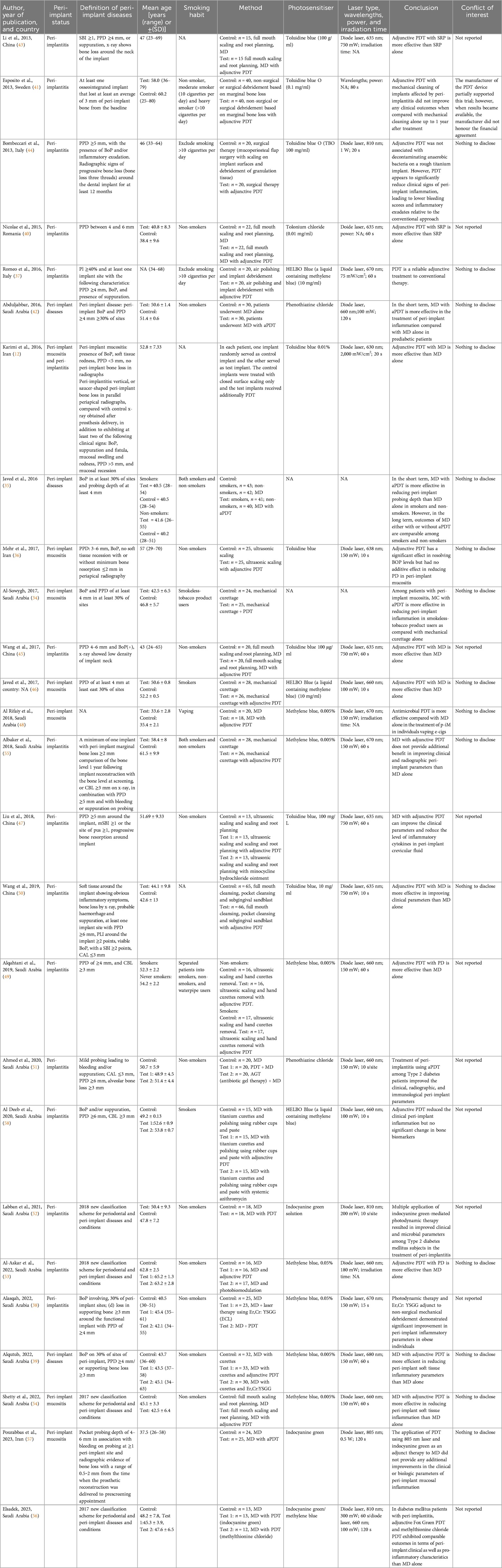
- 1Periodontology Unit, UCL Eastman Dental Institute, London, United Kingdom
- 2Periodontology Unit, University Vita-Salute San Raffaele, Milan, Italy
- 3Oral Sciences, University of Glasgow Dental School, School of Medicine, Dentistry and Nursing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom
- 4Biomaterials and Tissue Engineering, UCL Eastman Dental Institute, London, United Kingdom
Aim: This systematic review aimed to evaluate the antimicrobial efficacy of photodynamic therapy (PDT) in treating peri-implant diseases when combined with mechanical debridement (MD) compared with mechanical debridement alone.
Methods: A systematic review was completed according to PRISMA guidelines. The following databases, Cochrane Central Register for Controlled Trials (CENTRAL), Medline, Embase, Dentistry & Oral Sciences Source, Scopus, LILACS, and China Online, were searched based on the search strategies and hand search without language limitation until 15 June 2024. Only randomised controlled trials were included, assessing the efficacy of PDT used in combination with either surgical or non-surgical MD, compared with MD alone in participants with peri-implant diseases. Risk of bias for randomised controlled trials was assessed according to the recommendation of the Cochrane Reviewers' Handbook using the revised Cochrane tool. All outcomes were evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Results: A total of 26 studies were included in this study, of which 6 were categorised as low risk of bias, 9 were with some concern, and 11 were at high risk of bias. Nineteen studies were included in the quantitative analysis. At 3 months, PDT combined with non-surgical MD significantly reduced probing pocket depth (PPD) in peri-implant mucositis (−0.95 mm, 95% CI: −1.76 to −0.14) and peri-implantitis (−0.86 mm, 95% CI: −1.21 to −0.51) compared with MD alone. At 6 months, PPD reductions in peri-implantitis remained significant with non-surgical MD + PDT (−0.83 mm, 95% CI: −1.62 to −0.04) and surgical MD + PDT (−0.56 mm, 95% CI: −0.85 to −0.27). Non-surgical MD + PDT also reduced bleeding on probing (BoP) (−11.65% at 3 months, −6.76% at 6 months) and crestal bone loss (CBL) (−0.24 mm at 3 months, −0.28 mm at 6 months).
Conclusion: PDT enhances antimicrobial efficacy in peri-implant disease treatment, significantly improving PPD, CBL, and BoP when combined with MD. However, due to the overall moderate-to-low certainty of the evidence and some concerns regarding risk of bias in the included studies, these findings should be interpreted with caution. Further high-quality, well-designed randomised controlled trials are warranted to confirm these effects and optimise treatment protocols.
Systematic Review Registration: PROSPERO CRD42021262889.
1 Introduction
Peri-implant diseases, consisting of peri-implant mucositis and peri-implantitis, are increasingly prevalent due to the widespread use of dental implants for the replacement of missing teeth (1). Peri-implant mucositis is defined as an inflammatory disease that only involves soft tissue inflammation around dental implants, while peri-implantitis is characterised by inflammation in the mucosa as well as progressive loss of supporting bone tissue (2).
Aetiological evidence supports bacterial accumulation as a key determinant of the onset and progression of peri-implant diseases. However, the association between a specific bacterial cluster and peri-implantitis remains unclear. Bacteria commonly linked to periodontitis, such as Bacteroides, Campylobacter, Eubacterium, Fusobacterium, and Treponema species, are frequently identified in peri-implantitis (3, 4). Higher proportions of the genera Fusobacterium and Bacteroides have been identified in the peri-implant samples who are smokers when compared with never smokers (5). In addition, less common oral species, including staphylococci, enteric bacteria, and yeasts, have been recovered from failing implants, highlighting the increased complexity of the microbiota in peri-implantitis (6).
The plaque biofilm can trigger inflammation around dental implants and would result in soft and hard tissue destruction if left untreated (7). Treatment strategies of peri-implant diseases primarily focus on the decontamination of the implant surface and the reduction of bacteria in peri-implant tissues. Both non-surgical and surgical treatments for peri-implantitis are considered therapeutic regimens to manage bacterial biofilm (8). Studies have reported that mechanical debridement (MD) alone has limited effectiveness in the non-surgical treatment of peri-implantitis (9). Moreover, decontamination of a threaded metal surface, as that of dental implants, is more challenging than root dental surfaces; hence, surgical treatment facilitates access (10). Bacterial biofilms are intrinsically resistant to the metabolic activity required for standard antibiotic regimens to be effective. Therefore, it is imperative to develop highly effective alternative antimicrobial strategies to address and eliminate chronic or recurrent infections associated with biofilms. Ideally, these new treatment approaches should with killing mechanisms that minimise or prevent the development of microbial resistance (11). As a promising alternative to antibiotics, photodynamic therapy (PDT) is a non-invasive treatment using molecular energy produced by specific wavelength laser lights and photosensitive medication and resulting in the production of reactive oxygen species (ROS) with high chemical reactivity (12). PDT has a history in the management of periodontitis, where it has been used as an adjunctive antimicrobial approach to enhance the effects of conventional therapy (13). More recently, it has been used to treat peri-implant diseases. Previous published evidence, however, showed contradicting results on the effectiveness of PDT in managing peri-implant diseases, and comparisons between different PDT treatment protocols have not been adequately explored (13–15). Therefore, this systematic review and meta-analysis was designed to perform a comprehensive appraisal of all the evidence to date reporting on whether PDT combined with surgical or non-surgical MD improves clinical outcomes compared with mechanical debridement alone in adults with peri-implant mucositis or peri-implantitis, with a minimum follow-up of 3 months.
2 Methods
2.1 Protocol
A rigorous review protocol was developed and implemented in alignment with the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) 2020 guidelines (16) and registered on PROSPERO (reference no: CRD42021262889).
2.2 Eligibility criteria
2.2.1 Focused question
The focused research question was: “Can antimicrobial photodynamic therapy combined with surgical or non-surgical MD improve the clinical outcomes in peri-implant mucositis or peri-implantitis compared with MD alone, with a minimum of 3 months follow-up?”.
