- 1Department of Pediatric Gastroenterology, Hepatology and Nutrition, Beatrix Children’s Hospital, University of Groningen, University Medical Centre Groningen, Groningen, Netherlands
- 2Department of Clinical Pharmacy and Pharmacology, University Medical Centre Groningen, Groningen, Netherlands
Background: Ustekinumab is used off-label in pediatric Crohn’s disease refractory to anti-tumor necrosis factor. Data on optimal dosing, target trough levels, and potential benefit of therapeutic drug monitoring in children treated with ustekinumab are limited.
Materials and Methods: We describe a series of six adolescents who consented to be treated with ustekinumab. We measured their trough levels, C-reactive protein, and fecal calprotectin before every administration.
Results: Standard adult dosing was effective to achieve biochemical remission (fecal calprotectin < 250 mg/kg) in one patient and clinical remission (resolution of symptoms) in another. The other four patients failed to respond on standard dosing and underwent intravenous re-induction and interval shortening to increase ustekinumab trough levels. This resulted in biochemical remission in one patient and clinical remission in another, suggesting an exposure–response relationship. The remaining two patients had no therapeutic benefit, and ustekinumab was discontinued.
Conclusion: In this report, we show that ustekinumab can induce remission in pediatric patients with anti-tumor necrosis factor refractory Crohn’s disease. It is worth escalating the dose before abandoning the drug as ineffective. Prospective studies in children are needed to determine long-term efficacy of ustekinumab, usefulness of therapeutic drug monitoring strategies, and, if applicable, optimal target trough levels.
1 Introduction
The incidence of pediatric Crohn’s disease (CD) has increased rapidly over the past decades (Kuenzig et al., 2022). During this same period, anti-tumor necrosis factor (TNF) has become a treatment option which has positively altered the natural disease course of CD. While the cumulative five-year exposure rate to anti-TNF has increased to more than 50%, fewer patients now develop stricturing complications or require ileal resections (L et al., 2022). Despite these recent advances, therapeutic challenges arise in children refractory or intolerant to anti-TNF therapy.
According to the European guideline on the medical management of pediatric CD, ustekinumab can be considered in patients who fail to achieve or maintain clinical remission on adequately dosed anti-TNF agents (infliximab or adalimumab) in combination with immunomodulator use (van Rheenen et al., 2020). Ustekinumab is a fully human monoclonal antibody that targets the p40 protein subunit of interleukin-12 and -23 (European Medicines Agency, 2022). In a systematic review of 63 real-world studies in mostly adult refractory CD patients, ustekinumab was found to be safe and effective. Response was achieved in 64% and remission in 45% after ∼1 year (Rubín de Célix et al., 2022). Ustekinumab is not authorized for the treatment of CD in children (European Medicines Agency, 2022). It is, therefore, prescribed off-label, and data on optimal dosing, target drug levels, and attainment of adequate trough levels in children are limited (Carman et al., 2018).
Therapeutic drug monitoring (TDM) of monoclonal antibodies is based on observations that higher trough drug concentrations are associated with higher efficacy and that loss of response is primarily attributed to either low drug levels or to the development of anti-drug antibodies (i.e., immunogenicity). TDM has been proven to increase the efficacy and decrease the toxicity of anti-TNF therapy (Kapoor and Crowley, 2021). Studies on adults suggest that ustekinumab may be suitable for TDM, based on an extensive inter- and intraindividual variability in pharmacokinetics and the presence of an exposure–response relationship (Vande Casteele et al., 2017; Adedokun et al., 2018; Gómez Espín et al., 2021; Alsoud et al., 2022; Liefferinckx et al., 2022; Proietti et al., 2022). Development of anti-ustekinumab antibodies is uncommon (Bots et al., 2021).
There is paucity of information on the relationship between drug exposure levels and response in children. We here report on our experiences with off-label use of ustekinumab in six pediatric CD patients refractory to anti-TNF.
2 Materials and methods
This is a prospective case series in which patient care and data collection were planned ahead of time. In accordance with the European guideline, children with CD who were refractory to anti-TNF therapy were treated with a single intravenous dose of ustekinumab (6 mg/kg rounded to 130 mg, maximum 520 mg), followed by a subcutaneous injection of 90 mg every 8 weeks at our out-patient department (van Rheenen et al., 2020). 30 min before each ustekinumab administration, ustekinumab trough concentration and C-reactive protein (CRP) were measured. Ustekinumab trough concentrations were measured in serum with the use of an enzyme-linked immunosorbent assay (ELISA) by Sanquin Diagnostic Services (Amsterdam, Netherlands), and the detection range was 0.005–20 μg/mL (Menting et al., 2015). Fecal calprotectin was measured every 2–3 months or when a patient developed symptoms of a flare. Baseline characteristics, including disease and treatment history, fecal calprotectin, and CRP before ustekinumab initiation, were extracted from the patients’ medical records. Clinical remission was defined as a complete resolution of symptoms. Biochemical remission was defined as a fecal calprotectin below 250 mg/kg (van Rheenen et al., 2020). Patients who turned 18 continued their treatment at the adult gastroenterology department where routine trough level measurements were not performed.
This project concerned the evaluation of existing healthcare. When participating in a healthcare evaluation, patients were exposed to neither additional research procedures nor additional risks. Written informed consent was obtained for the off-label use of ustekinumab and for the publication of any potentially identifiable data included in this article.
