- Department of Pulmonary, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
Background: Anlotinib is a novel anti-angiogenesis drug. In non-small cell lung cancer (NSCLC), high body mass index (BMI) was not associated with worse survival in patients treated with bevacizumab compared with those with normal or low BMI. However, it remains unknown whether such an association still exists in NSCLC patients receiving anlotinib therapy. Hence, we conducted this study to investigate whether BMI is associated with clinical outcomes in patients treated with anlotinib for advanced NSCLC.
Methods: Data of 554 patients from the ALTER-0302 and the ALTER-0303 trials were analyzed in this study. The patients were classified into non-obesity (BMI <28 kg/m2) and obesity (BMI ≥28 kg/m2) subgroups. The primary endpoint was overall survival (OS). The secondary endpoints included progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR). OS was defined as the interval between the first drug administration and death. PFS was defined as the time span from the date of initiating the treatment to the first documented progression or death from any cause, whichever occurred first. ORR included complete response (CR) and partial response (PR).
Results: There were 354 patients (63.9%) who received anlotinib in this study. Restricted cubic spline model showed a U-shaped relation between BMI and the risk of death in the anlotinib group. In a multivariable Cox regression model, a trend of worse overall survival was observed in obese patients who received anlotinib compared with placebo (HR, 2.33; 95% CI, 0.77–7.06; p = 0.136). The interaction between BMI stratification and treatment was significant for OS (P for interaction = 0.038).
Conclusion: Our results revealed a U-shaped relationship between BMI and risk of death in patients receiving anlotinib for advanced NSCLC. More importantly, obesity (BMI ≥28 kg/m2) might be a potential predictor of use of anlotinib in advanced NSCLC.
Background
Obesity is widely considered as a poor prognostic factor in cancer (Lennon et al., 2016). However, the relationship between obesity and favorable prognosis has been reported in patients with early or advanced lung cancer who received different treatment including surgery, chemotherapy, and radiotherapy (Dahlberg et al., 2013; Yap et al., 2018; Icard et al., 2020), which is called the “obesity paradox” (Zhang et al., 2017). This phenomenon was seen in lung cancer patients treated with tyrosine kinase inhibitor (TKI) before. A retrospective study has demonstrated that patients with a body mass index (BMI) ≥ 25 kg/m2 had longer progression-free survival (PFS) and overall survival (OS) compared with those with BMI <18.5 kg/m2 when they received epidermal growth factor receptor (EGFR-TKI) for non-small cell lung cancer (NSCLC). Moreover, BMI was an independent predictor of outcomes in this study (Park et al., 2016). In addition, a trend to prolonged PFS and OS was observed in advanced NSCLC patients with EGFR-TKI therapy and with higher body weight, though it was not significant (Imai et al., 2017).
As a novel TKI, anlotinib suppresses tumor proliferation by inhibiting tumor angiogenesis (Sun et al., 2016). Due to the results of the ALTER-0303 trial, anlotinib has been approved as a third-line or further therapy for advanced NSCLC in China (Zhou et al., 2019). Nevertheless, the objective response rate (ORR) of anlotinib was about 10% and adverse events occurred more frequently in patients treated with anlotinib than placebo (Han et al., 2018a). Therefore, great efforts have been made to find potential predictive biomarkers so that patients who are more likely to response to anlotinib could be identified earlier (Lu et al., 2019a; Lu et al., 2019b; Lu et al., 2019c). However, these predictors are relatively complex and expensive to use in clinical practice. Thus, researchers are trying to find simple and convenient predictive factors of anlotinib use.
BMI is a readily available index to measure weight. A previous meta-analysis has reported that no association is observed between obesity and worse clinical outcomes compared with normal or low BMI in patients treated with bevacizumab for advanced NSCLC (Shukla et al., 2021). However, it remains unknown whether there is a relationship between obesity and poor clinical outcomes in patients receiving anlotinib for NSCLC.
Therefore, we conducted this study to investigate whether BMI is associated with clinical outcomes in patients treated with anlotinib for advanced NSCLC.