2.2.2 PICOS
The PICOS framework was applied as follows:
P (Population)—adult population diagnosed with peri-implant mucositis or peri-implantitis.
I (Intervention)—photodynamic therapy combined with surgical or non-surgical MD.
C (Comparison)—surgical or non-surgical MD alone.
O (Outcome)—primary outcome: probing pocket depth (PPD) reduction. Secondary outcomes: improvement of (1) clinical attachment level (CAL); (2) bleeding on probing (BoP); (3) gingival index (GI); (4) plaque index (PI); (5) sulcus bleeding index (SBI); (6) modified sulcus bleeding index (mSBI); (7) crestal bone loss (CBL); (8) plaque score (PS); (9) modified plaque index (mPI); and (10) modified gingival index (mGI) reduction.
S (study): Only randomised controlled trials (RCT) with at least a 3-month follow-up were included.
2.3 Search strategy
Broad and inclusive electronic search strategies were applied to include citations until 15 June 2024. The following electronic databases were searched without language limitation using medical subject headings and free-text terms: Cochrane Central Register for Controlled Trials (CENTRAL), Medline, Embase, Web of Science, Dentistry & Oral Sciences Source, Scopus, LILACS, and China Online. The following journals were searched by hand since 2005: Journal of Periodontology, Journal of Clinical Periodontology, Clinical Oral Implants Research, Journal of Oral Implantology, and Clinical Implant Dentistry and Related Research. Two trial registers, namely, ClinicalTrials.gov and WHO ICTRP, were searched. The SIGLE database was searched for grey literature (Appendix Table A1). Search results retrieved from the electronic searches were imported into a reference management software, and duplicates were removed (EndNote, version 20).
2.4 Study selection
Titles and abstracts were screened independently by two reviewers (YY and RR). Full-text articles were obtained for studies where there was insufficient information in the title and abstract to make a clear decision. The full reports were assessed independently, in duplicate, by the same two reviewers to establish eligibility for inclusion. If manuscripts were lacking information necessary for analysis, authors were contacted to retrieve missing data. Disagreement was resolved by discussion, and if necessary, a third reviewer was consulted (MO).
2.5 Risk of bias evaluation of selected studies
Data were extracted into evidence tables. The extracted data included study characteristics, peri-implant status, definition of peri-implant diseases, mean age, smoking habit, intervention, photosensitiser type, laser properties, irradiation time, and conclusion. Quality assessment and risk of bias for randomised controlled trials were assessed according to the recommendation of the Cochrane Reviewers' Handbook using the revised Cochrane tool (RoB 2) (17). A qualitative review with descriptive analysis was performed to determine the quality of data, checking for the level of risk of bias for the studies and selecting studies suitable for inclusion in quantitative analyses.
2.6 Data synthesis and grading
All data retrieved that could be used in quantitative analyses were analysed using STATA statistical software 18.0MP Parallel Edition (StataCorp LLC, College Station, TX, USA), including PPD reduction and improvement of CAL, BoP, GI, PI, SBI, mSBI, CBL, PS, mPI, and mGI at a minimum of 3-month follow-up. Mean differences were calculated with a 95% confidence interval (CI). The chi-square-based Q-statistic method and I2 measurements were employed to assess the heterogeneity. The pooled estimates were calculated using random-effects models because of the expected heterogeneity between studies. The pooled effect was considered significant if p < 0.05. Egger's test (18) was generated to assess whether small studies generate larger treatment effects (19). All outcomes were then evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (20).
2.7 Subgroup analyses
Subgroup analyses were generated to explore the sources of heterogeneity for the studies based on the following factors:
1) Smoking habit: smokers and non-smokers were analysed separately.
2) Case definition: studies were grouped by case definition and analysed separately.
3) Treatment protocol of PDT: PDT with different kinds of photosensitisers were analysed.
3 Results
3.1 Selection and characteristics of included studies
The electronic search identified 7,444 hits. After removing 1,846 duplicates and incorporating results from the hand search, the title and abstract screening identified 39 articles eligible for full-text assessment. Two studies were excluded due to a lack of sufficient follow-up time (21, 22), seven studies used control groups with other adjunctive therapy (23–29), and four were non-RCTs (30–33). A total of 26 articles were eligible for qualitative analysis (Figure 1 and Table 1). PPD was assessed in 25 studies, BoP in 14 studies, CBL in 10 studies, PI in 12 studies, mPI in 4 studies, SBI in 2 studies, mSBI in 2 studies, mGI in 1 study, GI in 1 study, PS in 4 studies, CAL in 3 studies, and BI in 2 studies. After assessment of available data, seven studies were not eligible for meta-analysis due to a lack of mean or standard deviation (34–40). Another study was not included in the quantitative analysis due to the limited number of studies with a 12-month follow-up time point (41). Two studies were excluded from quantitative analysis due to merged peri-implantitis and peri-implant mucositis patients in one group (12, 42). A total of 16 randomised controlled trials with 1,205 participants (43–58) were deemed eligible for quantitative analysis.
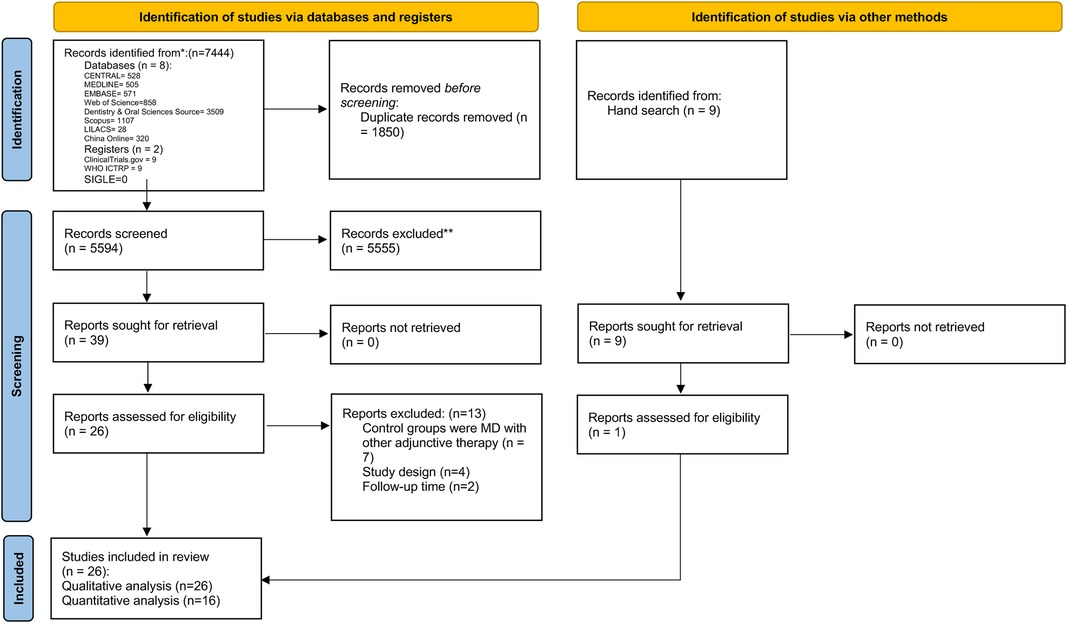
Figure 1. PRISMA flowchart of the study selection process, each stage of identifying and selecting studies.
For the quantitative analysis, eleven studies investigated patients with peri-implantitis (43–45, 47, 49–53, 55, 56), while five studies identified patients with peri-implant mucositis (46, 48, 54, 57, 58). MD performed in two studies was surgical debridement (44, 55), while in all of the other included studies, MD was non-surgical debridement.
Subgroup analysis based on smoking habits, case definition, and photosensitisers was conducted. Photosensitisers reported in the studies include toluidine blue, methylene blue, phenothiazine chloride, and indocyanine green solution. The light source type used in all studies was a diode laser with wavelengths ranging from 635 to 810 nm while irradiation timings ranged from 10 to 120 s. For the treatment procedure, the intervention in one study was MD with multiple sessions of PDT (52). Interventions in all other included studies were MD with a single session of PDT.
Four studies only include non-smokers (45, 47, 53, 54). Two studies only (46, 58) analysed smoking patients. One study (49) divided patients into both smokers and non-smokers, and another study investigated vaping patients (48). The remaining studies did not provide a clear definition of the smoking habits of the participants.
