- 1Department of Medicine, University of Tennessee Health Science Center College of Medicine, Knoxville, TN, United States
- 2Department of General Dentistry, University of Tennessee Health Science Center College of Medicine, Knoxville, TN, United States
- 3Knoxville Periodontics, Knoxville, TN, United States
- 4College of Dentistry, University of Tennessee Health Science Center, Memphis, TN, United States
- 5Department of Surgery, Graduate School of Medicine, University of Tennessee Health Science Center College of Medicine, Knoxville, TN, United States
Background: Incorporating gum disease assessment into epidemiologic studies would facilitate investigations of disease etiology.
Objective: We evaluated the accuracy and inter-rater reliability of experienced dental health professionals' visual assessments of digital photographs to determine inflammatory gum disease.
Methods: Raters viewed anonymized photographs of the teeth and gums of 30 adult patients and were asked to distinguish “healthy” gingiva from “gum disease” and to assess disease severity. Frequency, percentage, and cross-tabulation statistics were used to perform diagnostic calculations including sensitivity, specificity, and overall accuracy. Fleiss' Kappa, with a 95% confidence interval, was used to test for interrater reliability amongst the four raters. Cohen's Kappa was then calculated for each potential pairing of the four raters.
Results: The accuracy of determining active inflammatory gum disease from digital photographs ranged from 76.7% to 96.7% (mean 85.9%) across the four raters. Sensitivity ranged from 70% to 95% (mean 82.5%), and specificity ranged from 80% to 100% (mean 92.5%). However, inter-rater reliability for disease severity was only fair, with Fleiss's Kappa for gingivitis and periodontitis 0.25 (0.00–0.51) and 0.28 (0.03–0.54), respectively.
Conclusion: Our findings show that digital photographs could be useful for assessing inflammatory gum disease in epidemiologic studies of inflammation-mediated chronic systemic diseases.
Introduction
Gingivitis is gum inflammation caused by bacterial plaque. Signs of gingivitis include red, swollen gums that can easily bleed, for example, when brushing (1). This early-stage inflammatory gum disease can progress to periodontitis, where plaque below the gum causes the inner layer of the gum and bone to pull away from the teeth, often resulting in bone and tooth loss (2). Inflammatory gum disease remains a major public health concern in the U.S., with little overall improvement in the past 20 years. According to the National Institute of Dental and Craniofacial Research, 42% of adults are currently affected by periodontal disease (3). The prevalence of gingivitis is even higher, with most adults affected to varying degrees (4).
Inflammatory gum disease increases systemic inflammation and the risk of several chronic diseases (5). For example, adults with periodontitis have a higher risk of cardiovascular disease (6, 7). Periodontitis, and the systemic inflammation associated with it, also appear to promote diabetes, which, in turn, can worsen periodontitis in what has been theorized to be an inflammatory “vicious cycle.” (7, 8) Evidence of a vicious cycle includes a three-fold increased risk of periodontal disease in diabetics (8, 9) and improved glycemic control after periodontal treatment (10, 11). Periodontal disease is associated with rheumatoid arthritis occurrence and severity (12), Alzheimer's disease (13), and may contribute to cancer development and growth (14), for example, through an impaired immune surveillance system (15). Although less evidence exists for gingivitis alone, this inflammatory condition of the gingival tissue experimentally increased measures of systemic inflammation (16). Therefore, epidemiologic studies of chronic disease etiology, treatment and/or prevention may increasingly seek to incorporate measures of dental health into risk factor assessments and data analyses.
Epidemiologic studies focused on gum disease traditionally rely on direct clinical examination (17), which can be prohibitively expensive and burdensome in large-scale population-based studies, especially when gum disease is not the primary focus of the research. Therefore, assessment of dental health via digital photographs may have considerable advantages for large-scale epidemiology studies. Because the utility of digital photographs to assess inflammatory gum disease in epidemiologic studies remains unclear, we evaluated the accuracy and inter-rater reliability of visual assessment of digital photographs by experienced dentists to determine inflammatory gum disease status in a group of anonymized adult patients.
