- 1Faculty of Dentistry, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 2Key Laboratory of Oral Diseases Research of Anhui Province, College & Hospital of Stomatology, Anhui Medical University, Hefei, China
Objective: Porphyromonas gingivalis, a major periodontal pathogen, interacts with other oral microbes through quorum sensing, enhancing its growth and virulence, which contributes to periodontitis. This scoping review aims to examine the role of quorum sensing in the interactions between P. gingivalis and other oral microbial species.
Methods: Two independent researchers conducted a systematic search using the keywords {[(quorum sensing) OR QS] AND [(Porphyromonas gingivalis) OR P. gingivalis]} for English publications prior to 2025 from Medline, Scopus, and Web of Science databases. They screened titles and abstracts, retrieving full texts of original studies to identify key concepts and findings regarding the quorum sensing of P. gingivalis in oral microbial ecosystems.
Results: A total of 205 publications were identified, of which 26 were included in the review. These studies demonstrated quorum sensing of P. gingivalis and other bacteria through signal molecules Autoinducer-1 and Autoinducer-2. Autoinducer-1 enhances the pathogenicity of P. gingivalis, facilitating its integration into complex oral microbial communities. Autoinducer-2 fosters cooperative or competitive relationships between P. gingivalis and other periodontal pathogens, modifying the structure of oral biofilms. Additionally, researchers are studying the use of quorum sensing inhibitors to reduce the virulence of P. gingivalis for managing periodontitis and restoring microbial balance in the oral cavity.
Conclusion: Quorum sensing enhances the pathogenicity of P. gingivalis in the oral environment. Through the modulation of Autoinducer-1 and Autoinducer-2, quorum sensing regulates interactions between P. gingivalis and other oral microbes. This study demonstrates the need for further research into quorum sensing-targeted interventions in periodontal therapy.
1 Introduction
Oral cavity is a highly complex and dynamic microbiome, consisting of diverse bacterial species that coexist and engage in complex interactions (1). Porphyromonas gingivalis (P. gingivalis) stands out among these as a clinically significant gram-negative anaerobe, primarily found in biofilms on the surfaces of teeth and soft tissues. The interactions of P. gingivalis with other microorganisms are of particular interest because they are implicated in disrupting the homeostasis (the stable balance of microorganisms and conditions in the oral environment) and are crucial in the pathogenesis of periodontal disease, a prevalent chronic inflammatory condition (2).
Bacteria rely on quorum sensing a sophisticated mechanism to perceive and respond to changes in cell-population density through the production and detection of signaling molecules (3). When bacterial populations reach a critical density, this regulatory system triggers coordinated changes in gene expression and physiological behavior, such as virulence factors expression, biofilm formation, survival and colonization (4). Quorum sensing mechanisms are commonly studied using methodologies, including molecular detection techniques such as LC-MS/MS for quantitative analysis of autoinducers and reporter strain assays (e.g., Vibrio harveyi) for functional validation, genetic approaches such as LuxS/luxR gene knockout, omics technologies such as RNA-seq transcriptomics and mass spectrometry-based metabolomics, and in vitro biofilm models. In the context of oral microbiology, quorum sensing emerges as a significant mechanism influencing the pathogenic traits of P. gingivalis, enhancing its ability to form resilient and complex biofilms and to engage in interspecies interactions that can affect the overall microbial community structure (5, 6). Two quorum sensing systems are involved in P. gingivalis (3). The Autoinducer-1 (AI-1) system predominantly regulates intraspecies communication. Recent studies suggest that AI-1 also plays a role in facilitating interactions between P. gingivalis and other species, such as Streptococcus mutans (S. mutans), within mixed-species biofilms, thus highlighting the nuanced roles AI-1 plays in both maintaining species-specific networks and mediating broader ecological interactions (7). On the other hand, Autoinducer-2 (AI-2) serves as a universal signal molecule involved in interspecies communication, bridging both gram-positive and gram-negative bacteria (8). This signaling capability allows P. gingivalis to engage synergistically with various pathogens, thereby exacerbating the severity of periodontal diseases and complicating the microbial landscape of the oral cavity. The modulation of its interactions through AI-1 and AI-2 not only impacts its own pathogenicity but also significantly alters the ecological balance of the oral microbial community.
Considering the critical role of quorum sensing in influencing both cooperative and competitive interactions of P. gingivalis with other microbial species in oral, there is increasing interest in strategically targeting quorum sensing mechanisms using inhibitors. These inhibitors disrupt the intercellular communication pathways mediated by quorum sensing, thereby representing a potential therapeutic approach for combating P. gingivalis-associated diseases. Despite the burgeoning literature on quorum sensing and its implications in P. gingivalis, a cohesive understanding of how quorum sensing regulate microbial interactions with P. gingivalis remains limited. Therefore, this scoping review aims to elucidate the role of quorum sensing in regulating the interactions between Porphyromonas gingivalis and other oral microbial species. Ultimately, this review will provide insights into the potential of targeting quorum sensing pathways as a means to combat periodontal disease and enhance overall oral health (Figure 1).
