Abstract
Copaíba oil-resin is extracted from the trunk of the Copaíba tree and has medicinal, cosmetic, and industrial properties. As a result, widespread knowledge about the use of Copaíba oil-resin has evolved, attracting the scientific community’s attention. This paper aims to map the global knowledge production regarding the biological activities of Copaíba (Copaifera spp.). Bibliometric methodological instruments were used to conduct a search of the Web of Science-Core Collection database. The search resulted in 822 references. After screening titles and abstracts, 581 references did not meet the eligibility criteria, leaving 246 references for full-text examination. Subsequently, 15 studies were excluded, resulting in a final set of 232 records for the bibliometric analysis. In vitro was the most published study type, mainly from Brazil, from 2010 to 2020. Regarding the authors, Bastos, JK, and Ambrosio, SR were the ones with the most significant number of papers included. The most frequent keywords were Copaíba oil, Copaíba, and Copaifera. Our findings revealed global study trends about Copaíba, mainly related to its various effects and use over time. In general, all countries have conducted more research on antimicrobial and anti-inflammatory activities, also exposing its antioxidant and healing properties. Copaifera reticulata was the most investigated, followed by Copaifera langsdorffi and Copaifera multijuga in both in vitro and in vivo studies. Therefore, there is a need for human reports, given the promising results that Copaíba oils have been demonstrating.
1 Introduction
Humans have used plants to treat several diseases for centuries, but only recently traditional medicine has encouraged novel research with natural products, either bioactive compounds or complex mixtures such as extracts, fixed oils, essential oils, and oil-resin (Petrovska, 2012). Some plant species such as the genus Copaifera have different classes of secondary metabolites that may have potential pharmacological applications (Maciel et al., 2002; Palombo, 2011; Mauro et al., 2021). The Copaiba tree (Figure 1) is a member of the Fabaceae family, the Caesalpinioideae subfamily, and the Copaifera genus.
FIGURE 1

Copaifera tree. (A) Image of a copaíba tree trunk with (B) highlighting the oil-resin collection site. The source of the photo is attributed to the authors.
Although this genus has several species distributed worldwide (Africa, Central America, and South America), the greatest biodiversity of Copaifera is found in Brazil (Arruda et al., 2019). Sixteen out of 28 species of Copaiba cataloged by the Brazilian Agricultural Research Corporation (EMBRAPA) as native from Brazil. These species predominantly grow in the Cerrado and Amazon biomes, which comprise dry and flooded lands, banks of lakes, and streams (Arruda et al., 2019; Dini et al., 2019).
The Brazilian indigenous tribes have benefited from the medicinal properties of Copaifera oil-resin since the 16th century (Dini et al., 2019), while this genus was first documented in 1,534 and recorded in the Brazilian Descriptive Pact in 1,587 (Pieri et al., 2009). The pharmacological properties of Copaifera oil-resin are attributed to sesquiterpenes and diterpenes components (Cox-Georgian et al., 2019; da Trindade et al., 2018). Although the use of Copaifera products decreased in the 18th century, there is a growing demand for herbal medicines and Copaifera has gained renewed attention due to potential health benefits (Veiga Junior and Pinto, 2002). This genus has been evaluated by several authors that suggested the oil-resin obtained from these species may benefit human health (Guimarães-Santos et al., 2012; Arruda et al., 2019; Menezes et al., 2022). The Copaiba oil-resin has been used for centuries as a potent analgesic and anti-inflammatory by traditional Amazonians since the 16th century (Dini et al., 2019).
Copaiba has a variety of functions and has proven to be a valuable bioactive, mainly attributed to its anti-inflammatory characteristics (Símaro et al., 2021). It was demonstrated to be twice as effective as diclofenac sodium (Masson et al., 2013), which is the most used anti-inflammatory in the world. Moreover, the oil-resin obtained from the Copaiba tree is a raw material for the European industry, which produces varnishes, perfumes, antitetanics, urinary system antiseptics, antitussives, and cicatrizants. Furthermore, there is a worldwide trend to use Copaiba as biodiesel, which is a less pollutant fuel and preserves the environment. Copaiba oil is an excellent alternative to several medications due to its efficiency, low toxicity, biocompatibility, and low cost (Santos et al., 2011).
Therefore, the widespread knowledge about Copaiba oil-resin has attracted the attention of the scientific community, which progressively seeks answers and solutions to several diseases. The Amazon rainforest’s environmental wealth and size have progressively drawn the world’s attention to what it can offer. A reduced population purchasing power can justify the use of medicinal plants as alternative therapies when drug prices are high and access to medical healthcare is restricted (Battisti et al., 2013). Oils obtained from Copaifera species have shown great chemical diversity and potential biological activities (Veiga et al., 2007). Therefore, this study aimed to retrieve the up-to-date published knowledge regarding the biological activities of Copaiba (Copaifera spp.).
2 Methodology
2.1 Search method and data source
A bibliometric analysis was used to retrieve global knowledge on the biological activities of Copaiba; thus, articles were searched in the Web of Science Core Collection (WoS-CC), a database that indexes peer-reviewed articles published in high-quality journals and provides a detailed series of accurate information.
To avoid daily update bias, a search was conducted on a single day in June 2022 by two independent examiners by using the following terms: “Copaiba” OR “Copaiba oil” OR “Copaiba oils” OR “Copaiba oil resin” OR “Copaiba oil-resin” OR “Copaiba oleoresin” OR “Copaiba oil resins” OR “Copaiba oil-resins” OR “Copaiba oleoresins” OR “Copaifera” OR “Copaifera oil resin” OR “Copaifera oil-resin” OR “Copaifera oleoresin” OR “Copaifera oil resins” OR “Copaifera oil-resins” OR “Copaifera oleoresins” or “Copaifera species” OR “Copaifera spp” OR “Copaifera sp” OR “Copaifera genus.” Articles were searched from 1945 to 2022 without language restrictions.
2.2 Inclusion and exclusion criteria
The inclusion criteria comprised original and complete research articles (in vitro, in situ, in vivo, clinical trials, and narrative/systematic/bibliometric reviews) that investigated oil-resins and essential oil obtained from Copaiba and their biological activities. Letters to the editor and opinion editorials were excluded since they express personal perceptions on a publication or opinions to provide a novel point of view. Moreover, studies that did not primarily investigate the biological activities of Copaiba and studies that exclusively evaluated isolated compounds of Copaiba were excluded.
2.3 Article selection
The articles were sorted in descending order of citation number and were independently reviewed by two examiners (DRF and MSO), while a third examiner (RRL) was consulted in case of disagreement.
2.4 Data extraction
The following data of the articles were extracted: title, authors, country of origin (based on the corresponding author affiliation), year of publication, number of citations, study design, citation density (number of citations divided by the years since publication), journal, DOI/URL, and keywords. In the case of two articles with identical citation numbers, the article with the highest citation density was upper-ranked.
2.5 Data analysis and visualization
Bibliometric networks regarding author co-authorship and author keyword co-occurrences were created by using the Visualization of Similarities Viewer (VOSviewer) software (version 1.6.16) (Center for Science and Technology Studies, University of Leiden, Netherlands) (van Eck and Waltman, 2010). Furthermore, the distribution of articles by continent and country was graphically represented by using the MapChart website (mapchart.net).
2.6 Content analysis
The articles were read in full to map all the current knowledge by identifying different species and the biological activity that each publication investigated. All data were manually tabulated on Microsoft Excel In addition, Microsoft Excel, PowerPoint, and Adobe Photoshop were used to rank the most frequent study designs, Copaifera species, and biological activities investigated.
