- 1Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pharmacy, General Hospital of Central Theater Command, Wuhan, China
Prunus mume is one of the most ancient medicinal herbs and health foods commonly used in Asian countries. It is widely used as a constituent of many medicinal preparations and as a food ingredient for its beneficial health effects. In this review, we retrieved reports from PubMed, embase, Scopus, and SciFinder databases, to collect extensive scientific evidence on the phytochemical constituents, pharmacological properties, and clinical applications of Prunus mume. The literature review revealed that approximately 192 compounds have been isolated from different parts of the plant, and their molecular structures have been identified. The pharmacological properties of the plant, including anti-diabetic, liver-protective, antitumor, antimicrobial, antioxidant, and anti-inflammatory activities, as well as their underlying mechanisms, have been clarified by in vitro and in vivo studies. Clinical studies, although very limited, have been highlighted in this review to provide a reference for further exploration on therapeutic applications of the plant.
Introduction
Prunus mume (Siebold) Siebold&Zucc (P. mume) (=Armeniaca mume) is an Asian plum species belonging to the Rosaceae family. It is known as wu mei (Chinese:乌梅) in China, Japanese apricot or ume in Japan, and maesil or oumae in Korea. The plant is commonly cultivated throughout most of China, and is native to Japan and Korea. The fruit of the plant has been used as a medicinal herb and health food in East Asian countries for more than 2000°years. In China, the dried fruit of P. mume is listed in the earliest pharmacopoeia of traditional Chinese medicine (TCM), Shen Nong Ben Cao Jing, compiled during the Han Dynasty, in approximately AD 220. In Japan, the earliest record is found in a medical monograph called Ishinho (published in AD 984). According to the Chinese pharmacopoeia records, the dried ripening fruit of P. mume can be taken to relieve various physical disorders, such as chronic cough caused by lung deficiency, chronic infectious diarrhea, vomiting, or abdominal pain caused by Ascaris infection, dysfunctional uterine bleeding, inadequate secretion of saliva or body fluids. It is also used as a component of many formulas, such as wu mei wan (first recorded in Shang Han Za Bing Lun, compiled during AD200–210), Er Chen Tang and Chang Shan Yin (recorded in Tai Ping Hui Min He Ji Ju Fang, compiled during AD 1078–1085) to treat different kinds of diseases based on classical theories of TCM. As a common commercial food product, the fruit of P. mume is used to prepare pickled plums, plum sauce, plum juice, and plum liquor, which can be consumed as a snack, condiment, or beverage. To date, phytochemical studies have discovered numerous chemical components of the plant, mainly phenolics (Xia et al., 2011; Mitani et al., 2013), flavonoids (Yan et al., 2014a), and organic acids (Gao, 2012). Modern pharmacological studies have disclosed various biological activities and bioactive mechanisms of P. mume and its formulas, including antidiabetic (Kishida et al., 2013; Ko et al., 2013), hepatoprotective (Hokari, 2012; Beretta et al., 2016), antitumoral (Hattori et al., 2013; Cho et al., 2019), anti-inflammatory (Morimoto et al., 2009; Mitani et al., 2013), and antimicrobial (Lee and Stein, 2011; Seneviratne et al., 2011) activities. Bailly (2020) reviewed anticancer properties of P. mume extracts, however, no comprehensive review on the phytochemical and pharmacological properties of P. mume is available.
Herein, we conducted a comprehensive and systematic review to summarize the scattered studies on the phytochemical and pharmacological properties and the clinical applications of P. mume to provide a scientific basis for future research directions and better utilization of the plant.
Data Sources and Search Strategies
A comprehensive literature search was conducted through electronic databases, including PubMed, embase, Scopus, and SciFinder databases. The search time interval was from database inception to 31, Mar 2020. The search strategy used for the PubMed database was “Prunus mume” [tiab] or “Fructus mume” [tiab] or “Chinese plum” [tiab] or “Japanese apricot” [tiab] or “Asian plum” [tiab] or “Oriental plum” [tiab] or “MK 615” [tiab] or maesil [tiab] or oumae [tiab] or ume [tiab] or“Armeniaca mume” [tiab]. The search was limited to English-language and Chinese publications. A PubMed alert was set up for new relevant results.
Database searches initially identified 361 records from Pubmed, 273 records from embase, 540 records from Scopus, and 282 records from the SciFinder database. The duplicate references were removed by Endnote. Only the studies focusing on the phytochemical constituents, pharmacological properties, and clinical applications were selected by two independent reviewers.
Phytochemical Constituents
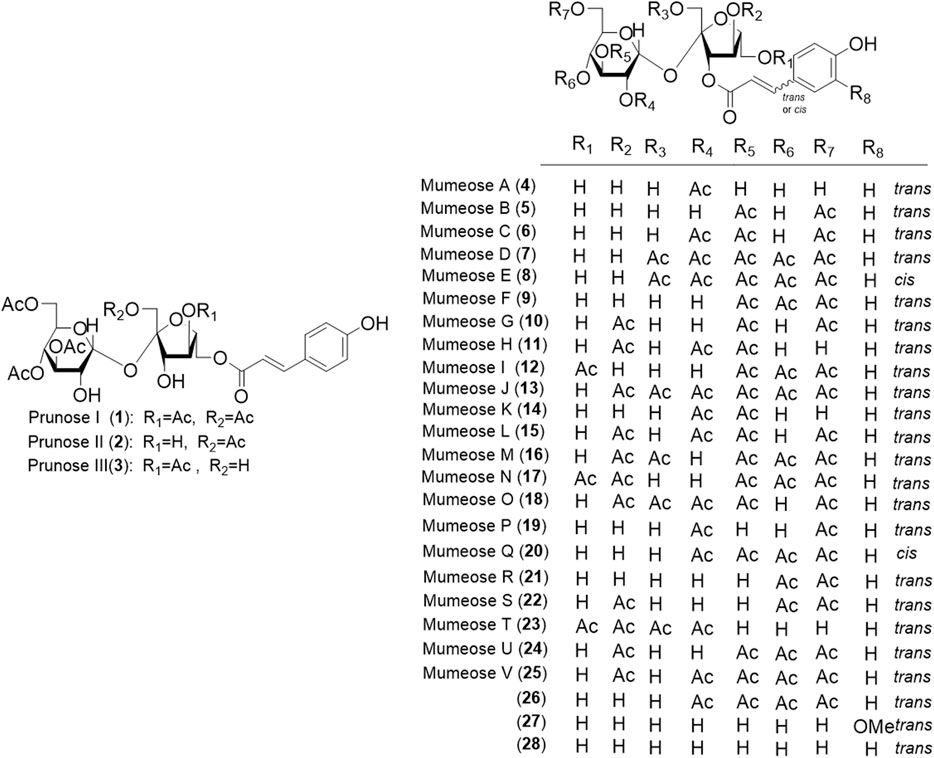
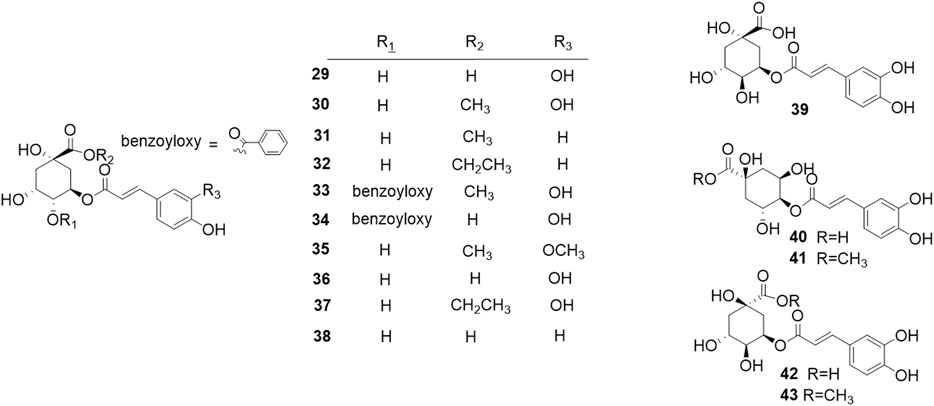
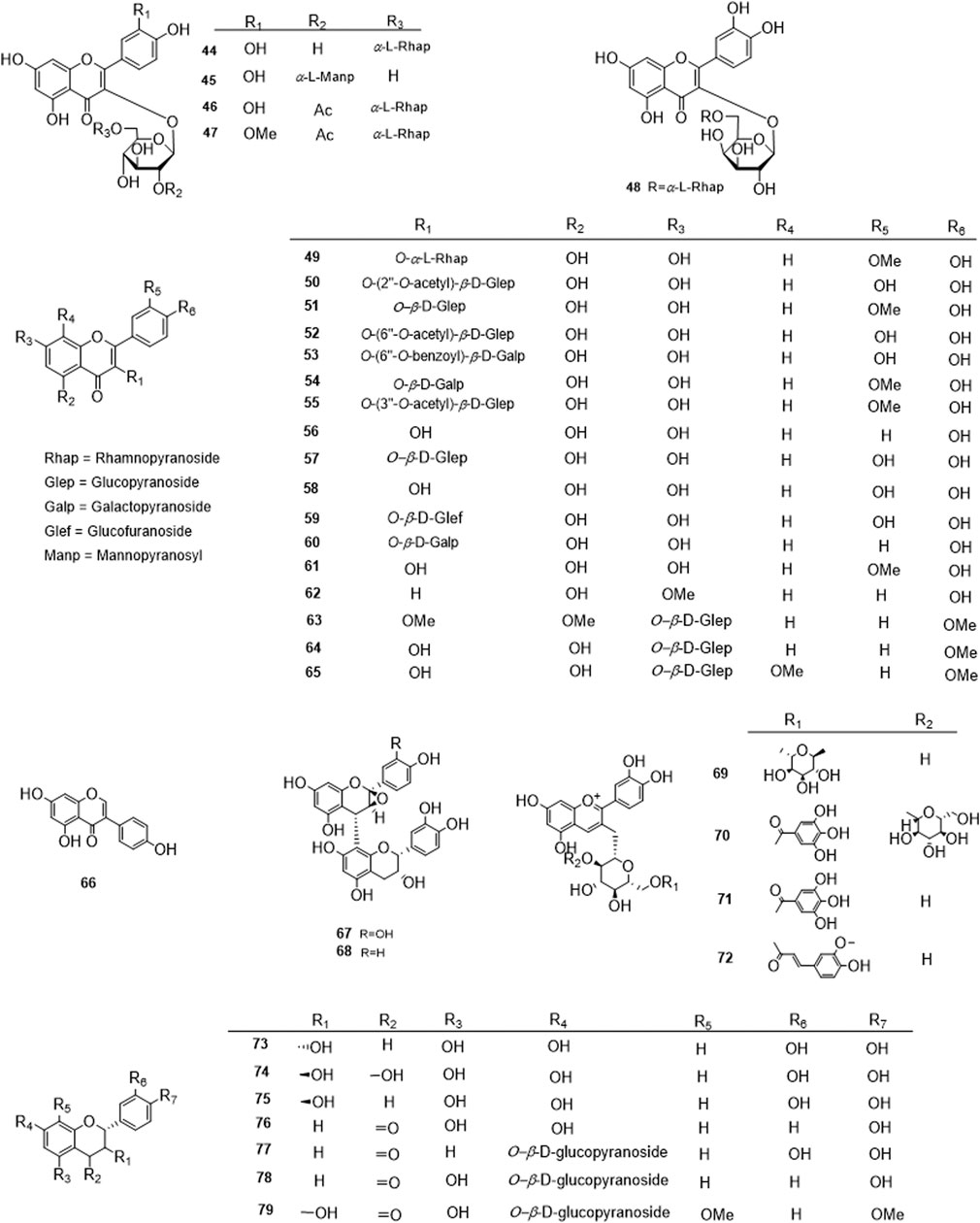
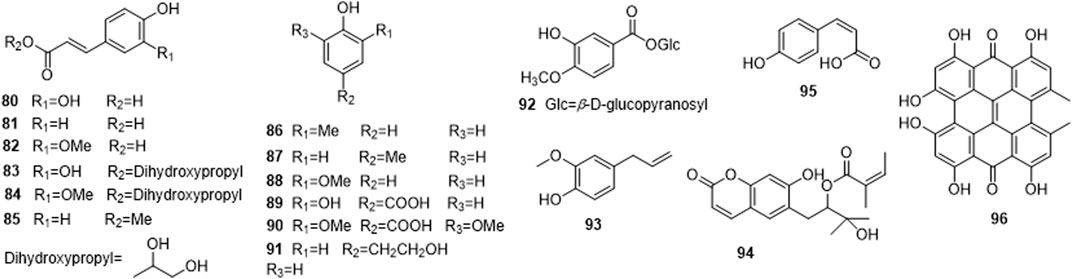
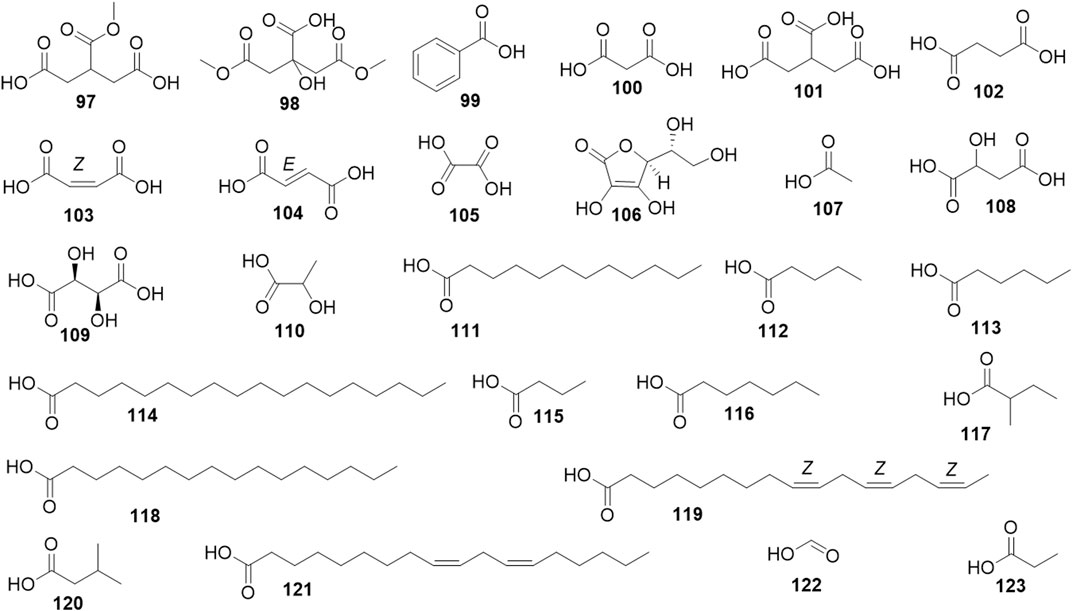
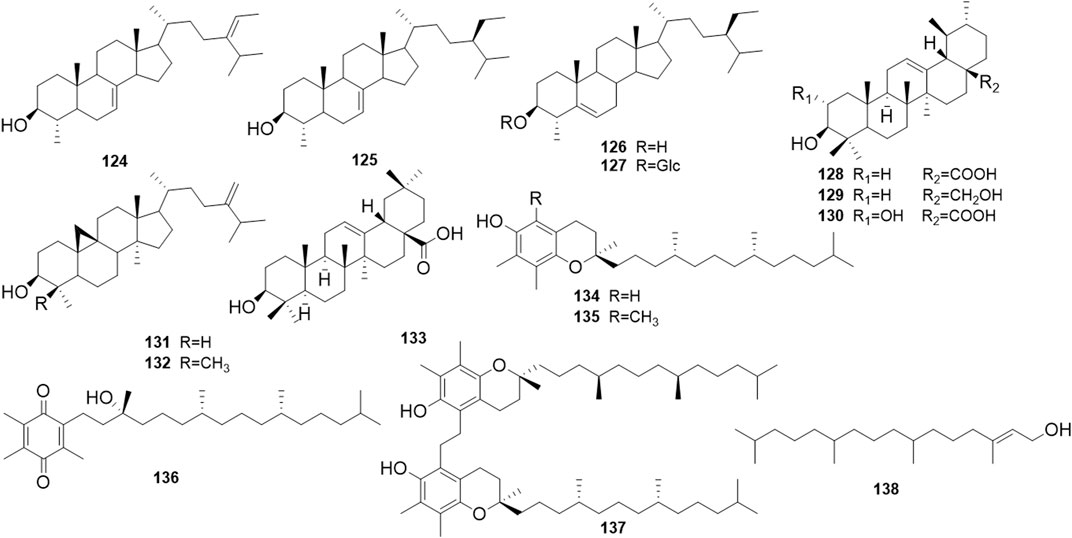
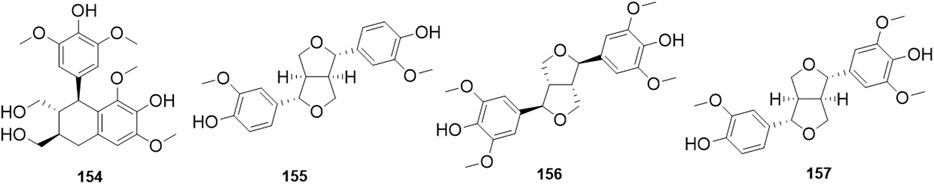
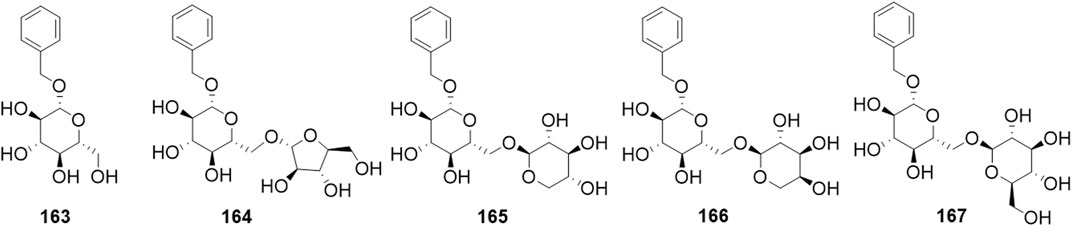
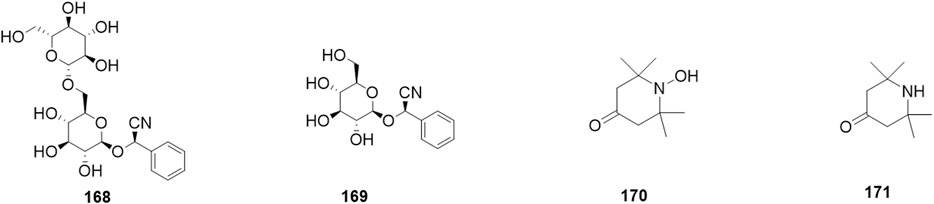
Phytochemical studies of P. mume have led to the isolation and identification of many types of natural products, including phenolics, organic acids, steroids, terpenes, lignans, furfurals, benzyl glycosides, cyanogenic glycosides, and alkaloids from different parts of P. mume. In total, 192 chemical compounds found in the P. mume were listed in Table 1. The phenolic components, abundantly present in P. mume flower, flower bud, fruit, wood, petal, and seed, are complex, and can be subdivided into phenylpropanoid sucrose esters, hydroxycinnamoylquinic acid derivatives, flavonoids, and other phenolics; the structures of these compounds (1–96) are shown in Figures 1–4. The fruit is the most studied part of the plant, contains mainly therapeutic chemical compounds including organic acid (compounds 92–123), steroids and terpenes (compounds 124–138), lignans (compounds 154–157), furfurals (compounds 158–162), benzyl glycosides (compounds 163–167), cyanogenic glycosides and alkaloid (compounds 168–171). The structures of these compounds are shown in Figures 5–10, respectively.
Pharmacological Properties and Clinical Applications
Metabolic Diseases
Diabetes Mellitus
