- 1Faculty of Graduate Studies, AL-Quds University, Jerusalem, Palestine
- 2Faculty of Medicine, Al-Quds University, Jerusalem, Palestine
- 3Faculty of Medicine and Health Sciences, An-Najah National University, Nablus, Palestine
- 4Oral Health Research and Promotion Unit, Al-Quds University, Jerusalem, Palestine
Objectives: This study aimed to assess the impact of knowledge, attitudes, and practices (KAP) related to oral health on oral health-related quality of life (OHRQoL) among patients with type 2 diabetes mellitus (T2DM).
Methods: A cross-sectional study was conducted from July 2023 to July 2024 in primary healthcare centers in the West Bank, using cluster sampling to select participants from three geographic regions A convenience sample was drawn from participants aged 40 years and older who were diagnosed with (T2DM). A structured validated Arabic questionnaire was employed to collect data on socio-demographic characteristics, oral health knowledge, attitudes, practices, and OHRQoL, using validated scales such as the OHIP-14.
Results: The results showed that the mean OHRQoL score was 17.84 ± 11.65 (range 0–50), the primary domains negatively impacting participants' oral health-related quality of life were psychological discomfort, social disability, and handicap. Key oral health problems reported included dry mouth (62.2%), tooth loss (48.6%), and caries (46.1%). Knowledge scores averaged 6.53 ± 2.07 (range 1–10) attitudes scores were 4.88 ± 1.65 (range 0–6), and practices scores were 1.99 ± 1.02 (range 0–6). Spearman's correlation analysis revealed significant positive correlations between practice and knowledge (ρ = 0.160, P = 0.000), practice and attitude (ρ = 0.171, P = 0.000), and Knowledge and attitude (ρ = 0.238, P = 0.000). In the final model, predicting factors to improve OHRQoL were full-time employment, better income, and positive attitude, while poorer OHRQoL was predicted by pain reason to visit dentist, discussion with a dentist about diabetes and oral complications, poor general health status, poor oral health status, lower educational level, no history of diabetes and long duration of to do HbA1c test.
Conclusion: This study highlights that positive attitudes significantly improve OHRQoL in diabetic patients, while poor outcomes relate to socioeconomic and health system barriers. Despite good knowledge, practices remain inadequate. Integrating oral health into diabetes care, improving access, and addressing social determinants are essential for enhancing overall quality of life in this population.
Introduction
Oral health is closely connected to overall health, as both share common risk factors such as tobacco use, poor diet, physical inactivity, and alcohol consumption (1, 2). Oral diseases are strongly linked to the four major non-communicable diseases (NCDs)—cardiovascular disease, cancer, diabetes, and chronic respiratory illnesses (1, 2). Recognizing this, the World Health Organization (WHO), in its 2022 Global Oral Health Status Report, emphasized the critical need to integrate oral health into strategies addressing NCDs and Universal Healthcare Coverage (UHC) (3). This integration is vital to ensuring comprehensive healthcare that addresses broader determinants of health, improves outcomes for individuals with NCDs, and reduces the global burden of oral diseases.
Diabetes Mellitus (DM) is a chronic metabolic condition that develops when the pancreas cannot produce insulin or when the body cannot effectively use the insulin produced (4). Insulin regulates blood glucose levels (4), and the aetiology of DM is multifactorial, involving both genetic and environmental factors (5). The American Diabetes Association (ADA) classifies diabetes into different types, including type 1 diabetes (insulin deficiency) and type 2 diabetes (insulin resistance) (6). In 2021, the International Diabetes Federation (IDF) reported that 537 million people aged 20–79 had diabetes worldwide, a number projected to increase to 643 million by 2030, 783 million by 2045 (7), and 1.31 billion by 2050 (8). Type 2 diabetes mellitus (T2DM) accounts for 90%–95% of all cases globally (9).
For most adults with diabetes, uncontrolled disease is indicated by an HbA1c of 7% or higher, while values above 9% represent very poor control (10). Poorly managed diabetes can result in severe complications such as kidney disease, cardiovascular disease, and diabetic foot ulcers (11). Oral health complications are also common, including gingivitis, periodontitis, xerostomia, fungal infections, plaque accumulation, delayed wound healing, and altered taste (11). These issues are largely associated with diabetes-related microvascular and macrovascular damage (11). Hyperglycaemia has been shown to share a bidirectional relationship with periodontal disease: poor glycaemic control worsens periodontal status, while periodontal inflammation can impair diabetes management (12). Preventive measures—such as regular toothbrushing, flossing, dental visits, and smoking cessation—can mitigate these complications (9, 13). Nevertheless, adherence to such practices is often hindered by limited knowledge, financial barriers, and restricted access to dental care, particularly in underserved populations (14).
Health-related quality of life (HRQoL) reflects how illness and its management affect patients' physical, psychological, and social well-being (15). Within this framework, oral health-related quality of life (OHRQoL) has emerged as an important yet underexplored dimension, highlighting the impact of oral conditions on daily life (16). Most studies suggest a negative association between diabetes and OHRQoL (17, 18), though some report no significant effect (19, 20). Effective dental management and patient education remain essential for reducing oral complications and improving OHRQoL.
Patients' knowledge, attitudes, and practices (KAP) related to oral health are key determinants of OHRQoL, offering valuable insights for preventive strategies and awareness programs (21). A scoping review of diabetes in South Asia found that strengthening knowledge and fostering positive attitudes can enhance oral health practices (22). Moreover, lower education levels and poorer general health are linked to reduced OHRQoL among the elderly (23), while higher education correlates with better health knowledge in diabetic populations (24). Despite this, evidence shows that many individuals with diabetes have limited awareness of oral complications and receive inadequate oral health guidance from their care providers (22). Knowledge of periodontal risk is often lower than knowledge of other complications (25), and patients are generally more informed about systemic complications than oral manifestations (12).
Although studies in Palestine and neighbouring regions have examined diabetes-related KAP, general quality of life (26–28), and oral health in diabetic patients (29, 30), these aspects have largely been studied in isolation. No research in Palestine has specifically assessed how oral health-related KAP influences OHRQoL among patients with T2DM. Addressing this gap is critical, as better understanding these associations can guide targeted health education, improve preventive strategies, and inform Ministry of Health (MoH) policies.
Therefore, the present study aims to evaluate the relationship between oral health-related KAP, oral health behaviours, and OHRQoL among T2DM patients attending MoH primary healthcare centers in the West Bank. Additionally, it seeks to explore the influence of demographic, socioeconomic, and dental care access factors on these outcomes, and to analyse different domains of the OHIP-14 in relation to overall OHRQoL.
Methods
Study design and setting
A cross-sectional study was conducted in the primary healthcare centers (PHCs) of the Palestinian Ministry of Health (MoH) in the West Bank from July 2023 to July 2024. The West Bank was selected due to logistical feasibility, the concentration of MoH PHCs, and the availability of reliable patient records, which facilitated standardized data collection. While this limits generalizability to other Palestinian regions such as Gaza, findings are expected to be broadly indicative of the West Bank population.
Sampling strategy
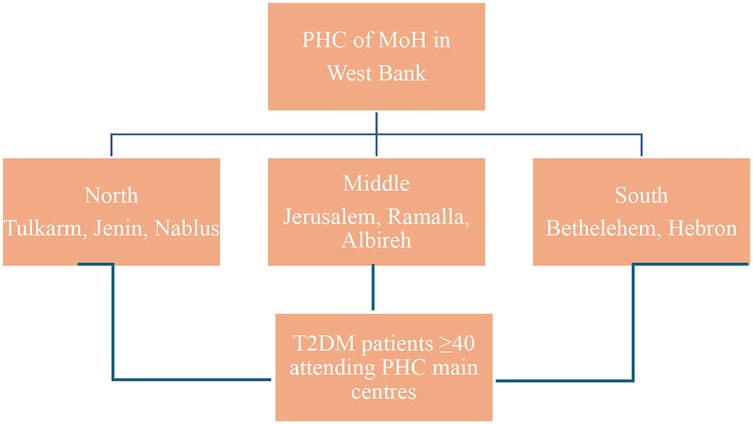
A cluster sampling approach was used to select governorates representing the three main geographic areas of the West Bank: North, Central, and South. The selected governorates were Jenin, Nablus, and Tulkarm (North); Jerusalem, Ramallah, and Al-Bireh (Central); and Bethlehem and Hebron (South) (Figure 1). PHCs within each governorate were purposively selected based on patient volume, prioritizing clinics with the highest number of registered type 2 diabetes mellitus (T2DM) patients to ensure adequate recruitment.