3 Results
3.1 Study selection
After the title and abstract reading, 581 out of 822 references were excluded by following exclusion criteria, and 246 references were selected for full-text reading. Then, 8 studies were excluded since only evaluated isolated compounds of Copaiba essential oil or oil-resin of (Geris et al., 2008; Idippily et al., 2017; Silva et al., 2017; de Carvalho et al., 2018; Pereira et al., 2018; Souza et al., 2018; Farias et al., 2019; Oliveira et al., 2020), 4 studies did not analyze Copaiba (Parreira et al., 2019; Kawakami et al., 2021; do Rosário et al., 2017; Rodrigues da Silva et al., 2020), and 2 articles did not investigate biological activity (Lovelock et al., 1999; Silva et al., 2001). Finally, 232 records were eligible for the bibliometric analysis (Figure 2) and their primary attributes are shown in Supplementary Table S1.
FIGURE 2

Flow diagram of the study selection process.
3.2 Metrics results
3.2.1 Year of publication
The 232 studies were published between 1983 and 2022. The most productive year in terms of publications was 2020 and 2017, with 24 publications each. The year with the most citations was 2012, with 446 citations, followed by 2007 and 2015, with 373 and 306 citations, respectively. The decade with the most publications (n = 142) and citations (n = 2,380) was 2010.
Both 2017 and 2020 were the most productive years in terms of number of publications (24 studies each). The highest number of citations (446) was observed in 2012, followed by 2007 and 2015 (373 and 306 citations, respectively). The 2010s was the decade with the most publications (n = 142) and citations (n = 2,380).
3.2.2 Authors
Among 1,237 authors, Bastos JK (ORCID iD: 0000–0001–8641–9686; n = 37), Ambrosio SR (ORCID iD: 0000–0001–5032–3930; n = 23), da Veiga VF (ORCID iD: 0000–0003–1365–7602; n = 19), and Veneziani RCS (ORCID iD: Not registered; n = 17) contributed to the majority of publications on Copaifera spp. (Figure 3). The most cited author was Da Veiga VF was the most cited author (1,055 citations), followed by Pinto AC (754 citations), Bastos JK (571 citations), and Ambrosio SR (383 citations) (Figure 4).
FIGURE 3
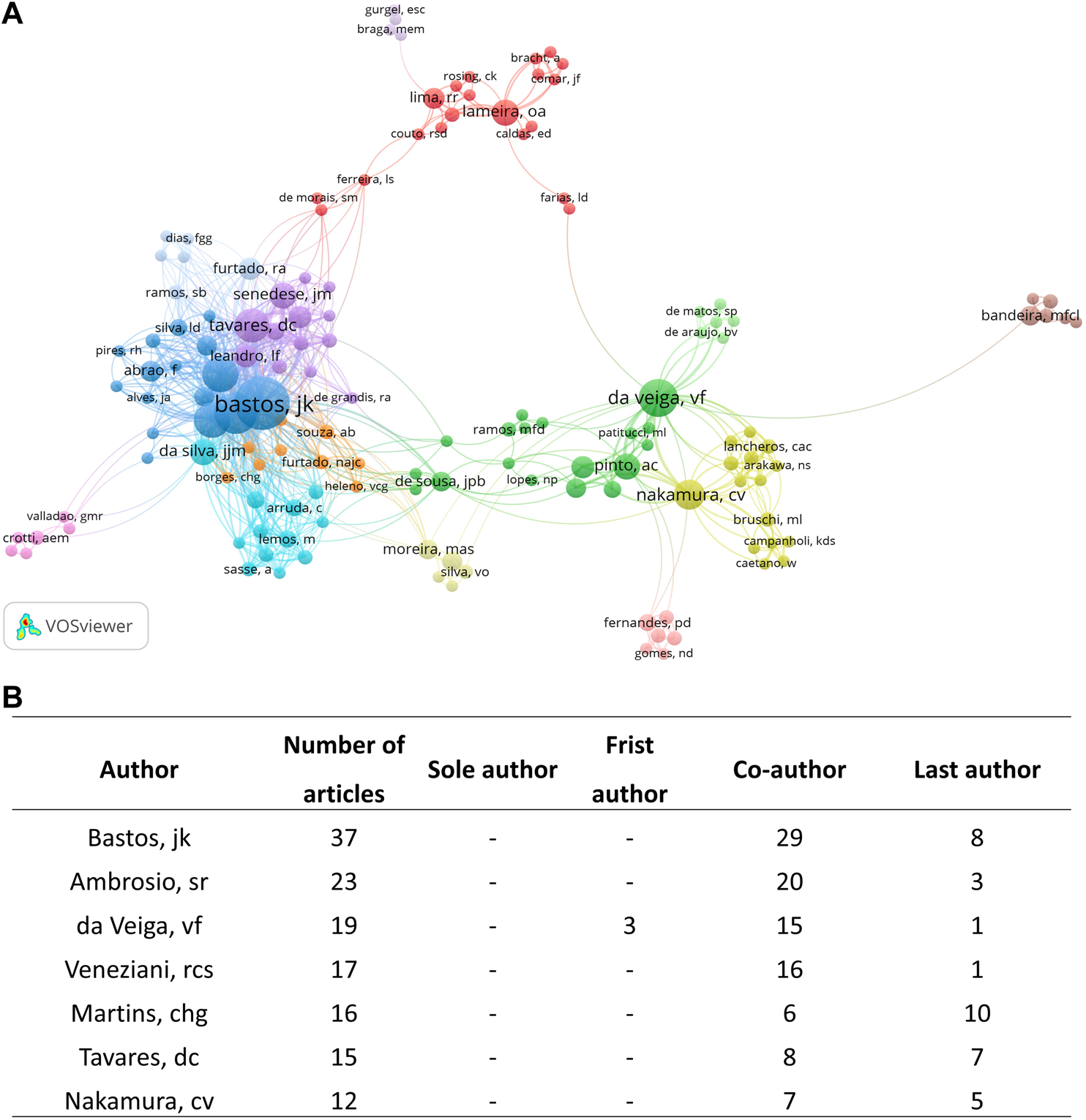
(A) Netmap of part of authors’ contribution with at least two published articles. The circles represent the number of articles, and the lines demonstrate the link strength of one author to another. (B) Table describing the author’s coauthorship in their manuscripts.
FIGURE 4

Netmap of part of authors’ citations and integration with other researchers. The circles represent the number of citations, and the lines demonstrate the link strength of one author to another. (B) Table describing the Top 10 most cited authors and the number of manuscripts.
3.2.3 Countries
Although the selected articles were published by authors from North America, South America, Europe, Africa, and Asia, only 15 countries of origin were observed: the United States of America (United States), Canada, Brazil, Argentina, Spain, France, Germany, Italy, Sweden, Greece, Portugal, Nigeria, Egypt, Japan, and Taiwan. The highest number of articles originated from Brazil (n = 206; 3,883 citations), followed by the United States (n = 6; 102 citations), Spain (n = 2; 67 citations), and Italy (n = 2; 66 citations) (Figure 5).
FIGURE 5

Map of the countries that investigated Copaíba ’s biological activities.
3.2.4 Keywords
A total of 650 keywords were identified (Figure 6): Copaiba oil (n = 40), Copaiba (n = 24), Copaifera (n = 13), Copaifera reticulata (n = 13), Copaifera langsdorffii (n = 12), rats (n = 12), Copaifera multijuga (n = 11), Fabaceae (n = 11), oil-resin (n = 11), phytotherapy (n = 10), antimicrobial activity (n = 9), and others.
FIGURE 6

Netmap of keywords found in the included studies organized through the year of use in the last 10 years.
3.2.5 Citation count and citation density
The citation count varied from 0 to 171 (mean 18.53) and the total citation count was 4,289. The review entitled “Medicinal plants: The need for multidisciplinary scientific studies” (Maciel et al., 2002), published in Quimica Nova was the most cited article (171 citations) and its citation density (total citations/mean citations per year) was 8.55. The top 20 most cited articles are shown in Table 1.