Methods
Patients
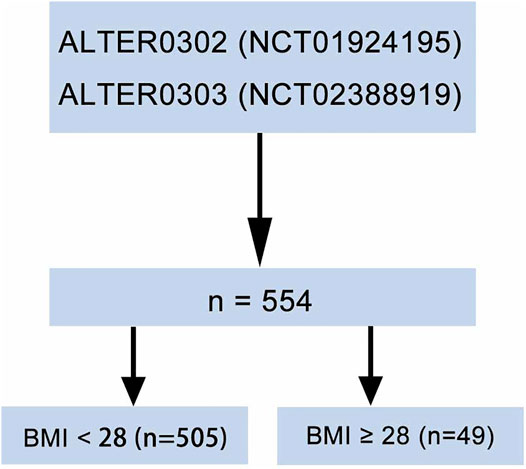
A total of 554 patients with evaluable data were obtained from the ALTER-0302 (Han et al., 2018a) and the ALTER-0303 (Han et al., 2018b) trials (Figure 1). The number of the ethical approval was LS1504. All the researchers have agreed on this study. These were multicenter, double-blind, randomized clinical trials that investigated the efficacy of anlotinib as a third-line or further treatment in patients with advanced NSCLC. Patients were randomly assigned to receive either anlotinib or placebo in a 1:1 ratio. Oral anlotinib was administrated with a dosage of 12 mg per day on Days 1–14 of a 21-d cycle. Informed consent was obtained from all patients in the two studies.
The trials were conducted according to the principles of the International Conference of Harmonisation Good Clinical Practice, the Declaration of Helsinki, and local institutional review board requirements.
Endpoints
The primary endpoint was OS. The secondary endpoints included PFS, ORR, and disease control rate (DCR). OS was measured from the date of randomization to the date of death from any cause or the last follow-up. PFS was defined as the time span between randomization to disease progression or mortality due to any cause, which occurred first. ORR was measured as the proportion of patients experiencing a complete response (CR) or a partial response (PR) as the best response to therapy. DCR consisted of CR, PR, and stable disease (SD). Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1.
Statistical Analysis
Associations between BMI and OS were flexibly modeled using restricted cubic spline (RCS) curves based on Cox proportional hazards models in anlotinib and placebo treatment groups. These analyses helped to define a reference BMI value. A reference value of 28 was chosen due to the BMI standard in China (China, 2004) and patients were subsequently stratified into non-obesity group (BMI <28 kg/m2) and obesity group (BMI ≥28 kg/m2). The differences of baseline characteristics between the two groups were examined using Pearson’s χ2 test. χ2 test was also used to compare ORR and DCR between non-obesity and obesity groups. The Kaplan-Meier method and log-rank test were used to analyze OS and PFS. The hazard ratios (HRs) of OS and PFS were estimated by utilizing the Cox proportional hazards model. Stratified Cox model containing variables of therapy, BMI, and treatment by BMI interaction were used to evaluate the p value for interaction. All p values were two sided and a p value < 0.05 was considered statistically significant. Data analysis was carried out using R, version 3.6.1 (R Project for Statistical Computing) and SPSS version 25.0 (IBM, Armonk, NY).
Results
Baseline Characteristics
A total of 554 patients were included in this study. In Figure 2, RCS was developed to smoothly model and display the relation between BMI and HR for OS. According to the BMI standard in China, 28 was chosen as a reference value. In patients receiving placebo, the RCS model showed a negative relation between BMI and the risk of mortality. High BMI was associated with low risk of death, which is in accordance with the obesity paradox. Regarding the relation between BMI and the risk of mortality in the anlotinib treatment group, a U-shaped association was seen. The risk of mortality decreased until around the BMI value of 28 and then turned to increase afterward. Above 28, the HRs of mortality related to BMI values elevated sharply.
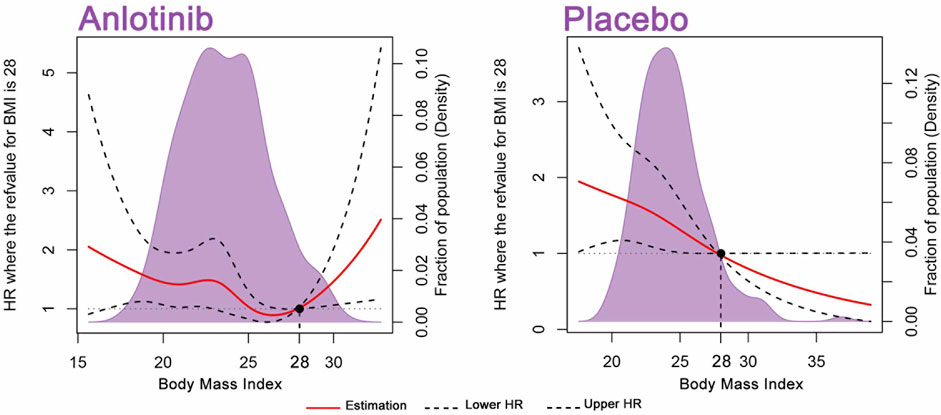
FIGURE 2. BMI on a continuous scale and the risk of mortality in 554 patients. Analyses were conducted using restricted cubic splines, with hazard ratios and 95% confidence intervals from multiple-event Cox proportional hazards regression. The BMI value of 28 was chosen as a reference. The purple areas indicate the distribution of concentrations of BMI. Body mass index (BMI).
