- 1School of Nursing, University of Rochester, Rochester NY, United States
- 2Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester NY, United States
- 3Departments of Psychiatry, Neuroscience, Obstetrics and Gynecology, University of Rochester, Rochester, NY, United States
- 4Department of Pediatrics, University of California, Irvine, CA, United States
- 5Craniofacial Biology and Bioengineering, ADA Forsyth Institute, Somerville, MA, United States
Background: Although the mechanisms underlying tooth eruption are not fully understood, the prenatal maternal milieu, particularly stress exposures, appears to play an important role in dental development. Yet, limited research has investigated the influence of prenatal stress and stress-related hormones on tooth eruption.
Methods: This study included 142 mother-child dyads from a birth cohort to examine associations between prenatal stress, stress-related hormones, and primary tooth eruption. The number of erupted teeth was assessed by dentists at child visits through 24 months of age. Maternal prenatal depression and anxiety diagnoses were extracted from medical records as a proxy for stress. Stress-related hormone concentrations, including cortisol, estradiol, progesterone, testosterone, triiodothyronine (T3), and thyroxine (T4), were measured from salivary samples collected in late pregnancy. Generalized linear models were used to assess associations between prenatal stress, stress-related hormones, and tooth eruption, adjusting for relevant covariates.
Results: Eruption timing varied within our cohort: 15.2% of children had at least one erupted tooth by 6 months, and 25% had all 20 primary teeth by 24 months. Correlations in tooth counts across visits ranged from 0.15 to 0.57. Several prenatal maternal hormones, including cortisol, estradiol, progesterone, testosterone, and T3, were significantly and positively associated with the number of erupted teeth at individual visits (p < 0.05). Particularly, higher prenatal cortisol levels were associated with more erupted teeth at 6 months, corresponding to an average difference of ∼4 teeth between the lowest and highest cortisol levels.
Conclusion: Maternal salivary hormone levels in late pregnancy may contribute to variations in primary tooth eruption during the first two years of life.
Introduction
The timing of tooth eruption is critical for oral health. Tooth eruption varies widely (1–3), with most children exhibiting between 2 and 11 erupted teeth by 12 months of age (2). Early and delayed eruption can disrupt dental alignment, contribute to malocclusion (4–6), compromise enamel quality, and increase the risk of dental caries (3, 7). Beyond oral health, early tooth eruption has also been proposed as a biomarker for accelerated biological aging in children (8).
Despite its clinical importance, the biological mechanisms regulating tooth eruption remain incompletely understood, though both genetic and environmental factors play critical roles (9, 10). Primary tooth development begins in utero (11, 12), making pregnancy a sensitive window when maternal and environmental exposures may influence dental outcomes. Previous studies have linked deviations in eruption timing to prenatal maternal smoking, suboptimal nutrition, and socioeconomic adversity (3, 13–15). Socioeconomic adversity is closely tied to chronic stress (16, 17), and prenatal stress exposure has also been associated with other dental outcomes, including enamel defects and dental caries (18, 19). However, few studies have directly examined whether prenatal stress specifically modulates the timing of tooth eruption.
This study aimed to address this research gap. Mechanistically, stress activates the hypothalamic-pituitary axis and alters concentrations of cortisol (20, 21) as well as other hormones, such as sex steroids (22) and thyroid hormones (23). These hormones are involved in bone development and the metabolism of vitamin D and calcium (24–28), both of which are implicated in dental development and tooth eruption (29–32). This prior evidence suggests a plausible pathway linking stress-related hormones to dental development. Accordingly, in this study we evaluated both maternal prenatal stress and stress-related hormone levels in relation to tooth eruption.
Method
Study design and cohort
The current study uses data from a prospective birth cohort that recruited 186 socioeconomically disadvantaged pregnant women during late pregnancy and subsequently enrolled their newborn children between 8/2017 and 11/2022 (33). The inclusion criteria for the current study were: 1) mothers provided saliva samples during the late second or third trimester, and 2) tooth eruption information was available for children at any of the 6-, 12-, 18-, or 24-month study visits. The final analytic sample included 142 mother-child dyads. The birth cohort study was approved by the university's Research Subject Review Board (#1248). Written informed consent was obtained from all participants, and consent for minors was obtained from their legal guardians.
Tracking tooth eruption
Comprehensive oral examinations were performed on children at multiple visits (1 week, 2, 4, 6, 12, 18, & 24 months) up to 24 months of age by one of three calibrated dentists using standard equipment. A tooth was considered erupted if its cusp was visible above the gum line. The number and type of erupted teeth were recorded at each visit for each child.
Prenatal stress and hormone measures
Prenatal stress was not directly measured in our participants; instead, a proxy measure was used, based on a documented diagnosis of depression or anxiety during pregnancy in the medical record, according to previous studies (34, 35). The variable was coded as the presence or absence of depression or anxiety. Saliva samples were collected once from each woman during late pregnancy. A detailed description of salivary hormone assessment has been provided in our previous publication (36). Briefly, prior to sample collection, participants were instructed to refrain from eating, drinking, or brushing their teeth for 2 h. Each woman provided approximately 3–5 ml of unstimulated saliva during the late 2nd or 3rd trimester by spitting into a sterile centrifuge tube. Salivary hormone levels of cortisol, estradiol, progesterone, testosterone, triiodothyronine (T3), and thyroxine (T4) were measured using the MILLIPLEX MAP Multi-Species Hormone Magnetic Bead Panel (Cat# MSHMAG-21K, Merck Millipore, Darmstadt, Germany). Results were obtained on a Luminex 200 instrument (Luminex, USA) and reported based on standard curve values.
