- Acupuncture and Moxibustion College, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
This review outlined evidence that purinergic signaling is involved in the modulation of blood-brain barrier (BBB) permeability. The functional and structural integrity of the BBB is critical for maintaining the homeostasis of the brain microenvironment. BBB integrity is maintained primarily by endothelial cells and basement membrane but also be regulated by pericytes, neurons, astrocytes, microglia and oligodendrocytes. In this review, we summarized the purinergic receptors and nucleotidases expressed on BBB cells and focused on the regulation of BBB permeability by purinergic signaling. The permeability of BBB is regulated by a series of purinergic receptors classified as P2Y1, P2Y4, P2Y12, P2X4, P2X7, A1, A2A, A2B, and A3, which serve as targets for endogenous ATP, ADP, or adenosine. P2Y1 and P2Y4 antagonists could attenuate BBB damage. In contrast, P2Y12-mediated chemotaxis of microglial cell processes is necessary for rapid closure of the BBB after BBB breakdown. Antagonists of P2X4 and P2X7 inhibit the activation of these receptors, reduce the release of interleukin-1 beta (IL-1β), and promote the function of BBB closure. In addition, the CD39/CD73 nucleotidase axis participates in extracellular adenosine metabolism and promotes BBB permeability through A1 and A2A on BBB cells. Furthermore, A2B and A3 receptor agonists protect BBB integrity. Thus, the regulation of the BBB by purinergic signaling is complex and affects the opening and closing of the BBB through different pathways. Appropriate selective agonists/antagonists of purinergic receptors and corresponding enzyme inhibitors could modulate the permeability of the BBB, effectively delivering therapeutic drugs/cells to the central nervous system (CNS) or limiting the entry of inflammatory immune cells into the brain and re-establishing CNS homeostasis.
1 Introduction
A well-developed central nervous system (CNS) barrier is very important for maintaining the homeostasis of the neural microenvironment. The blood-brain barrier (BBB) is a critical component of the CNS barrier and is composed of continuous endothelial cells within brain microvessels, which outline the physical structure of the BBB along with the end-feet of astrocytic glial cells, pericytes, and microglia (Kadry et al., 2020) (Figure 1). The BBB is the major site of blood-CNS exchange, controlling substances that can enter or leave the nervous tissue in a precise and tight manner. It also prevents harmful substances such as pathogens and toxins from entering the brain while allowing circulating nutrient substances from the blood to enter. BBB provides strong support for synaptic function, information processing, and neural communication, which explains why BBB is essential for maintaining the homeostasis of the intracerebral environment and peripheral blood.
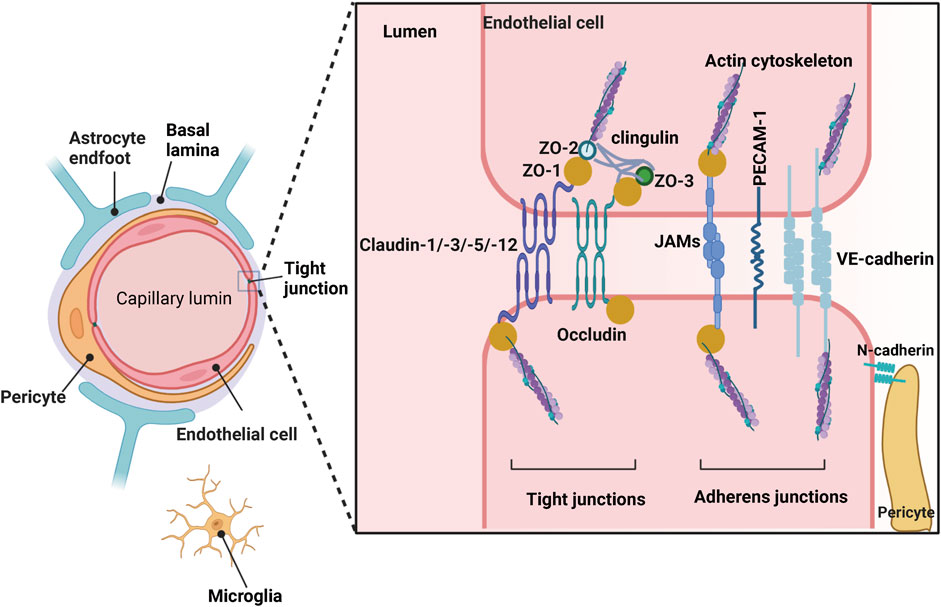
FIGURE 1. Structure of the blood-brain barrier (BBB) depicted graphically. Created with BioRender.com.
BBB is highly selective for substances to cross; these distinct properties tightly control the delivery of ions, molecules, and cells between the blood and the neural microenvironment. BBB endothelial cells are connected by various molecular junctional complexes, including tight junctions and adherens junctions. Tight junctions (TJ) involve occludin and claudin-1, claudin-3, claudin-5, and claudin-12, ZO-1, ZO-2, and ZO-3, and adherens junctions involve cadherins, the platelet endothelial cell adhesion molecule (PECAM-1), and the junctional adhesion molecules (JAMs): JAMA, JAMB, and JAMC (Sweeney et al., 2019) (Figure 1). Oxygen, carbon dioxide, and small lipid-soluble molecules (weight <400 Da) or containing <8 hydrogen bonds (such as ethanol) could cross the BBB in transmembrane diffusion manner (Sweeney et al., 2019). Additionally, the BBB also provides a combination of specific ion channels and transporters on the abluminal membrane of the BBB to regulate the balance of ions in the brain, such as the sodium pump (Na+, K+-ATPase), regulating sodium influx into and exchange for potassium efflux out. In addition, ATP-binding cassette (ABC) transporters are concentrated on the luminal side of the BBB, mediating the movement of molecules such as drugs and xenobiotic agents (Dean et al., 2022). The existence of solute carrier-mediated transport (CMT) and receptor-mediated transcytosis (RMT) ensures the entry of macromolecules into the brain. CMT improves the transport of macromolecules like carbohydrates, amino acids, fatty acids, hormones, vitamins, et al., while RMT ensures the exchange of proteins and peptides between the blood and brain (Zhao et al., 2015; Sweeney et al., 2019).
Although the presence of the BBB protects the CNS from neurotoxic substances circulating in the blood, the BBB also prevents the transfer of most macromolecules (e.g., peptides, proteins, and nucleic acids), severely limiting the treatment of CNS diseases (e.g., neurodegenerative diseases, brain tumors, brain infections, and strokes). BBB breakdown has been identified as a critical component in several neurological conditions. It is reported that in clinical trials and animal experiments, BBB dysfunction promoted the progression of various CNS disorders such as Alzheimer’s disease (AD) (Cai et al., 2018; Zhao et al., 2021), multiple sclerosis (MS) (Niu et al., 2019), hypoxia and ischemia (Yang et al., 2018), and traumatic brain injury (TBI) (Dinet et al., 2019; van Vliet et al., 2020). Therefore, an in-depth dissection of the mechanisms to understand the basic properties of BBB is necessary to elucidate the development of the physiology and pathology of the CNS.
Purinergic signaling is essential in the CNS for maintaining the function of neurons, astrocytes, and microglia and controlling their homeostasis, consequently influencing synaptic transmission and higher cognitive processes (Burnstock, 2017; Burnstock, 2020; Illes et al., 2020). It has been demonstrated that several purinergic receptors are broadly dispersed throughout the CNS, being present in neurons, glial cells, and endothelial cells (Burnstock, 2007; Muhleder et al., 2020). The integrity of the endothelial barrier is affected by the action of extracellular adenosine triphosphate (ATP) and its metabolites adenosine diphosphate (ADP) and adenosine (ADO) on the purinoceptors of endothelial cells (Communi et al., 2000). Endothelial cells are capable of releasing nucleotides in response to a variety of physiological or pathological stimuli. Endothelial cells produce nucleotides in reaction to pathological stimuli including inflammation, hypoxia, blood flow fluctuations, shear stress, and changes in osmotic pressure (Gunduz et al., 2006; Hartel et al., 2007). Meanwhile, a growing number of studies have shown significant modulation of the endothelial barrier by purinergic substances or purinergic receptors, including modulation of BBB permeability (Wang et al., 2015; Chen et al., 2020; Wu et al., 2022). Here, we present current in vivo and in vitro investigations that implicate purinergic receptors and major metabolic enzymes as crucial regulatory routes for BBB permeability. Modulation of purinergic signaling, on the one hand, has effects on promoting its integrity and protecting the CNS from damage by peripheral harmful substances and, on the other hand, is expected to allow therapeutic drugs to cross the BBB effectively to reach the brain parenchyma and optimize the treatment of CNS diseases.
2 Purinergic system
Purinergic signaling was proposed by Geoffrey Burnstock in 1972, with the theory indicating that virtually all extracellular purines were involved in cell communication in all animals and humans (Burnstock, 1972). The four major purines, as important components of the purine system, are ATP, ADP, adenosine monophosphate (AMP), and adenosine. ATP supports intracellular energy storage and also serves as a neurotransmitter and signaling molecule for intercellular communication (Burnstock, 1972). The purinergic system also includes three key enzymes, named the ectonucleoside triphosphate diphosphohydrolases (E-NTPDases: NTPDase1/CD39), ectonucleotide pyrophosphatase/phosphodiesterases (E-NPP), and ecto-5′-nucleotidase (E-5′-nucleotidase/CD73). Extracellular ATP is broken down by metabolic enzymes to produce ADP, AMP, and adenosine, which may stimulate a series of purinergic receptors expressed on the cell surface (Abbracchio and Burnstock, 1994). Purinergic receptors are divided into two main categories, P1 and P2 receptors. The P1 receptor is composed of four adenosine-selective receptor subtypes: A1, A2A, A2B, and A3 receptors. P2 receptors mainly include P2X receptors and P2Y receptors. In detail, P2X receptors include P2X1-7, seven ligand-gated cation channel subtypes, and P2Y receptors contain P2Y1, 2, 4, 6 and 11–14, eight metabolic G-protein-coupled receptor (GPCR) subtypes. Notably, P2X receptors respond only to ATP, whereas P2Y receptors respond to multiple nucleotides, including ATP/ADP, UTP/UDP, or UDP-glucose. Adenine-based nucleotides such as ATP are actively released by cells in the neurovascular unit, particularly astrocytes, microglia, and endothelial cells, which activate a variety of nearby purinergic receptors and induce changes in BBB barrier function (Burnstock and Knight, 2017; Lee et al., 2021).
The focus of purinergic research has been on adenine-based nucleotides and adenosine, while guanine-based components of this system have received comparatively less attention. Until now, there has been growing evidence of the extracellular effects of guanine-based purines. The nucleotides guanosine 5′-triphosphate (GTP), guanosine 5′-diphosphate (GDP), and guanosine 5′-monophosphate (GMP) constitute the guanine-based purines (GBPs). These purines are metabolized to guanosine by extracellular nucleotidases, and conversely, guanosine is converted to guanine by purine nucleoside phosphorylases (Rathbone et al., 2008).
Guanosine has been demonstrated to be neuroprotective in numerous in vitro and in vivo models of CNS illnesses, such as ischemic stroke, AD, Parkinson’s disease (PD), etc. (Su et al., 2009; Hansel et al., 2015; Lanznaster et al., 2017). The neuroprotective mechanisms of guanosine may involve the decrease of glutamatergic excitotoxicity to influence astrocyte function (Schmidt et al., 2007; Schmidt et al., 2008); modulation of the adenosinergic system (Almeida et al., 2017); as well as impacts on the inflammatory cascade response and oxidative stress (Paniz et al., 2014; Kovacs et al., 2015). However, there are few reports of guanosine-related purines directly regulating the integrity of the blood-brain barrier. In a rat model of cerebral ischemia, intranasal guanosine was delivered 3 h after stroke to prevent ischemia-induced motor impairment, brain cell death, and blood-brain barrier permeability (Muller et al., 2021).
Notably, it has been demonstrated that 3′-5′-cyclic guanosine monophosphate (cGMP), produced by GTP catalyzed by guanylate cyclase, disrupts the integrity of the BBB (Choi et al., 2018; Janigro et al., 1994; Chi et al., 1999). Although guanosine effects could open a new window in therapeutic approaches toward purinergic signaling in the CNS (Massari et al., 2021), due to the limited data on the effects of guanosine on the blood-brain barrier, this review focuses primarily on determining the association between adenine-based nucleotides, their receptors, and BBB permeability.
3 P2X receptors and signaling
P2X receptors belong to the family of ligand-gated ion channels, and seven subunits have been identified, namely P2XR (1–7). P2X receptors direct the inward flow of Ca2+, Na+, and K+ cations upon activation by extracellular nucleotides such as ATP (Shieh et al., 2006; Bernier et al., 2018). P2X receptors are widely distributed in tissues. P2X receptors in smooth muscle cells mediate fast excitatory junctional potentials, while in the central nervous system, activation of P2X receptors causes calcium ions to enter neurons and elicit neuromodulatory responses. Although the ATP-binding sites of P2X receptors are highly conserved, there are differences in ATP potency among the different isoforms (Illes et al., 2021). P2XR (1–6) receptors are active at low micromolar to submicromolar concentrations of ATP, whereas P2X7 receptors require hundreds of micromolar concentrations of ATP to activate.
3.1 P2X receptors in neurovascular unit (NVU)
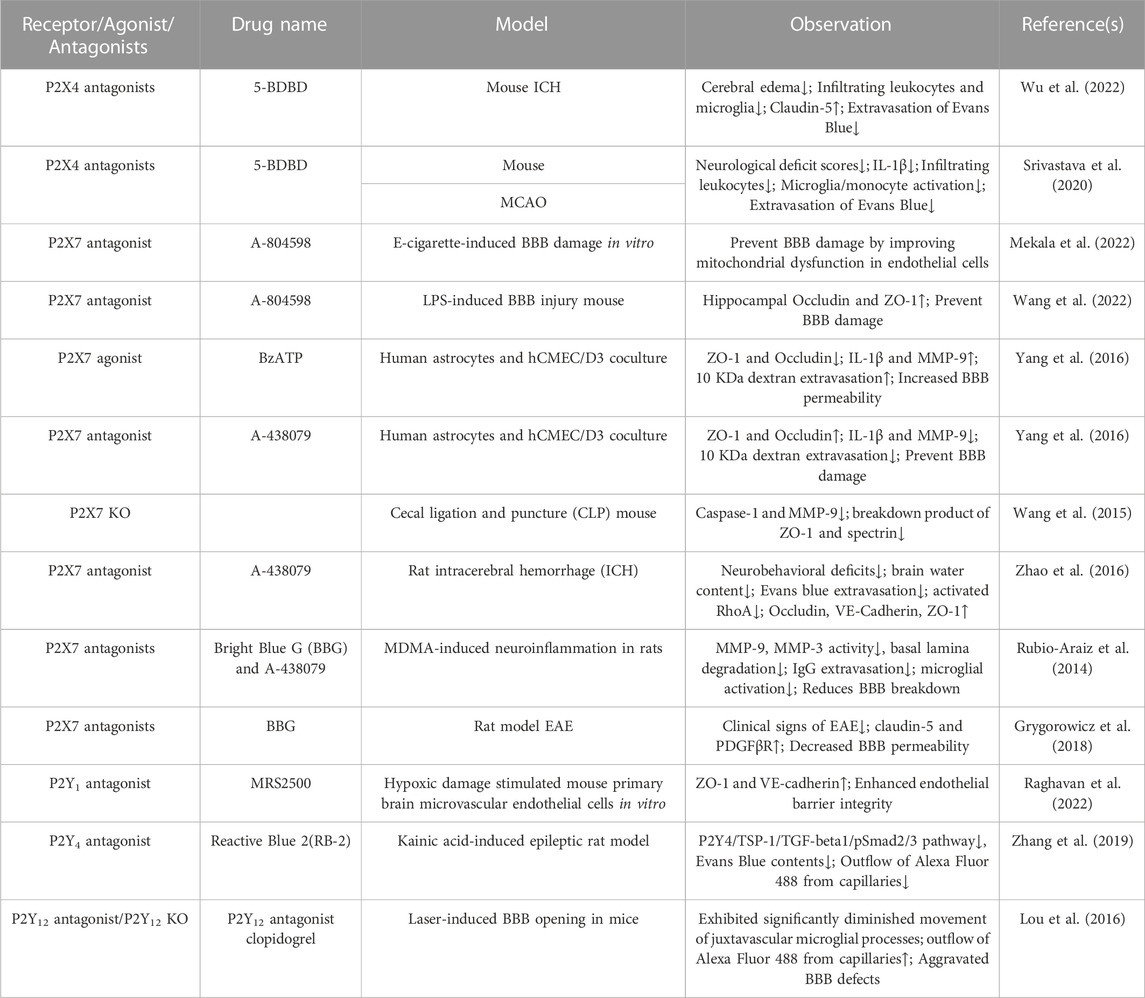
Neuronal and perivascular microglia are in touch with endothelial cells, pericytes, and astrocytes, which form the neurovascular unit. All P2X receptor mRNAs and proteins have been detected in the endothelium of multiple vessels (Loesch and Burnstock, 2000; Glass et al., 2002; Ramirez and Kunze, 2002; Wilson et al., 2007), but the expression of these receptors is not completely uniform in the neurovascular units of the brain. Using immunocytochemistry and transmission electron microscopy, Andrzej Loesch found that P2X1 was predominantly expressed in the astrocyte end-foot of the rat cerebellar vascular neural unit, with no significant expression in cerebellar endothelial cells and pericytes (Loesch, 2021). Similarly, the perivascular component of glial cells in the cerebellum showed P2X4 receptor immunoreactivity, while it was unlabeled in endothelial and pericytes (Loesch, 2021). However, both microvascular endothelial and perivascular astrocytes in the hypothalamus were immunoreactive for P2X4 receptors, but positive expression of P2X4 receptors in pericytes was not observed (Loesch, 2021). In addition, P2X6 receptors were expressed mainly in rat paraventricular nucleus microvascular endothelial cells and perivascular astrocytes end-foot (Loesch, 2021). Human brain microvascular endothelial cells (HBMECs) express P2X7 receptors (Wang et al., 2022). After stimulation by LPS, intracellular mitochondria produced a large amount of ATP and activated P2X7R, which further mediated the activation of the intracellular Omi/HtrA2 apoptosis signaling pathway and promoted cell apoptosis (Wang et al., 2022). Although the expression of these receptors in neurovascular units is partially understood, how P2X receptors regulate BBB permeability is less well studied, and the regulation of BBB by receptors other than P2X4 and P2X7 is unclear. Table 1 shows the effects of P2X receptor agonists and antagonists on the BBB.
