- Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, China
Objective: Polypharmacy increases the prevalence of potentially inappropriate drugs potentially inappropriate medications among older persons, lowering their quality of life. PIMs use can lead to higher mortality in older patients. This study aimed to compare the prevalence of PIMs in older Chinese outpatients according to the Beers criteria and the Chinese criteria and to analyze the risk factors. Second, we describe the differences between the two criteria, focusing on the inappropriate prescription of drugs in older outpatients.
Methods: In Chengdu, Southwest China, a cross-sectional study was undertaken using electronic medical data from 9 general hospitals s. Outpatients above the age of 60 who were treated in the Geriatrics Center of these medical institutions were included. The 2019 Beers criteria and the 2017 Chinese criteria were used to evaluate the PIM status of older outpatients, and binary logistic regression was used to identify potential risk factors for PIMs.
Results: There were 44,458 prescriptions from 2016 to 2018. The prevalence of PIMs among older outpatients was 30.05% (according to the Beers criteria) and 35.38% (according to the Chinese criteria), with statistical difference. Estazolam, hydrochlorothiazide and alprazolam were the top three PIMs in the Beers criteria, while the top three PIMs in the Chinese criteria were clopidogrel, estazolam and insulin. The prevalence of PIMs was associated with age, the number of diseases and the number of drugs. PIMs were shown to be more common in patients aged 70 and above, with more than 2 kinds of diseases and more than 4 kinds of drugs.
Conclusion: PIMs were shown to be common among older outpatients in China, according to this study. The detection rate of the Chinese criteria was higher than that of the Beers criteria.
Introduction
Comorbidity and polypharmacy in older persons are becoming more common as global aging becomes more serious. Polypharmacy is widespread in older adults, although it is not always avoided. It frequently involves inappropriate medications and can result in negative health effects, such as adverse drug reactions (ADRs), drug–drug interactions or drug-disease interactions (Rieckert et al., 2020). These adverse events often reduce the quality of life of older adults, increase the hospitalization rate and disability rate, and increase the economic burden on the social medical system. (Hamilton et al., 2011; Cahir et al., 2014; Davies and O’Mahony, 2015). Thus, polypharmacy and potentially inappropriate medications (PIMs) become major concerns among older patients.
Potentially inappropriate medication is used when the actual or potential harms of therapy outweigh its benefits. (Lau et al., 2020). Previous studies had showed that the prevalence of PIMs was high, and more adverse drug events, longer hospital stays, more resource use, higher hospital readmission rates and higher health-care expenses were linked to PIMs. (Spinewine et al., 2007; Dalleur et al., 2012; O’Connor et al., 2012; Reich et al., 2014; Hagstrom et al., 2015; Endres et al., 2016; Ma et al., 2018).
Different screening tools are available to assess the level of PIMs in older patients. The most extensively used and cited tools for PIMs were the Beers criteria published in the USA and the STOPP/START criteria published in Europe. The Beers criteria were first published in 1991, and last updated in 2019 (Salbu and Feuer, 2017; The 2019 American Geriatrics Society Beers Criteria ® Update Expert Panel, 2019). The STOPP/START criteria were published in 2008, and updated in 2014 (O'Mahony et al., 2015). They were both explicit criteria, which were used worldwide. In 2017, the Geriatrics Branch of Chinese Medical Association, along with several societies published criteria of potentially inappropriate medications for older adults in China (the Chinese criteria), which were also explicit criteria and widely used in Chinese older patients. (Rational Drug Use Branch of Chinese Association of Geriatric Research, Geriatrics Branch of Chinese Medical Association, Geriatric Medication Committee of Chinese Pharmaceutical Association, Anti-aging and Alzheimer Diseases Committee of Chinese Pharmacological Society, Division of Drug-induced Disease of Chinese Pharmacological Society, 2018).
To date, some studies have investigated the PIMs among Chinese patients based on the Beers criteria or the Chinese criteria, but their studies mainly focused on single hosiptal, or single disease (Ma et al., 2018; Li et al., 2021; Tian et al., 2022). No studies have analyzed the prevalence and risk factors for PIMs in older Chinese outpatients of multiple medical institutions for three consecutive years, and at the same time, reported the differences in the prevalence of PIMs using the 2019 Beers criteria and the 2017 Chinese criteria. Therefore, in this study, we compared the prevalence of PIMs in older Chinese outpatients using the Beers criteria and the Chinese criteria for three consecutive years, investigated relevant risk factors for PIMs, and listed the top drugs discovered using the two sets of criteria. We hope that this study will provide relevant evidence for further research.
Materials and methods
Sample and data collection
The prescriptions in this study were from the hospital prescription analytic cooperation project, which was organized by the Chinese Pharmaceutical Association. This project was started in 1997, and was carried out every 3 years. The purpose of the project is to learn about the use of drugs, improve the management of drug use, and enhance the rationality of drug use. The hospitals are invited in this project through project cooperation, and they can share data. The hospitals included in the project were voluntary participants, and provided their prescription data to the project every year. Through the network system, a computer was used to extract prescriptions from hospitals that volunteered to participate in the project. The data extraction rules of the project were that prescriptions of each hospital were randomly selected for 3–4 days every month, and a total of 40 days of prescriptions were randomly selected in 1 year. In Chengdu, nine hospitals volunteered to participate in the project. All nine hospitals were tertiary medical institutions rather than community service centers. The treatment of patients admitted by community service centers is relatively simple. Tertiary medical institutions are comprehensive hospitals, which receive severe patients and use complex drugs, so they are good choices to study PIMs. The project randomly selected prescriptions of all nine hospitals according to the extraction rules. Prescriptions of outpatients aged 60 and above were randomly selected from geriatric departments of the nine hospitals in Chengdu from 1 January 2016, to 31 December 2018. All the information extracted from the computer included the year of prescription, prescription code, drug name, drug specification, administration route, drug price, dosing frequency, dosage, gender, age, and clinical diagnosis. The identities of the hospitals and patients were kept confidential. One prescription had one prescription code, and one prescription code represented one patient. Sample size was calculated by the following formula.
