- Department of Orthodontics and Dentofacial Orthopedics, Sri Ramachandra Dental College, Chennai, India
Introduction: Crowding or spacing of teeth will impair oral hygiene maintenance and therefore lead to poor oral health and nutrition. Orthodontic treatment aligns the teeth and establish a proper occlusion, both of which are essential in the integrity of oral health and nutrition. Despite advancements in orthodontic treatment, relapse (teeth returning to the previous position) remains a major challenge. Previously, collagen turnover was considered the main factor, but aligns studies suggested extracellular matrix proteins such as tyrosine protein kinase (TEC protein) play a more significant role due to their exclusive presence during the retention phase. While extensive research exists on OTM, few studies have explored biochemical mediators during retention. The primary objective of this scoping review is to identify biochemical mediators at different timelines during OTM and relapse to consolidate findings and address gaps so that orthodontists may attempt to alter the mediators thereby restoring oral health and nutrition.
Method: This scoping review complied with the PRISMA-ScR guidelines. The search terms used were MESH terms and Boolean terminology. The search was conducted until July 2023 across five databases; PubMed, Scopus, Medline, Embase, and Google Scholar, including gray literature and unpublished data. The resulting numbers of articles (120) were chosen for the scoping review after matching with the framed inclusion and exclusion criteria (distributed as 113 and 7 studies for active and retentive phases respectively). Each reviewer stored the retrieved list of articles in separate folders designated for each database. The two reviewers resolved discrepancies through discussion. Any points of disagreement or conflict in the conducted search were escalated to the third senior reviewer, whose judgment was final.
Results: The extraction of relevant data was independently performed by the two reviewers. The following data were analyzed: author name, journal, year of publication, type of study, sample size, sample site, type of biomarker assessed, and stage of orthodontic treatment. Queries pertaining to a particular study were clarified by contacting the lead author. The data were compiled individually by each reviewer into a draft chart and then discussed to reach a consensus. These data were then shared with a third senior reviewer to streamline and finalize the data.
Conclusions: The literature on biomarkers of tooth movement is exhaustive. However, studies on biomarkers during the retention phase are limited, and more exploration is needed.
Systematic Review Registration: https://osf.io/sh6u5.
Introduction
Rationale
In recent decades, the mechanism of orthodontic tooth movement has been limited to the pressure tension theory, wherein, upon orthodontic force, there is bone resorption on the pressure side and bone apposition on the tension side, thus initiating the remodeling process to move teeth (1). During these events, biological mediators are released by the cells involved in remodeling. The predominant mediators are cytokines, growth factor enzymes and hormones, which initiate a cascade of orchestrated events leading to alterations in the nuclear protein matrix and eventually gene modulation, resulting in mechanotransduction. The most commonly expressed and documented cytokines include interleukins (ILs) (IL-1α, IL-1β, IL-1RA, IL-8, IL-2, IL-6, and IL-15), tumor necrosis factors (TNFs), interferons (IFNs), growth factors (GFs), and colony stimulating factors (CSFs). IL-1β, IL-2, IL-6, IL-8 and TNF-α are some of the cytokines reported by authors to be elevated during the first month of orthodontic force application (2–8). The earliest identified marker of bone resorption is IL-1β, followed by prostaglandin E2 (PGE2) (9, 10), nitric oxide, IL-6, and other inflammatory cytokines. An increase in the concentration of matrix metalloproteinases (MMPs), such as MMPs 1, 2, 3, 8 and 9, was also reported by other authors (11–15). Ariffin SHZ et al. reported elevated tartrate-resistant acid phosphatase (TRAP) levels during the initial days of active orthodontic tooth movement (16). Elevated myeloperoxidase (MPO) is observed at the second hour of activation of a fixed orthodontic appliance (17).
Despite advances in orthodontic appliances and treatment modalities, relapse remains an issue. Earlier, it was believed that the major contributor to relapse was collagen turnover in the gingiva and periodontal ligament. This notion was subsequently disproved by Maltha et al. (18) and Nakanishi (19), who reported that the rate of collagen fiber turnover in gingival and periodontal tissues were high at 7 days, which was too short to contribute to long-term relapse. Hence, other extracellular matrix proteins have been suggested to be responsible. Although many studies have explored the biological mechanisms and markers of orthodontic tooth movement, very few studies have investigated the biochemical mediators involved during the retentive phase of orthodontic treatment. Several animal studies have evaluated RANKL, Alkaline Phosphatase (ALP) and TRAP levels (20, 21). Few human studies have evaluated biochemical markers during relapse at a single time point. In 2019, Awang-Kechik et al. (22) determined the levels of biochemical markers during retention after treatment with a fixed appliance. Among the many markers studied, tyrosine–protein kinase (TEC protein) was detected only during the six-month retentive phase but, surprisingly, not during active tooth movement.
The literature on the mediators of OTM and relapse is numerous and scattered, i.e., a combination of animal and human studies and in vitro or in vivo studies with many other influential factors that play a role in the expression of these mediators. In addition, studies on the molecular events and biochemical mediators that prevail during relapse are limited and in the primitive stage. Despite the abundant literature on the molecular events leading to orthodontic tooth movement and the relatively few reports on relapse, the extent of the roles of the mediators across the various phases of orthodontic treatment, including retention, is still unclear. For these reasons, a scoping review was deemed mandatory to streamline the events and to identify the lacunae in the literature.
Objective
To identify biochemical mediators at different times during orthodontic tooth movement and during relapse. The broader research question of this review was “What are the biochemical mediators of tooth movement in orthodontic patients during and after orthodontic treatment?” The sub question was “Are the biochemical mediators that are expressed during orthodontic tooth movement similar to those expressed during relapse?”
Methods
Protocol and registration
The protocol was drafted on the basis of the PRISMA-ScR guidelines constructed by Tricco et al. (23) and was registered in the Open Science Framework (https://osf.io/sh6u5).
Eligibility criteria
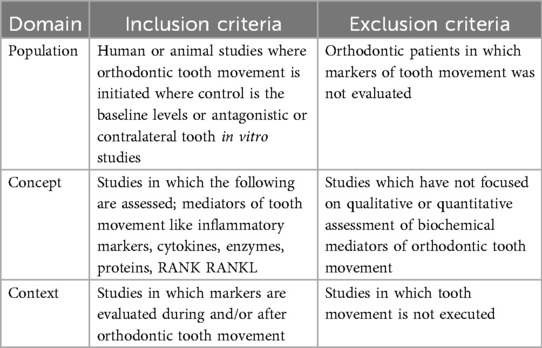
The inclusion criteria were designed on the basis of the population concept context (PCC) format recommended by Tricco et al. (23) and are outlined in Table 1 below.
Information sources
The search was conducted until July 2023 across five databases, namely, PubMed, Scopus, Medline, Embase, and Google Scholar. Studies published only in English were considered. The searches were rerun prior to the final analysis. The gray literature and unpublished data, if retrieved, were also included.