3.2 P2X receptors and blood-brain barrier
3.2.1 P2X4
P2X4 is a typical P2X receptor, which can bind to P2X2, P2X5, and/or P2X6 to form heterotrimers (Antonio et al., 2014). It is expressed on the plasma membrane and in intracellular compartments. Meanwhile, P2X4 is a highly sensitive purinergic receptor that recognizes extracellular free ATP produced by dying cells following tissue injury, and is located in central and peripheral neurons, microglia, astrocytes, endothelial cells, and epithelial tissues (Montilla et al., 2020). The function of the P2X4 receptor in microglia has received extensive attention because of the relatively high level of expression of this receptor in these cells (Stokes et al., 2017). Microglia rely on migration and motility for active surveillance of the brain. P2X4 receptor activation drives microglia movement mainly through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Ohsawa et al., 2007). Thus, microglia that move to the injured brain area express high levels of the P2X4 receptor (Domercq et al., 2013). P2X4 also contributes to the immune response of microglia, which affects BBB permeability in neuroinflammatory and degenerative diseases (Vazquez-Villoldo et al., 2014). After intracerebral hemorrhage (ICH), microglia activation and immune cell infiltration exacerbate cell death and BBB damage. P2X4R was shown to be overexpressed in the brains of ICH patients as well as in ICH animals. Its activation inhibited the secretion of anti-inflammatory cytokines from microglia after cerebral hemorrhage, which exacerbated inflammatory brain injury. Concomitantly, P2X4R inhibition with the selective inhibitor (5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD)) dramatically reduced cerebral edema, blood-brain barrier leakage in ICH animals by decreasing pro-inflammatory activity of microglia (Wu et al., 2022). In addition, 5-BDBD treatment significantly inhibited P2X4R expression in monocytes and microglia after ischemic stroke, while reducing neurological deficit scores, interleukin-1 beta (IL-1β) levels, and BBB permeability (Srivastava et al., 2020). Furthermore, P2X4R knockout reduced leukocyte infiltration into brain tissue and improved neurological function in an ischemic stroke model (Verma et al., 2017). Thus, P2X4 receptor regulation of microglia activity may be partially involved in the regulation of BBB permeability, but the in-depth mechanisms need further investigation.
3.2.2 P2X7
ATP-induced proinflammatory effects of the P2X7 receptor have been extensively studied. The P2X7 receptor is best known for its effects on proliferation, apoptosis, and inflammation (Gu and Wiley, 2018). In addition, the P2X7 receptor (P2X7R) is of particular interest because of its association with BBB disruption (Andrejew et al., 2019). Recently, P2X7R has been implicated in alcohol and nicotine-induced BBB damage (Le Dare et al., 2019; Le Dare et al., 2021). Electronic-cigarette (E-Cig) vape (0% or 1.8% nicotine) decreased occludin and glucose transporter 1 (Glut1) protein expression in brain tissue and increased BBB permeability in vivo (Heldt et al., 2020). In vitro (Mekala et al., 2022), treatment of brain microvascular endothelial cells with ethanol (ETH), acetaldehyde (ALD), or 1.8% e-Cig elevated P2X7R and TRPV1 channel gene expression. Meanwhile, the P2X7R antagonist A804598 (10 µM) restored mitochondrial oxidative phosphorylation levels and played a protective role in preventing extracellular ATP release. BBB functional assays using trans-endothelial electrical resistance showed that blocking P2X7R channels enhanced barrier function. P2X7R antagonist may prevent alcohol or e-cigarette-induced BBB damage by improving mitochondrial dysfunction in endothelial cells. In addition, P2X7R plays a key role in LPS-induced BBB injury in mice. LPS significantly upregulated hippocampus P2X7R expression, whereas treatment with the P2X7R inhibitor A-438079 prevented the LPS-induced decrease in hippocampal Occludin and ZO-1 expression in mice (Wang et al., 2022).
Microglia also contribute to the structural and functional integrity of the BBB. Although P2X7 receptors are expressed in a variety of cells in the brain, such as oligodendrocytes and astrocytes, their expression is highest in microglia (Illes et al., 2012; Illes, 2020). P2X7 is a receptor involved in microglial activation, which is significantly upregulated in the postmortem brain of Alzheimer’s patients and in animal models of various neurodegenerative diseases, promoting central neuroimmune and inflammatory responses that exacerbate disease progression (Takenouchi et al., 2010). Stimulation of P2X7Rs on the surface of microglia by high concentrations of ATP molecules activates NLRP3, which initiates the cleavage of pro-caspase-1 to caspase-1, followed by caspase-1-induced protein hydrolysis to convert pro-IL-1β to mature IL-1β. Studies have shown that P2X7 receptor activation of microglia leads to the release of the pro-inflammatory cytokine IL-1β (Ferrari et al., 2006; Takenouchi et al., 2009), which promotes the production of matrix metalloproteinase-9 (MMP-9) (Gu and Wiley, 2006) and decreases the expression of the tight junction protein ZO-1, thereby disrupting the blood-brain barrier in vivo and vitro (Harkness et al., 2000; Mori et al., 2002; Oliveira-Giacomelli et al., 2021). A breakdown in the blood-brain barrier resulted in neuroinflammation, ion dysregulation, and cerebral edema, leading to increased intracranial pressure, neuronal malfunction, and neurodegeneration (Profaci et al., 2020). For instance, extracellular aggregation of amyloid (Aβ) peptides is a key characteristic of AD, serving as an important trigger for glial cell activation and ATP release, thereby activating P2X7 receptors. Under AD pathology, high concentrations of ATP or Aβ peptides promoted the activation of P2X7 in microglia, which in turn induced increased release of chemokines such as CCL3 and the recruitment of CD8+ T cells into the hippocampus and choroid plexus and exacerbated the development of central inflammation (Martin et al., 2019) (Figure 2).
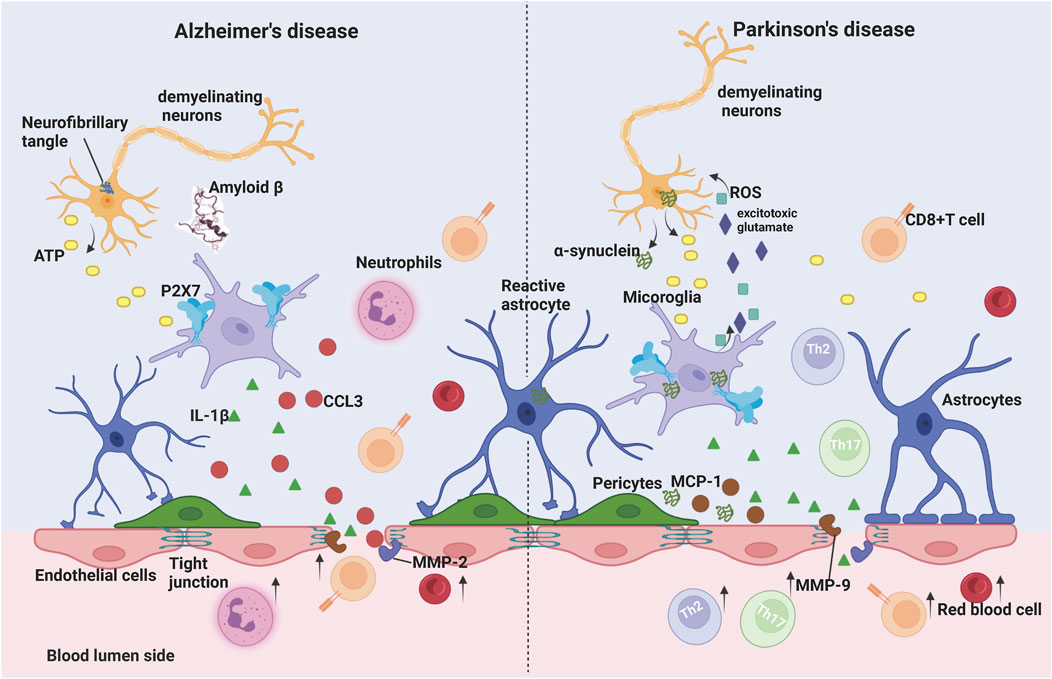
FIGURE 2. Microglia P2X7 receptor signaling on blood-brain barrier permeability in Alzheimer’s disease (AD) and Parkinson’s disease (PD). In AD, the process of neuronal death induces high levels of ATP release into the extracellular space, which activates microglia P2X7 receptors and leads to the release of IL-1β. In addition, the ATP/P2X7 receptor pathway enhances metalloproteinase (MMP) activity, leading to degradation of tight junction (TJ) protein, which results in increased blood-brain barrier permeability. Aβ peptide synergistically promotes P2X7 activation in microglia, which further induces increased release of chemokines such as CCL3 and recruitment of neutrophils and CD8+ T cells into the central nervous system (CNS) in the presence of BBB injury, promoting disease progression. In PD, α-synuclein aggregation leads to dopaminergic neuron death, increased ATP levels and P2X7 hyperactivation. On the other hand, α-synuclein activates microglia, leading to the release of excitatory glutamate and reactive oxygen species (ROS) from microglia to damage dopaminergic neurons, while α-synuclein binds to and stimulates the transcription of P2X7 receptors in microglia. α-synuclein and P2X7 receptors both increase the release of IL-1β and chemokines, increase MMP activity, disrupt the BBB, and lead to extravasation of red blood cells, which leads to cerebral microhemorrhage, as well as causing parenchymal infiltration of peripheral immune cells and exacerbating the pathological process of PD. Created with BioRender.com.
In addition, postmortem patients with PD have more phagocytically active reactive microglia in the brain (Toulorge et al., 2016), as well as increased microglia activation in the striatum and substantia nigra in a rat model (Carmo et al., 2014), accompanied by elevated P2X7 receptor gene expression (Oliveira-Giacomelli et al., 2021). α-synuclein protein is a crucial component of PD pathogenesis, and its aggregation is believed to be connected with disruption of the blood-brain barrier. Administration of α-synuclein dramatically increased the permeability of endothelium co-cultured with rat brain pericyte cells, while inducing the release of IL-1β, IL-6, TNF-α, MCP-1, and MMP-9 (Dohgu et al., 2019). Furthermore, α-synuclein protein activates microglia, causes the release of excitotoxic glutamate from microglia, and releases reactive oxygen species (ROS) to damage dopaminergic neurons (Dos-Santos-Pereira et al., 2018), as well as also binding to and stimulating transcription of P2X7 receptors in microglia (Jiang et al., 2015). Interestingly, P2X7 receptor activation increases IL-1β release, which in turn promotes MMP-9 secretion and disrupts the blood-brain barrier’s tight junctions (Yang et al., 2016). In PD disease, breakdown of the BBB causes extravasation of erythrocytes (Pienaar et al., 2015), which leads to cerebral microhemorrhages, as well as causing brain infiltration of peripheral immune cells and exacerbating the PD pathological process (Sweeney et al., 2018) (Figure 2).
P2X7 signaling in endothelial cells also plays a key role in BBB permeability regulation. Increased P2X7 receptor signaling in brain microvascular endothelial cells of septic encephalopathy (SE) mice induced by cecal ligation and puncture (CLP) enhanced the adhesion of Mac-1-expressing leukocytes to endothelial cells via intercellular cell adhesion molecule-1 (ICAM-1) and upregulated endothelial cell chemokine (C-X3-C motif) ligand 1 (CX3CL1), which triggered microglia activation. In addition, activation of NLRP3/caspase-1/IL-1β signaling via P2X7 receptor signaling in endothelial cells also accelerates BBB breakdown and neurovascular damage during SE (Wang et al., 2015) (Figure 3).
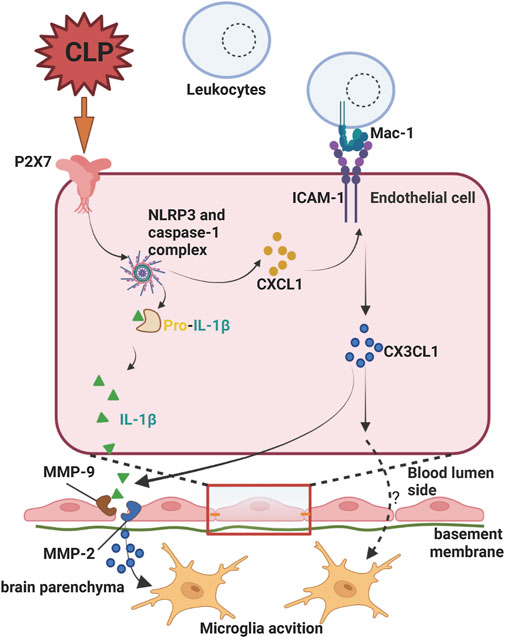
FIGURE 3. Endothelial P2X7 signaling on the permeability of the blood-brain barrier in septic encephalopathy (SE). Increased P2X7 receptor signaling in brain microvascular endothelial cells of SE mice generated by cecal ligation and puncture (CLP) increased adherence of Mac-1 expressing leukocytes to endothelial cells intercellular cell adhesion molecule-1(ICAM-1) and upregulated endothelial cell chemokine (C-X3-C motif) ligand 1 (CX3CL1), which activated microglia. The uncertainty exists as to whether CX3CL1 directly alters blood-brain barrier integrity during this process. P2X7 receptor signaling also promoted BBB breakdown and neurovascular injury during SE by activating NLRP3/caspase-1/IL-1β signaling. Created with BioRender.com.