Data inclusion criteria and evaluation criteria
From 1 January 2016 to 31 December 2018, outpatients aged 60 and above in the geriatric department or geriatrics center were included. Two researchers (YZ, FT) independently check prescriptions through an excel. Any inconsistencies between the two researchers were submitted to a third expert, and then resolved through collective discussion. Prescriptions with missing or incomplete information were excluded, such as blank gender, blank age, incomplete diagnosis, blank diagnosis, blank drug, blank dosage, blank administration route, and blank dosing frequency. The prescription was also ruled out if the patient’s gender was uncertain or the patient’s age was inconsistent. When calculating the number of drugs, solvent substances such as water for injection and 0.9% sodium chloride were not included.
Evaluation of potentially inappropriate medications
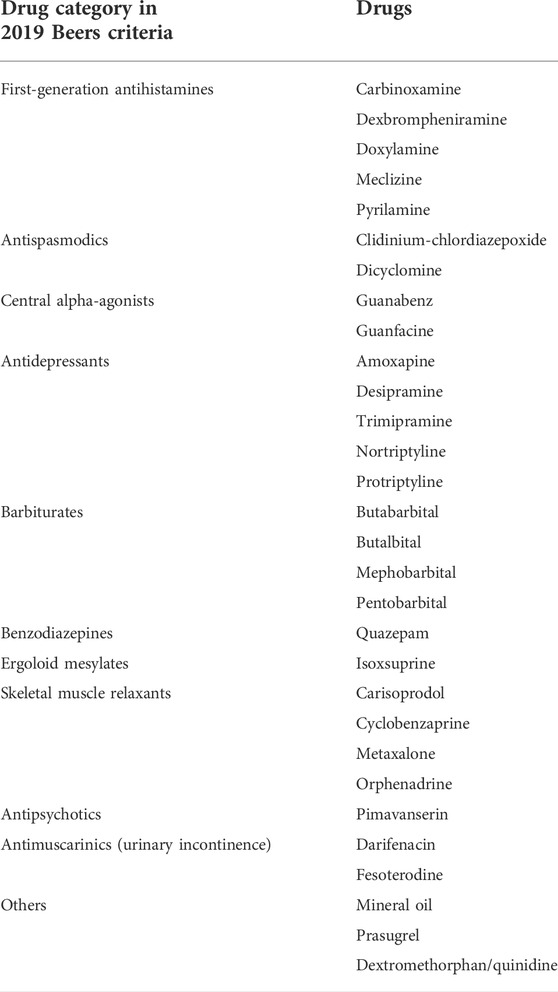
The 2019 Beers criteria and the 2017 Chinese criteria were used to evaluate the PIMs of older patients. (The 2019 American Geriatrics Society Beers Criteria ® Update Expert Panel, 2019; Rational Drug Use Branch of Chinese Association of Geriatric Research, Geriatrics Branch of Chinese Medical Association, Geriatric Medication Committee of Chinese Pharmaceutical Association, Anti-aging and Alzheimer Diseases Committee of Chinese Pharmacological Society, Division of Drug-induced Disease of Chinese Pharmacological Society, 2018). The 2019 Beers criteria included potentially inappropriate medication use in older adults, potentially inappropriate medication use in older adults due to drug-disease or drug-syndrome interactions that may exacerbate the disease or syndrome, drugs to be used with caution in older adults, potentially clinically important drug–drug interactions that should be avoided in older adults, and medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. (The 2019 American Geriatrics Society Beers Criteria ® Update Expert Panel, 2019). The 2017 Chinese criteria included potentially inappropriate medication use in older adults and potentially inappropriate medication use in older adults in a state of disease or syndrome. (Rational Drug Use Branch of Chinese Association of Geriatric Research, Geriatrics Branch of Chinese Medical Association, Geriatric Medication Committee of Chinese Pharmaceutical Association, Anti-aging and Alzheimer Diseases Committee of Chinese Pharmacological Society, Division of Drug-induced Disease of Chinese Pharmacological Society, 2018). Both criteria focus on the medications of central nervous system, cardiovascular system, endocrine system, gastrointestinal system, and urinary system, as well as anticholinergics, antithrombotics, anti-infective medications and pain medications. However, they also have differences. The Beers criteria also look at proton-pump inhibitors, mineral oils, desmopressin, androgens and drugs that should be avoided or used in reduced doses based on renal function. The Chinese criteria give additional attention to clopidogrel, theophylline, gatifloxacin, vancomycin, clindamycin and PIMs of gout, insomnia, glaucoma, constipation, chronic obstructive pulmonary disease, osteoporosis and hypertension.
Because the patients’ creatinine clearance rates could not be available, we did not assess medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. As a result, this study mainly studied the first four groups of the 2019 Beers criteria and the two groups of the 2017 Chinese criteria.
Statistical analysis
The chi-square test was used to analyze the differences in the characteristics. It was assumed that there was no difference between the compared groups. When the p-value was less than 0.05, the hypothesis was not valid, that is, there was a statistical difference between the compared groups. According to the prescription information extracted, sex, age, number of diseases and number of drugs were included in univariate survival analysis. Variables with statistical differences after univariate analysis were included in the binary logistic regression analysis to assess the possible risk factors affecting PIMs in older outpatients. SPSS version 26.0 software was used to perform the statistical analysis.
Ethics approval
This study protocol was approved by the Sichuan University West China Hospital Research Ethics Board (2020/651). The informed consent is waived by the ethics committee of Sichuan University West China Hospital Research Ethics Board. All procedures performed in this study conformed to the standards of the 1964 Helsinki Declaration and subsequent relevant ethics.
