- 1Institute of Marine Biotechnology, Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia
- 2Department of Biomedical Sciences, Faculty of Medicine and Health sciences, University of Putra Malaysia, Seri Kembangan, Malaysia
Cancer is a multifactorial, multi-stage disease, including complex cascades of signaling pathways—the cell growth governed by dysregulated and abrupt cell division. Due to the complexity and multi-regulatory cancer progression, cancer is still a challenging disease to treat and survive. The screening of extracts and fractions from plants and marine species might lead to the discovery of more effective compounds for cancer therapeutics. The isolated compounds and reformed analogs were known as future prospective contenders for anti-cancer chemotherapy. For example, Taxol, a potent mitotic inhibitor discovered from Taxus brevifolia, suppresses cell growth and arrest, induces apoptosis, and inhibits proliferation. Similarly, marine sponges show remarkable tumor chemo preventive and chemotherapeutic potential. However, there is limited research to date. Several plants and marine-derived anti-cancer compounds having the property to induce apoptosis have been approved for clinical trials. The anti-cancer activity kills the cell and slows the growth of cancer cells. Among cell death mechanisms, apoptosis induction is a more profound mechanism of cell death triggered by naturally isolated anti-cancer agents. Evading apoptosis is the major hurdle in killing cancer cells, a mechanism mainly regulated as intrinsic and extrinsic. However, it is possible to modify the apoptosis-resistant phenotype of the cell by altering many of these mechanisms. Various extracts and fractions successfully induce apoptosis, cell-cycle modulation, apoptosis, and anti-proliferative activity. Therefore, there is a pressing need to develop new anti-cancer drugs of natural origins to reduce the effects on normal cells. Here, we’ve emphasized the most critical elements: i) A better understanding of cancer progression and development and its origins, ii) Molecular strategies to inhibit the cell proliferation/Carcino-genesis, iii) Critical regulators of cancer cell proliferation and development, iv) Signaling Pathways in Apoptosis: Potential Targets for targeted therapeutics, v) Why Apoptosis induction is mandatory for effective chemotherapy, vi) Plants extracts/fractions as potential apoptotic inducers, vii) Marine extracts as Apoptotic inducers, viii) Marine isolated Targeted compounds as Apoptotic inducers (FDA Approved/treatment Phase). This study provides a potential therapeutic option for cancer, although more clinical studies are needed to verify its efficacy in cancer chemotherapy.
1 Cancer initiation and development: understanding cancer progression
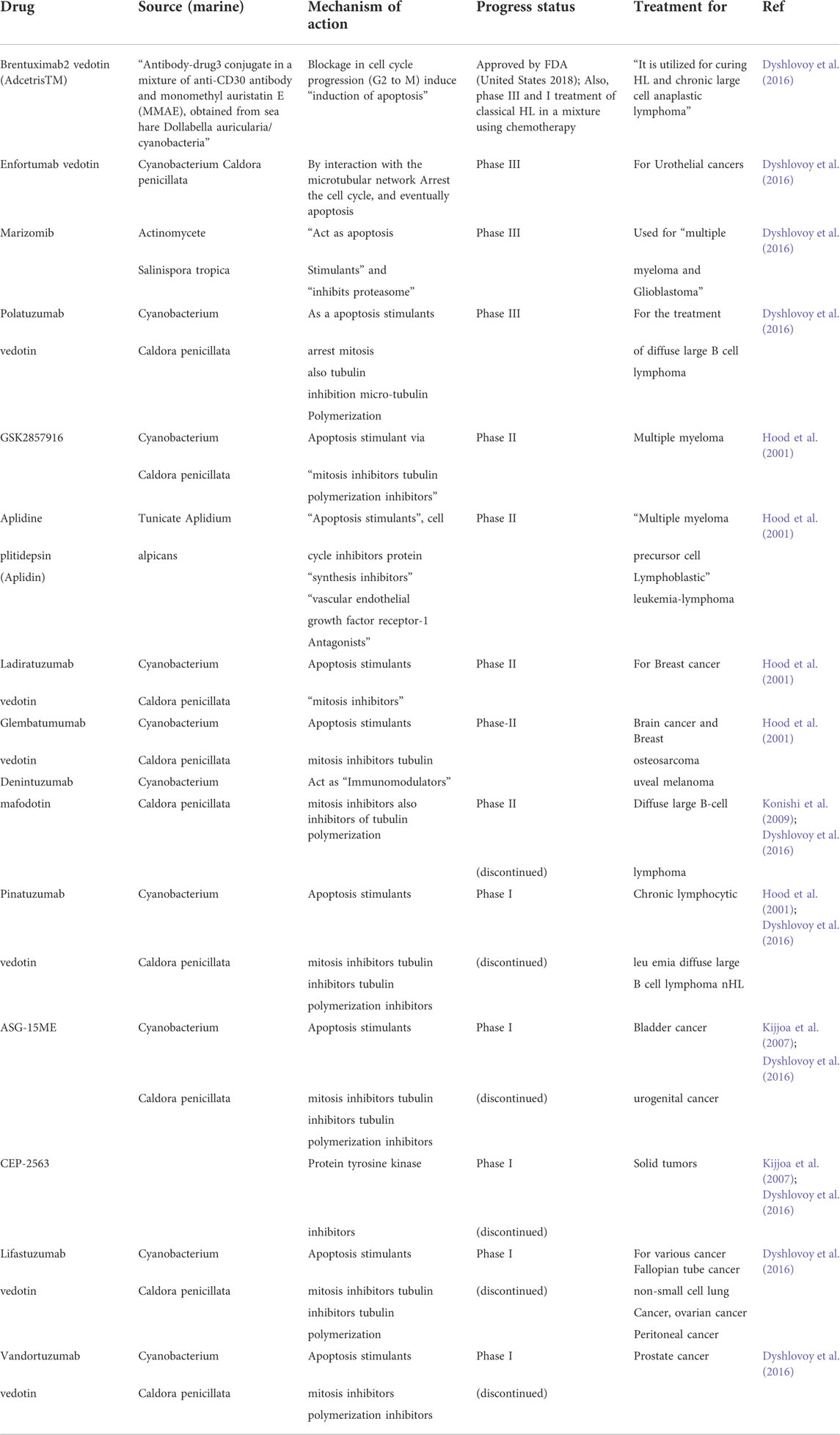
Cancer is the uncontrolled growth of specific cells possessed mutated genes or dys-regulatory cell division and growth. The word cancer means carcinoma, derived from a Greek word—cancer well-defined by the unregulated and uncontrolled multiplication of the cell. The specific alteration in genotype classified the normal cells as cancer cells responsible for uncontrol growth, re-location, and cancer metastasis (Sudhakar, 2009; Nithya et al., 2014; Fouad and Aanei, 2017). The cancer cell has a tremendous ability to divide and re-divide and metastatic abilities. The unchecked and over multiplication of cells leads to cancer tissue development and tends to move to various locations, resulting in metastasis development. In cancer, the different proteins involved in cell-cell attachment and regulation play a crucial role in regulating cancer cell movement. The matrix metalloproteases regulate the initiation and development of metastasis and enhancement of tumor spreading. The dysregulation in the expression of cell-cell attachment receptors fails cancer cell attachment. It encourages the ability to circulate in the bloodstream and re-locate to other locations inside the body—the abnormality in cell division, growth, and development results in cancer formation. The process of cancer development mainly comprises two fundamental alterations; i) genetical alternation and ii) Cellular alteration. The dys-regulatory genetic or cellular mechanism detected in cancer development. Genetically, the essential genes involved in the onset of cancer mainly comprised oncogenes and tumor suppression genes. The activation and deactivation of various “tumor suppressor genes” regulate the activation of cancer initiation and development. Furthermore, the variation in established genetic mechanisms involves genetic mutation, chromosomal abrasion or alteration, i.e., addition or deletion, and changes in cell cycling. The cellular alteration involves the dysregulated signaling pathways involved in the regulatory mechanism of dividing cells (Fearon and Vogelstein, 1990; Vogelstein and Kinzler, 2004). However, the hallmarks of cancer cells include: Selective proliferative advantage. i) Vascularization, immune modulation. ii) Metabolic rewiring. iii) Abetting microenvironment. iv) Tissue invasion/metastasis. v) Altered stress response. The cancer development process or carcinogenesis is mainly composed of diversified steps. Due to its rapidly dividing ability, the cell forms several cells or tumors and translocates to another site, leading to metastasis. Apart from general genetic and cellular alterations, epigenetic variations include; i) hypomethylation of oncogenes, ii) hypermethylation of tumor suppressor genes, iii) depletion of hydroxymethylation, iv) changes of histone acetylation, and v) methylation patterns and vi) miRNA expression level variations, are known to be associated with many cancers. Apoptosis is the primary mechanism that plays an essential role in balancing cells growth and cell division to prevent uncontrol cancer (Hassan et al., 2014). Carcinogenesis occurs due to damage, insult, or induction of alteration via various modes, including; physical, chemical, genetic, and biological. The cancer development and establishment process comprises cancer initiation, promotion, and progression (Figure 1). Various carcinogens act as initiators of cancer, which results in cancer formation along with promotor molecules. As in cancer initiation, most carcinogens cause irreversible damage to DNA. This damage causes the mutation in a particular genetic code that could be transferred to daughter cells and rapidly dividing cells with a mutated pattern. The cancer initiation trigger point could be an internal route or external via direct interaction with receptors on the cell surface or via various unspecified means. Thus, cancer initiation is based on irreversible genetic changes via the different genetic modes, including; i) simple mutations, small deletions, transitions, and transversions in DNA molecules. In the second stage of cancer development, there are no changes in the structure of DNA. The promotion of cancer is a reversible stage of advancement but rather in the expression of the genome mediated through promoter-receptor interactions. Interestingly, cancer progression depends on the protein involved in the migration of cancer. The extracellular environment (ECM) of cells is a pool of substances that could act like inducers that target the particular site and activate the cancer development or progression mechanisms. The last stage is characterized by cancer progression and migration, and karyotypic instability is considered an irreversible stage. However, the previous stage provides in-depth knowledge of molecular alteration, genetic changes in various tumor suppression genes, oncogenes, protooncogenes, and chromosomal aberrations (Faya et al., 2014). A better understanding of cancer etiology, initiation, and development of cancer has thoughtful consequences for targeting cancer biomarkers in developing therapeutics and considering agents/chemicals as carcinogens. In the cell death context, the fundamental process controlling the number of cells in tissue or organ development is apoptosis; however, in most cancers, the activation of oncogenes and dys-regulatory tumor suppressor genes occurs. The absence of apoptosis found in primary cancers makes the rapidly dividing cells unable to undergo the fundamental process of cell death.
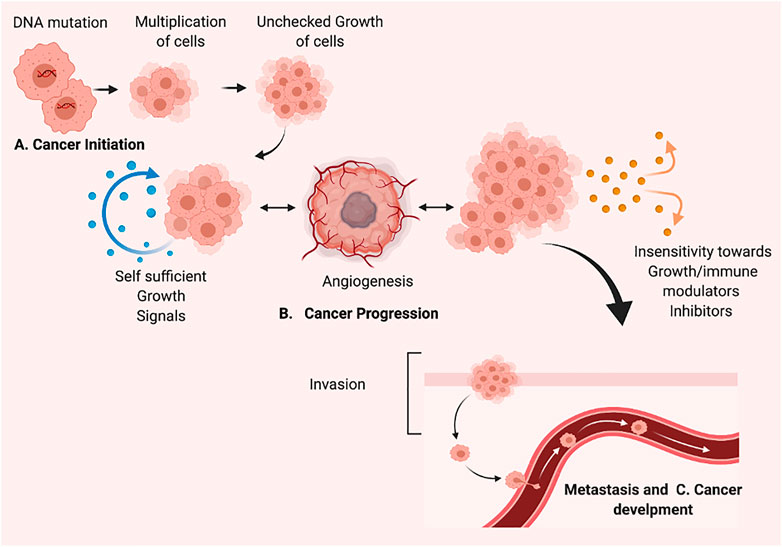
FIGURE 1. The process of cancer development/carcinogenesis, (A) Cancer initiation (B). Cancer Progression (C). Metastasis and Cancer development. Created with BioRender.com.
2 Molecular strategies to inhibit the cell proliferation/carcinogenesis
Various potential players play an active role in inhibiting cell proliferation. Here, we highlighted apoptosis regulating proteins/elements and other than apoptosis-inducing factors as a targeted strategy to regulate the process of carcinogenesis or cell proliferation. The apoptosis regulating strategy begins with the cell cycle—the regulators involved in cell division, cell arrest, and induction of apoptosis. The various genetic regulators of cell proliferation hold a remarkable role in cancer cell division and growth (as shown in Figure 2). The cell cycle plays a critical function in cellular genomic integrity and cell progression (Hanahan and Weinberg, 2000). It is important to know that the cell cycle comprises various stages such as the first one, G1 (gap 1), as well as the second, G2 (gap 2), and the last one, M (mitosis). For genetic information to be passed down across generations, DNA synthesis and genome replication must occur during the S phase. The M-phase is characterized by the separation of genetic information, the development of sister chromatids, and cell division. The G1 phase is the transition from M to S, while the G2 phase is the transition from S to M. These breaks (G1 and G2) are necessary to ensure that each phase is completed before moving on to the next. (Jacobson et al., 1997; Fulda et al., 2010). Cell cycle checkpoints are frequently activated in response to replication stress and DNA damage. Cycle-dependent kinase activation and inactivation are critical for cell growth and cell cycle regulation. (Hanahan and Weinberg, 2000; Fulda et al., 2010). In normal cell division, there is tight regulation at each checkpoint. Any dysregulation in those checkpoints results in the rapid division of cells without the hindering. The rapid division increases cell growth. Similarly, up-regulating the various pro-apoptosis proteins/elements and down-regulating the anti-apoptosis proteins might be a positive regulators of apoptosis. The signaling pathway is described in Section 3. The p53 role in induction apoptosis could be enhanced by up-regulating the transcription of mRNA and translated product. The use of inhibitors to suppress the activation of inhibitors of apoptosis (IAPs) also aids in the successful establishment of apoptosis in cancer cells. The other strategy involves various regulator proteins involved in cell progression and growth, such as MMPs (matrix metalloproteinases), play a vital role in cancer progression. MMP inhibition or blockade is a critical target for reducing metastatic potential. In addition to MMPs, metastasis suppressor genes such as MKK4 (mitogen-activated protein kinase 4), BRMS1 (breast cancer metastasis suppressor 1), and NM23 (non-metastasis gene 23) play important roles in metastasis inhibition. (Kerr et al., 1972; Neuman et al., 2002). Conventional therapy options are exceedingly tough, particularly in patients with metastatic disease. Invasion, intravasation, and extravasation are part of the metastatic pathway. The spread of cancer cells to distant places via the circulatory system marks the invasion phase. On the other hand, extravasation necessitates cancer cells penetrating the endothelium and the basement membrane. Cancer cells can develop in a secondary focus in extravasation. (Neuman et al., 2002; Fulda et al., 2010). Additionally, regulation of uPA and uPAR expression and TIMP expression are critical for metastasis prevention. (Kanduc et al., 2002). A complex net of signaling molecule cascades largely transduced several “pro-survival signaling pathways” that determined the fate of a cancer cell. Several cytokines and growth factors trigger pro-survival signaling cascades such as IP3K-PKB/Akt and MAPK. Nuclear factor-B (NFB) is a protein that plays a crucial function in controlling inflammation and immunological responses (Hakem and Harrington, 2005). The importance of blocking these pro-survival signaling pathways in various cancers has been thoroughly researched. Angiogenesis, autophagy, reduction of ER expression, intracellular ROS decrease production, and activation of “mitochondrial membrane potential” are among the signaling pathways that cause cytotoxicity in cancer cells.
