- 1Department of Nephrology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 4Tianjin Jinnan Traditional Chinese Medicine Hospital, Tianjin, China
Tripterygium wilfordii Hook. f. (TwHF) is a Chinese botanical drug containing a large number of metabolites. The discovered and recognized anti-inflammatory and immune-regulating effects have made it attract more and more attentions in trials and clinical researches. The extraction and processing of TwHF for pharmaceuticals is a manifestation of the role of traditional Chinese medicine. However, TwHF is toxic. Optimization of TwHF preparations has become a requirement for the development of TwHF pharmaceuticals. Our article introduces the main preparations of TwHF on the Chinese market and their characteristics. In particular, we summarize the clinical applications and influential mechanisms of TwHF and its preparations in kidney diseases. Considering that nephropathy is closely related to immune inflammation and TwHF is a botanical drug with a high number of metabolites, the application of TwHF in kidney diseases may be much more complicated. By revealing the role and mechanisms of TwHF in kidney diseases, this study aims to provide more insights to basic and clinical studies about nephropathy.
Introduction
Tripterygium wilfordii Hook. f. (Celastraceae) (known in Chinese as Leigongteng) (TwHF) (Figure 1), the traditional botanical drug in China, was first recorded in one of the four classics of traditional Chinese medicine-the Divine Farmer’s Materia Medica and has been used in China for hundreds of years (Xiao et al., 2019). TwHF is a species of Tripterygium Hook. f. (Celastraceae), but the phylogenetic relationship within Tripterygium Hook. f. was unclear, which led to the taxonomic controversy of the genus. Previous perspectives showed that TwHF, Tripterygium hypoglaucum (H.Lév.) Hutch. (Celastraceae) (known in Chinese as kunmiminshanhaitang) (THH) are two species of the genus (Law et al., 2011). In recent years, based on the DNA sequencing technology, researchers have found that TwHF and THH are actually the same species (Law et al., 2011; Zhang X. et al., 2016). Si (2008) also hold the view that THH was a variant of TwHF. Several taxonomic listings [WCVP (WCVP, 2022), WFO (The World Flora Online, 2022)] have already showed that THH is a synonym of TwHF. However, the composition distribution and toxicity of the two are different (Xia et al., 1994). TwHF and THH have long been regarded as two species in traditional Chinese medicine, and various pharmacognostic methods have been developed to tell them apart (Law et al., 2011). Moreover, Chinese patent medicines that clearly use THH as ingredients have been marketed and used in China. We agree that TwHF and THH are a single species. But based on the above reasons, our review still uses the name THH to distinguish typical TwHF.
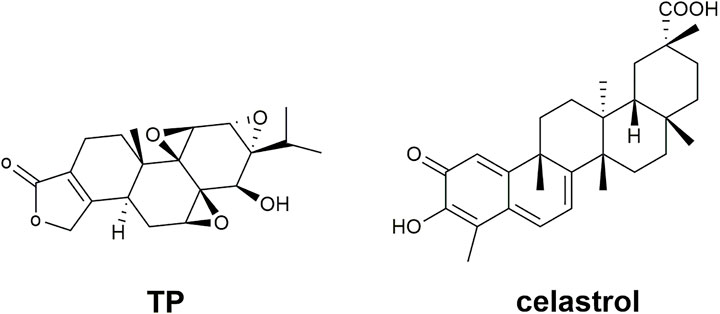
TwHF preparations include single TwHF preparations and compound preparations compatible with other Chinese medicines. TwHF itself is a botanical drug with complex and diverse chemical components. At present, there are more than 400 natural metabolites isolated and characterized from TwHF, mainly alkaloids, triterpenoids, diterpenoids and sesquiterpenes (Xiao et al., 2019). The most commonly studied natural active metabolites in TwHF are listed in Table 1, including their molecular formulas, molecular weights, toxicity, and toxic target organs. In particular, the metabolites that have anti-inflammatory and immunomodulatory effects concentrate in diterpenoids and alkaloids, which are also the main toxic ingredients in TwHF that usually cause side effects (Xue et al., 2010; Nong et al., 2019). Previous studies have shown that TwHF preparations have multiple toxicities such as gastrointestinal toxicity, reproductive toxicity, liver toxicity, nephrotoxicity, and skin toxicity (Huang et al., 2016; Nong et al., 2019; Ru et al., 2019). The study of Ru et al. (2019) showed that gastrointestinal toxicity is the most commonly observed toxicity of TwHF preparations, followed by reproductive toxicity. More importantly, the toxic dosage and the therapeutic dosage are similar, which makes the safety of TwHF more concerned (Liu LL. et al., 2019). Among the diterpenoids, triptolide (TP), a triepoxide, with its structural formula showing in Figure 2, has the strongest activity and toxicity. In addition to anti-inflammatory and immune suppression, TP is also used for anti-tumor, anti-osteoporosis and other diseases (Tong et al., 2021). In addition, many constituents of alkaloids are also believed to have the effects of anti-human immunodeficiency virus (Horiuch et al., 2006), anti-tumor (Jiang et al., 2014), analgesic (Liu L. et al., 2019), etc. Among the triterpenoid, Celastrol is currently the most extensively studied component-its structural formula displaying in Figure 2. Besides the anti-tumor effect (Lim et al., 2021), it can regulate lipid metabolism and is an effective anti-obesity agent (Feng et al., 2019). It also has the potential in central nervous system diseases’ treatment (Schiavone et al., 2021). As the chemical composition of TwHF is complex, and it contains toxic ingredients, toxin reduction and efficiency enhancement have always been the goal of TwHF preparations.
In recent years, research on the extraction and dosage forms of TwHF has gained more and more attentions. TwHF can be viewed as a “Chinese medicinal hormone”, with anti-inflammatory and immune regulation as its main pharmacological effects. Previous studies on TwHF preparations focused more on rheumatoid arthritis. However, there are relatively limited studies about TwHF preparations on kidney diseases. The glomerulus is a capillary globule. Circular immune complexes deposition and its own antigenic properties, such as glomerular basement membrane, can form in situ immune complexes, which determines that kidney diseases are closely related to immune inflammation response (Kant et al., 2021). Therefore, TwHF preparations should also have a broader application in the field of kidney diseases. Our research summarizes the development of TwHF preparations in recent years and their applications and mechanisms in nephropathy, looking forward to providing more insights to basic and clinical researches.
TwHF Preparations
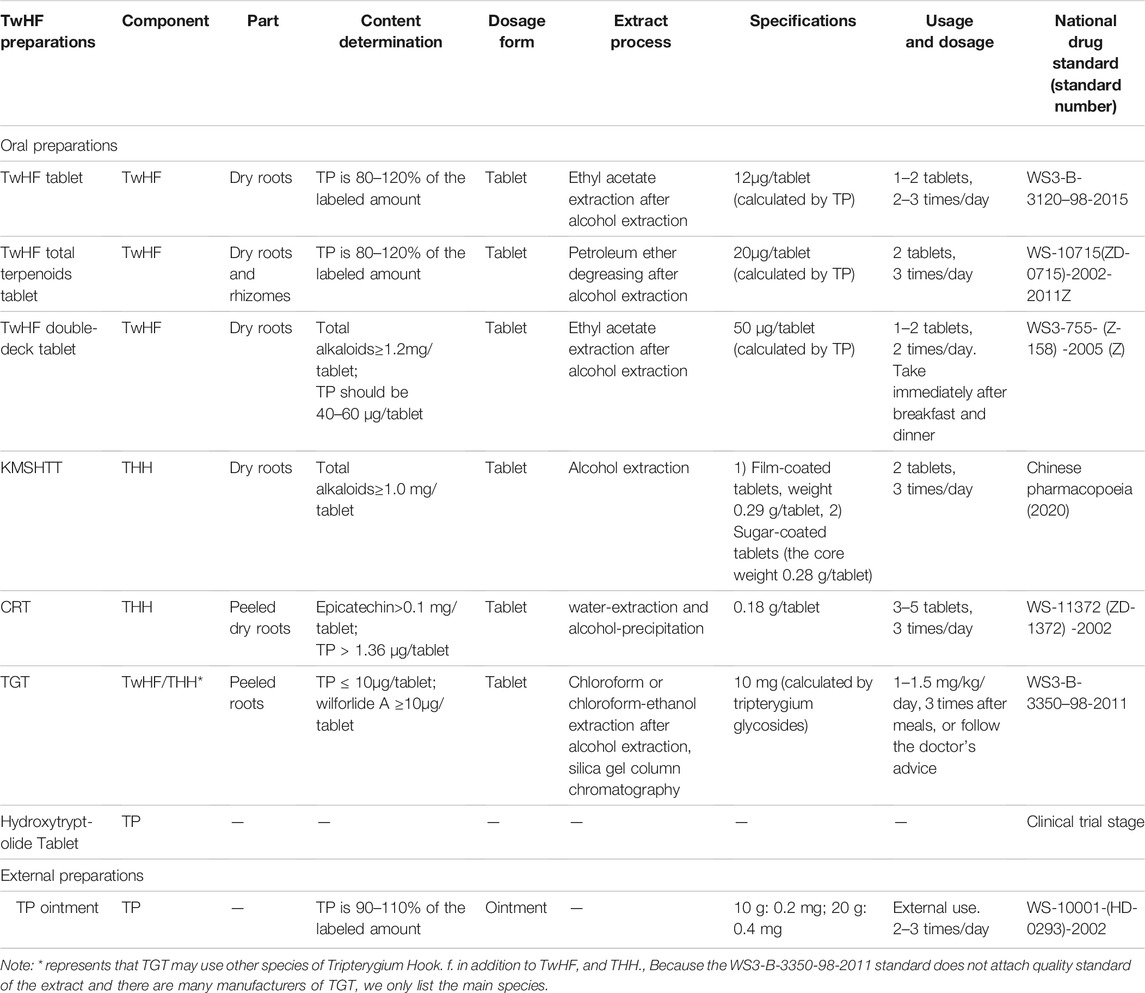
TwHF preparations include single TwHF preparations and compound TwHF preparations. Table 2 displays the most commonly used single TwHF preparations on the Chinese market. TwHF tablet and kunmingshanhaitang tablet (KMSHTT) are the representatives of single TwHF preparations. Both TwHF tablet and TwHF double-deck tablet use the ethanol-ethyl acetate extraction process, so their ingredients are similar, and the content of terpenoids is relatively high (Wang et al., 2019). Unlike TwHF tablet, TwHF double-deck tablet has two decks of drug releases, fast release and sustained release, with 30% released quickly in the stomach and 70% released slowly in the intestine. This feature reduces the side effects mainly based on gastrointestinal tract and prolongs the blood concentration (Li, 2002). TwHF total terpenoids tablet is extracted with ethanol and then treated with a low-polar solvent. This method retains the terpenoids and removes the oil that irritates to gastrointestinal tract, and also reduces the side effects after taking the medicine (Wang, 1996). KMSHTT uses the dried roots of THH as a medicine. In terms of chemical composition, the roots of typical TwHF and THH are similar (Huang et al., 2010), but there are certain differences in content distribution. Xia et al. (1994) used thin-layer chromatography to compare the components of TwHF and THH. They found that the content of diterpene lactones and celastrol in TwHF is higher than that in THH, and the content in the root bark is higher than the root core in both botanical drugs. In addition, the content of the remaining triterpenoids in TwHF is less than that in THH, and the content in the root bark is less than the root core in both botanical drugs. Moreover, the content of alkaloid is the same between TwHF and THH, and for both botanical drugs, the content in the root bark is higher than the root core (Xia et al., 1994). That is to say, TwHF, especially the root bark, is more toxic, the dosage of which should be paid higher attentions clinically. Tripterygium glycosides tablet (TGT) uses peeled root of typical TwHF/THH and maybe other species of the same genus. After procedures including alcohol extraction, chloroform or chloroform-ethanol extraction, and silica gel column chromatography, the composition of TGT is relatively simple, which consists a targeted collection of terpenes ingredients and hence reduces the toxicity and side effects (Wang et al., 2019; Sun et al., 2021). THH is also called colquhounia. Colquhounia root tablet (CRT) adopts peeled roots of THH, using water-extraction and alcohol-precipitation extract technology, which reduces the toxicity and side effects as well (Lin and Qi, 2005).