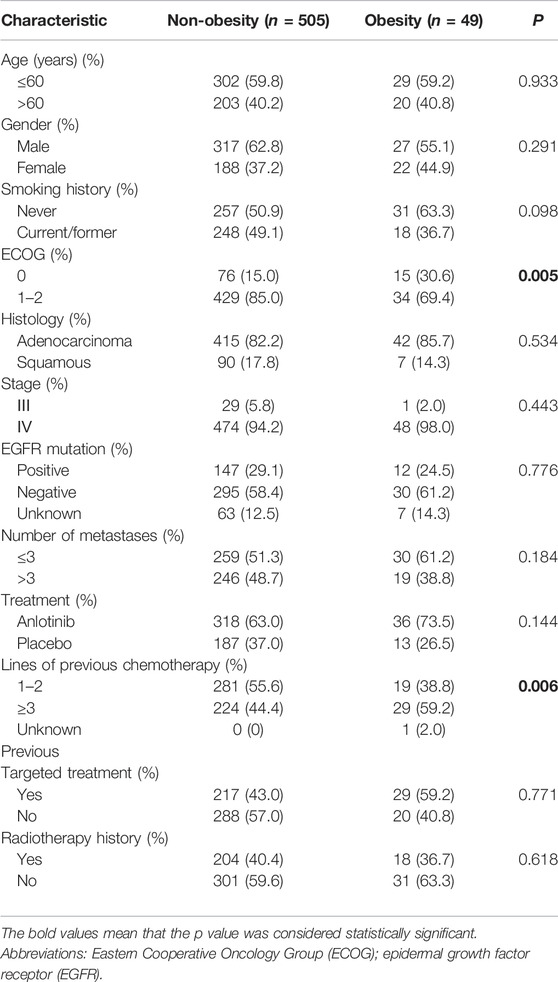
Due to the relationship between BMI and the risk of mortality depicted above, the 554 patients were classified into non-obesity (BMI <28 kg/m2) and obesity (BMI ≥28 kg/m2) subgroups. The mean values of BMI were 22.8 kg/m2 (15.6–27.9) and 30.0 kg/m2 (28.1–39.0) in the non-obese and obese groups, respectively. There were 49 patients (8.8%) in the obesity group. Baseline demographic and clinical characteristics were balanced between non-obesity and obesity subgroups except for Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) score and lines of previous chemotherapy. Obese patients had lower PS score (p = 0.005) and received more lines of previous chemotherapy (p = 0.006) (Table 1).
Primary and Secondary Outcomes
In the non-obesity group, multivariate analysis demonstrated that anlotinib therapy (p = 0.002), PS score of 0 (p = 0.005), EGFR mutation (p = 0.022), number of metastases ≤3 (p < 0.001), and less lines of previous chemotherapy (p = 0.013) were independent predictors of improved OS (Table 2).
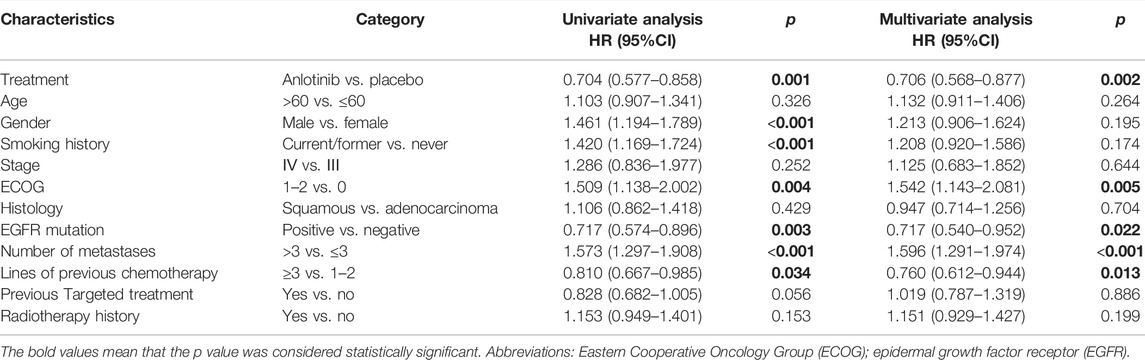
TABLE 2. Univariate and multivariable analyses for variables associated with OS in non-obesity (n = 505).
In the obesity group, only the number of metastases >3 (p = 0.033) was an independent factor of poor OS (Table 3).