Methods
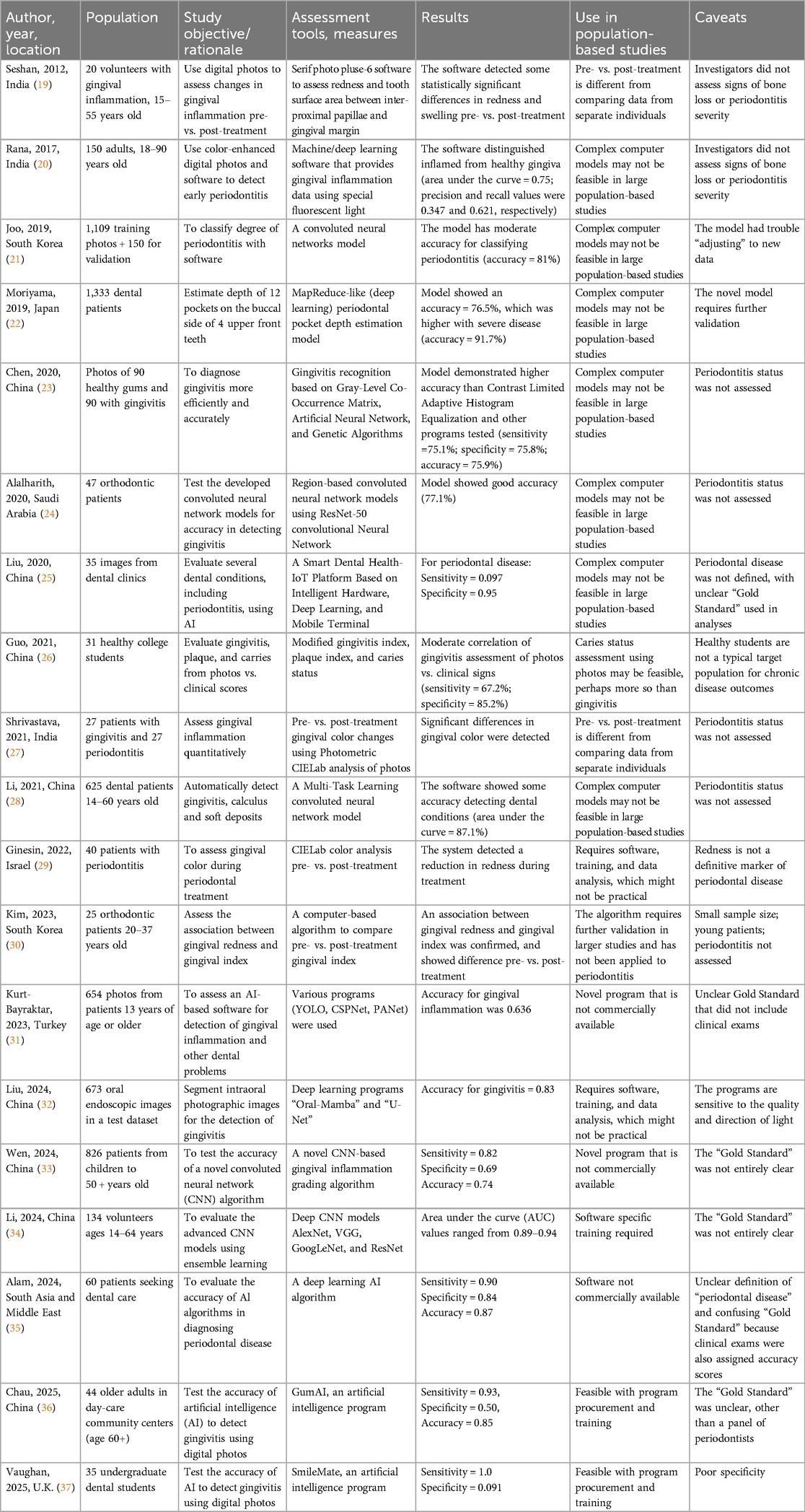
The research was undertaken at the University of Tennessee Medical Center (UTMC) in Knoxville, Tennessee, and was approved by the UT Graduate School of Medicine's Institutional Review Board. Anonymized digital photographs of the teeth and gums of 30 adult patients were provided by a dental practice in Knoxville, Tennessee, that was not affiliated with the UTMC Department of General Dentistry. Photos of patients were taken during the course of normal clinical care by a local periodontist with decades of clinical experience. Diagnoses were based on clinical examinations and radiographic techniques indicating key distinctions between healthy gums, gingivitis, and more advanced periodontitis. At the time the photographs were taken, each patient was diagnosed clinically and radiographically as having either no current gum disease (n = 10), gingivitis only (n = 10), or periodontitis (n = 10) (Figure 1).