3 Results
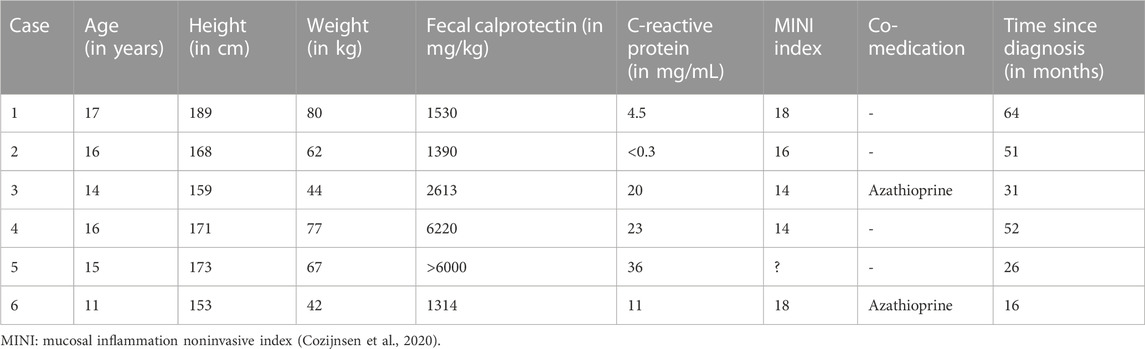
A total of six patients, five of which were male, were initiated on ustekinumab between November 2019 and June 2022 at a median age of 15.5 years (range 11–17). The median fecal calprotectin at ustekinumab initiation was 2072 mg/kg (range 1314–6000). Median follow-up after ustekinumab initiation was 29 months (range 4–35). None of the patients underwent endoscopy during follow-up on ustekinumab. No side effects of ustekinumab were observed.
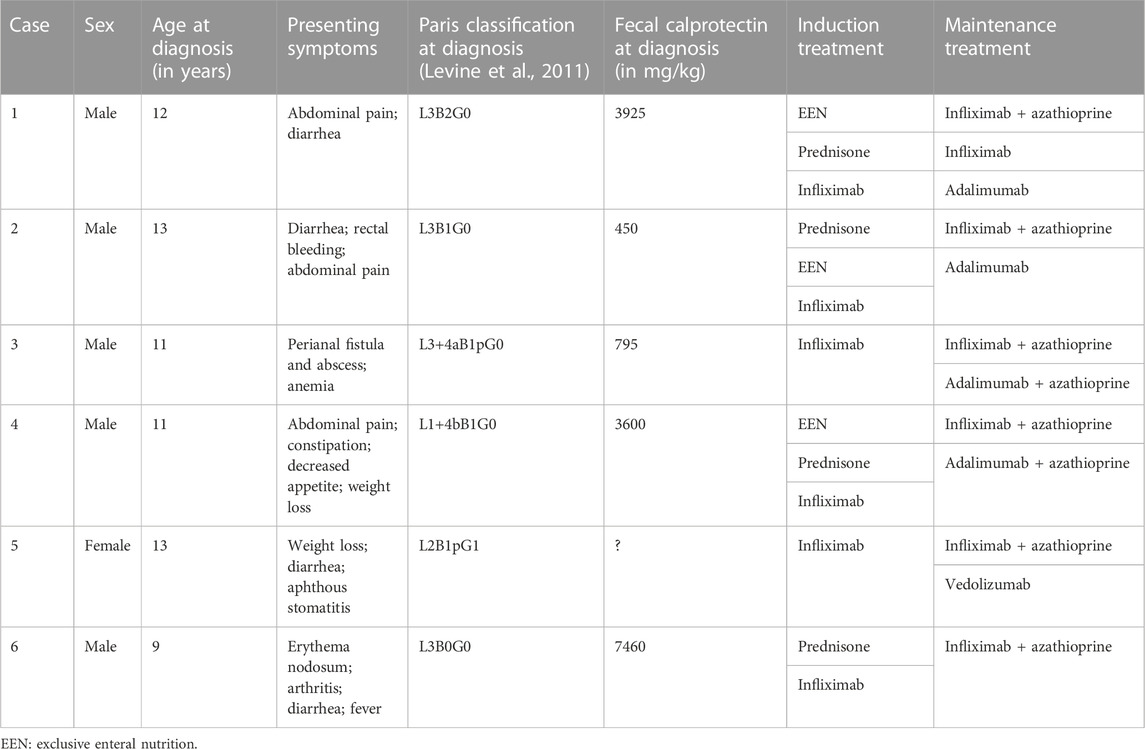
A summary of the clinical information before and at initiation of ustekinumab is presented in Table 1 and Table 2. Figure 1 shows ustekinumab trough levels, fecal calprotectin, and CRP plotted over time. An overview of all ustekinumab administrations, dosing, and measurements is provided in the Supplementary Material. A detailed description of the cases is provided in the following.
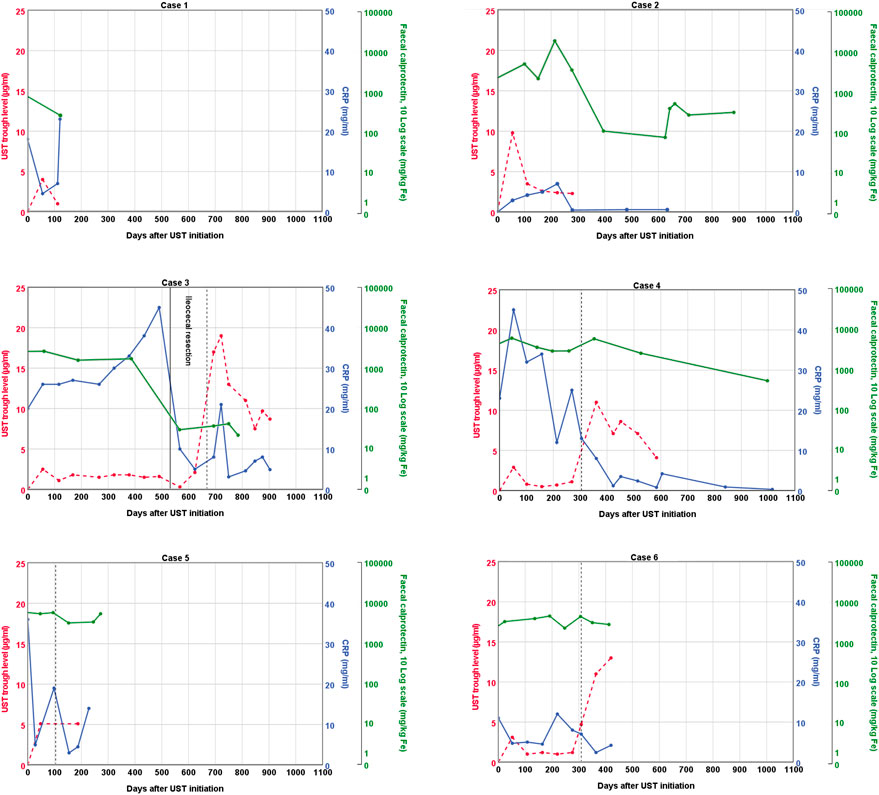
FIGURE 1. Graphical representation of ustekinumab trough levels, fecal calprotectin, and C-reactive protein over time. The vertical dashed line represents a second intravenous loading dose of ustekinumab followed by a shortened subcutaneous ustekinumab interval of 4 weeks. UST: ustekinumab; CRP; C-reactive protein.