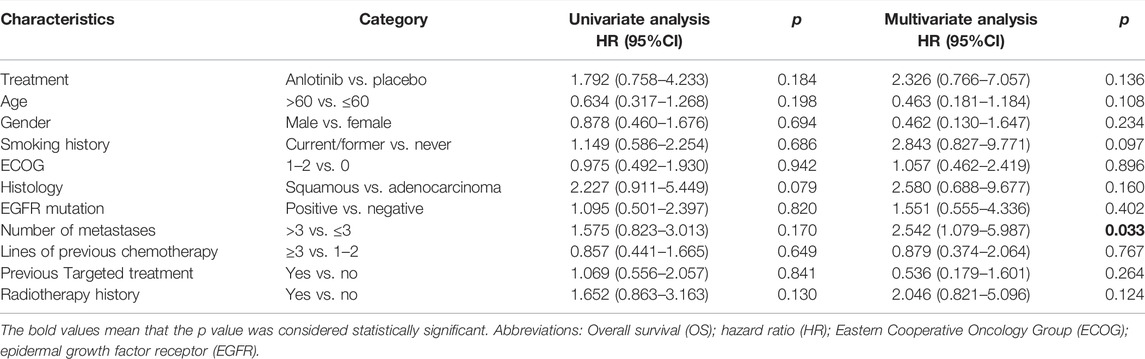
TABLE 3. Univariate and multivariable analyses for variables associated with OS in the obesity group (n = 49).
PFS was significantly longer in patients treated with anlotinib compared with placebo in both non-obesity (p < 0.001) and obesity (p = 0.025) groups. Anlotinib was also significantly associated with significantly higher DCR in the non-obesity (p < 0.001) and obesity (p = 0.046) groups. Moreover, anlotinib treatment resulted in significantly higher ORR in the non-obesity group (p < 0.001) (Supplementary Tables S1 and S2).
Obesity (BMI ≥28 kg/m2) Predicted Short OS in Patients Receiving Anlotinib
In the non-obesity group (BMI <28 kg/m2), patients treated with anlotinib had a significantly improved OS compared with placebo (adjusted hazard ratio [HR], 0.71; 95% CI, 0.57–0.88; p = 0.002), while a trend of poorer OS was observed in patients receiving anlotinib compared with placebo (HR, 2.33; 95% CI, 0.77–7.06; p = 0.136). The interaction between BMI classification and treatment was significant for OS (P for interaction = 0.038; Figure 3).
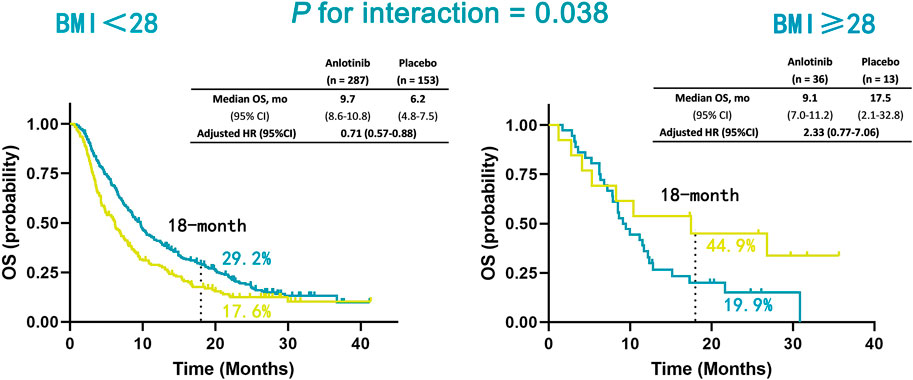
FIGURE 3. Kaplan-Meier estimates of OS in non-obesity (BMI <28 kg/m2) and obesity (BMI ≥28 kg/m2) patients in anlotinib and placebo treatment groups. Overall survival (OS); body mass index (BMI).
Discussion
In this study, a U-shaped association between BMI and risk of death was observed in patients receiving anlotinib for advanced NSCLC. An increased risk was observed in all BMI values equal to or above 28 kg/m2 for death. After classifying patients into non-obesity (BMI <28 kg/m2) and obesity (BMI ≥28 kg/m2) groups, we found that obesity was a predictor of worse efficacy of anlotinib in advanced NSCLC.
