- 1Department of Internal Medicine I, University Medical Center Schleswig-Holstein (UKSH), Kiel, Germany
- 2Institute of Diabetes and Clinical Metabolic Research, Kiel University and UKSH, Kiel, Germany
- 3Institute of Clinical Molecular Biology, Kiel University and UKSH, Kiel, Germany
- 4CONARIS Research Institute AG, Kiel, Germany
- 5Department of Cardiology and Angiology, Philipps-University, Marburg, Germany
- 6Department of Nuclear Medicine, Molecular Imaging Diagnostics and Therapy, UKSH, Kiel, Germany
- 7Department of Pathology, Otto-von-Guericke-University, Magdeburg, Germany
- 8Biochemical Institute, Kiel University, Kiel, Germany
Inflammation is a strong driver of atherosclerotic cardiovascular disease (ASCVD). There is a large unmet need for therapies that prevent or reduce excessive inflammation while avoiding systemic immunosuppression. We showed previously that selective inhibition of pro-inflammatory interleukin-6 (IL-6) trans-signalling by the fusion protein olamkicept (sgp130Fc) prevented and reduced experimental murine atherosclerosis in low-density lipoprotein receptor-deficient (Ldlr−/−) mice on a high-fat, high-cholesterol diet independently of low-density lipoprotein (LDL) cholesterol metabolism. Therefore, we allowed compassionate use of olamkicept (600 mg intravenously biweekly for 10 weeks) in a patient with very-high-risk ASCVD. Despite optimal LDL cholesterol under maximum tolerated lipid-lowering treatment, the patient had a remaining very high risk for future cardiovascular events related to significant arterial wall inflammation with lipoprotein (a) [Lp(a)]-cholesterol as the main contributor. 18Fluorodeoxyglucose positron emission tomography/computed tomography (18FDG PET/CT) measurements were performed before and after the treatment period. Olamkicept reduced arterial wall inflammation in this patient without interfering with lipoprotein metabolism. No clinical or laboratory side effects were observed during or after treatment with olamkicept. Our findings in this patient matched the results from our mechanistic study in Ldlr−/− mice, which were extended by additional analyses on vascular inflammation. Olamkicept may be a promising option for treating ASCVD independently of LDL cholesterol metabolism. A Phase II trial of olamkicept in ASCVD is currently being prepared.
Introduction
Anti-cytokine therapy is a promising option for treating ASCVD that is progressive despite lifestyle modification and optimizing plasma lipid levels (Schuett and Schieffer 2012; Ait-Oufella et al., 2019; Soehnlein and Libby 2021). The challenge in ASCVD is to diminish local, LDL cholesterol-driven, self-perpetuating metabolic inflammation in atherosclerotic plaques and, at the same time, to preserve systemic immune defense. The CANTOS trial investigated the anti-interleukin-1β (IL-1β) antibody canakinumab in established human inflammatory ASCVD and demonstrated the challenge of significant benefits through lowering the rate of recurrent cardiovascular events at the expense of a higher incidence of fatal infections (Ridker et al., 2017). Downstream of IL-1β, IL-6 signalling is centrally involved in atherogenesis (Libby 2017). IL-6 exerts its multiple functions through two main signalling pathways, which both require signal transduction by the transmembrane co-receptor gp130 (Scheller et al., 2014). In classic signalling, IL-6 uses the membrane-bound IL-6 receptor (IL-6R), which is mainly expressed by hepatocytes and some leukocyte subsets. In the trans-signalling pathway, circulating soluble IL-6R (sIL-6R) recruits IL-6 to form IL-6/sIL-6R complexes, which could activate the ubiquitously expressed gp130 on nearly any cell in the body (Garbers et al., 2018). However, such ubiquitous trans-signalling is physiologically prevented by an excess of soluble gp130 isoforms (sgp130) acting as a buffer in the blood (Zhou et al., 2020). While classic IL-6 signalling has many physiological and anti-infectious functions, excessive trans-signalling is seen in many chronic inflammatory conditions (Rose-John et al., 2017; Garbers et al., 2018). Specific trans-signalling inhibition instead of blocking IL-6 or its receptor has therefore been proposed to treat chronic inflammation without the negative effect of systemic immunosuppression (Rose-John et al., 2017; Garbers et al., 2018).
We developed the selective IL-6/sIL-6R complex trap sgp130Fc consisting of two sgp130 domains fused to the crystallisable fragment of human immunoglobulin G1 (Jostock et al., 2001). Olamkicept (optimized sgp130Fc) was efficacious in a large series of animal models (Rose-John et al., 2017; Garbers et al., 2018) and successfully passed Phase I trials without safety issues (EudraCT no.s 2012-005142-38 and 2013-004208-20). In a recently published Phase IIa trial in patients with inflammatory bowel diseases, the mechanism of action of olamkicept known from animal models of colitis was confirmed and olamkicept demonstrated clinical efficacy (Schreiber et al., 2021), which was recently confirmed in a larger Phase IIb trial in ulcerative colitis (NCT03235752). Patients with ASCVD show a dysregulated IL-6/sIL-6R/sgp130 system with decreased sgp130 levels (Schuett et al., 2012). Olamkicept specifically blocks IL-6 trans-signalling and expands the serum sgp130 buffer (Rose-John et al., 2017; Garbers et al., 2018). Our previous investigations in a standard murine atherosclerosis model (LDL receptor-deficient [Ldlr−/−] mice on a high-fat, high-cholesterol diet) showed both preventive and therapeutic efficacy of olamkicept, notably with a significant regression of established atherosclerotic lesion size (Schuett et al., 2012). Importantly, it has recently been shown in a rat myocardial infarction (MI) model that rat sgp130Fc but not an anti-IL-6-antibody attenuated neutrophil and macrophage infiltration into the myocardium, reduced infarct size, and preserved cardiac function 28 days after MI (George et al., 2021).
