- 1College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3College of Ethnomedicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
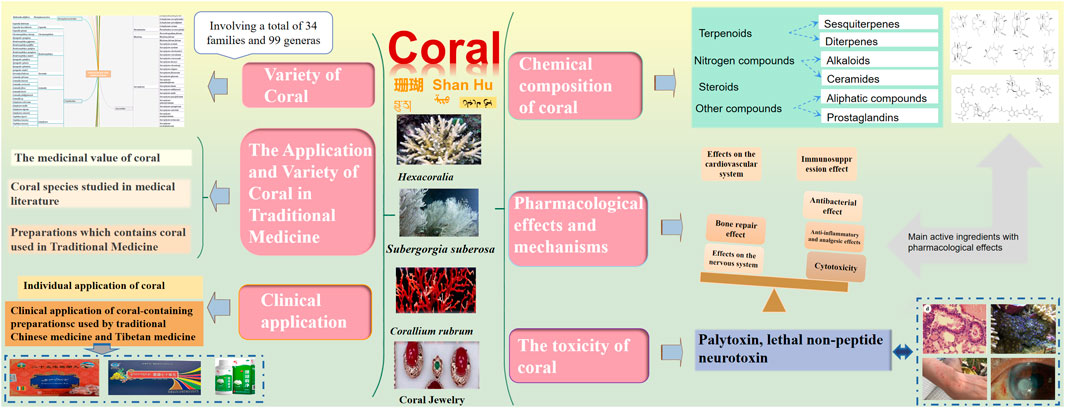
This review discusses the variety, chemical composition, pharmacological effects, toxicology, and clinical research of corals used in traditional medicine in the past two decades. At present, several types of medicinal coral resources are identified, which are used in 56 formulas such as traditional Chinese medicine, Tibetan medicine, Mongolian medicine, and Uyghur medicine. A total of 34 families and 99 genera of corals are involved in medical research, with the Alcyoniidae family and Sarcophyton genus being the main research objects. Based on the structural types of compounds and the families and genera of corals, this review summarizes the compounds primarily reported during the period, including terpenoids, steroids, nitrogen-containing compounds, and other terpenoids dominated by sesquiterpene and diterpenes. The biological activities of coral include cytotoxicity (antitumor and anticancer), anti-inflammatory, analgesic, antibacterial, antiviral, immunosuppressive, antioxidant, and neurological properties, and a detailed summary of the mechanisms underlying these activities or related targets is provided. Coral toxicity mostly occurs in the marine ornamental soft coral Zoanthidae family, with palytoxin as the main toxic compound. In addition, nonpeptide neurotoxins are extracted from aquatic corals. The compatibility of coral-related preparations did not show significant acute toxicity, but if used for a long time, it will still cause toxicity to the liver, kidneys, lungs, and other internal organs in a dose-dependent manner. In clinical applications, individual application of coral is often used as a substitute for orthopedic materials to treat diseases such as bone defects and bone hyperplasia. Second, coral is primarily available in the form of compound preparations, such as Ershiwuwei Shanhu pills and Shanhu Qishiwei pills, which are widely used in the treatment of neurological diseases such as migraine, primary headache, epilepsy, cerebral infarction, hypertension, and other cardiovascular and cerebrovascular diseases. It is undeniable that the effectiveness of coral research has exacerbated the endangered status of corals. Therefore, there should be no distinction between the advantages and disadvantages of listed endangered species, and it is imperative to completely prohibit their use and provide equal protection to help them recover to their normal numbers. This article can provide some reference for research on coral chemical composition, biological activity, chemical ecology, and the discovery of marine drug lead compounds. At the same time, it calls for people to protect endangered corals from the perspectives of prohibition, substitution, and synthesis.
1 Introduction
Marine biological resources are abundant, and coral is a common organism in the ocean. It is a low-level invertebrate of the ocean, belonging to the phylum Coelenterata and the class Coralis. Coral mainly lives in tropical oceans and has a wide variety and distribution. There are over 6,100 species of coral worldwide and 719 species in China (Li T. T., 2010). Corals can be divided into Hexacorallia and Octocorallia (Xu, 2016). Corals are known as “sea flowers” and are a type of aquatic coelenterate. Their population is dendritic, branching and spreading like fans, with fine branches. Their surface contains many hydra bodies called anthozoan polyps. Their body is hemispherical in shape, with eight feathered tentacles on top. The tentacles have a mouth in the center, and the insect body can secrete limestone to form bones. White is better than snow; red is similar to blood; green is similar to jade, and yellow is similar to gold. Coral naturally grows in the sea, with strange shapes and unparalleled beauty (National Compilation of Chinese Herbal Medicine, 1996). The main compound of coral is calcium carbonate, which also contains a series of elements such as iron, manganese, copper, and strontium, as well as chitin and organic acids. Corals are commonly white, whereas gemstone-grade corals are red, pink, and orangey red, with a small amount of black and blue. The color of coral is due to its content of approximately 1% iron oxide and organic matter. With red as the top grade, red coral is as red as fire, known as the “fire tree” in ancient times. Its origin is in the deep sea of the Mediterranean and Atlantic oceans, and it is primarily used for jewelry, with the largest being used for carving figures, flowers, and birds, and other handicrafts (Wen, 2007). In India and Tibet of China, people use coral as a mascot for worship, often used to make Buddhist beads and decorate deities. In the West, coral is one of the three major organic gemstones, whereas in the East, coral symbolizes auspiciousness and happiness since ancient times. It also represents nobility and power and symbolizes happiness and eternity (Wen et al., 2007). The ancient Romans believed that coral played roles in disaster prevention, intelligence, hemostasis, and heat dissipation, which continued until this century (Hong, 2009).
Corals are distributed in the South China Sea, North China Sea, and East China Sea, among which the South China Sea is located in a tropical and subtropical zone and contains abundant coral biological resources. Since the 1980s, chemists have conducted in-depth research on corals in the South China Sea. At the beginning of the 20th century, the utilization of coral resources includes human bone substitutes, feed calcium filler, and a good source of calcium supply for the human body (Huang et al., 1997). With the rapid development of modern separation and identification methods and the increasing maturity of biotechnology, a large number of active substances have been isolated from marine organisms (Zhang G. et al., 2013), such as salicin with antibacterial activity and alkaloids with cytotoxic activity, which have been isolated from Sinularia suberosa on the side of the South China Sea (Qi et al., 2005). In addition, antitumor alkaloids have been obtained from Ellisella curvata on the side of the South China Sea (Zhang J. R., 2012). With the deepening of chemical research on natural products of soft coral and gorgonians※, thousands of compounds with dozens of structural skeletons have been discovered, including steroids, terpenoids, nitrogen-containing compounds, long-chain fatty acids, and long-chain alcohols. The diverse structures, unique molecular frameworks, and significant pharmacological activities of coral secondary metabolites fully demonstrate their potential medicinal value (Zhang W. et al., 2006; Shao et al., 2009a).
In the late 1960s, scholars and others discovered prostaglandin precursors with unique structures and strong physiological activity from gorgonian※, which further promoted coral chemistry research. The pharmacological activity of coral is also gradually being explored, which is primarily manifested in various aspects such as antitumor, anticancer, antioxidant, and anti-cardiovascular and in cerebrovascular system diseases. These pharmacological effects are mostly exerted by a single active substance extracted from coral bodies, while the bones of corals are mostly used as materials for bone transplantation and other applications (Wang et al., 2002b). Coral, as a medicinal material, has been recorded in detail in The Compendium of Materia Medica (1578 AD). It tastes sweet, and the property of the medicine is flat. It can improve eyesight, tranquilize the mind, and stop epilepsy. Coral is primarily used to treat corneal opacity. It also dissipates blood stasis. Coral powder can stop epistaxis. In the clinic, coral is also used in various compound preparations, such as Ershiwuwei Shanhu pills and Shanhu Qishiwei pills, which can restore nerve function and relieve pain. It is used in the treatment of albichoriasis, unconsciousness, body numbness, dizziness, brain pain, irregular blood pressure, headache, epilepsy, and various types of neuropathic pain. The Compendium of Materia Medica (1578 AD) records that corals are nontoxic, but according to literature reports, corals can release toxins, which are the second largest known deadly gas in the world, ultimately leading to toxic reactions such as muscle pain, four-limb weakness, and fainting. By contrast, in compound use, short-term use does not produce acute toxic reactions, but long-term use can cause damage to the liver, kidneys, and other organs.
In this review, we first conducted keyword searches on coral on academic websites such as PubMed, ScienceDirect, and CNKI and screened thousands of literature works related to medicine. Second, we conducted data mining to establish a database and finally extracted effective information for organization and analysis. This review discusses the use of coral in traditional medicine and its application in chemical composition, pharmacology, toxicology, and clinical research in the past two decades to provide important research data for the comprehensive development of marine biological resources, the discovery of drug lead compounds, the chemical ecological research of marine invertebrates, and the determination of organic synthetic chemical target compounds.
2 The application and variety of coral in traditional medicine
2.1 Coral species studied in the medical literature
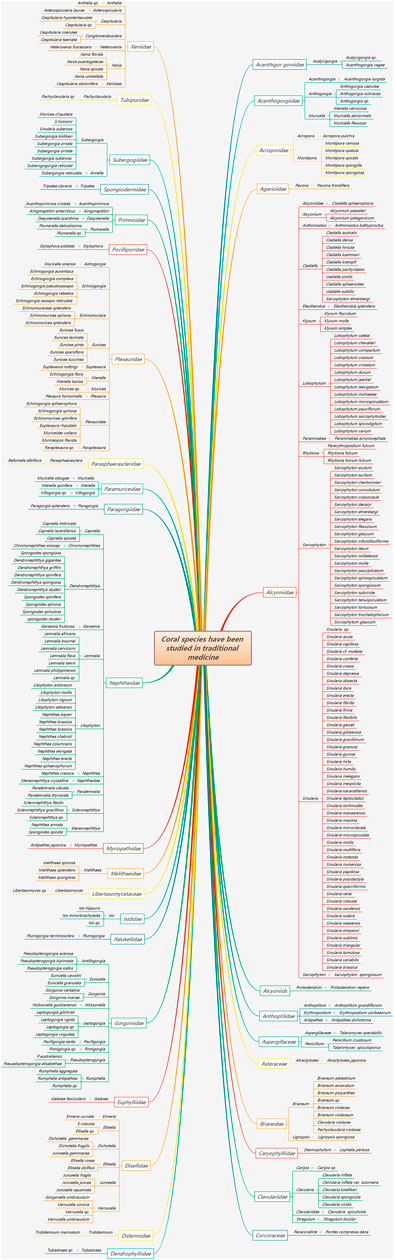
Tang Sujing mentioned in the Newly Revised Materia Medica in 659 AD that Corallium rubrum (Linnaeus), also known as red coral, Hong shan, Huo shu, and Corallium japonicum※, belongs to the genus Corallium in the family Coralliidae. In addition, Corallium japonicum Kishinouye※ was included in the genus Corallium in the family Coralliidae in the National Compilation of Chinese Herbal Medicine (Second Edition). Zhuru, Ulan Shuru, and Shuru are recorded as Mongolian medicines. Fossilia corrallium is recorded as a Uyghur medicine in the Dictionary of Chinese Ethnic Medicine, which is mostly distributed in the Baihe Mahle River. It is commonly used to treat diarrhea, gastrointestinal bleeding, and neurasthenia. The Dictionary of Traditional Chinese Medicine also records Corallium japonicum Kishinouye※, which is recorded as Corallium konojoi※ with the same name as that recorded in the Chinese Traditional Chinese Medicine Resources. Corallium secundum Dana※ and Corallium elatius Ridley are also recorded. The Records of Chinese Traditional Chinese Medicine Resources (Part 2) also records six species of coral, namely, Porites nigrescens Dana in the Poritidae family, Porites genus; Antipathes sp.; and the national first-class protected wild animals Corallium japonicum Kishinouye※, Corallium elatius Ridley, and Corallium konojoi Kishinouye※. In the past two decades, most of the coral species that have been studied in medicine belong to Alcyoniidae, Gorgonacea※, and Scleractinia※. After sorting, red coral is mostly used in medical records. Modern research on coral species is diverse, involving a total of 34 families and 99 genera. Corals in the Alcyoniidae, Nephtheidae, Plexauridae, Gorgoniidae※, Xeniidae, Elisellidae, Briareidae, Subergorgiidae, and Clavulariidae families are more common. Sarcophyton and Sinularia are research hotspots in Alcyconiidae, followed by Dendronephthya, Litophyton, and Lemnalia corals in the Nephtheidae family and by Echinogorgia, Plexauridae, and Eunicea corals in the Plexauridae family (Figure 1).
2.2 The medicinal value of coral
The records of coral can be traced back to the Three Kingdoms period (226–231 AD). Kangtai and Zhu Ying of the Eastern Wu Dynasty mentioned in their Biography of Fu Nan that “In the rising sea, the coral reef falls, and there is a rock at the bottom of the reef, and the coral grows on it” (Cao, 2012). Coral is used as a medicinal material, which was first recorded in the Newly Revised Materia Medica (659 AD) as “sweet, flat, and nontoxic” and primarily used for blood retention and corneal opacity. In addition, coral is ground to a powder and used to stop epistaxis. It grew in the South China Sea, resembling jade red, with many pores in the middle and some without pores. It can also be found in Persia and Sri Lanka. “The General Introduction to the Essential Prescriptions of Zengguang and Zhiju” (1208 AD) records that coral is effective in the removal of corneal opacity and cessation of bleeding in epistaxis. Yue Hau zi (908–923 AD) describes that coral can tranquilize the mind and stop epilepsy. Oversea Materia Medica (907–960 AD) records that coral is the main cause of blood stasis and wind epilepsy. The classic work Compendium of Materia Medica (1578 AD) points out that coral can treat corneal opacity. Materia Medica Yanyi (1116 AD) records that coral can be used to remove corneal opacity. Compendium of Selected Essentials of Materia Medica (1,644–1911 AD) describes that coral is primarily used for corneal opacity, blood stasis, and epistaxis. It can also improve eyesight, tranquilize mind, stop epilepsy, and drop and remove flying silk. In the Second Edition of the National Compilation of Chinese Herbal Medicine (Volume 2; compiled by the Compilation Team of the National Compilation of Chinese Herbal Medicine, 1996), coral is mentioned as red coral, with sweet and flat properties; it can tranquilize the mind, stop epilepsy, and improve eyesight, and it is primarily used in treating convulsions, stopping epilepsy, and removing corneal opacity. Traditional Chinese medicine books such as Taiping Holy Prescriptions for Universal Relief (992 AD), Fangmai Zhengzong (1749 AD), Peng Family Miao Prescription, and Aquatic Product Nutrition and Medicinal Manual all contain prescriptions made from red coral, which can remove corneal opacity in children, dizziness, epilepsy or palpitations, heart and lung congestion, persistent vomiting and bleeding, and water and fire burns (Lai et al., 2016).
Tibetans, Mongols, and Uyghurs also often use coral as a medicinal material for compatibility treatment. Coral Tibetan medicine, namely, Qiwuru, also known as Pazhuma, can clear liver heat and detoxify various toxins. It is primarily used to treat encephalopathy, liver disease, various fevers, and poisoning. The Mongolian medicine, namely, Shuru, which is also known as Zhuru and Ulan Shuru, can clear heat, detoxify toxins, and tranquilize the mind. It is primarily used to treat liver heat, lung heat, detoxify, toxic heat, stroke, and brain disease. The Uyghur medicine, namely, Bihe Marjiang, which is also known as Busai, can restore function and astringing sores, clear heat and inflammation, traete loose teeth, refresh the heart, please the mind, and stop bleeding and diarrhea. It is primarily used to treat damp heat or blood-related diseases. Li et al. (2015) found through experimental research that Mongolian Jiegu Medicine Water Pills have good therapeutic effects on fractures. A’naer Vigills can clear heat, restore function, and relieve itching. It has been used for various symptoms, such as itching, redness, swelling, and excessive vaginal discharge, caused by bacterial and fungal vaginitis in women. It is a commonly used Uyghur medicine preparation in clinical practice (Chen, 2011). Ershiwuwei Shanhu pills can intervene in the treatment of neurological diseases such as Alzheimer’s disease, cerebral infarction, and migraine (Zhou et al., 2019; Zhu et al., 2020; Jiaojia et al., 2022).
The use of coral in modern medicine is no longer limited to red coral. Jiang (2013) conducted an extraction experiment on the active ingredients of Dichotella gemmacea※ and found that some of its diterpenoid compounds showed cytotoxicity to human lung pancreatic cancer cells (A549) and human osteosarcoma cells (MG63), and some of the compounds had antibacterial activity. Wu (2013) conducted a study on Echinogorgia flora and found that its sesquiterpene active ingredients showed a weak antiviral activity against influenza virus. Mahmoud et al. (2022) showed that the steroids and sesquiterpene of Red Sea soft corals showed evident activity on A549, MCF-7, and HepG2 cell lines. The chemical compounds in Scleractinia※ (Zhao et al., 2016) exhibit good biological activities, such as cytotoxic, antibacterial, insecticidal, and toxic effects on fish. At present, the corals used as medicinal materials include soft corals, gorgonians※, Scleractinia※, and red corals (Ai et al., 2006). Scleractinia※ have received little attention from chemists because they are primarily composed of calcareous bones, and the scarcity of red coral resources also limits their utilization. Therefore, active soft corals and gorgonians※ have become the first option for coral reef benthic research, and they are increasingly becoming popular biological species in modern marine natural product research (Xue, 2014).
2.3 Preparations that contain coral used in traditional medicine
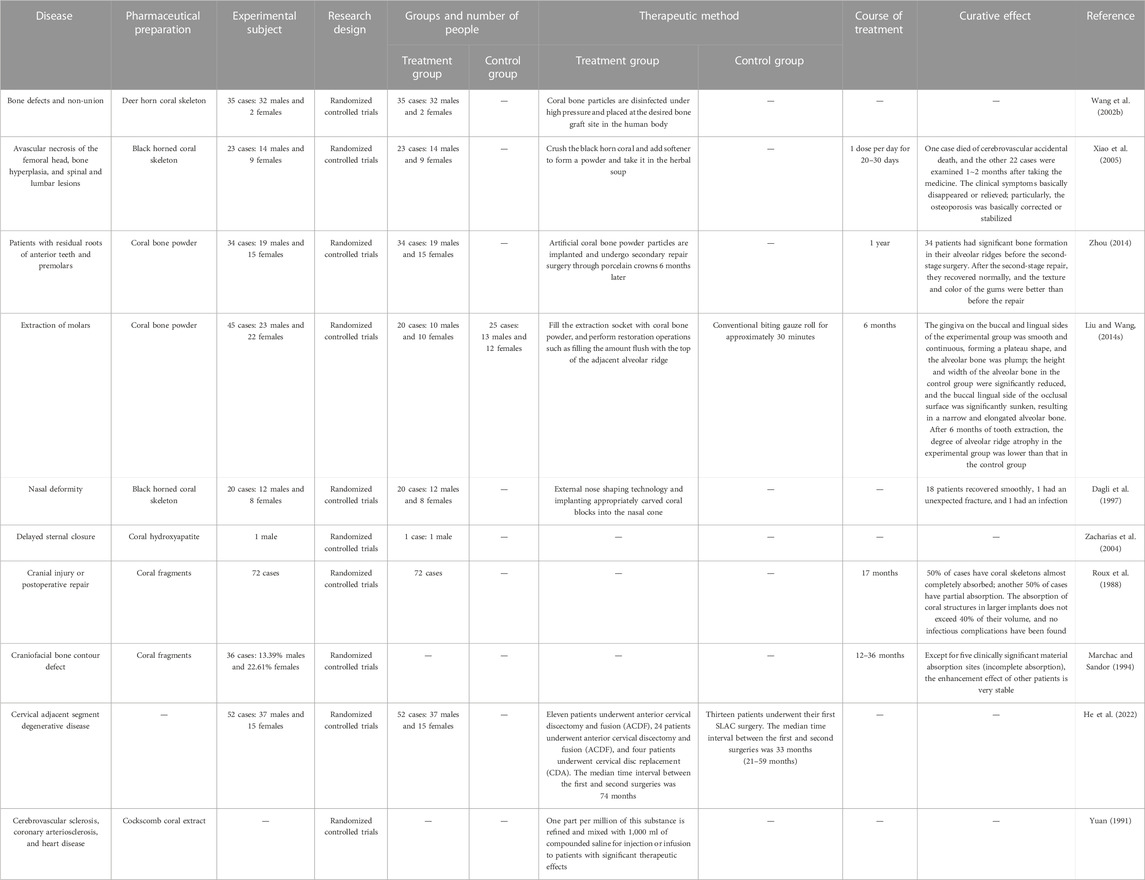
Coral is used as a medicinal material, which has a long history in China. Ancient Chinese ancestors recognized the medicinal value of coral. Coral is primarily used in traditional Chinese medicine, Tibetan medicine, and Mongolian medicine, but the specific variety of coral is not clearly specified in the prescription. Red coral is primarily used as medicine, and the method of medicine includes the following steps: take the original medicinal material, remove impurities, wash and grind it into a fine powder, sieve to obtain an extremely fine powder, and dry it. The compatibility of its medication is shown in Table 1. It is primarily used to treat nervous system disease, chronic ulcers, and various heat syndromes. Traditional Chinese herbs and formulas often play a role in clearing heat, treating eye diseases, relieving chest and hypochondriac swelling and pain caused by diseases, and dissipating heat in the liver and gallbladder. Tibetan medicine is used to treat headache, epilepsy, and various types of neuropathic pain caused by albichoriasis. Apart from traditional Chinese medicine and Tibetan medicine, Mongolian medicine has a wide range of treatments, including various new and old fractures, soft tissue injuries, femoral head necrosis, and various edemas. Records in Uyghur medicine provide evidence for the treatment of various bacterial and fungal infections and trichomonal vulvovaginitis, causing itching, redness, and swelling of the genital area, as well as excessive vaginal discharge, in women.
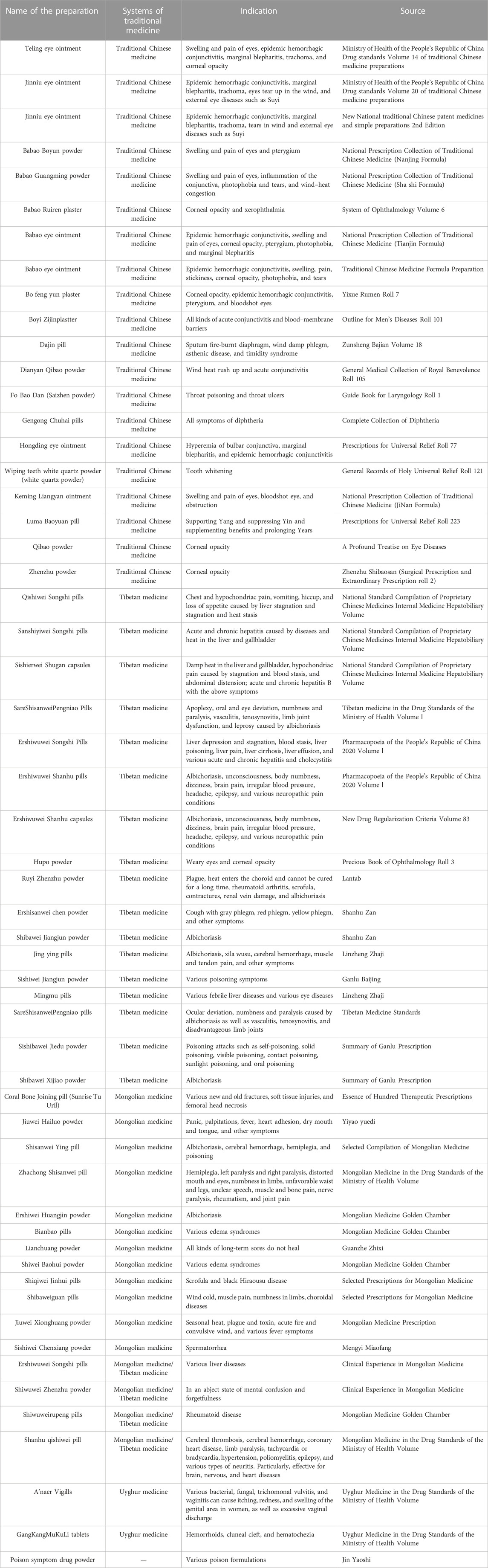
TABLE 1. Preparations that contain coral used in traditional medicine (Lai et al., 2016).
3 Chemical composition of coral
In recent years, Chinese scholars have made important contributions to the research of international marine natural products. In 1980, Su Jingyu first isolated two new types of diterpenoid dimers with double fourteen-membered cyclic carbon frameworks from soft corals (Xue, 2014). Weinheimer and Washecheck (1969) first discovered abundant and highly active prostaglandin-like compounds from gorgonians※. These research results have aroused great interest in the study of coral chemical composition. After decades of research exploration and development, a large number of structurally novel and biologically active compounds have been discovered and determined from corals. Each type of compound contains many compounds with different structures, such as terpenoids, alkaloids, steroids, macrolides, quinones, polyethers, flavonoids, and peptides (Li R., 2012). The following sections provide an explanation of the chemical composition of corals based on different structural types.
3.1 Terpenoids
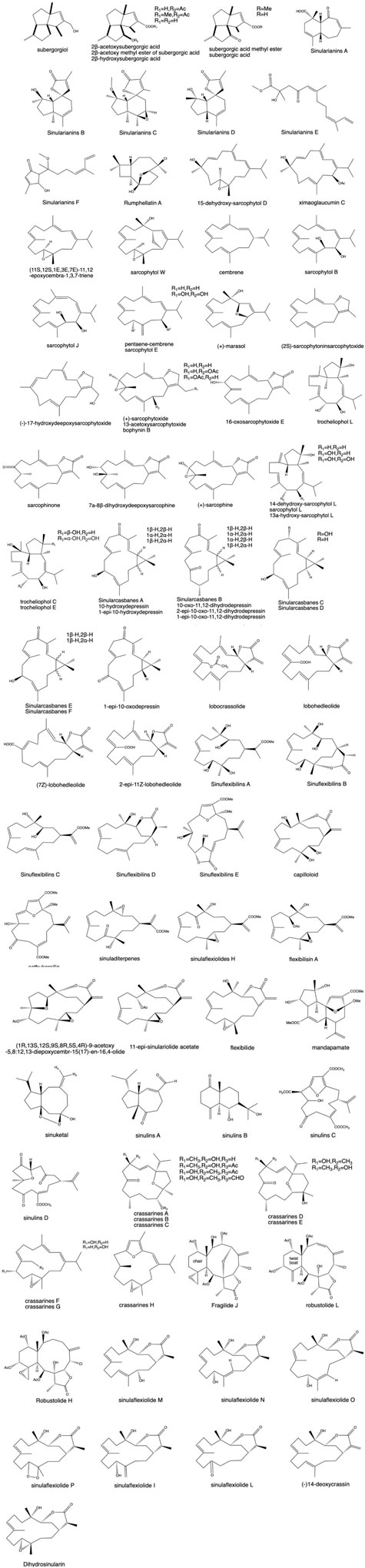
Terpenoids are the most abundant and diverse class of compounds in coral, and terpenoids with a new skeleton are constantly being discovered. Its pharmacological screening shows strong biological activity (Zhang and Guo, 2003; Liu, 2017). Therefore, the isolation and identification of terpenoids have always been the focus and hotspot of coral chemistry research. After sorting out and analyzing the literature, the primary terpenoid compounds are sesquiterpene and diterpenes, in addition to semiterpenoids and triterpenes.
3.1.1 Sesquiterpenes
Sesquiterpenes are an important class of terpenoids that are widely distributed in terrestrial fungi, higher plants, insects, and marine organisms such as soft corals. In addition to the earlier discovery of guaiacane and furan sesquiterpenes, sesquiterpene also contains africanne, capnellane, and illudalane (He, 2013). Wang et al. (2002a) isolated subergorgiol and 2β-acetyl subergorgic acid with a unique angular triquetane structure from the Taiwanese soft coral S. suberosa, in which subergorgiol exhibited moderate cytotoxicity against HeLa tumor cells. Menecubebane B, the known compound analog isolated from gorgonian※ Menella sp., showed moderate cytotoxicity against Eca9706 and HeLa cell lines with semi-inhibitory concentration values of 20.8 and 30.6 μM, respectively. In the coming year, Ngoc et al. (2017a) extracted and identified four sesquiterpenes, namely, nanolobatols A and B and sinularianins B and D, in the Vietnamese soft coral Sinularia nanolobata. Sinularianins B and D were similarly extracted from Sinularia sp. (Chao et al., 2006; Yang et al., 2013). A novel chlorine-containing carbon-deficient sesquiterpene was isolated from Taiwan gorgonian※, and this compound showed inhibitory effects on Gram-negative bacteria (Figure 2) (Sung et al., 2007).