DM is one of the most common metabolic diseases in the world. It is characterized by hyperglycemia as result of abnormal insulin secretion and insulin resistance. Obesity, which is normally associated with hyperglycemia and insulin resistance, is a high-risk factor for DM. One study found that a water extract of P. mume fruit and Lithospermum erythrorhizon root synergistically improved insulin sensitivity and prevented visceral adiposity in a high-fat diet (HFD)-fed ovariectomized rats model (Ko et al., 2013). A 70% ethanol extract of P. mume fruit was described as able to increas glucose uptake in C2C12 myotubes by activating the peroxisome proliferator-activated receptor (PPAR)-γ. The extract also significantly improved fasting glucose levels and glucose intolerance, reduced body weight, liver and adipose tissue weight without affecting food intake in HFD-fed mice (Hwang et al., 2012; Shin et al., 2013). A 70% ethanol extract of P. mume leaves also decreased blood glucose levels in a dose-dependent manner, and the polyphenol compounds were conjectured to account for these activities (Lee et al., 2016). In another study, phenolic extracts of P. mume inhibited small intestinal disaccharidase activity and suppressed postprandial elevation of blood glucose levels in rats (Kishida et al., 2013). A TCM formula called “wumei wan” in Chinese has also been reported to improve insulin resistance (IR) in type 2 DM rats, which might be related to up-regulation of protein and mRNA expression levels of the insulin receptor (Insr), insulin receptor substrate 1 (Irs-1), glucose transporter4 (Glut-4), and β-arrestin-2 in the liver and skeletal muscle (Li et al., 2013).
Clinically, in a multicenter randomized controlled pilot trial, 85 subjects diagnosed with type 2 DM, were randomized to receive either wumei wan or metformin. After a12-weeks intervention, the P. mume formula wumei wan decreased the fasting plasma glucose (FPG), postprandial glucose (PPG), and glycosylated hemoglobin (HbAlc) levels as effectively as the hypoglycemic agent metformin. In addition, the formula could significantly decrease the body mass index (BMI) when the patient’s BMI was greater than 23, but not when the BMI was below 23 (Tu et al., 2013).
Furthermore, the phenolic compounds 9∼13, 29, 30, 33, 34, 47 extracts from the flower buds of P. mume inhibited aldose reductase, reduced sorbitol accumulation in eye lenses and retinas, and had the potential to prevent diabetic complications such as cataract (Yoshikawa et al., 2002; Fujimoto et al., 2013).
Altogether, these studies demonstrated that P. mume could prevent obesity, maintain glucose metabolism, prevent diabetic complications, and bring therapeutic benefit to the patients with type 2 DMs.
Hypolipidemic Effects
Squalene synthase plays an important role in the cholesterol biosynthesis pathway. Inhibiting this enzyme in hypercholesterolemia can lower not only plasma cholesterol but also plasma triglyceride levels. Chlorogenic acid isolated from P. mume fruit inhibited squalene synthase in pig liver homogenate with an IC50 level of 100 nm (Choi et al., 2007). In HFD-fed mice, the 70% ethanol extract of P. mume fruit decreased serum triglyceride (TG) levels significantly (Hwang et al., 2012; Shin et al., 2013). Furthermore, in HFD-fed rats fed with P. mume concentrate for four°weeks, the total lipid, total cholesterol, and TG serum levels, and the atherogenic index decreased significantly compared with the HFD model control group; while, the serum level of high-density lipoprotein (HDL)-cholesterol was significantly higher than the HFD model control group (Chyun et al., 2012). Therefore, the plant may act as a potential therapeutic agent for hypercholesterolemia.
Gout
Gout is a metabolic disorder characterized by recurrent acute arthritis, hyperuricemia, and deposition of sodium urate in and around the joints, sometimes with the formation of uric acid calculi. The enzyme xanthine oxidase (XO) can oxidize hypoxanthine and xanthine to uric acid, thus playing an important role in the catabolism of purines, which are associated with the metabolic disorders of hyperuricemia and gout (Wang et al., 2010). P. mume can be used to treat gouty arthritis in combination with other herbal medicines based on clinical experience in TCM (Chen et al., 2007). Animal studies have also shown that a methanol extract of P. mume fruit, with the seeds removed (70 and 140 mg/kg, 7°days) decreased serum and liver uric acid levels, elevated urinary uric acid levels, and reduced hepatic XO activity in mice with potassium oxonate induced hyperuremia (Yi et al., 2012).
Osteoporosis
Osteoporosis is a metabolic disease that frequently occurs in aging communities. This degenerative disease is characterized by a progressive loss of bone mineral density (BMD) and deterioration of the bone micro-architecture, causing an increased risk of fracture (Bi et al., 2006). Currently, therapies for osteoporosis are focused on inhibiting osteoclastic activity, stimulating osteoblastic activity, and decreasing oxidative stress (Arai et al., 2007). MC3T3-E1 is a classic cell model of the osteoblastic phenotype. Treatment of MC3T3-E1 cells with the water-soluble fraction of P. mume increased osteogenic mRNA expression of bone morphogenetic protein (BMP-2), osteopontin (OPN), RUNX2, and increased alkaline phosphatase (ALP) activity, which is a marker of the early period of osteoblastic differentiation, and therefore induced cell proliferation and differentiation. Moreover, the Alizarin Red staining assay demonstrated that P. mume increased calcium deposition, and therefore had an accelerative effect on the mineralization of cells (Kono et al., 2011). Other research groups have explored the antioxidant and anti-osteoporosis activities of compounds isolated from P. mume fruit using murine pre-osteoblastic MC3T3-E1 cells and pre-osteoclastic RAW 264.7cells. These studies showed that phenolic and lignans compounds such as compounds 19∼21, 30, 39, 154∼156 exhibited peroxyl radical-scavenging activities in a dose-dependent manner. The benzyl glycoside compound 166 and flavonoid compounds 44, 45, 57, 67, 68, 73 significantly stimulated the differentiation of pre-osteoblastic MC3T3-E1 cells by increasing collagen synthesis and mineralization (Yan et al., 2014a; 2014b). Moreover, some phenylpropanoid sucrose esters, organic acids, lignans and glycoside compounds 30, 39, 98, 154, 163, 169 possessed significant inhibitory activity against osteoclast differentiation by suppressing tartrate-resistant acid phosphatase (TRAP) activity in pre-osteoclastic RAW 264.7cells (Yan et al., 2015). These results show that P. mume may be an excellent source of anti-osteoporosis activity that can be used to prevent osteoporosis.