In addition, the case definitions employed in the included studies exhibited variations, leading to distinct disease severity in the inclusion criteria among these studies. Five studies enrolled patients with PPD of at least 4 mm (42, 43, 46, 49), three studies included patients with PPD of ≥5 mm (44, 47, 55), two studies recruited patients with PPD ≥6 mm (50–52), and two studies recruited patients with PPD ranging from 4 to 6 mm (45, 57). Another three studies employed the case definition from the 2017 Classification of Periodontal and Peri-implant Diseases and Conditions (53, 54, 56). One study defined an average bone loss of 3 mm compared with baseline (41). Two studies omitted the PPD from their case definitions and inclusion criteria (48, 58).
Regarding systemic conditions, two studies specifically recruited participants with diabetes (51, 52), while another study included individuals with prediabetes (42). No other systemic diseases were involved in any of the included studies.
3.2 Risk of bias
Among the 26 trials, 6 were categorised as low risk of bias, 9 were with some concern, and 11 were at high risk of bias (Appendix Figure A1). Due to the limited number of studies available, it was not appropriate to assess publication bias.
3.3 Results of the clinical measurements
3.3.1 Probing pocket depth (PPD)
A greater reduction in PPD was detected after MD combined with PDT when compared with MD alone at 3-month follow-up with non-surgical therapy. Five studies with peri-implant mucositis patients confirmed a reduction of 0.95 mm [95% confidence interval (CI): −1.76 to −0.14, I2 = 98.62%, Figure 2A], and four out of five studies were using methylene blue as the photosensitiser. In addition, a mean difference of −0.86 mm (95% CI: −1.21 to −0.51, I2 = 97.38%) was observed among peri-implantitis patients (Figure 2B), five out of the nine studies used toluidine blue as the photosensitiser, while two studies used methylene blue.
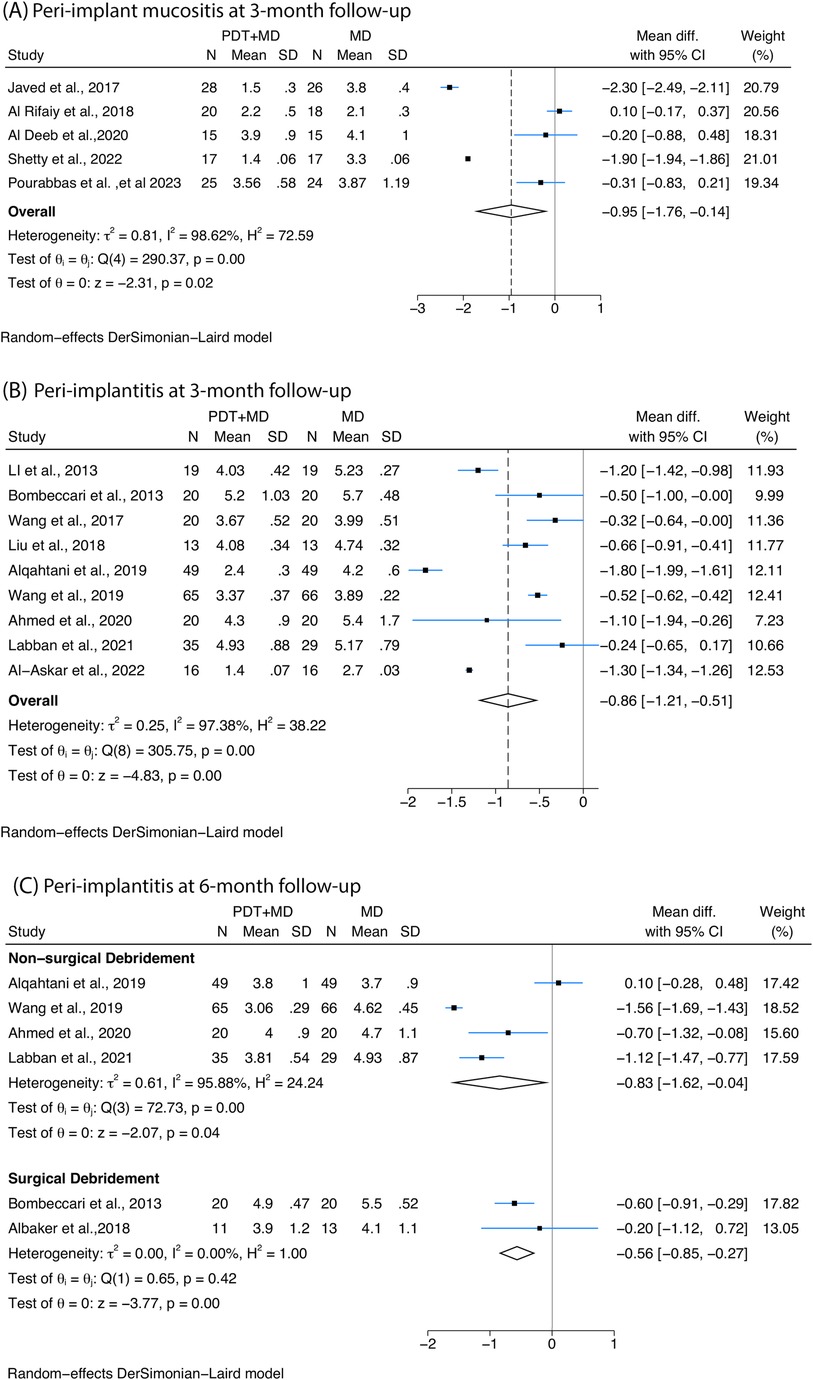
Figure 2. Forest plots illustrating the mean difference with 95% confidence interval (CI) in probing pocket depth (PPD) reduction posttreatment between photodynamic therapy (PDT) combined with mechanical debridement (MD) vs. MD alone. Patients receiving PDT + MD demonstrated a greater reduction in PPD than those receiving MD alone (A) at 3 months among peri-implant mucositis patients treated with non-surgical therapy; (B) at 3 months among peri-implantitis patients treated with non-surgical therapy; and (C) at 6 months among peri-implantitis patients receiving PDT combined with non-surgical or surgical treatment.
Six studies with peri-implantitis assessed PPD at 6-month follow-up. Among them, four studies investigating non-surgical therapy confirmed a greater reduction of −0.83 mm (95% CI: −1.62 to −0.04, I2 = 95.88%) in patients who underwent PDT combined protocol compared with control, and four different kind of photosensitisers were employed (phenothiazine chloride, indocyanine green, methylene blue, toluidine blue). Two studies with different photosensitisers (methylene blue, toluidine blue) underwent surgical MD and also showed a significant difference of −0.56 mm (95% CI: −0.85 to −0.27, I2 = 0%) (Figure 2C) in PDT combined with MD compared with MD alone.
3.3.1.1 Subgroup analyses based on case definition for non-surgical mechanical debridement
Firstly, at 3-month follow-up, subgroup analyses based on different PPD thresholds used in the case definition of peri-implantitis, including PPD ≥4 or 6 mm, all confirmed significant differences between PDT combined with MD compared with MD alone. For PPD ≥4 mm, a reduction of −1.5 mm (95% CI: −2.09 to −0.92, I2 = 93.8%) was observed. For PPD ≥6 mm, a reduction of −0.5 mm (95% CI: −0.78 to −0.22, I2 = 43.7%) was observed (Figure 3A). Secondly when evaluating studies of peri-implantitis with 6-month follow-up, subgroup analyses based on PPD threshold of PPD ≥6 mm showed greater reduction of −1.2 mm (95% CI: −1.67 to −0.73, I2 = 82.72%) in PPD with the treatment of MD combined with PDT compared with MD alone (Figure 3F).