Hydroxytriptolide tablet, developed by shanghai institute of materia medica, consists of (5R)-5-hydroxytriptolide (LLDT-8), which is a hydroxylated derivative of TP (Xi et al., 2017) (the structural formula is shown in Figure 3). TP is the main active ingredient of TwHF, however, its strong and extensive toxicity restricts its clinical application (Zhou et al., 2005). How to maintain the biological activity while reduce the toxicity has gained increasing research popularity in recent years. LLDT-8, as a derivative of TP, remains to show effective anti-inflammatory and immunosuppressive activities (Tang and Zuo, 2012), and its toxicity is greatly reduced (Xi et al., 2017). Until now, its phase 1 + 2 clinical trials on the effectiveness and safety of rheumatoid arthritis, which is insufficient response to methotrexate, have been completed (Registration number: CTR20140491). There are also two clinical trials still in progress (Registration number: CTR20191397 and CTR20201397). LLDT-8 is expected to be the best derivative of TP.
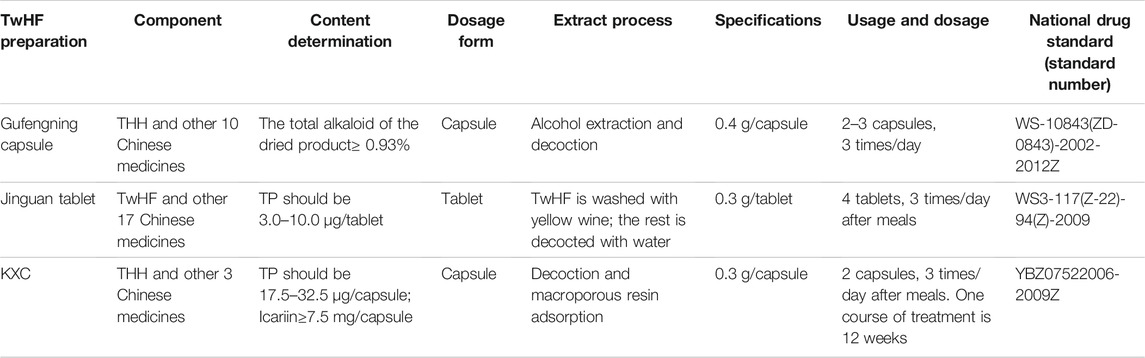
Table 3 lists the commonly used compound TwHF preparations on the Chinese market. Gufengning capsule and Jinguan tablet are early compound TwHF preparations, which work together with other Chinese medicines that can tonify liver and kidney or promote blood circulation and dredging collaterals. There are relatively few literatures on these two drugs. Kunxian capsule (KXC) is a new generation of compound TwHF preparations, composed of THH, Epimedium brevicornu Maxim (Berberidaceae), Lycium barbarum L. (Solanaceae) and Cuscuta chinensis Lam. (Convolvulaceae). Each component is extracted with the macroporous resin adsorption technology, and the high performance liquid chromatography technology is adopted to detect their active ingredients, which reduce the toxicity and side effects of KXC (Lu and Yuan, 2021). A controlled experiment of KXC and TGT on the treatment of chronic kidney disease (CKD) showed that both can reduce the proteinuria level, but KXC took effects more quickly and treated more pathological types (Tu et al., 2021). Based on a Network pharmacology analysis, KXC can regulate the proliferation of inflammatory cells via a variety of signaling pathways. Meanwhile, Epimedium brevicornu Maxim, Lycium barbarum L., and Cuscuta chinensis Lam. can enhance the therapeutic effect of THH and weaken its side effects (Tang Y. et al., 2020).
Clinical Applications and Mechanisms of TwHF and its Preparations in Kidney Diseases
Clinical Applications
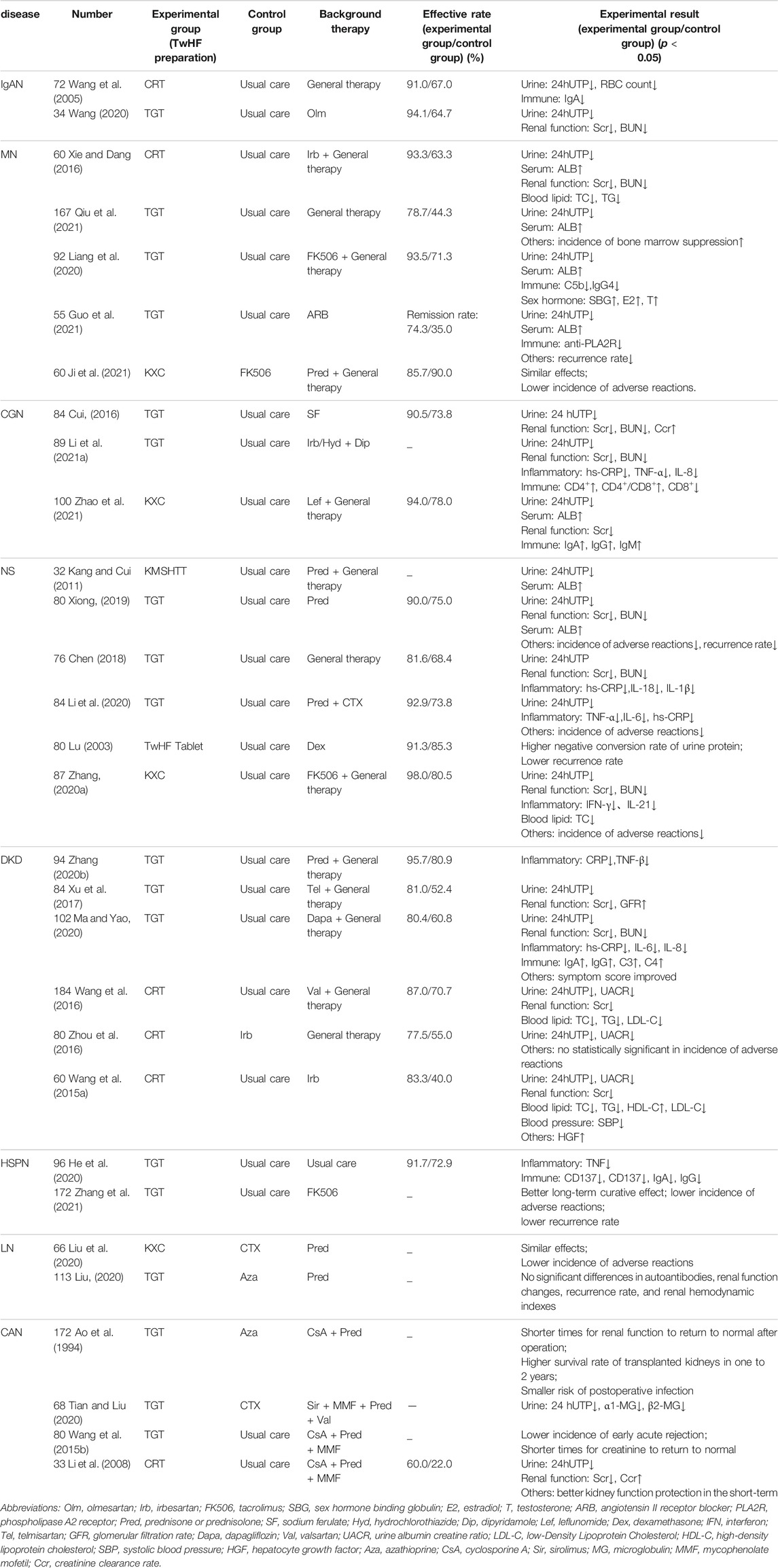
The bibliometric analysis shows that research and applications of TwHF preparations have focused on kidney diseases, rheumatic immune diseases, and immune-related skin diseases nowadays (Wang H. Y. et al., 2021). Table 4 summarizes the clinical studies of various TwHF preparations on the treatment of different kidney diseases in recent years. From the table, it can be seen that kidney diseases concerns IgA nephropathy (IgAN), membranous nephropathy (MN), chronic glomerulonephritis (CGN), nephrotic syndrome (NS), diabetic kidney disease (DKD), henoch-schonlein purpura nephritis (HSPN), lupus nephritis (LN) and chronic allograft nephropathy (CAN). The results generally show that compared with the control group, the experimental group that receives the treatments of TwHF preparations show the decline of 24-h urine total protein (24hUTP) quantity, the decline of renal function indexes such as blood urea nitrogen (BUN) and serum creatinine (Scr), the decline of blood lipid indexes such as total cholesterol (TC) and triglycerides (TG), the increase of serum albumin (ALB), the decrease of inflammatory factors [e.g., hypersensitive c-reactive protein (hs-CRP), interleukin-(IL-) 6 and tumor necrosis factor (TNF)], the up and down regulations of immune indicators [e.g., immunoglobulin (Ig) G and complement C3], as well as the lower incidence of adverse reactions and lower recurrence rate. The effective rate can be statistically significant or insignificant, suggesting that the efficacy of TwHF preparations alone or in combination with other drugs is higher than or equivalent to control group. There are also studies evaluating the effectiveness of TwHF preparations in kidney diseases using meta-analysis. For instance, Jin et al. (2021) conducted a meta-analysis of animal experiments on the treatment of DKD with TwHF, and found the adoption of TwHF in animals was also effective and safe. Chen et al. (2013) carried out a meta-analysis of TwHF preparations on the treatment of primary NS. The results showed that TwHF preparations had an additional effect on the remission of primary NS, but there was insufficient evidence to prove that they were as effective as prednisone (Pred) and cyclophosphamide (CTX). Therefrom they proposed TwHF can be directly compared with the widely used immunosuppressive agents in the future (Chen et al., 2013).
TwHF preparations can also be applied to CAN, the chronic kidney injury after transplantation, which is an important factor affecting the effectiveness of kidney transplantation. CAN is a complex multi-causal process. Chronic rejection, recurrence of original kidney disease, newly acquired conditions and toxicity of immunosuppressive agents can all result in graft loss (Kielar et al., 2021). The pathology of kidney biopsy can indicate non-specific features such as chronic immune damage, interstitial fibrosis or renal tubular atrophy (Sayin et al., 2017). With the advancement of matching technology, surgical technology and the use of immunosuppressive agents, short-term outcome of kidney transplantation has been improved, but long-term outcome still remains a challenge (Gokhale et al., 2021). How to reduce the occurrence of CAN is currently the most concerned issue. New immunosuppressive agents are generally expensive and can cause serious side effects (Zhang et al., 2015). TwHF, as an immunosuppressant in Chinese medicine, has relatively small side effects and multiple targets due to its complex ingredients, which is consistent with the multi-factorial pathogenic characteristics of CAN (Zhang et al., 2015). A study had shown that although the immunosuppressive effect of tripterygium glycosides on kidney transplanted mice was not as good as cyclosporin A, it had a synergistic effect with cyclosporin A (Min, 1999). Therefore, the combination of TwHF preparations with immunosuppressive agents may increase the immune suppression effect and bring excess benefits such as renal function protection and side effects reduction according to table 4. The conclusion needs to be supported by large-scale clinical data.