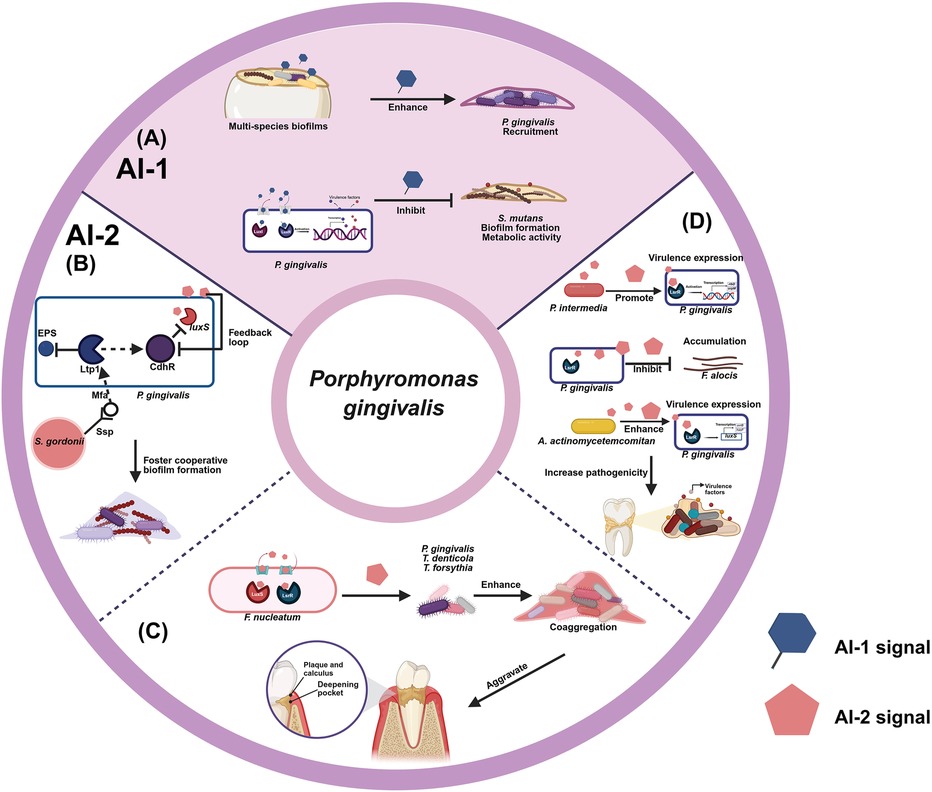
Figure 1. Diagrams of quorum sensing in P. gingivalis. (A) Modulation of P. gingivalis interactions with other bacterial species by AI-1 (B) commensality of P. gingivalis interactions with S. gordonii by AI-2 (C) promotion of P. gingivalis interactions with F. nucleatum by AI-2 (D) collaboration of P. gingivalis interactions with other bacterial species by AI-2. Created in BioRender. Zhao, Z. (2025) https://BioRender.com/o95s786.
2 Methods
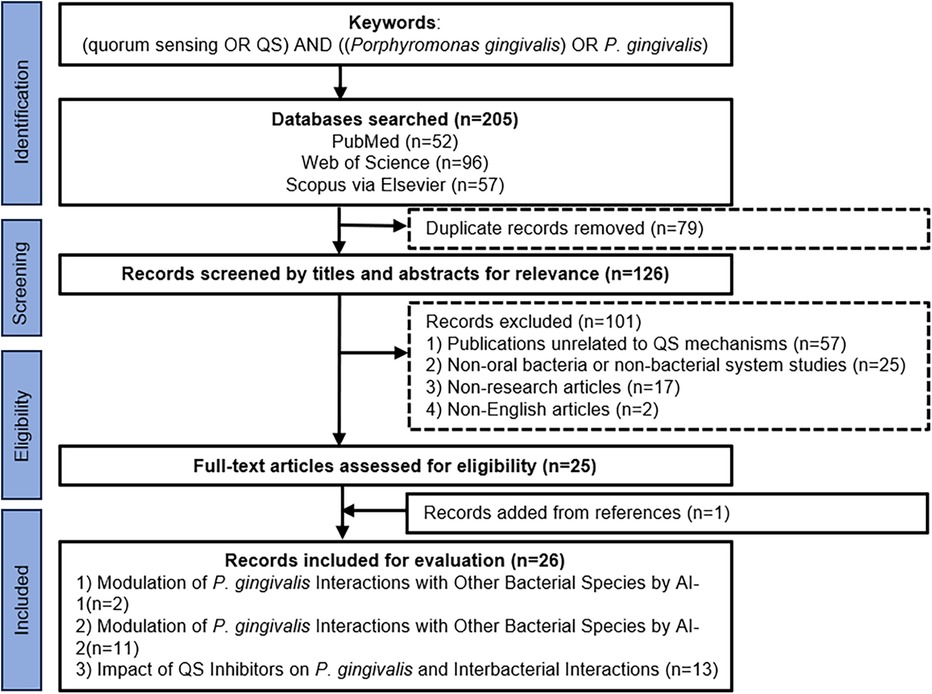
This scoping review followed Arksey & O'Malley's framework and PRISMA-ScR guidelines. To avoid selection biases, a comprehensive search strategy with explicit methodology and transparent reporting was conducted. Two independent researchers systematically searched three databases (PubMed, Web of Science and Scopus via Elsevier) for English-language articles published prior to 31 Dec 2024. The keywords used for relevant articles were “(quorum sensing OR QS) AND [(Porphyromonas gingivalis) OR P. gingivalis]”. Eligibility criteria were defined as: (i) population: studies investigating P. gingivalis and its interactions within oral microbial communities; (ii) concept: studies focusing on quorum sensing mechanisms or quorum sensing inhibitors; (iii) context: oral microbiome studies; (iv) study types: in vitro experiments, animal models, clinical trials or human observational studies; (v) language/date: English-language publications prior to 31 December 2024. Exclusion criteria included: (i) studies lacking direct investigation of quorum sensing mechanisms; (ii) studies focusing on non-oral bacteria or non-bacterial systems; (iii) non-research articles, e.g., reviews without novel data, editorials, conference abstracts. A complete PRISMA-ScR flow diagram showed the screening process and reasons for exclusion at each stage. From 205 initially identified records, 26 articles met all inclusion criteria and were included in the final stage (Figure 2).