These data in rodents could lead to closing a therapeutic gap in humans. About 50% of ASCVD patients are left with an untreated residual inflammatory risk, a crucial part of the ASCVD treatment paradigm (Ridker et al., 2017), and atherosclerotic plaque burden. Thus, clinical cases with high inflammatory ASCVD and plaque burden remain untreated with licensed anti-ASCVD medical treatment. Crucial mediators of arterial wall inflammation are proinflammatory oxidized phospholipids carried by Lp(a) (van der Valk et al., 2016). Therefore, Lp(a) is an independent and causal risk factor for ASCVD (Tsimikas 2017; Stiekema et al., 2019), related to cardiovascular death, myocardial infarction, and stroke when high-sensitivity C-reactive protein (hsCRP) levels are 2 mg/L or more (Puri et al., 2020). Today, there is no medical option to treat Lp(a) and hsCRP in ASCVD patients (Tsimikas and Stroes 2020). Although anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) therapy can reduce Lp(a) by about 20–30%, the anti-PCSK9 antibody evolocumab did not influence arterial wall inflammation (Stiekema et al., 2019). Only Lp(a) apheresis seemed to lower Lp(a) and hsCRP levels, resulting in reduction of the atherosclerotic burden (Pokrovsky et al., 2017).
Case Description
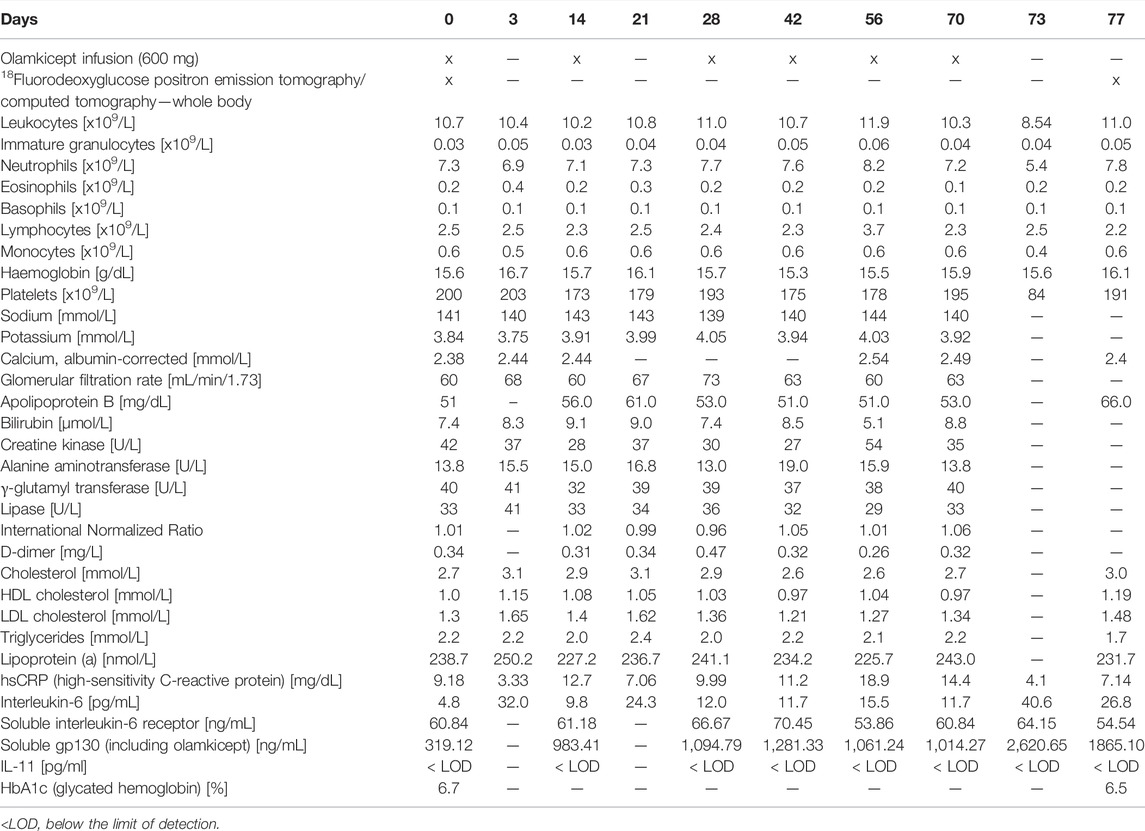
Due to the large unmet need for adding anti-cytokine treatments to lipid reduction therapies for ASCVD (Ridker 2017), we allowed compassionate use of olamkicept (600 mg intravenously biweekly for 10 weeks) in a patient with very-high-risk ASCVD (Mach et al., 2020), elevated Lp(a) and high inflammatory vascular risk (Ridker 2016) under maximum tolerated lipid-lowering treatment. The patient was a Caucasian female aged 64 years (body mass index: 37 kg/m2, blood pressure 135/90 mmHg), with very-high-risk ASCVD (ANA/ANCA-negative). She had a history of coronary artery disease and had previously undergone right carotid endarterectomy. The patient’s therapy consisted of evolocumab, aspirin, metoprolol, amlodipine, hydrochlorothiazide, candesartan, pantoprazole, vitamin D and a healthy lifestyle. Type 2 diabetes mellitus was controlled without medication as a choice of the patient at this stage. Non-alcoholic fatty liver disease (NAFLD) seemed unlikely in this patient due to low alanine aminotransferase (Ma et al., 2020) and non-detectable IL-11 serum levels (Dong et al., 2021) (Table 1) as well as the absence of any signs of NAFLD in the low dose CT. Despite maximum tolerated lipid-lowering treatment (evolocumab monotherapy because of statin-associated muscle symptoms and ezetimibe intolerance), the patient still had a remaining very high risk for future vascular events related to the advanced stage of ASCVD because of elevated hsCRP and Lp(a). The patient’s characteristics are detailed in Table 1. Olamkicept was administered open-label at the clinical trial dose—600 mg intravenously (i.v.) within 1 h, biweekly (Schreiber et al., 2021)—for 10 weeks (6 infusions) from January to March 2018 (Table 1; Figure 1). Olamkicept’s half-life is 4.7 days. The patient was monitored for infusion reactions for 3 h (first two infusions) or 1 h (subsequent infusions).