TABLE 1
| Authors (year) | Article title | Times cited, WoS core |
|---|---|---|
| MACIEL, MAM; PINTO, AC; VEIGA, VF; GRYNBERG, NF; ECHEVARRIA, A (2002) | Medicinal plants: The need for multidisciplinary scientific studies | 171 |
| VEIGA, VF; ROSAS, EC; CARVALHO, MV; HENRIQUES, MGMO; PINTO, AC (2007) | Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne - A comparative study | 165 |
| LEANDRO, LM; VARGAS, FD; BARBOSA, PCS; NEVES, JKO; DA SILVA, JA; DA VEIGA, VF (2012) | Chemistry and Biological Activities of Terpenoids from Copaiba (Copaifera spp.) Oleoresins | 150 |
| DOS SANTOS, AO; UEDA-NAKAMURA, T; DIAS, BP; VEIGA, VF; PINTO, AC; NAKAMURA, CV (2008) | Antimicrobial activity of Brazilian copaiba oils obtained from different species of the Copaifera genus | 109 |
| GOMES, NM; REZENDE, CM; FONTES, SP; MATHEUS, ME; FERNANDES, PD (2007) | Antinociceptive activity of Amazonian Copaiba oils | 107 |
| SANTOS, AO; UEDA-NAKAMURA, T; DIAS, BP; VEIGA, VF; PINTO, AC; NAKAMURA, CV (2008) | Effect of Brazilian copaiba oils on Leishmania amazonensis | 99 |
| ANDRADE, BFMT; BARBOSA, LN; PROBST, ID; FERNANDES, A (2014) | Antimicrobial activity of essential oils | 91 |
| LIMA, SRM; VEIGA, VF; CHRISTO, HB; PINTO, AC; FERNANDES, PD (2003) | In vivo and in vitro studies on the anticancer activity of Copaifera multijuga Hayne and its fractions | 88 |
| VEIGA, VF; ZUNINO, L; CALIXTO, JB; PATITUCCI, ML; PINTO, AC (2001) | Phytochemical and antioedematogenic studies of commercial copaiba oils available in Brazil | 84 |
| DE MENDONCA, FAC; DA SILVA, KFS; DOS SANTOS, KK; JUNIOR, KALR; SANT'ANA, AEG (2005) | Activities of some Brazilian plants against larvae of the mosquito Aedes aegypti | 83 |
| SOUZA, AB; MARTINS, CHG; SOUZA, MGM; FURTADO, NAJC; HELENO, VCG; DE SOUSA, JPB; ROCHA, EMP; BASTOS, JK; CUNHA, WR; VENEZIANI, RCS; AMBROSIO, SR (2011) | Antimicrobial Activity of Terpenoids from Copaifera langsdorffii Desf. Against Cariogenic Bacteria | 82 |
| FERNANDES, FD; FREITAS, EDP (2007) | Acaricidal activity of an oleoresinous extract from Copaifera reticulata (Leguminosae: Caesalpinioideae) against larvae of the southern cattle tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) | 80 |
| BASILE, AC; SERTIE, JAA; FREITAS, PCD; ZANINI, AC (1988) | Anti-Inflammatory Activity of Oleoresin from Brazilian Copaifera | 75 |
| DE LIMA, MRF; LUNA, JD; DOS SANTOS, AF; DE ANDRADE, MCC; SANT'ANA, AEG; GENET, JP; MARQUEZ, B; NEUVILLE, L; MOREAU, N (2006) | Anti-bacterial activity of some Brazilian medicinal plants | 75 |
| PAIVA, LAF; RAO, VSN; GRAMOSA, NV; SILVEIRA, ER (1998) | Gastroprotective effect of Copaifera langsdorffii oleo-resin on experimental gastric ulcer models in rats | 74 |
| SOUZA, AB; DE SOUZA, MGM; MOREIRA, MA; MOREIRA, MR; FURTADO, NAJC; MARTINS, CHG; BASTOS, JK; DOS SANTOS, RA; HELENO, VCG; AMBROSIO, SR; VENEZIANI, RCS (2011) | Antimicrobial Evaluation of Diterpenes from Copaifera langsdorffii Oleoresin Against Periodontal Anaerobic Bacteria | 72 |
| POHLIT, AM; REZENDE, AR; BALDIN, ELL; LOPES, NP; NETO, VFD (2011) | Plant Extracts, Isolated Phytochemicals, and Plant-Derived Agents Which Are Lethal to Arthropod Vectors of Human Tropical Diseases - A Review | 66 |
| OHSAKI, A; YAN, LT; ITO, S; EDATSUGI, H; IWATA, D; KOMODA, Y (1994) | The Isolation and in vivo Potent Antitumor-Activity of Clerodane Diterpenoid from the Oleoresin of the Brazilian Medicinal Plant, Copaifera langsdorfii Desf | 66 |
| TINCUSI, BM; JIMENEZ, IA; BAZZOCCHI, IL; MOUJIR, LM; MAMANI, ZA; BARROSO, JP; RAVELO, AG; HERNANDEZ, BV (2002) | Antimicrobial terpenoids from the oleoresin of the Peruvian medicinal plant Copaifera paupera | 64 |
| BONAN, RF; BONAN, PRF; BATISTA, AUD; SAMPAIO, FC; ALBUQUERQUE, AJR; MORAES, MCB; MATTOSO, LHC; GLENN, GM; MEDEIROS, ES; OLIVEIRA, JE (2015) | In vitro antimicrobial activity of solution blow spun poly (lactic acid)/polyvinylpyrrolidone nanofibers loaded with Copaiba (Copaifera sp.) oil | 61 |
Top 20 most cited studies.
3.2.6 Journal ranking
The highest number of articles on the biological activities of Copaiba was published by the Journal of Ethnopharmacology (n = 16), followed by the Brazilian Journal of Pharmacognosy (n = 10). The top 20 journals in terms of number of published articles are shown in Figure 7.
FIGURE 7

Journal rankings of Copaíba’s biological activities knowledge production.
3.3 Content results
3.3.1 Study design
The majority of studies on the biological activities of Copaiba were in vitro (n = 132; 59.70%), followed by in vivo (n = 91; 39.39%), review articles (n = 15; 6.49%), and clinical trials (n = 6; 2.59%) (Figure 8). It must be addressed that 17 studies combined in vivo and in vitro experiments.
FIGURE 8

Ranking of study types on the global knowledge production on Copaíba ’s biological activities.
3.3.2 Copaifera species
Although 12 Copaifera spp. Species were described in the articles, most papers reviewed multiple species or did not detail them (n = 55). Nevertheless, C. reticulata (n = 53), Copaifera langsdorffi (n = 48), C. multijuga (n = 37), and Copaifera officinalis (n = 27) were the most investigated species. Figure 9 shows that other species were found in less than one-tenth of the articles.