Results
Basic characteristics of patients
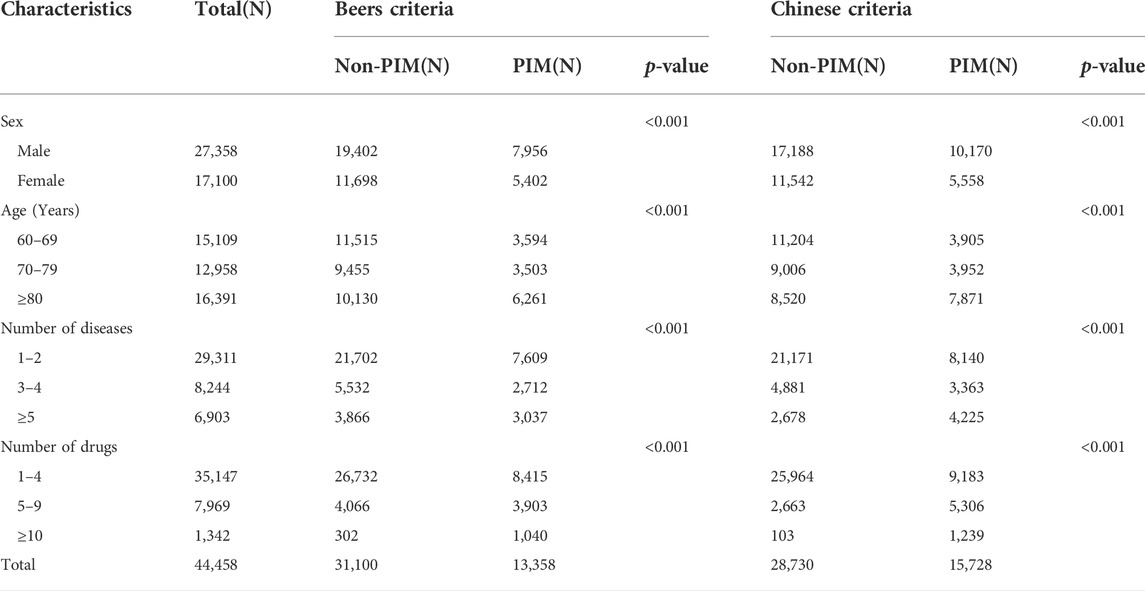
In this study, all data were extracted into an excel for analysis. One prescription code corresponded to one patient. From 2016 to 2018, a total of 44,857 prescriptions were acquired. Among them, 312 prescriptions with no diagnosis, 77 prescriptions with incomplete diagnosis, 4 prescriptions with missing gender, and 6 prescriptions with only the water for injection were excluded. Finally, 44,458 prescriptions were included. There were 27,358 prescriptions for male patients, representing 61.54% of the total, and 17,100 prescriptions for female patients, accounting for 38.46%. Patients ranged in age from 60 to 119 years old. The number of drugs ranged from 1 to 24, while the number of diseases ranged from 1 to 19. The basic characteristics of the patients are listed in Table 1.
Detection of potentially inappropriate medications
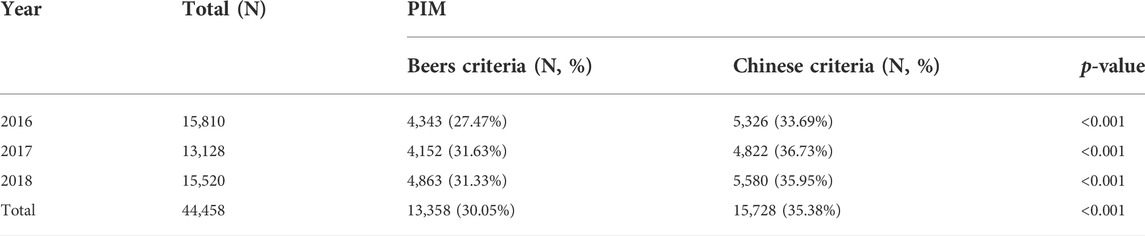
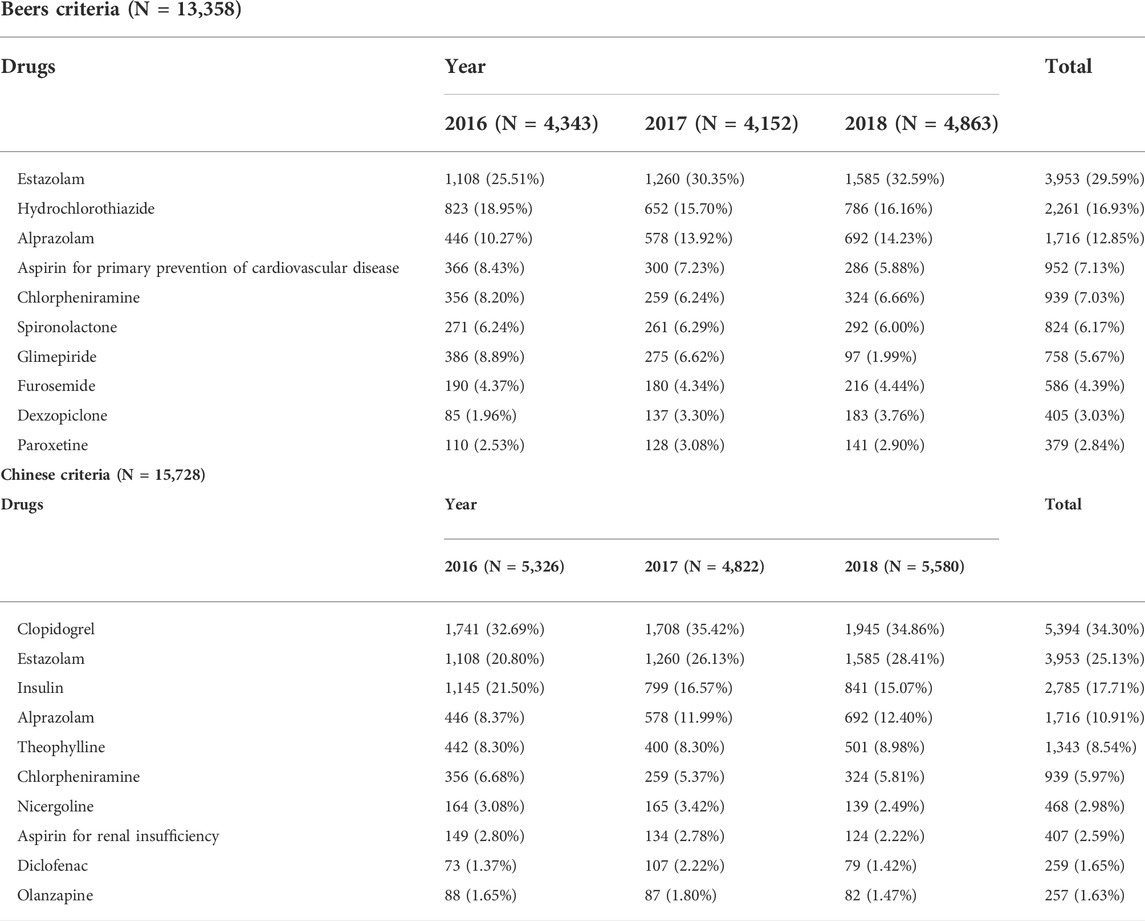
According to the Beers criteria, 13,358 (30.05%) of the 44,458 prescriptions had at least one PIM. According to the Chinese criteria, 15,728 prescriptions (35.38%) contained at least one PIM. There was a significant difference in PIM detection between the two groups. The prevalence rates of PIMs are listed in Table 2. Estazolam, hydrochlorothiazide and alprazolam were the top three PIMs in the Beers criteria, while clopidogrel, estazolam and insulin were the top three PIMs in the Chinese criteria. The top ten PIMs are listed in Table 3.