The deteriorated dyslipidemia caused by anlotinib might provide an explanation for the poor prognosis of the patients in the obesity group. The elimination half-life (t1/2) of anlotinib was considerably longer than other tyrosine kinase inhibitors (Li, 2021). Previous studies have reported that the decreased mRNA expression and activity of the main metabolic enzymes of anlotinib are found in obese individuals (Morrish et al., 2011; Zhong et al., 2018). Moreover, a significant inductive effect was not observed on the metabolic enzymes of anlotinib (Sun et al., 2017). Thus, obesity might lead to the accumulation of anlotinib, which might cause increased incidence of adverse events (AEs). Dyslipidemia is a common complication of obesity and one of the AEs of anlotinib (Han et al., 2018a; Vekic et al., 2019). An association between elevated pretreatment blood lipid and poor prognosis was observed in patients receiving anlotinib for advanced NSCLC (Poirier et al., 2006; Lijnen et al., 2007; Wells et al., 2012; Ebadi and Mazurak, 2014; Jang et al., 2016; Tang et al., 2021). Therefore, we supposed that the administration of anlotinib might cause worsened dyslipidemia and lead to a decreased survival time of the obese patients.
To our knowledge, this is the first time when a U-shaped relationship was found between BMI and efficacy of an anti-angiogenesis drug. Previous studies have reported that obesity is associated with improved OS in patients with lung cancer (Leung et al., 2011; Chen et al., 2021). In accordance with the conclusion, in our study, obese patients receiving a placebo have a median longer OS than the other three groups. Such a result might indicate that obese patients with lung cancer have an increased OS than those with a normal or low BMI. Nevertheless, the administration of anlotinib might lead to an inferior prognosis in this population. Thus, careful consideration should be given to the issue of whether obese patients need to receive anlotinib treatment for refractory NSCLC. However, to our best knowledge, this is the first time a U-shaped relationship was reported between BMI and effectiveness of anlotinib in advanced NSCLC patients. What is more, the sample size in the obese group was quite small in our study. Therefore, the finding must be interpreted with caution.
Although the study about the relationship between obesity and the effectiveness of anlotinib was still absent, the association of BMI and the efficacy of bevacizumab, another anti-angiogenesis drug, has been investigated before. Previous studies have suggested that elevated BMI was associated with poor prognosis in patients receiving bevacizumab for advanced CRC (Faruk Aykan et al., 2013) and epithelial ovarian cancer (Slaughter et al., 2014), whereas the association of increased BMI with improved OS was observed in advanced CRC patients treated with chemotherapy rather than those with the combination of chemotherapy and bevacizumab (Simkens et al., 2011). Hence, it is still controversial whether and how high BMI influences the prognosis of patients treated with anti-angiogenesis drugs for different cancers. Further investigation is clearly warranted.
Our result demonstrated that BMI was an independent predictor of survival for advanced NSCLC patients treated with anlotinib. Anlotinib is an inhibitor targeting multiple molecules included in tumor progression, which has been approved in NSCLC patients having disease progression after two or more lines of chemotherapy (Zhou et al., 2019). Compared with other potential predictors (Lu et al., 2019a; Lu et al., 2019b; Lu et al., 2019c), BMI is more available, convenient, and with acknowledged classification. Thus, BMI might be a promising predictive factor for anlotinib treatment.
There are some limitations in our study. First, the study was limited by its retrospective nature. A previous study has demonstrated that weight change during treatment provided more important information than a simple comparison of pre-treated BMI (Patel et al., 2016). Nevertheless, post-treatment BMI values were not available in our study. Second, the sample size of our study was relatively small, which might cause the considerable difference of the patient numbers in the non-obesity group and the obesity group. Thus, the result must be interpreted with caution. Third, it is controversial whether BMI is an appropriate measure of obesity (Griggs and Sabel, 2008). The predictive role of other body composition measures has been reported in cancer risk, such as waist circumference, waist-to-hip ratio (Pischon et al., 2006), and visceral fat area (Guiu et al., 2010; Patel et al., 2015). Hence, our results need further validation in prospective studies based on a larger population.
Conclusion
Our findings revealed a U-shaped relationship between BMI and risk of death in patients receiving anlotinib for advanced NSCLC. More importantly, the interaction between BMI classification and treatment was significant for OS. Therefore, obesity (BMI ≥28 kg/m2) might be a poor predictor of the efficacy of anlotinib in NSCLC.
Clinical Practice Points
The relationship between obesity and favorable prognosis has been reported in lung cancer patients, which is called the “obesity paradox”. In non-small cell lung cancer (NSCLC) patients receiving epidermal growth factor receptor tyrosine kinase inhibitor (TKI), better prognosis has been observed in overweight and obese patients. As a novel TKI, anlotinib suppresses tumor proliferation by inhibiting tumor angiogenesis. Some studies have demonstrated that elevated body mass index (BMI) is associated with poor prognosis in metastatic colorectal cancer patients receiving anti-angiogenesis treatment. However, it remains unclear whether increased BMI is associated with poor efficacy of anlotinib in NSCLC. In this study, our results revealed a U-shaped relationship between BMI and risk of death in patients receiving anlotinib for NSCLC. More importantly, obesity (BMI ≥28 kg/m2) might be a poor predictor of the efficacy of anlotinib in NSCLC.
