- Department of Abdominal Oncology, West China Hospital, Sichuan University, Chengdu, China
Immune-related myocarditis is a severe and even life-threatening immune-related adverse event (irAE) which may also be underestimated due to the challenge in diagnosis. The inherent difference between individuals with immune-associated myocarditis has received little attention. Our study aimed to identify which baseline characteristics could contribute to distinguishing mild from severe ICI myocarditis. A retrospective analysis was conducted between March 2019 and June 2020 in West China Hospital, and 18 patients with immune-related myocarditis were studied. Patients were classified as having mild (n = 12) or severe myocarditis (n = 6), according to the clinical manifestations and hemodynamic complications. Factors associated with severe myocarditis were identified by comparing covariates derived from medical records in various groups. In this retrospective analysis, the median age of the 18 patients was 60 years old. Most myocarditis cases occur early and approximately after the first or second ICI infusion. The severity of myocarditis may be correlated with lactate dehydrogenase (LDH) (p = 0.04) and troponin levels (p = 0.0057). The relationship between troponin and myocarditis was further confirmed in another cohort, which included 30 patients. In addition, patients are more likely to develop multi-irAEs, and myositis was the most common second irAE. Those who experience multi-irAEs usually had significantly higher LDH (p = 0.02) and myoglobin levels (p = 0.02) than those who did not experience them. All patients were treated with steroids timely, and the mortality rate was 5.6% in our study. In this study, we explored risk factors for severe myocarditis and emphasized the importance of a multidisciplinary team in assisting diagnosis and treatment options. It is critical to initiate corticosteroid therapy, regardless of the severity of the myocarditis.
Introduction
The advent of immune-checkpoint inhibitors (ICIs) which block cytotoxic T lymphocyte-associated protein-4 (CTLA-4) and the programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) axis, both as monotherapy or in combination strategies, have revolutionized cancer treatment (Pardoll, 2012; Postow et al., 2015; Gong et al., 2018; Liu and Guo, 2018). However, as the use of ICIs in clinical practice has increased, immune-mediated toxicities have been observed, which can impact numerous organ systems, including the heart (Escudier et al., 2017; Zhu et al., 2021). Immune-related myocarditis is the most serious life-threatening toxicity, and the underlying pathomechanism is poorly understood (Liu et al., 2021). The incidence of immune-related myocarditis caused by ICI is approximately 1%, but the mortality rate can be as high as 46% (Mahmood et al., 2018; Moslehi et al., 2018). Because of its rarity, information on this unusual disease is limited, and further research is needed to fully understand the devastating irAEs. Furthermore, patients’ data are scarce on the clinical characteristics, results of pertinent examinations, and outcomes of ICI-related cardiotoxicity.
To gain better knowledge of this disease, we analyzed and described the characteristics of patients with immune-related myocarditis, including baseline clinical characteristics, drug-related data, relevant examination results, and outcomes. Our research looked into the internal disparities between patients and identified baseline variables that might assist in differentiating between low- and high-severity ICI myocarditis.
Materials and Methods
Study Population
In this single-center retrospective study, data were collected from 18 patients with ICI-related myocarditis in West China Hospital from March 2019 to June 2020. According to the clinical manifestations and whether they experienced hemodynamic consequences such as heart failure, cardiogenic shock, or arrhythmia, the patients were divided into mild (n = 12) and severe (n = 6) myocarditis groups. Inclusion criteria were listed as follows: 1) a histologically confirmed diagnosis of solid malignancy in West China Hospital; 2) age ≥18 years at ICI initiation; 3) received at least one cycle of ICI; 4) met the diagnostic criteria through multidisciplinary panel decision; and 5) exclude the possible diagnosis of myocarditis caused by other reasons such as COVID. The study was approved by the institutional review board, and the requirement for written informed consent was waived. The present study was approved by the institutional review board of West China Hospital and was exempted from informed consent requirements owing to its retrospective design.
This article does not contain any studies with human participants or animals performed by any of the authors. All methods were performed in accordance with the relevant guidelines and regulations.
Patient Data Collection
The following characteristics of patients were recorded: 1) clinical baseline characteristics: age, gender, smoking and alcohol status, comorbid conditions, disease stage, cancer type, and histological subtype; 2) ICI-related information: type of ICI, treatment start date, the date of diagnosis, therapy line, combination with other drugs, and response patterns; 3) relevant examination results: laboratory data, including myocardial markers and infection-related indicators, echocardiography, coronary angiography, and coronary computed tomography (CT); and 4) treatment and outcome.
Statistical Analysis
Study variables were presented as number (n)—percentage (%) and mean ± standard deviation. Wilcoxon rank-sum test was used for continuous variables, and χ2 or Fisher’s exact test was used for categorical variables. Missing data were not analyzed in this study. The statistical analyses were conducted via R 4.0.3 and GraphPad Prism 8. p < 0.05 was a statistically significant standard.
Results
Baseline Characteristics
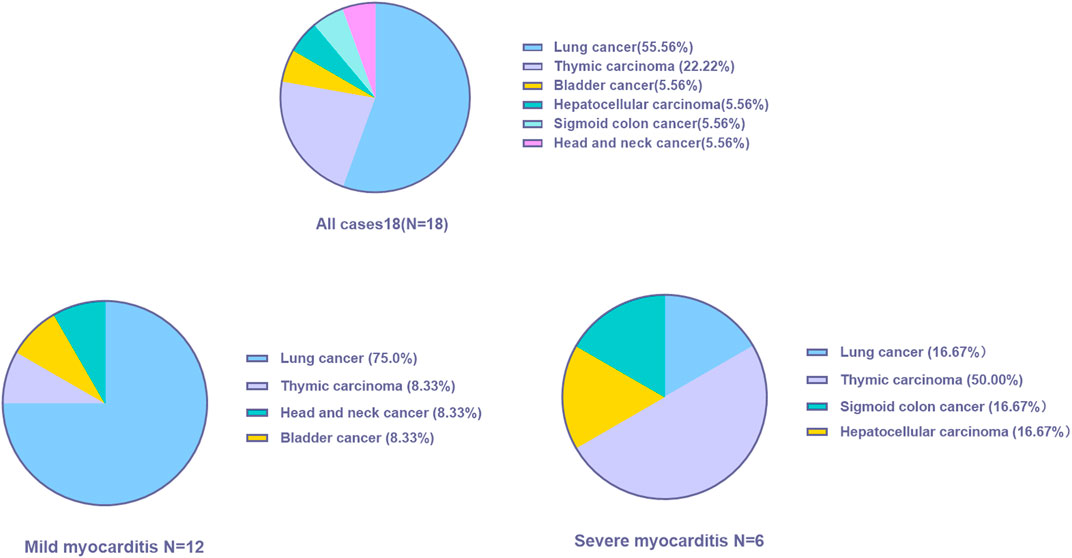
A total of 18 patients who met the selection criteria were retrospectively identified in this study, and the patients were classified into mild (n = 12) and severe myocarditis (n = 6) according to the clinical manifestation and whether they had hemodynamic complications, such as heart failure, cardiogenic shock, or arrhythmia. The clinical characteristics of the 18 patients with immune-related myocarditis are shown in Table 1. The median age among the 18 patients was 60 years old (range from 29.0 to 79.0 years), 83.3% were male, and a history of smoking was reported in 33.3%. Alcohol consumption was reported in 16.7% of the patients. 27.8% of patients had a history of diabetes, and 38.9% of patients had hypertension. Lung cancer was the most common cancer diagnosis (55.6%), and thymic carcinoma ranked next (22.2%), followed by bladder cancer (5.56%), sigmoid colon cancer (5.56%), and head and neck cancer (5.56%) (Figure 1). In addition, the majority of patients (72.2%) were in stage IV cancer at the time of ICI commencement. Half of the patients were asymptomatic. The most common clinical manifestations included dyspnea and fatigue.