Factors associated with potentially inappropriate medications
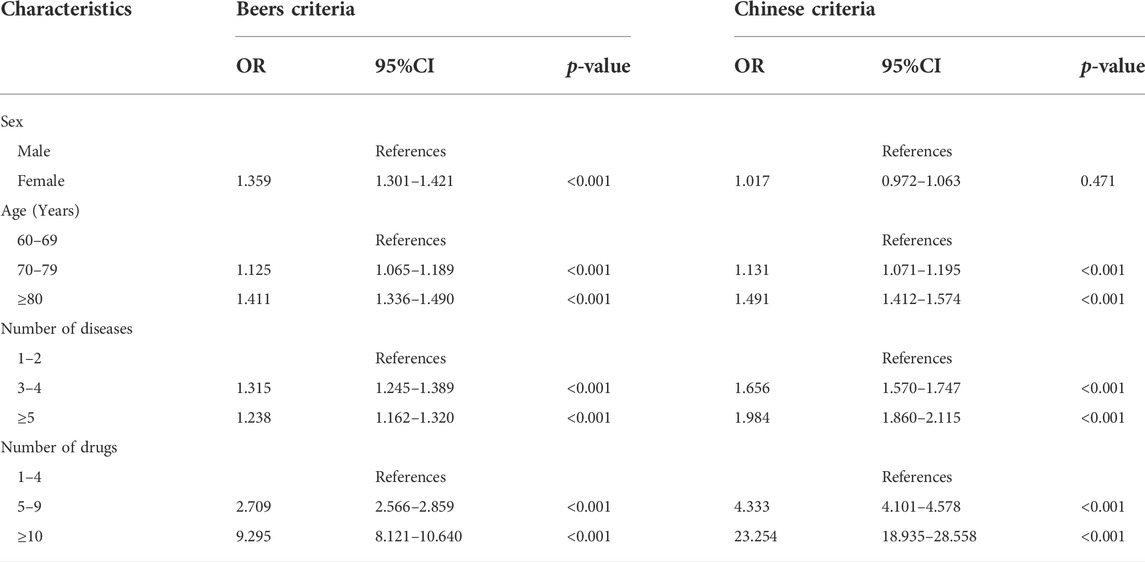
Sex, age, number of diseases and number of drugs were included in univariate analysis, and they were all statistically different. Then, they were included in the binary logistic regression analysis. The reference groups were males, 60–69 years old, 1–2 kinds of diseases and 1–4 kinds of drugs. According to the Beers criteria, gender, age, the number of diseases and the number of drugs all affected the prevalence of PIMs. PIMs were more common in patients who were female (OR 1.359, 95% CI 1.301–1.421), aged 70 and above (OR 1.125, 95% CI 1.065–1.189), had more than 2 kinds of diseases (OR 1.315, 95% CI 1.245–1.389), and had more than 4 kinds of drugs (OR 2.709, 95% CI 2.566–2.859). According to the Chinese criteria, age, the number of diseases and the number of drugs were all linked to an increased risk of PIM use. PIMs were more common in patients aged 70 and above (OR 1.131 95% CI 1.071–1.195), as well as those with more than 2 kinds of diseases (OR 1.656, 95% CI 1.570–1.747) and more than 4 kinds of drugs (OR 4.333, 95% CI 4.101–4.578). Patients who took more than 9 kinds of drugs had the highest risk of PIM use, according to both criteria, as shown in Table 4.