Digestive System Diseases
Liver Protection
In recent years, many preclinical studies have demonstrated the antioxidant (Xia et al., 2010; Kang et al., 2016) and anti-inflammatory (Morimoto et al., 2009; Mitani et al., 2013) effects of P. mume. Oxidative stress and inflammatory reactions are key risk factors of some chronic liver diseases, such as alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), and viral hepatitis (Oyanagi et al., 1999; Lieber, 2001; Ekstedt et al., 2006), thus the hepatoprotective effects of P. mume have been investigated in both animal and clinical models.
MK615 is a commercial product extract from the fruit of P. mume that is rich in hydrophobic substances. Hokari (2012) revealed that d-galactosamine hydrochloride (D-GalN) (600 mg/kg, single intraperitoneal injection) induced hepatopathy in a rat model. MK615 treatment (4 ml/kg per day for 7°days) significantly decreased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) plasma levels and reduced hepatic injury. In the same report, a case series study was carried out to evaluate the clinical effects of MK615. Fifty-eight enrolled patients with liver disorders, including hepatitis C, NAFLD, and autoimmune liver disease, orally took MK615 solution 13 g every day for 12°weeks. After a 12-weeks intervention, the serum ALT and AST levels of these patients decreased significantly compared with the pretreatment baseline levels.
In another randomized double-blind placebo-controlled study, 45 healthy subjects with transaminase levels between 20 and 40°UI/L were enrolled, and two doses of a food supplement containing a standardized extract of P. mume were administered. After 3°months of treatment, the liver enzyme (ALT, AST, and gamma-glutamyl transferase [γGT]) levels, lipid profile parameters (HDL cholesterol, LDL/HDL ratio, and triglycerides), glycemia, oxidative parameters (reduced or oxidized plasma cysteine (Cys), plasma CysGly, erythrocyte glutathione (GSH), plasma GSH, and plasma neopterin/creatinine ratio) were significantly improved vs. the placebo group and the pretreatment baseline (Beretta et al., 2016).
In the alcoholic liver injury mouse model, the P. mume formula also exhibited hepatoprotective effects (Chen et al., 2016). Khan et al. (2017) investigated the molecular mechanism using a metabolic approach. The three-way hierarchical cluster analysis showed that 101 features were statistically different among the alcohol and P. mume pretreatment groups. The relative concentrations of compounds such as phosphatidylcholine and Saikosaponin BK1 increased significantly in the P. mume treatment group. These compounds are responsible for the hepatoprotective effects of P. mume by inhibiting the reactive oxygen species (ROS)-mediated p53 and mitogen-active protein kinase (MAPK) signaling pathways.
Helicobacter pylori-Related Chronic Gastritis
Epidemiological evidence has indicated a significant relationship between Helicobacter pylori (H. pylori) infection and chronic gastritis (Uemura et al., 2001). Some studies have found that P. mume extract has direct bactericidal activity against H. pylori both in vitro and in vivo (Fujita et al., 2002; Otsuka et al., 2005; Miyazawa et al., 2006). A clinical case series study, which enrolled 18 H. pylori-positive subjects, demonstrated that drinking 130 ml 1% concentrated fruit juice of P. mume twice a day for two°weeks, resulted in a slight fall in the urea breath test (UBT) values (Nakajima et al., 2006). Enomoto et al. (2010) carried out a study to examine the associations between P. mume intake and H. pylori-related chronic gastritis. The results showed that in the 458 non-elderly H. pylori-positive subjects (age range 30–64°years), the H. pylori antibody titers and serum PG-II levels were significantly lower in the high dose P. mume intake group compared with the low dose intake group. Thus, P. mume extract was shown to have a potential protective effect against H. pylori related chronic gastritis.
Dysmotility Disease
P. mume is believed to improve gastrointestinal dysmotility and dyspepsia in traditional medicine in Eastern countries. Some scientific studies have provided evidence for the efficacy of such folk remedies. Tamura et al. (2011) found that P. mume contains both soluble and insoluble fibers and can increase fecal output and fecal lipid excretion significantly. A methanol extract of P. mume was reported to modulate the pacemaker activities of interstitial cells of Cajal (ICCs) and was proposed as a potential gastroprokinetic agent for regulating gastrointestinal motility (Lee et al., 2017). The improvement in gastrointestinal motility also brought benefits to constipation and gastroesophageal reflux diseases (GERD), according to several animal and clinical experiments (Na et al., 2012). Jung et al. (2014) carried out a double-blind, randomized, placebo-controlled trial, in which patients experiencing constipation consumed P. mume fruit extract 7.2 g (n = 28) or a placebo (n = 29) twice a day for eight°weeks. The colon transit time and defecation function were evaluated by questionnaire. The results showed a significant decrease in total colon transit time and abdominal pain during defecation in the group that consumed P. mume compared with the placebo group. In a community cohort study, the frequency scale for symptom of GERD (FSSG) questionnaire was used to investigate the effects of P. mume consumption on GERD symptoms. Of a total of 1303 subjects, 392 were categorized into the P. mume daily intake group, 911 were included in the no or occasional intake group. The results showed that the total FSSG score and FSSG dysmotility score were significantly lower in the P. mume daily intake group compared with no or occasional intake (Maekita, 2015).
Inflammatory Bowel Disease
Oxidative stress and inflammatory reactions are the major etiologies of IBD. Oxidative stress due to excessive ROS triggers inflammatory reactions of the gut wall and causes tissue-disruptive disease (Ko and Auyeung, 2014). Many studies have reported the free radical-scavenging (Matsuda et al., 2003; Xia et al., 2010), antioxidant (Karakaya et al., 2001; Kang et al., 2016), and anti-inflammatory properties of P. mume (Choi et al., 2007; Morimoto et al., 2009). Some studies have assessed further beneficial effects of P. mume extract or formulation on different IBD mouse models. The results showed that P. mume treatment decreased immunoglobulin M (IgM) and immunoglobulin E (IgE) levels, reduced COX-2, tumor necrosis factor alpha (TNF-α), interferon (IFN-γ), interleukin (IL)-12, and IL-17 levels in the colon tissue of colitis mouse models, alleviated dextran sulfate sodium (DSS) or 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced histological changes and inflammatory responses (Liu et al., 2009; Zhang et al., 2011; Lee et al., 2014; Lee S. Y. et al., 2017; Kim et al., 2021). All these studies show that P. mume may represent a potential new therapeutic agent for IBD treatment.
Nervous System Diseases
The human hippocampus is associated with cognitive function such as learning, memory, and emotional control (Burgess et al., 2002). A mixture of P. mume concentrate, disodium succinate and Span80 (3.6:4.6:1 ratios) improved the spatial memory of normal rats in the Morris water maze test, the effects being linked to the MAPK/ERK (extracellular signal-regulated kinase) signaling pathway that results in the phosphorylation of cyclic adenosine monophosphate (cAMP)-response-element-binding protein (CREB) through tropomyosin receptor kinase B (TrkB) and/or the NR2B subunit of the N-methyl-d-aspartate (NMDA) receptor (Kim et al., 2008).
Chronic cerebral hypoperfusion (CCH) can cause white matter and hippocampal damage and is a key etiological factor in vascular dementia (VaD). The permanent bilateral common carotid artery occlusion (BCCAo) animal model has been widely used to study CCH-relevant nervous system diseases (Farkas et al., 2007). Jeon et al. (2012) used the BCCAo rat model to study the effects of P. mume extract on cognitive deficits caused by CCH. The results showed that an aqueous extract of P. mume (200 mg/kg, 40°days) reduced microglial activation, decreased p-ERK expression, prevented nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation in rat hippocampus, and improved the spatial learning of rats in the Morris water maze task. Likewise, an ethanol extract of P. mume alleviated inflammatory responses and cholinergic dysfunction by attenuating white matter lesions, decreasing expression of pro-inflammatory mediators, inhibiting microglial and astrocytic activation, and down-regulating toll-like receptor 4 (TLR4) and p38MAPK signaling (Jeon, 2015; Lee et al., 2015; Kim et al., 2016).
In addition, P. mume can also benefit neurodegenerative diseases such as Alzheimer’s disease. Kim et al. (2015) found that an ethanol extract of P. mume attenuated memory impairment in the scopolamine-induced mouse model. Park et al. (2016) examined the effects of P. mume on cognitive impairments in 5XFAD transgenic mice with five typical Alzheimer mutations. After a 90-days treatment, the P. mume group performed better in the Morris water maze task, the object/location novelty recognition test, and contextual fear-conditioning compared with the model group.
Cardiovascular Disease
Different study groups have found that the fruitjuice concentrate of P. mume markedly improved blood fluidity in different micro-channel instruments (Chuda et al., 1999; Kubo et al., 2005). The polyacylated sucrose, citric acid, and mumefural derivatives of P. mume showed inhibitory effects on collagen-, arachidonic acid-, and ADP-induced platelet aggregation in vitro (Yoshikawa et al., 2002; Kubo et al., 2005). An herbal mixture of Phyllostachys pubescens leaves and P. mume fruits also showed inhibitory effects on platelet aggregation in vitro (Dong-Seon et al., 2013; Son et al., 2017). In the arteriovenous shunt thrombosis rat model and the carrageenan-induced mice tail thrombosis model, the mixture dose-dependently reduced the weight or length of tail thrombosis, respectively. The mechanism study showed that the mixture upregulated intracellular cAMP levels, inhibited the release of granule contents containing serotonin, platelet-activating factor (PAF), and thromboxane A2 (TXA2), and decreased the intracellular concentration of calcium ion. The mixture also exerted inhibitory effects by deactivating the collagen receptor glycoprotein VI (GPVI), blocking ligand binding to the receptor, inhibiting the downstream signaling pathway and the ERK activation pathway, and inhibiting the conversion of fibrinogen to fibrin (Dong-Seon et al., 2013; Son et al., 2017).
Utsunomiya et al. (2002) found that a fruit juice concentrate of P. mume markedly inhibited angiotensin II and H2O2-induced epidermal growth factor (EGF) receptor transactivation, inhibited ERK activation, and mitigated angiotensin II-induced vascular remodeling. The chlorogenic acid derived from P. mume decreased angiotensin converting enzyme (ACE) levels in rat plasma (Ina et al., 2003). Jo et al. (2019) studied the vasodilatory effects of a 70% ethanol extract of P. mume branches on isolated rat aortic rings. The authors showed the vasorelaxant effect of the extract was endothelium dependent. The extract affected the nitric oxide (NO) cyclic guanosine monophosphate (cGMP) pathway, the prostacyclin pathway, the muscarinic receptor pathway, potassium channels, and might represent a promising anti-hypertensive treatment.
Clinically, a 12-weeks double-blind randomized placebo-controlled pilot trial evaluated the anti-hypertensive effects of P. mume. The study recruited 15 participants with normal or normal high blood pressure (BP) (systolic blood pressure [SBP],130–139 mmHg; diastolic blood pressure [DBP], 85–89 mmHg) or hypertension grade 1 (SBP, 140–159 mmHg; DBP, 90–99 mmHg) and taking no anti-hypertensive agents. After a 12°weeks-intervention, the P. mume group showed a lower, albeit not significant, SBP compared with the control group (Takemura et al., 2014). These results require confirmed in clinical trials with a larger patient sample.
These results suggest that P. mume may useful as an herbal remedy to treat and prevent some cardiovascular diseases.
Antitumor Effects
The antitumor effects of P. mume have been an important focus of pharmacological studies of the plant in recent years. MK615 and other compounds extracted from P. mume have exhibited anti-proliferative activity in vitro on many human cancer cell lines (Jeong et al., 2006), for example, the human hepatocellular carcinoma cell lines HuH7, HepG2, and Hep3B (Okada et al., 2007; Sakuraoka et al., 2010); human colon cancer cell lines SW480, COLO, and WiDr (Mori et al., 2007; Cho et al., 2019); human pancreatic cancer cell lines PANC-1, PK-1, PK45H, and MIAPaCa-2 cells (Toshie, 2008; Hattori et al., 2013); human malignant melanoma cell lines SK-MEL28 and A375 cells (Tada et al., 2012); human breast cancer cell lines MDA-MB-468 and MCF-7 cells (Nakagawa et al., 2007); human lung cancer cell lines A549 and PC14 cells (Sunage et al., 2011); and human leukemia cell lines HIMeg, HL-60, and Su9T01 cells (Shen et al., 1995; Kai et al., 2011). The proposed antitumor mechanisms, involved directly suppressing Aurora A and Aurora B kinase activity, inhibition of NF-κB activation (Toshie, 2008), triggering of apoptosis and autophagy (Mori et al., 2007), inducing accumulation of ROS in cancer cells but not in normal endothelial cells (Hattori et al., 2013), inhibition of the ERK1/2 and DNA binding-1 (Id-1) pathways, decreasing Bcl-2 expression (Tada et al., 2012), and suppressing hypoxia tolerance by up-regulation of E-cadherin in cancer cells with mutant KRAS (Nishi et al., 2020).
Anti-neoplastic in vivo studies have also shown that MK615 significantly inhibited the growth of human cancer cells in xenograft mice. The effects might be associated with the antioxidant capacity of MK615 (Hattori et al., 2013). Fermented P. mume and probiotic treatment also alleviated the 12-dimethylbenz [a]anthracene and 12-O-tetradecanoyl phorbol-13-acetate-induced skin carcinogenesis by mitigating oxidative stress (Lee et al., 2013). In addition, a recent study found that MK615 activated T cell-mediated immunity through programmed death-ligand 1(PD-L1) down-regulation (Yanaki et al., 2018).