Mechanisms
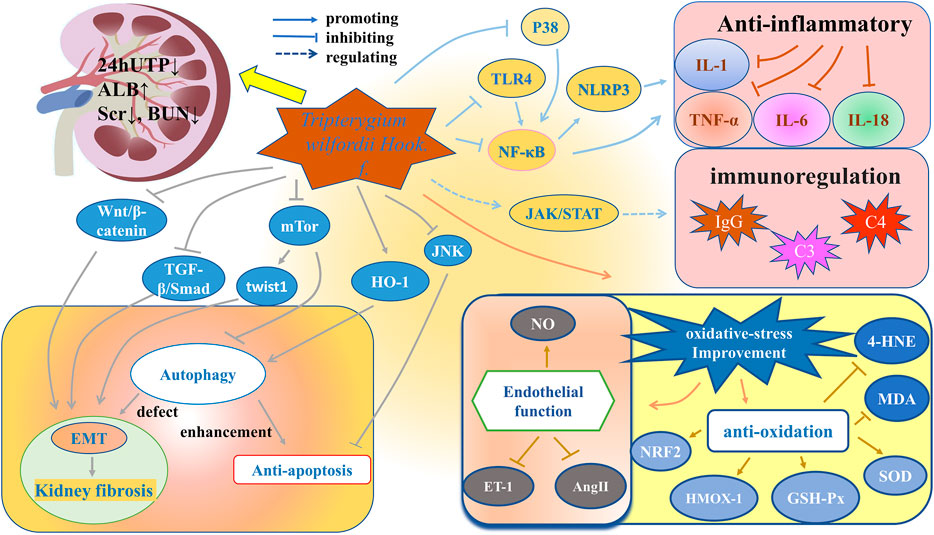
Anti-inflammatory and immune regulation are the main pharmacological effects of TwHF. In addition, TwHF has other pharmacological effects. For instance, Qin studied the effect of TP on podocyte pathology in Heymann nephritis model. He found that together with the improvement in the epithelial immune complex deposition, spikes, and basement membrane reactive proliferation, the dense on the epithelial side existed all along, even if proteinuria was significantly reduced (Qin et al., 2007). That is to say, besides the classic anti-inflammatory and immune regulation function, TwHF also has some other mechanisms to repair damage. Figure 4 generalizes the targets and signaling pathways of TwHF and its preparations in treating nephropathy.
Anti-inflammatory and Regulating Immunity
Inflammation and immunity are crucial pathogenesis of kidney diseases (Zhang and Sun, 2015). By stimulating human proximal tubular epithelial cells with inflammatory factors including IFN-γ (200 μg/L) and TNF-α (20 μg/L) for 24 h, Li et al. (2001) found that the expressions of major histocompatibility complex-Ⅱ (MHC-Ⅱ), intercellular adhesion molecule-1 (ICAM-1), and costimulatory molecules B7-1 and B7-2 were significantly increased. However, when TP was added in addition to IFN-γ and TNF-α at concentrations of 0.4 μg/L, 2.0 μg/L and 10.0 μg/L for 24 h, they found that the expressions of MHC-II and B7 decreased in a dose-dependent manner. The experimental results showed that TP weakened the ability of human proximal tubular epithelial cells to activate T cells as antigen-presenting cells and inhibited the inflammatory immune response (Li et al., 2001).
Nuclear factor- (NF-) κB signaling is a main signal pathway regulating inflammation and immunity (Morgan and Liu, 2011), which can be divided into classic and alternative pathways. The classic pathway is related to the response to pro-inflammatory cytokines-TNF-α and IL-1, involving the degradation of IκB protein. The alternative pathway does not involve the degradation of IκB, and plays an important role in immune organogenesis and lymphocyte function (Lawrence, 2009). It has been proven that in IgAN patients, NF-κB-mediated monocyte chemoattractant protein-1, granulocyte-macrophage colony stimulating factor and ICAM-1 are up-regulated in varying degrees in glomerulus and interstitium (Ashizawa et al., 2003). In LN and non-proliferative proteinuric glomerulopathy, podocyte NF-κB overactivation paralleled podocyte expression of TNF-α and IL-1β (Zheng et al., 2006). Based on the above findings about NF-κB signaling, a network pharmacology study of Liu et al. (2022) showed that the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt)/NF-κB/TNF-α/IL-1β pathway was the molecular mechanism of KXC to interfere DKD, which was confirmed by in vitro and in vivo experiments. Specifically, Liu et al. (2021) used high-fat diet combined with intraperitoneal injection of 35 mg kg−1 streptozotocin (STZ) to make DKD model in Sprague-Dawley (SD) rats. The KXC group was treated by KXC at a dosage of 480 mg kg−1, equivalent to 3 times of the clinical dosage for 4 weeks. The experimental results showed that compared with the control group without any treatment, the KXC treatment group can not only improve blood glucose, blood lipids, and urinary protein levels, but also significantly reduce phosphorylation-PI3K/PI3K, phosphorylation-AKT/AKT, phosphorylation-NF-κB/NF-κB, TNF-α and IL-1β levels (p < 0.01). The same results were also observed in vitro test (Liu et al., 2021).
Toll-like receptor 4 (TLR4) is the receptor of lipopolysaccharide, which is the outer wall component of gram-negative bacteria. TLR4 can activate the Toll/IL-1 receptor (TIR) and then activate the classic NF-κB pathway via adaptor protein with the TIR domain (Kawai and Akira, 2007; Zinatizadeh et al., 2021). Ma et al. (2015) also used high-fat diet combined with STZ to make diabetic model in SD rats. The diabetic rats were then randomly divided into four groups: diabetic rats without drug treatment and diabetic rats treated with TGT at a low-dose group (1 mg/kg/d), medium-dose group (3 mg/kg/d), and high-dose group (6 mg/kg/d), respectively. The administration time was 8 weeks. Through the detection of factors such as TLR4, NF-κB, and α-smooth muscle actin, they speculated that TGT can ameliorate renal tubulointerstitial fibrosis in diabetic rats through the TLR4/NF-κB signaling pathway (Ma et al., 2015).
The nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing protein 3 (NLRP3), which expresses predominantly in macrophages, is also closely related to inflammation. It binds to the adaptor molecule ASC and controls the activation of caspase-1, and then caspase-1 cleaves pro-IL-1β and pro-IL-18. The activation process of NLRP3 inflammasome is also regulated by NF-κB signaling (Bauernfeind et al., 2009; Islam et al., 2020). A study showed that TP, intragastrically administered at a dosage of 200 mg/kg/d for 16 weeks, can down-regulate the expression of NLRP3 and TLR4 in rats with IgAN, thereby reducing serum IL-1β and IL-18 (He et al., 2015).
P38 is a member of mitogen-activated protein kinase (MAPK) family, and p38MAPK is another important pathway that mediates inflammation (Awasthi et al., 2021). The activation of MAPK can also activate NF-κB signaling pathway (Zhou et al., 2006). Zhang et al. (2019) evaluated the effect and influential mechanisms of celastrol on renal injury in diabetic rats. They established diabetic rat model with the intraperitoneal injection of STZ. Based on the administration dosage of celastrol, the celastrol group was divided into the low-dose group (50 μg/kg, 4 weeks) and the high-dose group (100 μg/kg, 4 weeks). The results showed that compared with the control group without the celastrol treatment, the celastrol group facilitates the protection of renal function, the improvement of renal histological lesions and the reduction of inflammatory factors. In addition, by comparing the levels of p38MAPK and NF-κB p65 with the MAPK/NF-κB inhibitor group, they proved that the function of celastrol can be attributed to the regulation of the MAPK/NF-κB pathway (Zhang et al., 2019).
Nephropathy is closely related to the disorder of immune system. IgAN, MN, focal segmental glomerulosclerosis and LN are all related to immune dysfunction (Kant et al., 2021). In an in vitro study of Hong et al. (2002), TNF-α (2.5 ng/ml)-induced human proximal tubular epithelial cells were exposed to TP, CsA, or FK506 for 24 h, and then the levels of C3, CD40, and B7h were tested. They found that TP at the concentration of 4–8 ng/ml can inhibit the expression of C3, CD40 and B7h, and it is even more effective than CsA and FK506 in inhibiting C3 (Hong et al., 2002). Zhang et al. (2017) treated MRL/lpr mice with LLDT-8 (0.125 mg/kg/2 days) for 9 weeks and found that LLDT-8 can reduce the levels of inflammatory factors such as IL-6, inhibit the expressions of chemokines such as IP-10 and the infiltration of renal T cells, and reduce the proportion of macrophages and neutrophils in MRL/lpr mice.
Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway maintains the normal immune function. LN is a classic immune complex deposition disease (Alunno et al., 2019). It has been proven that JAK2-STAT1 inhibitor AG-490 can reduce the expression of IgG and C3, and reduce the inflammation of kidneys in LN rats, which suggests that the up-regulated JAK/STAT pathway is related to LN (Wang et al., 2010). Tang et al. (2021) intervened MRL/lpr mice with tail vein injection of TP (100 μg/kg) and found that the levels of JAK, p-JAK1, STAT3, and p-STAT3 decreased, while the levels of C3 and C4 and the positive rate of regulatory cells increased, which indicates that TP can reduce immune inflammatory response by inhibiting the JAK/STAT pathway.
Improvement of Oxidative Stress
Oxidative stress, inflammation and autophagy are closely related. Oxidative stress can increase the expression of pro-inflammatory IL-6, ICAM-1 and NF-κB (Prabhakar, 2013). Inflammation can induce oxidative stress, and oxidative stress can also up-regulate autophagy (Sureshbabu et al., 2015). Reactive oxygen species (ROS) can be divided into free radicals and non-free radicals. Although they play an important role in signal transduction, they have a strong binding capacity that can cause injuries to cellular components, such as lipids, proteins, and DNA (Tejchman et al., 2021). Oxidative stress is an important factor in inducing the progression of kidney diseases (Coppolino et al., 2018). It has been proven that oxidative stress can damage kidneys via increasing the synthesis of NADPH oxidases (Nlandu Khodo et al., 2012), regulating kelch-like ECH-associated protein1- (KEAP1-) nuclear factor erythroid 2-related factor 2 (NRF2) (Nezu et al., 2017), promoting endothelial mesenchymal (EnMT) (Li S. et al., 2021) and disrupting the balance with autophagy (Sureshbabu et al., 2015). Podocyte injury (Nagata, 2016), renal tubular fibrosis (Duni et al., 2019), CKD (Coppolino et al., 2018) and acute kidney injury (Sureshbabu et al., 2015) are all related to oxidative stress.
