Abstract
Aim: To systematically compare the efficacy and safety of biologics [tumor necrosis factor inhibitors (TNFi), interleukin (IL) inhibitors, phosphodiesterase-4 inhibitors (PDE4i), and Janus kinase inhibitors (JAKi)] for biological-naïve patients with psoriatic arthritis (PsA).
Methods: PubMed, Web of Science, Embase, and Cochrane Library were comprehensively searched until 12 March 2023. Only head-to-head active comparison studies were included, and placebo-controlled studies without active biologic comparators were excluded. Outcomes included musculoskeletal endpoint [American College of Rheumatology (ACR) 20/50/70, resolution of enthesitis, resolution of dactylitis], function endpoint [Health Assessment Questionnaire-Disability Index (HAQ-DI) change, ∆ HAQ-DI ≥ 0.35], composite index endpoint [ACR 50 + Psoriasis Area Severity Index (PASI) 100], and adverse events. The Jadad scale and Newcastle-Ottawa scale (NOS) were adopted to evaluate the quality of eligible studies.
Results: Totally 17 studies with head-to-head comparisons of these biologics were included in this systematic review and network meta-analysis. Compared with IL-17A inhibitors (IL-17Ai), TNFi were associated with a lower rate of achieving ACR 20 response [pooled risk ratios (RR) = 0.92, 95% credibility interval (CrI): 0.86, 0.98]. JAKi had the greatest possibility of achieving ACR 20 (50.25%) and ACR 50 (83.03%). The JAKi group had a higher rate of achieving ACR 70 response than the IL-17Ai group (pooled RR = 1.25, 95%CrI: 1.00, 1.57); TNFi were less effective than JAKi in terms of ACR 70 (pooled RR = 0.77, 95%CrI: 0.64, 0.94). ACR 70 was most likely to be achieved in patients using JAKi (97.48%). The IL-17Ai group had a higher rate of enthesitis resolution than the TNFi group [pooled RR = 1.22, 95% confidence interval (CI): 1.02, 1.47]. Compared with IL-17Ai, TNFi were associated with a lower rate of enthesitis resolution (pooled RR = 0.80, 95%CrI: 0.72, 0.88). Patients receiving IL-17Ai had the highest likelihood of achieving enthesitis resolution (82.76%), dactylitis resolution (58.66%) and the greatest HAQ-DI change (59.74%). IL-17Ai had a similar impact in achieving ∆ HAQ-DI ≥ 0.35 to TNFi (pooled RR = 1.15, 95%CI: 0.93, 1.41). Individuals receiving IL-17Ai had a higher rate of achieving combined ACR 50 and PASI 100 response than those receiving TNFi (pooled RR = 1.56, 95%CI: 1.29, 1.88). Patients receiving PDE4i were least likely to have adverse events (41.59%).
Conclusion: In 2023, considering both efficacy and safety, IL-17Ai may be the better treatment option for biological-naïve patients with PsA requiring biological therapy.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disorder characterized by joint and enthesis inflammation, and influences multiple organ systems, including peripheral and axial joints, entheses, skin, and nails (Ocampo and Gladman, 2019; López-Ferrer et al., 2022). This disease develops in approximately 30% of patients with psoriasis, and is a primary comorbidity of psoriasis (Gottlieb and Merola, 2020). An important concern for PsA patients is pain relief, as well as their ability to engage in social activities, fatigue, and psychological distress (Gudu and Gossec, 2018). PsA is related to decreased quality of life and a great economic burden (D'Angiolella et al., 2018; Kishimoto et al., 2021).
Nowadays, biologic disease-modifying antirheumatic drugs (bDMARDs) have been applied in the treatment of PsA due to reported efficacy and safety (Kamata and Tada, 2020; Ruyssen-Witrand et al., 2020). The main types of biological agents include tumor necrosis factor inhibitors (TNFi) (e.g., adalimumab), interleukin (IL) inhibitors (e.g., ixekizumab), Janus kinase inhibitors (JAKi) (e.g., upadacitinib), and phosphodiesterase-4 inhibitors (PDE4i) (e.g., apremilast) (Raychaudhuri et al., 2017; Singh et al., 2019). Several studies were conducted to explore the role of biologics in PsA. For example, Kristensen et al. (2022) found that compared with adalimumab, ixekizumab was more effective in achieving American College of Rheumatology (ACR) 50 and Psoriasis Area Severity Index (PASI) 100 simultaneously. According to another study, IL-17A inhibitors (IL-17Ai) exhibited similar impacts on the health assessment questionnaire (HAQ) score and ACR 20 achievement to TNFi in PsA (Izumiyama et al., 2021). For biological-naïve PsA patients, secukinumab appeared to be associated with greater ACR 20/50 response than infliximab in the medium to long term (Strand et al., 2019). Based on the existing direct and indirect evidence, increasing network meta-analyses have investigated the efficacy and safety of specific biologics (e.g., adalimumab, ustekinumab, apremilast) in PsA (Migliore et al., 2021). Nevertheless, comprehensive comparisons among broad categories of biologics, such as TNFi, IL inhibitors and JAKi, for biological-naïve patients with PsA are lacking. Besides, more studies on biologics for PsA patients are carried out in recent years. Updated network meta-analyses are needed to provide a reference for biologics selection in PsA treatment.
This latest study aimed to systematically evaluate and compare the efficacy and safety of biologics [TNFi, IL inhibitors, PDE4i, and JAKi] for biological-naïve patients with PsA via a systematic review and network meta-analysis.
Methods
Search strategy
PubMed, Web of Science, Embase, and Cochrane Library were comprehensively searched by two independent investigators (JX Lin, YG Ren) from inception to 12 March 2023. Disagreements were addressed via discussion. English search terms included “biological agent” OR “abatacept” OR “adalimumab” OR “apremilast” OR “certolizumab” OR “etanercept” OR “golimumab” OR “Infliximab” OR “Ixekizumab” OR “Secukinumab” OR “Tofacitinib” OR “Ustekinumab” OR “apremilast” OR “Tumor Necrosis Factor-alpha” OR “Monoclonal Antibodies” OR “Phosphodiesterase 4 Inhibitors) AND (Arthritis, Psoriatic” OR “Psoriasis, Arthritic” OR “Arthritic Psoriasis” OR “Psoriatic Arthritis” OR “Psoriasis Arthropathica” OR “Psoriatic Arthropathy” OR “Arthropathies, Psoriatic” OR “Arthropathy, Psoriatic” OR “Psoriatic Arthropathies”. Preliminary screening was conducted based on the titles and abstracts of the retrieved studies, followed by subsequent screening via full texts. This systematic review and network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension statement for network meta-analyses (Hutton et al., 2015).
Eligibility criteria
Inclusion criteria: 1) studies on biological-naïve patients with PsA (population); 2) studies on TNFi (adalimumab, golimumab, certolizumab, infliximab, etanercept), IL-17Ai (ixekizumab, secukinumab, bimekizumab) and IL-12/23 inhibitors (IL-12/23i) (ustekinumab), PDE4i (apremilast), and JAKi (upadacitinib) biologics (intervention and comparator); 3) head-to-head active comparison studies; 4) studies on any of the following outcomes: musculoskeletal endpoint [ACR 20/50/70, resolution of enthesitis evaluated by the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC) and Leeds Enthesitis Index (LEI), resolution of dactylitis evaluated by the Leeds Dactylitis Index-Basic (LDI-B)], function endpoint [Health Assessment Questionnaire-Disability Index (HAQ-DI) change (pre-treatment HAQ-DI minus post-treatment HAQ-DI), ∆ HAQ-DI ≥ 0.35], composite index endpoint (ACR 50 + PASI 100), adverse events, arthritis activity endpoint [disease activity score (DAS)], skin endpoint (PASI 90, PASI 100, PASI), and drug retention (outcome); 5) randomized controlled trials (RCTs) and cohort studies (study design). Adverse events included infections, injection site reactions, malignancies, cerebrocardiovascular events, allergic reactions/hypersensitivity, inflammatory bowel disease, depression, hepatic laboratory changes, cytopenia, and neutropenia.
Exclusion criteria: 1) animal trials; 2) studies on patients who had previously received treatment with relevant biologics, or on a mixed population of biological-naïve and biological-experienced patients; 3) studies without a control group or placebo-controlled studies without active biologic comparators; 4) studies of which data were incomplete or could not be extracted; 5) case reports, meeting abstracts, protocols, letters, reviews, meta-analysis; 6) non-English literature.
Data extraction and quality assessment
Extracted data from qualified studies included first author, year of publication, country, study design, group, sample size, age (years), sex (male/female), duration of PsA, PASI, DAS28, HAQ-DI, comorbidity, concomitant conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), concomitant nonsteroidal anti-inflammatory drugs (NSAIDs), concomitant glucocorticoids, follow-up time (months), and outcomes. Two investigators (JX Lin, YG Ren) collected the above data independently.
The Jadad scale was adopted to evaluate the quality of RCTs from four dimensions: randomization, concealment of allocation, double blinding, and withdrawals and dropouts. This scale had a total score of 7, with 1–3 as low quality and 4–7 as high quality (Jadad et al., 1996). For the quality assessment of cohort studies, the Newcastle-Ottawa scale (NOS) was employed and measured three sections: population selection, intergroup comparability, and outcome measurement. The NOS had a total score of 9, with 0–3 as poor quality, 4–6 as fair quality, and 7–9 as good quality (Wells et al., 2000).