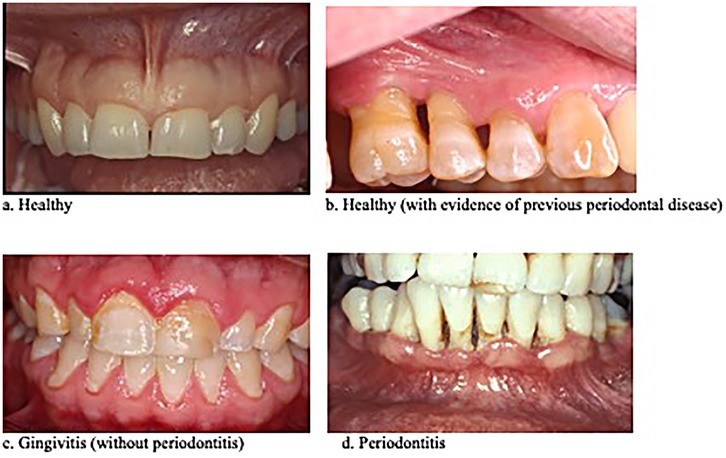
Figure 1. Four categories of photograph used in the study based on the patient's clinical diagnosis.
Patients' underlying diagnoses were blinded and the photographs were displayed in random order for four dental health professionals, a periodontist and two dentists at UTMC-Knoxville, and a third dentist at UTHSC College of Dentistry in Memphis, TN. Each evalutor had ties to both community-based and academic dentistry and had decades of experience diagnosing and treating patients with various forms and stages of inflammatory gum disease. Although each assessment relied primarily on the extensive clinical experience and training of the viewing dentist, gingival redness, edema, flattening of papillae, gum recession, and signs of periodontal bone loss, were considered when assessing the photographs. The photographs were viewed by each rater separately to help ensure independent assessments. In the first round, raters were asked to distinguish between currently “healthy” and “inflamed” gum tissue. Then, considering only the digital photographs of patients diagnosed with inflammatory gum disease, raters were asked to further distinguish between “gingivitis only” and “periodontitis.” Because some of the “healthy” patients had previous gum disease that was in remission, the raters were asked to distinguish “healthy” (Figure 1A) from “currently healthy with evidence of previous periodontal disease” (Figure 1B). The latter category was deemed important because of the high risk of periodontitis relapse in those individuals, which would be a consideration in epidemiologic studies.
To obtain previous studies that assessed gum disease from digital photographs, searches were conducted of the PubMed database using search-terms such as “oral disease,” “gum disease,” “gingivitis,” “periodontal disease,” “periodontitis,” “digital photographs,” and by cross-referencing citations in identified studies that were available in print or online before July 1, 2025. Although some of the retained studies included adolescents in their study populations, we did not consider studies that focused primarily on children. It was not our aim to conduct a full systematic review due to the lack of previous studies that relied on visual assessment by trained dental care providers.
Frequency, percentage, and cross-tabulation statistics were used to perform diagnostic calculations including sensitivity, specificity, and overall accuracy. Fleiss' Kappa with a 95% confidence interval was used to test for interrater reliability amongst the four raters. Cohen's Kappa was then calculated for each potential pairing of the four raters. Statistical significance was assumed at an alpha value of 0.05 and all analyses were performed using SPSS Version 29 (Armonk, NY: IBM Corp.).