The West Bank has approximately 493 in total primary healthcare centers (PHCs), accounting for 64.3% of all healthcare centers in the country (31), with 375 PHCs in the selected governorates;10 centers were purposively selected based on the highest patient volume of type 2 diabetes mellitus (T2DM) patients, to ensure efficient recruitment and adequate sample size. The selected centers included:
• North: Jenin main PHC, Nablus main PHC and another PHC clinic, Tulkarm main PHC.
• Central: Ramallah (Al-Bireh) main PHC, Jerusalem main PHC.
• South: Bethlehem main PHC, three main PHC clinics in Hebron.
Participants were recruited consecutively from these centers. Inclusion criteria were patients aged 40 years or older, diagnosed with T2DM for at least 6 months, and able to provide informed consent. Exclusion criteria were patients with type 1 diabetes mellitus (T1DM), prediabetes, gestational diabetes, secondary diabetes, or undiagnosed diabetes were excluded.
Sample size calculation
Based on an estimated T2DM prevalence of 20% in the West Bank [Markov model study (32)] and a population of 3.25 million (Palestinian Central Bureau of Statistics, 2023), the minimum required sample size was calculated using the Epitools online calculator with a 5% margin of error and 95% confidence level. The estimated sample size was 457, which was increased to 508 to account for a potential 10% nonresponse rate.
Conceptual framework
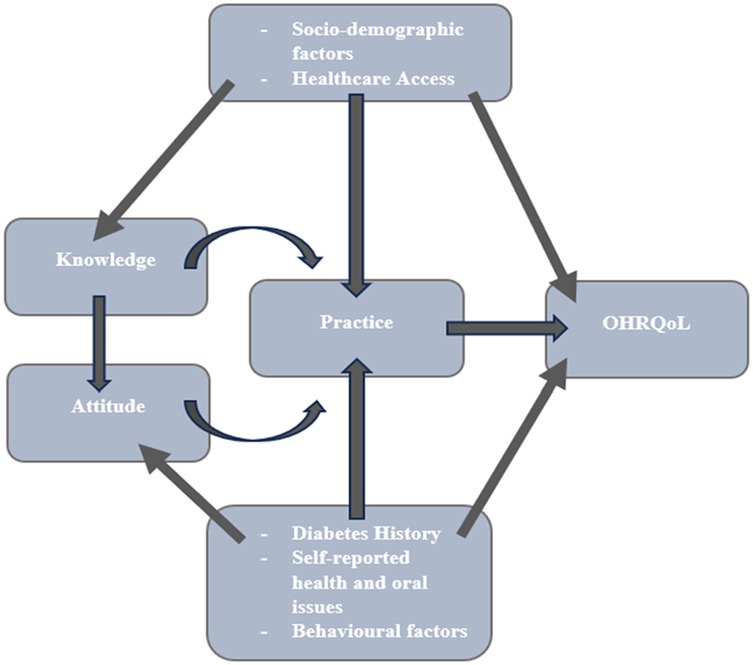
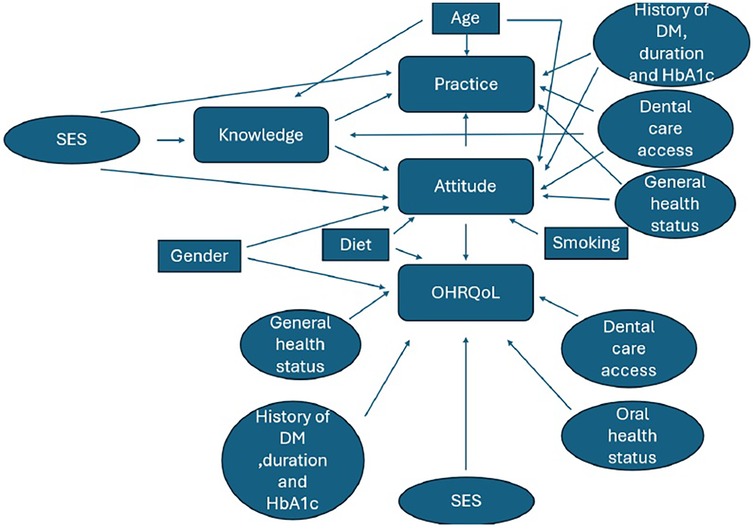
The study's conceptual framework (Figure 2) was adapted from previously published models on oral health-related quality of life (OHRQoL), diabetes knowledge, attitudes, and practices (KAP) (33–35). It was not newly developed but contextualized to the Palestinian setting, with cultural and demographic adjustments. All adaptations were based on validated instruments referenced in the literature.
Data collection and study tool
Data were collected through in-person structured interviews using a pre-tested Arabic questionnaire implemented via Google Forms. Participants were briefed on study aims, benefits, and confidentiality, and verbal informed consent was obtained. The study was approved by the Al-Quds University Ethics Committee and the Palestinian MoH.
The questionnaire had five sections covering demographics, diabetes history, KAP, dietary habits, oral hygiene practices, complications, and OHRQoL. The OHIP-14 instrument, previously validated in Arabic (36, 37), measured OHRQoL. Forward-backward translation ensured cultural and linguistic accuracy, and expert review (two dentists, a nurse researcher, and a public health researcher) refined the questions. Pilot testing with 45 patients demonstrated readability, comprehension, and internal consistency (Cronbach's α = 0.87).
HbA1c measurement
HbA1c data were obtained from patient medical records at each PHC, reflecting the most recent laboratory measurement within the past 3 months. This ensured standardized and accurate glycaemic control data for all participants.
Data collector training and qualifications
Field researchers were selected based on prior experience in healthcare data collection, educational background in medical, research and public health, and proximity to target governorates. All were trained in standardized interview techniques through two Zoom calibration sessions led by the principal investigator. Ongoing supervision was provided via phone and messaging to ensure consistency in data collection, ethical conduct, and adherence to study protocols.
Data analysis
Descriptive & normality testing
Means, SDs, frequencies, and percentages summarized participants' demographics, diabetes history, health status, and healthcare access. Normality of KAP (Knowledge, Attitude, Practice) and OHRQoL was tested using Kolmogorov–Smirnov and Shapiro–Wilk; p < 0.05 indicated non-normality, so non-parametric tests were applied. Analyses were conducted in IBM SPSS 26.0.
Scoring & composite variables
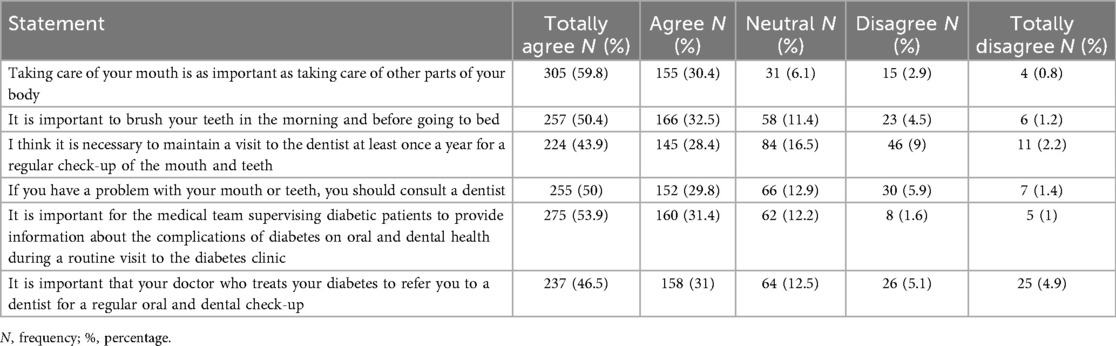
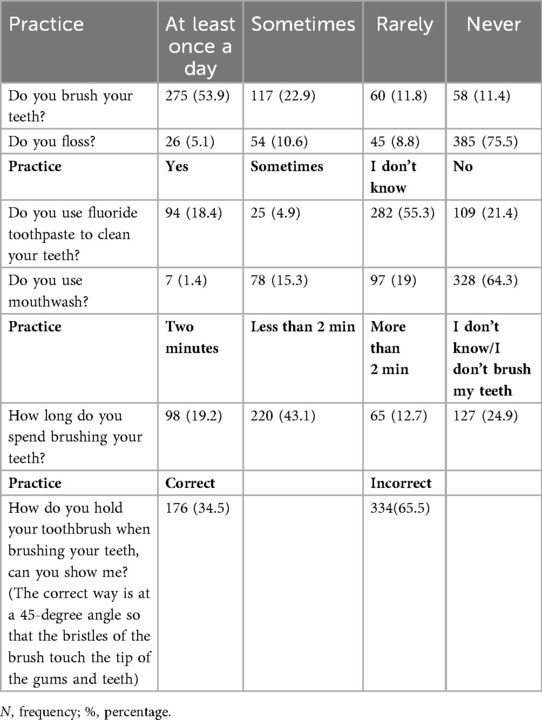
Knowledge: Correct = 1, Incorrect = 0. Total 0–10 (Higher = better knowledge). Attitude: “Agree/Totally agree” = 1; others = 0. Total 0–6 (Higher = positive attitude). Oral Hygiene Practices: Toothbrushing/flossing ≥1/day, correct technique, 2 min brushing, and fluoride/mouthwash use = 1; others = 0. Total 0–6 (Higher = better hygiene). Dietary Habits: Recoded to reflect poorer habits with higher scores (0 = good, 1 = fair, 2 = poor). Oral & General Complications: Yes = 1, No/Not sure = 0. (Higher = more complications). OHRQoL (OHIP-14): Score 0–56 (Higher = poorer quality of life), for the 7 domains, each 0–8 scores (Higher = poorer quality of life).
