- 1Department of Dental Biomaterials, School of Dentistry, Alborz University of Medical Sciences, Karaj, Iran
- 2Department of Dental Biomaterials, School of Dentistry, Iran University of Medical Sciences, Tehran, Iran
- 3Private Clinic, Tehran, Iran
- 4Research Center for Prevention of Oral and Dental Diseases, Baqiyatallah University of Medical Sciences, Tehran, Iran
Background and aim: Dentin hypersensitivity (DH) has long been a challenging condition, with many treatment methods showing limited success. However, the emergence of laser therapy, particularly the significant potential of diode laser (DL) and sodium fluoride (NaF) varnish, has sparked new hope. This research is a significant step towards a more effective treatment for DH, aiming to evaluate the promising potential of DL in treating DH, both independently and in combination with fluoride varnish. By delving into this research, you are investing your time in understanding a crucial advancement in the field of dentistry.
Methods: A comprehensive search was conducted across the PubMed, Scopus, and Web of Science databases, including studies published up until May 2024. Randomized clinical trials that assessed DH using a visual analog scale (VAS) score were included. Data on participant demographics, treatment types, and VAS scores were extracted by two reviewers. The risk of bias was assessed using the revised Cochrane risk-of-bias instrument for randomized trials (RoB-2).
Result: Three studies met the inclusion criteria, comparing NaF varnish, DL, and their combination. Both DL and the combination of DL and NaF varnish were more effective than NaF varnish alone in reducing DH. The combined treatment showed marginally superior outcomes compared to DL alone. Significant reductions in DH were observed across all treatment groups, with the combination therapy demonstrating the most substantial and consistent improvement.
Conclusion: Diode laser therapy, particularly when combined with NaF varnish, represents a promising treatment approach for DH, offering superior efficacy over NaF varnish alone. These findings suggest that combination therapy may provide longer-lasting relief, with implications for improving clinical outcomes in DH management.
1 Introduction
Dentin hypersensitivity (DH) is a common condition that affects approximately 20% of people. It is characterized by sharp, rapid, and temporary or prolonged pain in response to various triggers (1–4). These stimuli, including thermal (TS), evaporative (ES), tactile, osmotic, or chemical, typically do not elicit any response in healthy teeth (1). Significantly, DH cannot be attributed to other forms of dental pathology (1, 3). In addition, DH may be considered an adverse effect of dental bleaching procedures (2).
DH can be attributed to several contributing factors. Gingival recession is a key factor, as it exposes the cervical dentin and root surface, making them vulnerable to various stimuli. Aging can also increase dentin sensitivity, as the protective enamel layer may wear down over time. Dehiscence of the soft tissue, or the separation of the gingiva from the tooth surface, can also contribute to dentin exposure and increased sensitivity. Furthermore, overly aggressive tooth brushing has been identified as a potential cause of DH (5).
Several concepts have been proposed to clarify the underlying mechanisms of DH. These theories, including the transducer, gate control, direct receptor mechanism, and modulation theories, attempt to explain how and why DH occurs. However, the hydrodynamic hypothesis is widely accepted as the most plausible explanation for DH (3, 6).
Various assessment measures may be used to quantify the severity of pain related to DH. These include a descriptive scale that classifies the pain as mild, moderate, or severe, and a visual analog scale (VAS) that allows patients to assess the pain on a scale from 0 to 10 (7).
Desensitizing agents are commonly used in dental practice to manage DH. These agents typically contain compounds such as sodium fluoride (NaF), nanohydroxyapatite, amorphous calcium phosphate, calcium, and sodium monofluorophosphate (2). The effectiveness of fluoride-based compounds in alleviating DH has been well-established (8–10). Fluoride promotes the formation of calcium fluoride (CaF₂) crystals within the dentinal tubules, which reduces dentin permeability. These crystals are highly resistant to dissolution in saliva, thus temporarily occluding the tubules (11). However, despite the widespread use of 2% NaF varnish in clinical settings, the calcium deposits formed can be easily removed through routine brushing and the flow of saliva.