FIGURE 9
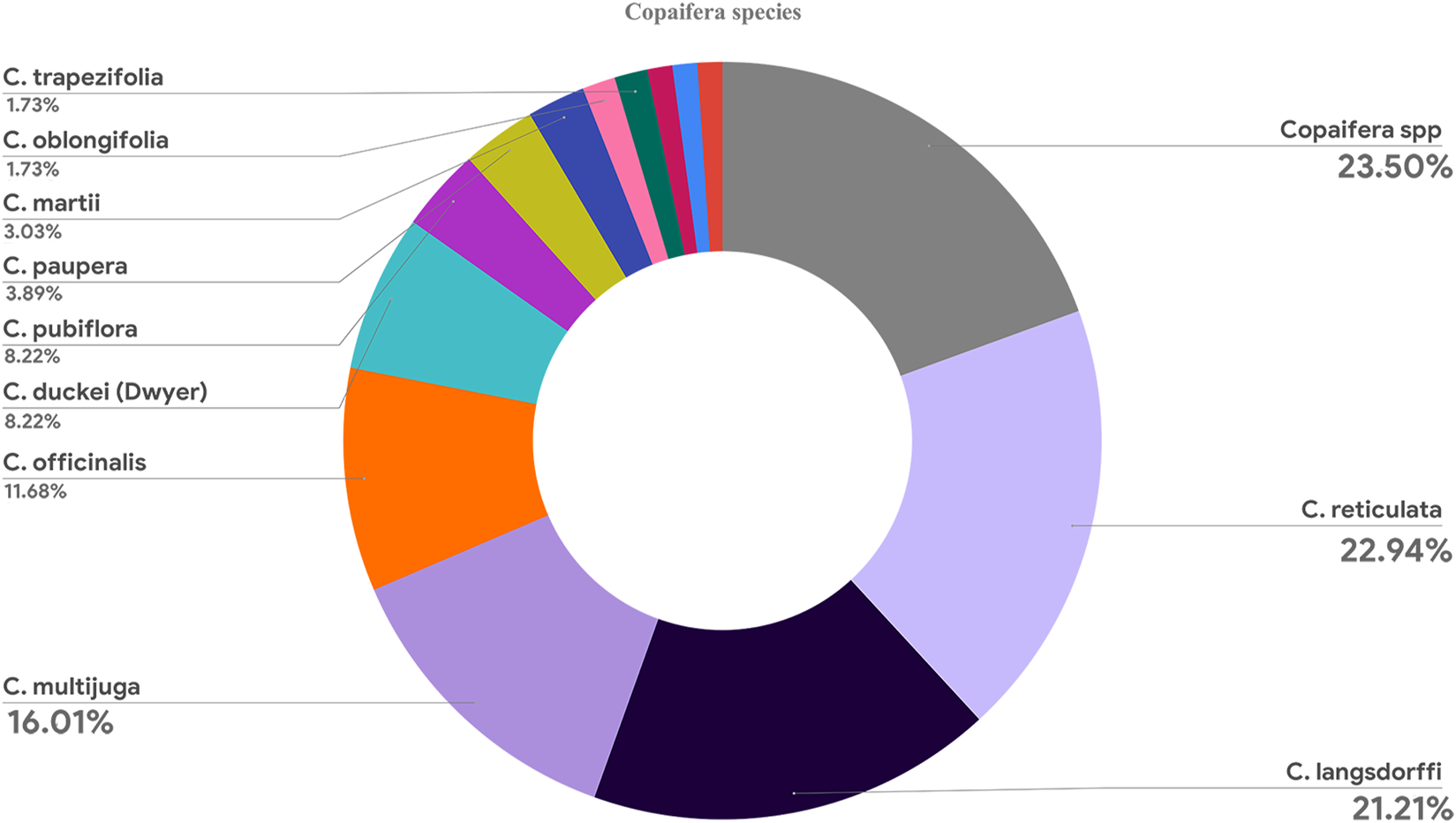
Graphic showing all Copaifera species investigated in the articles.
3.3.3 Biological activities
The articles revealed 13 biological activities of Copaiba: antimicrobial (antibacterial and antifungal) (30%), anti-inflammatory (13.71%), antioxidant (6.19%), healing/cicatrization (5.75%), antilarval (5.75%), antiparasitic (4.42%), gastroprotective (3.09%), insecticidal (1.76%), anticancer/anti-tumor (1.76%), and antinociceptive (1.76%) (Figure 10). Surprisingly, toxicological studies (5.3%) revealed that Copaiba does not harm human organisms.
FIGURE 10

Graphic showing all biological activities investigated in the articles.
4 Discussion
This literature review was based on a bibliometric analysis method, which addressed the global knowledge regarding the biological activities of Copaiba. Although most articles did not detail the investigated species, C. reticulata was the most frequently reported. In addition, in vivo and antimicrobial were respectively the most common study design and biological activity studied. Although Copaiba has been investigated since only the 1980s, this relatively recent topic was investigated by a great number of authors from several countries from almost all continents.
More than 11 Copaiba species were detailed in the selected studies. Furthermore, some studies investigated more than one species since their phytochemical profile changes in accordance with region, time, plant part, and detection method (Arruda et al., 2019). Nevertheless, Copaiba oil-resins and essential oils contain common components responsible for biological activities.
The literature indicates two major groups of components: sesquiterpenes and diterpenes (Figure 11). Terpenes are secondary metabolites with varied molecular structures that play a key role in essential sensory characteristics such as smell, taste, and color. β-Caryophyllene, trans-α-bergamotene, and β-bisabolene are sesquiterpenes present in Copaiba oil-resin. β-Caryophyllene is known as a significant marker of Copaiba oil-resin obtained from some species (Abrão et al., 2018). Copalic acid, polyalthic acid, and kaurenoic acid are diterpenes found in several species such as C. paupera (Herzog) Dwyer and C. reticulata Ducke (Lameira et al., 2009; Guimarães-Santos et al., 2012). The literature demonstrates that a wide range of biological processes can be attributed to these components found in both oil and extracts derived from different plant parts.
FIGURE 11

Compounds found in some copaiba species.
Most of the oils obtained from Copaifera duckei contain β-bisabolene, α-bergamotene, and kaur-16-en19-oic, and their biological activity is usually associated with the synergistic effect of all compounds. β-Bisabolene can be used as a flavoring agent (Dionisio et al., 2018) and presents a high antioxidant capacity (up to 14 ± 0.8 mg/mL in accordance with the DPHH method). Even though this major compound is mixed with other chemicals for essential oil production, β-bisabolene has antimicrobial effects against Gram-positive and Gram-negative bacteria (Dionisio et al., 2018). α-Bergamotene is a volatile substance combined with other compounds in several essential oils and is related to antioxidant capacity without toxic effects (Moraes et al., 2022). Kaur-16-en19-oic has antimicrobial activity against Gram-positive bacteria (Vieira et al., 2002).
The oil obtained from C. multijuga contains β-caryophyllene, α-humulene, and α-bergamotene as major components. β-Caryophyllene is a food additive that can modulate inflammatory processes. It can be consumed alone or contained in certain edible plant species such as black pepper (Gertsch et al., 2008). Topical application or oral administration of α-humulene can reduce inflammation and pain as demonstrated in an in vivo study with rats (Chaves et al., 2008). α-Copaene, which is one of the primary compounds of oils obtained from C. officinalis, is associated with antioxidant properties and did not show cytotoxicity through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenlytetrazolium bromide test (Turkez et al., 2014).
These several compounds found in Copaiba have come to the attention of researchers from medicine, dentistry, and other fields. Sixteen of 77 medical studies indicated promising effects of C. langsdorffi against several diseases or conditions such as Alzheimer’s (Penido et al., 2017)–47], urolithiasis (Brancalion et al., 2012), acne (da Silva et al., 2012), microorganism infections (Tincusi et al., 2002; de Lima et al., 2006; Tobouti et al., 2017), cancer (Abrão et al., 2015; Senedese et al., 2019), intestinal injury (Paiva et al., 1998; Adzu et al., 2015). Nevertheless, the first medical study investigated the anti-inflammatory effect of C. reticulata Ducke on carrageenin-induced pedal edema in rats (Basile et al., 1988). This model was widely used between 1960 and 1980 to investigate the anti-inflammatory potential of natural products such as African spices (Fernández-Moriano et al., 2019).
Moreover, in vivo (mainly animal models) was the most common type of medical study (n = 36). It is also important to mention that 3 out of 6 clinical trials found in this review originated from the medical field (da Silva et al., 2012; Bahr et al., 2018; Waibel et al., 2021). These trials have a considerably higher certainty of evidence than in vitro and in vivo studies; however, a low number of clinical trials were published (Murad et al., 2016). The placebo-controlled clinical trial conducted in 2012 showed that Copaiba essential oil reduced the face area affected by acne (da Silva et al., 2012). In 2018, another clinical trial showed the analgesic and anti-inflammatory effects of Copaiba essential oil used to hand massage individuals with arthritis and osteoarthritis (Bahr et al., 2018). Recently in 2021, a prospective, randomized, double-blind, and placebo-controlled clinical trial showed that the use of a Copaiba oil-containing silicone-based gel for 84 days improved the color, contour, distortion, and texture of different types of scars (Vasconcelos et al., 2008).