3 Modulation of P. gingivalis interactions with other bacterial species by AI-1
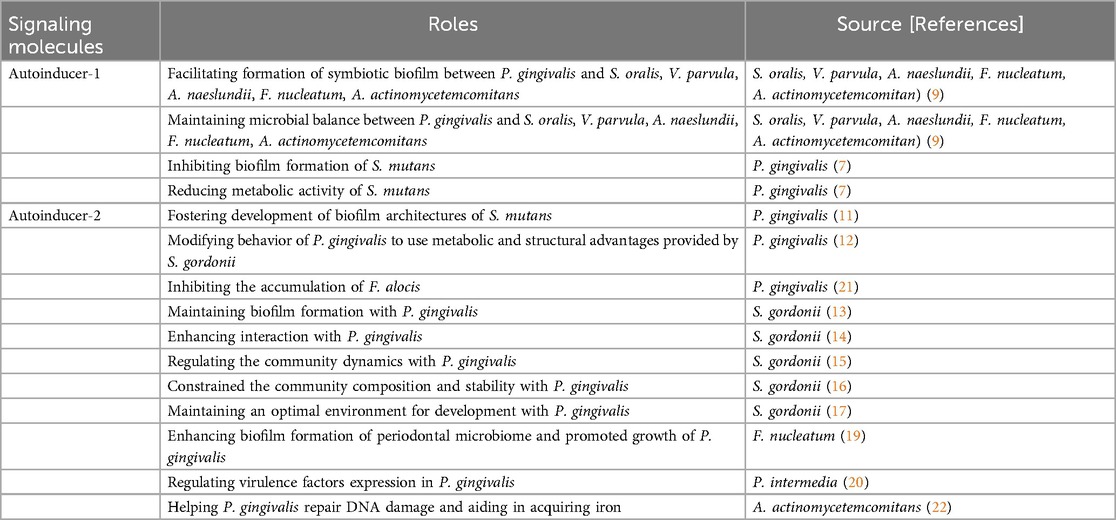
AI-1 is predominantly produced by several periodontal bacteria, including species within the genera Aggregatibacter, Fusobacterium, and Treponema (Muras et al. 2020). AI-1 enables bacteria to sense the density, thereby allowing bacterial survival and pathogenicity. The AI-1 signal N-acyl homoserine lactone (AHL), was also detected in a multi-species oral biofilms including Streptococcus oralis, Veillonella parvula, Actinomyces naeslundii, Fusobacterium nucleatum (F. nucleatum), Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) and P. gingivalis. In oral polymicrobial biofilms, the presence of AI-1 can enhance the recruitment of P. gingivalis, allowing it to take advantage of the protective and nutrient-rich milieu afforded by biofilm formation (9). AI-1 produced by P. gingivalis reduced the biofilm formation and metabolic activity of S. mutans when these two bacteria were grown together. Thus, the interaction between P. gingivalis and S. mutans through AI-1 not only reduced the pathogenic potential of S. mutans but may also indicated that AI-1 signaling plays a regulatory role between periodontal and cariogenic pathogens (7).
In summary, AI-1, predominantly produced by various periodontal bacteria, plays a crucial role in bacterial communication and survival within oral biofilms. AI-1 facilitates the recruitment and virulence of P. gingivalis in multi-species biofilms, enhancing its pathogenic potential. Furthermore, the interaction between P. gingivalis and S. mutans through AI-1 signaling reduces the pathogenicity of S. mutans, suggesting a regulatory role between periodontal and cariogenic pathogens. Understanding AI-1's influence on microbial dynamics and biofilm stability is crucial for grasping the complexities of oral microbial ecology and disease progression.