Statistical analysis
Through constructing a Bayesian framework and a Monte Carlo Markov Chain (MCMC), this network meta-analysis was performed, with the number of model chains of 4, the number of initial iterations of 20000, the number of updated iterations of 50000, and the step size of 1. Heterogeneity indicated the overall degree of difference in the same pair of comparisons. The I2 statistic was the primary indicator of statistical heterogeneity, with values < 25%, 25%–50% and >50% representing low, moderate and high heterogeneity, separately. Network plots were depicted to show direct and indirect comparisons of biologics for each outcome. A larger node indicated a larger sample size for the biologics represented by the node, while a thicker line indicates a larger number of studies for the comparison of biologics at both ends of the line. The influences of biologics on the outcomes were illustrated via forest plots and league tables. Rank probabilities exhibited the probability of different biologics ranking at a certain position (e.g., ranking first, second, third). For HAQ-DI change, weighted mean differences (WMDs) and 95% credibility intervals (CrIs) were described for different biological agents; for ACR 20/50/70, resolution of enthesitis, resolution of dactylitis, and adverse events, risk ratios (RRs) and 95%CrIs were estimated. Statistical analysis was completed by applying STATA 15.1 (Stata Corporation, College Station, TX, United States) and R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the included studies
A total of 16,031 studies were identified through database searching, with 2,916 from PubMed, 6,196 from Web of Science, 6,914 from Embase, and 5 from Cochrane Library. Subsequently, 10,417 studies left after duplicate removal, followed by screening with titles and abstracts, and then full texts based on the eligibility criteria. Ultimately, 17 studies (Atteno et al., 2010; Araujo et al., 2019; Strand et al., 2019; McInnes et al., 2020; Mease et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; Izumiyama et al., 2021; Lindström et al., 2021; McInnes et al., 2021; Eviatar et al., 2022; Kristensen et al., 2022; McInnes et al., 2022; Pina Vegas et al., 2022; Reich et al., 2022; Silva et al., 2022; McInnes et al., 2023) including head-to-head comparisons of the biologics were included in this systematic review and network meta-analysis. Figure 1 presents the detailed process of study selection. These included studies were published from 2010 to 2023. Most of the studies came from the United States. Ten studies (Araujo et al., 2019; McInnes et al., 2020; Mease et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; McInnes et al., 2021; Kristensen et al., 2022; McInnes et al., 2022; Reich et al., 2022; McInnes et al., 2023) were RCTs, of which 2 (Mease et al., 2020; Reich et al., 2022) had low quality, and 8 (Araujo et al., 2019; McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; McInnes et al., 2021; Kristensen et al., 2022; McInnes et al., 2022; McInnes et al., 2023) had high quality; 7 (Atteno et al., 2010; Strand et al., 2019; Izumiyama et al., 2021; Lindström et al., 2021; Eviatar et al., 2022; Pina Vegas et al., 2022; Silva et al., 2022) studies were cohort studies, with 6 (Atteno et al., 2010; Izumiyama et al., 2021; Lindström et al., 2021; Eviatar et al., 2022; Pina Vegas et al., 2022; Silva et al., 2022) of fair quality, and 1 (Strand et al., 2019) of good quality. Characteristics and quality assessment of the included studies are illustrated in Tables 1, 2, respectively.
FIGURE 1
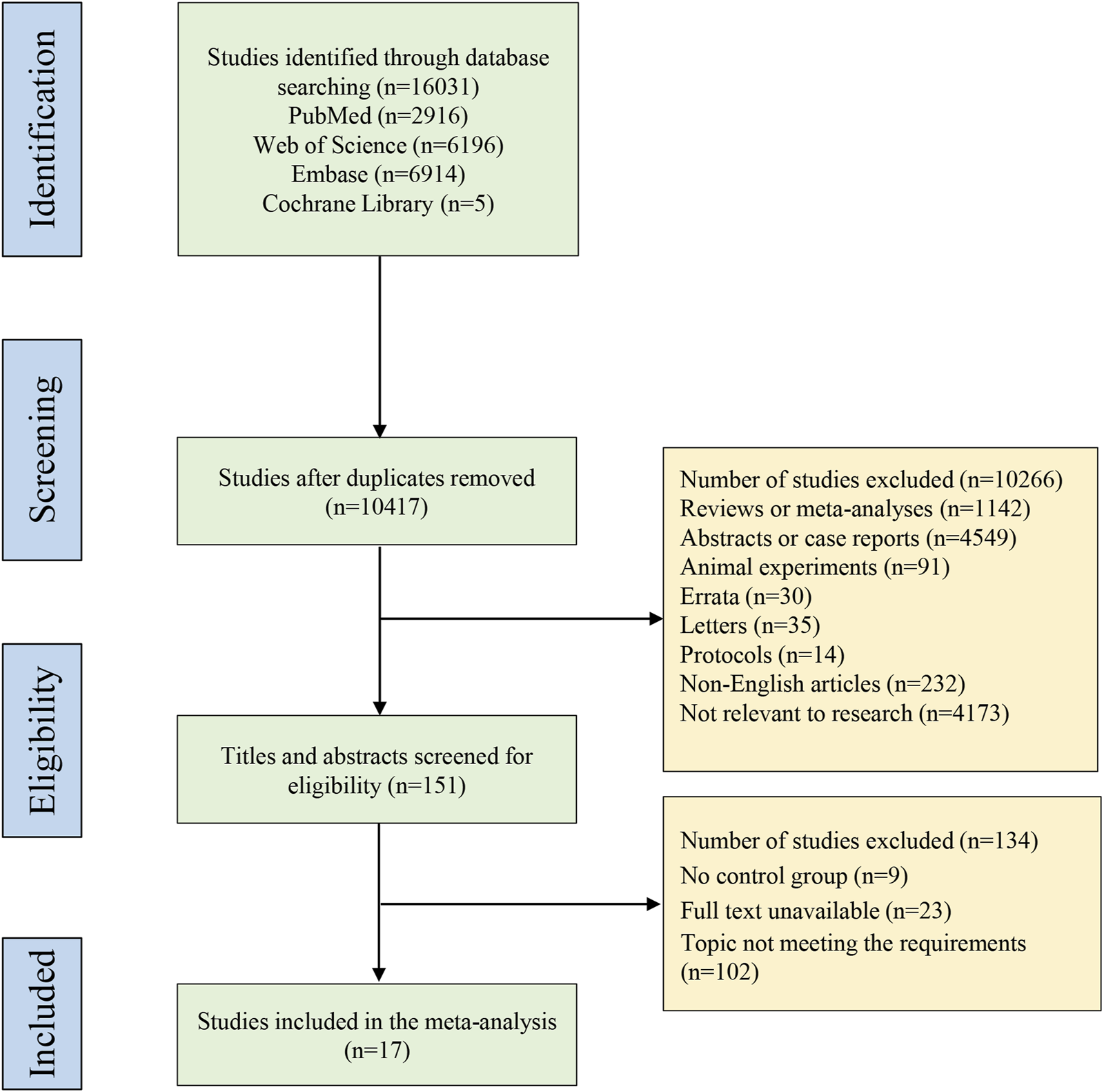
PRISMA flow diagram of study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
TABLE 1
| Author | Year | Country | Study design | Group | Sample size | Age, years | Sex (male/female), n | Duration of PsA, years | Basic PASI | Basic DAS28 | Basic HAQ-DI | Comorbidity, n | Concomitant csDMARDs, n | Concomitant NSAIDs, n | Concomitant glucocorticoids, n | Follow up, months | Outcomes measured |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Araujo | 2018 | Germany | RCT | Ustekinumab | 23 | 62 ± 18 | 10/13 | 2 ± 6.0 | 3.0 ± 6.6 | 4.0 ± 1.09 | 0.87 ± 0.63 | NA | MTX, 19 | NA | 0 | 6 | DAS28, PASI 90, PASI 100, HAQ-DI |
| TNFi | 24 | 58 ± 21 | 18/6 | 3 ± 4.8 | 2.8 ± 3.6 | 4.4 ± 1.24 | 1.17 ± 0.62 | NA | MTX, 24 | NA | 1 | ||||||
| Atteno | 2010 | Italy | Cohort study | Etanercept | 36 | 49.3 ± 13.4 | 15/21 | 80 (20–140) m | 26 ± 18.5 | NA | 1.2 ± 0.4 | NA | NA | NA | NA | 12 | PASI, HAQ-DI |
| Adalimumab | 34 | 47.5 ± 11.5 | 14/20 | 18 ± 16.5 | NA | 1.2 ± 0.3 | NA | NA | NA | NA | |||||||
| Infliximab | 30 | 48.5 ± 12.9 | 11/19 | 15 ± 14.8 | NA | 1.5 ± 0.5 | NA | NA | NA | NA | |||||||
| da Silva | 2022 | Brazil | Cohort study | Adalimumab | 91 | 50.92 ± 11.89 | 40/51 | 5.36 ± 7.27 | NA | NA | 1.23 ± 0.74 | 68 | 43 | 25 | 27 | 12 | HAQ, EQ-5D |
| Etanercept | 52 | 51.50 ± 12.90 | 19/33 | 4.61 ± 6.25 | NA | NA | 1.21 ± 0.71 | 40 | 15 | 9 | 9 | ||||||
| Eviatar | 2022 | Israel | Cohort study | Secukinumab | 13 | 41.2 ± 14.4 | NA | 13.7 ± 13.1 | NA | NA | NA | NA | NA | NA | NA | 12 | Drug retention |
| Etanercept | 130 | 42.6 ± 14.5 | NA | 7.8 ± 8.6 | NA | NA | NA | NA | NA | NA | NA | ||||||