NO is a vasodilator, which can react with superoxide anion (O2−) to generate a strong oxidant: peroxynitrite (ONOO−), making NO relatively deficient. The lack of NO can trigger hypertension and subsequent proteinuria and glomerulosclerosis (Modlinger et al., 2004). Previous in vivo study showed that TGT can up-regulate the level of NO, down-regulate the levels of serum endothelin-1 (ET-1) and angiotensin-Ⅱ (AngⅡ), and lower TNF-α, IL-6, transforming growth factor- (TGF-) β1 levels to improve the vascular endothelial injury of CKD rats (Xu et al., 2021). Song et al. (2020) used tripterygium glycosides (50 mg/kg/d) to intervene DKD rats for 12 weeks and found that tripterygium glycosides can inhibit oxidation such as the decreased expression of malondialdehyde (MDA), and enhance the activity of antioxidant molecules such as the increased expression of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and NRF2. 4-Hydroxynonenal (4-HNE) is the production of lipid peroxidation (Gao et al., 2010). Gao et al. (2010) found that both low-dose (25 μg/kg/d) and high-dose (50 μg/kg/d) TP for 12 weeks can reduce the expression of 4-HNE in db/db diabetic mice, especially for the high-dose group.
Oxidative stress is also related to vascular calcification, which is very common in patients with CKD (Li et al., 2019). Yang et al. (2021) first demonstrated that celastrol can inhibit vascular calcification from isolated vascular smooth muscle cells in SD rats, rat aortic rings and human arterial rings, respectively. Then they established CKD rat model by 5/6 nephrectomy. Alizarin red staining and Micro-CT analysis showed reduced aortic calcification in CKD rats treated with celastrol. Further, they found that vascular smooth muscle cells treated with celastrol significantly down-regulated ROS and MDA levels, while up-regulated the heme oxygenase-1 (HMOX-1) level. In addition, treatment with HMOX-1 inhibitor and HMOX-1 siRNA antagonized and blocked the effect of celastrol on vascular calcification. The results were verified in Vitamin D3-induced aortic calcification in mice. Based on the above findings, they concluded that celastrol attenuates oxidative stress and vascular calcification in CKD by upregulating HMOX-1 (Yang et al., 2021).
Enhancement of Autophagy and Anti-apoptosis
Autophagy is a cytoprotective mechanism, responsible for the degradation of long-lived or damaged proteins and dysfunctional organelles (Mariño et al., 2010). The maintenance of kidney structure and function relies on normal autophagy, the defect of which can lead to acute kidney injury and various primary or secondary nephropathy, and eventually result in fibrosis (Tang C. et al., 2020). Mammalian target of rapamycin (mTOR) is an important negative regulator of autophagy and is one of the downstream target proteins of Akt signaling pathway. An in vivo study conducted by Li et al. (2017) showed that 12 weeks of TP (200 μg/kg/d) treatment can up-regulate autophagy via miR-141–3p/PTEN/Akt/mTOR pathway to alleviate diabetic renal fibrosis.
Podocytes are a fundamental part of glomerular filtration membrane, the damage of which can directly lead to various kidney diseases (Afsar et al., 2021). Under external stimuli, podocytes can respond through hypertrophy, epithelial-mesenchymal transition (EMT) and apoptosis (Liu, 2010). Autophagy defects can trigger EMT (Li et al., 2015). Although the specific mechanism has not been clarified, EMT plays a critical role in interstitial fibrosis (Sheng and Zhuang, 2020). As a process of dedifferentiation, EMT is manifested by the disappearance of epithelial characteristics, such as the decreased or disappeared expression of nephrin, P-cadherin and zonula occludens-1, and the appearance of mesenchymal characteristics, such as the up-regulated expression of fibroblast-specific protein-1 and α-smooth muscle actin (Zhang et al., 2011). Tao et al. (2021) found that DKD mouse serum-induced podocytes in vitro decreased autophagy, and increased EMT and the expression of Twist1. When silencing Twist1, the results were reversed, so they believed that Twist1 was an important molecule that reduced autophagy and induced mesenchymal expression. Then, they found that TGT (1.25 μg/ml) was able to reduce Twist1 expression. However, after adding 3BDO, a mTOR activator, the expression of Twist1 increased, and the therapeutic effect of TGT was suppressed. Therefore, they concluded that TGT can suppress EMT in podocytes of DKD mice by targeting autophagy through the mTOR/Twist1 pathway (Tao et al., 2021).
In CKD, three signaling pathways (i.e., TGF-β/Smad, integrin/integrin-linked kinase (ILK), and Wnt/β-catenin) have been shown to be essential for tubular and podocyte EMT (Liu, 2010). Based on the above findings, a clinical study of Shen et al. (2021) found that TGT (20 mg, 3 times a day) combined with Pred for 12 months can reduce urinary TGF-β1 and Smad2 levels in patients with IgAN. Chang et al. (2007) found that the TGF-β1/Smad3 pathway is activated in rats with mesangial proliferative glomerulonephritis, and CRT (equivalent to clinical dosage) for 14 days can suppress the expression of TGF-β1 and Smad3. In addition, Chang et al. (2018) found that after 8 weeks of intervention in diabetic rats with 8 mg/kg and 16 mg/kg dosage of TGT, levels of Wnt-1, β-catenin, NF-κB p65 and TGF-β1 mRNA and protein were significantly reduced, so they speculated that the Wnt/β-catenin was involved in the effect of TGT on renal injury in diabetic rats, effectively preventing matrix accumulation and glomerulosclerosis.
Autophagy and apoptosis are two essential catabolic processes that maintain cell homeostasis and function. Despite significant differences between the two pathways, they are highly correlated in determining cell fate (Wu et al., 2014). Heme oxygenase-1 (HO-1) was proven to prevent high glucose-induced podocyte apoptosis via autophagy (Dong et al., 2015). Under high glucose condition, HO-1 is inactivated. Celastrol can restore the activity of HO-1 in vitro to protect podocytes from apoptosis, which is considered to be a new strategy for the treatment of DKD (Zhan et al., 2018). There are more findings about the anti-apoptotic effect of TwHF. For example, TP at the concentration of 2–4 mg/L can reverse the increased apoptosis rate of podocytes caused by PM2.5 (200 mg/L), which is manifested by the up-regulated protein expression of Bcl-2, nephrin and podocin, and the down-regulated protein expression of Bax (Wan et al., 2020). In terms of apoptosis, TP has also been shown to protect renal tubular epithelial cells from apoptosis caused by ischemia-reperfusion via c-Jun N-terminal kinase (JNK) signaling cascade reaction (Bao et al., 2018).
Toxicity of TwHF Preparations
TwHF also called “Gelsemium elegans”. Leigong is the god of thunder in Chinese mythology. Thus, it can be seen that Chinese ancestors have long recognized the virulent toxicity of TwHF (Ren et al., 2021). The molecular basis of TwHF toxicity are diterpenoids, alkaloids, triterpenes and glycosides. Besides, TwHF also contains a relatively high amount of harmful elements, such as lead, arsenic and cadmium (Xue et al., 2010). According to statistics, the incidence of adverse reactions of TwHF preparations was 26.7% (Zhang C. et al., 2016). The adverse reactions included gastrointestinal toxicity (e.g., stomach pain, nausea, vomiting, diarrhea and anorexia), reproductive toxicity (e.g., decreased sperm count, menstrual disorders and amenorrhea), liver toxicity (e.g., jaundice and abnormal liver function), nephrotoxicity (e.g., hematuria, edema and oliguria), hematopoietic system toxicity (e.g., leukopenia), skin toxicity (e.g., scall), etc., among which gastrointestinal toxicity is the most commonly observed, followed by reproductive toxicity (Huang et al., 2016; Ru et al., 2019). Ding et al. (2021) found that 48 components and 78 key targets of TwHF were related to reproductive toxicity, which was mainly caused through regulating vasoconstriction. It is important to note that the therapeutic dosage and the toxic dosage of TwHF are very similar, that is to say, the efficacy and toxicity coexist, which aggravates the occurrence of adverse reactions (Liu LL. et al., 2019). A study showed that TwHF decoction in the range of 0.29–1.17 g/kg (equivalent to 4.5–18.2 times the clinical dose of 70 kg adults) has anti-inflammatory effects in mice and shows a dose-effect relationship. However, at the drug concentration of 0.585 g/kg, adverse reactions of liver toxicity have already occurred, and after reaching 2.34 g/kg, liver parenchyma damage occurs (Feng et al., 2014). In addition, Xu et al. (2001) found that for most of the people, by taking TGT at the dosage of 60 mg/day, the average cumulative dosage of inappetence, nausea, abdominal pain, and diarrhea were 7632 mg, 3780 mg, 3150 mg, and 6,750 mg, respectively; the average cumulative dosage of menstrual irregularity and menopause were 4,340 mg and 7710 mg, respectively; the average cumulative dosage of liver damage and rash were 15310 and 8,029 mg, respectively.
The toxicity of TwHF preparations is also related to dosage forms, pharmaceutical factories and whether they are combined with other drugs. For example, TwHF tablet, TwHF total terpenoids tablet and TwHF double-deck tablet are supplied by exclusive manufacturers, so their product contents and qualities are relatively stable. In contrast, TGT is currently supplied by 11 manufacturers1, and the current national drug standards for TGT do not include the quality standards of Leigongteng extract, so the contents and quality of the products produced by each manufacturer are different (Wang Y. D. et al., 2021), which increase the unsafety of clinical medication.
Therefore, for the use of TwHF preparations, special emphasis should be placed on the rationality of clinical medication (Kang et al., 2021), including grasping the medication population and indications, strictly following the usage and dosage, and closely monitoring the occurrence of adverse reactions. Moreover, strengthening drug quality supervision can also reduce the incidence of TwHF preparations’ adverse reactions. It is necessary to specify the drug standards of TwHF in different places of production, different seasons, and different parts when formulating drug standards to ensure the quality of the same preparation from different manufacturers (Sheng et al., 2017).
Discussion
Our study introduces the development of main active components of TwHF and TwHF preparations and summarizes their applications and influential mechanisms in nephropathy. Considering the adverse reactions of TwHF and its preparations, we draw the following conclusions. First, the goal of “reducing toxicity and increasing efficiency” of TwHF preparations requires not only the discovery of new compounds and derivatives or the emergence of new dosage forms, but also the need to strengthen the quality management of existing preparations and the rational use of the drugs. Second, TwHF preparations combined with ARB or immunosuppressive agents may be better in reducing urine protein, protecting kidney function, reducing the incidence of adverse reactions, and maintaining long-term effects, which requires further verification by clinical large data. Third, TwHF treats nephropathy and protects kidneys mainly through anti-inflammatory and immune regulations, improving oxidative stress, and enhancing autophagy and anti-apoptosis.
Our research also has some limitations. For example, researches about the application of TwHF preparations in nephropathy is relatively limited. Most of the data are concentrated in China and focus on certain types of kidney diseases, so the application and mechanism of TwHF in nephropathy may not be comprehensive. As the “Chinese medicinal hormone”, TwHF has many components, which is quite likely to become a potential drug for the treatment of certain diseases in the future. It would be interesting to conduct more studies about the application of TwHF and its preparations in nephropathy and other fields.
Author Contributions
XT and YQ wrote the manuscript. YY and HL supplied materials and assembled the tables. ZC prepared the figures. BY, LW, and HY conceived and revised the article. XT and YQ have contributed equally to this work. All authors have read and approved the final submission.
Funding
This research was funded by the National Key R&D Program of China (Grant number, 2019YFC1709401).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1The data comes from National Pharmaceuticals Supervision Administration, web link: https://www.nmpa.gov.cn/datasearch/search-result.html.