Covariates
Covariates included in the model were selected based on previous literature (3, 15, 37). Maternal and child demographic and socioeconomic information (maternal age, educational level, marital status, employment status, parity, smoking status, child sex, child race/ethnicity, birthweight, and breastfeeding status) was obtained through self-reported questionnaires and medical records. Maternal medical conditions (diabetes and hypertension) were self-reported and confirmed using electronic health records. Gestational age at sample collection was calculated from the due date and the sample collection date. Breastfeeding status was determined primarily from the 6-month visit. If data at the 6-month visit were missing, information from earlier visits (2- and 4-month) was used; if unavailable, breastfeeding data from the 12-month visit were used.
Statistical analysis
All hormone levels were natural log-transformed to approximate a normal distribution. To reduce model complexity and avoid overfitting, covariates were selected for the final models through univariate analyses between each covariate and the number of erupted teeth at each visit, using a p-value threshold of 0.2. The final models included maternal age, educational level, employment status, nulliparity, smoking status, diabetes, hypertension, and child sex, race/ethnicity, birthweight, and breastfeeding status. Pearson and Spearman correlations were used to assess relationships among stress-related hormones and, separately, among the number of erupted teeth across visits. Group-based trajectory modeling was applied to identify distinct patterns of tooth eruption over time.
Because the outcome variable, the number of erupted teeth, was a count variable, generalized linear models were used to examine associations between prenatal stress, stress-related hormones, and tooth eruption at each visit, adjusting for covariates. A Poisson distribution with a log link function was used for the 12-, 18-, and 24-month visits, while a negative binomial distribution was applied at the 6-month visit to account for overdispersion. Overall associations across all visits were assessed using negative binomial mixed-effects models. Sensitivity analyses excluded infants born with low birthweight (<2,500 grams; n = 1), and cases with maternal hormone levels identified as outliers, defined as values beyond the median ± 4 × interquartile range. All statistical analyses were conducted using STATA 18.0 (College Station, TX, USA).
Results
Characteristics of participants
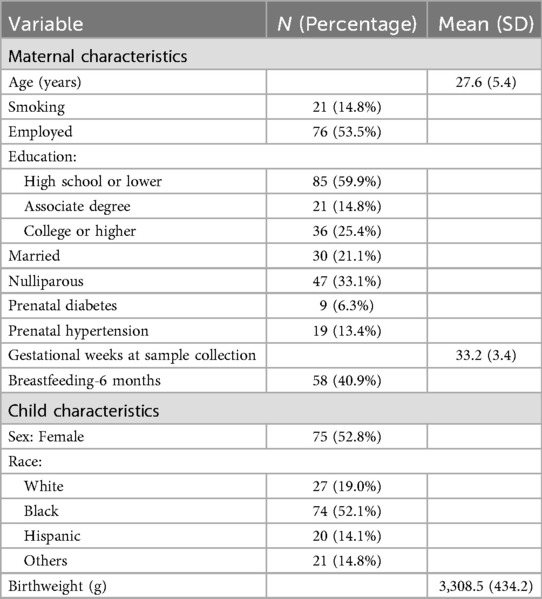
The characteristics of the mother-child dyads (n = 142) are summarized in Table 1. Most mothers were employed (>53%), had a high school education or lower (60%), had more than one pregnancy (>76%), and did not breastfeed around 6 months postpartum (59%). All children were born full-term, with an average birthweight of 3,308 grams. More than half of the children were African Americans (>52%).
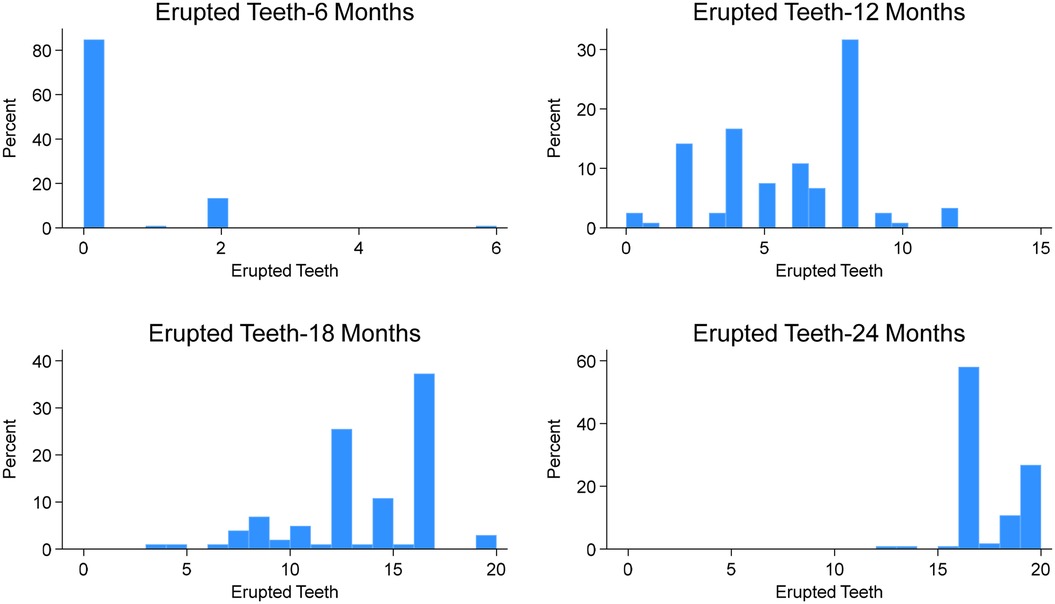
By 6 months of age (Figure 1), 17 children (15.2%) had erupted teeth, ranging from 1 to 6 (mean = 0.33, SD = 0.87). The lower central incisors (12%) were the most common teeth erupted at this age (Figure 2A). By 12 months, 3 children (2.5%) had no erupted teeth, while the median number of erupted teeth was 6 (range: 0–12; mean = 5.76, SD = 2.69). More than 95% of children had erupted lower central incisors, and approximately 80% had erupted upper central incisors (Figure 2B). At 18 months, all children attending the study visit (n = 102) had erupted teeth, with a median of 14 (range: 3–20; mean = 13.13, SD = 3.39). By 24 months, the median number of erupted teeth was 16 (mean = 17.18, SD = 1.85), and 25% of children had a full set of 20 primary teeth (Figure 1). Fewer than 40% had erupted upper and lower second molars (Figure 2D).