Results
The accuracy of determining active inflammatory gum disease (gingivitis and/or periodontitis) from digital photographs ranged from 76.7% to 96.7% (mean = 85.9%) across the four raters (Table 1). Sensitivity ranged from 70% to 95% (mean = 82.5%), and specificity ranged from 80% to 100% (mean = 92.5%). Approximately half of the currently “healthy” patients showed signs of periodontal disease in remission. In two of these cases, a rater incorrectly diagnosed active gum disease (data not shown).
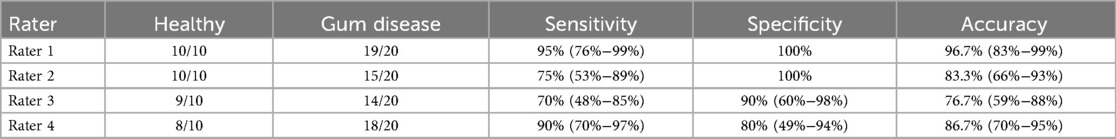
Table 1. Sensitivity and specificity of visual assessment of gum disease from digital photographs: “healthy” vs. “gum disease” (gingivitis and/or periodontitis).
When considering only patients with inflammatory gum disease, inter-rater reliability for disease severity was only fair (Table 2), with Fleiss's Kappa for gingivitis and periodontitis 0.25 (0.00–0.51) and 0.28 (0.03–0.54), respectively. The accuracy of distinguishing gingivitis from periodontitis ranged from 50.0% to 66.7% (mean = 62.5%).
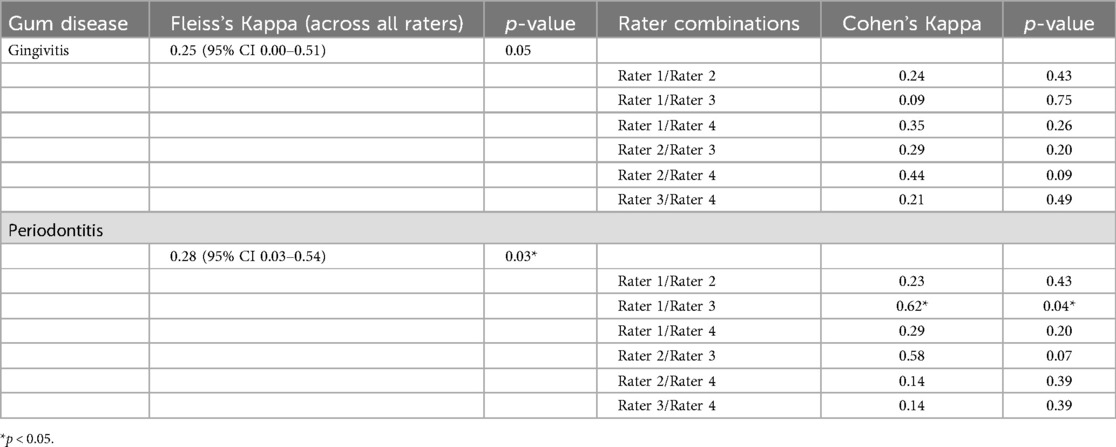
Table 2. Inter-rater reliability for inflammatory gum disease severity (gingivitis vs. periodontitis).
When considering patients currently without inflammatory gum disease, raters identified approximately half of these 10 “healthy” patients as having periodontitis in remission. However, only the patients' diagnoses at the time the photographs were taken were ultimately known, with no “gold standard” diagnosis previous to that time available, so we could not calculate measures of accuracy for distinguishing gum disease in remission. Nonetheless, a rater incorrectly assessed active inflammatory gum disease in two “healthy” patients that the other raters considered “currently healthy with evidence of previous periodontal disease.” The rater's errors in diagnosing active gum disease in currently healthy patients were incorporated into the estimates of accuracy shown in Table 1.
Discussion
We found that experienced dentists could distinguish healthy from inflamed gum tissue with good accuracy even though individuals with periodontitis in remission were included in the healthy group, reflecting real world applications. As expected, however, the inter-rater reliability regarding “gingivitis only” vs. “periodontitis,” as measured by Fleiss's Kappa, was only fair. The latter result was expected due to the well-recognized inability of digital photographs to expose subtle changes in bone density and structure, which otherwise can be discerned with good accuracy from clinical examination and x-rays. Nonetheless, our data support the utility of distinguishing inflammatory gum disease from healthy gums using digital photographs.