References
Afsar, B., Afsar, R. E., Demiray, A., Covic, A., and Kanbay, M. (2021). Deciphering Nutritional Interventions for Podocyte Structure and Function. Pharmacol. Res. 172, 105852. doi:10.1016/j.phrs.2021.105852
Alunno, A., Padjen, I., Fanouriakis, A., and Boumpas, D. T. (2019). Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: Integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells 8 (8), 898. doi:10.3390/cells8080898
Ao, J. H., Li, Y. T., Xiao, X. R., Gao, J. P., Dong, J., and Qian, Y. Y. (1994). Clinical Study of Tripterygium Wilfordii Polyglycosides after Kidney Transplantation. Chin. J. Surg. 32 (03), 175–177.
Ashizawa, M., Miyazaki, M., Abe, K., Furusu, A., Isomoto, H., Harada, T., et al. (2003). Detection of Nuclear Factor-kappaB in IgA Nephropathy Using Southwestern Histochemistry. Am. J. Kidney Dis. 42 (1), 76–86. doi:10.1016/s0272-6386(03)00411-6
Awasthi, A., Raju, M. B., and Rahman, M. A. (2021). Current Insights of Inhibitors of P38 Mitogen-Activated Protein Kinase in Inflammation. Med. Chem. 17 (6), 555–575. doi:10.2174/1573406416666200227122849
Bao, Z. Y., Liao, J., Li, X. F., and Zhu, C. F. (2018). Mechanism of Triptolide Inhibits Renal Tubular Epithelial Cell Apoptosis in Renal Ischemia Reperfusion Rats. Chinj. Mod. Appl. Pharm. 35 (07), 1041–1045. doi:10.13748/j.cnki.issn1007-7693.2018.07.021
Bauernfeind, F. G., Horvath, G., Stutz, A., Alnemri, E. S., MacDonald, K., Speert, D., et al. (2009). Cutting Edge: NF-kappaB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 183 (2), 787–791. doi:10.4049/jimmunol.0901363
Chang, B., Chen, W., Zhang, Y., Yang, P., and Liu, L. (2018). Tripterygium Wilfordii Mitigates Hyperglycemia-Induced Upregulated Wnt/β-Catenin Expression and Kidney Injury in Diabetic Rats. Exp. Ther. Med. 15 (4), 3874–3882. doi:10.3892/etm.2018.5901
Chang, W., Sun, H. Y., Zeng, H. B., and Lv, Y. M. (2007). Effect of Colquhoumia Root on TGF-Β1 and Smad3 in Experimental Mesangial Proliferative Glomerulonephritis. Chin. J. Hemorh. 17 (03), 364–366+407. doi:10.3969/j.issn.1009-881X.2007.03.006
Chen, Y., Gong, Z., Chen, X., Tang, L., Zhao, X., Yuan, Q., et al. (2013). Tripterygium Wilfordii Hook F (A Traditional Chinese Medicine) for Primary Nephrotic Syndrome. Cochrane Database Syst. Rev. 8, CD008568. doi:10.1002/14651858.CD008568.pub2
Chen, Y. S. (2018). Effect of Glycosides Tablets on the Patients with Primary Nephrotic Syndrome and its Influence on Inflammatory Factors. Hainan Med. J. 29 (06), 763–766. doi:10.3969/j.issn.1003-6350.2018.06.008
Cheng, Y., Zhao, Y., and Zheng, Y. (2021). Therapeutic Potential of Triptolide in Autoimmune Diseases and Strategies to Reduce its Toxicity. Chin. Med. 16 (1), 114. doi:10.1186/s13020-021-00525-z
Coppolino, G., Leonardi, G., Andreucci, M., and Bolignano, D. (2018). Oxidative Stress and Kidney Function: A Brief Update. Curr. Pharm. Des. 24 (40), 4794–4799. doi:10.2174/1381612825666190112165206
Cui, Y. H. (2016). Clinical Observation of Tripterygium Glycosides Tablets Combined with Sodium Ferulate in Treatment of Chronic Glomerulonephritis. Drugs & Clinic 31 (01), 33–36. doi:10.7501/j.issn.1674-5515.2016.01.008
Ding, Q., Wu, Y., and Liu, W. (2021). Molecular Mechanism of Reproductive Toxicity Induced by Tripterygium Wilfordii Based on Network Pharmacology. Medicine (Baltimore) 100 (27), e26197. doi:10.1097/md.0000000000026197
Dong, C., Zheng, H., Huang, S., You, N., Xu, J., Ye, X., et al. (2015). Heme Oxygenase-1 Enhances Autophagy in Podocytes as a Protective Mechanism against High Glucose-Induced Apoptosis. Exp. Cel Res. 337 (2), 146–159. doi:10.1016/j.yexcr.2015.04.005
Dong, Y. Z., Zhang, B., Lin, Z. J., and Zhang, X. M. (2019). Pharmacodynamic Effect and Virulent Effect of Tripterygium Wilfordii Based on Network Pharmacology. Zhongguo Zhong Yao Za Zhi 44 (16), 3460–3467. doi:10.19540/j.cnki.cjcmm.20181119.001
Duni, A., Liakopoulos, V., Roumeliotis, S., Peschos, D., and Dounousi, E. (2019). Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne's Thread. Int. J. Mol. Sci. 20 (15), 3711. doi:10.3390/ijms20153711
Feng, Q., Sun, R., Huang, W., Zhao, Q. H., and Li, X. Y. (2014). Assessment of Efficacy-Toxicity Correlation Based on the Anti-inflammatory of Different Doses of Tripterygium Wilfordii on Mice. zhong Yao Yao Li Yu Lin Chuang 30 (1), 75–79. doi:10.13412/j.cnki.zyyl.2014.01.026
Feng, X., Guan, D., Auen, T., Choi, J. W., Salazar Hernández, M. A., Lee, J., et al. (2019). IL1R1 Is Required for Celastrol's Leptin-Sensitization and Antiobesity Effects. Nat. Med. 25 (4), 575–582. doi:10.1038/s41591-019-0358-x
Gao, Q., Shen, W., Qin, W., Zheng, C., Zhang, M., Zeng, C., et al. (2010). Treatment of Db/db Diabetic Mice with Triptolide: a Novel Therapy for Diabetic Nephropathy. Nephrol. Dial. Transpl. 25 (11), 3539–3547. doi:10.1093/ndt/gfq245
Gokhale, A., Chancay, J., Shapiro, R., Randhawa, P., and Menon, M. C. (2021). Chronic Transplant Glomerulopathy: New Insights into Pathogenesis. Clin. Transpl. 35 (3), e14214. doi:10.1111/ctr.14214
Guo, Y., Guo, N., Wang, J., Wang, R., and Tang, L. (2021). Retrospective Analysis of Tripterygium Wilfordii Polyglycoside Combined with Angiotensin Receptor Blockers for the Treatment of Primary Membranous Nephropathy with Sub-nephrotic Proteinuria. Ren. Fail. 43 (1), 729–736. doi:10.1080/0886022x.2021.1918555
He, C. M., Liu, J. X., Han, H., Fu, G. H., Liu, X. D., Zhou, H. L., et al. (2020). Effects and Safety Analysis of Tripterygium Glycosides on Child Patients with Purpura Nephritis. World Chin. Med. 15 (20), 3094–3096. doi:10.3969/j.issn.1673-7202.2020.20.019
He, L., Peng, X., Liu, G., Tang, C., Liu, H., Liu, F., et al. (2015). Anti-inflammatory Effects of Triptolide on IgA Nephropathy in Rats. Immunopharmacol Immunotoxicol 37 (5), 421–427. doi:10.3109/08923973.2015.1080265
He, L., Y., Lin, Z. C., Lu, J. D., Xiong, G. L., and Wang, S. H. (2021). Detoxification and Sustained Effects of Tripterygium Wilfordii Based on Ganoderma Lucidum Bi-directional Solid Fermentation. J. Beijing Univ. Chem. Tech. (Natural Science) 48, 48–56. doi:10.13543/j.bhxbzr.2021.04.006
Hong, Y., Zhou, W., Li, K., and Sacks, S. H. (2002). Triptolide Is a Potent Suppressant of C3, CD40 and B7h Expression in Activated Human Proximal Tubular Epithelial Cells. Kidney Int. 62 (4), 1291–1300. doi:10.1111/j.1523-1755.2002.kid586.x
Horiuch, M., Murakami, C., Fukamiya, N., Yu, D., Chen, T. H., Bastow, K. F., et al. (2006). Tripfordines A-C, Sesquiterpene Pyridine Alkaloids from Tripterygium Wilfordii, and Structure Anti-HIV Activity Relationships of Tripterygium Alkaloids. J. Nat. Prod. 69 (9), 1271–1274. doi:10.1021/np060124a
Hou, W., Liu, B., and Xu, H. (2020). Celastrol: Progresses in Structure-Modifications, Structure-Activity Relationships, Pharmacology and Toxicology. Eur. J. Med. Chem. 189, 112081. doi:10.1016/j.ejmech.2020.112081
Huang, N., Liu, Z. Y., Zhang, Z. H., Huang, X. J., and Cheng, H. (2016). Analysis of Literature about Tripterygium Wilfordi Poissoning Reasons and Detoxicifying Methods. Asia-pac. Trad. Med. 12 (21), 48–51. doi:10.11954/ytctyy.201621018
Huang, Y. F., Gong, S. J., Huang, L., Qin, X. Y., and Pei, L. (2010). Comparative Study of Chemical Constituents in the Roots of Tripterygium Wilfordii Hook.F. And Tripterygium Hypoglaucum (H. lév.) Hutch by Thin-Layer Chromatography. Shi Zhen Guo Yi Guo Yao 21 (08), 1973–1974. doi:10.3969/j.issn.1008-0805.2010.08.057
Islam, M. T., Bardaweel, S. K., Mubarak, M. S., Koch, W., Gaweł-Beben, K., Antosiewicz, B., et al. (2020). Immunomodulatory Effects of Diterpenes and Their Derivatives through NLRP3 Inflammasome Pathway: A Review. Front. Immunol. 11, 572136. doi:10.3389/fimmu.2020.572136
Ji, M., Ma, Y. Y., Guo, Z. S., Wu, Q. T., Meng, Y. Y., Ma, D. H., et al. (2021). Comparison of the Effects of Kunxian Capsule and Tacrolimus in the Treatment of Idiopathic Membranous Nephropathy. J. Xinxiang Med. Univ. 38 (05), 472–476. doi:10.7683/xxyxyxb.2021.05.016
Jiang, X., Huang, X. C., Ao, L., Liu, W. B., Han, F., Cao, J., et al. (2014). Total Alkaloids of Tripterygium Hypoglaucum (levl.) Hutch Inhibits Tumor Growth Both In Vitro and In Vivo. J. Ethnopharmacol 151 (1), 292–298. doi:10.1016/j.jep.2013.10.045
Jin, D., Yu, M., Li, X., and Wang, X. (2021). Efficacy of Tripterygium Wilfordii Hook F on Animal Model of Diabetic Kidney Diseases: A Systematic Review and Meta-Analysis. J. Ethnopharmacol 281, 114536. doi:10.1016/j.jep.2021.114536
Kang, B. Y., Li, C. X., Dong, P. Y., Zhu, X. L., Zhang, H., Chen, Y. H., et al. (2021). Analysis on the Rationality of Clinical Medication of Tripterygium Wilfordii Polyglycosides Tablets. Chin. Tradit. Pat. Med. 43 (09), 2597–2599. doi:10.3969/j.issn.1001-1528.2021.09.062
Kang, T., and Cui, T. L. (2011). The Clinical Analysis of Tripterygium Hypoglaucum and Prednisone Treatment of Primary Nephrotic Syndrome. Guide Chin. Med. 9 (02), 38–39. doi:10.15912/j.cnki.gocm.2011.02.085
Kant, S., Kronbichler, A., Sharma, P., and Geetha, D. (2021). Advances in Understanding of Pathogenesis and Treatment of Immune-Mediated Kidney Disease: A Review. Am. J. Kidney Dis. doi:10.1053/j.ajkd.2021.07.019
Kawai, T., and Akira, S. (2007). Signaling to NF-kappaB by Toll-like Receptors. Trends Mol. Med. 13 (11), 460–469. doi:10.1016/j.molmed.2007.09.002
Kielar, M., Gala-Błądzińska, A., Dumnicka, P., Ceranowicz, P., Kapusta, M., Naumnik, B., et al. (2021). Complement Components in the Diagnosis and Treatment after Kidney Transplantation-Is There a Missing Link? Biomolecules 11 (6), 773. doi:10.3390/biom11060773
Law, S. K., Simmons, M. P., Techen, N., Khan, I. A., He, M. F., Shaw, P. C., et al. (2011). Molecular Analyses of the Chinese Herb Leigongteng (Tripterygium Wilfordii Hook.f.). Phytochemistry 72 (1), 21–26. doi:10.1016/j.phytochem.2010.10.015
Lawrence, T. (2009). The Nuclear Factor NF-kappaB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 1 (6), a001651. doi:10.1101/cshperspect.a001651
Li, G., Li, C. X., Xia, M., Ritter, J. K., Gehr, T. W., Boini, K., et al. (2015). Enhanced Epithelial-To-Mesenchymal Transition Associated with Lysosome Dysfunction in Podocytes: Role of p62/Sequestosome 1 as a Signaling Hub. Cell Physiol. Biochem. 35 (5), 1773–1786. doi:10.1159/000373989
Li, H., Liu, Z. H., Dai, C. S., and Li, H. S. (2001). Triptolide Down-Regulates Induced Antigen-Presenting Cell Function of Human Proximal Tubular Epithelial Cells. Chin. J. Nephrol. Dial. Transpl. 10 (04), 303–308. doi:10.3969/j.issn.1006-298X.2001.04.002
Li, Q., Huang, Y., Liu, P., Yuan, H., and Zhao, J. (2021a). Effect of Tripterygium Wilfordii Polyglycoside Tablets on Serum Inflammatory Factors and T Cells in Patients with Chronic Nephritis. Am. J. Transl. Res. 13 (7), 8385–8390.