Abbreviations
BMI, body mass index; CR, complete response; DCR, disease control rate; ECOG, Eastern cooperative oncology group; EGFR-TKI, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; mCRC, metastatic colorectal cancer; PR, partial response; SD, stable disease; RCS, restricted cubic spline; HRs: hazard ratios; PS, performance status; VEGFR, vascular endothelial growth factor receptor.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: 10.1038/bjc. 2017.478 10.1001/jamaoncol. 2018.3039.
Author Contributions
Conceptualization, AX and WN; literature search, YS and FP; figures, HZ, RZ, and TC; data collection and analysis, JL, SW, and JX; writing—original draft, AX and LC; writing—review and editing, BH and XZ.
Funding
This study was financially supported in part by Shanghai Chest Hospital Project of Collaborative Innovation, Grant Number: YJXT20190102, National Natural Science Foundation of China, Grant Number: 81601988 and and Nurture projects for basic research of Shanghai Chest Hospital, Grant Number: 2021YNJCM02.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.812555/full#supplementary-material
References
Chen, Y. M., Lai, C. H., Lin, C. Y., Tsai, Y. H., Chang, Y. C., Chen, H. C., et al. (2021). Body Mass Index, Weight Loss, and Mortality Risk in Advanced-Stage Non-small Cell Lung Cancer Patients: A Focus on EGFR Mutation. Nutrients 13. doi:10.3390/nu13113761
China, W. G. o. O. i. (2004). Guidelines for the Prevention and Control of Overweight and Obesity in Chinese Adults (Excerpt). ACTA Nutrimenta SINICA 1-4.
Dahlberg, S. E., Schiller, J. H., Bonomi, P. B., Sandler, A. B., Brahmer, J. R., Ramalingam, S. S., et al. (2013). Body Mass index and its Association with Clinical Outcomes for Advanced Non-small-cell Lung Cancer Patients Enrolled on Eastern Cooperative Oncology Group Clinical Trials. J. Thorac. Oncol. 8, 1121–1127. doi:10.1097/JTO.0b013e31829cf942
Ebadi, M., and Mazurak, V. C. (2014). Evidence and Mechanisms of Fat Depletion in Cancer. Nutrients 6, 5280–5297. doi:10.3390/nu6115280
Faruk Aykan, N., Yildiz, I., Sen, F., Kilic, L., Keskin, S., Ciftci, R., et al. (2013). Effect of Increased Body Mass index (BMI) on Time to Tumour Progression (TTP) in Unresectable Metastatic Colorectal Cancer (mCRC) Patients Treated with Bevacizumab-Based Therapy. Med. Oncol. 30, 679. doi:10.1007/s12032-013-0679-4
Griggs, J. J., and Sabel, M. S. (2008). Obesity and Cancer Treatment: Weighing the Evidence. J. Clin. Oncol. 26, 4060–4062. doi:10.1200/JCO.2008.17.4250
Guiu, B., Petit, J. M., Bonnetain, F., Ladoire, S., Guiu, S., Cercueil, J. P., et al. (2010). Visceral Fat Area Is an Independent Predictive Biomarker of Outcome after First-Line Bevacizumab-Based Treatment in Metastatic Colorectal Cancer. Gut 59, 341–347. doi:10.1136/gut.2009.188946
Han, B., Li, K., Zhao, Y., Li, B., Cheng, Y., Zhou, J., et al. (2018). Anlotinib as a Third-Line Therapy in Patients with Refractory Advanced Non-small-cell Lung Cancer: a Multicentre, Randomised Phase II Trial (ALTER0302). Br. J. Cancer 118, 654–661. doi:10.1038/bjc.2017.478
Han, B., Li, K., Wang, Q., Zhang, L., Shi, J., Wang, Z., et al. (2018). Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients with Advanced Non-small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial.
