- 1Department of Pharmacy, Life Science School, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj (Dhaka), Bangladesh
- 2Laboratory of Theoretical and Computational Biophysics, Ton Duc Thang University, Ho Chi Minh City, Vietnam
- 3Faculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City, Vietnam
- 4Department of Nutrition and Dietetics, Faculty of Pharmacy, and Centre for Healthy Living, University of Concepción, Concepción, Chile
- 5Universidad de Concepción, Unidad de Desarrollo Tecnológico, UDT, Concepción, Chile
- 6Department of Toxicology, University of Medicine and Pharmacy of Craiova, Craiova, Romania
- 7Department of Botany, University of Fort Hare, Alice, South Africa
- 8Phytochemistry Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 9Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, Craiova, Romania
The COVID-19 pandemic represents an unprecedented challenge for the researchers to offer safe, tolerable, and effective treatment strategies for its causative agent known as SARS-CoV-2. With the rapid evolution of the pandemic, even the off-label use of existing drugs has been restricted by limited availability. Several old antivirals, antimalarial, and biological drugs are being reconsidered as possible therapies. The effectiveness of the controversial treatment options for COVID-19 such as nonsteroidal antiinflammatory drugs, angiotensin 2 conversion enzyme inhibitors and selective angiotensin receptor blockers was also discussed. A systemic search in the PubMed, Science Direct, LitCovid, Chinese Clinical Trial Registry, and ClinicalTrials.gov data bases was conducted using the keywords “coronavirus drug therapy,” passive immunotherapy for COVID-19’, “convalescent plasma therapy,” (CPT) “drugs for COVID-19 treatment,” “SARS-CoV-2,” “COVID-19,” “2019-nCoV,” “coronavirus immunology,” “microbiology,” “virology,” and individual drug names. Systematic reviews, case presentations and very recent clinical guidelines were included. This narrative review summarizes the available information on possible therapies for COVID-19, providing recent data to health professionals.
Introduction
The contemporary century has witnessed the outbreak of several corona viral intimidations that cause a spotlight on public health, education, economy, and travels and respond to the threat of a global pandemic. The ongoing viral infection is caused by SARS-CoV-2, which establishes a novel coronavirus disease 2019 (COVID-19). Analogous (79.6% similar) to SARS-CoV, the SARS-CoV-2 is one of the members of a relatively largest family of the RNA viruses and contains four important structural proteins, such as the surface spike (S) glycoprotein, membrane (M) protein, small envelope (E) glycoprotein, and the nucleocapsid (N) protein that help for its development completely (Figure 1A) (Schoeman and Fielding, 2019; Risitano et al., 2020). The positive-sense, single-stranded RNA genome of SARS-CoV-2 contains a cap at 5’ end and polyadenylated (A) sequence at 3’ end, serves as mRNA for replicase polyprotein translation (Figure 1B) (Wu et al., 2020).
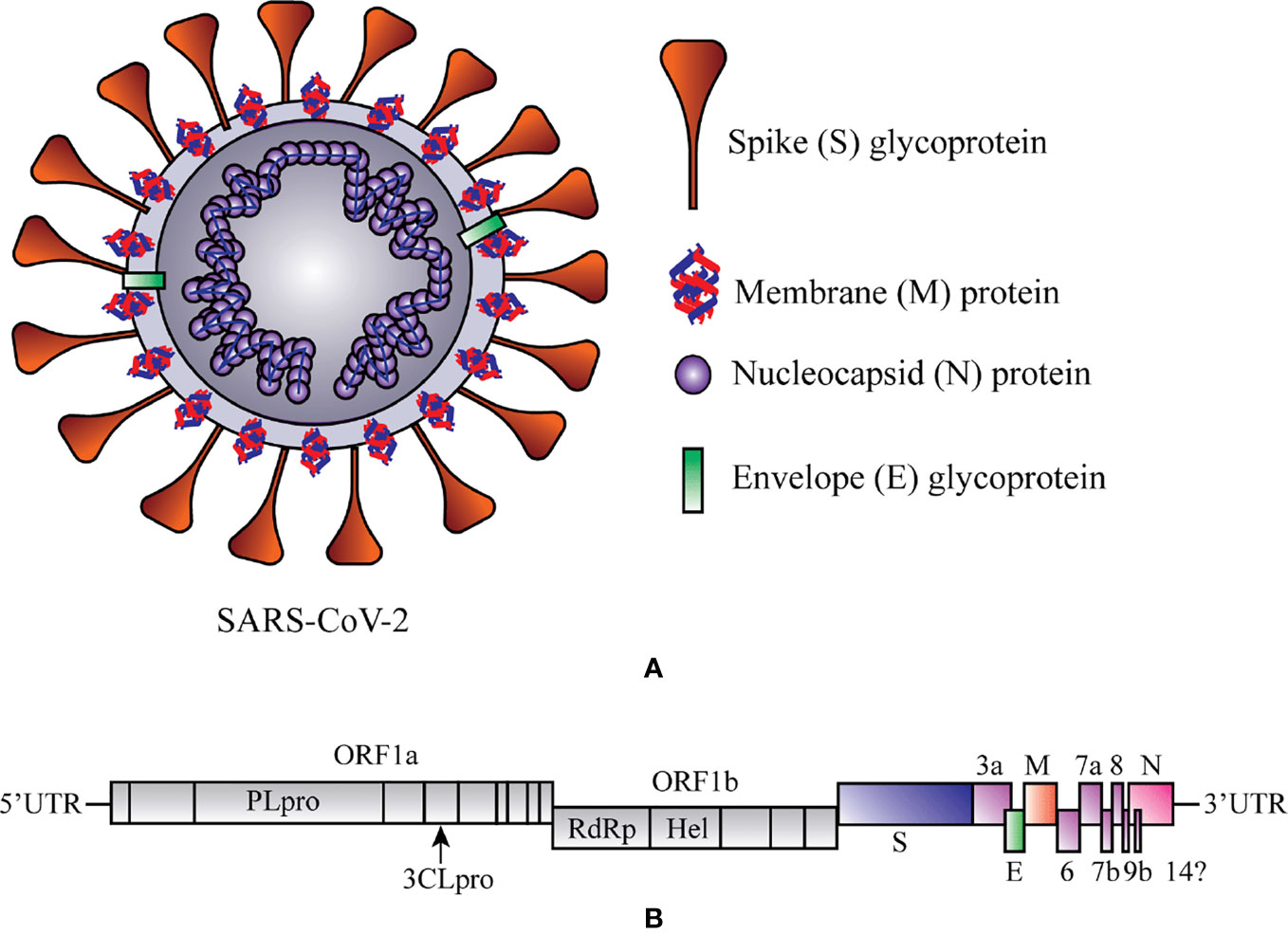
Figure 1 Schematic representation of structure and RNA genome of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). (A) Structure of SAR-CoV-2. (B) RNA genome sequence of SARS-CoV-2. 3CLPRO, 3-Chymotrypsin-like protease; Hel, helicase; ORF1a/b, Open reading frame 1a/b; PLPRO, Papain-like protease; RdRp, RNA-dependent RNA polymerase. At 5ʹ end 67% viral genome contains two open reading frames (ORF1a and ORF1b) that encode two significant replicase genes (rep1a and rep1b), and which helps to express large replicase polyprotein 1a/ab (pp1a and pp1ab) (Islam et al., 2020). These polyproteins produce nonstructural proteins (e.g., RNA-dependent RNA polymerase (RdRp) and helicase) after the cleavage with the help of two enzymes, papain-like cysteine protease (PLPRO) and 3-chymotrypsin-like serine protease (3CLPRO) (Zumla et al., 2016). At 3ʹ end 33% viral genome encodes the structural proteins (e.g., S, M, E, and N), which are required for the attachment of virus particle and entry of the viral genome into the host cell (Peiris et al., 2004).
From the beginning of the outbreak of SARS-CoV-2, it spreads immediately in most of the countries around the world and causes severe human diseases or death (Goumenou et al., 2020). The lack of effective drug therapy, and along with the high morbidity and mortality rates and its pandemic highlights the need for novel drug discovery for the treatment of COVID-19 (Tsatsakis et al., 2020).
Several national and international institutions and research groups are working collaboratively on a diversity of preemptive and beneficial interventions.
Cheap and widely available, dexamethasone is a steroid commonly used to treat allergic reactions, but also rheumatoid arthritis and asthma (Mititelu et al., 2020). British researchers who researched an effective treatment for COVID-19 reported that dexamethasone reduced deaths by a third among the most severely ill patients compared to regular treatment (Horby et al., 2020). It is currently conducting an analysis of the results obtained from the RECOVERY study arm regarding the use of dexamethasone-containing drugs in the treatment of hospitalized patients with COVID-19 infection. This component of the study looked at the effects of adding dexamethasone to regular therapeutic measures taken in adults who are being given invasive ventilation, those who are being given oxygen, or those who are not being given extra oxygen (Horby et al., 2020).
In the RECOVERY study, deaths occurred within 28 days of starting dexamethasone treatment. According to the preliminary results, in comparison with the routine measures, the administration of dexamethasone obtained the following: i) reduction by approximately 35% of the mortality rate in patients with invasive mechanical ventilation; ii) reduction by about 20% of the mortality rate in patients who were given oxygen without invasive ventilation; iii) nonreduction of the mortality rate in patients without oxygen therapy (Horby et al., 2020). As a result, in the UK, doctors have announced that patients will start receiving the first drug that has been shown to reduce COVID-19-associated death. While researchers believe that dexamethasone could save the lives of one in eight ventilator-connected patients, it has been shown to have few clinical benefits in less severe cases (Lu et al., 2020).
At least two major studies in the United States have shown that the antiviral drug remdesivir can reduce hospitalization period for patients with COVID-19. The results of these studies showed that remdesivir injections - originally intended as a treatment for Ebola - accelerated the patient’s recovery compared to placebo (Beigel et al., 2020; Goldman et al., 2020). Therefore, the US has authorized the emergency use of Remdesivir, an initiative followed by the European Union and several Asian nations, including Japan and South Korea (Gilead.Com, 2020).
China has completed clinical research on Favipiravir, an antiviral drug that has been shown to be clinically effective against the disease caused by the new coronavirus. Favipiravir, a flu medicine approved for clinical use in Japan in 2014, did not show any obvious side effects in the clinical trial (Heng et al., 2020).
More than 80 patients participated in the clinical trial, 35 of these patients received treatment with Favipiravir, and 45 were included in a control group. The results showed that patients treated with Favipiravir had negative results in testing for this virus in a shorter time compared to patients in the control group (Clinicaltrials.Gov, 2020a). Another randomized clinical trial also suggested that the therapeutic effect of Favipiravir was much better than that seen in the control group (Clinicaltrials.Gov, 2020bm). So, Favipiravir has been recommended by Chinese physicians and should be included in the diagnosis and treatment plan for COVID-19.
Prospective opportunities being reconnoitered include vaccine development, monoclonal antibodies (mAbs), interferon-based therapies, CPT, small-molecular drug therapies, and cell-based therapies (Li and De Clercq, 2020). (Calina et al., 2020).
In this comprehensive narrative review, we have sketched a current scenario on the most recent or ongoing clinical trials along with the remaining challenges and future perspectives of COVID-19 therapies.
Methodology
Since there is little information about these drug candidates in the peer-reviewed literature, aimed at this review, we also collected data from the publicly available websites and electronic and print media. In order to obtain all registered therapeutic and preventative interventions under clinical investigation, a systemic search (up to 10th June 2020) in the PubMed, Science Direct, LitCovid, Chinese Clinical Trial Registry, and ClinicalTrials.gov databases was conducted using the keywords “coronavirus drug therapy,” “passive immunotherapy for COVID-19,” “CPT,” “drugs for COVID-19 treatment,” “SARS-CoV-2,” “COVID-19,” “2019-nCoV,” “coronavirus immunology,” “microbiology,” “virology,” and individual drug names (Table 1). No language, country or study design restrictions were imposed. All information was evaluated in the knowledge about the treatment candidates, characteristics, dose/conc. (route of admin.), study systems, mechanism of action, and the stage of development of the COVID-19 therapies. The inclusion and exclusion criteria are given below.
Inclusion Criteria
1. Studies on current COVID-19 drug therapy performed in SARS-CoV-2 infected patients;
2. Studies that exploited single and/or multiple animals;
3. Registered clinical trials on the proposed, repurposed or experimental candidates for the COVID-19 treatment that are recorded in online registries such as ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP) of the WHO;
4. Therapeutic candidates with beneficial consideration after clinical trials;
5. Therapeutic candidates exhibited auspicious effectiveness in contrast to COVID-19;
6. Studies with or without proposing mechanism of actions of the therapeutic candidates in COVID-19;
7. The most recent or ongoing clinical trial(s) on the individual treatment candidate.
Exclusion Criteria
1. Data duplication, titles or abstracts not meeting the inclusion criteria;
2. Studies on antiviral drug candidates other than SARS-CoV-2;
3. Reports on treatment candidates that encode membrane (M), envelope (E), nucleocapsid (N) and spike (S) protein of genomic RNA other than SARS-CoV-2;
4. Active clinical trials were identified other than the ClinicalTrials.gov and the Chinese Clinical Trial Registry;
5. Previous clinical trial(s) on the individual candidate other than SARS-CoV-2 outbreak.
Old and New Drugs Potentially Purposed for COVID-19 Treatment
From December 2019, several clinical trials (including those not yet recruiting, recruiting, active, or completed) of the proposed or repurposed drugs in several countries around the world are continuously proceeding to deliver real-world clinical data for the COVID-19 challenges. We selected a total of 72 most current or ongoing clinical trials of the COVID-19 drug candidates with their mechanism of actions after refining through inclusion and exclusion criteria that might be helpful to screen, therefore, considered as starting points to discover and develop antiviral drug candidates for COVID-19 (Table 1 and Figure 2).
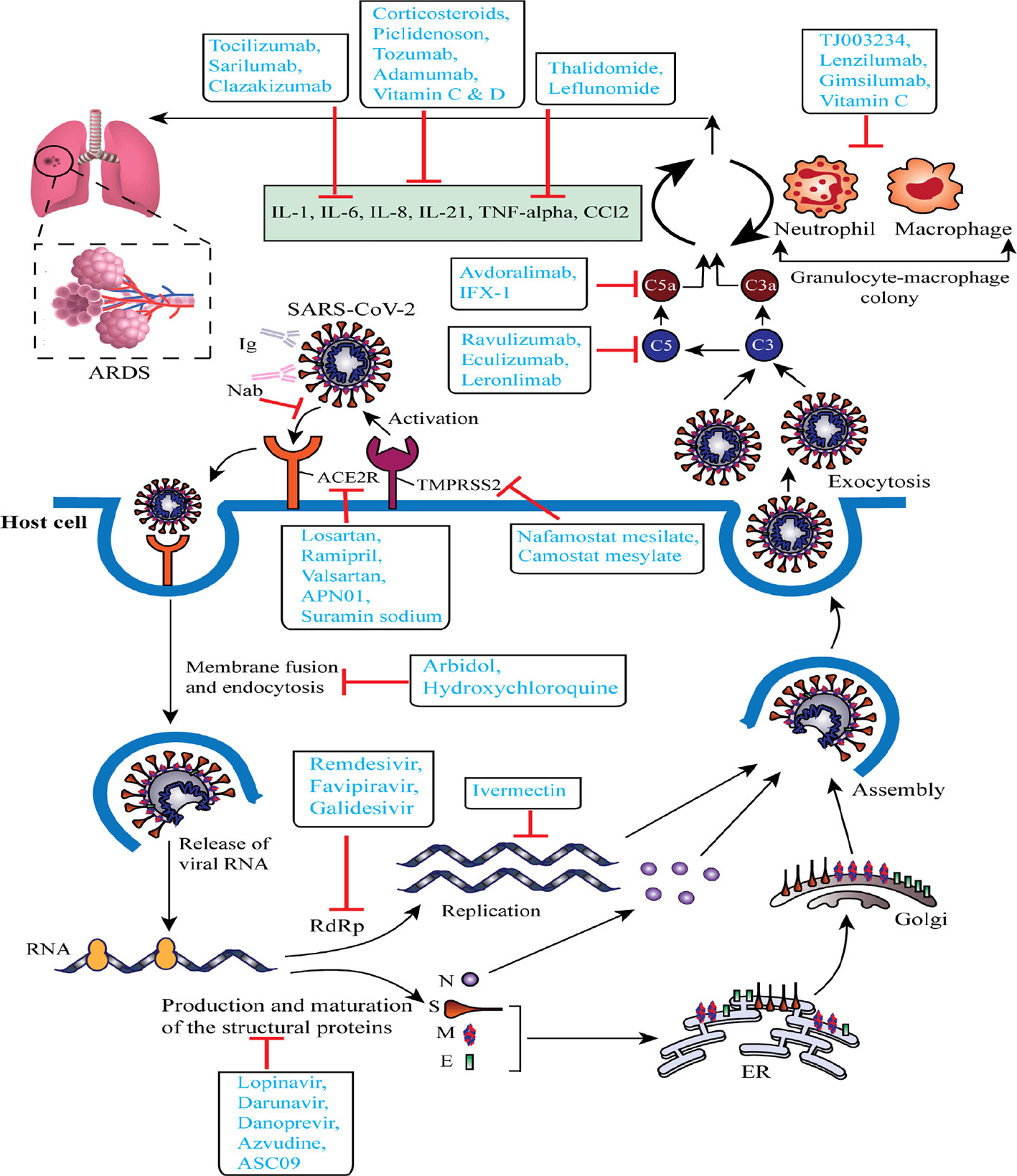
Figure 2 Schematic representation of virus-based treatment responses by targeting the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) replication cycle and SARS-CoV-2 associated acute respiratory distress syndrome. The proposed targets of most important candidates are noted. ACE2R, angiotensin-converting enzyme 2 receptor; ARDS, acute respiratory distress syndrome; ER, endoplasmic reticulum; E, envelope protein; Ig, immunoglobulin; M, membrane protein; N, nucleocapsid protein; Nab, neutralizing antibody; RdRp, RNA-dependent RNA polymerase; S, spike glycoprotein; TMPRSS2, type 2 transmembrane serine protease.
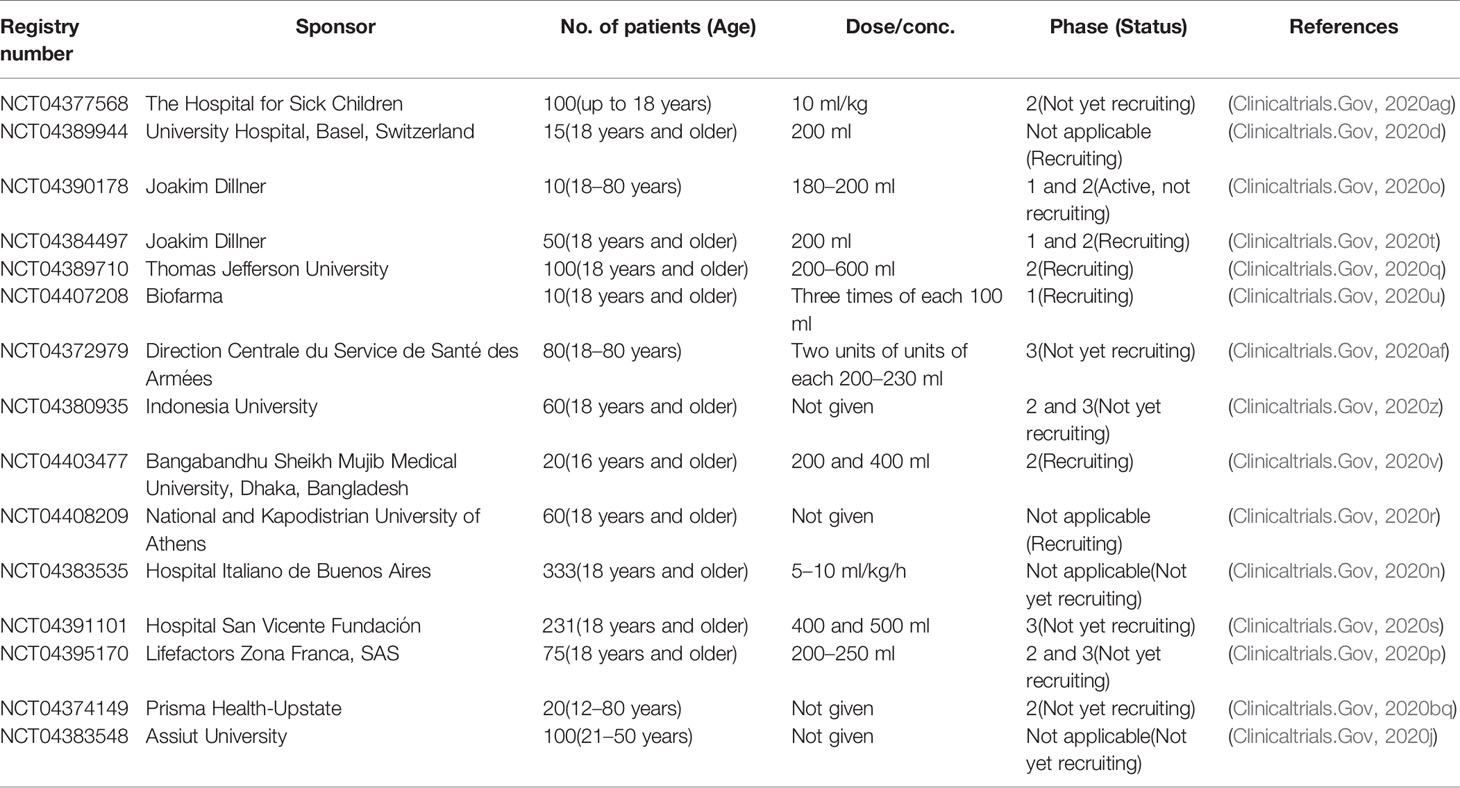
Table 1 shows i) antiviral drugs (nonspecific), ii) antiviral drugs (broad-spectrum), iii) antiviral drugs (antiretrovirals), iv) antimalarial drugs, v) antibiotics and antiparasitics, vi) nonspecific antiinflammatory and immunosuppressive drugs, vii) kinase inhibitors, viii) monoclonal antibodies, ix) hormonal preparations, x) cardiovascular drugs, and xi) blood and blood-forming organs.