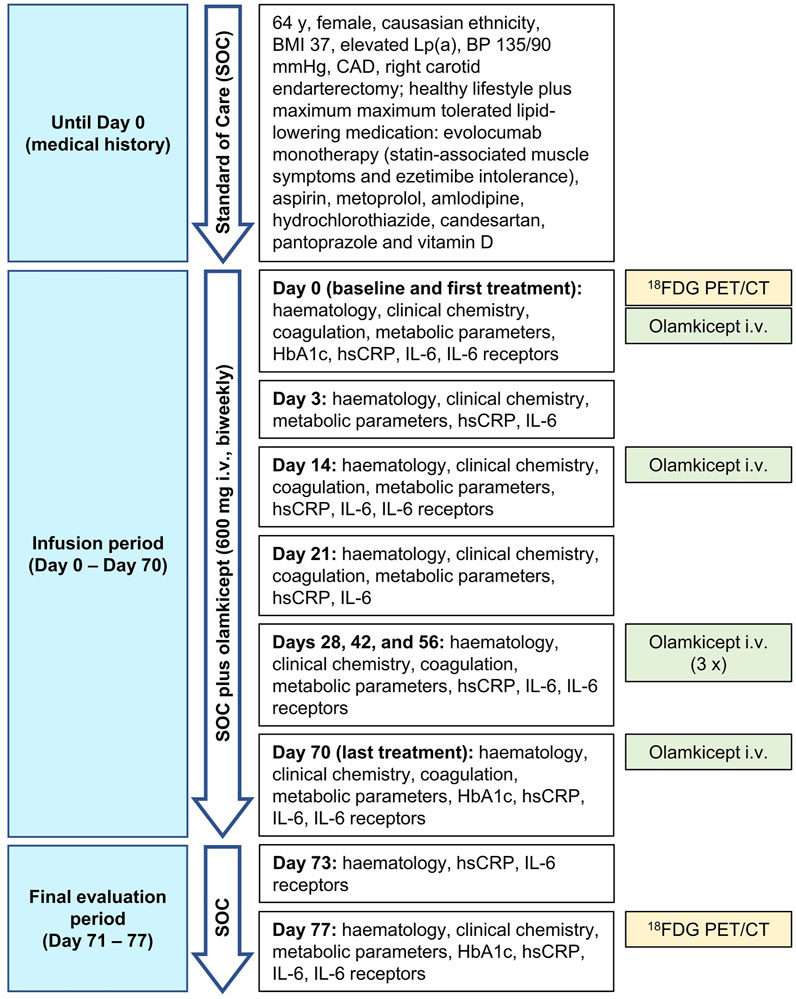
FIGURE 1. Case report timeline. BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; 18FDG PET/CT, 18Fluorodeoxyglucose positron emission tomography/computed tomography; HbA1c, glycated hemoglobin; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; i.v., intravenously.
For clinical assessment and non-invasive imaging, we used 18FDG PET/CT. In the patient, screening for inflammatory ASCVD consisted of a whole body 18FDG PET/CT examination. 18FDG PET/CT has shown great potential in visualizing, quantifying and characterizing atherosclerotic inflammation and plaque stability non-invasively, emerging as a suitable surrogate endpoint for clinical testing of novel anti-atherosclerotic therapeutics (Rudd et al., 2007; Rudd et al., 2008; Moreno Velasquez et al., 2015; Ali and Tawakol 2016; Hyafil and Vigne 2019). For example, arterial wall inflammation measured by 18FDG PET/CT decreased by testing statins (Tawakol et al., 2013), lipoprotein apheresis (van Wijk et al., 2014) and IL-1β inhibition (Hsue et al., 2018), whereas arterial wall inflammation persisted under anti-PCSK9 antibody treatment (Stiekema et al., 2019) and increased under glucocorticoid therapy (van der Valk et al., 2015). The target-to-background ratio (TBR) was calculated as described previously (van Wijk et al., 2014).