| Infliximab | 28 | 41.2 ± 4.2 | NA | 8.5 ± 9.4 | NA | NA | NA | NA | NA | NA | NA | ||||||
| Adalimumab | 103 | 41.5 ± 14.0 | NA | 8.7 ± 9.0 | NA | NA | NA | NA | NA | NA | NA | ||||||
| Golimumab | 25 | 39.9 ± 15.7 | NA | 12.1 ± 11.3 | NA | NA | NA | NA | NA | NA | NA | ||||||
| Gottlieb | 2021 | United States | RCT | Secukinumab | 110 | 48.9 ± 12.2 | 66/44 | 6.1 ± 8.9 | 16.2 ± 9.6 | 4.7 ± 0.9 | 1.3 ± 0.6 | NA | Included but not limited to MTX | Leflunomide | Prednisone, 8 | 13 | ACR 20/50/70, ACR 50 + PASI 100, HAQ-DI change, HAQ-DI ≥ 0.35, resolution of enthesitis, resolution of dactylitis |
| Adalimumab | 101 | 46.9 ± 12.3 | 57/44 | 6.7 ± 8.4 | 15.0 ± 8.9 | 4.8 ± 1.0 | 1.3 ± 0.7 | NA | Prednisone, 5 | ||||||||
| Izumiyama | 2021 | Japan | Cohort study | TNFi | 13 | 44.3 ± 9.5 | 9/4 | 57.7 ± 57.7 m) | NA | 3.1 ± 1.1 | 0.26 ± 0.44 | NA | MTX, 10 | NA | NA | 13 | HAQ-DI change, ACR20 |
| IL-17Ai | 18 | 55.2 ± 9.2 | 9/9 | 85.6 ± 67.7 m) | NA | 3.0 ± 1.9 | 0.47 ± 0.46 | NA | MTX, 7 | NA | NA | ||||||
| Kristensen | 2022 | Denmark | RCT | Ixekizumab | 234 | 48.0 ± 12.1 | 132/102 | 6.5 ± 7.4 | 4.7 ± 3.5 | NA | 1.2 ± 0.6 | NA | MTX, 47 | NA | 47 | 13 | HAQ-DI ≥ 0.35, resolution of enthesitis, resolution of dactylitis |
| Adalimumab | 231 | 48.7 ± 12.5 | 116/115 | 5.9 ± 6.4 | 4.9 ± 2.9 | NA | 1.2 ± 0.7 | NA | MTX, 42 | NA | 42 | ||||||
| Lindstrom | 2021 | Sweden | Cohort study | Adalimumab | 579 | 48 ± 13 | 300/279 | 10 ± 10 | NA | 27 ± 17 | NA | 30 | MTX, 13; Sulphasalazine, 3 | NA | NA | 6 | Drug retention, adverse event |
| Secukinumab | 165 | 52 ± 13 | 79/86 | 12 ± 10 | NA | 30 ± 17 | NA | 66 | MTX, 5; Sulphasalazine, 2 | NA | NA | ||||||
| Etanercept | 1786 | 49 ± 13 | 811/975 | 10 ± 10 | NA | 26 ± 16 | NA | 36 | MTX, 7; Sulphasalazine, 2 | NA | NA | ||||||
| Infliximab | 1,006 | 49 ± 13 | 464/542 | 10 ± 10 | NA | 27 ± 16 | NA | 33 | MTX, 35; Sulphasalazine, 7 | NA | NA | ||||||
| Golimumab | 215 | 46 ± 13 | 103/112 | 9 ± 7 | NA | 24 ± 13 | NA | 38 | MTX, 10; Sulphasalazine, 4 | NA | NA | ||||||
| Certolizumab | 272 | 47 ± 13 | 124/148 | 10 ± 9 | NA | 29 ± 14 | NA | 27 | MTX, 33; Sulphasalazine, 9 | NA | NA | ||||||
| McInnes | 2020 | United States | RCT | Secukinumab | 426 | 48.5 ± 12.38 | 208/218 | 5.1 ± 7.60 | 10.6 ± 9.00 | 4.7 ± 1.00 | 1.3 ± 0.64 | NA | NA | NA | 61 | 13 | ACR 20/50/70, HAQ-DI change, ACR 50 + PASI 100, HAQ-DI ≥0.35, adverse event, resolution of enthesitis, resolution of dactylitis |
| Adalimumab | 427 | 49.5 ± 12.44 | 229/198 | 5.7 ± 7.29 | 10.0 ± 8.15 | 4.7 ± 0.94 | 1.2 ± 0.64 | NA | NA | NA | 58 | ||||||
| McInnes | 2021 | United States | RCT | Upadacitinib | 429 | 51.6 ± 12.2 | 191/238 | 6.2 ± 7.4 | 9.8 ± 10.0 | NA | 1.2 ± 0.7 | NA | 353 | NA | 73 | 6 | ACR20, adverse event, resolution of enthesitis, resolution of dactylitis |
| Adalimumab | 429 | 51.4 ± 12.0 | 207/222 | 5.9 ± 7.1 | 9.4 ± 8.5 | NA | 1.1 ± 0.6 | NA | 347 | NA | 72 | ||||||
| McInnes | 2022 | United States | RCT | Upadacitinib | 429 | 51.6 ± 12.2 | 191/238 | 6.2 ± 7.4 | 9.8 ± 10.0 | NA | 1.2 ± 0.7 | NA | 353 | NA | 73 | 24 | ACR 20/50/70 |
| Adalimumab | 429 | 51.4 ± 12.0 | 207/222 | 5.9 ± 7.1 | 9.4 ± 8.5 | NA | 1.1 ± 0.6 | NA | 347 | NA | 72 | ||||||
| McInnes | 2023 | United Kingdom | RCT | Bimekizumab | 431 | 48.5 ± 12.6 | 201/230 | 6.0 ± 7.3 | 8.2 ± 6.8 | NA | 0.82 ± 0.59 | NA | 301 | NA | NA | 6 | ACR 20/50/70, HAQ-DI change, HAQ-DI ≥0.35, resolution of enthesitis, resolution of dactylitis, adverse event |
| Adalimumab | 140 | 49.0 ± 12.8 | 71/69 | 6.1 ± 6.8 | 8.5 ± 7.6 | NA | 0.86 ± 0.54 | NA | 99 | NA | NA | ||||||
| Mease | 2020 | United States | RCT | Ixekizumab | 283 | 47.5 ± 12.0 | 162/121 | 6.6 ± 7.4 | 7.9 ± 8.7 | 5.8 ± 0.9 | 1.2 ± 0.6 | NA | 193 | NA | NA | 6 | ACR 50+PASI 100, ACR 20/50/70, HAQ-DI ≥0.35 |
| Adalimumab | 283 | 48.3 ± 12.3 | 150/133 | 5.9 ± 6.4 | 7.7 ± 7.3 | 5.8 ± 1.0 | 1.3 ± 0.7 | NA | 199 | NA | NA | ||||||
| Reich | 2022 | United States | RCT | Ixekizumab | 49 | 45.3 ± 11.5 | 30/19 | 7.0 ± 7.4 | 22.9 ± 10.5 | NA | NA | NA | MTX, 25 | NA | NA | 13 | ACR 20/50/70, adverse event |
| Adalimumab | 51 | 46.3 ± 11.3 | 33/18 | 5.7 ± 6.2 | 20.5 ± 7.3 | NA | NA | NA | MTX, 28 | NA | NA | ||||||
| Smolen | 2020 | Austria | RCT | Ixekizumab | 283 | 47.5 ± 12.0 | 162/121 | 6.6 ± 7.4 | 7.9 ± 8.7 | 5.8 ± 0.9 | 1.2 ± 0.6 | NA | 193 | NA | NA | 13 | ACR 50+PASI 100, ACR 20/50/70, HAQ-DI ≥0.35, adverse event, resolution of enthesitis, resolution of dactylitis |
| Adalimumab | 283 | 48.3 ± 12.3 | 150/133 | 5.9 ± 6.4 | 7.7 ± 7.3 | 5.8 ± 1.0 | 1.3 ± 0.7 | NA | 199 | NA | NA | ||||||
| Strand | 2019 | United States | Cohort study | Secukinumab | 238 | 48.6 ± 11.8 | 116/122 | NA | 11.4 ± 12.7 | NA | 1.1 ± 0.6 | 83 | MTX, 47 | NA | NA | 12 | ACR 20/50/70 |
| Infliximab | 100 | 47.1 ± 12.8 | 71/29 | NA | 12.2 ± 11.3 | NA | 1.2 ± 0.6 | 126 | MTX, 105 | NA | NA | ||||||
| Vegas | 2022 | France | Cohort study | TNFi | 7,289 | 48.2 ± 12.8 | 3,002/4,287 | NA | NA | NA | NA | 3,150 | 2,992 | 1,473 | NA | 12 (6–25) | MACE |
| IL-12/23i | 1,058 | 49.8 ± 12.8 | 475/583 | NA | NA | NA | NA | 708 | 305 | 144 | NA | ||||||
| IL-17Ai | 1,163 | 49.2 ± 12.2 | 482/681 | NA | NA | NA | NA | 639 | 336 | 188 | NA | ||||||
| Apremilast | 1885 | 54.0 ± 12.5 | 835/1,050 | NA | NA | NA | NA | 1,175 | 653 | 357 | NA |
Characteristics of the included studies.