Discussion
There are four major findings in our study. First, the Chinese criteria revealed a higher prevalence of PIMs in older Chinese outpatients (35.38%) than the Beers criteria (30.05%). Second, the prevalence of PIMs in older Chinese outpatients gradually increased over three consecutive years, regardless of the Chinese criteria or Beers criteria. Third, Estazolam was the most common PIM according to the Beers criteria, while clopidogrel was the most common PIM according to the Chinese criteria. Finally, Patients aged 70 and above, with more than 2 kinds of diseases, and with more than 4 kinds of drugs were the common risk factors according to two sets of criteria.
Other studies reported the prevalence of PIMs in older outpatients based on the same version of the Beers criteria. Wang et al. investigated 604 hospitalized patients, and the prevalence of PIMs was 55.0%. (Wang et al., 2020). He et al. extracted prescriptions from hospitalized patients for the study, and the prevalence of inappropriate prescriptions was 64.80%. (He et al., 2021). Abdelwahed et al. included 502 older patients, and the PIM prevalence was 34.7%. (Abdelwahed et al., 2021). Achterhof et al. analyzed data of 300 patients, and 53% had at least one PIM. (Achterhof et al., 2020). Li et al. examined 8,235 patients in Chinese communities, and the prevalence of PIMs was 32.16%. (Li et al., 2021). Huang et al. assessed 8477 medications among 1874 patients, and 35.0% of them were PIM-users. (Huang et al., 2020). In our study, the prevalence of PIMs based on the Beers criteria was lower than that based on the Chinese criteria. This might be because the medications in the two criteria differed. In China, 30 of the medications listed in the Beers criteria were not available, as shown in Table 5. Furthermore, the Chinese criteria were biased because they were designed for Chinese patients. At the same time, we found that the prevalence of PIMs in older Chinese outpatients increased gradually from 2016 to 2018, regardless of whether the Chinese criteria or the Beers criteria were used. As a result, enhancing the sensible use of medications in older Chinese outpatients to reduce PIMs remains a major challenge.
Estazolam, hydrochlorothiazide and alprazolam were the top three PIMs in the Beers criteria, while clopidogrel, estazolam and insulin were the top three PIMs in the Chinese criteria. The difference in results was primarily attributable to the disparity between the two criteria. Diuretics were included in the Beers criteria as drugs to be used with caution in older adults due to the risk of hyponatremia, and regular monitoring of sodium was recommended when starting or altering dosages in older adults. (Hix et al., 2011; Correia et al., 2014; Filippatos et al., 2017; Grattagliano et al., 2018; Zhang and Li, 2020). Diuretics were excluded from the Chinese criteria; however, clopidogrel was included because of the hematologic and neurological adverse reactions associated with clopidogrel. (Zakarija et al., 2004; Tiaden et al., 2005; Balamuthusamy and Arora, 2007; Pflumm et al., 2008; Zakarija et al., 2009). On the other hand, the Beers criteria did not include clopidogrel. Despite the fact that insulin was included in both criteria, there were still discrepancies. The Beers criteria only included short- or rapid-acting insulins that were not used in conjunction with basal or long-acting insulins, while the Chinese criteria included all insulins. This led to a dramatic increase in the detection of insulins as PIMs.
Although the detection of insulin differed between the two criteria, both of them included insulin as PIM due to higher risk of hypoglycemia without improvement in hyperglycemia management. However, it is important to note that insulin is also necessary in some older patients with diabetes. In older patients with type 1 diabetes, insulin therapy is necessary. In addition, insulin treatment is also needed in older patients with type 2 diabetes, who fail to achieve glycemic target after lifestyle intervention and non-insulin therapy. To reduce the risk of hypoglycemia in older patients, we can do as follows: 1) Before starting insulin, the benefit of insulin therapy and the risk of hypoglycemia in older diabetic patients should be fully considered, and individualized treatment regimen should be developed. 2) When starting insulin therapy, basal insulins with stable serum concentration are preferred (American Diabetes Association, 2021a). 3) Older patients who are taking insulin should be evaluated whether insulin therapy is necessary or can be simplified. For older patients whose blood glucose can be controlled by non-insulin therapy, insulin should be gradually reduced and stopped. Besides, reducing injection frequency and adding short-acting insulin when the postprandial blood glucose is not satisfied after the use of long-acting insulin should be considered in older patients who need insulin to achieve satisfactory blood glucose control (LeRoith et al., 2019; American Diabetes Association, 2021b).
Estazolam and alprazolam, both benzodiazepines, were highly frequent PIMs according to the Beers criteria and the Chinese criteria. Some studies have shown that benzodiazepines are commonly used in older adults, and there is a long-term use phenomenon. (Kurko et al., 2015; Olfson et al., 2015; Maust et al., 2016). Benzodiazepines can lead to serious injuries such as falls, fractures, cognitive impairment and car accidents, putting a strain on society’s finances and judicial system. (Madhusoodanan and Bogunovic, 2004; Schroeck et al., 2016). Older adults are at risk from long-term benzodiazepine use. In addition, studies have shown that benzodiazepines are linked to the development of dementia or cognitive impairment; (Crowe and Stranks, 2018; Lucchetta et al., 2018; Picton et al., 2018); thus, patients with dementia or cognitive impairment should avoid taking them. Regarding the risks associated with benzodiazepines in older adults, several alternative therapies have been proposed to reduce their use. (Markota et al., 2016).
Potentially independent factors associated with PIMs were identified as patients aged 70 and above, with more than 2 kinds of diseases, and with more than 4 kinds of drugs (p < 0.001). Patients who took more than 9 kinds of drugs had the highest risk of PIM use. Polypharmacy was linked to not only PIMs but also medication compliance. Age, medication classes and medication knowledge were found to be associated with medication compliance in a Chinese study of older adults with polypharmacy. (He et al., 2021).