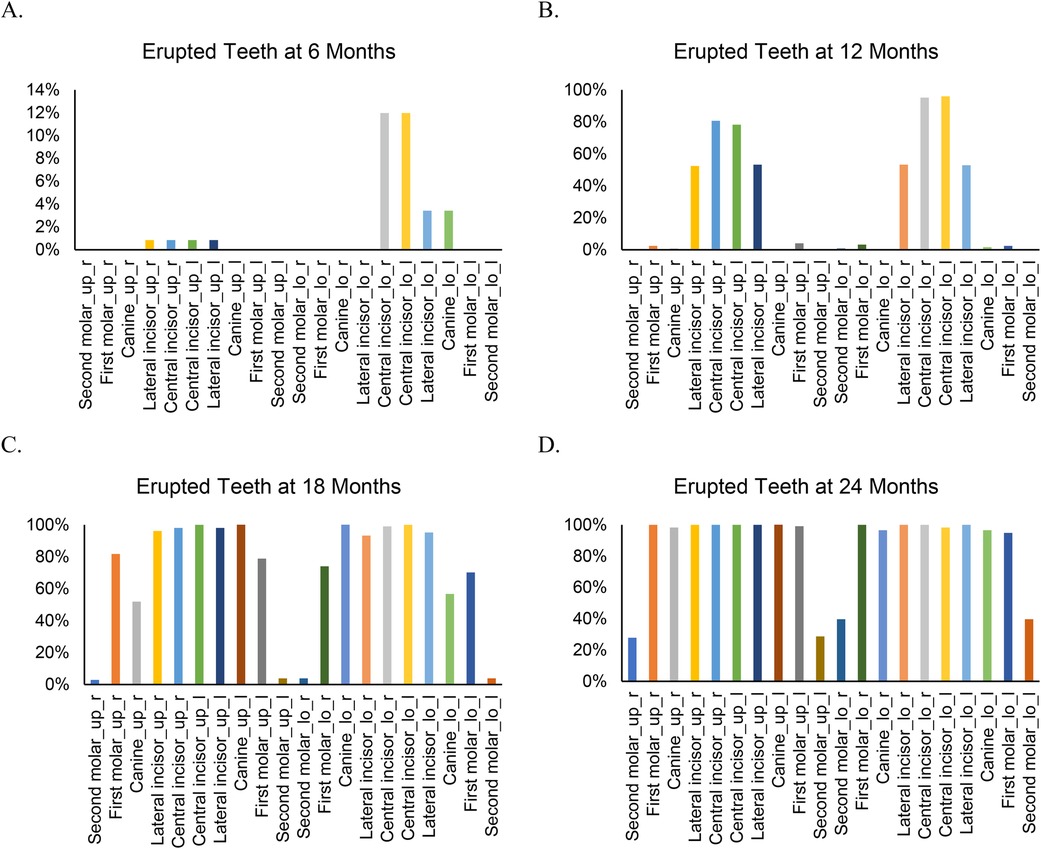
Figure 2. Type of erupted teeth at different visits from 6 to 24 months. “up” indicates upper and “lo” indicates lower. “r” indicates right and “l” indicates left.
Spearman's rank-order correlation was used to assess associations among tooth counts across visits. Significant correlations were observed (p < 0.01) for all pairs except between 6 and 18 months. Significant correlation coefficients were weak to modest, ranging from 0.30 to 0.57 (Figure 3A). However, the intraclass correlation coefficient (ICC) across visits was close to “0” (ICC = 7.24 × 10−10), suggesting limited stability in individual eruption patterns (Figure 3B). Group-based trajectory modeling identified two distinct tooth eruption patterns during the first two years of life, based on the lowest Bayesian information criterion (BIC, Figure 3C): a continuous growth pattern (97.3% of children) and a rapid growth pattern between 12 and 18 months (2.7% of children).