4 Modulation of P. gingivalis interactions with other bacterial species by AI-2
Quorum sensing molecule, AI-2, a process that bacteria use to communicate with each other and coordinate their behavior based on population density. AI-2 is synthesized by the enzyme LuxS and considered as a universal signaling molecule both in gram-positive and gram-negative bacterial species, making it as a key player in interspecies community (10). By producing and detecting AI-2, P. gingivalis can sense the presence of other bacterial cells in its environment and adjust its behavior in interaction with Streptococcus species. The luxS gene is responsible for producing an AI-2 signaling molecule, which is essential to establish complex biofilm architectures in P. gingivalis with S. mutans (11). AI-2 facilitates P. gingivalis in modifying its behavior to capitalize on the metabolic and structural advantages offered by S. gordonii (12). When the LuxS mutant in S. gordonii reduced AI-2 production, S. gordonii maintained individual growth and biofilm formation but failed to form a cooperative biofilm with P. gingivalis, indicting AI-2 favors a cooperative interaction between S. gordonii and P. gingivalis (13). Furthermore, contact between S. gordonii and P. gingivalis triggers a signaling cascade that can regulate AI-2 activity (14). In this interaction, the Mfa-Ssp binding event of S. gordonii is necessary for initiation of the Ltp1(cytoplasmic eukaryotic-type Low Molecular Weight Tyrosine Phosphatase) -CdhR (LuxR-family transcriptional regulator) pathway of P. gingivalis. CdhR of P. gingivalis modulates community dynamics by repressing luxS, forming a feedback loop with AI-2, which influences the composition and stability of the P. gingivalis-S. gordonii community (15). Additionally, Ltp1 of P. gingivalis plays a crucial role in the downregulation exopolysaccharide, which contributes to maintaining an optimal anaerobic environment favorable for P. gingivalis. This regulatory mechanism supports the stability and composition of the P. gingivalis-S. gordonii community by influencing AI-2-mediated signaling pathways, which are integral to the feedback loop modulating both microbial interaction and community dynamics (16, 17). Overall, the ability of P. gingivalis to detect AI-2 allows it to adapt its behavior to exploit the advantages presented by S. gordonii, fostering cooperative biofilm formation. By facilitating interactions between P. gingivalis and S. gordonii, AI-2 enhances the formation of bacterial communities that can significantly shift the balance between health and disease in the oral microbiome. These findings highlight the importance of AI-2 in facilitating interspecies cooperation, emphasizing the complex dynamics of bacterial communities and their reliance on biochemical signaling for survival and interaction.
AI-2 as a crucial interspecies signaling molecule significantly influences the interactions between P. gingivalis and other periodontal microbiome within the complex ecosystem (18). F. nucleatum is a major coaggregation bridge organism that links early colonising commensals and late pathogenic colonisers in dental biofilms, possesses the ability to co-aggregate with P. gingivalis. The external addition of partially purified AI-2 derived from F. nucleatum significantly influenced biofilm formation in both monospecific and multispecies cultures, including P. gingivalis, Treponema denticola (T. denticola), and Tannerella forsythia (T. forsythia). In the presence of AI-2, these biofilms demonstrated increased biomass, greater average depth, and enhanced coaggregation among bacterial species (19). In this synergistic interaction, F. nucleatum not only offers a structural framework for P. gingivalis adhesion but also contributes vital nutrients that support its growth through AI-2.
The collaborative dynamic established by AI-2 signaling can lead to a heightened inflammatory response, exacerbating periodontal tissue destruction and overall disease severity. This ability to engage in competitive signaling also allows P. gingivalis to disrupt the balance of commensal and pathogenic species in the oral cavity, shifting the prevailing microbial community towards a more pathogenic state. AI-2 mediates interactions with additional oral bacteria, such as Prevotella intermedia (P. intermedia), which are prevalent components of the subgingival microbiota. P. intermedia exerted synergistic effects with P. gingivalis W83 but antagonistic effects with strain ATCC33277 through AI-2 in regulating the virulence expression of ribD and orpM (riboflavin metabolism) (20). Filifactor alocis (F. alocis) is a gram-positive anaerobe that is emerging as an important periodontal pathogen. It interacts variably with other oral pathogens, with its colonization influenced by the presence of P. gingivalis, which utilizes AI-2 signaling to modulate community dynamics. While P. gingivalis can inhibit F. alocis accumulation, it simultaneously benefited from their association, highlighting the complexity of interspecies interactions. Ultimately, the presence of F. nucleatum enhanced F. alocis accumulation, suggesting that the spatial composition and signaling mechanisms, including AI-2 production, were critical for determining the microbial microenvironments in which F. alocis thrived (21). AI-2 of A. actinomycetemcomitans affected the neighboring periodontal pathogen, P. gingivalis, by modulating the expression of luxS-regulated genes, such as uvrB and hasF which helped P. gingivalis repair DNA damage and aided in acquiring iron enabling it to survive under stressful conditions and effectively establish infections in periodontal diseases (22). These multifaceted relationships illustrate how interspecies communication through AI-2 can lead to increased pathogenicity and the advancement of periodontal disease.