Bivariate analysis
Spearman's correlation, Mann–Whitney U and Kruskal–Wallis tests were used.
Multivariate analysis
Stepwise multiple linear regression assessed predictors of OHRQoL (continuous). Predictors: significant bivariate variables + theoretically relevant ones (Knowledge, Practice, Age, Smoking, Family history, Last dental visit, Education program attendance). Categorical predictors were dummy-coded; 51 predictors included. Stepwise results confirmed with forward regression.
Collinearity & model fit
No multicollinearity (VIF < 1.21, Tolerance > 0.82). Model fit evaluated via adjusted R2. Significance set at p < 0.05 (two-tailed).
Results
Socio-demographic and behavioural characteristics of participants
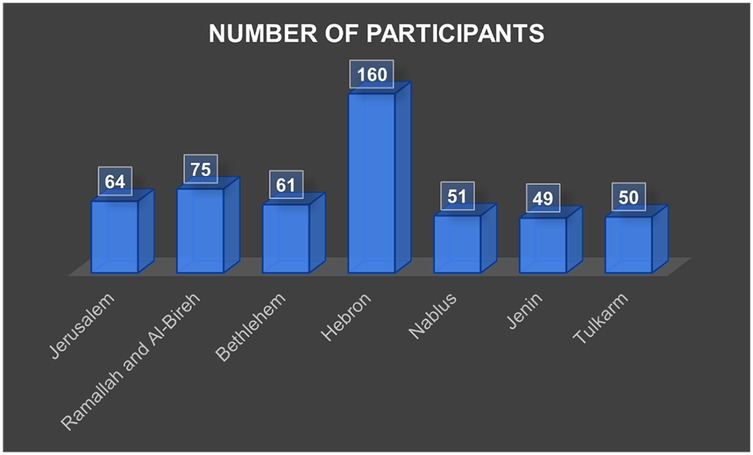
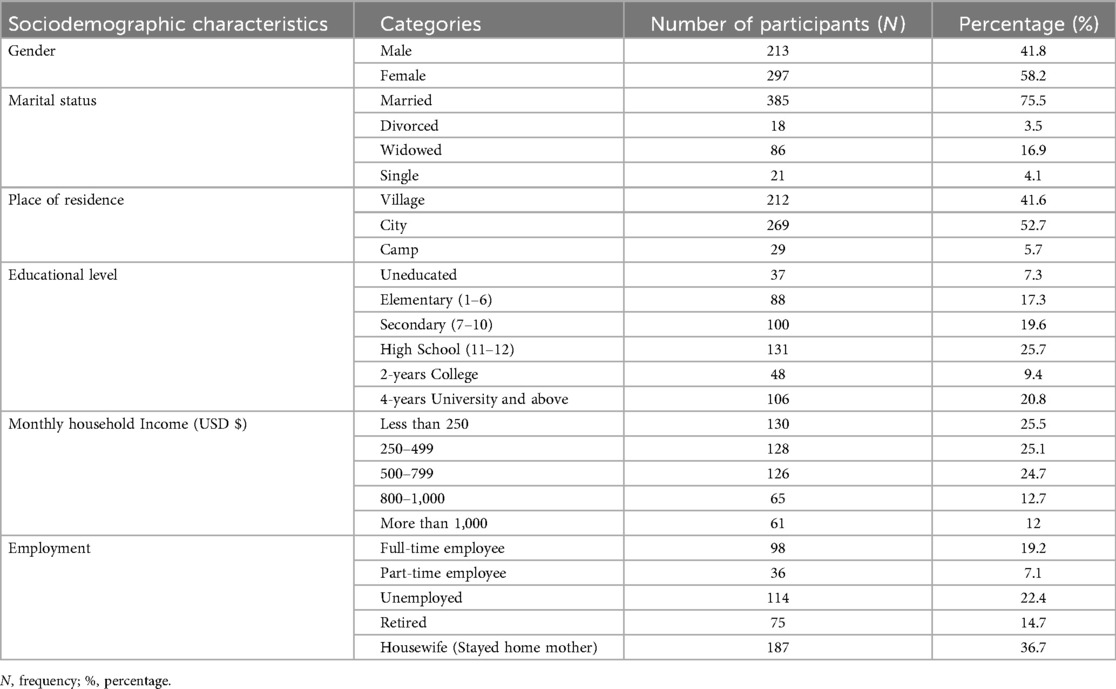
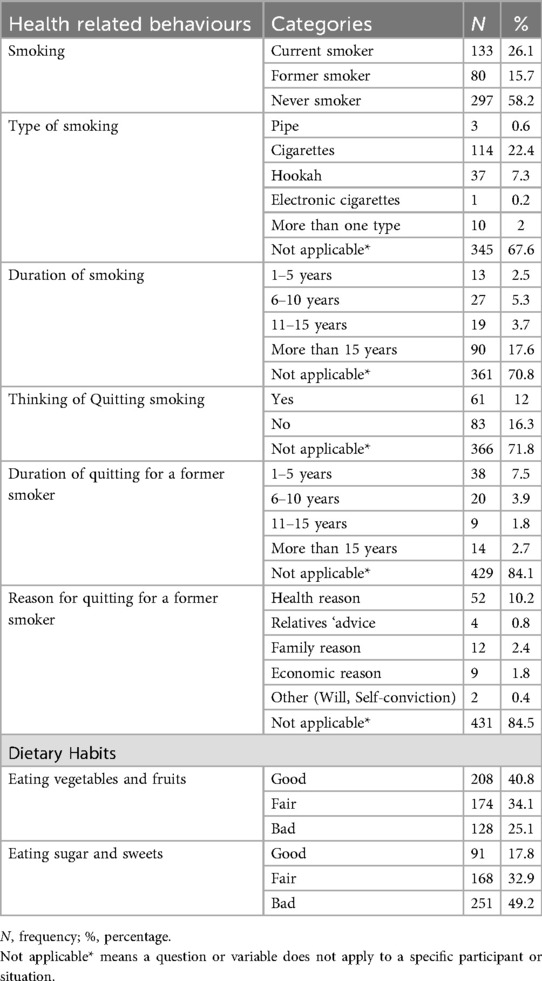
A total of 510 participants completed the study. The mean age was 60.4 ± 9.3 years (range 40–85), with 58.2% females. Hebron contributed the largest proportion of participants (31.4%), consistent with its size and distribution of PHCs (Figure 3). Most participants were married (75.5%), lived in cities (52.7%), and had completed high school or higher education (46.5%). Monthly household income was below $500 for half of the sample (50.6%). Current smokers comprised 26.1%, and 40.8% reported regular consumption of fruits and vegetables, whereas 49.2% reported frequent sugar intake (Tables 1, 2).