RCT, randomized controlled trials; PsA, psoriatic arthritis; PASI, psoriasis area severity index; DAS28, disease activity score 28; HAQ-DI, health assessment questionnaire disability index; MTX, methotrexate; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; NSAIDs, nonsteroidal anti-inflammatory drugs; TNFi, tumor necrosis factor inhibitors; IL-17Ai, interleukin-17A, inhibitors; EQ-5D, european quality of life five dimensions; MACE, major adverse cardiac event; ACR, american college of rheumatology; NA, not applicable.
TABLE 2
| Author | Year | Country | Study design | Randomization | Concealment of allocation | Double blinding | Withdrawals and dropouts | Total score |
|---|---|---|---|---|---|---|---|---|
| Araujo | 2018 | Germany | RCT | 1 | 2 | 1 | 1 | 5 |
| Gottlieb | 2021 | United States | RCT | 1 | 2 | 1 | 0 | 4 |
| Kristensen | 2022 | Denmark | RCT | 2 | 2 | 1 | 1 | 6 |
| McInnes | 2020 | United States | RCT | 1 | 2 | 1 | 0 | 4 |
| McInnes | 2021 | United States | RCT | 1 | 2 | 0 | 1 | 4 |
| McInnes | 2022 | United States | RCT | 2 | 1 | 0 | 1 | 4 |
| McInnes | 2023 | United Kingdom | RCT | 2 | 2 | 1 | 1 | 6 |
| Mease | 2020 | United States | RCT | 1 | 1 | 1 | 0 | 3 |
| Reich | 2022 | United States | RCT | 2 | 0 | 0 | 0 | 2 |
| Smolen | 2020 | Austria | RCT | 1 | 2 | 0 | 1 | 4 |
| Author | Year | Country | Study design | Selection | Comparability | Outcome | Total score | |
| Atteno | 2010 | Italy | Cohort study | ⟷⟷ | ⟷ | ⟷⟷ | 5 | |
| da Silva | 2022 | Brazil | Cohort study | ⟷⟷ | ⟷ | ⟷⟷ | 5 | |
| Eviatar | 2022 | Israel | Cohort study | ⟷⟷ | ⟷ | ⟷⟷ | 5 | |
| Izumiyama | 2021 | Japan | Cohort study | ⟷⟷ | ⟷ | ⟷⟷ | 5 | |
| Lindstrom | 2021 | Sweden | Cohort study | ⟷⟷ | ⟷⟷ | ⟷⟷ | 6 | |
| Strand | 2019 | United States | Cohort study | ⟷⟷⟷ | ⟷ | ⟷⟷⟷ | 7 | |
| Vegas | 2022 | France | Cohort study | ⟷⟷ | ⟷⟷ | ⟷⟷ | 6 | |
Quality assessment of the included studies.
The Jadad scale was adopted to evaluate the quality of RCTs from four dimensions: randomization, concealment of allocation, double blinding, and withdrawals and dropouts. For the quality assessment of cohort studies, the Newcastle-Ottawa scale (NOS) was employed and measured three sections: population selection, intergroup comparability, and outcome measurement.
RCT, randomized controlled trials.
Different biologics for the musculoskeletal endpoint
ACR 20
Eight studies (Strand et al., 2019; McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; Izumiyama et al., 2021; McInnes et al., 2021; Reich et al., 2022; McInnes et al., 2023) with 3,358 patients were eligible for ACR 20 evaluation, involving IL-17Ai, TNFi and JAKi biologics. TNFi and IL-17Ai were directly compared in more studies. TNFi were the most frequently used agent (Figure 2A). No significant differences were found in ACR 20 response among the TNFi, IL-17Ai and JAKi groups according to the forest plot (Figure 3A) and the league table (Table 3). The rank probabilities indicated that JAKi had the greatest possibility of achieving ACR 20 (68.79%), followed by IL-17Ai and TNFi (Table 4).
FIGURE 2
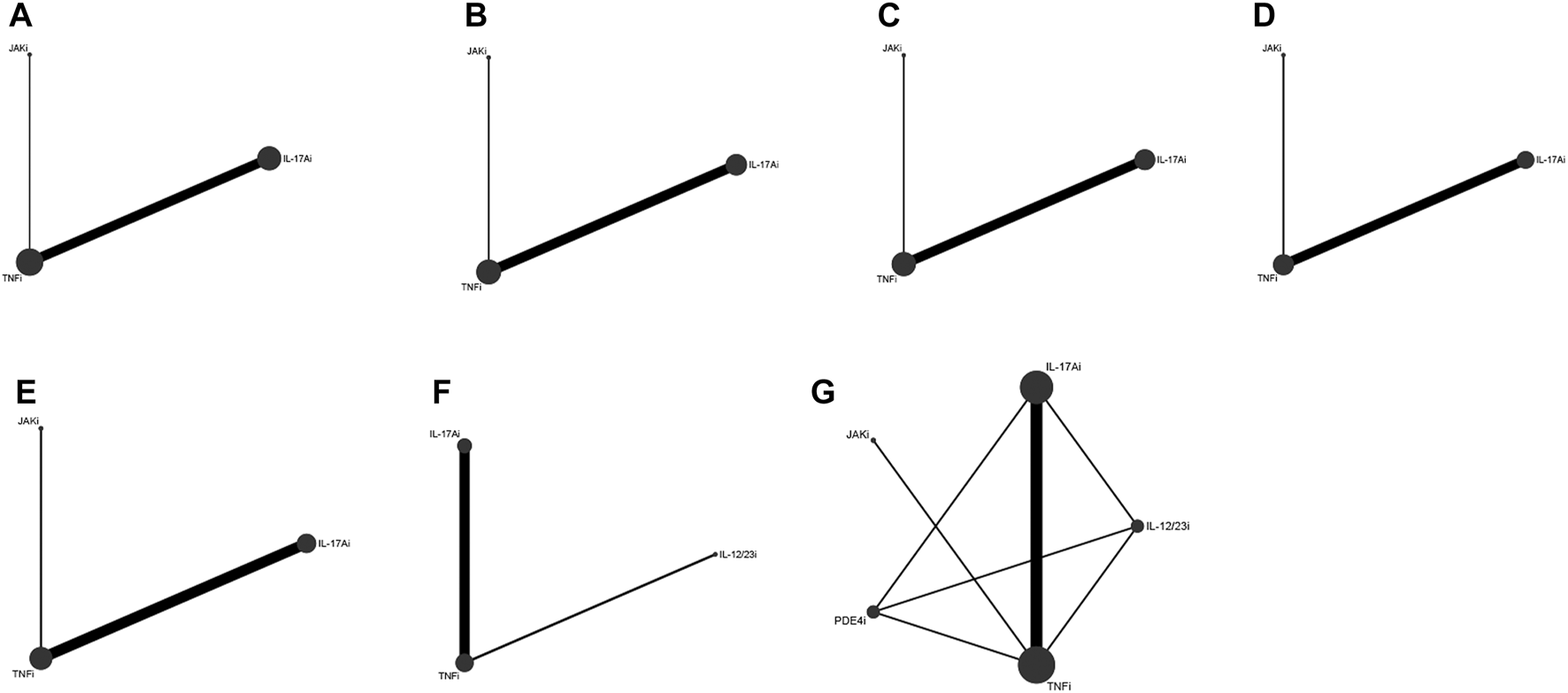
Network plots of various biologics for different outcomes in biological-naïve patients with PsA. (A), ACR 20; (B), ACR 50; (C), ACR 70; (D), resolution of enthesitis; (E), resolution of dactylitis; (F), HAQ-DI change; (G), adverse events. PsA, psoriatic arthritis; ACR, American College of Rheumatology; HAQ-DI, Health Assessment Questionnaire-Disability Index; TNFi, tumor necrosis factor inhibitors; IL-17Ai, interleukin-17A inhibitors; IL-12/23i, interleukin-12/23 inhibitors; PDE4i, phosphodiesterase-4 inhibitors; JAKi, Janus kinase inhibitors.