When a P. mume extract was combined with other anticancer drugs, the drugs showed additive and synergistic effects in different pharmacological models. For example, MK615 enhanced the apoptosis activity of bendamustine in lymphoma cell lines (Inoue et al., 2017). The triterpene extract from P. mume augmented the suppressive effects of 5-fluorouracil on esophageal cancer cell xenografts in the peritoneal cavity of a severe combined immunodeficient (SCID) mouse (Yamai et al., 2012). A MK615 and gemcitabine combined treatment was more effective than single treatments in inhibiting the growth of human pancreatic cancer cell xenografts in athymic nude mice (Hattori et al., 2013).
In a clinical setting, Matsushita et al. (2010) described a patient with malignant melanoma who was administered 13 g daily oral doses of MK615 for 5°months, and whose cutaneous in-transit metastatic lesions were significantly reduced, and the apoptotic index of tumor cells significantly increased. In another case report, a hepatocellular carcinoma (HCC)-recurrent patient was administered 6.15 g MK615 twice daily. After 3°months of treatment, the alpha-fetoprotein level decreased, and both the lymph nodes and pulmonary metastases decreased in size (Hoshino et al., 2013). A phase I clinical trial found that patients showed good tolerance to gemcitabine when it was combined with MK615 (Moriyama et al., 2018). A randomized placebo-controlled clinical trial recruited 208 breast cancer patients with diarrhea caused by lapatinib and capecitabine (Xing et al., 2018). The patients were randomized and assigned to two groups given either 100 mg ethanol extract of P. mume or placebo, respectively. Diarrhea and gastrointestinal symptoms were assessed using the seven-point Likert scale, two scale forms assessed quality of life of patients, and the SF-36 questionnaire, and Hospital Anxiety and Depression Scale (HADS) were used to evaluate the effects of P. mume on diarrhea of those patients. After six°weeks of treatment, the average scores of the Likert scale and HADS were reduced and SF-36 scores were improved significantly in P. mume extract treated group when compared to the control group. The results demonstrated that the ethanol extract of P. mume relieved diarrhea and gastrointestinal symptoms and improved life quality of breast cancer patients with diarrhea caused by lapatinib and capecitabine. Choi et al. (2002) found that consumption of P. mume extracts with a nitrate- and amine-rich diet inhibited endogenous nitrosamine formation in humans, and thus resulted in a lower cancer risk. Overall, numerous studies have shown that P. mume possesses antitumor properties and can be used as complimentary therapy for malignant tumors, but the effective constituents and the mechanism of action are worthy of further confirmation.
Antimicrobial and Antiviral Activity
Several studies have suggested that P. mume possesses a wide range of antibacterial activities. Two independent research groups found that P. mume extracts inhibited common periodontal bacteria, such as Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and cariogenic bacteria such as Streptococcus mitis, S. sanguis, and S. mutans in vitro (Wong et al., 2010; Seneviratne et al., 2011). It also inhibited bacteria biofilm formation in the mouth cavity (Morimoto-Yamashita et al., 2011). In a six-month randomized single-blinded parallel-controlled clinical trial, a mouthrinse containing P. mume showed beneficial effects in patients with fixed orthodontic appliances by decreasing the bleeding index (Chen et al., 2012).
Some studies showed that the herbal combination of P. mume, Schizandrae Fructus, and Coptidis Rhizoma inhibited some Salmonella and Escherichia coli (E. coli) strains in vitro and in vivo (Kwon et al., 2008; Lee and Stein, 2011). P. mume also inhibited vero-toxin release from some E. coli strains (Sakagami, 2001). Mitani et al. (2018) attributed the antimicrobial activity of P. mume on enterobacteria to the phenolic compounds it contains, but not to the free citric acid. Besides its effects on enterobacteria, P. mume also inhibited the growth of Klebsiella pneumoniae strains, partly by down-regulating the mRNA levels of the capsular polysaccharide (CPS) biosynthesis genes, decreasing CPS production, and reducing bacterial resistance to the host’s immune system (Lin et al., 2013).
In antiviral studies, a fruitjuice concentrate of P. mume inhibited human influenza A virus infection before viral adsorption in Mard in Darby canine kidney (MDCK) cells, presumably through activity of a heat-stable lectin-like molecule (Yingsakmongkon et al., 2008). Furan derivatives and phenolics extract might be the active antiviral components of P. mume relevant to the inhibition of multiplication of influenza pandemic virus and several other RNA and DNA viruses (Sriwilaijaroen et al., 2011; Ikeda et al., 2019). A recent study showed that the umesu phenolics obtained from P. mume inhibited the multiplication of herpes simplex virus (HSV) and might prevent superficial HSV infections (Nishide et al., 2019).
Immunomodulatory Effects
Jung et al. (2010) found that continuous feeding with fermented P. mume and probiotics for four°weeks increased the macrophage ratio in peripheral blood and the T lymphocyte ratio in the spleen in Institute of Cancer Research (ICR)-bred mice. This specific diet also significantly increased antibody production and enhanced the mRNA expression of TNF-α and INF-γ in the splenocytes of experimentally infected mice by killing Bordetella bronchiseptica. The immune-enhancing effect of the diet has also been proven in broiler chicks infected with Salmonella gallinarum (Jung et al., 2010a). In another study, an ethanol extract of P. mume increased the IL-12p40 concentration in the serum and the T cell ratio in the spleen in C57BL/6 J mice (Tsuji et al., 2014). Furthermore, in the tumor-bearing mouse, MK615 treatment enhanced the CD4+/CD8+ ratios following irradiation and reduced tumor volume compared with the irradiated only group (Al-Jahdari et al., 2010). In the field of organ transplantation rejection, Lu et al. (2018) found that an herbal formula containing P. mume inhibited both the mammalian target of rapamycin (mTOR) and the NF-κB signaling pathways and significantly inhibited murine skin allograft rejection. The results of all these studies indicated that P. mume has dual-directional regulatory effects on mammalian immune system.
Anti-Inflammatory and Antioxidant Activities
P. mume was found to have anti-inflammatory activity in various investigations. Extracellular high-mobility group box-1 protein (HMGB1) is a potent inflammatory agent that can promote the release of pro-inflammatory mediators such as TNF-α. The triterpenoid compounds extracted from P. mume, such as oleanolic acid (compound 133), inhibited HMGB1 release from lipopolysaccharide (LPS)-stimulated RAW246.7 cells via the Nrf2/HO-1 pathway (Kawahara et al., 2009). MK615 or a water extract of P. mume inhibited the production of cytokines induced by LPS in RAW246.7 cells and in gingival fibroblast cells. The action was mediated by inhibiting the phosphorylation of ERK1/2, p38MAPK, and c-Jun N-terminal kinases (JNK), and blocking LPS-triggered NF-κB activation (Choi H-J. et al., 2007; Morimoto et al., 2009; Morimoto-Yamashita et al., 2015). In the atopic dermatitis animal model, treatment with fermented P. mume with probiotics significantly inhibited the development of skin lesions and decreased the peripheral eosinophil ratio and serum concentrations of IgE. In addition, the mRNA expression levels of IL-4, and TNF-α in the spleen were reduced, while the serum concentrations of IL-10 increased (Jung et al., 2010b).
Different radical-scavenging tests, such as the 2,2-diphenyl-1-picrylhydrazyl (DPHH) test and the superoxide anion radical
Inflammatory and oxidative stress are the etiology of many diseases. The anti-inflammatory and antioxidant activities of P. mume may be the underlying cause of its pharmacological properties. However, most of the antioxidant studies we collected are in vitro-based studies. Only a clinical experiment assessed oxidative parameters in the study (Beretta et al., 2016), thus the antioxidative properties of P. mume need to be confirmed by more studies in vivo in the future.
Other Pharmacological Activities
Besides the pharmacological effects mentioned above, some scattered studies have reported additional activities induced by P. mume. Compounds in the P. mume extract, especially acylated quinic acid, inhibited melanogenesis and showed no cytotoxicity in theophylline-stimulated B16 melanoma cells (Nakamura et al., 2013a; Pi and Lee, 2017), indicating that P. mume might possess a skin-whitening effect. Interestingly, the effect can be strengthened by fermentation with Poria cocos mycelium (Kang et al., 2019). In folk remedies, P. mume has been reported to promote salivation. Some formulations, such as San Gan Hua Yin, which contain P. mume, can significantly improve the salivary flow rate and mitigate the severity of xerostomia in cancer patients (Murakami et al., 2009). Diets supplemented with P. mume extract significantly reduced serum ammonia concentration, elevated hepatic and muscle glycogen concentrations, increased lactate dehydrogenase, citrate synthase, and glutathione peroxidase activities, and decreased creatine kinase activity in skeletal muscles, and as a result, ameliorated exercise-induced fatigue, improved running endurance in rats. The function might relate to enhancing the oxidative capacity of skeletal muscle and inducing the muscle to prefer fatty acids for fuel use rather than amino acids or carbohydrates (Kim et al., 2008; Kim et al., 2020). A cross-sectional pilot study found that patients who receiving P. mume regularly had a significantly lower odds ratio (OR) for the presence of allergy symptoms. In the same study, oral treatment with P. mume extract attenuated the passive cutaneous anaphylaxis (PCA) reaction and mast cell degranulation in IgE-sensitized mice. The anti-allergic activity might relate to compounds including vanillin, syringic acid, protocatechuic aldehyde, lyoniresinol, and p-coumaric acid (Kono et al., 2018). Ina et al. (2002) found that the chlorogenic acid extract from P. mume reduced bradykinin and prostaglandin E2 production, inhibited acetic acid-induced writhing behavior in mice, and showed analgesic effects. The compound also relieved the tension caused by ether stresses in menopausal model rats, because it recovered catecholamine levels and decreased the adrenocorticotropic hormone (ACTH) levels in the plasma of model rats (Ina et al., 2004).
Toxicity and Safety
A systematic toxicology study evaluated the safety of the ethanol extract of P. mume. The oral acute test showed no lethal effects in rats and mice at the maximum tolerated dose of 20 g/kg. In the subacute toxicity test, no adverse effects were observed at doses greater than 3.33 g/kg body weight for 30°days. In addition, no mutagenic or genotoxic effects were observed in the experiments, including the Ames test, the micronucleus test, and the sperm abnormality test (Lu et al., 2009). The safety profile of mumefural, a bioactive compound derived from the heated fruit of P. mume, was also investigated by acute and subacute oral toxicity experiments. The results indicated that the approximate lethal dose of mumefural in ICR mice was >5 g/kg (Kim et al., 2020).
On the other hand, a few clinical studies evaluated the safety of P. mume during the experiments, and no adverse events were observed (Hoshino et al., 2013; Takemura et al., 2014). These studies demonstrated that P. mume could be used safely as a dietary supplement.
However, in recent years, several studies have found that P. mume peamaclein (also known as gibberellin-regulated protein or GRP) is a cross-reactive allergen between P. mume and peach (P. persica), and could cause food-dependent exercise-induced anaphylaxis (Iijima et al., 2015; Inomata et al., 2016; Yamanaka et al., 2019). It might be necessary to remind individuals who are allergic to peaches to avoid eating P. mume.
Conclusions and Future Perspectives
As an important medicinal herb and food commodity, the Japanese apricot or Chinese plum (P. mume) has aroused the interest of numerous researchers. In this review, we conducted an exhaustive search of the literature describing the phytochemical and pharmacological properties of P. mume. We found that 192 compounds have been isolated from different parts of the plant, including phenolics, organic acids, steroids, terpenes, benzyl glycosides, cyanogenic glycosides, furfurals, lignans, alkaloid, amino acids, and some other compounds. Numerous studies disclosed the pharmacological activities of P. mume, including its anti-diabetic, antihyperlipidemic, lowering uric acid, anti-osteoporosis, hepatoprotection, anti-H. pylori, stimulating intestinal motility, anti-inflammatory, antioxidant, improving blood fluidity effects, as well as its inhibiting platelet aggregation, anti-tumor, antimicrobial, antiviral, immunomodulation, skin whitening, stimulating salivary secretion, anti-fatigue, anti-allergic, and analgesic properties.
Several studies have established connections between the chemical compositions and the pharmacological properties of the plant. For example, the phenolic compounds confer its antidiabetic (Lee et al., 2016), antimicrobial (Mitani et al., 2018), antiviral (Ikeda et al., 2019), and anti-oxidative (Xia et al., 2010) activities; organic acid components exert hypolipidemic (Choi et al., 2007) and antibacterial (Gao, 2012) effects; and steroids and terpenes inhibit osteoclast differentiation (Yan et al., 2015). However, most of the pharmacological studies of P. mume are based on crude extracts, refined preparations such as MK615, and formulas containing P. mume such as wu mei wan or TCM decoctions. Thus, studies elucidating the relationships between the pharmacodynamics and the bioactive constituents of the plant still require further investigation.