It has been determined that astrocytes participate in BBB function and can affect the permeability of the BBB (Bang et al., 2017; Heithoff et al., 2021). Formation and maintenance of the blood-brain barrier require astrocyte endfeet on the abluminal side of endothelial cells. In primary astrocytes, BzATP activates the P2X7 receptor and generates a 2.5-fold increase in RhoA activity, which is reduced by the P2X7 antagonist BBG, demonstrating that RhoA is activated in the signaling pathway downstream of the P2X7 receptor (Henriquez et al., 2011; Beckel et al., 2014). In vivo, increased expression of P2X7 receptors was observed in astrocytes and endothelial cells surrounding the hematoma 24 h after ICH in rats (Zhao et al., 2016). Disruption of the blood-brain barrier is one of the most significant pathophysiological alterations early in the course of ICH, leading to the production of vasogenic brain edema, which can result in a poor prognosis for the disease (Zhou et al., 2014). By suppressing RhoA activation, A438079, and P2X7R siRNA alleviated neurological impairments, brain edema, and minimized BBB degradation (Zhao et al., 2016). Numerous studies have identified RhoA, a small guanosine triphosphatase (GTPase), as a key regulator of barrier formation and disruption. RhoA governs endothelium actin cytoskeleton dynamics and contraction, affecting intercellular junctional complexes, vascular permeability, and signal transduction (Amado-Azevedo et al., 2014; Ramos and Antonetti, 2017). Activation of RhoA in endothelial cells promotes the onset of BBB barrier disruption, as evidenced by the fact that its activation leads to stress fiber formation associated with disruption of interendothelial junctions, thereby increasing paracellular flux (Beckers et al., 2010; Ramos and Antonetti, 2017).
The basement membrane (BM) is the extracellular matrix (ECM) that provides structural support for the BBB and also serves as a link between NVU intercellular communication and signaling pathways. It consists of structural proteins including type IV collagen, fibronectin, laminin, and other glycoproteins (Langen et al., 2019). P2X7 receptors are involved in the degradation of laminin and type IV collagen by the neurotoxic compound 3,4-methyldioxymethamphetamine (MDMA) (Rubio-Araiz et al., 2014). The intervention with MDMA activated microglia in the hippocampus and increased microglial P2X7 receptor expression. MDMA increased matrix metalloproteinase-3 (MMP-3) and MMP-9 activity in the hippocampus, which was accompanied by a decrease in laminin and collagen IV expression, an increase in IgG extravasation into the brain parenchyma, and finally lead to higher BBB permeability. Following treatment with P2X7 antagonists (Bright Blue G (BBG) and A-438079), BBB damage was minimized and its integrity was maintained (Rubio-Araiz et al., 2014; Perez-Hernandez et al., 2017).
Pericytes are in close contact with endothelial cells via “peg and socket” junctions in the common basal lamina, which are necessary for the formation and maintenance of the BBB (Cheslow and Alvarez, 2016; Liebner et al., 2018). One study has shown that P2X7R is co-expressed with PDGFβR, a pericyte marker localized to microvascular units (Grygorowicz et al., 2018). In a rat model of experimental autoimmune encephalomyelitis (EAE), P2X7 receptor expression was increased in capillaries, which correlated with low levels of expression of PDGFβR protein and Claudin-5. Treatment of P2X7R antagonists with immunized rats significantly reduced the clinical signs of EAE and enhanced the expression of claudin-5 and PDGFβR. These results suggest that P2X7 receptors located on pericytes may be involved in pathological mechanisms in brain microvessels that affect BBB integrity during EAE (Grygorowicz et al., 2018).
4 P2Y receptors and signaling
P2Y receptors are GPCRs with eight isoforms that respond to extracellular adenine and uracil nucleotides. P2Y receptors contain two subfamilies, the Gq protein-coupled P2Y1-like receptors P2Y1, 2, 4, 6, 11, and the Gi protein-coupled P2Y12-like receptors P2Y12−14. Almost all cells contain P2Y receptors, which are implicated in pathophysiological reactions like pain, inflammation, platelet aggregation, and neuroprotective effects (von Kugelgen, 2021). P2Y receptors are one of the most extensively researched therapeutic targets in the treatment of clinical diseases; e.g., clopidogrel, an antagonist targeting platelet P2Y12, is an anti-thrombogenic drug, and diclofosfamide, a nucleotide agonist targeting P2Y2 receptor, is used to treat dry eye disease (Guo et al., 2021).
4.1 P2Y receptors in NVU
P2Y receptors are mostly expressed in neurons, glial cells, and microvasculature in the brain, where they co-mediate neurotransmission, neuroprotection, neuron-glia interactions, and cerebral blood flow regulation alongside P2X receptors (Weisman et al., 2012; Toth et al., 2015; Burnstock, 2017). P2Y1 and P2Y2 receptors are present in brain pericapillary cells, and extracellular ATP causes pericyte contraction by stimulating these two receptors and causing intracellular Ca2+ concentrations to rise (Horlyck et al., 2021). P2Y1, P2Y2, and P2Y13 receptors, which are present in neurons, are involved in the regulation of neuronal differentiation and neuroprotection (Perez-Sen et al., 2015; Miras-Portugal et al., 2019). Notably, P2Y1, P2Y2, P2Y4, and P2Y6 receptors in endothelial cells have an induced vasodilation effect (Jacobson et al., 2020). Astrocytes in the hippocampus, cortex, striatum, cerebellum, and spinal cord express multiple P2Y receptors, such as P2Y1, 4, 6, 13 (Franke et al., 2012). Indeed, P2Y12 is relatively restricted in distribution, is mainly expressed in microglia, and has an important role in inflammation and neuropathic pain (Tozaki-Saitoh et al., 2008). The effects of P2Y receptors agonists or antagonists on BBB are shown in Table 1.
4.2 P2Y receptors and blood-brain barrier
Multiple P2Y receptor subtypes are expressed by endothelial cells throughout the vascular system. P2Y receptors in endothelial cells have been studied mainly in the context of their NO-mediated vasodilatory properties. Therefore, there are fewer findings on the role of P2Y receptors in maintaining the BBB. Bowden and Patel have identified the importance of the tyrosine kinase/mitogen-activated protein kinase (MAPK) cascades in P2Y receptor regulation of prostacyclin production in major vascular endothelial cells (Bowden et al., 1995; Patel et al., 1996). MAPK cascades are essential for cell adhesion, and there is substantial evidence that tyrosine-phosphorylated proteins are involved in maintaining the BBB integrity. Coexisting P2Y receptors in brain endothelial cells may variably control phosphoinositide hydrolysis, cyclic AMP, and MAPK, resulting in various effects on the BBB (Albert et al., 1997). The P2Y1 receptor exacerbates leukocyte recruitment and induces inflammation, and the P2Y1 inhibitor MRS2500 is able to reduce vascular inflammation. Meanwhile, in P2Y1-deficient mouse, monocyte adherence to inflammatory factor-stimulated mouse endothelial cell monolayers was drastically reduced in vitro (Zerr et al., 2011). Additionally, it has been discovered that primary cultured brain microvascular endothelial cells have minimal P2Y1 receptor expression and that hypoxic damage stimulated elevation of this receptor expression, which resulted in degradation of endothelial cell junctional proteins and increased endothelial permeability (Raghavan et al., 2022). Similarly, the P2Y1 receptor antagonist MRS2500 enhanced endothelial barrier integrity.
The development of epilepsy is accompanied by a disruption of BBB (Sweeney et al., 2019). The kainic acid-induced epileptic rat model presented angiogenesis and disruption of BBB integrity, along with a significant increase in the expression of TSP-1, TGF-β1, and pSmad2/3. Treatment with pyridoxal phosphate-6-azophenyl-2′, 4′-disulfonic acid (a broad P2 receptor antagonist) or Reactive Blue 2 (a P2Y4 receptor antagonist) inhibited TSP-1 expression and Smad2/3 phosphorylation level, while significantly reducing acute seizure severity, decreasing Evans Blue contents, and attenuating BBB damage (Zhang et al., 2019).
The P2Y12 receptor is a unique purinergic receptor expressed only by microglia in the central nervous system (CNS) (Gu et al., 2016). As a chemotactic receptor, it is highly expressed in microglia (Sasaki et al., 2003) and drives microglial migration to areas of CNS damage (Ransohoff and Cardona, 2010; Sipe et al., 2016). Following central capillary injury, perivascular microglia, a component of the neurovascular unit, rapidly generate dense aggregates of microglial protrusions at the site of injury. In addition, P2Y12 receptor-mediated chemotaxis of microglia processes is necessary for the rapid closure of the BBB after its rupture (Lou et al., 2016). Movement of paravalvular microglial protrusions was significantly reduced and failed to close the opening of the laser-induced BBB in mice intervened with the P2Y12 receptor inhibitor clopidogrel and in mice knocked out with the P2RY12 gene (Lou et al., 2016). Given that P2Y12 receptor antagonists are commonly used as platelet inhibitors in patients with coronary heart disease and cerebrovascular disease, who are at increased risk for stroke with impaired BBB destruction, these findings may have clinical implications.
5 Adenosine and adenosine (P1) receptors
Adenosine is a bioactive compound that has been shown to possess strong neuromodulatory effects. It is able to function as a signaling molecule between the body’s periphery and the brain since it can easily penetrate the BBB (Chiu and Freund, 2014). AMP is a major source of intracellular and extracellular adenosine. Intracellular adenosine is a synergistic intermediary between nucleic acids and ATP, which is generated by AMP metabolism via 5′-nucleotidase and synthesized by adenosine kinase. Adenosine can also be produced outside of the cells by the breakdown of ATP or ADP that has been released by the cell. In this pathway, CD39 or E-NTPDase converts ATP/ADP to AMP, while CD73 or 5′nucleotidase converts AMP to adenosine (Yegutkin, 2008). Adenosine exerts its effect by acting on four expressed G-protein-coupled adenosine receptors (A1, A2A, A2B, and A3) on cell surfaces, and these receptors are expressed in some combination on almost all CNS cells. Under physiological conditions, extracellular adenosine levels range between 20 and 300 nM (Newby, 1985; Newby et al., 1985); however, local adenosine concentrations in the brain increase nearly 1000-fold under stress and inflammatory conditions (Hagberg et al., 1987). A1 and A2A receptors have a higher affinity for adenosine, in contrast to A2B and A3 receptors, which have a lower affinity for adenosine, indicating that A1 and A2A receptors in the CNS could be activated by reasonable levels of extracellular adenosine (Carman et al., 2011).
5.1 Adenosine receptors in NVU
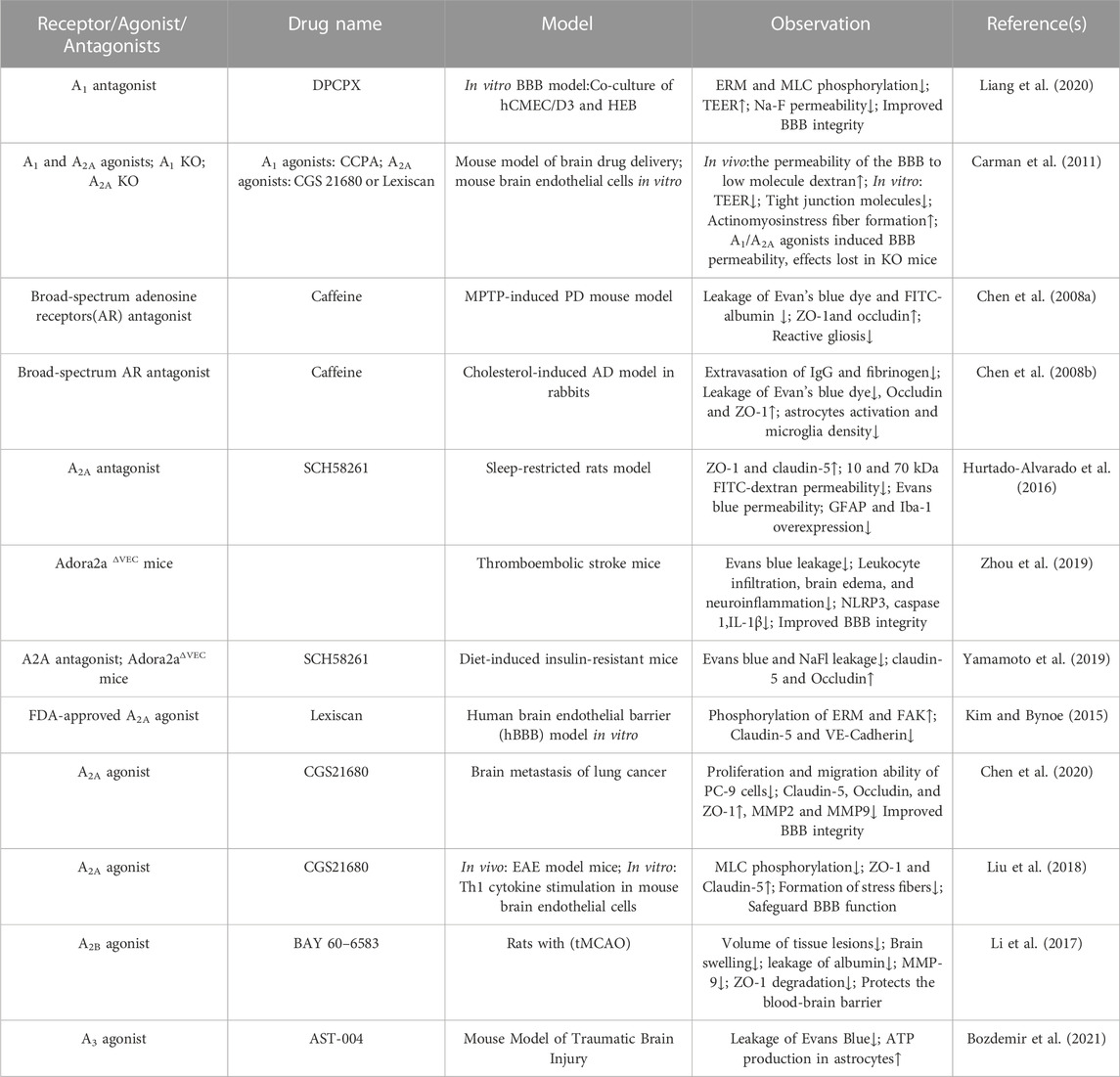
The human brain endothelial cell line hCMEC/D3 exhibited A1, A2A, and A2B receptors (Mills et al., 2011). A1 and A2A receptors were also expressed in primary human brain endothelial cells and in Bend.3 mouse brain endothelial cells (Carman et al., 2011). In addition, in vivo immunofluorescence reveals that A1 and A2A receptor proteins are expressed in mouse cortical brain endothelial cells, while in vitro, the two receptor proteins are present in primary mouse brain endothelial cells (Carman et al., 2011). Four P1 receptors (A1, A2A, A2B, and A3) have been identified in astrocytes (Dare et al., 2007). In astrocytes, cyclic adenosine monophosphate (cAMP) synthesis is inhibited by A1 receptors, while A2 receptors enhance cAMP synthesis, and A2B receptors are able to lead to a dose-dependent accumulation of cAMP (Peakman and Hill, 1994). Adenosine receptors govern various features of astrocytes, including A2A receptors that regulate glutamate uptake (Matos et al., 2012), but also A1 receptors that preserve cell integrity (Ciccarelli et al., 2001; D'Alimonte et al., 2007), and A3 receptors that protect against hypoxia-induced cell death and regulate CCL2 chemokine production (Wittendorp et al., 2004; Bjorklund et al., 2008). In physiological conditions, A1, A2A, A2B and A3 receptors are moderately expressed in glial cells, but their levels are upregulated in a central inflammatory environment (Hasko et al., 2005). The blood-brain barrier could be altered with disruptive changes in endothelial cells and tight junctions during central chronic inflammation, mediated mainly by adenosine receptors and CD39/CD73 expression (Selmi et al., 2016). The effects of adenosine receptors agonists or antagonists on BBB are shown in Table 2.