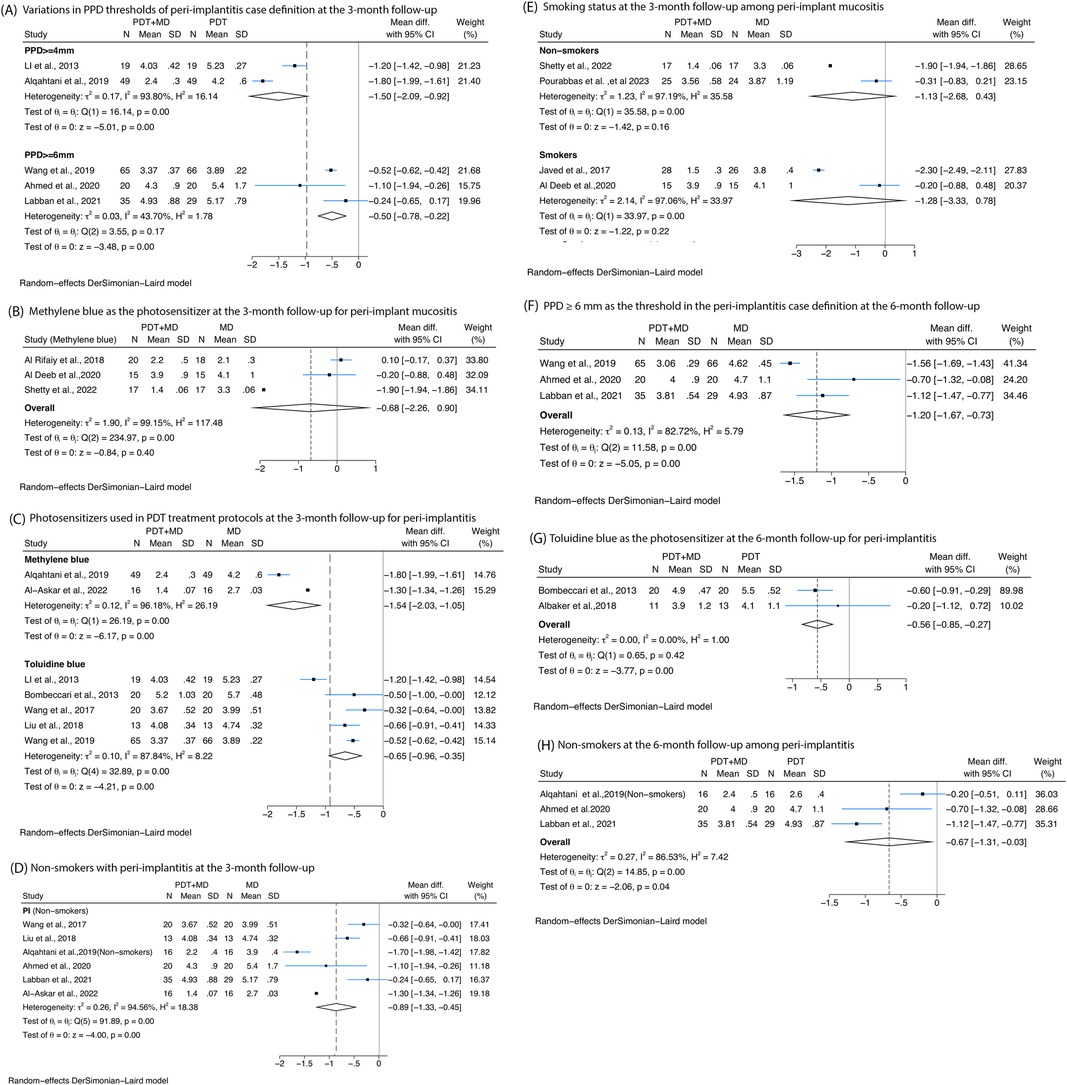
Figure 3. Forest plots presenting the posttreatment differences in probing pocket depth (PPD) between photodynamic therapy combined with mechanical debridement (PDT + MD) vs. mechanical debridement (MD) alone, categorised by specific variables at 3- and/or 6-month follow-ups. (A) Variations in PPD thresholds used in the peri-implantitis case definition at the 3-month follow-up. (B) Methylene blue as the photosensitiser in PDT treatment protocols at the 3-month follow-up for peri-implant mucositis. (C) Photosensitisers used in PDT treatment protocols at the 3-month follow-up for peri-implantitis. (D) Non-smokers at the 3-month follow-up among peri-implantitis patients. (E) Smoking status at the 3-month follow-up among peri-implant mucositis patients. (F) PPD ≥6 mm as the threshold in the peri-implantitis case definition at the 6-month follow-up. (G) Toluidine blue is the photosensitiser used in PDT protocols at the 6-month follow-up for peri-implantitis. (H) Non-smokers at the 6-month follow-up among peri-implantitis patients.
3.3.1.2 Subgroup analyses based on the treatment protocol of PDT for non-surgical mechanical debridement
Four types of photosensitisers at 3-month follow-up were evaluated. Toluidine blue showed no significant reductions in PPD among peri-implant mucositis patients (Figure 3B). In peri-implantitis patients, methylene blue showed a reduction of −1.54 mm (95% CI: −2.03 to −1.05, I2 = 99.18%) and then toluidine blue with a reduction of −0.65 mm (95% CI: −0.96 to −0.35, I2 = 87.84%). At 6-month follow-up, toluidine blue showed a significant reduction of −0.56 mm (95% CI: −0.85 to −0.27, I2 = 0%) (Figure 3G).
3.3.1.3 Subgroup analyses based on smoking habit for non-surgical mechanical debridement
A statistically significant reduction in PPD was observed in non-smoking peri-implantitis patients treated with MD and PDT compared with MD alone at the 3-month follow-up, with a mean difference of −0.89 mm (95% CI: −1.33 to −0.45, I2 = 94.56%) (Figure 3C). However, no significant reduction in PPD was found among peri-implant mucositis patients, irrespective of smoking status (Figure 3E). Additionally, three studies evaluating non-smoking peri-implantitis patients at the 6-month follow-up reported greater PPD reductions following PDT (−0.67 mm, 95% CI: −1.31 to −0.03, I2 = 86.53%) (Figure 3H).
3.3.2 Bleeding on probing (BoP)
All the studies that measured BoP used non-surgical therapy. Reductions in BoP of cases with peri-implantitis were detected at 3-month follow-up (−11.65%, 95% CI: −21.64% to −1.66%, I2 = 93.78%), while studies on peri-implant mucositis confirmed no significant difference in BoP at 3-month follow-up (Figure 4A). For peri-implantitis at 6 months, a significant reduction of −6.76% (95% CI: −11.31% to −2.21%, I2 = 71.19%) was observed (Figure 4B).
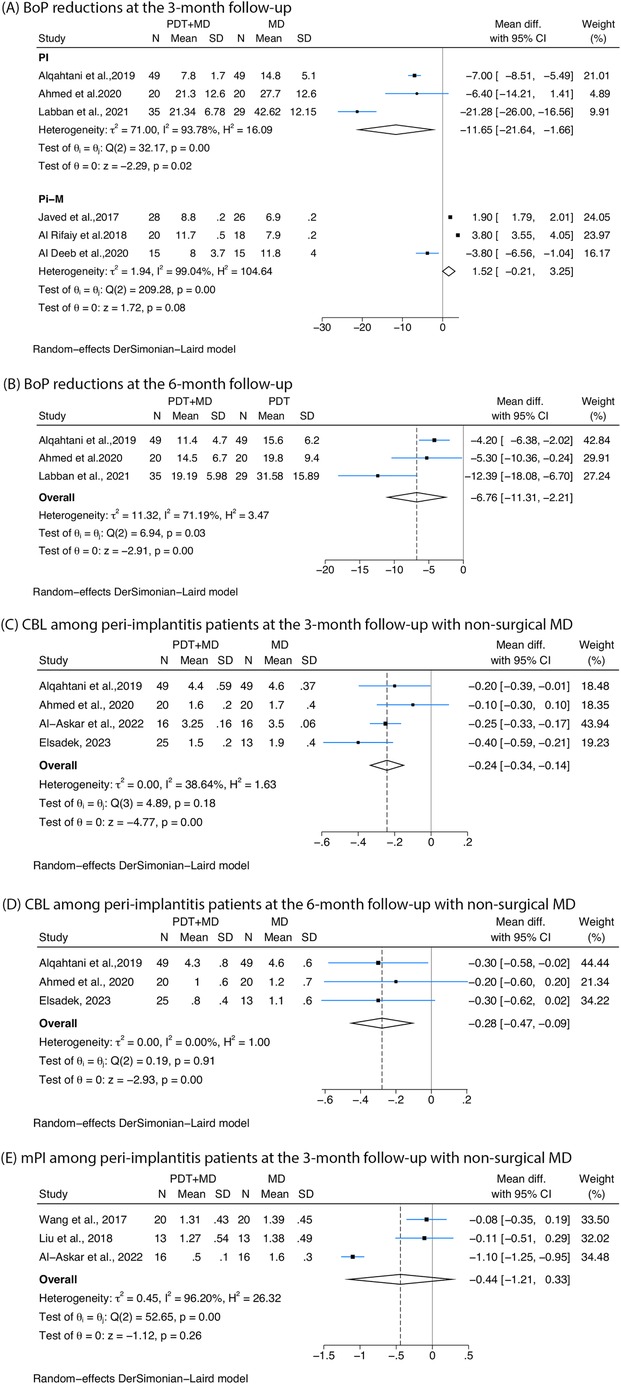
Figure 4. Forest plots showing the reduction in bleeding on probing (BoP), crestal bone loss (CBL), and modified plaque index (mPI) following treatment with photodynamic therapy combined with mechanical debridement (PDT + MD) vs. mechanical debridement (MD) alone. (A) BoP reductions in peri-implantitis (PI) and peri-implant mucositis (Pi-M) at the 3-month follow-up. (B) BoP reductions in peri-implantitis at the 6-month follow-up. (C) CBL among peri-implantitis patients at the 3-month follow-up with non-surgical MD. (D) CBL among peri-implantitis patients at the 6-month follow-up with non-surgical MD. (E) mPI among peri-implantitis patients at the 3-month follow-up with non-surgical MD.