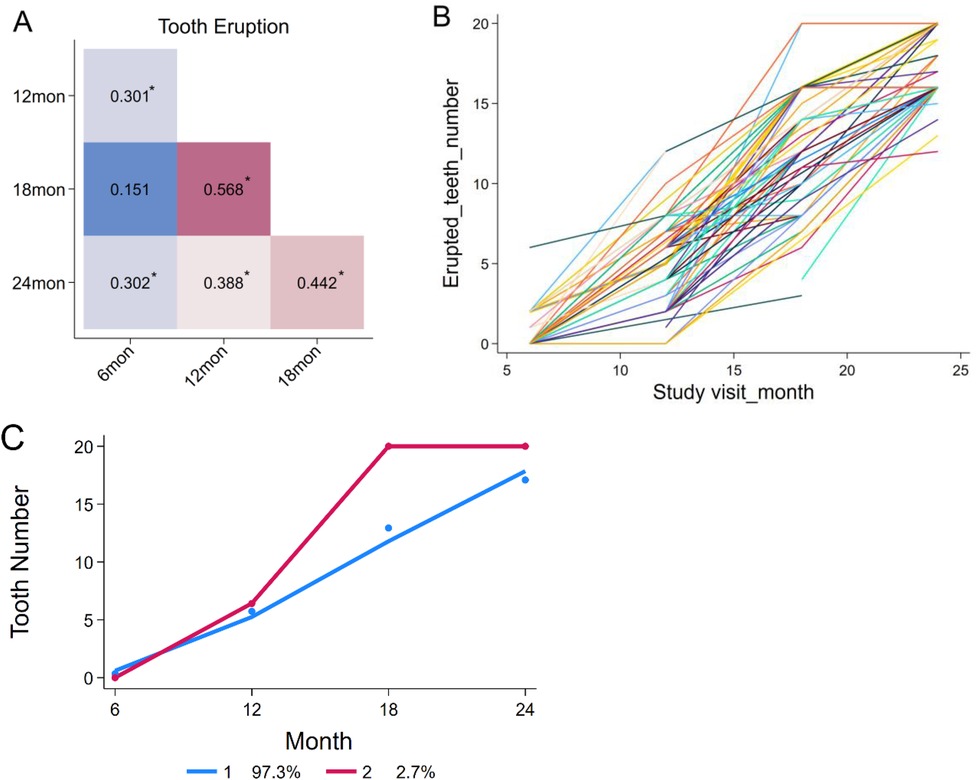
Figure 3. Correlations among the number of erupted teeth at different visits and individual growth trends. (A) Correlations among tooth counts from 6 to 24 months of age. (B) Variation in tooth eruption patterns among individual children. (C) Two distinct tooth eruption patterns identified by group-based trajectory modeling.
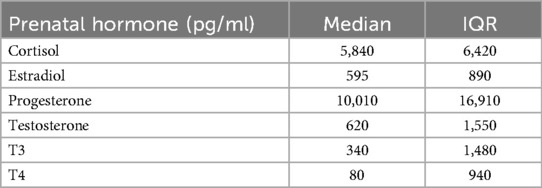
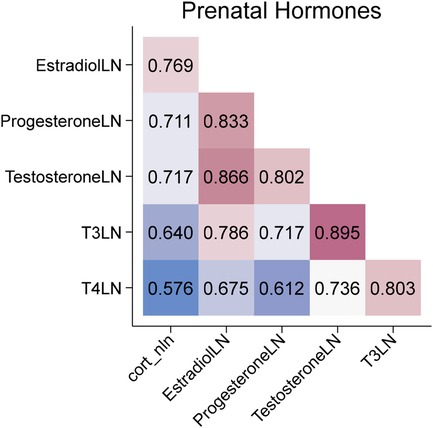
During pregnancy, 36.6% of women had a documented diagnosis of depression or anxiety. Levels of stress-related hormones measured in maternal salivary samples collected during the late 2nd or 3rd trimester are presented in Table 2. All hormones were significantly correlated (p < 0.001), with correlation coefficients ranging from 0.58 to 0.89 (Figure 4). No significant associations were observed between depression or anxiety diagnoses and hormone levels.
The relationship between prenatal depression/anxiety, salivary hormones, and tooth eruption
Due to the small-to-moderate correlations and low ICC for the number of erupted teeth across visits, associations between prenatal depression/anxiety, salivary hormones, and tooth eruption were first assessed separately at each child visit. Prenatal maternal depression/anxiety was not significantly associated with the number of erupted teeth at any time point (p > 0.05). In contrast, several prenatal stress-related hormones, including cortisol, estradiol, progesterone, testosterone, and T3, were positively associated with tooth counts (Table 3).
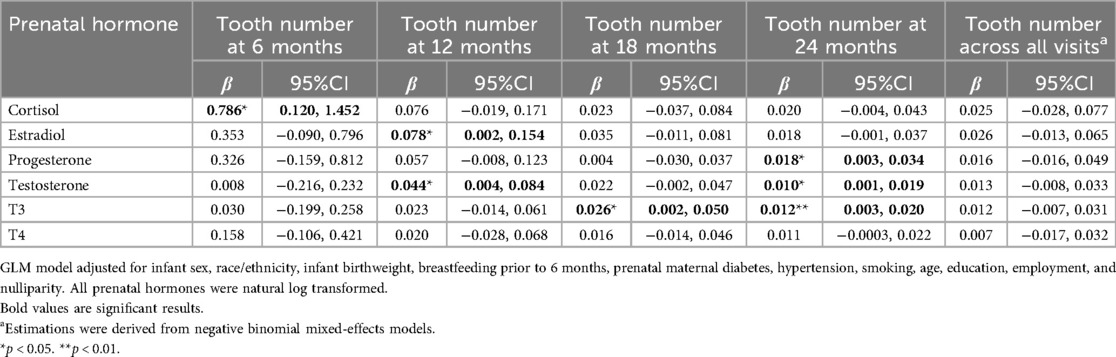
Table 3. The associations between prenatal maternal hormones and tooth eruption longitudinally and cross-sectionally.
Specifically, higher prenatal cortisol levels were associated with a greater number of erupted teeth at 6 months (β = 0.79, 95% CI: 0.12–1.45), corresponding to an average difference of ∼4 teeth between the lowest and highest cortisol levels. At 12 months, higher estradiol (β = 0.08, 95% CI: 0.002–0.15) and testosterone (β = 0.04, 95% CI: 0.004–0.08) were positively associated with tooth count, corresponding to average differences of 0.4 and 0.3 teeth, respectively. At 24 months, progesterone (β = 0.02, 95% CI: 0.003–0.03) and testosterone (β = 0.01, 95% CI: 0.001–0.02) were associated with tooth count, corresponding to average differences of 0.1 and 0.07 teeth. T3 levels were also positively associated with tooth counts at 18 months (β = 0.03, 95% CI: 0.002–0.05) and 24 months (β = 0.01, 95% CI: 0.003–0.02), corresponding to average differences of 0.2 and 0.09 teeth, respectively, between the lowest and highest hormone levels.