The number of studies in the oral science field has been constantly growing due to the potential antimicrobial effect of Copaiba, especially against oral pathogens. Although the first investigation of Copaiba in the dental field was published in the 2000s (Bandeira et al., 2000), this subject was highlighted in the scientific community only in the 2010s due to the need for safe, effective, and low-cost alternative methods to prevent and treat diseases (Palombo, 2011). Therefore, 17 out of the 25 studies (68%) on the use of Copaiba in the oral cavity and orofacial region were published between 2010 and 2020.
In the 2000s, the increase in the number of oral diseases, financial issues in underdeveloped countries, and bacteria resistance to regular antibiotics intensified research on adjuvant treatments such as phytotherapeutic compounds that could treat severe infections caused by multiresistant bacteria (Palombo, 2011). Phytomedicine has been used in dentistry as an anti-inflammatory, antibacterial, analgesic, sedative, and endodontic irrigant (Groppo et al., 2008). Copaiba’s anti-inflammatory and antimicrobial effects observed in the medical field suggested its use on the oral cavity.
Therefore, several studies investigated the effect of Copaiba on endodontic materials (Bandeira et al., 2000; Vasconcelos et al., 2008; Couto et al., 2020; Reiznautt et al., 2021), periodontal anaerobic bacteria (Souza et al., 2011a), periodontitis (Ohsaki et al., 1994), antimicrobial effect on plaque (Pieri et al., 2010; Pieri et al., 2013), cariogenic (Souza et al., 2011b; Moraes et al., 2016; Moraes et al., 2020; dos Santos et al., 2021), and anti-inflammatory and healing effects on tongue lesion (Teixeira et al., 2017; Wagner et al., 2017; Alvarenga et al., 2020). One study reported the lack of the Copaiba effect on mandibular bone, which could considerably benefit buccomaxillofacial surgery and orthodontics (Silva et al., 2015). The unique clinical trial that evaluated the effects of a Copaiba-containing varnish on children’s teeth to decrease the risk of dental caries (Rocha Valadas et al., 2021). Moreover, the unique in situ study found in this bibliometric analysis originated from the dentistry field and evaluated the antiproteolytic activity of Copaiba oil-based emulsion at the resin/dentin adhesive interface (Araújo et al., 2021).
A global distribution analysis showed that most studies were conducted in Brazil, which is the country that presents the most significant number of species worldwide. The Brazilian climate, particularly in the Amazon region, favors the growth of Copaiba trees. The hot and humid Amazon biome has abundant rainfall, a diverse range of ecosystems, and represents 49% of the Brazilian territory (Moraes et al., 2020; dos Santos et al., 2021). Nevertheless, the first research on Copaiba was conducted in 1983 at the University of California (United States) and published in Biochemistry and Molecular Biology (Arrhenius and Langenheim, 1983). Then in 1988, researchers from the University of São Paulo (Brazil) showed that dose-dependent administration of Copaiba oil-resin prevented the development of carrageenin-induced pedal edema in female rats; in addition, repeated administration of the oil-resin at 1.26 mL/kg for 6 days considerably reduced the permeability increase caused by histamine (Basile et al., 1988). Despite the promising results, the second Brazilian article on Copaiba was published only 10 years later and showed that oral administration of the oil-resin obtained from C. langsdorffii at 200 and 400 mg/kg provided dose-dependent protection against ethanol-induced gastric damage. Moreover, the administration of C. langsdorffii oil-resin at 400 mg/kg protected against indomethacin-induced gastric ulceration. In addition, the oil-resin significantly increased the accumulation of gastric juice volume and mucus secretion and suppressed overall acidity in pylorus-ligated rats after 4 h (Paiva et al., 1998).
In the meantime, a Japanese research investigated the anti-tumor potential of C. langsdorffii and its compounds (particularly the diterpenoid clerodane) (Ohsaki et al., 1994), while another in vitro study in Argentina demonstrated that C. reticulata inhibited free radical-mediated DNA damage (Desmarchelier et al., 1997). In 2002, a study originated from Spain determined the significant leishmanicidal, antimicrobial, cytotoxic, and aldose reductase inhibitory activities of compounds isolated from C. paupera (Senedese et al., 2019).
Henceforth, Brazil stood out in the research on biological activities of Copaiba for 10 years through the publication of in vitro studies as well as in vivo studies with animals. Furthermore, the first clinical trial on the effects of Copaiba against acne was conducted. Between 2003 and 2013, there was also an increase in the number of studies on antimicrobial activities, including bacteria, fungi, and viruses.
The studies usually investigate antibacterial activity through the minimum inhibitory concentration (MIC) method, which determines the in vitro sensitivity or resistance of specific bacterial strains to an antibiotic. The MIC of an antibiotic is the lowest concentration at which the growth of a particular strain of bacteria is completely inhibited under strictly controlled conditions. Recently, this method has been routinely reported for standard testing (Desmarchelier et al., 1997).
This bibliometric analysis found that most of the studies were conducted in vitro and very few clinical trials were published. Although in vitro and in vivo studies are needed, the level of evidence of the effects of Copaiba must be validated by studies with humans; in addition, there is a lack of either in vivo and in vitro investigations on mineralized tissues. Furthermore, most studies did not detail the species of Copaiba, probably due to the use of a commercial sample. However, some variations in the compounds among species may alter the biological activities induced in the same target.
5 Conclusion
This bibliometric analysis explored the global knowledge developed over the years regarding the biological activities of Copaiba. The comprehensive evaluation of 11 species was mainly based on in vivo investigations that emphasized antimicrobial activity. There is a growing interest in Copaiba within medical and dental fields, in which numerous studies explored potential applications across several specialties. Nevertheless, further research is required to validate the effects of Copaiba in humans, as well as the comparison among different Copaiba species. Overall, this review highlights the extensive potential of Copaiba and the importance of advanced research to support its evidence-based use.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: DF and RL; methodology: DF, DB-S, and MS; formal analysis: DF; DB-S, and RL; investigation: DF, DB-S, and MS; resources: RL; data curation: DF, DB-S, and RL; writing original draft preparation: DF, DB-S, JC, RF, and MF; writing, review, and editing: DF, JC, MS, RS-R, and RL; visualization: DF, JC, and RL; supervision: RL. All authors contributed to the article and approved the submitted version.
Funding
RL is a Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) researcher and received a grant under number 312275/2021-8. The APC was funded by Pró-Reitoria de Pesquisa e Pós-graduação from Federal University of Pará (PROPESP-UFPA).