Li, S., Wang, F., and Sun, D. (2021b). The Renal Microcirculation in Chronic Kidney Disease: Novel Diagnostic Methods and Therapeutic Perspectives. Cell Biosci 11 (1), 90. doi:10.1186/s13578-021-00606-4
Li, T. S. (2002). Tripterygium Wilfordii Double-Deck Tablet, a Sustained-Release Traditional Chinese Medicine, Come Out. Ethnic Med. Newspaper. 2002-09-27.
Li, X. Y., Fan, Y. S., He, X. L., He, Q., Qu, L. H., Wang, S. Y., et al. (2008). Clinical Observation on Treatment of Chronic Allograft Nephropathy with Colquhounia Root Tablet Combined with Immunosuppressive Protocol. Zhongguo Zhong Xi Yi Jie He Za Zhi 28 (09), 810–812. doi:10.3321/j.issn:1003-5370.2008.09.011
Li, X. Y., Wang, S. S., Han, Z., Han, F., Chang, Y. P., Yang, Y., et al. (2017). Triptolide Restores Autophagy to Alleviate Diabetic Renal Fibrosis through the miR-141-3p/PTEN/Akt/mTOR Pathway. Mol. Ther. Nucleic Acids 9, 48–56. doi:10.1016/j.omtn.2017.08.011
Li, Y. N., Duan, C. Y., Zong, H. T., and Huang, X. Y. (2020). Clinical Observation of Tripterygium Wilfordii Polyglycosides Combined with Prednisone and Cyclophosphamide Pulse Therapy in the Treatment of Nephrotic Syndrome. Jiangxi Med. J. 55 (07), 872–873+888. doi:10.3969/j.issn.1006-2238.2020.07.025
Li, Z., Wu, J., Zhang, X., Ou, C., Zhong, X., Chen, Y., et al. (2019). CDC42 Promotes Vascular Calcification in Chronic Kidney Disease. J. Pathol. 249 (4), 461–471. doi:10.1002/path.5334
Liang, Y., Yan, X. H., Wang, X. M., Jin, G., and Feng, T. (2020). Efficacy of Tripterygium Wilfordii Polyglycoside Combined with Taprolimus in the Treatment of Idiopathic Membranous Nephropathy and its Effect on Urinary C5b-9 and IgG4. Hebei Med. 26 (02), 211–215. doi:10.3969/j.issn.1006-6233.2020.02.009
Lim, H. Y., Ong, P. S., Wang, L., Goel, A., Ding, L., Li-Ann Wong, A., et al. (2021). Celastrol in Cancer Therapy: Recent Developments, Challenges and Prospects. Cancer Lett. 521, 252–267. doi:10.1016/j.canlet.2021.08.030
Lin, L. M., and Qi, X. M. (2005). Comparative Observation on the Effects of Radix Tripterygium Hypoglaucum Tablet and Tripterygium Glycosides Tablet in Treating Erosive Oral Lichen Planus. Chin J Integr Med 11 (02), 149–150. doi:10.1007/BF02836474
Liu, B. (2020). Effect of Tripterygium Glycoside Combined with Azathioprine in Treatment of Lupus Nephritis and Influence on Inflammatory Factors and Renal Hemodynamics. Chin. J. Integr. Tradit. West. Nephrol. 21 (11), 1012–1014. doi:10.3969/j.issn.1009-587X.2020.11.024
Liu, L. L., Tian, Y. G., Su, X. H., Fan, Y. F., Li, C., Zhu, X. X., et al. (2019b). Comparative Study on Dose-Toxicity-Effect of Tripterygium Glycosides Tablets and Tripterygium Wilfordii Tablets on CIA Model Rats. Zhongguo Zhong Yao Za Zhi 44 (16), 3502–3511. doi:10.19540/j.cnki.cjcmm.20190703.401
Liu, L., Yan, J., Shu, J. C., and Liu, J. Q. (2019a). Advance on Alkaloids from Tripterygium Wilfordii and Their Bioactivities. Nat. Prod. Res. Dev. 31 (12), 2170–2181. doi:10.16333/j.1001-6880.2019.12.022
Liu, M., Pan, W. Y., Meng, D. X., Jiang, Z., Li, J., Li, Y. S., et al. (2020). Clinical Study on Kunxian Capsule Combined with Glucocorticoids in the Treatment of Lupus Nephritis. Chin. J. Integr. Tradit. West. Med. 40 (08), 919–922. doi:10.7661/j.cjim.20200627.074
Liu, Y. (2010). New Insights into Epithelial-Mesenchymal Transition in Kidney Fibrosis. J. Am. Soc. Nephrol. 21 (2), 212–222. doi:10.1681/asn.2008121226
Liu, Y. D., Ma, Z. C., Li, C. C., Lin, Y., Zhang, Y. Q., and Lin, N. (2022). Investigation on Therapeutic Potential and Molecular Mechanisms of Kunxian Capsule against Diabetic Kidney Disease via Reversing the Imbalance of “Immune-inflammation” Network. Acta Pharm. Sin 57 (2), 375–384. doi:10.16438/j.0513-4870.2021-1109
Lu, F. C., and Yuan, H. L. (2021). A Review on Treating Kidney Diseases with the Kunxian Capsules. Clin. J. Chin. Med. 13 (13), 141–145. doi:10.3969/j.issn.1674-7860.2021.13.045
Lu, X. T. (2003). Treatment of Nephrotic Syndrome with Tripterygium Tablets and Hormone. Mod. J. Integr. Tradit. Chin. West. Med. 12 (10), 1058. doi:10.3969/j.issn.1008-8849.2003.10.034
Ma, A. J., and Yao, X. (2020). Effects of Tripterygium Wilfordii Polyglycoside Tablet Combined with Natrium-Glucose Cotransporter 2 Inhibitor on Renal Function and Proteinuria in Patients with Diabetic Nephropathy. Chin. Arch. Tradi. Chin. Med. 38 (08), 200–203. doi:10.13193/j.issn.1673-7717.2020.08.048
Ma, Z. J., Zhang, X. N., Li, L., Yang, W., Wang, S. S., Guo, X., et al. (2015). Tripterygium Glycosides Tablet Ameliorates Renal Tubulointerstitial Fibrosis via the Toll-like Receptor 4/Nuclear Factor Kappa B Signaling Pathway in High-Fat Diet Fed and Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2015, 390428. doi:10.1155/2015/390428
Mariño, G., Fernández, A. F., Cabrera, S., Lundberg, Y. W., Cabanillas, R., Rodríguez, F., et al. (2010). Autophagy Is Essential for Mouse Sense of Balance. J. Clin. Invest. 120 (7), 2331–2344. doi:10.1172/jci42601
Miao, Y. Y., Luo, L., Shu, T., Wang, H., Jiang, Z. Z., and Zhang, L. Y. (2019). Study on Difference of Liver Toxicity and its Molecular Mechanisms Caused by Tripterygium Wilfordii Multiglycoside and Equivalent Amount of Triptolid in Rats. Zhongguo Zhong Yao Za Zhi 44 (16), 3468–3477. doi:10.19540/j.cnki.cjcmm.20190301.002
Min, Z. L. (1999). Application of Traditional Chinese Medicine in Kidney Transplantation. Chin. J. Organ. Transpl. 20 (03), 49–50.