Antiviral Drugs (Nonspecific)
Immunoglobulin (Ig)
It is an inhibitor of viral fragment crystallizable (Fc) receptor activation, which prevents antibody-dependent enhancement of infection and provides boosting effects of endogenous neutralizing antibodies (Nabs) (Cao W. et al., 2020). Intravenous Ig is used to investigate to improve the treatment outcome of SARS-CoV-2 infection over the global pandemic with its capacity of proving passive immunity and antiinflammatory, and immunomodulatory effects. For this purpose, in phase 2/3 clinical trial (NCT04261426), 80 participants are treated with IV Ig at 0.5 g/kg/day dose for 5 days to understand the safety and efficacy of it in COVID-19 (Clinicaltrials.Gov, 2020ah).
Interferon (IFN)-β1a and -β1b
Interferon (IFN)-β1a is a cytokine signaling molecule used in the treatment of several chronic viral infections (e.g., HBV, HCV) that activates cytoplasmic enzymes, thereby, prevents mRNA translation and protein synthesis (Hensley et al., 2004; Clerico et al., 2007; Docea et al., 2016; Kamal et al., 2017). Recently, a research team of MJM Bontenprovides an adaptable research platform for the evaluation of treatment efficacy of IFN-β1a against the ongoing global pandemic in phase 4 clinical trial (NCT02735707) applying 10 μg intravenous (IV) dose once daily for 6 days in COVID-19 patients (n = 7,100) (Clinicaltrials.Gov, 2020bf). On the other hand, IFN-β1b, a cytokine used in the treatment of multiple sclerosis, is studied on 80 infected patients in phase 2 clinical trial (NCT04276688) with 0.25 mg subcutaneous (SC) dose for 3 days to evaluate the reduction of mortality rate (Hung et al., 2020). The combined therapy (lopinavir/ritonavir, ribavirin, and IFN-β1b) was found to suppress the viral load and reduce the mortality rate in the infected patients compared with the lopinavir/ritonavir (Clinicaltrials.Gov, 2020at).
Interleukin (IL)-2
It is another cytokine signaling molecule used in the immunotherapy treatment, especially in cancer (e.g., melanoma) (Rosenberg et al., 1994) and in the prevention of viral infection (e.g., HIV) (Kovacs et al., 1996; Boda et al., 2018). IL-2 is lymphocytotrophic hormone that is recognized and characterized as a fundamental for the generation and regulation of the immune response (Smith, 1988). It is a T lymphocyte product that stimulates T cells for the progression of the cell cycle via a finite number of interactions with its specific membrane receptors (Smith, 1988). A controlled phase 1 intervention (ChiCTR2000030167) increases the production of CD4+ T, CD8+ T and NK cell numbers in 20 infected patients at a low dose intramuscularly (IM) (Chictr.Org.Cn, 2020b).
CYNK-001
According to Celularity, the investigational new drug (IND) has been cleared by the authority of US Food and Drug Administration (FDA) for the use of CYNK-001 as an experimental allogeneic shelf cell (e.g., NK cell) therapy derived from the human placental CD34+ cells to treat COVID-19 patients (Celularity, 2020). Recently, a world-leading company “Celularity Incorporated” has experimented with the efficacy and safety of CYNK-001 (NCT04365101) on 86 participants, suggesting it has enriched for CD56+/CD3-NK cells (Clinicaltrials.Gov, 2020av).
Baloxavir Marboxil (S-033188)
Previously, baloxavir marboxil (S-033188) is used as a first-in-class antiviral prodrug that is converted as to its active form (baloxavir acid) through hydrolysis (Koshimichi et al., 2018) and in turn acts as a selective inhibitor of the cap-dependent endonuclease (Hayden et al., 2018) and the neuraminidase (NA) inhibitors (NAI) (O’hanlon and Shaw, 2019), which is specially approved for influenza. In a recent investigation (ChiCTR 2000029544), it is reported that the baloxavir marboxil selectively inhibits cap-dependent endonuclease of SARS-CoV-2 in 10 infected patients with 80 mg (once a day) oral dose (Lou et al., 2020).
Antiviral Drugs (Broad-Spectrum, Inhibitors of RNA-Dependent RNA Polymerase)
Remdesivir (GS-5734)
Remdesivir (GS-5734), an approved HIV reverse transcriptase inhibitor, is a monophosphoramidate prodrug of an adenosine C-nucleoside with a similar chemical structure to the tenofovir alafenamide that consequently demonstrates as an active energetic C-adenosine nucleoside triphosphate analog and prevents RdRp as a broad-spectrum antiviral drug of several RNA viruses including as Coronaviridae and Flaviviridae (Agostini et al., 2018; Gordon et al., 2020; Ko et al., 2020). The first clinical use of remdesivir was for the treatment of Ebola. Based on the current pandemic, a report has recently been demonstrated an adaptive, randomized, placebo-controlled, double blind phase 3 (NCT04257656) clinical trial to evaluate the efficacy and safety of this drug (200 mg on day 1 and 100 mg once daily for 9 days) combined with the supportive care in the hospitalized 237 COVID-19 patients (Scavone et al., 2020; Wang et al., 2020b).
But this initial studies with remdesivir showed no benefit as underpowered (Davies et al., 2020; Wang et al., 2020a), this changed with the NIH study called COVID-19 Adaptive Treatment Trial (ACTT 3) in which the safety and efficacy of a treatment regimen consisting of remdesivir plus the interferon beta-1a immunomodulator in patients with coronavirus 2019 (COVID-19) will be evaluated (National Institutes of Health, 2020). Remdesivir has recently been granted a conditional marketing authorization in the European Union countries by the European Commission (Agency, 2020). A very recent study showed that this antiviral, originally developed against Ebola hemorrhagic fever, slightly reduces the recovery time of patients hospitalized with Covid-19 (15 to 11 days, on average). In contrast, this drug has not been shown to reduce mortality. The European Medicines Agency (EMA) has recommended the authorization of remdesivir (Velkury, Gilead Company) for patients infected with the new coronavirus, at EU level, by “conditional placing on the market.” The EMA has recommended the use of remdesivir in adults and adolescents over 12 years of age who have pneumonia and need oxygen supplementation in critically ill patients (Agency, 2020).
The FDA has also authorized the use of remdesivir in infection with the new SARS-CoV-2 coronavirus, through the Special Emergency Use Authorization (EUA). This approval allows doctors to administer remdesivir to patients with suspected or confirmed infection, severe form (have blood oxygen saturation SpO2 ≤ 94%, require oxygen therapy, mechanical ventilation or extracorporeal membrane-to-arterial oxygenation/ECMO), even outside of clinical trials. However, EUA is not a complete approval, as further studies are needed to confirm the effectiveness of this treatment. Urgent approval follows the publication of encouraging results from two studies involving remdesivir:
i. Adaptive COVID-19 Treatment Trial (ACTT), organized by the US National Institute of Allergic and Infectious Diseases (NIAID): Phase III, randomized, placebo-controlled; 1,063 patients included; patients treated with remdesivir showed clinical improvement after a 31% shorter period; the study group had a median recovery time of 11 days, compared to 15 days in the control group; the study group had a mortality of 8%, compared to 11.6% in the control group (Health, 2020b).
ii. The SIMPLE study, organized by Gilead (the company producing remdesivir, veklury):
Phase III, without control group - patients receive a remdesivir treatment for 5 or 10 days;
Clinical improvement was similar in the two groups; half of the patients showed an improvement in the disease in the first 10 days, in the case of 5 days of treatment, and in the first 11 days (10 days of treatment); after 14 days, 60% of patients receiving remdesivir for 5 days were discharged, and 52.3% of those receiving 10 days were discharged (Gilead Sciences, 2020).
Favipiravir
Favipiravir (previously known as T-705 and Avigan) is a selective inhibitor of nonnucleoside RNA polymerase, which was developed and approved to treat influenza in Japan whereas it is already popular as a prodrug of purine nucleotide that is converted as to an active form namely favipiravir-ribofuranosyl-5′-triphosphate (RTP) by phosphoribosylation through the cellular enzymes (Furuta et al., 2013; Furuta et al., 2017). In response to the current global pandemic, with the help of a sponsor (Giuliano Rizzardini), a study (NCT04336904) on this drug is ongoing on 100 adult COVID-19 patients with 1800 mg/BID for day 1 and 600 mg/TID for day 2 and after that for a maximum of 14 days to evaluate the safety and efficacy of it combined with adequate supportive care (Clinicaltrials.Gov, 2020l).
Arbidol or Umifenovir
It is a selective broad-spectrum antiviral drug, which is initially licensed in Russia and China as a small indole-derivative molecule for the treatment of enveloped and nonenveloped virus infections (commonly influenza) through inhibiting the membrane haemagglutinin fusion (Blaising et al., 2014). Recently, a phase 4 clinical trial (NCT04246242) of arbidol is performed by the Xiangya Hospital of Central South University for determining the treatment efficacy and safety of it against the COVID-19 by applying the adaptable oral doses (e.g., 200 or 400 mg, TID) on 500 participants (Smartpatients.Com, 2020).
Galidesivir (BCX4430)
Galidesivir is another adenosine analog that demonstrates broad-spectrum antiviral activity against several types of viruses (e.g., togaviruses, filoviruses, arenaviruses, paramyxoviruses, orthomyxovirus bunyaviruses, CoVs, picornavirus, flaviviruses) and initially developed for the treatment of hepatitis C virus (HCV) (Westover et al., 2018). The first in-patient phase 1 clinical trial, is a randomized, placebo-controlled, and double-blind study to assess the safety, efficacy, pharmacokinetics, and tolerability of IV administration of galidesivir vs. placebo in hospitalized patients (n = 66) with either Group A (Yellow Fever) or Group B (COVID-19) (Clinicaltrials.Gov, 2020bp).
Antiviral Drugs (Antiretrovirals, Protease Inhibitors)
Lopinavir/Ritonavir
Lopinavir/ritonavir, a US FDA approved co-formulated antiretroviral therapy for treating HIV protease, established selective in vitro antiviral activity against 3CLPRO and PLPRO proteases of the SARS-CoV-2 (Chu et al., 2004; De Wilde et al., 2014). Based on liver cytochrome P450 inhibition, the simultaneous use of ritonavir may upsurge the plasma half-life of lopinavir (Barragan and Podzamczer, 2008). More recently, it has been cited that an improved clinical outcome of patients (n = 199) with SARS-CoV-2 is appeared to be associated with a randomized open-label trial (ChiCTR2000029308) of orally administered lopinavir/ritonavir (100 and 400 mg) vs. standard care (Cao B. et al., 2020; Dorward and Gbinigie, 2020).
Azvudine
It is an experimental nucleoside analog that may inhibit the reverse enzyme transcriptase for viral transcription and show the potential against COVID-19 (Wang et al., 2014). Nucleoside analogs (e.g., azvudine, remdesivir, galidesivir) are the adenine or guanine derivatives that prevent the viral RNA synthesis and inhibit RdRp by encoding viral replication of several RNA viruses, including hCoVs (De Clercq, 2019). A phase 3 clinical trial (ChiCTR2000029853) on azvudine is ongoing at the People’s Hospital of Guangshan County to determine its better effectiveness against COVID-19 (Chictr.Org.Cn, 2020e).
Danoprevir
It is an orally available hepatitis C virus (HCV)NS3/4A protease inhibitor and recently approved for treating noncirrhotic genotype 1b chronic hepatitis C in China (Moucari et al., 2010). Danoprevir (100 mg/tablet) combined with ritonavir (100 mg/tablet) is currently in phase 4 clinical trial (NCT04291729) evaluating its safety and efficacy in COVID-19 patients (n = 11) and has shown the potential prevention against SARS-CoV-2 transcription and replication (Chen H. et al., 2020).
Darunavir
It is a US FDA approved nonpeptidic protease inhibitor (PI) for the treatment of HIV-1 infections, which is generally applied as a part of antiretroviral therapy (ART) together with a low boosting dose of ritonavir (Mckeage et al., 2009). The darunavir boosted with ritonavir (low dose) is swiftly absorbed and reaches peak plasma concentrations within 2.5–4 h (Rittweger and Arastéh, 2007). Darunavir is comprehensively and nearly absolutely metabolized by the hepatic cytochrome P450 (CYP) 3A4 enzymes (Rittweger and Arastéh, 2007). Patients with SARS-CoV-2 are being recruited in a randomized phase 3 clinical trial (NCT04252274) to evaluate the safety and efficacy of this drug and cobicistat (a potent human cytochrome P-450 3A (CYP3A) enzyme inhibitors used in the treatment of HIV/acquired immune deficiency syndrome (AIDS) infections) (Clinicaltrials.Gov, 2020ab).
TMC-310911(ASC09)
Structurally comparable to the darunavir, the TMC-310911 is a potent protease inhibitor that has been initially demonstrated for treating human immunodeficiency virus (HIV)-1 infections due to its proteolytic cleavage protection (Mina et al., 2020). Nowadays, several multinational companies are trying to develop the antiviral activity of this drug as a discerning agent against SARS-CoV-2 in combination with other HIV therapies, including ritonavir and lopinavir (Mina et al., 2020). Ascletis Pharmaceuticals Co., Ltd. is one of these multinational companies that provides information about an open-label trial (NCT04261907) of ASC09/ritonavir (300 mg/100 mg tablet) and lopinavir/ritonavir (200 mg/50 mg tablet), which are experimented on 160 participants to evaluate and compare their effectiveness in COVID-19 (Clinicaltrials.Gov, 2020ak).
Antimalarial Drugs
The antimalarial drugs (e.g., hydroxychloroquine, chloroquine, quinacrine) are considered for a long time as effective therapies against malaria, and are also believed to have selective antiinflammatory effects against chronic inflammatory diseases (e.g., systemic lupus erythematosus, rheumatoid arthritis), have antiviral effects against different types of RNA viruses (e.g., dengue, chikungunya, HIV, SARS-CoVs, MERS-CoV), and also have immunomodulatory effects by inhibiting autophagy and lysosomal activity in host cells via cytokine signaling (Canadian Hydroxychloroquine Study Group, 1991; Rogoveanu et al., 2018; Vijayvargiya et al., 2020). A randomized and placebo-controlled phase 3 clinical study (NCT04308668) is being recruited for the treatment of COVID-19 patients (n = 3000) to evaluate the effectiveness of postexposure prophylaxis and preemptive therapy with hydroxychloroquine (200 mg tablet; 800 mg once, followed in 6 to 8 h by 600 mg, then 600 mg once daily for 4 days) (Clinicaltrials.Gov, 2020bb).
The World Health Organization recently announced that it is discontinuing clinical trials with hydroxychloroquine and the Lopinavir-Ritonavir combination due to failure to reduce mortality in patients infected with the novel coronavirus (WHO, 2020). Preliminary results of the SOLIDARITY study showed that hydroxychloroquine and the Lopinavir-Ritonavir combination reduced little or no mortality in hospitalized COVID-19 patients compared to the therapeutic standard (WHO, 2020). Also, according to RECOVERY, the first major clinical trial conducted by Oxford University in the UK, has been stopped because the delivered results, they showed that hydroxychloroquine has no beneficial effect on COVID-19 (Torjesen, 2020).
Antibiotics and Antiparasitics
Carrimycin
On June 24, 2019, an interesting antibiotic (carrimycin) with a trade name of ‘Bite’ is originally developed for the treatment of upper respiratory infections approved by the country’s National Medical Products Administration in China (Trialsitenews, 2020).
A randomized (1:1), multicenter, open-controlled phase 3 clinical trial (NCT04286503) on 520 COVID-19 patients with carrimycin (experimental group) and lopinavir/ritonavir or arbidol or chloroquine phosphate (active comparator group) was launched by Beijing Youan Hospital to analysis the safety and efficacy of carrimycinin COVID-19 (Clinicaltrials.Gov, 2020k).
Suramin Sodium
Since the 1920s, suramin sodium (polysulfonated naphthylurea) has significantly been used to treat trypanosomiasis and onchocerciasis in humans and has also been seen as a potent inhibitor of reverse transcriptase enzyme of various types of retroviruses including HIV/AIDS, and various autocrine growth factors including tumor growth factor-beta (TGF-β), insulin-like growth factor I (IGF-I), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), essential fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) (Hemady et al., 1996). It has also been used as an effective inhibitor of (Na+-K+)-activated ATPase and some hydrolytic and oxidative enzymes (Fortes et al., 1973). In retort to the ongoing pandemic, the hospitalized patients (n = 20) with proven SARS-CoV-2 infections at the First Affiliated Hospital of Zhejiang University School of Medicine are recruited to treat with it to evaluate its safety and efficacy in COVID-19 (Chictr.Org.Cn, 2020d).
Ivermectin
A macrocyclic lactone originally derived from an actinomycete (Streptomyces avermitilis) approved as a broad-spectrum antiparasitic and anthelmintic agent, is a 22,23-dihydro derivative of avermectin B1 with almost a similar structure to its naturally occurring precursor (abamectin) that is significantly used for the treatment of river blindness (onchocerciasis) and ectoparasitic disease, and also used against different types of nematode and arthropod parasites (Campbell et al., 1983; Campbell, 1985; Meinking et al., 1995). A randomized phase 2 (NCT04374279) clinical study has been applied in 60 severe COVID-19 patients to treat with standard care or standard care plus bicalutamide (150 mg once daily for 7 days) or ivermectin (600 µg/kg once daily for 3 days) (Clinicaltrials.Gov, 2020bv).
Dihydroartemisinine/Piperaquine
It is a fixed-dose combination antimalarial that contains 40 mg of dihydroartemisinin (potent and short-acting) and a 320 mg of partner drug, namely piperaquine (less-potent and long-acting) generally recommended by the World Health Organization (WHO) to treat uncomplicated malaria caused by Plasmodium falciparum (Amaratunga et al., 2016). On the other hand, the antiviral activity of dihydroartemisinin/piperaquine may underlie through interaction between its peroxide bridge and haem iron. Lately, a phase 4 clinical trial (ChiCTR2000030082) sponsored by the First Affiliated Hospital of Nanchang University is aimed to assess the anti-COVID-19 activity of this combination medicine on 40 COVID-19 patients (Chictr.Org.Cn, 2020a).
Azithromycin
The broad-spectrum antibiotic azithromycin is an orally administered acid-stableazalide antibacterial drug, which is an erythromycin derivative with a similar range of antimicrobial activity and developed pharmacokinetic physiognomies comparative to erythromycin (Peters et al., 1992; Zlatian et al., 2018). It is noted that the action of this drug is expanded significantly with a wide range of Gram-positive organisms, particularly Haemophilus Influenza-related with respiratory tract infections (Dunn and Barradell, 1996; Călina et al., 2017; Ungureanu et al., 2017). Most lately, azithromycin (500 mg) oral tablet has experimented as a prophylactic treatment following a randomized, single-blinded, placebo-controlled phase 2 trial (NCT04369365) in cancer patients (n = 200) undergoing antineoplastic therapy during the COVID-19 pandemic (Tran et al., 2019; Mohammad et al., 2020; Clinicaltrials.Gov, 2020bh).
Doxycycline
Doxycycline is a second-generation tetracycline that rapidly absorbed into the systemic circulation, distributed throughout the organism due to its function of lipophilicity, and eliminated through feces and urine (Saivin and Houin, 1988; Calina et al., 2016; Blejan et al., 2020). Xa study reports that doxycycline acts as a potent inhibitor of dengue viral replication and diminishesserum IL-6 levels at the time of viral infection (Sargiacomo et al., 2020). Patients (n = 330) with severe COVID-19 are recruited in a randomized, prospective, multicenter, double-blind phase 3 clinical study (NCT04371952) to evaluate the efficacy of doxycycline (200 mg/day) vs. a placebo (lactose 380 mg/capsule) (Clinicaltrials.Gov, 2020y).
NonSpecific AntiInflammatory and Immunosuppressive Drugs
Corticosteroids
Glucocorticosteroid hormones corticosteroids are repeatedly used to treat acute respiratory distress syndrome (ARDS) and severe lung injury due to their capacity of diminishing inflammatory and fibrotic phenomena and defeating deposition of collagen (Claman, 1972). There also have some controversial for the therapeutic efficiency of corticosteroids despite the popularity of their administering. To study an anti-ARDS efficacy against COVID-19, 86 COVID-19 patients were treated in a randomized, prospective, and placebo-controlled fashion with methylprednisolone, 1 mg/kg/day (IV) for 7 days, or placebo (Clinicaltrials.Gov, 2020aa).
Fingolimod
A first-in-class orally administered compound fingolimod (FTY720) is a frequent immunology modulator of sphingosine-1-phosphate–a receptor that has exposed clinical efficacy and expansion on imaging in a nonrandomized phase 2 intervention (NCT04280588) against 30COVID-19 participants (Clinicaltrials.Gov, 2020al). It is initially used in multiple sclerosis thanks to its function of sequestering lymphocytes in lymph nodes (Chun and Hartung, 2010).