The ability of P. gingivalis to sense and respond to AI-2 further extends to its influence on establishing the overall composition of the microbial community. By altering its virulence factor production and gene expression profiles in response to AI-2 signaling, P. gingivalis can reshape the dynamics of its interactions with various oral bacteria, favoring the establishment of a pathogenic community. This capacity to engage in complex signaling networks through AI-2 highlights the profound impact of interspecies communication on the pathogenicity of P. gingivalis in periodontal (Table 1).
5 Impact of QS inhibitors on P. gingivalis with interbacterial interactions
QS inhibitors have shown significant promise in mitigating the pathogenic effects of P. gingivalis and its interactions with other bacteria within the oral microbiome. By disrupting the intercellular communication essential for coordinating behaviors in bacterial communities, these inhibitors can impair biofilm formation, virulence factor expression, and the overall dynamics of microbial interactions. The two most studied categories of QS inhibitors are those targeting autoinducer-1 (AI-1) and autoinducer-2 (AI-2), each exhibiting distinct mechanisms of action and effects on P. gingivalis and its interbacterial relationships.
5.1 AI-1 inhibitors
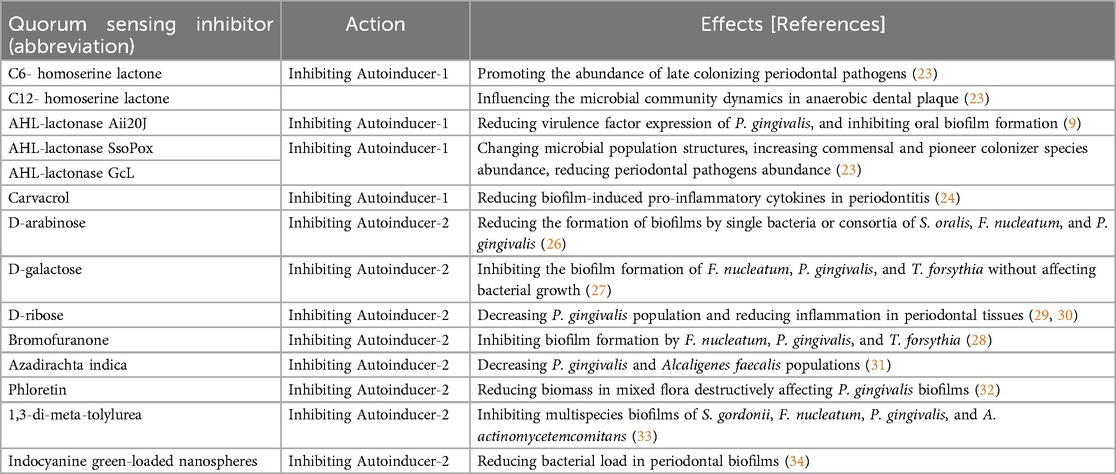
Inhibitors targeting AI-1 function by disrupting this signaling process, effectively hindering the ability of bacteria to communicate and cooperate. For instance, the addition of acyl-homoserine lactones (AHLs), such as C6-HSL and C12-HSL, in anaerobic dental plaque cultures significantly influenced the microbial community dynamics, promoting the abundance of late colonizing periodontal pathogens like P. gingivalis. Specifically, C6-HSL enhanced Veillonella abundance while C12-HSL reduced Fusobacterium levels (23). The implications of AI-1 inhibition extend beyond individual bacterial interactions; they also affect the structure and composition of biofilms.
Notably, enzymatic inhibitors, such as lactonases, have been characterized as effective agents that degrade the AI-1 signaling molecules, preventing them from reaching their receptors. By hydrolyzing the AHLs, the wide spectrum AHL-lactonase Aii20J effectively dismantled the communication network, leading to decreased expression of virulence factors in P. gingivalis. Furthermore, it significantly inhibited oral biofilm formation in different in vitro biofilm models and caused important changes in bacterial composition (9). Another two AHL lactonases, SsoPox from the Phosphotriesterase-like Lactonase (PLL) family and GcL from the Metallo β-Lactamase (MLL) family, was reported to change microbial population structures in both planktonic and biofilm states, resulting in the increase in the abundance of commensal and pioneer colonizer species (e.g., Lactobacillales, Streptococcus, Actinomyces) and reduce in the abundance of periodontal pathogens including P. gingivalis, T. forsythia and T. denticola (23).
The potential of active substances was also demonstrated. A recent study reported that a hydrogel combining carvacrol and magnolol significantly reduced pro-inflammatory cytokines in diabetic Wistar rats with periodontitis (24). The carvacrol was verified to competitively block AI-1 pathway by binding with LuxI-type AHL synthases and/or LuxR-type AHL receptor proteins (25). By disrupting QS, AI-1 inhibitors can alter the balance of bacterial species within the biofilm, potentially fostering a switch from a pathogenic to a more beneficial biofilm composition.