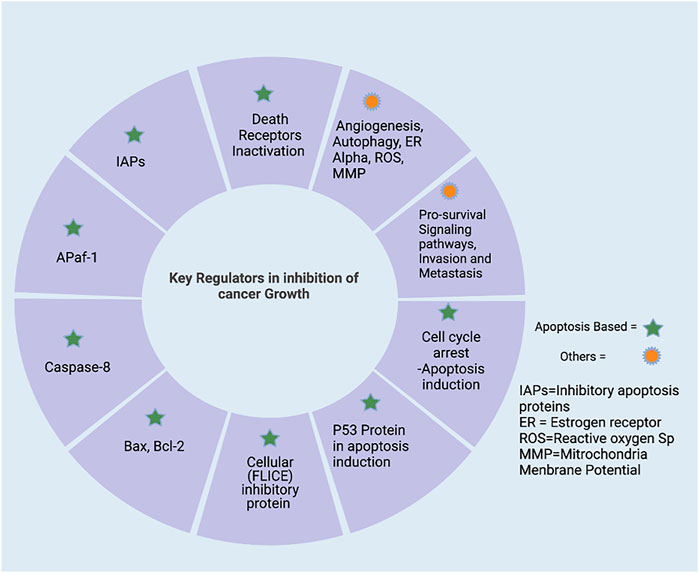
FIGURE 2. Targeting regulators in inhibition of cell progression. It is mainly divided into apoptosis regulators and others. Targeting Apoptosis includes i) up-regulation of pro-apoptotic (caspases protein, Bax, and pro-apoptotic member of Bcl-2 family) ii) down-regulation of anti-apoptotic proteins (IAPs). Also, by targeting the pro-survival signaling pathways, invasion and metastasis proteins dysregulated in cancer cells, created with BioRender.com.
3 Signaling pathways in apoptosis: Potential strategy for targeted therapeutics
Apoptosis is an essential physiological process of cell death that is meant to occur without the discharge of intracellular contents, and subsequent no activation of an inflammatory response as apoptosis is a crucial process in embryonic development, regulation of the immune system, and the response to DNA damage. However, dysregulation of apoptosis results in significant consequences in carcinogenesis. The imbalance between cell proliferation and cell death is considered the malignant tumor’s hallmark (Giorgi et al., 2008). Therefore, maintaining cellular homeostasis between proliferation and cell death is essential for normal physiological processes.
The signaling molecules of the apoptotic pathway played an essential role in the regulation of the apoptosis process. Therefore, these molecular proteins might be considered potential apoptotic biomarkers that can be targeted in advanced cancer treatment and therapeutics. Due to the development of potential biomarkers in cancer, targeted therapy research has emerged as an effective tool for cancer treatment compared to the conventional method. The standard chemotherapies act on rapidly dividing normal and cancerous cells, whereas targeted therapy plays an influential role as they act upon specific molecular targets related to specific cancer. The targeted apoptotic biomarkers in cancer are in clinical trials. However, the targeted therapies are designed so that they interact with their target, whereas many standard chemotherapies were identified because they kill cells. Furthermore, targeted therapies are often cytostatic as they block tumor cell proliferation, whereas standard chemotherapy agents are cytotoxic and kill tumor cells). Therefore, the apoptotic biomarkers and chemotherapy could combine targeted drug therapy to get the maximized results. Apoptosis has been extensively investigated over the last two decades. It is now a critical process of controlled death activated in response to cell injury and during normal “development and morphogenesis.” For instance, the apoptotic death of nearly half of newly produced peripheral neurotransmitters throughout formation forms the nervous system of vertebrates to regulate their quantity, so it matches the requirements of their target organs in the peripheral (Alberts et al., 2002). Induction of apoptosis is essential for complex organisms’ embryonic development and differentiation equilibrium. It occurs in a controlled manner, with characteristic morphologic markers such as “cell shrinkage, “chromatin condensation,” and “blabbing of the cytoplasmic membrane.” Apoptosis dysregulation has indeed been linked to several pathologies, notably tumor growth and the formation of cancerous cells’ chemoresistance (Gorski and Marra, 2002). Among mammals, apoptosis is triggered by two well-known pathways (Figure 3). Extrinsic signals, such as TNF “tumor necrosis factor,” Fas (CD95/APO1), and TRAIL (TNF-related apoptosis-inducing ligand receptors), might induce apoptosis. In contrast, internal stimuli, including mitochondrial transduction, also can trigger apoptosis. For example, the activation of cysteine aspartyl proteases (caspases) causes permeabilization of mitochondrial membranes, chromatin condensation, and DNA fragmentation, resulting in cell death (Zornig et al., 2001).
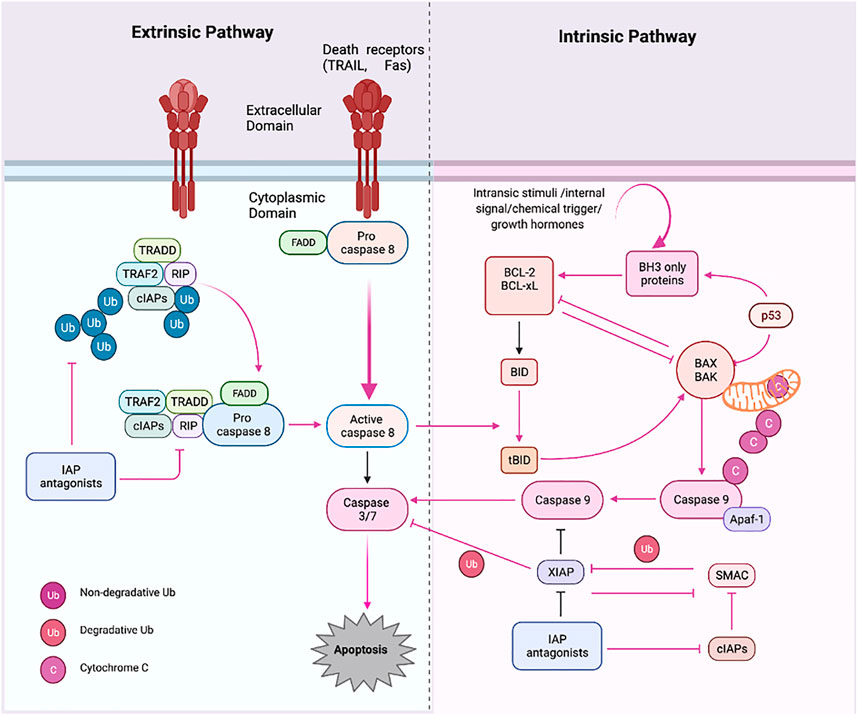
FIGURE 3. Apoptosis pathways. Apoptosis has two primary routes known as the i) extrinsic and ii) intrinsic pathway. First, external stimuli or ligand molecules activate the transduction, including death receptors (SDRs), leading to caspase 3/7 activation via/or activated caspase 8. Intrinsic pathway; via insertion of proapoptotic molecules BAX (protein) into the mitochondrial membrane results in the generation of cytochrome c, forming an apoptosome, which further triggers the apoptotic cascades beginning with proapoptotic activation caspase 9 and or then caspase 3. Created with BioRender.com.
The intrinsic route is triggered by physical and chemical inputs, including hypoxic conditions, growth factor shortages, cell detachment, and stress signals. The initiator caspases activation activates effector caspases; these are “aspartate-specific proteases” regulated post-translationally. Caspase inside as inactive proenzyme consists of a prodomain (a small and large subunit), which must be oligomerized and cleaved to activate. On the other hand, Caspases-independent apoptosis has been documented (LeBlanc, 2003; Bredesen et al., 2006). The death ligand binds the intracellular death domain of death receptors to begin the extrinsic pathway (Duckett et al., 1998; Venderova and Park, 2012). Many apoptotic signals are relayed to cell death machinery by p53, which interacts with other proteins like TNF, Fas, and TRAIL receptors, which are particular physiologic mediators of the extrinsic signaling route of apoptosis. Mitochondria have a role in several vital processes, including the caspase activators (cytochrome C), alterations in electron transport, mitochondrial membrane potential regulation, and the involvement of both pro-and antiapoptotic Bcl-2 family proteins (Bibel and Barde, 2000; Johnstone et al., 2002).
The proteins involved in the intrinsic route are “SMAC/DIABLO, Caspase-9, Bcl-2, Bcl-w, and Nox (Green, 2005). The intrinsic mechanism involves “Bax/Bak membrane insertion and cytochrome c release from the mitochondrial intermembrane gap into the cytosol (Anichini et al., 2006). The apoptosome is a multi-protein complex consisting of a “seven-spoke ring-shaped complex” that activates caspase 9 and then the caspase-3 signaling cascade, which leads to cell destruction and apoptosis (Hengartner, 2000). According to experimental findings, caspases have an overwhelming role in apoptosis (Ashkenazi and Dixit, 1998). There is a hopeful future for cancer therapy techniques that induce apoptosis in cancer cells by disrupting mitochondrial biogenesis, outflowing matrix calcium glutathione, or releasing membrane proteins due to mitochondrial failure. Anti-apoptotic proteins Bcl-2 and Bcl-xL block the cytochrome c release (Block et al., 1992). The development of a death-inducing signaling complex DISC which is made up of the adaptor molecule Fas-associated death domain (DD), procaspase-8, procaspase-10, and the cellular FLICE inhibitory proteins, is caused by the activation of SDRs by specific death ligands (DLs) (Heinrich et al., 1998). This Caspase 8 is activated in a real way that the “prodomain of caspase 8″ remains attached to the DISC. In contrast, the “active caspase 8 dissociates from the DISC” to initiate the cascade of caspase activation that occurs during the “execution phase of apoptosis (Kreuger et al., 2012). Traditionally, “caspases have been divided into i) initiator, ii) effector, and iii) executioner” types based on where they are located in an apoptotic signaling chain. At the same point, both extrinsic and intrinsic paths converge (execution phase). The execution phase is the last step in the apoptotic process (Kim, 2005; Elmore, 2007). Certain caspases have lengthy pro-domains that contain specific motif sequences, such as the death effector domain (DED) and caspase recruitment domains (CARD), which enable them to interact with other proteins and connect to signaling pathways. Caspases-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase-11, and caspase-12 are involved in DED, whereas caspase-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase-11, and caspase-12 are involved in CARD (Yuan and Akey, 2013). Caspases are critical apoptosis initiators and executors, and their function is strongly linked to their structure, which has distinct substrate preferences. The “Caspase-8 and 9 are initiator caspases, while Caspase-3, Caspase-6, Caspase-7, Caspase-10, CAD, and PARP” are effector or executioner caspases (Stergiou and Hengartner, 2004; Ghobrial et al., 2005). Caspase initiators are triggered by autocleavage, activating executioner caspases, which later proteolyze certain substrates, resulting in apoptosis. They have extended pro-domains that link big adaptor molecules, allowing them to multimerize and activate other caspases. However, effector caspases, on the other hand, have “short pro-domains” that execute apoptosis when activated by initiator caspases. As a result of the cytoplasmic endonuclease activated by executor caspases, chromatin condenses, cytoplasmic blebs develop, and apoptotic bodies are formed. Numerous target proteins are cleaved by caspases to regulate apoptotic cell death (Green, 2005). Caspases 3 and 4 are the essential executioner caspases, and an initiator caspase can activate them. Caspases-3 specifically activates endonuclease CAD, resulting in chromosomal DNA degradation and chromatin condensation inside nuclei. The cytoskeletal rearrangement and generation of cytoplasmic blebs and apoptotic bodies are both dependent on execution caspases (Medema et al., 1997). Execution caspases are activated first, followed by cytoplasmic endonuclease activation. Interestingly, nuclear material is degraded by cytoplasmic endonuclease, and proteases then degrade nuclear and cytoskeletal proteins.
4 Why apoptosis induction is mandatory for effective chemotherapy
An anti-cancer treatment mainly kills cancer cells and may or may not cause side effects to healthy cells. Currently accepted cancer therapy methods include a combination of medications, surgery, radiation, or a combination of all of these. Chemotherapeutic drugs can relieve symptoms, extend life, and even cure cancer in some cases. One of the best cancer drugs has a low risk of harming healthy cells in treating patients. Apoptosis substantially impacts both the lifespan of healthy cells and the prognosis of malignant cells. As a result, regulating default apoptosis could benefit cancer treatment and prevention. The aberrant response of malignant cells to apoptosis induction is caused by excessive cellular proliferation, which can also be described as overexpression of inhibitors or IAP family members. The Cell cycle-regulating genes are inactivated in cancer cells, and Bcl-2) adjusts their expression in tumors. Different malignancies, inflammatory illnesses, viral infections, and autoimmune diseases are caused by inhibiting or suppressing apoptosis.