Among the pharmacological properties of P. mume, the antidiabetic (Tu et al., 2013) and hepatoprotective effects (Hokari, 2012; Beretta et al., 2016), and the inhibitory effects on chronic gastritis (Enomoto et al., 2010) and gastroesophageal reflux (Maekita, 2015), blood pressure lowering effects (Takemura et al., 2014), and antitumor activities (Matsushita et al., 2010; Hoshino et al., 2013)are particularly notable. Because these pharmacological properties have been proven not only by in vitro and in vivo experiments but also by several clinical studies. However, most clinical studies have only involved small-sample clinical trials or case reports. Thus, to provide strong evidence of clinical applications, well-designed randomized controlled trials, cohort studies, nested case-control studies, and real-world studies need to be carried out appropriately in the future.
With regard to the safety profile of P. mume, existing studies have provided only limited information. More systematic toxicology studies still need to be carried out in the future on the aqueous extraction of P. mume, refined products such as MK 615, and pharmacodynamic components of the plant. The side-effect associating to the P. mume usage observed in the clinical experiments also needs to be identified and reported in future clinical studies.
In terms of quality control, the information about the harvest season and the maturity level of the fruit, the quantitative studies of the index components are scarcely in the existing studies. These should be emphasized in the future to promote the reproducibility of the studies.
In summary, this review provided a comprehensive information regarding P. mume, raised limitations of existing studies, and proposed future research directions, and has established a groundwork for further utilization and development of the plant.
Author Contributions
GD and JL conceived the manuscript, X-PG and YT wrote and revised the manuscript, Y-YS collected the date and revised the manuscript. All authors approved the final version of the manuscript for submission.
Funding
This study was supported by two grants from the Natural Science Foundation of Hubei Province, China (Grant Nos. 2014CKB516 and 2017CFB782).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Al-Jahdari, W. S., Sakurai, H., Yoshida, Y., Mobaraki, A., Suzuki, Y., and Nakano, T. (2010). MK615, a Prospective Anti-proliferative Agent, Enhances CD4/CD8 Ratio after Exposure to Irradiation. Int. J. Radiat. Biol. 87, 81–90. doi:10.3109/09553002.2010.518202
Arai, M., Shibata, Y., Pugdee, K., Abiko, Y., and Ogata, Y. (2007). Effects of Reactive Oxygen Species (ROS) on Antioxidant System and Osteoblastic Differentiation in MC3T3-E1 Cells. Tbmb 59, 27–33. doi:10.1080/15216540601156188
Bailly, C. (2020). Anticancer Properties of Prunus Mume Extracts (Chinese Plum, Japanese Apricot). J. Ethnopharmacology 246, 112215. doi:10.1016/j.jep.2019.112215
Beretta, A., Accinni, R., Dellanoce, C., Tonini, A., Cardot, J.-M., and Bussière, A. (2016). Efficacy of a Standardized Extract ofPrunus Mumein Liver Protection and Redox Homeostasis: A Randomized, Double-Blind, Placebo-Controlled Study. Phytother. Res. 30, 949–955. doi:10.1002/ptr.5597
Bi, Y., Nielsen, K. L., Kilts, T. M., Yoon, A., A. Karsdal, M. M., Wimer, H. F., et al. (2006). Biglycan Deficiency Increases Osteoclast Differentiation and Activity Due to Defective Osteoblasts. Bone 38, 778–786. doi:10.1016/j.bone.2005.11.005
Burgess, N., Maguire, E. A., and O'Keefe, J. (2002). The Human hippocampus and Spatial and Episodic Memory. Neuron 35, 625–641. doi:10.1016/s0896-6273(02)00830-9
Chen, C., Wen, D.-C., Gao, S.-d., Hu, X.-y., and Yi, C. (2016). The Protective Effects of Buzui on Acute Alcoholism in Mice. Evidence-Based Complement. Altern. Med. 2016, 1–8. doi:10.1155/2016/3539748
Chen, L., Wen, Y. Q., and Shi, Y. L. (2007). Comparison of External Application of Liuhedan and Diclofenac Treatment of Acute Gouty Arthritis. West. China Med. J. 22, 361–362.
Chen, Y., Wong, R. W. K., Seneviratne, C. J., Hagg, U., McGrath, C., Samaranayake, L. P., et al. (2012). The Effects of Natural Compounds-Containing Mouthrinses on Patients with Fixed Orthodontic Appliance Treatment: Clinical and Microbiological Outcomes. Int. J. Paediatric Dentistry 23 (6), 452–459. doi:10.1111/ipd.12018
Chen, Z. G., En, B. T., and Zhang, Z. Q. (2006). [Simultaneous Determination of Eight Organic Acids in Fructus Mume by RP-HPLC]. Zhongguo Zhong Yao Za Zhi 31, 1783–1786. dio: doi:10.3321/j.issn:1001-5302.2006.21.010
Cho, H. D., Kim, J. H., Won, Y. S., Moon, K. D., and Seo, K. I. (2019). Inhibitory Effects of Pectinase‐Treated Prunus Mume Fruit Concentrate on Colorectal Cancer Proliferation and Angiogenesis of Endothelial Cells. J. Food Sci. 84, 3284–3295. doi:10.1111/1750-3841.14824
Choi, H.-J., Kang, O.-H., Park, P.-S., Chae, H.-S., Oh, Y.-C., Lee, Y.-S., et al. (2007). Mume Fructus Water Extract Inhibits Pro-inflammatory Mediators in Lipopolysaccharide-Stimulated Macrophages. J. Med. Food 10, 460–466. doi:10.1089/jmf.2006.198
Choi, S. W., Hur, N. Y., Ahn, S. C., Kim, D. S., Lee, J. K., Kim, D. O., et al. (2007). Isolation and Structural Determination of Squalene Synthase Inhibitor from Prunus Mume Fruit. J. Microbiol. Biotechnol. 17, 1970–1975.
Choi, S. Y., Chung, M. J., and Sung, N. J. (2002). Volatile N-Nitrosamine Inhibition after Intake Korean Green Tea and Maesil (Prunus Mume SIEB. Et ZACC.) Extracts with an Amine-Rich Diet in Subjects Ingesting Nitrate. Food Chem. Toxicol. 40, 949–957. doi:10.1016/S0278-6915(02)00025-X
Chuda, Y., Ono, H., Ohnishi-Kameyama, M., Matsumoto, K., Nagata, T., and Kikuchi, Y. (1999). Mumefural, Citric Acid Derivative Improving Blood Fluidity from Fruit-Juice Concentrate of Japanese Apricot (PrunusmumeSieb. et Zucc). J. Agric. Food Chem. 47, 828–831. doi:10.1021/jf980960t
Chyun, J. H., Na, J. H., Park, H. J., and Yang, Y. (2012). Effect of the Supplementation of Prunus Mume Concentrates on Lipid Peroxide Levels and Antioxidative Enzyme Activities in Hyperlipidemic Rats. FASEB J. 26.
Dong-Seon, K., Seung-Hyung, K., Wen Yi, J., and Ho Kyoung, K. (2013). Antiplatelet and Antithrombotic Effect of Phyllostachys Pubescens Leaves and Mume Fructus Combination. Planta Med. 79. doi:10.1055/s-0033-1351959
Ekstedt, M., Franzén, L. E., Mathiesen, U. L., Thorelius, L., Holmqvist, M., Bodemar, G., et al. (2006). Long-term Follow-Up of Patients with NAFLD and Elevated Liver Enzymes. Hepatology 44, 865–873. doi:10.1002/hep.21327
Enomoto, S., Yanaoka, K., Utsunomiya, H., Niwa, T., Inada, K., Deguchi, H., et al. (2010). Inhibitory effects of Japanese apricot (Prunus mume Siebold et Zucc.; Ume) on Helicobacter pylori-related chronic gastritis. Eur. J. Clin. Nutr. 64, 714–719. doi:10.1038/ejcn.2010.70
Farkas, E., Luiten, P. G. M., and Bari, F. (2007). Permanent, Bilateral Common Carotid Artery Occlusion in the Rat: A Model for Chronic Cerebral Hypoperfusion-Related Neurodegenerative Diseases. Brain Res. Rev. 54, 162–180. doi:10.1016/j.brainresrev.2007.01.003
Fujimoto, K., Nakamura, S., Matsumoto, T., Ohta, T., Ogawa, K., Tamura, H., et al. (2013). Medicinal Flowers. XXXVIII. Structures of Acylated Sucroses and Inhibitory Effects of Constituents on Aldose Reducatase from the Flower Buds of Prunus Mume. Chem. Pharm. Bull. 61, 445–451. doi:10.1248/cpb.c12-01068
Fujimoto, K., Nakamura, S., Matsumoto, T., Ohta, T., Yoshikawa, M., Ogawa, K., et al. (2014). Structures of Acylated Sucroses from the Flower Buds of Prunus Mume. J. Nat. Med. 68, 481–487. doi:10.1007/s11418-014-0818-z
Fujita, K., Hasegawa, M., Fujita, M., Kobayashi, I., Ozasa, K., and Watanabe, Y. (2002). [Anti-Helicobacter pylori Effects of Bainiku-Ekisu (Concentrate of Japanese Apricot Juice)]. Nihon Shokakibyo Gakkai Zasshi 99, 379–385.
Gao, Z. (2012). Evaluation of Different Kinds of Organic Acids and Their Antibacterial Activity in Japanese Apricot Fruits. Afr. J. Agric. Res. 7, 4911–4918. doi:10.5897/AJAR12.1347
Guo, C. H., Hou, X., Wang, H., and Xiao, C. P. (2009). Separation and Identification of Flavone Components in Prunus Mume. Zhong Cheng Yao31 13, 1613–1614. doi:10.3969/j.issn.1001-1528.2009.10.041
Guo, L., Xie, F. W., Liu, H. M., Xia, Q. L., Zhao, X. D., and Liu, K. J. (2007). Analysis of Volatile, Semi-volatile and Non-volatile Organic Acids in Fructus Mume Concrete. Yan Cao Ke Ji 41, 33–37. doi:10.3969/j.issn.1002-0861.2007.12.008
Hasegawa, M. (1969). Flavonoids of Various Prunus Species IX. Shokubutsugaku Zasshi 82, 148–154. doi:10.15281/jplantres1887.82.148
Hasegawa, M. (1959). Notes- Flavonoids of Various Prunus Species. VIII. The Flavonoids in the Wood of Prunus Mume. J. Org. Chem. 24, 408–409. doi:10.1021/jo01085a036
Hattori, M., Kawakami, K., Akimoto, M., Takenaga, K., Suzumiya, J., and Honma, Y. (2013). Antitumor Effect of Japanese Apricot Extract (MK615) on Human Cancer Cells In Vitro and In Vivo through a Reactive Oxygen Species-dependent Mechanism. Tumori J. 99, 239–248. doi:10.1177/030089161309900220
Hokari, A. (2012). Efficacy of MK615 for the Treatment of Patients with Liver Disorders. Wjg 18, 4118. doi:10.3748/wjg.v18.i31.4118
Hoshino, T., Takagi, H., Naganuma, A., Koitabashi, E., Uehara, S., Sakamoto, N., et al. (2013). Advanced Hepatocellular Carcinoma Responds to MK615, a Compound Extract from the Japanese Apricot "Prunus Mume ". Wjh 5, 596. doi:10.4254/wjh.v5.i10.596
Hwang, J., Yang, H., Hur, H., and Park, J. (2012). Anti-diabetic Effect and Mechanism of Action of MaEsil (Prunus Mume) Extract. Planta Med. 78. doi:10.1055/s-0032-1320572
Ichikawa, K., Kinoshita, T., and Sankawa, U. (1989). The Screening of Chinese Crude Drugs for Ca2+ Antagonist Activity: Identification of Active Principles from the Aerial Part of Pogostemon Cablin and the Fruits of Prunus Mume. Chem. Pharm. Bull. 37, 345–348. doi:10.1248/cpb.37.345
Iijima, S., Ito, M., Makabe, K., Murakami, Y., Yokooji, T., and Matsuo, H. (2015). Case of Food-dependent Exercise-Induced Anaphylaxis Due to Japanese Apricot and Peach: Detection of Causative Antigens. J. Dermatol. 42, 916–917. doi:10.1111/1346-8138.12951
Ikeda, K., Nishide, M., Tsujimoto, K., Nagashima, S., Kuwahara, T., Mitani, T., et al. (2020). Antiviral and Virucidal Activities of Umesu Phenolics on Influenza Viruses. Jpn. J. Infect. Dis. 73, 8–13. doi:10.7883/yoken.JJID.2018.522
Ina, H., Yamada, K., Matsumoto, K., and Miyazaki, T. (2004). Effects of Benzyl Glucoside and Chlorogenic Acid from Prunus Mume on Adrenocorticotropic Hormone (ACTH) and Catecholamine Levels in Plasma of Experimental Menopausal Model Rats. Biol. Pharm. Bull. 27, 136–137. doi:10.1248/bpb.27.136
Ina, H., Yamada, K., Matsumoto, K., and Miyazaki, T. (2003). Effects of Benzyl Glucoside and Chlorogenic Acid from Prunus Mume on Angiotensin Converting Enzyme, Aldosterone and Corticosterone Levels in Rat Plasma. Nat. Medicines 57, 178–180.
Ina, H., Yamada, K., Matsumoto, K., and Miyazaki, T. (2002). Inhibitory Effects of Benzyl Glucoside and Chlorogenic Acid from Prunus Mume on Bradykinin and Prostaglandin E2 Production in the Abdominal Cavities of Mice. Nat. Medicines 56, 184–186.