3.1.2 Diterpenes
Many diterpenes show strong biological activities, so diterpenoids have remained a focus and hotspot for research in the past few years. Diterpenes are the most abundant and diverse structural types in corals, and the most common and diverse diterpene is cembrane, which is characterized by an isopropyl and three methyl substitutions in the tetradecane ring. Other diterpenes include eunicellin, casbane, biflorane, briarellin, dolabellane, lobane, sarcodictyins, and xenia (Shao et al., 2009b). Li J. F. et al. (2022) extracted 20 sissonane-type diterpenes from Sarcophyton glaucum. The Sinularia genus is rich in diterpenes. As isolated from the extract of CH2Cl2/EtOH in it, 18 sesquiterpenes such as sinoflexibilins A–F were identified (Yin et al., 2013; Jiang et al., 2019a), and two sinulins C and D (Qin et al., 2018) were isolated from the CH2Cl2/C2H5OH extract of Sinularia sp. Some of the compounds exhibit some degree of cytotoxicity against A549 and HL-60 cells or exert anti-inflammatory effects through inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression (Chao et al., 2011a). Wang et al. (2010) first discovered a new chlorinated briarane (fragilide J) and two chlorinated briaranes (robustolide L and robustolide H) from Junceella fragilis※ and Ellisella robusta※. Jiao-Jiao Xu isolated 10 sissonane diterpenes from soft coral Sinularia flexibilis samples, and the inhibitory effects of each monomer compound on LPS-induced NO release from RAW 264.7 cells were examined using the Griess method at noncytotoxic doses. The results showed that the compounds had some inhibitory effects on NO production (Xu, 2016).
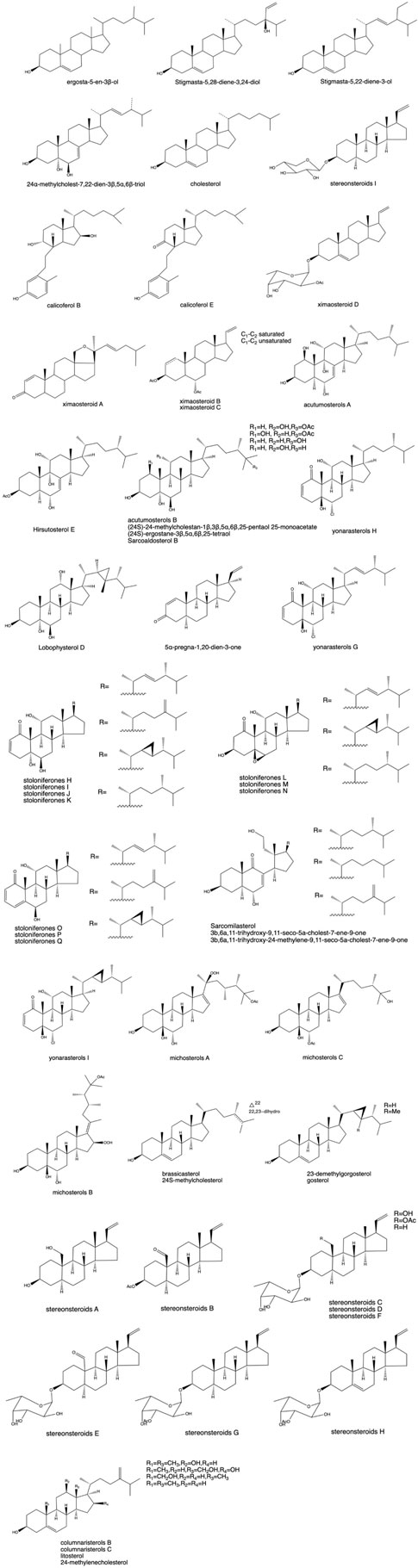
3.2 Steroids
As shown in Figure 3, steroids are a class of biologically active compounds in corals, particularly pregnane, cholestane, and ergosterone. It has received considerable attention because of its structural diversity and remarkable biological activity (Liu, 2017). Sterols are abundant in corals, and the structure is more complex because of the diversification of the sterol side chain structure and the different degrees of oxidation (Ai et al., 2006). Seven new cleaved ring sterols with C9,11 breaks and C22 hydroxylation were isolated for the first time from Tripalea clavaria collected from the South Atlantic Ocean in 2006, as determined by wave spectroscopy and the Mosher method, and further studies revealed that some of their substances showed some inhibitory activity against Staphylococcus aureus. Four bioactive sterols with anti-inflammatory, antibacterial, antioxidant, antitumor, and antitubercular properties were isolated from J. fragilis※ from Sanya, Hainan (Wen et al., 2007). Subsequently, two sterols were isolated from the CH2Cl2/C2H5OH extract of this coral (Qi et al., 2004). For the first time, two B-ring open-loop sterols were isolated from the Chinese small pointed gorgonian Muricella sinensis (Verril1) ※ from the South China Sea. During bioactivity screening, calicoferol E was found to show inhibitory activity against protein tyrosine phospholipase 1B (PTP1B), with an IC50 value of 27.28 μM (Yan et al., 2005).
3.3 Nitrogen compounds
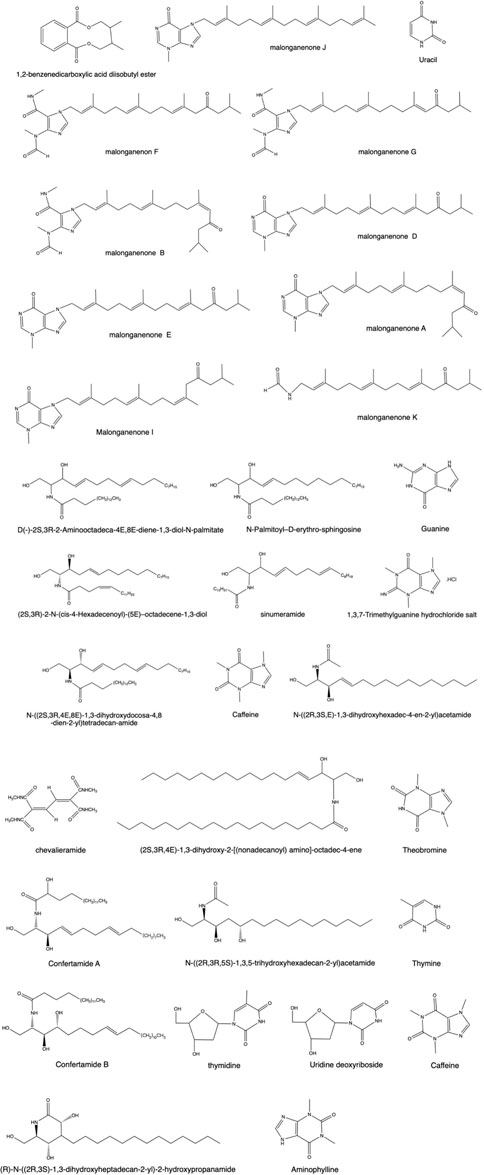
The nitrogenous compounds in corals primarily include ceramides and alkaloids (deoxythymidine, thymine, methyluracil, and urea). They generally exhibit antifungal, antibacterial, and cytotoxic activities. Such compounds can also inhibit acetylcholestan-converting protease, thereby providing an alternative lead compound for the development of therapeutic drugs for atherosclerosis and other cardiovascular diseases (Ai et al., 2006). Zhang J. R. (2012) isolated 16 alkaloids (nine diterpene alkaloids, including three new diterpene alkaloid compounds) and five ceramides from E. robusta and E. curvata of gorgonian※. A preliminary evaluation of the antitumor activity at the cellular level was carried out, from which four diterpene alkaloids were screened to show strong cytotoxicity against HeLa and K562 cancer cells, and the enzymatic activity inhibition was evaluated by enzyme-linked immunosorbent assay (ELISA). The activity results showed that diterpene alkaloid malonganenone D had a strong inhibitory effect on the enzymatic activity of c-Met. The ceramide N-1-hydroxymethyl-2-hydroxy-(E, E)-3,7-heptadecadienylhexadecanoamide (Liu et al., 2001), thymine, and uracil were isolated from Acropora pulchra※ (Brook; Xu et al. (2003)). In addition to different corals, such as Litophyton arboreum (Abou El-Kassem et al., 2018) and Junceella juncea※ (Pallas; Krishna et al. (2004)), Lobophytum chevalieri (Li et al., 1989) has bioactive ceramides. The structure diagram is shown in Figure 4.
3.4 Other compounds
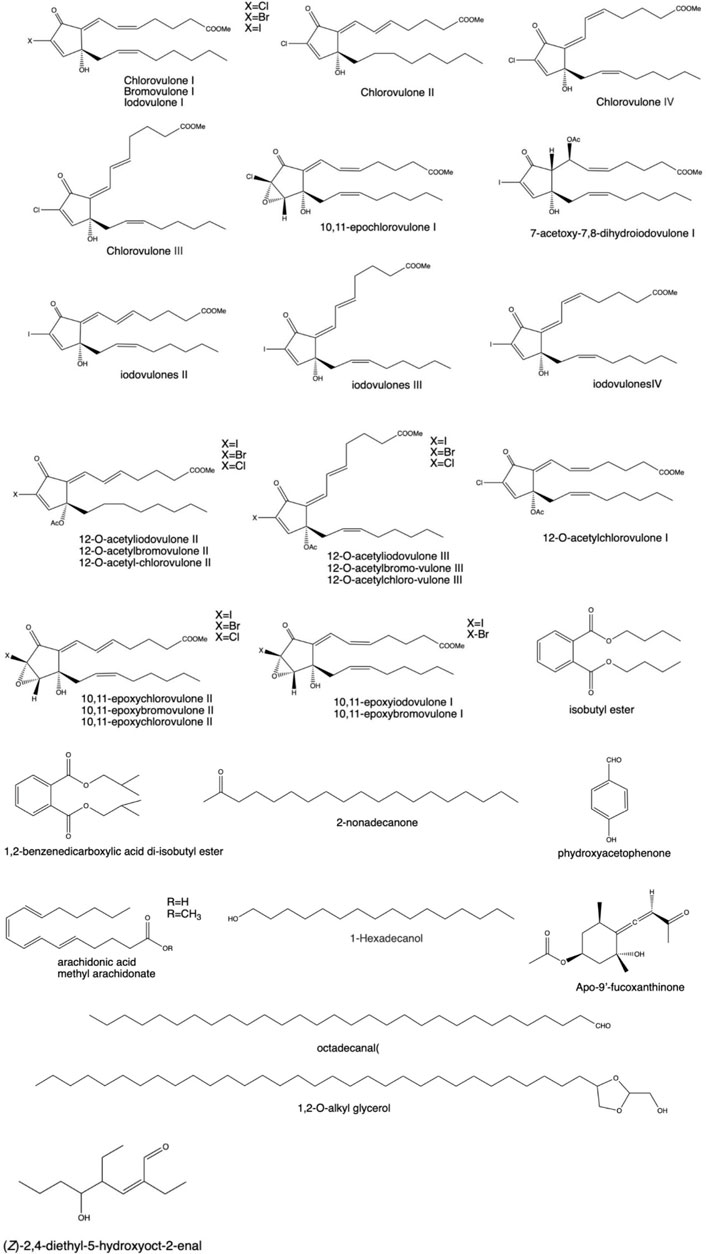
As shown in Figure 5, aliphatic compounds (long-chain fatty acids, long-chain aliphatic alcohols, and the aldehydes and esters they form) and prostaglandins were also extracted from different corals (Watanabe et al., 2003; Reina et al., 2013; Hurtado et al., 2020). According to the literature, a large amount of batyl alcohol has a pharmacological effect of raising leukocytes, which is extracted from coral and has been widely used in clinical practice (Ma, 2008; Zhao et al., 2011; Sun, 2012; Xue et al., 2014). Watanabe et al. (2003) tested 15 new halogenated prostaglandins isolated from the Okinawan soft coral Clavularia viridis. Among these prostaglandins, three belong to iodovulone; seven belong to 12-O-acetyliodovulones, 12-O-acetylbromovulones, and 12-O-acetylchlorovulones; and the rest belong to 10,11-epoxy congeners of iodovulone, bromovulone, and chlorovulone. A simple compound, p-hydroxybenzaldehyde, was obtained from crude extracts of Sinularia dissecta (Jin, 2005) and Muriceides collaris※ (Zhu et al., 2013). In addition, some simple aldehydes were isolated from Antipathes dichotoma Pallas※ (Ge et al., 2010), Sinularia notanda (Xu et al., 2017), Scleronephthya sp. (Huo et al., 2011), D. gemmacea※ (valenciennes; Liu (2008)), Dendronephthya sp. (Li, 2004), and Hicksonella guishanensis Zou※ (Yu et al., 2004). p-Hydroxybenzoic acid can be extracted from Subergorgia reticulata (Xie et al., 2013) and red coral (Lai, 2017). Zou (2015) sorted out olefins from the crude extract of Sinularia sp. Subsequently, Li R. (2012) extracted ketones and alcohols from this coral. Esters such as methyl arachidonic acid (Liang et al., 2017), dibutyl phthalate, diisobutyl (Wang et al., 2009), and 1,2-benzenedicarboxylate (Lv et al., 2012) are also found in this coral.
4 Pharmacological effects and mechanisms
Many structurally active unique secondary metabolites, such as terpenoids, steroids, ceramides, and prostaglandins, have been extracted from corals, and their significant pharmacological activities, such as cytotoxic and antiviral activities, have been widely noticed and studied by natural product chemists and other researchers. Meanwhile, the pharmacological activities of coral bone powder and various coral preparations in the cardiovascular system have been explored. This article focuses on their cytotoxic effects on a variety of tumor cells and cancer cells as well as their restorative effects on bone injury diseases and their biological activities, such as antioxidant, anti-inflammatory, analgesic, and antiviral activities, on tissues of the nervous system and respiratory system. Accumulating evidence suggests that they have significant therapeutic effects on diseases of the nervous system.
4.1 Bone repair effect
The key to the treatment of bone defects is the suitability of the repair material. Autologous bone grafts cannot meet clinical needs for various reasons, and allogeneic and xenogeneic bones are limited in clinical application because of their antigenic nature (Zhou et al., 1993). The microstructure of coral and skeleton is also very close, specifically in its internal structure. Corals are divided into pinnate, laminate, branching and pith-like structures depending on the arrangement of the calcification centers. According to the skeleton body, the tiny tube traffic is divided into interlocking and interoperable traffic. Depending on whether the microscopic tubes in the skeleton are in traffic or not, they are divided into interlocking and interoperable. The interconnected coral skeleton has longitudinally and horizontally arranged tiny tubes, with pore diameters of 0.05–2.0 mm. Regardless of the section, these pores are interconnected. The coral artificial bone is widely valued as a promising material for bone repair (Roux et al., 1988). Animal experiments have shown that artificial bones made from horned honeycomb coral (favites) have good biocompatibility and osteocompatibility. When it is implanted in the mandible, the femoral cortical defect site after 8 months can be repaired, resulting in complete restoration (Zeng et al., 1997). Zhou et al. (1993) used Hainan Cheng Huang Bin Coral (Hainan Coral, Porites Iutea; HNC) composite implant material as the material graft in the side mandibular defect model and affirmed that the coral group led to the formation of new bone tissue, wrapped with phenanthrene fibrous tissue, followed by its better bone repair effect when used with BMG. In addition, it led to osseous healing, bone marrow cavity formation, and clear visualization of new bone tissue. Lai (2017) concluded that red coral can promote fracture healing and reduce the fracture healing period. According to the literature (Souyris et al., 1985; Dagli et al., 1997; Zhou, 2014), coral can also be used to correct saddle nose deformities, oral implants, skull injuries or postoperative repairs, and other orthopedic disorders.
In addition, coral transplants in the human body do not cause rejection; countless fine pores in the coral facilitate the gradual growth of microscopic blood vessels and synthesis of living cells of bone (Ma, 1994). Guillemin et al. showed that the resorption of corals starts with the growth of granulation tissue and blood vessels from the bone marrow into the coral. Then, the coral is progressively resorbed by many osteoclasts near its edges, while the woven bone formed with osteoclasts gradually grows into the resorbed void; finally, bone marrow cavity is formed, and the newly formed bone tissue system is clear and visible (Guillemin et al., 1989; Lu and Chen, 1994).
Chemical compounds extracted from corals also play a role in bone injury diseases. Lin Y. F. et al. (2013) isolated Ya-s11 (9 mg/kg) from the Taiwanese soft coral Sinularia querciformis, which not only attenuated AIA-induced ankle joint pathological changes but also significantly reduced the expression of osteoclast-related proteins.
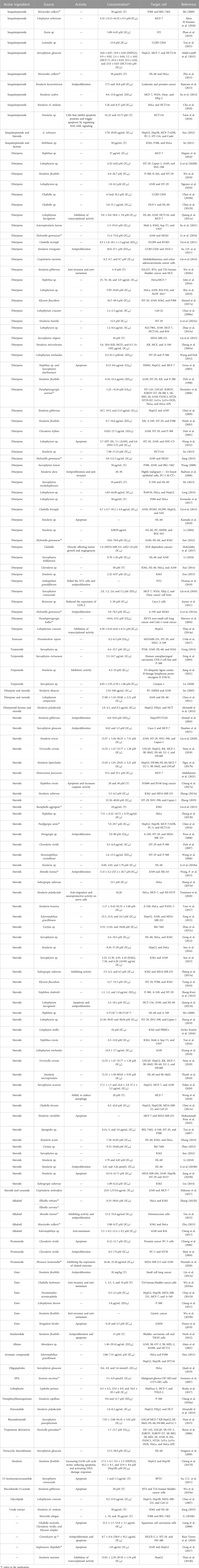
4.2 Cytotoxicity
As shown in Table 2, studies in the literature in the last two decades have found that compounds extracted from coral have good cytotoxicity, particularly diterpenes, sesquiterpenes, sterols, and a small number of alkaloids, prostaglandins, and esters as active substances that also have some biological activity. These compounds are mostly extracted from corals of the genera Sinularia, Lobophytum, and Sarcophyton all belonging to the family Alcyoniidae. Corals of the family Gorgoniidae※ are also used as a source of active natural substances. The evaluation of their cytotoxic activity against tumor cells such as A549, HL-60, MCF-7, colon cancer cells, K562, and HeLa, followed by HepG2, Hep3B, MDA-MB-231, P-388, HT-29, MCF-7, Sup-T1, U937 and other cells, has become the hotspots of research. Shaaban et al. (2021) evaluated the in vitro anticancer effects of hydroazulenes, an extract of the soft coral S. glaucum, on colon (Caco-2) and breast (MCF-7) cell lines by MTT assays and showed that its antiproliferative or antiangiogenic effects were ultimately achieved by inhibiting the migration of MCF-7 cells and significant inactivation of VEGFR2 enzymes. Interestingly, the growth inhibitory concentrations of 5α-3β,6α,11-trihydroxy-24-methyl-9,11-seco-5a-cholest-7-en-9-one on colon (Caco-2) and breast (MCF-7) cell lines were 0.62 and 2.3 mM, respectively, but no toxicity was recorded against RPE-1 cells at a high concentration of 10 mM. The team also studied for the first time the anticancer properties of the sterol 10-epicatechin methyl ether. The first study of Sarcophyton acutum extract activity by Sabry A. H. Zidan studied for the first time the cytotoxic activity of Sarcophyton acutum extract and showed that polyhydroxylated steroid compounds had significant cytotoxicity to the HepG2 cell line (semi-inhibitory concentration 17.2 ± 1.5 μg/mL) and MCF-7 (semi-inhibitory concentration 33.2 and 25.1 mM) (Zidan et al., 2020; Abdelkarem et al., 2021) and that the side chains of polyhydroxylated sterols play an important role in the cytotoxic activity of such sterols. The researchers also demonstrated using the SRB method that the gorgonian of Euplexaura rhipidalis※ has a significant apoptosis-inducing effect on A549 and HepG2 cells (Gong et al., 2017); in other words, prostaglandins with hydroxyl and carboxylic acids possess good cytotoxic properties and that they may have potential inhibitory effects on certain types of cancer (Hurtado et al., 2020). In fact, more than a decade ago, studies showed that the structure of compounds could influence cytotoxicity. A free hydroxyl group at C-12 or C-22 is important for enhancing the cytotoxic activity of a sterol against HeLa cell lines. In addition, the introduction of hydroxyl groups at C-20 decreased the inhibitory potency against HeLa cell lines, while the presence of acetoxy groups at C-18 seemed to enhance the cytotoxic activity (Zhang J. et al., 2013).
4.3 Anti-inflammatory and analgesic effects
Inflammatory processes usually constitute the initial activation of the mammalian immune system and the body’s normal defense or protective mechanisms against microbial infections or stimuli, tissue, or organ damage. Accumulating evidence shows a critical link between inflammation and the chronic promotion/progression of various human diseases, including atherosclerosis, diabetes, arthritis, inflammatory bowel disease, cancer, and Alzheimer’s disease (Wei et al., 2013). Different types of cells, such as monocytes/macrophages, neutrophils, and lymphocytes, are involved in the inflammatory process (Serhan and Savill, 2005). Several marine biology and chemistry researchers have systematically screened the in vitro anti-inflammatory activity of several marine natural products isolated from corals, and lipopolysaccharide-stimulated mouse macrophage models have been widely used as a system for assessing the anti-inflammatory activity of secondary metabolites of marine and terrestrial origin (Lin et al., 2015). Yen-You Lin’s study showed that the diterpene compound excavatolide B from the gorgonian of Briareum excavatum※ produced potent anti-inflammatory activity in vitro and in vivo and inhibited the expression of iNOS and COX-2 mRNA. Gyrosanols A and B show significant anti-inflammatory activity by reducing COX-2 protein levels in RAW 264.7 macrophages (Cheng et al., 2010a). Lee et al. (2013) found that soft coral-derived leminalol attenuated monosodium urate-induced gouty arthritis in rats by inhibiting leukocyte infiltration and the expression of iNOS and COX-2 proteins, among others.
The inflammatory process also involves the peripheral and central nervous system (CNS) and is thought to be involved in the pathogenesis of neuropathic pain (Ellis and Bennett, 2013s). Chen N. F. et al. (2014) investigated flexibilide, extracted from cultured soft corals, as a possible drug for neuropathic pain, and its anti-neuritis and analgesic mechanisms of action may be related to spinal TGF-β1 inhibition. The sphingosine derivative obtained from soft corals also has anti-inflammatory and analgesic effects (Radhika et al., 2005). After compiling nearly 100 studies, it was found that the anti-inflammatory activity of coral extracts is mainly attributed to diterpene compounds, followed by sterols, prostaglandins, and alkaloids. Its anti-inflammatory activity is mainly mediated by the inhibition of lipopolysaccharide-induced expression of iNOS and COX-2 in mouse macrophages (RAW 264.7) or by the inhibition of superoxide anion release from human neutrophils FMLP/CB and elastin. The specific functions of anti-inflammatory and analgesic effects in corals are shown in Table 3.
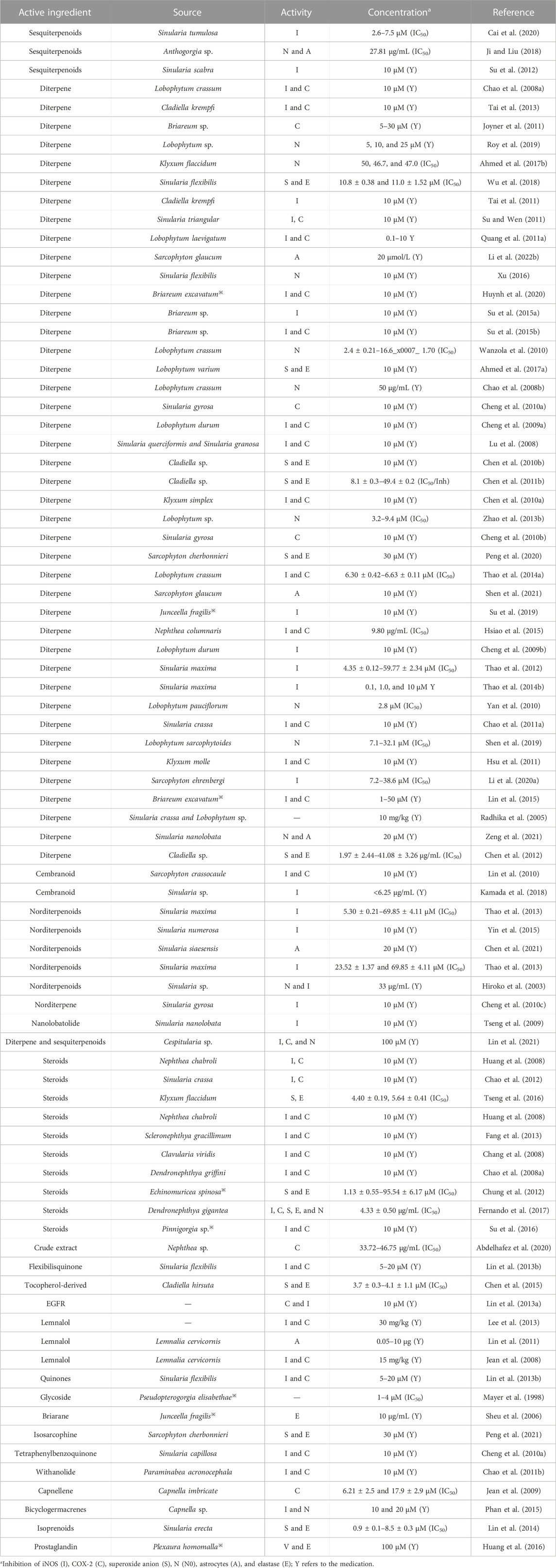
TABLE 3. Classification statistics for anti-inflammatory and analgesic effects of active substances extracted from the coral.
4.4 Antiviral
Viruses are infectious entities that use the cellular biosynthetic machinery to replicate their own nucleic acids, synthesize the proteins encoded by their nucleic acids, and finally assemble into complete, infectious viral particles. In most cases, viruses can cause disease and even death in infected hosts (Li W. et al., 2022). Almost all clinical and public health outbreaks over the decades have been due to emerging viruses, including coronavirus (SARS), which causes severe acute respiratory distress syndrome, influenza A virus subtype H1N1 (IAV-H1N1), which caused an influenza pandemic in 2009, human cytomegalovirus (HCMV), which can cause visceral disease, and the SARS CoV-2, which caused a widespread outbreak worldwide in 2019 (Chen et al., 2023). The widespread outbreak of the virus not only poses a great threat to the lives and health of people across the country but also severely hinders global economic development. Marine organisms have been shown to be a rich source of antiviral drugs (Cao et al., 2014). Chun-Kuang demonstrated that lobohedleolide isolated from the Taiwanese soft coral Lobophytum crassum significantly reduced HCV replication in replicon cells and JFH-1-infected systems with EC50 values of 10 ± 0.56 and 22 ± 0.75 μM at nontoxic concentrations, respectively. Their study also concluded that the inhibitory effect on HCV replication was due to the inhibition of HCV-induced COX-2 expression (Lin et al., 2018). Gong et al. (2013) showed for the first time thatspecific types of steroids were active against influenza viruses. The antiviral effect of coral is mainly achieved through the inhibition of viral replication and expression of antigens. As summarized, coral mainly has antiviral activity against pathogens such as HCMV and H1N1, and some studies have also found antiviral activity against pathogens such as HBV and HCV, as shown in Table 4.