Interestingly, apoptotic induction has emerged as a novel target for novel mechanism-based drug discovery (Ghavami et al., 2009; Sankari et al., 2012). Previously, it was thought that an increase in cellular proliferation was only linked to the rise in the number of cells accumulating in the body; however, it has now been discovered that a decrease in cell death is also responsible for cell proliferation. As a result, it is crucial to screen apoptotic inducers from natural products, either as crude extracts (plants or animal/marine organisms) or as separated components. Various studies have found evidence that compounds derived from natural sources effectively treat and prevent cancer. The isolated secondary metabolites from multiple natural sources, and mechanistic study (in-vitro), give better molecular fundamental knowledge of the future therapeutic agent. Various plants extracts, fractions, synthetic palladium, and other complexes are used to screen cytotoxicity and mode of cell death, which reduces the cost of isolation of phytochemicals and synthetic compounds and gives insight into a potential therapeutic agent. The ability to trigger apoptosis may serve as a unifying principle for many distinct types of chemo preventive drugs, each with a unique mode of action. Also, insight into the mechanisms of action of these chemicals could lead to new cancer-preventive and treatment strategies. Synthesis or modification of previously recognized medications in cancer treatment is still a significant research focus. Despite massive amounts of synthetic effort, the final products have improved slightly over a few prototypes. Thus, there is a constant demand for new chemotherapeutic agent prototypes–templates to develop more effective chemotherapy. Natural products (NPs) serve as models for new prototypes in the search for drug discovery. Apart from chemotherapy treatment, epidemiological studies suggest that eating lots of fruits and vegetables, rich in phytochemicals and other nutrients, may lower one’s risk of getting cancer (Jan and Chaudhry, 2019). In addition, it has been shown that natural product-derived tumor inhibitory chemicals have revealed a remarkable array of novel structural forms.
5 Plants extracts/fractions as potential regulator of pro-apoptotic and anti-apoptotic agents
Apoptosis is a cell death process that is conserved and well-controlled. It is essential to complex organisms’ development and homeostasis. Apoptosis inducers work very well in causing cytotoxicity in cancer cells. Due to their selective targeting of cancer cells, dys-regulatory apoptosis machinery only targets normal cells compared to cytotoxic agents. Therefore, they can work along with potential chemotherapy and reduce the risk of re-occurrence via killing the cell in a natural suicidal way. One of the essential factors in combination medication therapy is awareness of diverse phytochemicals and their synergistic approach. By indicating the presence of a potential agent, the extracts/fractions screening lowers the cost of phytochemical separation. Apoptosis’s external and intrinsic triggers include; radiation, growth factor withdrawal, ligands, tumor suppressor protein (p53), and oxidative stress (Hengartner, 2000; Hu et al., 2013). Additional pathological activities associated with the physiologic apoptosis pathway include cancer, inflammatory, and neurological diseases (Cairrao and Domingos, 2010). As shown in a substantial body of research, many cancerous tumors have a decreased capacity to die by apoptosis in reaction to physiological stimuli (Los et al., 1995). Therefore, it is critical to broadening the chemical repertory of agents that might induce or sensitize resistant cells to apoptosis to combat cancer. Furthermore, employing natural compounds as anti-carcinogenic or cytotoxicity-inducing compounds may be promising cancer prevention and therapy strategy. An in vitro investigation of secondary metabolites from various natural sources provides a better understanding of the molecular fundamentals of the therapeutic drug in the future (Israels and Israels, 2000).
5.1 Alkaloids based apoptosis induction
Alkaloids are chemical compounds found in nature that are predominantly made up of basic nitrogen atoms, neutral groups, and mildly acidic (Pucci et al., 2000; Khazaei et al., 2017a). Alkaloid Solamargine, located in the Chinese Plant Solanum incanum, has been demonstrated to trigger apoptosis in cancer cells (Hep-3B) and standard (“skin fibroblast cells”). Also, the level of TNF receptor I significantly elevated after solamargine treatment (Martin et al., 2000). Necrophilia guyanensis and Genipa Americana extract fractionation contain alkaloid cryptolepine, which appeared as the predominant active component. The cryptolepine compounds demonstrated substantial cytotoxic effects in human cells via the underlying mechanisms of apoptosis (Decock et al., 2011). Camptothecin (CPT), an inhibitor of topoisomerase I, causes triggered Oxygen radicals which cause DNA fragmentation and eventually induction and completion of apoptosis. (Cox et al., 1999).
5.2 Terpenes based apoptosis induction
The monoterpenes D-limonene and perillyl alcohol were found to have anti-carcinogenic properties in various cancer cell lines by exhibiting apoptosis. For example, perillyl alcohol was very effective in causing cell death of breast cancer cells (Klener et al., 2006). Similarly, Limonene inhibits stomach carcinogenesis caused by sodium chloride by enhancing apoptosis (Reddy et al., 1997). Taxol is a cytotoxic diterpene discovered in Taxus brevifolia that suppresses cell growth and proliferation. The mechanism of action is triggered via stabilizing power of Taxol during the spindle fiber formation in the cell division stage (mitosis). The polymerization of the Microtubule and improved stimulation of the formation of microtubule (MT) bundle impeded entry into the S phase, limiting cell division and apoptosis and also triggering necrosis at higher concentrations of Taxol (Fisher David, 1994). However, Paclitaxel-induced cell death also occurs via a signaling pathway not dependent on G2/M arrest (Pistritto et al., 2016).
5.3 Polyphenols based apoptotic inducers
Polyphenols obtained from Plant are abundant in the human diet and are deemed healthy at the recommended dose (one G/day) (Ren and Tang, 1999; Chaudhry et al., 2021a). A range of mutated cell lines (Schultze et al., 1991) show that gallic acid predominantly induces cell death. Similarly, in HL-60 cells, caffeine and tannic acids induce DNA breakage (Hajto et al., 1989). CAPE, the main ingredient in traditional medicinal propolis, has been shown to have particular cytotoxic activity against mutated cloned rat embryo fibroblast (CREF) cells by apoptotic cell death (Liu et al., 2000). CAPE, the main ingredient in traditional medicinal propolis, has been shown to have particular cytotoxic activity against mutated cloned rat embryo fibroblast (CREF) cells by apoptotic cell death (Liu et al., 2000). It has been established and demonstrated that curcumin, the primary color in turmeric, is a phenolic compound that causes apoptosis—a beneficial chemotherapeutic addition since it does not affect normal cells. The activation of apoptosis was boosted by curcumin’s ability to block cyclooxygenase (COX) products (Buhagiar et al., 1998; Bussing et al., 1999). Moreover, chilli pepper and hot red pepper contain capsaicin, triggering HL-60 cells to undergo apoptosis (Reed and Green, 2002).
5.4 Xanthones based apoptotic inducers
Xanthones are found in the naturally occurring substances in both plants and microbes. This new species from marine fungi contains anti-cancer xanthone compounds, which have been noticed recently. This is established that toxic compounds and anti-carcinogenic medication breakdown and elimination affect certain proteins. Xanthone compounds with anti-carcinogenic characteristics may be found in aquatic species and microalgae. In mangostana’s pericarp -mangostin, the xanthone induced apoptosis via up-regulation of caspase-3 (Thompson, 1995; Ashkenazi and Dixit, 1998).
5.5 Plants extracts regulate pro-apoptotic and anti-apoptosis proteins
The Table 1 shows the list of potential plant extracts as apoptotic inducers. Pepino, the extract was essential for the onset of DNA fragmentation and PARP cleavage (Ng and Bonavida, 2002). Similarly, Viscum album L. (Loranthaceae) extract in human lymphocytes causes cytotoxicity, more probably via apoptosis (Gorczyca et al., 1993; McNaught and Andrew, 1997). Salvia miltiorrhiza is often used to cure liver problems throughout Asia for centuries. Investigators found that sage salvia miltiorrhiza extracts showed significant cytotoxic activity and could have reduced the human hepatoma (HepG2) cell line (Manske Richard Helmuth, 1965). Apoptosis was shown to drive the cytotoxic activity of Conifer Tetraclinis articulate (essential oil) on several human cancers, including blood lymphocytes (Shu-Hui et al., 1996). Bussing et al. employed a seed extract of these and discovered that it produced oxygen radical mediators and induction of apoptosis. The “tumor necrosis factor” (alpha) and “interleukin-6" (IL-6) constitute pro-inflammatory factors, whereas “interleukin-5" (IL-5) and “interferon-gamma” (IFN-g) are T-cell associated cytokines (Shu-Wei, 1999). Genistein (a hydroxyisoflavone) was found to induce cell death in human promyelocytic (HL-60) leukemic cells according to flow cytometric analysis (Hiraoka et al., 1998). Vitex rotundifolia (leave) fraction remarkably induces apoptosis in MCF-7 & T47D via extrinsic and intrinsic pathways (Ariazi et al., 1999; Yano et al., 1999; Yeung et al., 1999). Squamous cell carcinoma was inhibited by an ethanolic extract of Azadirachta indica (neem) leaves in a hamster cancer model. The presence of extract increased the pro-apoptotic proteins (caspase-3 and -8, BIM, and Bcl-2), indicating that both internal and external mechanisms were significantly impacted. The Bax and Bcl-2 are up-regulated in breast cancer cell lines (MCF-7 and MDA-MB-231) by Phaseolus vulgaris (Fabaceae) extract treatment in cells, respectively (Bcl-2, Bcl-xL) (Weimin, 1999). In addition, a methanolic extract of Fragaria ananassa (Strawberry) increased Bax, Bid, and p73 expression in T-47D cells while decreasing Bcl-xL expression (King-Thom, 1997). According to apoptosis-inducing meisoindigo, the receptors on the cell surface and mitochondria are involved, according to the study. The colorectal cancer cell lines, methyl ferulate, a Tamarix aucheriana plant product, enhanced caspase-2, -3, -6, -7, -8, and -9 expression while decreasing the anti-apoptotic proteins Bcl2 and FLIP (Meyer et al., 2005). Inula racemosa (also known as pushkarmool) roots induced apoptosis and necrosis in human leukaemia cell line HL-60 when treated with an ethanolic extract of Inula racemosa roots. While cytochrome C (release) and Bax (translocation) dramatically reduced, this substance boosted the activity of Caspase-9, -3, and -8 (Makoto et al., 1994). The activation of intrinsic caspase-6, -8, and-9 by Oenocarpus bacaba aqueous extract promoted apoptosis in MCF-7 cells (Sakagami et al., 1994). Solanum lyratum (known as nightshades) chloroform extract is triggered by the extrinsic and intrinsic apoptotic mechanism in HSC-3, CAL-27 & SAS cancer cells. Among the cell lines studied, the extract decreased anti-apoptotic proteins (Bcl-2 and Bcl-xl) while elevating pro-apoptotic (Bax and Caspases) (Su et al., 1994). The cardiac glycoside (Ouabain) significantly decreased ROS and MMP levels while increasing Ca2+, activation of pro-apoptotic members, and reduced expression of Bcl-2 levels (Rao Chinthalapally et al., 1995). The cardiac glycoside (Ouabain) significantly decreased ROS and MMP levels while increasing Ca2+, activation of “pro-apoptotic” members, and reduced expression of Bcl-2 levels (Rao Chinthalapally et al., 1995). Cell cycle arrest and apoptosis were caused by “Rosamultic acid,” a triterpenoid, which cleaves PARP and activates caspase-3, -8, and -9 in SGC-7901 cells (Rao et al., 1995). A flavonoid produced from plants, Acacetin triggers an apoptotic cascade in stomach cancer cells (AGS cells) and kills them. “When ROS are produced, MMP collapses and “Bax and p53″ are increased “Bcl-2 is decreased”. Extrinsic activation of “caspase-8 and Bid proteins” is facilitated by an increase in the expressions of Fas and FasL (Wolvetang Ernst et al., 1996). Caspase-8 and Caspase-7 were activated in breast cancer (MCF-7) cells after exposure to extracts (methanol and & butanol) of “Oldenlandia diffusa” (Willd) Roxb. As a result, Bax expression was increased, and Bcl-2 levels were decreased (Matsumoto et al., 2003). Cell death was prevented in MCF-7 cells by Cucurbita ficifolia (chloroform) extract. There was an increase in the expression of FADD, BAK, BAX, and caspase-8, -9, and -3 (Nakagawa et al., 2007). Lycorine, an alkaloid molecule (from the Amaryllidaceae family), has been shown to enhance the “Bax/Bcl-2″ protein ratio and the activity of caspase-(8, -9, and -3) enzymes in cells compared to untreated cells (Chaudhry et al., 2019a). A mango peel ethanolic extract (Mangifera indica) has been shown to cause the death of HeLa cells. As a result of activating pro-apoptotic proteins (caspase-3, 7, 8 & 9) and the suppression of Bcl-2 (Chaudhry et al., 2019b). Celastrol, a pentacyclic triterpenoid, induces apoptosis via acting on receptor protein (death receptor) and internal pathways. Celastrol stimulated the signal transduction in mitochondria and increased Bax while decreasing Bcl-2 protein levels in the intrinsic route. To increase the expression of FAS and FASL, it induced FASL expression in the extrinsic pathway. Because celastrol activated caspases 9, 8, 3, and PARP, these enzymes began to break down proteins. Using a mouse model of cancer, Celastrol prevents the development of human glioblastoma xenografts by suppressing angiogenesis. It also induces apoptosis in osteosarcoma cells and increases the activities of pro-apoptotic caspase-3, -8, and 9 (Verdine, 1996; Chaudhry et al., 2020a). An ethanolic extract of Brucea javanica fruits was discovered to have apoptotic properties when tested on colon cancer (HT29) cells. The drug’s mode of action involves both receptor- and mitochondrial-mediated processes. The extract also increased TNF2 and DR6 in the extrinsic route. Caspase-8 and TRAIL-4 were also increased. Caspase-9 was also activated, which led to an increase in Bax, Bad, and cytochrome-c while decreasing Bcl-2 (Subapriya et al., 2005). Experiments on human cancer cell lines have shown that plant extracts can activate intrinsic and extrinsic pathways to cause cell death. They were also beneficial in vivo, killing cancer cells and delaying cancer progression. Because of this, even though these plant extracts have long been used in traditional cancer treatments, western science and medicine need proof of their anti-cancer properties using pure plant compounds isolated from plants. The intrinsic and external mechanisms in adult tumor cells can be affected by an N, N-dimethyl plant sphinganine (DMPS) derivative known as phytosphingosine (PS) (HL-60 cells). -Caspase-8 activation is a major element at the beginning of the intrinsic pathway, followed by the cytochrome C release and activation of pro-apoptotic proteins (caspase-9 and caspase-3, and inhibition of Bcl-2 (anti-apoptotic members of the family (Abadio Finco et al., 2016). Anthocyanins, a kind of flavonoid, are well-known for their medicinal qualities. Anthocyanin components produced from Vitis coignetiae destroy human leukaemia (U937) cells by apoptosis induction in these cells. Bax levels rise when Bid and caspases 3, 9, and 8 are activated, followed by a decrease in MMP, Bcl-2, and other MMPs, cIAP-1, and cIAP-2 levels well. Anthocyanins, which induce apoptosis, did not affect U937 cells with elevated Bcl-2 protein levels (Abaza et al., 2016). breast cancer (“MDA-MB-468″) cells treated with extracts (“Vatica diospyroides”) exhibit apoptosis as a result of Bax upregulation (Pal et al., 2010). An Anemone raddeana triterpenoid molecule, raddeanin A, is shown to activate apoptosis in stomach cancer cells. Raddeanin A upregulation pro-apoptotic protein (Bax) while decreasing the expressions of anti-apoptotic (BCL-xL) using molecular biology techniques. Additionally, in all three cell lines, this terpenoid increased the activity of caspase-3, -8, and -9, and the PARP enzyme (Orangi et al., 2016a). Non-small cell lung cancer cells” can be made to die by LA, which inhibits Bcl-2, FLIP, and XIAP while increasing “Bid” and caspase-3, -,8-9 (Chiu et al., 2015). LA is derived from the leaves of the Pinus koraiensis tree. The sBax and protein caspases up-regulated treated with Momordica cochinchinensis fruit extract, inducing apoptosis in MCF-7 (Chou et al., 2018). Using 34 HL-60 cells, ethyl acetate extract from Uncaria tomentoa (ethyl acetate (EA) extract) was found to activate the intrinsic pathway by compressing Matrix Metallo Protein and lowering anti-apoptotic (Bcl-XL protein), as well as by raising the membrane-bound Fas and activating caspase-8 (Sui et al., 2015). Protoberberine activated pro-apoptotic caspases, promoting cell death (apoptosis) in tongue cancer cells. Increased ratio (Bax/Bcl-2) and altered matrix protein were also observed (Pan et al., 2005). Two breast cancer cells were injected into athymic nude mice, and wogonin was found to have an anti-cancer effect in vivo. After 4 weeks of treatment, the xenograft burden was reduced by 88 percent (Chung et al., 2017a). Apoptosis in “breast cancer” (MCF-7) cells is caused by the methanolic fractions of Scrophularia oxystepala (Alshammari et al., 2020a). oleandrin, a carcinogenic glycoside, kills cancer cells in the U2OS and SaOS-2 lines by inducing cell apoptosis through the ROS Generation as well as the damage of Mitochondrial membrane potential, which discharges hydrolytic Enzymes into the cytosol and regulates the proteins (decreases the Bcl-2 level and increase Bax) along with caspases (caspases 9 and 8 and -3 (Liu et al., 2004).