Search strategy
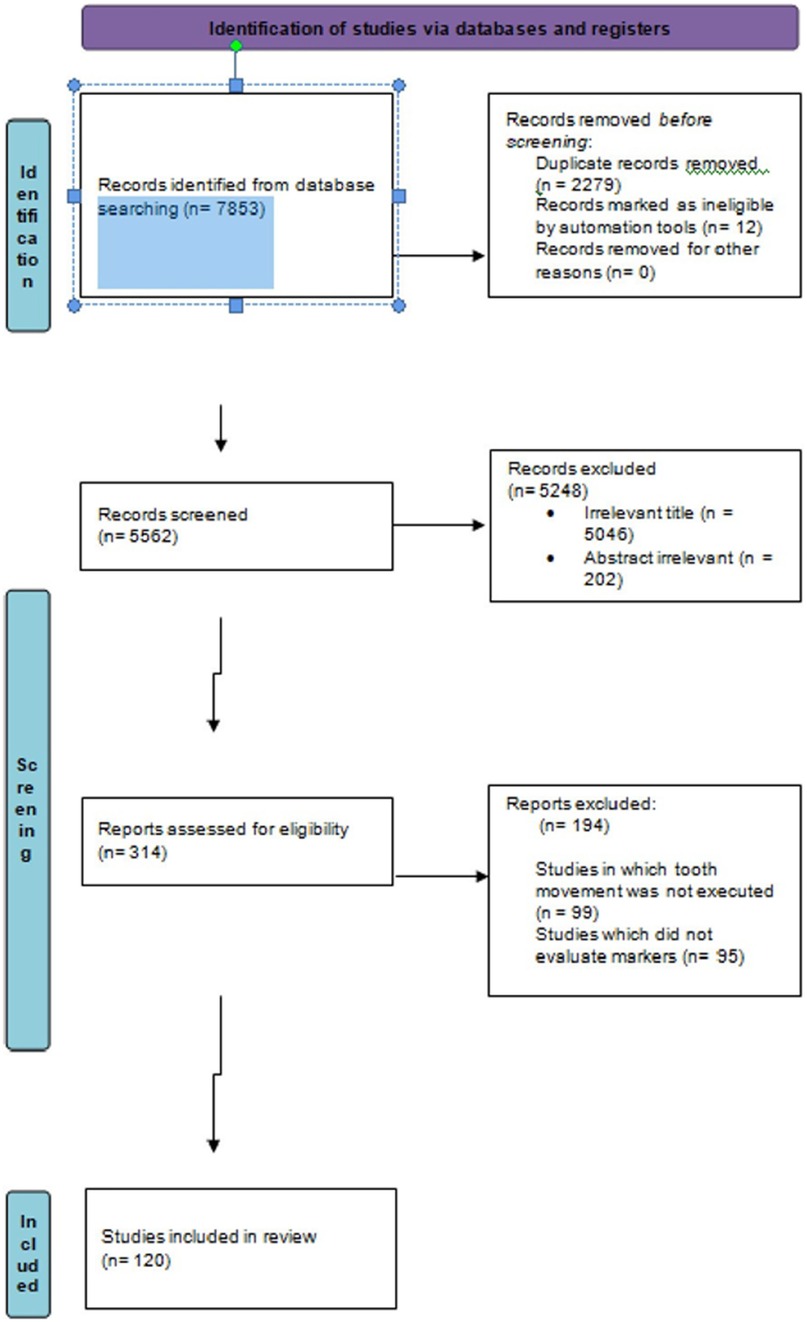
The search terms used were the following MeSH terms and Boolean terminology: “biochemical markers” OR “biomarkers” OR “mediators” OR “tissue reactions” OR “proteins” OR “biology” OR “molecular changes” OR “cytokines” OR “enzymes” OR “GCF” OR “saliva” AND “force” OR “tooth movement” OR “teeth movement” OR “relapse” OR “retention”. The search words were reviewed with the Peer Review of Electronic Search Strategies (PRESS) checklist [McGowan et al. (24)] Search words were initially selected for PubMed and were later modified to suit the preferences of the other databases (Figure 1).
Risk of bias & quality considerations
Consistent with the guidance of scoping reviews, we did not deem it mandatory to perform a risk of bias assessment prioritising a comprehensive identification and pooling of evidence over formal risk of bias appraisal. However, the quality of grey literature was assessed using the AACODS checklist (Authority, Accuracy, Coverage, Objectivity, Date, Significance). Both the reviewers appraised the included data but did not exclude them. The search was conducted across five indexed data bases to mitigate publication bias and strengthen the quality of included articles. Gray literature such as theses, conference proceedings, preprints, and trial registries was also backed up with backward/forward citation searches and deduplicated records in order to reduce positive—result bias.
Selection of sources of evidence
The publications obtained from the electronic search were imported into Mendeley (https://www.mendeley.com), with which duplicates were removed. The first level of screening excluded articles with irrelevant titles. The abstracts of the remaining articles were read and excluded if not relevant. The resulting number of articles was chosen for the scoping review after matching with the framed inclusion and exclusion criteria. Venkateswaran Ananthanarayanan (VA) stored the retrieved list of articles at each screening level in separate folders designated for each database in the Mendeley software. VA resolved discrepancies through discussion with the senior reviewer Sridevi Padmanabhan (SP) whose judgment was considered final.
Data charting process
VA developed a table to extract the relevant information from the included sources of evidence. The data were filled in by VA and crosschecked by the SP. Both reviewers discussed the results, and the form was constantly updated.
Data items
The following data were analyzed and charted from the selected articles: author name, journal, year of publication, type of study, sample size, sample site, characteristics of intervention (stage of orthodontic treatment), and outcome measures (type of biomarker assessed). In the process, any queries pertaining to a particular study will be clarified by contacting the lead author.
Synthesis of results
The data were compiled individually by VA into a draft form and then discussed with SP to reach a consensus. These data were then shared with SP to streamline and finalize the data. A detailed explanation of the studies across the above domains of assessment was provided. The results are presented via descriptive statistics such as percentages, tables, charts and flow diagrams, as appropriate. Narrative analysis was used to summarize the findings of the review.
Results
Selection of sources of evidence
The search was conducted in accordance with the PRISMA 2020 flow diagram and is illustrated in Figure 2 (PRISMA flowchart depicting search strategy) below. Of the 120 included studies, 113 evaluated biomarkers during active tooth movement, whereas 7 studied molecular changes after active tooth movement, i.e., the retentive phase.
Characteristics of the sources of evidence
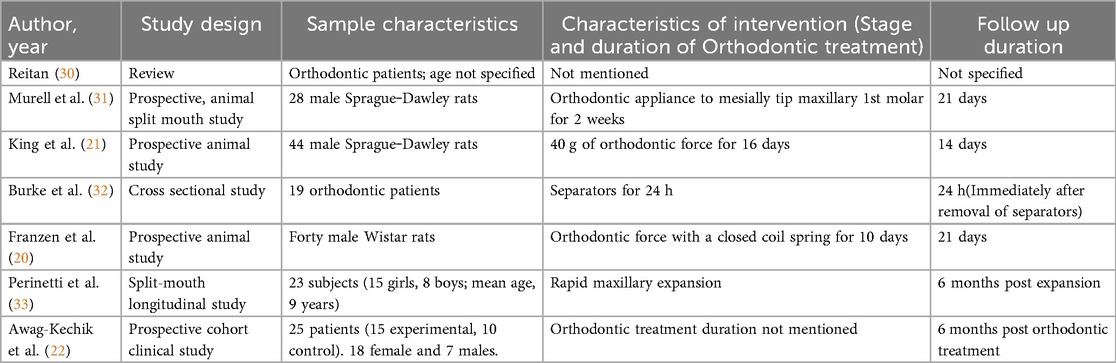
The existing sources of evidence can be categorized into those related to active tooth movement and those related to the retention phase. The categories in which each study was described were the author and year of study, sample characteristics, characteristics of the intervention, and follow-up duration. The domains of description with respect to the studies of active tooth movement have been described by Kapoor et al. (25, 26), Vansant et al. (27), Alhadlaq (28), Allen et al. (29) over the past decade. Similar domains were used to describe the characteristics of the studies of biomarkers during relapse and are depicted in Table 2.