Leflunomide
FDA approved immunomodulatory prodrug leflunomide to treat rheumatoid arthritis as a disease-modifying antirheumatic drug that is rapidly converted to its active metabolite (A771726) after oral administration (Fox, 1998; Prakash and Jarvis, 1999). The immunosuppressant leflunomide causes inhibition of dihydro-orotate dehydrogenase and tyrosine kinases and degradation of intracellular transcription factors (Rozman, 2002). In order to find out the tolerability of this drug with a high dose (300 mg once daily), the University of Chicago recruited a single-center tolerability phase 1 clinical trial (NCT04361214) with leflunomide in the ambulatory patients (n = 20) with mild COVID-19 (Clinicaltrials.Gov, 2020ar).
Thalidomide
Firstly, the CIBA pharmaceutical company manufactured thalidomide in 1954 thanks to the prescribed drug as a sedative, antiemetic, and tranquillizer for the morning sickness (Franks et al., 2004). Then it is profoundly marketed and endorsed throughout the world due to having its multi-purposes functions such as antiangiogenesis, antifibrotic, immune regulation effects, and antiinflammatory (Shannon et al., 2008). It inhibits excess production of tumor necrosis factor-alpha (TNF-α) and suppresses the leukocyte migration. A research group of the First Affiliated Hospital of Wenzhou Medical University randomly allocated 100 patients to receive thalidomide (100 mg, orally for 14 days) or placebo at the same dose of thalidomide in a first prospective, multi-center, placebo-controlled, double-blind phase 2 intervention (NCT04273529) to evaluate the safety and efficacy of this drug in COVID-19 (Clinicaltrials.Gov, 2020ac).
Colchicine
It is an alkaloid derivative derived from a plant source, namely Colchicum autumnale (Liliaceae) (Terkeltaub, 2009). Colchicine is standing with a long history for its application in inflammatory diseases, including familial Mediterranean fever, and severe gout and Behçet’s disease (Niel and Scherrmann, 2006). In retort to the COVID-19, a research team of the Montreal Heart Institute assigned 6,000 COVID-19 patients to be given either colchicine or placebo (1:1 allocation ratio) for 30 days in a randomized, multi-center, double-blind, placebo, parallel controlled phase 3 clinical study to examine the reduction of mortality rate and lung difficulties associated with COVID-19 (Clinicaltrials.Gov, 2020m).
Ibuprofen
It is a nonsteroidal antiinflammatory drug (NSAID) involved in the class of 2 aryl propionic acid (2-APA) that was first announced in England in 1967 (Davies, 1998). It inhibits the production of prostaglandins by decreasing the activity of the enzyme cyclooxygenase (Cole and Frautschy, 2010; Rogoveanu et al., 2018). Ibuprofen is also familiar for the advanced treatment of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, gout, and Bartter’s syndrome (Kantor, 1979; Mititelu et al., 2020; Salehi et al., 2020). After registration (NCT04334629), the King’s College London initiated a phase 4 clinical trial (multicenter, randomized, controlled trial) of this drug at a daily dose of 200 mg in 230 patients to examine the reduction in the austerity and advancement of lung difficulties associated with COVID-19 (Clinicaltrials.Gov, 2020as).
Naproxen
Stereochemically naproxen is a potent nitric oxide‐releasing NSAID that is usually administered orally or rectally for the treatment of severe rheumatic disease and several nonrheumatic circumstances (Todd and Clissold, 1990). A study has been reported that a nitroxybutyl ester derivative of naproxen shows the less ulcerogenic in the gastrointestinal tract (GIT) than its mother NSAID (Davies et al., 1997). A randomized phase 3 clinical trial (NCT04325633) is established at Assistance Publique - Hôpitaux de Paris in hospitalized patients (n=584)with severe COVID-19 to treat with the standard of care plus naproxen(250 mg BID) and lansoprazole (30 mg daily) in order to determine the effectiveness of this drug in COVID-19 (Knights et al., 2010; Clinicaltrials.Gov, 2020ae).
Piclidenoson (CF101)
According to the report from the website of Can-Fite BioPharma, piclidenoson, commonly known as IB-MECA (methyl 1-[N6-(3-iodobenzyl)-adenin-9-yl]-b-D-ribofuronamide) is an active antiinflammatory agent that has been experimented in different types of experimental models. It acts after binding to the G protein associated A3AR, which induces a robust antiinflammatory effect by inhibiting IL-17 and -23.Patients (n = 40) with COVID-19 are assigned in an open-label, randomized, control phase 2 clinical trial (NCT04333472) to receive either piclidenoson (2 mg Q12H orally) with standard care as an experimental arm or standard care alone as a control arm (1:1 allocation ratio) on empty stomach of patients to evaluate the safety and efficacy against COVID-19 (Clinicaltrials.Gov, 2020az).
Kinase Inhibitors
Jakotinib Hydrochloride
Jakotinib hydrochloride, an AP2-associated protein kinase 1 (AAK1) inhibitor as well as a Janus kinase (JAK) inhibitor, was recommended a conceivable candidate, making an allowance for its high rate of persistent virological response in COVID-19 patients (Zhang et al., 2020). To determine the antiviral and antiinfective activity of this drug (50 mg/BID, orally), a randomized phase 2 clinical intervention (NCT04312594) sponsored by Suzhou Zelgen Biopharmaceuticals Co., Ltd is assigned in 90 COVID-19 patients with idiopathic pulmonary fibrosis (Clinicaltrials.Gov, 2020ap).
Ruxolitinib
Ruxolitinib, formally known as INC424 or INCB18424, is a US FDA approved orally bioavailable JAK1/2 inhibitor usually used in the treatment of myelofibrosis as an effective and discerning inhibitor (Harrison et al., 2012; Stebbing et al., 2020). Ruxolitinib, a more auspicious repurposed antiviral agent to examine its safety and efficacy against randomized patients (n = 402) with COVID-19 a multicenter, double-blind, controlled, phase 3 clinical intervention (NCT04362137) has been recruited to treat with either ruxolitinib at a dose of 5 mg/BID plus standard of carein 2:1 allocation ratio or oral matching-image placebo plus standard of care for 14 days (Clinicaltrials.Gov, 2020ax).
Baricitinib
Baricitinib, orally bioavailable, is another potent and selective inhibitor of AAK1 and JAK1/2 (Richardson et al., 2020). It is a more auspicious repurposed antiviral agent with a unique mechanism of action targetingAAK1 and JAK1/2, and reducing SARS-CoV-2 endocytosis through binding to the cyclin g-associated kinase (GAK) (Cantini et al., 2020). Treatment with this drug is accompanied with a high rate of continuous virological response in patients (n = 200) with mild to moderate SARS-CoV-2 infection in the response-guided nonrandomized, prospective, open-label, 2-week, phase 2 and 3 interventions (NCT04320277) conducted in the Fabrizio Cantini, Hospital of Prato (Clinicaltrials.Gov, 2020f).
Tofacitinib
Tofacitinib, a persuasive oral inhibitor of the JAK1/2/3 (family: kinases), can alleviate alveolar inflammation thorough blocking interleukins signal such as IL-2, -4, -6, -7, -9, -15, and -21, which is generally approved as an immunomodulator and disease-modifying therapeutic agent for rheumatoid arthritis (Fleischmann et al., 2012; Sandborn et al., 2012). To examine the primary outcome of this drug in COVID-19, a single group assignment, prospective cohort, phase 2 study (NCT04332042) is being arranged by the Armando Gabrielli, Università Politecnica delle Marche to treat SARS-CoV-2 related interstitial pneumonia in patients (n = 50) with it at a dose of 10 mg/BID for 14 days (Clinicaltrials.Gov, 2020bs).
Imatinib
Imatinib, an approved agent for chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST), can potentially inhibit the fusion protein Bcr-Abl and platelet-derived growth factor receptors (e.g., PDGFRα and PDGFRβ) (Peng et al., 2005; Moen et al., 2007). To study the antiviral effect of this drug, a research team of the Versailles Hospital randomly assigned 99 patients with nonsevere COVID-19 in phase 2, randomized, open-label, parallel clinical trial (NCT04357613), in a 1:1 ratio, to receive imatinib (800 mg/day) or standard therapy (Clinicaltrials.Gov, 2020am).
Monoclonal Antibodies
Tozumab/Adamumab
Tozumab is used as an immunotherapy for the treatment of bilateral lung lesions, whereas adamumab is used in rheumatoid arthritis (Ying et al., 2020). A combination therapy (tozumab combined with adamumab) is applied in severe and critical COVID-19 patients (n = 60) having pneumonia in phase 4, randomized, single-center, prospective, controlled parallel trial (ChiCTR2000030580) to evaluate its safety and efficacy in COVID-19 (Chictr.Org.Cn, 2020c).
Ravulizumab (Ultomiris or ALXN1210)
Ravulizumab (also called Ultomiris and ALXN1210), a humanized monoclonal antibody, is firstly manufactured by the Alexion Pharmaceuticals as a new inhibitor of complement C5 for paroxysmal nocturnal haemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) treatment (Mckeage, 2019). It was first approved intravenous drug for paroxysmal nocturnal hemoglobinuria (PNH) in USA in December 2018, which is developed from eculizumab to have a considerably higher terminal half-life (Röth et al., 2018). To determine the safety and efficacy of its in COVID-19,270 patients with severe pneumonia are randomly assigned to receive weight-based doses of ravulizumab (intravenously on Days 1, 5, 10, and 15) with the best supportive care by applying phase 3 open-label, randomized, controlled study (NCT04369469) (Clinicaltrials.Gov, 2020ad).
Leronlimab
FDA approved CCR5 (G protein-coupled receptor) antagonist, leronlimab (also called PA14 and PRO-140) is a humanized IgG4 and a monoclonal antibody (mAb) to CCR5 that significantly prevents CoV entry and inhibits viral infection of CD4 T-cells by blocking the CCR5 (Clinicaltrials.Gov, 2020bo). The unique mechanism of binding to CCR5, leronlimab may improve the activities of DDR-based treatments for different types of cancer (e.g., prostate, pancreatic, breast, colon, and melanoma), permitting the reduction in dose of standard chemotherapy (Pestell et al., 2020). Researchers in CytoDyn, Inc. designed a randomized, double blind, adaptive, placebo controlled phase 2b/3 clinical trial (NCT04347239) to assess the efficacy, safety, and tolerability of subcutaneous leronlimab (weekly doses of 700 mg) in 390patients with severe COVID-19 (Clinicaltrials.Gov, 2020bo).
TJ003234
TJ003234, also known as TJM2 and TJ-003234RAR101, is an antigranulocyte macrophage–colony-stimulating factor (anti-GM-CSF) monoclonal antibody that produces a high level of mAbs against GM-CSF (Clinicaltrials.Gov, 2020bk). It is first discovered by a dynamic and global biotech company I-Mab Biopharma Co. Ltd. Again, this company arranged a multi-center, randomized, double-blind, placebo-controlled phase 1b/2 clinical trial (NCT04341116) to assess the safety and efficacy of its in COVID-19 (Clinicaltrials.Gov, 2020bl). In this study, 144 patients are assigned and divided into three groups to receive IV TJ003234 (3 mg/kg for 1st group and 6 mg/kg for 2nd group) and placebo (3rd group).
Nivolumab
Nivolumab (Optivo®), a human IgG4 monoclonal antibody, is a programmed death 1 (PD-1)immune checkpoint inhibitor (ICI) that directly binds to the PD-1 ligand 1 (PD-L1) receptor and blocks it’s interaction capacity with the PD-L1 and -L2 (Wolchok et al., 2013). It is an approved drug, reversing T-cell anergy and boosting immune responses in several cancers, including metastatic melanoma, skin and lung cancer, and virus-associated tumors (CheckMate 358) and against the viral infections, including HIV (Le Garff et al., 2017; Topalian et al., 2017). To assess the safety, efficacy, and tolerability of intravenous nivolumab, a total of 92 patients with severe SARS-CoV-2 disease are randomly assigned in a randomized, multi-center, 2 parallel arms, open-label phase 2 clinical study and allocated in a 1:1 ratio for which they receive either nivolumab at a dose of 3 mg/kg on day 1 or standard care (Clinicaltrials.Gov, 2020bu).
Meplazumab
It is a humanized mAb that acts against host-cell-expressed CD147. Meplazumab blocks the infection of SARS-CoV-2 through binding with the S protein of SARS-CoV-2 (Bian et al., 2020). In a recent study, 20 COVID-19 patients with pneumonia are assigned in a single center, single-arm, open-label phase 2 clinical trial to receive meplazumab at an IV dose of 10 mg at 1st, 2nd, and 5th day to evaluate the therapeutic safety, efficacy, and tolerability of this drug in COVID-19 (Bian et al., 2020).
Eculizumab
Eculizumab (Soliris), another approved humanized mAb for the inhibition of intravascular hemolysis of PNH, is a potent terminal complement inhibitor that directly binds to the C5 complement protein and inhibits the cleavage of C5a and C5b-9 (Legendre et al., 2013). In order to evaluate the safety and efficacy of this drug in COVID-19, the researchers of the Assistance Publique - Hôpitaux de Paris conducted a cohort multiple randomized controlled phase 2 trial (NCT04346797) where 120 patients with moderate or severe pneumonia were allocated to receive either eculizumab at a dose of 1,200 mg on days 1st, 4th, 8th then 1,200 mg or 900 mg on day 12th or best standard of care (Clinicaltrials.Gov, 2020w).
Clazakizumab
Clazakizumab is an anti-IL-6 monoclonal, which is a hereditarily engineered high affinity humanized monoclonal antibody (IgG1) that directly binds to IL-6 and averts its interaction and signaling through IL-6R (Eskandary et al., 2019). To determine the sustainable rate of virological response of this drug in COVID-19, patients with COVID-19 with signs of pulmonary involvement are randomly conducted in a phase 2, randomized, placebo-controlled intervention (NCT04348500) clazakizumab at the dose of 25 mg in 50 cc NS has been given by IV infusion x 1 dose and placebo at the dose of 50 cc NS given by IV infusion x 1 dose (Clinicaltrials.Gov, 2020i).
Avdoralimab
Avdoralimab (IPH5401), an immunoglobulin G1-kappa, is a selective anti-C5aR antibody that potentially reduces the inflammatory response in the lungs (World Health Organization, 2019). In response to current viral infection, 108 COVID-19 patients with severe pneumonia are included in a randomized, double-blind, placebo-controlled phase 2 clinical study (NCT04371367) to receive IV avdoralimab and placebo to improve the proportion of infected patients (Clinicaltrials.Gov, 2020e).
Lenzilumab
The humaneered recombinant antihuman granulocyte-macrophage colony- stimulating factor (anti-hGM-CSF) antibody lenzilumabis an IgG1 kappa monoclonal antibody that targets human GM-CSF to treat chronic myelomonocytic leukemia (Patnaik et al., 2019). To appraise the supportable rate of safety, efficacy and tolerability of virological response of it in COVID-19, a phase 3 randomized, double-blind, placebo-controlled intervention (NCT04351152) involving 238 patients with pneumonia are randomized in a 1:1 ratio of this drug plus standard care vs. standard care (Clinicaltrials.Gov, 2020ay).
LY3127804
LY3127804, a selective mAb firstly developed by an American pharmaceutical company Eli Lilly and Company, is engineered high affinity humanized monoclonal antibody (IgG4 isotype) that discerningly targets to angiopoietin-2 (Ang-2) and counteracts phospho-Tie2, tumor growth and metastasis (Chintharlapalli et al., 2016; Pestana et al., 2018). Eli Lilly and Company initiates a randomized, double-blind, placebo-controlled, phase 2 intervention (NCT04342897) of LY3127804 in April 13, 2020, to evaluate the effectiveness of LY3127804 against ongoing viral infection. In this study, 200 hospitalized patients with COVID-19 pneumonia have been receipted IV LY3127804 or placebo (Clinicaltrials.Gov, 2020bj).
IFX-1
It is applied as a first-in-class monoclonal antibody that serves as a C5a antagonist, which is being developed for the advanced treatment in COVID-19 patients by a German biopharmaceutical firm InflaRx in collaboration with Beijing (Clinicaltrialsarena.Com, 2020a). IFX-1 is one of the currently under development drugs generally used for the treatment as a skin disorder therapy, antiviral, antiinfective, antiinflammatory, and vascular disorder therapy. For the better advancement of this drug against COVID-19, the InflaRx has been assigned 130 COVID-19 patients with severe pneumonia in a two-arm (arm A: best supportive care plus IFX-1; arm B: best supportive care alone), randomized, open-label, pragmatic, adaptive, phase 2/3 clinical trial (NCT04333420) (Clinicaltrials.Gov, 2020aw).
Gimsilumab
A fully mAb gimsilumab that is developed by a pharmaceutical company Roivant Sciences Ltd. as a selective inhibitor of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Clinicaltrialsarena.Com, 2020b). In case of COVID-19, the Roivant Sciences Ltd. has been included 270COVID-19 participants having ARDS and lung complication secondary in a phase 2, adaptive, randomized, double-blind, placebo-controlled, multi-center study (NCT04351243) to check its safety and efficacy. In this study, subjects receive either gimsilumab at a higher dose on day 1 and a low dose on day 8 or saline solution as placebo on day 1 and day 8 (Clinicaltrials.Gov, 2020bn).
Tocilizumab
A humanized mAb tocilizumab (Actemra®) is an FDA approved IL-6 inhibitor that selectively inhibits IL-6-mediated proinflammatory signaling by blocking both soluble and membrane-expressed IL-6 receptors (Schiff et al., 2011) and also interrupts the process of cytokine release syndrome (CRS) (Le et al., 2018). Additionally, it has already been approved for the treatment of several types of arthritis, including rheumatoid, polyarticular juvenile idiopathic, systematic juvenile idiopathic and polyarticular juvenile idiopathic arthritis (Oldfield et al., 2009; Le et al., 2018). In response to the ongoing pandemic COVID-19, the University Hospital Inselspital, Berneinitiates is conducting a randomized, multicenter, double-blind, placebo-controlled phase 2 clinical trial(NCT04335071) in collaboration with the Roche Pharma to check its safety and efficacy at a dose of 8 mg/kg body weight, with a maximum single dose 800 mg patients (n=100) with severe pneumonia compared to a placebo group (Clinicaltrials.Gov, 2020br).
Sarilumab
The first fully human mAb sarilumab (Kevzara®, REGN88, and SAR153191) developed by jointly Sanofi and Regeneron Pharmaceuticals is an inhibitor of IL-6Rα that is firstly approved for the treatment of rheumatoid arthritis (Sieper et al., 2015). Sarilumab has a potent ability to bind directly to the soluble and membrane‐bound IL‐6R with the selective affinity, in that way preventing IL‐6–mediated cis and trans-signaling, thus may inhibit overactive inflammatory immune response associated with COVID-19 by inhibiting IL-6-mediated signaling (Genovese et al., 2015). Based on its IL-6 inhibitory capacity, the Regeneron Pharmaceuticals in collaboration with Sanofi starts have been started an adaptive phase 2/3, randomized, double-blind, placebo-controlled study (NCT04315298) to check out its clinical safety and efficacy in comparison to the control arm (Arrytown and Paris, 2020). For this, 2500 COVID-19 hospitalized patients with severe and critical phase are randomized to IV placebo or sarilumab at a single dose.
Bevacizumab
It is an anti-VEGF monoclonal IgG1 antibody that inhibits viral proliferation, migration, and survival by producing high levels of IgG1 antibody (Kazazi-Hyseni et al., 2010). In a randomized, open-label, controlled phase 2 clinical trial (NCT04344782), a most extensive hospital system in Europe (Assistance Publique - Hôpitaux de Paris) randomly assigns 130 patients with COVID-19 infection to receive either bevacizumab (7.5 mg/kg in 100 ml saline) as the experimental arm or standard of care as the control arm to examine safety and efficacy of this drug in COVID-19 (Clinicaltrials.Gov, 2020bt).
Hormonal Preparations and Related Drugs
Aviptadil
Aviptadil, an injectable formulation of the vasoactive intestinal peptide (VIP), is usually used in PAH (Al-Saikhan et al., 2015). It has also been awarded by FDA Orphan Drug Designation for the ARDS treatment and admitted to the FDA CoronaVirus Technology Accelerator Program. Some nonclinical studies reported that the aviptadil selectively prevents N-methyl-D-aspartate (NMDA)-induced caspase-3 activation in lung and constrains the production of IL-6 and TNF-α (Clinicaltrials.Gov, 2020ao). In 20 year history, aviptadil shows the safety and efficacy for sarcoid, pulmonary fibrosis, bronchospasm, and erectile dysfunction in phase 2 trials and ARDS in phase 1 trial. For further assuring the safety and efficacy of this drug against ARDS, the multi-national company NeuroRx, Inc. initiates a randomized, placebo-controlled, multicenter phase 2 clinical trial (NCT04311697) in hospitalized patients (n = 120) with COVID-19 associated ARDS (Clinicaltrials.Gov, 2020ao). In this study, patients are assigned randomly to receive an IV infusion of aviptadil (50–150 pmol/kg/h over 12 h) plus maximal intensive care or standard saline infusion plus maximal intensive care.