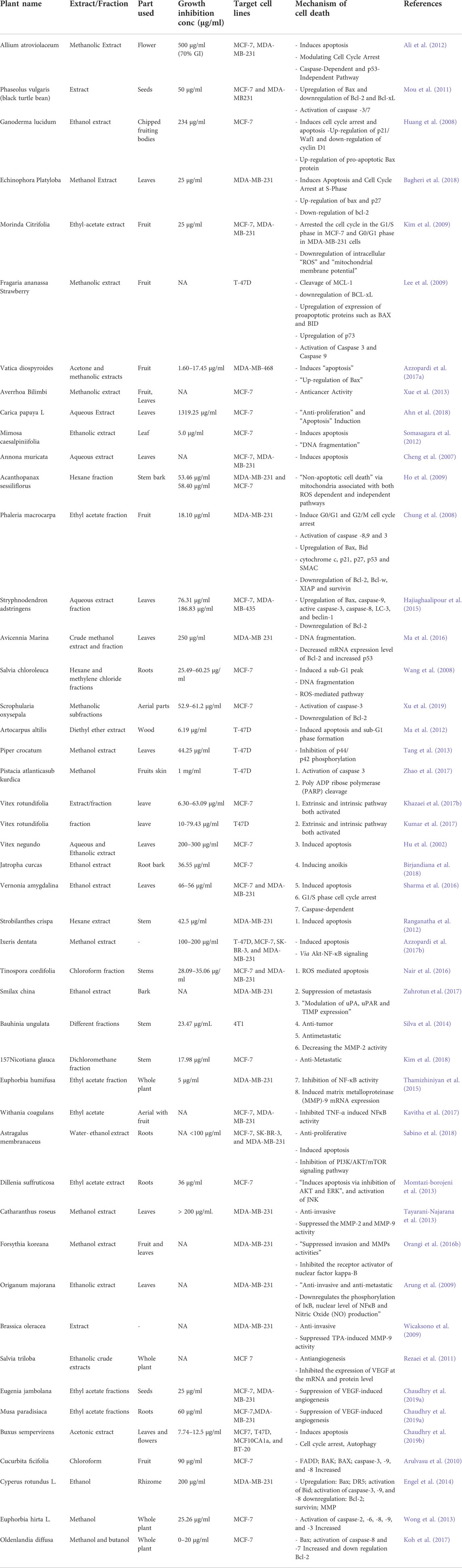
TABLE 1. Plant extracts/fractions as apoptotic inducers for breast cancer (in vitro). updated and improved from (Israels and Israels, 2000).
6 Marine extracts/fractions as apoptosis regulator
Aquatic environments, especially marine species, contain abundant various natural compounds which may have therapeutic significance. However, recent marine biomaterials experience demonstrates that ocean life seems to be an untapped resource. Bioactive compounds found naturally are the most effective. Apart from a few exceptions, most compounds used in medicines originate through biological and chemical modifications rather than from the initial natural substance. Our previous studies confirm apoptosis induction in the cancer cell (in-vitro) by marine extracts and fractions. The Aaptos sp., marine (fraction) causes the DNA fragmentation in the MCF-7 cell line, which is a remarkable feature of apoptosis (late). Similarly, marine sponges (whole) extracts and fractions also induce the fragmentation in DNA in breast cancer (MCF-7) cell lines. The details information mention in Table 2. The “Stichopus chloronotus” and “Holothuria nobilis” fractions induced cytotoxicity via apoptosis induction in cervical cancer (HeLa) cells shows the remarkable potential of marine phytochemicals in the induction of apoptosis. The “Bruguiera gymnorrhiza” extracts potential phytochemicals that trigger DNA fragmentation, leading to the induction of apoptosis in MCF-7 cells. The other marine species, “Acanthaster planci” sp., and “Diadema setosum” sp., fractions Induction of apoptosis in HeLa cells. The “Xylocarpus moluccensis” induce the triggering of the Extrinsic pathway of apoptosis which causes the primary factor for cytotoxicity of fractions in human hepatocellular carcinoma (HepG2) and human cervical cancer cell line (HeLa). Some alkaloid chemicals discovered in traditional medicines, tetrandrine (TET) and cepharanthine (CEP), operate as apoptotic agents. There is reduction in the progression of malignant tumors. When viewing human leukemia cells treated with alkaloids, Xu et al. found that both initiator and effector caspases were dramatically increased (caspase-3 and& -6). Saikosaponin A, a triterpenoid saponin, was studied by Kim and colleagues to see if it could kill human colon cancer cell lines. According to the researchers’ findings, the activation of Bax and Bid proteins and the activation of caspase-2, -8, and -9 in saikosaponin A increased apoptosis in cells. Emodin (40 mg/kg/day) suppressed tumor weight in “nude mice xenografts” carrying “LS1034” colon cancer cells in vivo, according to in vitro findings. “Anti-apoptotic” proteins “Bcl-2” and Bcl-xL, Bax, XIAP and cFLIP are reduced in “HT-29”, “HCT-116” and “SW480” cells by the flavonoid compound Casticin. The “OVCAR-3” and “SKOV-3” (ovarian cancer) cell lines were exposed to kaempferol (kaempferol), a flavonoid, the levels of apoptosis-inducing factors like proteins (Bax, caspase-3, -8, & -9) were significantly increased. In contrast, apoptosis inhibiting proteins decreased in these cells. Similar outcomes were found when combined with the anti-inflammatory agent TRAIL.
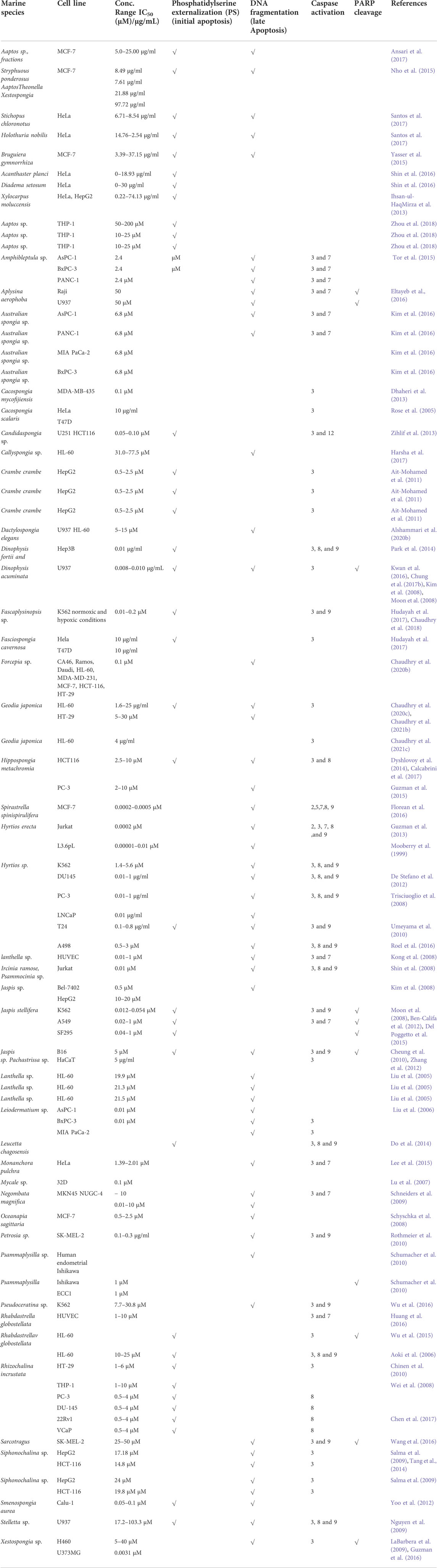
TABLE 2. Marine (sponges) extracts/fractions as apoptotic inducers for various cancer cell lines (in vitro). Improved and updated from (Shin et al., 2017) (Calcabrini et al., 2017).
7 Marine isolated targeted compounds as apoptotic inducers (FDA approved/treatment phase)
Besides extracts/fractions, the various marine-based drug also show apoptosis-inducing potential. And over 50 years since cytarabine was authorized, four marine-derived drugs have been approved (Hu et al., 2013). Some other items have been included in the marine anticancer medicinal route in clinical studies in different phases of development as we focused on apoptotic inducers. Table 3 shows the list of respective drugs (approved or in clinical trials) having the potential for inducing apoptosis. These include; Brentuximab2 vedotin (AdcetrisTM), Brentuximab2 vedotin (AdcetrisTM), as “Antibody-drug3 conjugate in a mixture of anti-CD30 antibody and monomethyl auristatin E (MMAE), isolated from sea hare Dollabella auricularia/cyanobacteria” used for Blockage in cell cycle progression (G2 to M) induce induction of apoptosis phase III and I treatment of classical HL in a mixture using chemotherapy for curing HL and chronic large cell anaplastic lymphoma. Enfortumab vedotin, Marizomib, Polatuzumab Vedotin, use in phase III. Besides, GSK2857916, Aplidine, plitidepsin (Aplidin), Ladiratuzumab vedotin, Glembatumumab vedotin, Denintuzumab mafodotin, in phase II trials. However, Pinatuzumab vedotin, ASG-15ME, CEP-2563, Lifastuzumab vedotin, and Vandortuzumab vedotin were discontinued after phase I used for lymphoma, bladder, ovarian, as Peritoneal cancer. Vandortuzumab isolated from Cyanobacterium. (Caldora penicillata) acts as apoptotic inducer and mitotic inhibitor for Prostate cancer.
8 Conclusion
The potential use of natural substances as either a combination treatment with standard therapies or as an individual derivative molecule as anticancer medicine requires an understanding of cancer initiation and activation of cancer development, the role of signalling pathway in activation of cancer initiation and progression, pro-oncogenes, and oncogenes. Since apoptosis is a crucial regulating mechanism for normal cells, any disruption in this process might lead to unchecked cell growth. An in-depth mechanistic knowledge of apoptotic signalling pathways is necessary for the creation of efficient cancer therapies. Plant extracts, marine extracts/fractions, and the chemicals described in this research may represent a promising and efficient approach to cancer treatment. Researchers found that natural product derivatives may have an important role in the prevention and treatment of several different types of cancer. To verify their overall usefulness as potent chemotherapeutic drugs, however, more preclinical and clinical research are required. It is interesting to note that natural compounds produced from plant extracts/marine can be used in combination with possible anticancer treatment to enhance drug sensitivity in aggressive and resistant kind of cancer. Due to the promising results seen when using natural goods in conjunction with cancer medications, intensive study into the therapeutic uses of natural products is required to get the best possible outcomes in individualized, targeted cancer care. Similarly, the use of more than one compounds as multitargeted signaling pathway inhibitors or activator should be consider with one signaling target as apoptosis pathway. However, natural chemical derivatives that induce apoptosis, along with cutting-edge drug delivery methods like polymeric nanoparticles, may yield impressive results. Compounds with low solubility and bioavailability are less likely to be effective in the development of chemotherapy drugs. In conclusion, this study aims to bring to the notice of scientists and researchers the numerous beneficial impacts of natural products in the development of novel and safe pharmaceuticals or in combine delivery technique with anticancer treatments for probable sensitization of cancer therapy. It might also serve as a solid basis for further research into natural compounds in cancer therapeutics.