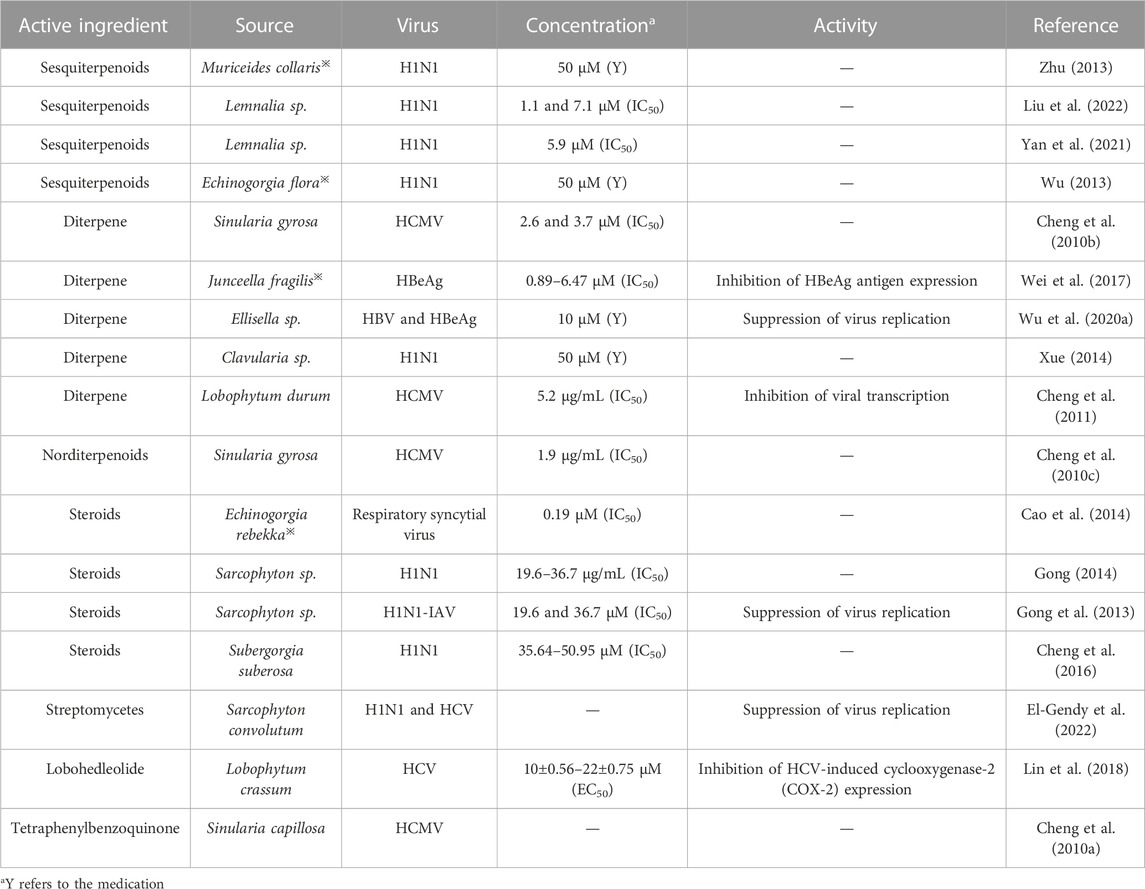
TABLE 4. Classification statistics for antiviral effects of active substances extracted from the coral.
4.5 Antibacterial
As shown in Table 5, according to the literature, the antimicrobial activity of coral is mainly exhibited in terms of activity against bacteria (Gram-negative and Gram-positive bacteria, etc.) and fungi. Its antibacterial activity is mainly attributed to terpene compounds extracted from coral, particularly sesquiterpenes and diterpenes, followed by steroidal active substances. In 1997, Badria’s team demonstrated the antibacterial activity of sarcophytolide extracted from soft corals using reagents such as dimethyl sulfoxide and showed that the compound had broad activity against Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, and oenococcus oeni. Mohamed N. Gomaa not only tested the antibacterial activity of the soft coral of the Sarcophyton genus but also compared the differences in the antibacterial activity of different extracts. The results showed that the hexane extract had a strong antibacterial effect. The antibacterial activity of nerve sphingolipids and sterols extracted from A. dichotoma※ was also demonstrated using the disc diffusion technique (Al-Lihaibi et al., 2010). The diterpenoids isolated from Lemnalia sp. also showed antibacterial activity with MICs of 4–64 μg/mL for Bacillus subtilis and Staphylococcus aureus (Yan et al., 2021). The antibacterial mechanism has not been specifically reported.
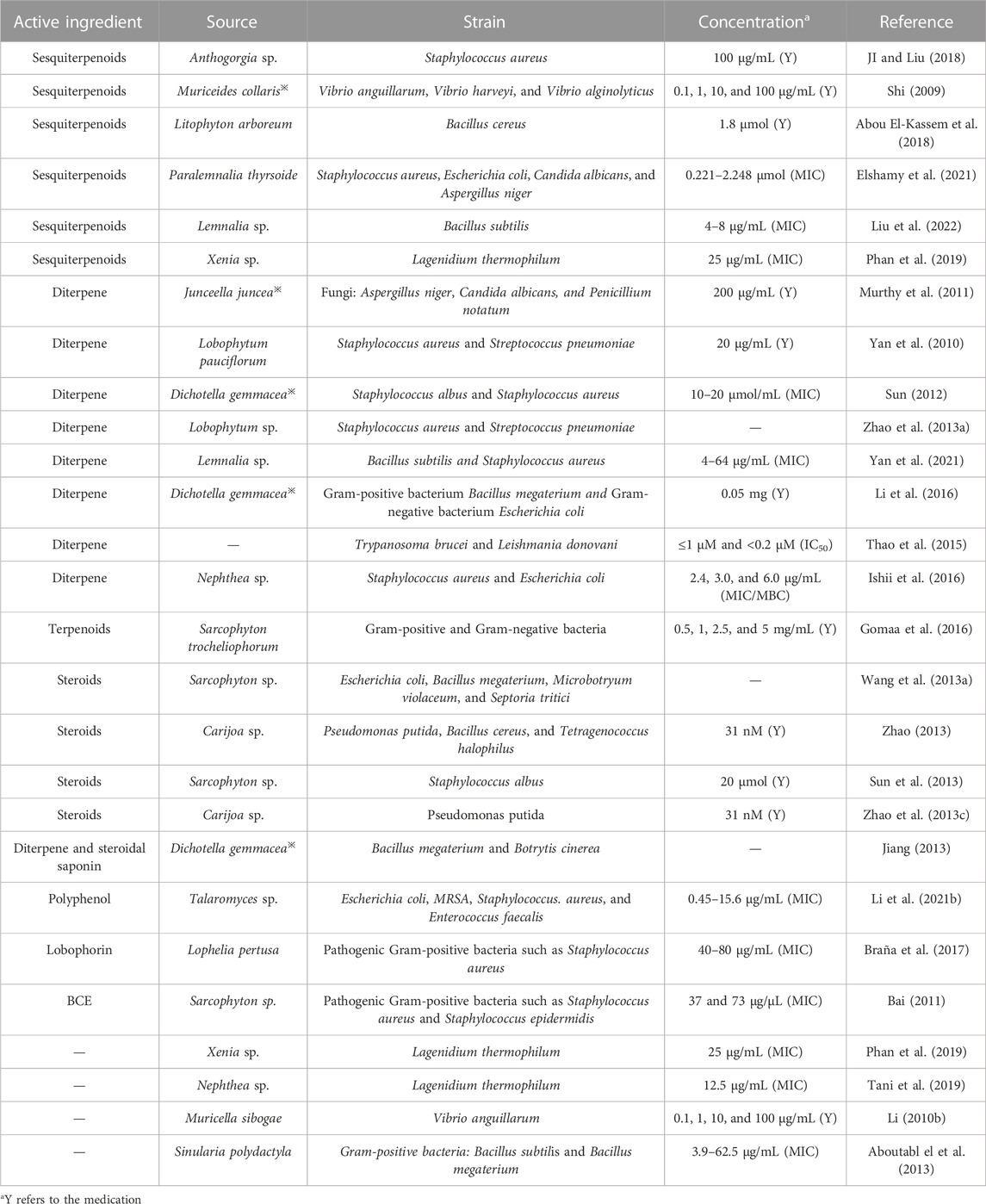
TABLE 5. Classification statistics for antibacterial effects of active substances extracted from the coral.
4.6 Antioxidant activity
Altered oxidative status may have peroxidative effects on lipids, proteins, and RNA and regulate cellular responses, signal transduction, and metabolism, thereby impairing their biological functions. At present, few reports on the antioxidant effect of coral can be retrieved, and the antioxidant effect mostly works through free radical scavenging, oxidative free radicals, and lipid peroxidation. In general, common free radicals include −OH, O2−, DPPH, and ABTS−/+. The coral derivatives sinularin and dihydrosinularin showed general radical scavenging activity against the free radicals 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS), and hydroxyl (-OH), as well as the induction of Fe+3 reduction and Fe+2-chelating ability, all of which enhanced their antioxidant activity. Sinularin exhibited higher antioxidant properties than dihydrosinularin. Further ATP assays showed that the different antioxidant properties contributed to the antiproliferative effect on different cancer cells as well (Wang et al., 2021). The in vitro antioxidant results of the active ingredients BCE (alkanes, terpenoids, esters, fatty acids, and aromatic compounds) extracted from black horn coral※ indicated that some of them have scavenging effects on DPPH- and OH-. The in vivo antioxidant effect not only induces a morphological protective effect on lung tissue but also effectively increases SOD activity in vivo and reduces the MDA content, thereby reducing the damage to lung tissue caused by the large amount of oxygen free radicals in tobacco (Bai, 2011).
4.7 Antimalarial
Malaria, caused by Plasmodium vivax, poses a major health threat to the majority of the world’s population (Thao et al., 2015). Various marine natural products with anti-protozoal activity have been reported in the literature (Watts et al., 2010; Sanchez et al., 2013; Mohyeldin et al., 2017). Thao et al. (2015) identified laevigatol A in Vietnamese soft corals, which showed inhibition of the Plasmodium falciparum (Pf) NF54 strain with IC50 < 5.0 µM. The antimalarial activity of sesquiterpene extracts of the octocoral coral Eunicea sp.※ (Plexauridae: Octocorallia: Cnidaria) was demonstrated against chloroquine-resistant strains of Plasmodium falciparum by inserting fluorochromes into the parasite DNA. The results revealed that compounds showed a significant inhibition of Plasmodium falciparum growth (Garzón et al., 2005). Ospina et al. (2005) conducted an experiment and showed that caucanolide A, a diterpene compound extracted from anise coral, exhibited significant in vitro antiplasmodial activity against Plasmodium falciparum W2 at an IC50 of 17 μg/mL, and caucanolide D was equally effective at an IC50 of 15 μg/mL. Please refer to Table 6 for details.
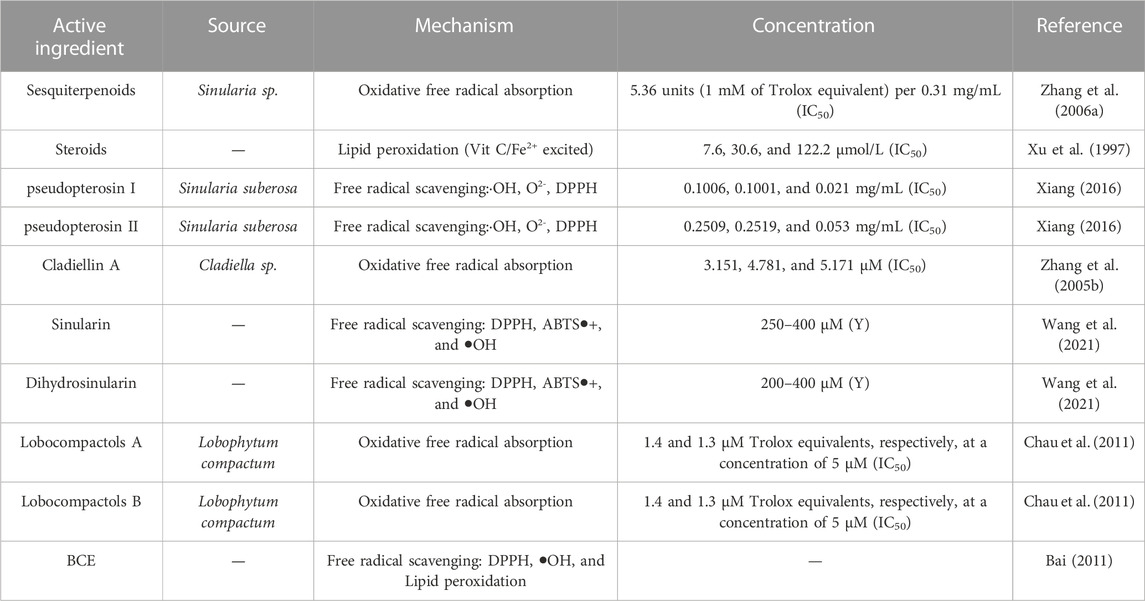
TABLE 6. Classification statistics for antioxidant effects of active substances extracted from the coral.
4.8 Immunosuppressive effect
According to incomplete statistics, terpenoid and sterol active substances extracted mainly from the soft coral Sinularia scabra, Sinularia polydactyla, Sinularia sp., Libertasomyces sp., and gorgonian※ Verrucella umbraculum※ have immunosuppressive effects in vitro. Sun et al. (2017) reported for the first time the immunomodulatory activity of new polyketide and trans-fused decane ring system-like metabolites by inducing the proliferation of CD3+ T cells. Further structure–activity analysis revealed a key role of the Δ7 and terminal OH groups in the regulation of CD3+ T-cell proliferation. Yang et al. (2020) revealed that the sterol compound yalongsterol A, 5α,8α-epidioxy-24-methyl-cholesta-6,24 (28)-dien-3β-ol and (22E,24S)-5α,8α-epidioxy-24-methylcholesta-6,22 -dien-3β-ol, exhibited moderate immunosuppressive activity against T and/or B lymphocytes with semi-inhibitory concentration values of 19.30–59.49 µM. Subsequently, Cui et al. (2020) showed that polycyclic furanobutenolide-derived norditerpenoids exhibited strong inhibitory effects on ConA-induced T lymphocyte and/or LPS-induced B lymphocyte proliferation. Diterpenoids of different membrane types isolated from the South China Sea soft coral S. scabra have the same biological activity (Yang et al., 2019). A recent report revealed that metabolites containing the 9,10-secosteroid structure extracted from the South China Sea gorgonian V. umbraculum※ showed immunomodulatory activity by inhibiting the differentiation of CD4+ T lymphocytes (Li J. et al., 2021).
4.9 Enzymatic activity
As summarized in Table 7, reports on coral enzyme activity are rare, but from the collected literature, it can be seen that some terpene masses isolated from coral have enzyme inhibitory activity. In addition, some steroid, polyketide, and alkaloid active substances may also have enzyme activity. In-depth research has led to the understanding of the significant role of enzymes in the regulation of diseases, not only for the adjuvant treatment of important organs such as the brain, heart, liver, and kidneys but also in the selective treatment of tumors with remarkable results. The diterpenes sinupol and sinulacetate exhibit good inhibitory activity against protein tyrosine phosphatase 1B (PTP1B), which in turn is a potential drug target for the treatment of type II diabetes and obesity (Ye et al., 2018). Cespine diterpenes isolated from the soft coral Sinularia crassa in the South China Sea are used as alpha-glucosidase inhibitors for antidiabetic treatment. This provides a different way of thinking for developing new drugs (Wu et al., 2020b).
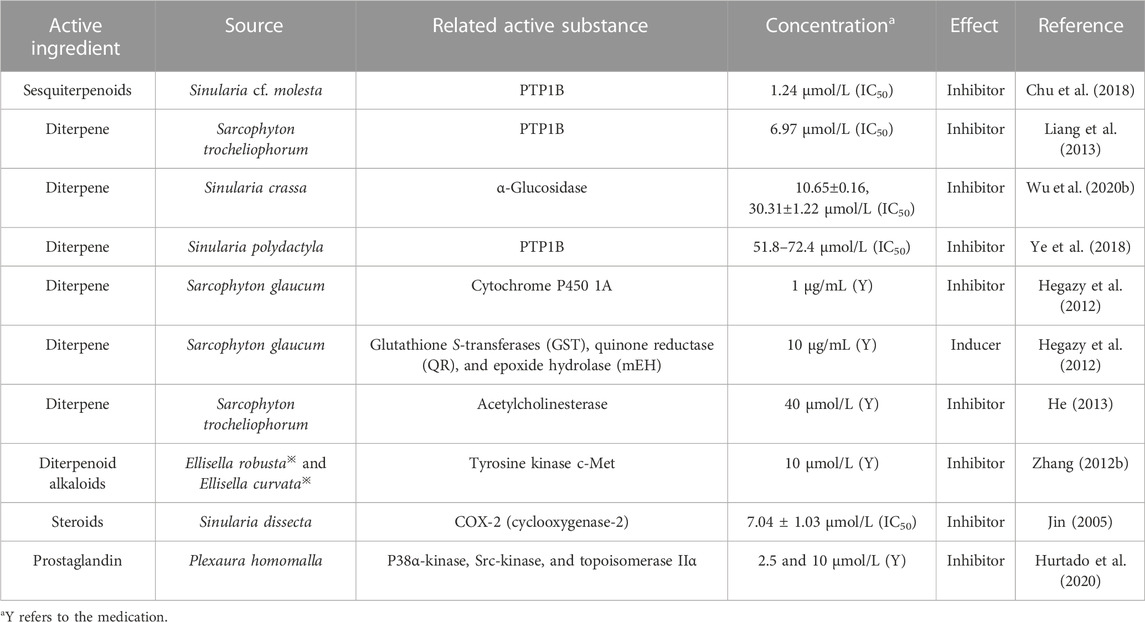
TABLE 7. Classification statistics for enzymatic activity of active substances extracted from the coral.
4.10 Effects on the nervous system
The neuroprotective effects of coral are manifested in two ways. On the one hand, they exhibit anticonvulsant and antiepileptic effects. As early as 1984, preliminary pharmacological experimental studies on the soft coral Lemnalia exilis showed that its extract had a significant antispasmodic effect on the isolated ileum of guinea pigs (Fang and Zhang, 1984). Eltahawy et al. (2015) measured the anticonvulsant activity of ceramide isolated from the Red Sea soft coral Sarcophyton auritum using a pentylenetetrazol (PTZ)-induced seizure model, and the mechanism may be through the modulation of CNS inhibitory activity through GABA and serotonin receptors. Some sterols also exhibited neuroprotective activity against neuron-like SH-SY5Y cells (Tammam et al., 2020). On the contrary, it has a sedative–hypnotic effect (Liao et al., 1992). Finally, the coral derivative excavatolide B can enhance long-term induction by suppressing the delayed rectifier potassium current, which lowers the action potential onset threshold and ultimately enhances situational memory retrieval in mice, resulting in enhanced memory extraction.
The effects of formulated preparations of coral on the nervous system have also been documented. First, Ershiwuwei Shanhu pills can prolong the latency period of epileptic seizures, shorten the duration of epileptic seizures, reduce the level of epileptic seizures, decrease the number of clonic seizures, and suppress epileptic discharges. At a certain dose, its effect was significantly better than that of the positive control drug sodium valproate (Luo, 2012; Luo et al., 2013). Second, Li et al. (2014) explored the protective effects of Ershiwuwei Shanhu pills on senescent hippocampal cells. The drug inhibited D-lactose-induced neuronal degeneration and excessive activation of astrocytes, thereby reducing neuronal and astrocyte damage. Finally, Ershiwuwei Shanhu capsules can increase adenosine levels in secondary spinal cord injury, thereby increasing the ability of nerve cells to repair themselves (Jiao et al., 2013).
4.11 Effects on the cardiovascular system
Fang and Zhang (1984) found that soft coral extract has high physiological activity on the cardiovascular system. The extract of soft coral can not only delay the time of arrhythmia in isolated hearts of rats and shorten the duration of arrhythmia but also increase rabbit’s heart coronary flow and slow down the heart rate. Lai (2017) also pointed out that red coral could regulate TXB2/6-keto-PGF1α levels, reduce plasma PF4/β-TG levels, and lower plasma ET-1 levels in a blood stasis rat model, ultimately reducing vascular injury in rats. 15-Hydroxy-tetracosa-6,9,12,16,18-pentaenoic acid and sesquiterpenes isolated from the soft coral S numerosa and Lemnalia sp. exhibit anti-tubulinogenic and pro-angiogenic activities, respectively, in a dose-dependent manner (Yao et al., 2007; Yamashita et al., 2009; Wang et al., 2020).
4.12 Other effects
Other effects of corals include antihypertensive, hypolipidemic, and antiulcer activities. The diterpene glucoside isolated from the soft coral Cespitularia turgida in the South China Sea has a significant acute antihypertensive effect, and it has an obvious quantity–effect relationship; its antihypertensive effect has no rapid tolerance phenomenon, and at the same time, it has little effect on the heart rate when used as antihypertensives. The formulated preparation of coral, Shanhu Qishiwei pill, may reduce blood lipid levels in HLP model rats by inhibiting the LKB1/AMPK signaling pathway (Chun et al., 2022). Elshamy et al. (2017) demonstrated the antiulcer activity in a rat ulcer model induced by ethanol and acetic acid.
5 The toxicity of coral
Many corals, such as animal corals, also known as soft corals, are very popular in aquariums (home or public) because of their appreciation value and low maintenance costs. The soft corals of genera Palythoa, Protopalythoa, Zoanthus, and Parazoanthus in the Zoanthidae family contain a highly toxic and potentially lethal compound, palytoxin (Hoffmann et al., 2008). Therefore, the toxic compound of coral is mainly palytoxin. Ciminiello et al. (2011) extracted palytoxin and 42-hydroxy palytoxin at levels up to 25–450 ng per kg of Zoanthid. Palytoxin is a potent vasoconstrictor, and its neurotoxicity and cardiotoxicity are primarily due to dysregulation of the transmembrane pump Na/K-ATP enzyme, which can lead to serious human disease, causing gastrointestinal symptoms, myalgia, muscle spasms, respiratory and cardiac problems, and even death (Wieringa et al., 2014). The toxin is heat-resistant, and conventional boiling inactivation operations are not effective against it. Reports of human exposure to palytoxin consumption have described significant morbidity and mortality (Sud et al., 2013).
Palytoxin exposure and the production of toxic compounds through corals are primarily associated with toxin poisoning from inhalation of toxin-dissolved water aerosols during cleaning, scrubbing, or eradication of corals in home/public aquariums. Thus, aquarium store staff and home aquarium hobbyists face a consequent elevated risk of exposure. The data we collected showed that people aged less than 80 years and children exposed to palytoxin nebulized from coral had immediate symptoms such as cough, dyspnea, chest pain, myalgia, tachycardia, and gastrointestinal symptoms, and in severe cases, acute reactions such as burning or stinging and erythema also occur. Coral injuries may also have complications such as foreign body reactions, bacterial infections, or local eczema reactions (Na et al., 2008). Examples of poisoning due to prolonged and unprotected exposure to corals have also been reported (Smith et al., 2003; Hoffmann et al., 2008). A patient who placed his right hand on a Zoanthid colony while cleaning a seawater aquarium at home developed myalgia, symptoms of general weakness in limbs, and, subsequently, signs of poisoning such as speech impairment, dull eyes, and fainting. The degree of poisoning is closely related to the contact time, contact distance, and contact method. Subsequently, corneal toxicity due to exposure to Zoanthid corals has been documented. Seven patients presented with corneal manifestations ranging from superficial punctate epithelial lesions to bilateral corneal melting and subsequent perforation, with some patients presenting with progressive corneal melting even requiring therapeutic penetrating corneal transplantation. Fortunately, more than half of these case reports show that short-term minor injuries are reversible with medication or emergency measures, with only a few disabilities or a significant reduction in quality of life due to sequelae (Chang et al., 2020).
In 2014, water extracts from water corals were first reported to contain a lethal nonpeptide neurotoxin (García-Arredondo et al., 2015). The investigators administered 5.3 µg protein/g body weight of the extract to mice intravenously, which caused violent convulsions and death in the range of 1 min and histopathological damage to the kidneys and lungs at doses below the LD50 (LD50 = 4.62 µg protein/g body weight). After incubation under heat denaturing conditions, this histopathological damage was completely eliminated. However, the denatured extracts maintained their lethal effect. Second, in the process of researching the anti-neurotoxic active ingredients of the side flat soft willow coral, it was found that water-insoluble parts of alkali extracts of S. suberosa can make the animal produce a whole body soft, heavy limb tremor, turn positive reflex disappear, and cause other reactions (Liao et al., 1992).
Coral is often used as medicine in combination. Ershiwuwei Shanhu pills and others are classic Tibetan remedies consisting coral preparations. In the acute toxicity test of Ershiwuwei Shanhu pills, there were no obvious acute toxic reactions, but in the subacute toxicity test, toxic damage to liver, kidney, and lung pathological sections was observed (Liu F. L. et al., 2016). Long-term doses of Ershiwuwei Shanhu pills lead to accumulation of copper, mercury, and lead in the internal organs of the rats, with few rats developing symptoms of the vegetative nervous system, such as increased salivary gland secretion (LI, 2011). It can cause toxic reactions, manifested in immune function, and liver, kidney, and lung tissues are affected and damaged to varying degrees. The main toxic target organs are the liver, kidney, and lung, and damage due to toxicity occurs in a dose-dependent manner (Liu F. L. et al., 2016). However, given the complexity of its compounds, specific toxic substances remain to be investigated.
6 Clinical application
6.1 Individual application of coral
Coral’s good stability, ease of use, and low cost contribute to its use as main material in the treatment of orthopedic diseases. In addition, coral contains 11 kinds of trace elements, namely, Zn0.05, Cu0.6, Pb0.0025, Ni0.004, Ti0.005, Mn0.004, Fe0.7, Al0.35, Mg3, Si > 1.0, and Sr0.1, and most of these trace elements are indispensable to the human body (Wang et al., 2002b). Xiao et al. (2005) systematically reported on black horn coral for the treatment of bone injury diseases. After taking the medicine for 5–7 days in mild cases and 1–2 months in severe cases, patients’ clinical symptoms were basically relieved, and X-ray films showed that the bone changes were basically corrected or in a stable state. In the clinical method of immediate implant placement, artificial coral bone powder particles were placed in the bone defect area near the crest of the alveolar fossa, where significant osteogenesis was observed after 6 months. The gingival texture and color were better than before the restoration (Zhou, 2014).
Coral clinical applications are detailed in Table 8. It is often processed into powder for punching or used directly to treat bone injury diseases. It is also very effective in the treatment of cerebral vascular sclerosis and coronary artery sclerosis (Yuan, 1991). In 1990, the School of Medicine of Kyoto University in Japan extracted a substance from the coral of the cockle and used one 100th of a gram of it to mix into 1,000 mL of compound saline for injection or infusion. In difficult cases, it is also often used in combination with restorative dental tablets. However, the mechanism of action of coral is still unknown to us. In the available literature, it has been reported that it may be related to the absorption of coral by osteoclast-associated proteins (Lin Y. Y. et al., 2013) and bone marrow granulation tissue and blood vessels (Guillemin et al., 1989). However, it is also only a vague term, and a clearer and more explicit mechanism has to be studied.
6.2 Clinical application of preparations that contain coral
In clinical practice, the compound prescription of coral is mainly composed of Ershiwuwei Shanhu pills, Ershiwuwei Shanhu capsules, and Shanhu Qishiwei pills. Ershiwuwei Shanhu pills are a traditional, famous prescription and proven recipe for Tibetan medicine to treat albichoriasis and epilepsy. It uses coral as the monarch drug, together with pearl, Terminalia chebula and so on. It restores nerve function and relieves pain. It is mainly used to treat albichoriasis, unconsciousness, body numbness, dizziness, brain pain, irregular blood pressure, headache, epilepsy, and various types of neuropathic pain. Based on the collected literature, Ershiwuwei Shanhu pills has satisfactory clinical efficacy in the treatment of neurological diseases (epilepsy, primary headache, etc.), cardiovascular diseases (cerebral infarction, hypertension, etc.), and orthopedic system (neurogenic cervical spondylosis, lumbar myofasciitis, etc.). In acute and severe cases, the combination of drugs is often used clinically to promote a synergistic effect and relief (Table 9).
6.2.1 Clinical application of preparations that contain coral for nervous system disease
Neurological disorders consist of two main areas. First, it is manifested in the treatment of epilepsy disorders. Epilepsy is a chronic disease of sudden, transient, recurrent central nervous system malfunction caused by abnormal over discharge of neurons in the brain (Xu et al., 2009). Ershiwuwei Shanhu pills can cause a significant reduction in the number of seizures, shorten the duration of seizures, improve the type of seizures, reduce the symptoms of headache after seizures, and reduce the degree of cognitive impairment, with significant anti-seizure and anticonvulsant effects. Clinically, 112 patients were randomly divided into a treatment group and a control group, and the treatment group was given 25 coral pills, whereas the control group was treated with Western standardized AEDs. The results showed that the total effective rate of the treatment group was 91.07%, whereas that of the control group was only 67.86% (Wang et al., 2014a). In the treatment of patients with epileptic tonic–clonic seizures, the total effective rate of the treatment group (taking Ershiwuwei Shanhu pills alone) was 88.23% (Wang et al., 2013b). The effects of combination drug treatment regimens have also been reported. Patients were treated orally with Ershiwuwei Shanhu pills in combination with oral levetiracetam tablets or carbamazepine, and the results showed that the therapeutic effect was higher than that of conventional Western medical treatment, reducing the levels of serum IL-2, TNF-α, sICAM-1, IL-6, and CRP. The combination of drugs has better clinical efficacy in the treatment of epilepsy, while improving the immune function of patients and reducing the inflammatory response (Huang and Zhao, 2017; Yuan et al., 2018).