3.1 Case 1
Case 1 presented at age 12 with abdominal pain and diarrhea. At endoscopy, inflammation of the terminal ileum and the right-sided colon was seen. Initial induction treatment included EEN and azathioprine maintenance (2 mg/kg). Because of lack of response to EEN, a corticosteroid-tapering scheme was followed. Despite an initial clinical improvement, symptoms quickly reappeared after steroid withdrawal. A third induction treatment, this time with infliximab (5 mg/kg at week 0, 2, and 6 followed by every 8 weeks) was started and azathioprine was continued. After the third infusion, a symptomatic stenosis of the terminal ileum was found, which necessitated ileocecal resection. Infliximab monotherapy was given postoperatively to reduce the risk of recurrent Crohn’s inflammation of the anastomosis. Azathioprine was discontinued after shared decision making. Two years later, low titer anti-infliximab antibodies were detected. Infliximab treatment escalation made the antibodies disappear. Unfortunately, after another year, an anaphylactic reaction occurred during infliximab administration and treatment was switched to adalimumab (80 mg, followed by 40 mg every 2 weeks). Because of persistently subtherapeutic trough levels (2.4–3 μg/mL), the adalimumab dose was increased (80 mg every 2 weeks). This resulted in adequate trough levels (7.5 μg/mL) and biochemical remission. One year after the initiation of adalimumab, the patient developed immunogenic loss of response due to the formation of anti-adalimumab antibodies. Ileocolonoscopy revealed active disease in the neo-terminal ileum and ascending colon. Adalimumab was discontinued, and ustekinumab was initiated. An intravenous loading dose of 390 mg was administered, followed by 90 mg subcutaneously every 8 weeks. Currently, 5 months after the initiation of ustekinumab, the patient is in clinical and biochemical remission, with an ustekinumab trough level of 1 μg/mL.
3.2 Case 2
Case 2 presented at age 12 with diarrhea, rectal bleeding, and abdominal pain. At endoscopy, Crohn’s colitis and inflammation of the ileocecal valve were seen. Initial induction treatment included a corticosteroid-tapering regimen and azathioprine (2 mg/kg). After an initial clinical improvement, rectal bleeding reappeared as soon as steroids were withdrawn. A second induction treatment, this time with exclusive enteral nutrition (EEN), was also unsuccessful, and a step-up to infliximab was made (5 mg/kg at week 0, 2 and 6, followed by maintenance every 8 weeks). The patient reached biochemical remission before the fourth infusion and maintained remission until 1.5 years later. Despite dose and interval escalation (to 10 mg/kg every 6 weeks, with a trough level of 4.9 μg/m), rectal bleeding occurred and fecal calprotectin was >5000 mg/kg. Infliximab was switched to adalimumab (80 mg followed by 40 mg every 2 weeks), azathioprine was stopped, and remission was re-achieved. Eighteen months later, loss of response was observed. Dose escalation (to 80 mg) and interval shortening (to weekly dosing) did not yield any clinical benefit anymore. An intravenous loading dose of ustekinumab of 390 mg was administered, followed by 90 mg subcutaneously every 8 weeks. Ustekinumab trough levels in the maintenance phase were stable around 2.5 μg/mL. Symptoms of distal inflammation (feeling of urgency and worries of incontinence) improved after a concurrent course of local budesonide. Clinical remission was reached 5 months after the initiation of ustekinumab, but fecal calprotectin levels remain mildly elevated.
3.3 Case 3
Case 3 presented at age 11 with perianal disease (fistula and abscess) and anemia. At endoscopy, terminal ileitis and aphthous lesions of the stomach and duodenum were seen in addition to perianal disease. He was treated with upfront high-dose infliximab (10 mg/kg at week 0, 2, and 6), followed by infliximab maintenance (5 mg/kg) every 8 weeks and azathioprine (2 mg/kg). Clinical remission was achieved, but fecal calprotectin, erythrocyte sedimentation rate, and CRP remained elevated. After 1.5 years of infliximab and azathioprine combination therapy, both luminal and perianal disease recurred. A within-class switch to adalimumab was to no avail, despite high adalimumab trough levels, and azathioprine co-medication continued. Fecal calprotectin was 2613 mg/kg when ustekinumab was introduced. An intravenous loading dose of 260 mg was administered, followed by 90 mg subcutaneously every 8 weeks. Almost 1.5 years after ustekinumab was initiated, the terminal ileum was still severely inflamed and stenotic, and a new perianal abscess had formed. Ustekinumab trough levels at that time were between 1.5 and 1.8 μg/mL. Ileocecal resection with primary ileocolic anastomosis was complicated by an anastomotic leak, which resulted in a temporary diverting ileostomy. Ustekinumab and methotrexate were continued after surgery. Four months later, a small bowel obstruction was observed, consistent with active inflammation in the loop in front of ileostomy. A second intravenous ustekinumab loading dose was administered, followed by subcutaneous ustekinumab (90 mg) every 4 weeks. Ustekinumab trough levels increased dramatically (between 7.5 and 19 μg/mL). Currently, fecal calprotectin is normal, perianal fistulae have closed, and the ileostomy has successfully been reversed.