Laser therapy has emerged as an alternative method for treating DH. Clinical studies report significant variability in treatment outcomes, with success rates in the range of 5%–100% (12, 13). Many patients experience immediate relief from sensitivity after laser treatment, and the effectiveness of the therapy is influenced by various factors, including the wavelength, irradiation mode (continuous or pulsed), exposure time, and power output (14). Although the precise mechanism of action is not fully understood, it is widely believed that lasers alleviate sensitivity by sealing dentinal tubules through a process of melting and recrystallization of the dentin, which reduces fluid movement and nerve stimulation (15).
Several types of lasers, including neodymium-doped yttrium aluminum garnet (Nd:YAG), erbium-doped yttrium aluminum garnet (Er:YAG), CO₂ lasers, and diode lasers, have been investigated for DH treatment. Among these, diode lasers have shown considerable effectiveness, often achieving results comparable to or better than conventional methods. In addition, low-level laser therapy (LLLT) – also known as cold laser therapy or photobiomodulation therapy – has attracted attention for its non-invasive approach and minimal thermal effects (2, 4, 7, 16). LLLT typically uses wavelengths in the red (630–690 nm) and near-infrared (810–980 nm) spectrum. It stimulates odontoblasts, promoting the formation of tertiary dentin, narrowing of dentinal tubules, and modulation of inflammation and pain through cellular activation (17). The therapeutic effectiveness of LLLT is dose-dependent and usually requires multiple sessions spaced over time. The response to LLLT varies among individuals, with outcomes influenced by laser parameters, treatment frequency, and the underlying cause of hypersensitivity. Notably, lasers can also be combined with conventional desensitizing agents to enhance clinical outcomes, offering a synergistic approach to managing DH effectively (4).
In this regard, this systematic review aimed to evaluate and compare the effectiveness of NaF varnish, diode laser therapy (DL), and their combination in reducing DH.
2 Methods
2.1 Search strategy
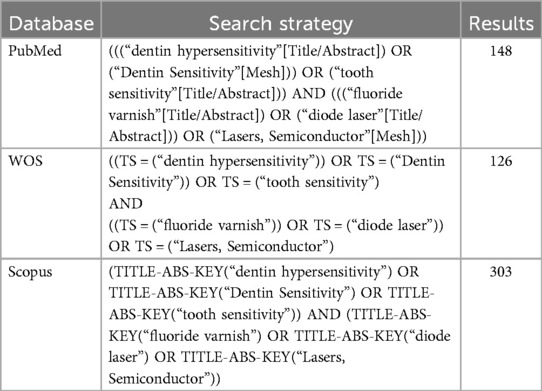
Following the PRISMA criteria (9), two researchers conducted thorough searches of several electronic databases, including PubMed, Scopus, Web of Science, and Google Scholar, covering publications published up to May 2024. The search methodology used a combination of Medical Subject Headings (MeSH) terms and text-based keywords. Alongside the electronic database searches, the researchers manually reviewed the reference lists of the selected articles and related review papers and meta-analyses to identify any potentially relevant publications. Search terms include: [(“fluoride varnish” OR “NaF varnish” OR “sodium fluoride” OR “diode laser” OR “sodium monofluorophosphate”) AND “dentin hypersensitivity”] (Table 1).
2.2 Inclusion criteria and study selection
Studies were included if they were clinical trials published in English, comparing NaF varnish, diode laser, or their combination for treating dentin DH, and used a VAS score. PICO criteria were
• Population: individuals with DH
• Intervention: sodium fluoride varnish, diode laser, or their combination
• Comparison: placebo or comparisons between the interventions
• Outcome: reduction in DH, measured by VAS score
Once duplicate publications were removed, the remaining papers were screened based on their titles and abstracts to exclude irrelevant themes or articles that did not match the inclusion requirements. Subsequently, one reviewer (A) conducted a thorough assessment of the whole texts of the remaining publications. Any uncertainties were addressed through discussion with a second reviewer (B), ensuring a rigorous and transparent selection process. When multiple publications were found from the same study, the most comprehensive and/or latest paper was considered for inclusion.