When overall associations across all visits were assessed, the results were non-significant, likely reflecting the low ICC and weak correlations across visits. Most findings remained robust in sensitivity analyses; however, associations between cortisol and tooth count at 24 months (β = 0.03, 95% CI: 0.0002–0.05) and between estradiol and tooth count at 24 months (β = 0.02, 95% CI: 0.0005–0.04) became significant. In contrast, associations with estradiol (p = 0.09) and testosterone (p = 0.05) at 12 months were attenuated.
Discussion
Overall, our findings indicate that correlations in the number of erupted teeth across visits during the first two years of life were weak to modest, suggesting variability in dental growth patterns among individual children. Several prenatal salivary hormones were positively associated with the number of primary teeth at specific time points, with more associations observed at 24 months. However, the strongest associations emerged earlier, particularly between prenatal cortisol levels and tooth count at 6 months. These findings may suggest potential biological mechanisms through which prenatal stress may influence the timing of tooth eruption.
In our cohort, the sequence of primary tooth eruption aligned with prior studies (38), yet children exhibited relatively delayed tooth eruption by 12 months and more advanced development between 12 and 24 months compared to previous studies (3, 38). Such differences may be explained by variability in race/ethnicity and socioeconomic status across study populations. Interestingly, we observed heterogeneous tooth eruption patterns, with some children experiencing rapid tooth emergence in infancy and others showing accelerated eruption during toddlerhood. Two primary eruption trajectories were identified using group-based trajectory modeling. However, the weak to modest correlations across visits suggest that additional, unidentified eruption trajectories may exist. Future studies with larger, more diverse samples are needed to characterize these potential subgroups and to assess predictors of interindividual variation in eruption timing.
To date, evidence linking prenatal hormone levels with tooth eruption timing in offspring remains sparse. Vucic and colleagues found no significant associations between maternal thyroid hormones and dental development at age 9 (31), consistent with our null findings for T4. However, we observed significant associations between prenatal maternal T3 levels in late pregnancy and the number of erupted teeth at 18 and 24 months. Because T3 was not measured in the prior study (31), our findings may highlight a novel association. Biologically, T3 plays a critical role in bone development (26) and mineral homeostasis (39, 40), which may similarly influence odontogenesis and help explain its relationship with tooth eruption.
Given the relatively high correlations among the measured hormones, it remains unclear which ones play a key role in modifying eruption timing. Cortisol, estradiol, progesterone, testosterone, and T3 are all implicated in bone development and calcium/vitamin D metabolism (24–28). Among these, prenatal cortisol showed the strongest association with tooth count at 6 months. Biologically, cortisol has been shown to impair osteoclastogenesis and osteoblastogenesis and promote apoptosis of osteoblasts and osteocytes (41), although its role in odontogenesis remains unclear. Cortisol also affects vitamin D and calcium availability through malabsorption, hypercalciuria, and bone resorption (24, 42, 43). Experimental studies have demonstrated that calcium and vitamin D supplementation during pregnancy accelerates tooth eruption in mice (44), yet evidence in humans remains inconsistent (15, 45, 46). Our observation that high prenatal cortisol was linked to earlier tooth eruption may also parallel findings that prenatal stress accelerates biological aging processes such as epigenetic modifications and telomere shortening (47–49).
Although associations between sex steroid hormones (estradiol, progesterone, and testosterone) and tooth eruption were statistically significant, effect sizes were small, suggesting limited clinical impact. These hormones are essential for fetal development and birthweight, both linked to eruption timing (9, 15, 50). Sex steroid hormone levels may also indirectly reflect vitamin D sufficiency, which regulates placental hormone production (27). Estradiol additionally modulates osteoblasts partly through the RANKL-OPG-RANK pathway (28), which may also influence odontogenesis. Nevertheless, given the small magnitude of effects observed, these associations should be interpreted cautiously.
Several limitations in this study warrant consideration. First, the ICC for erupted tooth counts across visits was close to zero, indicating low stability of eruption over time within individuals. In practical terms, having more teeth in early infancy did not reliably predict having more teeth later. Therefore, interpretations of mixed-effects and cross-sectional models should be made with caution. Second, prenatal stress was assessed during maternal diagnoses of depression or anxiety, which likely underestimated stress exposure, particularly in a cohort characterized by socioeconomic adversity. While salivary cortisol provided an objective biomarker of stress (51), future studies should incorporate validated stress questionnaires, such as perceived stress scale and stressful life events, alongside biomarkers to capture stress more comprehensively. Third, hormone levels were measured from a single salivary sample. This approach does not account for diurnal cortisol fluctuations, which better reflect overall activity of the hypothalamic-pituitary-adrenal axis (52). Therefore, future studies are recommended to collect multiple saliva samples to capture cortisol diurnal variation. For the other hormones, prior studies indicate that salivary and blood concentrations show moderate to strong correlations (53, 54). suggesting that salivary hormone levels may reflect systemic levels. Finally, maternal biospecimens were collected in late pregnancy, a period corresponding to crown formation. However, primary tooth development begins as early as ∼6 weeks of gestation (55), and the influence of hormones may vary by gestational stage. Future studies should consider assessing hormone levels at multiple time points throughout pregnancy to better examine potential time-specific effects on tooth development.