5.2 AI-2 inhibitors
AI-2 represents a more universal QS signaling molecule, synthesized by a wide range of bacterial species. This characteristic makes AI-2 inhibitors important players in modulating the interactions of P. gingivalis with various oral bacterial communities.
D-arabinose significantly reduced the formation of biofilms by single bacteria or consortia of S. oralis, F. nucleatum, and P. gingivalis by inhibiting the activity of AI-2 (26). Similarly, D-galactose markedly inhibited the biofilm formation of F. nucleatum, P. gingivalis, and T. forsythia induced by the AI-2 of F. nucleatum without affecting F. nucleatum growth (27). Apart from the monosaccharides, the bromofuranone analogs, such as 3-(dibromomethylene)isobenzofuran-1(3H)-one derivatives, also exerted inhibitory activities against biofilm formation of F. nucleatum, P. gingivalis, and T. forsythia (28). Moreover, the combination of (5Z)-4-bromo-5-(bromomethylene)-2(5H)-furanone (furanone compound) and D-ribose inhibited AI-2-induced biofilm formation and coaggregation of single and dual species and coaggregation between F. nucleatum and each species of the “red complex” including P. gingivalis, T. forsythia, and T. denticola. The complex also inhibited the expression of the representative adhesion molecules of the periodontopathogens, FadA of F. nucleatum, RgpA of P. gingivalis, Msp of T. denticola, and BspA of T. forsythia (19). The in vivo effects of the complex were showed in the mice infection model which demonstrated a reduction of bone destruction and a decrease in the number of periodontal bacteria (29, 30).
Azadirachta indica (Neem), a unique and traditional source of antioxidant and antibacterial compounds from India contains catechin. It demonstrated a significant reduction in LuxS activity, with decreases in P. gingivalis and Alcaligenes faecalis, indicating its capacity to disrupt intercellular communication among biofilm-forming strains (31). Phloretin and its analogs inhibited biofilm formation and intercellular communication mediated by AI-2 in mixed bacterial communities, particularly those involving P. gingivalis, F. nucleatum, and S. mitis. Structural modifications of these flavonoids could enhance their anti-biofilm efficacy by targeting AI-2 production among these pathogenic oral bacteria (32). DMTU (1,3-di-m-tolyl-urea), a biocompatible aromatic compound effectively inhibited and disrupted multispecies oral biofilms composed of S. gordonii, F. nucleatum, P. gingivalis, and A. actinomycetemcomitans without exhibiting bactericidal activity. The study also revealed significant downregulation of biofilm and virulence-related genes in P. gingivalis, particularly the AI-2 signalling luxS gene, in multispecies biofilm contexts, suggesting that multispecies interactions influence gene expression in AI-2 pathway (33). Antimicrobial photodynamic therapy (aPDT) using indocyanine green-loaded nanospheres (ICG-Nano/c) effectively reduces the bacterial load in polymicrobial periodontal biofilms, showcasing significant potential as an alternative or adjunctive treatment to conventional antibiotics. Additionally, the observed decrease in luxS expression in both P. gingivalis and S. gordonii indicated that aPDT may influence QS pathways, further supporting its role as a promising strategy to combat biofilm-related infections and address the increasing health challenges posed by antimicrobial resistance (34).
The application of AI-2 inhibitors disrupts interspecies communication, inhibiting pathogenic species like P. gingivalis to grow while simultaneously fostering the recovery of commensal organisms that can suppress its virulence. These inhibitors not only directly target bacterial populations but also enhance therapeutic strategies aimed at restoring a balanced oral microbiome (Table 2). Challenges for QS inhibitors application in clinical trials include variability in patient response, difficulty in achieving effective concentrations at infection sites, and potential side effects. Highlighted gaps include a lack of large-scale human studies and long-term efficacy data. Hence, QS study limitations often involve small sample sizes and single in vitro experiments. Despite these challenges, QS inhibitors show clinical potential as adjuncts to conventional periodontal therapies by targeting bacterial communication, reducing virulence, and enhancing treatment outcomes.
6 Conclusion
In conclusion, the interaction between P. gingivalis and oral microbial community highlights the significance of QS as a critical regulatory mechanism in microbial communication and ecological dynamics. Through AI-1/AI-2 signaling, P. gingivalis not only enhances its virulence and promotes biofilm persistence but also drives dysbiosis and reshapes microenvironments. QS inhibitors present a promising therapeutic strategy to rebalance microbial communities rather than eradicate pathogens. Combination of QS inhibitors with conventional periodontal treatments may offer innovative and microbiome-preserving approaches to manage periodontal diseases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZZ: Writing – original draft. WS: Writing – review & editing. LG: Investigation, Writing – original draft. CC: Supervision, Writing – review & editing. JZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Research Fund of Anhui Institute of translational medicine (No: 2022zhyx-C58) and Scientific Research Funding of Anhui Province Health Commission (AHWJ2023A20161). The funding body had no involvement in the writing of the manuscript and in the decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer IXY declared a shared affiliation with the authors ZZZ, CHC, JZ to the handling editor at the time of [Faculty of Dentistry, The University of Hong Kong, Sai Ying Pun, Hong Kong SAR, China].