Modlinger, P. S., Wilcox, C. S., and Aslam, S. (2004). Nitric Oxide, Oxidative Stress, and Progression of Chronic Renal Failure. Semin. Nephrol. 24 (4), 354–365. doi:10.1016/j.semnephrol.2004.04.007
Morgan, M. J., and Liu, Z. G. (2011). Crosstalk of Reactive Oxygen Species and NF-Κb Signaling. Cell Res 21 (1), 103–115. doi:10.1038/cr.2010.178
Nagata, M. (2016). Podocyte Injury and its Consequences. Kidney Int. 89 (6), 1221–1230. doi:10.1016/j.kint.2016.01.012
Nezu, M., Suzuki, N., and Yamamoto, M. (2017). Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression. Am. J. Nephrol. 45 (6), 473–483. doi:10.1159/000475890
Nlandu Khodo, S., Dizin, E., Sossauer, G., Szanto, I., Martin, P. Y., Feraille, E., et al. (2012). NADPH-oxidase 4 Protects against Kidney Fibrosis during Chronic Renal Injury. J. Am. Soc. Nephrol. 23 (12), 1967–1976. doi:10.1681/asn.2012040373
Nong, C., Wang, X. Z., Jiang, Z. Z., and Zhang, L. Y. (2019). Progress of Effect and Mechanisms of Tripterygium Wilfordii on Immune System. Zhongguo Zhong Yao Za Zhi 44 (16), 3374–3383. doi:10.19540/j.cnki.cjcmm.20190419.401
Prabhakar, O. (2013). Cerebroprotective Effect of Resveratrol through Antioxidant and Anti-inflammatory Effects in Diabetic Rats. Naunyn Schmiedebergs Arch. Pharmacol. 386 (8), 705–710. doi:10.1007/s00210-013-0871-2
Qi, A. R., Wen, S. X., Fu, B., Zhou, L., Liu, Y. J., and Li, S. M. (2018). Effects of Different Tripterygium Wilfordii on Apoptosis of Hamster Ovary Cells. Acta Chin. Med. 33, 1510–1514. doi:10.16368/j.issn.1674-8999.2018.08.358
Qin, W. S., Liu, Z. H., Zeng, C. H., Zheng, C. X., Jia, Z. H., Wang, S. Y., et al. (2007). Therapeutic Effect of Triptolide on Podocyte Injury in Passive Heymann Nephritis. Chin. J. Nephrol. Dial. Transpl. 16 (02), 101–109. doi:10.3969/j.issn.1006-298X.2007.02.001
Qin, W. Z. (2018). Discussion on the Chemical Active Monomers and Derivatives of Leigongteng. Chin. J. Integr. Tradit. West. Med. 38 (03), 265–266. doi:10.7661/j.cjim.20180105.031
Qiu, Y., Zhu, B., Cui, C. M., Tang, X. L., Wang, W. W., Mao, L. M., et al. (2021). A Prospective Randomized Controlled Study of Tripterygium Glycoside Tablets in the Treatment of Idiopathic Membranous Nephropathy with Middle Amount Albuminuria. Chin. J. Integr. Tradit. West. Nephrol. 22 (04), 337–340.
Ren, L. W., Zheng, X. J., Li, W., Wang, J. H., and Du, G. H. (2021). Historical Cognition and Evaluation of Tripterygium Wilfordii Toxicity. Herald Med. 40 (05), 637–641. doi:10.3870/j.issn.1004-0781.2021.05.012
Ru, Y., Luo, Y., Zhou, Y., Kuai, L., Sun, X., Xing, M., et al. (2019). Adverse Events Associated with Treatment of Tripterygium Wilfordii Hook F: A Quantitative Evidence Synthesis. Front. Pharmacol. 10, 1250. doi:10.3389/fphar.2019.01250
Sayin, B., Canver, B., Gurlek Demirci, B., Colak, T., Ozdemir, B. H., and Haberal, M. (2017). Renin-Angiotensin System Blockage and Avoiding High Doses of Calcineurin Inhibitors Prevent Interstitial Fibrosis and Tubular Atrophy in Kidney Transplant Recipients. Exp. Clin. Transpl. 15 (Suppl. 1), 32–36. doi:10.6002/ect.mesot2016.O19
Schiavone, S., Morgese, M. G., Tucci, P., and Trabace, L. (2021). The Therapeutic Potential of Celastrol in Central Nervous System Disorders: Highlights from In Vitro and In Vivo Approaches. Molecules 26 (15), 4700. doi:10.3390/molecules26154700
Shen, S. Z., Yang, Z. M., Cai, J. Y., Sun, L. Y., and Wang, J. (2021). Clincal Effects of Tripterygium Glycosides Combined with Prednisone on IgA Nephropathy and its Effect on Urinary Smad2 and TGF-Β1. Chin. J. Mod. Appl. Pharm. 38 (09), 1094–1098. doi:10.13748/j.cnki.issn1007-7693.2021.09.014
Sheng, A. Q., Shi, N., Fu, H. Y., and Shi, S. L. (2017). Comparison of Cell Activity from Different Parts of Tripterygium Wilfordill Based on xCELLigence System. Zhong Cao Yao 48 (06), 1172–1177. doi:10.7501/j.issn.0253-2670.2017.06.019
Sheng, L., and Zhuang, S. (2020). New Insights into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front. Physiol. 11, 569322. doi:10.3389/fphys.2020.569322
Si, J. P. (2008). “A Study on Classification of Leigongteng and Kunmingshanhaitang,” in Proceedings of the Fifth National Symposium on Leigongteng, Chongqing, July 2008.
Song, Q. M., Liu, Y., Xu, X. Y., and Xu, L. S. (2020). Tripterygium Wilfordii Polyglycosides Reduce Inflammation and Oxidative Stress in Kidney Tissues of Diabetic Rats via AMPK Pathway. J. Clin. Exp. Med. 19 (05), 464–468. doi:10.3969/j.issn.1671-4695.2020.05.005
Sun, Y., Wang, Y. D., Wen, H. R., Wang, Q., and Ma, S. C. (2021). Considerations on Establishing Clinically Relevant Specifications of Traditional Chinese Medicine Preparations with Tripterygium Glycoside Tablets as an Example. Mod. Chin. Med. 23, 1331–1334. doi:10.13313/j.issn.1673-4890.20200903002
Sureshbabu, A., Ryter, S. W., and Choi, M. E. (2015). Oxidative Stress and Autophagy: Crucial Modulators of Kidney Injury. Redox Biol. 4, 208–214. doi:10.1016/j.redox.2015.01.001
Tang, C., Livingston, M. J., Liu, Z., and Dong, Z. (2020a). Autophagy in Kidney Homeostasis and Disease. Nat. Rev. Nephrol. 16 (9), 489–508. doi:10.1038/s41581-020-0309-2
Tang, W., and Zuo, J. P. (2012). Immunosuppressant Discovery from Tripterygium Wilfordii Hook F: the Novel Triptolide Analog (5R)-5-Hydroxytriptolide (LLDT-8). Acta Pharmacol. Sin. 33 (9), 1112–1118. doi:10.1038/aps.2012.108
Tang, Y., Zhang, Y., Li, L., Xie, Z., Wen, C., and Huang, L. (2020b). Kunxian Capsule for Rheumatoid Arthritis: Inhibition of Inflammatory Network and Reducing Adverse Reactions through Drug Matching. Front. Pharmacol. 11, 485. doi:10.3389/fphar.2020.00485
Tang, Y. H., Liu, L., Liu, J., Jiang, M., and Yu, L. (2021). Effect of Triptolide Regulating JAK/STAT Signal Pathway on Inflammation and Immunity in Mice with Systemic Lupus Erythematosus. J. Chin. Med. Mater. 44 (08), 1986–1990. doi:10.13863/j.issn1001-4454.2021.08.037
Tao, M., Zheng, D., Liang, X., Wu, D., Hu, K., Jin, J., et al. (2021). Tripterygium Glycoside Suppresses Epithelial-to-mesenchymal T-ransition of D-iabetic K-idney D-isease P-odocytes by T-argeting A-utophagy through the mTOR/Twist1 P-athway. Mol. Med. Rep. 24 (2), 592. doi:10.3892/mmr.2021.12231
Tejchman, K., Kotfis, K., and Sieńko, J. (2021). Biomarkers and Mechanisms of Oxidative Stress-Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 22 (15), 8010. doi:10.3390/ijms22158010
The World Flora Online (2022). Tripterygium Hypoglaucum (H. Lév.) Hutch. Published on the Internet. Available at: http://www.worldfloraonline.org/taxon/wfo-0001253208 (Accessed on Jan 13, 2022).
Tian, Y., and Liu, Y. H. (2020). Study on the Clinical Effect of Tripterygium Polyglycoside in the Treatment of Proteinuria in Kidney Transplantation Patients Taking Sirolimus. J. Bengbu Med. Coll. 45 (08), 1060–1063. doi:10.13898/j.cnki.issn.1000-2200.2020.08.018
Tong, L., Zhao, Q., Datan, E., Lin, G. Q., Minn, I., Pomper, M. G., et al. (2021). Triptolide: Reflections on Two Decades of Research and Prospects for the Future. Nat. Prod. Rep. 38 (4), 843–860. doi:10.1039/d0np00054j
Tu, X., Yang, M. D., Li, Y. Y., and Zhang, S. J. (2021). Comparison of Efficacy and Safety of Kunxian Capsule and Tripterygium Glycoside Tablets in the Treatment of Chronic Kidney Disease. J. Zhejiang Chin. Med. Univ. 45 (06), 582–587+602. doi:10.16466/j.issn1005-5509.2021.06.003
Wan, Q., Liu, Z., Yang, M., Deng, P., Tang, N., and Liu, Y. (2020). Triptolide Ameliorates fine Particulate Matter-Induced Podocytes Injury via Regulating NF-Κb Signaling Pathway. BMC Mol. Cel Biol. 21 (1), 4. doi:10.1186/s12860-020-0248-6
Wang, H. Y., Tu, S. J., Wang, X. Q., and Zhou, X. R. (2021a). Analysis of Research Trends and Hotspots of Tripterygium Wilfordii Multiglucoside in Recent 20 Years Based on Knowledge Graph. Chin. Med. Herald 18 (17), 18–24.
Wang, K. M., Lai, X. H., and Chen, S. S. (2005). Study on Colquhounia Root in Treating IgA Nephropathy. Mod. J. Integr. Tradit. Chin. West. Med. 14 (14), 1819–1820. doi:10.3969/j.issn.1008-8849.2005.14.008
Wang, L. J., Yu, J. Y., Luo, M., and Fan, L. P. (2015a). The Effect of Huobahuagen Tablets in Intervening Diabetic Nephropathy in Clinical Stage and the Influence on the Level of Serum HGF. J. Nanjing Univ. Tradit. Chin. Med. 31 (05), 424–427. doi:10.14148/j.issn.1672-0482.2015.0424
Wang, S., Yang, N., Zhang, L., Huang, B., Tan, H., Liang, Y., et al. (2010). Jak/STAT Signaling Is Involved in the Inflammatory Infiltration of the Kidneys in MRL/lpr Mice. Lupus 19 (10), 1171–1180. doi:10.1177/0961203310367660
Wang, X. Q., Pan, Z. B., Gao, R. H., Han, S. H., and Wang, S. Y. (2016). Influence of HuobaHuagen Tablets Combined with Valsartan on Renal Function and Lipid Metabolism in Patients with Diabetic Nephropathy. Chin. J. Gen. Pract. 14 (11), 1860–1862. doi:10.16766/j.cnki.issn.1674-4152.2016.11.022
Wang, Y. D., Wang, Q., Zhang, J. B., Dai, Z., Lin, N., Wu, X. F., et al. (2019). Research Progress on Chemical Constituents and Quality Control of Tripterygium Wilfordii Preparations. Zhongguo Zhong Yao Za Zhi 44 (16), 3368–3373. doi:10.19540/j.cnki.cjcmm.20190606.501
Wang, Y. D., Yan, J. G., Chen, M. H., Pang, Y., Dai, Z., and Ma, S. C. (2021b). Study on UPLC Fingerprint of Tripterygium Glycosides Tablets from Different Manufacturers. Chin. J. Pharmacov. https://kns.cnki.net/kcms/detail/11.5219.R.20210608.1017.008.html
Wang, Y. H. (2020). Clinical Efficacy of Tripterygium Wilfordii Polyglycoside Combined with Omesartan in the Treatment of Patients with Primary IgA Nephropathy. Guide Chin. Med. 18 (29), 132–133. doi:10.15912/j.cnki.gocm.2020.29.063
Wang, Y. T., Gao, B. S., Yao, L. Y., Wang, W. G., Zhou, H. L., Zhang, W. L., et al. (2015b). Effect of Tripterygium Glycoside Tablets on Anti-rejection in the Early Stage after Kidney Transplantation. Chin. J. Gerontol. 35 (21), 6190–6191. doi:10.3969/j.issn.1005-9202.2015.21.090
WCVP (2022). World Checklist of Vascular Plants. version 2.0. Facilitated by the Royal Botanic Gardens, Kew. Available at: Retrieved http://wcvp.science.kew.org/ January 13, 2022).