Despite these limitations, this study has several strengths. As a prospective cohort, maternal and child data were collected longitudinally, reducing recall bias. Dental assessments were performed by calibrated dentists using standardized protocols, ensuring data quality and consistency. Moreover, we examined a broad panel of hormones related to stress and fetal development, enabling exploratory analyses of potential biological mechanisms linking prenatal stress with primary tooth eruption.
Conclusion
Maternal salivary hormone levels, particularly cortisol, measured in late pregnancy were potentially associated with tooth eruption in children during the first two years of life. Larger, more diverse cohorts with repeated biomarker assessments are needed to clarify the biological mechanisms linking prenatal stress to tooth eruption.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Rochester Research Subject Review Board (study #1248). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants. For minor participants, written informed consent for participation was provided by the participants' legal guardians/next of kin.
Author contributions
YM: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. RY: Data curation, Project administration, Writing – review & editing. NA: Data curation, Project administration, Writing – review & editing. TO: Writing – review & editing. JR: Writing – review & editing. FB: Writing – review & editing. JX: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Institutes of Health (R01DE031025). JR is funded by the National Institutes of Health (R00 HD-100593 and R01 MH-138481).
Acknowledgments
We acknowledge the valuable contributions of all participants and study coordinators involved in our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT and grammarly were used to check grammar mistakes of the manuscript. No other preparations of this manuscript involved usage of Generative AI tools.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1663817/full#supplementary-material
References
1. Muthu MS, Vandana S, Akila G, Anusha M, Kandaswamy D, Aswath Narayanan MB. Global variations in eruption chronology of primary teeth: a systematic review and meta-analysis. Arch Oral Biol. (2024) 158:105857. doi: 10.1016/j.archoralbio.2023.105857
2. Martins RFM, Dos Santos AM, Saraiva M, Ribeiro CCC, Alves CMC, da Silva AAM, et al. Prediction of deciduous teeth eruption in Brazilian children: a cross-sectional study nested in a prospective birth cohort (BRISA). BMC Oral Health. (2024) 24(1):61. doi: 10.1186/s12903-023-03823-0
3. Ntani G, Day PF, Baird J, Godfrey KM, Robinson SM, Cooper C, et al. Maternal and early life factors of tooth emergence patterns and number of teeth at 1 and 2 years of age. J Dev Orig Health Dis. (2015) 6(4):299–307. doi: 10.1017/S2040174415001130
4. Yaseen SM, Naik S, Uloopi KS. Ectopic eruption—a review and case report. Contemp Clin Dent. (2011) 2(1):3–7. doi: 10.4103/0976-237X.79289
5. El Osta N, Chambon P, Dol G, Soulier-Peigue D, Hennequin M. Does malocclusion affect ingestion: a systematic review. Clin Oral Investig. (2024) 28(1):111. doi: 10.1007/s00784-024-05508-6
6. Roulias P, Kalantzis N, Doukaki D, Pachiou A, Karamesinis K, Damanakis G, et al. Teeth eruption disorders: a critical review. Children (Basel). (2022) 9(6):771. doi: 10.3390/children9060771
7. Alrashdi M, Alotaiby F, Almutlaq K, Alrkabee N, Almulhim B, Alduwayghiri E. Effect of early tooth eruption on the development of dental caries in children: a cross-sectional study. BMC Oral Health. (2025) 25(1):247. doi: 10.1186/s12903-025-05449-w
8. McDermott CL, Hilton K, Park AT, Tooley UA, Boroshok AL, Mupparapu M, et al. Early life stress is associated with earlier emergence of permanent molars. Proc Natl Acad Sci U S A. (2021) 118(24):e2105304118. doi: 10.1073/pnas.2105304118
9. Wu H, Chen T, Ma Q, Xu X, Xie K, Chen Y. Associations of maternal, perinatal and postnatal factors with the eruption timing of the first primary tooth. Sci Rep. (2019) 9(1):2645. doi: 10.1038/s41598-019-39572-w
10. Hughes TE, Bockmann MR, Seow K, Gotjamanos T, Gully N, Richards LC, et al. Strong genetic control of emergence of human primary incisors. J Dent Res. (2007) 86(12):1160–5. doi: 10.1177/154405910708601204
11. Lacruz RS, Habelitz S, Wright JT, Paine ML. Dental enamel formation and implications for oral health and disease. Physiol Rev. (2017) 97(3):939–93. doi: 10.1152/physrev.00030.2016
12. Jernvall J, Thesleff I. Tooth shape formation and tooth renewal: evolving with the same signals. Development. (2012) 139(19):3487–97. doi: 10.1242/dev.085084
13. Suri L, Gagari E, Vastardis H. Delayed tooth eruption: pathogenesis, diagnosis, and treatment. A literature review. Am J Orthod Dentofacial Orthop. (2004) 126(4):432–45. doi: 10.1016/j.ajodo.2003.10.031
14. Alvarez JO. Nutrition, tooth development, and dental caries. Am J Clin Nutr. (1995) 61(2):410S–6. doi: 10.1093/ajcn/61.2.410S
15. Georgiadou AI, Ritsas A, Arhakis A. The impact of maternal, perinatal, and early infancy period on the eruption timing of the first primary tooth. Eur J Dent Oral Health. (2021) 2(3):28–33. doi: 10.24018/ejdent.2021.2.3.63
16. Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. (2006) 68(3):414–20. doi: 10.1097/01.psy.0000221236.37158.b9
17. Businelle MS, Mills BA, Chartier KG, Kendzor DE, Reingle JM, Shuval K. Do stressful events account for the link between socioeconomic status and mental health? J Public Health (Oxf). (2014) 36(2):205–12. doi: 10.1093/pubmed/fdt060