Acknowledgments
We would like to thank the Brazilian National Council for Scientific and Technological Development (CNPq) and the Programa Nacional de Cooperação Acadêmica na Amazônia—PROCAD/Amazônia from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1215437/full#supplementary-material
References
1
AbrãoF.AlvesJ. A.AndradeG.de OliveiraP. F.AmbrósioS. R.VenezianiR. C. S.et al (2018). Antibacterial effect of Copaifera duckei Dwyer oleoresin and its main diterpenes against oral pathogens and their cytotoxic effect. Front. Microbiol.9, 201. Epub ahead of print 21 February 2018. 10.3389/fmicb.2018.00201
2
AbrãoF.de Araújo CostaL. D.AlvesJ. M.SenedeseJ. M.de CastroP. T.AmbrósioS. R.et al (2015). Copaifera langsdorffii oleoresin and its isolated compounds: Antibacterial effect and antiproliferative activity in cancer cell lines. BMC Complement. Altern. Med.15, 443. Epub ahead of print 21 December 2015. 10.1186/s12906-015-0961-4
3
AdzuB.BalogunS. O.PavanE.AscêncioS. D.SoaresI. M.AguiarR. W. S.et al (2015). Evaluation of the safety, gastroprotective activity and mechanism of action of standardised leaves infusion extract of Copaifera malmei Harms. J. Ethnopharmacol.175, 378–389. 10.1016/j.jep.2015.09.027
4
AlvarengaM. O. P.BittencourtL. O.MendesP. F. S.RibeiroJ. T.LameiraO. A.MonteiroM. C.et al (2020). Safety and effectiveness of copaiba oleoresin (C. reticulata ducke) on inflammation and tissue repair of oral wounds in rats. Int. J. Mol. Sci.21, 3568. Epub ahead of print 2 May 2020. 10.3390/ijms21103568
5
AraújoE. A. M.LimaG. R.Santos De MeloL. A. dosde SousaL. B.de VasconcellosM. C.CondeN. C. d. O.et al (2021). Effect of a copaiba oil-based dental biomodifier on the inhibition of metalloproteinase in adhesive restoration. Adv. Pharmacol. Pharm. Sci.2021, 8840570. Epub ahead of print 2021. 10.1155/2021/8840570
6
ArrheniusS. P.LangenheimJ. H. (1983). Inhibitory effects of Hymenaea and Copaifera leaf resins on the leaf fungus, Pestalotia subcuticularis. Biochem. Syst. Ecol.11, 361–366. 10.1016/0305-1978(83)90037-6
7
ArrudaC.Aldana MejíaJ. A.RibeiroV. P.Gambeta BorgesC. H.MartinsC. H. G.Sola VenezianiR. C.et al (2019). Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—a review. Biomed. Pharmacother.109, 1–20. 10.1016/j.biopha.2018.10.030
8
BahrT.AllredK.MartinezD.RodriguezD.WintertonP. (2018). Effects of a massage-like essential oil application procedure using Copaiba and Deep Blue oils in individuals with hand arthritis. Complement. Ther. Clin. Pract.33, 170–176. 10.1016/j.ctcp.2018.10.004
9
BandeiraM. F.OliveriaM.PizzolittoA. C. (2000). Antibacterial activity of the copaiba oil associated to the Ca(OH)(2) and to the zinc oxide. J. Dent. Res.79, 1070.
10
BasileA. C.SertiiJ. A. A.FreitavP. C. D. (1988). Anti-inflammatory activity of oleoresin from Brazilian Copaifera. J. Ethnopharmacol.22, 81.
11
BattistiC.GarletT. M. B.EssiL.HorbachR. K.de AndradeA.BadkeM. R. (2013). Plantas medicinais utilizadas no município de Palmeira das Missões, RS, Brasil. Rev. Bras. Biociências11, 338–348.
12
BrancalionA. P. S.OliveiraR. B.SousaJ. P. B.GroppoM.BerrettaA. A.BarrosM. E.et al (2012). Effect of hydroalcoholic extract from Copaifera langsdorffii leaves on urolithiasis induced in rats. Urol. Res.40, 475–481. 10.1007/s00240-011-0453-z
13
ChavesJ. S.LealP. C.PianowiskyL.CalixtoJ. B. (2008). Pharmacokinetics and tissue distribution of the sesquiterpene alpha-humulene in mice. Planta Med.74, 1678–1683. 10.1055/s-0028-1088307
14
CoutoR. S. D.RodriguesM. F. S. D.FerreiraL. S.DinizI. M. A.SilvaF. d. S.LopezT. C. C.et al (2020). Evaluation of resin-based material containing copaiba oleoresin (Copaifera reticulata ducke): Biological effects on the human dental pulp stem cells. Biomolecules10, 972–1014. 10.3390/biom10070972
15
Cox-GeorgianD.RamadossN.DonaC. (2019). Therapeutic and medicinal uses of terpenes. undefined2019, 333–359. 10.1007/978-3-030-31269-5_15
16
da SilvaA. G.PuziolP. de F.LeitãoR. N.GomesT. R.SchererR.MartinsM. L. L.et al (2012). Application of the essential oil from copaiba (Copaifera langsdorffii desf) for acne vulgaris: A double-blind, placebo controlled clinical trial. Altern. Med. Rev. a J. Clin. Ther.17, 69–75.
17
da TrindadeR.da SilvaJ. K.SetzerW. N. (2018). Copaifera of the neotropics: A review of the phytochemistry and pharmacology. Int. J. Mol. Sci.19, 1511. Epub ahead of print 18 May 2018. 10.3390/IJMS19051511
18
de CarvalhoC.Ferreira-D. A.WedgeD.CantrellC. L.RosaL. H. (2018). Antifungal activities of cytochalasins produced by Diaporthe miriciae, an endophytic fungus associated with tropical medicinal plants. Can. J. Microbiol.64, 835–843. 10.1139/cjm-2018-0131
19
de LimaM. R. F.de Souza LunaJ.dos SantosA. F.de AndradeM. C. C.Sant'AnaA. E. G.GenetJ. P.et al (2006). Anti-bacterial activity of some Brazilian medicinal plants. J. Ethnopharmacol.105, 137–147. 10.1016/j.jep.2005.10.026
20
DesmarchelierC.CoussioJ.CicciaG. (1997). Extracts of Bolivian plants, Copaifera reticulata and heisteria pallida inhibit in vitro free radical-mediated DNA damage. Phytotheraphy Res.11, 460–462. 10.1002/(sici)1099-1573(199709)11:6<460::aid-ptr125>3.0.co;2-x
21
DiniV. S. Q.FurtadoS. da C.BarcellosJ. F. M.de CostaO. S. T. (2019). Ação anti-inflamatória do óleo de copaíba em artrite induzida em modelo animal: Uma revisão sistemática - PDF download grátis. Sci. Amazon.8, CB1.
22
DionisioK. L.PhillipsK.PriceP. S.GrulkeC. M.WilliamsA.BiryolD.et al (2018). The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data5, 180125–180129. 10.1038/sdata.2018.125
23
do RosárioM. M. T.NoletoG. R.de Oliveira PetkowiczC. L. (2017). Degalactosylation of xyloglucans modify their pro-inflammatory properties on murine peritoneal macrophages. Int. J. Biol. Macromol.105, 533–540. 10.1016/j.ijbiomac.2017.07.068
24
dos SantosA. C. M.OliveiraV. C.MacedoA. P.BastosJ. K.OgasawaraM. S.WatanabeE.et al (2021). Effectiveness of oil-based denture dentifrices-organoleptic characteristics, physicochemical properties and antimicrobial action. Antibiotics10, 813. Epub ahead of print 1 July 2021. 10.3390/antibiotics10070813
25
FariasC. L. A.MartinezG. R.CadenaS. M. S. C.MercêA. L. R.de Oliveira PetkowiczC. L.NoletoG. R. (2019). Cytotoxicity of xyloglucan from Copaifera langsdorffii and its complex with oxovanadium (IV/V) on B16F10 cells. Int. J. Biol. Macromol.121, 1019–1028. 10.1016/j.ijbiomac.2018.10.131
26
Fernández-MorianoC.González-BurgosE.Gómez-SerranillosM. P. (2019). Curcumin: Current evidence of its therapeutic potential as a lead candidate for anti-inflammatory drugs—An overview. Discov. Dev. Anti-inflammatory Agents Nat. Prod.2019, 7–59.