FIGURE 3
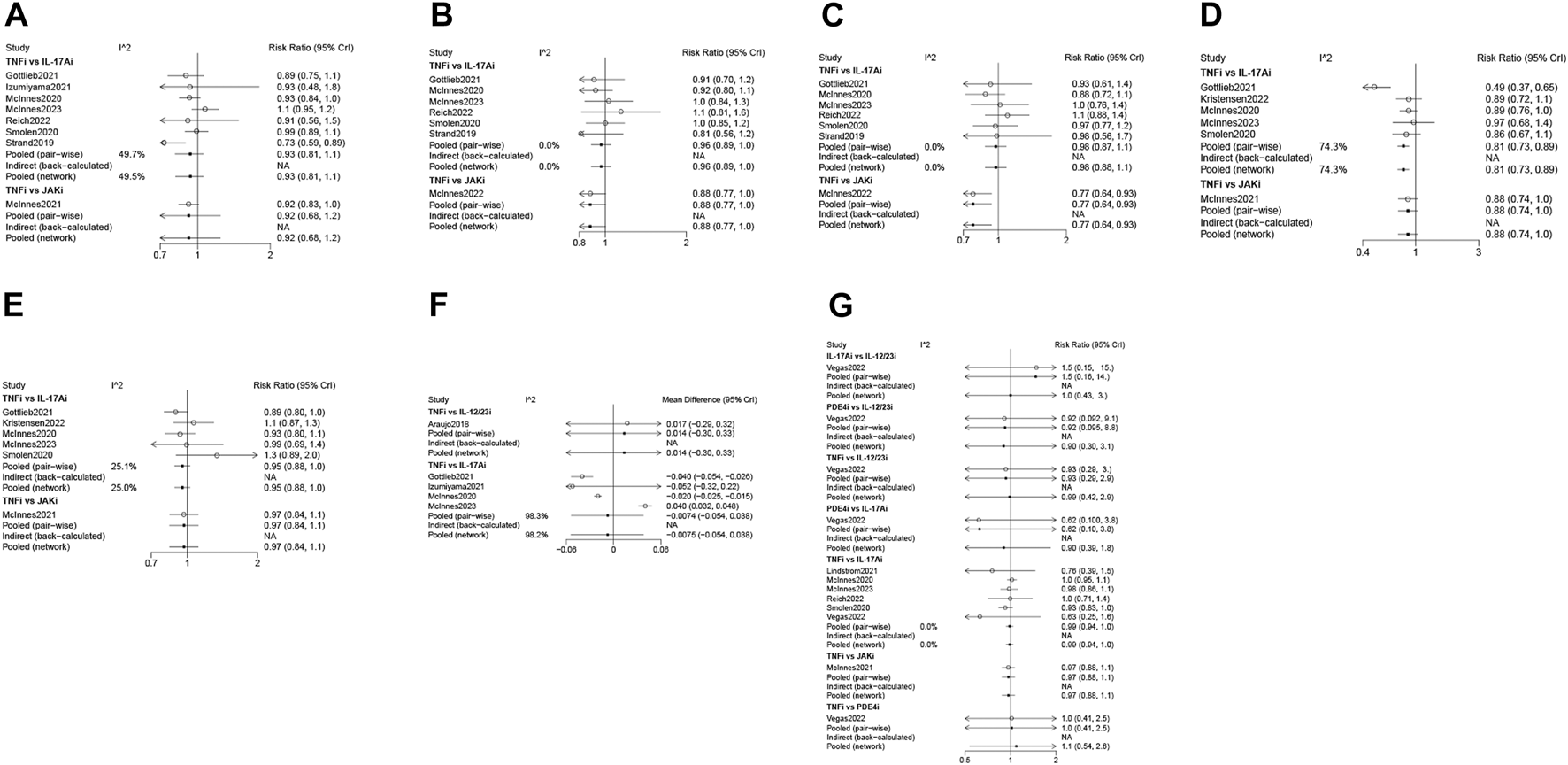
Forest plots of various biologics for different outcomes in biological-naïve patients with PsA. (A), ACR 20; (B), ACR 50; (C), ACR 70; (D), resolution of enthesitis; (E), resolution of dactylitis; (F), HAQ-DI change; (G), adverse events. PsA, psoriatic arthritis; ACR, American College of Rheumatology; HAQ-DI, Health Assessment Questionnaire-Disability Index; TNFi, tumor necrosis factor inhibitors; IL-17Ai, interleukin-17A inhibitors; IL-12/23i, interleukin-12/23 inhibitors; PDE4i, phosphodiesterase-4 inhibitors; JAKi, Janus kinase inhibitors; CrI, credibility interval.
TABLE 3
| ACR 20 [RR (95%CrI)] | ||||
|---|---|---|---|---|
| IL-17Ai | 1.03 (0.92, 1.15) | 0.95 (0.89, 1.00) | ||
| 0.97 (0.87, 1.09) | JAKi | 0.92 (0.83, 1.01) | ||
| 1.06 (1.00, 1.12) | 1.09 (0.99, 1.20) | TNFi | ||
| ACR 50 [RR (95%CrI)] | ||||
| IL-17Ai | 1.10 (0.94, 1.29) | 0.96 (0.89, 1.05) | ||
| 0.91 (0.78, 1.07) | JAKi | 0.88 (0.77, 1.00) | ||
| 1.04 (0.95, 1.13) | 1.14 (1.00, 1.30) | TNFi | ||
| ACR 70 [RR (95%CrI)] | ||||
| IL-17Ai | 1.26 (1.01, 1.57) | 0.98 (0.88, 1.09) | ||
| 0.79 (0.64, 0.99) | JAKi | 0.77 (0.64, 0.93) | ||
| 1.02 (0.92, 1.14) | 1.29 (1.07, 1.57) | TNFi | ||
| Resolution of enthesitis [RR (95%CrI)] | ||||
| IL-17Ai | 0.92 (0.76, 1.12) | 0.81 (0.73, 0.89) | ||
| 1.08 (0.89, 1.32) | JAKi | 0.88 (0.74, 1.04) | ||
| 1.23 (1.12, 1.36) | 1.14 (0.96, 1.35) | TNFi | ||
| Resolution of dactylitis [RR (95%CrI)] | ||||
| IL-17Ai | 0.98 (0.84, 1.16) | 0.95 (0.88, 1.03) | ||
| 1.02 (0.86, 1.19) | JAKi | 0.97 (0.84, 1.11) | ||
| 1.05 (0.97, 1.13) | 1.03 (0.90, 1.19) | TNFi | ||
| HAQ-DI change [WMD (95%CrI)] | ||||
| IL-12/23i | 0.02 (−0.30, 0.34) | 0.02 (−0.30, 0.33) | ||
| −0.02 (−0.34, 0.30) | IL-17Ai | −0.01 (−0.05, 0.04) | ||
| 0.02 (−0.33, 0.30) | 0.01 (−0.04, 0.05) | TNFi | ||
| Adverse events [RR (95%CrI)] | ||||
| IL-12/23i | 1.01 (0.43, 2.96) | 1.03 (0.44, 3.02) | 0.91 (0.30, 3.04) | 0.99 (0.43, 2.92) |
| 0.99 (0.34, 2.31) | IL-17Ai | 1.02 (0.91, 1.14) | 0.90 (0.38, 1.84) | 0.99 (0.94, 1.04) |
| 0.97 (0.33, 2.28) | 0.98 (0.88, 1.10) | JAKi | 0.88 (0.38, 1.82) | 0.97 (0.88, 1.07) |
| 1.10 (0.33, 3.34) | 1.11 (0.54, 2.60) | 1.13 (0.55, 2.66) | PDE4i | 1.09 (0.54, 2.57) |
| 1.01 (0.34, 2.35) | 1.01 (0.96, 1.07) | 1.03 (0.94, 1.14) | 0.91 (0.39, 1.86) | TNFi |
League table of various biologics for different outcomes in biological-naïve patients with PsA.
Note: The data on the upper right (with the diagonal line as the dividing line) were used. These data were presented with the agent on the left as the reference group and the agent on the lower as the intervention group. For example, 1.03 (0.92, 1.15) indicated the RR and 95%CrI of the JAKi group versus the IL-17Ai group.
PsA, psoriatic arthritis; ACR, american college of rheumatology; HAQ-DI, Health Assessment Questionnaire-Disability Index; TNFi, tumor necrosis factor inhibitors; IL-17Ai, interleukin-17A inhibitors; IL-12/23i, interleukin-12/23 inhibitors; PDE4i, phosphodiesterase-4 inhibitors; JAKi, Janus kinase inhibitors; WMD, weighted mean difference; RR, risk ratio; CrI, credibility interval.
TABLE 4
| ACR 20 | |||||
|---|---|---|---|---|---|
| Ranking first | Ranking second | Ranking third | |||
| IL-17Ai | 0.310970 | 0.661085 | 0.027945 | ||
| JAKi | 0.687935 | 0.271005 | 0.041060 | ||
| TNFi | 0.001095 | 0.067910 | 0.930995 | ||
| ACR 50 | |||||
| Ranking first | Ranking second | Ranking third | |||
| IL-17Ai | 0.119795 | 0.678310 | 0.201895 | ||
| JAKi | 0.874495 | 0.100485 | 0.025020 | ||
| TNFi | 0.005710 | 0.221205 | 0.773085 | ||
| ACR 70 | |||||
| Ranking first | Ranking second | Ranking third | |||
| IL-17Ai | 0.018030 | 0.654235 | 0.327735 | ||
| JAKi | 0.980615 | 0.016120 | 0.003265 | ||
| TNFi | 0.001355 | 0.329645 | 0.669000 | ||
| Resolution of enthesitis (LEI = 0) | |||||
| Ranking first | Ranking second | Ranking third | |||
| IL-17Ai | 0.788715 | 0.21128 | 0.000005 | ||
| JAKi | 0.211285 | 0.72443 | 0.064285 | ||
| TNFi | 0.000000 | 0.06429 | 0.935710 | ||
| Resolution of dactylitis (LDI-B = 0) | |||||
| Ranking first | Ranking second | Ranking third | |||
| IL-17Ai | 0.554005 | 0.368845 | 0.07715 | ||
| JAKi | 0.412945 | 0.273585 | 0.31347 | ||
| TNFi | 0.033050 | 0.357570 | 0.60938 | ||
| HAQ-DI change | |||||
| Ranking first | Ranking second | Ranking third | |||
| IL-12/23i | 0.40363 | 0.045975 | 0.524465 | ||
| IL-17Ai | 0.596365 | 0.443720 | 0.181825 | ||
| TNFi | 0.000005 | 0.510305 | 0.293710 | ||
| Adverse events | |||||
| Ranking first | Ranking second | Ranking third | Ranking fourth | Ranking fifth | |
| IL-12/23i | 0.394440 | 0.091285 | 0.021970 | 0.149435 | 0.342870 |
| IL-17Ai | 0.110960 | 0.287290 | 0.333315 | 0.206310 | 0.062125 |
| JAKi | 0.227275 | 0.319630 | 0.251960 | 0.142000 | 0.059135 |
| PDE4i | 0.242450 | 0.138225 | 0.027840 | 0.173755 | 0.417730 |
| TNFi | 0.024875 | 0.163570 | 0.364915 | 0.328500 | 0.118140 |
Rank probabilities of various biologics for different outcomes in biological-naïve patients with PsA.