18. Mountain RV, Zhu Y, Pickett OR, Lussier AA, Goldstein JM, Roffman JL, et al. Association of maternal stress and social support during pregnancy with growth marks in children’s primary tooth enamel. JAMA Netw Open. (2021) 4(11):e2129129. doi: 10.1001/jamanetworkopen.2021.29129
19. Auger N, Low N, Lee G, Lo E, Nicolau B. Maternal mental disorders before delivery and the risk of dental caries in children. Caries Res. (2020) 54(3):242–9. doi: 10.1159/000505906
20. de Kloet ER, Joëls M. The cortisol switch between vulnerability and resilience. Mol Psychiatry. (2024) 29(1):20–34. doi: 10.1038/s41380-022-01934-8
21. Anjum A, Anwar H, Sohail MU, Ali Shah SM, Hussain G, Rasul A, et al. The association between serum cortisol, thyroid profile, paraoxonase activity, arylesterase activity and anthropometric parameters of undergraduate students under examination stress. Eur J Inflamm. (2021) 19:20587392211000884. doi: 10.1177/20587392211000884
22. Assad S, Khan HH, Ghazanfar H, Khan ZH, Mansoor S, Rahman MA, et al. Role of sex hormone levels and psychological stress in the pathogenesis of autoimmune diseases. Cureus. (2017) 9(6):e1315. doi: 10.7759/cureus.1315
23. Anifantaki F, Pervanidou P, Lambrinoudaki I, Panoulis K, Vlahos N, Eleftheriades M. Maternal prenatal stress, thyroid function and neurodevelopment of the offspring: a mini review of the literature. Front Neurosci. (2021) 15:692446. doi: 10.3389/fnins.2021.692446
24. Ng JS, Chin KY. Potential mechanisms linking psychological stress to bone health. Int J Med Sci. (2021) 18(3):604–14. doi: 10.7150/ijms.50680
25. Naguib R, Elkemary EZ, Elsharkawi KM. The severity of bone loss: a comparison between cushing’s disease and cushing’s syndrome. J Endocrinol Metab. (2023) 13(1):33–8. doi: 10.14740/jem857
26. Kim H-Y, Mohan S. Role and mechanisms of actions of thyroid hormone on the skeletal development. Bone Res. (2013) 1(1):146–61. doi: 10.4248/BR201302004
27. Barrera D, Avila E, Hernández G, Halhali A, Biruete B, Larrea F, et al. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol. (2007) 103(3–5):529–32. doi: 10.1016/j.jsbmb.2006.12.097
28. Kovacs CS. Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol Rev. (2014) 94(4):1143–218. doi: 10.1152/physrev.00014.2014
29. Choukroune C. Tooth eruption disorders associated with systemic and genetic diseases: clinical guide. J Dentofacial Anom Orthod. (2017) 20(4):402. doi: 10.1051/odfen/2018129
30. Durá-Travé T, Gallinas-Victoriano F. Dental caries in children and vitamin D deficiency: a narrative review. Eur J Pediatr. (2024) 183(2):523–8. doi: 10.1007/s00431-023-05331-3
31. Vucic S, Korevaar TIM, Dhamo B, Jaddoe VWV, Peeters RP, Wolvius EB, et al. Thyroid function during early life and dental development. J Dent Res. (2017) 96(9):1020–6. doi: 10.1177/0022034517708551
32. Boyce WT, Den Besten PK, Stamperdahl J, Zhan L, Jiang Y, Adler NE, et al. Social inequalities in childhood dental caries: the convergent roles of stress, bacteria and disadvantage. Soc Sci Med. (2010) 71(9):1644–52. doi: 10.1016/j.socscimed.2010.07.045
33. Xiao J, Fogarty C, Wu TT, Alkhers N, Zeng Y, Thomas M, et al. Oral health and candida carriage in socioeconomically disadvantaged US pregnant women. BMC Pregnancy Childbirth. (2019) 19(1):480. doi: 10.1186/s12884-019-2618-7
34. Murphy HR, Gu Y, Wu Q, Brunner J, Panisch LS, Best M, et al. Prenatal diurnal cortisol: normative patterns and associations with affective symptoms and stress. Psychoneuroendocrinology. (2022) 143:105856. doi: 10.1016/j.psyneuen.2022.105856
35. Feng G, Xu X, Lei J. Tracking perceived stress, anxiety, and depression in daily life: a double-downward spiral process. Front Psychol. (2023) 14:1114332. doi: 10.3389/fpsyg.2023.1114332
36. Yang R, Lu X, Alomeir N, Quataert S, Wu T, Xiao J. Association between salivary hormones, dental caries, and cariogenic microorganisms during pregnancy. J Clin Med. (2024) 13(11):3183. doi: 10.3390/jcm13113183
37. Żądzińska E, Sitek A, Rosset I. Relationship between pre-natal factors, the perinatal environment, motor development in the first year of life and the timing of first deciduous tooth emergence. Ann Hum Biol. (2016) 43(1):25–33. doi: 10.3109/03014460.2015.1006140
38. Ogodescu E, Popa M, Isac C, Pinosanu R, Olaru D, Cismas A, et al. Eruption timing and sequence of primary teeth in a sample of Romanian children. Diagnostics. (2022) 12(3):606. doi: 10.3390/diagnostics12030606
39. Psoter W, Gebrian B, Prophete S, Reid B, Katz R. Effect of early childhood malnutrition on tooth eruption in Haitian adolescents. Community Dent Oral Epidemiol. (2008) 36(2):179–89. doi: 10.1111/j.1600-0528.2007.00386.x
40. Hermans F, Hemeryck L, Lambrichts I, Bronckaers A, Vankelecom H. Intertwined signaling pathways governing tooth development: a give-and-take between canonical wnt and shh. Front Cell Dev Biol. (2021) 9:758203. doi: 10.3389/fcell.2021.758203
41. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. (1998) 102(2):274–82. doi: 10.1172/JCI2799
42. Al Refaie A, Baldassini L, De Vita M, Gonnelli S, Caffarelli C. Vitamin D and adrenal gland: myth or reality? A systematic review. Front Endocrinol (Lausanne). (2022) 13:1001065. doi: 10.3389/fendo.2022.1001065
43. Kaltsas G, Makras P. Skeletal diseases in Cushing's syndrome: osteoporosis versus arthropathy. Neuroendocrinology. (2010) 92(Suppl 1):60–64. doi: 10.1159/000314298
44. Sitosari H, Sari FT, Jonarta AL, Haniastuti T. Consumption of nano calcium and vitamin D affects the day of eruption and eruption level of sprague dawley maxillary and mandibular incisors. Mal J Med Health Sci. (2020) 16:67–72.