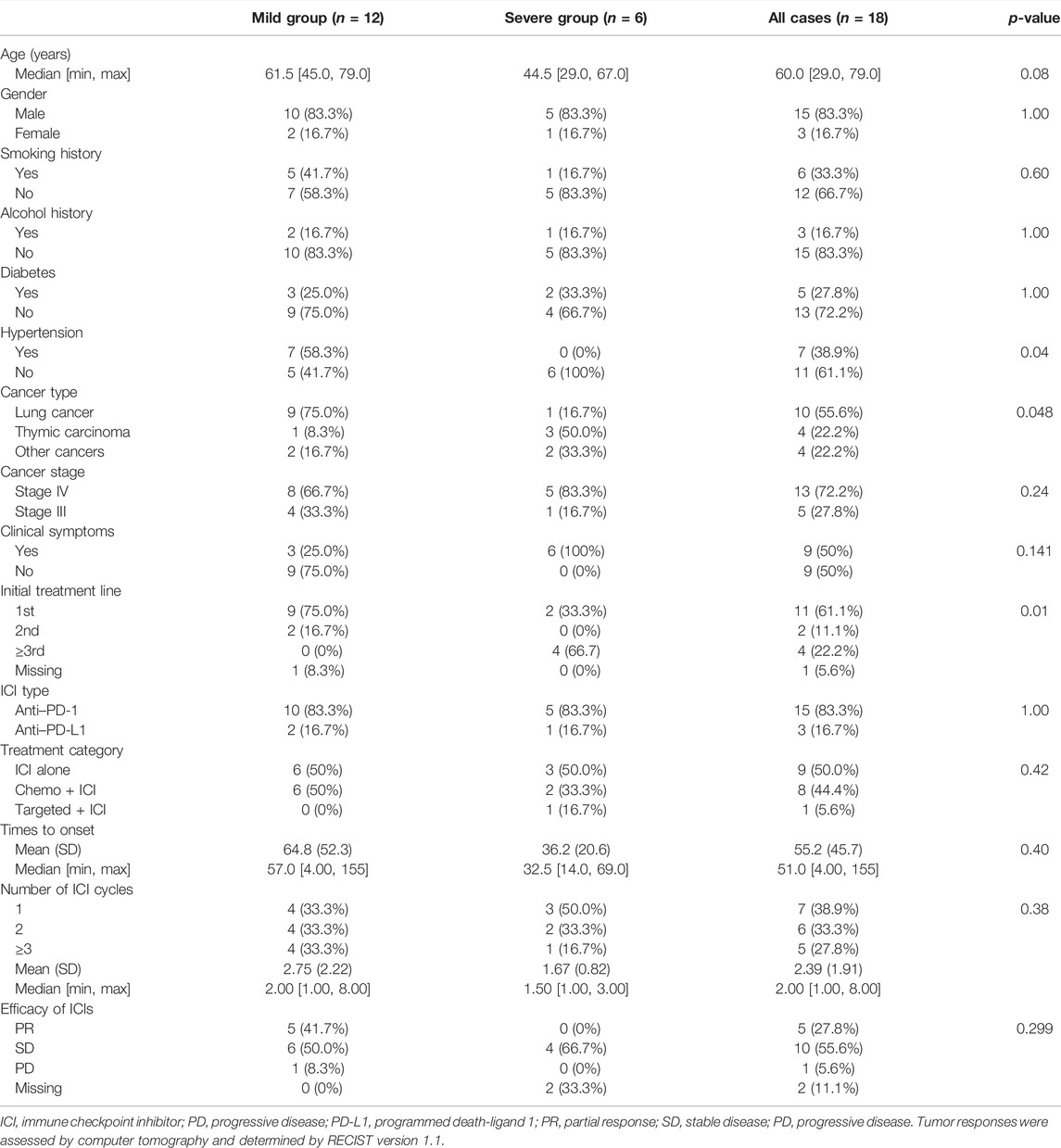
TABLE 1. Clinical characteristics of the patients and information on ICI in patients with immune-related myocarditis.
In the mild myocarditis group, the mean age was 63.3 years old (range from 45.0 to 79.0 years), and in the severe group, the mean age was 46.8 years old (range from 29.0 to 67.0 years). Lung cancer was the most prevalent cancer type in the mild group (75.0%), but thymic carcinoma was the most common cancer type in the younger age group (50.0%). Furthermore, none of the patients with severe myocarditis had a history of hypertension.
Clinical signs of severe ICI-related myocarditis include arrhythmia, heart failure, and cardiogenic shock. Only one of the six patients with severe myocarditis in this study did not have an arrhythmia; the other five out of six had all of the clinical symptoms listed earlier.
Treatment-Related Characteristics
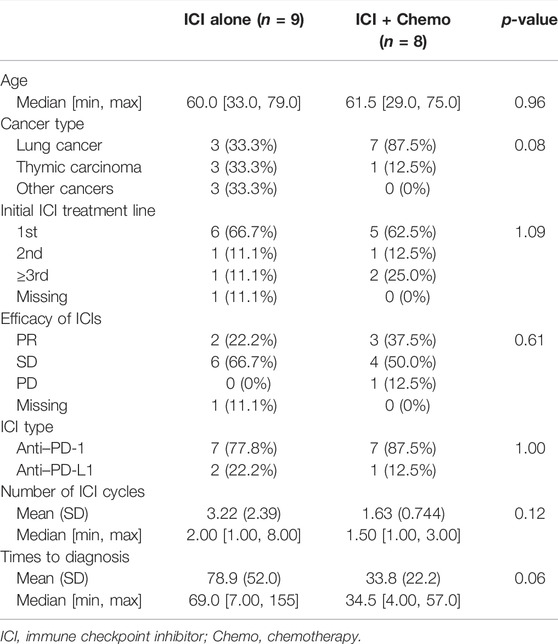
The majority of patients (61.1%) received ICIs as first-line therapy, while chemotherapy, not immunotherapy, has been used as first- or second-line therapy in the severe myocarditis group. In 66.7% of patients with severe myocarditis, ICIs were used as a third-line or later treatment (Table 1). According to the distinct mechanisms of action of ICIs, 15 patients (83.3%) received anti-PD-1 therapy, and the remaining three patients (16.7%) received anti-PD-L1 treatment. The percentage distribution of 18 cases was as follows: pembrolizumab 33.3%, sintilimab 22.2%, camrelizumab 16.7%, and other ICIs 27.8%, according to various ICI administered.
The median cycles of the onset of immune-related myocarditis from the initiation of ICI infusion were 2 (range, 1–8 cycles), and the median time was 51.0 days (range, 4–155 days). The number of days from the initiation of ICI infusion to the presentation of myocarditis in the mild group was longer than in the severe group. The mean time was 64.8 and 36.2 days in the mild and severe groups, respectively. Approximately 72.2% of patients developed myocarditis after 1 or 2 ICI infusion cycles. For patients with severe myocarditis, only one patient received more than 3 cycles at the time of diagnosis. Tumor responses were assessed using computer tomography and determined using the RECIST version 1.1. Overall, five patients experienced partial responses, ten patients exhibited stable disease, and one patient had progressive disease after ICI administration.