Now, several tools for PIMs identification have been developed to help doctors and pharmacists reduce PIMs. PIM dashboard is a clinical decision-making tool embedded in medical system. It can identify patients who use PIMs and show the type and number of PIMs (Zullo et al., 2018; Richter Lagha et al., 2020). Electronic medical record (EMR) alerts is a system of pop-up alerts in EMRs. It can inform PIMs, adverse drug reactions and drug interactions (Alagiakrishnan et al., 2019). MedStopper webpage is a tool for frail older patients. It can assess PIMs and drug overuse (Cassels, 2017).
In addition to the above tools, multidisciplinary medication makes a contribution to reducing PIMs (Krause, 2019). Clinical pharmacist is an important member of the multidisciplinary team. Clinical pharmacists carry out drug reorganization, drug counseling and pharmaceutical care, find inappropriate medication, give professional advices on patient treatment, reduce avoidable adverse drug reactions and adverse interactions. As a full member, clinical pharmacists can reduce PIMs in elderly patients by 20% or even more (Stuhec et al., 2021; Stuhec and Lah, 2021; Stuhec, 2021).
Several limitations of this study should be noted. First, this study was a retrospective study including a specific region of China, so the results could not be applied to other countries. Second, this study only included outpatients, which could not reflect the characteristics of hospitalized patients. Third, this study only included older patients in geriatric departments, so it could not represent patients in other departments. Next, patients’ creatinine clearance rates could not be available, and this limited us assess medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. Besides, prescriptions were only extracted for 40 days in 1 year, which did not represent the whole year. Finally, the adverse outcomes caused by PIMs were not available, so we could not determine which criteria were more suitable for the evaluation of PIMs.
Conclusion
This study showed that the prevalence of PIMs according to the Chinese criteria was higher than that according to the Beers criteria, and there was a statistical difference between them. The prevalence of PIMs in older Chinese outpatients gradually increased from 2016 to 2018, and benzodiazepines were the most common PIMs. In view of this, we suggest that doctors and pharmacists cooperate to identify the indications of drugs, improve patient compliance, and reduce PIMs in older adults.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Developing design: YZ and FT. Literature search: YZ, FT, and ZC. Manuscript writing: YZ and FT. Analysis of results: YZ, FT, and ZC.
Acknowledgments
We thank the participants in our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.991087/full#supplementary-material
References
Abdelwahed, A. A., El-Dahiyat, F., Aljawamis, D., Al Ajimi, J., and Bin Rafeea, K. J. (2021). Potentially inappropriate medications in older adults according to Beers criteria 2019: Prevalence and risk factors. Int. J. Clin. Pract. 75, e14715. doi:10.1111/ijcp.14715
Achterhof, A. B., Rozsnyai, Z., Reeve, E., Jungo, K. T., Floriani, C., Poortvliet, R. K. E., et al. (2020). Potentially inappropriate medication and attitudes of older adults towards deprescribing. PLoS One 15, e0240463. doi:10.1371/journal.pone.0240463
Alagiakrishnan, K., Ballermann, M., Rolfson, D., Mohindra, K., Sadowski, C. A., Ausford, A., et al. (2019). Utilization of computerized clinical decision support for potentially inappropriate medications. Clin. Interv. Aging 14, 753–762. doi:10.2147/CIA.S192927
American Diabetes Association (2021a). 12. Older adults: Standards of medical care in diabetes-2021. Diabetes Care 44, S168–S179. doi:10.2337/dc21-S012
American Diabetes Association (2021b). 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2021. Diabetes Care 44, S111–S124. doi:10.2337/dc21-S009
Balamuthusamy, S., and Arora, R. (2007). Hematologic adverse effects of clopidogrel. Am. J. Ther. 14, 106–112. doi:10.1097/01.mjt.0000212708.81034.22
Cahir, C., Bennett, K., Teljeur, C., and Fahey, T. (2014). Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br. J. Clin. Pharmacol. 77, 201–210. doi:10.1111/bcp.12161
Cassels, A. (2017). Can I stop even one of these pills?' the development of a tool to make deprescribing easier. Eur. J. Hosp. Pharm. 24, 3–4. doi:10.1136/ejhpharm-2016-001011
Correia, L., Ferreira, R., Correia, I., Lebre, A., Carda, J., Monteiro, R., et al. (2014). Severe hyponatremia in older patients at admission in an internal medicine department. Arch. Gerontol. Geriatr. 59, 642–647. doi:10.1016/j.archger.2014.08.002
Crowe, S. F., and Stranks, E. K. (2018). The residual medium and long-term cognitive effects of benzodiazepine use: An updated meta-analysis. Arch. Clin. Neuropsychol. 33, 901–911. doi:10.1093/arclin/acx120
Dalleur, O., Spinewine, A., Henrard, S., Losseau, C., Speybroeck, N., and Boland, B. (2012). Inappropriate prescribing and related hospital admissions in frail older persons according to the STOPP and START criteria. Drugs Aging 29, 829–837. doi:10.1007/s40266-012-0016-1
Davies, E. A., and O’Mahony, M. S. (2015). Adverse drug reactions in special populations-the elderly. Br. J. Clin. Pharmacol. 80, 796–807. doi:10.1111/bcp.12596