45. Jairam LS, Konde S, Raj NS, Kumar NC. Vitamin D deficiency as an etiological factor in delayed eruption of primary teeth: a cross-sectional study. J Indian Soc Pedod Prev Dent. (2020) 38(3):211–5. doi: 10.4103/JISPPD.JISPPD_184_18
46. Dhamo B, Miliku K, Voortman T, Tiemeier H, Jaddoe VW, Wolvius EB, et al. The associations of maternal and neonatal vitamin D with dental development in childhood. Curr Dev Nutr. (2019) 3(4):nzy100. doi: 10.1093/cdn/nzy100
47. McGill MG, Pokhvisneva I, Clappison AS, McEwen LM, Beijers R, Tollenaar MS, et al. Maternal prenatal anxiety and the fetal origins of epigenetic aging. Biol Psychiatry. (2022) 91(3):303–12. doi: 10.1016/j.biopsych.2021.07.025
48. Suh DI, Kang MJ, Park YM, Lee JK, Lee SY, Sheen YH, et al. Leukocyte telomere length reflects prenatal stress exposure, but does not predict atopic dermatitis development at 1 year. Allergy Asthma Immunol Res. (2019) 11(3):357–66. doi: 10.4168/aair.2019.11.3.357
49. Suarez A, Lahti J, Czamara D, Lahti-Pulkkinen M, Knight AK, Girchenko P, et al. The epigenetic clock at birth: associations with maternal antenatal depression and child psychiatric problems. J Am Acad Child Adolesc Psychiatry. (2018) 57(5):321–8. doi: 10.1016/j.jaac.2018.02.011
50. Meng Y, Thornburg LL, Dreisbach C, Orzolek C, Kautz A, Murphy HR, et al. The role of prenatal maternal sex steroid hormones in weight and adiposity at birth and growth trajectories during infancy. Int J Obes (Lond). (2025) 49(5):954–64. doi: 10.1038/s41366-025-01743-3
51. Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. (2009) 34(2):163–71. doi: 10.1016/j.psyneuen.2008.10.026
52. Dmitrieva NO, Almeida DM, Dmitrieva J, Loken E, Pieper CF. A day-centered approach to modeling cortisol: diurnal cortisol profiles and their associations among U.S. adults. Psychoneuroendocrinology. (2013) 38(10):2354–65. doi: 10.1016/j.psyneuen.2013.05.003
53. Huang T, Howse FM, Stachenfeld NS, Usselman CW. Correlations between salivary- and blood-derived gonadal hormone assessments and implications for inclusion of female participants in research studies. Am J Physiol Heart Circ Physiol. (2023) 324(1):H33–46. doi: 10.1152/ajpheart.00399.2022
54. Sawicka-Gutaj N, Glinicki P, Nijakowski K, Bromińska B, Ostrowska M, Szatko A, et al. Measurement of salivary thyroid hormones using the LC-MS/MS technique in a clinical setting. Ther Adv Endocrinol Metab. (2024) 15:20420188241277414. doi: 10.1177/20420188241277414
Keywords: tooth eruption, prenatal stress, cortisol, sex steroid hormones, thyroid hormones
Citation: Meng Y, Yang R, Alomeir N, O’Connor TG, Rasmussen JM, Bidlack FB and Xiao J (2025) Prenatal maternal salivary hormones and timing of tooth eruption in early childhood: a prospective birth cohort study. Front. Oral Health 6:1663817. doi: 10.3389/froh.2025.1663817
Received: 11 July 2025; Accepted: 9 October 2025;
Published: 18 November 2025.
Edited by:
Kitty Jieyi Chen, Sun Yat-sen University, ChinaReviewed by:
Md Atiqul Haque, Hajee Mohammad Danesh Science & Technology University, BangladeshMurad Alrashdi, Qassim University, Saudi Arabia
Copyright: © 2025 Meng, Yang, Alomeir, O’Connor, Rasmussen, Bidlack and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Meng, eWluZ19tZW5nQHVybWMucm9jaGVzdGVyLmVkdQ==
 Ying Meng
Ying Meng Ruqian Yang
Ruqian Yang Nora Alomeir
Nora Alomeir Thomas G. O’Connor
Thomas G. O’Connor Jerod M. Rasmussen
Jerod M. Rasmussen Felicitas B. Bidlack
Felicitas B. Bidlack Jin Xiao
Jin Xiao