2.3 Data extraction
Two researchers (A and B) independently reviewed each potentially eligible article and extracted the relevant information. A data extraction template was developed specifically for this review. The extracted data included participant age, gender, type of treatment (fluoride varnish, diode laser, and their combination), VAS score (before each treatment, after each treatment, and after combined treatment), and history of received DH treatments.
2.4 Risk of bias assessment
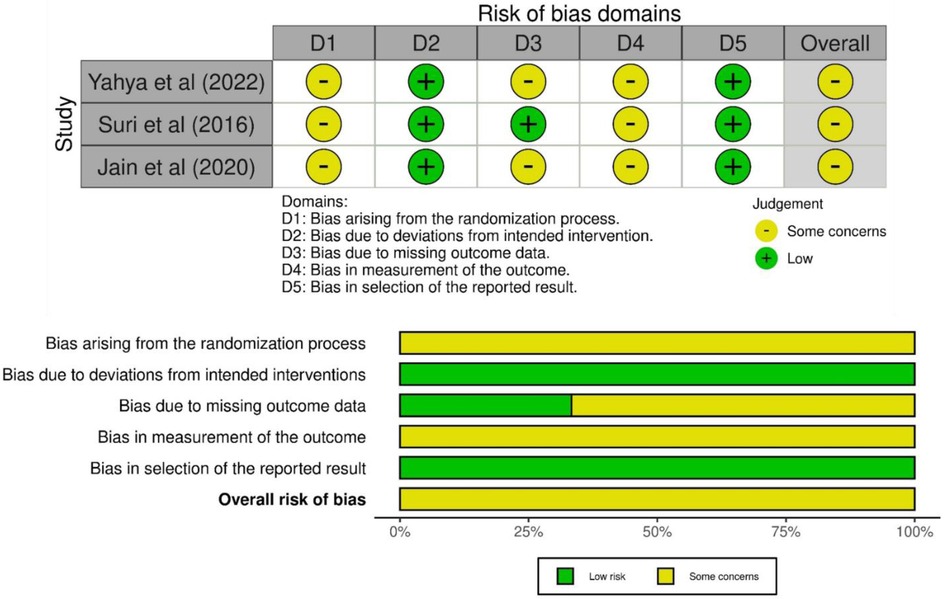
The methodological quality of the included studies was assessed using the RoB-2 tool (Figure 1), which evaluates five domains: randomization, deviations from planned interventions, missing outcome data, outcome measurement, and selection of reported outcomes. Each domain was rated as “high,” “unclear,” or “low” risk of bias (18). All three studies clearly described randomization methods; however, allocation concealment was not adequately detailed, resulting in an unclear risk. Deviations from planned interventions were low. The risk of bias due to missing data was unclear due to insufficient reporting in two studies. Bias in outcome measurement was unclear in studies since none of them mentioned whether the outcome assessors were blinded to the intervention groups. The risk of selective reporting was low in all studies. Two separate reviewers undertook the quality assessment for all the papers in the review, referred to as reviewer A and reviewer B. Any discrepancies between the two reviewers' assessments were discussed to reach an agreement. In cases where the two reviewers could not resolve the disagreement, a third reviewer, reviewer C, was consulted to intervene and help determine the final quality rating for the disputed study.
