunohistochemical evaluation, performed by an expert surgical pathologist using an Olympus BX41 optical microscope and NanoZoomer S60 C13210 digital scanner, confirmed the diagnosis. The granular cells showed strong cytoplasmic positivity for vimentin and NSE, and were negative for S100, cytokeratin AE1/AE3, smooth muscle actin, desmin, and CD1a. CD68 positivity was limited to scattered background histiocytes and not present in the granular cell component. This combined histological and immunohistochemical profile is diagnostic for congenital granular cell epulis, clearly distinguishing it from granular cell tumors of neural origin.
2.3.4 Therapeutic intervention
A prompt surgical dental consultation was undertaken. Based on the multidisciplinary assessment, the decision was made to proceed with early surgical excision under sedation using electrosurgical technique, both to relieve functional obstruction and to obtain tissue for definitive histopathological diagnosis.
2.3.5 Follow-up and outcomes
Residual electrocoagulative effects were observed at the surgical resection margin, characterized by localized tissue changes indicative of thermal injury. The patient underwent a follow-up examination one month after the surgical procedure. Clinical assessment demonstrated excellent healing of the surgical site, with no signs of infection, dehiscence, or other postoperative complications. The mucosal and tissue regeneration appeared optimal, indicating effective wound healing and satisfactory tissue integration.
2.3.6 Patient perspective
The patient's parents expressed satisfaction with the clinical outcome and appreciated the prompt diagnosis and treatment provided.
2.3.7 Informed consent
Written informed consent was obtained from the patient's legal guardians for publication of this case report and accompanying images.
3 Discussion
Histologically, CGCE is composed of large polygonal cells with granular eosinophilic cytoplasm and small, bland nuclei, typically arranged in nests or sheets within a vascular stroma (6). These features resemble adult granular cell tumors (GCTs), which also affect the oral cavity—most commonly the tongue—and are more frequently observed in women aged 30–60 years (7) (Figure 5 Supplementary Data).
However, important differences exist between CGCE and GCT. The most significant differential diagnostic factor lies in their immunohistochemical profile. GCTs are typically S100-positive, reflecting their Schwannian origin, while CGCE generally lacks S100 expression (Figure 6 Supplementary Data) (7). Furthermore, CD68 expression, typically strong and diffuse in GCTs due to their lysosome-rich cytoplasm, is often weak or absent in CGCE (Figure 7 Supplementary Data) (8, 9). In the present case, immunohistochemical analysis confirmed these expected patterns, with CGCE cells testing negative for S100, cytokeratin AE1/AE3, desmin, smooth muscle actin, and CD1a, but positive for vimentin and neuron-specific enolase (NSE).
Another key histological distinction lies in the relationship with the overlying epithelium: GCTs frequently show pseudoepitheliomatous hyperplasia (PEH), a reactive epithelial proliferation that can mimic squamous cell carcinoma, whereas CGCE typically lacks this feature (10, 11). The absence of PEH in CGCE further supports its benign and distinct nature.
From a clinical standpoint, although CGCE is benign, its presence can interfere with essential functions such as breastfeeding, as observed in our case. In rare instances, spontaneous regression has been documented (12), but in most cases, conservative surgical excision is the preferred management approach due to potential mechanical obstruction or aesthetic concerns. Recurrence after complete excision is exceedingly rare (13).
While the etiopathogenesis of CGCE remains unclear, occasional familial cases suggest a possible genetic predisposition (14). Further research is needed to elucidate the molecular pathways involved in its development and to better define its origin within the spectrum of congenital oral lesions (15).
Finally, the postoperative outcome in this case showed excellent healing, without complications. This is in line with recent studies indicating that optimal wound healing depends on factors such as the surgical technique, tissue characteristics, and postoperative care (16, 17).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
PG: Investigation, Writing – original draft. RS: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. LF: Investigation, Writing – original draft. GT: Investigation, Writing – original draft. GM: Data curation, Writing – original draft. MP: Formal Analysis, Methodology, Writing – original draft. GP: Conceptualization, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Funding acquisition, Project administration, Resources, Validation, Writing – original draft. RZ: Formal analysis, Supervision, Writing – review & editing. GM: Funding acquisition, Project administration, Resources, Validation, Writing – original draft. ID: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The author recognizes the financial contribution of the European Union—NextGenerationUE as part of PNRR MUR—M4C2—Investimento 1.3—Public Call “PartenariatiEstesi”—D.D. n. 341/2022, “HEAL ITALIA—Health Extended Alliancefor Innovative Therapies, Advanced Lab-research and Integrated Approaches ofPrecision Medicine” Cod. PE_00000019, and “la pubblicazione è stata realizzata con il cofinanziamento dell'Unione europea—Next Generation EU nell'ambito del PNRR MUR—M4C2—Investimento 1.3—Avviso ‘Partenariati Estesi’ —D.D. n. 341/2022”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1548291/full#supplementary-material
References
1. Conrad R, Perez MC. Congenital granular cell epulis. Arch Pathol Lab Med. (2014) 138(1):128–31. doi: 10.5858/arpa.2012-0306-RS
2. Dzieniecka M, Komorowska A, Grzelak-Krzymianowska A, Kulig A. Multiple congenital epulis (congenital granular cell tumours) in the newborn: a case report and review of literature. Pol J Pathol. (2011) 62(1):69–71.21574109
3. Finkelstein M, Ozdemir C. Congenital granular cell epulis: a case report and review of the literature. J Clin Pediatr Dent. (2011) 35(3):303–7.