In this patient, 600 mg olamkicept administered i. v. biweekly over 10 weeks was safe. No clinical or laboratory side effects were observed during or after treatment (Table 1). Also from the patient’s perspective, the infusions were well tolerated. While sIL-6R levels remained unchanged, concentrations of serum IL-6 increased slightly, reflecting olamkicept’s additional sgp130 buffering capacity for IL-6/sIL-6R complexes (Table 1). Administration of olamkicept transiently decreased elevated high-sensitivity C-reactive protein (hsCRP) by 64% 3 days after infusion (Table 1). A missing long-lasting effect on hsCRP suppression may be explained by the pharmacokinetics of olamkicept and by the fact that CRP production is controlled by classic IL-6 signalling, which is not inhibited by olamkicept (Hoge et al., 2013; Garbers et al., 2018). Therefore, the reduction in hsCRP observed after olamkicept treatment does not represent a mechanistic reduction by blocking IL-6-mediated CRP production, but rather reflects a reduced inflammatory burden. As expected for selective inhibition of IL-6 trans-signalling, serum levels of total cholesterol, high-density lipoprotein (HDL) cholesterol, LDL cholesterol, triglycerides and Lp(a) did not show any clear trends or changes under olamkicept treatment (Table 1). This is in contrast to the common anabolic side effects (increased serum triglyceride and cholesterol levels as well as body weight) observed with anti-IL-6 or anti-IL-6R, which inhibit both classic and trans-signalling (Garbers et al., 2018).
We had previously shown that olamkicept reduced cellular infiltration of plaques by cells positive for the monocyte/macrophage marker MOMA-2 (mainly macrophages) early in the course of experimental atherosclerosis in mice (Schuett et al., 2012). Therefore, we compared 18FDG PET/CT images of arterial wall inflammation in the carotid arteries in this patient before and after compassionate use of olamkicept (6 biweekly infusions; Table 1; Figure 1). The density of plaque macrophages has been shown to correlate with the uptake of 18FDG measured by PET (Moreno Velasquez et al., 2015), and the resulting signal is expressed as mean and maximum target-to-background ratio (TBRmean and TBRmax). The patient presented with an atherosclerosis characterized by Lp(a)-cholesterol and hsCRP. The arterial wall inflammation detected by 18FDG PET/CT at baseline was strongly reduced after 3 months by 6 infusions of olamkicept (Figure 2A) without observing changes in lipoprotein distribution. This is in contrast to evolocumab treatment, where a large reduction in LDL cholesterol and a small reduction in Lp(a) levels did not lead to a decrease in arterial wall inflammation (Stiekema et al., 2019). Similar to the 18FDG PET/CT findings in this patient, olamkicept decreased the amount of MOMA-2-positive plaque macrophages in atherosclerotic lesions also in samples from mice with established experimental atherosclerosis which had been produced in our previous mechanistic trial (Schuett et al., 2012) but not yet been analysed in this regard (Figures 2B,C).
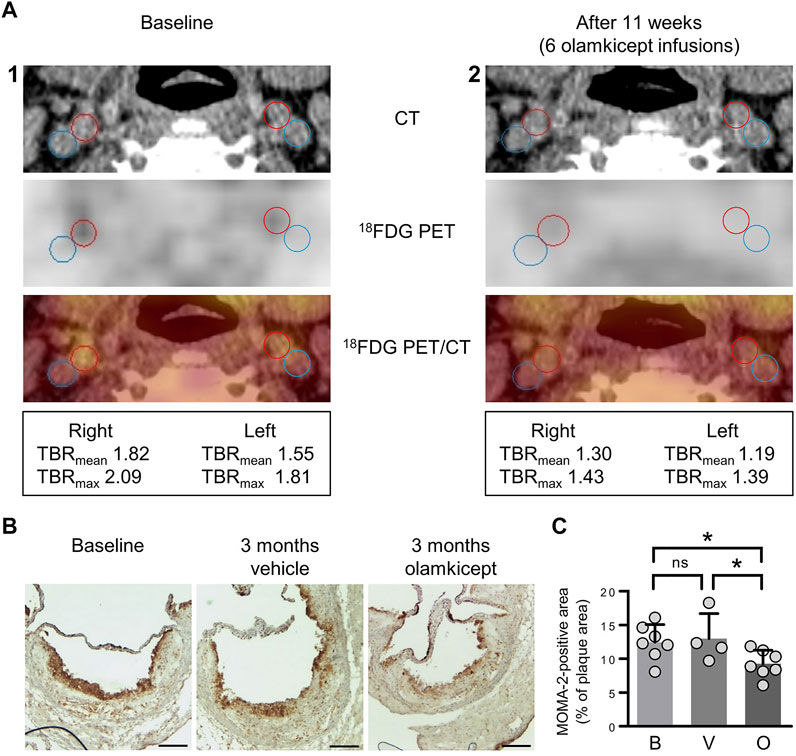
FIGURE 2. Inhibition of IL-6 trans-signalling reduces arterial wall inflammation and macrophage infiltration of atherosclerotic plaques in end-stage atherosclerosis. (A) Arterial wall inflammation in the carotid arteries of the patient (1) at baseline and (2) 11 weeks after the beginning of olamkicept treatment (6 infusions of 600 mg i. v. biweekly; Table 1). In the representative axial computed tomography (CT), 18fluorodeoxyglucose positron emission tomography (18FDG PET), and fused images (18FDG PET/CT), regions of interest are highlighted by red (artery) and blue circles (vein). Mean and maximum target-to-background ratio (TBRmean and TBRmax) are listed below. (B,C) New analyses of samples from a previous study in a murine model of experimental atherosclerosis (Schuett et al., 2012) (B) Representative MOMA-2-stained (macrophage-positive) lesions in aortic root sections (scale bars = 250 µm) of Ldlr−/− mice (n = 4 to n = 7 per group) after 12 weeks on a high-fat, high-cholesterol diet followed by another 12 weeks on the same diet combined with vehicle or olamkicept treatment (0.5 mg/kg intraperitoneally, twice weekly); (C) MOMA-2 levels expressed as percentage (mean, standard deviation) of the MOMA-positive (+) total plaque area at baseline (B) or after vehicle (V) or olamkicept treatment (O); *, p < 0.05; ns, not significant.