Patient's diabetes history, self-reported health issues and self-reported oral complications
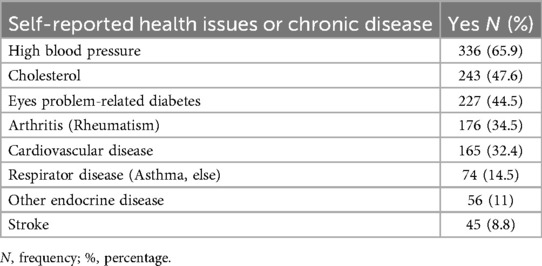
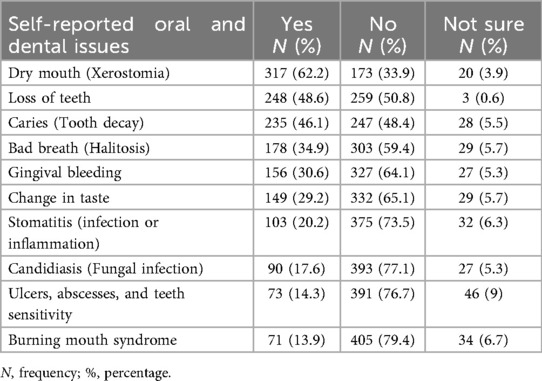
A majority (70.2%) had a first-degree relative with diabetes. Mean HbA1C was 8.12 ± 1.78, with 64.5% of participants classified as uncontrolled. Most participants were on diabetes medication (92.9%), primarily oral hypoglycaemics. Common comorbidities included hypertension (65.9%) and hypercholesterolemia (47.6%). Oral complications were frequent, with xerostomia (62.2%), tooth loss (48.6%), and caries (46.1%) being most reported (Tables 3, 4).
Access to dental care and to information about oral health
Nearly all participants had insurance (95.9%), predominantly public. Satisfaction with PHC services was moderate (72.2%), with medication availability and waiting times as main concerns. Only 17.6% attended diabetes education programs, and 43.5% discussed diabetes with their dentist (Table 5).
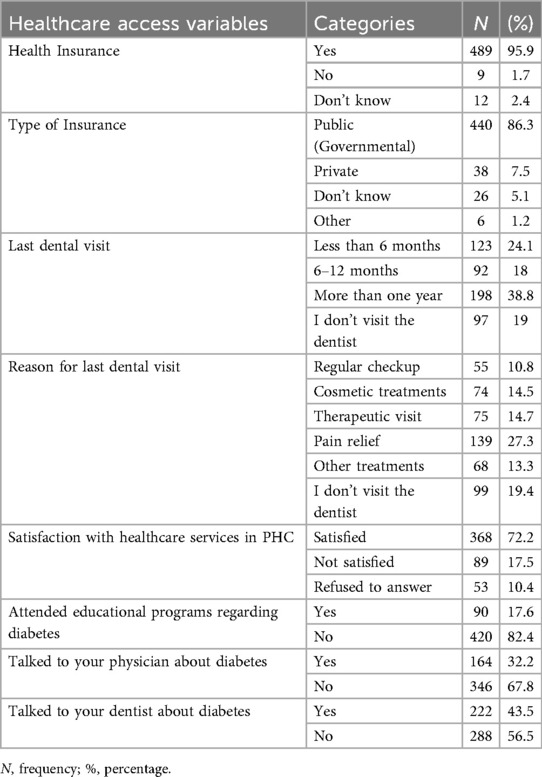
Table 5. Access to healthcare service, satisfaction with provided services and access to health and oral health information.
Knowledge, attitude, and hygiene practice (KAP) related to diabetes
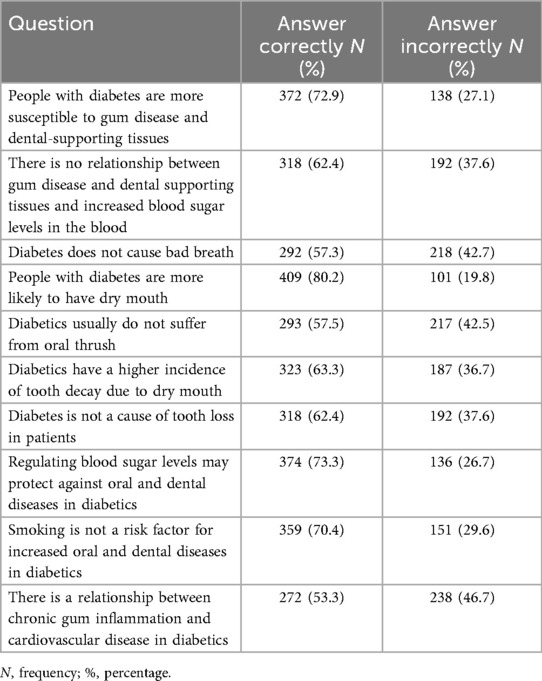
Mean composite scores were knowledge 6.53 ± 2.07, attitude 4.88 ± 1.65, and oral hygiene practices 1.99 ± 1.02. Higher knowledge and positive attitudes were significantly correlated with better oral hygiene practices (ρ = 0.160–0.238, p < 0.001). Education, income, city residence, regular dental visits, and attending educational programs were the strongest predictors of better KAP (Tables 6–8).
Oral health-related quality of life (OHRQoL)
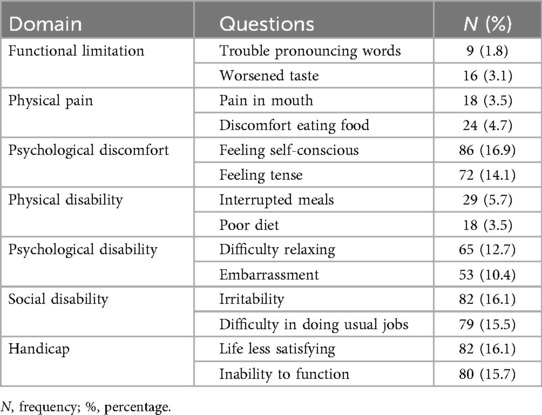
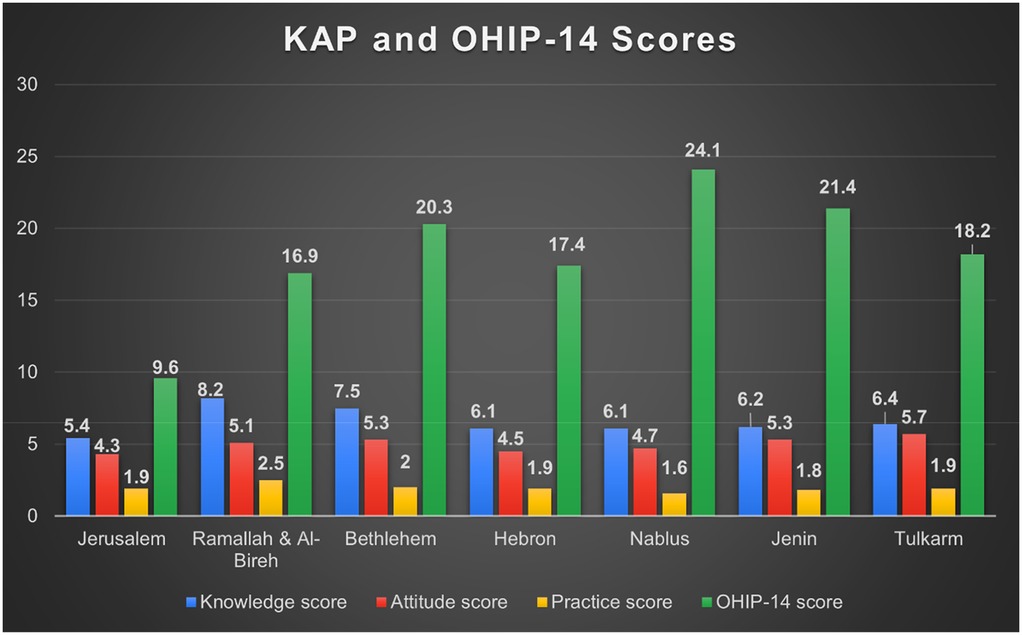
Mean OHIP-14 score was 17.84 ± 11.65, indicating moderate impact. Psychological discomfort, social disability, and handicap domains were most affected (Table 9). While (Figure 4) illustrates the distribution of KAP and OHIP-14 scores across governorates.