3 Result
3.1 Study selection and characteristics
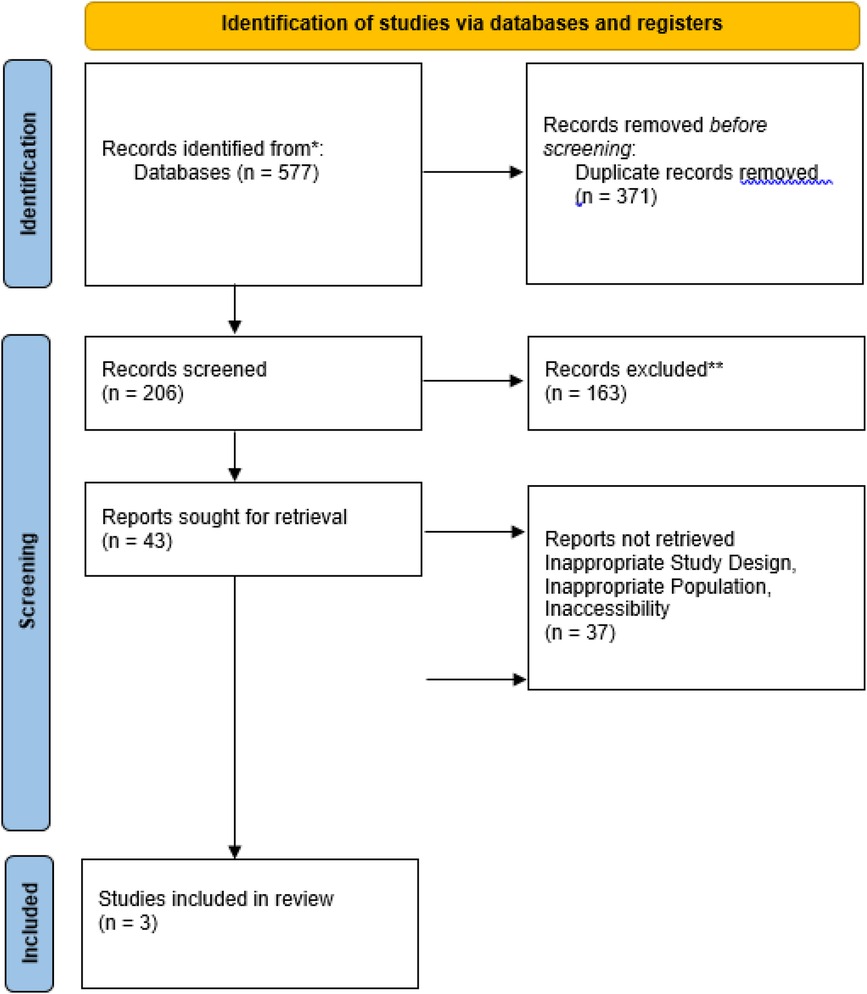
The search strategy identified 577 studies across the selected databases (Figure 2). After removing 371 duplicates, 206 articles remained for title and abstract screening. Of these, 203 were excluded, resulting in the inclusion of three studies in the systematic review. Table 2 presents the characteristics and key findings of the included studies.
3.2 Demographic characteristics
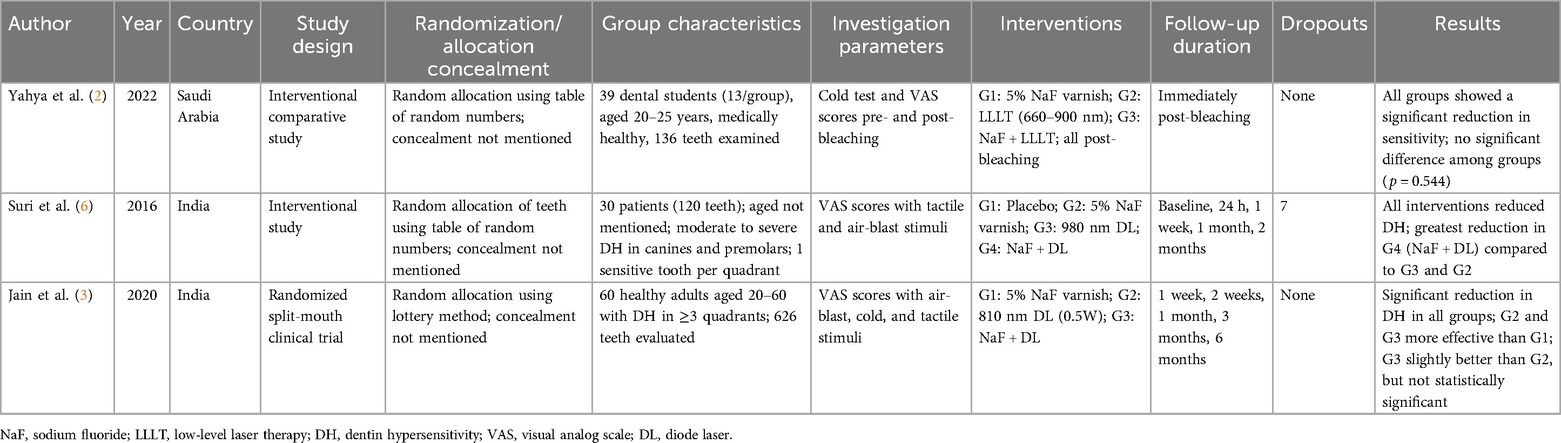
A total of 150 participants were evaluated across the included studies. The study by Yahya et al. (2) involved 30 participants, though specific demographic details were not reported. Jain et al. (3) studied 60 patients, comprising 33 women and 27 men, with a mean age of 36 years. Baseline hypersensitivity scores did not differ significantly between groups (p ≥ 0.05). In the study by Suri et al. (6), 30 patients (120 teeth) were followed over a 2-month period. Although there was an equal gender distribution in the 40–49-year-old age group, there were more men than women in the other age categories (20–29, 30–39, and 50–59 years), this variation was not statistically significant. No adverse events were reported during the observation period in any study.
3.3 Intragroup changes in DH
All studies demonstrated significant reductions in DH within each group. In Yahya et al. (2), mean VAS scores rose from 4.80 ± 2.41 before bleaching to 6.00 ± 2.23 after bleaching, then declined to 3.72 ± 2.31 after treatment (p < 0.05). For the NaF varnish, DL, and combination groups, VAS scores decreased from 6.32 ± 2.21 to 3.89 ± 2.41, 5.83 ± 2.33 to 3.90 ± 2.38, and 5.83 ± 2.21 to 3.44 ± 2.16, respectively (p < 0.05 for all). Jain et al. (3) observed significant DH reductions at intervals of 1, 3, and 6 months across all groups (p ≤ 0.05) for air-blast, cold, and tactile stimuli, respectively. Similarly, Suri et al. (6) reported significant declines in tactile stimulation (TS) scores from baseline to 2 months: from 5.60 to 1.23 in the NaF group, 6.23 to 0.73 in the DL group, and 6.00 to 0.43 in the combination group. Air-blast scores also decreased significantly: from 6.70 to 1.80 in the NaF group, 6.30 to 1.27 in the DL group, and 6.27 to 0.87 in the combination group (p < 0.001 for all within-group changes).