Taken together, the specific therapeutic inhibition of IL-6 trans-signalling in established atherosclerosis reduced local inflammatory activity and thus the atherosclerotic burden in a patient with very-high-risk ASCVD despite maximum tolerated lipid-lowering medical treatment. The patient has been regularly visiting our outpatient clinic until today (last visit in week 16, 2022). So far, no clinical progression of the very-high-risk ASCVD or any cardiovascular events occurred.
Discussion
ASCVD is primarily caused by an interplay of lipid metabolism disorder and inflammatory atherosclerosis (Ross 1999). Patients with very-high-risk ASCVD and high inflammatory load despite state-of-the-art medical treatment have a large unmet need for effective anti-inflammatory therapies. Specific inhibition of IL-1β can be beneficial in patients with inflammatory atherosclerosis (Ridker et al., 2017), in contrast to general immunosuppression (e.g., by methotrexate) (Ridker et al., 2019), which does not reduce cardiovascular events.
IL-6 is a pleiotropic cytokine produced in response to infection and tissue damage. Patients with ASCVD show increased levels of circulating IL-6, which are correlated with clinical activity (Ridker 2016). High IL-6 plasma levels are associated with a higher risk of future cardiovascular events (Kaptoge et al., 2014). In murine models, lifetime IL-6 deficiency was atheroprotective (Madan et al., 2008) and inhibition of IL-6R reduced atherosclerotic lesions (Akita et al., 2017). Selective IL-6 trans-signalling inhibition, but not global IL-6 blockade, is efficacious in ameliorating the consequences of MI in a rat model and preserving cardiac function (George et al., 2021). In human atherosclerosis, inhibition of IL-1β by canakinumab led to a significantly lower rate of recurrent cardiovascular events and lowered IL-6 levels. However, side effects due to the systemic immunosuppression by canakinumab led to an unfavourable risk/benefit ratio for the therapy of ASCVD (Ridker et al., 2017; Palmer and Vaccarezza 2019). These results are in line with the increased rate of opportunistic and severe infections that is observed with the anti-IL-6R antibody tocilizumab (Rose-John et al., 2017). Another potential limitation of complete IL-6 inhibition is the potential increase in triglycerides and LDL cholesterol (Garbers et al., 2018; Soehnlein and Libby 2021).
We have previously shown in experimental atherosclerosis in Ldlr–/– mice that inhibiting IL-6 trans-signalling by olamkicept can both prevent atherogenesis and reduce established atherosclerosis, which is a rare finding in a disease model (Schuett et al., 2012). Of note, we have now used samples from this study showing that olamkicept reduced plaque macrophage contents in a setting of established atherosclerosis. In our patient, histological validation of atherosclerotic plaque morphology was not available. In future clinical trials, coronary burden and atherosclerotic plaque characteristics shall be investigated by appropriate imaging techniques. Therefore, the planned placebo-controlled, randomized, double-blinded Phase II trial shall investigate not only the effects of olamkicept on inflammatory burden in an index vessel (carotid arteries or aorta, evaluated by 18FDG PET/CT), but also changes in intima-media thickness (IMT), plaque size and calcification in the carotid arteries or aorta (evaluated by 18-MHz ultrasound) as well as changes in the endothelial layer, IMT, plaque size, calcification and necrotic core in radial arteries (evaluated by 70-MHz high-resolution ultrasound).
Ziegler et al. recently demonstrated that the ratio between proinflammatory IL-6/sIL-6R and neutralized IL-6/sIL-6R/sgp130 complexes was associated with the risk of cardiovascular events, indicating that a deficit in trans-signalling buffer capacity may contribute to disease risk (Ziegler et al., 2019). This is in line with our findings that sgp130 levels are reduced in patients with coronary artery disease (Schuett et al., 2012). The slightly elevated IL-6 levels observed in our present patient mainly represent buffered IL-6 in IL-6/sIL-6R/olamkicept complexes (Garbers et al., 2018). Nevertheless, the anti-cytokine treatment olamkicept reduced arterial wall inflammation burden. It may be speculated whether a high hsCRP level would be a necessary biomarker for patient selection for a clinical trial of olamkicept in ASCVD or whether the proteins involved in IL-6 trans-signalling would be more suitable in this regard. In the planned Phase II trial, patients with CRP levels above 2 mg/dl shall be included.