Increasing evidence suggests that inflammatory gum disease can fuel the development, progression, and treatment intransigence, of several common and debilitating chronic diseases, likely through pathways related to systemic inflammation (5–16). Whereas epidemiologic investigations of chronic diseases are likely to assess data on tobacco smoking, for example, and other known or suspected chronic disease risk factors, the assessment of inflammatory gum disease in epidemiologic studies has been rare. There are several reasons for this, including a general lack of awareness of how important gum disease may be in the occurrence, development and treatment efficacy of several chronic diseases, and the logistic and financial burdens of assessing gum disease in large-scale population-based studies. Clinical oral examination and x-rays, the gold standard for assessing gum disease, is consequently rarely done in large scale epidemiologic studies, especially when gum disease is not the primary focus of the research. Examiner fatigue, low patient participation, high dropout rates, and high risk of observer bias, are other problems noted with clinical oral examinations in epidemiologic studies (17). Therefore, assessment of dental health using digital photographs has advantages for large-scale epidemiologic studies, where costs, risk of observer bias, and burdens on study participants and staff, are greatly reduced. Moreover, study participants, as well as people in the general population, are often not aware of the status of their dental health and/or do not report it accurately (18). Given all of these considerations, our study's findings may have implications for the widescale incorporation of gum disease assessment in population-based epidemiologic studies of chronic diseases.
Our literature search yielded 19 previous studies that assessed gum disease using digital photographs (Table 3) (19–37), with most published in the past four to five years. Data from over 5,000 patients were analyzed in these studies from East (n = 11) and South (n = 3) Asia, the Middle East (n = 4), and Europe (n = 1). Sample sizes ranged in from n = 20 to n = 1,333 participants, among whom a minority were children and young adults. Estimates of accuracy in assessing inflammatory gum disease from digital photographs generally ranged from approximately 0.7–0.9 in those studies. One study (25) calculated the sensitivity and specificity of visual assessment of gingivitis (sensitivity = 67.2%, specificity = 85.2%). Of note, the estimates of sensitivity and specificity obtained for the visual assessment (25) regarding gum disease were similar to those obtained for the complex algorithms and computer software (Table 3). As noted earlier, our estimates of accuracy were also consistent with those from AI-based software.
All except one previous study used software to assess gum disease. Those studies showed reasonable accuracy discerning inflammatory gum disease from digital photographs, for example, using powerful “deep learning” or similar types of software (Table 3). However, the computer algorithms appear to be specific to each study, require development and maintenance by highly skilled personnel, and may be proprietary and expensive to purchase. Our data suggest that experienced dental health professionals can achieve similar accuracy in diagnosing inflammatory gum disease without the use of complex and costly computer algorithms. In our study, raters were more accurate in discerning patients with active inflammatory gum disease than in categorizing disease severity, i.e., gingivitis vs. periodontitis. However, the latter distinction may be less important because both conditions increase measures of systemic inflammation (16, 38).
Our study has four noteworthy limitations, including its sample size. Each of four raters assessed gum disease in 30 digital photographs, which was sufficient to generate estimates of accuracy and inter-rater reliability with moderate precision. Nonetheless, a larger sample size likely will be needed to increase the precision of these estimates in future studies.
Second, the digital photographs we obtained from an unaffiliated dental practice were not taken using a standardized protocol and, hence, were not uniform in image perspective or lighting (Figure 1). Greater accuracy in gum disease diagnosis may result from using a standardized series of photographs for each patient, for example, a frontal photograph showing labial surfaces of anterior teeth; lateral photographs showing buccal surfaces of left and right posterior teeth; a maxillary dentition photograph showing palatal and occlusal surfaces of maxillary dentition; and a mandibular dentition photograph showing lingual and occlusal surfaces of mandibular dentition, using established protocols regarding photography equipment, lighting, and camera angle. This need not be overly burdensome on study staff or resources, however, because study coordinators could be trained by study dentists to follow such data collection protocols at participant enrollment.