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL. The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol 2000. (2021) 87(1):107–31. doi: 10.1111/prd.12393
2. Xu W, Zhou W, Wang H, Liang S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol. (2020) 120:45–84. doi: 10.1016/bs.apcsb.2019.12.001
3. Nobbs AH, Jenkinson HF. Interkingdom networking within the oral microbiome. Microbes Infect. (2015) 17(7):484–92. doi: 10.1016/j.micinf.2015.03.008
4. Mukherjee S, Bassler BL. Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol. (2019) 17(6):371–82. doi: 10.1038/s41579-019-0186-5
5. Dabdoub SM, Ganesan SM, Kumar PS. Comparative metagenomics reveals taxonomically idiosyncratic yet functionally congruent communities in periodontitis. Sci Rep. (2016) 6:38993. doi: 10.1038/srep38993
6. Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, et al. Microbial interactions in building of communities. Mol Oral Microbiol. (2013) 28(2):83–101. doi: 10.1111/omi.12012
7. Kriswandini IL, Sidarningsih S, Hermanto AC, Tyas PR, Aljunaid MA. The influence of Streptococcus mutans biofilm formation in a polymicrobial environment (Streptococcus gordonii & Porphyromonas gingivalis). Eur J Dent. (2024) 18(4):1085–9. doi: 10.1055/s-0044-1782215
8. Muras A, Mallo N, Otero-Casal P, Pose-Rodríguez JM, Otero A. Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral Dis. (2022) 28(2):307–13. doi: 10.1111/odi.13689
9. Muras A, Otero-Casal P, Blanc V, Otero A. Acyl homoserine lactone-mediated quorum sensing in the oral cavity: a paradigm revisited. Sci Rep. (2020) 10(1):9800. doi: 10.1038/s41598-020-66704-4
10. Sánchez MC, Romero-Lastra P, Ribeiro-Vidal H, Llama-Palacios A, Figuero E, Herrera D, et al. Comparative gene expression analysis of planktonic Porphyromonas gingivalis ATCC 33277 in the presence of a growing biofilm versus planktonic cells. BMC Microbiol. (2019) 19(1):58. doi: 10.1186/s12866-019-1423-9
11. Yoshida A, Ansai T, Takehara T, Kuramitsu HK. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl Environ Microbiol. (2005) 71(5):2372–80. doi: 10.1128/AEM.71.5.2372-2380.2005
12. Muras A, Mayer C, Otero-Casal P, Exterkate RAM, Brandt BW, Crielaard W, et al. Short-chain N-acylhomoserine lactone quorum-sensing molecules promote periodontal pathogens in in vitro oral biofilms. Appl Environ Microbiol. (2020) 86(3):e01941–19. doi: 10.1128/AEM.01941-19
13. McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J Bacteriol. (2003) 185(1):274–84. doi: 10.1128/JB.185.1.274-284.2003
14. Whitmore SE, Lamont RJ. The pathogenic persona of community-associated oral streptococci. Mol Microbiol. (2011) 81(2):305–14. doi: 10.1111/j.1365-2958.2011.07707.x
15. Chawla A, Hirano T, Bainbridge BW, Demuth DR, Xie H, Lamont RJ. Community signalling between Streptococcus gordonii and Porphyromonas gingivalis is controlled by the transcriptional regulator CdhR. Mol Microbiol. (2010) 78(6):1510–22. doi: 10.1111/j.1365-2958.2010.07420.x
16. Maeda K, Tribble GD, Tucker CM, Anaya C, Shizukuishi S, Lewis JP, et al. A Porphyromonas gingivalis tyrosine phosphatase is a multifunctional regulator of virulence attributes. Mol Microbiol. (2008) 69(5):1153–64. doi: 10.1111/j.1365-2958.2008.06338.x
17. Paramonov N, Rangarajan M, Hashim A, Gallagher A, Aduse-Opoku J, Slaney JM, et al. Structural analysis of a novel anionic polysaccharide from Porphyromonas gingivalis strain W50 related to arg-gingipain glycans. Mol Microbiol. (2005) 58(3):847–63. doi: 10.1111/j.1365-2958.2005.04871.x
18. Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. (2001) 69(5):3431–4. doi: 10.1128/IAI.69.5.3431-3434.2001
19. Jang YJ, Choi YJ, Lee SH, Jun HK, Choi BK. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch Oral Biol. (2013) 58(1):17–27. doi: 10.1016/j.archoralbio.2012.04.016
20. Zhang Y, Shi W, Song Y, Wang J. Metatranscriptomic analysis of an in vitro biofilm model reveals strain-specific interactions among multiple bacterial species. J Oral Microbiol. (2019) 11(1):1599670. doi: 10.1080/20002297.2019.1599670