Synthesis of results of individual sources of evidence
For ease of comprehension, the results of this scoping review were broadly tabulated into studies that focused on the changes occurring during orthodontic tooth movement and those that evaluated the changes during the retentive phase of orthodontic treatment (Table 3).
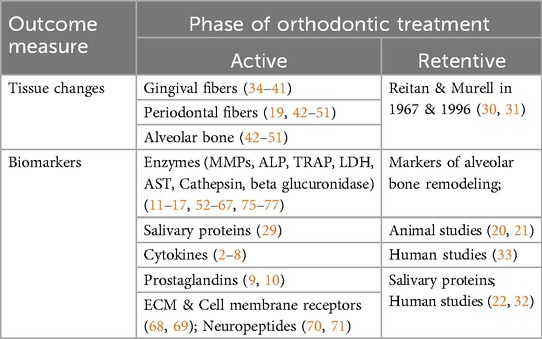
Table 3. Classification of studies that evaluated changes during and after orthodontic tooth movement.
Biochemical markers of orthodontic tooth movement
The literature on the molecular basis of orthodontic tooth movement is exhaustive, and four systematic reviews have been conducted by Kapoor et al., Vansant et al., Alhadlaq and Allen et al. (25–29).
Kapoor et al. conducted systematic reviews exclusively on cytokine and enzyme expression during orthodontic tooth movement in 2014 and 2019, respectively. Most of the cytokines are released during the first 24 h and gradually decrease after the application of orthodontic force, suggesting that remodeling occurs only during initial tooth movement. These levels increase immediately after every reactivation. The RANKL/OPG ratio increased during initial tooth movement, especially in growing subjects, indicating the potential for accelerated tooth movement immediately after an orthodontic force, which is amplified in young patients. However, none of the studies showed a clear distinction in biomarker expression between the sexes, and several confounding factors were either ignored or not explored in the literature (25). The enzymatic expression varied depending upon the stage and site of orthodontic force application. The compression sites presented early increases in the levels of MMP1, MMP2, TIMP1, and MMP9 from 1 to 4 h and late peaks in the levels of TIMP2, TRAP, and AST after 7 days, 4–5 weeks and 8–12 weeks, respectively. Tension sites showed a significant increase in ALP after 7 days and in MMP1 between the first 1st and third hours (25, 26).
Vansant et al. published a systematic review on the biological mediators of orthodontic tooth movement in 2018. In addition to the human and animal studies, they also reported few in vitro studies and realized that the setup of the latter was considerably different, which raised concerns about the validity of the studies (27). Osteoclast formation and recruitment are induced by factors such as IL-6, TNFα and chemokines (especially CCL2, CCL3 CCL5 and CXCL2), which are increased within the first week of OTM. Bone apposition at tension sites is mediated by runt-related transcription factor (RUNX2), osterix (OSX) and osteocalcin (OCN), which are expressed in vivo within the first 2 weeks (27). The MMPs and cathepsins increased on the compression side within 1 week of OTM and decreased by the 2nd week. These enzymes help degrade the extracellular matrix, which is then replaced by a new matrix. Transforming growth factor (TGF)-β levels increase gradually until 4 weeks, which is consistent with the synthesis of new extracellular matrix (27).
Allen et al. performed a systematic review of salivary protein biomarkers during orthodontic tooth movement and reported that these biomarkers are useful not only for studies pertaining to tooth movement but also for future research on their role in root resorption (29).
Tissue changes and biochemical markers during orthodontic retention
The results of the included studies are described in Table 4.
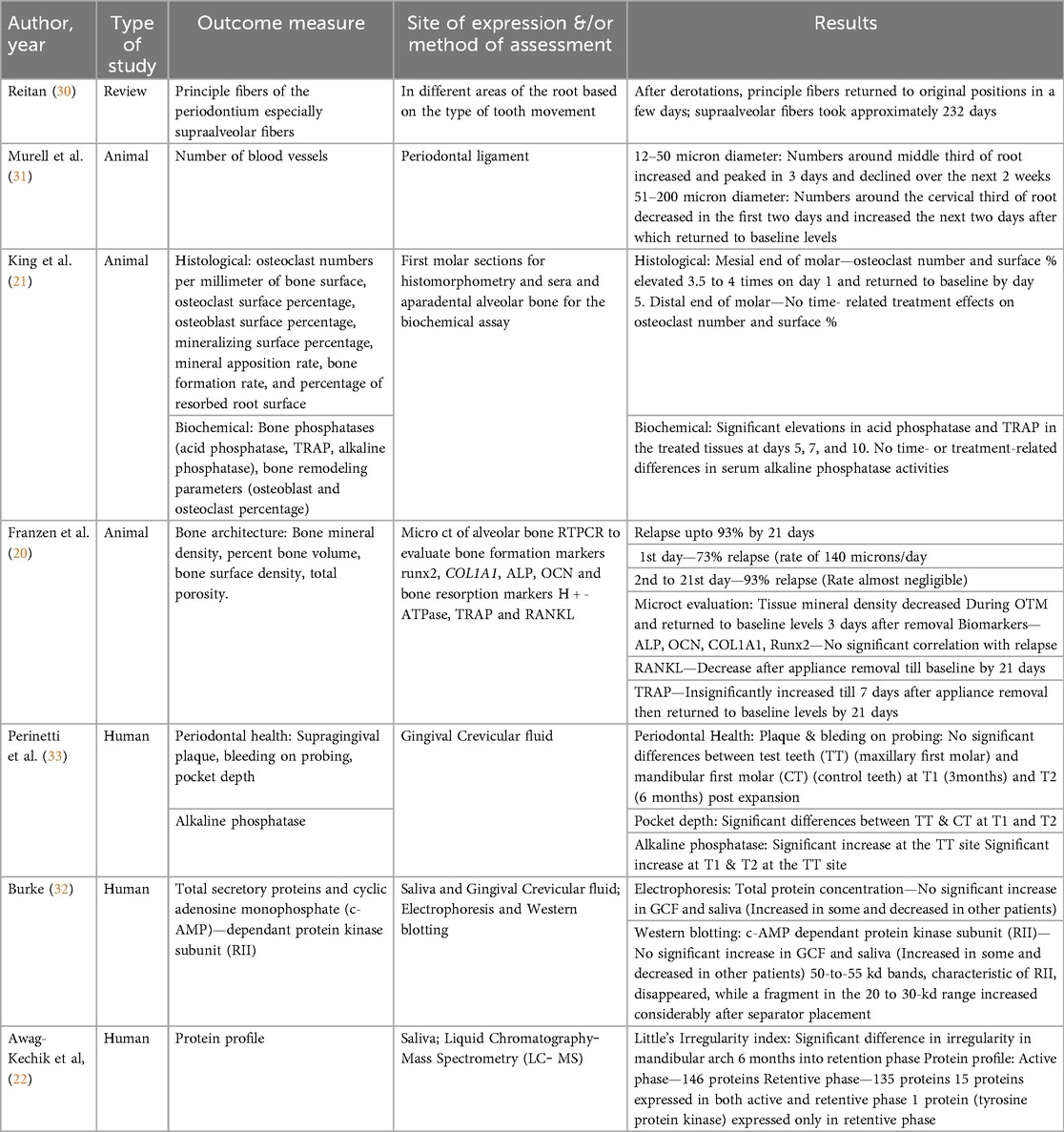
Table 4. Description of the results of the studies included in the systematic review demonstrating tissue and biochemical changes during orthodontic retention.