Third, the primary aim of our study was to assess the sensitivity, specificity, and accuracy of the remote assessment of inflammatory gum disease from digital photographs by experienced dentists using a common set of criteria. We did not concurrently assess gum disease from digital photographs using computer software, so we can not directly compare our study results with those of an algorithm-based assessment in our study population. However, based on our results and those of the previous studies we reviewed here, there seems to be no clear evidence of greater diagnostic accuracy of algorithm-based assessment over visual assessment. Likewise, no previous study performed a direct comparison of assessment methods. A comparison between algorithm-based assessments of digital photographs with visual assessments by experienced dentists would be a reasonable aim of future studies.
Finally, although we searched two well-known extensive online databases for published literature related to the assessment of dental health via photographs, and cross-referenced citations in the identified studies in search of additional citations, our review was not a systematic review (39). Therefore, it is possible that we did not obtain one or more of the relevant previous studies.
Conclusion
Incorporating the assessment of inflammatory gum disease into epidemiologic studies would facilitate investigations of chronic disease etiology as well as those to determine the effect of treating gum disease on the course of several chronic systemic diseases, such as diabetes (11, 12). However, an ongoing question with such studies is how to accurately discern the presence of inflammatory gum disease when clinical examinations and x-rays, the gold standard, are not feasible. Several previous studies assessed the accuracy of discerning gum disease in digital photographs using complex computer algorithms. Our study's findings support the utility of a simpler method that yields similar results and could be readily applied in population-based field studies and large-scale epidemiologic investigations.
Data availability statement
The datasets presented in this article are not readily available because No personal information can be shared without permission. Requests to access the datasets should be directed tocHRlcnJ5QHV0bWNrLmVkdQ==.
Ethics statement
The studies involving humans were approved by University of Tennessee Graduate School of Medicine IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PT: Conceptualization, Investigation, Writing – original draft, Methodology, Writing – review & editing. OW: Investigation, Writing – review & editing, Project administration, Writing – original draft. MH: Writing – review & editing, Writing – original draft, Investigation. OT: Writing – original draft, Investigation, Writing – review & editing. RH: Writing – review & editing, Writing – original draft, Formal analysis, Investigation. RD: Investigation, Conceptualization, Writing – review & editing, Writing – original draft, Supervision, Methodology.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gingivitis. Mayo Clinic. Available online at: https://www.mayoclinic.org/diseases-conditions/gingivitis/symptoms-causes/syc-20354453 (Accessed August 30, 2024).
2. Periodontitis. Mayo Clinic. Available online at: https://www.mayoclinic.org/diseases-conditions/periodontitis/symptoms-causes/syc-20354473 (Accessed August 30, 2024).
3. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: national health and nutrition examination survey 2009–2014. J Am Dent Assoc. (2018) 149:576–88.e6. doi: 10.1016/j.adaj.2018.04.023
4. Oral health across the lifespan: working-age adults. In: Oral Health in America: Advances and Challenges. Bethesda, MD: National Institute of Dental and Craniofacial Research (US) (2021). Available online at: https://www.nidcr.nih.gov/research/oralhealthinamerica/section-3a-summary (Accessed August 30, 2024).
5. Kaplia YL. Oral health's Inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. (2021) 87(1):11–6. doi: 10.1111/prd.12398
6. Sanz M, del Castillo AM, Jepsen S, Gonzalez-Jaunatey JR, D’Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodont. (2020) 47:268–8. doi: 10.1111/jcpe.13189
7. Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. (2019) 20:1414–27. doi: 10.3390/ijms20061414
8. Wu C-Z, Yuan Y-H, Liu H-H, Li S-S, Zhang B-W, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. (2020) 20:204–19. doi: 10.1186/s12903-020-01180-w
9. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. (2011) 7:738–48. doi: 10.1038/nrendo.2011.106
10. Baeza M, Moarales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. (2020) 28:e20190248. doi: 10.1590/1678-7757-2019-0248
11. Teeuw WJ, Gerdes VEA, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients. Diabetes Care. (2010) 33:421–7. doi: 10.2337/dc09-1378