Progesterone
The steroid hormone progesterone has traditionally been considered as the mammalian pregnancy hormone, which also reduces inflammation and promotes repair of the respiratory epithelium (Lydon et al., 1995; Hall and Klein, 2017). To evaluate safety and efficacy of progesterone against SARS-CoV-2, a phase 1 randomized, single center, controlled trial (NCT04365127) is conducted in which 40 patients (men) with COVID-19 who are 18 years of age or older receive either subcutaneous (SC) progesterone (100 mg/BID) plus standard care or standard care alone (Clinicaltrials.Gov, 2020bc).
Sildenafil
Sildenafil is an orally administered phosphodiesterase type 5 (PDE5) inhibitor that permits corpus cavernosum smooth muscle to relax and potentiating erections during sexual stimulation (Langtry and Markham, 1999; Georgiadis et al., 2020; Iordache et al., 2020a). It is also reported that the sildenafil can inhibit the breakdown of cyclic guanosine monophosphate (cGMP) through binding at the phosphodiesterase binding site (Iordache et al., 2020b; Rogosnitzky et al., 2020). A pilot study of sildenafil is designed in phase 3 clinical trial (NCT04304313) to check its citrate form tablet’s safety, efficacy, and tolerability at a dose of 0.1g/day for 14 days in 10 COVID-19 patients (Clinicaltrials.Gov, 2020ba).
Triiodothyronine (T3)
It is a thyroid hormone that usually impedes the activation of p38 mitogen-activated protein kinase (MAPK) and promotes tissue repair through controlled protein kinase B (Akt) activation (Pantos et al., 2020). To evaluate its anti-COVID-19 activity, a phase 2, parallel, prospective, randomized, double-blind, placebo-controlled trial (NCT04348513) is designed to explore the probable effect of IVT3 solution (0.8 g/kg within 1 h and then followed by 0.113 g/kg/h for 48 h) in critically ill patients admitted in the intensive care unit (ICU) due to COVID-19 (Clinicaltrials.Gov, 2020bw).
Estradiol Patch
It is a nuclear hormone that interacts with a target cell receptor (Erα or Erβ) within the cytoplasm of the cell. The in vivo and in vitro studies have been demonstrated that estrogen acts in different types of viral infections and wound repair processes (Clinicaltrials.Gov, 2020aj). Thus, the it can be used in viral infections in the lung. To reduce the severity of SARS-CoV-19 disease, COVID-19 positive and probable COVID-19 positive patients (n = 110) are randomly assigned to receive estradiol patchat the dose of 100 µg/day for 7 days on the skin (Clinicaltrials.Gov, 2020aj).
Cardiovascular Drugs
Losartan
Losartan, a selective, orally available ACE inhibitor, which was developed to treat heart failure that acts through blocking the vasoconstrictor and aldosterone-secreting effects of angiotensin II via inhibiting the binding of it to the angiotensin II type 1 receptor (AT1R) (Tsatsakis et al., 2019; Tignanelli et al., 2020). Around 14% dose of losartan is converted to its 10 to 40 fold more active metabolite E3174 after an oral administration of it with its 6 to 9 h estimated terminal half-life. A research group led by the University of Minnesota has recently initiated a randomized, placebo-controlled, multi-center, double-blinded, phase 2 study (NCT04312009) for COVID-19 treatment (Clinicaltrials.Gov, 2020au). In this study, investigators assigned 200COVID-19 patients in a 1:1 ratio to get this drug at an oral dose of 50 mg/day or placebo for 7 days or hospital discharge (Clinicaltrials.Gov, 2020au).
Valsartan
A highly selective angiotensin II (Ang II) type 1 (AT1) receptor blockers that potentially increases pulmonary vascular permeability through blocking AT1R activation and down-regulating the activity ofACE2 (Markham and Goa, 1997). It is evident that the COVID-19 is a high burden of morbidity and mortality because of the development of ARDS. The renin-angiotensin-system (RAS) is also related to developing ARDS and in the meantime, ACE2 is one of the enzymes involved in the RAS cascade (Trifirò et al., 2020). According to this perspective, some scientists of Radboud University initiate a double-blind, placebo-controlled 1:1 randomized phase 4 intervention (NCT04335786) in a total of 651 COVID-19 patients to treat them with valsartan in a dosage titrated to blood pressure up to a maximum of 160 mg/BID or placebo (80 or 160 mg) for 14 days or hospital discharge (Clinicaltrials.Gov, 2020bx).
Ramipril
Oral capsule ramipril is suggested as another RAS blocker that averts diabetes in people with hypertension or cardiovascular disease (Bosch et al., 2006). Exhibiting comparable pharmacodynamic responses to captopril and enalapril, ramipril is also considered as a long-acting ACE inhibitor (Todd and Benfield, 1990). It is a prodrug that is converted to its pharmacologically active metabolite ramiprilat after absorption through hydrolysis with a long estimated terminal half-life (Todd and Benfield, 1990). In response to ongoing infectious disease, the University of California, San Diego in collaboration with Pfizer is planning to initiate a randomized, double-blind, placebo-controlled, phase 2 trial (NCT04366050) to treat 560 COVID-19 patients with ramipril (2.5 mg/day) or placebo for 14 days (Clinicaltrials.Gov, 2020bd).
APN01
It is a recombinant humanACE2 (rhACE2), which is currently used for COVID-19 patients due to its ability to block viral entry and decreasing viral replication in the host cells (Taylor, 2020). APN01 is being tried to develop by the Apeiron Biologics for advanced treatment of COVId-19. For this reason, recently, a randomized, double-blind, phase 2 trial (NCT04335136) of APN01 is assigned in 200 COVID-19 participants (Clinicaltrials.Gov, 2020bg).
Spironolactone
It is an antagonist of aldosterone often used to treat patients with low renin essential hypertension, primary aldosteronism, hypokalemia, and diuretic (Loriaux et al., 1976). It acts as a competitive aldosterone antagonist through binding at the aldosterone-dependent Na1+/K1+ exchange site in the distal convoluted renal tubule. A phase 4 clinical study (NCT04345887) is designed by Istanbul University-Cerrahpasa to assess the effects of spironolactone on oxygenation in COVID-19 ARDS patients (Clinicaltrials.Gov, 2020bi).
Agents Acting on Blood and Blood-Forming Organs
Nafamostat Mesilate
It is a proven serine protease inhibitor that is initially approved in Japan for the treatment of disseminated intravascular coagulation, acute pancreatitis, and anticoagulation in extracorporeal circulation (Tu et al., 2020). It inhibits different types of enzymatic systems, including coagulation and fibrinolytic systems (e.g., thrombin, Xa, XIIa), complement system, and kallikrein-kinin system (Bittmann et al., 2020). It has also been recognized that the nafamostat mesilate is an inhibitor of MERS-CoV S glycoprotein mediated viral membrane fusion through the inhibition of transmembrane protease, serine 2 (TMPRSS2) activity (Yamamoto et al., 2016). Based on previous experiments, a randomized, double-blind, placebo-controlled parallel-group, phase 2/3 trial (NCT04352400) of nafamostat mesilate is designed to evaluate its efficacy against 256 COVID-19 patients (Clinicaltrials.Gov, 2020ai).
Camostat Mesilate
It is another synthetic serine protease inhibitor that is initially used to treat dystrophic epidermolysis, chronic pancreatitis, and oral squamous cell carcinoma (Tu et al., 2020). It was first manufactured by the Nichi-Iko Pharmaceutical Co., Ltd. in combination with Ono Pharmaceutical, Japan (Ohkoshi and Oka, 1984). It has experimented that the camostat mesilate showed the inhibition effect of SARS-COV-2 replication in the in vitro study. Based on this previous preclinical study, a phase 2 clinical study is designed with 114 COVID-19 patients to treat them either with camostat mesylate at a dose of 200mg/TID or with placebo/TID for 7 days (Clinicaltrials.Gov, 2020g).
Vitamins
Vitamin C
Vitamin C (also known as L-ascorbic acid, ascorbic acid, and ascor, sodium ascorbate), a six-carbon lactone, is popular for its antioxidant properties that plays an essential role in reducing the inflammatory process, preventing from respiratory failure, deterring common cold, inhibiting the neutrophils accumulation in the lung, and also modulating the immune system (May and Harrison, 2013; Wilson, 2013; Carr and Maggini, 2017; Salehi et al., 2019b; Sharifi-Rad M. et al., 2020). In addition, some previous studies have been highlighted that the higher dose of IV vitamin C may be beneficial for the patients with acute lung injury, ARDS, and sepsis (Salehi et al., 2019a; Clinicaltrials.Gov, 2020c). It has also been reported that the deficiency of this vitamin may increase the risk and severity of influenza infections (Clinicaltrials.Gov, 2020by). Based on the previous reports, some clinical trials on this vitamin have been registered in order to evaluate its effectiveness in COVID-19.
A research group led by the Hunter Holmes Mcguire Veteran Affairs Medical Center has registered a nonrandomized, open-label, parallel, phase 1/2 clinical trial (NCT04357782) to evaluate the safety, tolerability, and efficacy of the IV vitamin C against SARS-CoV-2 infection and decreased oxygenation (Clinicaltrials.Gov, 2020c). In response to this study, 20 hospitalized patients (age: 18-99 years) are designed to receive this vitamin at a dose of 50 mg/kg given 6 hourly for 4 days (16 total doses) (Clinicaltrials.Gov, 2020c). Another research team of Zhongnan Hospital is actively functioning on vitamin C to evaluate its therapeutic efficacy against the severe SARS-CoV-2 infected pneumonia patients (n = 140) of 18 years and older-aged humans (Clinicaltrials.Gov, 2020by). This study is assigned in a randomized, placebo-controlled, phase 2 clinical trial (NCT04264533) to treat the patients with either vitamin C (12 g/BID for 7 days) plus sterile water (50 ml) or sterile water alone (50 ml/BID for 7 days) (Clinicaltrials.Gov, 2020by). In addition, vitamin C is also introduced in another randomized, multi-center phase 2 (NCT04395768) intervention in 200 COVID-19 patients (18 years and older), which is led by the National Institute of Integrative Medicine, Australia (Clinicaltrials.Gov, 2020an). In this study, the recommended dose of vitamin C is 50 mg/kg 6 hourly on day 1 followed by 100 mg/kg 6 hourly for 7 days (Clinicaltrials.Gov, 2020an).
Vitamin D
Vitamin D may provide the boosting and priming effects against the viral replication caused by several microbial peptides including cathelicidins and defensins (Grant et al., 2020), dysregulation of the renin-angiotensin system, and cytokine storm in the host (Clinicaltrials.Gov, 2020x) through modulating the innate and adaptive immune system (Aranow, 2011). According to the various pre-clinical studies, it is found that the SARS-CoV-2 replication in the host cell leads to severe ARDS by leading to a cytokine storm (Clinicaltrials.Gov, 2020x). Meanwhile, various studies (in vivo and in vitro) on vitamin D have been established that clearly highlight the activity of vitamin D against ARDS and COVID-19-associated coagulopathy (Clinicaltrials.Gov, 2020aq; Clinicaltrials.Gov, 2020x). Based on this critical information on vitamin D, some research groups are continuously working on clinical trials of vitamin D to evaluate its response against COVID-19 patients.
Recently, the University Hospital, Angers has registered a multicenter, randomized, phase 3 clinical trial (NCT04344041) to check out the efficacy of vitamin D for COVID-19 patients (Clinicaltrials.Gov, 2020x). In this instance, 260 patients with life-threatening COVID-19 are randomly allocated to get a high dose of oral vitamin D3 (400,000 IU) or a standard dose of vitamin D3 (50,000 IU) (Clinicaltrials.Gov, 2020x). Another institution Louisiana State University Health Sciences Center in New Orleans has also decided to initiate a multi-center, prospective, randomized, phase 2 intervention (NCT04363840) on SARS-CoV-2 infected patients (n = 1,080) to appraise the efficiency of the vitamin D (50,000 IU, once weekly for 2 weeks) in combination with aspirin (81 mg, once daily for 14 days) against the growing global health crisis (Clinicaltrials.Gov, 2020aq).
At the current crisis of COVID-19 pandemic, the investigators from the Hospital de Alta Complejidad en Red El Cruce Florencio Varela, Buenos Aires, Argentina design a phase 4, randomized, placebo-controlled clinical trial (NCT04411446) of vitamin D in 1,265 hospitalized COVID-19 patients to identify the outcome of vitamin D at a dose of 100.000 UI (total five capsules) compared with placebo at the similar dosage of vitamin D (Clinicaltrials.Gov, 2020h). Besides, to determine the therapeutic efficiency of vitamin D3, a randomized, double-blind, placebo-controlled, proof-of-concept, phase 2 intervention (NCT04400890) has been registered. In response to this study, 200 participants are randomly apportioned in 2 arms (100 for active comparator and 100 for placebo comparator) to treat them with either resveratrol plus vitamin D3 (100,000 IU on day 1) or placebo with vitamin D3 (100,000 IU on day 1) for 15 days (Clinicaltrials.Gov, 2020be).
Convalescent Plasma Therapy
Passive immunotherapy is one of the effective therapeutic approaches in the endemic or pandemic infectious disease, which is still used in ongoing pandemic expending polyclonal antibodies, rather as a hyperimmune preparation from the convalescent patient’s sera who have already recovered from the infection (Dodd, 2012). Convalescent sera or immunoglobulin obtained from the donor is very effective in SARS-CoV-2 infected patients by emerging immediate immune responses in the host system will be possible to neutralize the viral particles in the host (Figure 3) (Rojas et al., 2020).
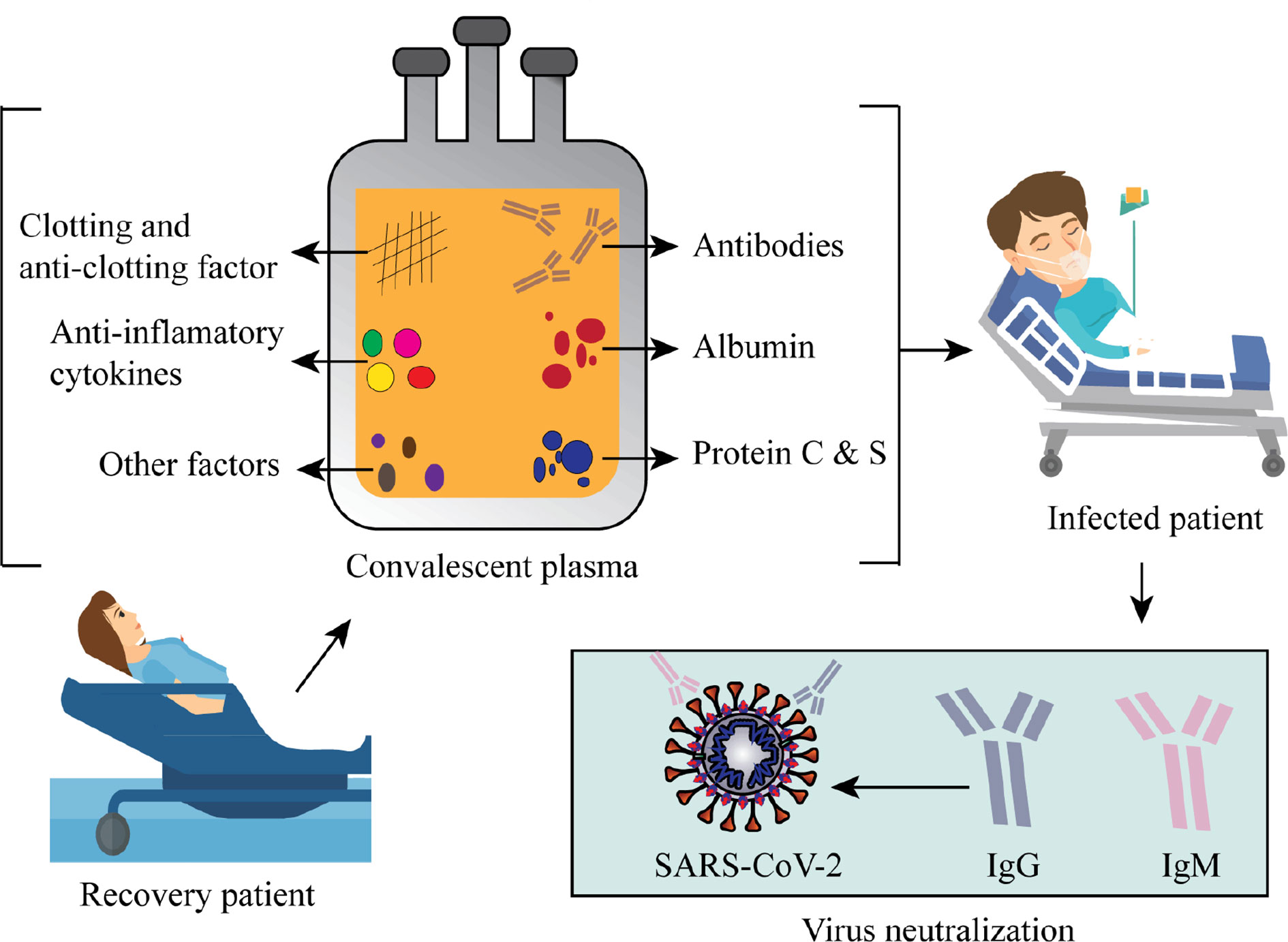
Figure 3 Schematic represents convalescent plasma components and the viral neutralizing activity of convalescent plasma.
Passive immunotherapy has been used as a reliable treatment option for many infectious outbreaks, including the 2003 SARS-CoV-1 epidemic, 2009-2010 H1N1 influenza virus pandemic, 2012 MERS-CoV epidemic, and 2014 Ebola virus epidemic (Chen L. et al., 2020). Based on the previous experiences of CPT in viral infections, the clinical trials of convalescent plasma (CP) in different countries have been assigned to evaluate the safety, efficacy, and immunogenicity of passive immunotherapy for the treatment of COVID-19. As of now on 9th June 2020, approximately 40 clinical studies on the CP have been registered with the clinicaltrial.gov for SARS-Cov-19 infection. Based upon inclusion and exclusion criteria, we select a total of 15 clinical trials that are registered from May 2020 to Jun 2020 (Table 2).
A research team led by the Hospital for Sick Children in Canada registered a multi-centered, open-label, randomized controlled phase 2 clinical trial (NCT04377568) to evaluate the efficacy and safety of COVID-19 CP (C19-CP) for the treatment of COVID-19 in hospitalized children (Clinicaltrials.Gov, 2020ag). In this study, 100 hospitalized children (age up to 18 years) are randomized (1:2 ratio) to receive either C19-CP at the dose of 10 ml/kg plus standard care or standard care. Another research team of the University Hospital, Basel, Switzerland is energetically functioning on a pathogen-inactivated CP addition to best supportive care and antiviral therapy on experimental worsening in participants (n = 15) of 18 years and older age with COVID-19 (Clinicaltrials.Gov, 2020d).
An open-label, nonrandomized, controlled, phase 1/2 clinical trial (NCT04390178) is being carried out by the Joakim Dillner to evaluate the efficacy, safety, and tolerability of plasma collected from the donors who have recovered from the SARS-Cov-19 infection. In response to this study, 10 participants with varying degrees of COVID-19 illness are assigned nonrandomly to receive 180–200 ml of CP (Clinicaltrials.Gov, 2020o). Another nonrandomized, phase 1/2 clinical trial (NCT04384497) is designed by Joakim Dillner with 50 participants (age: 18 years and older) to treat them with CP (200 ml, up to a maximum of 7 CP infusions) for further investigation (Clinicaltrials.Gov, 2020t).
The Thomas Jefferson University registered an open-label, phase 2 clinical intervention (NCT04389710) with 100 SARS-CoV-2 infected participants who have severe or life-threatening COVID-19. The participants typically receive 1–2 units (200–600 ml) of ABO compatible donor’s CP administrating at a rate of 100–250 ml/h, which has the anti-SARS-CoV-2 antibody (Clinicaltrials.Gov, 2020q).
Most recently, a pilot study led by the Biofarma has enrolled 10 participants(age: 18 years and older) with severe COVID-19 at Gatot Soebroto Central Army Presidential Hospital Jakarta Pusat, Indonesia has undergone with the CP administrating at the 3 times of each 100 ml on day 0, 3, and 6, which has the minimum titer (1:80) of anti-SARS-CoV-2 antibody (Clinicaltrials.Gov, 2020u). On the other hand, a phase 3 clinical study involved in PlasCoSSA (randomized, controlled, triple-blinded, parallel study) is registered to evaluate the efficacy of the transfusion of SARS-CoV-2 CP as an early treatment of the COVID-19 (Clinicaltrials.Gov, 2020af). In this instance, 80 participants of 18-80 years aged are randomly conducted to receive an amotosalen inactivated IV injection of 2 units SARS-CoV-2 CPof each 200–230 ml (Clinicaltrials.Gov, 2020af).
Recently, the Indonesia University has registered for initiating a phase 2/3, randomized, open-label, controlled clinical study (NCT04380935) in the Referral Hospitals in Indonesia to evaluate the effectiveness and safety of CPT in COVID-19 patients with ARDS (Clinicaltrials.Gov, 2020z). In response to this study, the research group of Indonesia University is planning to assign 60 patients randomly to get either CP plus standard care or standard care (Clinicaltrials.Gov, 2020z).
The Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh has recently registered a phase 2, randomized, three-arm clinical trial with 20 participants testing positive for SARS-CoV-2 (Clinicaltrials.Gov, 2020v). Of interest, the apheretic CP is collected from donors who have recovered from COVID-19, which has the antibody titre >1:320. In this study, the intervention model is designed as the three arms (arm-A, B, and C) in which the participants are conducted to receive standard supportive treatment alone as the arm-A, standard supportive treatment plus 200 ml apheretic CP as the arm-B, and standard supportive treatment plus 400 ml apheretic CP as the arm-C to evaluate effectiveness, safety, and efficacy of the dose-depended CPT (Clinicaltrials.Gov, 2020v).