4. Rao PR, Chava K. Congenital granular cell epulis: a rare entity of the oral cavity in a neonate. J Clin Diagn Res. (2015) 9(2):201–2.
5. Bianchi PR, de Araujo VC, Ribeiro JW, Passador-Santos F, Soares de Araujo N, Soares AB. Multiple congenital granular cell epulis: case report and immunohistochemical profile with emphasis on vascularization. Case Rep Dent. (2015) 2015:878192. doi: 10.1155/2015/878192
6. Lifshitz MS, Flotte TJ, Greco MA. Congenital granular cellepulis. Immunohistochemical and ultrastructural observations. Cancer. (1984) 53(9):1845–8. doi: 10.1002/1097-0142(19840501)53:9%3C1845::AID-CNCR2820530908%3E3.0.CO;2-L
7. Filie AC, Lage JM, Azumi N. Immunoreactivity of S100 protein, alpha-1-antitrypsin, and CD68 in adult and congenital granular cell tumors. Mod Pathol. (1996) 9(9):888–92.8878020
8. Vered M, Dobriyan A, Buchner A. Congenital granular cell epulis presents an immunohistochemical profile that distinguishes it from the granular cell tumor of the adult. Virchows Arch. (2009) 454:303–10. doi: 10.1007/s00428-009-0733-y
9. Avalos HS, Manci E, Mulekar M, Finnegan A, Barui S, Galliani C, et al. Congenital granular cell epulis: 24 new cases with more differences than similarities to granular cell tumor. Ultrastruct Pathol. (2022) 46(4):388–400. doi: 10.1080/01913123.2022.2107750
10. Atarbashi-Moghadam S, Lotfi A, Eftekhari-Moghadam P. Oral granular cell tumor: a case report with emphasis on pseudoepitheliomatous hyperplasia in oral lesions. J Dent (Shiraz). (2024) 25(1):91–4. doi: 10.30476/dentjods.2023.98784.2108
11. Vered M, Carpenter WM, Buchner A. Granular cell tumor of the oral cavity: updated immunohistochemical profile. J Oral Pathol Med. (2009) 38(1):150–9. doi: 10.1111/j.1600-0714.2008.00725.x
12. Bhatia SK, Goyal A, Ritwik P, Rai S. Spontaneous regression of a congenital epulis in a newborn. J Clin Pediatr Dent. (2013) 37(3):297–9. doi: 10.17796/jcpd.37.3.m1n1425821896807
13. Ritwik P, Brannon RB, Musselman RJ. Spontaneous regression of congenital epulis: a case report and review of the literature. J Med Case Rep. (2010) 4:331. doi: 10.1186/1752-1947-4-331
14. Scully C, Bagan JV. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Oxford: Oxford University Press (2009).
15. Su L, Zhang C. Congenital granular cell epulis: histopathologic and immunohistochemical study of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. (2019) 127(5):e144–8.
16. Lucas RB, Cawson RA. Lucas’s Pathology of Tumors of the Oral Tissues. 5th ed. London: Churchill Livingstone (1998). p. 339.
Keywords: benign tumors, congenital epulis, granular cell tumor, infancy tumor, oral tumor, histopathology, immunohistochemistry
Citation: Guidone PC, Seccia R, Fabrocini LA, Troiano G, Maffei G, Pedicillo MC, Pannone G, Lo Muzio L, Zamparese R, Mori G and De Stefano IS (2025) Congenital granular cell epulis in a neonate: a case report and review of diagnosis, treatment, and prognosis. Front. Oral Health 6:1548291. doi: 10.3389/froh.2025.1548291
Received: 19 December 2024; Accepted: 26 June 2025;
Published: 11 August 2025.
Edited by:
Felipe Silveira, University of the Republic, UruguayReviewed by:
Madhu Shrestha, Texas A&M University, United StatesJessica Maldonado-Mendoza, Metropolitan Autonomous University, Mexico
Copyright: © 2025 Guidone, Seccia, Fabrocini, Troiano, Maffei, Pedicillo, Pannone, Lo Muzio, Zamparese, Mori and De Stefano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilenia Sara De Stefano, aWxlbmlhLmRlc3RlZmFub0B1bmlmZy5pdA==
 P. C. Guidone1
P. C. Guidone1 G. Troiano
G. Troiano G. Maffei
G. Maffei Giuseppe Pannone
Giuseppe Pannone Lorenzo Lo Muzio
Lorenzo Lo Muzio Giorgio Mori
Giorgio Mori