3.4 Intergroup comparisons
Combination therapy consistently showed the greatest reduction in DH. In the study by Yahya et al. (2), mean post-treatment VAS scores were lowest in the combination group (3.44 ± 2.16) compared to the NaF (3.89 ± 2.41) and DL groups (3.90 ± 2.38), though not statistically significant (p = 0.544). Jain et al. (3) found significantly greater reductions in the DL and combination groups compared to the NaF group at all follow-ups (p < 0.05); however, differences between the DL and combination groups were not significant. Suri et al. (6) reported significant intergroup differences at multiple timepoints, with the combination group outperforming others as early as 24 h (p < 0.05), and at 1 week, 1 month, and 2 months (p < 0.001).
4 Discussion
This systematic review aimed to evaluate and compare the effectiveness of NaF varnish, DL, and their combination in reducing DH. Across the three included studies, all interventions demonstrated significant reductions in DH. These findings support the overall efficacy of NaF, DL, and their combined use in mitigating DH.
The mechanism of action for NaF varnish is attributed to the formation of calcium fluoride (CaF₂) crystals, which temporarily occlude the dentinal tubules. However, due to their small size (approximately 0.05 µm), these crystals are prone to dissolution or mechanical removal through brushing, salivary flow, and exposure to dietary acids, which can eventually reopen tubules and lead to a recurrence of symptoms. In contrast, diode laser therapy offers a potentially longer-lasting effect by inducing nerve desensitization and promoting internal obliteration of tubules through the stimulation of secondary dentin formation. This secondary dentin is less susceptible to mechanical wear, thereby extending the duration of desensitization (19).