Bivariate analysis
Spearman's correlation revealed significant positive relationships between Knowledge, Attitudes, and Practices:
Knowledge ↔ Oral hygiene practices (ρ = 0.160, p < 0.001)
Attitudes ↔ Oral hygiene practices (ρ = 0.171, p < 0.001)
Knowledge ↔ Attitudes (ρ = 0.238, p < 0.001)
These findings suggest that better knowledge and positive attitudes are linked to improved oral health practices.
Predictors of better oral health knowledge
Clinically relevant predictors of higher knowledge included:
Younger age (ρ = −0.190, p < 0.001)
Urban residence [H(2) = 11.4, p < 0.001]
Higher education (ρ = 0.263, p < 0.001) and higher income (ρ = 0.121, p < 0.05)
Full-time employment [H(4) = 12.1, p < 0.05]
Regular dental visits, especially routine check-ups (H = 38.6–56.2, p < 0.001)
Attending diabetes education programs (U = 23,034.5, p < 0.001)
Discussing oral health with healthcare providers (physician: U = 33,341.5, dentist: U = 39,829, p < 0.001)
Lower knowledge was associated with longer diabetes duration and extended intervals since the last dental visit.
Predictors of positive attitudes
Positive attitudes toward oral health were more likely among:
Younger participants (ρ = −0.142, p < 0.001) and females (U = 35,820.5, p < 0.05)
Urban residents [H(2) = 20.9, p < 0.001]
Higher education and income (ρ = 0.189, H = 21.1–23.7, p < 0.001)
Full-time employment [H(4) = 12.4, p < 0.05]
Healthy diet and non-smoking (H = 9.48–28.3, p < 0.001)
Controlled diabetes and family history of diabetes [U = 25,484.5; H(3) = 13.59, p < 0.05]
Regular dental visits and satisfaction with healthcare services (H = 7.06–48.7, p < 0.05)
Engagement in educational programs and discussions with providers (U = 22,485.5–38,038, p < 0.001)
Negative predictors included older age, longer diabetes duration, higher HbA1C, cumulative sugar results, and poorer general health.
Predictors of favourable oral hygiene practices
Better oral hygiene practices were associated with:
Higher education and income (ρ = 0.188–0.282, p < 0.001)
Full-time employment [H(4) = 34.7, p < 0.001]
Younger age (ρ = −0.278, p < 0.001)
Regular dental visits, particularly for check-ups or therapeutic reasons (H = 26.4, p < 0.001)
Controlled diabetes (U = 25,922.5, p < 0.05)
Negative predictors included longer diabetes duration, poorer general health, higher cumulative sugar test results, and infrequent dental visits.
Predictors of oral health-related quality of life (OHRQoL)
Poorer OHRQoL (higher OHIP-14 scores) was associated with:
Female gender (U = 36,287.5, p < 0.05)
Unemployment [H(4) = 25.35, p < 0.001]
Uncontrolled diabetes (higher HbA1C, U = 34,421.5, p < 0.05)
Worse general health and more diabetes-related oral health issues (ρ = 0.252–0.407, p < 0.001)
Dental visits for pain relief or discussions about diabetes with dentists/physicians (U = 32,919–36,925, H = 25.65, p < 0.05)
Better OHRQoL was observed in participants with higher education and income, positive attitudes, better oral hygiene practices, and controlled diabetes.
OHIP-14 domains
Across the seven OHIP-14 domains, clinically relevant findings included:
Functional Limitation & Physical Pain: Worse outcomes in participants who had not attended educational programs or consulted healthcare providers, and those visiting dentists for pain relief or never visiting.
Psychological Discomfort & Disability: Associated with higher HbA1C, more oral health issues, and poorer general health.
Social Disability & Handicap: Worse outcomes in participants attending educational programs, discussing diabetes with providers, or visiting for pain relief, but better outcomes among those with higher education, income, and positive attitudes.
Bivariate analysis of the different domains of OHIP-14
Significant differences (p < 0.05) in mean scores across the seven domains of OHIP-14 were identified using Mann–Whitney and Kruskal–Wallis tests. These differences were associated with participants' engagement in diabetes-related educational programs, discussions with healthcare providers about their condition, and the reason for their recent dental visit.
OHIP-14 domain outcomes
Analysis of OHIP-14 domains revealed several consistent predictors of oral health-related quality of life (OHRQoL) among participants.
Functional limitation was significantly worse among individuals who had not attended diabetes education programs, had not discussed their condition with healthcare providers, or sought dental care only for pain. Elevated HbA1c, self-reported diabetes-related oral complications, and poorer general health were also linked to greater limitations, whereas positive attitudes toward oral health were protective.
Physical pain was more pronounced in participants with uncontrolled diabetes, oral complications, and poor health status, while higher education and preventive dental visits were associated with less pain.
Psychological discomfort was unexpectedly higher among those who attended education programs or discussed their condition with physicians or dentists, likely reflecting heightened awareness of oral health risks. Discomfort was further exacerbated by poor glycemic control, oral complications, and poor health status, but mitigated by higher income and more frequent dental visits.
Physical disability was strongly associated with poor glycemic control, oral complications, and lower socioeconomic status. Protective factors included higher income, positive attitudes, and better oral hygiene practices.
Psychological disability was more common among participants with higher HbA1c, poor general health, and oral complications, but was significantly reduced in those with higher education and income.
Social disability was elevated among participants with uncontrolled diabetes, oral complications, or lower socioeconomic status. By contrast, higher education and income were associated with reduced social disability.
Handicap scores were higher among older participants and those with poor glycemic control, poor health status, or oral complications. In contrast, higher education, better income, positive attitudes, and healthier oral practices were protective.
Overall predictors of OHRQoL
Across domains, three predictors consistently emerged as the most clinically relevant for poorer OHRQoL:
• Poor glycemic control (higher HbA1c levels).
• Presence of diabetes-related oral complications.
• Lower socioeconomic status (education and income).
Conversely, positive attitudes, healthier oral practices, and proactive dental care were protective across multiple domains.
Multivariable analysis for OHRQoL variable (OHIP-14 scores)
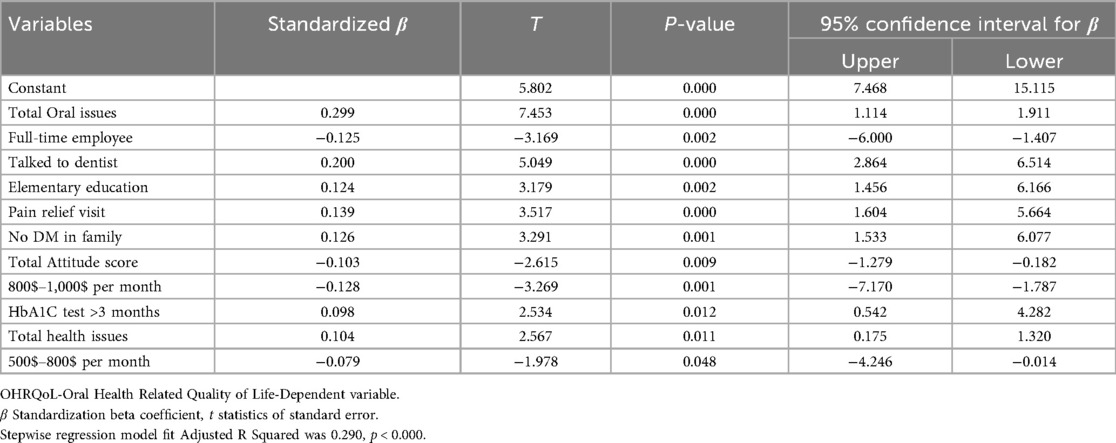
A total of 51 variables were entered into multiple linear regression with stepwise method and confirmed with forward method. Eleven models were produced, and model number 11 with eleven variables was selected with R2 was 0.306 and adjusted R2 was 0.290, p value < 0.000 and F change was 19.95. Predicted variables for poor or good oral health related quality of life are shown in Table 10.
Factors associated with poorer OHRQoL (higher OHIP-14 scores)
• Presence of diabetes-related oral complications (β = 0.299, p < 0.001).