3.3.3 Crestal bone loss (CBL)
Four studies reported greater reductions in CBL at 3 months among peri-implantitis cases treated with non-surgical debridement combined with PDT compared with controls (−0.24, 95% CI: −0.34 to −0.14, I2 = 38.64%) (Figure 4C). In two studies (53, 56), CBL was defined as the linear distance from 2 mm below the implant-abutment interface to the most crestal point of the adjacent alveolar bone. However, the remaining two studies (49, 51) did not clearly specify the starting or ending points used for the measurement, which may compromise the accuracy and comparability of the CBL measurements across studies. Three studies investigating peri-implantitis treated with non-surgical therapy measured CBL at 6 months, and a greater reduction was found in patients undergoing PDT (−0.28, 95% CI: −0.47 to −0.09, I2 = 0%) (Figure 4D).
3.3.4 Modified plaque index (mPI)
Three studies on non-surgical therapy for peri-implantitis reported mPI at 3 months, showing no significant improvement in mPI when PDT was combined with MD compared with MD alone (Figure 4E).
3.4 Grading of available evidence
Although all the included studies were randomised controlled trials, resulting in a high category of the GRADE system, the overall certainty of evidence for each outcome was rated as moderate due to concerns about inconsistency. For peri-implant mucositis at 3-month follow-up, serious inconsistency was noted (I2 = 88.62%). Similarly, for peri-implantitis at 3-month follow-up, the evidence was downgraded due to high heterogeneity (I2 = 87.37%). In the case of non-surgical treatment for peri-implantitis with a 6-month follow-up, moderate certainty was assigned as well, with inconsistency explained after subgroup analysis (I2 = 85.68%). For surgical therapy at 6-month follow-up, the certainty of evidence remained moderate. These moderate-certainty ratings suggest that adjunctive PDT may be beneficial, although some caution is warranted when interpreting the results due to variability and potential reporting bias (Appendix Figure A2).
4 Discussion
This review indicated that PDT with MD is effective in producing greater reductions of PPD in both peri-implantitis and peri-implant mucositis, which showed a promising antibacterial efficiency. PDT with MD was superior to MD alone with regards to BoP and CBL reduction in peri-implantitis patients. However, PDT showed no significant improvement in BoP reduction among peri-implant mucositis. PDT combined with surgical MD also demonstrated better outcomes compared with surgical MD alone. However, the reduction in PPD was less pronounced than that observed with non-surgical MD, likely due to the inherently greater effectiveness of surgical MD over non-surgical approaches.
Inconclusive evidence on the topic was published before. Indeed, a previous systematic review demonstrated a reduction in both CBL and BoP at 6-month follow-up when PDT was combined with MD in patients with diabetes, as compared with MD alone. However, no substantial changes in PPD were observed (59). A similar review involved patients with peri-implant mucositis, confirming improvements in PPD when PDT was combined with MD but differences in BoP (60). When analysing the impact of treatment in patients who smoked and had peri-implant diseases, the combination of MD and PDT resulted in a greater reduction in both PPD and PI (61) than the control.
A previous study indicated that increased PPD is associated with a pathogenic bacterial boost (62). Moreover, a systematic review demonstrated that heterogeneous mixed infection was detected around inflamed implants with predominantly non-culturable Gram-negative species compared with periodontitis (63). PDT has been suggested as an alternative therapy for peri-implant diseases in eliminating Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia (64). Gram-positive species, including staphylococci, were also detected (6). Gram-positive bacteria are more susceptible to anionic and neutral photosensitisers due to their porous cell wall, while Gram-negative bacteria are more resistant because of their outer membrane (65). However, cationic photosensitisers are effective against both types, showing enhanced phototoxicity in various studies (66, 67).
An increasing number of studies have focused on the use of systemically administered antimicrobials for managing peri-implantitis; however, the findings on their antimicrobial effectiveness have been inconsistent. Two studies have shown improvements in peri-implantitis in patients who received systemic antibiotics, including amoxicillin, azithromycin, and metronidazole combined with non-surgical treatment (68, 69). But this approach seemed to have failed to reduce the need for additional surgical therapy (70, 71). In addition, a study revealed that only 50% of the cases with systemic antibiotics combined with surgical treatment showed improvement after 12 months (72). Given the risk of antibiotic resistance and the uncertain effectiveness of antibiotics in treating peri-implantitis, caution should be exercised in their administration. Overuse or unnecessary use of antibiotics can contribute to the development of resistant bacterial strains, posing a potential risk to public health (72). Unlike antibiotics, PDT does not pose a risk of resistance and can be administered without dose limitations, making it unlikely for resistance to develop even with repeated treatments. PDT also exhibits several additional advantages. It is straightforward to use in clinical settings as a portable device, and the procedure typically lasts only a few minutes. With this, PDT treatment is considered relatively cost-effective compared with other approaches, such as surgical debridement. Given its demonstrated effectiveness when combined with non-surgical therapy, PDT may reduce the need for more invasive surgical interventions, thereby potentially lowering the overall cost of managing peri-implant disease. However, current evidence comparing the cost of PDT with other adjunctive treatments remains limited, and further economic evaluations are needed to substantiate its cost-effectiveness.
This review poses an important question of whether it should be used more, combined with MD protocols for controlling bacteria, to reduce the need for surgical access procedures.
The long-term efficiency of PDT should be explored in future studies. One randomised clinical study that evaluated PPD at 1-year follow-up revealed that no additional benefits were observed in the PDT group, and recurrence of peri-implantitis affected groups was found either with or without PDT (41). Another study from Javed et al. (35) demonstrated no significant difference at 12-month follow-up of PPD, also providing evidence for the hypothesis. These findings may be attributed to several factors. First, the antimicrobial effects of PDT are transient, and without regular maintenance therapy or repeated PDT sessions, the clinical benefits may not be sustained over time due to its limited capacity to fully eliminate the complex biofilms on implant surfaces (35). Secondly, host factors, such as the patient's immune response and systemic health status, may influence the long-term response to therapy (73).
Smoking habit is considered a factor impacting the efficacy of PDT. Previously conducted reviews illustrated the efficacy of PDT in the treatment of peri-implant diseases among smokers and indicated that PDT improves the condition of peri-implant diseases even if excluding non-smoking patients (61, 74). Other studies also documented that tobacco use contributes to a poor response to supportive periodontal treatment (75, 76). However, it is noteworthy that smoking exerts a continuous and strong suppressive effect on gingival bleeding (77). Therefore, caution should be taken in the analysis of BoP, since the efficacy of PDT on smokers can be masked with regard to BoP assessment. Accordingly, more studies should be conducted to explore whether smoking could reduce the efficacy of PDT on peri-implant diseases.
A difference between PDT treatment protocols was noted in this review. Although all the test groups of the trials included in this review adopted protocols of MD with PDT, several photosensitisers (including toluidine blue, methylene blue, phenothiazine chloride, and indocyanine green solution), different illumination times, and various wavelengths were utilised. The antimicrobial efficacy is closely related to the photosensitisers, and an appropriate light source must be matched to the delivery device and photosensitiser to achieve the most effective outcome (78). This review revealed a decreasing efficacy in reducing PPD at the 3-month follow-up among peri-implantitis, with the order being methylene blue and then toluidine blue. However, it is important to interpret these results with caution, given the limited number of studies available; further research should be conducted to investigate which photosensitiser and which light wavelength yield the highest effectiveness in PDT. Moreover, there is a requirement to develop a specific protocol for applying PDT in the treatment of peri-implant diseases.