27
GerisR.SilvaI. G.SilvaH. H. G.BarisonA.Rodrigues-FilhoE.FerreiraA. G. (2008). Diterpenoids from Copaifera reticulata ducke with larvicidal activity against Aedes aegypti (L) (Diptera, Culicidae). Rev. Inst. Med. Trop. S Paulo50, 25–28. 10.1590/s0036-46652008000100006
28
GertschJ.LeontiM.RadunerS.RaczI.ChenJ. Z.XieX. Q.et al (2008). Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U. S. A.105, 9099–9104. 10.1073/pnas.0803601105
29
GroppoF. C.de Cássia BergamaschiC.CogoK.Franz-MontanM.MottaR. H. L.de AndradeE. D. (2008). Use of phytotherapy in dentistry. Phytother. Res.22, 993–998. 10.1002/ptr.2471
30
Guimarães-SantosA.SantosD. S.SantosI. R.LimaR. R.PereiraA.de MouraL. S.et al (2012). Copaiba oil-resin treatment is neuroprotective and reduces neutrophil recruitment and microglia activation after motor cortex excitotoxic injury. Evidence-based Complementary Altern. Med.2012, 918174. Epub ahead of print 2012. 10.1155/2012/918174
31
IdippilyN. D.ZhengQ.GanC.QuamineA.AshcraftM. M.ZhongB.et al (2017). Copalic acid analogs down-regulate androgen receptor and inhibit small chaperone protein. Bioorg Med. Chem. Lett.27, 2292–2295. 10.1016/j.bmcl.2017.04.046
32
KawakamiM. Y. M.ZamoraL. O.AraújoR. S.FernandesC. P.RicottaT. Q. N.de OliveiraL. G.et al (2021). Efficacy of nanoemulsion with Pterodon emarginatus Vogel oleoresin for topical treatment of cutaneous leishmaniasis. Biomed. Pharmacother.134, 111109. Epub ahead of print 1 February 2021. 10.1016/j.biopha.2020.111109
33
LameiraO. A.Martins-Da-SilvaR. C. V.ZoghbiM. D. G. B.OliveiraE. C. (2009). Seasonal variation in the volatiles of Copaifera duckei dwyer growing wild in the state of Pará—Brazil. Taylor Francis online21, 105–107. 10.1080/10412905.2009.9700124
34
LovelockC. E.PosadaJ.WinterK. (1999). Effects of elevated CO2 and defoliation on compensatory growth and photosynthesis of seedlings in a tropical tree, Copaifera aromatica1. Biotropica31, 279–287. 10.1111/j.1744-7429.1999.tb00139.x
35
MacielM. A. M.PintoA. C.VeigaV. F.GrynbergN. F.EchevarriaA. (2002). Plantas medicinais: A necessidade de estudos multidisciplinares. Quim Nova25, 429–438. 10.1590/s0100-40422002000300016
36
MassonD. S.SalvadorS. L.PolizelloA. C. M.FradeM. (2013). Atividade antimicrobiana do óleo-resina de copaíba (Copaifera langsdorffii) em bactérias de significância clínica em úlceras cutâneas. Rev. Bras. Plantas Med.15, 664–669. 10.1590/s1516-05722013000500006
37
MauroM.da SilvaR. M.de CamposM. L.BauermeisterA.LopesN. P.de MoraesN. V. (2021). In vitro metabolism of copalic and kaurenoic acids in rat and human liver microsomes. Quim Nova44, 700–708. 10.21577/0100-4042.20170724
38
MenezesA. C. dos S.AlvesL. D. B.GoldembergD. C.de MeloA. C.AntunesH. S. (2022). Anti-inflammatory and wound healing effect of copaiba oleoresin on the oral cavity: A systematic review. Heliyon8, e08993. 10.1016/j.heliyon.2022.e08993
39
MoraesÂ. A. B. deFerreiraO. O.CostaL. S. daAlmeidaL. Q.VarelaE. L. P.CascaesM. M.et al (2022). Phytochemical profile, preliminary toxicity, and antioxidant capacity of the essential oils of myrciaria floribunda (H west ex willd) O berg and myrcia sylvatica (G.mey) DC (myrtaceae). Antioxidants11, 2076. 10.3390/antiox11102076
40
MoraesT. da S.LeandroL. F.SantiagoM. B.de Oliveira SilvaL.BianchiT. C.VenezianiR. C. S.et al (2020). Assessment of the antibacterial, antivirulence, and action mechanism of Copaifera pubiflora oleoresin and isolated compounds against oral bacteria. Biomed. Pharmacother.129, 110467. Epub ahead of print 1 September 2020. 10.1016/j.biopha.2020.110467
41
MoraesT. da S.LeandroL. F.SilvaL. de O.SantiagoM. B.SouzaA. B.FurtadoR. A.et al (2016). In vitro evaluation of Copaifera oblongifolia oleoresin against bacteria causing oral infections and assessment of its cytotoxic potential. Curr. Pharm. Biotechnol.17, 894–904. 10.2174/1389201017666160415155359
42
MuradM. H.AsiN.AlsawasM.AlahdabF. (2016). New evidence pyramid. BMJ Evid. Based Med.21, 125–127. 10.1136/ebmed-2016-110401
43
OhsakiA.YanL. T.ItoS.EdatsugiH.IwataD.KomodaY. (1994). The isolation and in vivo Potent Antitumor activity of clerodane diterpenoid from the oleoresin of the brazilian medicinal plant, Copaifera langsdorfi desfon. Bioorg Med. Chem. Lett.4, 2889–2892. 10.1016/s0960-894x(01)80834-9
44
OliveiraL. C.PortoT. S.JuniorA. H. C.SantosM. F. C.RamosH. P.BraunG. H.et al (2020). Schistosomicidal activity of kaurane, labdane and clerodane-type diterpenes obtained by fungal transformation. Process Biochem.98, 34–40. 10.1016/j.procbio.2020.07.020
45
PaivaL. A. F.RaoV. S. N.GramosaN. v.SilveiraE. R. (1998). Gastroprotective effect of Copaifera langsdorffii oleo-resin on experimental gastric ulcer models in rats. J. Ethnopharmacol.62, 73–78. 10.1016/s0378-8741(98)00058-0
46
PalomboE. A. (2011). Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Altern. Med.2011, 680354. Epub ahead of print 2011. 10.1093/ECAM/NEP067
47
ParreiraD. S.Alcántara-de la CruzR.Rodrigues DimatéF. A.BatistaL. D.RibeiroR. C.Rigueira FerreiraG. A.et al (2019). Bioactivity of ten essential oils on the biological parameters of Trichogramma pretiosum (hymenoptera: Trichogrammatidae) adults. Ind. Crops Prod.127, 11–15. 10.1016/j.indcrop.2018.10.063
48
PenidoA. B.de MoraisS. M.RibeiroA. B.AlvesD. R.RodriguesA. L. M.Dos SantosL. H.et al (2017). Medicinal plants from northeastern Brazil against Alzheimer’s disease. Evidence-based Complementary Altern. Med.2017, 1753673. Epub ahead of print 2017. 10.1155/2017/1753673
49
PereiraD. L.da CunhaA. P. S.CardosoC. R. P.RochaC. Q. D.VilegasW.SinhorinA. P.et al (2018). Antioxidant and hepatoprotective effects of ethanolic and ethyl acetate stem bark extracts of Copaifera multijuga (Fabaceae) in mice. Acta Amaz.48, 347–357. 10.1590/1809-4392201704473
50
PetrovskaB. B. (2012). Historical review of medicinal plants’ usage. Pharmacogn. Rev.6, 1–5. 10.4103/0973-7847.95849
51
PieriF.SilvaV.VargasF. (2013). Antimicrobial activity of Copaifera langsdorffii oil and evaluation of its most bioactive fraction against bacteria of dog’s dental plaque. Pak Vet. J.34, 165–169.