21. Wang Q, Wright CJ, Dingming H, Uriarte SM, Lamont RJ. Oral community interactions of Filifactor alocis in vitro. PLoS One. (2013) 8(10):e76271. doi: 10.1371/journal.pone.0076271
22. Fong KP, Chung WO, Lamont RJ, Demuth DR. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect Immun. (2001) 69(12):7625–34. doi: 10.1128/IAI.69.12.7625-7634.2001
23. Sikdar R, Beauclaire MV, Lima BP, Herzberg MC, Elias MH. N-acyl homoserine lactone signaling modulates bacterial community associated with human dental plaque. bioRxiv [Preprint]. (2024). doi: 10.1101/2024.03.15.585217
24. Potra Cicalău GI, Marcu OA, Ghitea TC, Ciavoi G, Iurcov RC, Beiusanu C, et al. Study of periodontal bacteria in diabetic wistar rats: assessing the anti-inflammatory effects of carvacrol and magnolol hydrogels. Biomedicines. (2024) 12(7):1445. doi: 10.3390/biomedicines12071445
25. Deryabin D, Galadzhieva A, Kosyan D, Duskaev G. Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: modes of action. Int J Mol Sci. (2019) 20(22):5588. doi: 10.3390/ijms20225588
26. An SJ, Namkung JU, Ha KW, Jun HK, Kim HY, Choi BK. Inhibitory effect of d-arabinose on oral bacteria biofilm formation on titanium discs. Anaerobe. (2022) 75:102533. doi: 10.1016/j.anaerobe.2022.102533
27. Ryu EJ, Sim J, Sim J, Lee J, Choi BK. D-Galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens. J Microbiol. (2016) 54(9):632–7. doi: 10.1007/s12275-016-6345-8
28. Park JS, Ryu EJ, Li L, Choi BK, Kim BM. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur J Med Chem. (2017) 137:76–87. doi: 10.1016/j.ejmech.2017.05.037
29. Cho YJ, Song HY, Amara B, Choi H, Eunju BK, Cho R, et al. In vivo inhibition of Porphyromonas gingivalis growth and prevention of periodontitis with quorum-sensing inhibitors. J Periodontol. (2016) 87(9):1075–82. doi: 10.1902/jop.2016.160070
30. Ben Amara H, Song HY, Ryu E, Park JS, Schwarz F, Kim BM, et al. Effects of quorum-sensing inhibition on experimental periodontitis induced by mixed infection in mice. Eur J Oral Sci. (2018) 126(6):449–57. doi: 10.1111/eos.12570
31. Lahiri D, Nag M, Dutta B, Mukherjee I, Ghosh S, Dey A, et al. Catechin as the most efficient bioactive compound from Azadirachta indica with antibiofilm and anti-quorum sensing activities against dental biofilm: an in vitro and in silico study. Appl Biochem Biotechnol. (2021) 193(6):1617–30. doi: 10.1007/s12010-021-03511-1
32. Wu D, Hao L, Liu X, Li X, Zhao G. The anti-biofilm properties of phloretin and its analogs against Porphyromonas gingivalis and its complex flora. Foods. (2024) 13(13):1994. doi: 10.3390/foods13131994
33. Kalimuthu S, Cheung BPK, Yau JYY, Shanmugam K, Solomon AP, Neelakantan P. A novel small molecule, 1,3-di-m-tolyl-urea, inhibits and disrupts multispecies oral biofilms. Microorganisms. (2020) 8(9):1261. doi: 10.3390/microorganisms8091261
Keywords: quorum sensing, microbial interaction, Porphyromonas gingivalis, biofilms, periodontal disease
Citation: Zhao ZZ, Shan W, Guo L, Chu CH and Zhang J (2025) Quorum sensing in Porphyromonas gingivalis and oral microbial interactions: a scoping review. Front. Oral Health 6:1573863. doi: 10.3389/froh.2025.1573863
Received: 10 February 2025; Accepted: 26 May 2025;
Published: 6 June 2025.
Edited by:
Cleber Machado-Souza, Pelé Pequeno Príncipe Research Institute, BrazilReviewed by:
Dana Carmen Zaha, University of Oradea, RomaniaGeorgios Charalampakis, National and Kapodistrian University of Athens, Greece
Iris Xiaoxue Yin, The University of Hong Kong, Hong Kong SAR, China
Marta Mazur, Sapienza University of Rome, Italy
Copyright: © 2025 Zhao, Shan, Guo, Chu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhang, amluZzgxN0Boa3UuaGs=
†These authors share first authorship
 Zelda Ziyi Zhao
Zelda Ziyi Zhao Wenwen Shan2,†
Wenwen Shan2,† Chun Hung Chu
Chun Hung Chu Jing Zhang
Jing Zhang