All three studies affirmed the therapeutic effects of NaF and the diode laser, both individually and in combination. The reduction in sensitivity in the NaF-only groups may result from the interaction between fluoride and calcium ions in the dentinal fluid, forming a superficial layer of CaF₂ that partially blocks tubules.
The combined use of NaF and the diode laser showed promising outcomes in the studies by both Suri et al. and Jain et al., suggesting a synergistic effect between NaF's remineralizing capacity and the laser's biostimulatory properties. Previous studies have reported comparable findings, indicating that the diode laser, whether applied alone or in combination with fluoride varnish, demonstrated a significantly higher effectiveness compared to fluoride varnish alone (20–22). Similar findings were reported by Umberto et al. (23) and Kumar and Mehta (24), who observed a greater reduction in sensitivity scores (VAS and cold air-blast index) when both treatments were used together compared to either alone. The laser likely enhances desensitization by stimulating odontoblasts, promoting secondary dentin formation, and increasing pain thresholds via nerve depolarization at the dentin–pulp interface (25).
Despite these encouraging results, Yahya et al. (2) reported no significant differences between treatment groups immediately after bleaching, contrasting with the findings of Suri et al. (6) and Jain et al. (3), who demonstrated superior outcomes in the DL and combined therapy groups over time. This discrepancy may stem from differences in follow-up duration; Yahya et al. (2) conducted only an immediate post-treatment assessment, while Jain et al. (3) followed participants for up to 6 months, allowing for observation of longer-term effects.
The incremental benefit of combining NaF with DL, although evident in some studies, was not statistically significant in the study by Jain et al. (3), suggesting the possibility of a modest additive effect. Variability in laser parameters (e.g., wavelength and power), baseline DH severity, and application protocols may have influenced these results. For instance, Suri et al. (6) used a 980 nm DL at 2 W continuous wave (CW) – a setting supported by Liu et al. (26) for effective tubule sealing – while Jain et al. employed an 810 nm DL at 0.5 W CW and Yahya et al. (2) used a laser in the 660–900 nm range at 90 mW with no cooling. These methodological inconsistencies hinder direct comparisons and may contribute to the variation in outcomes.
Several other factors may account for discrepancies among the studies. First, all three studies used the VAS to assess pain, which is inherently subjective and highly dependent on individual pain thresholds (27). In addition, examiner-dependent factors – such as pressure applied during tactile testing, variability in air-blast force, and fluctuations in temperature during cold testing – could have contributed to result variability. A further limitation was the lack of placebo control in two studies (2, 3), which complicates interpretation of treatment-related effects relative to natural desensitization or placebo responses. Sample size limitations also warrant consideration. Although each study showed statistically significant findings, larger and more diverse samples would enhance the generalizability and statistical power of future research.