In addition, to determine the therapeutic efficacy of the CP, the titer of neutralizing anti-SARS-CoV-2 antibodies (IgG) obtained from the CP of the fully recovered patients from COVID-19 is administered on days 1–7, 14, 21, 28, and 35 from the start of treatment in 60 patients (age: 18 years and older) with severe SARS-Cov-19 infection (Clinicaltrials.Gov, 2020r). Another multi-center randomized, double-blind, placebo-controlled clinical trial (NCT04383535)has been also registered by the Hospital Italiano de Buenos Aires to evaluate the effect of CP vs. placebo (Clinicaltrials.Gov, 2020n). For this study, 333 patients with severe COVID-19 are conducted in a 2:1 ratio, to administer CP (222 patients) or placebo (111 patients). On the other hand, a phase 3 clinical study (NCT04391101) with 231 participants in a 2:1 ratio (CP:standard management), registered from Hospital San Vicente Fundación is running to evaluate the safety, efficacy, and tolerability of CP (400–500 ml) (Clinicaltrials.Gov, 2020s).
At the current crisis of the nCoV-19 pandemic, the investigators from Lifefactors Zona Franca, SAS designs a randomized, multicenter, phase 2/3 clinical trial (NCT04395170) of CP in 75 hospitalized patients with COVID-19 to assess the efficacy of CP at a dose of 200–250 ml on days 1 and 3 of the intervention compared to the intravenous anti-COVID-19 human immunoglobulin at a dose of immunoglobulin 10% IgG solution on days 1 and 3 of treatment (Clinicaltrials.Gov, 2020p).
Therapeutic plasma exchange (TPE) is an important intervention that helps instantly and scientifically to remove pathogenic antibodies and toxic candidates by using centrifugal separation of plasma or plasma membrane filtration. Sometimes, TPE in combination with tocilizumab and steroids has been used efficaciously for the treatment of severe 2, 3, 4 CRS following CAR-T treatment. To evaluate the efficacy of TPE, the Prisma Health-Upstate has registered a pilot study, where 20 patients are enrolled in a nonrandomized, open-label phase 2 clinical trial receive either TPE alone and or in combination with ruxolitinib (Clinicaltrials.Gov, 2020bq). In another study, the hyper immunoglobulins containing anti-SARS-CoV-2 immunoglobulin is being investigated in order to assess its efficacy as a passive immunization as well as treatment of early disease before the development of lower respiratory tract disease (e.g., pneumonia) (Clinicaltrials.Gov, 2020j).
Discussion: Challenges and Clinical Perspectives on COVID-19 Pharmacotherapy
It is the greatest challengeable for the rapid identification of effective therapy developmental technologies and interventions for the COVID-19 associated paramount global public health crisis.
Compared with other viral infection, SARS-CoV-2 causes high proinflammatory disease state associated with COVID-19 through inducing lower levels of IFN–I and –III expression with a moderate reaction of IFN-stimulated genes (ISGs) and raising chemokine expression (Figure 4) (Blanco-Melo et al., 2020).
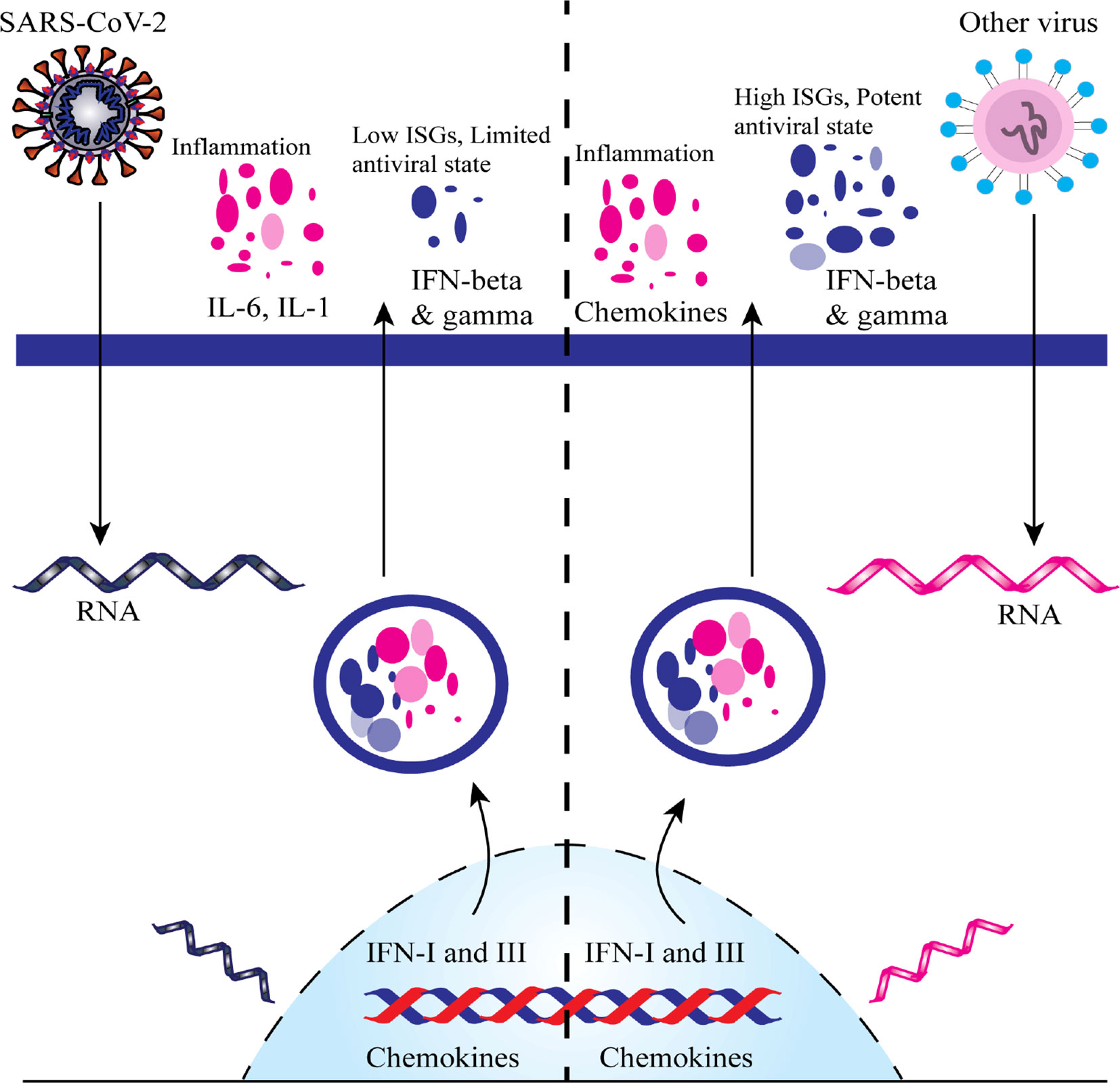
Figure 4 A comparative scheme regarding the imbalanced host response of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection versus other common respiratory virus infections. ISGs, IFN-stimulated genes.
Mild forms of COVID-19 can be treated at home if the infection is not very symptomatic and the person can be properly isolated (Godman, 2020). Patient care in these cases focuses on preventing transmission to others and monitoring the clinical condition to detect damage that could lead to hospitalization (Gao et al., 2020). Patient care in these cases is purely symptomatic (antipyretic), and preventive - the use of a mask in contact with other people, surface disinfection, hand hygiene, isolation of other people (Gao et al., 2020).
In other more severe clinical forms of COVID-19, patient care consists of the following aspects (Wiersinga et al., 2020):
i. Initially, symptomatic treatment is used - antipyretics to control fever (Jamerson and Haryadi, 2020)
ii. In case of hypoxia - oxygen therapy to maintain saturation> 94% - in patients who have signs of aggravation (apnea or severe dyspnea, central cyanosis, shock, coma, convulsions) - airway management, oxygen therapy minimum 5 L/min up to 10–15 L/min per mask; after stabilization SpO2 (blood oxygen saturation levels)> 90% is maintained; in some cases noninvasive ventilation is recommended (Dondorp et al., 2020).
iii. Co-infection treatment - even in case of suspicion of COVID-19, empirical antibiotic therapy is administered, especially in case of sepsis (1 h after the identification of sepsis), based on the clinical diagnosis (Chang and Chan, 2020).
iv. In case of ARDS (acute respiratory distress syndrome) - mechanical ventilation; with extreme care during the intubation maneuver which has a high risk of contamination; in severe ARDS it is recommended to use the sitting position; hydroelectrolytic rebalancing
v. Septic shock - hydroelectrolytic rebalancing antibiotic in the first hour, installation of central venous/arterial catheter (Fan et al., 2020).
vi. Prevention of complications is essential - maneuvers specific to each type of complication, especially in the case of those determined by prolonged immobilization and parenteral nutrition(Phua et al., 2020);
vii. The next supportive medicines will not be administered (due to the fact that they can aggravate the patient’s condition) - hypotonic crystalloids, corticosteroids (no benefits have been proven so far, but many side effects and increased mortality due to secondary infections and side effects, to be used only if there are comorbidities that would may require such therapy) (Singh et al., 2020).
The reason for using corticosteroids in COVID-19 therapy is based on their ability to reduce the host’s inflammatory responses in the lungs, inflammatory responses that could cause acute lung damage and acute respiratory distress syndrome. However, this benefit may be outweighed by their side effects, including delayed viral clearance and increased risk of secondary infection. Although direct evidence for the use of corticosteroids in COVID-19 is limited, analyzes of results in other viral pneumonias are relevant (Health, 2020a).
Observational studies in patients with SARS and MERS did not report any association between corticosteroid use and increased survival (Arabi et al., 2018), but showed an association of their use with delayed viral clearance in the respiratory tract and blood and the high frequency of complications, including hyperglycemia and psychosis (Keller et al., 2020).
In addition, a 2019 meta-analysis of 10 observational studies with 6,548 patients with influenza pneumonia found that corticosteroid therapy was associated with an increased risk of mortality (risk ratio [RR], 1.75 [95% CI, 1.3–2.4]; P <0.001) and a twice as high risk of secondary infections (RR, 1.98 [95% CI, 1.0–3.8]; P = 0.04)) (Ni et al., 2019).
Although the effectiveness of corticosteroids in acute respiratory distress syndrome and septic shock remains generally controversial, Russell and colleagues have argued that they are more effective in bacterial infections than in viral ones. A recent retrospective study of 201 COVID-19 patients in China found that for those who developed acute respiratory distress syndrome, methylprednisolone treatment was associated with a lower risk of death (23/50 [46%] with steroids vs. 21/34 [62%] without; HR, 0.38 [95% CI, 0.20–0.72]) (Russell et al., 2020).
Therefore, the potential adverse reactions and lack of proven benefits for corticosteroids in COVID-19 are arguments against their routine use in patients with COVID-19, unless there is a concomitant convincing indication, such as chronic exacerbation of obstructive disease or refractory shock.
Very recently, according to preliminary results from the RECOVERY study, dexamethasone, a common steroidal antiinflammatory drug, could reduce the death rate by one-third among patients severely affected by the new coronavirus. Dexamethasone is a glucocorticoid used since the 1960s in the treatment of many inflammatory conditions, but also in oncology (Bucolo et al., 2018).
Launched in March 2020, RECOVERY (Randomized Evaluation of COVid-19 Therapy) is one of the largest studies exploring potential treatments for COVID-19, and has included approximately 11,500 patients from over 175 hospitals NHS (National Health Service) in the United Kingdom. The results were surprising: one of the arms of the study, in which 2,104 patients were treated with six milligrams of dexamethasone per day (orally or intravenously) for ten days, was compared with 4,321 patients who received treatment considered be the current standard for SARS-CoV-2 infection (RECOVERY Collaborative Group, 2020).
In patients receiving standard treatment, mortality at 28 days was 41% in patients who required invasive ventilation, 25% in those who required only oxygen, and was lower (13%) in those who did not have need any intervention. It has been found that the use of dexamethasone reduces mortality by one third in ventilated patients - ratio 0.65; 95% confidence interval (0.48–0.88); p = 0.0003 - and one-fifth in patients with additional oxygen requirements - 0.80 (0.67–0.96); p = 0.0021, no significant benefit was observed in patients who did not require respiratory support - 1.22 (0.86 to 1.75); p = 0.14. These results demonstrate that if patients with COVID-19 requiring additional oxygen or invasive ventilation are given dexamethasone, it could save lives at extremely low cost. However, the WHO warns that the use of dexamethasone should only be used in severe cases of SARS-CoV-2 infection, these being the situations in which benefits were noticed significant (Villar et al., 2020).
Thus, dexamethasone should become the new standard in the treatment of COVID-19, especially in severe cases and the WHO will soon update the therapeutic protocol guidelines for COVID-19 (WHO, 2020).
In the case of drug development, the shorter time period insisted health providers to focus on identifying existing drugs or drug candidates intended for other indications that may have efficacy against COVID-19 and put them into accelerated clinical trials. Further dose assessments can be combined into an extended phase 3 trial using a combination of clinical, viral load decline and immune response as endpoints. This kind of accelerated procedure will place an extensive load on controlling agencies that only the pandemic itself can rationalize. To solve this, WHO launched the harmonized “Solidarity Trial” in different countries to rapidly assess in thousands of COVID-19 patients to evaluate the effectiveness of current antiviral and antiinflammatory agents not yet evaluated specifically for COVID-19 (Cheng et al., 2020). Similarly, The US National Institute of Allergy and Infectious Diseases (NIAID) commenced an adaptive design for international phase 3 trial called “ACTT” to include up to 800 hospitalized COVID-19 persons at 100 places in numerous countries (Clinicaltrials.Gov, 2020b).
However, the more international arrangement would necessitate maintaining the high degree of regulatory coordination and normalization of clinical operations across many diverse settings. Another aspect of regarding drug development in COVID-19 is repurposed drugs, an accepted drug for the treatment of different ailments or medical conditions than that for which it was initially developed. Conversely, COVID-19 is a novel disease, the repurposed drugs will not totally effective, and extensive research will be needed to optimize them (Shi et al., 2020).
Like the other two therapies, CPT was a promising treatment for serious COVID-19, though it has hidden risks such as aggravating hyperimmune attacks. Moreover, this therapy is more effective in the earlier stage of disease and researches on SARS confirmed it. Therefore, the ideal timing of administering CPon COVID-19 patient needs to be cautiously measured (Zhao and He, 2020). Another challenging factor of CPT is SARS-CoV-2 neutralizing antibody titer. An investigation on SARS verified that the precise IgG began to upsurge about week 3 later of the onset, and reached at a high level at week twelve (Li et al., 2003). In addition, another study on influenza advocated that CPT with a neutralizing antibody titer level of 1:160 and more reduced mortality. Thus, CP from donors who have recovered at week 12 after onset with a neutralizing antibody titer level of not less than 1:160 is estimated to be more effective (Hung et al., 2011). Besides, the most common adverse reaction of CPT are transfusion-related problems, including fever, anaphylactic shocks, transfusion-related acute lung injury, circulatory overload and hemolysis (Maclennan and Barbara, 2006). Considering all these challenges, healthcare providers may use CPT for hospitalized patients to reduce morbidity and mortality.
Herd immunity (HdI) is the indirect protection from infection conferred to susceptible individuals when a sufficiently large proportion of immune individuals exist in a population. The time to reach HdI of a community depends on the reproduction number (R0). It means the average number of people that a single infected person with the virus can infect those aren’t already immune. The higher the R0, the more people need to be resistant to reach HdI (Randolph and Barreiro, 2020). According to the scientific reports, the R0 for COVID-19 is within 2 to 6 (Sanche et al., 2020). This means that one infected person can infect two to six other persons. It also means 17 to 50% of the population would need to be resistant before HdI kicks in and the infection rates start to go down. However, a single pathogen may have multiple R0 values depending on the characteristics and transmission dynamics of the population. Therefore, the HdI threshold value (1 - 1/R0) may vary between populations (Anderson and May, 1985).
The communicability of an infectious disease depends on many factors, such as population density and age structure, cultural behaviors, underlying comorbidity rates, differences in contact rates across demographic groups, which may affect the HdI threshold (Sharifi-Rad J. et al., 2020). The effective reproduction number (Re or Rt) is also important to understand the population-level immunity. It is the average number of secondary cases generated by a single index case over an infectious period in a partially immune population. Thus, the goal of vaccination programs is to bring the value of Re below 1 will be possible only when the HdI threshold exceeded. The pathogen spread cannot be maintained, therefore, a decline in the number of infected individuals will be seen within the population (Randolph and Barreiro, 2020).
The challenges of HdI in case of COVID-19 are: (i) less effectiveness, periodic outbreaks can still occur, (ii) unevenly distributed within a population, clusters of susceptible hosts that frequently contact one another may remain, (iii) the proportion of immunized individuals surpasses the HdI threshold, susceptible individuals will be found in the risk zone for local outbreaks, (iv) nonrelevant infection fatality rate (IFR) and case fatality rate (CFR). Still there is no straightforward, ethical path to reach the goal with HdI in case of COVID-19, due to the societal consequences of achieving it are devastating. A nonuniform COVID-19 case fatality rate (CFR) has been reported across age groups, with the vast majority of deaths occurring among individuals 60 years old or greater. Sex- and ethnicity-specific CFRs suggest that genetic, environmental, and social determinants may affect in susceptibility to COVID-19 and the severity of SARS-CoV-19 infections.
Sodium chloride (NaCl) also called ‘table salt’ as coating material on the fiber surface of the filtration unit of surgical mask effectively deactivated a number influenza virus species, suggesting a new strategy in the protective measures to avoid primary/secondary infection and transmission of many viruses, including SARS-CoV-19 (Quan et al., 2017). On the other hand, the natural adsorbents, including clay, charcoal, and clay minerals showed 99.99% adsorption of CoVs (Robson, 2020). Some minerals that may act against CoVs are selenium (Ma et al., 2019), copper (Rupp et al., 2017), iron (Jayaweera et al., 2019), chromium (Terpiłowska and Siwicki, 2017), potassium (Punch et al., 2018), zinc (Te Velthuis et al., 2010), and so on. Moreover, medicinal plants or their derivatives are also evident to act against hCoVs (Kim et al., 2010; Aanouz et al., 2020; Islam et al., 2020).
Limitations
The main limiting aspects of this comprehensive review emerge from the studies that have been performed on these drugs that are still only experimental. Many of the studies analyzed included a relatively small number of patients and as a result data that are not statistically significant. That is why many old drugs with a well-known mechanism of action are re-proposed for the treatment of COVID-19. As no vaccine against COVID_19 has been approved yet, vaccine types have not been included in this paper. In addition, this review did not consider the cases of special patients such as the pediatric population and pregnant women, as they are excluded from clinical trials for ethical reasons.
The strength of this review is providing of the recent data with regard to the management of the COVID-19, within the environment in which information is rapidly changing and being made available; and it will be beneficial to health professionals.
Conclusion
The ongoing SARS-CoV-2 associated COVID-19 pandemic is continuously emerging worldwide and signifying the greatest spotlight on public health, education, travels, and economic conditions in the current world. The swiftness and dimensions of emerging therapeutic interventions hurled to explore potential treatments for COVID-19 highlight both the necessity and competence to produce superior evidence even at the time of a pandemic. Still, there is no single specific therapy that may give effective responses toward COVID-19. We believe, this paper will be able to provide sufficient information regarding the current treatment strategies and future directions for the pandemic SARS-CoV-2 infection.
Drug treatment is individualized according to the patient’s symptoms. The patient receives adequate care to relieve and treat symptoms. Patients suffering from serious illnesses and complications (such as pneumonia, severe respiratory problems, diabetes, cancer, cardiovascular disease) receive optimal care to support vital functions. Throughout this period, since the beginning of the pandemic, drugs already used in other diseases have been used, the safety of which has already been tested on humans. However, there is a drug that has already been tested for the treatment of people infected with Ebola and MERS, Remdesivir and seems to be an option for a treatment that is available globally.
In spite of the pandemic condition, we cannot forsake the prerequisite for well-designed clinical trials. Therefore, the current situation highlights the urgency for adhering to clinical pharmacology and model-informed drug development to optimize COVID-19 therapies, designing adaptive solidarity trials to decrease therapeutic dilemmas in clinical trial settings, as well as implementing the right patient, right drug, right dosage, and right timing approach to maximize trial success. Finally, adaptive designs for COVID-19 will lead to the development of more vigorous infectious disease research infrastructure and funding to help mitigate future pandemics.
Author Contributions
CS, MMo, MTI: conceptualization. MMa, AOD, and MTI: validation investigation. DC, AOD, JS-R, and MMa: resources. MMo, AOD, MMa, AM, DC, and JS-R: data curation. MMa, DC, and JS-R: review and editing. All authors: writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by CONICYT PIA/APOYO CCTE AFB170007.
References
(WHO), W.H.O (2020). WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients. Available at: https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients. Accesed on July 23, 2020.
Aanouz, I., Belhassan, A., El-Khatabi, K., Lakhlifi, T., El-Ldrissi, M., Bouachrine, M. (2020). Moroccan Medicinal plants as inhibitors against SARS-CoV-2 main protease: Computational investigations. J. Biomol. Struct. Dyn. 1–9. doi: 10.1080/07391102.2020.1758790
Agency, E. M. (2020). Veklury. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/veklury. (Accesed on July 30, 2020).