Another contributing factor to the heterogeneity is the variability in case definitions across the included studies. Most of these studies mentioned PPD in their case definitions; lower heterogeneity was observed when categorising the studies based on different PPD thresholds. Therefore, standardising case definitions is crucial to ensure more accurate and consistent results. It is highly recommended to adopt the case definition outlined in the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions (2) in future studies.
This review has several limitations that should be considered. Firstly, a moderate-to-high risk of bias was observed in a substantial number of clinical trials based on the RoB 2 assessment tool. When considered alongside the GRADE evaluation, which rated the certainty of evidence as moderate primarily due to inconsistency, it becomes evident that the strength and reliability of the conclusions are limited. Although the available evidence suggests a potential benefit of PDT as an adjunctive therapy, these methodological concerns highlight the need for more robust, low-risk studies to confirm its efficacy. Secondly, the meta-analysis included studies with a maximum of 3- and 6-month follow-ups; hence, it is unclear whether the greater reductions observed with PDT are sustained beyond these short-term periods. Thirdly, high levels of heterogeneity of the results were reported, which appeared to be influenced by factors such as smoking status, case definitions, and variations in the protocols used for the application of photosensitisers. However, the possibility of additional unassessed confounding factors cannot be excluded. Additionally, the inclusion of patients with diabetes in the analysed studies may have impacted treatment efficacy, as diabetes is known to impair healing and modulate inflammatory responses. This could have introduced variability in the observed outcomes and potentially affected the accuracy of efficacy assessments. On the other hand, the strengths of this systematic review should be considered. The approach in reviewing this topic followed rigorous methods following a preregistered protocol, with a well-defined methodology and process. Confounding factors were considered, and subgroup analyses were developed to explore the reason of high heterogeneity. Lower levels of heterogeneity were detected after the subgroup analysis based on the PPD thresholds defined in the study case definitions and based on different photosensitisers.
5 Conclusion
This review emphasises the effectiveness of PDT in managing peri-implant diseases. When combined with MD, PDT led to greater reductions in PPD and CBL, along with significant improvements in BoP. PDT seems to be an accessible, low-cost strategy to target polymicrobial communities without contributing to antimicrobial resistance, making it a promising adjunctive treatment that can be widely implemented by trained dental professionals. However, these conclusions should be interpreted with caution due to the moderate certainty of evidence rated by the GRADE approach and the moderate to high risk of bias observed in several included studies. Moreover, the long-term effects of PDT on the microbial composition of peri-implant biofilms remain underexplored. Although this review highlights the antimicrobial potential of PDT, the included studies did not directly evaluate the decontaminating effects. Future research is needed to clarify the impact of PDT on microbial ecology over time, standardise treatment protocols, and explore potential synergistic effects with other antimicrobial strategies through well-designed, low-bias clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
YY: Software, Investigation, Writing – review & editing, Writing – original draft, Conceptualization, Formal analysis, Data curation, Methodology. RR: Conceptualization, Writing – review & editing, Investigation, Data curation, Methodology. JS: Writing – review & editing, Methodology, Investigation, Data curation. MO: Writing – review & editing, Methodology. AP: Writing – review & editing. FD’A: Writing – original draft, Writing – review & editing, Project administration, Formal analysis, Supervision, Data curation, Methodology, Investigation, Conceptualization.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was completed at UCL as part of the Biomedical Research Centre funded by the NIHR. FD’A received funding from the Biomedical Research Centre. Debora Marletta helped with the search strategy. The funder of the study had no role in study design, data extraction, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1614982/full#supplementary-material
References
1. Dreyer H, Grischke J, Tiede C, Eberhard J, Schweitzer A, Toikkanen SE, et al. Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res. (2018) 53(5):657–81. doi: 10.1111/jre.12562
2. Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions. J Periodontol. (2018) 89(S1):S313–8. doi: 10.1002/JPER.17-0739
3. Tabanella G, Nowzari H, Slots J. Clinical and microbiological determinants of ailing dental implants. Clin Implant Dent Relat Res. (2009) 11(1):24–36. doi: 10.1111/j.1708-8208.2008.00088.x
4. Casado PL, Otazu IB, Balduino A, de Mello W, Barboza EP, Duarte MEL. Identification of periodontal pathogens in healthy periimplant sites. Implant Dent. (2011) 20(3):226–35. doi: 10.1097/ID.0b013e3182199348
5. Pimentel SP, Fontes M, Ribeiro FV, Corrêa MG, Nishii D, Cirano FR, et al. Smoking habit modulates peri-implant microbiome: a case-control study. J Periodontal Res. (2018) 53(6):983–91. doi: 10.1111/jre.12597
6. Koyanagi T, Sakamoto M, Takeuchi Y, Maruyama N, Ohkuma M, Izumi Y. Comprehensive microbiological findings in peri-implantitis and periodontitis. J Clin Periodontol. (2013) 40(3):218–26. doi: 10.1111/jcpe.12047
7. Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol. (2018) 89(S1):S304–12. doi: 10.1002/JPER.17-0588
8. Renvert S, Hirooka H, Polyzois I, Kelekis-Cholakis A, Wang H-L. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants – consensus report of working group 3. Int Dent J. (2019) 69(S2):12–7. doi: 10.1111/idj.12490
9. Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontology. (2014) 66(1):255–73. doi: 10.1111/prd.12049
10. Lindhe J, Meyle J. Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol. (2008) 35(8 Suppl):282–5. doi: 10.1111/j.1600-051X.2008.01283.x
11. Luke-Marshall NR, Hansen LA, Shafirstein G, Campagnari AA. Antimicrobial photodynamic therapy with chlorin e6 is bactericidal against biofilms of the primary human otopathogens. mSphere. (2020) 5(4):e00492-20. doi: 10.1128/msphere.00492-20
12. Karimi MR, Hasani A, Khosroshahian S. Efficacy of antimicrobial photodynamic therapy as an adjunctive to mechanical debridement in the treatment of peri-implant diseases: a randomized controlled clinical trial. J Lasers Med Sci. (2016) 7(3):139–45. doi: 10.15171/jlms.2016.24
13. Chambrone L, Wang H-L, Romanos GE. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: an American Academy of Periodontology best evidence review. J Periodontol. (2018) 89(7):783–803. doi: 10.1902/jop.2017.170172
14. Sculean A, Deppe H, Miron R, Schwarz F, Romanos G, Cosgarea R. Effectiveness of photodynamic therapy in the treatment of periodontal and peri-implant diseases. Monogr Oral Sci. (2020) 29:133–43. doi: 10.1159/000510189
15. Zeza B, Farina R, Pilloni A, Mongardini C. Clinical outcomes of experimental gingivitis and peri-implant mucositis treatment with professionally administered plaque removal and photodynamic therapy. Int J Dent Hyg. (2018) 16(2):e58–64. doi: 10.1111/idh.12302
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
17. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
18. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
19. Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3(2):98–110. doi: 10.1002/jrsm.1044
20. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015
21. Mongardini C, Pilloni A, Farina R, Di Tanna G, Zeza B. Adjunctive efficacy of probiotics in the treatment of experimental peri-implant mucositis with mechanical and photodynamic therapy: a randomized, cross-over clinical trial. J Clin Periodontol. (2017) 44(4):410–7. doi: 10.1111/jcpe.12689
22. Ohba S, Sato M, Noda S, Yamamoto H, Egahira K, Asahina I. Assessment of safety and efficacy of antimicrobial photodynamic therapy for peri-implant disease. Photodiagnosis Photodyn Ther. (2020) 31:101936. doi: 10.1016/j.pdpdt.2020.101936
23. Al-Khureif AA, Mohamed BA, Siddiqui AZ, Hashem M, Khan AA, Divakar DD. Clinical, host-derived immune biomarkers and microbiological outcomes with adjunctive photochemotherapy compared with local antimicrobial therapy in the treatment of peri-implantitis in cigarette smokers. Photodiagnosis Photodyn Ther. (2020) 30:101684. doi: 10.1016/j.pdpdt.2020.101684
24. Almohareb T, Alhamoudi N, Al Deeb M, Bin-Shuwaish MS, Mokeem SA, Saad Shafqat S, et al. Clinical efficacy of photodynamic therapy as an adjunct to mechanical debridement in the treatment of per-implantitis with abscess. Photodiagnosis Photodyn Ther. (2020) 30:101750. doi: 10.1016/j.pdpdt.2020.101750
25. Birang E, Talebi Ardekani MR, Rajabzadeh M, Sarmadi G, Gutknecht N. Evaluation of effectiveness of photodynamic therapy with low-level diode Laser in nonsurgical treatment of peri-implantitis. J Lasers Med Sci. (2017) 8(3):136–42. doi: 10.15171/jlms.2017.25
26. Rakašević D, Lazić Z, Rakonjac B, Soldatović I, Janković S, Magić M, et al. Efficiency of photodynamic therapy in the treatment of peri-implantitis – a three-month randomized controlled clinical trial. Srp Arh Celok Lek. (2016) 144(9-10):478–84. doi: 10.2298/SARH1610478R
27. Schär D, Ramseier CA, Eick S, Arweiler NB, Sculean A, Salvi GE. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res. (2013) 24(1):104–10. doi: 10.1111/j.1600-0501.2012.02494.x
28. Hui F, Yi C, Zong-kai L. Effect of tea polyphenols combined with photodynamic therapy on the efficacy and RANKL and Shh levels in patients with early peri-implantitis. J Hainan Med Univ. (2019) 25(17):76–9.