Tissue changes during retention phase
In 1967, Reitan (30) was one of the first people to observe changes in the PDL of teeth that experienced orthodontic force. Clinical and histologic observations were made both during and immediately after orthodontic treatment. In terms of relapse, he attributed it to the muscular imbalance caused by moving the dentition. In addition, another major contributing factor was the rearrangement of the principal fibers of the gingiva and PDL. He explained that the rearrangement of the principle fibers might not take long, whereas the supra-alveolar fibers might take a longer time and remain stretched. He concluded that this might have a greater role in the relapse of orthodontic treatment. Murrell et al. (31) reported that during two weeks of orthodontic force application, the number and density of blood vessels increased. Immediately after the removal of orthodontic force, there was an immediate decrease in the blood vessel profile, after which it increased and then returned to the baseline levels by the end of 3 weeks posttreatment.
Biochemical markers during retention phase markers of alveolar bone remodelling
Animal studies
King et al. (21) studied alveolar bone turnover after orthodontic tooth movement in rats. Turnover was evaluated with both histomorphometric and biochemical changes in the oral tissues. One group was subjected to 40 g of force applied to move the maxillary first molars mesially, whereas the other group was subjected to all procedures except spring placement to actively move the tooth. The treatment was active until 16 days, after which it was discontinued. The following parameters were assessed at both the mesial and distal roots of the maxillary first molars in both groups: bone phosphatases (acid phosphatase, TRAP, alkaline phosphatase), bone remodeling parameters (osteoblast and osteoclast percentage) and root resorption (percentage of resorbed root surface). The biochemical changes revealed a sustained increase in the level of bone phosphatases at the mesial roots (previously pressure sites). In 2014, Franzen et al. were the first and probably the only authors who have studied relapse three-dimensionally via micro-CT. Orthodontic force was applied to rat first molars to move them mesially, and the biochemical markers of alveolar bone remodeling, both during and after orthodontic treatment, were estimated. One day after appliance removal, the molars started to relapse; the levels of ALP, osteocalcin, and COL1A1 started to increase. By the end of 3 weeks, the levels had returned to baseline. There was no significant correlation between TRAP, RANKL or HTPase and relapse.
Human studies
Perinetti et al. (33) estimated the levels of ALP in the GCF of teeth, which are used to support the appliance for rapid maxillary expansion required in prepubertal patients with constricted maxillary arches. The samples were procured from the tension sites of the teeth at 3-month and 6-month intervals. The results revealed that there was an increase in ALP activity at the tension sites of the tooth PDL, which decreased by the end of the 6 months postexpansion. This was a split-mouth longitudinal study in which the antagonist teeth served as the control. The control side also presented increased ALP activity, but it was not greater than that of the experimental side. The authors concluded that bone formation postexpansion might be complete by the end of 6 months postexpansion, thereby indicating the need for retention for a minimum of 6 months postexpansion.
Salivary proteins
Human studies
Burke et al. (32) evaluated the expression of secretory proteins in the GCF and saliva from patients for whom separators had been placed. The separator placement was considered to be the source of force application. The samples were collected before and 1 day after separator placement, and total secretory proteins and cyclic adenosine monophosphate (c-AMP)-dependent protein kinase subunit (RII) were measured via electrophoresis and western blotting. The results revealed that there was no significant increase in the total protein concentrations in the GCF or saliva. However, mechanical strain causes a significant increase in the RII subunit, indicating that a systemic response through the AMP signaling pathway might be initiated. Awang-Kechik et al. (22) conducted one of the few protein profile analysis studies in patients during retention after orthodontic treatment. The retention phase was 6 months, and the protein profile was analyzed via liquid chromatography‒mass spectrometry (LC‒MS). Relapse was quantified via Little's Irregularity Index. The results revealed 146 proteins that were expressed in the control group (untreated) and 135 proteins expressed in the retention group. The protein that was detected only during the retention phase and not in the control was the immune response protein tyrosine-protein kinase (TEC). This result encouraged the authors to conclude that TEC protein might be a good potential biomarker for predicting relapse.
Although a certain degree of heterogeneity across the included studies remains, a relatively consistent pattern of biomarker expression was observed across the included studies. During active tooth movement, there was a transient increase in the ALP at tension sites within the 1st 1–2 weeks followed by the increase in OCN (2–4 weeks later).
During the retention phase, animal model studies reveal an initial increase in the ALP/OCN/ COL1A1 immediately after debonding. This is followed up with a return to the baseline levels by 3 weeks.
Discussion
This systematic review aimed primarily to thoroughly review the literature, which has explored the changes that occur during the active and retentive phases of orthodontic tooth movement. The biomarkers that have been studied include cytokines; enzymes; RANK, RANKL, OPG, MMP, and TRAP; salivary proteins; osteocalcin; TGF-β; ALP; TNFa; cathepsin B; epidermal growth factor; beta-2-microglobulin; glycosaminoglycans; PGE2; LDH; AST; neurotransmitters; metabolites of arachidonic acid; and growth factors, among many others. Among the mediators, IL-1β and TNF-α have been the most studied (78). During active tooth movement, studies have shown a common consensus on the increased levels of bone resorptive mediators, which peak by the 24th hour and return to baseline levels within 3 weeks of orthodontic force application. However, the pattern is not sustained across the studies and is rather reversed in some studies. This could be explained by the fact that the sites of compression and tension are in close proximity.
During the first days of force application, the levels of apoptotic mediators such as heat shock protein, caspases, BCL-2-associated X protein (BAX), B-cell lymphoma 2 (BCL-2), death domain-containing protein (CRADD) and the Bcl-2-associated death promoter (BAD) tend to increase in osteoblasts after compressive stimulation (27). There was also a significant increase in the GCF levels of the MMP inhibitors TIMP-1 at 4 h and TIMP-2 after 7 day during canine retraction (26). Both of these findings indicate a lag phase of tooth movement. Apoptotic signals are then followed by cell strain signals such as connexin 43, marking mechanotransduction, which eventually activates intracellular signaling pathways, leading to cell differentiation and the activation of osteoclastic precursors. This initiates the RANKL—OPG pathway, wherein there is an increase in the RANKL protein levels at compression sites and an increase in the OPG levels on the tension side immediately after force application (27, 72). This finding was not consistent across the studies; rather, the sRANKL/OPG ratio seemed more closely associated with OTM (29). An interesting observation was made in a comprehensive salivary protein analysis by Awang-Kechik et al., who compared protein expression in the active and retentive phases of orthodontic treatment. There was the presence of an immune system protein [identified as tyrosine-protein kinase TEC (TEC protein)] in the retention phase that was not expressed in the active phase. This protein, in addition to having a role in immunity, is also involved in RANKL-induced osteoclastogenesis. Therefore, it is only logical to assume that the TEC protein may have an important role in relapse (22).