Agostini, M. L., Andres, E. L., Sims, A. C., Graham, R. L., Sheahan, T. P., Lu, X., et al. (2018). Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 9, e00221–e00218. doi: 10.1128/mBio.00221-18
Al-Saikhan, F. I., Al-Shdefat, R. I., Abd-Elaziz, M. A., Adam, E. H. K., Ahsan, F. (2015). Development and Validation of RP-HPLC Method for Quantitation of Aviptadil Acetate and its Degradation Products. Asian J. Biol. Life Sci. 4 1, 76–80.
Amaratunga, C., Lim, P., Suon, S., Sreng, S., Mao, S., Sopha, C., et al. (2016). Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect. Dis. 16, 357–365. doi: 10.1016/S1473-3099(15)00487-9
Anderson, R. M., May, R. M. (1985). Vaccination and herd immunity to infectious diseases. Nature 318, 323–329. doi: 10.1038/318323a0
Arabi, Y. M., Mandourah, Y., Al-Hameed, F., Sindi, A. A., Almekhlafi, G. A., Hussein, M. A., et al. (2018). Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 197, 757–767. doi: 10.1164/rccm.201706-1172OC
Aranow, C. (2011). Vitamin D and the immune system. J. Invest. Med. Off. Publ. Am. Fed. Clin. Res. 59, 881–886. doi: 10.231/JIM.0b013e31821b8755
Arrytown, N. Y., Paris (2020). Regeneron and Sanofi Provide Update on U.S. Phase 2/3 AdaptiveDesigned Trial of Kevzara® (Sarilumab) in Hospitalized Covid-19 Patients. Available at: https://investor.regeneron.com/news-releases/news-release-details/regeneron-andsanofi-provide-update-us-phase-23-adaptive (Accessed 2020 Apr 27).
Barragan, P., Podzamczer, D. (2008). Lopinavir/ritonavir: a protease inhibitor for HIV-1 treatment. Expert Opin. Pharmacother. 9, 2363–2375. doi: 10.1517/14656566.9.13.2363
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al (2020). Remdesivir for the treatment of Covid-19—preliminary report. New Engl. J. Med. doi: 10.1056/NEJMoa2007764
Bian, H., Zheng, Z.-H., Wei, D., Zhang, Z., Kang, W.-Z., Hao, C.-Q., et al. (2020). Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. 2020.2003.2021.20040691. doi: 10.1101/2020.03.21.20040691
Bittmann, S., Luchter, E., Weissenstein, A., Villalon, G., Moschuring-Alieva, E. (2020). TMPRSS2-Inhibitors Play a role in Cell Entry Mechanism of COVID-19: An Insight into Camostat and Nafamostat. J. Regener. Biol. Med. 2, 1–3.
Blaising, J., Polyak, S. J., Pécheur, E.-I. (2014). Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 107, 84–94. doi: 10.1016/j.antiviral.2014.04.006
Blanco-Melo, D., Nilsson-Payant, B. E., Liu, W.-C., Uhl, S., Hoagland, D., Møller, R., et al. (2020). Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181, 1036–1045.e1039. doi: 10.1016/j.cell.2020.04.026
Blejan, I. E., Diaconu, C. C., Letiția, A., Arsene, D. I. U., Ghica, M., Drăgănescu, D., et al. (2020). ANTIBIOTIC RESISTANCE IN COMMUNITY-ACQUIRED PNEUMONIA. A ROMANIAN PERSPECTIVE. FARMACIA 68, 512–520.
Boda, D., Docea, A. O., Calina, D., Ilie, M. A., Caruntu, C., Zurac, S., et al. (2018). Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int. J. Oncol. 52, 637–655. doi: 10.3892/ijo.2018.4256
Bosch, J., Yusuf, S., Gerstein, H. C., Pogue, J., Sheridan, P., Dagenais, G., et al. (2006). Effect of ramipril on the incidence of diabetes. N Engl. J. Med. 355, 1551–1562. doi: 10.1056/NEJMoa065061
Bucolo, C., Gozzo, L., Longo, L., Mansueto, S., Vitale, D. C., Drago, F. (2018). Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J. Pharmacol. Sci. 138, 219–232. doi: 10.1016/j.jphs.2018.11.001
Calina, D., Rosu, L., Rosu, A. F., Ianosi, G., Ianosi, S., Zlatian, O., et al. (2016). Etiological diagnosis and pharmacotherapeutic management of parapneumonic pleurisy. Farmacia 64, 946–952.
Călina, D., Docea, A. O., Rosu, L., Zlatian, O., Rosu, A. F., Anghelina, F., et al. (2017). Antimicrobial resistance development following surgical site infections. Mol. Med. Rep. 15, 681–688. doi: 10.3892/mmr.2016.6034
Calina, D., Docea, A. O., Petrakis, D., Egorov, A. M., Ishmukhametov, A. A., Gabibov, A. G., et al. (2020). Towards effective COVID19 vaccines: Updates, perspectives and challenges (Review). Int. J. Mol. Med. 46 (1), 3–16. doi: 10.3892/ijmm.2020.4596
Campbell, W. C., Fisher, M. H., Stapley, E. O., Albers-Schönberg, G., Jacob, T. A. (1983). Ivermectin: a potent new antiparasitic agent. Science 221, 823–828. doi: 10.1126/science.6308762
Campbell, W. C. (1985). Ivermectin: An update. Parasitol. Today 1, 10–16. doi: 10.1016/0169-4758(85)90100-0
Canadian Hydroxychloroquine Study Group (1991). A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl. J. Med. 324, 150–154. doi: 10.1056/NEJM199101173240303
Cantini, F., Niccoli, L., Matarrese, D., Nicastri, E., Stobbione, P., Goletti, D. (2020). Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 81 (2), 318–356. doi: 10.1016/j.jinf.2020.04.017
Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., et al. (2020). A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. New Engl. J. Med. 382, 1787–1799. doi: 10.1056/NEJMoa2001282
Cao, W., Liu, X., Bai, T., Fan, H., Hong, K., Song, H., et al. (2020). High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect. Dis. 7, ofaa102. doi: 10.1093/ofid/ofaa102
Carr, A. C., Maggini, S. (2017). Vitamin C and Immune Function. Nutrients 9, 1211. doi: 10.3390/nu9111211
Celularity (2020). Celularity announces FDA clearance of IND application for CYNK-001 in coronavirus, first in cellular therapy. Available at: https://clinicaltrials.gov/ct2/show/NCT04365101. (Accessed 2020 June 30).
Chang, C. Y., Chan, K. G. (2020). Underestimation of co-infections in COVID-19 due to non-discriminatory use of antibiotics. J. Infect. 81, e29–e30. doi: 10.1016/j.jinf.2020.06.077
Chen, H., Zhang, Z., Wang, L., Huang, Z., Gong, F., Li, X., et al. (2020). First Clinical Study Using HCV Protease Inhibitor Danoprevir to Treat Naive and Experienced COVID-19 Patients. medRxiv. 2020.2003.2022.20034041. doi: 10.1101/2020.03.22.20034041
Chen, L., Xiong, J., Bao, L., Shi, Y. (2020). Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 20, 398–400. doi: 10.1016/S1473-3099(20)30141-9
Cheng, M. P., Lee, T. C., Tan, D. H. S., Murthy, S. (2020). Generating randomized trial evidence to optimize treatment in the COVID-19 pandemic. Can. Med. Assoc. J. 192, E405. doi: 10.1503/cmaj.200438
Chictr.Org.Cn (2020a). Cancelled by the investigator Randomized controlled trial for the efficacy of dihydroartemisinine piperaquine in the treatment of mild/common novel coronavirus pneumonia (COVID-19). Available at: http://www.chictr.org.cn/showproj.aspx?proj=49915 (Accessed 2020 Mar 15).
Chictr.Org.Cn (2020b). Clinical Trial for Recombinant Human Interleukin-2 in the Treatment of Novel Coronavirus Pneumonia (COVID-19). Available at: http://www.chictr.org.cn/showprojen.aspx?proj=49567 (Accessed 2020 Feb 24).
Chictr.Org.Cn (2020c). Efficacy and safety of tozumab combined with adamumab (Qletli) in severe and critical patients with novel coronavirus pneumonia (COVID-19). Available at: http://www.chictr.org.cn/showprojen.aspx?proj=50693 (Accessed 2020 Mar 15).
Chictr.Org.Cn (2020d). A multi-center study on the efficacy and safety of suramin sodium in adult patients with novel coronavirus pneumonia (COVID-19). Available at: http://www.chictr.org.cn/showprojen.aspx?proj=49824 (Accessed 2020 Feb 21).
Chictr.Org.Cn (2020e). A randomized, open-label, controlled clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID-19). Available at: http://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000030424 (Accessed 2020 May 14).
Chintharlapalli, S. R., Croy, J. E., Leung, D., Gerald, D., Lu, J., Iversen, P. W., et al. (2016). Abstract 3259: LY3127804, a novel anti-Angiopoietin-2 antibody in combination with an anti-VEGFR2 antibody potently inhibits angiogenesis, tumor growth and metastasis. Cancer Res. 76, 3259. doi: 10.1158/1538-7445.AM2016-3259
Chu, C. M., Cheng, V. C. C., Hung, I. F. N., Wong, M. M. L., Chan, K. H., Chan, K. S., et al. (2004). Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59, 252–256. doi: 10.1136/thorax.2003.012658
Chun, J., Hartung, H.-P. (2010). Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33, 91–101. doi: 10.1097/WNF.0b013e3181cbf825
Claman, H. N. (1972). Corticosteroids and Lymphoid Cells. New Engl. J. Med. 287, 388–397. doi: 10.1056/NEJM197208242870806
Clerico, M., Contessa, G., Durelli, L. (2007). Interferon-β1a for the treatment of multiple sclerosis. Expert Opin. Biol. Ther. 7, 535–542. doi: 10.1517/14712598.7.4.535
Clinicaltrials.Gov (2020aa). Efficacy and Safety of Corticosteroids in COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04273321 (Accessed 2020 May 11).
Clinicaltrials.Gov (2020ab). Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID-19 (DC-COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04252274 (Accessed 2020 Apr 13).
Clinicaltrials.Gov (2020ac). The Efficacy and Safety of Thalidomide in the Adjuvant Treatment of Moderate New Coronavirus (COVID-19) Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04273529 (Accessed 2020 Feb 21).
Clinicaltrials.Gov (2020ad). Efficacy and Safety Study of IV Ravulizumab in Patients with COVID-19 Severe Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04369469 (Accessed 2020 Apr 30).
Clinicaltrials.Gov (2020ae). Efficacy of Addition of Naproxen in the Treatment of Critically Ill Patients Hospitalized for COVID-19 Infection (ENACOVID). Available at: https://clinicaltrials.gov/ct2/show/NCT04325633 (Accessed 2020 Apr 14).
Clinicaltrials.Gov (2020af). Efficacy of Convalescent Plasma Therapy in the Early Care of COVID-19 Patients. (PLASCOSSA). Available at: https://clinicaltrials.gov/ct2/show/NCT04372979 (Accessed 2020 May 15).
Clinicaltrials.Gov (2020ag). Efficacy of Human Coronavirus-immune Convalescent Plasma for the Treatment of COVID-19 Disease in Hospitalized Children (CONCOR-KIDS). Available at: https://clinicaltrials.gov/ct2/show/NCT04377568 (Accessed 2020 May 6).
Clinicaltrials.Gov (2020ah).The Efficacy of Intravenous Immunoglobulin Therapy for Severe 2019-nCoV Infected Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT02543567 (Accessed 2020 Feb 7).
Clinicaltrials.Gov (2020ai). Efficacy of Nafamostat in Covid-19 Patients (RACONA Study) (RACONA). Available at: https://clinicaltrials.gov/ct2/show/NCT04352400 (Accessed 2020 Apr 20).
Clinicaltrials.Gov (2020aj). Estrogen Patch for COVID-19 Symptoms. Available at: https://clinicaltrials.gov/ct2/show/NCT04359329 (Accessed 2020 Apr 24).
Clinicaltrials.Gov (2020ak). Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection. Available at: https://clinicaltrials.gov/ct2/show/NCT04261907 (Accessed 2020 Feb 10).
Clinicaltrials.Gov (2020al). Fingolimod in COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04280588 (Accessed 2020 Feb 21).
Clinicaltrials.Gov (2020am). Imatinib in Covid-19 Disease in Aged Patients (IMAGE-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04357613 (Accessed 2020 Apr 22).
Clinicaltrials.Gov (2020an). International ALLIANCE Study of Therapies to Prevent Progression of COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04395768 (Accessed 2020 May 20).
Clinicaltrials.Gov (2020ao). Intravenous Aviptadil for COVID-19 Associated Acute Respiratory Distress (COVID-AIV). Available at: https://clinicaltrials.gov/ct2/show/NCT04311697 (Accessed 2020 Apr 24).
Clinicaltrials.Gov (2020ap). Jaktinib Dihydrochloride Monohydrate in Idiopathic Pulmonary Fibrosis. Available at: https://clinicaltrials.gov/ct2/show/NCT04312594 (Accessed 2020 Apr 30).
Clinicaltrials.Gov (2020aq). The LEAD COVID-19 Trial: Low-risk, Early Aspirin and Vitamin D to Reduce COVID-19 Hospitalizations (LEAD COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04363840 (Accessed 2020 May 6).
Clinicaltrials.Gov (2020ar). Leflunomide in Mild COVID-19 Patients. Available at: https://clinicaltrials.gov/ct2/show/NCT04361214 (Accessed 2020 May 11).
Clinicaltrials.Gov (2020as). LIBERATE Trial in COVID-19 (LIBERATE). Available at: https://clinicaltrials.gov/ct2/show/NCT04334629 (Accessed 2020 May 1).
Clinicaltrials.Gov (2020at). Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment. Available at: https://clinicaltrials.gov/ct2/show/NCT04276688 (Accessed 2020 Apr 15).
Clinicaltrials.Gov (2020au). Losartan for Patients with COVID-19 Requiring Hospitalization. Available at: https://clinicaltrials.gov/ct2/show/NCT04312009 (Accessed 2020 May 11).
Clinicaltrials.Gov (2020av). Natural Killer Cell (CYNK-001) Infusions in Adults with COVID-19 (CYNK-001-COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04365101 (Accessed 2020 Apr 28).
Clinicaltrials.Gov (2020aw). Open-label, Randomized Study of IFX-1 in Patients with Severe COVID-19 Pneumonia (PANAMO). Available at: https://clinicaltrials.gov/ct2/show/NCT04333420 (Accessed 2020 Apr 16).
Clinicaltrials.Gov (2020ax). Phase 3 Randomized, Double-blind, Placebo-controlled Multicenter Study to Assess the Efficacy and Safety of Ruxolitinib in Patients with COVID-19 Associated Cytokine Storm (RUXCOVID) (RUXCOVID). Available at: https://clinicaltrials.gov/ct2/show/NCT04362137 (Accessed 2020 May 7).
Clinicaltrials.Gov (2020ay). Phase 3 Study to Evaluate Efficacy and Safety of Lenzilumab in Hospitalized Patients with COVID-19 Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04351152 (Accessed 2020 May 12).
Clinicaltrials.Gov (2020az). Piclidenoson for Treatment of COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04333472 (Accessed 2020 Apr 3).
Clinicaltrials.Gov (2020b). Adaptive COVID-19 Treatment Trial (ACTT). Available at: https://clinicaltrials.gov/ct2/show/NCT04280705 (Accessed 2020 May 12).
Clinicaltrials.Gov (2020ba). A Pilot Study of Sildenafil in COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04304313 (Accessed 2020 Mar 17).
Clinicaltrials.Gov (2020bb). Post-exposure Prophylaxis / Preemptive Therapy for SARSCoronavirus-2 (COVID-19 PEP). Available at: https://clinicaltrials.gov/ct2/show/NCT04308668 (Accessed 2020 May 1).
Clinicaltrials.Gov (2020bc). Progesterone for the Treatment of COVID-19 in Hospitalized Men. Available at: https://clinicaltrials.gov/ct2/show/NCT04365127 (Accessed 2020 Apr 28).
Clinicaltrials.Gov (2020bd). Ramipril for the Treatment of COVID-19 (RAMIC). Available at: https://clinicaltrials.gov/ct2/show/NCT04366050 (Accessed 2020 Apr 28).
Clinicaltrials.Gov (2020be). Randomized Proof-of-Concept Trial to Evaluate the Safety and Explore the Effectiveness of Resveratrol for COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04400890 (Accessed 2020 May 26).
Clinicaltrials.Gov (2020bf). Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia (REMAP-CAP). Available at: https://clinicaltrials.gov/ct2/show/NCT02735707 (Accessed 2020 Apr 16).
>Clinicaltrials.Gov (2020bg). Recombinant Human Angiotensin-converting Enzyme 2 (rhACE2) as a Treatment for Patients with COVID-19 (APN01-COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04335136 (Accessed 2020 May 5).
Clinicaltrials.Gov (2020bh). A Single-blinded, Randomized, Placebo Controlled Phase II Trial of Prophylactic Treatment with Oral Azithromycin Versus Placebo in Cancer Patients Undergoing Antineoplastic Treatment During the Corona Virus Disease 19 (COVID-19) Pandemic. Available at: https://clinicaltrials.gov/ct2/show/NCT04369365 (Accessed 2020 May 1).
Clinicaltrials.Gov (2020bi). Spironolactone in Covid-19 Induced ARDS. Available at: https://clinicaltrials.gov/ct2/show/NCT04345887 (Accessed 2020 Apr 15).
Clinicaltrials.Gov (2020bj). A Study of LY3127804 in Participants with COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04342897 (Accessed 2020 May 5).
Clinicaltrials.Gov (2020bk). Study of TJ003234 (Anti-GM-CSF Monoclonal Antibody) in Healthy Adult Subjects. Available at: https://clinicaltrials.gov/ct2/show/NCT03794180 (Accessed 2019 Dec 18).
Clinicaltrials.Gov (2020bl). Study of TJ003234 (Anti-GM-CSF Monoclonal Antibody) in Subjects with Severe Coronavirus Disease 2019 (COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04341116 (Accessed 2020 May 14).
Clinicaltrials.Gov (2020bm). Study on Safety and Efficacy of Favipiravir (Favipira) for COVID-19 Patient in Selected Hospitals of Bangladesh. (Accessed 2020 May 28).
Clinicaltrials.Gov (2020bn). A Study to Assess the Efficacy and Safety of Gimsilumab in Subjects with Lung Injury or Acute Respiratory Distress Syndrome Secondary to COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04351243 (Accessed 2020 May 13).
Clinicaltrials.Gov (2020bo). Study to Evaluate the Efficacy and Safety of Leronlimab for Patients with Severe or Critical Coronavirus Disease 2019 (COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04347239 (Accessed 2020 May 01).
Clinicaltrials.Gov (2020bp). A Study to Evaluate the Safety,Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT03891420 (Accessed 2020 Apr 15).
Clinicaltrials.Gov (2020bq). Therapeutic Plasma Exchange Alone or in Combination With Ruxolitinib in COVID-19 Associated CRS. Available at: https://clinicaltrials.gov/ct2/show/NCT04374149 (Accessed 2020 May 5).
Clinicaltrials.Gov (2020br). Tocilizumab in the Treatment of Coronavirus Induced Disease (COVID-19) (CORON-ACT). Available at: https://clinicaltrials.gov/ct2/show/NCT04335071 (Accessed 2020 Apr 28).
Clinicaltrials.Gov (2020bs). TOFAcitinib in SARS-CoV2 Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04332042 (Accessed 2020 Apr 2).
Clinicaltrials.Gov (2020bt). Trial Evaluating Efficacy and Safety of Bevacizumab (Avastin®/Zeribev®) in Patients With COVID-19 Infection, Nested in the Corimmuno-19 Cohort (CORIMMUNO-BEVA). Available at: https://clinicaltrials.gov/ct2/show/NCT04344782 (Accessed 2020 Apr 17).
Clinicaltrials.Gov (2020bu). Trial Evaluating Efficacy and Safety of Nivolumab (Optivo®) in Patients With COVID-19 Infection, Nested in the Corimmuno-19 Cohort (CORIMUNONIVO). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04343144 (Accessed 2020 Apr 14).
Clinicaltrials.Gov (2020bv). Trial to Promote Recovery from COVID-19 with Ivermectin or Endocrine Therapy (RECOVER). Available at: https://clinicaltrials.gov/ct2/show/NCT04374279 (Accessed 2020 May 5).
Clinicaltrials.Gov (2020bw). Triiodothyronine for the Treatment of Critically Ill Patients with COVID-19 Infection (Thy-Support). Available at: https://clinicaltrials.gov/ct2/show/NCT04348513 (Accessed 2020 Apr 16).
Clinicaltrials.Gov (2020bx). Valsartan for Prevention of Acute Respiratory Distress Syndrome in Hospitalized Patients with SARS-COV-2 (COVID-19) Infection Disease. Available at: https://clinicaltrials.gov/ct2/show/NCT04335786 (Accessed 2020 May 8).
Clinicaltrials.Gov (2020by). Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04264533 (Accessed 2020 Mar 10).
Clinicaltrials.Gov (2020c). Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO). Available at: https://clinicaltrials.gov/ct2/show/NCT04357782 (Accessed 2020 Apr 22).
Clinicaltrials.Gov (2020d). Amotosalen-Ultraviolet A Pathogen-Inactivated Convalescent Plasma in Addition to Best Supportive Care and Antiviral Therapy on Clinical Deterioration in Adults Presenting With Moderate to Severe COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04389944 (Accessed 2020 May 15).