Endres, H. G., Kaufmann-Kolle, P., Steeb, V., Bauer, E., Böttner, C., and Thürmann, P. (2016). Association between potentially inappropriate medication (PIM) use and risk of hospitalization in older adults: An observational study based on routine data comparing PIM use with use of PIM alternatives. PLoS One 11, e0146811. doi:10.1371/journal.pone.0146811
Filippatos, T. D., Makri, A., Elisaf, M. S., and Liamis, G. (2017). Hyponatremia in the elderly: Challenges and solutions. Clin. Interv. Aging 12, 1957–1965. doi:10.2147/CIA.S138535
Grattagliano, I., Mastronuzzi, T., and D'Ambrosio, G. (2018). Hyponatremia associated with long-term medication use in the elderly: An analysis in general practice. J. Prim. Health Care 10, 167–173. doi:10.1071/HC17084
Hagstrom, K., Nailor, M., Lindberg, M., Hobbs, L., and Sobieraj, D. M. (2015). Association between potentially inappropriate medication use in elderly adults and hospital-related outcomes. J. Am. Geriatr. Soc. 63, 185–186. doi:10.1111/jgs.13229
Hamilton, H., Gallagher, P., Ryan, C., Byrne, S., and O'Mahony, D. (2011). Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch. Intern. Med. 171, 1013–1019. doi:10.1001/archinternmed.2011.215
He, D., Zhu, H., Zhou, H., Dong, N., and Zhang, H. (2021). Potentially inappropriate medications in Chinese older adults: A comparison of two updated Beers criteria. Int. J. Clin. Pharm. 43, 229–235. doi:10.1007/s11096-020-01139-5
Hix, J. K., Silver, S., and Sterns, R. H. (2011). Diuretic-associated hyponatremia. Semin. Nephrol. 31, 553–566. doi:10.1016/j.semnephrol.2011.09.010
Huang, Y., Zhang, L., Huang, X., Liu, K., Yu, Y., and Xiao, J. (2020). Potentially inappropriate medications in Chinese community-dwelling older adults. Int. J. Clin. Pharm. 42, 598–603. doi:10.1007/s11096-020-00980-y
Krause, O., Wiese, B., Doyle, I. M., Kirsch, C., Thürmann, P., Wilm, S., et al. (2019). Multidisciplinary intervention to improve medication safety in nursing home residents: Protocol of a cluster randomised controlled trial (HIOPP-3-iTBX study). BMC Geriatr. 19, 24. doi:10.1186/s12877-019-1027-0
Kurko, T. A., Saastamoinen, L. K., Tähkäpää, S., Tuulio-Henriksson, A., Taiminen, T., Tiihonen, J., et al. (2015). Long-term use of benzodiazepines: Definitions, prevalence and usage patterns - a systematic review of register-based studies. Eur. Psychiatry 30, 1037–1047. doi:10.1016/j.eurpsy.2015.09.003
Lau, S., Lun, P., Ang, W., Tan, K. T., and Ding, Y. Y. (2020). Barriers to effective prescribing in older adults: Applying the theoretical domains framework in the ambulatory setting – a scoping review. BMC Geriatr. 20, 459. doi:10.1186/s12877-020-01766-7
LeRoith, D., Biessels, G. J., Braithwaite, S. S., Casanueva, F. F., Draznin, B., Halter, J. B., et al. (2019). Treatment of diabetes in older adults: An endocrine society* clinical practice guideline. J. Clin. Endocrinol. Metab. 104, 1520–1574. doi:10.1210/jc.2019-00198
Li, Y., Hu, J., Gao, Y. Z., Zhou, F., Zhu, Z. H., Zhang, B. F., et al. (2021). Prevalence and determinants of potentially inappropriate medications prescribing in elderly patients in Chinese communities. Ann. Palliat. Med. 10, 2072–2079. doi:10.21037/apm-21-32
Lucchetta, R. C., da Mata, B. P. M., and Mastroianni, P. C. (2018). Association between development of dementia and use of benzodiazepines: A systematic review and meta-analysis. Pharmacotherapy 38, 1010–1020. doi:10.1002/phar.2170
Ma, Z., Zhang, C., Cui, X., and Liu, L. (2018). Comparison of three criteria for potentially inappropriate medications in Chinese older adults. Clin. Interv. Aging 14, 65–72. doi:10.2147/CIA.S190983
Madhusoodanan, S., and Bogunovic, O. J. (2004). Safety of benzodiazepines in the geriatric population. Expert Opin. Drug Saf. 3, 485–493. doi:10.1517/14740338.3.5.485
Markota, M., Rummans, T. A., Bostwick, J. M., and Lapid, M. I. (2016). Benzodiazepine use in older adults: Dangers, management, and alternative therapies. Mayo Clin. Proc. 91, 1632–1639. doi:10.1016/j.mayocp.2016.07.024
Maust, D. T., Kales, H. C., Wiechers, I. R., Blow, F. C., and Olfson, M. (2016). No end in sight: Benzodiazepine use in older adults in the United States. J. Am. Geriatr. Soc. 64, 2546–2553. doi:10.1111/jgs.14379
O’Connor, M. N., Gallagher, P., and O’Mahony, D. (2012). Inappropriate prescribing: Criteria, detection and prevention. Drugs Aging 29, 437–452. doi:10.2165/11632610-000000000-00000
Olfson, M., King, M., and Schoenbaum, M. (2015). Benzodiazepine use in the United States. JAMA Psychiatry 72, 136–142. doi:10.1001/jamapsychiatry.2014.1763
O’Mahony, D., O’Sullivan, D., Byrne, S., O’Connor, M. N., Ryan, C., and Gallagher, P. (2015). STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 44, 213–218. doi:10.1093/ageing/afu145
Pflumm, J. E., Pomykaj, T., and Heintzen, M. P. (2008). Secondary prevention after myocardial infarction. Internist (Berl) 49, 1052–1060. doi:10.1007/s00108-008-2077-0
Picton, J. D., Marino, A. B., and Nealy, K. L. (2018). Benzodiazepine use and cognitive decline in the elderly. Am. J. Health. Syst. Pharm. 75, e6–e12. doi:10.2146/ajhp160381