Patients were divided into three groups based on their treatment regimen. The proportion of participants who used ICI alone, chemotherapy + ICI, and targeted therapy + ICI were 50.0%, 44.4%, and 5.60%, respectively. Due to the small number of participants in this study, people treated with ICI alone (n = 9) and Chemo + ICI (n = 8) will be discussed further (Table 2). The median onset time was earlier in the Chemo + ICI group compared with ICI alone cohort (34.5 vs. 69.0 days). In the mild-myocarditis group, the onset time of ICI alone was 109 days, which was longer than patients who received Chemo + ICI (onset time:34.5 days). Timing of the development of immune-related myocarditis with a mean delay of 78.9 days (range, 7–155 days) and a median of 2 doses from the first ICI administration in the monotherapy group. Details of other information in each subgroup are listed in Table 2.
Laboratory and Other Relevant Examination Results
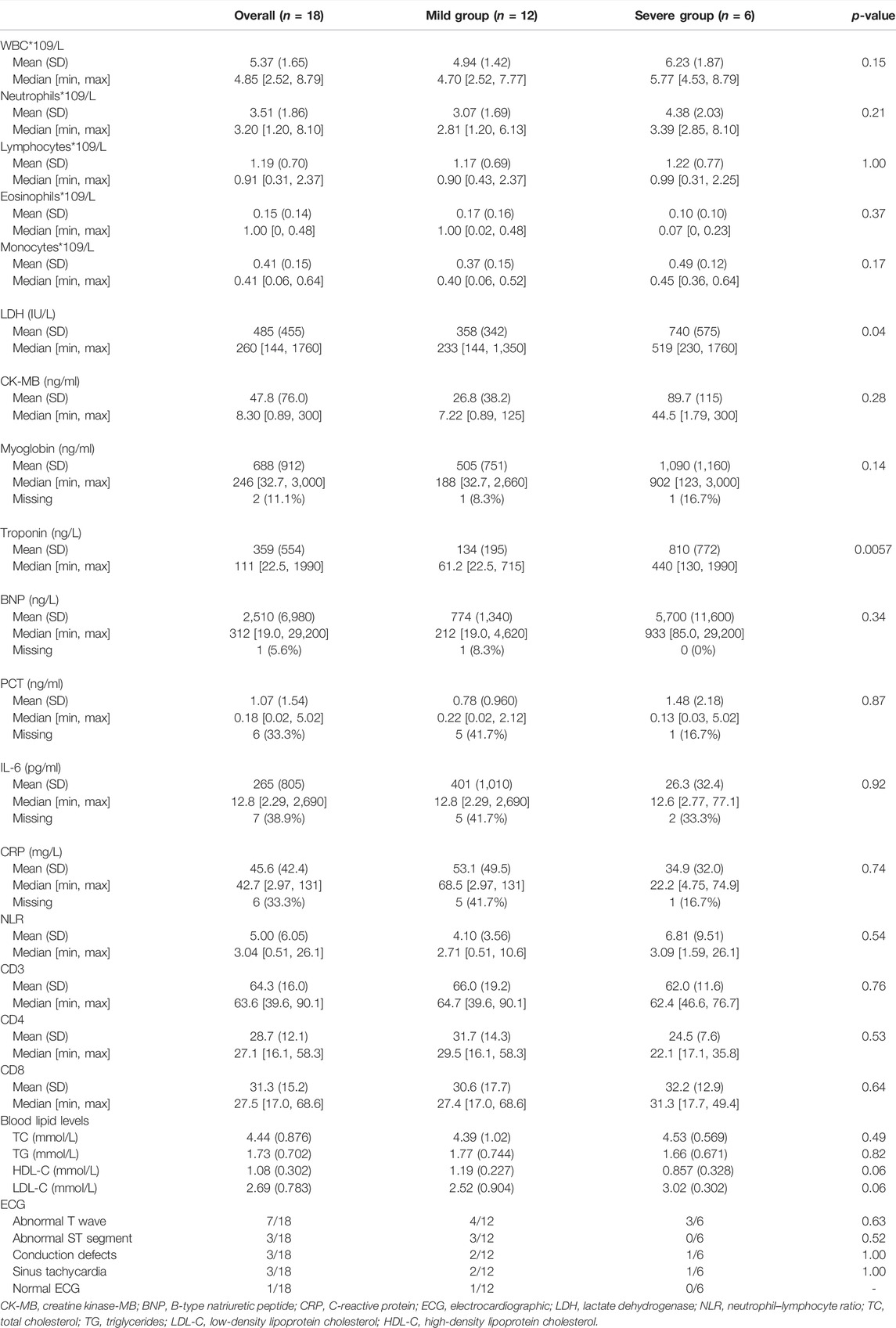
Baseline laboratory results were available in the majority of our patients. Troponin was elevated in all of the patients. Troponin values in most patients did not return to normal after 6 months, and the levels fluctuated considerably during therapy. The median troponin levels were significantly higher in the severe myocarditis group than in patients with mild myocarditis (61.2 ng/L vs. 440 ng/L, respectively, p = 0.0057). B-type natriuretic peptide (BNP), creatine kinase-MB (CK-MB), and myoglobin in the severe cohort were higher than in the mild cohort, but the difference was not statistically significant. A significant elevation in lactate dehydrogenase (LDH) was observed between those two groups (p = 0.04). The median value of LDH was 233 IU/L and 519 IU/L in the mild and severe myocarditis groups, respectively. White blood cells (WBC), neutrophils, lymphocytes, eosinophils, and monocytes were higher in the severe group than in the mild group, but those parameters were not statistically significant (Table 3). CD3, CD8, and CD4 serum levels were also examined prior to ICI therapy, but no significant differences were seen. Blood lipid levels, including total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), were also measured in our study. No statistical significance was detected between the severity of myocarditis and blood lipid levels. Higher TC and LDL-C levels and lower HDL-C levels were found in the severe group, and large-scale studies considering the relationship are needed for further exploration.
Every patient received an electrocardiographic (ECG) examination, and nearly every case of myocarditis showed an irregular ECG (94.4%). It was found that seven cases had abnormal T waves, three cases had an abnormal ST segment, and conduction defects were also found in three patients. In addition, sinus tachycardia showed in three cases. But such a difference in ECG between those two groups was not statistically significant.
Echocardiography was performed on 17 patients. Two patients were discovered to have a ventricular wall movement disorder, and two patients had left ventricular systolic dysfunction (LVEF ≤ 50%). One of those two patients was diagnosed with severe myocarditis and third-degree atrioventricular block, with an LVEF of 40%. Coronary angiography was also performed on this patient, which revealed the anterior descending branch, circumflex branch, and right coronary artery were stenosed, and the first diagonal branch had the most severe stenosis of nearly 40%. However, the patient had no history of coronary heart disease. Coronary angiography results were also obtained from the other four patients. One patient in the severe group had stenosis in the left anterior descending branch, circumflex branch, and right coronary artery. The diagnosis of myocarditis in these two cases was made by a multidisciplinary team, and they all matched the inclusion criteria. Nevertheless, there were no meaningful findings in the other three patients in the mild group. A cardiac magnetic resonance imaging (MRI) investigation was performed in six cases; however, we were unable to find meaningful data in five of them. Only one patient showed myocardial edema on T2-weighted imaging. Furthermore, one patient with severe myocarditis received cardiac 18F-fluorodeoxyglucose (18F-FDG) position emission tomography magnetic resonance imaging (PET-MRI), which revealed enhanced FDG metabolism at the left ventricle. Once myocarditis was diagnosed, patients received steroid treatment. The initial steroid dose was an equivalent of methylprednisolone (1–2 mg/kg), which was sustained for 3–5 days and followed by a long-term oral steroid taper. In four patients, intravenous gamma globulin was used to relieve their symptoms. Unfortunately, one patient died as a result of myocarditis-related complications.