Clinicaltrials.Gov (2020e). Avdoralimab an Anti-C5aR Antibody, in Patients with COVID-19 Severe Pneumonia (FORCE). Available at: https://clinicaltrials.gov/ct2/show/NCT04371367 (Accessed 2020 May 1).
Clinicaltrials.Gov (2020f). Baricitinib in Symptomatic Patients Infected by COVID-19: an Open-label, Pilot Study. Available at: https://clinicaltrials.gov/ct2/show/NCT04320277 (Accessed 2020 Apr 22).
Clinicaltrials.Gov (2020g). Camostat Mesylate in COVID-19 Outpatients. Available at: https://clinicaltrials.gov/ct2/show/NCT04353284 (Accessed 2020 May 20).
Clinicaltrials.Gov (2020h). Cholecalciferol to Improve the Outcomes of COVID-19 Patients (CARED). Available at: https://clinicaltrials.gov/ct2/show/NCT04411446 (Accessed 2020 Jun 4).
Clinicaltrials.Gov (2020i). Clazakizumab (Anti-IL- 6 Monoclonal) Compared to Placebo for COVID19 Disease. Available at: https://clinicaltrials.gov/ct2/show/NCT04348500 (Accessed 2020 Apr 16).
Clinicaltrials.Gov (2020j). Clinical Study for Efficacy of Anti-Corona VS2 Immunoglobulins Prepared From COVID19 Convalescent Plasma Prepared by VIPS Mini-Pool IVIG Medical Devices in Prevention of SARS-CoV-2 Infection in High Risk Groups as Well as Treatment of Early Cases of COVID19 Patients. Available at: https://clinicaltrials.gov/ct2/show/NCT04383548 (Accessed 2020 May 12).
Clinicaltrials.Gov (2020k). Available at: https://clinicaltrials.gov/ct2/show/NCT04286503 (Accessed The Clinical Study of Carrimycin on Treatment Patients with COVID-192020 Feb 27).
Clinicaltrials.Gov (2020l). Clinical Study to Evaluate the Performance And Safety of Favipiravir in COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04336904 (Accessed 2020 Apr 8).
Clinicaltrials.Gov (2020m). Colchicine Coronavirus SARS-CoV2 Trial (COLCORONA) (COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04322682 (Accessed 2020 Apr 24).
Clinicaltrials.Gov (2020n). Convalescent Plasma and Placebo for the Treatment of COVID-19 Severe Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04383535 (Accessed 2020 May 13).
Clinicaltrials.Gov (2020o). Convalescent Plasma as Treatment for Acute Coronavirus Disease (COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04390178 (Accessed 2020 May 15).
Clinicaltrials.Gov (2020p). Convalescent Plasma Compared to Anti-COVID-19 Human Immunoglobulin and Standard Treatment (TE) in Hospitalized Patients. Available at: https://clinicaltrials.gov/ct2/show/NCT04395170 (Accessed 2020 May 20).
Clinicaltrials.Gov (2020q). Convalescent Plasma for the Treatment of COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04389710 (Accessed 2020 May 15).
Clinicaltrials.Gov (2020r). Convalescent Plasma for the Treatment of Patients with SevereCOVID-19 Infection. Available at: https://clinicaltrials.gov/ct2/show/NCT04408209 (Accessed 2020 June 2).
Clinicaltrials.Gov (2020s). Convalescent Plasma for the Treatment of Severe SARS-CoV-2 (COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04391101 (Accessed 2020 May 20).
Clinicaltrials.Gov (2020t). Convalescent Plasma for Treatment of COVID-19: An Exploratory Dose Identifying Study. Available at: https://clinicaltrials.gov/ct2/show/NCT04384497 (Accessed 2020 May 27).
Clinicaltrials.Gov (2020u). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04407208 (Accessed Convalescent Plasma Therapy in Patients with COVID-192020 June 2).
Clinicaltrials.Gov (2020v). Convalescent Plasma Therapy in Severe COVID-19 Infection. Available at: https://clinicaltrials.gov/ct2/show/NCT04403477 (Accessed 2020 May 27).
Clinicaltrials.Gov (2020w). CORIMUNO19-ECU: Trial Evaluating Efficacy and Safety of Eculizumab (Soliris) in Patients with COVID-19 Infection, Nested in the CORIMUNO-19 Cohort (CORIMUNO19-ECU). Available at: https://clinicaltrials.gov/ct2/show/NCT04346797 (Accessed 2020 Apr 27).
Clinicaltrials.Gov (2020x). COvid-19 and Vitamin D Supplementation: a Multicenter Randomized Controlled Trial of High Dose Versus Standard Dose Vitamin D3 in Highrisk COVID-19 Patients (CoVitTrial). Available at: https://clinicaltrials.gov/ct2/show/NCT04344041 (Accessed 2020 May 6).
Clinicaltrials.Gov (2020y). DYNAMIC Study (DoxycYcliNe AMbulatoIre COVID-19) (DYNAMIC). Available at: https://clinicaltrials.gov/ct2/show/NCT04371952 (Accessed 2020 May 7).
Clinicaltrials.Gov (2020z). Effectiveness and Safety of Convalescent Plasma Therapy on COVID-19 Patients with Acute Respiratory Distress Syndrome. Available at: https://clinicaltrials.gov/ct2/show/NCT04380935 (Accessed 2020 May 8).
Clinicaltrialsarena.Com (2020a). InflaRx starts dosing Covid-19 patients in Europe. Available at: https://www.clinicaltrialsarena.com/news/inflarx-covid-19-dosing-europe (Accessed 2020 Apr 1).
Clinicaltrialsarena.Com (2020b). Roivant starts gimsilumab dosing in Covid-19 trial. Available at: https://www.clinicaltrialsarena.com/news/roivant-gimsilumab-covid-19-trial (Accessed 2020 Apr 16).
Cole, G. M., Frautschy, S. A. (2010). Mechanisms of action of non-steroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 9, 140–148. doi: 10.2174/187152710791011991
Davies, N. M., Røseth, A. G., Appleyard, C. B., Mcknight, W., Del Soldato, P., Calignano, A., et al. (1997). NO-naproxen vs. naproxen: ulcerogenic, analgesic and anti-inflammatory effects. Aliment Pharmacol. Ther. 11 (1), 69–79. doi: 10.1046/j.1365-2036.1997.115286000.x
Davies, M., Osborne, V., Lane, S., Roy, D., Dhanda, S., Evans, A., et al. (2020). Remdesivir in Treatment of COVID-19: A Systematic Benefit–Risk Assessment. Drug Saf. 1. doi: 10.1007/s40264-020-00952-1
Davies, N. M. (1998). Clinical Pharmacokinetics of Ibuprofen. Clin. Pharmacokinet. 34, 101–154. doi: 10.2165/00003088-199834020-00002
De Clercq, E. (2019). New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem. Asian J. 14, 3962–3968. doi: 10.1002/asia.201900841
De Wilde, A. H., Jochmans, D., Posthuma, C. C., Zevenhoven-Dobbe, J. C., Van Nieuwkoop, S., Bestebroer, T. M., et al. (2014). Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrobial Agents Chemother. 58, 4875–4884. doi: 10.1128/AAC.03011-14
Docea, A. O., Gofita, E., Calina, D., Ioan, Z. S., Valcea, D. I., Mitrut, P. (2016). Autoimmune disorders due to double antiviral therapy with peginterferon and ribavirin in patients with hepatitis C virus infection. Farmacia 64, 605–611.
Dodd, R. Y. (2012). Emerging pathogens and their implications for the blood supply and transfusion transmitted infections. Br. J. Haematol. 159, 135–142. doi: 10.1111/bjh.12031
Dondorp, A. M., Hayat, M., Aryal, D., Beane, A., Schultz, M. J. (2020). Respiratory Support in COVID-19 Patients, with a Focus on Resource-Limited Settings. Am. J. Trop. Med. Hyg. 102, 1191–1197. doi: 10.4269/ajtmh.20-0283
Dorward, J., Gbinigie, K. (2020). Lopinavir/ritonavir: A rapid review of effectiveness in COVID-19. Oxford: Centre for Evidence Based Medicine, University of Oxford. Available at: https://www.cebm.net/covid-19/lopinavir-ritonavir-a-rapid-review-of-the-evidence-for-effectiveness-in-treating-covid. (Accessed 2020 May 15).
Dunn, C. J., Barradell, L. B. (1996). Azithromycin. Drugs 51, 483–505. doi: 10.2165/00003495-199651030-00013
Eskandary, F., Dürr, M., Budde, K., Doberer, K., Reindl-Schwaighofer, R., Waiser, J., et al. (2019). Clazakizumab in late antibody-mediated rejection: study protocol of a randomized controlled pilot trial. Trials 20, 37. doi: 10.1186/s13063-018-3158-6
Fan, E., Beitler, J. R., Brochard, L., Calfee, C. S., Ferguson, N. D., Slutsky, A. S., et al. (2020). COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir. Med. 8, 816–821. doi: 10.1016/S2213-2600(20)30304-0
Fleischmann, R., Kremer, J., Cush, J., Schulze-Koops, H., Connell, C. A., Bradley, J. D., et al. (2012). Placebo-Controlled Trial of Tofacitinib Monotherapy in Rheumatoid Arthritis. New Engl. J. Med. 367, 495–507. doi: 10.1056/NEJMoa1109071
Fortes, P. A., Ellory, J. C., Lew, V. L. (1973). Suramin: a potent ATPase inhibitor which acts on the inside surface of the sodium pump. Biochim. Biophys. Acta 318, 262–272. doi: 10.1016/0005-2736(73)90119-3
Fox, R. I. (1998). Mechanism of action of leflunomide in rheumatoid arthritis. J. Rheumatol. Suppl. 53, 20–26.
Franks, M. E., Macpherson, G. R., Figg, W. D. (2004). Thalidomide. Lancet 363, 1802–1811. doi: 10.1016/S0140-6736(04)16308-3
Furuta, Y., Gowen, B. B., Takahashi, K., Shiraki, K., Smee, D. F., Barnard, D. L. (2013). Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 100, 446–454. doi: 10.1016/j.antiviral.2013.09.015
Furuta, Y., Komeno, T., Nakamura, T. (2017). Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 93, 449–463. doi: 10.2183/pjab.93.027
Gao, Z., Xu, Y., Sun, C., Wang, X., Guo, Y., Qiu, S., et al. (2020). A Systematic Review of Asymptomatic Infections with COVID-19. J. Microbiol. Immunol. Infect. doi: 10.1016/j.jmii.2020.05.001
Genovese, M. C., Fleischmann, R., Kivitz, A. J., Rell-Bakalarska, M., Martincova, R., Fiore, S., et al. (2015). Sarilumab Plus Methotrexate in Patients With Active Rheumatoid Arthritis and Inadequate Response to Methotrexate: Results of a Phase III Study. Arthritis Rheumatol. 67, 1424–1437. doi: 10.1002/art.39093
Georgiadis, G., Zisis, I.-E., Docea, A. O., Tsarouhas, K., Fragkiadoulaki, I., Mavridis, C., et al. (2020). Current Concepts on the Reno-Protective Effects of Phosphodiesterase 5 Inhibitors in Acute Kidney Injury: Systematic Search and Review. J. Clin. Med. 9, 1284. doi: 10.3390/jcm9051284
Gilead Sciences, I. (2020). Gilead Announces Results From Phase 3 Trial of Remdesivir in Patients With Moderate COVID-19. https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/gilead-announces-results-from-phase-3-trial-of-remdesivir-in-patients-with-moderate-covid-19
Godman, B. (2020). Combating COVID-19: Lessons learnt particularly among developing countries and the implications. Bangladesh J. Med. Sci. 103-S, 108. doi: 10.1056/NEJMoa2015301
Goldman, J. D., Lye, D. C., Hui, D. S., Marks, K. M., Bruno, R., Montejano, R., et al (2020). Remdesivir for 5 or 10 days in patients with severe Covid-19. New Engl. J. Med. doi: 10.1056/NEJMoa2015301
Gordon, C. J., Tchesnokov, E. P., Feng, J. Y., Porter, D. P., Götte, M. (2020). The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 295, 4773–4779. doi: 10.1074/jbc.AC120.013056
Goumenou, M., Sarigiannis, D., Tsatsakis, A., Anesti, O., Docea, A. O., Petrakis, D., et al. (2020). COVID19 in Northern Italy: An integrative overview of factors possibly influencing the sharp increase of the outbreak (Review). Mol. Med. Rep. 22, 20–32. doi: 10.3892/mmr.2020.11079
Grant, W. B., Lahore, H., Mcdonnell, S. L., Baggerly, C. A., French, C. B., Aliano, J. L., et al. (2020). Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 12, 988. doi: 10.3390/nu12040988
Hall, O. J., Klein, S. L. (2017). Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 10, 1097–1107. doi: 10.1038/mi.2017.35
Harrison, C., Kiladjian, J. J., Al-Ali, H. K., Gisslinger, H., Waltzman, R., Stalbovskaya, V., et al. (2012). JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl. J. Med. 366, 787–798. doi: 10.1056/NEJMoa1110556
Hayden, F. G., Sugaya, N., Hirotsu, N., Lee, N., De Jong, M. D., Hurt, A. C., et al. (2018). Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl. J. Med. 379, 913–923. doi: 10.1056/NEJMoa1716197
Health, N. I. O. (2020a). Corticosteroids. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/
Health, N. I. O. (2020b). NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19.
Hemady, R. K., Sinibaldi, V. J., Eisenberger, M. A. (1996). Ocular symptoms and signs associated with suramin sodium treatment for metastatic cancer of the prostate. Am. J. Ophthalmol. 121, 291–296. doi: 10.1016/S0002-9394(14)70277-6
Heng, L., Li, Y., Fei-Fei, L., Xin-Na, M., Pei-Lan, H., Wei, T., et al (1996). Overview of Therapeutic Drug Research for COVID-19 in China. Acta Pharmacol. Sin. 41 (9), 1133–1140. doi: 10.1038/s41401-020-0438-y
Hensley, L. E., Fritz, L. E., Jahrling, P. B., Karp, C. L., Huggins, J. W., Geisbert, T. W. (2004). Interferon-beta 1a and SARS coronavirus replication. Emerg. Infect. Dis. 10, 317–319. doi: 10.3201/eid1002.030482
Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., Linsell, L., et al. (2020). Dexamethasone in hospitalized patients with Covid-19—preliminary report. New Engl. J. Med. doi: 10.1056/NEJMoa2021436
Hung, I. F., To, K. K., Lee, C. K., Lee, K. L., Chan, K., Yan, W. W., et al. (2011). Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 52, 447–456. doi: 10.1093/cid/ciq106
Hung, I. F.-N., Lung, K.-C., Tso, E. Y.-K., Liu, R., Chung, T. W.-H., Chu, M.-Y., et al. (2020). Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395, 1695–1704. doi: 10.1016/S0140-6736(20)31042-4
Iordache, A. M., Buga, A. M., Albulescu, D., Vasile, R. C., Mitrut, R., Georgiadis, G., et al. (2020a). Phosphodiesterase-5 inhibitors ameliorate structural kidney damage in a rat model of contrast-induced nephropathy. Food Chem. Toxicol. 111535 doi: 10.1016/j.fct.2020.111535
Iordache, A. M., Docea, A. O., Buga, A. M., Zlatian, O., Ciurea, M. E., Rogoveanu, O. C., et al. (2020b). Sildenafil and tadalafil reduce the risk of contrast-induced nephropathy by modulating the oxidant/antioxidant balance in a murine model. Food Chem. Toxicol. 135, 111038. doi: 10.1016/j.fct.2019.111038
Islam, M. T., Sarkar, C., El-Kersh, D. M., Jamaddar, S., Uddin, S. J., Shilpi, J. A., et al. (2020). Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. doi: 10.1002/ptr.6700
Jamerson, B. D., Haryadi, T. H. (2020). The use of ibuprofen to treat fever in COVID-19: A possible indirect association with worse outcome? Med. Hypotheses 144, 109880. doi: 10.1016/j.mehy.2020.109880
Jayaweera, J., Reyes, M., Joseph, A. (2019). Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci. Rep. 9, 12637. doi: 10.1038/s41598-019-49122-z
Kamal, A. M., Mitru, P., Docea, A. O., Sosoi, S. S., Kamal, C. K., Mitrut, R., et al. (2017). Double therapy with pegylated interferon and ribavirin for chronic hepatitis C. A pharmacogenetic guide for predicting adverse events. Farmacia 65, 877–884.