Author contributions
GC prepared the concept, wrote and reviewed the manuscript. MA, YS, and TS provided valuable input during the review process of this manuscript.
Acknowledgments
Authors would like to acknowledge the UMT for TAPERG/2021/UMT/807 grant provided to GC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abadio Finco, F. D. B., Kloss, L., and Graeve, L. (2016). Bacaba (Oenocarpus bacaba) phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS J. 5, 5–15. doi:10.1016/j.nfs.2016.11.001
Abaza, M. S., Afzal, M., Al-Attiyah, R. J., and Guleri, R. (2016). Methylferulate from Tamarix aucheriana inhibits growth and enhances chemosensitivity of human colorectal cancer cells: Possible mechanism of action. BMC Complement. Altern. Med. 16, 384. doi:10.1186/s12906-016-1358-8
Ahn, D. S., Lee, H. J., Hwang, J., Han, H., Kim, B., Shim, B., et al. (2018). Lambertianic acid sensitizes non-small cell lung cancers to TRAIL-induced apoptosis via inhibition of XIAP/NF-κB and activation of caspases and death receptor 4. Int. J. Mol. Sci. 19, 1476. doi:10.3390/ijms19051476
Ait-Mohamed, O., Battisti, V., Joliot, V., Fritsch, L., Pontis, J., Medjkane, S., et al. (2011). Acetonic extract of buxus sempervirens induces cell cycle arrest, apoptosis and autophagy in breast cancer cells. PLoS One 6 (9), e24537. doi:10.1371/journal.pone.0024537
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and Walter, P. (2002). Programmed cell death (apoptosis) - molecular biology of the cell. 4th ed. New York: Garland Science.
Ali, M. R., Yong, M. J., Gyawali, R., Mosaddik, A., Ryu, Y. C., Cho, S. K., et al. (2012). Mango (Mangifera indica L.) peel extracts inhibit proliferation of HeLa human cervical carcinoma cell via induction of apoptosis. J. Korean Soc. Appl. Biol. Chem. 55, 397–405. doi:10.1007/s13765-012-1024-x
Alshammari, G. M., Balakrishnan, A., Alshatwi, A. A., and Al-Khalifa, A. (2020). Cucurbita ficifolia fruit extract induces tp53/caspase- mediated apoptosis in MCF-7 breast cancer cells. Biomed. Res. Int. 2020, 3712536. doi:10.1155/2020/3712536
Alshammari, G. M., Balakrishnan, A., Alshatwi, A. A., and Al-Khalifa, A. (2020). Cucurbita ficifolia fruit extract induces tp53/caspase- mediated apoptosis in MCF-7 breast cancer cells. Biomed. Res. Int., 3712536. doi:10.1155/2020/3712536
Anichini, A., Mortarini, R., Sensi, M., and Zanon, M. (2006). APAF-1 signaling in human melanoma. Cancer Lett. 238, 168–179. doi:10.1016/j.canlet.2005.06.034
Ansari, J. A., Rastogi, N., Ahmad, M. K., Khan, A. R., and Thakur, R. (2017). ROS mediated pro-apoptotic effects of Tinospora cordifolia on breast cancer cells. Front. Biosci. 9, 89–100. doi:10.2741/e788
Aoki, S., Cho, S. H., Ono, M., Kuwano, T., Nakao, S., Kuwano, M., et al. (2006). Bastadin 6, a spongean brominated tyrosine derivative, inhibits tumor angiogenesis by inducing selective apoptosis to endothelial cells. Anticancer. Drugs 17, 269–278. doi:10.1097/00001813-200603000-00005
Ariazi, E. A., Satomi, Y., Ellis, M. J., Haag, J. D., Shi, W., Sattler, C. A., et al. (1999). Activation of the transforming growth factor β signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 59 (8), 1917–1928.
Arulvasu, C., Prabhu, D., Manikandan, R., and Srinivasan, P. (2010). Induction of apoptosis by the aqueous and ethanolic leaf extract of Vitex negundo L. in MCF-7 human breast cancer cells. Int. J. Drug Disc. 2 (1), 1–7. doi:10.9735/0975-4423.2.1.1-7
Arung, E. T., Wicaksono, B. D., Handoko, Y. A., Kusuma, I. W., Yulia, D., and Sandra, F. (2009). Anti-cancer properties of diethylether extract of wood from sukun (artocarpus altilis) in human breast cancer (T47D) cells. Trop. J. Pharm. Res. 8.
Ashkenazi, A., and Dixit, V. M. (1998). Death receptors: Signaling and modulation. Science 281, 1305–1308. doi:10.1126/science.281.5381.1305
Azzopardi, M., Farrugia, G., and Balzan, R. (2017). Cell-cycle involvement in autophagy and apoptosis in yeast. Mech. Ageing Dev. 161 (2), 211–224. doi:10.1016/j.mad.2016.07.006
Azzopardi, M., Farrugia, G., and Balzan, R. (2017). Cell-cycle involvement in autophagy and apoptosis in yeast. Mechanisms of Ageing and Development, 211–224.
Bagheri, E., Hajiaghaalipour, F., Nyamathulla, S., and Salehen, N. (2018). The apoptotic effects of Brucea javanica fruit extract against HT29 cells associated with p53 upregulation and inhibition of NF-κB translocation. Drug Des. devel. Ther. 12, 657–671. doi:10.2147/DDDT.S155115
Ben-Califa, N., Bishara, A., Kashman, Y., and Neumann, D. (2012). Salarin c, a member of the salarin superfamily of marine compounds, is a potent inducer of apoptosis. Investig. New Drugs 30, 98–104.
Bibel, M., and Barde, Y. A. (2000). Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14, 2919–2937. doi:10.1101/gad.841400
Birjandiana, E., Motameda, N., and Yassab, N. (2018). Crude methanol extract of echinophora platyloba induces apoptosis and cell cycle arrest at S-phase in human breast cancer cells. Iran. J. Pharm. Res. 17 (1), 307–316.
Block, G., Patterson, B., and Subar, A. (1992). Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 18, 1–29. doi:10.1080/01635589209514201
Bredesen, D. E., Rao, R. V., and Mehlen, P. (2006). Cell death in the nervous system. Nature 443 (7113), 796–802. doi:10.1038/nature05293
Buhagiar, J. A., Podesta, M. T., Wilson, A. P., Micallef, M. J., and Ali, S. (1998). The induction of apoptosis in human melanoma, breast and ovarian cancer cell lines using an essential oil extract from the conifer Tetraclinis articulata. Anticancer Res. 19.6B, 5435–5443.
Bussing, A., HerterIch-AkInpelu, I., and Pfuller, U. (1999). Apoptosis-associated generation of reactive oxygen intermediates and release of pro- inflammatory cytokines in human lymphocytes and granulocytes by extracts from the seeds of Acalypha wilkesiana. J. Ethnopharmacol. 66 (3), 301–309. doi:10.1016/s0378-8741(98)00227-x
Cairrao, F., and Domingos, P. M. (2010). “Apoptosis: Molecular mechanisms,” in Encyclopedia of life sciences (ELS) (Chichester: John Wiley & Sons).
Calcabrini, C., Catanzaro, E., Bishayee, A., Turrini, E., and Fimognari, C. (2017). Marine sponge natural products with anticancer potential: An updated review. Mar. Drugs 15 (10), E310. doi:10.3390/md15100310
Chaudhry, G. E., Islamiah, M., Zafar, M. N., Bakar, K., Aziz, N., Saidin, J., et al. (2021). Induction of apoptosis by Acanthaster planci sp., and Diadema setosum sp., fractions in human cervical cancer cell line, HeLa. Asian pac. J. Cancer Prev. 22 (5), 1365–1373. doi:10.31557/APJCP.2021.22.5.1365
Chaudhry, G. E., Jan, R., Mohammad, H., and Muhammad, T. S. T. (2019a). Vitex rotundifolia fractions induce apoptosis in human breast cancer cell line, MCF-7, via extrinsic and intrinsic pathways. Res. Pharm. Sci. 14 (3), 273–285. doi:10.4103/1735-5362.258496
Chaudhry, G. E., Jan, R., Zafar, M. N., Mohammad, H., and Muhammad, T. S. T. (2020). Phytochemistry and biological activity of Vitex rotundifolia L. Res. J Pharm Tech 13 (11), 5534–5538.
Chaudhry, G. E., Jan, R., Zafar, M. N., Mohammad, H., and Muhammad, T. S. T. (2019b). Vitex rotundifolia fractions induced apoptosis in human breast cancer T-47D cell line via activation of extrinsic and intrinsic pathway. Asian pac. J. Cancer Prev. 20 (12), 3555–3562. doi:10.31557/APJCP.2019.20.12.3555
Chaudhry, G. E., Kassim, M. N. I., Zafar, M. N., Mohammad, H., Andriani, Y., Ismail, N., et al. (2020). Induction of apoptosis by Stichopus chloronotus and Holothuria nobilis fractions in human cervical cancer cell line, HeLa. Int. J. Res. Pharm. Sci. 11 (1), 1238–1247.
Chaudhry, G. E., Sohimi, N. K. A., Mohamad, H., Zafar, M. N., Ahmed, A., Sung, Y. Y., et al. (2021). Xylocarpus moluccensis induces cytotoxicity in human hepatocellular carcinoma HepG2 cell line via activation of the extrinsic pathway. Asian pac. J. Cancer Prev. 22 (S1), 17–24. doi:10.31557/APJCP.2021.22.S1.17
Chaudhry, G., Islamiah, M., Ismail, N., Mohamad, H., Sung, Y. Y., and Muhammad, T. S. T. (2018). Induction of apoptosis by aaptos sp., fractions in human breast cancer cell line, mcf-7. Int. J. Res. Pharm. Sci. 9 (2), 328–337.
Chaudhry, G. S., Jan, R., Akim, A., Zafar, M. N., Muhammad, T. S. T., et al. (2021). Breast cancer: A global concern, diagnostic and therapeutic perspectives, mechanistic targets in drug development. Adv. Pharm. Bull. 11 (4), 580–594. doi:10.34172/apb.2021.068
Chaudhry, G. S., Rahman, N. H., Vigneswari, S., Aziz, A., Habsah, M., et al. (2020). Cytotoxicity effect and cell death mechanism of Bruguiera gymnorrhiza extracts on human breast cancer cell line (MCF-7). J. Adv. Pharm. Technol. Res. 11 (4), 233–237.
Chen, Y., Zhou, Q., Zhang, L., Zhong, Y., Fan, G., Zhang, Z., et al. (2017). Stellettin b induces apoptosis in human chronic myeloid leukemia cells via targeting pi3k and stat5. Oncotarget 8, 28906–28921. doi:10.18632/oncotarget.15957
Cheng, A. C., Jian, C. B., Huang, Y. T., Lai, C. S., Hsu, P. C., Pan, M. H., et al. (2007). Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrome c release, and caspases activation in human leukemia cells. Food Chem. Toxicol. 45, 2206–2218. doi:10.1016/j.fct.2007.05.016
Cheung, F. W., Li, C., Che, C. T., Liu, B. P., Wang, L., and Liu, W. K. (2010). Geoditin a induces oxidative stress and apoptosis on human colon HT29 cells. Mar. Drugs 8, 80–90.
Chinen, T., Nagumo, Y., Watanabe, T., Imaizumi, T., Shibuya, M., Kataoka, T., et al. (2010). Irciniastatin a induces jnk activation that is involved in caspase-8-dependent apoptosis via the mitochondrial pathway. Toxicol. Lett. 199, 341–346.
Chiu, C. H., Chou, Y. C., Lin, J. P., Kuo, C. L., Lu, H. F., Huang, Y. P., et al. (2015). Chloroform extract of Solanum lyratum induced G0/G1 arrest via p21/p16 and induced apoptosis via reactive oxygen species, caspases and mitochondrial pathways in human oral cancer cell lines. Am. J. Chin. Med. 43, 1453–1469. doi:10.1142/S0192415X15500822
Chou, W. H., Liu, K. L., Shih, Y. L., Chuang, Y. Y., Chou, J., Lu, H. F., et al. (2018). Ouabain induces apoptotic cell death through caspase- and mitochondria-dependent pathways in human osteosarcoma U-2 OS cells. Anticancer Res. 38, 169–178. doi:10.21873/anticanres.12205
Chung, H., Jung, Y. M., Shin, D. H., Lee, J. Y., Oh, M. Y., Kim, H. J., et al. (2008). Anticancer effects of wogonin in both estrogen receptor-positive and -negative human breast cancer cell lines in vitro and in nude mice xenografts. Int. J. Cancer 122, 816–822. doi:10.1002/ijc.23182
Chung, T. W., Choi, H., Lee, J. M., Ha, S. H., Kwak, C. H., Abekura, F., et al. (2017). Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J. Ethnopharmacol. 195, 309–317.
Chung, T. W., Choi, H., Lee, J. M., Ha, S. H., Kwak, C. H., Abekura, F., et al. (2017). Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J. Ethnopharmacol. 195, 309–317. doi:10.1016/j.jep.2016.11.036
Cox, G., Steward, W. P., and O’Byrne, K. J. (1999). The plasmin cascade and matrix metalloproteinases in non-small cell lung cancer. Thorax 54 (2), 169–179. doi:10.1136/thx.54.2.169
De Stefano, D., Tommonaro, G., Malik, S. A., Iodice, C., De Rosa, S., Maiuri, M. C., et al. (2012). Cacospongionolide and scalaradial, two marine sesterterpenoids as potent apoptosis-inducing factors in human carcinoma cell lines. PLoS One 7, e33031. doi:10.1371/journal.pone.0033031
Decock, J., Thirkettle, S., Wagstaff, L., and Edwards, D. R. (2011). Matrix metalloproteinases: Protective roles in cancer. J. Cell. Mol. Med. 15 (6), 1254–1265. doi:10.1111/j.1582-4934.2011.01302.x
Del Poggetto, E., Tanturli, M., Ben-Califa, N., Gozzini, A., Tusa, I., Cheloni, G., et al. (2015). Salarin C inhibits the maintenance of chronic myeloid leukemia progenitor cells. Cell Cycle 14, 3146–3154. doi:10.1080/15384101.2015.1078029
Dhaheri, Y. A., Attoub, S., Arafat, K., AbuQamar, S., and Viallet, J. (2013). Anti-metastatic and anti-tumor growth effects of origanum majorana on highly metastatic human breast cancer cells: Inhibition of NFκB signaling and reduction of nitric oxide production. PLoS One 8 (7), e68808. doi:10.1371/journal.pone.0068808
Do, M. T., Na, M., Kim, H. G., Khanal, T., Choi, J. H., Jin, S. W., et al. (2014). Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to trail-induced apoptosis through activation of ros-erk/p38 mapk-chop signaling pathways. Food Chem. Toxicol. 71, 51–59.