Concomitant irAEs and Outcomes
66.7% of patients (12 cases) experienced at least one irAE (Figure 2A). A total of nine patients developed another irAE: six patients were diagnosed with immune-related myositis, two acquired hypothyroidism, and one suffered pneumonitis. One patient developed another two irAEs, and two patients were concurrent with three irAEs. Overall, there was no difference in the prevalence between the mild and severe groups (p > 0.5). Myositis (n = 8) and hypothyroidism (n = 4) were the most common irAEs in our cohorts.
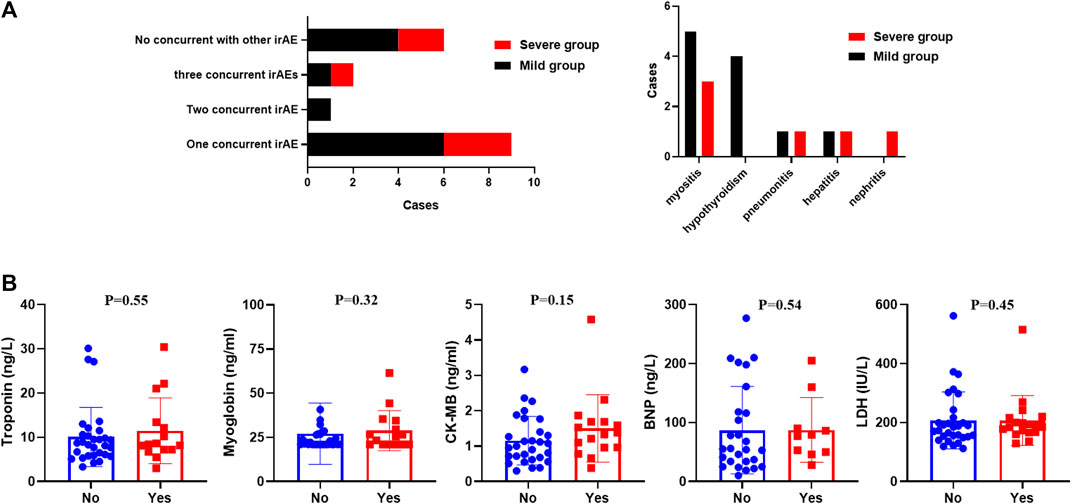
FIGURE 2. (A) Distribution of concurrent irAEs in our study. (B) Serum myocardial enzymes and LDH between patients with or without immune-related myocarditis.
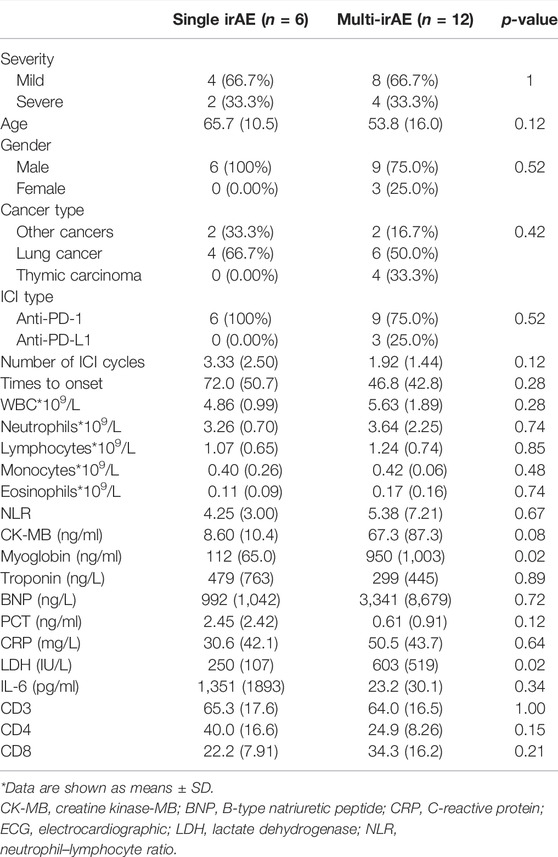
The difference between those who had additional irAE and those who did not was also discussed in our research (Table 4). Patients without concomitant irAEs (n = 6) were all males and treated with PD-1 antibodies. Patients with a single irAE had a delayed onset time than those with multiple irAEs. Baseline characteristics were explored, and we identified that the alcohol history was significantly different. All patients with concomitant irAEs did not have an alcohol history (p = 0.03). However, this result should be validated in a larger cohort study. There were also statistically significant changes in LDH and myoglobin. Patients with multi-irAEs had a higher levels of LDH (250 IU/L vs. 603 IU/L; p = 0.02) and myoglobin (112 ng/ml vs. 950 ng/ml; p = 0.02). There was also a trend of higher CK-MB, BNP, and a lower troponin in patients with multi-irAEs, although these changes were not statistically significant.
Comparison of the Baseline Levels of Serum Myocardial Enzymes and Lactate Dehydrogenase Between Different Groups
Our results revealed that the serum myocardial enzymes and LDH might play a suggestive role in the early diagnosis of immune-related myocardial injury. However, baseline values of those markers did not differ statistically between patients with severe and mild myocarditis in our study (p > 0.05, Table 5). To further explore the difference in those markers between patients with and without myocarditis, we retrospectively screened 30 patients without myocarditis in our hospital who were treated with ICIs. There was no statistical significance in the differences in serum myocardial enzymes and LDH between patients who experienced immune-related myocarditis and those who did not experience them (p > 0.05, Figure 2B). We also analyzed the changes in those markers during treatment with ICIs in patients without immune-associated myocarditis. The comparison of LDH, troponin, CK-MB, BNP, and myoglobin levels between baseline and after one or two ICI cycles were not statistically significant (p > 0.05).
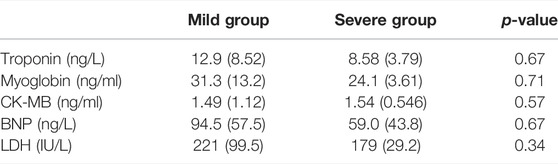
TABLE 5. Comparison of the baseline levels of serum myocardial enzymes and LDH between different groups.
Role of Troponin and Lactate Dehydrogenase Levels in Diagnosing Severe Myocarditis
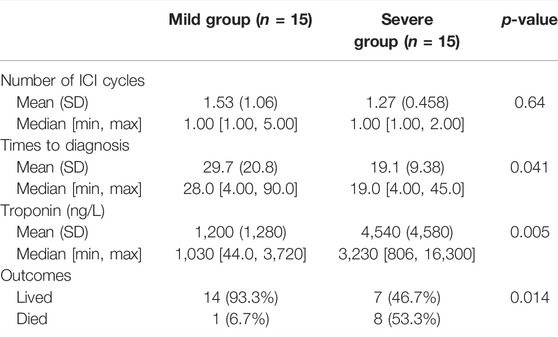
We searched PubMed for relevant articles to validate our findings and selected 30 patients for analysis (Katsume et al., 2018; Khoury et al., 2019; Wang and Hu, 2019; Balanescu et al., 2020; Diamantopoulos et al., 2020; Hardy et al., 2020; Hyun et al., 2020; Leaver et al., 2020; Szuchan et al., 2020; Tu et al., 2020; Valenti-Azcarate et al., 2020; Wang et al., 2020; Xie et al., 2020; Xing et al., 2020; Arangalage et al., 2021; Cham et al., 2021; Chen et al., 2021; Elder et al., 2021; Iwasaki et al., 2021; Komatsu et al., 2021; Liang et al., 2021; Tanabe et al., 2021; Yanase et al., 2021; Zhang et al., 2021). The results are coincident with ours. Patients with severe myocarditis have a higher troponin (p = 0.005) and an earlier onset time (19.1 vs. 29.7 days, p = 0.014) than patients in the mild group. In addition, a significant difference was found in the outcome between the two groups (Table 6). Patients in the severe group have a higher mortality (53.3% vs. 6.7%, p = 0.041). The LDH in most studies were not eligible and, thus, we did not analyze it.