Icard, P., Schussler, O., Loi, M., Bobbio, A., Lupo, A. M., Wislez, M., et al. (2020). Pre-Disease and Pre-surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage. Cancers (Basel) 12. doi:10.3390/cancers12020266
Imai, H., Kuwako, T., Kaira, K., Masuda, T., Miura, Y., Seki, K., et al. (2017). Evaluation of Gefitinib Efficacy According to Body Mass index, Body Surface Area, and Body Weight in Patients with EGFR-Mutated Advanced Non-small Cell Lung Cancer. Cancer Chemother. Pharmacol. 79, 497–505. doi:10.1007/s00280-016-3232-2
Jang, S., Zheng, C., Tsai, H. T., Fu, A. Z., Barac, A., Atkins, M. B., et al. (2016). Cardiovascular Toxicity after Antiangiogenic Therapy in Persons Older Than 65 Years with Advanced Renal Cell Carcinoma. Cancer 122, 124–130. doi:10.1002/cncr.29728
Lennon, H., Sperrin, M., Badrick, E., and Renehan, A. G. (2016). The Obesity Paradox in Cancer: a Review. Curr. Oncol. Rep. 18, 56. doi:10.1007/s11912-016-0539-4
Leung, C. C., Lam, T. H., Yew, W. W., Chan, W. M., Law, W. S., and Tam, C. M. (2011). Lower Lung Cancer Mortality in Obesity. Int. J. Epidemiol. 40, 174–182. doi:10.1093/ije/dyq134
Li, S. (2021). Anlotinib: A Novel Targeted Drug for Bone and Soft Tissue Sarcoma. Front. Oncol. 11, 664853. doi:10.3389/fonc.2021.664853
Lijnen, H. R., Van Hoef, B., Kemp, D., and Collen, D. (2007). Inhibition of Vascular Endothelial Growth Factor Receptor Tyrosine Kinases Impairs Adipose Tissue Development in Mouse Models of Obesity. Biochim. Biophys. Acta 1770, 1369–1373. doi:10.1016/j.bbagen.2007.06.001
Lu, J., Shi, Q., Zhang, L., Wu, J., Lou, Y., Qian, J., et al. (2019). Integrated Transcriptome Analysis Reveals KLK5 and L1CAM Predict Response to Anlotinib in NSCLC at 3rd Line. Front. Oncol. 9, 886. doi:10.3389/fonc.2019.00886
Lu, J., Zhong, H., Chu, T., Zhang, X., Li, R., Sun, J., et al. (2019). Role of Anlotinib-Induced CCL2 Decrease in Anti-angiogenesis and Response Prediction for Nonsmall Cell Lung Cancer Therapy. Eur. Respir. J. 53. doi:10.1183/13993003.01562-2018
Lu, J., Zhong, H., Wu, J., Chu, T., Zhang, L., Li, H., et al. (2019). Circulating DNA-Based Sequencing Guided Anlotinib Therapy in Non-small Cell Lung Cancer. Adv. Sci. (Weinh) 6, 1900721. doi:10.1002/advs.201900721
Morrish, G. A., Pai, M. P., and Green, B. (2011). The Effects of Obesity on Drug Pharmacokinetics in Humans. Expert Opin. Drug Metab. Toxicol. 7, 697–706. doi:10.1517/17425255.2011.570331
Park, S., Park, S., Lee, S. H., Suh, B., Keam, B., Kim, T. M., et al. (2016). Nutritional Status in the Era of Target Therapy: Poor Nutrition Is a Prognostic Factor in Non-small Cell Lung Cancer with Activating Epidermal Growth Factor Receptor Mutations. Korean J. Intern. Med. 31, 1140–1149. doi:10.3904/kjim.2015.062
Patel, G. S., Ullah, S., Beeke, C., Hakendorf, P., Padbury, R., Price, T. J., et al. (2015). Association of BMI with Overall Survival in Patients with mCRC Who Received Chemotherapy versus EGFR and VEGF-Targeted Therapies. Cancer Med. 4, 1461–1471. doi:10.1002/cam4.490
Patel, J. D., Pereira, J. R., Chen, J., Liu, J., Guba, S. C., John, W. J., et al. (2016). Relationship between Efficacy Outcomes and Weight Gain during Treatment of Advanced, Non-squamous, Non-small-cell Lung Cancer Patients. Ann. Oncol. 27, 1612–1619. doi:10.1093/annonc/mdw211
Pischon, T., Lahmann, P. H., Boeing, H., Friedenreich, C., Norat, T., Tjønneland, A., et al. (2006). Body Size and Risk of colon and Rectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 98, 920–931. doi:10.1093/jnci/djj246
Poirier, P., Giles, T. D., Bray, G. A., Hong, Y., Stern, J. S., Pi-Sunyer, F. X., et al. (2006). Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: an Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113, 898–918. doi:10.1161/CIRCULATIONAHA.106.171016