MMPs and their inhibitors tend to increase during tooth movement, peaking a few days later and returning to the control levels within a week (28). There are different types of MMPs, and all of them are responsible for OTM at different phases or for various durations depending on the type and magnitude of force. MMP-9 is responsible for the cleavage of denatured collagen, i.e., gelatin; MMP-13 dissolves native fibrillar collagen; MMP-1 is an interstitial collagenase that hydrolyzes mainly type III collagen;61 and MMP-3 is responsible for the activation of MMPs 8 and 9 (73). The peak for MMP9 and MMP3 ranged from 8 to 72 h after force application. MMP expression is also site specific, although not significant. Bone remodeling is predominantly mediated by the two enzymes ACP and ALP, which are responsible for bone resorption and apposition, respectively. In animal studies, the ACP is increased in the first two weeks, followed by an increase in the ALP in the subsequent couple of weeks, specifically at the tension site. This is followed by a decrease in ALP levels corresponding to hyalinized tissue removal and the initiation of the postlag phase (26). The only long-term human study that evaluated ALP levels postexpansion was by Perinetti et al. in 2015. These patients presented significantly elevated ALP levels at 3 months and 6 months postexpansion. The elevation starts within the first 2–3 weeks, which is also the time taken for bone apposition to begin after a phase of bone resorption. There is no significant difference in the ALP levels at 3 and 6 months (33). Although ALP is one of the most commonly researched biomarkers, it is also frequently associated with inflammation; therefore, an increased ALP level might not be completely attributed to tooth movement. To avoid this, either standardization of oral hygiene regimens should be practiced or split-mouth studies can be designed. TRAP is a potent osteoclast biomarker expressed in areas of compression. TRAP peaked at 4 weeks. LDH peaks around the 14th day and varies depending on the type, magnitude and direction of force. Other inflammatory mediators, such as MPO, which is a sensitive marker for inflammation and pain associated with OTM, showed early increases at 2 h until 1 day (26).
Salivary sample collection is less technique sensitive than GCF collection is. However, across the studies, the time of salivary collection and method of processing differed. The time of salivary sample collection becomes critical because of the spikes in salivary composition attributed to the circadian cycle. During saliva retrieval, care should be exercised to avoid food contaminants that may alter biomarker expression. Most of the studies, however, preferred convenience in collection time. Centrifugation and fast freezing are the most common and accepted methods of processing, and most, if not all, have similar processing methods (29, 74). Proteins can be detected both in saliva and GCF during the active and retentive phases of tooth movement, and authors such as Burke et al. in 2002 have used both sources. One of the benefits of studying biomarkers in GCF is the specificity of the results, but they cannot detect proteins in GCF, which is probably due to the generalized increase in the concentration of protein in GCF (32). Similar results were reported in one of the recent systematic reviews to explore the presence of salivary proteins during OTM by Allen et al. in 2019; interestingly, the most common cytokines were not detectable because they were diluted in saliva or disappeared too quickly before they were detected (29).
Vansant et al. compared and contrasted in vivo and in vitro studies that assessed the mediators of OTM in their systematic review. Despite the disparity in animal studies, most of them used rodents, which gave some credibility to the results of the studies (27). The results of a 1997 study by King et al. revealed that 13.9 µm of the distal molar moved with time in rats. Interestingly, however, biochemical changes included a sustained increase in bone phosphatases at the mesial roots (previously pressure sites), suggesting continued mesial molar movement after appliance removal. However, this finding does not indicate that there might be continued alveolar bone remodeling several days after removal of force application, which is consistent with the direction of loading of force but returns to baseline levels at the end of 14 days (21).
Vascular endothelial growth factor (VEGF) and endothelin, which mediate vascular alterations and angiogenesis, gradually increase on the tension side during the first week and remain elevated during the second week. Vascularity during relapse was first explained in terms of its number and density by Murell EF et al. in 1996. Changes in vascularity always occur in the direction of tooth movement (either during orthodontic tooth movement or during relapse). They concluded that vascular changes, especially when the orthodontic force is increased after removal, could contribute to relapse by modulating interstitial tissue pressure during alveolar remodeling (31).
There were many confounding factors among the studies, such as patient characteristics (sample size, age and growth status), orthodontic force application (type, time, duration), retention protocol (type, and duration of wear), detection source (GCF or saliva), and oral hygiene maintenance regimen. Although an adequate sample size is more credible to the outcome of results, it is not very critical in orthodontic studies for two reasons: during orthodontic treatment, it makes more sense to monitor a patient for a longer period than to follow up many patients once, and most of the biomarkers have high interpatient variability; therefore, minimizing the sample size and increasing the time intervals to study biomarkers of orthodontic tooth movement will be more logical (29). Depending on growth status, growing and nongrowing individuals express different mediators (25). Heavy orthodontic forces increase ALP levels, indicating that hyalinization is also characterized by increased aspartate transaminase (AST) levels, all of which occur within the first 4 weeks of force application (26).
Clinical implications
Comprehension of the mediator expression could give an insight to the following;
• Vulnerability of the tissues at various time points and how retention can be tailored accordingly.
• Though presumptive and not yet trial tested, RANKL/OPG, MMP/TIMP modulation may be investigated for reducing the relapse.
Animal to human studies (translational considerations)
• Saliva is the most common medium used for the assay in humans in comparison with the animal models where pdl/ bone samples are used.
• Oral biotope for humans and animal models cannot be equated
• Experimental forces and remodeling rates differ in animal and human models
Limitations
• Human studies are exhaustive, and the available literature is skewed toward the study of only some biomarkers, ignoring the remaining biomarkers, increasing the need for more research on several other unexplored mediators of OTM.
• Even with the literature, various results have been reported, which can be attributed primarily to differences in methodologies and participant characteristics, ultimately making comparisons difficult. There was a need to strike the right balance between homogeneity and specificity since any attempt to minimize heterogeneity could lead to population-confined results (29).
• Studies with GCF as a source of biomarkers require excellent handling skills, and only some studies mention the conditions under which the GCF is handled; therefore, the results should be interpreted with caution (26).
• With respect to animal studies, most have used rodents that most closely mimic the biology of humans, thereby making comparisons easier. However, the force levels used are interestingly higher than what is expected. For example, a smaller surface area of the roots of rat molars demands the use of less force to allow for frontal resorptive activity. Therefore, the results from animal studies need to be interpreted with caution (27). Rodents, unlike humans, have a physiological distal drift, and studies involving rodents apply force to the molars mesially, and relapse, if any, is evaluated at the distal aspect of molars. Four animal studies have assessed relapse, and all of them revealed a rapid increase in the rate of distal movement followed by a gradual decline over the next 21 days until it reached rates close to physiological drift, indicating that there was no further relapse. There was also a concurrent increase in the m—RNA expression of COL1A1, ALP, and OCN during the initial relapse phase. Franzen et al. demonstrated increased tissue mineral density and % bone volume in the microcts of rats in the direction of relapse at compression sites (20).
• Controlling or standardizing the variables has been easier in in vitro studies. However, its clinical translation remains questionable (27).
Conclusions
Active phase
Inflammatory mediators
There is a release of inflammatory mediators such as cytokines and interleukins within the first hour, reaching a peak approximately 24 h after which they disappear.
Osteogenic/enzymatic mediators
ALP and TRAP peak at 1 week and 4 weeks, respectively. OCN and COL1A1 increase later reflecting the consolidation of the newly formed tissues.
ECM remodeling mediators
The commonly released enzymes (MMP1 and MMP2) are released within the first 4 h coordinating periodontal ligament and matrix turnover.
Retentive phase
• The relapse rate is the maximum immediately after the removal of orthodontic force.
• Animal models report an increase in the signalling molecules immediately after unloading, which return to baseline levels within 3 weeks.