The specificity and efficacy of olamkicept as a trans-signalling inhibitor was underlined by the absence of changes in general lipid profiling, especially of Lp(a) (Table 1). The data at hand from clinical trials (see Introduction) suggest to expect minimal side effects of olamkicept also in patients with very-high-risk ASCVD. In addition to high-dose i.v. induction treatment periods to quickly reduce cardiovascular inflammation and reduce plaques, also before planned cardiovascular interventions, this would also warrant permanent maintenance treatment scenarios—possibly with low-dose subcutaneous applications or more widely spaced high-dose i.v. administrations. As olamkicept does not directly inhibit the induction of acute phase proteins like CRP (Hoge et al., 2013), the decrease of hsCRP in the patient might even reflect the reduction of the systemic inflammatory state including the amelioration of inflammatory disease activity in the atherosclerotic lesions. Therefore, instead of moving upstream from IL-1β and IL-6 to identify novel targets for atheroprotection (Ridker 2016), future clinical studies should investigate specific inhibition of IL-6 trans-signalling by olamkicept. As many animal models (Garbers et al., 2018) and current human clinical data suggest that inhibition of trans-signalling does not lead to systemic immunosuppression, olamkicept might deliver a more attractive risk/benefit ratio than canakinumab for a cardiovascular benefit by directly targeting the inflammatory component of atherosclerosis independently of lipid profiles.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
This compassionate use of olamkicept in very-high-risk ASCVD at the Department of Internal Medicine I of the University Medical Center Schleswig-Holstein (UKSH) in Kiel, Germany, received full, unconditional approval by the clinical ethics consultancy of the UKSH. The patient was fully informed and provided written consent before starting the unapproved therapy as well as written consent to the publication of her anonymized data in a scientific journal. Mice were maintained in the Central Animal Facility at Hannover Medical School. All experiments were approved by the governmental animal ethics committee and performed according to the guidelines of the Federation of European Animal Science Associations.
Author Contributions
DMS, BSchu, JS, AKS, and MS treated the patient; MM and UL performed and interpreted imaging analyses; HS, A-KV, BSchi, and KG performed and interpreted supporting mechanistic animal experiments; CG and JL produced and interpreted molecular data; SR-J and GHW constructed olamkicept and interpreted data; SS, PR, and ML interpreted data; GHW, DMS, and SS wrote the paper, and all co-authors revised it critically for important intellectual content. All authors contributed to and approved of the final paper.
Funding
This work was supported by grants from the German Excellence Cluster “Inflammation at Interfaces” to DMS, PR, SR-J, and SS. The animal study was supported by a grant from the German Federal Ministry of Education and Research (01GU0711) to KG.
Conflict of Interest
GHW is employed by CONARIS Research Institute AG, a company that owns the intellectual property (IP) rights to olamkicept, and is an inventor of such IP. SR-J and SS are consultants to CONARIS and members of CONARIS’ supervisory board. SR-J is a direct shareholder of CONARIS. CONARIS’ IP regarding olamkicept has been licensed to Ferring Pharmaceuticals (Saint-Prex, CH). None of the other authors have any conflicts of interest, financial or otherwise, to disclose in connection to this work. In particular, the clinicians who decided on the compassionate use of olamkicept and treated the patient, had no conflict of interest. The results of the present work have been protected by a European patent application of Kiel University with the inventors DMS, GHW, ML, and SS.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Katharina Hartmann (Department of Internal Medicine I, UKSH, Campus Kiel) for her expert technical assistance.
References
Ait-Oufella, H., Libby, P., and Tedgui, A. (2019). Anticytokine Immune Therapy and Atherothrombotic Cardiovascular Risk. Arterioscler. Thromb. Vasc. Biol. 39, 1510–1519. doi:10.1161/ATVBAHA.119.311998
Akita, K., Isoda, K., Sato-Okabayashi, Y., Kadoguchi, T., Kitamura, K., Ohtomo, F., et al. (2017). An Interleukin-6 Receptor Antibody Suppresses Atherosclerosis in Atherogenic Mice. Front. Cardiovasc Med. 4, 84. doi:10.3389/fcvm.2017.00084
Ali, A., and Tawakol, A. (2016). FDG PET/CT Imaging of Carotid Atherosclerosis. Neuroimaging Clin. N. Am. 26, 45–54. doi:10.1016/j.nic.2015.09.004
Dong, J., Viswanathan, S., Adami, E., Singh, B. K., Chothani, S. P., Ng, B., et al. (2021). Hepatocyte-specific IL11 Cis-Signaling Drives Lipotoxicity and Underlies the Transition from NAFLD to NASH. Nat. Commun. 12, 66. doi:10.1038/s41467-020-20303-z