Inomata, N., Miyakawa, M., Hotta, A., and Aihara, M. (2016). Identification of Japanese Apricot Peamaclein as a New Allergen Related to Food-dependent Exercise-Induced Anaphylaxis Due to Japanese Apricot: Cross-Reactivity to PRU P 7. J. Allergy Clin. Immunol. 137, AB236. doi:10.1016/j.jaci.2015.12.956
Inoue, M., Honma, Y., Urano, T., and Suzumiya, J. (2017). Japanese Apricot Extract (MK615) Potentiates Bendamustine-Induced Apoptosis via Impairment of the DNA Damage Response in Lymphoma Cells. Oncol. Lett. 14, 792–800. doi:10.3892/ol.2017.6219
Jang, A. J., Lee, J.-H., Yotsu-Yamashita, M., Park, J., Kye, S., Benza, R. L., et al. (2018). A Novel Compound, "FA-1" Isolated from Prunus Mume, Protects Human Bronchial Epithelial Cells and Keratinocytes from Cigarette Smoke Extract-Induced Damage. Sci. Rep. 8. doi:10.1038/s41598-018-29701-2
Jeon, W. (2015). Fructus Mume Alleviates Chronic Cerebral Hypoperfusion-Induced Hippocampal Damage via Inhibition of Inflammation and Downregulation of TLR4 and P38 MAPK Signaling. Neurodegenerative Dis. 15, 1948. doi:10.1159/000381736
Jeon, W. K., Ma, J., Choi, B.-R., Han, S.-H., Jin, Q., Hwang, B. Y., et al. (2012). Effects ofFructus mumeExtract on MAPK and NF-Κb Signaling and the Resultant Improvement in the Cognitive Deficits Induced by Chronic Cerebral Hypoperfusion. Evidence-Based Complement. Altern. Med. 2012, 1–13. doi:10.1155/2012/450838
Jeong, J. T., Moon, J.-H., Park, K.-H., and Shin, C. S. (2006). Isolation and Characterization of a New Compound fromPrunus mumeFruit that Inhibits Cancer Cells. J. Agric. Food Chem. 54, 2123–2128. doi:10.1021/jf0523770
Jin, Q. H., Lee, C., Lee, J. W., Lee, I. S., Lee, M. K., Jeon, W. K., et al. (2012). Chemical Constituents from the Fruits of Prunus Mume. Nat. Product. Sci. 18, 200–203.
Jo, C., Kim, B., Lee, S., Ham, I., Lee, K., and Choi, H.-Y. (2019). Vasorelaxant Effect of Prunus Mume (Siebold) Siebold & Zucc. Branch through the Endothelium-dependent Pathway. Molecules 24, 3340. doi:10.3390/molecules24183340
Jung, B.-G., Cho, S.-J., Koh, H.-B., Han, D.-U., and Lee, B.-J. (2010). Fermented Maesil (Prunus Mume) with Probiotics Inhibits Development of Atopic Dermatitis-like Skin Lesions in NC/Nga Mice. Vet. Dermatol. 21, 184–191. doi:10.1111/j.1365-3164.2009.00796.x
Jung, B.-G., Ko, J.-H., Cho, S.-J., Koh, H.-B., Yoon, S.-R., Han, D.-U., et al. (2010b). Immune-Enhancing Effect of Fermented Maesil (Prunus Mume Siebold & Zucc.) with Probiotics against Bordetella Bronchiseptica in Mice. J. Vet. Med. Sci. 72, 1195–1202. doi:10.1292/jvms.09-0555
Jung, B.-G., Ko, J.-H., and Lee, B.-J. (2010a). Dietary Supplementation with a Probiotic Fermented Four-Herb Combination Enhances Immune Activity in Broiler Chicks and Increases Survivability against salmonella Gallinarum in Experimentally Infected Broiler Chicks. J. Vet. Med. Sci. 72, 1565–1573. doi:10.1292/jvms.10-0152
Jung, E. S., Ha, K. C., Choi, E. K., Jung, S. Y., and Na, J. R. (2014). The Effect of Defecate Function in Constipation by a 8-week Supplementation of Prunus Mume Fruit Extract: A Randomized Double-Blind, Placebo-Controlled Trial. FASEB J. 28.
Kai, H., Akamatsu, E., Torii, E., Kodama, H., Yukizaki, C., Sakakibara, Y., et al. (2011). Inhibition of Proliferation by Agricultural Plant Extracts in Seven Human Adult T-Cell Leukaemia (ATL)-related Cell Lines. J. Nat. Med. 65, 651–655. doi:10.1007/s11418-011-0510-5
Kameoka, H., and Kitagawa, C. (1976).Constituents of the Fruits of Prunus Mume Sieb. And Zucc. Nippon Nogei Kagaku Kaishi. 50, 389–393. doi:10.1271/nogeikagaku1924.50.9_389
Kang, J. S., Kim, D. J., Kim, G.-Y., Cha, H.-J., Kim, S., Kim, H.-S., et al. (2016). Ethanol Extract of Prunus Mume Fruit Attenuates Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis Involving Nrf2/Ho-1 Activation in C2C12 Myoblasts. Revista Brasileira de Farmacognosia 26, 184–190. doi:10.1016/j.bjp.2015.06.012
Kang, K.-Y., Hwang, Y.-H., Lee, S.-J., Jang, H.-Y., Hong, S.-G., Mun, S.-K., et al. (2019). Verification of the Functional Antioxidant Activity and Antimelanogenic Properties of Extracts of Poria Cocos Mycelium Fermented with Freeze-Dried Plum Powder. Int. J. Biomater. 2019, 1–8. doi:10.1155/2019/9283207
Karakaya, S., El, S. N., and Ta, A. A. (2001). Antioxidant Activity of Some Foods Containing Phenolic Compounds. Cijf 52, 501–508. doi:10.1080/713671810
Kawahara, K., Hashiguchi, T., Masuda, K., Saniabadi, A. R., Kikuchi, K., Tancharoen, S., et al. (2009). Mechanism of HMGB1 Release Inhibition from RAW264.7 Cells by Oleanolic Acid in Prunus Mume Sieb. Et Zucc. Int. J. Mol. Med. 23, 615–620. doi:10.3892/ijmm_00000172
Khan, A., Pan, J. H., Cho, S., Lee, S., and Kim, Y. J. (2017). Investigation of the Hepatoprotective Effect of Prunus mume Sieb. et Zucc Extract in a Mouse Model of Alcoholic Liver Injury Through High-Resolution MetabolomicsInvestigation of the hepatoprotective effect ofPrunus mume sieb. Et zucc extract in a mouse model of alcoholic liver injury through High-Resolution metabolomics. J. Med. Food 20, 734–743. doi:10.1089/jmf.2016.3874
Kim, H., Park, Y. H., Dhungana, S. K., Kim, M., and Shin, D. (2014). Comparative Assessment of Physicochemical Properties of Unripe Peach (Prunus Persica) and Japanese Apricot (Prunus Mume). Asian Pac. J. Trop. Biomed. 4, 97–103. doi:10.1016/S2221-1691(14)60216-1
Kim, J.-H., Cho, H.-D., Won, Y.-S., Hong, S.-M., Moon, K.-D., and Seo, K.-I. (2020). Anti-Fatigue Effect of Prunus Mume Vinegar in High-Intensity Exercised Rats. Nutrients 12, 1205. doi:10.3390/nu12051205
Kim, J.-H., Won, Y.-S., Cho, H.-D., Hong, S.-M., Moon, K.-D., and Seo, K.-I. (2021). Protective Effect of Prunus Mume Fermented with Mixed Lactic Acid Bacteria in Dextran Sodium Sulfate-Induced Colitis. Foods 10, 58. doi:10.3390/foods10010058
Kim, J., Han, M., and Jeon, W. K. (2020). Acute and Subacute Oral Toxicity of Mumefural, Bioactive Compound Derived from Processed Fruit of Prunus Mume Sieb. Et Zucc., in ICR Mice. Nutrients 12, 1328. doi:10.3390/nu12051328
Kim, M.-S., Bang, J. H., Lee, J., Han, J.-S., Kang, H. W., and Jeon, W. K. (2016). Fructus Mume Ethanol Extract Prevents Inflammation and Normalizes the Septohippocampal Cholinergic System in a Rat Model of Chronic Cerebral Hypoperfusion. J. Med. Food 19, 196–204. doi:10.1089/jmf.2015.3512
Kim, M.-S., Jeon, W. K., Lee, K. W., Park, Y. H., and Han, J.-S. (2015). Ameliorating Effects of Ethanol Extract ofFructus Mumeon Scopolamine-Induced Memory Impairment in Mice. Evidence-Based Complement. Altern. Med. 2015, 1–8. doi:10.1155/2015/102734
Kim, S.-W., Ha, N.-Y., Kim, K.-I., Park, J.-K., and Lee, Y.-H. (2008). Memory-improving Effect of Formulation-MSS by Activation of Hippocampal MAPK/ERK Signaling Pathway in Rats. BMB Rep. 41, 242–247. doi:10.5483/bmbrep.2008.41.3.242
Kim, S., Park, S.-H., Lee, H.-N., and Park, T. (2008). Prunus mumeExtract Ameliorates Exercise-Induced Fatigue in Trained Rats. J. Med. Food 11, 460–468. doi:10.1089/jmf.2007.0097
Kishida, K., Heya, Y., Yamazaki, Y., Horinishi, A., and Ozaki, Y. (2013). Inhibitory Effects of Phenolic Extracts Derived from Japanese Apricot Fruit (Prunus Mume) on Rat Small Intestinal Disaccharidase Activities. 63, 1570. doi:10.1159/000354245
Ko, B.-S., Kim, D. S., Kang, S., Ryuk, J. A., and Park, S. (2013). Prunus mumeandLithospermum erythrorhizonExtracts Synergistically Prevent Visceral Adiposity by Improving Energy Metabolism through Potentiating Hypothalamic Leptin and Insulin Signalling in Ovariectomized Rats. Evidence-Based Complement. Altern. Med. 2013, 1–9. doi:10.1155/2013/750986
Ko, J., and Auyeung, K. (2014). Inflammatory Bowel Disease: Etiology, Pathogenesis and Current Therapy. Cpd 20, 1082–1096. doi:10.2174/13816128113199990416
Kono, R., Nakamura, M., Nomura, S., Kitano, N., Kagiya, T., Okuno, Y., et al. (2018). Biological and Epidemiological Evidence of Anti-allergic Effects of Traditional Japanese Food Ume (Prunus Mume). Sci. Rep. 8. doi:10.1038/s41598-018-30086-5
Kono, R., Nomura, S., Okuno, Y., Nakamura, M., Maeno, A., Kagiya, T., et al. (2014). 3,4-Dihydroxybenzaldehyde Derived from Prunus Mume Seed Inhibits Oxidative Stress and Enhances Estradiol Secretion in Human Ovarian Granulosa Tumor Cells. Acta Histochem. Cytochem. 47, 103–112. doi:10.1267/ahc.14003
Kono, R., Okuno, Y., Inada, K.-i., Tokuda, A., Hashizume, H., Yoshida, M., et al. (2011). APrunus mumeExtract Stimulated the Proliferation and Differentiation of Osteoblastic MC3T3-E1 Cells. Biosci. Biotechnol. Biochem. 75, 1907–1911. doi:10.1271/bbb.110264
Kubo, M., Yamazaki, M., Matsuda, H., Gato, N., and Kotani, T. (2005). Effect of Fruit-Juice Concentrate of Japanese Apricot (Prunus Mume SEIB. Et ZUCC.) on Improving Blood Fluidity. Nat. Medicines 59, 22–27.