Wu, H., Che, X., Zheng, Q., Wu, A., Pan, K., Shao, A., et al. (2014). Caspases: a Molecular Switch Node in the Crosstalk between Autophagy and Apoptosis. Int. J. Biol. Sci. 10 (9), 1072–1083. doi:10.7150/ijbs.9719
Xi, C., Peng, S., Wu, Z., Zhou, Q., and Zhou, J. (2017). WITHDRAWN: Toxicity of Triptolide and the Molecular Mechanisms Involved. Environ. Toxicol. Pharmacol. 90, 531–541. doi:10.1016/j.biopha.2017.04.00310.1016/j.etap.2017.09.013
Xia, Z. L., Xu, R. Q., Guo, S. M., and Deng, F. X. (1994). Thin-Layer Chromatography Examination of Tripterygium Wilfordii and Tripterygium Hypoglaucum (H. Lév.) Hutch. Zhong Cao Yao 25 (09), 464–465. doi:10.3321/j.issn:0253-2670.1994.09.011
Xiao, Z. J., Liu, C. X., Yang, X. X., Li, Y. Q., Wang, Z. X., Zhang, Z. K., et al. (2019). Research Progress on Chemical Composition and Pharmacological Activity of Tripterygium Wilfordii and Predictive Analysis on Q-Marker. Zhong Cao Yao 50 (19), 4752–4768. doi:10.7501/j.issn.0253-2670.2019.19.028
Xie, Q. K., and Dang, Y. (2016). Effect of Colquhounia Root Tablets Combined with Irbesartan in the Treatment of Idiopathic Membranous Nephropathy with Middle Amount Albuminuria. Chin. J. Integr. Tradit. West. Nephrol. 17 (12), 1070–1071. doi:10.3969/j.issn.1009-587X.2016.12.012
Xiong, X. R. (2019). Clinical Effect of Tripterygium Wilfordii Polyglycoside Tablets Combined with Prednisone in the Treatment of Nephrotic Syndrome. Chin. Mod. Med. 26 (19), 100–102. doi:10.3969/j.issn.1674-4721.2019.19.030
Xu, B. Z., Wu, M. J., Hu, X. H., Wang, T., and Gao, Y. W. (2021). Protective Effect of Multiglycoside of Tripterygium Wilfordii on Vascular Endothelial Injury in Rats with Chronic Kidney Disease and its Mechanism. J. Jilin Univ. (Medicine Edition) 47 (02), 377–383. doi:10.13481/j.1671-587x.20210217
Xu, J. Z., Zeng, Q. Y., Huang, S. B., and Xiao, Z. Y. (2001). Observation of Adverse Reactions of Tripterygium Glycosides. Chin. J. Clin. 29 (6), 47–48. doi:10.3969/j.issn.1008-1089.2001.06.038
Xu, P., Chen, B. W., and Chen, W. D. (2017). Effect of Tripterygium Glycoside Tablets in the Treatment of Diabetic Nephropathy and its Influence on Patients' UTP, GFR and SCr Levels. Dang Dai Yi Yao Lun Cong 15 (21), 107–108. doi:10.3969/j.issn.2095-7629.2017.21.077
Xue, J., Jia, X. B., Tan, X. B., Chen, Y., and Zhang, L. Y. (2010). Chemical Constituents of Triptergium Wilfordii Hook.F and its Toxicity. Chin. J. Tradit. Chin. Med. Pharm. 25 (05), 726–733.
Yang, X., Chen, A., Liang, Q., Dong, Q., Fu, M., Liu, X., et al. (2021). Up-regulation of Heme Oxygenase-1 by Celastrol Alleviates Oxidative Stress and Vascular Calcification in Chronic Kidney Disease. Free Radic. Biol. Med. 172, 530–540. doi:10.1016/j.freeradbiomed.2021.06.020
Zhan, X., Yan, C., Chen, Y., Wei, X., Xiao, J., Deng, L., et al. (2018). Celastrol Antagonizes High Glucose-Evoked Podocyte Injury, Inflammation and Insulin Resistance by Restoring the HO-1-Mediated Autophagy Pathway. Mol. Immunol. 104, 61–68. doi:10.1016/j.molimm.2018.10.021
Zhang, C., Sun, P. P., Guo, H. T., Liu, Y., Li, J., He, X. J., et al. (2016a). Safety Profiles of Tripterygium Wilfordii Hook F: A Systematic Review and Meta-Analysis. Front. Pharmacol. 7, 402. doi:10.3389/fphar.2016.00402
Zhang, C., Xia, M., Boini, K. M., Li, C. X., Abais, J. M., Li, X. X., et al. (2011). Epithelial-to-mesenchymal Transition in Podocytes Mediated by Activation of NADPH Oxidase in Hyperhomocysteinemia. Pflugers Arch. 462 (3), 455–467. doi:10.1007/s00424-011-0981-y
Zhang, C., Wang, G. C., Wang, S. G., and Chen, J. (2015). New Idea of TCM Prevention and Treatment of Chronic Allograft Nephropathy. Chin. Med. Mod. Distance Educ. Chin. 13 (03), 148–150. doi:10.3969/j.issn.1672-2779.2015.03.079
Zhang, H., Li, X., Xu, H., Ran, F., and Zhao, G. (2021). Effect and Safety Evaluation of Tacrolimus and Tripterygium Glycosides Combined Therapy in Treatment of Henoch-Schönlein Purpura Nephritis. Int. J. Urol. 28 (11), 1157–1163. doi:10.1111/iju.14665
Zhang, H., and Sun, S. C. (2015). NF-κB in Inflammation and Renal Diseases. Cel Biosci 5, 63. doi:10.1186/s13578-015-0056-4
Zhang, J. (2020a). Clinical Effect of Kunxian Capsules in the Treatment of Nephrotic Syndrome and its Effect on the Levels of Serum IFN-Γand IL-21 Levels in Patients. Shaanxi Med. J. 49 (03), 345–348. doi:10.3969/j.issn.1000-7377.2020.03.021
Zhang, L. Y., Li, H., Wu, Y. W., Cheng, L., Yan, Y. X., Yang, X. Q., et al. (2017). (5R)-5-hydroxytriptolide Ameliorates Lupus Nephritis in MRL/lpr Mice by Preventing Infiltration of Immune Cells. Am. J. Physiol. Ren. Physiol. 312 (4), F769–f777. doi:10.1152/ajprenal.00649.2016
Zhang, M., Chen, Y., Yang, M. J., Fan, X. R., Xie, H., Zhang, L., et al. (2019). Celastrol Attenuates Renal Injury in Diabetic Rats via MAPK/NF-κB Pathway. Phytother. Res. 33 (4), 1191–1198. doi:10.1002/ptr.6314
Zhang, X., Chen, C., Zhong, Y., and Zeng, X. (2020). lncRNA Involved in Triptonide-Induced Cytotoxicity in Mouse Germ Cells. Reprod. Toxicol. 98, 218–224. doi:10.1016/j.reprotox.2020.10.004
Zhang, X., Li, N., Yao, Y., Liang, X., Qu, X., Liu, X., et al. (2016b). Identification of Species in Tripterygium (Celastraceae) Based on DNA Barcoding. Biol. Pharm. Bull. 39 (11), 1760–1766. doi:10.1248/bpb.b15-00956
Zhang, X. L. (2020b). Effect Observation of Tripterygium Wilfordii Glycosides Tablets in Treatment of Diabetic Nephropathy and its Influence on Inflammatory Factors. Sichuan J. Anat. 28 (04), 56–57. doi:10.3969/j.issn.1005-1457.2020.04.026
Zhao, L. N., Liu, L., Li, H. Y., Wu, W., Sun, W. C., Zhang, W., et al. (2021). Study on the Effects and Immune Mechanisms of Kunxian Capsule in the Treatment of Chronic Glomerulonephritis. Chin. J. Integr. Tradit. West. Nephrol. 22 (04), 332–334. doi:10.3969/j.issn.1009-587X.2021.04.014
Zhen, Q. S., Ye, X., and Wei, Z. J. (1995). Recent Progress in Research on Tripterygium: a Male Antifertility Plant. Contraception 51 (2), 121–129. doi:10.1016/0010-7824(94)00018-r
Zheng, L., Sinniah, R., and Hsu, S. I. (2006). In Situ glomerular Expression of Activated NF-kappaB in Human Lupus Nephritis and Other Non-proliferative Proteinuric Glomerulopathy. Virchows Arch. 448 (2), 172–183. doi:10.1007/s00428-005-0061-9
Zhou, J. B., Wang, L. J., Sun, X. Y., Yuan, Y., Yu, J. Y., and Xu, L. (2016). Comparison of Efficacy of Colquhounia Root Tablets and Irbesartan Tablets in Treatment of DN. Xinan Guo Fang Yi Yao 26 (09), 961–963. doi:10.3969/j.issn.1004-0188.2016.09.001
Zhou, R., Zhang, F., He, P. L., Zhou, W. L., Wu, Q. L., Xu, J. Y., et al. (2005). (5R)-5-hydroxytriptolide (LLDT-8), a Novel Triptolide Analog Mediates Immunosuppressive Effects In Vitro and In Vivo. Int. Immunopharmacol. 5 (13-14), 1895–1903. doi:10.1016/j.intimp.2005.06.009
Zhou, R., Zheng, S. X., Tang, W., He, P. L., Li, X. Y., Yang, Y. F., et al. (2006). Inhibition of Inducible Nitric-Oxide Synthase Expression by (5R)-5-Hydroxytriptolide in Interferon-Gamma- and Bacterial Lipopolysaccharide-Stimulated Macrophages. J. Pharmacol. Exp. Ther. 316 (1), 121–128. doi:10.1124/jpet.105.093179
Keywords: tripterygium wilfordii hook. f., tripterygium wilfordii hook. f. preparations, kidney diseases, clinical applications, mechanisms, toxicity
Citation: Tong X, Qiao Y, Yang Y, Liu H, Cao Z, Yang B, Wei L and Yang H (2022) Applications and Mechanisms of Tripterygium Wilfordii Hook. F. and its Preparations in Kidney Diseases. Front. Pharmacol. 13:846746. doi: 10.3389/fphar.2022.846746
Received: 31 December 2021; Accepted: 01 March 2022;
Published: 21 March 2022.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Yibin Feng, The University of Hong Kong, Hong Kong SAR, ChinaXin Huang, China Pharmaceutical University, China
Peng Liu, Beijing Traditional Chinese Medicine Hospital, China
Copyright © 2022 Tong, Qiao, Yang, Liu, Cao, Yang, Wei and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Yang, eWI4MjAzQDEyNi5jb20=
†These authors have contributed equally to this work
 Xue Tong
Xue Tong Yanheng Qiao1,2†
Yanheng Qiao1,2† Yuanjian Yang
Yuanjian Yang Haizhao Liu
Haizhao Liu Lijuan Wei
Lijuan Wei Hongtao Yang
Hongtao Yang