• Human cohorts report ALP activity lasting for 3–6 months suggesting an extended period of retention post expansion.
• One of the human studies revealed a distribution of proteins that are detected in both the active and retentive phases, with the exception of tyrosine protein kinase (TEC) which was observed only during the retention phase.
Although there is a consistent temporal pattern of biomarker expression, there is still a lacuna in the mediator specific mechanisms notably during the retention phase. This uncertainty is the key conclusion of our review emphasizing the need for future research focusing both on quantification of relapse and mediator expression during retention phase.
Knowledge gaps
• Scarce literature on human longitudinal studies during retention phase.
• Limited standardization of matrices, assays and sampling schedules across the existing literature.
• No literature on objective correlation between biomarker expression and the quantitative amount of relapse.
Scope for future research
• The biomarker studies during retention can adopt standardized time points and correlate the biomarker expression with the amount of relapse measured by the existing relapse indices.
• The evidence on biomarkers can be taken a step further by executing studies that focus on their correlation with the type of archwire, bracket system and microbial colonization.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SP: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding is provided by Sri Ramachandra Institute of Higher Education & Research, Chennai, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kitaura H, Kimura K, Ishida M, Sugisawa H, Kohara H, Yoshimatsu M, et al. Effect of cytokines on osteoclast formation and bone resorption during mechanical force loading of the periodontal membrane. Sci World J. (2014) 2014:617032. doi: 10.1155/2014/617032
2. Iwasaki LR, Haack JE, Nickel JC, Reinhardt RA, Petro TM. Human interleukin-1β and interleukin-1 receptor antagonist secretion and velocity of tooth movement. Arch Oral Biol. (2001) 46(2):185–9. doi: 10.1016/S0003-9969(00)00088-1
3. Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop. (2001) 119 (3):307–12. doi: 10.1067/mod.2001.110809
4. Başaran G, Özer T, Kaya FA, Hamamci O. Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. Am J Orthod Dentofacial Orthop. (2006) 130(1):7-e1. doi: 10.1016/j.ajodo.2005.12.027
5. Grant M, Wilson J, Rock P, Chapple I. Induction of cytokines, MMP9, TIMPs, RANKL and OPG during orthodontic tooth movement. Eur J Orthod. (2013) 35(5):644–51. doi: 10.1093/ejo/cjs057
6. Saadi N, Ghaib NH. Effect of orthodontic tooth movement on salivary levels of interleukin-1beta, tumor necrosis factor-alpha, and C-reactive protein. J Baghdad Coll Dent. (2013) 325(2209):1–6. doi: 10.12816/0015078
7. Saloom HF. Evaluation of Salivary Levels of Proinflammatory Cytokines (IL-1α, IL-8 and GM-CSF) in Adult Orthodontic Patients. Ghaziabad: International Organization Of Scientific Research (IOSR) (2014). doi: 10.9790/0853-13317578
8. Castroflorio T, Gamerro EF, Caviglia GP, Deregibus A. Biochemical markers of bone metabolism during early orthodontic tooth movement with aligners. Angle Orthod. (2017) 87(1):74–81. doi: 10.2319/022416-159.1
9. Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. (1984) 85(6):508–18. doi: 10.1016/0002-9416(84)90091-5
10. Grieve WG III, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1β (IL-1β) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (1994) 105(4):369–74. doi: 10.1016/S0889-5406(94)70131-8
11. Cantarella G, Cantarella R, Caltabiano M, Risuglia N, Bernardini R, Leonard R. Levels of matrix metalloproteinases 1 and 2 in human gingival crevicular fluid during initial tooth movement. Am J Orthod Dentofacial Orthop. (2006) 130(5):568.e11–6. doi: 10.1016/j.ajodo.2006.04.020
12. Ingman T, Apajalahti S, Mäntylä P, Savolainen P, Sorsa T. Matrix metalloproteinase-1 and -8 in gingival crevicular fluid during orthodontic tooth movement: a pilot study during 1 month of follow-up after fixed appliance activation. Eur J Orthod. (2005) 27(2):202–7. doi: 10.1093/ejo/cjh097
13. Canavarro C, Teles RP, Capelli Júnior J. Matrix metalloproteinases-1, -2, -3, - 7, -8, -12, and-13 in gingival crevicular fluid during orthodontic tooth movement: a longitudinal randomized split-mouth study. Eur J Orthod. (2013) 35(5):652–8. doi: 10.1093/ejo/cjs053
14. Capelli Junior J, Kantarci A, Haffajee A, Teles RP, Fidel Jr R, & Figueredo CM. Matrix metalloproteinases and chemokines in the gingival crevicular fluid during orthodontic tooth movement. Eur J Orthod. (2011) 33(6):705–11. doi: 10.1093/ejo/cjq148
15. Takahashi I, Nishimura M, Onodera K, Bae JW, Mitani H, Okazaki M, et al. Expression of MMP-8 and MMP-13 genes in the periodontal ligament during tooth movement in rats. J Dent Res. (2003) 82(8):646–51. doi: 10.1177/154405910308200815
16. Ariffin SHZ, Elias MF, Wahab RMA, Bakar Y, Senafi S. Profiles of lactate dehydrogenase, tartrate resistant acid phosphatase and alkaline phosphatase in saliva during orthodontic treatment. Sains Malaysiana. (2010) 39(3):405–12. Available online at: https://www.researchgate.net/publication/285966029_Profiles_of_Lactate_Dehydrogenase_Tartrate_Resistant_Acid_Phosphatase_and_Alkaline_Phosphatase_in_Saliva_during_Orthodontic_Treatment
17. Marcaccini AM, Amato PA, Leão FV, Gerlach RF, Ferreira JT. Myeloperoxidase activity is increased in gingival crevicular fluid and whole saliva after fixed orthodontic appliance activation. Am J Orthod Dentofacial Orthop. (2010) 138(5):613–6. doi: 10.1016/j.ajodo.2010.01.029
18. Maltha JC, Vandevska-Radunovic V, Kuijpers-Jagtman AM. The biological background of relapse of orthodontic tooth movement. In: Krishnan V, Davidovitch Z, editors. Biological Mechanisms of Tooth Movement. NJ: Wiley (2015). p. 297–307. doi: 10.1002/9781119608912.ch19
19. Nakanishi H, Seki Y, Kohno T, Muramoto T, Toda K, Soma K. Changes in response properties of periodontal mechanoreceptors after experimental orthodontic tooth movement in rats. Angle Orthod. (2004) 74(1):93–9. doi: 10.1043/0003-3219(2004)074%3C0093:CIRPOP%3E2.0.CO;2
20. Franzen TJ, Monjo M, Rubert M, Vandevska-Radunovic V. Expression of bone markers and micro-CT analysis of alveolar bone during orthodontic relapse. Orthod Craniofac Res. (2014) 17(4):249–58. doi: 10.1111/ocr.12050
21. King GJ, Latta L, Rutenberg J, Ossi A, Keeling SD. Alveolar bone turnover and tooth movement in male rats after removal of orthodontic appliances. Am J Orthod Dentofacial Orthop. (1997) 111(3):266–75. doi: 10.1016/S0889-5406(97)70184-7