This study suggests that diode laser therapy, particularly when combined with 5% NaF varnish, may offer superior and longer-lasting relief from DH compared to either modality alone. These findings support the clinical utility of combination therapy for DH management. However, future clinical trials should aim for including placebo-controlled groups, standardizing laser parameters, and using objective pain assessment tools. Consistent follow-up intervals extending beyond 6 months are recommended to determine the longevity of therapeutic effects. In additionally, studies should consider controlling for confounding factors such as plaque levels and baseline oral hygiene. Larger multicenter trials would also be valuable to validate findings across broader populations and clinical settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MM: Conceptualization, Data curation, Writing – original draft. NJ: Data curation, Investigation, Methodology, Software, Writing – original draft. MY: Data curation, Investigation, Methodology, Writing – original draft. EK: Data curation, Investigation, Writing – original draft, Writing – review & editing. SH: Data curation, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moeintaghavi A, Ahrari F, Nasrabadi N, Fallahrastegar A, Sarabadani J, Rajabian F. Low level laser therapy, Er, Cr: YSGG laser and fluoride varnish for treatment of dentin hypersensitivity after periodontal surgery: a randomized clinical trial. Lasers Med Sci. (2021) 36:1949–56. doi: 10.1007/s10103-021-03310-4
2. Yahya G, AlAlwi A, Shurayji F, Baroom W, Rajeh M, AbdelAleem N. Effectiveness of sodium fluoride varnish and/or diode laser in decreasing post-bleaching hypersensitivity: a comparative study. Saudi Dent J. (2022) 34(1):62–7. doi: 10.1016/j.sdentj.2021.09.024
3. Jain A, Rao J, Pal N, Singh A. Effectiveness of fluoride varnish, diode laser, and their combination in treatment of dentin hypersensitivity: a randomized split-mouth clinical trial. J Indian Soc Periodontol. (2020) 24(4):369–74. doi: 10.4103/jisp.jisp_494_19
4. Forouzande M, Rezaei-Soufi L, Yarmohammadi E, Ganje-Khosravi M, Fekrazad R, Farhadian M, et al. Effect of sodium fluoride varnish, gluma, and Er, Cr: YSGG laser in dentin hypersensitivity treatment: a 6-month clinical trial. Lasers Med Sci. (2022) 37(7):2989–97. doi: 10.1007/s10103-022-03583-3
5. Cattoni F, Ferrante L, Mandile S, Tetè G, Polizzi EM, Gastaldi G. Comparison of lasers and desensitizing agents in dentinal hypersensitivity therapy. Dent J. (2023) 11(3):63. doi: 10.3390/dj11030063
6. Suri I, Singh P, Shakir QJ, Shetty A, Bapat R, Thakur R. A comparative evaluation to assess the efficacy of 5% sodium fluoride varnish and diode laser and their combined application in the treatment of dentin hypersensitivity. J Indian Soc Periodontol. (2016) 20(3):307–14. doi: 10.4103/0972-124X.181243
7. Jain PR, Naik GD, Uppor SA, Kamath DG. Diode laser and fluoride varnish in the management of dentin hypersensitivity. J Interdiscip Dent. (2015) 5(2):71–4. doi: 10.4103/2229-5194.173226
8. Antoniazzi RP, Machado ME, Grellmann AP, Santos R, Zanatta FB. Effectiveness of a desensitizing agent for topical and home use for dentin hypersensitivity: a randomized clinical trial. Am J Dent. (2014) 27(5):251–7.25842457
9. Shah FA. Fluoride-containing bioactive glasses: glass design, structure, bioactivity, cellular interactions, and recent developments. Mater Sci Eng C. (2016) 58:1279–89. doi: 10.1016/j.msec.2015.08.064
10. Han L, Okiji T. Dentin tubule occluding ability of dentin desensitizers. Am J Dent. (2015) 28(2):90–4.26087574
11. Chen C, Parolia A, Pau A, Celerino de Moraes Porto I. Comparative evaluation of the effectiveness of desensitizing agents in dentine tubule occlusion using scanning electron microscopy. Aust Dent J. (2015) 60(1):65–72. doi: 10.1111/adj.12275
12. Lopes AO, de Paula Eduardo C, Aranha ACC. Clinical evaluation of low-power laser and a desensitizing agent on dentin hypersensitivity. Lasers Med Sci. (2015) 30:823–9. doi: 10.1007/s10103-013-1441-z
13. Yilmaz H, Bayindir H. Clinical and scanning electron microscopy evaluation of the Er, Cr: YSGG laser therapy for treating dentine hypersensitivity: short-term, randomised, controlled study. J Oral Rehabil. (2014) 41(5):392–8. doi: 10.1111/joor.12156