• Poorer general health status (β = 0.104, p < 0.05).
• Having discussed diabetes with a dentist (β = 0.200, p < 0.001).
• Lower educational level (elementary only) (β = 0.124, p < 0.001).
• Dental visits primarily for pain relief (β = 0.139, p < 0.001).
• Lack of family history of diabetes (β = 0.126, p < 0.001).
• Delayed HbA1c monitoring (>3 months) (β = 0.098, p < 0.05).
Factors associated with better OHRQoL (lower OHIP-14 scores)
• Full-time employment (β = −0.125, p < 0.05).
• Positive attitudes toward oral health (β = −0.103, p < 0.05).
• Higher monthly income ($500–800: β = −0.079, p < 0.05; $800–1,000: β = −0.128, p < 0.001).
These findings highlight the interplay of clinical (oral and general health, HbA1c control), psychosocial (attitudes, education, family history), and structural (employment, income, healthcare-seeking behavior) factors in shaping OHRQoL in diabetic patients.
Figure 5 depicts the newly proposed conceptual model, which was constructed according to the significant findings and interrelationships among the study variables.
Discussion
This study is the first to assess OHRQoL among diabetic patients in the West Bank and examine its relationship with knowledge, attitudes, practices, and sociodemographic and health-related factors. It employed a robust methodology, including a validated tool, standardized data collection, and a representative national sample, ensuring the findings are reliable and generalizable.
This study applied the Knowledge-Attitude-Practice (KAP) model to explore oral health and OHRQoL in diabetic patients. In line with previous studies (38–40), our findings show that even though diabetic patients demonstrated moderate knowledge and positive attitudes toward oral health, these did not consistently translate into proper oral hygiene practices. This suggests that knowledge and attitude alone are insufficient to change behavior. Interventions should therefore go beyond education to include strategies that facilitate behavior change, such as structured oral health programs, reminders, and improved access to dental care, to effectively promote better oral health practices among diabetic patients. A positive attitude was strongly linked to higher OHRQoL, over 60% of participants acknowledged the importance of oral health, this result is similar to other research on diabetic patients (25, 41).
Sociodemographic factors influenced outcomes: younger, educated, and higher-income participants had better oral health knowledge, as shown in previous literature about KAP in Tanzania and Pakistan (39, 42), while employed, educated females demonstrated more positive attitudes, consistent with results of previous study about KAP of diabetic patients in Saudi Arabia (43). However, knowledge did not always translate into good practices, likely due to competing diabetes management, lack of motivation, and limited access to dental care (39, 42). Better practices were linked to higher income and employment, similar to global findings (43), while older age was associated with poorer hygiene, possibly due to denture use, impaired motor skills, or cumulative oral health challenges (44, 45).
Controlled diabetes correlated with better attitudes and practices, reflecting the bidirectional relationship between diabetes and periodontal disease. Poor glycemic control exacerbates oral inflammation, periodontitis, and xerostomia, which in turn can worsen systemic glycemic control, as reported in other research for Genco et al. about infection and inflammation in periodontal and cardiovascular diseases (46). Despite this, only 43.5% discussed diabetes with a dentist and 32.2% with a physician, emphasizing the need for interprofessional collaboration. Local and global literature highlight that integrated care improves both oral health outcomes and glycemic control (47, 48), reducing risks of cardiovascular, kidney, and other complications (49).
Only 18% of participants attended diabetes education programs, highlighting a critical gap in public health outreach, consistent with previous study by Abbasi et al. in Malaysia (50). Access to dental care improved knowledge, attitudes, and practices (51), while preventive and aesthetic treatments motivated better hygiene (52). Positive lifestyles, including healthy diet and non-smoking, were linked to better outcomes, whereas current smokers had poorer attitudes, consistent with previous study in Tehran, Iran by Sadeghi et al. (53). These observations suggest that public health strategies targeting lifestyle modification, smoking cessation, and dietary counseling are essential for improving oral health behaviors in diabetic populations.
Poor OHRQoL in this study was mostly attributed to psychological discomfort, handicap, and social disability. Higher HbA1c levels, poor general and oral health, and low socioeconomic status were associated with worse disability domains (51, 52). Age, poor practices, and low knowledge further increased disability. These results highlight the need to address both biological and social determinants of health through health education, behavioral interventions, and socioeconomic support.
Participants who had not attended educational programs or communicated with providers had worse outcomes across OHIP−14 domains (54, 55). Interestingly, those who did attend reported higher psychological distress, possibly due to increased awareness of complications without adequate support, as also reported in prior studies (56, 57). Crisis-driven dental visits for pain or cosmetic reasons were linked to worse OHRQoL, reflecting reactive care-seeking patterns seen locally and globally (44).
Poor glycemic control (high HbA1c) correlated with worse OHRQoL across all domains (47), whereas higher education, income, positive attitudes, and better practices predicted improved outcomes (58, 59). Sociodemographic disadvantages—older age, unemployment, low income, and low education—were associated with poorer OHRQoL, consistent with both Palestinian and global studies (23, 49, 59). Self-reported oral issues and poor glycemic control worsened OHRQoL, while milder, non-medicated diabetes was linked to better outcomes and these results the same in UAE and Kuwait (60, 61). Interestingly, lack of family history predicted poorer OHRQoL—a novel finding. This may reflect reduced awareness, lower engagement in preventive care, and missed early interventions.
Overall, this study demonstrates that oral health in diabetic patients is influenced by clinical, behavioral, and socioeconomic factors. Integrating oral health into routine diabetes care, expanding education, and addressing social inequalities are critical, as supported by literature. Recommended public health interventions include structured diabetes and oral health education programs, improved access to preventive dental care and hygiene tools, lifestyle interventions such as healthy diet and smoking cessation, and policies promoting interprofessional collaboration between dentists and physicians. Implementing these strategies can enhance oral health practices, reduce complications, and improve OHRQoL among diabetic patients, particularly in low-resource settings like Palestine.
Importantly, these recommendations must be adapted to the realities of the Palestinian health system. Primary healthcare centers managed by the Ministry of Health (MoH) already serve as first-line providers for diabetes care and thus represent a practical entry point for integrating oral health screening and education. Low-cost interventions—such as training diabetes educators and nurses to deliver brief oral health messages, incorporating oral health checklists into diabetes visits, and engaging community health workers in rural areas—would be feasible within existing structures. Partnerships with local universities and dental schools could further support preventive outreach and patient education campaigns. Given financial and resource constraints, prioritizing preventive care, task-shifting, and interprofessional collaboration offer realistic pathways for improving oral health outcomes among Palestinian diabetic patients.
Limitations and future research
This study's cross-sectional design limits causal inference, capturing associations at a single time point. Reliance on self-reported data may introduce recall or social desirability bias. As the study was conducted only in West Bank PHCs, findings may not generalize to Gaza or underserved populations outside PHC coverage. Future research should use longitudinal or mixed method designs and include diverse geographic and socioeconomic groups to better understand the determinants of oral health behaviors and OHRQoL among diabetic patients in Palestine.
Conclusion
This study provides important insight into the OHRQoL of diabetic patients in the West Bank. While knowledge and positive attitudes were common, they did not consistently translate into optimal oral hygiene behaviors. Positive attitudes were associated with better OHRQoL, whereas poor outcomes correlated with limited healthcare access, low socioeconomic status, poor glycemic control, and psychological distress. Improving OHRQoL requires a holistic, patient-centered approach that integrates oral health into chronic disease management, promotes healthy lifestyles, and addresses structural and social barriers. Findings should be interpreted cautiously due to limited generalizability and modest explanatory power, suggesting that additional clinical, psychosocial, and structural factors likely influence OHRQoL.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Al-Quds University School of Public Health Ethical Research Committee and AL- Quds University Graduate Studies Committee. Approval was obtained from the Ministry of Health to access the healthcare centres. For the potential participants, field researchers explained the aim of this study then verbal consent from them and finishing the questionnaire. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IW: Conceptualization, Methodology, Project administration, Investigation, Data curation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. AE: Investigation, Writing – review & editing. IH: Investigation, Writing – review & editing. MAm: Investigation, Writing – review & editing. MAb: Investigation, Writing – review & editing. YH: Investigation, Writing – review & editing. EK: Conceptualization, Methodology, Validation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1670923/full#supplementary-material
Abbreviations
KAP, knowledge, attitude, practice; OHRQoL, oral health related quality of life; T2DM, type 2 diabetes mellitus; MoH, ministry of health; PHC, primary health care centers. HbA1c, glycated haemoglobin/cumulative sugar rate; OHIP, oral health impact profile; SPSS, statistical package software for social science.