3.4 Case 4
Case 4 presented at age 11 with abdominal pain, constipation, decreased appetite, weight loss, and increased fecal calprotectin. At endoscopy, Crohn’s inflammation was seen in the stomach and terminal ileum. Initial induction treatment included EEN and azathioprine maintenance (2.5 mg/kg), but remission was not achieved. A second induction treatment with a corticosteroid-tapering scheme was also unsuccessful. A third induction treatment, this time with infliximab (5 mg/kg at week 0, 2, and 6 followed by every 8 weeks) resulted in complete resolution of symptoms, but fecal calprotectin levels remained high. Ileocolonoscopy confirmed persistent active inflammation in the terminal ileum. Infliximab trough levels were low (<1 μg/mL) and remained low despite dose increase (to 10 mg/kg). A switch to adalimumab did not change the situation. Terminal ileitis progressed to a significant stenosis, which necessitated an ileocecal resection. Postoperative treatment consisted of adalimumab (80 mg every 2 weeks) and azathioprine. Eight months after surgery, mild stenotic symptoms reoccurred. Adalimumab and azathioprine were discontinued, and an ustekinumab intravenous loading dose of 390 mg was administered, followed by 90 mg subcutaneously every 8 weeks. Trough levels varied between 0.5 and 1.1 μg/mL in the maintenance phase. Ten months after the first administration of ustekinumab, clinical remission was achieved, but fecal calprotectin levels remained high. After a second intravenous loading dose of ustekinumab, followed by subcutaneous ustekinumab (90 mg) every 4 weeks, ustekinumab trough levels were between 4.1 and 8.6 μg/mL. Currently, fecal calprotectin levels still remain high, but the patient refuses further colonoscopic evaluation and treatment adjustments because of the absence of symptoms.
3.5 Case 5
Case 5 presented at age 13 with weight loss, diarrhea, and aphthous stomatitis. At colonoscopy, colonic inflammation and perianal fistulae were seen. Because of perianal involvement, a starting dose of 10 mg/kg infliximab was administered. At week 2 and 6, a dose of 5 mg/kg was used, followed by a maintenance dose of 5 mg/kg every 8 weeks. Because of primary non-response, empiric dose intensification and interval shortening were employed, but remission was not achieved. Infliximab and azathioprine co-medication were discontinued, and vedolizumab was initiated. Multiple hospitalizations were required to manage disease flares with corticosteroids or exclusive enteral nutrition. High fecal calprotectin levels persisted, and endoscopically confirmed panenteric ulcerations necessitated a switch to ustekinumab. An intravenous loading dose of 390 mg was administered, followed by 90 mg subcutaneously every 8 weeks. Sixteen weeks after the loading dose, neither clinical nor biochemical response was observed. The ustekinumab trough level at that time was 5.1 μg/ml. A second intravenous ustekinumab loading dose was administered, followed by 90 mg subcutaneously every 4 weeks. Despite treatment intensification, ustekinumab trough levels did not increase, fecal calprotectin remained high, and perianal disease persisted. Ustekinumab was discontinued because of primary non-response, and adalimumab (160 mg followed by 80 mg every 2 weeks) and azathioprine (1.5 mg/kg) were initiated. Currently, 4 months after the initiation of adalimumab, remission has still not been achieved.
3.6 Case 6
Case 6 presented at age 9 with erythema nodosum, arthritis of the ankle, diarrhea, and fever. At endoscopy, extensive inflammation primarily affecting the terminal ileum and cecum was seen. Initial induction treatment included a corticosteroid tapering regimen and azathioprine (2.3 mg/kg). After an initial clinical improvement, symptoms reoccurred during steroid tapering. A second induction treatment, this time with infliximab (5 mg/kg at week 0, 2, and 6 followed by every 8 weeks), was started. Remission was not achieved despite optimizing infliximab trough levels. Infliximab was discontinued, and ustekinumab was initiated. An intravenous loading dose of 260 mg was administered, followed by 90 mg subcutaneously every 8 weeks. Azathioprine was continued. After five subcutaneous administrations of ustekinumab and trough levels between 1.0 and 1.5 μg/mL, a second intravenous loading dose was administered, followed by subcutaneous injections every 4 weeks. This resulted in a dramatic rise in trough levels (11–13 μg/mL). Nevertheless, colonic inflammation persisted which resulted in an endoscopically impassable stenosis of the ascending colon. Ustekinumab was discontinued, and ileocolic resection was performed. Postoperative treatment consisted of adalimumab (80 mg, followed by 40 mg every 2 weeks) and azathioprine (2 mg/kg) to reduce the risk of postoperative recurrence. Moreover, all fecal calprotectin levels were in the target range (<250 mg/kg).
4 Discussion
We presented six pediatric cases with luminal CD who were refractory to at least one anti-TNF agent. Loss of response to anti-TNF occurs commonly. Four out of the six cases (Carman et al., 2018; van Rheenen et al., 2020; European Medicines Agency, 2022; Kuenzig et al., 2022) developed stricturing complications at a relative young age and required bowel resection. Case 3 and 5 had additional perianal involvement. All six cases represent a CD clinical phenotype with a less favorable prognosis. In patients with this CD phenotype, the traditional step-wise or “pyramidal” approach almost inevitably leads to the top of the pyramid with no remaining medication options unless an off-label drug, such as ustekinumab, is used. We acknowledge that the anti-TNF treatment in the two patients with perianal involvement may have been suboptimal. Higher postinduction infliximab trough levels have been associated with better response in perianal fistulizing disease (El-Matary et al., 2019). Although these two patients did receive an initial starting dose of 10 mg/kg infliximab, this higher dosing was de-escalated to standard dosing after only 1 or 3 infusions.
Ustekinumab therapy using standard adult dosing was effective to achieve biochemical remission in case 1 and clinical remission in case 2. Intravenous re-induction and interval shortening of ustekinumab in the other four patients resulted in biochemical remission in case 3 and clinical remission in case 4. Case 5 and 6 had no therapeutic benefit of ustekinumab, despite dose escalation, and ustekinumab was discontinued.
4.1 Effectiveness of ustekinumab in pediatric Crohn’s disease
As previously summarized, two out of the six patients achieved biochemical remission on ustekinumab, another two achieved clinical remission, and in the remaining two, ustekinumab was discontinued for lack of response. In the latter two, case 5 and 6, treatment was withdrawn after 9 and 15 months, respectively, which was sufficiently long to observe a therapeutic response. According to the updated therapeutic targets in IBD, a mean of 11–14 weeks after ustekinumab initiation is required to achieve the medium-term treatment target clinical remission and normalization of fecal calprotectin (to <250 mg/kg) and C-reactive protein (Turner et al., 2021). Endoscopic remission, which was not measured in our patients, represents an even deeper form of remission and is considered a long-term treatment outcome which can be achieved after 19 weeks (Turner et al., 2021).