Garbers, C., Heink, S., Korn, T., and Rose-John, S. (2018). Interleukin-6: Designing Specific Therapeutics for a Complex Cytokine. Nat. Rev. Drug Discov. 17, 395–412. doi:10.1038/nrd.2018.45
George, M. J., Jasmin, N. H., Cummings, V. T., Richard-Loendt, A., Launchbury, F., Woollard, K., et al. (2021). Selective Interleukin-6 Trans-signaling Blockade Is More Effective Than Panantagonism in Reperfused Myocardial Infarction. JACC Basic Transl. Sci. 6, 431–443. doi:10.1016/j.jacbts.2021.01.013
Hoge, J., Yan, I., Jänner, N., Schumacher, V., Chalaris, A., Steinmetz, O. M., et al. (2013). IL-6 Controls the Innate Immune Response against Listeria Monocytogenes via Classical IL-6 Signaling. J. Immunol. 190, 703–711. doi:10.4049/jimmunol.1201044
Hsue, P. Y., Li, D., Ma, Y., Ishai, A., Manion, M., Nahrendorf, M., et al. (2018). IL-1β Inhibition Reduces Atherosclerotic Inflammation in HIV Infection. J. Am. Coll. Cardiol. 72, 2809–2811. doi:10.1016/j.jacc.2018.09.038
Hyafil, F., and Vigne, J. (2019). Imaging Inflammation in Atherosclerotic Plaques: Just Make it Easy! J. Nucl. Cardiol. 26, 1705–1708. doi:10.1007/s12350-018-1289-5
Jostock, T., Müllberg, J., Ozbek, S., Atreya, R., Blinn, G., Voltz, N., et al. (2001). Soluble Gp130 Is the Natural Inhibitor of Soluble Interleukin-6 Receptor Transsignaling Responses. Eur. J. Biochem. 268, 160–167. doi:10.1046/j.1432-1327.2001.01867.x
Kaptoge, S., Seshasai, S. R., Gao, P., Freitag, D. F., Butterworth, A. S., Borglykke, A., et al. (2014). Inflammatory Cytokines and Risk of Coronary Heart Disease: New Prospective Study and Updated Meta-Analysis. Eur. Heart J. 35, 578–589. doi:10.1093/eurheartj/eht367
Libby, P. (2017). Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 70, 2278–2289. doi:10.1016/j.jacc.2017.09.028
Ma, X., Liu, S., Zhang, J., Dong, M., Wang, Y., Wang, M., et al. (2020). Proportion of NAFLD Patients with Normal ALT Value in Overall NAFLD Patients: a Systematic Review and Meta-Analysis. BMC Gastroenterol. 20, 10. doi:10.1186/s12876-020-1165-z
Mach, F., Baigent, C., Catapano, A. L., Koskinas, K. C., Casula, M., Badimon, L., et al. (2020). 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 41, 111–188. doi:10.1093/eurheartj/ehz455
Madan, M., Bishayi, B., Hoge, M., and Amar, S. (2008). Atheroprotective Role of Interleukin-6 in Diet- And/or Pathogen-Associated Atherosclerosis Using an ApoE Heterozygote Murine Model. Atherosclerosis 197, 504–514. doi:10.1016/j.atherosclerosis.2007.02.023
Moreno Velásquez, I., Golabkesh, Z., Källberg, H., Leander, K., de Faire, U., and Gigante, B. (2015). Circulating Levels of Interleukin 6 Soluble Receptor and its Natural Antagonist, Sgp130, and the Risk of Myocardial Infarction. Atherosclerosis 240, 477–481. doi:10.1016/j.atherosclerosis.2015.04.014
Palmer, R. D., and Vaccarezza, M. (2019). New Promises and Challenges on Inflammation and Atherosclerosis: Insights From CANTOS and CIRT Trials. Front. Cardiovasc Med. 6, 90. doi:10.3389/fcvm.2019.00090
Pokrovsky, S. N., Afanasieva, O. I., Safarova, M. S., Balakhonova, T. V., Matchin, Y. G., Adamova, I. Y. U., et al. (2017). Specific Lp(a) Apheresis: A Tool to Prove Lipoprotein(a) Atherogenicity. Atheroscler. Suppl. 30, 166–173. doi:10.1016/j.atherosclerosissup.2017.05.004
Puri, R., Nissen, S. E., Arsenault, B. J., St John, J., Riesmeyer, J. S., Ruotolo, G., et al. (2020). Effect of C-Reactive Protein on Lipoprotein(a)-Associated Cardiovascular Risk in Optimally Treated Patients With High-Risk Vascular Disease: A Prespecified Secondary Analysis of the ACCELERATE Trial. JAMA Cardiol. 5, 1136. doi:10.1001/jamacardio.2020.2413
Ridker, P. M., Everett, B. M., Pradhan, A., MacFadyen, J. G., Solomon, D. H., Zaharris, E., et al. (2019). Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 380, 752–762. doi:10.1056/NEJMoa1809798
Ridker, P. M., Everett, B. M., Thuren, T., MacFadyen, J. G., Chang, W. H., Ballantyne, C., et al. (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 377, 1119–1131. doi:10.1056/NEJMoa1707914
Ridker, P. M. (2016). From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 118, 145–156. doi:10.1161/CIRCRESAHA.115.306656
Ridker, P. M. (2017). How Common Is Residual Inflammatory Risk? Circ. Res. 120, 617–619. doi:10.1161/CIRCRESAHA.116.310527
Rose-John, S., Winthrop, K., and Calabrese, L. (2017). The Role of IL-6 in Host Defence against Infections: Immunobiology and Clinical Implications. Nat. Rev. Rheumatol. 13, 399–409. doi:10.1038/nrrheum.2017.83
Ross, R. (1999). Atherosclerosis--an Inflammatory Disease. N. Engl. J. Med. 340, 115–126. doi:10.1056/NEJM199901143400207
Rudd, J. H., Myers, K. S., Bansilal, S., Machac, J., Pinto, C. A., Tong, C., et al. (2008). Atherosclerosis Inflammation Imaging with 18F-FDG PET: Carotid, Iliac, and Femoral Uptake Reproducibility, Quantification Methods, and Recommendations. J. Nucl. Med. 49, 871–878. doi:10.2967/jnumed.107.050294