Rational Drug Use Branch of Chinese Association of Geriatric Research, Geriatrics Branch of Chinese Medical Association, Geriatric Medication Committee of Chinese Pharmaceutical Association, Anti-aging and Alzheimer Diseases Committee of Chinese Pharmacological Society, Division of Drug-induced Disease of Chinese Pharmacological Society (2018). Criteria of potentially inappropriate medications for older adults in China. Adverse Drug React. J. 20, 2–8. (in Chinese). doi:10.3760/cma.j.issn.1008-5734.2018.01.002
Reich, O., Rosemann, T., Rapold, R., Blozik, E., and Senn, O. (2014). Potentially inappropriate medication use in older patients in Swiss managed care plans: Prevalence, determinants and association with hospitalization. PLoS One 9, e105425. doi:10.1371/journal.pone.0105425
Richter Lagha, R., Burningham, Z., Sauer, B. C., Leng, J., Peters, C., Huynh, T., et al. (2020). Usability testing a potentially inappropriate medication dashboard: A core component of the dashboard development process. Appl. Clin. Inf. 11, 528–534. doi:10.1055/s-0040-1714693
Rieckert, A., Reeves, D., Altiner, A., Drewelow, E., Esmail, A., Flamm, M., et al. (2020). Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: Cluster randomised controlled trial. BMJ 369, m1822. doi:10.1136/bmj.m1822
Salbu, R. L., and Feuer, J. (2017). A closer look at the 2015 Beers criteria. J. Pharm. Pract. 30, 419–424. doi:10.1177/0897190016663072
Schroeck, J. L., Ford, J., Conway, E. L., Kurtzhalts, K. E., Gee, M. E., Vollmer, K. A., et al. (2016). Review of safety and efficacy of sleep medicines in older adults. Clin. Ther. 38, 2340–2372. doi:10.1016/j.clinthera.2016.09.010
Spinewine, A., Schmader, K. E., Barber, N., Hughes, C., Lapane, K. L., Swine, C., et al. (2007). Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 370, 173–184. doi:10.1016/S0140-6736(07)61091-5
Stuhec, M. (2021). Clinical pharmacist consultant in primary care settings in Slovenia focused on elderly patients on polypharmacy: Successful national program from development to reimbursement. Int. J. Clin. Pharm. 43, 1722–1727. doi:10.1007/s11096-021-01306-2
Stuhec, M., Flegar, I., Zelko, E., Kovačič, A., and Zabavnik, V. (2021). Clinical pharmacist interventions in cardiovascular disease pharmacotherapy in elderly patients on excessive polypharmacy : A retrospective pre-post observational multicentric study. Wien. Klin. Wochenschr. 133, 770–779. doi:10.1007/s00508-020-01801-y
Stuhec, M., and Lah, L. (2021). Clinical pharmacist interventions in elderly patients with mental disorders in primary care focused on psychotropics: A retrospective pre-post observational study. Ther. Adv. Psychopharmacol. 11, 20451253211011007. doi:10.1177/20451253211011007
The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel (2019). American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694. doi:10.1111/jgs.15767
Tiaden, J. D., Wenzel, E., Berthold, H. K., and Müller-Oerlinghausen, B. (2005). Adverse reactions to anticoagulants and to antiplatelet drugs recorded by the German spontaneous reporting system. Semin. Thromb. Hemost. 31, 371–380. doi:10.1055/s-2005-916670
Tian, F., Li, H., Chen, Z., and Xu, T. (2021). Potentially inappropriate medications in Chinese older outpatients in tertiary hospitals according to Beers criteria: A cross-sectional study. Int. J. Clin. Pract. 75, e14348. doi:10.1111/ijcp.14348
Tian, F., Zhao, M., Chen, Z., and Yang, R. (2022). Prescription of potentially inappropriate medication use in older cancer outpatients with multimorbidity: Concordance among the Chinese, AGS/Beers, and STOPP criteria. Front. Pharmacol. 13, 857811. doi:10.3389/fphar.2022.857811
Wang, F., Ma, Z., Liu, M., and Wu, X. (2020). Potentially inappropriate medications at admission and discharge in older adults: A comparison of the Beers 2019 and 2015 criteria. Int. J. Clin. Pharmacol. Ther. 58, 299–309. doi:10.5414/CP203638
Zakarija, A., Bandarenko, N., Pandey, D. K., Auerbach, A., Raisch, D. W., Kim, B., et al. (2004). Clopidogrel-associated TTP: An update of pharmacovigilance efforts conducted by independent researchers, pharmaceutical suppliers, and the food and drug administration. Stroke 35, 533–537. doi:10.1161/01.STR.0000109253.66918.5E
Zakarija, A., Kwaan, H. C., Moake, J. L., Bandarenko, N., Pandey, D. K., McKoy, J. M., et al. (2009). Ticlopidine- and clopidogrel-associated thrombotic thrombocytopenic purpura (TTP): Review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989-2008). Kidney Int. 75 (112), S20–S24. doi:10.1038/ki.2008.613
Zhang, X., and Li, X. Y. (2020). Prevalence of hyponatremia among older inpatients in a general hospital. Eur. Geriatr. Med. 11, 685–692. doi:10.1007/s41999-020-00320-3
Keywords: Elderly patients, polypharmacy, potentially inappropriate medications, Beers criteria, Chinese criteria, outpatient
Citation: Zhang Y, Chen Z and Tian F (2022) Potentially inappropriate medications in older Chinese outpatients based on the Beers criteria and Chinese criteria. Front. Pharmacol. 13:991087. doi: 10.3389/fphar.2022.991087
Received: 11 July 2022; Accepted: 15 September 2022;
Published: 30 September 2022.
Edited by:
Chenyu Sun, AMITA Health Saint Joseph Hospital Chicago, United StatesReviewed by:
Joseph O. Fadare, Ekiti State University, NigeriaMatej Stuhec, University of Maribor, Slovenia
Copyright © 2022 Zhang, Chen and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangyuan Tian, dGlhbmZhbmd5dWFuMDYwOEAxNjMuY29t
 Ying Zhang
Ying Zhang Zhaoyan Chen
Zhaoyan Chen Fangyuan Tian
Fangyuan Tian