Discussion
Immune checkpoint inhibitors have drastically improved clinical outcomes, and they are increasingly being licensed for use in the early stage of cancer and in combination with other anti-tumor therapies, such as chemotherapy or targeted therapy (Postow et al., 2015; Zhang et al., 2018; Luo et al., 2021). However, due to the increased usage of these medications, immune-related adverse events in patients receiving ICIs are becoming more widely recognized, which may limit their clinical applicability. One of the most fatal irAEs is immune-related myocarditis. Due to its severity and high mortality, there is a growing interest in further study (Ganatra and Neilan, 2018). Because of its rarity, the diagnosis and management of ICI-related myocarditis represent a clinical challenge. The diagnostic algorithm may start with troponin screening or the onset of symptoms during ICI therapy (Ball et al., 2019; Hu et al., 2019).
The risk of myocarditis varies depending on the treatment regimen and the ICI drugs used. Overall, ICI-related myocarditis was reported to be around 1% of the incidence. Previous research showed that dual ICIs regimens caused a higher incidence than monotherapy (Rubio-Infante et al., 2021). The incidence of monotherapy, dual ICIs therapies, and ICI plus chemotherapy were found to be 3.1, 5.8, and 3.7%, respectively. There were also variations in the occurrence of myocarditis among the various classes of ICIs (Mahmood et al., 2018). Based on results from limited clinical studies, myocarditis is more likely to be caused by the CTLA-4 antibody (Mascolo et al., 2021). Furthermore, it has been observed that nivolumab (anti-PD-1) had a decreased incidence of cardiac irAEs. The underlying mechanism is still unclear. Many hypotheses have been formulated with regard to the relationship between ICIs and cardiotoxicity. T-cell infiltration into the myocardium, increased auto-antibodies acting on self-antigens, and increased T cells reacting to antigens shared by cancer and normal cells could all be contributors to the pathomechanism of the irAEs (Baik et al., 2021).
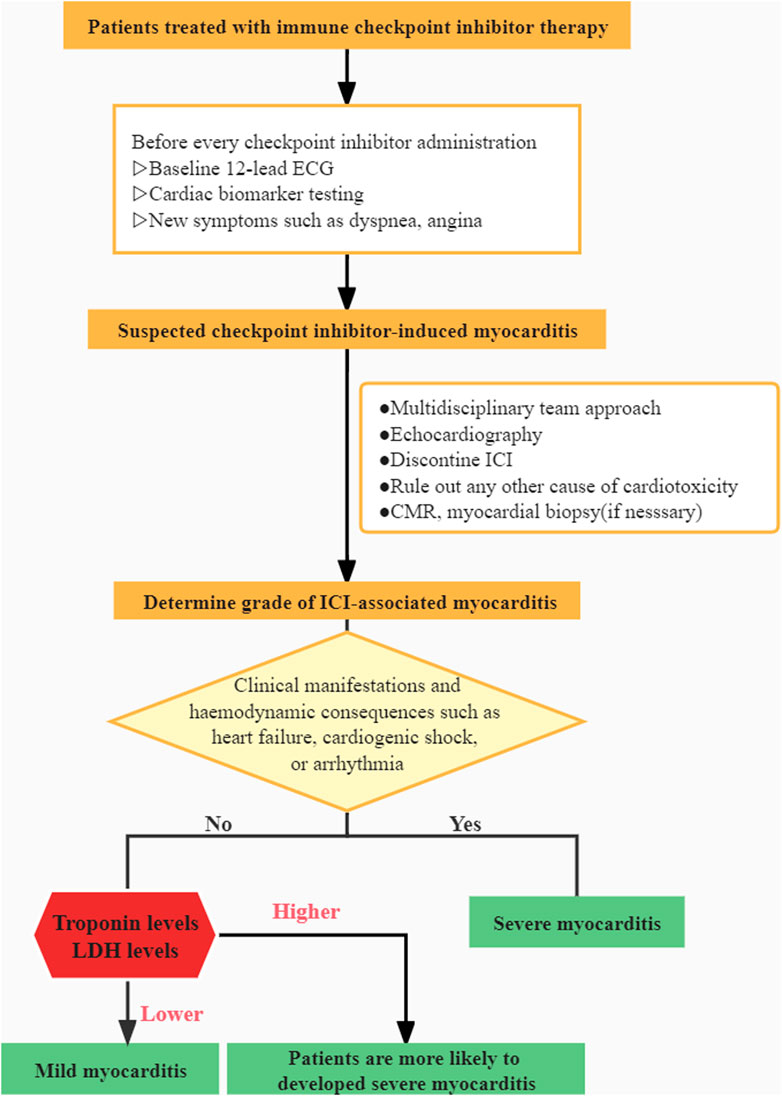
There is a scarcity of information on this potentially fatal adverse event. Our current understanding of the illness is limited, and patient-derived evidence is scarce. Recent research has concentrated on examining the disease’s overall characteristics while neglecting the individual variabilities. The goal of this retrospective study was to provide new insights about such a rare irAE and explore risk factors for severe myocarditis and multi-irAEs. According to Common Terminology Criteria for Adverse Events (CTCAE), AE grading is currently based on the outcomes of biomarkers, ECG, symptoms, and cardiac complications (Brahmer et al., 2018). Patients with grade 1 need to be closely monitored during therapy. The patient in grade 1 was asymptomatic but had aberrant cardiac biomarkers with an irregular ECG. Compared to grade 1, patients with grade 2 had minor symptoms. Symptoms in grade 3 patients were more severe and required the use of steroids to manage. Grade 4 was defined as moderate to severe decompensation of life-threatening situations that demand intravenous injection of medication or intervention. Patients were divided into two groups in our study based on their clinical manifestations and whether or not they had hemodynamic complications such as heart failure, cardiogenic shock, or arrhythmia, which may be more useful in assisting clinicians in determining a patient’s condition and prognosis. Patients in the severe group were categorized as grade 3–4 toxicity, and patients in the mild group were classified as grade 1–2 toxicity.
It is critical to distinguish between mild and severe myocarditis due to the high mortality rate. Several studies have identified treatment regimens, comorbidities such as hypertension and diabetes, and tobacco use as risk factors for cardiotoxicity (Patel et al., 2021). However, Subgroup analyses based on age, gender, tobacco usage, diabetes, and cancer stage revealed no differences in our study. According to our findings, hypertension and cancer type may not correlate to the severity of myocarditis. Contrary to earlier reports, our data have shown that none of the patients in the severe group had hypertension. In addition, none of the 18 patients had coronary artery disease or any other type of cardiac disease. No statistical significance was detected between the severity of myocarditis and blood lipid levels.