Duckett, C. S., Li, F., Wang, Y., Tomaselli, K. J., Thompson, C. B., Armstrong, R. C., et al. (1998). Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol. Cell. Biol. 18 (1), 608–615. doi:10.1128/mcb.18.1.608
Dyshlovoy, S. A., Fedorov, S. N., Shubina, L. K., Kuzmich, A. S., Bokemeyer, C., Keller-von Amsberg, G., et al. (2014). Aaptamines from the marine sponge Aaptos sp. Display anticancer activities in human cancer cell lines and modulate ap-1-, Nf-κB-, and p53-dependent transcriptional activity in mouse jb6 cl41 cells. Biomed. Res. Int. 2014, 469309.
Dyshlovoy, S. A., Otte, K., Alsdorf, W. H., Hauschild, J., Lange, T., Venz, S., et al. (2016). Marine compound rhizochalinin shows high in vitro and in vivo efficacy in castration resistant prostate cancer. Oncotarget 7, 69703–69717. doi:10.18632/oncotarget.11941
Elmore, S. (2007). Apoptosis: A review of programmed cell death. Toxicol. Pathol. 35 (4), 495–516. doi:10.1080/01926230701320337
Eltayeb, N. M., Ng, S. Y., Ismail, Z., and Salhimi, S. M. (2016). Anti-invasive effect of catharanthus roseus extract on EFFECT OF Catharanthus roseus extract on highly metastatic human breast cancer MDA-MB-231 cell. Jurnal Teknologi Sci. Eng. 78113, 35–40.
Engel, N., Falodun, A., Kühn, J., Kragl, U., Langer, P., Nebe, B., et al. (2014). Pro-apoptotic and anti-adhesive effects of four African plant extracts on the breast cancer cell line MCF-7. BMC Complement. Altern. Med. 14, 334. doi:10.1186/1472-6882-14-334
Faya, M., Millimouno, J. D., Liu, Y., Jiang, Li., and Xiaomeng, Li. (2014). Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother. Nat. Cancer Prev. Res. (Phila). 7 (11), 1081–1107.
Fearon, E. R., and Vogelstein, B. (1990). A genetic model for colorectal tumorigenesis. Cell 61, 759–767. doi:10.1016/0092-8674(90)90186-i
Fisher David, E. (1994). Apoptosis in cancer therapy: Crossing the threshold. Cell 78 (4), 539–542. doi:10.1016/0092-8674(94)90518-5
Florean, C., Schnekenburger, M., Lee, J. Y., Kim, K. R., Mazumder, A., Song, S., et al. (2016). Discovery and characterization of isofistularin-3, a marine brominated alkaloid, as a new DNA demethylating agent inducing cell cycle arrest and sensitization to trail in cancer cells. Oncotarget 7, 24027–24049. doi:10.18632/oncotarget.8210
Fouad, Y. A., and Aanei, C. (2017). Revisiting the hallmarks of cancer. Am. J. Cancer Res. 7 (5), 1016–1036.
Fulda, S., Galluzzi, L., and Kroemer, G. (2010). Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 9, 447–464. doi:10.1038/nrd3137
Ghavami, S., Hashemi, M., Ande, S. R., Yeganeh, B., Xiao, W., Eshraghi, M., et al. (2009). Apoptosis and cancer: Mutations within caspase genes. J. Med. Genet. 46 (8), 497–510. doi:10.1136/jmg.2009.066944
Ghobrial, I. M., Witzig, T. E., and Adjei, A. A. (2005). Targeting apoptosis pathways in cancer therapy. Ca. Cancer J. Clin. 55 (3), 178–194. doi:10.3322/canjclin.55.3.178
Giorgi, C., Romagnoli, A., Pinton, P., and Rizzuto, R. (2008). Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 8 (2), 119–130. doi:10.2174/156652408783769571
Gorczyca, W., Gong, J., Ardelt, B., TraganosF., , and DarZynkiewicZ, Z. (1993). The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res. 53 (13), 3186–3192.
Gorski, S., and Marra, M. (2002). Programmed cell death takes flight: Genetic and genomic approaches to gene discovery in Drosophila. Physiol. Genomics 9 (2), 59–69. doi:10.1152/physiolgenomics.00114.2001
Green, D. R. (2005). Apoptotic pathways: Ten minutes to dead. Cell 121, 671–674. doi:10.1016/j.cell.2005.05.019
Guzman, E. A., Maers, K., Roberts, J., Kemami-Wangun, H. V., Harmody, D., and Wright, A. E. (2015). The marine natural product microsclerodermin a is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells. Investig. New Drugs 33, 86–94.
Guzman, E. A., Xu, Q., Pitts, T. P., Mitsuhashi, K. O., Baker, C., Linley, P. A., et al. (2016). Leiodermatolide, a novel marine natural product, has potent cytotoxic and antimitotic activity against cancer cells, appears to affect microtubule dynamics, and exhibits antitumor activity. Int. J. Cancer 139, 2116–2126.
Guzman, E., Maher, M., Temkin, A., Pitts, T., and Wright, A. (2013). Spongiatriol inhibits nuclear factor kappa B activation and induces apoptosis in pancreatic cancer cells. Mar. Drugs 11, 1140–1151.
Hajiaghaalipour, F., Kanthimathi, M. S., Sanusi, J., and Rajarajeswaran, J. (2015). White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 169, 401–410. doi:10.1016/j.foodchem.2014.07.005
Hajto, T., Katarina, H., and Hans-Joachim, G. (1989). Modulatory potency of the β-galactoside specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res. 49 (17), 4803–4808.
Hakem, R., and Harrington, L. (2005). The McGraw-hill companies inc. Cell death. 4th ed. New York: .
Hanahan, D., and Weinberg, R. A. (2000). The hallmarks of cancer. Cell 100, 57–70. doi:10.1016/s0092-8674(00)81683-9
Harsha, R. M., Ghosh, D., Banerjee, R., and Salimath, B. P. (2017). Suppression of VEGF-induced angiogenesis and tumor growth by Eugenia jambolana, Musa paradisiaca, and Coccinia indica extracts. Pharm. Biol. 55, 1489–1499. doi:10.1080/13880209.2017.1307422
Hassan, M., Watari, H., AbuAlmaaty, A., Ohba, Y., and Sakuragi, N. (2014). Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 150845. doi:10.1155/2014/150845
Heinrich, M., Robles, M., West, J. E., Ortiz de Montellano, B. R., and Rodriguez, E. (1998). Ethnopharmacology of Mexican asteraceae (compositae). Annu. Rev. Pharmacol. Toxicol. 38, 539–565. doi:10.1146/annurev.pharmtox.38.1.539
Hiraoka, W., VazquezN., , Nieves-Neira, W., Chanock, S. J., and Pommier, Y. (1998). Role of oxygen radicals generated by NADPH oxidase in apoptosis induced in human leukemia cells. J. Clin. Invest. 102 (11), 1961–1968. doi:10.1172/JCI3437
Ho, Y. T., Lu, C. C., Yang, J. S., Chiang, J. H., Li, T. C., Ip, S. W., et al. (2009). Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 29, 4063–4070.
Hood, K. A., West, L. M., Northcote, P. T., Berridge, M. V., and Miller, J. H. (2001). Induction of apoptosis by the marine sponge (mycale) metabolites, mycalamide a and pateamine. Apoptosis 6, 207–219. doi:10.1023/a:1011340827558
Hu, H., Ahn, N. S., Yang, X., Lee, Y. S., and Kang, K. S. (2002). Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int. J. Cancer 102, 250–253. doi:10.1002/ijc.10707
Hu, Q., Wu, D., Chen, W., Yan, Z., and Shi, Y. (2013). Proteolytic processing of the caspase-9 zymogen is required for apoptosome-mediated activation of caspase-9. J. Biol. Chem. 288 (21), 15142–15147. doi:10.1074/jbc.M112.441568
Huang, H. H., Kuo, S. M., Wu, Y. J., and Su, J. H. (2016). Improvement and enhancement of antibladder carcinoma cell effects of heteronemin by the nanosized hyaluronan aggregation. Int. J. Nanomed. 11, 1237–1251.
Huang, Y., Zhou, Y., Fan, Y., and Zhou, D. (2008). Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 264, 101–106. doi:10.1016/j.canlet.2008.01.043
Hudayah, T., Chaudhry, G., Taib, M., Ismail, N., and Mohammad, T. S. T. (2017). Methanol extracts of four selected marine sponges induce apoptosis in human breast cancer cell line, MCF-7. Int. J. Res. Pharm. Sci. 8 (4), 667–675.
Ihsan-ul- Haq, , Mirza, B., Tamara, P., KondratyukEun-Jung, P., Burns, B. E., Marler, L. E., et al. (2013). Preliminary evaluation for cancer chemopreventive and cytotoxic potential of naturally growing ethnobotanically selected plants of Pakistan. Pharm. Biol. 51 (3), 316–328. doi:10.3109/13880209.2012.728612
Israels, E. D., and Israels, L. G. (2000). The cell cycle. Oncologist 5 (6), 510–513. doi:10.1634/theoncologist.5-6-510
Jacobson, M. D., Weil, M., and Raff, M. C. (1997). Programmed cell death in animal development. Cell 88, 347–354. doi:10.1016/s0092-8674(00)81873-5
Jan, R., and Chaudhry, G. E. (2019). Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 9 (2), 205–218. doi:10.15171/apb.2019.024
Johnstone, R. W., Ruefli, A. A., and Lowe, S. W. (2002). Apoptosis: A link between cancer genetics and chemotherapy. Cell 108, 153–164. doi:10.1016/s0092-8674(02)00625-6
Kanduc, D., Mittelman, A., Serpico, R., Sinigaglia, E., Sinha, A. A., Natale, C., et al. (2002). Cell death: Apoptosis versus necrosis (review). Int. J. Oncol. 21 (1), 165–170.
Kavitha, N., EinOon, C., Chen, Y., Kanwar, J. R., and SasidharanPhaleria, S. (2017). Macrocarpa (Boerl.) fruit induce G0/G1 and G2/M cell cycle arrest and apoptosis through mitochondria-mediated pathway in MDA-MB-231 human breast cancer cell. J. Ethnopharmacol. 201, 42–55.
Kerr, J. F., Wyllie, A. H., and Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26 (4), 239–257. doi:10.1038/bjc.1972.33
Khazaei, S., Abdul Hamid, R., Ramachandran, V., Mohd Esa, N., Pandurangan, A. K., Danazadeh, F., et al. (2017). Cytotoxicity and proapoptotic effects of Allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Evid. Based. Complement. Altern. Med. 2017, 1468957. doi:10.1155/2017/1468957
Khazaei, S., Hamid, R. A., Ramachandran, V., Esa, N. M., Pandurangan, A. K., Danazadeh, F., et al. (2017). Cytotoxicity and proapoptotic effects of allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Evid. Based. Complement. Altern. Med. 2017, 1468957. doi:10.1155/2017/1468957
Kijjoa, A., Wattanadilok, R., Campos, N., Nascimento, M. S., Pinto, M., and Herz, W. (2007). Anticancer activity evaluation of kuanoniamines a and c isolated from the marine sponge oceanapia sagittaria, collected from the gulf of Thailand. Mar. Drugs 5, 6–22.
Kim, B. M., Choi, Y. J., Han, Y., Yun, Y. S., and Hong, S. H. N. (2009). N, N-dimethyl phytosphingosine induces caspase-8-dependent cytochrome c release and apoptosis through ROS generation in human leukemia cells. Toxicol. Appl. Pharmacol. 239, 87–97. doi:10.1016/j.taap.2009.05.020
Kim, J. Y., Dao, T. T. P., Song, K., Park, S. B., Jang, H., Park, M. K., et al. (2018). Annona muricata leaf extract triggered intrinsic apoptotic pathway to attenuate cancerous features of triple negative breast cancer MDA-MB-231 cells. Evidence-Based Complementary Altern. Med. 10, 1. doi:10.1155/2018/7972916
Kim, M. O., Moon, D. O., Heo, M. S., Lee, J. D., Jung, J. H., Kim, S. K., et al. (2008). Pectenotoxin-2 abolishes constitutively activated Nf-κB, leading to suppression of Nf-κB related gene products and potentiation of apoptosis. Cancer Lett. 271, 25–33.
Kim, R. (2005). Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer 103 (8), 1551–1560. doi:10.1002/cncr.20947
Kim, Y. L., Lee, S. K., Park, K. K., and Chung, W. Y. (2016). The inhibitory effects of forsythia koreana extracts on the metastatic ability of breast cancer cells and bone resorption by osteoclasts. J. Cancer Prev. 21 (2), 88–94. doi:10.15430/JCP.2016.21.2.88
King-Thom, C. (1997). Gastrointestinal toxicology of monogastrics", gastrointestinal microbiology. Springer US., 511–582.
Klener, P., Andera, L., Klener, P., Necas, E., and Zivný, J. (2006). Cell death signalling pathways in the pathogenesis and therapy of haematologic malignancies: Overview of apoptotic pathways. Folia Biol. 52 (1-2), 34–44.
Koh, R. Y., Lim, F. P., Ling, L. S. Y., Liew, S. F., Yew, M. Y., et al. (2017). Anticancer mechanisms of Strobilanthes crispa Blume hexane extract on liver and breast cancer cell lines. Oncol. Lett. 14 (4), 4957–4964. doi:10.3892/ol.2017.6821
Kong, D., Aoki, S., Sowa, Y., Sakai, T., and Kobayashi, M. (2008). Smenospongine, a sesquiterpene aminoquinone from a marine sponge, induces g1 arrest or apoptosis in different leukemia cells. Mar. Drugs 6, 480–488.