22. Awang-Kechik NH, Ahmad R, Doustjalali SR, Sabet NS, Abd-Rahman AN. Liquid chromatography‒mass spectrometry (LC‒MS) analysis in determining the saliva protein of orthodontic patients during retention phase. J Clin Exp Dent. (2019) 11(3):e269–74. doi: 10.4317/jced.55546
23. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169(7):467–73. doi: 10.7326/M18-0850
24. McGowan J, Sampson M, Lefebvre C. An evidence-based checklist for the peer review of electronic search strategies (PRESS EBC). Evid Based Libr Inf Pract. (2010) 5(1):149–54. doi: 10.18438/B8SG8R
25. Kapoor P, Kharbanda OP, Monga N, Miglani R, Kapila S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog Orthod. (2014) 15(1):1–21. doi: 10.1186/s40510-014-0065-6
26. Kapoor P, Monga N, Kharbanda OP, Kapila S, Miglani R, Moganty R. Effect of orthodontic forces on levels of enzymes in gingival crevicular fluid (GCF): a systematic review. Dental Press J Orthod. (2019) 24:40–e1. doi: 10.1590/2177-6709.24.2.40.e1-22.onl
27. Vansant L, De Llano-Pérula MC, Verdonck A, Willems G. Expression of biological mediators during orthodontic tooth movement: a systematic review. Arch Oral Biol. (2018) 95:170–86. doi: 10.1016/j.archoralbio.2018.08.003
28. Alhadlaq AM, Patil S. Biomarkers of orthodontic tooth movement in gingival crevicular fluid: a systematic review. J Contemp Dent Pract. (2015) 16(7):578–87. doi: 10.5005/jp-journals-10024-1725
29. Allen RK, Edelmann AR, Abdulmajeed A, Bencharit S. Salivary protein biomarkers associated with orthodontic tooth movement: a systematic review. Orthod Craniofac Res. (2019) 22:14–20. doi: 10.1111/ocr.12258
30. Reitan K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am J Orthod. (1967) 53(10):721–45. doi: 10.1016/0002-9416(67)90118-2
31. Murrell EF, Yen EH, Johnson RB. Vascular changes in the periodontal ligament after removal of orthodontic forces. Am J Orthod Dentofacial Orthop. (1996) 110(3):280–6. doi: 10.1016/S0889-5406(96)80012-6
32. Burke JC, Evans CA, Crosby TR, Mednieks MI. Expression of secretory proteins in oral fluid after orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (2002) 121(3):310–5. doi: 10.1067/mod.2002.121011
33. Perinetti G, D'Apuzzo F, Contardo L, Primozic J, Rupel K, Perillo L. Gingival crevicular fluid alkaline phosphate activity during the retention phase of maxillary expansion in prepubertal subjects: a split-mouth longitudinal study. Am J Orthod Dentofacial Orthop. (2015) 148(1):90–6. doi: 10.1016/j.ajodo.2015.02.025
34. Chase SW, Revesz J. Reestablishment of transseptal fibers following extraction. J Dent Res. (1944) 23(5):333–6. doi: 10.1177/00220345440230050501
35. Boisson M, Gianelly AA. Collagen synthesis in rat gingiva during tooth movement. Am J Orthod. (1998) 80(3):289–99. doi: 10.1016/0002-9416(81)90291-8
36. Circuns AL, Tulloch JC. Gingival invagination in extraction sites of orthodontic patients: their incidence, effects on periodontal health, and orthodontic treatment. Am J Orthod. (1983) 83(6):469–76. doi: 10.1016/S0002-9416(83)90245-2
37. Redlich M, Palmon A, Zaks B, Geremi E, Rayzman S, Shoshan S. The effect of centrifugal force on the transcription levels of collagen type I and collagenase in cultured canine gingival fibroblasts. Arch Oral Biol. (1998) 43(4):313–6. doi: 10.1016/S0003-9969(97)00108-8
38. Redlich M, Shoshan S, Palmon A. Gingival response to orthodontic force. Am J Orthod Dentofacial Orthop. (1999) 116(2):152–8. doi: 10.1016/S0889-5406(99)70212-X
39. Redlich M, Roos HA, Reichenberg E, Zaks B, Mussig D, Baumert U, et al. Expression of tropoelastin in human periodontal ligament fibroblasts after simulation of orthodontic force. Arch Oral Biol. (2004) 49(2):119–24. doi: 10.1016/j.archoralbio.2003.08.002
40. Bolcato-Bellemin AL, Elkaim R, Abehsera A, Fausser JL, Haikel Y, Tenenbaum H. Expression of mRNAs encoding for α and β integrin subunits, MMPs, and TIMPs in stretched human periodontal ligament and gingival fibroblasts. J Dent Res. (2000) 79(9):1712–6. doi: 10.1177/00220345000790091201
41. Danciu TE, Gagari E, Adam RM, Damoulis PD, Freeman MR. Mechanical strain delivers anti-apoptotic and proliferative signals to gingival fibroblasts. J Dent Res. (2004) 83(8):596–601. doi: 10.1177/154405910408300803
42. Rygh P, Selvig KA. Erythrocytic crystallization in rat molar periodontium incident to tooth movement. Eur J Oral Sci. (1973) 81(1):62–73. doi: 10.1111/j.1600-0722.1973.tb01495.x
43. Garant PR, Cho MI. Autoradiographic evidence of the coordination of the genesis of Sharpey’s fibers with new bone formation in the periodontium of the mouse. J Periodontal Res. (1979) 14(2):107–14. doi: 10.1111/j.1600-0765.1979.tb00779.x
44. Rygh P, Bowling K, Hovlandsdal L, Williams S. Activation of the vascular system: a main mediator of periodontal fiber remodeling in orthodontic tooth movement. Am J Orthod. (1986) 89(6):453–68. doi: 10.1016/0002-9416(86)90001-1
45. Keeling SD, King GJ, McCoy EA, Valdez M. Serum and alveolar bone phosphatase changes reflect bone turnover during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (1993) 103(4):320–6. doi: 10.1016/0889-5406(93)70012-D
46. Rody WJ Jr., King GJ, Gu G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (2001) 120(5):477–89. doi: 10.1067/mod.2001.118623
47. Howard PS, Kucich U, Taliwal R, Korostoff JM. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J Periodontal Res. (1998) 33(8):500–8. doi: 10.1111/j.1600-0765.1998.tb02350.x
48. Bumann A, Carvalho RS, Schwarzer CL, Yen EH. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. (1997) 19(1):29–37. doi: 10.1093/ejo/19.1.29
49. Noxon SJ, King GJ, Gu G, Huang G. Osteoclast clearance from periodontal tissues during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (2001) 120(5):466–76. doi: 10.1067/mod.2001.117912
50. Attal U, Blaushild N, Brin I, Steigman S. Histomorphometric study of the periodontal vasculature during and after experimental tipping of the rat incisor. Arch Oral Biol. (2001) 46(10):891–900. doi: 10.1016/S0003-9969(01)00058-9
51. Verna C, Dalstra M, Lee TC, Cattaneo PM, Melsen B. Microcracks in the alveolar bone following orthodontic tooth movement: a morphological and morphometric study. Eur J Orthod. (2004) 26(5):459–67. doi: 10.1093/ejo/26.5.459
52. Perinetti G, Paolantonio M, D′Attilio M, D′Archivio D, Tripodi D, Femminella B, et al. Alkaline phosphatase activity in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (200) 122:548–6. doi: 10.1067/mod.2002.126154