5.2 Adenosine receptors and blood-brain barrier
5.2.1 A1 and A2A receptors
Adenosine A1 and A2A receptors are expressed on both human and murine brain microvascular endothelial cells (Kalaria and Harik, 1988; Carman et al., 2011; Mills et al., 2011). Two studies have shown that Ginkgo biloba extract increases BBB permeability by activating the A1 adenosine receptor signaling pathway (Guo et al., 2020; Liang et al., 2020). In these studies, BBB models were constructed by co-culture with human cerebral microvascular endothelial cells (hCMEC/D3) and human normal glial cells (HEB) in vitro. The hCMEC/D3 cell line is the first stable, well-differentiated human brain endothelial cell line that expresses CD73, a cell surface enzyme that converts extracellular AMP to adenosine, as well as adenosine receptor subtypes A1, A2A, and A2B (Mills et al., 2011). Intervention with Ginkgo biloba extract increased BBB permeability, as evidenced by an increased fluorescein sodium (Na-F) penetration rate, disruption of tight junction structures, and increased actin-binding proteins ezrin, radixin and moesin (ERM) and myosin light chain (MLC) phosphorylation levels in vitro (Guo et al., 2020; Liang et al., 2020). ERM (ezrin/radixin/moesin) has been shown to be an important actin-binding molecule and the target of threonine phosphorylation in a variety of signaling pathways (Garcia-Ponce et al., 2015). Increased phosphorylation of ERM can induce actin remodeling and increase vascular permeability. Meanwhile, the phosphorylation of myosin light chain kinase (MLCK) to MLC causes actin filaments at the tight junction of endothelial cells to contract, leading to the opening of the barrier. Indeed, administration of the A1 receptor antagonist DPCPX or adenosine A1 receptor siRNA inhibited ERM and MLC phosphorylation levels, altered TJ ultrastructure, and improved BBB integrity (Guo et al., 2020; Liang et al., 2020) (Figure 4).
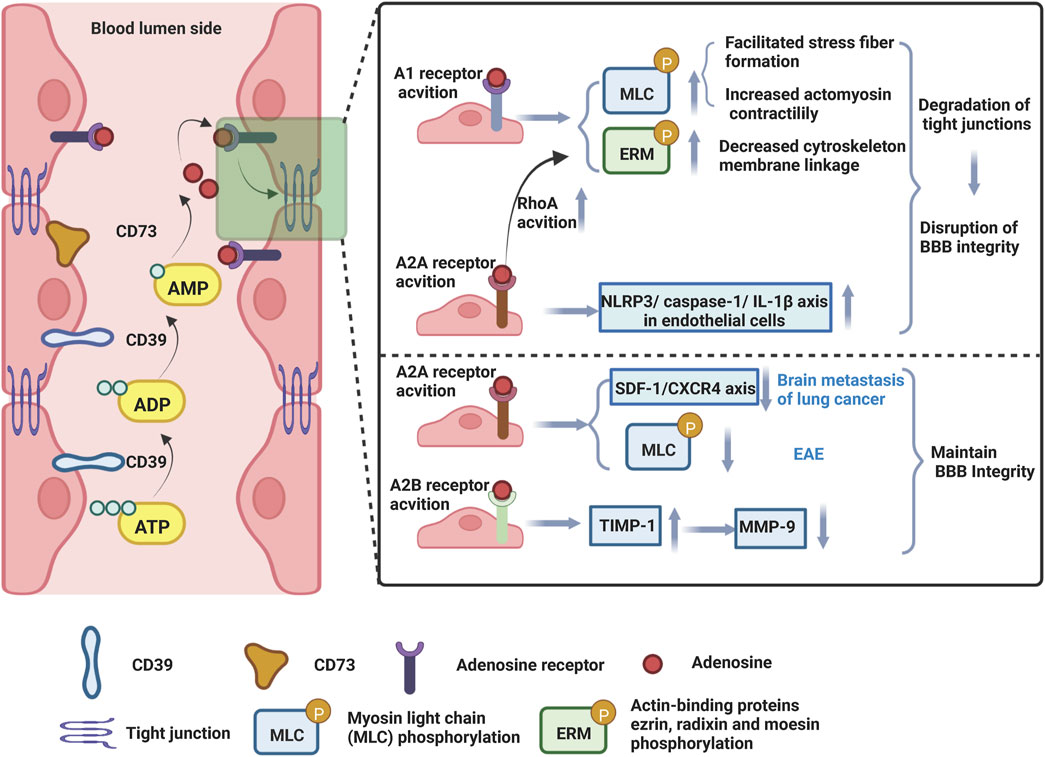
FIGURE 4. Adenosine receptors (ARs) modulate the permeability of blood-brain barrier. Endothelial cells of the microvasculature of the brain express adenosine receptors, CD39, and CD73. Under conditions of tissue damage, stress, and inflammation, ATP is released and converted by CD39 into ADP and AMP, which are then converted to adenosine by CD73. Adenosine binds to adenosine receptors on endothelial cells and operates to restructure the actin cytoskeleton, thereby disrupting tight junctions and adherens junctions and affecting BBB integrity. Created with BioRender.com.
A2A receptors are expressed at increased levels in brain tissue in multiple animal models, such as sleep restriction and thromboembolic stroke, accompanied by BBB damage (Hurtado-Alvarado et al., 2018; Zhou et al., 2019; Medina-Flores et al., 2020). Lack of sleep produces a low-grade inflammatory state that increases pro-inflammatory mediators, which regulate BBB function in a subtle but sustained manner (Hurtado-Alvarado et al., 2018). Sleep restriction increased the expression of A2A adenosine receptors in the hippocampus and basal nucleus. Blockade of A2A receptors by SCH58261 reversed sleep restriction-induced BBB dysfunction, including increased dextrans coupled to fluorescein (FITC-dextrans) and Evans blue permeability, degraded tight junction protein expression, and increased expression of neuroinflammatory markers Iba-1 and GFAP (Hurtado-Alvarado et al., 2016).
Besides, the human striatum contains high levels of A2A receptors, while the cerebral cortex, hippocampus, and immune cells contain lower levels (van Waarde et al., 2018). It was demonstrated that the broad AR agonist NECA [activates all ARs (A1, A2A, A2B, A3)], the selective A1 agonist CCPA and the A2A receptor agonists CGS 21680 or Lexiscan increased the permeability of the BBB to 10 kDa dextran, while the effect of these ARs on BBB permeability was attenuated in mice knocked out of A1 or A2A receptors (Carman et al., 2011). Notably, in vitro, NECA and Lexiscan intervention in mouse brain endothelial cells inhibited the expression of intercellular tight junctions ZO-1, claudin-5, and Occludin and reduced transendothelial electrical resistance (TEER) (Carman et al., 2011). Consistent with these results, in animal models of neurodegenerative disease, investigators observed that caffeine, a broad-spectrum AR antagonist, attenuated Parkinson and Alzheimer animal models-induced leakage of Evans blue dye, degradation of occludin and ZO-1, and ameliorated BBB dysfunction (Chen et al., 2008a; Chen et al., 2008b). Likewise, the FDA-approved A2A AR agonist Lexiscan, a selective A2A receptor agonist, increased the permeability of the human brain endothelial barrier (hBBB) model in vitro (Kim and Bynoe, 2015). A2A receptor activation mediated increase in cAMP and RhoA signaling activation, which in turn stimulated instability of the actin-cytoskeleton, decreased phosphorylation of factors involved in focal adhesion ERM and focal adhesion kinase (FAK) and degradation of Claudin-5 and VE-Cadherin, hence increasing the permeability of the hBBB (Figure 4). Notably, the high permeability of hBBB induced by A2A agonists is rapid, time-dependent and reversible.
Accordingly, expression of A2A receptors in endothelial cells was increased after thromboembolic stroke (Zhou et al., 2019). In mice specifically lacking endothelial Adora2a (Adora2a ΔVEC), Evans blue leakage, leukocyte infiltration, brain edema, and neuroinflammation were attenuated. In vitro silencing of the Adora2a gene using siRNA in cultured brain microvascular endothelial cells also attenuated endothelial inflammation by inhibiting the NLRP3 inflammasome and downregulating expression of cleaved caspase 1 and IL-1β (Zhou et al., 2019). These results suggest that A2A receptor-mediated NLRP3 activation may have a role in brain endothelial inflammation and need to be investigated in depth. It has also been shown that obesity and insulin resistance disrupt the BBB in both humans and animals (Banks, 2019; Banks and Rhea, 2021). The activation of A2A receptors in endothelial cells is also closely associated with cognitive impairment caused by obesity. In this study, diet-induced insulin-resistant mice exhibited elevated BBB permeability to low molecular weight sodium fluorescein (NaFl) and Evans blue; however, administration of the A2A antagonist SCH58261 restored the BBB barrier integrity (Yamamoto et al., 2019). SCH58261 is a selective adenosine A2A receptor antagonist that crosses the BBB (Mohamed et al., 2012). Further study showed that Adora2a activation in endothelial cells exacerbated BBB damage and cognitive dysfunction in diet-induced insulin-resistant mice, while mice specifically knockout of endothelial Adora2a protected BBB integrity after suffering from diet-induced insulin resistance (Yamamoto et al., 2019). These results indicate that Adora2a-mediated signaling in vascular endothelial cells, which resulted in BBB failure, may be a potential mechanism for cognitive deficiencies caused by obesity and insulin resistance.
Certain viruses and bacteria infiltrated the CNS through boosting the local expression of adenosine, which promoted the BBB permeability and quickly opened the BBB (Zhao et al., 2020). For instance, Haemophilus influenzae type a (Hia) infection stimulated A2A and A2B adenosine receptors in a model of BBB co-cultured with human brain microvascular endothelial cells (BMEC) and pericytes (BMPC) in vitro, which induced the release of large amounts of VEGF from pericytes. VEGF caused pericyte shedding and endothelial cell proliferation, which triggered the BBB disorder (Caporarello et al., 2018). In addition, adenosine produced by a surface enzyme (Ssads) of Streptococcus suis promotes its pathogen’s entrance into the brains of mice, hence causing meningitis. A1 AR activation increases S. suis BBB penetration, and A1 AR signaling exploitation may represent a generic virulence mechanism (Zhao et al., 2020).
Conversely, some studies have shown that A2A receptor activation also protects the integrity of BBB barrier. Brain metastases is the most common and lethal malignancy of the CNS, (Lowery and Yu, 2017; Singh et al., 2018), yet it is unknown how primary cancer cells traverse the BBB and metastasis to physiological regions of brain tissue. The A2A receptor agonist CGS21680 inhibited the proliferation and migration ability of PC-9 cells, a type of lung cancer cell, and suppressed brain metastasis (Chen et al., 2020). Notably, activation of A2A receptors in vitro increased the expression levels of Claudin-5, Occludin, and ZO-1, reduced the expression of MMP-2 and MMP-9, and increased the BBB integrity, whereas the opposite effect was obtained with the A2A receptor antagonist SCH58261 (Chen et al., 2020). Stromal cell-derived factor-1 (SDF-1) is an important chemokine in homeostasis that interacts with C-X-C motif chemokine receptor 4 (CXCR4), which is commonly classified as a GPCR (Janssens et al., 2018). The interaction between SDF-1 and CXCR4 has been identified to regulate several cellular physiological processes, such as transcription, energy metabolism, cell adhesion, and chemotaxis (Mao et al., 2017). Their findings also showed that A2A receptor stimulation inhibited CXCR4 expression and that A2A receptor agonists and CXCR4 antagonists protected nude mice from the metastasis of malignant tumor cells in vivo and prolonged their survival time (Chen et al., 2020). Mechanistically, A2A receptor activation maintained BBB integrity by regulating the SDF-1/CXCR4 axis, which in turn inhibited brain metastasis (Figure 4). Besides, specific A2A receptor agonist CGS-21680 improved pathological and clinical manifestations of EAE by decreasing BBB permeability, inhibiting neuroinflammation (Liu et al., 2018). Th1 cytokines are known to activate MLCK, which promotes phosphorylation of MLC (p-MLC) and disrupts actin-myosin interactions, thereby regulating endothelial cell morphology (Capaldo and Nusrat, 2009). Thus, activation of MLCK also leads to TJ injury (Rissanen et al., 2013). In vitro, brain endothelial cells treated with the Th1 cytokines IL-1β, TNF-α, and IFN-γ exhibited barrier failure. By inhibiting MLC phosphorylation and promoting ZO-1 and Claudin-5 expression, the A2A receptor-specific agonist CGS-21680 provided direct BBB protection (Liu et al., 2018). In addition, CGS-21680 helps maintain the shape of endothelial cells by reducing the formation of stress fibers in cells caused by Th1 cytokines (Liu et al., 2018). Activation of the A2A receptor may safeguard BBB function by suppressing MLCK-mediated MLC phosphorylation in EAE (Figure 4). According to tissue injury and associated pathological conditions, activating A2A receptors has both beneficial and detrimental effects in different diseases (Chen et al., 2013). It was shown that A2A inhibited specific lymphocyte proliferation, reduced infiltration of CD4+ T lymphocytes, and suppressed inflammatory cytokine production, thus inhibiting EAE progression. Additionally, Lexiscan, an A2A receptor-specific agonist, is an FDA-approved drug with proven therapeutic effects in inflammatory bowel disease, lung injury, and hepatic ischemia-reperfusion (Liu et al., 2016). Based on the complexity of the brain microenvironment, the activation of A2A receptors may also have a dual effect on the function of the BBB.
5.2.2 A2B and A3 receptors
The interaction of A2B receptors and A3 receptors with the BBB has been less reported. The A2B receptor agonist BAY 60-6583 reduced the volume of tissue plasminogen activator (tPA)-induced lesions and attenuated brain swelling and BBB disruption in rats with ischemic stroke (Li et al., 2017). BAY 60-6583 inhibited tPA-enhanced MMP-9 activation, possibly by increasing the tissue inhibitor matrix metalloproteinase 1 (TIMP-1), thereby reducing TJ protein degradation and protecting the blood-brain barrier (Li et al., 2017) (Figure 4).
In a mouse model of traumatic brain injury, the adenosine A3 receptor agonist AST-004 decreased the permeability of BBB and neuroinflammation, and enhanced spatial memory (Bozdemir et al., 2021). Intervention with AST-004 boosted ATP production in astrocytes and enhanced neuroprotective efficacy after brain injury; however, the precise mechanism of enhancing BBB function requires additional investigation.
5.2.3 CD39 and CD73
Ecto-5′-nucleotidase CD73 is an enzyme present on the cell surface that participates in the purine catabolism process and is capable of catalyzing the breakdown of AMP to adenosine. In the central nervous system, neurons, astrocytes, endothelium, and other cells release ATP, then CD39 catalyzes the conversion of ATP/ADP to AMP, and CD73 metabolizes AMP to adenosine (Burnstock and Boeynaems, 2014; Fuentes and Palomo, 2015). Multiple types of endothelial cells express CD39 and CD73 (Koszalka et al., 2004; Dudzinska et al., 2014), and expression of CD39/CD73 at the cellular level regulates tissue barrier function via modulating ATP levels (Colgan et al., 2006). CD73 is expressed in mouse (Bend.3) and hCMEC/D3 brain endothelial cell lines in vitro (Carman et al., 2011; Mills et al., 2011). Compared to human brain endothelial cells, mouse brain endothelial cell CD73 expression is extremely low in vivo (Mills et al., 2008). CD73 is highly expressed on choroid plexus epithelial cells that form the blood-cerebrospinal fluid barrier, but its expression is lower on brain endothelial barrier cells under steady-state conditions (Mills et al., 2008). However, CD73 was increased in the presence of cellular stress, local inflammation, or tissue injury that produced adenosine. Meanwhile, CD39 is widely expressed in brain endothelial cells (Wang and Guidotti, 1998), and CD39 on endothelial cells is conducive to reducing inflammatory cell transport and platelet reactivity, thereby reducing tissue damage after cerebral ischemia (Hyman et al., 2009). Extracellular adenosine is generated by ATP via metabolism of the CD39/CD73 extracellular nucleotidase axis and subsequently could regulate BBB permeability via adenosine receptor signaling expressed on BBB cells (Bynoe et al., 2015).