Note: The data indicated the probability of different biologics ranking at a certain position (e.g., ranking first, second, third).
PsA, psoriatic arthritis; ACR, american college of rheumatology; HAQ-DI, Health Assessment Questionnaire-Disability Index; TNFi, tumor necrosis factor inhibitors; IL-17Ai, interleukin-17A inhibitors; IL-12/23i, interleukin-12/23 inhibitors; PDE4i, phosphodiesterase-4 inhibitors; JAKi, Janus kinase inhibitors; LEI, leeds enthesitis index; LDI-B, Leeds Dactylitis Index-Basic.
ACR 50
ACR 50 was measured by 7 studies (Strand et al., 2019; McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; McInnes et al., 2022; Reich et al., 2022; McInnes et al., 2023) including 3,333 patients. IL-17Ai, TNFi and JAKi biologics were compared. More studies made direct comparison between TNFi and IL-17Ai, and more patients used TNFi (Figure 2B). Based on the forest plot (Figure 3B) and league table (Table 3), patients receiving IL-17Ai, TNFi and JAKi had similar ACR 50. The rank probabilities showed that JAKi were most likely to achieve ACR 50 (87.45%), followed by IL-17Ai and TNFi (Table 4).
ACR 70
Seven studies (Strand et al., 2019; McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; McInnes et al., 2022; Reich et al., 2022; McInnes et al., 2023) of 3,333 patients provided data on ACR 70, and IL-17Ai, TNFi and JAKi biologics were involved. More studies directly compared TNFi and IL-17Ai. TNFi were the most commonly used agent (Figure 2C). The forest plot found that compared with JAKi, TNFi were associated with a significantly lower rate of achieving ACR 70 response (pooled RR = 0.77, 95%CrI: 0.64, 0.93) (Figure 3C). The JAKi group had a significantly higher rate of achieving ACR 70 response than the IL-17Ai group (pooled RR = 1.26, 95%CrI: 1.01, 1.57); TNFi were less effective than JAKi in terms of ACR 70 (pooled RR = 0.77, 95%CrI: 0.64, 0.93), as demonstrated by the league table (Table 3). According to the rank probabilities, ACR 70 was most likely to be achieved in patients using JAKi (97.48%), followed by those using IL-17Ai and TNFi (Table 4).
Resolution of enthesitis
SPARCC
Three studies (McInnes et al., 2020; Smolen et al., 2020; Kristensen et al., 2022) involving 1,491 patients evaluated the role of IL-17Ai and TNFi biologics for enthesitis resolution defined by SPARCC = 0. As illustrated by pooled analysis, the IL-17Ai group had a significantly higher rate of enthesitis resolution than the TNFi group (pooled RR = 1.22, 95%CI: 1.02, 1.47, p = 0.032).
LEI
Enthesitis resolution defined by LEI = 0 was assessed by 6 studies (McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; McInnes et al., 2021; Kristensen et al., 2022; McInnes et al., 2023) in 2,633 patients who were treated with IL-17Ai, TNFi and JAKi biologics. More studies directly compared TNFi and IL-17Ai, and TNFi were the most commonly applied agent (Figure 2D). The forest plot (Figure 3D) and the league table (Table 3) showed that the rate of enthesitis resolution was significantly lower in the TNFi group than that in the IL-17Ai group (pooled RR = 0.81, 95%CrI: 0.73, 0.89). From the rank probabilities, patients receiving IL-17Ai were most likely to have enthesitis resolution (78.87%), followed by those receiving JAKi and TNFi (Table 4).
Resolution of dactylitis
LDI-B
Six studies (McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; McInnes et al., 2021; Kristensen et al., 2022; McInnes et al., 2023) compared IL-17Ai, TNFi and JAKi biologics for dactylitis resolution defined by LDI-B = 0 in 1950 patients. The direct comparison of TNFi and IL-17Ai was found in more studies, and the most patients used TNFi (Figure 2E). No significant differences were found in dactylitis resolution among IL-17Ai, TNFi and JAKi, according to the forest plot (Figure 3E) and league table (Table 3). The rank probabilities suggested that IL-17Ai had the greatest likelihood to achieve the resolution of dactylitis (55.40%), followed by JAKi and TNFi (Table 4).
Different biologics for the function endpoint
HAQ-DI change
Five studies (Araujo et al., 2019; McInnes et al., 2020; Gottlieb et al., 2021; Izumiyama et al., 2021; McInnes et al., 2023) with 1,535 patients were quantified for HAQ-DI change assessment, involving IL-17Ai, TNFi and IL-12/23i biologics. TNFi and IL-17Ai were directly compared in more studies. TNFi were the most frequently used agent (Figure 2F). No significant differences were observed in HAQ-DI change among IL-17Ai, TNFi and IL-12/23i according to the forest plot (Figure 3F) and league table (Table 3). The rank probabilities indicated that patients receiving IL-17Ai had the highest likelihood of achieving the greatest HAQ-DI change (59.64%), followed by those receiving TNFi and IL-12/23i (Table 4).
∆ HAQ-DI ≥ 0.35
The condition of ∆ HAQ-DI ≥ 0.35 was assessed in 5 studies (McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021; Kristensen et al., 2022; McInnes et al., 2023) including 2,617 patients, and IL-17Ai and TNFi biologics were compared. Pooled analysis showed that IL-17Ai had a similar impact in achieving ∆ HAQ-DI ≥ 0.35 to TNFi [pooled RR = 1.15, 95% confidence interval (CI): 0.93, 1.41, p = 0.194].
Different biologics for the composite index endpoint
ACR 50 + PASI 100
As for combined ACR 50 and PASI 100 response, 3 studies (McInnes et al., 2020; Smolen et al., 2020; Gottlieb et al., 2021) with 1,194 patients were included for comparison of IL-17Ai and TNFi biologics. Pooled analysis demonstrated that patients receiving IL-17Ai had a significantly higher rate of achieving combined ACR 50 and PASI 100 response than those receiving TNFi (pooled RR = 1.56, 95%CI: 1.29, 1.88, p < 0.001).
Different biologics for adverse events
Seven studies (McInnes et al., 2020; Smolen et al., 2020; Lindström et al., 2021; McInnes et al., 2021; Pina Vegas et al., 2022; Reich et al., 2022; McInnes et al., 2023) with 15,087 patients provided data on adverse events, involving IL-17Ai, TNFi, JAKi, IL-12/23i and PDE4i biologics. There were more studies directly comparing TNFi and IL-17Ai, and more patients receiving TNFi (Figure 2G). The incidences of adverse events were comparable among these biologics based on the forest plot (Figure 3G) and league table (Table 3). The rank probabilities showed that patients receiving PDE4i were least likely to have adverse events (41.77%), following by IL-17Ai, TNFi, JAKi, and IL-12/23i (Table 4).
According to the study of McInnes et al. (2021), the incidence of serious adverse events was higher in patients receiving 30-mg dose of upadacitinib versus those receiving adalimumab and 15-mg dose of upadacitinib (6.1% vs. 3.7% and 3.1%). The adalimumab group exhibited more serious adverse events than the ixekizumab group (9.8% vs. 0.0%) (Reich et al., 2022). By week 24, 17 (4%) patients in the bimekizumab group and five (4%) patients in the adalimumab group at baseline had serious adverse events (McInnes et al., 2023).
Different biologics for the arthritis activity endpoint
Araujo et al. (2019) randomized patients to receive IL-12/23i and TNFi in a 1:1 ratio. After 24 weeks of follow-up, DAS28 scores were compared between the IL-12/23i and TNFi groups, and no statistical difference was found between the two groups (p > 0.05).
Different biologics for the skin endpoint
According to the study of Araujo et al. (2019), PASI 100 was found in 59% of patients treated with ustekinumab and 29% of those treated with TNFi (p = 0.039), while 86% of patients receiving ustekinumab and 29% of patients receiving TNFi showed PASI 90 (p = 0.0001). Atteno et al. (2010) compared the efficacy of three kinds of TNFi (etanercept, adalimumab, and infliximab), and the difference in PASI was statistically significant at 1 year of follow-up (p < 0.01). Compared with patients receiving etanercept, those receiving adalimumab (p < 0.01) and infliximab (p < 0.001) showed greater improvement in the expansion of psoriasis rash.
Different biologics for drug retention
Eviatar et al. (2022) reported that as a first-line treatment, the drug retention of secukinumab (IL-17Ai) was similar to that of other TNFi, except for the poor drug retention of golimumab. Three years later, 76% of patients still used secukinumab, while 50% of patients used etanercept, 52% used infliximab, 56% used adalimumab, and 34% used golimumab. Lindström et al. (2021) showed that the 1-year treatment retention rates of secukinumab and adalimumab were similar. However, there were some differences between different TNFi, and the retention rates of infliximab and certolizumab pegol showed a decreasing trend.