Konishi, H., Kikuchi, S., Ochiai, T., Ikoma, H., Kubota, T., Ichikawa, D., et al. (2009). Latrunculin a has a strong anticancer effect in a peritoneal dissemination model of human gastric cancer in mice. Anticancer Res. 29, 2091–2097.
Kreuger, M. R., Grootjans, S., Biavatti, M. W., Vandenabeele, P., and D'Herde, K. (2012). Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anticancer. Drugs 23, 883–896. doi:10.1097/CAD.0b013e328356cad9
Kumar, S., Sharma, V. K., Yadav, S., and Dey, S. (2017). Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem. Cent. J. 20 (111), 56. doi:10.1186/s13065-017-0281-5
Kwan, Y. P., Saito, T., Ibrahim, D., Al-Hassan, F. M., Ein Oon, C., Chen, Y., et al. (2016). Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 54, 1–14. doi:10.3109/13880209.2015.1064451
LaBarbera, D. V., Modzelewska, K., Glazar, A. I., Gray, P. D., Kaur, M., Liu, T., et al. (2009). The marine alkaloid naamidine a promotes caspase-dependent apoptosis in tumor cells. Anticancer. Drugs 20, 425–436. doi:10.1097/CAD.0b013e32832ae55f
LeBlanc, A. C. (2003). Natural cellular inhibitors of caspases. Prog. Neuropsychopharmacol. Biol. Psychiatry 27 (2), 215–229. doi:10.1016/S0278-5846(03)00017-4
Lee, H. Y., Chung, K. J., Hwang, I. H., Gwak, J., Park, S., Ju, B. G., et al. (2015). Activation of p53 with ilimaquinone and ethylsmenoquinone, marine sponge metabolites, induces apoptosis and autophagy in colon cancer cells. Mar. Drugs 13, 543–557.
Lee, S. H., Park, S. M., Park, S. M., Park, J. H., Shin, D. Y., Kim, G. Y., et al. (2009). Induction of apoptosis in human leukemia U937 cells by anthocyanins through down-regulation of Bcl-2 and activation of caspases. Int. J. Oncol. 34, 1077–1083. doi:10.3892/ijo_00000234
Liu, J., Shen, H. M., Choon-Nam, , and Ong, C. N. (2000). Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG 2 cells. Cancer Lett. 153 (1), 85–93. doi:10.1016/s0304-3835(00)00391-8
Liu, J., Hu, W. X., He, L. F., Ye, M., and Li, Y. (2004). Effects of lycorine on HL 60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 578, 245–250. doi:10.1016/j.febslet.2004.10.095
Liu, W. K., Cheung, F. W., and Che, C. T. (2006). Stellettin a induces oxidative stress and apoptosis in hl-60 human leukemia and lncap prostate cancer cell lines. J. Nat. Prod. 69, 934–937.
Liu, W. K., Ho, J. C., and Che, C. T. (2005). Apoptotic activity of isomalabaricane triterpenes on human promyelocytic leukemia hl60 cells. Cancer Lett. 230, 102–110. doi:10.1016/j.canlet.2004.12.034
Los, M., Van de Craen, M., Penning, L. C., Schenk, H., Westendorp, M., Baeuerle, P. A., et al. (1995). Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature 375 (6526), 81–83. doi:10.1038/375081a0
Lu, P. H., Chueh, S. C., Kung, F. L., Pan, S. L., Shen, Y. C., and Guh, J. H. (2007). Ilimaquinone, a marine sponge metabolite, displays anticancer activity via gadd153-mediated pathway. Eur. J. Pharmacol. 556, 45–54.
Ma, Y. S., Weng, S. W., Lin, M. W., Lu, C. C., Chiang, J. H., Yang, J. S., et al. (2012). Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem. Toxicol. 50, 1271–1278. doi:10.1016/j.fct.2012.01.033
Ma, Y., Zhu, B., Yong, L., Song, C., Liu, X., Yu, H., et al. (2016). Regulation of intrinsic and extrinsic apoptotic pathways in osteosarcoma cells following oleandrin treatment. Int. J. Mol. Sci. 17, 1950. doi:10.3390/ijms17111950
Makoto, I., Suzuki, R., Koide, T., SakaguchiN., , Ogihara, Y., and Yabu, Y. (1994). Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem. Biophys. Res. Commun. 204 (2), 898–904. doi:10.1006/bbrc.1994.2544
Martin, T. A., Ye, L., Sanders, A. J., Lane, J., and Jiang, W. G. (2000). “Cancer invasion and metastasis: Molecular and cellular perspective,” in Madame curie bioscience database. Editors M. V. Blagosklonny, and A. B. Pardee (Austin, TX: Landes Bioscience), 2013.
Matsumoto, K., Akao, Y., Kobayashi, E., Ohguchi, K., Ito, T., Tanaka, T., et al. (2003). ―Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod. 66, 1124–1127. doi:10.1021/np020546u
McNaught, A. D., and Andrew, W. (1997). Compendium of chemical terminology. Oxford Blackwell: IUPAC recommendations.
Medema, J. P., Scaffidi, C., Kischkel, F. C., Shevchenko, A., Mann, M., et al. (1997). FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16 (10), 2794–2804. doi:10.1093/emboj/16.10.2794
Meyer, M., Schreck, R., and Patrick, A. B. (2005). H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 12 (5), 2005–2015. doi:10.1002/j.1460-2075.1993.tb05850.x
Momtazi-borojeni, A. A., Behbahani, M., and Sadeghi-aliabadi, H. (2013). Antiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia marina. Iran. J. Basic Med. Sci. 16 (11), 1203–1208.
Mooberry, S. L., Tien, G., Hernandez, A. H., Plubrukarn, A., and Davidson, B. S. (1999). Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 59, 653–660.
Moon, D. O., Kim, M. O., Kang, S. H., Lee, K. J., Heo, M. S., Choi, K. S., et al. (2008). Induction of G2/M arrest, endoreduplication, and apoptosis by actin depolymerization agent pextenotoxin-2 in human leukemia cells, involving activation of erk and jnk. Biochem. Pharmacol. 76, 312–321.
Mou, H., Zheng, Y., Zhao, P., Bao, H., Fang, W., Xu, N., et al. (2011). Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol. Vitro 25, 1027–1032. doi:10.1016/j.tiv.2011.03.023
Nair, M. S., Soren, K., Singh, V., and Boro, B. (2016). Anticancer activity of fruit and leaf extracts of averrhoa bilimbi on MCF-7 human breast cancer cell lines: A preliminary study. Austin J. Pharmacol. Ther. 4 (2), 1082.
Nakagawa, Y., Iinuma, M., Naoe, T., Nozawa, Y., and Akao, Y. (2007). Characterized mechanism of alpha-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg. Med. Chem. 15, 5620–5628. doi:10.1016/j.bmc.2007.04.071
Neuman, M. G., Katz, G. G., Malkiewicz, I. M., Mathurin, P., Tsukamoto, H., Adachi, M., et al. (2002). Alcoholic liver injury and apoptosis--synopsis of the symposium held at ESBRA 2001: 8th congress of the European society for biomedical research on alcoholism, paris, september 16, 2001. Alcohol 28 (2), 117–128. doi:10.1016/s0741-8329(02)00243-4
Ng, C. P., and Bonavida, B. (2002). A new challenge for successful immunotherapy by tumors that are resistant to apoptosis: Two complementary signals to overcome cross-resistance. Adv. Cancer Res. 85, 145–174. doi:10.1016/s0065-230x(02)85005-9
Nguyen, H. T., Chau, V. M., Tran, T. H., Phan, V. K., Hoang, T. H., Nguyen, T. D., et al. (2009). C29 sterols with a cyclopropane ring at C-25 and 26 from the Vietnamese marine sponge ianthella sp. in addition, their anticancer properties. Bioorg. Med. Chem. Lett. 19, 4584–4588.
Nho, K. J., Chun, J. M., and Kim, H. K. (2015). Anti-metastatic effect of Smilax China L. extract on MDA-MB-231 cells. Mol. Med. Rep. 11, 499–502. doi:10.3892/mmr.2014.2698
Nithya, M., Ambikapathy, V., Panneerselvam, A., and Thajuddin, N. (2014). Anti-tumour activity of different extracts of ganoderma lucidum (curt.: Fr.) p. karst. World J. Pharm. Res. 3 (4), 2204–2214.
Orangi, M., Ardalan, P., Shanehbandi, D., Kazemi, T., Yousefi, B., Behnaz-Alsadat, H., et al. (2016). Cytotoxic and apoptotic activities of methanolic sub fractions of Scrophularia oxysepala against human breast cancer cell line. Evidence-based complementary and alternative medicine.
Orangi, M., Pasdaran, A., Shanehbandi, D., Kazemi, T., Yousefi, B., Hosseini, B. A., et al. (2016). Cytotoxic and apoptotic activities of methanolic subfractions of Scrophularia oxysepala against human breast cancer cell line. Evid. Based. Complement. Altern. Med. 2016, 8540640. doi:10.1155/2016/8540640
Pal, H. C., Sehar, I., Bhushan, S., Gupta, B. D., and Saxena, A. K. (2010). Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol. Vitro 24, 1599–1609. doi:10.1016/j.tiv.2010.06.007
Pan, M. H., Lai, C. S., Hsu, P. C., and Wang, Y. J. (2005). Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J. Agric. Food Chem. 53, 620–630. doi:10.1021/jf048430m
Park, S. E., Shin, W. T., Park, C., Hong, S. H., Kim, G. Y., Kim, S. O., et al. (2014). Induction of apoptosis in MDA-MB-231 human breast carcinoma cells with an ethanol extract of Cyperus rotundus L. by activating caspases. Oncol. Rep. 32, 2461–2470.
Pistritto, G., Trisciuoglio, D., Ceci, C., Garufi, A., and D'Orazi, G. (2016). Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 8 (4), 603–619. doi:10.18632/aging.100934
Pucci, B., Kasten, M., and Giordano, A. (2000). Cell cycle and apoptosis. Neoplasia 2 (4), 291–299. doi:10.1038/sj.neo.7900101
Ranganatha, R. S., Mahesh, H., Kishore, K. C., Anjaneyulu, M., Bibha, C., and Sathees, C. (2012). Raghavan extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One 7 (10), e47021.
Rao, C. V., Rivenson, A., Simi, B., Zang, E., Kelloff, G., Steele, V., et al. (1995). Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti- inflammatory agent. Cancer Res. 55 (7), 1464–1472.
Rao Chinthalapally, V., Rivenson, A., Simi, B., and Reddy, B. S. (1995). Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 55 (2), 259–266.
Reddy, B. S., Wang, C. X., Samaha, H., Lubet, R., Steele, V. E., Kelloff, G. J., et al. (1997). Chemoprevention of colon carcinogenesis by dietary perillyl alcohol. Cancer Res. 57, 420–425.
Reed, J. C., and Green, D. R. (2002). Remodeling for demolition: Changes in mitochondrial ultrastructure during apoptosis. Mol. Cell 9, 1–3. doi:10.1016/s1097-2765(02)00437-9
Ren, W. E. I. P. I. N. G., and Tang, D. G. (1999). Extract of Solanum muricatum (Pepino/CSG) inhibits tumor growth by inducing apoptosis. Anticancer Res. 191A, 403–408.
Rezaei, P. F., Fouladdel, Sh., Cristofanon, S., Ghaffari, S. M., Amin, G. R., and Azizi, E. (2011). Comparative cellular and molecular analysis of cytotoxicity and apoptosis induction by doxorubicin and Baneh in human breast cancer T47D cells. Cytotechnology 63 (5), 503–512.
Roel, M., Rubiolo, J. A., Guerra-Varela, J., Silva, S. B., Thomas, O. P., Cabezas-Sainz, P., et al. (2016). Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget 7, 83071–83087. doi:10.18632/oncotarget.13068
Rose, P., Huang, Q., Ong, C. N., and Whiteman, M. (2005). Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 209 (2), 105–113. doi:10.1016/j.taap.2005.04.010
Rothmeier, A. S., Schneiders, U. M., Wiedmann, R. M., Ischenko, I., Bruns, C. J., Rudy, A., et al. (2010). The marine compound spongistatin 1 targets pancreatic tumor progression and metastasis. Int. J. Cancer 127, 1096–1105.
Sabino, A. P. L., Eustáquio, L. M. S., Miranda, A. C. F., Biojone, C., Mariosa, T. N., Gouvêa, C. M. C. P., et al. (2018). Stryphnodendron adstringens ("Barbatimão") leaf fraction: Chemical characterization, antioxidant activity, and cytotoxicity towards human breast cancer cell lines. Appl. Biochem. Biotechnol. 184 (4), 1375–1389. doi:10.1007/s12010-017-2632-z
Sakagami, H., KuribayashiN., , IidaM., , Sakagami, T., TakedaM., , FuKuchi, K., et al. (1994). Induction of DNA fragmentation by tannin-and lignin-related substances. Anticancer Res. 15 (5B), 2121–2128.
Salma, Y., Lafont, E., Therville, N., Carpentier, S., Bonnafe, M. J., Levade, T., et al. (2009). The natural marine anhydrophytosphingosine, jaspine b, induces apoptosis in melanoma cells by interfering with ceramide metabolism. Biochem. Pharmacol. 78, 477–485.
Sankari, S. L., Masthan, K. M., Babu, N. A., Bhattacharjee, T., and Elumalai, M. (2012). Apoptosis in cancer-an update. Asian pac. J. Cancer Prev. 13 (10), 4873–4878. doi:10.7314/apjcp.2012.13.10.4873
Santos, K. M., Gomes, I. N. F., Romao, W., Ribeiro, R. I. M. A., Silva-Oliveira, R. J., Pinto, F. E., et al. (2017). Bauhinia stem extracts as a possible new treatment for breast cancer and metastasis: Inhibition of migration, invasion and of the activity of matrix metalloproteinases. J. Biotechnol. Biomater. 7, 6.
Schneiders, U. M., Schyschka, L., Rudy, A., and Vollmar, A. M. (2009). Bh3-only proteins mcl-1 and bim as well as endonuclease g are targeted in spongistatin 1-induced apoptosis in breast cancer cells. Mol. Cancer Ther. 8, 2914–2925.