In BBB, CD73 expression is at a low level but is sensitive to cAMP through its promoter (Narravula et al., 2000). The released adenosine activates cell surface adenosine A2B receptors, leading to reorganization of endothelial junctions and promoting barrier function (Narravula et al., 2000). In addition, interferon (IFN)-β treatment increased CD73 expression in human blood-brain barrier endothelial cells (BBB-EC) and human astrocytes, and upregulation of CD73 and increased adenosine production may contribute to the beneficial effects of IFN-β on multiple sclerosis(MS) by enhancing endothelial barrier function (Niemela et al., 2008). Grunewald et al. also demonstrated that in vitro, CD73 increased adenosine production and maintained cell shape and actin cytoskeleton stability, thereby reducing endothelial barrier permeability (Grunewald and Ridley, 2010).
6 BBB-permeable A2A-related compounds under clinical trials in neurodegenerative diseases
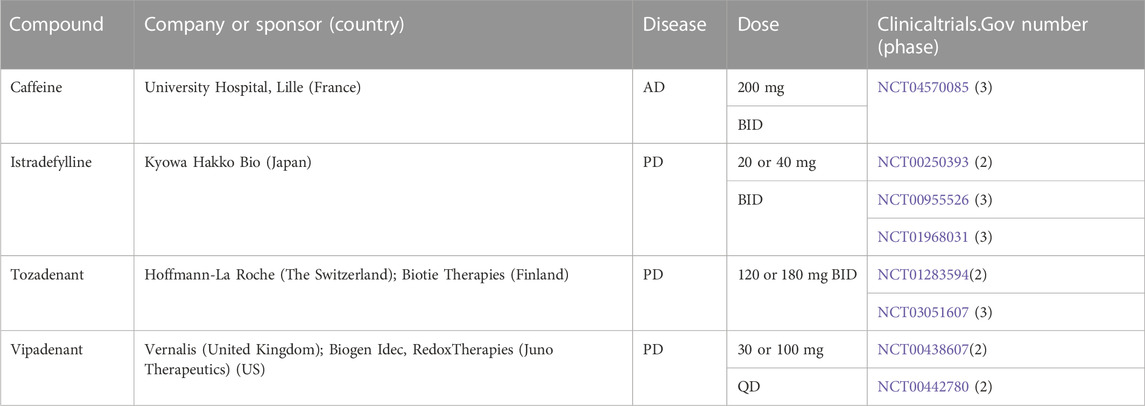
BBB-permeable purine-related compounds are primarily associated with A2A receptors in clinical trials of neurodegenerative diseases. Caffeine is the most commonly consumed A2A receptor antagonist that penetrates the blood-brain barrier. Caffeine enhances cognitive performance by decreasing hippocampus tau hyperphosphorylation, attenuating neuroinflammation, and reversing memory loss (Laurent et al., 2014; Carvalho et al., 2019; Jacobson et al., 2022), offering evidence for targeting A2A receptors in the therapy of AD. The effect of caffeine on cognitive function in AD is being investigated in the Phase 3 clinical trial NCT04570085. Istradefylline is a potent selective A2A antagonist that crosses the blood-brain barrier and has a high affinity for human A2A receptors, improving dyskinesia in PD patients (Muller, 2015; Torti et al., 2018). Indeed, it has been used as a combination therapy with Levodopa (L-DOPA) in PD treatment. It was approved early in Japan and Korea and passed various clinical safety and efficacy tests in the United States in August 2019. In addition, Tozadenant (SYN115) was originally developed for the treatment of PD and has been studied as a monotherapy for PD and as a combination therapy with L-DOPA or dopamine agonists (Pourcher and Huot, 2015; Shang et al., 2021). In the initial clinical studies for the therapeutic efficacy of the decreased closure time, no major adverse effects were observed; however, seven cases of sepsis and six deaths occurred in 890 patients in phase 3 clinical trial (Lewitt et al., 2020). As a result, clinical development of this drug was terminated in 2018. Noteblely, Tozadenant analogues, including 18F-labeled radiotracers for prospective positron emission tomography (PET) imaging, have recently been identified as A2A antagonists (Renk et al., 2021). Besides, vipadenant also belongs to a group of potent A2A antagonists, which was used for the treatment of PD (Pourcher and Huot, 2015), but its development was stopped due to safety concerns and later switched to cancer immunotherapy (Yu et al., 2020). Table 3 summarized the BBB-permeable A2A-related compounds under clinical trials in neurodegenerative diseases, and some of these clinical trials have been completed while others are still ongoing.
7 Prospects for improving drug delivery within the CNS
The BBB strictly regulates the movement of ions, molecules and cells between the blood and brain cells and is essential for neurological function and protection. Despite the BBB’s protective function, it restricts the availability of therapeutic compounds to the brain, making it more difficult to treat illnesses of the central nervous system (Profaci et al., 2020). The emergence of drug modification modalities based on receptor-mediated transcytosis, neurotropic virus-mediated transport, nanoparticles and exosomes all provide solutions for crossing the BBB, and drugs supported by these technologies are currently being evaluated in multiple clinical trials (Zhou et al., 2018; Liu et al., 2021; Terstappen et al., 2021). Our literature review demonstrates that antagonists of P2X7 and A2A receptors have beneficial therapeutic effects on brain damage, central inflammation, neurodegeneration etc., and attenuate the concurrent deterioration of the BBB barrier function. Up to now, a number of P2X7R antagonists that can cross the BBB have been developed (Bhattacharya, 2018; Wei et al., 2018). The compounds JNJ-47965567 and JNJ-42253432 demonstrated significant activity against P2X7R in rodents and humans, as well as effective BBB penetration. Likewise, novel blood-brain barrier permeable derivatives have been designed and synthesized as potential P2X7 antagonists known as compound 6 (2-(6-chloro-9h-purin-9-YL)-1-(2,4-dichlorophenyl) ethan 1-one), named ITH15004 (Calzaferri et al., 2021). It is a most potent, selective and highly BBB permeable antagonist and is considered to be the first non-nucleotide purine proposition for future drug optimization (Calzaferri et al., 2021). In addition, overexpression of A2A receptor leads to progressive neurodegeneration, and A2A antagonists have broad prospects for the treatment of CNS diseases. In the latest literature review, Merighi et al. used standard commercial software to calculate multi-parameter optimization (MPO) scores of CNS drugs for A2A antagonists in clinical trials to predict the likelihood of the compound crossing the blood-brain barrier with appropriate metabolic stability (Merighi et al., 2022). Meanwhile, their analysis suggested that alkylxanthines caffeine and DMPX have good ability to cross the blood-brain barrier and are expected to be potential drugs for the treatment of CNS diseases (Merighi et al., 2022).
In addition, researchers concentrated on developing strategies to control the BBB in order to facilitate access to the CNS (Rajadhyaksha et al., 2011). Determining how to accomplish this in a safe and effective manner has a profound impact on the treatment of a variety of neurological conditions. Current interventions include the use of drugs such as mannitol or bradykinin analog (Cereport/RMP7) to induce disruption of barrier function. Hypertonic mannitol, reduces tight junction integrity through endothelial cell contraction (Dabrowski et al., 2021), but its limitation is that it may cause seizures (Marchi et al., 2007). Cereport/RMP-7 has shown some potential to transiently increase BBB permeability (Borlongan and Emerich, 2003) and has shown some efficacy in animal models for the treatment of CNS pathology, but has not yielded satisfactory results in clinical trials (Prados et al., 2003).
Agonists of some receptors are able to open the BBB to allow large molecules or cells to enter. The observation of transiently increased BBB permeability upon A2A receptor activation suggests that exploiting this pharmacological effect holds the promise of facilitating drug delivery within the CNS (Carman et al., 2011; Kim and Bynoe, 2015). By labeling several copies of A2A receptor-activating ligands on dendrimers, a sequence of nanoagonists (NAs) was produced. NAs tagged with varying amounts of AR-activating ligands can adjust the BBB opening time-window within a range of 0.5–2.0 h (Gao et al., 2014). The FDA-approved A2A receptor agonist Lexiscan or the broad-spectrum agonist NECA increases BBB permeability and allows delivery of macromolecules to the CNS (Carman et al., 2011). Notably, there was a correlation between the duration of induced permeability and the half-life of the agonist. The half-life of BBB permeation induced by NECA intervention was 4 h. Its duration is substantially longer than that of Lexican, which induces BBB permeation with a half-life of 2.5 min (Carman et al., 2011). These findings suggest that the use of this agonist to briefly open the BBB may facilitate the delivery of therapeutic antibodies to the central nervous system. Since NECA is a broad-spectrum adenosine receptor agonist, additional research is necessary to better comprehend the mechanisms by which A1/A2A/A2B receptors specifically mediate signaling involved in BBB permeability regulation and to optimize the parameters for drug design.
8 Conclusion
Extracellular nucleotides acting on purinergic receptors of BBB cells modulate the permeability of the blood-brain barrier, with different types of receptors and concentrations of nucleotides affecting the specific modulatory effects. Inhibition or knockdown of P2X4, P2X7, P2Y1, or P2Y4 receptors protect BBB barrier integrity, limiting the entry of toxic substances, inflammatory immune cells, etc. into the brain and conversely, inhibition of P2Y12 receptor further exacerbates BBB permeability. The P2X7 receptor is highly investigated in the regulation of BBB integrity, although the distribution of other P2 receptors in the NVU and the pharmacological effects of inhibitors of these receptors are comparatively less explored. Additionally, specific subtypes of P1 receptors also variably influence BBB permeability, with A1 and A2A receptor activation boosting BBB permeability and A2B and A3 receptor agonists protecting BBB integrity. Although a small number of studies have demonstrated a protective effect of A2A agonists on BBB integrity, it cannot be overlooked that A1 and A2A agonists transiently open the BBB to facilitate the passage of large or small molecules and that this process is reversible, with the BBB closing as the drug’s half-life passes, offering great promise for drug delivery to the CNS. Therefore, purinergic signaling, as the gatekeeper of the BBB, has a switching regulatory function on the BBB, and the intervention of appropriate agonist/antagonist of purinergic receptors is beneficial to restore CNS homeostasis under pathological conditions. Furthermore, purinergic receptors are widely distributed in NVU, and their regulation of the blood-brain barrier is intricate. However, studies on the regulatory effects of purinergic receptors on the BBB have primarily focused on endothelial cells, and the targeting of these receptors on other BBB cells such as pericytes is not well understood. For validation purposes, more in vitro BBB models and in vivo research targeting purinergic receptors on cells other than endothelial cells are required.
Author contributions
YW retrieved the literature and drafted the manuscript. YZ drafted part of the manuscript. JW, LD, SHL, SIL, made the figures; QW proposed and revised the manuscript. All the authors reviewed the manuscript. Each of the authors agrees to be accountable for the content of the work.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82174512), the National Key R&D Program of China (No. 2022YFC3500703), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202003) and the Fund of Science and Technology Department of Sichuan Province, China (No. 2021ZYD0081, 2022ZDZX0033).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbracchio, M. P., and Burnstock, G. (1994). Purinoceptors: Are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 64 (3), 445–475. doi:10.1016/0163-7258(94)00048-4
Albert, J. L., Boyle, J. P., Roberts, J. A., Challiss, R. A., Gubby, S. E., and Boarder, M. R. (1997). Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen-activated protein kinase. Br. J. Pharmacol. 122 (5), 935–941. doi:10.1038/sj.bjp.0701453
Almeida, R. F., Comasseto, D. D., Ramos, D. B., Hansel, G., Zimmer, E. R., Loureiro, S. O., et al. (2017). Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems. Mol. Neurobiol. 54 (1), 423–436. doi:10.1007/s12035-015-9660-x
Amado-Azevedo, J., Valent, E. T., and Van Nieuw, A. G. (2014). Regulation of the endothelial barrier function: A filum granum of cellular forces, rho-GTPase signaling and microenvironment. Cell Tissue Res. 355 (3), 557–576. doi:10.1007/s00441-014-1828-6
Andrejew, R., Glaser, T., Oliveira-Giacomelli, A., Ribeiro, D., Godoy, M., Granato, A., et al. (2019). Targeting purinergic signaling and cell therapy in cardiovascular and neurodegenerative diseases. Adv. Exp. Med. Biol. 1201, 275–353. doi:10.1007/978-3-030-31206-0_14
Antonio, L. S., Stewart, A. P., Varanda, W. A., and Edwardson, J. M. (2014). Identification of P2X2/P2X4/P2X6 heterotrimeric receptors using atomic force microscopy (AFM) imaging. FEBS Lett. 588 (12), 2125–2128. doi:10.1016/j.febslet.2014.04.048
Bang, S., Lee, S. R., Ko, J., Son, K., Tahk, D., Ahn, J., et al. (2017). A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci. Rep. 7 (1), 8083. doi:10.1038/s41598-017-07416-0
Banks, W. A., and Rhea, E. M. (2021). The blood-brain barrier, oxidative stress, and insulin resistance. Antioxidants (Basel) 10 (11), 1695. doi:10.3390/antiox10111695
Banks, W. A. (2019). The blood-brain barrier as an endocrine tissue. Nat. Rev. Endocrinol. 15 (8), 444–455. doi:10.1038/s41574-019-0213-7