In a retrospective cohort study, effectiveness of ustekinumab was evaluated in 42 CD patients aged 21 years or below (median 16.9, interquartile range 13.9–18.2). 74% patients were still on ustekinumab after 1 year. 60% achieved steroid-free remission, and 67% achieved clinical remission (Dayan et al., 2019). Another cohort study in children showed similar results (Kim et al., 2021). In a third cohort study, only 39% of pediatric CD patients achieved clinical remission 1 year after ustekinumab initiation (Chavannes et al., 2019). Meta-analyses of studies among adult patients with active perianal CD suggested that ustekinumab may also be beneficial in this category of patients (Attauabi et al., 2021; Godoy Brewer et al., 2021). Prospective clinical trials are needed to confirm the long-term efficacy of ustekinumab in the treatment of refractory pediatric CD.
4.2 Treatment escalation
Four out of the six children failed on ustekinumab standard dosing and consequently underwent intravenous re-induction and interval shortening. This resulted in a substantial increase in ustekinumab levels at week 4 in three cases (>10 μg/mL) and a slight revert in the maintenance phase, but with a net increase compared to the standard dosing regimen. To the best of our knowledge, this is the first study in children in which ustekinumab drug levels were routinely measured. Higher drug levels resulted in two additional patients with a favorable response, which suggested an exposure–response relationship. In case 5, dose escalation had no effect on trough levels.
Our escalation strategy consisted of intravenous re-induction, followed by a shortened interval of subcutaneous ustekinumab of 4 weeks. This strategy was also described in a retrospective case study in which seven out of 10 children treated with ustekinumab required escalation, although the dosing interval was only shortened to 5 or 6 weeks (Do et al., 2020). In a retrospective cohort of 69 children that failed on ustekinumab standard dosing, escalation also mainly consisted of interval shortening (91%). A combination of intravenous re-induction and interval shortening was performed on only three patients. 42% of the pediatric cohort achieved clinical remission within 3 months after escalation. Adverse events were rare, mild, and did not result in ustekinumab discontinuation (Yerushalmy-Feler et al., 2022).
4.3 Target trough levels
Establishing an exposure–response relationship is the first step in developing a TDM strategy. Multiple studies have demonstrated such a relationship of ustekinumab in adult CD patients (Battat et al., 2017; Adedokun et al., 2018; Verstockt et al., 2019; Painchart et al., 2020; Thomann et al., 2020; Gómez Espín et al., 2021; Yao et al., 2021; Hirayama et al., 2022; Liefferinckx et al., 2022), and our report additionally could support an exposure–response relationship in children. Next, target trough levels must be determined. Unfortunately, those target levels for ustekinumab in children have not been described.
With the aim to expand our knowledge of ustekinumab (target) levels in children, we measured trough levels before each administration. Trough levels 8 weeks after the first intravenous loading dose (i.e., before the first subcutaneous administration) varied in our patients, ranging from 2.5 to 9.8 μg/mL (mean 4.6 μg/mL). Trough levels during maintenance (i.e., at 16 weeks or later) with standard ustekinumab dosing ranged from 0.3 to 4.7 μg/mL.
In the absence of specific target trough levels for children, adult targets were often used. For ustekinumab, a variety of target trough levels have been appointed in adults, partly depending on the timing of measurement and the outcome of interest (Battat et al., 2017; Adedokun et al., 2018; Verstockt et al., 2019; Painchart et al., 2020; Thomann et al., 2020; Gómez Espín et al., 2021; Yao et al., 2021; Hirayama et al., 2022; Liefferinckx et al., 2022). An overview of these target ustekinumab trough levels and therapeutic outcomes in adult patients with CD is presented in table 3.
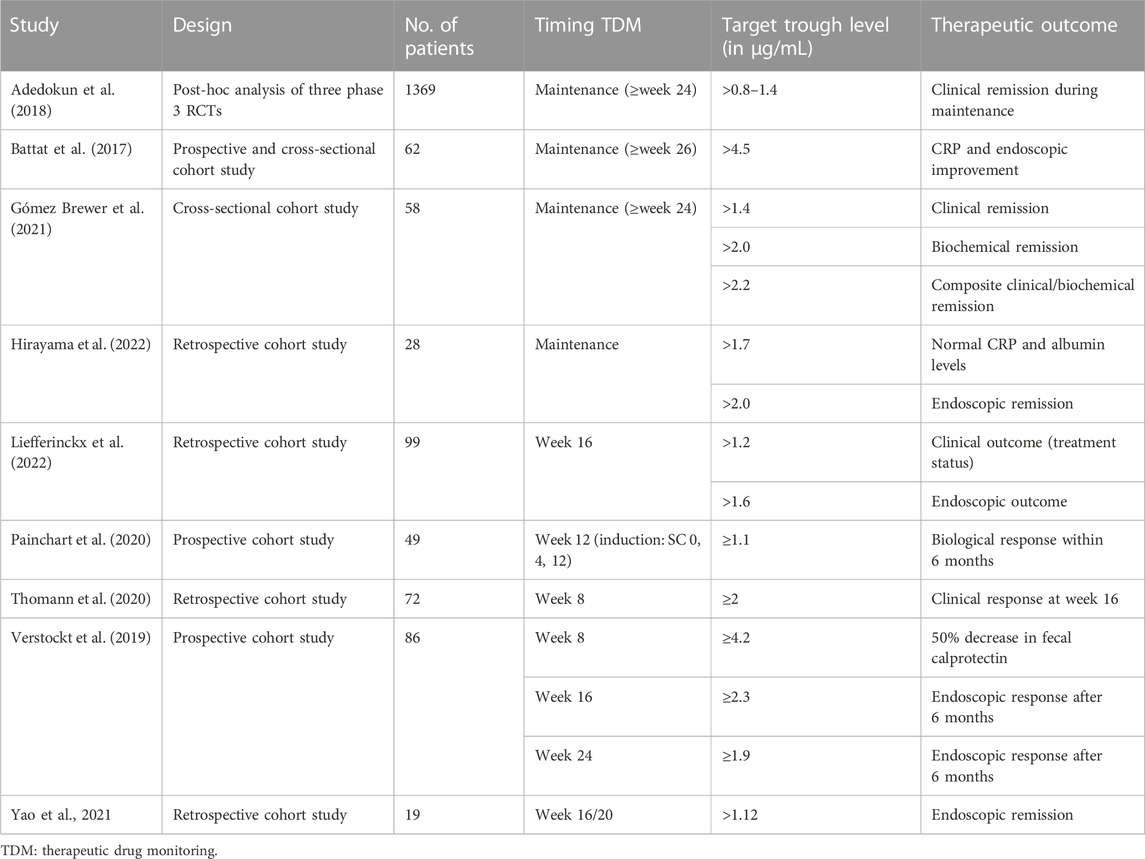
TABLE 3. Overview of ustekinumab concentration targets and therapeutic outcomes in adult patients with CD.