Schultze, J. L., Stettin, A., and Berg, P. A. (1991). Demonstration of specifically sensitized lymphocytes in patients trea ted with an aqueous mistletoe extract (Viscum album L.). Klin. Wochenschr. 69 (9), 397–403. doi:10.1007/BF01647413
Schumacher, M., Cerella, C., Eifes, S., Chateauvieux, S., Morceau, F., Jaspars, M., et al. (2010). Heteronemin, a spongean sesterterpene, inhibits tnf alpha-induced NF-κB activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharmacol. 79, 610–622.
Schyschka, L., Rudy, A., Jeremias, I., Barth, N., Pettit, G. R., and Vollmar, A. M. (2008). Spongistatin 1: A new chemosensitizing marine compound that degrades xiap. Leukemia 22, 1737–1745. doi:10.1038/leu.2008.146
Sharma, K., Pachauri, S. D., Khandelwal, K., Ahmad, H., AryA, A., Biala, P., et al. (2016). Anticancer effects of extracts from the fruit of Morinda citrifolia (noni) in breast cancer cell lines. Drug Res. 66 (3), 141–147. doi:10.1055/s-0035-1555804
Shin, D. Y., Kim, G. Y., Kim, N. D., Jung, J. H., Kim, S. K., Kang, H. S., et al. (2008). Induction of apoptosis by pectenotoxin-2 is mediated with the induction of DR4/DR5, EGR-1 and NAG-1, activation of caspases and modulation of the Bcl-2 family in p53-deficient Hep3B hepatocellular carcinoma cells. Oncol. Rep. 19, 517–526.
Shin, S. A., Lee, H. N., Choo, G. S., Kim, H. J., Che, J. H., Jung, J. Y., et al. (2017). Ixeris dentata (thunb. Ex thunb.) nakai extract inhibits proliferation and induces apoptosis in breast cancer cells through akt/NF-κB pathways. Int. J. Mol. Sci. 18 (2), E275. doi:10.3390/ijms18020275
Shin, S. Y., Kim, C. G., Jung, Y. J., Jung, Y., Jung, H., Im, J., et al. (2016). Euphorbia humifusa Willd exerts inhibition of breast cancer cell invasion and metastasis through inhibition of TNFα-induced MMP-9 expression. BMC Complement. Altern. Med. 16 (1), 413. doi:10.1186/s12906-016-1404-6
Shu-Hui, H., Tsai, T. R., Lin, C. N., Yen, M. H., and Kuo, K. W. (1996). Solamargine purified from Solanum incanum Chinese herb triggers gene expression of human TNFR I which may lead to cell apoptosis. Biochem. Biophys. Res. Commun. 229, 1–5. doi:10.1006/bbrc.1996.1748
Shu-Wei, Y. (1999). Synthesis and biological evaluation of analogues of cryptolepine, an alkaloid isolated from the Suriname rainforest 1. J. Nat. Prod. 62 (7), 976–983.
Silva, M. J. D., Carvalho, A. J. S., Rocha, C. Q., Vilegas, W., Silva, M. A., and Gouvêa, C. M. C. P. (2014). Ethanolic extract of Mimosa caesalpiniifolia leaves: Chemical characterization and cytotoxic effect on human breast cancer MCF-7 cell line. South Afr. J. Bot. 93, 64–69.
Somasagara, R. R., Hegde, M., Chiruvella, K. K., Musini, A., Choudhary, B., Raghavan, S. C., et al. (2012). Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One 7 (10), e47021. doi:10.1371/journal.pone.0047021
Stergiou, L., and Hengartner, M. O. (2004). Death and more: DNA damage response pathways in the nematode C. elegans. Cell Death Differ. 11 (1), 21–28. doi:10.1038/sj.cdd.4401340
Su, Z. Z., Lin, J., PrewettM., , Goldstein, N. I., and Fisher, P. B. (1994). Apoptosis mediates the selective toxicity of caffeic acid phenethyl ester (CAPE) toward oncogene-transformed rat embryo fibroblast cells. Anticancer Res. 15 (5), 1841–1848.
Subapriya, R., Bhuvaneswari, V., and Nagini, S. (2005). Ethanolic neem (Azadirachta indica) leaf extract induces apoptosis in the hamster buccal pouch carcinogenesis model by modulation of Bcl-2, Bim, caspase 8 and caspase 3. Asian pac. J. Cancer Prev. 6, 515–520.
Sudhakar, A. (2009). History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther. 1 (2), 1–4. doi:10.4172/1948-5956.100000e2
Sui, C. G., Meng, F. D., Li, Y., and Jiang, Y. H. (2015). Antiproliferative activity of rosamulticacidis associated with induction of apoptosis, cell cycle arrest, inhibition of cell migration and caspase activation in human gastric cancer (SGC-7901) cells. Phytomedicine 22, 796–806. doi:10.1016/j.phymed.2015.05.004
Tang, S. A., Zhou, Q., Guo, W. Z., Qiu, Y., Wang, R., Jin, M., et al. (2014). In vitro antitumor activity of stellettin b, a triterpene from marine sponge jaspis stellifera, on human glioblastoma cancer sf295 cells. Mar. Drugs 12, 4200–4213.
Tang, S. Y., Zhong, M. Z., Yuan, G. J., Hou, S. P., Yin, L. L., Jiang, H., et al. (2013). Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol. Rep. 29, 474–480. doi:10.3892/or.2012.2127
Tayarani-Najarana, Z., Asilib, J., Aioubib, E., and Emami, S. A. (2013). Growth inhibition and apoptosis induction of salvia chloroleuca on MCF-7 breast cancer cell line. Iran. J. Pharm. Res. 12 (4), 789–799.
Thamizhiniyan, V., Choi, Y. W., and Kim, Y. K. (2015). The cytotoxic nature of Acanthopanax sessiliflorus stem bark extracts in human breast cancer cells. Saudi J. Biol. Sci. 22 (6), 752–759.
Thompson, C. B. (1995). Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462. doi:10.1126/science.7878464
Tor, Y. S., Yazan, L. S., Foo, J. B., Wibowo, A., Ismail, N., Cheah, Y. K., et al. (2015). Induction of apoptosis in MCF-7 cells via oxidative stress generation, mitochondria-dependent and caspase-independent pathway by ethyl acetate extract of Dillenia suffruticosa and its chemical profile. PLoS One 10 (6), e0127441. doi:10.1371/journal.pone.0127441
Trisciuoglio, D., Uranchimeg, B., Cardellina, J. H., Meragelman, T. L., Matsunaga, S., Fusetani, N., et al. (2008). Induction of apoptosis in human cancer cells by candidaspongiolide, a novel sponge polyketide. J. Natl. Cancer Inst. 100, 1233–1246.
Umeyama, A., Matsuoka, N., Mine, R., Nakata, A., Arimoto, E., Matsui, M., et al. (2010). Polyacetylene diols with antiproliferative and driving Th1 polarization effects from the marine sponge Callyspongia sp. J. Nat. Med. 64, 93–97.
Venderova, K., and Park, D. S. (2012). Programmed cell death in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2 (8), a009365. doi:10.1101/cshperspect.a009365
Verdine, G. L. (1996). The combinatorial chemistry of nature. Nature 384 (1), 11–13. doi:10.1038/384011a0
Vogelstein, B., and Kinzler, K. W. (2004). Cancer genes and the pathways they control. Nat. Med. 10, 789–799. doi:10.1038/nm1087
Wang, M. K., Ding, L. S., and Wu, F. E. (2008). Antitumor effects of raddeanin A on S180, H22 and U14 cell xenografts in mice. Ai Zheng 27, 910–913.
Wang, R., Zhang, Q., Peng, X., Zhou, C., Zhong, Y., Chen, X., et al. (2016). Stellettin b induces g1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci. Rep. 6, 27071.
Wei, S. Y., Li, M., Tang, S. A., Sun, W., Xu, B., Cui, J. R., et al. (2008). Induction of apoptosis accompanying with g(1) phase arrest and microtubule disassembly in human hepatoma cells by jaspolide b, a new isomalabaricane-type triterpene. Cancer Lett. 262, 114–122. doi:10.1016/j.canlet.2007.11.039
Weimin, F. (1999). Possible mechanisms of paclitaxel-induced apoptosis. Biochem. Pharmacol. 57 (11), 1215–1221. doi:10.1016/s0006-2952(99)00006-4
Wicaksono, B. D., Handoko, Y. A., Arung, E. T., Kusuma, I. W., Yulia, D., Pancaputra, A. N., et al. (2009). Antiproliferative effect of the methanol extract of piper crocatum ruiz & pav leaves on human breast (T47D) cells in-vitro. Trop. J. Pharm. Res. 8 (4).
Wolvetang Ernst, J., Larm, J. A., Moutsoulas, P., and LAwen, A. (1996). Apoptosis induced by inhibitors of the plasma membrane NADH-oxidase involves Bcl-2 and calcineurin. Cell Growth Differ. 7 (10), 1315–1325.
Wong, F. C., Woo, C. C., Hsu, A., Kwong, B., and Tan, H. (2013). The anti-cancer activities of vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One 8 (10), e78021. doi:10.1371/journal.pone.0078021
Wu, J. C., Wang, C. T., Hung, H. C., Wu, W. J., Wu, D. C., Chang, M. C., et al. (2016). Heteronemin is a novel c-Met/STAT3 inhibitor against advanced prostate cancer cells. Prostate 76, 1469–1483. doi:10.1002/pros.23230
Wu, S. Y., Sung, P. J., Chang, Y. L., Pan, S. L., and Teng, C. M. (2015). Heteronemin, a spongean sesterterpene, induces cell apoptosis and autophagy in human renal carcinoma cells. Biomed. Res. Int. 2015, 738241.
Xu, W., Wang, X., Tu, Y., Masaki, H., Tanaka, S., Onda, K., et al. (2019). Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chem. Biol. Interact. 310, 108726. doi:10.1016/j.cbi.2019.108726
Xue, G., Zou, X., Zhou, J. Y., Sun, W., Wu, J., Xu, J. L., et al. (2013). Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro. Biochem. Biophys. Res. Commun. 439, 196–202. doi:10.1016/j.bbrc.2013.08.060
Yano, H., TatsutaM., , IisHi, H., BabaM., , SakaiN., , and UedoN., (1999). Attenuation by d‐limonene of sodium chloride‐enhanced gastric carcinogenesis induced by N‐methyl‐N′‐nitro‐N‐nitrosoguanidine in Wistar rats. Int. J. Cancer 82 (5), 665–668. doi:10.1002/(sici)1097-0215(19990827)82:5<665::aid-ijc8>3.0.co;2-e
Yasser, M. T., Saad, S. D., Loiy, E., Ahmed, H., Majed Ahmed, Al-M., Mohamad, T. A., et al. (2015). Vitro anti-metastatic and antioxidant activity of nicotiana glauca fraction against breast cancer cells. Adv. Biol. Res. 9 (2), 95–102.
Yeung, T. K., Germond, C., Chen, X., and Wang, Z. (1999). The mode of action of taxol: Apoptosis at low concentration and necrosis at high concentration. Biochem. Biophys. Res. Commun. 263 (2), 398–404. doi:10.1006/bbrc.1999.1375
Yoo, H., Lee, Y. S., Lee, S., Kim, S., and Kim, T. Y. (2012). Pachastrissamine from Pachastrissa sp. Inhibits melanoma cell growth by dual inhibition of Cdk2 and erk-mediated foxo3 downregulation. Phytother. Res. 26, 1927–1933.
Yuan, S., and Akey, C. W. (2013). Apoptosome structure, assembly, and procaspase activation. Structure 21 (4), 501–515. doi:10.1016/j.str.2013.02.024
Zhang, Y. W., Ghosh, A. K., and Pommier, Y. (2012). Lasonolide a, a potent and reversible inducer of chromosome condensation. Cell Cycle 11, 4424–4435. doi:10.4161/cc.22768
Zhao, Y., Tian, B., Wang, Y., and Ding, H. (2017). Kaempferol sensitizes human ovarian cancer cells-OVCAR-3 and SKOV-3 to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-Induced apoptosis via JNK/ERK-CHOP pathway and up-regulation of death receptors 4 and 5. Med. Sci. Monit. 23, 5096–5105. doi:10.12659/msm.903552
Zhou, R., Chen, H., Chen, J., Chen, X., Wen, Y., Xu, L., et al. (2018). Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 18 (1), 83. doi:10.1186/s12906-018-2148-2
Zihlif, M., Afifi, F., Abu-Dahab, R., Majid, A. M. S. A., Somrain, H., et al. (2013). The antiangiogenic activities of ethanolic crude extracts of four Salvia species.Salvia species. BMC Complement. Altern. Med. 13, 358. doi:10.1186/1472-6882-13-358
Zornig, M., Hueber, A., Baum, W., and Evan, G. (2001). Apoptosis regulators and their role in tumorigenesis. Biochim. Biophys. Acta 1551, 1–37. doi:10.1016/s0304-419x(01)00031-2
Keywords: apoptosis, marine drug, cancer, natural product, targeted therapeutic, apoptotic inducers
Citation: Chaudhry G-e-S, Md Akim A, Sung YY and Sifzizul TMT (2022) Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol. 13:842376. doi: 10.3389/fphar.2022.842376
Received: 23 December 2021; Accepted: 13 July 2022;
Published: 10 August 2022.
Edited by:
Nehad M. Ayoub, Jordan University of Science and Technology, JordanReviewed by:
Pamela Ovadje, Innovate Calgary, CanadaMrutyunjay Jena, Berhampur University, India
Huidi Liu, Harbin Medical University, China
Copyright © 2022 Chaudhry, Md Akim, Sung and Sifzizul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gul-e-Saba Chaudhry, c2FiYWJpb2NoZW1AZ21haWwuY29t, Z3VsLnNhYmFAdW10LmVkdS5teQ==
 Gul-e-Saba Chaudhry
Gul-e-Saba Chaudhry Abdah Md Akim
Abdah Md Akim Yeong Yik Sung
Yeong Yik Sung Tengku Muhammad Tengku Sifzizul
Tengku Muhammad Tengku Sifzizul