29. Bassetti M, Schär D, Wicki B, Eick S, Ramseier CA, Arweiler NB, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res. (2014) 25(3):279–87. doi: 10.1111/clr.12155
30. Aldosari LIN, Hassan SAB, Alshadidi AAF, Rangaiah GC, Divakar DD. Short-term influence of antimicrobial photodynamic therapy as an adjuvant to mechanical debridement in reducing soft-tissue inflammation and subgingival yeasts colonization in patients with peri-implant mucositis. Photodiagnosis Photodyn Ther. (2023) 42:103320. doi: 10.1016/j.pdpdt.2023.103320
31. Al Amri MD, Kellesarian SV, Ahmed A, Al-Kheraif AA, Romanos GE, Javed F. Efficacy of periimplant mechanical debridement with and without adjunct antimicrobial photodynamic therapy in patients with type 2 diabetes mellitus. Photodiagnosis Photodyn Ther. (2016) 14:166–9. doi: 10.1016/j.pdpdt.2016.04.015
32. Ahmed AR, Kamran MA, Suleman G, Sharif RA, Alamrey AAM, Sulaiman SA. Novel use of chloro-aluminum phthalocyanine assisted photodynamic therapy helps in periimplant healing among smoking patients. Photodiagnosis Photodyn Ther. (2023) 41:103193. doi: 10.1016/j.pdpdt.2022.103193
33. Al Deeb M, Alresayes S, Mokeem SA, Alhenaki AM, AlHelal A, Vohra F, et al. Clinical peri-implant health and biological bone marker levels in tobacco users treated with photodynamic therapy. Photodiagnosis Photodyn Ther. (2020) 31:101821. doi: 10.1016/j.pdpdt.2020.101821
34. Al-Sowygh ZH. Efficacy of periimplant mechanical curettage with and without adjunct antimicrobial photodynamic therapy in smokeless-tobacco product users. Photodiagnosis Photodyn Ther. (2017) 18:260–3. doi: 10.1016/j.pdpdt.2017.03.011
35. Javed F, Abduljabbar T, Carranza G, Gholamiazizi E, Mazgaj DK, Kellesarian SV, et al. Efficacy of periimplant mechanical debridement with and without adjunct antimicrobial photodynamic therapy in the treatment of periimplant diseases among cigarette smokers and non-smokers. Photodiagnosis Photodyn Ther. (2016) 16:85–9. doi: 10.1016/j.pdpdt.2016.09.001
36. Mehr AK, Pourabbas R, Khajeh MS. Effect of photodynamic therapy with toluidine blue photosensitizer on nonsurgical management of peri-implant mucosal inflammation. The effect of an educational intervention based on BASNEF model on nurses communication skills with patients in educational hospitals of Tehran in 2017, 2018. 2.
37. Romeo U, Nardi GM, Libotte F, Sabatini S, Palaia G, Grassi FR. The antimicrobial photodynamic therapy in the treatment of peri-implantitis. Int J Dent. (2016) 2016(1):7692387. doi: 10.1155/2016/7692387
38. Alasqah MN. Influence of adjunctive non-surgical peri-implant therapy on clinical and salivary cytokine profile in obese patients. Photodiagnosis Photodyn Ther. (2022) 37:102721. doi: 10.1016/j.pdpdt.2022.102721
39. Alqutub MN. Peri-implant parameters and cytokine profile among peri-implant disease patients treated with Er Cr YSGG laser and PDT. Photodiagnosis Photodyn Ther. (2022) 37:102641. doi: 10.1016/j.pdpdt.2021.102641
40. Nicolae V, Chiscop I, Cioranu VS, Martu MA, Luchian AI, Martu S, et al. The use of photoactivated blue-O toluidine for periimplantitis treatment in patients with periodontal disease. Rev Chim. (2015) 66:2121–3.
41. Esposito M, Grusovin MG, De Angelis N, Camurati A, Campailla M, Felice P. The adjunctive use of light-activated disinfection (LAD) with FotoSan is ineffective in the treatment of peri-implantitis: 1-year results from a multicentre pragmatic randomised controlled trial. Eur J Oral Implantol. (2013) 6(2):109–19.23926583
42. Abduljabbar T. Effect of mechanical debridement with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implant diseases in prediabetic patients. Photodiagnosis Photodyn Ther. (2016) 17:9–12. doi: 10.1016/j.pdpdt.2016.10.011
43. Li Y, Fa Y, Yang Y, Cai X, Li G. Treatment of peri-implantitis with photodynamic therapy. J Pract Stomatol. (2013) 29(6):848–51.
44. Bombeccari GP, Guzzi G, Gualini F, Gualini S, Santoro F, Spadari F. Photodynamic therapy to treat periimplantitis. Implant Dent. (2013) 22(6):631–8. doi: 10.1097/01.id.0000433592.18679.91
45. Wang Y, Ma Y, Liu X, Guo L. Short-term clinical effects on the treatment of peri-implantitis with photodynamic therapy. Henan Med Res. (2017) 26(16):2890–2.
46. Javed F, BinShabaib MS, Alharthi SS, Qadri T. Role of mechanical curettage with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implant mucositis in cigarette smokers: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. (2017) 18:331–4. doi: 10.1016/j.pdpdt.2017.04.015
47. Liu X, Ma Y, Yang S, Guo L. Efficacy of photodynamic therapy as an adjunct in the treatment of peri-implantitis. Acta Univ Med Anhui. (2018) 53(4):645–8.