The most common malignancy type in the mild group was lung cancer, while the most common type in the severe group was thymic carcinoma. The fundamental cause of the discrepancy in our study was unknown. This could be due to the fact that a large percentage of patients with thymic cancer have an autoimmune syndrome, which could be an independent risk factor for ICI-associated cardiotoxicity. The thymus plays an essential role in the development of T-cells. Although autoimmune disease patients are not the focus of our research, the function and composition of T-cells, which are important components of the immune system, are similarly aberrant in the thymoma microenvironment (Hung et al., 2019). In addition, the incidence of myocarditis was higher in two trials which evaluated pembrolizumab in thymic tumors, at 5 and 9.1%, respectively (Giaccone et al., 2018; Cho et al., 2019). Additional studies are needed to further explore the link between myocarditis, thymic tumors, and ICI therapy. Overall, monitoring the occurrence of myocarditis in thymic carcinoma patients receiving ICI is crucial.
According to the literature, immune-related myocarditis frequently develops soon after starting ICI therapy. The median time of onset was 34 days, with the majority of cases occurring within 3 months (Mahmood et al., 2018). In our studies, the median length was 2 cycles, and the onset time was 51 days (range: 4–155), which is longer than previous reports (Ma et al., 2021). Patients with severe myocarditis had a faster onset time than those with mild myocarditis. Myocarditis is more likely to present at an early age in patients in the mild group who were treated with combination therapy, although the results of onset time were not statistically significant. Treatment lines, ICI type, number of ICI cycles, and ICI efficacy all had no statistically significant differences. Recent research suggested that irAEs have been linked to a long-term response and therapeutic benefit (Dimitriou et al., 2021; Ma et al., 2021; Paderi et al., 2021). Among the 16 patients, five patients had a partial response (PR), ten patients had stable disease (SD), and one patient had progressive disease (PD).
Early identification of severe myocarditis followed by timely treatment is essential to reduce mortality and assist physicians in personalized medicine decision-making. Our findings showed that troponin and LDH might contribute to recognizing severe myocarditis as soon as possible. Previous studies have demonstrated that monitoring troponin during treatment is reasonable (Sarocchi et al., 2018; Liu et al., 2021; Puzanov et al., 2021), but the relationship between LDH and cardiotoxicity was never reported. Patients with severe myocarditis are more likely to have a higher LDH. Furthermore, real-time screening of concomitant irAE is essential in patients experiencing immune-related myocarditis, especially in patients with higher LDH and myoglobin levels. In addition, the level of troponin also correlated with outcomes.
The diagnosis and management of this disorder continue to be a clinical and research challenge. Myocardial biopsy is the gold standard for diagnosis. However, the complications may have an adverse effect on clinical utility and outcomes (Imran et al., 2019). The relevance of cardiac MRI, FDG-PET, and coronary angiography in the identification of myocarditis has also been highlighted in recent research (Nensa et al., 2018; Spallarossa et al., 2020; Ederhy et al., 2021). However, performing those examinations may miss the optimal treatment time and result in negative repercussions. A multidisciplinary team (MDT) approach is crucial for assessing suspected immune-related myocarditis since it aids decision-making and lowers death rates. The management of ICI-related myocarditis demands close collaboration between oncologists and cardiologists.
The mortality rate was reported to be as high as 46% (Mahmood et al., 2018), while in our study, it was 5.6% (1/18). Early identification by MDT and the use of steroids may have contributed to the lower mortality rate. For severe myocarditis, there is no question that corticosteroids should be used, but the treatment of mild myocarditis by steroids is still unknown and requires further research. Our study provides clinical relevance and rationale for initiating corticosteroid therapy regardless of the severity of myocarditis. All 18 patients in our study received steroid treatment. An equivalent dose of (methyl) prednisolone (1–2 mg/kg) was given for the first 3–5 days, followed by a long-term oral steroid taper. In addition to the use of steroids, intravenous gamma globulin should be considered to alleviate the symptoms of patients based on their clinical needs. After being diagnosed with myocarditis, no one disputes ICIs again.
Our research fills a gap in the literature and sheds new light on a rare irAE. A clinical algorithm is presented based on our clinical experience (Figure 3). However, there are a few flaws worth mentioning. It is worth noting the inherent bias in any single-institution retrospective analysis and also the limited sample population in our study. Due to the challenges in diagnosis, the vast majority of myocarditis events may be overlooked or misdiagnosed. Furthermore, extreme vigilance is essential in the therapy and the avoidance of excessive corticosteroid usage for biased results. Further investigation is required as an outcome of our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of West China Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ML: conceptualization, supervision, and data curation; YL: writing original draft, formal analysis, and validation; remaining authors: supervision and validation.
Funding
This work was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC21043).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arangalage, D., Pavon, A. G., Özdemir, B. C., Michielin, O., Schwitter, J., and Monney, P. (2021). Acute Cardiac Manifestations under Immune Checkpoint Inhibitors-Beware of the Obvious: a Case Report. Eur. Heart J. Case Rep. 5, ytab262. doi:10.1093/ehjcr/ytab262
Baik, A. H., Tsai, K. K., Oh, D. Y., and Aras, M. A. (2021). Mechanisms and Clinical Manifestations of Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors. Clin. Sci. (Lond) 135, 703–724. doi:10.1042/CS20200331
Balanescu, D. V., Donisan, T., Palaskas, N., Lopez-Mattei, J., Kim, P. Y., Buja, L. M., et al. (2020). Immunomodulatory Treatment of Immune Checkpoint Inhibitor-Induced Myocarditis: Pathway toward Precision-Based Therapy. Cardiovasc Pathol. 47, 107211. doi:10.1016/j.carpath.2020.107211