Kwon, H., Kwon, Y., Kwon, D., and Lee, J. (2008). Evaluation of Antibacterial Effects of a Combination of Coptidis Rhizoma, Mume Fructus, and Schizandrae Fructus against Salmonella. Int. J. Food Microbiol. 127, 180–183. doi:10.1016/j.ijfoodmicro.2008.06.020
Lee, J.-A., Ko, J.-H., Jung, B.-G., Kim, T.-H., Hong, J.-I., Park, Y.-S., et al. (2013). Fermented Prunus Mume with Probiotics Inhibits 7,12-dimethylbenz[a]anthracene and 12-O-Tetradecanoyl Phorbol-13-Acetate Induced Skin Carcinogenesis through Alleviation of Oxidative Stress. Asian Pac. J. Cancer Prev. 14, 2973–2978. doi:10.7314/apjcp.2013.14.5.2973
Lee, J. D., Lee, J. R., Park, B. A., and Hur, S. J. (2014). The Effect of Prunus Mume by the Biopolymer in Mice Model Presenting Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. (Australia) 29, 247–248. doi:10.1111/jgh.12766_2
Lee, J. H., and Stein, B. D. (2011). Antimicrobial Activity of a Combination of Mume Fructus, Schizandrae Fructus, and Coptidis Rhizoma on Enterohemorrhagic Escherichia coli O26, O111, and O157 and its Effect on Shiga Toxin Releases. Foodborne Pathog. Dis. 8, 643–646. doi:10.1089/fpd.2010.0710
Lee, K. M., Bang, J., Kim, B. Y., Lee, I. S., Han, J.-S., Hwang, B. Y., et al. (2015). Fructus Mume Alleviates Chronic Cerebral Hypoperfusion-Induced White Matter and Hippocampal Damage via Inhibition of Inflammation and Downregulation of TLR4 and P38 MAPK Signaling. BMC Complement. Altern. Med. 15, 125. doi:10.1186/s12906-015-0652-1
Lee, M. W., Kwon, J. E., Lee, Y.-J., Jeong, Y. J., Kim, I., Cho, Y. M., et al. (2016). Prunus Mumeleaf Extract Lowers Blood Glucose Level in Diabetic Mice. Pharm. Biol. 54, 2135–2140. doi:10.3109/13880209.2016.1147052
Lee, S. W., Kim, S. J., Kim, H., Yang, D., Kim, H. J., and Kim, B. J. (2017). Effects of Prunus Mume Siebold & Zucc. In the Pacemaking Activity of Interstitial Cells of Cajal in Murine Small Intestine. Exp. Ther. Med. 13, 327–334. doi:10.3892/etm.2016.3963
Lee, S. Y., Lee, S.-J., and Hur, S. J. (2017). Effects ofPrunus mumeSieb. et Zucc. extract and its biopolymer encapsulation on a mouse model of colitis. J. Sci. Food Agric. 97, 686–692. doi:10.1002/jsfa.7790
Li, J.-B., Xu, L.-j., Dong, H., Huang, Z.-y., Zhao, Y., Chen, G., et al. (2013). Effects of Chinese Fructus Mume Formula and its Separated Prescription Extract on Insulin Resistance in Type 2 Diabetic Rats. J. Huazhong Univ. Sci. Technol. [Med. Sci. 33, 877–885. doi:10.1007/s11596-013-1215-7
Lieber, C. S. (2001). Alcoholic Liver Injury: Pathogenesis and Therapy in 2001. Pathologie Biologie 49, 738–752. doi:10.1016/s0369-8114(01)00239-5
Lin, T.-H., Huang, S.-H., Wu, C.-C., Liu, H.-H., Jinn, T.-R., Chen, Y., et al. (2013). Inhibition ofKlebsiella pneumoniaeGrowth and Capsular Polysaccharide Biosynthesis byFructus Mume. Evidence-Based Complement. Altern. Med. 2013, 1–10. doi:10.1155/2013/621701
Liu, L., Yuan, S., Sun, Y., Long, Y., Li, Y., Niu, Y., et al. (2009). The Possible Mechanisms of Fructus Mume Pill in the Treatment of Colitis Induced by 2,4,6-trinitrobenzene Sulfonic Acid in Rats. J. Ethnopharmacology 126, 557–564. doi:10.1016/j.jep.2009.08.013
Lu, B., Wu, X., Dong, Y., Gong, J., and Zhang, Y. (2009). Mutagenicity and Safety Evaluation of Ethanolic Extract of Prunus Mume. J. Food Sci. 74, T82–T88. doi:10.1111/j.1750-3841.2009.01372.x
Lu, C., Liu, H., Jin, X., Chen, Y., Liang, C.-L., Qiu, F., et al. (2018). Herbal Components of a Novel Formula PSORI-CM02 Interdependently Suppress Allograft Rejection and Induce CD8+CD122+PD-1+ Regulatory T Cells. Front. Pharmacol. 9, 88. doi:10.3389/fphar.2018.00088
Maekita, T. (2015). Japanese Apricot Improves Symptoms of Gastrointestinal Dysmotility Associated with Gastroesophageal Reflux Disease. Wjg 21, 8170. doi:10.3748/wjg.v21.i26.8170
Matsuda, H., Morikawa, T., Ishiwada, T., Managi, H., Kagawa, M., Higashi, Y., et al. (2003). Medicinal Flowers. VIII. Radical Scavenging Constituents from the Flowers of Prunus Mume: Structure of Prunose III. Chem. Pharm. Bull. 51, 440–443. doi:10.1248/cpb.51.440
Matsushita, S., Tada, K.-I., Kawahara, K.-I., Kawai, K., Hashiguchi, T., Maruyama, I., et al. (2010). Advanced Malignant Melanoma Responds to Prunus Mume Sieb. Et Zucc (Ume) Extract: Case Report and In Vitro Study. Exp. Ther. Med. 1, 569–574. doi:10.3892/etm_00000089
Mitani, T., Horinishi, A., Kishida, K., Kawabata, T., Yano, F., Mimura, H., et al. (2013). Phenolics Profile of Mume, Japanese Apricot (Prunus mumeSieb. et Zucc.) Fruit. Biosci. Biotechnol. Biochem. 77, 1623–1627. doi:10.1271/bbb.130077
Mitani, T., Ota, K., Inaba, N., Kishida, K., and Koyama, H. A. (2018). Antimicrobial Activity of the Phenolic Compounds of Prunus Mume against Enterobacteria. Biol. Pharm. Bull. 41, 208–212. doi:10.1248/bpb.b17-00711
Miyazawa, M., Shirakawa, N., Utsunomiya, H., Inada, K.-i., and Yamada, T. (2009). Comparision of the volatile components of unripe and ripe Japanese apricot (Prunus mumeSieb. et Zucc.). Nat. Product. Res. 23, 1567–1571. doi:10.1080/14786410500462926
Miyazawa, M., Utsunomiya, H., Inada, K.-i., Yamada, T., Okuno, Y., Tanaka, H., et al. (2006). Inhibition of Helicobacter pylori Motility by (+)-Syringaresinol from Unripe Japanese Apricot. Biol. Pharm. Bull. 29, 172–173. doi:10.1248/bpb.29.172
Mori, S., Sawada, T., Okada, T., Ohsawa, T., Adachi, M., and Keiichi, K. (2007). New Anti-proliferative Agent, MK615, from Japanese Apricot "Prunus Mume " Induces Striking Autophagy in Colon Cancer Cells In Vitro. Wjg 13, 6512–6517. doi:10.3748/wjg.v13.i48.651210.3748/wjg.13.6512
Morimoto, Y., Kikuchi, K., Ito, T., Tokuda, M., Matsuyama, T., Noma, S., et al. (2009). MK615 Attenuates Porphyromonas Gingivalis Lipopolysaccharide-Induced Pro-inflammatory Cytokine Release via MAPK Inactivation in Murine Macrophage-like RAW264.7 Cells. Biochem. Biophysical Res. Commun. 389, 90–94. doi:10.1016/j.bbrc.2009.08.103
Morimoto-Yamashita, Y., Kawakami, Y., Tatsuyama, S., Miyashita, K., Emoto, M., Kikuchi, K., et al. (2015). A Natural Therapeutic Approach for the Treatment of Periodontitis by MK615. Med. Hypotheses 85, 618–621. doi:10.1016/j.mehy.2015.07.028
Morimoto-Yamashita, Y., Matsuo, M., Komatsuzawa, H., Kawahara, K.-i., Kikuchi, K., Torii, M., et al. (2011). MK615: A New Therapeutic Approach for the Treatment of Oral Disease. Med. Hypotheses 77, 258–260. doi:10.1016/j.mehy.2011.04.027
Moriyama, I., Ikejiri, F., Kawakami, K., Inoue, M., and Kumanomido, S. (2018). A Phase I Fixed-Dose Feasibility Study of MK615 and Gemcitabine in Patients with Advanced or Metastatic Pancreatic Cancer. J. Clin. Oncol. 36. doi:10.1200/JCO.2018.36.4-suppl.51010.1200/jco.2018.36.4_suppl.510
Murakami, M., Wei, M.-X., Ding, W., and Zhang, Q.-D. (2009). Effects of Chinese Herbs on Salivary Fluid Secretion by Isolated and Perfused Rat Submandibular Glands. Wjg 15, 3908–3915. doi:10.3748/wjg.15.3908
Na, J.-R., Oh, K.-N., Park, S.-U., Bae, D., Choi, E. J., Jung, M. A., et al. (2012). The Laxative Effects of Maesil (Prunus mumeSiebold & Zucc.) on Constipation Induced by a Low-Fibre Diet in a Rat Model. Int. J. Food Sci. Nutr. 64, 333–345. doi:10.3109/09637486.2012.738648
Nakagawa, A., Sawada, T., Okada, T., Ohsawa, T., Adachi, M., and Kubota, K. (2007). New Antineoplastic Agent, MK615, from UME (A Variety of) Japanese Apricot Inhibits Growth of Breast Cancer Cells In Vitro. Breast J. 13, 44–49. doi:10.1111/j.1524-4741.2006.00361.x
Nakajima, S., Fujita, K., Inoue, Y., Nishio, M., and Seto, Y. (2006). Effect of the Folk Remedy, Bainiku-Ekisu, a Concentrate of Prunus Mume Juice, on Helicobacter pylori Infection in Humans. Helicobacter 11, 589–591. doi:10.1111/j.1523-5378.2006.00463.x
Nakamura, S., Fujimoto, K., Matsumoto, T., Nakashima, S., Ohta, T., Ogawa, K., et al. (2013a). Acylated Sucroses and Acylated Quinic Acids Analogs from the Flower Buds of Prunus Mume and Their Inhibitory Effect on Melanogenesis. Phytochemistry 92, 128–136. doi:10.1016/j.phytochem.2013.04.012
Nakamura, S., Fujimoto, K., Matsumoto, T., Ohta, T., Ogawa, K., Tamura, H., et al. (2013b). Structures of Acylated Sucroses and an Acylated Flavonol Glycoside and Inhibitory Effects of Constituents on Aldose Reductase from the Flower Buds of Prunus Mume. J. Nat. Med. 67, 799–806. doi:10.1007/s11418-013-0750-7
Nishi, K., Tsunoda, T., Uchida, Y., Sueta, T., Sawatsubashi, M., Yamano, T., et al. (2020). MK615 Suppresses Hypoxia Tolerance by Up-Regulation of E-Cadherin in Colorectal Cancer Cells with Mutant KRAS. Anticancer Res. 40, 4687–4694. doi:10.21873/anticanres.14468
Nishide, M., Ikeda, K., Mimura, H., Yoshida, M., Mitani, T., and Hajime Koyama, A. (2019). Antiviral and Virucidal Activities against Herpes Simplex Viruses of Umesu Phenolics Extracted from Japanese Apricot. Microbiol. Immunol. 63, 359–366. doi:10.1111/1348-0421.12729
Okada, T., Sawada, T., Osawa, T., Adachi, M., and Kubota, K. (2007). A Novel Anti-cancer Substance, MK615, from Ume, a Variety of Japanese Apricot, Inhibits Growth of Hepatocellular Carcinoma Cells by Suppressing Aurora a Kinase Activity. Hepatogastroenterology 54, 1770–1774.
Otsuka, T., Tsukamoto, T., Tanaka, H., Inada, K., Utsunomiya, H., Mizoshita, T., et al. (2005). Suppressive Effects of Fruit-Juice Concentrate of Prunus Mume Sieb. Et Zucc. (Japanese Apricot, Ume) on Helicobacter Pylori-Induced Glandular Stomach Lesions in Mongolian Gerbils. Asian Pac. J. Cancer Prev. 6, 337–341.
Oyanagi, Y., Takahashi, T., Matsui, S., Takahashi, S., Boku, S., Takahashi, K., et al. (1999). Ehanced Expression of Interleukin-6 in Chronic Hepatitis C. Liver Int. 19, 464–472. doi:10.1111/j.1478-3231.1999.tb00078.x
Park, J.-C., Ma, J., Jeon, W. K., and Han, J.-S. (2016). Fructus Mume Extracts Alleviate Cognitive Impairments in 5XFAD Transgenic Mice. BMC Complement. Altern. Med. 16, 54. doi:10.1186/s12906-016-1033-0
Pi, K., and Lee, K. (2017). Prunus Mume Extract Exerts Antioxidant Activities and Suppressive Effect of Melanogenesis under the Stimulation by Alpha-Melanocyte Stimulating Hormone in B16-F10 Melanoma Cells. Biosci. Biotechnol. Biochem. 81, 1883–1890. doi:10.1080/09168451.2017.1365591
Poonam, V., Raunak, , Kumar, G., S. Reddy L.Kumar, C. C. S., Jain, R., K. Sharma, S., et al. (2011). Chemical Constituents of the Genus Prunus and Their Medicinal Properties. Cmc 18, 3758–3824. doi:10.2174/092986711803414386
Ren, S., Fu, Ln., Wang, H., and Xiao, C.(2004). [Isolation and Identification of Alkaloids from Armeniaca Mume Sieb]. Zhong Yao Cai, 27, 917, 8. dio: doi:10.13863/j.issn1001-4454.2004.12.013
Sakagami, Y. (2001). Inhibitory Effect of the Extract of Prunus mume Sieb. et Zucc. on Vero-toxin Production by Enterohemorrhagic Escherichia coli O157:H7. Biocontrol Sci. 6, 53–56. doi:10.4265/bio.6.53
Sakuraoka, Y., Sawada, T., Okada, T., Shiraki, T., Miura, Y., Hiraishi, K., et al. (2010). MK615 Decreases RAGE Expression and Inhibits Tage-Induced Proliferation in Hepatocellular Carcinoma Cells. Wjg 16, 5334–5341. doi:10.3748/wjg.v16.i42.5334
Seneviratne, C. J., Wong, R. W. K., Hägg, U., Chen, Y., Herath, T. D. K., Lakshman Samaranayake, P., et al. (2011). Prunus Mume Extract Exhibits Antimicrobial Activity against Pathogenic Oral Bacteria. Int. J. Paediatric Dentistry 21, 299–305. doi:10.1111/j.1365-263X.2011.01123.x
Shen, H., Cheng, T., Qiao, C., Su, Z., and Li, C. (1995). [Antitumor Effect In Vitro and Immuno-Response In Vivo of Fructus Mume]. Zhongguo Zhong Yao Za Zhi 20, 365–368. inside back cover.