Beckel, J. M., Argall, A. J., Lim, J. C., Xia, J., Lu, W., Coffey, E. E., et al. (2014). Mechanosensitive release of adenosine 5'-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: A mechanism for purinergic involvement in chronic strain. Glia 62 (9), 1486–1501. doi:10.1002/glia.22695
Beckers, C. M., van Hinsbergh, V. W., and van Nieuw, A. G. (2010). Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb. Haemost. 103 (1), 40–55. doi:10.1160/TH09-06-0403
Bernier, L. P., Ase, A. R., and Seguela, P. (2018). P2X receptor channels in chronic pain pathways. Br. J. Pharmacol. 175 (12), 2219–2230. doi:10.1111/bph.13957
Bhattacharya, A. (2018). Recent advances in CNS P2X7 physiology and pharmacology: Focus on neuropsychiatric disorders. Front. Pharmacol. 9, 30. doi:10.3389/fphar.2018.00030
Bjorklund, O., Shang, M., Tonazzini, I., Dare, E., and Fredholm, B. B. (2008). Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur. J. Pharmacol. 596 (1-3), 6–13. doi:10.1016/j.ejphar.2008.08.002
Borlongan, C. V., and Emerich, D. F. (2003). Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: Laboratory and preliminary clinical evidence from bradykinin receptor agonist, cereport. Cereport. Brain Res. Bull. 60 (3), 297–306. doi:10.1016/s0361-9230(03)00043-1
Bowden, A., Patel, V., Brown, C., and Boarder, M. R. (1995). Evidence for requirement of tyrosine phosphorylation in endothelial P2Y- and P2U- purinoceptor stimulation of prostacyclin release. Br. J. Pharmacol. 116 (6), 2563–2568. doi:10.1111/j.1476-5381.1995.tb17208.x
Bozdemir, E., Vigil, F. A., Chun, S. H., Espinoza, L., Bugay, V., Khoury, S. M., et al. (2021). Neuroprotective roles of the adenosine A(3) receptor agonist AST-004 in mouse model of traumatic brain injury. Neurotherapeutics 18 (4), 2707–2721. doi:10.1007/s13311-021-01113-7
Burnstock, G., and Boeynaems, J. M. (2014). Purinergic signalling and immune cells. Purinergic Signal 10 (4), 529–564. doi:10.1007/s11302-014-9427-2
Burnstock, G. (2020). Introduction to purinergic signalling in the brain. Adv. Exp. Med. Biol. 1202, 1–12. doi:10.1007/978-3-030-30651-9_1
Burnstock, G., and Knight, G. E. (2017). Cell culture: Complications due to mechanical release of ATP and activation of purinoceptors. Cell Tissue Res. 370 (1), 1–11. doi:10.1007/s00441-017-2618-8
Burnstock, G. (2007). Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87 (2), 659–797. doi:10.1152/physrev.00043.2006
Burnstock, G. (2017). Purinergic signalling and neurological diseases: An update. CNS Neurol. Disord. Drug Targets 16 (3), 257–265. doi:10.2174/1871527315666160922104848
Bynoe, M. S., Viret, C., Yan, A., and Kim, D. G. (2015). Adenosine receptor signaling: A key to opening the blood-brain door. Fluids Barriers CNS 12, 20. doi:10.1186/s12987-015-0017-7
Cai, Z., Qiao, P. F., Wan, C. Q., Cai, M., Zhou, N. K., and Li, Q. (2018). Role of blood-brain barrier in Alzheimer's disease. J. Alzheimers Dis. 63 (4), 1223–1234. doi:10.3233/JAD-180098
Calzaferri, F., Narros-Fernandez, P., de Pascual, R., de Diego, A., Nicke, A., Egea, J., et al. (2021). Synthesis and pharmacological evaluation of novel non-nucleotide purine derivatives as P2X7 antagonists for the treatment of neuroinflammation. J. Med. Chem. 64 (4), 2272–2290. doi:10.1021/acs.jmedchem.0c02145
Capaldo, C. T., and Nusrat, A. (2009). Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788 (4), 864–871. doi:10.1016/j.bbamem.2008.08.027
Caporarello, N., Olivieri, M., Cristaldi, M., Scalia, M., Toscano, M. A., Genovese, C., et al. (2018). Blood-brain barrier in a Haemophilus influenzae type a in vitro infection: Role of adenosine receptors A2A and A2B. Mol. Neurobiol. 55 (6), 5321–5336. doi:10.1007/s12035-017-0769-y
Carman, A. J., Mills, J. H., Krenz, A., Kim, D. G., and Bynoe, M. S. (2011). Adenosine receptor signaling modulates permeability of the blood-brain barrier. J. Neurosci. 31 (37), 13272–13280. doi:10.1523/JNEUROSCI.3337-11.2011
Carmo, M. R., Menezes, A. P., Nunes, A. C., Pliassova, A., Rolo, A. P., Palmeira, C. M., et al. (2014). The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 81, 142–152. doi:10.1016/j.neuropharm.2014.01.045
Carvalho, K., Faivre, E., Pietrowski, M. J., Marques, X., Gomez-Murcia, V., Deleau, A., et al. (2019). Exacerbation of C1q dysregulation, synaptic loss and memory deficits in tau pathology linked to neuronal adenosine A2A receptor. Brain 142 (11), 3636–3654. doi:10.1093/brain/awz288
Chen, J. F., Eltzschig, H. K., and Fredholm, B. B. (2013). Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 12 (4), 265–286. doi:10.1038/nrd3955
Chen, L., Li, L., Zhou, C., Chen, X., and Cao, Y. (2020). Adenosine A2A receptor activation reduces brain metastasis via SDF-1/CXCR4 axis and protecting blood-brain barrier. Mol. Carcinog. 59 (4), 390–398. doi:10.1002/mc.23161
Chen, X., Gawryluk, J. W., Wagener, J. F., Ghribi, O., and Geiger, J. D. (2008a). Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer's disease. J. Neuroinflammation 5, 12. doi:10.1186/1742-2094-5-12
Chen, X., Lan, X., Roche, I., Liu, R., and Geiger, J. D. (2008b). Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J. Neurochem. 107 (4), 1147–1157. doi:10.1111/j.1471-4159.2008.05697.x
Cheslow, L., and Alvarez, J. I. (2016). Glial-endothelial crosstalk regulates blood-brain barrier function. Curr. Opin. Pharmacol. 26, 39–46. doi:10.1016/j.coph.2015.09.010
Chi, O. Z., Liu, X., and Weiss, H. R. (1999). Effects of cyclic GMP on microvascular permeability of the cerebral cortex. Microvasc. Res. 58 (1), 35–40. doi:10.1006/mvre.1999.2152
Chiu, G. S., and Freund, G. G. (2014). Modulation of neuroimmunity by adenosine and its receptors: Metabolism to mental illness. Metabolism 63 (12), 1491–1498. doi:10.1016/j.metabol.2014.09.003
Choi, H. J., Kim, N. E., Kim, J., An, S., Yang, S. H., Ha, J., et al. (2018). Dabigatran reduces endothelial permeability through inhibition of thrombin-induced cytoskeleton reorganization. Thromb. Res. 167, 165–171. doi:10.1016/j.thromres.2018.04.019
Ciccarelli, R., Ballerini, P., Sabatino, G., Rathbone, M. P., D'Onofrio, M., Caciagli, F., et al. (2001). Involvement of astrocytes in purine-mediated reparative processes in the brain. Int. J. Dev. Neurosci. 19 (4), 395–414. doi:10.1016/s0736-5748(00)00084-8
Colgan, S. P., Eltzschig, H. K., Eckle, T., and Thompson, L. F. (2006). Physiological roles for ecto-5'-nucleotidase (CD73). Purinergic Signal 2 (2), 351–360. doi:10.1007/s11302-005-5302-5
Communi, D., Janssens, R., Suarez-Huerta, N., Robaye, B., and Boeynaems, J. M. (2000). Advances in signalling by extracellular nucleotides. the role and transduction mechanisms of P2Y receptors. Cell. Signal. 12 (6), 351–360. doi:10.1016/s0898-6568(00)00083-8
D'Alimonte, I., Ballerini, P., Nargi, E., Buccella, S., Giuliani, P., Di Iorio, P., et al. (2007). Staurosporine-induced apoptosis in astrocytes is prevented by A1 adenosine receptor activation. Neurosci. Lett. 418 (1), 66–71. doi:10.1016/j.neulet.2007.02.061
Dabrowski, W., Siwicka-Gieroba, D., Robba, C., Bielacz, M., Solek-Pastuszka, J., Kotfis, K., et al. (2021). Potentially detrimental effects of hyperosmolality in patients treated for traumatic brain injury. J. Clin. Med. 10 (18), 4141. doi:10.3390/jcm10184141
Dare, E., Schulte, G., Karovic, O., Hammarberg, C., and Fredholm, B. B. (2007). Modulation of glial cell functions by adenosine receptors. Physiol. Behav. 92 (1-2), 15–20. doi:10.1016/j.physbeh.2007.05.031
Dean, M., Moitra, K., and Allikmets, R. (2022). The human ATP-binding cassette (ABC) transporter superfamily. Hum. Mutat. 43 (9), 1162–1182. doi:10.1002/humu.24418
Dinet, V., Petry, K. G., and Badaut, J. (2019). Brain-immune interactions and neuroinflammation after traumatic brain injury. Front. Neurosci. 13, 1178. doi:10.3389/fnins.2019.01178
Dohgu, S., Takata, F., Matsumoto, J., Kimura, I., Yamauchi, A., and Kataoka, Y. (2019). Monomeric alpha-synuclein induces blood-brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc. Res. 124, 61–66. doi:10.1016/j.mvr.2019.03.005
Domercq, M., Vazquez-Villoldo, N., and Matute, C. (2013). Neurotransmitter signaling in the pathophysiology of microglia. Front. Cell. Neurosci. 7, 49. doi:10.3389/fncel.2013.00049
Dos-Santos-Pereira, M., Acuna, L., Hamadat, S., Rocca, J., Gonzalez-Lizarraga, F., Chehin, R., et al. (2018). Microglial glutamate release evoked by alpha-synuclein aggregates is prevented by dopamine. Glia 66 (11), 2353–2365. doi:10.1002/glia.23472
Dudzinska, D., Luzak, B., Boncler, M., Rywaniak, J., Sosnowska, D., Podsedek, A., et al. (2014). CD39/NTPDase-1 expression and activity in human umbilical vein endothelial cells are differentially regulated by leaf extracts from Rubus caesius and Rubus idaeus. Cell. Mol. Biol. Lett. 19 (3), 361–380. doi:10.2478/s11658-014-0202-8
Ferrari, D., Pizzirani, C., Adinolfi, E., Lemoli, R. M., Curti, A., Idzko, M., et al. (2006). The P2X7 receptor: A key player in IL-1 processing and release. J. Immunol. 176 (7), 3877–3883. doi:10.4049/jimmunol.176.7.3877
Franke, H., Verkhratsky, A., Burnstock, G., and Illes, P. (2012). Pathophysiology of astroglial purinergic signalling. Purinergic Signal 8 (3), 629–657. doi:10.1007/s11302-012-9300-0
Fuentes, E., and Palomo, I. (2015). Extracellular ATP metabolism on vascular endothelial cells: A pathway with pro-thrombotic and anti-thrombotic molecules. Vasc. Pharmacol. 75, 1–6. doi:10.1016/j.vph.2015.05.002
Gao, X., Qian, J., Zheng, S., Changyi, Y., Zhang, J., Ju, S., et al. (2014). Overcoming the blood-brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS Nano 8 (4), 3678–3689. doi:10.1021/nn5003375
Garcia-Ponce, A., Citalan-Madrid, A. F., Velazquez-Avila, M., Vargas-Robles, H., and Schnoor, M. (2015). The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb. Haemost. 113 (1), 20–36. doi:10.1160/TH14-04-0298
Glass, R., Loesch, A., Bodin, P., and Burnstock, G. (2002). P2X4 and P2X6 receptors associate with VE-cadherin in human endothelial cells. Cell. Mol. Life Sci. 59 (5), 870–881. doi:10.1007/s00018-002-8474-y
Grunewald, J. K., and Ridley, A. J. (2010). CD73 represses pro-inflammatory responses in human endothelial cells. . 7 (1), 10. doi:10.1186/1476-9255-7-10
Grygorowicz, T., Dabrowska-Bouta, B., and Struzynska, L. (2018). Administration of an antagonist of P2X7 receptor to EAE rats prevents a decrease of expression of claudin-5 in cerebral capillaries. Purinergic Signal 14 (4), 385–393. doi:10.1007/s11302-018-9620-9
Gu, B. J., and Wiley, J. S. (2018). P2X7 as a scavenger receptor for innate phagocytosis in the brain. Br. J. Pharmacol. 175 (22), 4195–4208. doi:10.1111/bph.14470
Gu, B. J., and Wiley, J. S. (2006). Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood 107 (12), 4946–4953. doi:10.1182/blood-2005-07-2994
Gu, N., Eyo, U. B., Murugan, M., Peng, J., Matta, S., Dong, H., et al. (2016). Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav. Immun. 55, 82–92. doi:10.1016/j.bbi.2015.11.007
Gunduz, D., Kasseckert, S. A., Hartel, F. V., Aslam, M., Abdallah, Y., Schafer, M., et al. (2006). Accumulation of extracellular ATP protects against acute reperfusion injury in rat heart endothelial cells. Cardiovasc. Res. 71 (4), 764–773. doi:10.1016/j.cardiores.2006.06.011
Guo, C., Wang, H., Liang, W., Xu, W., Li, Y., Song, L., et al. (2020). Bilobalide reversibly modulates blood-brain barrier permeability through promoting adenosine A1 receptor-mediated phosphorylation of actin-binding proteins. Biochem. Biophys. Res. Commun. 526 (4), 1077–1084. doi:10.1016/j.bbrc.2020.03.186
Guo, X., Li, Q., Pi, S., Xia, Y., and Mao, L. (2021). G protein-coupled purinergic P2Y receptor oligomerization: Pharmacological changes and dynamic regulation. Biochem. Pharmacol. 192, 114689. doi:10.1016/j.bcp.2021.114689
Hagberg, H., Andersson, P., Lacarewicz, J., Jacobson, I., Butcher, S., and Sandberg, M. (1987). Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J. Neurochem. 49 (1), 227–231. doi:10.1111/j.1471-4159.1987.tb03419.x
Hansel, G., Tonon, A. C., Guella, F. L., Pettenuzzo, L. F., Duarte, T., Duarte, M., et al. (2015). Guanosine protects against cortical focal ischemia. Involvement of inflammatory response. Mol. Neurobiol. 52 (3), 1791–1803. doi:10.1007/s12035-014-8978-0
Harkness, K. A., Adamson, P., Sussman, J. D., Davies-Jones, G. A., Greenwood, J., and Woodroofe, M. N. (2000). Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain 123, 698–709. doi:10.1093/brain/123.4.698
Hartel, F. V., Rodewald, C. W., Aslam, M., Gunduz, D., Hafer, L., Neumann, J., et al. (2007). Extracellular ATP induces assembly and activation of the myosin light chain phosphatase complex in endothelial cells. Cardiovasc. Res. 74 (3), 487–496. doi:10.1016/j.cardiores.2007.02.013
Hasko, G., Pacher, P., Vizi, E. S., and Illes, P. (2005). Adenosine receptor signaling in the brain immune system. Trends Pharmacol. Sci. 26 (10), 511–516. doi:10.1016/j.tips.2005.08.004