Migraine, tension headache, and intractable headache are common clinical primary headache disorders. Sixty-three patients with migraine were randomly divided and treated with either Ershiwuwei Shanhu capsules or Nao Zhen Ning. After 30 days, 30 out of 33 patients taking Ershiwuwei Shanhu capsules were effectively treated, with a total effective rate of 90.9%, and 22 out of 30 patients taking Nao Zhen Ning were effectively treated, with a total effective rate of only 73.3% (Renwang and Renqing, 2010). A total of 110 patients were selected for the study, and the efficiency of the treatment group (taking Ershiwuwei Shanhu pills alone) was 94.55%, which was significantly higher than the total efficiency of the control group (taking flunarizine hydrochloride capsules combined with amitriptyline hydrochloride tablets), which was 74.55%. Meanwhile, clinical efficacy observation shows that Ershiwuwei Shanhu pills can improve the clinical outcomes of headache by reducing the abnormal blood flow condition (Wang et al., 2013c). In addition to medication, acupuncture can also be combined with treatment. A total of 110 patients were randomly divided into two groups: the control group was treated with acupuncture, and the observation group was treated with Ershiwuwei Shanhu pills. The results showed that the total effective rate was 80% in the acupuncture group but 94.5% in the observation group. Further study found that β-EP, NO, and 5-HT levels in the observation group were higher than those in the acupuncture group, and ET levels in the observation group were lower than those in the acupuncture group, suggesting that Ershiwuwei Shanhu pills can improve neuro-endocrine factors and regulate cerebral blood flow rate in patients with migraine, thereby contributing to the improvement of migraine symptoms (Gu, 2014). As early as 2000, a study found that Ershiwuwei Shanhu pills combined with acupuncture could treat intractable headaches (Bai and You, 2000). Modern research has shown that Ershiwuwei Shanhu pills not only dilate blood vessels and improve the effect of microcirculation in the brain but also alleviate the symptoms of vascular smooth muscle spasm to restore local central cerebral area blood perfusion, thereby relieving headache symptoms (Li, 2007; Yang, 2010).
6.2.2 Clinical application of preparations that contain coral for cardiovascular and cerebrovascular diseases
In cardiovascular system diseases, it is effective in treating poststroke headache and cerebral infarction-related conditions. Sixty-four patients with poststroke headache were studied, and after 4 weeks of treatment, the efficiency of the treatment group who underwent conventional medical treatment in combination with Ershiwuwei Shanhu capsules was 93.75%, which was significantly higher than that of the control group who underwent only conventional medical treatment (56.25%). The patient’s headache level is reduced; the number of attacks is significantly reduced, and the duration of headache is significantly shortened during the treatment period (Shi and Zheng, 2018). In a study by Dongmei Guan, the clinical efficacy of Ershiwuwei Shanhu capsules given to patients with poststroke headache was higher than that of the reference group. Pharmacological analysis showed that the mechanism was similar to that of primary headache, which acted by dilating blood vessels, regulating cerebral blood flow, and improving neurological function (Wang and Li, 2014; Yang et al., 2015).
Regarding cerebral infarction disease, 90 patients were randomly divided into the control group and the observation group and were given Huoxue Tongmai Pian and Ershiwuwei Shanhu pills, respectively. The results showed that the efficacy of Erxuoyi Coral Pill was better, and its clinical application was more valuable (Zeng, 2019). On the basis of the study that Ershiwuwei Shanhu pills can significantly reduce infarct foci in rats with focal cerebral ischemia, researchers randomly selected 60 patients and tested their blood lipid, uric acid, homocysteine, and other levels. The results showed that the treatment group had elevated levels of glutamate transaminase, glutamic oxaloacetic transaminase, and other enzymes, which clearly demonstrated the efficacy of Ershiwuwei Shanhu pills in treating cerebral infarction, but such pills have a certain effect on heart, liver, and kidney function, and the mechanism may be related to the regulation of blood lipids (Zhu et al., 2020). Although aspirin can improve the hypercoagulable state of blood, the drug alone is not effective. Patients with acute cerebral infarction were observed after using Ershiwuwei Shanhu pills combined with aspirin, and the control group used aspirin combined with atorvastatin. The results showed that MMSE scores increased; NIHSS scores, FIB, D-dimer, and platelet aggregation index decreased, and the changes were large in the observation group (Tao et al., 2022). Pharmacological studies have further shown that Ershiwuwei Shanhu pills can inhibit cerebral thrombosis, reduce the area of cerebral infarction, reduce brain tissue edema, dilate cerebral blood vessels, and improve cerebral blood circulation and brain tissue metabolism, which coexist with the antithrombotic effect of aspirin to improve the therapeutic effect and have higher clinical application value (Tan, 2020). The total effective rate of Shanhu Qishiwei pills for the treatment of persistent heart failure also reached 88%, whereas no significant toxic side effects were found (He et al., 2007).
Ershiwuwei Shanhu pills cured 26 out of 30 cases of hypertension, with a total efficiency of 96.7%. The pharmacological study proved that the whole formula lowered blood viscosity, reduced water retention in the body, and changed blood rheology. It has a long-lasting and stable effect on lowering blood pressure level, which is more effective for unstable hypertension (Li J. G., 2010). In addition, combination treatment regimens not only improve treatment efficiency but also ensure treatment safety. The total effective rate of Ershiwuwei Shanhu pills combined with diphenhydramine drugs in the treatment of hypertensive patients was as high as 95.56%, which was higher than that of patients taking only diphenhydramine drugs, whose effective rate was only 77.78% (LI and Liu, 2021).
6.2.3 Clinical application of preparations that contain coral for orthopedic system diseases
Similar to the application of coral single medicine, compound prescription is also effective in orthopedic system diseases, and it has better efficacy in the clinical treatment of neurogenic cervical spondylosis, lumbar myofasciitis, and traumatic synovitis of the knee joint (Li W. H. et al., 2013; Jiao et al., 2013). In 65 clinical cases of neurogenic cervical spondylosis, after taking Ershiwuwei Shanhu pills orally combined with acupuncture based on the condition for one course of treatment, the pain symptoms were significantly reduced, and after two courses, the symptoms disappeared completely, and no recurrence was seen thus far (Zhang and Zhang, 2011). Ershiwuwei Shanhu pills have also been used in combination with conventional Western medical treatment. The researchers randomly assigned 84 patients to the control group who received flunarizine hydrochloride capsules orally and the observation group received Ershiwuwei Shanhu capsules in combination with flunarizine hydrochloride capsules. The results showed that the observation group could increase patients’ plasma neurohypophyseal hormone concentration, reduce pain, and improve blood flow velocity in the vertebral and basilar arteries, with a final total effective rate of 90.48%, which is significantly higher than the 69.05% of the control group (Ren et al., 2015). A patient with lumbar myofasciitis was treated with oral and external application of Ershiwuwei Shanhu pills for 20 days; all the symptoms were removed, and no recurrence was observed after 1 year of follow-up (Li, 2006).
6.2.4 Clinical applications of preparations that contain coral for other diseases
In addition, 25 flavored coral pills have shown clinical return in trauma, herpes zoster, and respiratory system. Seventeen patients with lumbar, hand, and foot sprains and smash injuries were cured within 7 days by using coral Ershiwuwei Shanhu pills alone, internally and externally on the affected area (Yang, 2003). In clinical practice, the efficacy of taking Ershiwuwei Shanhu pills as the monarch drug, together with Chouluo Gengsheng powder, in clearing heat and detoxifying, clearing, and moistening the lung in 54 cases of patients with lung fever obtained satisfactory results (Yang, 2003). Acyclovir is also used clinically in combination with Ershiwuwei Shanhu pills to treat herpes zoster, a neuropathic pain caused by damage after the activation of the herpes zoster virus, which belongs to the Tibetan medical term “albichoriasis” (Zhang H. Y., 2012). Therefore, the treatment of neuralgia of herpes zoster with Ershiwuwei Shanhu pills has unique effects and efficacy. Shanhu Qishiwei pills is also a common classical compound prescription containing coral and is used to treat cerebral hemorrhage, limb paralysis, epilepsy, and various neuritis. In four patients with cerebral hemorrhage, headache and vomiting were relieved after taking Shanhu Qishiwei pills once a day for 20 days, and round-like hypodense foci were observed in the skull. In addition, no other adverse effects were observed (Bian, 2012). Although the compound prescriptions are diverse and the ingredients that exert their medicinal effects may be multiple, the synergistic effect of the coral in treating the symptoms of the disease and improving the efficacy of the treatment is evident.
In clinical practice, we use one side to treat multiple diseases, identify the syndrome accurately, and use the right medicine for the syndrome. The conventional Western medical treatment package includes symptomatic treatment, such as improving the patient’s hemodynamics and pain relief, but the efficacy is not significant (Ren et al., 2015). The therapeutic rate of combined drugs is higher than that of single or compound drugs, and it can even produce additional therapeutic effects. Therefore, it has a higher promotion value and is an effective solution worth promoting in the clinic.
7 Discussion
Coral is an important marine biological resource, and species resources are extremely confusing and complex. In the Qing dynasty (1,616–1912 AD), red coral is a symbol of official status. In India and Tibet of China, people use coral as an auspicious object to worship Buddha, mostly to make Buddhist beads and decorate the statue of the deity in the temple (Hong, 2009). In ancient records, coral applications in medicine have also long been recorded, but only a few have pointed out that coral in medicine is a combination of coral species for the use of red coral. However, red coral has a broad range and many species, such as Corallium japonicum Kishinouye※, Corallium secundum Dana※, and Corallium elatius Ridley. Corallium japonicum Kishinouye※ (trade name: Aka) is mostly used in compounding. Arca is expensive, but no reports have been retrieved on whether other red corals can be substituted. In addition, the corals studied in modern pharmaceutical research involve a total of 34 families and 99 genera of corals, dominated by the families Alcyoniidae, Nephtheidae, and Plexauridae※. Coral species are confusing and complex, and sorting out their resource species not only helps us distinguish corals but also lays the foundation for developing new drugs and further research on corals.
Coral has a long history of medicinal value, which can remove corneal opacity, improve eyesight, tranquilize the mind, promote wound healing, and stop bleeding. Modern pharmacological studies have also gradually verified the medicinal value of coral and its mechanism of action. First, coral transplantation in the human body does not cause rejection; in coral, countless fine pores will gradually grow microscopic blood vessels and synthesize living cells of the bone. Numerous studies have reported that coral has become an alternative material to bone, and coral is often used in the fields of maxillofacial surgery and orthopedics (Guillemin et al., 1989; Zhu, 2001; Lai, 2017). Second, active ingredients such as terpenoids (diterpenes and sesquiterpenes) and steroids extracted from coral have evident pharmacological properties such as antiviral, antibacterial, antioxidant, and antimalarial activities. In addition, some of the active ingredients show not only good enzyme inhibition activity but also evident anticonvulsant, antiepileptic, and sedative–hypnotic effects in the nervous system; in the cardiovascular system, they show anti-tubular formation activity and proangiogenic activity as well as a certain amount–effect relationship. The antihypertensive, hypolipidemic (Chun et al., 2022), and antiulcer (Elshamy et al., 2017) activities have also been relevantly verified. Most of these chemical compounds were extracted from corals of Alcyoniidae and Gorgonidae※, and the compounds extracted from a particular coral may have multiple uses. Therefore, the study of active ingredients in corals has become the cornerstone of subsequent pharmacological studies, and exploring the mechanism of action of active substances can be a research direction to provide a basis for the elucidation of pharmacological effects and the design of clinical experiments (Wang, 2015).
Coral has various pharmacological activities, among which cytotoxic, anti-inflammatory, and analgesic pharmacological effects are more prominent. A549, HL-60, MCF-7, colon cancer cells, K562, HeLa, and other tumor cells are research hotspots. Scholars have mostly evaluated the inhibitory and apoptotic effects of different concentrations of active ingredients on different cells by MTT assay and SRB method. Studies have also shown that cytotoxicity can be influenced by compound structure. For example, prostaglandins with hydroxyl groups have good inhibitory properties (Hurtado et al., 2020); sterols introduced with hydroxyl groups decrease the inhibitory potency against HeLa cell lines; and acetyl groups increase the cytotoxic activity. Pro-inflammatory enzymes, particularly iNOS for nitric oxide production and prostaglandin-producing COX-2, play a central role in inflammatory mechanisms (Wei et al., 2013). In addition, glial cells and elastin are also important components of the anti-inflammatory mechanism. At present, pharmacological experiments of coral have identified its active ingredients. However, most of the results of pharmacological studies are derived from cellular or animal models, and they do not fully prove their effectiveness, so more clinical trials are needed to confirm these findings (Zhang X. L. et al., 2015).
Clinically, coral is often processed into powder for punching or used directly to treat bone diseases, in addition to showing good therapeutic effects in the treatment of epilepsy, primary headache, migraine, cerebral infarction, hypertension, neurogenic cervical spondylosis, and lumbar myofasciitis. In the face of complex diseases, obtaining the desired effect of a single drug is difficult, so coral is often used in combination with other drugs to treat the disease, which has satisfactory results in clinical applications. At a certain efficacy, compound prescriptions containing coral exhibit the same effects as when coral used alone. However, given the large number of herbs contained in the compound, the role played by coral remains unclear; the effect may be weakened; the effect may be synergistically enhanced; or another effect may be stimulated. Moreover, the mechanism of action of coral remains unknown; thus, further research is needed.
Although coral toxicity is not included in the Pharmacopoeia of the People’s Republic of China, studies have found that coral toxicity is mostly found in marine ornamental soft corals of the Zoanthidae family. Palytoxin is the main toxic compound. Nonpeptide neurotoxins were extracted from water coral all of which have toxic effects on the skin, cornea, etc. Short-term minor injuries are reversible with medication or emergency measures, with only a few disabilities or a significant decrease in quality of life because of sequelae. No significant acute toxicity was observed in coral-related compound preparations, but if applied for a long time, toxicity to the liver, kidneys, lungs, and other internal organs can still occur in a dose-dependent manner. The toxicity of coral is not yet generalized because of the complexity and diversity of its species. Coral insects are toxic, but whether coral is toxic after calcification is yet to be studied because of the special nature of coral.
The organic compounds in corals are remarkably studied, whereas other compounds, such as trace elements, are less studied. Coral as mineral medicine should strengthen the exploration and development of other compounds, such as trace elements, to pave the way for improving its quality standards and research on the basis of medicinal substances. Toxicological studies have also come to the forefront. The limited clinical trials are not perfect in quality, but they still have some reference value, and more scientific and representative clinical trials are needed in the future. At present, coral is used in several different fields, such as medical and apparel (Zhang Q. Y., 2013), with more areas still under development. Its value in medical care is particularly significant, which needs more attention and extensive research.
Coral reefs are one of the most diverse ecosystems on Earth, sensitive and fragile marine ecosystems, and one of the most sensitive environmental indicators of global climate change (Huang et al., 2023). Coral reefs not only provide a place for many fish and marine invertebrates to lay eggs, reproduce, and avoid predators, but also have extremely high biodiversity. It also plays a crucial role in homeland security. At present, habitat loss, diseases, bleach accidents, and species invasion are the main causes of coral death (Ma et al., 2018). Antipatharia, Scleractinia, Helioporacea, Gorgonaceae, Tubiporidae, Corallium, Corallium elatius, Corallium japonicum, Corallium konjou, and Corallium secundum are officially listed as endangered. Fishing for any coral species may have some impact on the ecosystem, as coral reefs are a complex ecosystem that is interdependent on many other organisms. So wild acquisition is strictly prohibited for species that have already been listed as extinct in the wild, regionally extinct, and critically endangered. Strengthening the protection and management of rare and endangered wild plants of vulnerable, near-endangered, and non-endangered species by building dynamic monitoring databases and strictly implementing on-site conservation measures is essential to maintain ecological balance and biodiversity (Wang, 2023). The most fundamental thing is that the coral species (extinct in the wild, regionally extinct, critically endangered, and rare and endangered wild plants of vulnerable, near-endangered, and non-endangered species) included in the list have no distinction between beneficial and insignificant and should receive equal protection. Strict implementation of the Endangered Species Law should help them restore to their normal numbers.
It is worth noting that some corals are currently listed as endangered on CITES and IUCN lists, such as Antipatharia※, Tubiporidae※, Corallium elatius※, Corallium japonicum※, Corallium konjou※, and Corallium secundum※, ensuring that a balance between the sustainability of scientific research and the protection of endangered species is a complex but crucial task. The development and research on the effectiveness of coral is likely to lead to the indiscriminate capture of coral, thereby exacerbating the endangered situation of coral. Ethically speaking, the application and development of coral should be prohibited. This article mainly discusses the endangerment caused by coral medicinal use. Therefore, we encourage scientists to use new technologies and methods, such as remote sensors, remote sensing technology, and genetic analysis, to reduce interference with endangered species while providing more valuable data (Wang et al.). The most effective measure is to avoid and prohibit the use of coral. We call on scholars to devise strategies for replacing coral in treatments to alleviate and solve the problems of endangerment of corals. First, search for alternatives based on similar biological species relationships. Species that are closely related often have similar physiological structures, as well as their chemical composition and pharmacological activities (Tian et al., 2023). Naemorhedus goral and Saiga tatarica have similar chemical components such as proteins, peptides, and amino acids (Liu et al., 2018), and as a substitute, Naemorhedus goral has a better sedative effect (Jiang and Zhai, 2006). Therefore, soft corals, sea fans, and sea yellows with similar chemical components have become one of the ways to replace endangered corals. Second, search for alternatives based on similar pharmacological effects. On the one hand, coral is usually used as an orthopedic material for the treatment of bone diseases. Therefore, we can achieve the same therapeutic effect by using other composite materials such as composite resin, ceramic, rubber, organic glass resin, and metal alloy instead of coral. This measure can not only achieve the effect of treating diseases but also reduce the use of coral. On the other hand, based on the bioactive substances extracted from coral, search for other organisms contain similar bioactive substances. For example, the pharmacological activity of cetosane diterpenoids can be extracted from Boswellia carterii (Xu et al., 2023). Medicinal compounds such as terpenoids and other substances can be extracted from Croton tiglium and Panax notoginseng (Wei et al., 2022). In modern research, replacing bile powder of Rhinoceros unicornis with that of Bubalus bubalis and using other animal bile powder instead of that of Selenarctos thibetanus as medicine have alleviated the endangerment problem of animals to a certain extent (Bai et al., 2018; Chen et al., 2022; Ye et al., 2022). Third, search for alternatives based on artificial domestication and breeding. Artificial cultivation of coral has become a feasible method (Wang, 2020). The artificial breeding technology of coral can be divided into sexual reproduction and asexual reproduction and can be classified into in situ cultivation technology and off-site cultivation technology according to the cultivation environment. The South China Sea Institute of Oceanography, Chinese Academy of Sciences, has carried out experiments on coral sexual reproduction and coral larva cultivation and proliferation in Sanya Bay and Yongxing Island in Xisha, Hainan Province. At present, it has mastered the reproductive law and larva development process of species such as Acropora gemmifera※ and Platygyra sinensis (Yu et al., 2022)※. Fourth, look for alternatives based on synthetic methods. The active ingredients with pharmacological activities can be directly synthesized by chemical synthesis and enzyme engineering technology. The development of artificial Moschus berezovskii can be described as a sword sharpened for decades, which has completely solved the problem of long-term shortage of Moschus berezovskii supply (Fu et al., 2023). Artificial Panthera tigris is similar to natural Panthera tigris in fingerprint, pharmacological and pharmacodynamic indexes, and clinical efficacy (Liu and Han, 2006). They are all used in a variety of Chinese patent medicines (Yan et al., 2023). Finally, find the alternative mode of biotechnology based on industrialization. The use of coral stem cells to cultivate medicinal parts and secrete metabolites is a biotechnology method that can meet the requirements of syngeneic and homogeneous substitutes. Animal stem cell research mainly focuses on the direct use of stem cells for disease treatment, repair of organ damage, and establishment of the drug screening platform (Shi, 2020). Taxus chinensis’s stem cells have been used to produce paclitaxel and its corresponding active substance (Lee et al., 2010), and Panax ginseng stem cells have been used to produce ginseng (Jang et al., 2023).
Although some developments have not been broken yet through due to incomplete research on the material foundation and mechanism of action, as well as immature artificial farming techniques, which have prevented the formation of large-scale farming, modern biotechnology and multi-omics detection methods have brought new avenues for the development and evaluation of animal drugs, using genomics, proteomics, transcriptomics, metabolomics, and other detection methods at the molecular and cellular levels. The balanced and comprehensive approaches such as implementing multidimensional and multi-level systematic evaluation at the animal level, establishing new approaches for alternative research, and providing support for the protection, development, and utilization of endangered medicinal animals help ensure the survival of endangered species while providing valuable knowledge for the scientific community (Chun et al.).
It is undeniable that effective scientific research can alleviate the problem of endangerment of species, but the decision to “protect” and/or “establish a recovery plan” does not depend only on science. At the same time, there is ambiguity among managers regarding governance issues, and the institutional management plan seems to have failed to address the vulnerability of endangered species. Therefore, a statutory plan should be established to fundamentally alert people. Unfortunately, only relatively few countries have enacted national legislation on endangered and threatened species. Internationally, the Endangered Species Act of 1973 is a legislative model. Its implementation has achieved significant results, and it is said that 90% of the species on the bill’s protected list have been restored (Teng and Zhang, 2022). So the government and relevant departments should strengthen regulations and policies to ensure that research on endangered coral species does not lead to abuse or overfishing, such as restrict or prohibit fishing and destructive activities and adopt sustainable fishing practices, including limiting fishing quantities, using selective fishing nets, and monitoring fishing activities, to protect marine ecosystems. Obviously, effective protection of endangered and threatened species in the ocean depends on appropriate legislation developed to protect them, and similarly, achieving the goals of these legislative tools depends on the political and social factors that affect their implementation. Since the 21st century, the debate between the protection of endangered species and economic benefits and ecological values has never stopped. Congress and the government always try to weaken the effectiveness of bills and tend to choose economic development. Opponents believe that some measures have harmed their own interests, and even more so, some are testing the “red line” for potential benefits. However, the public is more inclined to support the protection of endangered species, and the public support rate for the bill remains high. Obviously, the importance of protecting species is higher than economic growth and protecting private property, and maintaining biodiversity is a global cause (Jeffrey, 2016). Wildlife managers have a greater responsibility to ensure that their management actions reflect public values and attitudes. Third, the negative impact of social media comments on the public is very strong, which leads to significant differences in public beliefs in participating in endangered species management (Rodgers and Willcox, 2018). Some people, even with an urgent desire to protect endangered species, have low mobility. Therefore, it is important to raise awareness among the public, governments, and businesses about the importance of protecting coral reefs, encourage environmental action, and alleviate the pressure faced by these fragile ecosystems. In addition, with the development of industrialization and technology, the marine ecosystem is increasingly deteriorating, the ability to accommodate and rescue wildlife is weak, and habitats are gradually lost. Scientific research and monitoring are necessary. This includes ecosystem monitoring, which regularly monitors the health status of coral reef ecosystems and changes in environmental parameters such as temperature, salinity, and acidity, as well as research on coral diseases. Studying the pathogenesis of coral diseases will help develop prevention and control strategies (Yang, 2021). It is also necessary to reduce the flow of land pollution into the ocean, including agricultural and urban sewage discharge, as well as pollutants from rivers and streams (Gao, 2023; Zhu and Hu, 2023).
There is relatively little research on linking wildlife value orientations with attitudes toward T&E species and non-charismatic species, and even to a large extent, it has been overlooked (George et al., 2016). We encourage cooperation among scholars, conservation organizations, and governments to work together to achieve the common goals of scientific research and conservation (Lv et al., 2020; Zou and Jiang, 2023).
Coral is one of the marine species. The imminent extinction of coral reminds people to take immediate measures to protect endangered species and the ecological environment. The substitution principles are as follows: search for alternatives based on similar biological species relationships. Search for alternatives based on similar pharmacological effects. Search for alternatives based on artificial domestication and breeding. Look for alternatives based on synthetic methods. Search for the alternative mode of biotechnology based on industrialization. These principles should be applied to protect all organisms from natural sources and not restricted to corals. Among them, the alternative strategy of artificial domestication is one of the most fundamental and effective measures. For example, treatments using pearls, centipedes, and others should be supplemented and replaced by other resources to prevent their endangerment or even extinction. In addition, reducing pollution, which means taking measures to reduce marine pollution, especially plastic waste, agricultural and industrial emissions, oil pollution, and sustainable economic management methods including fisheries, tourism, and marine technology, can promote economic growth and create decent employment opportunities, thereby reducing people’s hunting of certain species, which is also one of the effective measures to protect ecosystems (Chinese and Foreign Experts and Scholars Talk Together on Marine Ecological Protection in the Process of Modernization, 2023). Protecting endangered species and protecting ecosystems is our mission and responsibility. These measures can balance the goal of protecting ecosystems and meeting human needs.
Recently, the signing of the High Seas Treaty in September 2023 and the Nagoya Protocol in October 2010 has pushed the protection of endangered species and ecological environmental issues onto the international stage, marking a significant shift in ecological governance from disorder to order. The Chinese government must strictly abide by the agreement, and in addition, it can educate the Chinese people, enterprises, and other relevant departments through media and policies to reduce and prohibit the use of coral. China’s participation should make good use of the platform for treaty and agreement consultations and be more proactive in clarifying the content and value of this theory to the international community, injecting new vitality into the protection of ecology and subsequent consultations. The feasibility of global action is low, and its effectiveness is difficult to assess and regulate. However, the ability of countries or regional international organizations to respond more quickly to environmental changes greatly contributes to the improvement of the marine environment. International treaties can not only regulate the behavior of contracting parties but also promote the practice of non-contracting parties. Conducting this work under international cooperation undoubtedly has the most legitimacy and credibility. This not only strengthens cooperation among countries and encourages them to jointly address high seas protection issues but also enhances people’s awareness of coral protection and ecosystem protection through universal participation. At the same time, these agreements also limit people’s indiscriminate killing of corals and causing damage to the environment, pointing the way for protecting the ecological environment. To a large extent, it is urgent to alert and call on people to protect corals and endangered species, but how to achieve from regional practice to universal participation is still a difficult implementation dilemma.
Warning: The coral species marked with "※" have been included in the rare species list of CITES and IUCN. We should respect life and nature. We should protect wild animals and create a safe home for them. In addition, we call on researchers to increase their attention and importance to the protection of endangered corals and to conduct limited and valuable coral-related research within the framework of domestic and foreign laws and regulations. We also call on non-researchers to refrain from illegally collecting and using endangered corals after reading this review article and to carry out collection and utilization activities within the framework of domestic and foreign laws and regulations.
8 Conclusion
Marine invertebrates, a rich potential source of drug precursors, have been a popular avenue for the international search for drugs or drug precursors in recent decades. In the last two decades, coral chemistry and pharmacology research has made some achievements and discovered some new compounds with unique structures and strong physiological activities, but the utilization of corals is limited to only a small number and species of families, such as Alcyoniidae, Nephtheidae, Plexauridae, and Gorgoniidae※. This article provides the first comprehensive account of six aspects of the medicinal history, species, chemical composition, pharmacological activity, toxicology, and clinical application of coral in China. Coral is a natural mineral medicine, and its active ingredients are mixed and difficult to extract and identify. At present, the effective compounds extracted from coral are terpenoids, steroids, and nitrogen-containing compounds, with sesquiterpenes and diterpenes being the main compounds of terpenoids. However, during extraction, extraction conditions, and the joint use of related techniques, such as ICP-MS and LC-MS, have not been reported. Exploring the best extraction of the active ingredients of coral is a breakthrough for future experiments. The pharmacological effects of most of the compounds isolated from coral have been developed. The pharmacological activities of terpenoids are relatively rich, including cytotoxicity, anti-inflammatory, antibacterial, and antiviral. Second, steroid compounds also play important roles in antitumor, anticancer, and anti-inflammatory activities. Finally, other compounds such as lipids and aromatic compounds play important roles in their pharmacological activities such as antioxidant and immunosuppressive effects. However, they mostly reside in superficial areas, which shows a long way to go in the study of the mechanism. In addition, scientists are encouraged to use new technologies and methods, such as remote sensors and gene analysis, to reduce interference with endangered species while providing more valuable data. Toxicological studies have shown that corals of the family Zoanthidae cause toxic reactions in people through contact and inhalation, but they can be treated with pharmacological relief. With regard to clinical application, coral is mostly used in combination with other drugs to treat diseases, with limited cases of coral alone, which may lead to the inability to prove the effectiveness of coral but is still informative. Pharmacological studies of coral are mostly about the monomer extracted from coral, whereas clinical studies are more about the compound prescription application of coral. Studies show that coral is often used as a substitute for orthopedic materials to treat diseases such as bone defects and bone hyperplasia. Compound preparations that contain coral are widely used in the treatment of neurological diseases such as migraine, primary headache, epilepsy, cerebral infarction, hypertension, and other cardiovascular and cerebrovascular diseases.