Shukla, S., Babcock, Z., Pizzi, L., and Brunetti, L. (2021). Impact of Body Mass index on Survival and Serious Adverse Events in Advanced Non-small Cell Lung Cancer Treated with Bevacizumab: a Meta-Analysis of Randomized Clinical Trials. Curr. Med. Res. Opin. 37, 811–817. doi:10.1080/03007995.2021.1900091
Simkens, L. H., Koopman, M., Mol, L., Veldhuis, G. J., Ten Bokkel Huinink, D., Muller, E. W., et al. (2011). Influence of Body Mass index on Outcome in Advanced Colorectal Cancer Patients Receiving Chemotherapy with or without Targeted Therapy. Eur. J. Cancer 47, 2560–2567. doi:10.1016/j.ejca.2011.06.038
Slaughter, K. N., Thai, T., Penaroza, S., Benbrook, D. M., Thavathiru, E., Ding, K., et al. (2014). Measurements of Adiposity as Clinical Biomarkers for First-Line Bevacizumab-Based Chemotherapy in Epithelial Ovarian Cancer. Gynecol. Oncol. 133, 11–15. doi:10.1016/j.ygyno.2014.01.031
Sun, H., Sun, X., Zhai, X., Guo, J., Liu, Y., Ying, J., et al. (2016). Body Mass index and Exon 19 Mutation as Factors Predicting the Therapeutic Efficacy of Gefitinib in Patients with Epidermal Growth Factor Receptor Mutation-Positive Non-small Cell Lung Cancer. Thorac. Cancer 7, 61–65. doi:10.1111/1759-7714.12275
Sun, W., Wang, Z., Chen, R., Huang, C., Sun, R., Hu, X., et al. (2017). Influences of Anlotinib on Cytochrome P450 Enzymes in Rats Using a Cocktail Method. Biomed. Res. Int. 2017, 3619723. doi:10.1155/2017/3619723
Tang, M., Song, C., Zhang, Y., Xu, X., Wang, C., Zhang, Z., et al. (2021). Levels of Pretreatment Blood Lipids Are Prognostic Factors in Advanced NSCLC Patients Treated with Anlotinib. Lipids Health Dis. 20, 165. doi:10.1186/s12944-021-01596-5
Vekic, J., Zeljkovic, A., Stefanovic, A., Jelic-Ivanovic, Z., and Spasojevic-Kalimanovska, V. (2019). Obesity and Dyslipidemia. Metabolism 92, 71–81. doi:10.1016/j.metabol.2018.11.005
Wells, S. A., Robinson, B. G., Gagel, R. F., Dralle, H., Fagin, J. A., Santoro, M., et al. (2012). Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: a Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. 30, 134–141. doi:10.1200/JCO.2011.35.5040
Yap, W. K., Shih, M. C., Kuo, C., Pai, P. C., Chou, W. C., Chang, K. P., et al. (2018). Development and Validation of a Nomogram for Assessing Survival in Patients with Metastatic Lung Cancer Referred for Radiotherapy for Bone Metastases. JAMA Netw. Open 1, e183242. doi:10.1001/jamanetworkopen.2018.3242
Zhang, X., Liu, Y., Shao, H., and Zheng, X. (2017). Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. J. Thorac. Oncol. 12, 1478–1488. doi:10.1016/j.jtho.2017.07.022
Zhong, C. C., Chen, F., Yang, J. L., Jia, W. W., Li, L., Cheng, C., et al. (2018). Pharmacokinetics and Disposition of Anlotinib, an Oral Tyrosine Kinase Inhibitor, in Experimental Animal Species. Acta Pharmacol. Sin 39, 1048–1063. doi:10.1038/aps.2017.199
Keywords: VEGFR-TKIs, body mass index, anti-angiogenesis, predictive biomarker, multi-targer drug
Citation: Xiong A, Nie W, Cheng L, Zhong H, Chu T, Zhong R, Lu J, Wang S, Xu J, Shen Y, Pan F, Han B and Zhang X (2022) Association Between Obesity and Poor Prognosis in Patients Receiving Anlotinib for Advanced Non-Small Cell Lung Cancer. Front. Pharmacol. 13:812555. doi: 10.3389/fphar.2022.812555
Received: 10 November 2021; Accepted: 22 February 2022;
Published: 30 March 2022.
Edited by:
Sonja M. Kessler, Martin Luther University of Halle-Wittenberg, GermanyCopyright © 2022 Xiong, Nie, Cheng, Zhong, Chu, Zhong, Lu, Wang, Xu, Shen, Pan, Han and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Nie, bmlld2VpLTEwMDFAMTYzLmNvbQ==; Baohui Han, MTg5MzA4NTgyMTZAMTYzLmNvbQ==; Xueyan Zhang, enh5Y2hlc3RAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Anning Xiong
Anning Xiong Wei Nie
Wei Nie Lei Cheng†
Lei Cheng† Hua Zhong
Hua Zhong Tianqing Chu
Tianqing Chu Runbo Zhong
Runbo Zhong Jun Lu
Jun Lu Shuyuan Wang
Shuyuan Wang Jianlin Xu
Jianlin Xu Yinchen Shen
Yinchen Shen Baohui Han
Baohui Han Xueyan Zhang
Xueyan Zhang