References
1. WHO B. Oral Health Programme. (2023). Available online at: https://www.who.int/teams/noncommunicable-diseases/ncds-management/oral-health-programme (Accessed September 23, 2024).
2. Wolf TG, Cagetti MG, Fisher JM, Seeberger GK, Campus G. Non-communicable diseases and oral health: an overview. Front Oral Health. (2021) 2:725460. doi: 10.3389/froh.2021.725460
3. World Health Organization. Global oral health status report: towards universal health coverage for oral health by 2030. (2022). Available online at: https://books.google.co.il/books?hl=en&lr=&id=XnwOEQAAQBAJ&oi=fnd&pg=PR5&dq=oral+health+and+general+health+-+what+are+the+connections&ots=DSCpeFidKV&sig=bIfx6eCLwECI3AXZF12fib83edQ&redir_esc=y#v=onepage&q=oral health and general health—what are the conn. (Accessed January 5, 2025).
4. Diabetes. NCD. (2024). Available online at: https://www.emro.who.int/noncommunicable-diseases/diabetes/index.html (Accessed June 4, 2024).
5. Imam A, Abdo N, Sharif E. Handbook of Healthcare in the Arab World (2020). [Epub ahead of print 2020]. doi: 10.1007/978-3-319-74365-3
6. Care D, Suppl SS. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43(January):S14–31. doi: 10.2337/dc20-S002
7. International Diabetes Federation. Diabetes Facts and Figures. (2024). Available online at: https://idf.org/about-diabetes/diabetes-facts-figures/ (Accessed July 8, 2024).
8. Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet. (2023) 402(10397):203–34. doi: 10.1016/S0140-6736(23)01301-6
9. Miller A, Ouanounou A. Diagnosis, management, and dental considerations for the diabetic patient. J Can Dent Assoc. (2020) 86:k8. PMID: 32543368
10. ADA. HbA1c. American Diabetes Association. (2024). Available online at: https://diabetes.org/about-diabetes/a1c (Accessed December 13, 2024).
11. AlMulhim AA, Zakaria OM, Daoud MY, Almulhim SA. Dental and Oral Problems among Diabetic Patients: A Developing Country–Local Perceptual Study.
12. Desai R, Khobaragade B, McCracken G, Wassall R, Taylor JJ, Bissett SM, et al. Impact of diabetes and periodontal status on life quality. BDJ Open. (2021) 7(1):1–7. doi: 10.1038/s41405-021-00061-w
13. Mark AM. Can diabetes affect my oral health? J Am Dent Assoc. (2018) 149(4):328. doi: 10.1016/j.adaj.2018.01.032
14. Alhazmi YA, Parveen S, Alfaifi WH, Najmi NM, Namazi SA, Abuzawah LH, et al. Assessment of knowledge, attitude and practice of diabetic patients towards oral health: a cross-sectional study. World J Dent. (2022) 13(3):192–8. doi: 10.5005/jp-journals-10015-1922
15. Sitlinger A, Zafar SY. Health-Related quality of life: the impact on morbidity and mortality. Surg Oncol Clin N Am. (2018) 27(4):675–84. doi: 10.1016/j.soc.2018.05.008
16. Al Shamrany M. Oral health-related quality of life: a broader perspective. East Mediterr Health J. (2006) 12(6):894–901. PMID: 17333837
17. Al Habashneh R, Khader YS, Salameh S. Use of the arabic version of oral health impact profile-14 to evaluate the impact of periodontal disease on oral health-related quality of life among Jordanian adults. J Oral Sci. (2012) 54(1):113–20. doi: 10.2334/josnusd.54.113
18. Mian RI, Rashidi FF, Alshammary TM, Al Zubaidi S, Al Shammary F, Amin J, et al. Oral health-related knowledge and assessment of oral health status of diabetic patients attending dental clinic at college of dentistry, hail, Kingdom of Saudi Arabia. J Contemp Dent Pract. (2017) 21:78–82. doi: 10.5005/jp-journals-10024-2729
19. Irani FC, Wassall RR, Preshaw PM. Impact of periodontal status on oral health-related quality of life in patients with and without type 2 diabetes. J Dent. (2015) 43(5):506–11. doi: 10.1016/j.jdent.2015.03.001
20. Mohsin SF, Fawwad A, Mustafa N, Shoaib A, Basit A. Impact of type 2 diabetes mellitus on oral health-related quality of life among adults in Karachi, Pakistan-a cross-sectional study. Br J Med Med Res. (2017) 20(1):1–7. doi: 10.9734/BJMMR/2017/31676
21. Sun J, Tong D, Sun C, Wang X, Zuo Z, Liu Y, et al. Knowledge, attitude, and practice toward self-control of dental plaque among patients with periodontal diseases: a cross-sectional study. BMC Oral Health. (2023) 23(1):1–10. doi: 10.1186/s12903-023-03352-w
22. Poudel P, Rawal LB, Kong A, Yadav UN, Sousa MS, Karmacharya B, et al. Oral health knowledge, attitudes and practices of people living with diabetes in south Asia: a scoping review. Int J Environ Res Public Health. (2022) 19(21):1–21. doi: 10.3390/ijerph192113851
23. Baniasadi K, Armoon B, Higgs P, Bayat AH, Mohammadi Gharehghani MA, Hemmat M, et al. The association of oral health Status and socio-economic determinants with oral health-related quality of life among the elderly: a systematic review and meta-analysis. Int J Dent Hyg. (2021) 19(2):153–65. doi: 10.1111/idh.12489
24. Alhajaji R. Oral health knowledge, attitude and practice among adults living with diabetes in Makkah city. J Res Med Dent Sci. (2022) 10(1):495.
25. Allen EM, Ziada HM, O’Halloran D, Clerehugh V, Allen PF. Attitudes, awareness and oral health-related quality of life in patients with diabetes. J Oral Rehabil. (2008) 35(3):218–23. doi: 10.1111/j.1365-2842.2007.01760.x
26. Ashour Y, Jlambo A, Abuzerr S. Patients in Gaza with chronic conditions need urgent interventions. Lancet. (2024) 403(10439):1847–8. doi: 10.1016/S0140-6736(24)00705-0
27. Asmar I, Almahmoud O, Qoud B, Assaf M, Saleh AD, Alyan A, et al. Diabetes knowledge, attitudes and practices among university students in a developing country. J Diabetes Nurs. (2024) 28(3):1–7.
28. Badran A, Bahar A, Tammam M, Bahar S, Khalil A, Koni AA, et al. The relationship between diabetes-related knowledge and kidney disease knowledge, attitudes, and practices: a cross-sectional study. BMC Public Health. (2023) 23(1):1–15. doi: 10.1186/s12889-023-15390-8
29. Alqedra E, Aljeesh Y. Oral health problems among patients with type 2 diabetes attending UNRWA health centres in Gaza governorates: a cross-sectional study. Lancet. (2022) 399:S20. doi: 10.1016/S0140-6736(22)01155-2
31. Palestinian Ministry of Health. Health Annual Report. Vol. 11. (2022). Available online at: http://link.springer.com/10.1007/978-3-319-59379-1%0A, http://dx.doi.org/10.1016/B978-0-12-420070-8.00002-7%0A, http://dx.doi.org/10.1016/j.ab.2015.03.024%0A, https://doi.org/10.1080/07352689.2018.1441103%0A, http://www.chile.bmw-motorrad.cl/sync/showroom/lam/es/ (Accessed January 5, 2025).