More extensive and in-depth research on the active ingredients of coral and its mechanism of action should be focused on deepening the understanding at genetic and molecular levels in the future to make it better applied in practice. In addition, whether the absorption, distribution, metabolism, and excretion as well as the blood concentration of coral change over time after administration in the body remains unclear. Coral is often used in the form of powder into the body, but whether coral powder can be taken orally as well as differences and similarities between its oral and external therapeutic effects remains unknown. Finally, diluted coral extracts have been derived as new drugs for the treatment of diseases. Therefore, the form of coral intake should not be limited to powder or as an orthopedic material. However, the development of its active ingredients is not a research strategy and prospect. It provides different ideas for the development of new drugs. We experienced pressure and challenge during the study of the clinical application of coral but offered us a good opportunity (Ai et al., 2006).
Author contributions
MH is responsible for collecting data and writing this article, ZoW is responsible for improving the chemical composition structure diagram and pharmacological activity content, and YL is responsible for the translation of the clinical application section. YS put forward relevant suggestions for the article, and ZaW directs the writing of the article and functions as our corresponding author. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Sichuan Province (2023NSFSC0654).
Acknowledgments
We thank all authors for their contributions to this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhafez, O. H., Ali, T. F. S., Fahim, J. R., Desoukey, S. Y., Ahmed, S., Behery, F. A., et al. (2020). Anti-inflammatory potential of green synthesized silver nanoparticles of the soft coral nephthea sp. Supported by metabolomics analysis and docking studies. Int. J. nanomedicine 15, 5345–5360. doi:10.2147/IJN.S239513
Abdelkarem, F. M., Desoky, E. K., Nafady, A. M., Allam, A. E., Mahdy, A., Ashour, A., et al. (2021). Two new polyhydroxylated steroids from Egyptian soft coral Heteroxenia fuscescens (Fam.; Xeniidae). Nat. Prod. Res. 35 (2), 236–243. doi:10.1080/14786419.2019.1624958
Abdel-Lateff, A., Alarif, W. M., Ayyad, S. E., Al-Lihaibi, S. S., and Basaif, S. A. (2015). New cytotoxic isoprenoid derivatives from the Red Sea soft coral Sarcophyton glaucum. Nat. Prod. Res. 29 (1), 24–30. doi:10.1080/14786419.2014.952637
Abou El-Kassem, L. T., Hawas, U. W., El-Desouky, S. K., and Al-Farawati, R. (2018). Sesquiterpenes from the Saudi Red Sea: Litophyton arboreum with their cytotoxic and antimicrobial activities. Zeitschrift fur Naturforschung. C, J. Biosci. 73 (1-2), 9–14. doi:10.1515/znc-2017-0037
Aboutabl el, S. A., Azzam, S. M., Michel, C. G., Selim, N. M., Hegazy, M. F., Ali, A. H., et al. (2013). Bioactive terpenoids from the Red Sea soft coral Sinularia polydactyla. Nat. Prod. Res. 27 (23), 2224–2226. doi:10.1080/14786419.2013.805333
Ahmed, A. F., Dai, C. F., Kuo, Y. H., and Sheu, J. H. (2003). 1alpha,3beta,5beta-trihydroxy-24-methylenecholestan-6-one: a novel steroid from a soft coral Sinularia gibberosa. Steroids 68 (4), 377–381. doi:10.1016/s0039-128x(03)00036-9
Ahmed, A. F., Teng, W. T., Huang, C. Y., Dai, C. F., Hwang, T. L., and Sheu, J. H. (2017a). Anti-inflammatory lobane and prenyleudesmane diterpenoids from the soft coral Lobophytum varium. Mar. drugs 15 (10), 300. doi:10.3390/md15100300
Ahmed, A. F. s., Tsai, C. R., Huang, C. Y., Wang, S. Y., and Sheu, J. H. (2017b). Klyflaccicembranols A-I, new cembranoids from the soft coral Klyxum flaccidum. Mar. drugs 15 (1), 23. doi:10.3390/md15010023
Ai, X. H., Chen, Y. X., and Qi, S. H. (2006). New advances in the study of chemical composition and biological activity of Chinese coral. J. Guangzhou Univ. Natur. Sci. Ed., 49–56. doi:10.3969/j.issn.1671-4229.2006.01.012
Alam, N., Bae, B. H., Hong, J., Lee, C. O., Im, K. S., and Jung, J. H. (2001). Cytotoxic diacetylenes from the stony coral Montipora species. J. Nat. Prod. 64 (8), 1059–1063. doi:10.1021/np010148b
Al-Lihaibi, S. S., Ayyad, S. E., Shaher, F., and Alarif, W. M. (2010). Antibacterial sphingolipid and steroids from the black coral Antipathes dichotoma. Chem. Pharm. Bull. 58 (12), 1635–1638. doi:10.1248/cpb.58.1635
Bai, S. J., and You, W. F. (2000). Summary of 8 cases of intractable headache treated with acupuncture in combination with twenty-five flavor coral pills western journal of traditional Chinese medicine, 50. 03.
Bai, X. T. (2011). Study on the volatile constituents and bioactivity of Black coral. Master’s dissertation. Shantou, China: Shantou university.
Bai, Z., Ni, H., Lian, H., Tie, L., Sa, R., and Bai, C. (2018). Effect of Mongolian medicine bear bile and several livestock bile on cyclophosphamide induced thrombocytopenic bleeding model. Res. Pract. Chin. Med. 32 (04), 31–35. doi:10.13728/j.1673-6427.2018.04.009
Bian, L. L., Liu, F., Gu, X. F., and Zhao, X. C. (2016). Ershiwuwei coral capsules combined with bovine pox vaccine-inflammatory rabbit skin extract for the treatment of headache. World Latest Med. Inf. 16 (79), 152. doi:10.3969/j.issn.1671-3141.2016.79.123
Bian, M. L. (2012). Clinical observation on the treatment of cerebral hemorrhage with Monk's Coral Seventy Pill. Chin. J. Ethnomedicine Ethnopharmacy 21 (03), 4. doi:10.3969/j.issn.1007-8517.2012.03.005
Braña, A. F., Sarmiento-Vizcaíno, A., Osset, M., Pérez-Victoria, I., Martín, J., de Pedro, N., et al. (2017). Lobophorin K, a new natural product with cytotoxic activity produced by streptomyces sp. M-207 associated with the deep-sea coral lophelia pertusa. Mar. drugs 15 (5), 144. doi:10.3390/md15050144
Cai, Y. S., Cui, W. X., Tang, W., and Guo, Y. W. (2020). Uncommon terpenoids with anti-inflammatory activity from the Hainan soft coral Sinularia tumulosa. Bioorg. Chem. 104, 104167. doi:10.1016/j.bioorg.2020.104167
Cao, F., Shao, C. L., Chen, M., Zhang, M. Q., Xu, K. X., Meng, H., et al. (2014). Antiviral C-25 epimers of 26-acetoxy steroids from the South China Sea gorgonian Echinogorgia rebekka. J. Nat. Prod. 77 (6), 1488–1493. doi:10.1021/np500252q
Cao, X. (2012). Studies on the chemical constitutents and their bioactivities of Sinularia sp. Master’s dissertation. Qingdao, China: Ocean University of China.
Chang, C. H., Wen, Z. H., Wang, S. K., and Duh, C. Y. (2008). New anti-inflammatory steroids from the Formosan soft coral Clavularia viridis. Steroids 73 (5), 562–567. doi:10.1016/j.steroids.2008.01.007
Chang, E., Deeds, J., and Spaeth, K. (2020). A case of long-term neurological and respiratory sequelae of inhalational exposure to palytoxin. Toxicon official J. Int. Soc. Toxinology 186, 1–3. doi:10.1016/j.toxicon.2020.07.018
Chao, C. H., Chou, K. J., Huang, C. Y., Wen, Z. H., Hsu, C. H., Wu, Y. C., et al. (2011a). Bioactive cembranoids from the soft coral Sinularia crassa. Mar. drugs 9 (10), 1955–1968. doi:10.3390/md9101955
Chao, C. H., Chou, K. J., Huang, C. Y., Wen, Z. H., Hsu, C. H., Wu, Y. C., et al. (2012). Steroids from the soft coral Sinularia crassa. Mar. drugs 10 (2), 439–450. doi:10.3390/md10020439
Chao, C. H., Chou, K. J., Wen, Z. H., Wang, G. H., Wu, Y. C., Dai, C. F., et al. (2011b). Paraminabeolides A-F, cytotoxic and asnti-inflammatory marine withanolides from the soft coral Paraminabea acronocephala. J. Nat. Prod. 74 (5), 1132–1141. doi:10.1021/np2000705
Chao, C. H., Hsieh, C. H., Chen, S. P., Lu, C. K., and Sheu, J. H. J. C. (2006). Sinularianins A and B, novel sesquiterpenoids from the Formosan soft coral Sinularia sp. Tetrahedron Lett. 47 (49), 5889–5891. doi:10.1002/chin.200649183
Chao, C. H., Huang, H. C., Wu, Y. C., Lu, C. K., Dai, C. F., and Sheu, J. H. (2007). Glycolipids from the formosan soft coral Lobophytum crassum. Chem. Pharm. Bull. 55 (12), 1720–1723. doi:10.1248/cpb.55.1720
Chao, C. H., Wen, Z. H., Su, J. H., Chen, I. M., Huang, H. C., Dai, C. F., et al. (2008a). Further study on anti-inflammatory oxygenated steroids from the octocoral Dendronephthya griffini. Steroids 73 (14), 1353–1358. doi:10.1016/j.steroids.2008.06.008
Chao, C. H., Wen, Z. H., Wu, Y. C., Yeh, H. C., and Sheu, J. H. (2008b). Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 71 (11), 1819–1824. doi:10.1021/np8004584
Chau, V. M., Phan, V. K., Nguyen, X., Nguyen, X. C., Nguyen, P. T., Nguyen, H. N., et al. (2011). Cytotoxic and antioxidant activities of diterpenes and sterols from the Vietnamese soft coral Lobophytum compactum. Bioorg. Med. Chem. Lett. 21 (7), 2155–2159. doi:10.1016/j.bmcl.2011.01.072
Chen, B. W., Chang, S. M., Huang, C. Y., Su, J. H., Wen, Z. H., Wu, Y. C., et al. (2011a). Hirsutosterols A-G, polyoxygenated steroids from a Formosan soft coral Cladiella hirsuta. Org. Biomol. Chem. 9 (9), 3272–3278. doi:10.1039/c1ob05106g
Chen, B. W., Chao, C. H., Su, J. H., Wen, Z. H., Sung, P. J., and Sheu, J. H. (2010a). Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 8 (10), 2363–2366. doi:10.1039/b926353e
Chen, B. W., Uvarani, C., Huang, C. Y., Hwang, T. L., Dai, C. F., and Sheu, J. H. (2015). New anti-inflammatory tocopherol-derived metabolites from the Taiwanese soft coral Cladiella hirsuta. Bioorg. Med. Chem. Lett. 25 (1), 92–95. doi:10.1016/j.bmcl.2014.11.002
Chen, D. X., Chu, J. F., Lin, S., Zhang, L., Chen, H. W., Sun, Z. W., et al. (2022). Therapeutic effects of different animal bile powders on lipid metabolism disorders and their composition analysis. Chin. J. Integr. Med. 28 (10), 918–923. doi:10.1007/s11655-021-3441-3
Chen, M., Han, L., Zhang, X. L., and Wang, C. Y. (2016). Cytotoxic 19-oxygenated steroids from the South China Sea gorgonian, Pacifigorgia senta. Nat. Prod. Res. 30 (12), 1431–1435. doi:10.1080/14786419.2015.1063055
Chen, M. F., Zhou, R. L., and Chen, X. Y. (2014a). Effects of ershiwuwei coral pill combined with flunarizine on plasma endothelin in patient with migraine. Intern. Med. 9 (02), 145–147. doi:10.16121/j.cnki.cn45-1347/r.2014.02.042
Chen, N. F., Huang, S. Y., Lu, C. H., Chen, C. L., Feng, C. W., Chen, C. H., et al. (2014b). Flexibilide obtained from cultured soft coral has anti-neuroinflammatory and analgesic effects through the upregulation of spinal transforming growth factor-β1 in neuropathic rats. Mar. drugs 12 (7), 3792–3817. doi:10.3390/md12073792
Chen, S. P., Su, J. H., Yeh, H. C., Ahmed, A. F., Dai, C. F., Wu, Y. C., et al. (2009). Novel norhumulene and xeniaphyllane-derived terpenoids from a formosan soft coral Sinularia gibberosa. Chem. Pharm. Bull. 57 (2), 162–166. doi:10.1248/cpb.57.162
Chen, Y. H., Hwang, T. L., Su, Y. D., Chang, Y. C., Chen, Y. H., Hong, P. H., et al. (2012). New 6-hydroxyeunicellins from a soft coral Cladiella sp. Chem. Pharm. Bull. 60 (1), 160–163. doi:10.1248/cpb.60.160
Chen, Y. H., Tai, C. Y., Hwang, T. L., Weng, C. F., Li, J. J., Fang, L. S., et al. (2010b). Cladielloides A and B: new eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar. drugs 8 (12), 2936–2945. doi:10.3390/md8122936
Chen, Y. H., Tai, C. Y., Kuo, Y. H., Kao, C. Y., Li, J. J., Hwang, T. L., et al. (2011b). Cladieunicellins A-E, new eunicellins from an Indonesian soft coral Cladiella sp. Chem. Pharm. Bull. 59 (3), 353–358. doi:10.1248/cpb.59.353
Chen, Y. P., Sun, X., Wang, S. Y., Ao, D. S., and Song, H. (2023). Application of proteomics technology in virology research. Chin. J. Virology 39 (02), 572–582. doi:10.13242/j.cnki.bingduxuebao.004212
Chen, Y. S. (2011). Analysis of vaginal discharge test results in 720 cases. Chongqing Med. 40 (36), 3718–3720. doi:10.3969/j.issn.1671-8348.2011.36.035
Chen, Z. H., Li, W. S., Zhang, Z. Y., Luo, H., Wang, J. R., Zhang, H. Y., et al. (2021). Sinusiaetone A, an anti-inflammatory norditerpenoid with a bicyclo[11.3.0]hexadecane nucleus from the hainan soft coral Sinularia siaesensis. Org. Lett. 23 (15), 5621–5625. doi:10.1021/acs.orglett.1c01601
Cheng, S. Y., Chen, P. W., Chen, H. P., Wang, S. K., and Duh, C. Y. (2011). New cembranolides from the Dongsha Atoll soft coral Lobophytum durum. Mar. drugs 9 (8), 1307–1318. doi:10.3390/md9081307
Cheng, S. Y., Chuang, C. T., Wang, S. K., Wen, Z. H., Chiou, S. F., Hsu, C. H., et al. (2010a). Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 73 (6), 1184–1187. doi:10.1021/np100185a
Cheng, S. Y., Chuang, C. T., Wen, Z. H., Wang, S. K., Chiou, S. F., Hsu, C. H., et al. (2010b). Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 18 (10), 3379–3386. doi:10.1016/j.bmc.2010.04.012
Cheng, S. Y., Huang, K. J., Wang, S. K., Wen, Z. H., Chen, P. W., and Duh, C. Y. (2010c). Antiviral and anti-inflammatory metabolites from the soft coral Sinularia capillosa. J. Nat. Prod. 73 (4), 771–775. doi:10.1021/np9008078
Cheng, S. Y., Wen, Z. H., Wang, S. K., Chiou, S. F., Hsu, C. H., Dai, C. F., et al. (2009a). Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 72 (1), 152–155. doi:10.1021/np800686k
Cheng, S. Y., Wen, Z. H., Wang, S. K., Chiou, S. F., Hsu, C. H., Dai, C. F., et al. (2009b). Anti-inflammatory cembranolides from the soft coral Lobophytum durum. Bioorg. Med. Chem. 17 (11), 3763–3769. doi:10.1016/j.bmc.2009.04.053
Cheng, W., Liu, Z., Yu, Y., van Ofwegen, L., Proksch, P., Yu, S., et al. (2017). An unusual spinaceamine-bearing pregnane from a soft coral Scleronephthya sp. inhibits the migration of tumor cells. Bioorg. Med. Chem. Lett. 27 (12), 2736–2741. doi:10.1016/j.bmcl.2017.04.058
Cheng, W., Ren, J., Huang, Q., Long, H., Jin, H., Zhang, L., et al. (2016). Pregnane steroids from a gorgonian coral Subergorgia suberosa with anti-flu virus effects. Steroids 108, 99–104. doi:10.1016/j.steroids.2016.02.003
Chiang, P. C., Kung, F. L., Huang, D. M., Li, T. K., Fan, J. R., Pan, S. L., et al. (2006). Induction of Fas clustering and apoptosis by coral prostanoid in human hormone-resistant prostate cancer cells. Eur. J. Pharmacol. 542 (1-3), 22–30. doi:10.1016/j.ejphar.2006.05.030
Chinese and Foreign Experts and Scholars Talk Together on Marine Ecological Protection in the Process of Modernization (2023). Chinese and foreign Experts and scholars Talk together on marine ecological protection in the process of modernization. Beijing, China: People's Daily. People's Daily. 017.
Chu, M. J., Tang, X. L., Han, X., Li, T., Luo, X. C., Jiang, M. M., et al. (2018). Metabolites from the paracel islands soft coral Sinularia cf. molesta. Mar. drugs 16 (12), 517. doi:10.3390/md16120517
Chun, H., Wei, D. D., Zhu, X. H., Wu, Z. Y., and Dawa, Z. B. (2022). Study on the hypolipidemic effect and mechanism of the Tibetan medicine Coral Shanhu Qishiwei Pills on rats with hyperlipidemia model. China Pharm. 33 (01), 46–50. doi:10.6039/j.issn.1001-0408.2022.01.08
Chung, H. M., Hong, P. H., Su, J. H., Hwang, T. L., Lu, M. C., Fang, L. S., et al. (2012). Bioactive compounds from a gorgonian coral Echinomuricea sp. (Plexauridae). Mar. drugs 10 (5), 1169–1179. doi:10.3390/md10051169
Chung, T. W., Lin, S. C., Su, J. H., Chen, Y. K., Lin, C. C., and Chan, H. L. (2017a). Sinularin induces DNA damage, G2/M phase arrest, and apoptosis in human hepatocellular carcinoma cells. BMC complementary Altern. Med. 17 (1), 62. doi:10.1186/s12906-017-1583-9
Chung, T. W., Su, J. H., Lin, C. C., Li, Y. R., Chao, Y. H., Lin, S. H., et al. (2017b). 24-Methyl-Cholesta-5,24(28)-Diene-3β,19-diol-7β-Monoacetate inhibits human small cell lung cancer growth in vitro and in vivo via apoptosis induction. Mar. drugs 15 (7), 210. doi:10.3390/md15070210
Ciminiello, P., Dell'Aversano, C., Dello Iacovo, E., Fattorusso, E., Forino, M., and Tartaglione, L. (2011). LC-MS of palytoxin and its analogues: state of the art and future perspectives. Toxicon official J. Int. Soc. Toxinology 57 (3), 376–389. doi:10.1016/j.toxicon.2010.11.002
Cui, W. X., Yang, M., Li, H., Li, S. W., Yao, L. G., Li, G., et al. (2020). Polycyclic furanobutenolide-derived norditerpenoids from the South China Sea soft corals Sinularia scabra and Sinularia polydactyla with immunosuppressive activity. Bioorg. Chem. 94, 103350. doi:10.1016/j.bioorg.2019.103350
Dagli, A. S., Akalin, Y., Bilgili, H., Seckin, S., and Ensari, S. (1997). Correction of saddle nose deformities by coral implantation. Eur. archives oto-rhino-laryngology official J. Eur. Fed. Oto-Rhino-Laryngological Soc. (EUFOS) Affil. Ger. Soc. Oto-Rhino-Laryngology - Head Neck Surg. 254 (6), 274–276. doi:10.1007/BF02905986
Dai, J. (2010). Clinical observation of the 25 wei coral pill treatment efficacy for tension headache. Master’s dissertation. Wuhan, China: Hubei University of Chinese medicine. doi:10.7666/d.y1712180
Duh, C. Y., Chia, M. C., Wang, S. K., Chen, H. J., El-Gamal, A. A., and Dai, C. F. (2001). Cytotoxic dolabellane diterpenes from the Formosan soft coral Clavularia inflata. J. Nat. Prod. 64 (8), 1028–1031. doi:10.1021/np010106n
Duh, C. Y., Lo, I. W., Wang, S. K., and Dai, C. F. (2007). New cytotoxic steroids from the soft coral Clavularia viridis. Steroids 72 (6-7), 573–579. doi:10.1016/j.steroids.2007.03.010
Duh, C. Y., Wang, S. K., Tseng, H. K., Sheu, J. H., and Chiang, M. Y. (1998). Novel cytotoxic cembranoids from the soft coral Sinularia flexibilis. J. Nat. Prod. 61 (6), 844–847. doi:10.1021/np980021v
El-Gendy, M., Yahya, S. M. M., Hamed, A. R., and El-Bondkly, A. M. A. (2022). Assessment of the phylogenetic analysis and antimicrobial, antiviral, and anticancer activities of marine endophytic Streptomyces species of the soft coral Sarcophyton convolutum. Int. Microbiol. official J. Span. Soc. Microbiol. 25 (1), 133–152. doi:10.1007/s10123-021-00204-x
Ellis, A., and Bennett, D. L. (2013). Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 111 (1), 26–37. doi:10.1093/bja/aet128
Elshamy, A. I., El-Kashak, W. A., Abdallah, H. M., Farrag, A. H., and Nassar, M. I. (2017). Soft coral Cespitularia stolonifera: new cytotoxic ceramides and gastroprotective activity. Chin. J. Nat. Med. 15 (2), 105–114. doi:10.1016/S1875-5364(17)30026-2
Elshamy, A. I., Mohamed, T. A., Elkady, E. M., Saleh, I. A., El-Beih, A. A., Alhammady, M. A., et al. (2021). Paralemnolins X and Y, new antimicrobial sesquiterpenoids from the soft coral Paralemnalia thyrsoide. Antibiot. (Basel, Switz. 10 (10), 1158. doi:10.3390/antibiotics10101158
Eltahawy, N. A., Ibrahim, A. K., Radwan, M. M., Zaitone, S. A., Gomaa, M., Elsohly, M. A., et al. (2015). Mechanism of action of antiepileptic ceramide from Red Sea soft coral Sarcophyton auritum.
Fang, H. Y., Hsu, C. H., Chao, C. H., Wen, Z. H., Wu, Y. C., Dai, C. F., et al. (2013). Cytotoxic and anti-inflammatory metabolites from the soft coral Scleronephthya gracillimum. Mar. drugs 11 (6), 1853–1865. doi:10.3390/md11061853
Fang, Z. S., and Zhang, M. Y. (1984). Pharmacological test of soft coral with weak scales. J. Trop. Oceanogr. (01), 72–76.
Fernando, I. P. S., Sanjeewa, K. K. A., Kim, H. S., Kim, S. Y., Lee, S. H., Lee, W. W., et al. (2017). Identification of sterols from the soft coral Dendronephthya gigantea and their anti-inflammatory potential. Environ. Toxicol. Pharmacol. 55, 37–43. doi:10.1016/j.etap.2017.08.003
Fu, L., Li, Z., Liu, L., and Li, K. (2023). Finding alternative paths for endangered Chinese medicinal herbs. Beijing, China: Science and Technology Daily. Science and Technology Daily. 005.
Gao, Js. (2023). Proposed implementation of the marine environmental protection target responsibility system and assessment and evaluation system. Beijing, China: Xinhua daily telegraph. Xinhua daily telegraph. 003.
García-Arredondo, A., Rojas-Molina, A., Bah, M., Ibarra-Alvarado, C., Gallegos-Corona, M. A., and García-Servín, M. (2015). Systemic toxic effects induced by the aqueous extract of the fire coral Millepora complanata and partial purification of thermostable neurotoxins with lethal effects in mice. Comp. Biochem. physiology. Toxicol. Pharmacol. CBP 169, 55–64. doi:10.1016/j.cbpc.2014.12.004
Garzón, S. P., Rodríguez, A. D., Sánchez, J. A., and Ortega-Barria, E. (2005). Sesquiterpenoid metabolites with antiplasmodial activity from a Caribbean gorgonian coral, Eunicea sp. J. Nat. Prod. 68 (9), 1354–1359. doi:10.1021/np0501684
Ge, J. H., Gao, C. H., Wang, P., Wen, L. J., and Qi, S. H. (2010). Study on the chemical constituents of Antipathes dichotoma. J. Chin. Med. Mater. 33 (09), 1403–1405. doi:10.13863/j.issn1001-A4454.2010.09.018
George, K. A., Slagle, K. M., Wilson, R. S., Moeller, S. J., and Bruskotter, J. T. (2016). Changes in attitudes toward animals in the United States from 1978 to 2014. Biol. Conserv. 201, 237–242. doi:10.1016/j.biocon.2016.07.013
Gomaa, M. N., Soliman, K., Ayesh, A., Abd El-Wahed, A., Hamza, Z., Mansour, H. M., et al. (2016). Antibacterial effect of the red sea soft coral Sarcophyton trocheliophorum. Nat. Prod. Res. 30 (6), 729–734. doi:10.1080/14786419.2015.1040991
Gong, K. K. (2014). Studies on the chemical constituents and bioactivities of three marine organisms. Doctor’s dissertation. Qingdao, China: Ocean University of China.
Gong, K. K., Tang, X. L., Zhang, G., Cheng, C. L., Zhang, X. W., Li, P. L., et al. (2013). Polyhydroxylated steroids from the South China Sea soft coral Sarcophyton sp. and their cytotoxic and antiviral activities. Mar. drugs 11 (12), 4788–4798. doi:10.3390/md11124788
Gong, W. X., Yuan, H. H., Tang, H., Zhang, W., and Lan, M. B. (2017). Study on the antitumor activity of acetone extract of Euplexaura rhipidalis. Lishizhen Med. Materia Medica Res. 28 (01), 4–7. doi:10.3969/j.issn.1008-0805.2017.01.002
Gross, H., Kehraus, S., Nett, M., König, G. M., Beil, W., and Wright, A. D. (2003). New cytotoxic cembrane based diterpenes from the soft corals Sarcophyton cherbonnieri and Nephthea sp. Org. Biomol. Chem. 1 (6), 944–949. doi:10.1039/b210039h
Gu, H. (2014). Clinical observation of Ershi Wuwei Shanhu pills combined with acupuncture on migraine with 55cases. Chin. J. Exp. Traditional Med. Formulae 20 (19), 210–213. doi:10.13422/j.cnki.syfjx.2014190210
Guan, D. M. (2020). Clinical efficacy of Tibetan medicine Ershiwuwei Shanhu capsules in the treatment of post-stroke headache. J. Med. Pharm. Chin. 26 (11), 26–28. doi:10.16041/j.cnki.cn15-1175.2020.11.017
Guillemin, G., Meunier, A., Dallant, P., Christel, P., Pouliquen, J. C., and Sedel, L. (1989). Comparison of coral resorption and bone apposition with two natural corals of different porosities. J. Biomed. Mater. Res. 23 (7), 765–779. doi:10.1002/jbm.820230708
Han, L. (2011). The secondary metabolites and chemical ecological effects of four corals and two symbiotic Fungi from South China Sea. Master’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.y1928324
He, D. H., Song, Q. X., and Xiong, X. Q. (2007). Clinical observation of 100 cases of persistent heart failure treated with heart failure combination with coral seventy. J. Med. Pharm. Chin. Minorities (09), 29–30. doi:10.16041/j.cnki.cn15-1175.2007.09.029
He, J. F. (2017). The efficacy of Tibetan medicine Ershiwuwei Shanhu Pills combined with nursing intervention in the treatment of vascular neurogenic headache. Electron. J. Pract. Clin. Nurs. Sci. 2 (17), 65.