14. He S, Wang Y, Li X, Hu D. Effectiveness of laser therapy and topical desensitising agents in treating dentine hypersensitivity: a systematic review. J Oral Rehabil. (2011) 38(5):348–58. doi: 10.1111/j.1365-2842.2010.02193.x
15. Schwarz F, Arweiler N, Georg T, Reich E. Desensitizing effects of an Er: YAG laser on hypersensitive dentine: a controlled, prospective clinical study. J Clin Periodontol. (2002) 29(3):211–5. doi: 10.1034/j.1600-051x.2002.290305.x
16. Aghanashini S, Puvvalla B, Nadiger S, Mundinamanae DB, Bhat D, Andavarapu S. Comparative evaluation of diode laser and fluoride varnish for treatment of dentin hypersensitivity: a clinical study. J Interdiscip Dent. (2018) 8(3):110–7. doi: 10.4103/jid.jid_3_18
17. Papadopoulou A, Vourtsa G, Tolidis K, Koliniotou-Koumpia E, Gerasimou P, Strakas D, et al. Clinical evaluation of a fluoride gel, a low-level laser, and a resin varnish at the treatment of dentin hypersensitivity. Lasers Dent Sci. (2019) 3:129–35. doi: 10.1007/s41547-019-00057-8
18. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Dantas EM, Amorim F, Nóbrega F, Dantas PMC, Vasconcelos RG, Queiroz LMG. Clinical efficacy of fluoride varnish and low-level laser radiation in treating dentin hypersensitivity. Braz Dent J. (2016) 27:79–82. doi: 10.1590/0103-6440201602422
20. Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E. Long-term effect of diode laser irradiation compared to sodium fluoride varnish in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized controlled clinical study. Photomed Laser Surg. (2011) 29(11):721–5. doi: 10.1089/pho.2010.2974
21. Tailor A, Shenoy N, Thomas B. To compare and evaluate the efficacy of bifluorid 12, diode laser and their combined effect in treatment of dentinal hypersensitivity-a clinical study. J Health Allied Sci NU. (2014) 4(02):054–8. doi: 10.1055/s-0040-1703764
22. Pesevska S, Nakova M, Ivanovski K, Angelov N, Kesic L, Obradovic R, et al. Dentinal hypersensitivity following scaling and root planing: comparison of low-level laser and topical fluoride treatment. Lasers Med Sci. (2010) 25:647–50. doi: 10.1007/s10103-009-0685-0
23. Umberto R, Claudia R, Gaspare P, Gianluca T, Alessandro DV. Treatment of dentine hypersensitivity by diode laser: a clinical study. Int J Dent. (2012) 2012(1):858950. doi: 10.1155/2012/858950
24. Kumar NG, Mehta D. Short-term assessment of the Nd: YAG laser with and without sodium fluoride varnish in the treatment of dentin hypersensitivity–a clinical and scanning electron microscopy study. J Periodontol. (2005) 76(7):1140–7. doi: 10.1902/jop.2005.76.7.1140
25. Walsh L. The current status of low level laser therapy in dentistry. Part 2. Hard tissue applications. Aust Dent J. (1997) 42(5):302–6. doi: 10.1111/j.1834-7819.1997.tb00134.x
26. Liu Y, Gao J, Gao Y, Xu S, Zhan X, Wu B. In vitro study of dentin hypersensitivity treated by 980-nm diode laser. J Lasers Med Sci. (2013) 4(3):111.25606318
Keywords: dentin hypersensitivity, diode laser, fluoride varnish, systematic review, randomized clinical trial
Citation: Mohammadian M, Jalouti N, Yazdanian M, Keykha E and Hajisadeghi S (2025) Comparison of the effectiveness of diode laser, fluoride varnish, and their combination in treatment of dentin hypersensitivity: a systematic review of randomized clinical trials. Front. Oral Health 6:1550127. doi: 10.3389/froh.2025.1550127
Received: 22 December 2024; Accepted: 14 May 2025;
Published: 5 June 2025.
Edited by:
Reisha Rafeek, University of the West Indies St. Augustine, Trinidad and TobagoReviewed by:
Naif Abogazalah, King Faisal University, Saudi ArabiaThamaraiselvan M., Saveetha Dental College and Hospitals, India
Copyright: © 2025 Mohammadian, Jalouti, Yazdanian, Keykha and Hajisadeghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samira Hajisadeghi, ZHIucy5oYWppc2FkZWdoaUBnbWFpbC5jb20=
 Manijeh Mohammadian1,2
Manijeh Mohammadian1,2 Nima Jalouti
Nima Jalouti Samira Hajisadeghi
Samira Hajisadeghi