52
PieriF. A.MussiM. C.FioriniJ. E.SchneedorfJ. (2010). Clinical and microbiological effects of copaiba oil (Copaifera officinalis) on dental plaque forming bacteria in dogs. Arq. Bras. Med. Vet. Zootec.62, 578–585. 10.1590/s0102-09352010000300012
53
PieriF. A.MussiM. C. MMoreiraM. A. (2009). Óleo de copaíba (Copaifera sp): Histórico, extração, aplicações industriais e propriedades medicinais. Rev. Bras. Pl. Med.11, 465–472. 10.1590/s1516-05722009000400016
54
ReiznauttC. M.RibeiroJ. S.KrepsE.da RosaW. L. O.de LacerdaH.PeraltaS. L.et al (2021). Development and properties of endodontic resin sealers with natural oils. J. Dent.104, 103538. Epub ahead of print 1 January 2021. 10.1016/j.jdent.2020.103538
55
Rocha ValadasL. A.Dantas LoboP. L.da Cruz FonsecaS. G.FechineF. V.Rodrigues NetoE. M.FontelesM. M. d. F.et al (2021). Clinical and antimicrobial evaluation of Copaifera langsdorffii desf. Dental varnish in children: A clinical study. Evidence-based Complementary Altern. Med.2021, 6647849. Epub ahead of print 2021. 10.1155/2021/6647849
56
Rodrigues da SilvaG. H.GeronimoG.García-LópezJ. P.RibeiroL. N. M.de MouraL. D.BreitkreitzM. C.et al (2020). Articaine in functional NLC show improved anesthesia and anti-inflammatory activity in zebrafish. Sci. Rep.10, 19733. Epub ahead of print 1 December 2020. 10.1038/s41598-020-76751-6
57
SantosR. L.GuimaraesG. P.NobreM. S. C.PortelaA. (2011). Análise sobre a fitoterapia como prática integrativa no Sistema Único de Saúde. Rev. Bras. Plantas Med.13, 486–491. 10.1590/s1516-05722011000400014
58
SenedeseJ. M.Rinaldi-NetoF.FurtadoR. A.NicollelaH. D.de SouzaL. D. R.RibeiroA. B.et al (2019). Chemopreventive role of Copaifera reticulata Ducke oleoresin in colon carcinogenesis. Biomed. Pharmacother.111, 331–337. 10.1016/j.biopha.2018.12.091
59
SilvaA. N.SoaresA. C. F.CabralM. M. W.de AndradeA. R. P.de SlivaM. B.MartinsC. H. G.et al (2017). Antitubercular activity increase in labdane diterpenes from Copaifera oleoresin through structural modification. J. Braz Chem. Soc.28, 1106–1112. 10.21577/0103-5053.20160268
60
SilvaJ. A.MacedoM. L. R.NovelloJ. C.MarangoniS. (2001). Biochemical characterization and N-terminal sequences of two new trypsin inhibitors from Copaifera langsdorffii seeds. J. Protein Chem.20, 1–7. 10.1023/a:1011053002001
61
SilvaP. F.BritoM. V. H.PontesF. S. C.RamosS. R.MendesL. C.OliveiraL. C. M. (2015). Copaiba oil effect on experimental jaw defect in Wistar rats. Acta Cir. Bras.30, 120–126. 10.1590/S0102-86502015002000006
62
SímaroG. V.LemosM.Mangabeira da SilvaJ. J.RibeiroV. P.ArrudaC.SchneiderA. H.et al (2021). Antinociceptive and anti-inflammatory activities of Copaifera pubiflora Benth oleoresin and its major metabolite ent-hardwickiic acid. J. Ethnopharmacol.271, 113883. Epub ahead of print 10 May 2021. 10.1016/j.jep.2021.113883
63
SouzaA. B.de SouzaM. G. M.MoreiraM. A.FurtadoN. A. J. C.MartinsC. H. G.et al (2011a). Antimicrobial evaluation of diterpenes from Copaifera langsdorffii oleoresin against periodontal anaerobic bacteria. Molecules16, 9611–9619. 10.3390/molecules16119611
64
SouzaA. B.MartinsC. H. G.SouzaM. G. M.FurtadoN. A. J. C.HelenoV. C. G.de SousaJ. P. B.et al (2011b). Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytotherapy Res.25, 215–220. 10.1002/ptr.3244
65
SouzaM. G. M. deLeandroL. F.MoraesT. da S.AbrãoF.VenezianiR. C. S.AmbrosioS. R.et al (2018). ent-Copalic acid antibacterial and anti-biofilm properties against Actinomyces naeslundii and Peptostreptococcus anaerobius. Anaerobe52, 43–49. 10.1016/j.anaerobe.2018.05.013
66
TeixeiraF. B.SilvaR. de B.LameiraO. A.WebberL. P.D'Almeida CoutoR. S.MartinsM. D.et al (2017). Copaiba oil-resin (Copaifera reticulata Ducke) modulates the inflammation in a model of injury to rats’ tongues. BMC Complement. Altern. Med.17, 313. Epub ahead of print 2017. 10.1186/s12906-017-1820-2
67
TincusiB. M.JiménezI. A.BazzocchiI. L.MoujirL. M.MamaniZ. A.BarrosoJ. P.et al (2002). Antimicrobial terpenoids from the oleoresin of the Peruvian medicinal plant Copaifera paupera. Planta Med.68, 808–812. 10.1055/s-2002-34399
68
ToboutiP. L.de Andrade MartinsT. C.PereiraT. J.MussiM. C. M. (2017). Antimicrobial activity of copaiba oil: A review and a call for further research. Biomed. Pharmacother.94, 93–99. 10.1016/j.biopha.2017.07.092
69
TurkezH.TogarB.TatarA.GeyıkogluF.HacımuftuogluA. (2014). Cytotoxic and cytogenetic effects of α-copaene on rat neuron and N2a neuroblastoma cell lines. Biol. Pol.69, 936–942. 10.2478/s11756-014-0393-5
70
van EckN. J.WaltmanL. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics84, 523–538. 10.1007/s11192-009-0146-3
71
VasconcelosK. R. F.Veiga JuniorV. F. daRochaW. C.BandeiraM. F. C. L. (2008). n vitro assessment of antibacterial activity of a dental cement constituted of a Copaifera multijuga Hayne oil-resin. Rev. Bras. Farmacogn.18, 733–738. 10.1590/s0102-695x2008000500017
72
Veiga JuniorV. F.PintoA. C. (2002). The Copaifera L. GENUS. This review details the history, chemistry and pharmacology of the Copaifera L. genus (Leguminosae- Caesalpinoideae), including copaiba oil. Quim Nova25, 273–286. 10.1590/s0100-40422002000200016
73
VeigaV. F.RosasE. C.CarvalhoM. v.HenriquesM. G. M. O.PintoA. C. (2007). Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne-A comparative study. J. Ethnopharmacol.112, 248–254. 10.1016/j.jep.2007.03.005
74
VieiraH. S.TakahashiJ. A.de OliveiraA. B.ChiariE.BoaventuraM. A. D. (2002). Novel derivatives of kaurenoic acid. J. Braz Chem. Soc.13, 151–157. 10.1590/s0103-50532002000200004
75
WagnerV. P.WebberL. P.OrtizL.RadosP. V.MeurerL.LameiraO. A.et al (2017). Effects of copaiba oil topical administration on oral wound healing. Phytotherapy Res.31, 1283–1288. 10.1002/ptr.5845
76
WaibelJ.PatelH.CullE.SidhuR.LupatiniR. (2021). Prospective, randomized, double-blind, placebo-controlled study on efficacy of copaiba oil in silicone-based gel to reduce scar formation. Dermatol Ther. (Heidelb)11, 2195–2205. 10.1007/s13555-021-00634-5
Summary
Keywords
Copaiba oil-resin, biological products, bibliometrics, medicinal plants, traditional medicine
Citation
Frazão DR, Cruz JN, Santana de Oliveira M, Baia-da-Silva DC, Nazário RMF, Rodrigues MFdL, Saito MT, Souza-Rodrigues RD and Lima RR (2023) Evaluation of the biological activities of Copaiba (Copaifera spp): a comprehensive review based on scientometric analysis. Front. Pharmacol. 14:1215437. doi: 10.3389/fphar.2023.1215437
Received
03 May 2023
Accepted
31 July 2023
Published
01 September 2023
Volume
14 - 2023
Edited by
Mohammad S. Mubarak, The University of Jordan, Jordan
Reviewed by
Francisco Isaac Fernandes Gomes, University of São Paulo, Brazil
Suzana Guimaraes Leitao, Federal University of Rio de Janeiro, Brazil
Updates
Copyright
© 2023 Frazão, Cruz, Santana de Oliveira, Baia-da-Silva, Nazário, Rodrigues, Saito, Souza-Rodrigues and Lima.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafael Rodrigues Lima, rafalima@ufpa.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.