He, L. L. (2013). Studies on two soft corals from South China sea: chemical consitituents and bioactivities. Master’s dissertation. Hefei, China: Hefei university of technology. doi:10.7666/d.Y2336497
He, W., He, D., Wang, Q. L., Tian, W., Liu, B., Liu, Y. J., et al. (2022). Longitudinal spinous-splitting laminoplasty with coral bone for the treatment of cervical adjacent segment degenerative disease: a 5-year follow-up study. Orthop. Surg. 14 (2), 435–442. doi:10.1111/os.13027
Hegazy, M. E., Gamal-Eldeen, A. M., Mohamed, T. A., Alhammady, M. A., Hassanien, A. A., Shreadah, M. A., et al. (2016). New cytotoxic constituents from the Red Sea soft coral Nephthea sp. Nat. Prod. Res. 30 (11), 1266–1272. doi:10.1080/14786419.2015.1055266
Hegazy, M. F., Eldeen, A. M. G., Shahat, A. A., Abdel-Latif, F. F., Mohamed, T. A., Whittlesey, B. R., et al. (2012). Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. drugs 10 (1), 209–222. doi:10.3390/md10010209
Hiroko, T., Ryoji, K., You, I., Ryuichi, H., Tomofumi, M. J. B., and bulletin, p. (2003). Inhibitory effect of norditerpenes on LPS-induced TNF-α production from the okinawan soft coral. Sinularia sp.
Hoffmann, K., Hermanns-Clausen, M., Buhl, C., Büchler, M. W., Schemmer, P., Mebs, D., et al. (2008). A case of palytoxin poisoning due to contact with zoanthid corals through a skin injury. Toxicon official J. Int. Soc. Toxinology 51 (8), 1535–1537. doi:10.1016/j.toxicon.2008.03.009
Hong, J. Y., Boo, H. J., Kang, J. I., Kim, M. K., Yoo, E. S., Hyun, J. W., et al. (2012). (1S,2S,3E,7E,11E)-3,7,11,15-Cembratetraen-17,2-olide, a cembrenolide diterpene from soft coral Lobophytum sp., inhibits growth and induces apoptosis in human colon cancer cells through reactive oxygen species generation. Biol. Pharm. Bull. 35 (7), 1054–1063. doi:10.1248/bpb.b11-00024
Hsiao, T. H., Sung, C. S., Lan, Y. H., Wang, Y. C., Lu, M. C., Wen, Z. H., et al. (2015). New anti-inflammatory cembranes from the cultured soft coral Nephthea columnaris. Mar. drugs 13 (6), 3443–3453. doi:10.3390/md13063443
Hsieh, P. W., Chang, F. R., McPhail, A. T., Lee, K. H., and Wu, Y. C. (2003). New cembranolide analogues from the formosan soft coral Sinularia flexibilis and their cytotoxicity. Nat. Prod. Res. 17 (6), 409–418. doi:10.1080/14786910310001617677
Hsu, F. J., Chen, B. W., Wen, Z. H., Huang, C. Y., Dai, C. F., Su, J. H., et al. (2011). Klymollins A-H, bioactive eunicellin-based diterpenoids from the formosan soft coral Klyxum molle. J. Nat. Prod. 74 (11), 2467–2471. doi:10.1021/np200589n
Huang, C. Y., Tseng, W. R., Ahmed, A. F., Chiang, P. L., Tai, C. J., Hwang, T. L., et al. (2018). Anti-inflammatory polyoxygenated steroids from the soft coral Lobophytum michaelae. Mar. drugs 16 (3), 93. doi:10.3390/md16030093
Huang, C. Y., Tseng, Y. J., Chokkalingam, U., Hwang, T. L., Hsu, C. H., Dai, C. F., et al. (2016). Bioactive isoprenoid-derived natural products from a dongsha atoll soft coral Sinularia erecta. J. Nat. Prod. 79 (5), 1339–1346. doi:10.1021/acs.jnatprod.5b01142
Huang, F., Zhang, Y., Zhang, J., Lin, S. Z., and Liu, M. D. (1997). Coral resources development pathway. Chengdu, China: Resource Development & Market, 140–141.
Huang, H., Yu, X., Huang, L., and Jiang, L. (2023). Current status and prospects of coral reef ecology research. J. Trop. Oceanogr., 1–11.
Huang, X. H., and Zhao, H. S. (2017). Clinical study on twenty-five ingredient coral pill combined with carbamazepine in treatment of epilepsy. Drugs & Clin. 32 (01), 120–123. doi:10.7501/j.issn.1674-5515.2017.01.029
Huang, Y. C., Wen, Z. H., Wang, S. K., Hsu, C. H., and Duh, C. Y. (2008). New anti-inflammatory 4-methylated steroids from the Formosan soft coral Nephthea chabroli. Steroids 73 (11), 1181–1186. doi:10.1016/j.steroids.2008.05.007
Huang, Z. Y. (2008). Clinical observation on 30 cases of migraine treated with twenty-five flavored coral pills. Chin. J. Mod. Drug Appl. (11), 100–101.
Huo, J., Tang, H., Li, l., Liu, B. S., Sun, P., Yi, Y. H., et al. (2011). Study on bioactive constituents of the South China Sea soft coral Scleronephthya sp. Acad. J. Nav. Med. Univ. 32 (01), 21–24. doi:10.3724/sp.j.1008.2011.00021
Hurtado, D. X., Castellanos, F. A., Coy-Barrera, E., and Tello, E. (2020). Prostaglandins isolated from the octocoral Plexaura homomalla: in silico and in vitro studies against different enzymes of cancer. Mar. drugs 18 (3), 141. doi:10.3390/md18030141
Huynh, T. H., Fang, L. S., Chen, Y. H., Peng, B. R., Chen, Y. Y., Zheng, L. G., et al. (2020). Briarenols I-K, new anti-inflammatory 8,17-epoxybriaranes from the octocoral Briareum excavatum (Briareidae). Mol. (Basel, Switz. 25 (6), 1405. doi:10.3390/molecules25061405
Ishii, T., Kamada, T., and Vairappan, C. S. (2016). Three new cembranoids from the Bornean soft coral Nephthea sp. J. Asian Nat. Prod. Res. 18 (5), 415–422. doi:10.1080/10286020.2016.1145670
Iwagawa, T., Hashimoto, K., Yokogawa, Y., Okamura, H., Nakatani, M., Doe, M., et al. (2009). Cytotoxic biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 72 (5), 946–949. doi:10.1021/np8003485
Iwamaru, A., Iwado, E., Kondo, S., Newman, R. A., Vera, B., Rodríguez, A. D., et al. (2007). Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Mol. cancer Ther. 6 (1), 184–192. doi:10.1158/1535-7163.MCT-06-0422
Jang, M. O., Lee, E. K., and Jin, Y. W. (2023). Plant stem cell line derived from cambium of herbaceous plant with storage root and method for isolating the same. US.
Jean, Y. H., Chen, W. F., Duh, C. Y., Huang, S. Y., Hsu, C. H., Lin, C. S., et al. (2008). Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 578 (2-3), 323–331. doi:10.1016/j.ejphar.2007.08.048
Jean, Y. H., Chen, W. F., Sung, C. S., Duh, C. Y., Huang, S. Y., Lin, C. S., et al. (2009). Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 158 (3), 713–725. doi:10.1111/j.1476-5381.2009.00323.x
Jeffrey, L. (2016). DDT wars: rescuing our national bird, preventing cancer, and creating the environmental defense fund. Oxford and New York: Oxford University Press.
Ji, Y. B., and Liu, Z. Y. (2018). Study on the chemical constituents of gorgonian Anthogorgia sp. Chin. J. Mar. Drugs 37 (02), 13–18. doi:10.13400/j.cnki.cjmd.2018.02.003
Jiang, C. S., Ru, T., Huan, X. J., Miao, Z. H., and Guo, Y. W. (2019a). New cytotoxic ergostane-type sterols from the Chinese soft coral Sinularia sp. Steroids 149, 108425. doi:10.1016/j.steroids.2019.108425
Jiang, C. S., Ru, T., Yao, L. G., Miao, Z. H., and Guo, Y. W. (2019b). Four new cembranoids from the Chinese soft coral Sinularia sp. and their anti-Aβ aggregation activities. Fitoterapia 136, 104176. doi:10.1016/j.fitote.2019.104176
Jiang, M. (2013). Study on the bioactive metabolits and structure-activity relationship of the South China sea gorgonian Dichotella gemmacea. Master’s dissertation. Shanghai, China: Naval Medical University. doi:10.7666/d.Y2339967
Jiang, M. M. (2015). Studies on the chemical constituents and bioactivities of the Xisha soft coral Sinularia cf. molesta. Master’s dissertation. Qingdao, China: Ocean University of China.
Jiang, Q., and Zhai, Y. (2006). Pharmacological action comparison between cornu antelopis and cornu caprae hircus. Shanxi Med. J. (07), 582–583. doi:10.3969/j.issn.0253-9926.2006.07.003
Jiang, W., Wang, D., Wilson, B. A. P., Voeller, D., Bokesch, H. R., Smith, E. A., et al. (2021). Sinularamides A-G, terpenoid-derived spermidine and spermine conjugates with casitas B-lineage lymphoma proto-oncogene B (Cbl-b) inhibitory activities from a Sinularia sp. soft coral. J. Nat. Prod. 84 (6), 1831–1837. doi:10.1021/acs.jnatprod.1c00367
Jiao, Y., Yao, B., Zhou, Q., and Luo, X. Y. (2013). Effect of Jinzhuyalong Ershiwuewei Coral Capsule on adenosine in secondary spinal cord injury. Mod. J. Integr. Traditional Chin. West. Med. 22 (28), 3090–3092. doi:10.3969/j.issn.1008-8849.2013.28.006
Jiaojia, C. R., Zhong, B. L., Li, H. H., Hu, X. D., Han, Y. N., Chen, Q. Q., et al. (2022). Regulation of intestinal flora in mice with Alzheimer's disease by the Tibetan medicine Ershiwuwei Shanhu Pill. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 24 (01), 158–167. doi:10.11842/wst.20210604005
Jin, P. F. (2005). Studies on the structure and biological activities of chemical constituents from the soft coral Sinularia dissecta and marine sponge Aplysina aerophoba in South China Sea. Doctor’s dissertation. Shenyang, China: Shenyang Pharmaceutical University.
Joyner, P. M., Waters, A. L., Williams, R. B., Powell, D. R., Janakiram, N. B., Rao, C. V., et al. (2011). Briarane diterpenes diminish COX-2 expression in human colon adenocarcinoma cells. J. Nat. Prod. 74 (4), 857–861. doi:10.1021/np100775a
Kamada, T., Kang, M. C., Phan, C. S., Zanil, , Jeon, Y. J., and Vairappan, C. S. (2018). Bioactive cembranoids from the soft coral genus Sinularia sp. in borneo. Mar. drugs 16 (4), 99. doi:10.3390/md16040099
Krishna, N., Muralidhar, P., Kumar, M. M., Rao, D. V., and Rao, C. H. (2004). A new sphingolipid from the gorgonian Junceella juncea of the Indian Ocean. Nat. Prod. Res. 18 (6), 551–555. doi:10.1080/14786410350001622031
Lai, Y. (2017). Study on chemical components and promote fracture healing of red coral. Master’s dissertation. Changchun, China: Jilin Agricultural University.
Lai, Y., Bao, H. Y., and Bao, Z. H. (2016). Progress of research on resources, chemical composition and pharmacological activity of red coral. Chin. J. Mar. Drugs 35 (03), 112–120. doi:10.13400/j.cnki.cjmd.2016.03.018
Lang, J. H. (2013). Chemical constituent and their bioactivities of one soft coral Lobophytum sp. Master’s dissertation. Shanghai, China: Naval Medical University. doi:10.7666/d.Y2339728
Lee, E. K., Jin, Y. W., Park, J. H., Yoo, Y. M., Hong, S. M., Amir, R., et al. (2010). Cultured cambial meristematic cells as a source of plant natural products. Nat. Biotechnol. 28 (11), 1213–1217. doi:10.1038/nbt.1693
Lee, H. P., Huang, S. Y., Lin, Y. Y., Wang, H. M., Jean, Y. H., Wu, S. F., et al. (2013). Soft coral-derived lemnalol alleviates monosodium urate-induced gouty arthritis in rats by inhibiting leukocyte infiltration and iNOS, COX-2 and c-Fos protein expression. Mar. drugs 11 (1), 99–113. doi:10.3390/md11010099
Li, C., Jiang, M., La, M. P., Li, T. J., Tang, H., Sun, P., et al. (2013a). Chemistry and tumor cell growth inhibitory activity of 11,20-epoxy-3Z,5(6)E-diene briaranes from the South China Sea gorgonian Dichotella gemmacea. Mar. drugs 11 (5), 1565–1582. doi:10.3390/md11051565
Li, C., La, M. P., Tang, H., Sun, P., Liu, B. S., Zhuang, C. L., et al. (2016). Chemistry and bioactivity of briaranes from the South China Sea gorgonian Dichotella gemmacea. Mar. drugs 14 (11), 201. doi:10.3390/md14110201
Li, G., Li, H., Tang, W., Yao, L. G., Liang, L. F., and Guo, Y. W. (2020a). Further polyoxygenated cembranoids from South China Sea soft coral Sarcophyton ehrenbergi. Bioorg. Chem. 101, 103993. doi:10.1016/j.bioorg.2020.103993
Li, G. Q. (2004). Compounds isolated from the soft coral Dendronephthya sp., Nephthea sp. and Sinularia sp. Master’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.y646707
Li, H. (2021). Analysis of the efficacy of the Tibetan medicine Twenty-five Flavored Coral Pills with nursing intervention for vasoneurotic headache. J. Med. Pharm. Chin. Minorities 27 (03), 78–80. doi:10.16041/j.cnki.cn15-1175.2021.03.041
Li, J., Huan, X. J., Wu, M. J., Chen, Z. H., Chen, B., Miao, Z. H., et al. (2022a). Chemical constituents from the South China sea soft coral Sinularia humilis. Nat. Prod. Res. 36 (13), 3324–3330. doi:10.1080/14786419.2020.1855645
Li, J., Sun, Y. L., Tang, H., Su, L., Zheng, G. L., and Zhang, W. (2021a). Immunosuppressive 9,10-secosteroids from the gorgonian Verrucella umbraculum collected in the South China sea. J. Nat. Prod. 84 (5), 1671–1675. doi:10.1021/acs.jnatprod.1c00200
Li, J. F., Yao, L. G., Zeng, Y. B., and Guo, Y. W. (2022b). Chemical constituents of soft coral Sarcophyton glaucum collected from Xisha and their anti-inflammatory activity. Acta Pharm. Sin. 57 (03), 741–749. doi:10.16438/j.0513-4870.2021-1292
Li, J. G. (2010a). Twenty-five flavored coral pills for the treatment of 30 cases of hypertension. J. Pract. Traditional Chin. Med. 26 (07), 497. doi:10.3969/j.issn.1004-2814.2010.07.049
Li, J. X., Lei, X. X., Tan, Y. H., Liu, Y. H., Yang, B., and Li, Y. Q. (2021b). Two new bioactive polyphenols from the soft coral-derived fungus Talaromyces sp. SCSIO 041201. Nat. Prod. Res. 35 (24), 5778–5785. doi:10.1080/14786419.2020.1836632
Li, L. (2007). 80 cases of tension headache treated with Dexin and Ershiwuwei Shanhu Pills. Chin. J. Pract. Nerv. Dis. (08), 104–105. doi:10.3969/j.issn.1673-5110.2007.08.067
Li, Q. Z., and Liu, Z. Q. (2021). Efficacy of the Tibetan medicine Ershiwuwei Shanhu Pills combined with diphenhydramine in the treatment of hypertension. J. Med. Pharm. Chin. Minorities 27 (09), 8–10. doi:10.16041/j.cnki.cn15-1175.2021.09.005
Li, R. (2012a). The secondery metabolites and their bioactivies of Sinularia sp. and Verrucella umbraculum. Master’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.y2158880
Li, R. S., Di, L., and Long, K. H. (1989). Chemical composition of soft corals from China (Chemical composition of soft corals from China). Acta Sci. Nat. Univ. Sunyatseni (01), 51–54. doi:10.1007/BF02943117
Li, S. W. (2018). Studies on the chemical constitutions and bioactivities of two soft corals from the South China Sea. Master’s dissertation. Nanchang, China: Nanchang University.
Li, S. W., Chen, W. T., Yao, L. G., and Guo, Y. W. (2018a). Two new cytotoxic steroids from the Chinese soft coral Sinularia sp. Steroids 136, 17–21. doi:10.1016/j.steroids.2018.05.009
Li, S. W., Cuadrado, C., Huan, X. J., Yao, L. G., Miao, Z. H., Hernandez Daranas, A., et al. (2020b). Rare new bicyclic cembranoid ethers and a novel trihydroxy prenylated guaiane from the Xisha soft coral Lobophytum sp. Bioorg. Chem. 103, 104223. doi:10.1016/j.bioorg.2020.104223
Li, S. W., Yao, L. G., Liang, L. F., and Guo, Y. W. (2018b). The study on the chemical constituents and bioactivities of soft coral Sinularia sp. from Ximao island. Chin. J. Mar. Drugs 37 (04), 1–6. doi:10.13400/j.cnki.cjmd.2018.04.001
Li, T. T. (2010b). Studies on chemical constituents and bioactivity of the Gorgonian Muricella sibogae. Master’s dissertation. Qingdao, China. Ocean University of Chsina. doi:10.7666/d.D326926
Li, W., Liu, Z. Y., and Wang, J. H. (2022c). Research progress of apoptosis in process of anti-virus. Chin. J. Immunol. 38 (23), 2933–2936+2940. doi:10.3969/j.issn.1000-484X.2022.23.022
Li, W. G. (2012b). Clinical observation of Ershiwuwei coral pill associated with a small dose of amitriptyline pills on frequent episodic tension type headache. J. Emerg. Traditional Chin. Med. 21 (03), 356–357. doi:10.3969/j.issn.1004-745X.2012.03.009
Li, W. H., Wang, Z. Y., Huang, Y. J., and Luo, X. Y. (2013b). Effect of neurotensin in vertebral artery type of cervical spondylosis by manipulation therapy and jinzhuyalong ershiwuwei coral capsule. Chin. Med. Mod. Distance Educ. China 11 (07), 13–15. doi:10.3969/j.issn.1672-2779.2013.07.007
Li, X. (2015). Preclinical pharmacology study on Mongolia bone setting medicine pill. Master’s dissertation. Changchun, China: Jilin Agricultural University.
Li, Z. H. (2006). Clinical study on the treatment of lumbar myofasciitis with twenty-five flavored coral pills. Lishizhen Med. Materia Medica Res. (12), 2509–2510.
Liang, C. H., Wang, G. H., Liaw, C. C., Lee, M. F., Wang, S. H., Cheng, D. L., et al. (2008). Extracts from Cladiella australis, Clavularia viridis and Klyxum simplex (soft corals) are capable of inhibiting the growth of human oral squamous cell carcinoma cells. Mar. drugs 6 (4), 595–606. doi:10.3390/md6040595
Liang, L. F., Kurtán, T., Mándi, A., Yao, L. G., Li, J., Zhang, W., et al. (2013). Unprecedented diterpenoids as a PTP1B inhibitor from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 15 (2), 274–277. doi:10.1021/ol303110d
Liang, L. F., Li, Y. F., Zheng, T. T., Yao, L. G., and Guo, Y. W. (2017). Chemical constituents from South China Sea Soft coral Sinularia sp. Chin. Traditional Herb. Drugs 48 (05), 868–873. doi:10.7501/j.issn.0253-2670.2017.05.005
Liao, K. W., Haung, J. Q., and Liu, W. S. (1992). Extraction and isolation of sedative-hypnotic active ingredients from Subergorgia suberosa and observation of their pharmacological effects. Mil. Med. Jt. Logist. (02), 41–43.
Li, M. (2011). A safety evaluation and a concomitant toxicokinetics study of Tibetan medicine:25-ingredient corals pills. Master’s dissertation . Chengdu, China: Chengdu University of Traditional Chinese Medicine.
Lin, C. K., Tseng, C. K., Liaw, C. C., Huang, C. Y., Wei, C. K., Sheu, J. H., et al. (2018). Lobohedleolide suppresses hepatitis C virus replication via JNK/c-Jun-C/EBP-mediated down-regulation of cyclooxygenase-2 expression. Sci. Rep. 8 (1), 8676. doi:10.1038/s41598-018-26999-w
Lin, W. Y., Su, J. H., Lu, Y., Wen, Z. H., Dai, C. F., Kuo, Y. H., et al. (2010). Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 18 (5), 1936–1941. doi:10.1016/j.bmc.2010.01.036
Lin, Y. C., Huang, S. Y., Jean, Y. H., Chen, W. F., Sung, C. S., Kao, E. S., et al. (2011). Intrathecal lemnalol, a natural marine compound obtained from Formosan soft coral, attenuates nociceptive responses and the activity of spinal glial cells in neuropathic rats. Behav. Pharmacol. 22 (8), 739–750. doi:10.1097/FBP.0b013e32834d0ecb
Lin, Y. C., Lin, C. C., Chu, Y. C., Fu, C. W., and Sheu, J. H. (2021). Bioactive diterpenes, norditerpenes, and sesquiterpenes from a formosan soft coral Cespitularia sp. Pharm. (Basel, Switz. 14 (12), 1252. doi:10.3390/ph14121252
Lin, Y. C., Wang, S. S., Chen, C. H., Kuo, Y. H., and Shen, Y. C. (2014). Cespitulones A and B, cytotoxic diterpenoids of a new structure class from the soft coral Cespitularia taeniata. Mar. drugs 12 (6), 3477–3486. doi:10.3390/md12063477
Lin, Y. F., Kuo, C. Y., Wen, Z. H., Lin, Y. Y., Wang, W. H., Su, J. H., et al. (2013a). Flexibilisquinone, a new anti-inflammatory quinone from the cultured soft coral Sinularia flexibilis. Mol. (Basel, Switz. 18 (7), 8160–8167. doi:10.3390/molecules18078160
Lin, Y. Y., Jean, Y. H., Lee, H. P., Chen, W. F., Sun, Y. M., Su, J. H., et al. (2013b). A soft coral-derived compound, 11-epi-sinulariolide acetate suppresses inflammatory response and bone destruction in adjuvant-induced arthritis. PloS one 8 (5), e62926. doi:10.1371/journal.pone.0062926
Lin, Y. Y., Lin, S. C., Feng, C. W., Chen, P. C., Su, Y. D., Li, C. M., et al. (2015). Anti-inflammatory and analgesic effects of the marine-derived compound excavatolide B isolated from the culture-type formosan gorgonian Briareum excavatum. Mar. drugs 13 (5), 2559–2579. doi:10.3390/md13052559
Li, P., Yang, C., Huang, F. K., Luo, Y. D., Hu, X. D., Shao, J., et al. (2014). Effect of 25 odors coral pills on pyramidal cell and expression of glial fibrillary acidic protein in hippocampus of -D-galactose induced Brain aging rats. Nat. Prod. Res. Dev. 26 (04), 606–609.
Liu, C. L., Tian, X. D., and Zhang, Y. (2004). Twenty-five flavored coral pills clinical study summary. Health Vocat. Educ. 20, 104–105. doi:10.3969/j.issn.1671-1246.2004.20.077
Liu, C. X., Li, P. L., Tang, X. L., and Li, G. Q. (2012). Studies on chemical constituents of the South China Sea gorgonian Rumphella aggregata. Chin. J. Mar. Drugs 31 (05), 5–10. doi:10.13400/j.cnki.cjmd.2012.05.005
Liu, F., Bian, L. L., Gu, X. F., and Zhao, X. C. (2016a). Ershiwuwei coral capsules combined with danzhen headache capsules for headache. World Latest Med. Inf. 16 (79), 157. doi:10.3969/j.issn.1671-3141.2016.79.128
Liu, F. L., Zha, S., Guo, W., Pu, Z., Yu, Z., Wang, J. L., et al. (2016b). Studies on the acute and subacute toxicology of Ershiwuwei-Shanhu Pills. Tibet. Med. 37 (02), 9–13.
Liu, H. Y. (2008). The allelopathicsubstances from gorgonian Dichotella gemmacea (valenciennes) and soft coral Sinularia sp. from the South China Sea. Master’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.y1337464
Liu, J., Wu, X., Yang, M., Gu, Y. C., Yao, L. G., Huan, X. J., et al. (2020). Erectsterates A and B, a pair of novel highly degraded steroid derivatives from the South China Sea soft coral Sinularia erecta. Steroids 161, 108681. doi:10.1016/j.steroids.2020.108681
Liu, J., Xia, F., Ouyang, H., Wang, W., Li, T., Shi, Y., et al. (2022). Nardosinane-related antimicrobial terpenoids from Lemnalia sp. soft coral. Phytochemistry 196, 113088. doi:10.1016/j.phytochem.2022.113088
Liu, L. L., and Wang, L. J. (2014). Clinical study on coral hydroxyapatite combined with acellular dermal Matrix in socket preservation. J. Mod. Stomatology 28 (06), 340–344.
Liu, M. (2014). Study of the secondary metabolits and bioactivities of Gorgonian S. suberosa and the gorgonian-derived fungus A. terreus. Master’s dissertation. Qingdao, China: Ocean University of China.
Liu, R., Zhu, Z., Wu, j., Qian, D., and Duan, J. (2018). Comparison on proteins of saigae tataricae Cornu and caprae hircus cornu. China J. Chin. Materia Medica 43 (16), 3329–3334. doi:10.19540/j.cnki.cjcmm.20180511.001
Liu, X., Zhang, J., Liu, Q., Tang, G., Wang, H., Fan, C., et al. (2015). Bioactive cembranoids from the South China Sea soft coral Sarcophyton elegans. Mol. (Basel, Switz. 20 (7), 13324–13335. doi:10.3390/molecules200713324
Liu, Y. P., Xu, S. H., Guo, S. H., and Li, X. L. (2001). Isolation and identification of chemical constituents from Acropora pulchra. J. Chin. Med. Mater. 24 (09), 640–641. doi:10.13863/j.issn1001-4454.2001.09.009
Liu, Z., and Han, D. (2006). Clinical research progress on Panthera tigris and artificial Panthera tigris. Chin. J. Traditional Med. Traumatology Orthop. (02), 73–75. doi:10.3969/j.issn.1005-0205.2006.02.030
Liu, Z. Y. (2017). Advances in the study of chemical composition of gorgonian. Sci. Technol. Innovation (21), 11–12.
Lu, C. H., and Chen, Z. Q., (1994). Animal experiments on coral and coral-hydroxyapatite composites Chinese Journal of Stomatology, 29(06), 357–359.
Lu, S. Q. (2020). Studies on the chemical composition of two soft corals from South China Sea. Master’s dissertation. Jinzhou, China: Jinzhou Medical University. doi:10.1007/BF02006258
Lu, Y., Huang, C. Y., Lin, Y. F., Wen, Z. H., Su, J. H., Kuo, Y. H., et al. (2008). Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 71 (10), 1754–1759. doi:10.1021/np8003563
Luo, Y. D. (2012). Retrospective clinical and animal experiment study on Ershiwuwei coral Pill in treating epilepsy. Master’s dissertation. Guangzhou, China: Southern Medical University.
Luo, Y. D., Zhen, L. F., Huang, X., du, W. B., Pan, J. H., Shao, J., et al. (2013). Effects of 25 Flavored Coral Pills on the behavior and EEG of pentazocine epileptogenic rats. Pharmacol. Clin. Chin. Materia Medica 29 (03), 169–172. doi:10.13412/j.cnki.zyyl.2013.03.063
Lv, F., Chi, K. Y., Dai, R. J., and Deng, Y. L. (2012). Isolation and Structural identification of chemical constituents from Sinularia sp. Trans. Beijing Inst. Technol. 32 (10), 1096–1100. doi:10.15918/j.tbit1001-0645.2012.10.016
Lv, Y., Lou, Q., Wu, P., Chen, Z., Fang, H., Chen, Y., et al. (2020). A review on the technologies of coral reef monitoring and development of an instiu monitoring. J. Ocean Technol. 39 (01), 84–90. doi:10.3969/j.issn.1003-2029.2020.01.013
Ma, K. (2008). Studies on the chemical connstituents and bioactivities of three marine organisms. Doctor’s dissertation. Beijing, China: Peking University. doi:10.11759/hykx20180424001
Ma, L., Yin, J., Wu, Y., and Feng, Z. (2018). Research status and knoeledge mapping analysis of international coral reefs. Mar. Sci. 42 (11), 118–125.