12. Venkataraman A, Almas K. Rheumatoid arthritis and periodontal disease. An update. NY State Dent J. (2015) 81:30–6.
13. Beydoun MA, Beydoun HA, Hossain S, El-Hajj ZW, Weiss J, Zonderman AB. Clinical and bacterial markers of periodontitis and their association with incident all-cause and Alzheimer’s disease dementia in a large national survey. J Alzheimers Dis. (2020) 75:157–72. doi: 10.3233/JAD-200064
14. Meyer MS, Joshipura K, Giovanucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. (2008) 19:895–907. doi: 10.1007/s10552-008-9163-4
15. Kajihara R, Sakai H, Han Y, Amari K, Kawamoto M, Hakoyama Y, et al. Presence of periodontitis may synergistically contribute to cancer progression via Treg and IL-6. Sci Rep. (2022) 12:11584. doi: 10.1038/s41598-022-15690-w
16. Eberhard J, Grote K, Luchtefeld M, Heuer W, Schuett H, Divchev D, et al. Experimental gingivitis induces systemic inflammatory markers in young healthy individuals: a single-subject interventional study. PLoS One. (2013) 8:e55265. doi: 10.1371/journal.pone.0055265
17. Hogan R, Goodwin M, Boothman N, Iafolla T, Pretty IA. Further opportunities for digital imaging in dental epidemiology. J Dent. (2018) 74(Suppl 1):S2–9. doi: 10.1016/j.jdent.2018.04.018
18. DentistryIQ. American Dental Association, Crest, Oral-B: national public opinion survey reveals findings on oral health-care perceptions. Available online at: https://www.dentistryiq.com/practice-management/industry/article/16369987/national-public-opinion-survey-reveals-findings-on-oral-health-care-perceptions (Accessed August 30, 2024).
19. Seshan H, Shwetha M. Gingival inflammation assessment: image analysis. J Ind Soc Periodontol. (2012) 16:231–4. doi: 10.4103/0972-124X.99267
20. Rana A, Yauney G, Wong LC, Gupta O, Muftu A, Shah P. Automated segmentation of gingival diseases oral images. 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT). doi: 10.1109/HIC.2017.8227605
21. Joo J, Jeong S, Jin H, Lee U, Yoon JY, Kim SC. Periodontal disease detection using convolutional neural networks. 2019 International Conference on Artificial Intelligence in Information and Communication (ICAIIC)). doi: 10.1109/ICAIIC.2019.8669021
22. Moriyama Y, Lee C, Date S, Kashiwagi Y, Narukawa Y, Nozaki K, et al. A MapReduce-like deep learning model for the depth estimation of periodontal pockets. Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019). p. 388–95. doi: 10.5220/0007405703880395
23. Chen Y, Chen X. Gingivitis identification via GLCM and artificial neural network. Conference Proceedings: Medical Imaging and Computer-aided diagnosis Lecture Notes in Electrical Engineering (2020). p. 95–106. doi: 10.1007/978-981-15-5199-4_10
24. Alalharith DM, Alharthi HM, Alghamdi WM, Alsenbel YM, Aslam N, Khan IU, et al. A deep learning-based approach for the detection of early signs of gingivitis in orthodontic patients using faster region-based convolutional neural networks. Int J Environ Res Public Health. (2020) 17:8447. doi: 10.3390/ijerph17228447
25. Liu L, Xu J, Huan Y, Zou Z, Yeh S-C, Zheng L-R. A smart dental health-iot platform based on intelligent hardware, deep learning, and mobile terminal. IEEE J Biomed Health Informatics. (2020) 24:898–906. doi: 10.1109/JBHI.2019.2919916
26. Guo SL, Chen Y, Mallineni SK, Huang SY, Liu BW, Zhang SY, et al. Feasibility of oral health evaluation by intraoral digital photography: a pilot study. Int Med Res. (2021) 49:300060520982841. doi: 10.1177/0300060520982841