48. Al Rifaiy MQ, Qutub OA, Alasqah MN, Al-Sowygh ZH, Mokeem SA, Alrahlah A. Effectiveness of adjunctive antimicrobial photodynamic therapy in reducing peri-implant inflammatory response in individuals vaping electronic cigarettes: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. (2018) 22:132–6. doi: 10.1016/j.pdpdt.2018.03.002
49. Alqahtani F, Alqhtani N, Alkhtani F, Divakar DD, Al-Kheraif AA, Javed F. Efficacy of mechanical debridement with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implantitis among moderate cigarette-smokers and waterpipe-users. Photodiagnosis Photodyn Ther. (2019) 28:153–8. doi: 10.1016/j.pdpdt.2019.09.003
50. Wang H, Li W, Zhang D, Li W. Adjunctive photodynamic therapy improves the outcomes of peri-implantitis: a randomized controlled trial. Aust Dent J. (2019) 64(3):256–62. doi: 10.1111/adj.12705
51. Ahmed P, Bukhari IA, Albaijan R, Sheikh SA, Vohra F. The effectiveness of photodynamic and antibiotic gel therapy as an adjunct to mechanical debridement in the treatment of peri-implantitis among diabetic patients. Photodiagnosis Photodyn Ther. (2020) 32:102077. doi: 10.1016/j.pdpdt.2020.102077
52. Labban N, Shibani NA, Al-Kattan R, Alfouzan AF, Binrayes A, Assery MK. Clinical, bacterial, and inflammatory outcomes of indocyanine green-mediated photodynamic therapy for treating periimplantitis among diabetic patients: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. (2021) 35:102350. doi: 10.1016/j.pdpdt.2021.102350
53. Al-Askar MH, Abdullatif FA, Alshihri AA, Ahmed A, Divakar DD, Almoharib H, et al. Comparison of photobiomodulation and photodynamic therapy as adjuncts to mechanical debridement for the treatment of peri-implantitis. Technol Health Care. (2022) 30(2):389–98. doi: 10.3233/THC-213062
54. Shetty B, Ali D, Ahmed S, Ibraheem WI, Preethanath RS, Vellappally S, et al. Role of antimicrobial photodynamic therapy in reducing subgingival oral yeasts colonization in patients with peri-implant mucositis. Photodiagnosis Photodyn Ther. (2022) 38:102803. doi: 10.1016/j.pdpdt.2022.102803
55. Albaker AM, ArRejaie AS, Alrabiah M, Al-Aali KA, Mokeem S, Alasqah MN, et al. Effect of antimicrobial photodynamic therapy in open flap debridement in the treatment of peri-implantitis: a randomized controlled trial. Photodiagnosis Photodyn Ther. (2018) 23:71–4. doi: 10.1016/j.pdpdt.2018.05.003
56. Elsadek MF. Effectiveness of two photosensitizer-mediated photodynamic therapy for treating moderate peri-implant infections in type-II diabetes mellitus patients: a randomized clinical trial. Photodiagnosis Photodyn Ther. (2023) 43:103643. doi: 10.1016/j.pdpdt.2023.103643
57. Pourabbas R, Khorramdel A, Sadighi M, Kashefimehr A, Mousavi SA. Effect of photodynamic therapy as an adjunctive to mechanical debridement on the nonsurgical treatment of peri-implant mucositis: a randomized controlled clinical trial. Dent Res J. (2023) 20:1–10. doi: 10.4103/1735-3327.367900
58. Al Deeb M, Alsahhaf A, Mubaraki SA, Alhamoudi N, Al-Aali KA, Abduljabbar T. Clinical and microbiological outcomes of photodynamic and systemic antimicrobial therapy in smokers with peri-implant inflammation. Photodiagnosis Photodyn Ther. (2020) 29:101587. doi: 10.1016/j.pdpdt.2019.101587
59. Afrasiabi S, Heidari M, Younespour S, Chiniforush N. Evaluating the effect of mechanical debridement with adjunctive antimicrobial photodynamic therapy in comparison with mechanical debridement alone on the peri-implant parameters in type 2 diabetic mellitus patients with peri-implantitis: a systematic review and meta-analysis. BMC Oral Health. (2023) 23(1):751. doi: 10.1186/s12903-023-03337-9
60. Guo J, Chen X, Xie H, Li T. Efficacy of adjunctive photodynamic therapy to conventional mechanical debridement for peri-implant mucositis. BMC Oral Health. (2024) 24(1):464. doi: 10.1186/s12903-024-04198-6
61. Shahmohammadi R, Younespour S, Paknejad M, Chiniforush N, Heidari M. Efficacy of adjunctive antimicrobial photodynamic therapy to mechanical debridement in the treatment of peri-implantitis or peri-implant mucositis in smokers: a systematic review and meta-analysis. Photochem Photobiol. (2022) 98(1):232–41. doi: 10.1111/php.13481
62. Loozen G, Ozcelik O, Boon N, De Mol A, Schoen C, Quirynen M, et al. Inter-bacterial correlations in subgingival biofilms: a large-scale survey. J Clin Periodontol. (2014) 41(1):1–10. doi: 10.1111/jcpe.12167
63. Lafaurie GI, Sabogal MA, Castillo DM, Rincón MV, Gómez LA, Lesmes YA, et al. Microbiome and microbial biofilm profiles of peri-implantitis: a systematic review. J Periodontol. (2017) 88(10):1066–89. doi: 10.1902/jop.2017.170123
64. Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res. (2001) 12(2):104–8. doi: 10.1034/j.1600-0501.2001.012002104.x
65. Liu Y, Qin R, Zaat SAJ, Breukink E, Heger M. Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. J Clin Transl Res. (2015) 1(3):140–67.30873451
66. Merchat M, Bertolini G, Giacomini P, Villaneuva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. (1996) 32(3):153–7. doi: 10.1016/1011-1344(95)07147-4
67. Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J Photochem Photobiol B. (1996) 32(3):159–64. doi: 10.1016/1011-1344(95)07148-2
68. Carrillo de Albornoz A, Montero E, Alonso-Español A, Sanz M, Sanz-Sánchez I. Treatment of peri-implantitis with a flapless surgical access combined with implant surface decontamination and adjunctive systemic antibiotics: a retrospective case series study. J Clin Periodontol. (2024) 51(8):968–80. doi: 10.1111/jcpe.13993
69. Riben Grundström C, Lund B, Kämpe J, Belibasakis GN, Hultin M. Systemic antibiotics in the surgical treatment of peri-implantitis: a randomized placebo-controlled trial. J Clin Periodontol. (2024) 51(8):981–96. doi: 10.1111/jcpe.13994
70. Parmar N. Can systemic antibiotics reduce the need for surgical intervention in treating peri-implantitis? Evid Based Dent. (2024) 25:182–3. doi: 10.1038/s41432-024-01071-x
71. Hakkers J, Vangsted TE, van Winkelhoff AJ, de Waal YCM. Do systemic amoxicillin and metronidazole during the non-surgical peri-implantitis treatment phase prevent the need for future surgical treatment? A retrospective long-term cohort study. J Clin Periodontol. (2024) 51(8):997–1004. doi: 10.1111/jcpe.14024
72. Teughels W, Seyssens L, Christiaens V, Temmerman A, Castro AB, Cosyn J. Adjunctive locally and systemically delivered antimicrobials during surgical treatment of peri-implantitis: a systematic review. J Clin Periodontol. (2023) 50(S26):359–72. doi: 10.1111/jcpe.13773
73. Săndulescu M, Sîrbu VD, Popovici IA. Bacterial species associated with peri-implant disease - a literature review. Germs. (2023) 13(4):352–61. doi: 10.18683/germs.2023.1405
74. Zhao Y, Yan Q, Wu X, Hua F, Shi B. The benefit of antimicrobial photodynamic therapy to mechanical debridement in the treatment of smokers with peri-implant diseases: a systematic review and meta-analysis. Lasers Med Sci. (2022) 37(8):3051–66. doi: 10.1007/s10103-022-03592-2
75. Heasman L, Stacey F, Preshaw PM, McCracken GI, Hepburn S. The effect of smoking on periodontal treatment response: a review of clinical evidence. J Clin Periodontol. (2006) 33(4):241–53. doi: 10.1111/j.1600-051X.2006.00902.x
76. Pretzl B, El Sayed S, Weber D, Eickholz P, Bäumer A. Tooth loss in periodontally compromised patients: results 20 years after active periodontal therapy. J Clin Periodontol. (2018) 45(11):1356–64. doi: 10.1111/jcpe.13010
77. Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigareté smoking on gingival bleeding. J Periodontol. (2004) 75(1):16–22. doi: 10.1902/jop.2004.75.1.16
Keywords: photodynamic therapy, antimicrobial, peri-implantitis, peri-implant disease, peri-implant mucositis
Citation: Yan Y, Rotundo R, Suvan J, Orlandi M, Poma A and D’Aiuto F (2025) Photodynamic therapy and peri-implant diseases: a systematic review and meta-analysis. Front. Oral Health 6:1614982. doi: 10.3389/froh.2025.1614982
Received: 20 April 2025; Accepted: 20 June 2025;
Published: 9 July 2025.
Edited by:
Selena Toma, Université Catholique de Louvain, BelgiumReviewed by:
Alexandre Henrique dos Reis-Prado, Federal University of Minas Gerais, BrazilGeorge Pelekos, The University of Hong Kong, Hong Kong SAR, China
Copyright: © 2025 Yan, Rotundo, Suvan, Orlandi, Poma and D’Aiuto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco D'Aiuto Zi5kYWl1dG9AdWNsLmFjLnVr
†ORCID:
Francesco D’Aiuto
orcid.org/0000-0001-8654-935X
 Yumeng Yan
Yumeng Yan Roberto Rotundo2
Roberto Rotundo2 Alessandro Poma
Alessandro Poma Francesco D’Aiuto
Francesco D’Aiuto