Kazazi-Hyseni, F., Beijnen, J. H., Schellens, J. H. M. (2010). Bevacizumab. Oncol. 15, 819–825. doi: 10.1634/theoncologist.2009-0317
Keller, M., Kitsis, E., Arora, S., Chen, J., Agarwal, S., Ross, M., et al. (2020). Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J. Hosp. Med. 15, 489–493. doi: 10.12788/jhm.3497
Kim, H. Y., Eo, E. Y., Park, H., Kim, Y. C., Park, S., Shin, H. J., et al. (2010). Medicinal herbal extracts of Sophorae radix, Acanthopanacis cortex, Sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro. Antivir. Ther. 15, 697–709. doi: 10.3851/IMP1615
Knights, K. M., Mangoni, A. A., Miners, J. O. (2010). Defining the COX inhibitor selectivity of NSAIDs: implications for understanding toxicity. Expert Rev. Clin. Pharmacol. 3, 769–776. doi: 10.1586/ecp.10.120
Ko, W.-C., Rolain, J.-M., Lee, N.-Y., Chen, P.-L., Huang, C.-T., Lee, P.-I., et al. (2020). Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents 55, 105933–105933. doi: 10.1016/j.ijantimicag.2020.105933
Koshimichi, H., Ishibashi, T., Kawaguchi, N., Sato, C., Kawasaki, A., Wajima, T. (2018). Safety, Tolerability, and Pharmacokinetics of the Novel Anti-influenza Agent Baloxavir Marboxil in Healthy Adults: Phase I Study Findings. Clin. Drug Invest. 38, 1189–1196. doi: 10.1007/s40261-018-0710-9
Kovacs, J. A., Vogel, S., Albert, J. M., Falloon, J., Davey, R. T., Jr., Walker, R. E., et al. (1996). Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl. J. Med. 335, 1350–1356. doi: 10.1056/NEJM199610313351803
Langtry, H. D., Markham, A. (1999). Sildenafil. Drugs 57, 967–989. doi: 10.2165/00003495-199957060-00015
Le, R. Q., Li, L., Yuan, W., Shord, S. S., Nie, L., Habtemariam, B. A., et al. (2018). FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 23, 943–947. doi: 10.1634/theoncologist.2018-0028
Le Garff, G., Samri, A., Lambert-Niclot, S., Even, S., Lavolé, A., Cadranel, J., et al. (2017). Transient HIV-specific T cells increase and inflammation in an HIV-infected patient treated with nivolumab. Aids 31, 1048–1051. doi: 10.1097/QAD.0000000000001429
Legendre, C. M., Licht, C., Muus, P., Greenbaum, L. A., Babu, S., Bedrosian, C., et al. (2013). Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic–Uremic Syndrome. New Engl. J. Med. 368, 2169–2181. doi: 10.1056/NEJMoa1208981
Li, G., De Clercq, E. (2020). Therapeutic options for the 2019 novel coronavirus, (2019-nCoV). Nat. Rev. Drug Discovery 19, 149–150. doi: 10.1038/d41573-020-00016-0
Li, G., Chen, X., Xu, A. (2003). Profile of specific antibodies to the SARS-associated coronavirus. N Engl. J. Med. 349, 508–509. doi: 10.1056/NEJM200307313490520
Loriaux, D. L., Menard, R., Taylor, A., Pita, J. C., Santen, R. (1976). Spironolactone and Endocrine Dysfunction. Ann. Internal Med. 85, 630–636. doi: 10.7326/0003-4819-85-5-630
Lou, Y., Liu, L., Qiu, Y. (2020). Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: an Exploratory Randomized, Controlled Trial. medRxiv. 2020.2004.2029.20085761. doi: 10.1101/2020.04.29.20085761
Lu, X., Chen, T., Wang, Y., Wang, J., Yan, F. (2020). Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit. Care 24, 1–4. doi: 10.1186/s13054-020-02964-w
Lydon, J. P., Demayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A., Jr., et al. (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266–2278. doi: 10.1101/gad.9.18.2266
Ma, X., Bi, S., Wang, Y., Chi, X., Hu, S. (2019). Combined adjuvant effect of ginseng stem-leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult. Sci. 98, 3548–3556. doi: 10.3382/ps/pez207
Maclennan, S., Barbara, J. (2006). Risks and side effects of therapy with plasma and plasma fractions. Best Pract. Res. Clin. Haematol. 19, 169–189. doi: 10.1016/j.beha.2005.01.033
May, J. M., Harrison, F. E. (2013). Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal 19, 2068–2083. doi: 10.1089/ars.2013.5205
Mckeage, K., Perry, C. M., Keam, S. J. (2009). Darunavir. Drugs 69, 477–503. doi: 10.2165/00003495-200969040-00007
Mckeage, K. (2019). Ravulizumab: First Global Approval. Drugs 79, 347–352. doi: 10.1007/s40265-019-01068-2
Meinking, T. L., Taplin, D., Herminda, J. L., Pardo, R., Kerdel, F. A. (1995). The Treatment of Scabies with Ivermectin. New Engl. J. Med. 333, 26–30. doi: 10.1056/NEJM199507063330105
Mina, F., Rahman, M., Das, S., Karmakar, S., Billah, M. (2020). Potential Drug Candidates Underway Several Registered Clinical Trials for Battling COVID-19. Preprints 2020040367. doi: 10.20944/preprints202004.0367.v1
Mititelu, R. R., Pădureanu, R., Băcănoiu, M., Pădureanu, V., Docea, A. O., Calina, D., et al. (2020). Inflammatory and Oxidative Stress Markers—Mirror Tools in Rheumatoid Arthritis. Biomedicines 8, 125. doi: 10.3390/biomedicines8050125
Moen, M. D., Mckeage, K., Plosker, G. L., Siddiqui, M. (2007). Imatinib. Drugs 67, 299–320. doi: 10.2165/00003495-200767020-00010
Mohammad, F. S., Karmakar, V., Irfan, Z. (2020). Hydroxychloroquine and azithromycin combination could be lethal to covid-19 patients. FARMACIA 68, 384–389. doi: 10.31925/farmacia.2020.3.2
Moucari, R., Forestier, N., Larrey, D., Guyader, D., Couzigou, P., Benhamou, Y., et al. (2010). Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut 59, 1694–1698. doi: 10.1136/gut.2010.219089
National Institutes of Health (2020). NIH clinical trial testing remdesivir plus interferon beta-1a for COVID-19 treatment begins. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. accesd July, 30,2020
Ni, Y. N., Chen, G., Sun, J., Liang, B. M., Liang, Z. A. (2019). The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit. Care 23, 99. doi: 10.1186/s13054-019-2395-8
Niel, E., Scherrmann, J.-M. (2006). Colchicine today. Joint Bone Spine 73, 672–678. doi: 10.1016/j.jbspin.2006.03.006
Ohkoshi, M., Oka, T. (1984). Clinical experience with a protease inhibitor [N,N-dimethylcarbamoylmethyl 4-(4-guanidinobenzoyloxy)-phenylacetate] methanesulfate for prevention of recurrence of carcinoma of the mouth and in treatment of terminal carcinoma. J. Maxillofac. Surg. 12, 148–152. doi: 10.1016/S0301-0503(84)80235-0
Oldfield, V., Dhillon, S., Plosker, G. L. (2009). Tocilizumab. Drugs 69, 609–632. doi: 10.2165/00003495-200969050-00007
O’hanlon, R., Shaw, M. L. (2019). Baloxavir marboxil: the new influenza drug on the market. Curr. Opin. Virol. 35, 14–18. doi: 10.1016/j.coviro.2019.01.006
Pantos, C., Tseti, I., Mourouzis, I. (2020). Use of triiodothyronine to treat critically ill COVID-19 patients: a new clinical trial. Crit. Care 24, 209. doi: 10.1186/s13054-020-02934-2
Patnaik, M. M., Sallman, D. A., Mangaonkar, A. A., Heuer, R., Hirvela, J., Zblewski, D., et al. (2019). A Phase 1 Study of Lenzilumab, a humaneered recombinant Anti-Human Granulocyte-Macrophage Colony- Stimulating Factor (anti-hGM-CSF) Antibody, for Chronic Myelomonocytic Leukemia (CMML). Blood 134, 4234–4234. doi: 10.1182/blood-2019-131181
Peiris, J. S. M., Guan, Y., Yuen, K. Y. (2004). Severe acute respiratory syndrome. Nat. Med. 10, S88–S97. doi: 10.1038/nm1143
Peng, B., Lloyd, P., Schran, H. (2005). Clinical Pharmacokinetics of Imatinib. Clin. Pharmacokinet. 44, 879–894. doi: 10.2165/00003088-200544090-00001
Pestana, R. C., Hassan, M. M., Abdel-Wahab, R., Abugabal, Y. I., Girard, L. M., Li, D., et al. (2018). Clinical and prognostic significance of circulating levels of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. Oncotarget 9, 37721–37732. doi: 10.18632/oncotarget.26507
Pestell, R. G., Cristofanilli, M., Rui, H., Jiao, X., Wang, M. (2020). Abstract PD4-04: Leronlimab, a humanized monoclonal antibody to CCR5, restrains breast cancer metastasis and enhances cell death induced by DNA damaging chemotherapies. Cancer Res. 80, PD4–P04. doi: 10.1158/1538-7445.SABCS19-PD4-04
Peters, D. H., Friedel, H. A., Mctavish, D. (1992). Azithromycin. Drugs 44, 750–799. doi: 10.2165/00003495-199244050-00007
Phua, J., Weng, L., Ling, L., Egi, M., Lim, CM., Divatia, J. V., et al (2020). Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir. Med. 8 (5), 506–517. doi: 10.1016/S2213-2600(20)30161-2
Prakash, A., Jarvis, B. (1999). Leflunomide. Drugs 58, 1137–1164. doi: 10.2165/00003495-199958060-00010
Punch, E. K., Hover, S., Blest, H. T. W., Fuller, J., Hewson, R., Fontana, J., et al. (2018). Potassium is a trigger for conformational change in the fusion spike of an enveloped RNA virus. J. Biol. Chem. 293, 9937–9944. doi: 10.1074/jbc.RA118.002494
Quan, F.-S., Rubino, I., Lee, S.-H., Koch, B., Choi, H.-J. (2017). Universal and reusable virus deactivation system for respiratory protection. Sci. Rep. 7, 39956. doi: 10.1038/srep39956
Randolph, H. E., Barreiro, L. B. (2020). Herd Immunity: Understanding COVID-19. Immunity 52, 737–741. doi: 10.1016/j.immuni.2020.04.012
RECOVERY Collaborative Group, Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., et al (2020). Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report [published online ahead of print, 2020 Jul 17]. New Engl. J. Med. doi: 1056/NEJMoa2021436
Richardson, P., Griffin, I., Tucker, C., Smith, D., Oechsle, O., Phelan, A., et al. (2020). Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395, e30–e31. doi: 10.1016/S0140-6736(20)30304-4
Risitano, A. M., Mastellos, D. C., Huber-Lang, M., Yancopoulou, D., Garlanda, C., Ciceri, F., et al. (2020). Complement as a target in COVID-19? Nat. Rev. Immunol. 20, 343–344. doi: 10.1038/s41577-020-0320-7
Rittweger, M., Arastéh, K. (2007). Clinical Pharmacokinetics of Darunavir. Clin. Pharmacokinet. 46, 739–756. doi: 10.2165/00003088-200746090-00002
Robson, B. (2020). Computers and viral diseases. Preliminary bioinformatics studies on the design of a synthetic vaccine and a preventative peptidomimetic antagonist against the SARS-CoV-2 (2019-nCoV, COVID-19) coronavirus. Comput. Biol. Med. 119, 103670. doi: 10.1016/j.compbiomed.2020.103670
Rogosnitzky, M., Berkowitz, E., Jadad, A. R. (2020). No Time to Waste: Real-world Repurposing of Generic Drugs as a Multifaceted Strategy against COVID-19. JMIR Publ. doi: 10.2196/preprints.19583
Rogoveanu, O. C., Calina, D., Cucu, M. G., Burada, F., Docea, A. O., Sosoi, S., et al. (2018). Association of cytokine gene polymorphisms with osteoarthritis susceptibility. Exp. Ther. Med. 16, 2659–2664. doi: 10.3892/etm.2018.6477
Rojas, M., Rodríguez, Y., Monsalve, D. M., Acosta-Ampudia, Y., Camacho, B., Gallo, J. E., et al. (2020). Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 19, 102554. doi: 10.1016/j.autrev.2020.102554
Rosenberg, S. A., Yannelli, J. R., Yang, J. C., Topalian, S. L., Schwartzentruber, D. J., Weber, J. S., et al. (1994). Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst. 86, 1159–1166. doi: 10.1093/jnci/86.15.1159
Röth, A., Rottinghaus, S. T., Hill, A., Bachman, E. S., Kim, J. S., Schrezenmeier, H., et al. (2018). Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies. Blood Adv. 2, 2176–2185. doi: 10.1182/bloodadvances.2018020644
Rozman, B. (2002). Clinical Pharmacokinetics of Leflunomide. Clin. Pharmacokinet. 41, 421–430. doi: 10.2165/00003088-200241060-00003
Rupp, J. C., Locatelli, M., Grieser, A., Ramos, A., Campbell, P. J., Yi, H., et al. (2017). Host Cell Copper Transporters CTR1 and ATP7A are important for Influenza A virus replication. Virol. J. 14, 11. doi: 10.1186/s12985-016-0671-7
Russell, C. D., Millar, J. E., Baillie, J. K. (2020). Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395, 473–475. doi: 10.1016/S0140-6736(20)30317-2
Saivin, S., Houin, G. (1988). Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 15, 355–366. doi: 10.2165/00003088-198815060-00001
Salehi, B., Capanoglu, E., Adrar, N., Catalkaya, G., Shaheen, S., Jaffer, M., et al. (2019a). Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 24 (10), 1854. doi: 10.3390/molecules24101854
Salehi, B., Lopez-Jornet, P., Pons-Fuster Lopez, E., Calina, D., Sharifi-Rad, M., Ramirez-Alarcon, K., et al. (2019b). Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee Properties. Biomolecules 9 (3), 106. doi: 10.3390/biom9030106
Salehi, B., Rescigno, A., Dettori, T., Calina, D., Docea, A. O., Singh, L., et al. (2020). Avocado-Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules 24 (10), 1854. doi: 10.3390/biom10010130
Sanche, S., Lin, Y. T., Xu, C., Romero-Severson, E., Hengartner, N., Ke, R. (2020). High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 26. doi: 10.3201/eid2607.200282
Sandborn, W. J., Ghosh, S., Panes, J., Vranic, I., Su, C., Rousell, S., et al. (2012). Tofacitinib, an Oral Janus Kinase Inhibitor, in Active Ulcerative Colitis. New Engl. J. Med. 367, 616–624. doi: 10.1056/NEJMoa1112168
Sargiacomo, C., Sotgia, F., Lisanti, M. P. (2020). COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging 12, 6511–6517. doi: 10.18632/aging.103001
Scavone, C., Brusco, S., Bertini, M., Sportiello, L., Rafaniello, C., Zoccoli, A., et al. (2020). Current pharmacological treatments for COVID-19: What’s next? Br. J. Pharmacol. 10.1111/bph.15072.
Schiff, M. H., Kremer, J. M., Jahreis, A., Vernon, E., Isaacs, J. D., Van Vollenhoven, R. F. (2011). Integrated safety in tocilizumab clinical trials. Arthritis Res. Ther. 13, R141. doi: 10.1186/ar3455
Schoeman, D., Fielding, B. C. (2019). Coronavirus envelope protein: current knowledge. Virol. J. 16, 69. doi: 10.1186/s12985-019-1182-0
Shannon, E., Noveck, R., Sandoval, F., Kamath, B. (2008). Thalidomide suppressed IL-1beta while enhancing TNF-alpha and IL-10, when cells in whole blood were stimulated with lipopolysaccharide. Immunopharmacol. Immunotoxicol. 30, 447–457. doi: 10.1080/08923970802135161
Sharifi-Rad, J., Rodrigues, C. F., Sharopov, F., Docea, A. O., Can Karaca, A., Sharifi-Rad, M., et al. (2020). Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 17 (7), 2326. doi: 10.3390/ijerph17072326
Sharifi-Rad, M., Kumar, N. V. A., Zucca, P., Varoni, E. M., Dini, L., Panzarini, E., et al. (2020). Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 11, 694. doi: 10.3389/fphys.2020.00694
Shi, J., Xiao, Y., Zhang, Y., Geng, D., Cong, D., Shi, K. X., et al. (2020). Challenges of drug development during the COVID-19 pandemic: key considerations for clinical trial designs. Preprints. doi: 10.22541/au.158705386.69295078
Sieper, J., Braun, J., Kay, J., Badalamenti, S., Radin, A. R., Jiao, L., et al. (2015). Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann. Rheum. Dis. 74, 1051–1057. doi: 10.1136/annrheumdis-2013-204963
Singh, A. K., Majumdar, S., Singh, R., Misra, A. (2020). Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes Metab. Syndr. 14, 971–978. doi: 10.1016/j.dsx.2020.06.054
Smartpatients.Com (2020). A Randomized Multicenter Controlled Clinical Trial of Arbidol in Patients with 2019 Novel Coronavirus (2019-nCoV). Available at: https://www.smartpatients.com/trials/NCT04246242 (Accessed 2020 Apr).
Smith, K. A. (1988). Interleukin-2: inception, impact, and implications. Science 240, 1169–1176. doi: 10.1126/science.3131876
Stebbing, J., Phelan, A., Griffin, I., Tucker, C., Oechsle, O., Smith, D., et al. (2020). COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 20, 400–402. doi: 10.1016/S1473-3099(20)30132-8
Taylor, P. (2020). Apeiron Biologics has been cleared to start a European phase 2 trial of a drug that it says could stop COVID-19 entering cells, blocking the infection. Available at: https://pharmaphorum.com/news/apeiron-starts-mid-stage-trial-of-drug-that-blockscoronavirus/ (Accessed 2020 Apr 5).
Te Velthuis, A. J., Van Den Worm, S. H., Sims, A. C., Baric, R. S., Snijder, E. J., Van Hemert, M. J. (2010). Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PloS Pathog. 6, e1001176. doi: 10.1371/journal.ppat.1001176
Terkeltaub, R. A. (2009). Colchicine Update: 2008. Semin. Arthritis Rheum. 38, 411–419. doi: 10.1016/j.semarthrit.2008.08.006
Terpiłowska, S., Siwicki, A. K. (2017). Chromium(III) and iron(III) inhibits replication of DNA and RNA viruses. Biometals 30, 565–574. doi: 10.1007/s10534-017-0027-9
Tignanelli, C. J., Ingraham, N. E., Sparks, M. A., Reilkoff, R., Bezdicek, T., Benson, B., et al. (2020). Antihypertensive drugs and risk of COVID-19? Lancet Respir. Med. 8, e30–e31. doi: 10.1016/S2213-2600(20)30158-2
Todd, P. A., Benfield, P. (1990). Ramipril. Drugs 39, 110–135. doi: 10.2165/00003495-199039010-00009
Todd, P. A., Clissold, S. P. (1990). Naproxen. Drugs 40, 91–137. doi: 10.2165/00003495-199040010-00006
Topalian, S. L., Bhatia, S., Hollebecque, A., Awada, A., Boer, J. P. D., Kudchadkar, R. R., et al. (2017). Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC). Cancer Res. 77, CT074. doi: 10.1158/1538-7445.AM2017-CT074
Torjesen, I. (2020). Covid-19: Hydroxychloroquine does not benefit hospitalised patients, UK trial finds (London, UK: British Medical Journal Publishing Group).
Tran, D. H., Sugamata, R., Hirose, T., Suzuki, S., Noguchi, Y., Sugawara, A., et al. (2019). Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J. Antibiotics 72, 759–768. doi: 10.1038/s41429-019-0204-x
Trialsitenews (2020). The Carrimycin Clinical Trial in China: An Obscure Drug Targeting Upper Respiratory Infection. Available at: https://www.trialsitenews.com/the-carrimycin-clinicaltrial-in-china-an-obscure-drug-targeting-upper-respiratory-infection/ (Accessed 2020 Apr 7).
Trifirò, G., Crisafulli, S., Andò, G., Racagni, G., Drago, F. (2020). Should Patients Receiving ACE Inhibitors or Angiotensin Receptor Blockers be Switched to Other Antihypertensive Drugs to Prevent or Improve Prognosis of Novel Coronavirus Disease 2019 (COVID-19)? Drug Saf. 43, 507–509. doi: 10.1007/s40264-020-00935-2
Tsatsakis, A., Docea, A. O., Calina, D., Tsarouhas, K., Zamfira, L. M., Mitrut, R., et al. (2019). A Mechanistic and Pathophysiological Approach for Stroke Associated with Drugs of Abuse. J. Clin. Med. 8 (9), 1295. doi: 10.3390/jcm8091295
Tsatsakis, A., Petrakis, D., Nikolouzakis, T. K., Docea, A. O., Calina, D., Vinceti, M., et al. (2020). COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 141, 111418. doi: 10.1016/j.fct.2020.111418
Tu, Y.-F., Chien, C.-S., Yarmishyn, A. A., Lin, Y.-Y., Luo, Y.-H., Lin, Y.-T., et al. (2020). A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int. J. Mol. Sci. 21, 2657. doi: 10.3390/ijms21072657
Ungureanu, A., Zlatian, O., Mitroi, G., Drocaş, A., Ţîrcă, T., Călina, D., et al. (2017). Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol. Med. Rep. 16, 8771–8780. doi: 10.3892/mmr.2017.7746
Vijayvargiya, P., Garrigos, Z. E., Castillo Almeida, N. E., Gurram, P. R., Stevens, R. W., Razonable, R. R. (2020). Treatment Considerations for COVID-19: A Critical Review of the Evidence (or Lack Thereof). Mayo Clin. Proc. 95, 7, 1454–1466. doi: 10.1016/j.mayocp.2020.04.027
Villar, J., Ferrando, C., Martínez, D., Ambrós, A., Muñoz, T., Soler, J. A., et al. (2020). Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 8, 267–276. doi: 10.1016/S2213-2600(19)30417-5
Wang, R. R., Yang, Q. H., Luo, R. H., Peng, Y. M., Dai, S. X., Zhang, X. J., et al. (2014). Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PloS One 9, e105617. doi: 10.1371/journal.pone.0105617
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., et al. (2020a). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. doi: 10.1371/journal.pone.0105617
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., et al. (2020b). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578. doi: 10.1016/S0140-6736(20)31022-9
Westover, J. B., Mathis, A., Taylor, R., Wandersee, L., Bailey, K. W., Sefing, E. J., et al. (2018). Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters. Antiviral Res. 156, 38–45. doi: 10.1016/j.antiviral.2018.05.013
WHO (2020). WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19.
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J., Prescott, H. C. (2020). Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. Jama. 9 (8), e105617. doi: 10.1001/jama.2020.12839
Wilson, J. X. (2013). Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid. Redox Signal 19, 2129–2140. doi: 10.1089/ars.2013.5401
Wolchok, J. D., Kluger, H., Callahan, M. K., Postow, M. A., Rizvi, N. A., Lesokhin, A. M., et al. (2013). Nivolumab plus Ipilimumab in Advanced Melanoma. New Engl. J. Med. 369, 122–133. doi: 10.1056/NEJMoa1302369
World Health Organization (2019). International nonproprietary names for pharmaceutical substances (INN): proposed INN: list 121. WHO Drug Inf. 33, 233–392.
Wu, A., Peng, Y., Huang, B., Ding, X., Wang, X., Niu, P., et al. (2020). Genome Composition and Divergence of the Novel Coronaviru-nCoV) Originating in China. Cell Host Microbe 27, 325–328. doi: 10.1016/j.chom.2020.02.001
Yamamoto, M., Matsuyama, S., Li, X., Takeda, M., Kawaguchi, Y., Inoue, J. I., et al. (2016). Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob. Agents Chemother. 60, 6532–6539. doi: 10.1128/AAC.01043-16
Ying, W., Qian, Y., Kun, Z. (2020). Drugs supply and pharmaceutical care management practices at a designated hospital during the COVID-19 epidemic. Res. Soc. Administrative Pharm. RSAP. S1551-7411 (1520), 30325–30329. doi: 10.1016/j.sapharm.2020.04.001
Zhang, W., Zhao, Y., Zhang, F., Wang, Q., Li, T., Liu, Z., et al. (2020). The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. (Orlando Fla.) 214, 108393–108393. doi: 10.1016/j.clim.2020.108393
Zhao, Q., He, Y. (2020). Challenges of Convalescent Plasma Therapy on COVID-19. J. Clin. Virol. 127, 104358. doi: 10.1016/j.jcv.2020.104358
Zlatian, O., Balasoiu, A. T., Balasoiu, M., Cristea, O., Docea, A. O., Mitrut, R., et al. (2018). Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp. Ther. Med. 16, 4499–4510. doi: 10.3892/etm.2018.6737
Keywords: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), pandemic, COVID-19 proposed therapy, convalescent plasma, therapeutic challenges
Citation: Sarkar C, Mondal M, Torequl Islam M, Martorell M, Docea AO, Maroyi A, Sharifi-Rad J and Calina D (2020) Potential Therapeutic Options for COVID-19: Current Status, Challenges, and Future Perspectives. Front. Pharmacol. 11:572870. doi: 10.3389/fphar.2020.572870
Received: 17 June 2020; Accepted: 24 August 2020;
Published: 15 September 2020.
Edited by:
Filippo Drago, University of Catania, ItalyReviewed by:
Mmamosheledi Elsie Mothibe, Sefako Makgatho Health Sciences University, South AfricaSunita Naira, Consultant, Mumbai, India
Copyright © 2020 Sarkar, Mondal, Torequl Islam, Martorell, Docea, Maroyi, Sharifi-Rad and Calina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Torequl Islam, bXVoYW1tYWQudG9yZXF1bC5pc2xhbUB0ZHR1LmVkdS52bg==; Javad Sharifi-Rad, amF2YWQuc2hhcmlmaXJhZEBnbWFpbC5jb20= ; Daniela Calina, Y2FsaW5hZGFuaWVsYUBnbWFpbC5jb20=
 Chandan Sarkar
Chandan Sarkar Milon Mondal1
Milon Mondal1 Miquel Martorell
Miquel Martorell Anca Oana Docea
Anca Oana Docea Alfred Maroyi
Alfred Maroyi Javad Sharifi-Rad
Javad Sharifi-Rad Daniela Calina
Daniela Calina