27. Shrivastava D, Srivastava KC, Ganji KK, Alam MK, Al Zoubi I, Sghaireen MG. Quantitative assessment of gingival inflammation in patients undergoing nonsurgical periodontal therapy using photometric CIELab analysis. BioMed Res Int. (2021) 2021(1):6615603. doi: 10.1155/2021/6615603
28. Li W, Liang Y, Zhang X, Liu C, He L, Miao L, et al. A deep learning approach to automatic gingivitis screening based on classification and localization in RGB photos. Sci Rep. (2021) 11:16831. doi: 10.1038/s41598-021-96091-3
29. Ginesin O, Zigdon-Giladi H, Gabay E, Machtei EE, Mijiritsky E, Mayer Y. Digital photometric analysis of gingival response to periodontal treatment. J Dent. (2022) 127:104331. doi: 10.1016/j.jdent.2022.104331
30. Kim H-N, Kim K, Lee Y. Intra-oral photograph analysis for gingivitis screening in orthodontic patients. Int J Environ Res Public Health. (2023) 20:3705. doi: 10.3390/ijerph20043705
31. Kurt-Bayrakdar S, Ugurlu M, Yavuz MB, Sali N, Bayrakdar IS, Celik O, et al. Detection of tooth numbering, frenulum attachment, gingival overgrowth, and gingival inflammation signs on dental photographs using convolutional neural network algorithms: a retrospective study. Quintessence Int. (2023) 54:680–93. doi: 10.3290/j.qi.b4157183
32. Liu Y, Cheng Y, Song Y, Cai D, Zhang N. Oral screening of dental calculus, gingivitis and dental caries through segmentation on intraoral photographic images using deep learning. BMC Oral Health. (2024) 24:1287. doi: 10.1186/s12903-024-05072-1
33. Wen C, Bai X, Yang J, Li S, Wang X, Yang D. Deep learning based approach: automated gingival inflammation grading model using gingival removal strategy. Sci Rep. (2024) 14:19780. doi: 10.1038/s41598-024-70311-y
34. Li W, Guo E, Zhao H, Li Y, Miao L, Liu C, et al. Evaluation of transfer ensemble learning-based convolutional neural network models for the identification of chronic gingivitis from oral photographs. BMC Oral Health. (2024) 24:814. doi: 10.1186/s12903-024-04460-x
35. Alam MK, Alanazi NH, Alshehri ADA, Chowdhury F. Accuracy of Al algorithms in diagnosing periodontal disease using intraoral images. J Pharm Bioallied Sci. (2024) 16(Suppl 1):S583–S5. doi: 10.4103/jpbs.jpbs_873_23
36. Chau RCW, Cheng ACC, Mao K, Thu KM, Ling Z, Tew IM, et al. External validation of an AI mHealth tool for gingivitis detection among older adults at daycare centers: a pilot study. Int Dental J. (2025) 75:1970–8. doi: 10.1016/j.identj.2025.01.008
37. Vaughan M, Mheissen S, Cobourne M, Ahmed F. Diagnostic accuracy of artificial intelligence for dental and occlusal parameters using standardized clinical photographs. Am J Orthod Dentofac Orthop. (2025) 167:733–40. doi: 10.1016/j.ajodo.2025.01.017
38. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. doi: 10.1038/nrdp.2017.38
Keywords: epidemiologic studies, inflammatory gum disease, dental health, chronic diseases, remote assessment
Citation: Terry PD, Wilson OL, Heaton ML, Triplett O, Heidel RE and Dhand R (2025) Accuracy of digital photographs for assessing inflammatory gum disease in epidemiologic studies. Front. Oral Health 6:1667604. doi: 10.3389/froh.2025.1667604
Received: 16 July 2025; Accepted: 28 July 2025;
Published: 2 September 2025.
Edited by:
Luis Proença, Instituto Universitário Egas Moniz, PortugalReviewed by:
Walter Y. H. Lam, The University of Hong Kong, Hong Kong SAR, ChinaShilpa Duseja, Narsinhbhai Patel Dental College & Hospital, India
Copyright: © 2025 Terry, Wilson, Heaton, Triplett, Heidel and Dhand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul D. Terry, cHRlcnJ5QHV0bWNrLmVkdQ==
 Paul D. Terry
Paul D. Terry O. Lee Wilson2
O. Lee Wilson2 R. Eric Heidel
R. Eric Heidel Rajiv Dhand
Rajiv Dhand