Discussion
To the best of our knowledge, the current network meta-analysis was the first to compare and rank TNFi, IL-17Ai, IL-12/23i, PDE4i, and JAKi biologics based on their efficacy and safety in biological-naïve patients with PsA. With 16 studies included, it was found that JAKi had the highest probability of achieving ACR 20/50/70 response, IL-17Ai were most likely to realize the resolution of enthesitis and dactylitis and the greatest HAQ-DI change and had a higher rate of achieving combined ACR 50 and PASI 100 response than TNFi, and patients receiving PDE4i were least likely to have adverse events. Taking into account both efficacy and safety, IL-17Ai may be the better biological agent for PsA treatment. These findings may facilitate understating of different categories of biologics, and assist in clinical decision-making for the treatment of biological-naïve patients with PsA.
Many network meta-analyses have been conducted to explore the efficacy and safety of specific biologics in PsA over the past 20 years or so (Migliore et al., 2021). Recently, infliximab, guselkumab, adalimumab, golimumab, secukinumab, and ustekinumab were shown by a network meta-analysis to be possibly safer and more effective than other targeted DMARDs in induction therapy of active PsA (Lu et al., 2019). Ruyssen-Witrand et al. (2020) also compared specific biologics in PsA via a network meta-analysis, and illustrated that infliximab was superior to other biologics for ACR and PASI response. Another network meta-analysis focused on comparison of 14 small-molecule biologics for PsA patients (Qiu et al., 2020), and reported that golimumab, etanercept and infliximab could be the optimum agents in terms of efficacy and safety. These network meta-analyses incorporated both biological-naïve and biological-experienced patients, and used placebo as the common comparator. This network meta-analysis exclusively paid attention to PsA patients who were naïve to biologics and compare broad categories of biologics (TNFi, IL-17Ai, IL-12/23i, PDE4i, and JAKi) as regards efficacy and safety. Of note, we only included studies with head-to-head comparisons of these biologics since the evidence strength of direct comparison is greater than that of indirect comparison. Hence, studies with biologics versus placebo or methotrexate (MTX) alone were not included for the current analysis.
In terms of efficacy, the musculoskeletal endpoint (ACR 20/50/70, resolution of enthesitis, resolution of dactylitis), function endpoint (HAQ-DI change, ∆ HAQ-DI ≥ 0.35), and composite index endpoint (ACR 50 + PASI 100) were quantitatively assessed. JAKi were identified to have the highest likelihood of achieving ACR 20/50/70 response, followed by IL-17Ai in biological-naïve patients. JAKi are the latest drug class of disease-modifying medication to emerge for the treatment of rheumatoid arthritis (RA). Recent evidence has provided support for the effectiveness of JAKi regarding ACR 20/50/70 (Harkins et al., 2023; Lee and Song, 2023). The JAK family, including JAKi, JAK2, JAK3, and tyrosine kinase (TYK) 2, is related to signal transducers and activators of transcription (STAT) and serves as a crucial role in mediating downstream signaling of many important pro-inflammatory cytokines involved in the pathogenesis of PsA (Kvist-Hansen et al., 2020; Crispino and Ciccia, 2021). The biological agent JAKi cause suppression of the JAK/STAT pathway, and adjusts several inflammatory pathways via influencing various cytokines, which improve clinical manifestations, thus enhancing ACR response (Jamilloux et al., 2019; El Jammal et al., 2020). JAKi also exhibit rapid onset of action, role in reducing central pain, and impact on structural damage (Harrington et al., 2020). IL-17Ai were reported to inhibit disease activity associated with the skin, joints and entheses in spondyloarthritides including PsA (McGonagle et al., 2019), which may relate to the positive role of IL-17Ai in ACR 20/50/70 response. In addition, IL-17Ai were most likely to achieve the resolution of enthesitis and dactylitis, as shown in this paper, which suggested that IL-17Ai may be beneficial for relieving the musculoskeletal symptoms of PsA. As for the other key domains for PsA, peripheral arthritis, axial disease and skin and nail psoriasis, relevant information was missing in the included studies. A recent review showed that many therapeutic options, including IL-17Ai, IL-12/23i and JAKi, had similar effects on peripheral arthritis in patients with PsA (Ayan et al., 2023). Lubrano et al. (2023) reported that IL-17Ai and JAKi could be applied for axial disease treatment in PsA. Besides, among 14% of patients, peripheral arthritis, skin disease and nail psoriasis are the most common combination of PsA domains, and IL-17 inhibitors exhibited effectiveness across all domains (Mease et al., 2023). Future studies should pay more attention to these PsA domains and improve reporting of corresponding data. With respect to the function endpoint, patients receiving IL-17Ai were most likely to obtain the greatest HAQ-DI change, although no significant difference was found among involved IL-17Ai, TNFi and IL-12/23i biologics. According to previous reviews, ixekizumab relieved joint symptoms, improves function, and impede development of structural damage in PsA (Toussirot, 2018), and individuals with PsA had enhanced physical function and health-related quality of life after secukinumab usage (Blair, 2021). Secukinumab was well tolerated in general (Blair, 2021). For the endpoint ACR 50 + PASI 100 response considering both musculoskeletal and skin manifestations, IL-17Ai were superior to TNFi, indicating that IL-17Ai may have comprehensive control of PsA in biological-naïve patients. However, no other biologics were assessed in this endpoint, which necessitates future studies to investigate more biologics for simultaneous ACR 50 and PASI 100 response in PsA.
In terms of safety, PDE4i had the lowest probability to cause adverse events among the 5 kinds of biologics despite no significant difference observed among IL-17Ai, TNFi, JAKi, IL-12/23i and PDE4i biologics. PDE4i including apremilast blocks PDE4 enzyme and elevates the levels of intracellular cyclic adenosine monophosphate (cAMP), leading to downregulated inflammatory reactions through suppressing IL-17, interferon-γ, TNF, and so forth (Nassim et al., 2020; Picchianti-Diamanti et al., 2021), which may explain the potential adventage of PDE4i over other biologics. To be noted, merely apremilast was evaluated as a representative of PDE4i, the information of which was provided by one qualified study in the current network meta-analysis. Consistently, the safety of apremilast has been identified in existing research (Mease, 2014; Qu et al., 2016; Kang et al., 2022). The reporting of PDE4i′s efficacy in the future should also be improved for inclusion to facilitate comprehensive assessment. Concerning serious adverse events, McInnes et al. (2021) and Reich et al. (2022) reported the higher incidences of serious adverse events in patients receiving upadacitinib (30-mg dose) and adalimumab, respectively. According to European Medicine Agency (EMA) and US Food and Drug Administration (FDA) recent warnings, JAKi were associated with increased risks of major adverse cardiac events, cancer, venous thromboembolic events, severe infections, and death (Kragstrup et al., 2022; Philippoteaux et al., 2022), and clinicians needs to carefully consider risk factors and assess corresponding risks of individual patients before use of JAKi. For the arthritis activity endpoint, skin endpoint, and drug retention, qualitative descriptions were provided since unsynthesizable data from the included studies. Studies should adopt standardized reporting and a great number of investigations are required for these outcomes.
Based on the head-to-head evidence of different biologics for both efficacy and safety, IL-17Ai may be the most favorable treatment option to improve musculoskeletal, skin and function outcomes with few adverse effects for biological-naïve patients with PsA. Several limitations should be mentioned in interpreting the results. Firstly, the dosage and course of treatment of same biologics may vary in different included studies, possibly leading to increased heterogeneity between studies. Some data on TNFi were collected more than 15 years ago when PsA was less known and biologic treated disease severity was higher as compared to more recent trials, which may also increase heterogeneity. Besides, some patients in the included studies had comorbidities, such as diabetes, chronic obstructive pulmonary disease, asthma, emotional disorders, etc., which may affect the treatment results. Secondly, other key efficacy domains for PSA (axial disease, skin nails and related conditions) (Coates et al., 2022) were not covered by the current paper because no relevant data were provided by the included studies, which necessitates future studies to explore the effect of biologics on these outcomes. As shown in Table 1, only five studies reported the combination therapy with MTX alone (in some or all patients), two studies reported the combination therapy with MTX or other csDMARDs (data on MTX could not be distinguished from data on other csDMARDs), the remaining nine studies did not report relevant information on MTX. Thus, the clear separation of treatments with biologics combined with and not combined with MTX could not be achieved. Thirdly, some outcomes were evaluated by limited literature, and a small sample size may influence the stability of the results. There were relatively limited safety data and population sizes by biologics in the original papers, which requires more patients in a group and with long-term follow up to enrich safety data in future research. Additionally, some outcomes could only be qualitatively described. Finally, due to the lack of relevant information on sponsor-supported open-label extensions (OLEs) and non-sponsored OLEs, sponsor-supported OLEs and non-sponsored OLEs could not be split. Studies should improve their reporting of these aspects to promote deeper comprehensive research.