Ball, S., Ghosh, R. K., Wongsaengsak, S., Bandyopadhyay, D., Ghosh, G. C., Aronow, W. S., et al. (2019). Cardiovascular Toxicities of Immune Checkpoint Inhibitors: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 74, 1714–1727. doi:10.1016/j.jacc.2019.07.079
Brahmer, J. R., Lacchetti, C., Schneider, B. J., Atkins, M. B., Brassil, K. J., Caterino, J. M., et al. (2018). Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 36, 1714–1768. doi:10.1200/JCO.2017.77.6385
Cham, J., Ng, D., and Nicholson, L. (2021). Durvalumab-induced Myocarditis, Myositis, and Myasthenia Gravis: a Case Report. J. Med. Case Rep. 15, 278. doi:10.1186/s13256-021-02858-7
Chen, Y., Jia, Y., Liu, Q., Shen, Y., Zhu, H., Dong, X., et al. (2021). Myocarditis Related to Immune Checkpoint Inhibitors Treatment: Two Case Reports and Literature Review. Ann. Palliat. Med. 10, 8512–8517. doi:10.21037/apm-20-2620
Cho, J., Kim, H. S., Ku, B. M., Choi, Y. L., Cristescu, R., Han, J., et al. (2019). Pembrolizumab for Patients with Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J. Clin. Oncol. 37, 2162–2170. doi:10.1200/JCO.2017.77.3184
Diamantopoulos, P. T., Tsatsou, K., Benopoulou, O., Bonou, M., Anastasopoulou, A., Mastrogianni, E., et al. (2020). Concomitant Development of Neurologic and Cardiac Immune-Related Adverse Effects in Patients Treated with Immune Checkpoint Inhibitors for Melanoma. Melanoma Res. 30, 484–491. doi:10.1097/CMR.0000000000000681
Dimitriou, F., Staeger, R., Ak, M., Maissen, M., Kudura, K., Barysch, M., et al. (2021). Frequency, Treatment and Outcome of Immune-Related Toxicities in Patients with Immune-Checkpoint Inhibitors for Advanced Melanoma: Results from an Institutional Database Analysis. Cancers 13, 931. doi:10.3390/cancers13122931
Ederhy, S., Salem, J. E., Dercle, L., Hasan, A. S., Chauvet-Droit, M., Nhan, P., et al. (2021). Role of Cardiac Imaging in the Diagnosis of Immune Checkpoints Inhibitors Related Myocarditis. Front. Oncol. 11, 640985. doi:10.3389/fonc.2021.640985
Elder, C. T., Davis, E. C., Jaipal, S., and Wight, C. E. (2021). Immune-checkpoint Inhibitor Toxicity during a Pandemic: Overcoming Patient Fears to Provide Care. A Case Report. J. Oncol. Pharm. Pract. 27, 2035–2040. doi:10.1177/10781552211012782
Escudier, M., Cautela, J., Malissen, N., Ancedy, Y., Orabona, M., Pinto, J., et al. (2017). Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 136, 2085–2087. doi:10.1161/CIRCULATIONAHA.117.030571
Ganatra, S., and Neilan, T. G. (2018). Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 23, 879–886. doi:10.1634/theoncologist.2018-0130
Giaccone, G., Kim, C., Thompson, J., McGuire, C., Kallakury, B., Chahine, J. J., et al. (2018). Pembrolizumab in Patients with Thymic Carcinoma: a Single-Arm, Single-Centre, Phase 2 Study. Lancet Oncol. 19, 347–355. doi:10.1016/S1470-2045(18)30062-7
Gong, J., Chehrazi-Raffle, A., Reddi, S., and Salgia, R. (2018). Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: a Comprehensive Review of Registration Trials and Future Considerations. J. Immunother. Cancer 6, 8. doi:10.1186/s40425-018-0316-z
Hardy, T., Yin, M., Chavez, J. A., Ivanov, I., Chen, W., Nadasdy, T., et al. (2020). Acute Fatal Myocarditis after a Single Dose of Anti-PD-1 Immunotherapy, Autopsy Findings: a Case Report. Cardiovasc Pathol. 46, 107202. doi:10.1016/j.carpath.2020.107202
Hu, J. R., Florido, R., Lipson, E. J., Naidoo, J., Ardehali, R., Tocchetti, C. G., et al. (2019). Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors. Cardiovasc Res. 115, 854–868. doi:10.1093/cvr/cvz026
Hung, C. T., Tsai, T. F., Chen, J. S., and Hsieh, M. S. (2019). Thymoma-associated Multiorgan Autoimmunity. BMJ Case Rep. 12, 229163. doi:10.1136/bcr-2018-229163
Hyun, J. W., Kim, G. S., Kim, S. H., Cho, J. Y., Kim, H. J., Lee, G. K., et al. (2020). Fatal Simultaneous Multi-Organ Failure Following Pembrolizumab Treatment for Refractory Thymoma. Clin. Lung Cancer 21, e74–e77. doi:10.1016/j.cllc.2019.10.008
Imran, M., Wang, L., McCrohon, J., Yu, C., Holloway, C., Otton, J., et al. (2019). Native T1 Mapping in the Diagnosis of Cardiac Allograft Rejection: A Prospective Histologically Validated Study. JACC Cardiovasc Imaging 12, 1618–1628. doi:10.1016/j.jcmg.2018.10.027
Iwasaki, S., Hidaka, H., Uojima, H., Hashimura, M., Nabeta, T., Sanoyama, I., et al. (2021). A Case of Immune Checkpoint Inhibitor-Associated Myocarditis after Initiation of Atezolizumab Plus Bevacizumab Therapy for Advanced Hepatocellular Carcinoma. Clin. J. Gastroenterol. 14, 1233–1239. doi:10.1007/s12328-021-01442-2
Katsume, Y., Isawa, T., Toi, Y., Fukuda, R., Kondo, Y., Sugawara, S., et al. (2018). Complete Atrioventricular Block Associated with Pembrolizumab-Induced Acute Myocarditis: The Need for Close Cardiac Monitoring. Intern Med. 57, 3157–3162. doi:10.2169/internalmedicine.0255-17
Khoury, Z. H., Hausner, P. F., Idzik-Starr, C. L., Frykenberg, M. R. A., Brooks, J. K., Dyalram, D., et al. (2019). Combination Nivolumab/Ipilimumab Immunotherapy for Melanoma with Subsequent Unexpected Cardiac Arrest: A Case Report and Review of Literature. J. Immunother. 42, 313–317. doi:10.1097/CJI.0000000000000282
Komatsu, M., Hirai, M., Kobayashi, K., Hashidate, H., Fukumoto, J., Sato, A., et al. (2021). A Rare Case of Nivolumab-Related Myasthenia Gravis and Myocarditis in a Patient with Metastatic Gastric Cancer. BMC Gastroenterol. 21, 333. doi:10.1186/s12876-021-01904-4
Leaver, P. J., Jang, H. S., Vernon, S. T., and Fernando, S. L. (2020). Immune Checkpoint Inhibitor-Mediated Myasthenia Gravis with Focal Subclinical Myocarditis Progressing to Symptomatic Cardiac Disease. BMJ Case Rep. 13, 232920. doi:10.1136/bcr-2019-232920
Liang, S., Yang, J., Lin, Y., Li, T., Zhao, W., Zhao, J., et al. (2021). Immune Myocarditis Overlapping with Myasthenia Gravis Due to Anti-PD-1 Treatment for a Chordoma Patient: A Case Report and Literature Review. Front. Immunol. 12, 682262. doi:10.3389/fimmu.2021.682262
Liu, M., and Guo, F. (2018). Recent Updates on Cancer Immunotherapy. Precis. Clin. Med. 1, 65–74. doi:10.1093/pcmedi/pby011
Liu, Q., Yu, Y., Lin, J., Wang, Y., Ai, L., Li, Q., et al. (2021). Treatment Strategy for Myocarditis in Patients Using Immune Checkpoint Inhibitors or Combined Anti-vascular Endothelial Growth Factor Therapy by Clinical Severity. Eur. J. Cancer 157, 10–20. doi:10.1016/j.ejca.2021.07.023
Luo, W., Wang, Z., Zhang, T., Yang, L., Xian, J., Li, Y., et al. (2021). Immunotherapy in Non-small Cell Lung Cancer: Rationale, Recent Advances and Future Perspectives. Precis. Clin. Med. 4, 258–270. doi:10.1093/pcmedi/pbab027