Shin, E. J., Hur, H. J., Sung, M. J., Park, J. H., Yang, H. J., Kim, M. S., et al. (2013). Ethanol Extract of the Prunus Mume Fruits Stimulates Glucose Uptake by Regulating PPAR-γ in C2C12 Myotubes and Ameliorates Glucose Intolerance and Fat Accumulation in Mice Fed a High-Fat Diet. Food Chem. 141, 4115–4121. doi:10.1016/j.foodchem.2013.06.059
Son, E., Kim, S.-H., Yang, W.-K., Kim, D.-S., and Cha, J. (2017). Antiplatelet Mechanism of an Herbal Mixture Prepared from the Extracts of Phyllostachys Pubescens Leaves and Prunus Mume Fruits. BMC Complement. Altern. Med. 17, 511–541. doi:10.1186/s12906-017-2032-5
Sriwilaijaroen, N., Kadowaki, A., Onishi, Y., Gato, N., Ujike, M., Odagiri, T., et al. (2011). Mumefural and Related HMF Derivatives from Japanese Apricot Fruit Juice Concentrate Show Multiple Inhibitory Effects on Pandemic Influenza a (H1N1) Virus. Food Chem. 127, 1–9. doi:10.1016/j.foodchem.2010.12.031
Sunage, N., Hiraishi, K., Ishizuka, T., Kaira, K., Iwasaki, Y., JImma, F., et al. (2011). MK615, a Compound Extract from the Japanese Apricot “Prunus Mume” Inhibits In Vitro Cell Growth and Interleukin-8 Expression in Non-small Cell Lung Cancer Cells. J. Cancer Sci. Ther. S11, 2. doi:10.4172/1948-5956.S11-002
Tada, K.-i., Kawahara, K.-i., Matsushita, S., Hashiguchi, T., Maruyama, I., and Kanekura, T. (2012). MK615, a Prunus Mume Steb. Et Zucc ('Ume') Extract, Attenuates the Growth of A375 Melanoma Cells by Inhibiting the ERK1/2-Id-1 Pathway. Phytother. Res. 26, 833–838. doi:10.1002/ptr.3645
Takemura, S., Yoshimasu, K., Fukumoto, J., Mure, K., Nishio, N., Kishida, K., et al. (2014). Safety and Adherence of Umezu Polyphenols in the Japanese Plum (Prunus Mume) in a 12-week Double-Blind Randomized Placebo-Controlled Pilot Trial to Evaluate Antihypertensive Effects. Environ. Health Prev. Med. 19, 444–451. doi:10.1007/s12199-014-0404-8
Tamura, M., Ohnishi, Y., Kotani, T., and Gato, N. (2011). Effects of New Dietary Fiber from Japanese Apricot (Prunus Mume Sieb. Et Zucc.) on Gut Function and Intestinal Microflora in Adult Mice. Ijms 12, 2088–2099. doi:10.3390/ijms12042088
Toshie, O. T. S. T. (2008). MK615 Inhibits Pancreatic Cancer Cell Growth by Dual Inhibition of Aurora a and B Kinases. World J. Gastroenterol. 14, 1378–1382. doi:10.3748/wjg.14.1378
Tsuji, R., Koizumi, H., and Fujiwara, D. (2014). Effects of a Plum (Prunus mumeSiebold and Zucc.) Ethanol Extract on the Immune Systemin Vivoandin Vitro. Biosci. Biotechnol. Biochem. 75, 2011–2013. doi:10.1271/bbb.100886
Tu, X., Xie, C., Wang, F., Chen, Q., Zuo, Z., Zhang, Q., et al. (2013). Fructus MumeFormula in the Treatment of Type 2 Diabetes Mellitus: A Randomized Controlled Pilot Trial. Evidence-Based Complement. Altern. Med. 2013, 1–8. doi:10.1155/2013/787459
Uemura, N., Okamoto, S., Yamamoto, S., Matsumura, N., Yamaguchi, S., Yamakido, M., et al. (2001). Helicobacter pyloriInfection and the Development of Gastric Cancer. N. Engl. J. Med. 345, 784–789. doi:10.1056/NEJMoa001999
Utsunomiya, H., Takekoshi, S., Gato, N., Utatsu, H., Motley, E. D., Eguchi, K., et al. (2002). Fruit-juice Concentrate of Asian Plum Inhibits Growth Signals of Vascular Smooth Muscle Cells Induced by Angiotensin II. Life Sci. 72, 659–667. doi:10.1016/S0024-3205(02)02300-7
Wang, X., Du, J., and Zhou, J. (2019). Antibiotic Activities of Extracts from Prunus Mume Fruit against Food-Borne Bacterial Pathogens and its Active Components. Ind. Crops Prod. 133, 409–413. doi:10.1016/j.indcrop.2019.02.050
Wang, X., Wang, C.-P., Hu, Q.-H., Lv, Y.-Z., Zhang, X., OuYang, Z., et al. (2010). The Dual Actions of Sanmiao Wan as a Hypouricemic Agent: Down-Regulation of Hepatic XOD and Renal mURAT1 in Hyperuricemic Mice. J. Ethnopharmacology 128, 107–115. doi:10.1016/j.jep.2009.12.035
Wong, R. W. K., Hägg, U., Samaranayake, L., Yuen, M. K. Z., Seneviratne, C. J., and Kao, R. (2010). Antimicrobial Activity of Chinese Medicine Herbs against Common Bacteria in Oral Biofilm. A Pilot Study. Int. J. Oral Maxillofac. Surg. 39, 599–605. doi:10.1016/j.ijom.2010.02.024
Xia, D., Shi, J., Gong, J., Wu, X., and Yang, Q. (2010). Antioxidant Activity of Chinese Mei (Prunus Mume) and its Active Phytochemicals. J. Med. Plants Res. 4, 1156–1160.
Xia, D., Wu, X., Shi, J., Yang, Q., and Zhang, Y. (2011). Phenolic Compounds from the Edible Seeds Extract of Chinese Mei (Prunus Mume Sieb. Et Zucc) and Their Antimicrobial Activity. LWT - Food Sci. Technol. 44, 347–349. doi:10.1016/j.lwt.2010.05.017
Xing, H., Zhang, L., Ma, J., Liu, Z., Song, C., and Liu, Y. (2018). Fructus Mume Extracts Alleviate Diarrhea in Breast Cancer Patients Receiving the Combination Therapy of Lapatinib and Capecitabine. Front. Pharmacol. 9, 516. doi:10.3389/fphar.2018.00516
Yamai, H., Yamamoto, Y., Furukita, Y., Seike, J., and Tangoku, A. (2012). Triterpenes Augment the Inhibitory Effects of Anticancer Drugs on Growth of Human Esophageal Carcinoma Cells In Vitro and Suppress Experimental Metastasis In Vivo. Dis. Esophagus 25, 141A. doi:10.1111/j.1442-2050.2012.01405.x
Yamanaka, M., Kato, A., Moriyama, T., Okazaki, F., Momma, K., and Narita, H. (2019). Food-dependent Exercise-Induced Anaphylaxis Due to Pickled Japanese Apricot. Allergol. Int. 68, 524–526. doi:10.1016/j.alit.2019.02.009
Yan, X.-T., Lee, S.-H., Li, W., Jang, H.-D., and Kim, Y.-H. (2015). Terpenes and Sterols from the Fruits of Prunus Mume and Their Inhibitory Effects on Osteoclast Differentiation by Suppressing Tartrate-Resistant Acid Phosphatase Activity. Arch. Pharm. Res. 38, 186–192. doi:10.1007/s12272-014-0389-2
Yan, X.-T., Lee, S.-H., Li, W., Sun, Y.-N., Yang, S.-Y., Jang, H.-D., et al. (2014a). Evaluation of the Antioxidant and Anti-osteoporosis Activities of Chemical Constituents of the Fruits of Prunus Mume. Food Chem. 156, 408–415. doi:10.1016/j.foodchem.2014.01.078
Yan, X.-T., Li, W., Sun, Y.-N., Yang, S.-Y., Lee, S.-H., Chen, J.-B., et al. (2014b). Identification and Biological Evaluation of Flavonoids from the Fruits of Prunus Mume. Bioorg. Med. Chem. Lett. 24, 1397–1402. doi:10.1016/j.bmcl.2014.01.028
Yan, X.-T., Li, W., Sun, Y.-N., Yang, S. Y., Song, G. Y., and Kim, Y. H. (2014c). A New Furfural Diglycoside and Other Carbohydrate Derivatives from Fermented Beverage of Prunus Mume Fruit. Bull. Korean Chem. Soc. 35, 2162–2164. doi:10.5012/bkcs.2014.35.7.2162
Yanaki, M., Kobayashi, M., Aruga, A., Nomura, M., and Ozaki, M. (2018). In vivo antitumor Effects of MK615 Led by PD-L1 Downregulation. Integr. Cancer Ther. 17, 646–653. doi:10.1177/1534735418766403
Yi, L.-T., Li, J., Su, D.-X., Dong, J.-F., and Li, C.-F. (2012). Hypouricemic Effect of the Methanol Extract fromPrunus Mumefruit in Mice. Pharm. Biol. 50, 1423–1427. doi:10.3109/13880209.2012.683115
Yingsakmongkon, S., Miyamoto, D., Sriwilaijaroen, N., Fujita, K., Matsumoto, K., Jampangern, W., et al. (2008). In vitro inhibition of Human Influenza a Virus Infection by Fruit-Juice Concentrate of Japanese Plum (Prunus Mume SIEB. Et ZUCC). Biol. Pharm. Bull. 31, 511–515. doi:10.1248/bpb.31.511
Yoshikawa, M., Murakami, T., Ishiwada, T., Morikawa, T., Kagawa, M., Higashi, Y., et al. (2002). New Flavonol Oligoglycosides and Polyacylated Sucroses with Inhibitory Effects on Aldose Reductase and Platelet Aggregation from the Flowers ofPrunus Mume1. J. Nat. Prod. 65, 1151–1155. doi:10.1021/np020058m
Zhang, M., Long, Y., Sun, Y., Wang, Y., Li, Q., Wu, H., et al. (2011). Evidence for the Complementary and Synergistic Effects of the Three-Alkaloid Combination Regimen Containing Berberine, Hypaconitine and Skimmianine on the Ulcerative Colitis Rats Induced by Trinitrobenzene-Sulfonic Acid. Eur. J. Pharmacol. 651, 187–196. doi:10.1016/j.ejphar.2010.10.030
Zhang, Q. H., Zhang, L., Shang, L. X., Shao, C. L., and Wu, Y. X. (2008). [Studies on the Chemical Constituents of Flowers of Prunus Mume]. Zhong Yao Cai 31, 1666–1668.
Zhang, X., Lin, Z., Fang, J., Liu, M., Niu, Y., Chen, S., et al. (2015). An On-Line High-Performance Liquid Chromatography-Diode-Array Detector-Electrospray Ionization-Ion-Trap-Time-Of-Flight-Mass Spectrometry-Total Antioxidant Capacity Detection System Applying Two Antioxidant Methods for Activity Evaluation of the Edible Flowers from Prunus Mume. J. Chromatogr. A 1414, 88–102. doi:10.1016/j.chroma.2015.08.033
Zhao, C. L., Guo, W. M., and Chen, J. Y. (2006). Isolation and Structural Identification of the Anthocyanins from the Flower Color Pigment of Prunus Mume 'Nanjing Hongxu'. Lin Ye Ke Xue 42, 29–36. doi:10.3321/j.issn:1001-7488.2006.01.00610.3901/jme.2006.12.036
Keywords: Prunus mume, pharmacological activities, clinical application, functional mechanism, phytochemical constituents
Citation: Gong X-P, Tang Y, Song Y-Y, Du G and Li J (2021) Comprehensive Review of Phytochemical Constituents, Pharmacological Properties, and Clinical Applications of Prunus mume. Front. Pharmacol. 12:679378. doi: 10.3389/fphar.2021.679378
Received: 11 March 2021; Accepted: 04 May 2021;
Published: 28 May 2021.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Syed Babar Jamal, National University of Medical Sciences (NUMS), PakistanAnthony Bussiere, Stragen Pharma SA, Switzerland
Copyright © 2021 Gong, Tang, Song, Du and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Du, dGp5eGJAc2luYS5jb20=; Juan Li, bGlqdWFuQHRqaC50am11LmVkdS5jbg==
†These authors share first authorship
 Xue-Peng Gong
Xue-Peng Gong Ying Tang
Ying Tang Yuan-Yuan Song
Yuan-Yuan Song Guang Du1*
Guang Du1* Juan Li
Juan Li