32. Abu-Rmeileh NM, Husseini A, O’Flaherty M, Shoaibi A, Capewell S. Forecasting prevalence of type 2 diabetes mellitus in Palestinians to 2030: validation of a predictive model. Lancet. (2012) 380:S21. doi: 10.1016/S0140-6736(13)60202-0
33. Zheng S, Zhao L, Ju N, Hua T, Zhang S, Liao S. Relationship between oral health-related knowledge, attitudes, practice, self-rated oral health and oral health-related quality of life among Chinese college students: a structural equation modeling approach. BMC Oral Health. (2021) 21(1):1–11. doi: 10.1186/s12903-021-01419-0
34. Zhu W, Liang D, Petersen JD, Zhang W, Huang J, Dong Y. Relationship between diabetic knowledge, attitudes and practices among patients with diabetes in China: a structural equation model. BMJ Open. (2023) 13(11):e076464. doi: 10.1136/bmjopen-2023-076464
35. Rav-Marathe K, Wan TTH, Marathe S. A systematic review on the KAP-O framework for diabetes education and research. Med Res Arch. (2016) 4(1):1–21.
36. Khalifa N, Allen PF, Abu-bakr NH, Abdel-Rahman ME. Psychometric properties and performance of the oral health impact profile (OHIP-14s-ar) among Sudanese adults. J Oral Sci. (2013) 55(2):123–32. doi: 10.2334/josnusd.55.123
37. Osman SM, Khalifa N, Alhajj MN. Validation and comparison of the arabic versions of GOHAI and OHIP-14 in patients with and without denture experience. BMC Oral Health. (2018) 18(1):157. doi: 10.1186/s12903-018-0620-5
38. Ansari Moghadam S, Derakhshan M, Asadi MR. Evaluation of knowledge, attitude and practice of diabetic patients towards periodontal diseases in Zahedan. J Health Rep Technol. (2024) 10(4). doi: 10.10.5812/jhrt-145398
39. Sohal KS, Kambole R, Owibingire SS. Oral health-related knowledge, attitudes, and practices of diabetic patients in Tanzania. Int Dent J. (2025) 75(1):256–62. doi: 10.1016/j.identj.2024.06.006
40. Elsous A, Fetaiha A, Radwan M. Exploring oral health related awareness, perceptions, practices and experiences among type 2 diabetes mellitus patients: a mixed method design. BMC Oral Health. (2025) 25(1):781. doi: 10.1186/s12903-025-06153-5
41. Wang CX, Ma LL, Yang Y, Xu MR, Wang X, Feng XP, et al. Oral health knowledge, attitudes, behaviour and oral health status of Chinese diabetic patients aged 55 to 74 years. Chin J Dent Res. (2018) 21(4):267–73. doi: 10.3290/j.cjdr.a41085
42. Shaikh H, Badar A. Poor oral health care practices by diabetic patients. Pakistan J Med Dent. (2023) 12(4):37–44. doi: 10.36283/PJMD12-4/008
43. Mahzari MA, Oraibi OH, Shami AM, Shami MO, Thobab TY, Awlaqi AA, et al. Knowledge, attitude, and practice regarding diabetes Mellitus among type 2 diabetic patients attending primary health care centers in the Jazan Region of Saudi Arabia. Cureus. (2022) 14(9):e28704. doi: 10.7759/cureus.28704
44. Schierz O, Baba K, Fueki K. Functional oral health-related quality of life impact: a systematic review in populations with tooth loss. J Oral Rehabil. (2021) 48(3):256–70. doi: 10.1111/joor.12984
45. Dibello V, Zupo R, Sardone R, Lozupone M, Castellana F, Dibello A, et al. Oral frailty and its determinants in older age: a systematic review. Lancet Healthy Longev. (2021) 2(8):e507–20. doi: 10.1016/S2666-7568(21)00143-4
46. Rahimi A, Afshari Z. Periodontitis and cardiovascular disease: A literature review. ARYA Atheroscler. (2021) 17(5):1–8. doi: 10.22122/arya.v17i0.2362
47. Preshaw PM, Bissett SM. Periodontitis and diabetes. Br Dent J. (2019) 227(7):577–84. doi: 10.1038/s41415-019-0794-5
48. Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. (2008) 14(3):191. doi: 10.1111/j.1601-0825.2008.01442.x
49. Usmani W, de Courten M, Hanna F. Can oral health care be a gateway to improve cardiovascular disease? A scoping review. Front Oral Health. (2024) 5(May):1364765. doi: 10.3389/froh.2024.1364765
50. Abbasi YF, See OG, Ping NY, Balasubramanian GP, Hoon YC, Paruchuri S. Diabetes knowledge, attitude, and practice among type 2 diabetes mellitus patients in Kuala Muda district, Malaysia—a cross-sectional study. Diabetes Metab Syndr Clin Res Rev. (2018) 12(6):1057–63. doi: 10.1016/j.dsx.2018.06.025
51. Alhuwais M, Alkanderi A, Jospeh B. Attitudes and awareness of diabetic patients in Kuwait towards their oral health. Int J Oral Dent Health. (2021) 7(3). doi: 10.23937/2469-5734/1510133
52. Malik R, K T, Singh V, Jain A, Mitra S, Singh S. Impact of dental treatment on oral health-related quality of life of patients. Cureus. (2023) 15(5):e38625. doi: 10.7759/cureus.38625
53. Sadeghi D, Motlagh MK, Darvish A, Daryaafzoon M, Mohamadnejad E, Molaei A, et al. Comparative effect of physical health training and psychological training of the theory of reasoned action (TRA) model on the life quality of patients with diabetes in Tehran, Iran: utilization of message texting. BMC Endocr Disord. (2024) 24(1):1–12. doi: 10.1186/s12902-024-01598-1
54. Bissett SM, Stone KM, Rapley T, Preshaw PM. An exploratory qualitative interview study about collaboration between medicine and dentistry in relation to diabetes management. BMJ Open. (2013) 3(2):e002192. doi: 10.1136/bmjopen-2012-002192
55. Luo H, Wu B, Kamer AR, Adhikari S, Sloan F, Plassman BL, et al. Oral health, diabetes, and inflammation: effects of oral hygiene behaviour. Int Dent J. (2022) 72(4):484. doi: 10.1016/j.identj.2021.10.001
56. Mubayrik AFB, Alhoqail RI, Alhoqail RI, Dous RAB. Oral health-related quality of life among diabetic patients: a cross-sectional controlled study. J Family Med Prim Care. (2024) 13(2):619–26. doi: 10.4103/jfmpc.jfmpc_1079_23
57. Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. (2000) 28(6):399–406. doi: 10.1034/j.1600-0528.2000.028006399.x
58. Sabbah W, Tsakos G, Chandola T, Sheiham A, Watt RG. Social gradients in oral and general health. J Dent Res Dent Clin Dent Prospects. (2007) 86(10):99. doi: 10.1177/154405910708601014
59. Locker D. Deprivation and oral health: a review. Community Dent Oral Epidemiol. (2000) 28(3):161–9. doi: 10.1034/j.1600-0528.2000.280301.x
60. Khalifa N, Rahman B, Gaintantzopoulou MD, Al-Amad S, Awad MM. Oral health status and oral health-related quality of life among patients with type 2 diabetes mellitus in the United Arab Emirates: a matched case-control study. Health Qual Life Outcomes. (2020) 18(1):182. doi: 10.1186/s12955-020-01418-9 32539861
Keywords: diabetes, oral health-related quality of life, knowledge, attitude, practice, oral hygiene, OHIP-14
Citation: Wahbeh I, Enairat ALE, Hemieid I, Amro M, Abueed M, Hirzallah YN and Kateeb E (2025) Non-communicable diseases and oral health: an overview. Front. Oral Health 6:1670923. doi: 10.3389/froh.2025.1670923
Received: 22 July 2025; Accepted: 8 October 2025;
Published: 31 October 2025.
Edited by:
Chandrashekar Janakiram, Amrita Vishwa Vidyapeetham University, IndiaReviewed by:
Aswini Y. Balappanavar, University of Delhi, IndiaPooja Latti, Annoor Dental College & Hospital, India
Copyright: © 2025 Wahbeh, Enairat, Hemieid, Amro, Abueed, Hirzallah and Kateeb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iman Wahbeh, aW1hbi53YWhiZWhAc3R1ZGVudHMuYWxxdWRzLmVkdQ==; Elham Kateeb, ZWthdGVlYkBzdGFmZi5hbHF1ZHMuZWR1
 Iman Wahbeh
Iman Wahbeh Aesha L. E. Enairat
Aesha L. E. Enairat Ihab Hemieid2
Ihab Hemieid2 Mahmoud Amro
Mahmoud Amro Elham Kateeb
Elham Kateeb