Ma, R., Wang, Q., Meng, D., Li, K., and Zhang, Y. (2021). Immune Checkpoint Inhibitors-Related Myocarditis in Patients with Cancer: an Analysis of International Spontaneous Reporting Systems. BMC cancer 21, 38. doi:10.1186/s12885-020-07741-0
Mahmood, S. S., Fradley, M. G., Cohen, J. V., Nohria, A., Reynolds, K. L., Heinzerling, L. M., et al. (2018). Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 71, 1755–1764. doi:10.1016/j.jacc.2018.02.037
Mascolo, A., Scavone, C., Ferrajolo, C., Rafaniello, C., Danesi, R., Del Re, M., et al. (2021). Immune Checkpoint Inhibitors and Cardiotoxicity: An Analysis of Spontaneous Reports in Eudravigilance. Drug Saf. 44, 957–971. doi:10.1007/s40264-021-01086-8
Moslehi, J. J., Salem, J. E., Sosman, J. A., Lebrun-Vignes, B., and Johnson, D. B. (2018). Increased Reporting of Fatal Immune Checkpoint Inhibitor-Associated Myocarditis. Lancet 391, 933. doi:10.1016/S0140-6736(18)30533-6
Nensa, F., Kloth, J., Tezgah, E., Poeppel, T. D., Heusch, P., Goebel, J., et al. (2018). Feasibility of FDG-PET in Myocarditis: Comparison to CMR Using Integrated PET/MRI. J. Nucl. Cardiol. 25, 785–794. doi:10.1007/s12350-016-0616-y
Paderi, A., Giorgione, R., Giommoni, E., Mela, M. M., Rossi, V., Doni, L., et al. (2021). Association between Immune Related Adverse Events and Outcome in Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Cancers (Basel) 13, 860. doi:10.3390/cancers13040860
Pardoll, D. M. (2012). The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Patel, R. P., Parikh, R., Gunturu, K. S., Tariq, R. Z., Dani, S. S., Ganatra, S., et al. (2021). Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 23, 79. doi:10.1007/s11912-021-01070-6
Postow, M. A., Callahan, M. K., and Wolchok, J. D. (2015). Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 33, 1974–1982. doi:10.1200/JCO.2014.59.4358
Puzanov, I., Subramanian, P., Yatsynovich, Y. V., Jacobs, D. M., Chilbert, M. R., Sharma, U. C., et al. (2021). Clinical Characteristics, Time Course, Treatment and Outcomes of Patients with Immune Checkpoint Inhibitor-Associated Myocarditis. J. Immunother. Cancer 9, e002553corr1. doi:10.1136/jitc-2021-002553
Rubio-Infante, N., Ramírez-Flores, Y. A., Castillo, E. C., Lozano, O., García-Rivas, G., and Torre-Amione, G. (2021). Cardiotoxicity Associated with Immune Checkpoint Inhibitor Therapy: a Meta-Analysis. Eur. J. Heart Fail 23, 1739–1747. doi:10.1002/ejhf.2289
Sarocchi, M., Grossi, F., Arboscello, E., Bellodi, A., Genova, C., Dal Bello, M. G., et al. (2018). Serial Troponin for Early Detection of Nivolumab Cardiotoxicity in Advanced Non-small Cell Lung Cancer Patients. Oncologist 23, 936–942. doi:10.1634/theoncologist.2017-0452
Spallarossa, P., Sarocchi, M., Tini, G., Arboscello, E., Toma, M., Ameri, P., et al. (2020). How to Monitor Cardiac Complications of Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 11, 972. doi:10.3389/fphar.2020.00972
Szuchan, C., Elson, L., Alley, E., Leung, K., Camargo, A. L., Elimimian, E., et al. (2020). Checkpoint Inhibitor-Induced Myocarditis and Myasthenia Gravis in a Recurrent/metastatic Thymic Carcinoma Patient: a Case Report. Eur. Heart J. Case Rep. 4, 1–8. doi:10.1093/ehjcr/ytaa051
Tanabe, J., Watanabe, N., Endo, A., Nagami, T., Inagaki, S., and Tanabe, K. (2021). Asymptomatic Immune Checkpoint Inhibitor-Associated Myocarditis. Intern Med. 60, 569–573. doi:10.2169/internalmedicine.5412-20
Tu, L., Liu, J., Li, Z., Liu, Y., and Luo, F. (2020). Early Detection and Management of Immune-Related Myocarditis: Experience from a Case with Advanced Squamous Cell Lung Carcinoma. Eur. J. Cancer 131, 5–8. doi:10.1016/j.ejca.2020.02.046
Valenti-Azcarate, R., Esparragosa Vazquez, I., Toledano Illan, C., Idoate Gastearena, M. A., and Gállego Pérez-Larraya, J. (2020). Nivolumab and Ipilimumab-Induced Myositis and Myocarditis Mimicking a Myasthenia Gravis Presentation. Neuromuscul. Disord. 30, 67–69. doi:10.1016/j.nmd.2019.10.006
Wang, F., Sun, X., Qin, S., Hua, H., Liu, X., Yang, L., et al. (2020). A Retrospective Study of Immune Checkpoint Inhibitor-Associated Myocarditis in a Single Center in China. Chin. Clin. Oncol. 9, 16. doi:10.21037/cco.2020.03.08
Wang, Q., and Hu, B. (2019). Successful Therapy for Autoimmune Myocarditis with Pembrolizumab Treatment for Nasopharyngeal Carcinoma. Ann. Transl. Med. 7, 247. doi:10.21037/atm.2019.04.73
Xie, X., Wang, F., Qin, Y., Lin, X., Xie, Z., Liu, M., et al. (2020). Case Report: Fatal Multiorgan Failure and Heterochronous Pneumonitis Following Pembrolizumab Treatment in a Patient with Large-Cell Neuroendocrine Carcinoma of Lung. Front. Pharmacol. 11, 569466. doi:10.3389/fphar.2020.569466
Xing, Q., Zhang, Z. W., Lin, Q. H., Shen, L. H., Wang, P. M., Zhang, S., et al. (2020). Myositis-myasthenia Gravis Overlap Syndrome Complicated with Myasthenia Crisis and Myocarditis Associated with Anti-programmed Cell Death-1 (Sintilimab) Therapy for Lung Adenocarcinoma. Ann. Transl. Med. 8, 250. doi:10.21037/atm.2020.01.79
Yanase, T., Moritoki, Y., Kondo, H., Ueyama, D., Akita, H., and Yasui, T. (2021). Myocarditis and Myasthenia Gravis by Combined Nivolumab and Ipilimumab Immunotherapy for Renal Cell Carcinoma: A Case Report of Successful Management. Urol. Case Rep. 34, 101508. doi:10.1016/j.eucr.2020.101508
Zhang, T., Armstrong, A. J., George, D. J., and Huang, J. (2018). The Promise of Immunotherapy in Genitourinary Malignancies. Precis. Clin. Med. 1, 97–101. doi:10.1093/pcmedi/pby018
Zhang, C., Qin, S., and Zuo, Z. (2021). Immune-related Myocarditis in Two Patients Receiving Camrelizumab Therapy and Document Analysis. J. Oncol. Pharm. Pract., 10781552211027339. official publication of the International Society of Oncology Pharmacy Practitioners. doi:10.1177/10781552211027339
Keywords: immune checkpoint inhibitors, immune-related myocarditis, immune-related myositis, troponin, lactate dehydrogenase
Citation: Lei Y, Zheng X, Huang Q, Li X, Qiu M and Liu M (2022) Intrinsic Differences in Immune Checkpoint Inhibitor-Induced Myocarditis: A Retrospective Analysis of Real World Data. Front. Pharmacol. 13:914928. doi: 10.3389/fphar.2022.914928
Received: 07 April 2022; Accepted: 06 June 2022;
Published: 05 July 2022.
Edited by:
Shuang Zhou, University of Houston, United StatesReviewed by:
Charis Liapi, National and Kapodistrian University of Athens, GreecePankti Reid, University of Chicago Medicine, United States
Copyright © 2022 Lei, Zheng, Huang, Li, Qiu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Liu, bGl1bWluZzYyOUB3Y2hzY3UuY24=
 Yanna Lei
Yanna Lei Xiufeng Zheng
Xiufeng Zheng Qian Huang
Qian Huang Xiaoying Li
Xiaoying Li Meng Qiu
Meng Qiu Ming Liu
Ming Liu