If ustekinumab trough level measurements are performed before the first subcutaneous injection (i.e., 8 weeks after intravenous induction), trough levels ≥2 μg/mL are associated with clinical response at week 16 and ≥4.2 with a 50% decrease in fecal calprotectin (Verstockt et al., 2019; Thomann et al., 2020). Ustekinumab levels of our patients at this time point ranged from 2.5 to 9.8 μg/mL.
During maintenance (i.e., ≥16 weeks after induction), target trough levels >0.8–1.4 μg/mL are associated with clinical remission (Adedokun et al., 2018; Gómez Espín et al., 2021). Ustekinumab trough levels ≥4.5 are associated with biochemical response (Battat et al., 2017), trough levels >1.7–2.0 with biochemical remission, (Gómez Espín et al., 2021; Hirayama et al., 2022), >1.6–4.5 with endoscopic response (Battat et al., 2017; Verstockt et al., 2019; Liefferinckx et al., 2022), and trough levels >1.12–2.0 with endoscopic remission (Yao et al., 2021; Hirayama et al., 2022). This is consistent with the trough levels found in case 2 of our series, who is in clinical remission on standard dosing. Case 1 from our series, who is in clinical and biochemical remission on standard dosing, has a slightly lower trough level of 1 μg/mL.
4.4 Timing of trough level measurement
Overall, two strategies for TDM are distinguished: proactive and reactive TDM. In proactive TDM, drug serum concentrations are routinely measured, irrespective of disease activity. Proactive TDM early in the treatment course allows for early dose optimization which can result in increased efficacy. Reactive TDM is performed if a disease flare occurs to help determine the cause of the flare and the next therapeutic step (such as increasing the dose or shortening the interval, or switching to an out-of-class biological) (van Rheenen et al., 2020; Shmais et al., 2022). Looking at the recent history of infliximab, the development of reactive TDM strategies will most likely precede that of proactive TDM strategies (Shmais et al., 2022). It has to be noted that within our six cases, we measured ustekinumab trough levels routinely before each subcutaneous injection, but the decision to escalate the dose was primarily based on clinical and biochemical parameters. Trough levels were measured without adapting dosing based on trough levels alone, which did not fully comply with the TDM criteria. Ustekinumab trough levels, however, were considered when deciding to discontinue ustekinumab.
Based on the currently available data, clear recommendations for the timing of trough level measurement of ustekinumab in pediatric CD cannot be given. Although substantiated by limited evidence, some recommendations for adults have been made. In a study by Cheifetz et al. a panel of 10 adult gastroenterology experts reached consensus that reactive TDM should be performed in patients with confirmed primary non-response to ustekinumab (prior to switching therapy) and in patients with confirmed secondary loss of response, but the panel did not formulate a target level or corresponding therapeutic step (Cheifetz et al., 2021). Shukla et al. proposed to proactively monitor ustekinumab levels, but the level of evidence to support this approach was low (Shukla and Ananthakrishnan, 2021).
Because of the potential exposure–response relationship in children, ustekinumab may be suitable for TDM in children. The findings in this report, together with contradicting results in adults, endorse the need for more data about ustekinumab trough levels in children. As an important first step, clinical centers that have the means should routinely measure ustekinumab drug levels in all children treated with ustekinumab and collect their findings in (multicenter) registries or databases.
4.5 Immunomodulator co-medication
In two of our patients, an immunomodulator was continued during ustekinumab treatment. The most important indication for an immunomodulator during treatment with a biological response is to prevent the formation of anti-drug antibodies. However, as mentioned before, immunogenicity in ustekinumab is less common than in anti-TNF therapy (Yarur et al., 2022). None of our patients developed anti-ustekinumab antibodies. Based on the currently available literature, combination treatment with an immunomodulator does not seem to have added value compared to ustekinumab monotherapy (Rosh et al., 2021; Yarur et al., 2022).
4.6 Limitations of this study
Shortcomings of case series, including a small sample size and lack of a control group, also apply to this case series. This limits generalizability and precludes drawing firm conclusions. Moreover, in a limited number of patients, follow-up time was relatively short for some patients.
Although the methods of ustekinumab prescription and monitoring of effectiveness were recorded in the local protocol, we had to rely on data accumulated in the electronic patient file. Since such records often are not complete, important data may be missing, which introduces a source of error into the report.
The intention of this report was to describe and discuss ustekinumab trough level measurement, not to prove that TDM is the way to go. Observations conducted in the six cases reported here cannot be generalized to other patients.