Heithoff, B. P., George, K. K., Phares, A. N., Zuidhoek, I. A., Munoz-Ballester, C., and Robel, S. (2021). Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia 69 (2), 436–472. doi:10.1002/glia.23908
Heldt, N. A., Seliga, A., Winfield, M., Gajghate, S., Reichenbach, N., Yu, X., et al. (2020). Electronic cigarette exposure disrupts blood-brain barrier integrity and promotes neuroinflammation. Brain Behav. Immun. 88, 363–380. doi:10.1016/j.bbi.2020.03.034
Henriquez, M., Herrera-Molina, R., Valdivia, A., Alvarez, A., Kong, M., Munoz, N., et al. (2011). ATP release due to Thy-1-integrin binding induces P2X7-mediated calcium entry required for focal adhesion formation. J. Cell Sci. 124, 1581–1588. doi:10.1242/jcs.073171
Horlyck, S., Cai, C., Helms, H., Lauritzen, M., and Brodin, B. (2021). ATP induces contraction of cultured brain capillary pericytes via activation of P2Y-type purinergic receptors. Am. J. Physiol. Heart Circ. Physiol. 320 (2), H699–H712. doi:10.1152/ajpheart.00560.2020
Hurtado-Alvarado, G., Becerril-Villanueva, E., Contis-Montes, D. O. A., Dominguez-Salazar, E., Salinas-Jazmin, N., Perez-Tapia, S. M., et al. (2018). The yin/yang of inflammatory status: Blood-brain barrier regulation during sleep. Brain Behav. Immun. 69, 154–166. doi:10.1016/j.bbi.2017.11.009
Hurtado-Alvarado, G., Dominguez-Salazar, E., Velazquez-Moctezuma, J., and Gomez-Gonzalez, B. (2016). A2A adenosine receptor antagonism reverts the blood-brain barrier dysfunction induced by sleep restriction. PLoS One 11 (11), e0167236. doi:10.1371/journal.pone.0167236
Hyman, M. C., Petrovic-Djergovic, D., Visovatti, S. H., Liao, H., Yanamadala, S., Bouis, D., et al. (2009). Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J. Clin. Invest. 119 (5), 1136–1149. doi:10.1172/JCI36433
Illes, P., Muller, C. E., Jacobson, K. A., Grutter, T., Nicke, A., Fountain, S. J., et al. (2021). Update of P2X receptor properties and their pharmacology: IUPHAR review 30. Br. J. Pharmacol. 178 (3), 489–514. doi:10.1111/bph.15299
Illes, P. (2020). P2X7 receptors amplify CNS damage in neurodegenerative diseases. Int. J. Mol. Sci. 21 (17), 5996. doi:10.3390/ijms21175996
Illes, P., Verkhratsky, A., Burnstock, G., and Franke, H. (2012). P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neuroscientist 18 (5), 422–438. doi:10.1177/1073858411418524
Illes, P., Xu, G. Y., and Tang, Y. (2020). Purinergic signaling in the central nervous system in health and disease. Neurosci. Bull. 36 (11), 1239–1241. doi:10.1007/s12264-020-00602-7
Jacobson, K. A., Delicado, E. G., Gachet, C., Kennedy, C., von Kugelgen, I., Li, B., et al. (2020). Update of P2Y receptor pharmacology: IUPHAR review 27. Br. J. Pharmacol. 177 (11), 2413–2433. doi:10.1111/bph.15005
Jacobson, K. A., Gao, Z. G., Matricon, P., Eddy, M. T., and Carlsson, J. (2022). Adenosine A(2A) receptor antagonists: From caffeine to selective non-xanthines. Br. J. Pharmacol. 179 (14), 3496–3511. doi:10.1111/bph.15103
Janigro, D., West, G. A., Nguyen, T. S., and Winn, H. R. (1994). Regulation of blood-brain barrier endothelial cells by nitric oxide. Circ. Res. 75 (3), 528–538. doi:10.1161/01.res.75.3.528
Janssens, R., Struyf, S., and Proost, P. (2018). The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 15 (4), 299–311. doi:10.1038/cmi.2017.107
Jiang, T., Hoekstra, J., Heng, X., Kang, W., Ding, J., Liu, J., et al. (2015). P2X7 receptor is critical in alpha-synuclein--mediated microglial NADPH oxidase activation. Neurobiol. Aging. 36 (7), 2304–2318. doi:10.1016/j.neurobiolaging.2015.03.015
Kadry, H., Noorani, B., and Cucullo, L. (2020). A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17 (1), 69. doi:10.1186/s12987-020-00230-3
Kalaria, R. N., and Harik, S. I. (1988). Adenosine receptors and the nucleoside transporter in human brain vasculature. J. Cereb. Blood Flow. Metab. 8 (1), 32–39. doi:10.1038/jcbfm.1988.5
Kim, D. G., and Bynoe, M. S. (2015). A2A adenosine receptor regulates the human blood-brain barrier permeability. Mol. Neurobiol. 52 (1), 664–678. doi:10.1007/s12035-014-8879-2
Koszalka, P., Ozuyaman, B., Huo, Y., Zernecke, A., Flogel, U., Braun, N., et al. (2004). Targeted disruption of cd73/ecto-5'-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ. Res. 95 (8), 814–821. doi:10.1161/01.RES.0000144796.82787.6f
Kovacs, Z., Kekesi, K. A., Dobolyi, A., Lakatos, R., and Juhasz, G. (2015). Absence epileptic activity changing effects of non-adenosine nucleoside inosine, guanosine and uridine in Wistar Albino Glaxo Rijswijk rats. Neuroscience 300, 593–608. doi:10.1016/j.neuroscience.2015.05.054
Langen, U. H., Ayloo, S., and Gu, C. (2019). Development and cell biology of the blood-brain barrier. Annu. Rev. Cell Dev. Biol. 35, 591–613. doi:10.1146/annurev-cellbio-100617-062608
Lanznaster, D., Mack, J. M., Coelho, V., Ganzella, M., Almeida, R. F., Dal-Cim, T., et al. (2017). Guanosine prevents anhedonic-like behavior and impairment in hippocampal glutamate transport following amyloid-β1-40 administration in mice. Mol. Neurobiol. 54 (7), 5482–5496. doi:10.1007/s12035-016-0082-1
Laurent, C., Eddarkaoui, S., Derisbourg, M., Leboucher, A., Demeyer, D., Carrier, S., et al. (2014). Beneficial effects of caffeine in a transgenic model of Alzheimer's disease-like tau pathology. Neurobiol. Aging. 35 (9), 2079–2090. doi:10.1016/j.neurobiolaging.2014.03.027
Le Dare, B., Ferron, P. J., and Gicquel, T. (2021). The purinergic P2X7 receptor-NLRP3 inflammasome pathway: A new target in alcoholic liver disease? Int. J. Mol. Sci. 22 (4), 2139. doi:10.3390/ijms22042139
Le Dare, B., Victoni, T., Bodin, A., Vlach, M., Vene, E., Loyer, P., et al. (2019). Ethanol upregulates the P2X7 purinergic receptor in human macrophages. Fundam. Clin. Pharmacol. 33 (1), 63–74. doi:10.1111/fcp.12433
Lee, N. T., Ong, L. K., Gyawali, P., Nassir, C., Mustapha, M., Nandurkar, H. H., et al. (2021). Role of purinergic signalling in endothelial dysfunction and thrombo-inflammation in ischaemic stroke and cerebral small vessel disease. Biomolecules 11 (7), 994. doi:10.3390/biom11070994
Lewitt, P. A., Aradi, S. D., Hauser, R. A., and Rascol, O. (2020). The challenge of developing adenosine A(2A) antagonists for Parkinson disease: Istradefylline, preladenant, and tozadenant. Park. Relat. Disord. 80, S54–S63. doi:10.1016/j.parkreldis.2020.10.027
Li, Q., Han, X., Lan, X., Hong, X., Li, Q., Gao, Y., et al. (2017). Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol. Dis. 108, 173–182. doi:10.1016/j.nbd.2017.08.011
Liang, W., Xu, W., Zhu, J., Zhu, Y., Gu, Q., Li, Y., et al. (2020). Ginkgo biloba extract improves brain uptake of ginsenosides by increasing blood-brain barrier permeability via activating A1 adenosine receptor signaling pathway. J. Ethnopharmacol. 246, 112243. doi:10.1016/j.jep.2019.112243
Liebner, S., Dijkhuizen, R. M., Reiss, Y., Plate, K. H., Agalliu, D., and Constantin, G. (2018). Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 135 (3), 311–336. doi:10.1007/s00401-018-1815-1
Liu, D., Zhu, M., Zhang, Y., and Diao, Y. (2021). Crossing the blood-brain barrier with AAV vectors. Metab. Brain Dis. 36 (1), 45–52. doi:10.1007/s11011-020-00630-2
Liu, Y., Alahiri, M., Ulloa, B., Xie, B., and Sadiq, S. A. (2018). Adenosine A2A receptor agonist ameliorates EAE and correlates with Th1 cytokine-induced blood brain barrier dysfunction via suppression of MLCK signaling pathway. Immun. Inflamm. Dis. 6 (1), 72–80. doi:10.1002/iid3.187
Liu, Y., Zou, H., Zhao, P., Sun, B., Wang, J., Kong, Q., et al. (2016). Activation of the adenosine A2A receptor attenuates experimental autoimmune encephalomyelitis and is associated with increased intracellular calcium levels. Neuroscience 330, 150–161. doi:10.1016/j.neuroscience.2016.05.028
Loesch, A., and Burnstock, G. (2000). Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in vascular endothelial cells in rat brain. Endothelium 7 (2), 93–98. doi:10.3109/10623320009072204
Loesch, A. (2021). On P2X receptors in the brain: Microvessels. Dedicated to the memory of the late professor Geoffrey Burnstock (1929-2020). Cell Tissue Res. 384 (3), 577–588. doi:10.1007/s00441-021-03411-0
Lou, N., Takano, T., Pei, Y., Xavier, A. L., Goldman, S. A., and Nedergaard, M. (2016). Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 113 (4), 1074–1079. doi:10.1073/pnas.1520398113
Lowery, F. J., and Yu, D. (2017). Brain metastasis: Unique challenges and open opportunities. Biochim. Biophys. Acta Rev. Cancer 1867 (1), 49–57. doi:10.1016/j.bbcan.2016.12.001
Mao, T. L., Fan, K. F., and Liu, C. L. (2017). Targeting the CXCR4/CXCL12 axis in treating epithelial ovarian cancer. Gene Ther. 24 (10), 621–629. doi:10.1038/gt.2017.69
Marchi, N., Angelov, L., Masaryk, T., Fazio, V., Granata, T., Hernandez, N., et al. (2007). Seizure-promoting effect of blood-brain barrier disruption. Epilepsia 48 (4), 732–742. doi:10.1111/j.1528-1167.2007.00988.x
Martin, E., Amar, M., Dalle, C., Youssef, I., Boucher, C., Le Duigou, C., et al. (2019). New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol. Psychiatr. 24 (1), 108–125. doi:10.1038/s41380-018-0108-3
Massari, C. M., Zuccarini, M., Di Iorio, P., and Tasca, C. I. (2021). Guanosine mechanisms of action: Toward molecular targets. Front. Pharmacol. 12, 653146. doi:10.3389/fphar.2021.653146
Matos, M., Augusto, E., Santos-Rodrigues, A. D., Schwarzschild, M. A., Chen, J. F., Cunha, R. A., et al. (2012). Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia 60 (5), 702–716. doi:10.1002/glia.22290
Medina-Flores, F., Hurtado-Alvarado, G., Contis-Montes, D. O. A., Lopez-Cervantes, S. P., Konigsberg, M., Deli, M. A., et al. (2020). Sleep loss disrupts pericyte-brain endothelial cell interactions impairing blood-brain barrier function. Brain Behav. Immun. 89, 118–132. doi:10.1016/j.bbi.2020.05.077
Mekala, N., Gheewala, N., Rom, S., Sriram, U., and Persidsky, Y. (2022). Blocking of P2X7r reduces mitochondrial stress induced by alcohol and electronic cigarette exposure in brain microvascular endothelial cells. Antioxidants (Basel) 11 (7), 1328. doi:10.3390/antiox11071328
Merighi, S., Borea, P. A., Varani, K., Vincenzi, F., Travagli, A., Nigro, M., et al. (2022). Pathophysiological role and medicinal chemistry of A2A adenosine receptor antagonists in Alzheimer's disease. Molecules 27 (9), 2680. doi:10.3390/molecules27092680
Mills, J. H., Alabanza, L., Weksler, B. B., Couraud, P. O., Romero, I. A., and Bynoe, M. S. (2011). Human brain endothelial cells are responsive to adenosine receptor activation. Purinergic Signal 7 (2), 265–273. doi:10.1007/s11302-011-9222-2
Mills, J. H., Thompson, L. F., Mueller, C., Waickman, A. T., Jalkanen, S., Niemela, J., et al. (2008). CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 105 (27), 9325–9330. doi:10.1073/pnas.0711175105
Miras-Portugal, M. T., Queipo, M. J., Gil-Redondo, J. C., Ortega, F., Gomez-Villafuertes, R., Gualix, J., et al. (2019). P2 receptor interaction and signalling cascades in neuroprotection. Brain Res. Bull. 151, 74–83. doi:10.1016/j.brainresbull.2018.12.012
Mohamed, R. A., Agha, A. M., and Nassar, N. N. (2012). SCH58261 the selective adenosine A(2A) receptor blocker modulates ischemia reperfusion injury following bilateral carotid occlusion: Role of inflammatory mediators. Neurochem. Res. 37 (3), 538–547. doi:10.1007/s11064-011-0640-x
Montilla, A., Mata, G. P., Matute, C., and Domercq, M. (2020). Contribution of P2X4 receptors to CNS function and pathophysiology. Int. J. Mol. Sci. 21 (15), 5562. doi:10.3390/ijms21155562
Mori, T., Wang, X., Aoki, T., and Lo, E. H. (2002). Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J. Neurotrauma 19 (11), 1411–1419. doi:10.1089/089771502320914642
Muhleder, S., Fuchs, C., Basilio, J., Szwarc, D., Pill, K., Labuda, K., et al. (2020). Purinergic P2Y2 receptors modulate endothelial sprouting. Cell. Mol. Life Sci. 77 (5), 885–901. doi:10.1007/s00018-019-03213-2
Muller, G. C., Loureiro, S. O., Pettenuzzo, L. F., Almeida, R. F., Ynumaru, E. Y., Guazzelli, P. A., et al. (2021). Effects of intranasal guanosine administration on brain function in a rat model of ischemic stroke. Purinergic Signal 17 (2), 255–271. doi:10.1007/s11302-021-09766-x
Muller, T. (2015). The safety of istradefylline for the treatment of Parkinson's disease. Expert Opin. Drug Saf. 14 (5), 769–775. doi:10.1517/14740338.2015.1014798
Narravula, S., Lennon, P. F., Mueller, B. U., and Colgan, S. P. (2000). Regulation of endothelial CD73 by adenosine: Paracrine pathway for enhanced endothelial barrier function. J. Immunol. 165 (9), 5262–5268. doi:10.4049/jimmunol.165.9.5262
Newby, A. C. (1985). The role of adenosine kinase in regulating adenosine concentration. Biochem. J. 226 (1), 343–344. doi:10.1042/bj2260343
Newby, A. C., Worku, Y., and Holmquist, C. A. (1985). Adenosine formation Evidence for a direct biochemical link with energy metabolism. Adv. Myocardiol. 6, 273–284.