Mahmoud, A. H., Zidan, S. A. H., Samy, M. N., Alian, A., Abdelmohsen, U. R., Fouad, M. A., et al. (2022). Cytotoxicity and chemical profiling of the Red Sea soft corals Litophyton arboreum. Nat. Prod. Res. 36 (16), 4261–4265. doi:10.1080/14786419.2021.1974437
Marchac, D., and Sandor, G. (1994). Use of coral granules in the craniofacial skeleton. J. craniofacial Surg. 5 (4), 213–217. doi:10.1097/00001665-199409000-00003
Marrero, J., Rodríguez, A. D., Baran, P., Raptis, R. G., Sánchez, J. A., Ortega-Barria, E., et al. (2004). Bielschowskysin, a gorgonian-derived biologically active diterpene with an unprecedented carbon skeleton. Org. Lett. 6 (10), 1661–1664. doi:10.1021/ol049495d
Mayer, A. M., Jacobson, P. B., Fenical, W., Jacobs, R. S., and Glaser, K. B. (1998). Pharmacological characterization of the pseudopterosins: novel anti-inflammatory natural products isolated from the Caribbean soft coral, Pseudopterogorgia elisabethae. Life Sci. 62 (26), Pl401–407. doi:10.1016/s0024-3205(98)00229-x
Mohammadi Pour, P., Yegdaneh, A., Aghaei, M., Ali, Z., Khan, I. A., and Ghanadian, M. (2022). Novel 16,17-epoxy-23-methylergostane derivative from Sinularia variabilis, a soft coral from the Persian Gulf, with apoptotic activities against breast cancer cell lines. Nat. Prod. Res. 36 (15), 3796–3805. doi:10.1080/14786419.2021.1887178
Mohyeldin, M. M., Akl, M. R., Siddique, A. B., Hassan, H. M., and El Sayed, K. A. (2017). The marine-derived pachycladin diterpenoids as novel inhibitors of wild-type and mutant EGFR. Biochem. Pharmacol. 126, 51–68. doi:10.1016/j.bcp.2016.12.003
Montalvo, D., Amade, P., Funel-Le Bon, C., Fernández, R., and Reyes, F. (2006). Acerolide, a new cytotoxic cembranolide from the soft coral Pseudopterogorgia acerosa. Nat. Prod. Res. 20 (6), 548–552. doi:10.1080/14786410500183274
Murthy, Y. L., Mallika, D., Rajack, A., and Reddy, G. D. (2011). A new antifungal briarane diterpenoid from the gorgonian Junceella juncea Pallas. Bioorg. Med. Chem. Lett. 21 (24), 7522–7525. doi:10.1016/j.bmcl.2011.06.087
Na, S. Y., Lee, H. Y., Baek, J. O., Roh, J. Y., and Lee, J. R. (2008). A case of cellulitis associated with coral injury. Ann. dermatology 20 (4), 212–215. doi:10.5021/ad.2008.20.4.212
Nam, N. H., Ngoc, N. T., Hanh, T. T. H., Cuong, N. X., Thanh, N. V., Thao, D. T., et al. (2018). Cytotoxic steroids from the Vietnamese gorgonian Verrucella corona. Steroids 138, 57–63. doi:10.1016/j.steroids.2018.06.006
Nam, N. H., Tung, P. T., Ngoc, N. T., Hanh, T. T., Thao, N. P., Thanh, N. V., et al. (2015). Cytotoxic biscembranoids from the soft coral Sarcophyton pauciplicatum. Chem. Pharm. Bull. 63 (8), 636–640. doi:10.1248/cpb.c15-00273
National Compilation of Chinese Herbal Medicine (1996). National Compendium of Chinese herbal medicine color atlas. Second Edition. Beijing: People's Medical Publishing House.
Neoh, C. A., Wang, R. Y., Din, Z. H., Su, J. H., Chen, Y. K., Tsai, F. J., et al. (2012). Induction of apoptosis by sinulariolide from soft coral through mitochondrial-related and p38MAPK pathways on human bladder carcinoma cells. Mar. drugs 10 (12), 2893–2911. doi:10.3390/md10122893
Ngoc, N. T., Hanh, T. T. H., Thanh, N. V., Thao, D. T., Cuong, N. X., Nam, N. H., et al. (2017a). Cytotoxic steroids from the Vietnamese soft coral Sinularia leptoclados. Chem. Pharm. Bull. 65 (6), 593–597. doi:10.1248/cpb.c17-00129
Ngoc, N. T., Huong, P. T. M., Thanh, N. V., Cuong, N. X., Nam, N. H., Thung, D. C., et al. (2017b). Sesquiterpene constituents from the soft coral Sinularia nanolobata. Nat. Prod. Res. 31 (15), 1799–1804. doi:10.1080/14786419.2017.1292508
Nguyen, H. T., Chau, V. M., Phan, V. K., Hoang, T. H., Nguyen, H. N., Nguyen, X. C., et al. (2010). Chemical components from the Vietnamese soft coral Lobophytum sp. Archives pharmacal Res. 33 (4), 503–508. doi:10.1007/s12272-010-0402-3
Nuzzo, G., Gomes, B. A., Gallo, C., Amodeo, P., Sansone, C., Pessoa, O. D. L., et al. (2019). Potent cytotoxic analogs of amphidinolides from the atlantic octocoral Stragulum bicolor. Mar. drugs 17 (1), 58. doi:10.3390/md17010058
Ospina, C. A., Rodríguez, A. D., Sánchez, J. A., Ortega-Barria, E., Capson, T. L., and Mayer, A. M. (2005). Caucanolides A-F, unusual antiplasmodial constituents from a colombian collection of the gorgonian coral Pseudopterogorgia bipinnata. J. Nat. Prod. 68 (10), 1519–1526. doi:10.1021/np050239z
Peng, C. C., Huang, C. Y., Ahmed, A. F., Hwang, T. L., and Sheu, J. H. (2020). Anti-inflammatory cembranoids from a formosa soft coral Sarcophyton cherbonnieri. Mar. drugs 18 (11), 573. doi:10.3390/md18110573
Peng, C. C., Huang, T. Y., Huang, C. Y., Hwang, T. L., and Sheu, J. H. (2021). Cherbonolides M and N from a formosan soft coral Sarcophyton cherbonnieri. Mar. drugs 19 (5), 260. doi:10.3390/md19050260
Phan, C. S., Kamada, T., Ishii, T., Hamada, T., and Vairappan, C. S. (2019). 12-Epi-9-deacetoxyxenicin, new cytotoxic diterpenoid from a Bornean soft coral, Xenia sp. Xenia sp. Nat. Prod. Res. 33 (6), 808–813. doi:10.1080/14786419.2017.1410812
Phan, C. S., Ng, S. Y., Kim, E. A., Jeon, Y. J., Palaniveloo, K., and Vairappan, C. S. (2015). Capgermacrenes A and B, bioactive secondary metabolites from a bornean soft coral, Capnella sp. Mar. drugs 13 (5), 3103–3115. doi:10.3390/md13053103
Poza, J. J., Fernández, R., Reyes, F., Rodríguez, J., and Jiménez, C. (2008). Isolation, biological significance, synthesis, and cytotoxic evaluation of new natural parathiosteroids A-C and analogues from the soft coral Paragorgia sp. J. Org. Chem. 73 (20), 7978–7984. doi:10.1021/jo801198u
Qi, S. H., Zhang, S., Huang, H., Xiao, Z. H., Huang, J. S., and Li, Q. X. (2004). New briaranes from the South China Sea gorgonian Junceella juncea. J. Nat. Prod. 67 (11), 1907–1910. doi:10.1021/np049848h
Qi, S. H., Zhang, S., Li, X., and Li, Q. X. (2005). A cytotoxic sesquiterpene alkaloid from the South China Sea gorgonian Subergorgia suberosa. J. Nat. Prod. 68 (8), 1288–1289. doi:10.1021/np050105l
Qin, G. F., Tang, X. L., Sun, Y. T., Luo, X. C., Zhang, J., van Ofwegen, L., et al. (2018). Terpenoids from the soft coral Sinularia sp. collected in yongxing island. Mar. drugs 16 (4), 127. doi:10.3390/md16040127
Quah, Y., Mohd Ismail, N. I., Ooi, J. L. S., Affendi, Y. A., Abd Manan, F., Teh, L. K., et al. (2019). Purification and identification of novel cytotoxic oligopeptides from soft coral Sarcophyton glaucum. J. Zhejiang Univ. Sci. B 20 (1), 59–70. doi:10.1631/jzus.B1700586
Quang, T. H., Ha, T. T., Minh, C. V., Kiem, P. V., Huong, H. T., Ngan, N. T., et al. (2011a). Cytotoxic and anti-inflammatory cembranoids from the Vietnamese soft coral Lobophytum laevigatum. Bioorg. Med. Chem. 19 (8), 2625–2632. doi:10.1016/j.bmc.2011.03.009
Quang, T. H., Ha, T. T., Minh, C. V., Kiem, P. V., Huong, H. T., Ngan, N. T., et al. (2011b). Cytotoxic and PPARs transcriptional activities of sterols from the Vietnamese soft coral Lobophytum laevigatum. Bioorg. Med. Chem. Lett. 21 (10), 2845–2849. doi:10.1016/j.bmcl.2011.03.089
Radhika, P., Rao, P. R., Archana, J., and Nalamolu, K. R. (2005). Anti-inflammatory activity of a new sphingosine derivative and cembrenoid diterpene (lobohedleolide) isolated from marine soft corals of Sinularia crassa TIXIER-DURIVAULT and Lobophytum species of the Andaman and Nicobar Islands. Biol. Pharm. Bull. 28 (7), 1311–1313. doi:10.1248/bpb.28.1311
Radwan, M. M., Manly, S. P., El Sayed, K. A., Wali, V. B., Sylvester, P. W., Awate, B., et al. (2008). Sinulodurins A and B, antiproliferative and anti-invasive diterpenes from the soft coral Sinularia dura. J. Nat. Prod. 71 (8), 1468–1471. doi:10.1021/np800208k
Rajaram, S., Ramulu, U., Ramesh, D., Srikanth, D., Bhattacharya, P., Prabhakar, P., et al. (2013). Anti-cancer evaluation of carboxamides of furano-sesquiterpene carboxylic acids from the soft coral Sinularia kavarattiensis. Bioorg. Med. Chem. Lett. 23 (23), 6234–6238. doi:10.1016/j.bmcl.2013.09.093
Reina, E., Ramos, F. A., Castellanos, L., Aragón, M., and Ospina, L. F. (2013). Anti-inflammatory R-prostaglandins from Caribbean Colombian soft coral Plexaura homomalla. J. Pharm. Pharmacol. 65 (11), 1643–1652. doi:10.1111/jphp.12138
Ren, G. L., Wang, H., Ma, L., Gao, L. X., and Zhou, X. J. (2015). 42 cases of vertebral artery type cervical spondylosis treated with Ershiwuewei coral capsules combined with western medicine. China Pharm. 24 (12), 118–119.
Renwang, C. R., and Renqing, W. J. (2010). The efficacy of Tibetan medicine Jinzhu Yalong Ershiwuwei Shanhu capsules in the treatment of vascular neuropathic headache. J. Med. Pharm. Chin. Minorities 16 (08), 22–23. doi:10.16041/j.cnki.cn15-1175.2010.08.005
Reyes, F., Martín, R., and Fernandez, R. (2006). Granulatamides A and B, cytotoxic tryptamine derivatives from the soft coral Eunicella granulata. J. Nat. Prod. 69 (4), 668–670. doi:10.1021/np050382s
Rodgers, K., and Willcox, A. (2018). Public attitudes toward threatened and endangered species and management options in the Southeastern United States. J. Biol. Conserv. 227, 104–111. doi:10.1016/j.biocon.2018.08.025
Roux, F. X., Brasnu, D., Loty, B., George, B., and Guillemin, G. (1988). Madreporic coral: a new bone graft substitute for cranial surgery. J. Neurosurg. 69 (4), 510–513. doi:10.3171/jns.1988.69.4.0510
Roy, P. K., Roy, S., and Ueda, K. (2019). New cytotoxic cembranolides from an Okinawan soft coral, Lobophytum sp. Lobophytum Sp. Fitoter. 136, 104162. doi:10.1016/j.fitote.2019.05.001
Ruiz-Torres, V., Rodríguez-Pérez, C., Herranz-López, M., Martín-García, B., Gómez-Caravaca, A. M., Arráez-Román, D., et al. (2019). Marine invertebrate extracts induce colon cancer cell death via ROS-mediated DNA oxidative damage and mitochondrial impairment. Biomolecules 9 (12), 771. doi:10.3390/biom9120771
Sanchez, L. M., Knudsen, G. M., Helbig, C., De Muylder, G., Mascuch, S. M., Mackey, Z. B., et al. (2013). Examination of the mode of action of the almiramide family of natural products against the kinetoplastid parasite Trypanosoma brucei. J. Nat. Prod. 76 (4), 630–641. doi:10.1021/np300834q
Serhan, C. N., and Savill, J. (2005). Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6 (12), 1191–1197. doi:10.1038/ni1276
Shaaban, M., Yassin, F. Y., and Soltan, M. M. (2021). Calamusins J-K: new anti-angiogenic sesquiterpenes from Sarcophyton glaucum. Nat. Prod. Res. 35 (24), 5720–5731. doi:10.1080/14786419.2020.1828404
Shang-Kwei, W., Shyh-Yueh, P., and Duh, C. Y. (2013). New steroids from the soft coral Nephthea chabrolii. Mar. Drugs 11, 571–580. doi:10.3390/md11020571
Shao, C. L., Fu, X. M., Wang, C. Y., Han, L., Liu, X., Fang, Y. C., et al. (2009a). Investigation on the status of coral reef resources and medicinal research in China Ⅱ. status of Flok medicinal usage and medicinal research. Periodical Ocean Univ. China 39 (04), 691–698. doi:10.3969/j.issn.1672-5174.2009.04.019
Shao, C. L., Fu, X. M., Wang, C. Y., Han, L., Liu, X., Fang, Y. C., et al. (2009b). Investigation on the status of mangrove resource and medicinal research in China Ⅲ. Status of foik medicinal usage and medicinal research. Periodical Ocean Univ. China 39 (04), 712–718. doi:10.3969/j.issn.1672-5174.2009.04.020
Shen, H., Liu, X., Jiang, M., Luo, G., Wu, Z., Chen, B., et al. (2019). Anti-inflammatory cembrane-type diterpenoids and prostaglandins from soft coral Lobophytum sarcophytoides. Mar. drugs 17 (8), 481. doi:10.3390/md17080481
Shen, S. M., Li, W. S., Ding, X., Luo, H., Zhang, H. Y., and Guo, Y. W. (2021). Ximaoglaucumins A - F, new cembranoids with anti-inflammatory activities from the South China Sea soft coral Sarcophyton glaucum. Bioorg. Med. Chem. 38, 116139. doi:10.1016/j.bmc.2021.116139
Shen, Y. C., Cheng, Y. B., Lin, Y. C., Guh, J. H., Teng, C. M., and Ko, C. L. (2004). New prostanoids with cytotoxic activity from Taiwanese octocoral Clavularia viridis. J. Nat. Prod. 67 (4), 542–546. doi:10.1021/np030435a
Sheu, J. H., Chen, Y. P., Hwang, T. L., Chiang, M. Y., Fang, L. S., and Sung, P. J. (2006). Junceellolides J-L, 11,20-epoxybriaranes from the gorgonian coral Junceella fragilis. J. Nat. Prod. 69 (2), 269–273. doi:10.1021/np058077u
Shi, G. J., and Zheng, P. H. (2018). Clinical observation 0n25 Flavor of coral Capsules in treating headache after stroke. J. Med. Inf. 31 (16), 137–138+141. doi:10.3969/j.issn.1006-1959.2018.16.043
Shi, M. (2020). Research on the promoting meural differentiation capacity of effective compounds of Chinese materia medica. Beijing, China: China Academy of Chinese Medical Sciences.
Shi, X. F. (2009). Studies on bioactive constituents of the gorgonian Muriceides collaris. Master’s dissertation. Qingdao, China: Ocean University of China.
Smith, S., Taylor, G. D., and Fanning, E. A. (2003). Chronic cutaneous Mycobacterium haemophilum infection acquired from coral injury. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. Am. 37 (7), e100–e101. doi:10.1086/377267
Souyris, F., Pellequer, C., Payrot, C., and Servera, C. (1985). Coral, a new biomedical material. Experimental and first clinical investigations on Madreporaria. J. Maxillofac. Surg. 13 (2), 64–69. doi:10.1016/s0301-0503(85)80018-7
Su, C. C., Su, J. H., Lin, J. J., Chen, C. C., Hwang, W. I., Huang, H. H., et al. (2011). An investigation into the cytotoxic effects of 13-acetoxysarcocrassolide from the soft coral Sarcophyton crassocaule on bladder cancer cells. Mar. drugs 9 (12), 2622–2642. doi:10.3390/md9122622
Su, J. H., Huang, C. Y., Li, P. J., Lu, Y., Wen, Z. H., Kao, Y. H., et al. (2012). Bioactive cadinane-type compounds from the soft coral Sinularia scabra. Archives pharmacal Res. 35 (5), 779–784. doi:10.1007/s12272-012-0503-2
Su, J. H., Liu, C. I., Lu, M. C., Chang, C. I., Hsieh, M. Y., Lin, Y. C., et al. (2021). New secondary metabolite with cytotoxicity from spawning soft coral Asterospicularia laurae in Taiwan. Nat. Prod. Res. 35 (6), 967–975. doi:10.1080/14786419.2019.1614579
Su, J. H., and Wen, Z. H. (2011). Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangular. Mar. drugs 9 (6), 944–951. doi:10.3390/md9060944
Su, L. L. (2011). Studies on bioactive constituents of the gorgonian Melithaea sp. and the soft coral Lobophytum sp. Master’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.y1927662
Su, T. P., Yuan, C. H., Jhu, Y. M., Peng, B. R., Wen, Z. H., Wu, Y. J., et al. (2019). Fragilides U-W: new 11,20-epoxybriaranes from the sea whip gorgonian coral Junceella fragilis. Mar. drugs 17 (12), 706. doi:10.3390/md17120706
Su, Y. D., Cheng, C. H., Wen, Z. H., Wu, Y. C., and Sung, P. J. (2016). New anti-inflammatory sterols from a gorgonian Pinnigorgia sp. Bioorg. Med. Chem. Lett. 26 (13), 3060–3063. doi:10.1016/j.bmcl.2016.05.015
Su, Y. D., Su, T. R., Wen, Z. H., Hwang, T. L., Fang, L. S., Chen, J. J., et al. (2015a). Briarenolides K and L, new anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. drugs 13 (2), 1037–1050. doi:10.3390/md13021037
Su, Y. D., Wu, T. Y., Wen, Z. H., Su, C. C., Chen, Y. H., Chang, Y. C., et al. (2015b). Briarenolides U-Y, new anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. drugs 13 (12), 7138–7149. doi:10.3390/md13127060
Sud, P., Su, M. K., Greller, H. A., Majlesi, N., and Gupta, A. (2013). Case series: inhaled coral vapor--toxicity in a tank. J. Med. Toxicol. official J. Am. Coll. Med. Toxicol. 9 (3), 282–286. doi:10.1007/s13181-013-0307-x
Sun, H., Liu, F., Feng, M. R., Peng, Q., Liao, X. J., Liu, T. T., et al. (2016). Isolation of a new cytotoxic polyhydroxysterol from the South China Sea soft coral Sinularia sp. Nat. Prod. Res. 30 (24), 2819–2824. doi:10.1080/14786419.2016.1166495
Sun, L. L. (2012). The secondary metabolites and chemical defensiveness of six coral from the South China Sea. Doctor’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.y2212435
Sun, L. L., Fu, X. M., Li, X. B., Xing, Q., and Wang, C. Y. (2013). New 18-oxygenated polyhydroxy steroid from a South China Sea soft coral Sarcophyton sp. Nat. Prod. Res. 27 (21), 2006–2011. doi:10.1080/14786419.2013.814053
Sun, Y. Z., Kurtán, T., Mándi, A., Tang, H., Chou, Y., Soong, K., et al. (2017). Immunomodulatory polyketides from a phoma-like fungus isolated from a soft coral. J. Nat. Prod. 80 (11), 2930–2940. doi:10.1021/acs.jnatprod.7b00463
Sung, P. J., Chuang, L. F., Kuo, J., Fan, T. Y., and Hu, W. P. J. C. (2007). Rumphellatin A, the first chloride-containing caryophyllane-type norsesquiterpenoid from Rumphella antipathies. Tetrahedron Lett. 48 (23), 3987–3989. doi:10.1016/j.tetlet.2007.04.040
Tai, C. J., Su, J. H., Huang, C. Y., Huang, M. S., Wen, Z. H., Dai, C. F., et al. (2013). Cytotoxic and anti-inflammatory eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. drugs 11 (3), 788–799. doi:10.3390/md11030788
Tai, C. J., Su, J. H., Huang, M. S., Wen, Z. H., Dai, C. F., and Sheu, J. H. (2011). Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. drugs 9 (10), 2036–2045. doi:10.3390/md9102036
Taira, J., Miyazato, H., and Ueda, K. (2018). Marine peroxy sesquiterpenoids induce apoptosis by modulation of nrf2-ARE signaling in HCT116 colon cancer cells. Mar. drugs 16 (10), 347. doi:10.3390/md16100347
Tammam, M. A., Rárová, L., Kvasnicová, M., Gonzalez, G., Emam, A. M., Mahdy, A., et al. (2020). Bioactive steroids from the Red Sea Soft coral Sinularia polydactyla. Mar. drugs 18 (12), 632. doi:10.3390/md18120632
Tan, J. Z. (2020). Analysis of the effect of twenty-five ingredients coral pill combined with Aspirin on 40 cases of oatients with acute cerebral infarction. Drug Eval. 17 (24), 41–43.
Tani, K., Kamada, T., Phan, C. S., and Vairappan, C. S. (2019). New cembrane-type diterpenoids from Bornean soft coral Nephthea sp. with antifungal activity against Lagenidium thermophilum. Nat. Prod. Res. 33 (23), 3343–3349. doi:10.1080/14786419.2018.1475387
Tao, L. Q., Huang, G. H., and Luo, Y. H. (2022). Application of Aspirin combined with Clopidogrel dual antiplatelet therapy in patients with acute cerebral infarction. China Mod. Med. 29 (25), 122–125.
Teng, H., and Zhang, X. (2022). The background and historical status of american endangered species Act of 1973. Collect. Pap. Hist. Stud. (04), 132–144. doi:10.19832/j.cnki.0559-8095.2022.0048
Thanh, N. V., Ngoc, N. T., Anh Hle, T., Thung do, C., Thao do, T., Cuong, N. X., et al. (2016). Steroid constituents from the soft coral Sinularia microspiculata. J. Asian Nat. Prod. Res. 18 (10), 938–944. doi:10.1080/10286020.2016.1173676
Thao, N. P., Luyen, B. T., Brun, R., Kaiser, M., Van Kiem, P., Van Minh, C., et al. (2015). Anti-Protozoal activities of cembrane-type diterpenes from Vietnamese soft corals. Mol. (Basel, Switz. 20 (7), 12459–12468. doi:10.3390/molecules200712459
Thao, N. P., Luyen, B. T., Ngan, N. T., Song, S. B., Cuong, N. X., Nam, N. H., et al. (2014a). New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorg. Med. Chem. Lett. 24 (1), 228–232. doi:10.1016/j.bmcl.2013.11.033
Thao, N. P., Nam, N. H., Cuong, N. X., Luyen, B. T., Tai, B. H., Kim, J. E., et al. (2014b). Inhibition of NF-κB transcriptional activation in HepG2 cells by diterpenoids from the soft coral Sinularia maxima. Archives pharmacal Res. 37 (6), 706–712. doi:10.1007/s12272-013-0230-3
Thao, N. P., Nam, N. H., Cuong, N. X., Quang, T. H., Tung, P. T., Dat le, D., et al. (2013). Anti-inflammatory norditerpenoids from the soft coral Sinularia maxima. Bioorg. Med. Chem. Lett. 23 (1), 228–231. doi:10.1016/j.bmcl.2012.10.129
Thao, N. P., Nam, N. H., Cuong, N. X., Quang, T. H., Tung, P. T., Tai, B. H., et al. (2012). Diterpenoids from the soft coral Sinularia maxima and their inhibitory effects on lipopolysaccharide-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Chem. Pharm. Bull. 60 (12), 1581–1589. doi:10.1248/cpb.c12-00756
Thomas, S. A. L., Sanchez, A., Kee, Y., Wilson, N. G., and Baker, B. J. (2019). Bathyptilones: terpenoids from an antarctic sea pen, Anthoptilum grandiflorum (verrill, 1879). Mar. drugs 17 (9), 513. doi:10.3390/md17090513
Tian, Y., Wang, C., Wu, Y., Liu, Z., Ye, X., Wang, C., et al. (2023). Rethinking the substitution strategy of rare and endangered animal and plant medicinal materials. Glob. Tradit. Chin. Med. 16 (03), 387–394. doi:10.3969/j.issn.1674-1749.2023.03.003
Tran, H. H. T., Nguyen Viet, P., Nguyen Van, T., Tran, H. T., Nguyen Xuan, C., Nguyen Hoai, N., et al. (2017). Cytotoxic steroid derivatives from the Vietnamese soft coral Sinularia brassica. J. Asian Nat. Prod. Res. 19 (12), 1183–1190. doi:10.1080/10286020.2017.1307192
Tsai, T. C., Huang, Y. T., Chou, S. K., Shih, M. C., Chiang, C. Y., and Su, J. H. (2016). Cytotoxic oxygenated steroids from the soft coral Nephthea erecta. Chem. Pharm. Bull. 64 (10), 1519–1522. doi:10.1248/cpb.c16-00426
Tseng, W. R., Huang, C. Y., Tsai, Y. Y., Lin, Y. S., Hwang, T. L., Su, J. H., et al. (2016). New cytotoxic and anti-inflammatory steroids from the soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 26 (14), 3253–3257. doi:10.1016/j.bmcl.2016.05.060
Tseng, Y. J., Wen, Z. H., Dai, C. F., Chiang, M. Y., and Sheu, J. H. (2009). Nanolobatolide, a new C18 metabolite from the Formosan soft coral Sinularia nanolobata. Org. Lett. 11 (21), 5030–5032. doi:10.1021/ol901990c
Urda, C., Fernández, R., Pérez, M., Rodríguez, J., Jiménez, C., and Cuevas, C. (2017). Protoxenicins A and B, cytotoxic long-chain acylated xenicanes from the soft coral Protodendron repens. J. Nat. Prod. 80 (3), 713–719. doi:10.1021/acs.jnatprod.7b00046
Wang, Y. H. (2003). 30 cases of cerebral infarction treated with the Tibetan medicine Ershwuwei Shanhu Coral Pills. New Chin. Med. (04), 57–58. doi:10.13457/j.cnki.jncm.2003.04.032
Wang, C. Y., Liu, H. Y., Sun, X. P., Li, X. B., Sun, L. L., Liu, X., et al. (2009). Studies on the chemical constituents of soft coral Sinularia sp. from the South China sea. Periodical Ocean Univ. China 39 (04), 735–738. doi:10.16441/j.cnki.hdxb.2009.04.029
Wang, D. J. (2016). Exploring the clinical efficacy of Twenty-five Flavored Coral Pills in the treatment of intractable headache. World Latest Med. Inf. 16 (71), 228+231. doi:10.3969/j.issn.1671-3141.2016.71.179
Wang, G. H., Ahmed, A. F., Kuo, Y. H., and Sheu, J. H. (2002). Two new subergane-based sesquiterpenes from a Taiwanese Gorgonian coral Subergorgia suberosa. J. Nat. Prod. 65 (7), 1033–1036. doi:10.1021/np0106586
Wang, G. L., and Li, Y. (2014). Clinical efficacy of headache capsules in the treatment of headache caused by nitrate drugs. Chin. J. Gerontology 34 (04), 878–880. doi:10.3969/j.issn.1005-9202.2014.04.006
Wang, P., Tang, H., Liu, B. S., Li, T. J., Sun, P., Zhu, W., et al. (2013). Tumor cell growth inhibitory activity and structure-activity relationship of polyoxygenated steroids from the gorgonian Menella kanisa. Steroids 78 (9), 951–958. doi:10.1016/j.steroids.2013.05.019
Wang, Q., Tang, X., Liu, H., Luo, X., Sung, P. J., Li, P., et al. (2020). Clavukoellians G-K, new nardosinane and aristolane sesquiterpenoids with angiogenesis promoting activity from the marine soft coral Lemnalia sp. Mar. drugs 18 (3), 171. doi:10.3390/md18030171
Wang, S. C., Li, R. N., Lin, L. C., Tang, J. Y., Su, J. H., Sheu, J. H., et al. (2021). Comparison of antioxidant and anticancer properties of soft coral-derived sinularin and dihydrosinularin. Mol. (Basel, Switz. 26 (13), 3853. doi:10.3390/molecules26133853
Wang, S. H., Chang, Y. C., Chiang, M. Y., Chen, Y. H., Hwang, T. L., Weng, C. F., et al. (2010). Chlorinated briarane diterpenoids from the sea whip gorgonian corals Junceella fragilis and Ellisella robusta (Ellisellidae). Chem. Pharm. Bull. 58 (7), 928–933. doi:10.1248/cpb.58.928
Wang, S. K., Dai, C. F., and Duh, C. Y. (2006). Cytotoxic pregnane steroids from the formosan soft coral Stereonephthya crystalliana. J. Nat. Prod. 69 (1), 103–106. doi:10.1021/np050384c
Wang, S. K., and Duh, C. Y. (2012). New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. drugs 10 (2), 306–318. doi:10.3390/md10020306
Wang, S. Q., Wang, S. L., Han, M., Liu, G. H., and Zhen, X. G. (2002). Clinical study of marine coral bone in the treatment of bone defects and osseous discontinuities. Harbin Med. J. (02), 14–15.