4.7 Conclusions
In this report, we show that ustekinumab can induce remission in pediatric CD patients refractory to anti-TNF therapy. Treatment escalation can induce remission in patients non-responsive to the standard treatment, supporting an exposure–response relationship. Treatment escalation should, therefore, be considered before the drug is abandoned as ineffective. Prospective clinical trials in children are needed to determine the long-term efficacy of ustekinumab, optimal target drug concentrations, and TDM strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participant(s) and minor(s) legal guardian/next of kin. Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization, methodology, and writing—review and editing: MB, PM, and PvR; investigation and writing—original draft preparation: MB. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1180750/full#supplementary-material
References
Adedokun, O. J., Xu, Z., Gasink, C., Jacobstein, D., Szapary, P., Johanns, J., et al. (2018). Pharmacokinetics and exposure response relationships of ustekinumab in patients with crohn's disease. Gastroenterology 154 (6), 1660–1671. doi:10.1053/j.gastro.2018.01.043
Alsoud, D., De Hertogh, G., Compernolle, G., Tops, S., Sabino, J., Ferrante, M., et al. (2022). Real-world endoscopic and histological outcomes are correlated with ustekinumab exposure in patients with ulcerative colitis. J. Crohns Colitis 16 (10), 1562–1570. doi:10.1093/ecco-jcc/jjac067
Attauabi, M., Burisch, J., and Seidelin, J. B. (2021). Efficacy of ustekinumab for active perianal fistulizing crohn's disease: A systematic review and meta-analysis of the current literature. Scand. J. Gastroenterol. 56 (1), 53–58. doi:10.1080/00365521.2020.1854848
Battat, R., Kopylov, U., Bessissow, T., Bitton, A., Cohen, A., Jain, A., et al. (2017). Association between Ustekinumab Trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with crohn's disease. Clin. Gastroenterol. Hepatol. 15 (9), 1427–1434. doi:10.1016/j.cgh.2017.03.032
Bots, S. J., Parker, C. E., Brandse, J. F., Löwenberg, M., Feagan, B. G., Sandborn, W. J., et al. (2021). Anti-drug antibody formation against biologic agents in inflammatory bowel disease: A systematic review and meta-analysis. BioDrugs 35 (6), 715–733. doi:10.1007/s40259-021-00507-5
Carman, N., Mack, D. R., and Benchimol, E. I. (2018). Therapeutic drug monitoring in pediatric inflammatory bowel disease. Curr. Gastroenterol. Rep. 20 (5), 18. doi:10.1007/s11894-018-0623-z
Chavannes, M., Martinez-Vinson, C., Hart, L., Kaniki, N., Chao, C. Y., Lawrence, S., et al. (2019). Management of paediatric patients with medically refractory crohn's disease using ustekinumab: A multi-centred cohort study. J. Crohns Colitis 13 (5), 578–584. doi:10.1093/ecco-jcc/jjy206
Cheifetz, A. S., Abreu, M. T., Afif, W., Cross, R. K., Dubinsky, M. C., Loftus, E. V., et al. (2021). A comprehensive literature review and expert consensus statement on therapeutic drug monitoring of biologics in inflammatory bowel disease. Am. J. Gastroenterol. 116 (10), 2014–2025. doi:10.14309/ajg.0000000000001396
Cozijnsen, M. A., Ben Shoham, A., Kang, B., Choe, B. H., Choe, Y. H., Jongsma, M. M. E., et al. (2020). Development and validation of the mucosal inflammation noninvasive index for pediatric crohn's disease. Clin. Gastroenterol. Hepatol. 18 (1), 133–140. doi:10.1016/j.cgh.2019.04.012
Dayan, J. R., Dolinger, M., Benkov, K., Dunkin, D., Jossen, J., Lai, J., et al. (2019). Real world experience with ustekinumab in children and young adults at a tertiary care pediatric inflammatory bowel disease center. J. Pediatr. Gastroenterol. Nutr. 69 (1), 61–67. doi:10.1097/MPG.0000000000002362
Do, P., Andersen, J., Patel, A., Semrin, G., Sifuentes-Dominguez, L., Luu, P., et al. (2020). Augmented ustekinumab dosing is needed to achieve clinical response in patients with anti-TNF refractory pediatric crohn's disease: A retrospective chart review. F1000Res 9, 316. doi:10.12688/f1000research.22673.2
El-Matary, W., Walters, T. D., Huynh, H. Q., deBruyn, J., Mack, D. R., Jacobson, K., et al. (2019). Higher postinduction infliximab serum Trough Levels are associated with healing of fistulizing perianal crohn's disease in children. Inflamm. Bowel Dis. 25 (1), 150–155. doi:10.1093/ibd/izy217
European Medicines Agency (2022). Stelara: european public assessment report - medicine overview. Available at: https://www.ema.europa.eu/en/documents/overview/stelara-epar-medicine-overview_en.pdf.
Godoy Brewer, G. M., Salem, G., Afzal, M. A., Limketkai, B. N., Haq, Z., Tajamal, M., et al. (2021). Ustekinumab is effective for perianal fistulising crohn's disease: A real-world experience and systematic review with meta-analysis. BMJ Open Gastroenterol. 8 (1), e000702. doi:10.1136/bmjgast-2021-000702
Gómez Espín, R., Nicolás De Prado, I., Gil Candel, M., González Carrión, M., Rentero Redondo, L., and Iniesta Navalón, C. (2021). Association between ustekinumab trough concentrations and biochemical outcomes in patients with Crohn's disease. A real life study. Rev. Esp. Enferm. Dig. 113 (2), 110–115. doi:10.17235/reed.2020.7124/2020
Hirayama, H., Morita, Y., Imai, T., Takahashi, K., Yoshida, A., Bamba, S., et al. (2022). Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn's disease. BMC Gastroenterol. 22 (1), 195. doi:10.1186/s12876-022-02271-4
Kapoor, A., and Crowley, E. (2021). Advances in therapeutic drug monitoring in biologic therapies for pediatric inflammatory bowel disease. Front. Pediatr. 9, 661536. doi:10.3389/fped.2021.661536
Kim, F. S., Patel, P. V., Stekol, E., Ali, S., Hamandi, H., Heyman, M. B., et al. (2021). Experience using ustekinumab in pediatric patients with medically refractory Crohn disease. J. Pediatr. Gastroenterol. Nutr. 73 (5), 610–614. doi:10.1097/MPG.0000000000003230