Niemela, J., Ifergan, I., Yegutkin, G. G., Jalkanen, S., Prat, A., and Airas, L. (2008). IFN-beta regulates CD73 and adenosine expression at the blood-brain barrier. Eur. J. Immunol. 38 (10), 2718–2726. doi:10.1002/eji.200838437
Niu, J., Tsai, H. H., Hoi, K. K., Huang, N., Yu, G., Kim, K., et al. (2019). Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat. Neurosci. 22 (5), 709–718. doi:10.1038/s41593-019-0369-4
Ohsawa, K., Irino, Y., Nakamura, Y., Akazawa, C., Inoue, K., and Kohsaka, S. (2007). Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia 55 (6), 604–616. doi:10.1002/glia.20489
Oliveira-Giacomelli, A., Petiz, L. L., Andrejew, R., Turrini, N., Silva, J. B., Sack, U., et al. (2021). Role of P2X7 receptors in immune responses during neurodegeneration. Front. Cell. Neurosci. 15, 662935. doi:10.3389/fncel.2021.662935
Paniz, L. G., Calcagnotto, M. E., Pandolfo, P., Machado, D. G., Santos, G. F., Hansel, G., et al. (2014). Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metab. Brain Dis. 29 (3), 645–654. doi:10.1007/s11011-014-9548-x
Patel, V., Brown, C., Goodwin, A., Wilkie, N., and Boarder, M. R. (1996). Phosphorylation and activation of p42 and p44 mitogen-activated protein kinase are required for the P2 purinoceptor stimulation of endothelial prostacyclin production. Biochem. J. 320, 221–226. doi:10.1042/bj3200221
Peakman, M. C., and Hill, S. J. (1994). Adenosine A2B-receptor-mediated cyclic AMP accumulation in primary rat astrocytes. Br. J. Pharmacol. 111 (1), 191–198. doi:10.1111/j.1476-5381.1994.tb14043.x
Perez-Hernandez, M., Fernandez-Valle, M. E., Rubio-Araiz, A., Vidal, R., Gutierrez-Lopez, M. D., O'Shea, E., et al. (2017). 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) produces edema due to BBB disruption induced by MMP-9 activation in rat hippocampus. Neuropharmacology 118, 157–166. doi:10.1016/j.neuropharm.2017.03.019
Perez-Sen, R., Queipo, M. J., Morente, V., Ortega, F., Delicado, E. G., and Miras-Portugal, M. T. (2015). Neuroprotection mediated by P2Y13 nucleotide receptors in neurons. Comput. Struct. Biotechnol. J. 13, 160–168. doi:10.1016/j.csbj.2015.02.002
Pienaar, I. S., Lee, C. H., Elson, J. L., Mcguinness, L., Gentleman, S. M., Kalaria, R. N., et al. (2015). Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson's disease. Neurobiol. Dis. 74, 392–405. doi:10.1016/j.nbd.2014.12.006
Pourcher, E., and Huot, P. (2015). Adenosine 2A receptor antagonists for the treatment of motor symptoms in Parkinson's disease. Mov. Disord. Clin. Pract. 2 (4), 331–340. doi:10.1002/mdc3.12187
Prados, M. D., Schold, S. J., Fine, H. A., Jaeckle, K., Hochberg, F., Mechtler, L., et al. (2003). A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 5 (2), 96–103. doi:10.1093/neuonc/5.2.96
Profaci, C. P., Munji, R. N., Pulido, R. S., and Daneman, R. (2020). The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 217 (4), e20190062. doi:10.1084/jem.20190062
Raghavan, S., Brishti, M. A., Collier, D. M., and Leo, M. D. (2022). Hypoxia induces purinergic receptor signaling to disrupt endothelial barrier function. Front. Physiology 13, 1049698. doi:10.3389/fphys.2022.1049698
Rajadhyaksha, M., Boyden, T., Liras, J., El-Kattan, A., and Brodfuehrer, J. (2011). Current advances in delivery of biotherapeutics across the blood-brain barrier. Curr. Drug Discov. Technol. 8 (2), 87–101. doi:10.2174/157016311795563866
Ramirez, A. N., and Kunze, D. L. (2002). P2X purinergic receptor channel expression and function in bovine aortic endothelium. Am. J. Physiol. Heart Circ. Physiol. 282 (6), H2106–H2116. doi:10.1152/ajpheart.00892.2001
Ramos, C. J., and Antonetti, D. A. (2017). The role of small GTPases and EPAC-Rap signaling in the regulation of the blood-brain and blood-retinal barriers. Tissue Barriers 5 (3), e1339768. doi:10.1080/21688370.2017.1339768
Ransohoff, R. M., and Cardona, A. E. (2010). The myeloid cells of the central nervous system parenchyma. Nature 468 (7321), 253–262. doi:10.1038/nature09615
Rathbone, M., Pilutti, L., Caciagli, F., and Jiang, S. (2008). Neurotrophic effects of extracellular guanosine. Nucleosides Nucleotides Nucleic Acids 27 (6), 666–672. doi:10.1080/15257770802143913
Renk, D. R., Skraban, M., Bier, D., Schulze, A., Wabbals, E., Wedekind, F., et al. (2021). Design, synthesis and biological evaluation of Tozadenant analogues as adenosine A(2A) receptor ligands. Eur. J. Med. Chem. 214, 113214. doi:10.1016/j.ejmech.2021.113214
Rissanen, E., Virta, J. R., Paavilainen, T., Tuisku, J., Helin, S., Luoto, P., et al. (2013). Adenosine A2A receptors in secondary progressive multiple sclerosis: A [(11)C]TMSX brain PET study. J. Cereb. Blood Flow. Metab. 33 (9), 1394–1401. doi:10.1038/jcbfm.2013.85
Rubio-Araiz, A., Perez-Hernandez, M., Urrutia, A., Porcu, F., Borcel, E., Gutierrez-Lopez, M. D., et al. (2014). 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) disrupts blood-brain barrier integrity through a mechanism involving P2X7 receptors. Int. J. Neuropsychopharmacol. 17 (8), 1243–1255. doi:10.1017/S1461145714000145
Sasaki, Y., Hoshi, M., Akazawa, C., Nakamura, Y., Tsuzuki, H., Inoue, K., et al. (2003). Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 44 (3), 242–250. doi:10.1002/glia.10293
Schmidt, A. P., Lara, D. R., and Souza, D. O. (2007). Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol. Ther. 116 (3), 401–416. doi:10.1016/j.pharmthera.2007.07.004
Schmidt, A. P., Tort, A. B., Souza, D. O., and Lara, D. R. (2008). Guanosine and its modulatory effects on the glutamatergic system. Eur. Neuropsychopharmacol. 18 (8), 620–622. doi:10.1016/j.euroneuro.2008.01.007
Selmi, C., Barin, J. G., and Rose, N. R. (2016). Current trends in autoimmunity and the nervous system. J. Autoimmun. 75, 20–29. doi:10.1016/j.jaut.2016.08.005
Shang, P., Baker, M., Banks, S., Hong, S. I., and Choi, D. S. (2021). Emerging nondopaminergic medications for Parkinson's disease: Focusing on A2A receptor antagonists and GLP1 receptor agonists. J. Mov. Disord. 14 (3), 193–203. doi:10.14802/jmd.21035
Shieh, C. C., Jarvis, M. F., Lee, C. H., and Perner, R. J. (2006). P2X receptor ligands and pain. Expert Opin. Ther. Pat. 16 (8), 1113–1127. doi:10.1517/13543776.16.8.1113
Singh, M., Venugopal, C., Tokar, T., Mcfarlane, N., Subapanditha, M. K., Qazi, M., et al. (2018). Therapeutic targeting of the premetastatic stage in human lung-to-brain metastasis. Cancer Res. 78 (17), 5124–5134. doi:10.1158/0008-5472.CAN-18-1022
Sipe, G. O., Lowery, R. L., Tremblay, M. E., Kelly, E. A., Lamantia, C. E., and Majewska, A. K. (2016). Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 7, 10905. doi:10.1038/ncomms10905
Srivastava, P., Cronin, C. G., Scranton, V. L., Jacobson, K. A., Liang, B. T., and Verma, R. (2020). Neuroprotective and neuro-rehabilitative effects of acute purinergic receptor P2X4 (P2X4R) blockade after ischemic stroke. Exp. Neurol. 329, 113308. doi:10.1016/j.expneurol.2020.113308
Stokes, L., Layhadi, J. A., Bibic, L., Dhuna, K., and Fountain, S. J. (2017). P2X4 receptor function in the nervous system and current breakthroughs in pharmacology. Front. Pharmacol. 8, 291. doi:10.3389/fphar.2017.00291
Su, C., Elfeki, N., Ballerini, P., D'Alimonte, I., Bau, C., Ciccarelli, R., et al. (2009). Guanosine improves motor behavior, reduces apoptosis, and stimulates neurogenesis in rats with parkinsonism. J. Neurosci. Res. 87 (3), 617–625. doi:10.1002/jnr.21883
Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14 (3), 133–150. doi:10.1038/nrneurol.2017.188
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2019). Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 99 (1), 21–78. doi:10.1152/physrev.00050.2017
Takenouchi, T., Sekiyama, K., Sekigawa, A., Fujita, M., Waragai, M., Sugama, S., et al. (2010). P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. . 58 (2), 91–96. doi:10.1007/s00005-010-0069-y
Takenouchi, T., Sugama, S., Iwamaru, Y., Hashimoto, M., and Kitani, H. (2009). Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells. Crit. Rev. Immunol. 29 (4), 335–345. doi:10.1615/critrevimmunol.v29.i4.40
Terstappen, G. C., Meyer, A. H., Bell, R. D., and Zhang, W. (2021). Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 20 (5), 362–383. doi:10.1038/s41573-021-00139-y
Torti, M., Vacca, L., and Stocchi, F. (2018). Istradefylline for the treatment of Parkinson's disease: Is it a promising strategy? Expert Opin. Pharmacother. 19 (16), 1821–1828. doi:10.1080/14656566.2018.1524876
Toth, P., Tarantini, S., Davila, A., Valcarcel-Ares, M. N., Tucsek, Z., Varamini, B., et al. (2015). Purinergic glio-endothelial coupling during neuronal activity: Role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 309 (11), H1837–H1845. doi:10.1152/ajpheart.00463.2015
Toulorge, D., Schapira, A. H., and Hajj, R. (2016). Molecular changes in the postmortem parkinsonian brain. J. Neurochem. 139, 27–58. doi:10.1111/jnc.13696
Tozaki-Saitoh, H., Tsuda, M., Miyata, H., Ueda, K., Kohsaka, S., and Inoue, K. (2008). P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J. Neurosci. 28 (19), 4949–4956. doi:10.1523/JNEUROSCI.0323-08.2008
van Vliet, E. A., Ndode-Ekane, X. E., Lehto, L. J., Gorter, J. A., Andrade, P., Aronica, E., et al. (2020). Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol. Dis. 145, 105080. doi:10.1016/j.nbd.2020.105080
van Waarde, A., Dierckx, R., Zhou, X., Khanapur, S., Tsukada, H., Ishiwata, K., et al. (2018). Potential therapeutic applications of adenosine A2A receptor ligands and opportunities for A2A receptor imaging. Med. Res. Rev. 38 (1), 5–56. doi:10.1002/med.21432
Vazquez-Villoldo, N., Domercq, M., Martin, A., Llop, J., Gomez-Vallejo, V., and Matute, C. (2014). P2X4 receptors control the fate and survival of activated microglia. Glia 62 (2), 171–184. doi:10.1002/glia.22596
Verma, R., Cronin, C. G., Hudobenko, J., Venna, V. R., Mccullough, L. D., and Liang, B. T. (2017). Deletion of the P2X4 receptor is neuroprotective acutely, but induces a depressive phenotype during recovery from ischemic stroke. Brain Behav. Immun. 66, 302–312. doi:10.1016/j.bbi.2017.07.155
von Kugelgen, I. (2021). Molecular pharmacology of P2Y receptor subtypes. Biochem. Pharmacol. 187, 114361. doi:10.1016/j.bcp.2020.114361
Wang, H., Hong, L. J., Huang, J. Y., Jiang, Q., Tao, R. R., Tan, C., et al. (2015). P2RX7 sensitizes Mac-1/ICAM-1-dependent leukocyte-endothelial adhesion and promotes neurovascular injury during septic encephalopathy. Cell Res. 25 (6), 674–690. doi:10.1038/cr.2015.61
Wang, K., Sun, M., Juan, Z., Zhang, J., Sun, Y., Wang, G., et al. (2022). The improvement of sepsis-associated encephalopathy by P2X7R inhibitor through inhibiting the omi/HtrA2 apoptotic signaling pathway. Behav. Neurol. 2022, 3777351. doi:10.1155/2022/3777351
Wang, T. F., and Guidotti, G. (1998). Widespread expression of ecto-apyrase (CD39) in the central nervous system. Brain Res. 790 (1-2), 318–322. doi:10.1016/s0006-8993(97)01562-x
Wei, L., Syed, M. S., Yan, J., Zhang, L., Wang, L., Yin, Y., et al. (2018). ATP-activated P2X7 receptor in the pathophysiology of mood disorders and as an emerging target for the development of novel antidepressant therapeutics. Neurosci. Biobehav Rev. 87, 192–205. doi:10.1016/j.neubiorev.2018.02.005
Weisman, G. A., Camden, J. M., Peterson, T. S., Ajit, D., Woods, L. T., and Erb, L. (2012). P2 receptors for extracellular nucleotides in the central nervous system: Role of P2X7 and P2Y- receptor interactions in neuroinflammation. Mol. Neurobiol. 46 (1), 96–113. doi:10.1007/s12035-012-8263-z
Wilson, H. L., Varcoe, R. W., Stokes, L., Holland, K. L., Francis, S. E., Dower, S. K., et al. (2007). P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Br. J. Pharmacol. 151 (1), 115–127. doi:10.1038/sj.bjp.0707213
Wittendorp, M. C., Boddeke, H. W., and Biber, K. (2004). Adenosine A3 receptor-induced CCL2 synthesis in cultured mouse astrocytes. Glia 46 (4), 410–418. doi:10.1002/glia.20016
Wu, S. T., Han, J. R., Yao, N., Li, Y. L., Zhang, F., Shi, Y., et al. (2022). Activation of P2X4 receptor exacerbates acute brain injury after intracerebral hemorrhage. CNS Neurosci. Ther. 28 (7), 1008–1018. doi:10.1111/cns.13831
Yamamoto, M., Guo, D. H., Hernandez, C. M., and Stranahan, A. M. (2019). Endothelial Adora2a activation promotes blood-brain barrier breakdown and cognitive impairment in mice with diet-induced insulin resistance. J. Neurosci. 39 (21), 4179–4192. doi:10.1523/JNEUROSCI.2506-18.2019
Yang, F., Zhao, K., Zhang, X., Zhang, J., and Xu, B. (2016). ATP induces disruption of tight junction proteins via IL-1 beta-dependent MMP-9 activation of human blood-brain barrier in vitro. Neural Plast. 2016, 8928530. doi:10.1155/2016/8928530
Yang, Y., Kimura-Ohba, S., Thompson, J. F., Salayandia, V. M., Cosse, M., Raz, L., et al. (2018). Vascular tight junction disruption and angiogenesis in spontaneously hypertensive rat with neuroinflammatory white matter injury. Neurobiol. Dis. 114, 95–110. doi:10.1016/j.nbd.2018.02.012
Yegutkin, G. G. (2008). Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783 (5), 673–694. doi:10.1016/j.bbamcr.2008.01.024
Yu, F., Zhu, C., Xie, Q., and Wang, Y. (2020). Adenosine A(2A) receptor antagonists for cancer immunotherapy. J. Med. Chem. 63 (21), 12196–12212. doi:10.1021/acs.jmedchem.0c00237
Zerr, M., Hechler, B., Freund, M., Magnenat, S., Lanois, I., Cazenave, J. P., et al. (2011). Major contribution of the P2Y₁receptor in purinergic regulation of TNFα-induced vascular inflammation. Circulation 123 (21), 2404–2413. doi:10.1161/CIRCULATIONAHA.110.002139
Zhang, Y., Zhu, W., Yu, H., Yu, J., Zhang, M., Pan, X., et al. (2019). P2Y4/TSP-1/TGF-β1/pSmad2/3 pathway contributes to acute generalized seizures induced by kainic acid. Brain Res. Bull. 149, 106–119. doi:10.1016/j.brainresbull.2019.04.004
Zhao, H., Zhang, X., Dai, Z., Feng, Y., Li, Q., Zhang, J. H., et al. (2016). P2X7 receptor suppression preserves blood-brain barrier through inhibiting RhoA activation after experimental intracerebral hemorrhage in rats. Sci. Rep. 6, 23286. doi:10.1038/srep23286
Zhao, M., Jiang, X. F., Zhang, H. Q., Sun, J. H., Pei, H., Ma, L. N., et al. (2021). Interactions between glial cells and the blood-brain barrier and their role in Alzheimer's disease. Ageing Res. Rev. 72, 101483. doi:10.1016/j.arr.2021.101483
Zhao, Z., Nelson, A. R., Betsholtz, C., and Zlokovic, B. V. (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163 (5), 1064–1078. doi:10.1016/j.cell.2015.10.067
Zhao, Z., Shang, X., Chen, Y., Zheng, Y., Huang, W., Jiang, H., et al. (2020). Bacteria elevate extracellular adenosine to exploit host signaling for blood-brain barrier disruption. Virulence 11 (1), 980–994. doi:10.1080/21505594.2020.1797352
Zhou, Y., Peng, Z., Seven, E. S., and Leblanc, R. M. (2018). Crossing the blood-brain barrier with nanoparticles. J. Control. Release. 270, 290–303. doi:10.1016/j.jconrel.2017.12.015
Zhou, Y., Wang, Y., Wang, J., Anne, S. R., and Yang, Q. W. (2014). Inflammation in intracerebral hemorrhage: From mechanisms to clinical translation. Prog. Neurobiol. 115, 25–44. doi:10.1016/j.pneurobio.2013.11.003
Keywords: purinergic signaling, blood-brain barrier, endothelial cells, P2Y receptors, P2X receptors, P1 receptors, CD39, CD73
Citation: Wang Y, Zhu Y, Wang J, Dong L, Liu S, Li S and Wu Q (2023) Purinergic signaling: A gatekeeper of blood-brain barrier permeation. Front. Pharmacol. 14:1112758. doi: 10.3389/fphar.2023.1112758
Received: 30 November 2022; Accepted: 27 January 2023;
Published: 07 February 2023.
Edited by:
Henning Ulrich, University of São Paulo, BrazilReviewed by:
Mariachiara Zuccarini, University of Studies G. d'Annunzio Chieti and Pescara, ItalyIvana Grković, University of Belgrade, Serbia
Copyright © 2023 Wang, Zhu, Wang, Dong, Liu, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiaofeng Wu, d3VxaWFvZmVuZ0BjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Yuemei Wang†
Yuemei Wang† Junmeng Wang
Junmeng Wang Qiaofeng Wu
Qiaofeng Wu