Rudd, J. H., Myers, K. S., Bansilal, S., Machac, J., Rafique, A., Farkouh, M., et al. (2007). (18)Fluorodeoxyglucose Positron Emission Tomography Imaging of Atherosclerotic Plaque Inflammation Is Highly Reproducible: Implications for Atherosclerosis Therapy trialsFluorodeoxyglucose Positron Emission Tomography Imaging of Atherosclerotic Plaque Inflammation Is Highly Reproducible: Implications for Atherosclerosis Therapy Trials. J. Am. Coll. Cardiol. 50, 892–896. doi:10.1016/j.jacc.2007.05.024
Scheller, J., Garbers, C., and Rose-John, S. (2014). Interleukin-6: from Basic Biology to Selective Blockade of Pro-inflammatory Activities. Semin. Immunol. 26, 2–12. doi:10.1016/j.smim.2013.11.002
Schreiber, S., Aden, K., Bernardes, J. P., Conrad, C., Tran, F., Höper, H., et al. (2021). Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease. Gastroenterology 160, 2354. doi:10.1053/j.gastro.2021.02.062
Schuett, H., Oestreich, R., Waetzig, G. H., Annema, W., Luchtefeld, M., Hillmer, A., et al. (2012). Transsignaling of Interleukin-6 Crucially Contributes to Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 32, 281–290. doi:10.1161/ATVBAHA.111.229435
Schuett, H., and Schieffer, B. (2012). Targeting Cytokine Signaling as an Innovative Therapeutic Approach for the Prevention of Atherosclerotic Plaque Development. Curr. Atheroscler. Rep. 14, 187–189. doi:10.1007/s11883-012-0246-z
Soehnlein, O., and Libby, P. (2021). Targeting Inflammation in Atherosclerosis - from Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 20, 589–610. doi:10.1038/s41573-021-00198-1
Stiekema, L. C. A., Stroes, E. S. G., Verweij, S. L., Kassahun, H., Chen, L., Wasserman, S. M., et al. (2019). Persistent Arterial Wall Inflammation in Patients with Elevated Lipoprotein(a) Despite Strong Low-Density Lipoprotein Cholesterol Reduction by Proprotein Convertase Subtilisin/kexin Type 9 Antibody Treatment. Eur. Heart J. 40, 2775–2781. doi:10.1093/eurheartj/ehy862
Tawakol, A., Fayad, Z. A., Mogg, R., Alon, A., Klimas, M. T., Dansky, H., et al. (2013). Intensification of Statin Therapy Results in a Rapid Reduction in Atherosclerotic Inflammation: Results of a Multicenter Fluorodeoxyglucose-Positron Emission Tomography/computed Tomography Feasibility Study. J. Am. Coll. Cardiol. 62, 909–917. doi:10.1016/j.jacc.2013.04.066
Tsimikas, S. (2017). A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 69, 692–711. doi:10.1016/j.jacc.2016.11.042
Tsimikas, S., and Stroes, E. S. G. (2020). The Dedicated "Lp(a) Clinic": A Concept Whose Time Has Arrived? Atherosclerosis 300, 1–9. doi:10.1016/j.atherosclerosis.2020.03.003
van der Valk, F. M., Bekkering, S., Kroon, J., Yeang, C., Van den Bossche, J., van Buul, J. D., et al. (2016). Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation 134, 611–624. doi:10.1161/CIRCULATIONAHA.116.020838
van der Valk, F. M., van Wijk, D. F., Lobatto, M. E., Verberne, H. J., Storm, G., Willems, M. C., et al. (2015). Prednisolone-containing Liposomes Accumulate in Human Atherosclerotic Macrophages upon Intravenous Administration. Nanomedicine 11, 1039–1046. doi:10.1016/j.nano.2015.02.021
van Wijk, D. F., Sjouke, B., Figueroa, A., Emami, H., van der Valk, F. M., MacNabb, M. H., et al. (2014). Nonpharmacological Lipoprotein Apheresis Reduces Arterial Inflammation in Familial Hypercholesterolemia. J. Am. Coll. Cardiol. 64, 1418–1426. doi:10.1016/j.jacc.2014.01.088
Zhou, M., Dai, W., Cui, Y., Liu, H., and Li, Y. (2020). Associations between the IL-6-neutralizing sIL-6r-Sgp130 Buffer System and Coronary Artery Disease in Postmenopausal Women. Ann. Transl. Med. 8, 379. doi:10.21037/atm.2020.02.27
Keywords: atherosclerosis, case report, interleukin-6, olamkicept, sgp130Fc
Citation: Schulte DM, Waetzig GH, Schuett H, Marx M, Schulte B, Garbers C, Lokau J, Vlacil A-K, Schulz J, Seoudy AK, Schieffer B, Rosenstiel P, Seeger M, Laudes M, Rose-John S, Lützen U, Grote K and Schreiber S (2022) Case Report: Arterial Wall Inflammation in Atherosclerotic Cardiovascular Disease is Reduced by Olamkicept (sgp130Fc). Front. Pharmacol. 13:758233. doi: 10.3389/fphar.2022.758233
Received: 13 August 2021; Accepted: 09 May 2022;
Published: 09 June 2022.
Edited by:
Ali H. Eid, Qatar University, QatarReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalyYvonne Baumer, National Heart, Lung, and Blood Institute (NIH), United States
Jürgen Scheller, Heinrich Heine University of Düsseldorf, Germany
Copyright © 2022 Schulte, Waetzig, Schuett, Marx, Schulte, Garbers, Lokau, Vlacil, Schulz, Seoudy, Schieffer, Rosenstiel, Seeger, Laudes, Rose-John, Lützen, Grote and Schreiber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Schreiber, cy5zY2hyZWliZXJAbXVjb3NhLmRl
†These authors have contributed equally to this work and share first authorship
 Dominik M. Schulte
Dominik M. Schulte Georg H. Waetzig
Georg H. Waetzig Harald Schuett5
Harald Schuett5 Christoph Garbers
Christoph Garbers Juliane Lokau
Juliane Lokau Ann-Kathrin Vlacil
Ann-Kathrin Vlacil Bernhard Schieffer
Bernhard Schieffer Philip Rosenstiel
Philip Rosenstiel Matthias Laudes
Matthias Laudes Stefan Rose-John
Stefan Rose-John Stefan Schreiber
Stefan Schreiber