Kuenzig, M. E., Fung, S. G., Marderfeld, L., Mak, J. W. Y., Kaplan, G. G., Ng, S. C., et al. (2022). Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology 162 (4), 1147–1159.e4. doi:10.1053/j.gastro.2021.12.282
Ley, D., Leroyer, A., Dupont, C., Sarter, H., Bertrand, V., Spyckerelle, C., et al. (2022). New therapeutic strategies have changed the natural history of pediatric crohn's disease: A two-decade population-based study. Clin. Gastroenterol. Hepatol. 20 (11), 2588–2597.e1. doi:10.1016/j.cgh.2022.01.051
Levine, A., Griffiths, A., Markowitz, J., Wilson, D. C., Turner, D., Russell, R. K., et al. (2011). Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm. Bowel Dis. 17 (6), 1314–1321. doi:10.1002/ibd.21493
Liefferinckx, C., Hubert, A., Thomas, D., Bottieau, J., Minsart, C., Cremer, A., et al. (2022). Predictive models assessing the response to ustekinumab highlight the value of therapeutic drug monitoring in Crohn's disease. Dig. Liver Dis. 55, 366–372. doi:10.1016/j.dld.2022.07.015
Menting, S. P., van den Reek, J. M., Baerveldt, E. M., de Jong, E. M., Prens, E. P., Lecluse, L. L., et al. (2015). The correlation of clinical efficacy, serum trough levels and antidrug antibodies in ustekinumab-treated patients with psoriasis in a clinical-practice setting. Br. J. Dermatol 173 (3), 855–857. doi:10.1111/bjd.13834
Painchart, C., Brabant, S., Duveau, N., Nachury, M., Desreumaux, P., Branche, J., et al. (2020). Ustekinumab serum Trough Levels may identify suboptimal responders to ustekinumab in crohn's disease. Dig. Dis. Sci. 65 (5), 1445–1452. doi:10.1007/s10620-019-05865-3
Proietti, E., Pauwels, R. W. M., van der Woude, C. J., Doukas, M., Oudijk, L., Peppelenbosch, M. P., et al. (2022). Ustekinumab tissue and serum levels in patients with crohn's disease are closely correlated though not consistently associated with objective response after induction. Inflamm. Bowel Dis. 29, 1038–1046. doi:10.1093/ibd/izac169
Rosh, J. R., Turner, D., Griffiths, A., Cohen, S. A., Jacobstein, D., Adedokun, O. J., et al. (2021). Ustekinumab in paediatric patients with moderately to severely active crohn's disease: pharmacokinetics, safety, and efficacy results from UniStar, a phase 1 study. J. Crohns Colitis 15 (11), 1931–1942. doi:10.1093/ecco-jcc/jjab089
Rubín de Célix, C., Chaparro, M., and Gisbert, J. P. (2022). Real-world evidence of the effectiveness and safety of ustekinumab for the treatment of crohn's disease: systematic review and meta-analysis of observational studies. J. Clin. Med. 11 (14), 4202. doi:10.3390/jcm11144202
Shmais, M., Regueiro, M., and Hashash, J. G. (2022). Proactive versus reactive therapeutic drug monitoring: why, when, and how? Inflamm. Intest. Dis. 7 (1), 50–58. doi:10.1159/000518755
Shukla, R., and Ananthakrishnan, A. (2021). Therapeutic drug monitoring of non-anti-tumor necrosis factor biologics. Clin. Gastroenterol. Hepatol. 19 (6), 1108–1110. doi:10.1016/j.cgh.2021.01.002
Thomann, A. K., Schulte, L. A., Globig, A. M., Hoffmann, P., Klag, T., Itzel, T., et al. (2020). Ustekinumab serum concentrations are associated with clinical outcomes in Crohn's disease - a regional multi-center pilot study. Z Gastroenterol. 58 (5), 439–444. doi:10.1055/a-1088-1461
Turner, D., Ricciuto, A., Lewis, A., D'Amico, F., Dhaliwal, J., Griffiths, A. M., et al. (2021). STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 160 (5), 1570–1583. doi:10.1053/j.gastro.2020.12.031
van Rheenen, P. F., Aloi, M., Assa, A., Bronsky, J., Escher, J. C., Fagerberg, U. L., et al. (2020). The medical management of paediatric crohn's disease: an ECCO-ESPGHAN guideline update. J. Crohns Colitis 15, 171–194. doi:10.1093/ecco-jcc/jjaa161
Vande Casteele, N., Herfarth, H., Katz, J., Falck-Ytter, Y., and Singh, S. (2017). American gastroenterological association institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 153 (3), 835–857. doi:10.1053/j.gastro.2017.07.031
Verstockt, B., Dreesen, E., Noman, M., Outtier, A., Van den Berghe, N., Aerden, I., et al. (2019). Ustekinumab exposure-outcome analysis in crohn's disease only in Part Explains limited endoscopic remission rates. J. Crohns Colitis 13 (7), 864–872. doi:10.1093/ecco-jcc/jjz008
Yao, J. Y., Zhang, M., Wang, W., Peng, X., Zhao, J. Z., Liu, T., et al. (2021). Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory crohn's disease: A Chinese real-world study. BMC Gastroenterol. 21 (1), 380. doi:10.1186/s12876-021-01946-8
Yarur, A. J., McGovern, D., Abreu, M. T., Cheifetz, A., Papamichail, K., Deepak, P., et al. (2022). Combination therapy with immunomodulators improves the pharmacokinetics of infliximab but not vedolizumab or ustekinumab. Clin. Gastroenterol. Hepatol. doi:10.1016/j.cgh.2022.10.016
Yerushalmy-Feler, A., Pujol-Muncunill, G., Martin-de-Carpi, J., Kolho, K. L., Levine, A., Olbjorn, C., et al. (2022). Safety and potential efficacy of escalating dose of ustekinumab in pediatric Crohn disease (the speed-up study): A multicenter study from the pediatric IBD porto group of espghan. J. Pediatr. Gastroenterol. Nutr. 75 (6), 717–723. doi:10.1097/MPG.0000000000003608
Keywords: ustekinumab, therapeutic drug monitoring, Crohn’s disease, inflammatory bowel disease, pediatrics
Citation: Bouhuys M, Mian P and van Rheenen PF (2023) Ustekinumab trough levels in children with Crohn’s disease refractory to anti-tumor necrosis factor agents: a prospective case series of off-label use. Front. Pharmacol. 14:1180750. doi: 10.3389/fphar.2023.1180750
Received: 06 March 2023; Accepted: 11 September 2023;
Published: 25 September 2023.
Edited by:
Jessica K. Roberts, Cognigen, United StatesReviewed by:
Manuela Neuman, University of Toronto, CanadaRuggiero Francavilla, University of Bari Aldo Moro, Italy
Copyright © 2023 Bouhuys, Mian and van Rheenen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick F. van Rheenen, cC5mLnZhbi5yaGVlbmVuQHVtY2cubmw=
 Marleen Bouhuys
Marleen Bouhuys Paola Mian2
Paola Mian2 Patrick F. van Rheenen
Patrick F. van Rheenen