Wang, T. (2023). Investigation and protection suggestions on rare and endangered wild plant resources in qilian mountain national nature reserve. Rural Sci. Technol. 14 (13), 115–117. doi:10.19345/j.cnki.1674-7909.2023.13.022
Wang, W. W. (2015). Talking about the significance and development of pharmacokinetic research of Chinese medicine. Health guide (17), 1.
Wang, X. J. (2008). Studies on cytotoxic cembranoid of soft coral Sinularia sp. from south China Sea. Master’s dissertation. Qingdao, China: Qingdao University of Science and Technology. doi:10.7666/d.y1399920
Wang, Y. G. (2020). Study on artificial breeding of reef building corals in land based systems. Master’s dissertation. Nanning, China: Guangxi University.
Wang, Z., Tang, H., Wang, P., Gong, W., Xue, M., Zhang, H., et al. (2013). Bioactive polyoxygenated steroids from the South China sea soft coral, Sarcophyton sp. Mar. drugs 11 (3), 775–787. doi:10.3390/md11030775
Wang, Z. S., Gao, F., Liu, S. J., and Zhao, X. C. (2014a). Clinical efficacy of twenty-five flavored coral pills in treating 64 cases of migrain. World J. Integr. Traditional West. Med. 9 (08), 870–872. doi:10.13935/j.cnki.sjzx.140825
Wang, Z. S., Zhao, X. C., Wang, K. M., Chen, B. L., and Gao, F. (2014b). The clinical curative effect observation of with 25 ingredients coral pill for epilepsy 68 cases. J. Basic Chin. Med. 20 (02), 276–277. doi:10.19945/j.cnki.issn.1006-3250.2014.02.061
Wang, Z. S., Zhao, X. C., Wang, K. M., and Gao, F. (2013). Clinical curative effect of twenty five ingredient coral pill on tension headache. Res. Integr. Traditional Chin. West. Med. 5 (05), 225–227. doi:10.3969/j.issn.1674-4616.2013.05.00
Wang, Z. S., Zhao, X. C., Wang, K. M., Wu, C. H., Zhang, C. L., and Gao, F. (2014c). Effect of twenty-five ingredient coral pill in thes treatment of complex partial epilepsy. Res. Integr. Traditional Chin. West. Med. 6 (04), 169–171. doi:10.3969/j.issn.1674-4616.2014.04.001
Wang, Z. S., Zhao, X. C., Zhao, Z. P., Chen, B. L., and Gao, F. (2013). The effect of twenty-five ingredient coral pill in the treatment of epilepsy with tonic-clonic seizures. Res. Integr. Traditional Chin. West. Med. 5 (06), 285–287. doi:10.3969/j.issn.1674-4616.2013.06.002
Wanzola, M., Furuta, T., Kohno, Y., Fukumitsu, S., Yasukochi, S., Watari, K., et al. (2010). Four new cembrane diterpenes isolated from an Okinawan soft coral Lobophytum crassum with inhibitory effects on nitric oxide production. Chem. Pharm. Bull. 58 (9), 1203–1209. doi:10.1248/cpb.58.1203
Watanabe, K., Sekine, M., and Iguchi, K. (2003). Isolation and structures of new halogenated prostanoids from the Okinawan soft coral Clavularia viridis. J. Nat. Prod. 66 (11), 1434–1440. doi:10.1021/np030181t
Watts, K. R., Tenney, K., and Crews, P. (2010). The structural diversity and promise of antiparasitic marine invertebrate-derived small molecules. Curr. Opin. Biotechnol. 21 (6), 808–818. doi:10.1016/j.copbio.2010.09.015
Wei, C., Cui, P., Xiao, J., Luo, J., Jiang, S., and Liu, X. (2022). Research progress in natural diterpenoid compounds with antibacterial activity. Chin. Tradit. Pat. Med. 44 (07), 2240–2249. doi:10.3969/j.issn.1001-1528.2022.07.030
Wei, C., Ming, J., Li, X., Ren, J., Lin, W. J. T., van Ofwegen, L., et al. (2017). Fragilolides A-Q, norditerpenoid and briarane diterpenoids from the gorgonian coral Junceella fragilis. Tetrahedron 73 (17), 2518–2528. doi:10.1016/j.tet.2017.03.037
Wei, W. C., Sung, P. J., Duh, C. Y., Chen, B. W., Sheu, J. H., and Yang, N. S. (2013). Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. drugs 11 (10), 4083–4126. doi:10.3390/md11104083
Weinheimer, A. J., and Washecheck, P. H. (1969). The structure of the marine benzofuran, furoventalene, a non-farnesyl sesquiterpene chemistry or coelenterates. XIV. Tetrahedron Lett. 10 (39), 3315–3318. doi:10.1016/s0040-4039(00)99750-8
Wen, Y. M., Qi, S. H., and Zhang, C. (2007). Studies on chemical constituents of gorgonian Juncee fragilis from South China Sea. J. Trop. Oceanogr. 01, 73–77. doi:10.3969/j.issn.1009-5470.2007.01.013
Weng, J. R., Chiu, C. F., Hu, J. L., Feng, C. H., Huang, C. Y., Bai, L. Y., et al. (2018). A sterol from soft coral induces apoptosis and autophagy in MCF-7 breast cancer cells. Mar. drugs 16 (7), 238. doi:10.3390/md16070238
Wieringa, A., Bertholee, D., Ter Horst, P., van den Brand, I., Haringman, J., and Ciminiello, P. (2014). Respiratory impairment in four patients associated with exposure to palytoxin containing coral. Clin. Toxicol. Phila. Pa 52 (2), 150–151. doi:10.3109/15563650.2013.878867
Wu, C. H., Chao, C. H., Huang, T. Z., Huang, C. Y., Hwang, T. L., Dai, C. F., et al. (2018). Cembranoid-related metabolites and biological activities from the soft coral Sinularia flexibilis. Mar. drugs 16 (8), 278. doi:10.3390/md16080278
Wu, C. R. (2013). Studies on chemical constituents and bioactivities of the gorgonian Echinogorgia flora. Ocean University of China. doi:10.7666/d.D326926
Wu, J., Li, X., Guo, X., Cheng, Z., Meng, J., Cheng, W., et al. (2020). Briarane-type diterpenoids from a gorgonian coral Ellisella sp. with anti-HBV activities. Bioorg. Chem. 105, 104423. doi:10.1016/j.bioorg.2020.104423
Wu, M. J., Wang, H., Jiang, C. S., and Guo, Y. W. (2020). New cembrane-type diterpenoids from the South China Sea soft coral Sinularia crassa and their α-glucosidase inhibitory activity. Bioorg. Chem. 104, 104281. doi:10.1016/j.bioorg.2020.104281
Wu, Y. J., Lin, S. H., Din, Z. H., Su, J. H., and Liu, C. I. (2019a). Sinulariolide inhibits gastric cancer cell migration and invasion through downregulation of the EMT process and suppression of FAK/PI3K/AKT/mTOR and MAPKs signaling pathways. Mar. drugs 17 (12), 668. doi:10.3390/md17120668
Wu, Y. J., Su, T. R., Dai, G. F., Su, J. H., and Liu, C. I. (2019b). Flaccidoxide-13-Acetate-Induced apoptosis in human bladder cancer cells is through activation of p38/JNK, mitochondrial dysfunction, and endoplasmic reticulum stress regulated pathway. Mar. drugs 17 (5), 287. doi:10.3390/md17050287
Wu, Y. J., Wei, W. C., Dai, G. F., Su, J. H., Tseng, Y. H., and Tsai, T. C. (2020). Exploring the mechanism of flaccidoxide-13-acetate in suppressing cell metastasis of hepatocellular carcinoma. Mar. drugs 18 (6), 314. doi:10.3390/md18060314
Xiang, J. B. (2016). Separation, purification and characterization of active substance from gorgonian and Caulerpa lentillifera. Master’s dissertation. Shanghai, China: Shanghai Ocean University.
Xiao, Z. M., Wang, R. Z., and Li, W. X. (2005). Clinical observation of black coral in the treatment of bone injury diseases. J. Hainan Med. Univ., 135. doi:10.3969/j.issn.1007-1237.2005.02.022
Xie, W. P., Lin, L., Long, B., He, B. J., and Gao, C. H. (2013). Chemical constituents from the beibu gulf gorgonian Suberogogorgian reticulat. Guangxi Sci. 20 (02), 165–167. doi:10.13656/j.cnki.gxkx.2013.02.010
Xu, C. M., Tang, X. L., Li, P. L., and Li, G. Q. (2017). Study on chemical commponents of the soft coral Sinularia notanda. Chin. J. Mar. Drugs 36 (01), 87–91. doi:10.13400/j.cnki.cjmd.2017.01.014
Xu, J. J. (2016). Study on the anti-inflammatory constituents from two marine organisms. Master’s dissertation. Tianjin, Chian: Tianjin medical university.
Xu, L., Chen, F., Chen, Z., Yang, B., and Luo, Y. (2023). Progress in the study of cedarane type diterpenoids in the frankincense genus. J. Chin. Med. Mater. (03), 780–788. doi:10.13863/j.issn1001-4454.2023.03.042
Xu, S. B., Zhao, J. H., Hu, Q., Wang, Z. L., and Li, R. S. (1997). Study on the antioxidant effect of soft coral cholestero. Chin. Pharmacol. Bull. (01), 87–90.
Xu, S. F., Abulikemu, L. W. Y. D., and Zhuang, L. X. (2009). Evaluation of clinical efficacy of acupoint embedding with drugs in the treatment of generalized tonic-clonic seizures in epilepsy. ournal Sichuan Traditional Chin. Med. 27 (06), 114–116.
Xu, S. H., Yang, K., Guo, S. H., and Liu, Y. P. (2003). Studies on chemical constituents from Acropora pulchra. Master’s dissertation. Chengdu, China: Natural Product Research and Development, 109–112. doi:10.3969/j.issn.1001-6880.2003.02.005
Xu, Y. (2013). Studies on the bioactive constituents of Acanthella carvernosa and Sinularia sp. Doctor’s dissertation. Shenyang, China: Shenyang Pharmaceutical University.
Xue, L. (2014). Studies on the chemical constituents and boactivities of two corals from South China Sea. Master’s dissertation. Qingdao, China: Ocean University of China.
Xue, L., Guan, T. W., Yu, Y. J., Li, P. L., Tang, X. L., and Li, G. Q. (2014). Studies on chemical constituents of the South China Sea gorgonian Echinogorgia sassapo reticulata. Chin. J. Mar. Drugs 33 (05), 43–47.
Yamashita, T., Nakao, Y., Matsunaga, S., Oikawa, T., Imahara, Y., and Fusetani, N. (2009). A new antiangiogenic C24 oxylipin from the soft coral Sinularia numerosa. Bioorg. Med. Chem. 17 (6), 2181–2184. doi:10.1016/j.bmc.2008.10.083
Yan, L., Wang, B., Wang, L., Ma, W., Jia, X., and Feng, L. (2023). Substitution model of rare and endangered Chinese medicinal materials and research strategy of multicomponent replacement. China J. Chin. Materia Medica 48 (01), 30–38. doi:10.19540/j.cnki.cjcmm.20220918.103
Yan, P., Deng, Z., van Ofwegen, L., Proksch, P., and Lin, W. (2010). Lobophytones O-T, new biscembranoids and cembranoid from soft coral Lobophytum pauciflorum. Mar. drugs 8 (11), 2837–2848. doi:10.3390/md8112848
Yan, X., Ouyang, H., Wang, W., Liu, J., Li, T., Wu, B., et al. (2021). Antimicrobial terpenoids from South China Sea Soft coral Lemnalia sp. Mar. drugs 19 (6), 294. doi:10.3390/md19060294
Yan, X. H., Guo, Y. W., and Zhu, X. Z. (2005). Chemical composition of Chinese small pointed gorgonian Muricellasinensis (Verrill). Chengdu, China: Natural Product Research and Development, 412–414. doi:10.3969/j.issn.1001-6880.2005.04.006
Yan, X. H., Lin, L. P., Ding, J., and Guo, Y. W. (2007). Methyl spongoate, a cytotoxic steroid from the Sanya soft coral Spongodes sp. Bioorg. Med. Chem. Lett. 17 (9), 2661–2663. doi:10.1016/j.bmcl.2007.01.095
Yang, B., Liao, S., Lin, X., Wang, J., Liu, J., Zhou, X., et al. (2013). New sinularianin sesquiterpenes from soft coral Sinularia sp. Mar. drugs 11 (12), 4741–4750. doi:10.3390/md11124741
Yang, C., Li, P., Luo, Y. D., Du, Y. K., Huang, F. K., Zheng, L. F., et al. (2015). Effect of 25 odors coral pills on pyramidal cell and the expression of NSE in hippocampus of D-galactose induced brain aging rats. Nat. Prod. Res. Dev. 27 (01), 45–49. doi:10.16333/j.1001-6880.2015.01.009
Yang, L. (2021). Scientists call for global joint protection of coral reefs. Fish. Inf. Strategy 36 (04), 305.
Yang, M., Li, H., Zhang, Q., Wu, Q. H., Li, G., Chen, K. X., et al. (2019). Highly diverse cembranoids from the South China Sea soft coral Sinularia scabra as a new class of potential immunosuppressive agents. Bioorg. Med. Chem. 27 (15), 3469–3476. doi:10.1016/j.bmc.2019.06.030
Yang, M., Liang, L. F., Li, H., Tang, W., and Guo, Y. W. (2020). A new 5α,8α-epidioxysterol with immunosuppressive activity from the South China Sea soft coral Sinularia sp. Nat. Prod. Res. 34 (13), 1814–1819. doi:10.1080/14786419.2018.1561683
Yang, Q. F. (2003). New clinical use of Tibetan medicine coral ershiwuwei Shanhu pills. J. Med. Pharm. Chin. Minorities (01), 14. doi:10.16041/j.cnki.cn15-1175.2003.01.011
Yang, Z. Q. (2010). The efficacy of twenty-five flavored coral pills in the treatment of migraine. China Med. Eng. 18 (04), 67.
Yao, G., Vidor, N. B., Foss, A. P., and Chang, L. C. (2007). Lemnalosides A-D, decalin-type bicyclic diterpene glycosides from the marine soft coral Lemnalia sp. J. Nat. Prod. 70 (6), 901–905. doi:10.1021/np0603424
Yao, J. E., Yang, Y., Sun, H. Y., Tang, L., Wu, F. J., Wei, W., et al. (2015). Chemical compositions of alkalodis from marine gorgonian Menella kanisa and their activities of Anti-proliferation on Human osteosarcoma cell U2OS. Guangxi Sci. 22 (03), 341–345. doi:10.13656/j.cnki.gxkx.20150401.004
Ye, F., Zhu, Z. D., Gu, Y. C., Li, J., Zhu, W. L., and Guo, Y. W. (2018). Further new diterpenoids as PTP1B inhibitors from the Xisha soft coral Sinularia polydactyla. Mar. drugs 16 (4), 103. doi:10.3390/md16040103
Ye, X., Wu, X., He, L., Liu, Z., Sun, X., Wang, H., et al. (2022). Substitutes of endangered animal medicinial materials:a review. Chin. J. Exp. Traditional Med. Formulae 28 (20), 226–231. doi:10.13422/j.cnki.syfjx.20220247
Yin, C. T., Wen, Z. H., Lan, Y. H., Chang, Y. C., Wu, Y. C., and Sung, P. J. (2015). New anti-inflammatory norcembranoids from the soft coral Sinularia numerosa. Chem. Pharm. Bull. 63 (9), 752–756. doi:10.1248/cpb.c15-00414
Yin, J., Zhao, M., Ma, M., Xu, Y., Xiang, Z., Cai, Y., et al. (2013). New casbane diterpenoids from a South China Sea soft coral, Sinularia sp. Mar. drugs 11 (2), 455–465. doi:10.3390/md11020455
Yu, J. L., Yan, X. H., Hou, H. X., and Guo, Y. W. (2004). Study on the chemical composition of Hicksonella guishanensis Zou. Nat. Prod. Res. Dev., 118–120. doi:10.3969/j.issn.1001-6880.2004.02.006
Yu, Y., Zhao, Y., Lang, S., Wei, Z., and Lu, H. (2022). Analysis on the development demand of coral artificial breeding equipment industry. Shenzhen China: China Fisheries, 78–81.
Yuan, (1991). Extraction from coral for the treatment of cerebral vascular sclerosis. Sci. Technol. Today (06), 28.
Yuan, F. L., Ji, X., Xing, X. R., and Zuo, Z. Z. (2018). Clinical study on Ershiwuwei Shanhu pills combined with levetiracetam in treatment of epilepsy. Drugs & Clin. 33 (06), 1347–1351. doi:10.7501/j.issn.1674-5515.2018.06.012
Zacharias, C., Pörner, M., Seybold, S., Haas, F., Eicken, A., and Martinoff, S. (2004). Coral as a substitute for bone grafts in delayed sternal closure in neonates. Pediatr. Radiol. 34 (12), 1028. doi:10.1007/s00247-004-1287-4
Zeng, L. M., Lan, W. J., Su, J. Y., Zhang, G. W., Feng, X. L., Liang, Y. J., et al. (2004). Two new cytotoxic tetracyclic tetraterpenoids from the soft coral Sarcophyton tortuosum. J. Nat. Prod. 67 (11), 1915–1918. doi:10.1021/np0400151
Zeng, T. J. (2019). A comparative observation on the treatment of cerebral infarction patients with Tibetan Medicine twenty-five-flavor coral pill and Huoxue Tongmai tablet. Smart Healthc. 5 (25), 126–127. doi:10.19335/j.cnki.2096-1219.2019.25.056
Zeng, Z. R., Li, W. S., Nay, B., Hu, P., Zhang, H. Y., Wang, H., et al. (2021). Sinunanolobatone A, an anti-inflammatory diterpenoid with bicyclo[13.1.0]pentadecane carbon scaffold, and related casbanes from the Sanya soft coral Sinularia nanolobata. Org. Lett. 23 (19), 7575–7579. doi:10.1021/acs.orglett.1c02772
Zeng, Z. R., Ren, C. N., and Li, C. Y. (1997). Experimental study on the repair of cortical bone defects by dense coral bone. Zhonghua Kou Qiang Yi Xue Za Zhi (01), 17–19+68.
Zhang, C. X., Yan, S. J., Zhang, G. W., Lu, W. G., Su, J. Y., Zeng, L. M., et al. (2005a). Cytotoxic diterpenoids from the soft coral Sinularia microclavata. J. Nat. Prod. 68 (7), 1087–1089. doi:10.1021/np058006v
Zhang, G. (2013a). Studies on the chernical constituents and bioactivities of the South China Sea gorgonian Subergorgia suberosa. Doctor’s dissertation. Qingdao, China: Ocean University of China.
Zhang, G., Tang, X., Cheng, C., Gong, K., Zhang, X., Zhu, H., et al. (2013a). Cytotoxic 9,11-secosteroids from the South China Sea gorgonian Subergorgia suberosa. Steroids 78 (9), 845–850. doi:10.1016/j.steroids.2013.05.009
Zhang, G. W., Ma, X. Q., Kurihara, H., Zhang, C. X., Yao, X. S., Su, J. Y., et al. (2005b). New hemiketal steroid from the soft coral Cladiella sp. Org. Lett. 7 (6), 991–994. doi:10.1021/ol0477475
Zhang, G. W., Ma, X. Q., Su, J. Y., Zhang, K., Kurihara, H., Yao, X. S., et al. (2006a). Two new bioactive sesquiterpenes from the soft coral Sinularia sp. Nat. Prod. Res. 20 (7), 659–664. doi:10.1080/14786410500183233
Zhang, H. Y. (2012a). Acyclovir in combination with 25 coral pills for herpes zoster. Chin. J. Dermatovenereology Integr. Traditional West. Med. 11 (01), 44–45. doi:10.3969/j.issn.1672-0709.2012.01.019
Zhang, J., Liao, X. J., Wang, K. L., Deng, Z., and Xu, S. H. (2013b). Cytotoxic cholesta-1,4-dien-3-one derivatives from soft coral Nephthea sp. Steroids 78 (4), 396–400. doi:10.1016/j.steroids.2012.12.012
Zhang, J., Liu, L. L., Zhong, B. L., Liao, X. J., and Xu, S. H. (2015a). 9,11-Secosteroids with cytotoxic activity from the South China Sea gorgonian coral Subergorgia suberosa. Steroids 98, 100–106. doi:10.1016/j.steroids.2015.03.004
Zhang, J. R. (2012b). Study on chemical constituents and bioactivities of a marine sponge and two gorgonian corals from South China Sen. Master’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.y2212439
Zhang, N. X. (2014). Studies on the chemical constitutents and their bioactivties of Sinularia acuta. Master’s dissertation. Qingdao, China: Ocean University of China.
Zhang, Q. (2019). Studies on chemical components and bioactivities of one species of soft corals and one species of marine sponges collected from South China Sea. Master’s dissertation. Hangzhou, China: Zhejiang University.
Zhang, Q., Liang, L. F., Miao, Z. H., Wu, B., and Guo, Y. W. (2019). Cytotoxic polyhydroxylated steroids from the South China Sea soft coral Lobophytum sp. Steroids 141, 76–80. doi:10.1016/j.steroids.2018.11.015
Zhang, Q. Y. (2013b). The present situation & creative design of coral ornament in Taiwan. Master’s dissertation. Beijing, China: China University of Geosciences Beijing.
Zhang, w., and Guo, Y. W. (2003). Advances in chemical composition and biological activity of marine organism gorgonian. Chin. J. Nat. Med. (02), 8–14.
Zhang, W., Guo, Y. W., and Gu, Y. (2006b). Secondary metabolites from the South China Sea invertebrates: chemistry and biological activity. Curr. Med. Chem. 13 (17), 2041–2090. doi:10.2174/092986706777584960
Zhang, X. L., Mao, D. J., Ge, Z. Y., and Tang, Y. E. (2015b). The efficacy of adding aloe vera pearl capsule in the treatment of melasma. Dermatol. Bull. 32 (05), 545. doi:10.3969/j.issn.1002-1256.2006.04.032
Zhang, Y. S., and Zhang, Y. (2011). Clinical efficacy of Twenty-five Flavored Coral Pills in treating 65 cases of cervical spondylosis. J. Med. Pharm. Chin. Minorities 17 (05), 16. doi:10.16041/j.cnki.cn15-1175.2011.05.014
Zhao, H. Y. (2013). The secondary metabolites and their bioactivies of a gorgonian coral and two sponge-derived Fungi. Master’s dissertation. Qingdao, China: Ocean University of China. doi:10.7666/d.D326921
Zhao, H. Y., Shao, C. L., Li, Z. Y., Han, L., Cao, F., and Wang, C. Y. (2013c). Bioactive pregnane steroids from a South China Sea gorgonian Carijoa sp. Mol. (Basel, Switz. 18 (3), 3458–3466. doi:10.3390/molecules18033458
Zhao, J., Hou, G. F., Li, X. B., Shao, C. L., Liu, Y., Guan, H. S., et al. (2011). Secondary metabolites of Echinogorgia sassapo reticulata (esper) from the South China sea. Periodical Ocean Univ. China 41 (04), 103–108. doi:10.16441/j.cnki.hdxb.2011.04.017
Zhao, M., Li, X., Zhao, F., Cheng, S., Xiang, Z., Dong, J., et al. (2013a). Four New 7,8-epoxycembranoids from a Chinese soft coral Lobophytum sp. Chem. Pharm. Bull. 61 (12), 1323–1328. doi:10.1248/cpb.c13-00612
Zhao, M., Yin, J., Jiang, W., Ma, M., Lei, X., Xiang, Z., et al. (2013b). Cytotoxic and antibacterial cembranoids from a South China Sea soft coral, Lobophytum sp. Lobophytum Sp. Mar. drugs 11 (4), 1162–1172. doi:10.3390/md11041162
Zhao, S. Y., Zhang, J. R., and Yan, X. J. (2016). Progress in the study of chemical composition and biological activity of stony coral. Nat. Prod. Res. Dev. 28 (05), 800–809. doi:10.16333/j.1001-6880.2016.5.029
Zhao, X. C. (2011). Clinical observation on migraine treated with combination of 25-ingredient coral pills and fluorine. Med. Recapitulate 17 (16), 2544–2545. doi:10.3969/j.issn.1006-2084.2011.16.054
Zhou, C. L., Liu, Q., Mei, D., and Chen, Y. (2019). Clinical observation of twenty-five-flavor coral pills associated with a small dose of trazodone hydrochloride tablets on chronic tension-type headache. Contemp. Med. 25 (30), 35–37. doi:10.3969/j.issn.1009-4393.2019.30.014
Zhou, C. Y., Zheng, C. T., Lin, T. C., Fu, Q. X., Yang, H. Y., Lu, C. X., et al. (1993). Pre-experimental study of hainan coral, Porites iutea as an artificial bone substitute hainan medical journal(01), 51–66.
Zhou, D. Y. (2009). A controlled clinical study on the treatment of migraine by swallowing large doses of Ershiwei Shanhu Pills. Sichuan Med. J. 30 (05), 657–659. doi:10.16252/j.cnki.issn1004-0501-2009.05.005
Zhou, Y. (2014). Clinical analysis of coral bone powder and oral restorative film applied to immediate implants. Contemp. Med. 20 (20), 93–94. doi:10.3969/j.issn.1009-4393.2014.20.063
Zhu, H., and Hu, S. (2023). Countries should take consistent measures to protect coral reefs in the South China Sea. Beijing, China: China SCIENCE DAILY. CHINA SCIENCE DAILY, 1.
Zhu, H. Y. (2013). Re-study on constituents and bioactivities of thr gorgonian Muriceides collaris. Master’s dissertation. Qingdao, China: Ocean University of China.
Zhu, H. Y., Li, P. L., Tang, X. L., and Li, G. Q. (2013). Studies on chemical constituents of the South China sea gorgonian Muriceides Collaris. Chin. J. Mar. Drugs 32 (05), 21–25.
Zhu, L. (2001). Advances in research on coral, a bone-derived material. J. Clin. Orthop. (03), 233–235.
Zhu, Y., Zhu, W. M., Li, L. F., Liu, G. Z., Li, X. J., Huang, C. Y., et al. (2020). Clinical observation on treatment of 30 cases of cerebral infarction with Ershiwuwei Shanhu pill. Chin. J. Ethnomedicine Ethnopharmacy 29 (09), 118–122.
Zidan, S. A. H., Abdelhamid, R. A., Al-Hammady, M., Fouad, M. A., Matsunami, K., and Orabi, M. A. A. (2020). Cytotoxic polyhydroxy sterols from the Egyptian Red Sea soft coral Sarcophyton acutum. Fitoterapia 147, 104765. doi:10.1016/j.fitote.2020.104765
Zou, L., and Jiang, W. (2023). Analysis of the applicability of the compensation system for wildlife-related Ecological enviroment sdamage. Chin. J. Wildl., 1–7.
Zou, X. (2015). Studies on the chemical constituents and bioactivities of Sinularia sp. from Paracel islands. Master’s dissertation. Qingdao, China: Ocean University of China.
Keywords: coral, traditional medicine of China, chemical constituents, pharmacology, toxicology, clinical application
Citation: Han M, Wang Z, Li Y, Song Y and Wang Z (2024) The application and sustainable development of coral in traditional medicine and its chemical composition, pharmacology, toxicology, and clinical research. Front. Pharmacol. 14:1230608. doi: 10.3389/fphar.2023.1230608
Received: 29 May 2023; Accepted: 14 November 2023;
Published: 03 January 2024.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Wen-Chi Wei, National Research Institute of Chinese Medicine, TaiwanMd. Moklesur Rahman Sarker, State University of Bangladesh, Bangladesh
Run-Lan Wan, The Affiliated Hospital of Southwest Medical University, China
Copyright © 2024 Han, Wang, Li, Song and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Wang, d2FuZ3poYW5nY3FjZEBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Mengtian Han1,2†
Mengtian Han1,2† Zhang Wang
Zhang Wang