53. Batra P, Kharbanda OP, Duggal R, Singh N, Parkash H. Alkaline phosphatase activity in gingival crevicular fluid during canine retraction. Orthod Craniofac Res. (2006) 9(1):44–51. doi: 10.1111/j.1601-6343.2006.00358.x
54. Flórez-Moreno GA, Marín-Restrepo LM, Isaza-Guzmán DM, Tobón-Arroyave SI. Screening for salivary levels of deoxypyridinoline and bone-specific alkaline phosphatase during orthodontic tooth movement: a pilot study. Eur J Orthod. (2013) 35(3):361–8. doi: 10.1093/ejo/cjr138
55. Megat Abdul Wahab R, Dasor M M, Senafi S, Abang Abdullah AA, Yamamoto Z, Jemain AA, et al. Crevicular alkaline phosphatase activity and rate of tooth movement of female orthodontic subjects under different continuous force applications. Int J Dent. (2013) 2013(1):245818. doi: 10.1155/2013/245818
56. Wahab RM, Dasor MM, Senafi S, Abdullah AA, Jemain AA, Kasim NA, et al. Crevicular tartrate resistant acid phosphatase activity and rate of tooth movement under different continuous force applications. Afr J Pharm Pharmacol. (2011) 5(20):2213–9. doi: 10.5897/AJPP11.304
57. Serra E, Perinetti G, D’Attilio M, Cordella C, Paolantonio M, Festa F, et al. Lactate dehydrogenase activity in gingival crevicular fluid during orthodontic treatment. Am J Orthod Dentofacial Orthop. (2003) 124(2):206–11. doi: 10.1016/S0889-5406(03)00407-4
58. Perinetti G, Serra E, Paolantonio M, Bruè C, Meo SD, Filippi MR, et al. Lactate dehydrogenase activity in human gingival crevicular fluid during orthodontic treatment: a controlled, short-term longitudinal study. J Periodontol. (2005) 76(3):411–7. doi: 10.1902/jop.2005.76.3.411
59. Rohaya MA, Muda JR. The activity of aspartate aminotransferase during canine retraction (bodily tooth movement) in. J Med Sci. (2008) 8(6):553–8. doi: 10.3923/jms.2008.553.558
60. Wahab RMA, Hisham S, Khazlina K. Preliminary study of aspartate aminotransferase activity in the gingival crevicular fluids during orthodontic tooth movement. J App Sci. (2009) 9(7):1393–6. doi: 10.3923/jas.2009.1393.1396
61. Hayashi K, Igarashi K, Miyoshi K, Shinoda H, Mitani H. Involvement of nitric oxide in orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. (2002) 122(3):306–9. doi: 10.1067/mod.2002.126151
62. Sugiyama Y, Yamaguchi M, Kanekawa M, Yoshii M, Nozoe T, Nogimura A, et al. The level of cathepsin B in gingival crevicular fluid during human orthodontic tooth movement. Eur J Orthod. (2003) 25(1):71–6. doi: 10.1093/ejo/25.1.71
63. Yoo SK, Warita H, Soma K. Duration of orthodontic force affecting initial response of nitric oxide synthase in rat periodontal ligaments. J Med Dent Sci. (2004) 51(1):83–8. PMID: 1513746915137469
64. D'Attillio M, Di Maio F, D'Arcangela C, Rita Filippi M, Felaco M, Lohinai Z, et al. Gingival endothelial and inducible nitric oxide synthase levels during orthodontic treatment: a cross-sectional study. Angle Orthod. (2004) 74(6):851–8. doi: 10.1043/0003-3219(2004)074%3C0851:GEAINO%3E2.0.CO;2
65. Zhang J, Zhou S, Zheng H, Zhou Y, Chen F, Lin J. Magnetic bead-based salivary peptidome profiling analysis during orthodontic treatment durations. Biochem Biophys Res Commun. (2012) 421(4):844–9. doi: 10.1016/j.bbrc.2012.04.100
66. Elias MF, Zainal Ariffin SH, Karsani SA, Abdul Rahman M, Senafi S, Megat Abdul Wahab R. Proteomic analysis of saliva identifies potential biomarkers for orthodontic tooth movement. Sci World J. (2012) 2012(1):647240. doi: 10.1100/2012/647240
67. Hussian SF, Ghaib NH. Expression of secretary proteins in whole unstimulated saliva before and after placement of orthodontic elastic separators in Iraqi samples (clinical study). Iraqi Orthod J. (2005) 1:xxi-i.
68. Waddington RJ, Embery G. Proteoglycans and orthodontic tooth movement. J Orthod. (2001) 28(4):281–90. doi: 10.1093/ortho/28.4.281
69. Talic N, Evans CA, Daniel JC, George A, Zaki AM. Immunohistochemical localization of αvβ3 integrin receptor during experimental tooth movement. Am J Orthod Dentofacial Orthop. (2004) 125(2):178–84. doi: 10.1016/j.ajodo.2003.03.005
70. Norevall LI, Forsgren S, Matsson L. Expression of neuropeptides (CGRP, substance P) during and after orthodontic tooth movement in the rat. Eur J Orthod. (1995) 17(4):311–25. doi: 10.1093/ejo/17.4.311
71. Yamaguchi M, Yoshii M, Kasai K. Relationship between substance P and interleukin-1β in gingival crevicular fluid during orthodontic tooth movement in adults. Eur J Orthod. (2006) 28(3):241–6. doi: 10.1093/ejo/cji100
72. Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. (2009) 12(2):113–9. doi: 10.1111/j.1601-6343.2009.01444.x
73. Redlich M, Reichenberg E, Harari D, Zaks B, Shoshan S, Palmon A. The effect of mechanical force on mRNA levels of collagenase, collagen type I, and tissue inhibitors of metalloproteinases in gingivae of dogs. J Dent Res. (2001) 80(12):2080–4. doi: 10.1177/00220345010800121101
74. Apajalahti S, Sorsa T, Ingman T. Matrix metalloproteinase -2, -8, -9, and -13 in gingival crevicular fluid of short root anomaly patients. Eur J Orthod. (2003) 25(4):365–9. doi: 10.1093/ejo/25.4.365
75. Insoft M, King GJ, Keeling SD. The measurement of acid and alkaline phosphatase in gingival crevicular fluid during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (1996) 109(3):287–96. doi: 10.1016/S0889-5406(96)70152-X
76. Alfaqeeh SA, Anil S. Lactate dehydrogenase activity in gingival crevicular fluid as a marker in orthodontic tooth movement. Open Dent J. (2011) 5:105–9. doi: 10.2174/1874210601105010105
77. Rhee SH, Kang J, Nahm DS. Cystatins and cathepsin B during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. (2009) 135(1):99–105. doi: 10.1016/j.ajodo.2006.10.029
Keywords: mediators, relapse, tooth movement, bone remodelling, cytokines
Citation: Ananthanarayanan V and Padmanabhan S (2025) Biochemical mediators during active and retentive phases of tooth movement in orthodontic patients—a scoping review. Front. Oral Health 6:1681304. doi: 10.3389/froh.2025.1681304
Received: 7 August 2025; Accepted: 15 October 2025;
Published: 17 November 2025.
Edited by:
Aswini Y. Balappanavar, University of Delhi, IndiaReviewed by:
Janvier Habumugisha, Okayama University, JapanAnshika Gandhi, University of Delhi, India
Copyright: © 2025 Ananthanarayanan and Padmanabhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sridevi Padmanabhan, c3JpZGV2aXBhZG1hbmFiaGFuQHNyaXJhbWFjaGFuZHJhLmVkdS5pbg==
 Venkateswaran Ananthanarayanan
Venkateswaran Ananthanarayanan Sridevi Padmanabhan
Sridevi Padmanabhan