Conclusion
In 2023, JAKi had the highest probability of achieving ACR 20/50/70 response, and IL-17Ai were most likely to realize the resolution of enthesitis and dactylitis and the greatest HAQ-DI change and had a higher rate of combined ACR 50 and PASI 100 response. Patients receiving PDE4i were least likely to have adverse events, despite no significant difference among different biologics. Considering both efficacy and safety, IL-17Ai may be the better option for biological-naïve patients with PsA requiring biological therapy. Future studies are warranted for validation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing–original draft, Writing–review and editing. YR: Data curation, Formal Analysis, Investigation, Software, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Araujo E. G. Englbrecht M. Hoepken S. Finzel S. Kampylafka E. Kleyer A. et al (2019). Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin. Arthritis Rheum.48 (4), 632–637. 10.1016/j.semarthrit.2018.05.011
2
Atteno M. Peluso R. Costa L. Padula S. Iervolino S. Caso F. et al (2010). Comparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugs. Clin. Rheumatol.29 (4), 399–403. 10.1007/s10067-009-1340-7
3
Ayan G. Ribeiro A. Macit B. Proft F. (2023). Pharmacologic treatment strategies in psoriatic arthritis. Clin. Ther.45 (9), 826–840. 10.1016/j.clinthera.2023.05.010
4
Blair H. A. (2021). Secukinumab: a review in psoriatic arthritis. Drugs81 (4), 483–494. 10.1007/s40265-021-01476-3
5
Coates L. C. Soriano E. R. Corp N. Bertheussen H. Callis Duffin K. Campanholo C. B. et al (2022). Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol.18 (8), 465–479. 10.1038/s41584-022-00798-0
6
Crispino N. Ciccia F. (2021). JAK/STAT pathway and nociceptive cytokine signalling in rheumatoid arthritis and psoriatic arthritis. Clin. Exp. Rheumatol.39 (3), 668–675. 10.55563/clinexprheumatol/e7ayu8
7
D'Angiolella L. S. Cortesi P. A. Lafranconi A. Micale M. Mangano S. Cesana G. et al (2018). Cost and cost effectiveness of treatments for psoriatic arthritis: a systematic literature review. Pharmacoeconomics36 (5), 567–589. 10.1007/s40273-018-0618-5
8
El Jammal T. Gerfaud-Valentin M. Sève P. Jamilloux Y. (2020). Inhibition of JAK/STAT signaling in rheumatologic disorders: the expanding spectrum. Jt. Bone Spine87 (2), 119–129. 10.1016/j.jbspin.2019.09.005
9
Eviatar T. Zisman D. Gendelman O. Reitblat T. Balbir-Gurman A. Mashiach T. et al (2022). Secukinumab real world drug retention compared to TNF-alpha inhibitors in psoriatic arthritis. Clin. Exp. Rheumatol.40 (1), 15–23. 10.55563/clinexprheumatol/1sx5yk
10
Gottlieb A. Merola J. F. (2020). Psoriatic arthritis for dermatologists. J. Dermatol. Treat.31 (7), 662–679. 10.1080/09546634.2019.1605142
11
Gottlieb A. B. Merola J. F. Reich K. Behrens F. Nash P. Griffiths C. E. M. et al (2021). Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study. Br. J. Dermatol185 (6), 1124–1134. 10.1111/bjd.20413
12
Gudu T. Gossec L. (2018). Quality of life in psoriatic arthritis. Expert Rev. Clin. Immunol.14 (5), 405–417. 10.1080/1744666x.2018.1468252
13
Harkins P. Burke E. Swales C. Silman A. Conway R. (2023). Are Janus kinase inhibitors safe and effective in treating the key clinical domains of psoriatic arthritis? A systematic review and meta-analysis. Int. J. Rheum. Dis.26 (1), 31–42. 10.1111/1756-185x.14447
14
Harrington R. Al Nokhatha S. A. Conway R. (2020). JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J. Inflamm. Res.13, 519–531. 10.2147/jir.S219586
15
Hutton B. Salanti G. Caldwell D. M. Chaimani A. Schmid C. H. Cameron C. et al (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med.162 (11), 777–784. 10.7326/m14-2385
16
Izumiyama T. Mori Y. Oizumi I. Hamada S. Kurishima H. Terui H. et al (2021). Effect of interleukin-17A inhibitor in Japanese patients with psoriatic arthritis compared with tumor necrosis factor-alpha inhibitor. J. Orthop. Surg. Hong. Kong29 (2), 23094990211012286. 10.1177/23094990211012286
17
Jadad A. R. Moore R. A. Carroll D. Jenkinson C. Reynolds D. J. Gavaghan D. J. et al (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin. Trials17 (1), 1–12. 10.1016/0197-2456(95)00134-4
18
Jamilloux Y. El Jammal T. Vuitton L. Gerfaud-Valentin M. Kerever S. Sève P. (2019). JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev.18 (11), 102390. 10.1016/j.autrev.2019.102390
19
Kamata M. Tada Y. (2020). Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int. J. Mol. Sci.21 (5), 1690. 10.3390/ijms21051690
20
Kang Q. Chen J. S. Yang H. (2022). Efficacy and safety profile of phosphodiesterase 4 inhibitor in the treatment of psoriasis: a systematic review and meta-analysis of randomized controlled trials. Front. Immunol.13, 1021537. 10.3389/fimmu.2022.1021537
21
Kishimoto M. Deshpande G. A. Fukuoka K. Kawakami T. Ikegaya N. Kawashima S. et al (2021). Clinical features of psoriatic arthritis. Best. Pract. Res. Clin. Rheumatol.35 (2), 101670. 10.1016/j.berh.2021.101670
22
Kragstrup T. W. Glintborg B. Svensson A. L. McMaster C. Robinson P. C. Deleuran B. et al (2022). Waiting for JAK inhibitor safety data. RMD open8 (1), e002236. 10.1136/rmdopen-2022-002236
23
Kristensen L. E. Okada M. Tillett W. Leage S. L. El Baou C. Sapin C. et al (2022). Ixekizumab demonstrates consistent efficacy versus adalimumab in biologic disease-modifying anti-rheumatic drug-naïve psoriatic arthritis patients regardless of psoriasis severity: 52-week post hoc results from SPIRIT-H2H. Rheumatol. Ther.9 (1), 109–125. 10.1007/s40744-021-00388-8
24
Kvist-Hansen A. Hansen P. R. Skov L. (2020). Systemic treatment of psoriasis with JAK inhibitors: a review. Dermatol Ther. (Heidelb)10 (1), 29–42. 10.1007/s13555-019-00347-w
25
Lee Y. H. Song G. G. (2023). Relative efficacy and safety of Janus kinase inhibitors for the treatment of active psoriatic arthritis: a network meta-analysis. Z Rheumatol.82 (5), 408–416. 10.1007/s00393-021-01119-8
26
Lindström U. Glintborg B. Di Giuseppe D. Schjødt Jørgensen T. Gudbjornsson B. Lederballe Grøn K. et al (2021). Comparison of treatment retention and response to secukinumab versus tumour necrosis factor inhibitors in psoriatic arthritis. Rheumatol. Oxf.60 (8), 3635–3645. 10.1093/rheumatology/keaa825
27
López-Ferrer A. Laiz A. Puig L. (2022). Psoriatic arthritis. Med. Clin. Barc.159 (1), 40–46. 10.1016/j.medcli.2022.01.024
28
Lu C. Wallace B. I. Waljee A. K. Fu W. Zhang Q. Liu Y. (2019). Comparative efficacy and safety of targeted DMARDs for active psoriatic arthritis during induction therapy: a systematic review and network meta-analysis. Semin. Arthritis Rheum.49 (3), 381–388. 10.1016/j.semarthrit.2019.06.001
29
Lubrano E. Chan J. Queiro-Silva R. Cauli A. Goel N. Poddubnyy D. et al (2023). Management of axial disease in patients with psoriatic arthritis: an updated literature review informing the 2021 GRAPPA treatment recommendations. J. Rheumatol.50 (2), 279–284. 10.3899/jrheum.220309
30
McGonagle D. G. McInnes I. B. Kirkham B. W. Sherlock J. Moots R. (2019). The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann. Rheum. Dis.78 (9), 1167–1178. 10.1136/annrheumdis-2019-215356
31
McInnes I. B. Anderson J. K. Magrey M. Merola J. F. Liu Y. Kishimoto M. et al (2021). Trial of upadacitinib and adalimumab for psoriatic arthritis. N. Engl. J. Med.384 (13), 1227–1239. 10.1056/NEJMoa2022516
32
McInnes I. B. Asahina A. Coates L. C. Landewé R. Merola J. F. Ritchlin C. T. et al (2023). Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet401 (10370), 25–37. 10.1016/S0140-6736(22)02302-9
33
McInnes I. B. Behrens F. Mease P. J. Kavanaugh A. Ritchlin C. Nash P. et al (2020). Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet395 (10235), 1496–1505. 10.1016/s0140-6736(20)30564-x
34
McInnes I. B. Kato K. Magrey M. Merola J. F. Kishimoto M. Haaland D. et al (2022). Efficacy and safety of upadacitinib in patients with psoriatic arthritis: 2-year results from the phase 3 SELECT-PsA 1 study. Rheumatol. Ther.10 (1), 275–292. 10.1007/s40744-022-00499-w
35
Mease P. J. (2014). Apremilast: a phosphodiesterase 4 inhibitor for the treatment of psoriatic arthritis. Rheumatol. Ther.1 (1), 1–20. 10.1007/s40744-014-0005-4
36
Mease P. J. O'Brien J. Middaugh N. Kricorian G. Stryker S. Collier D. H. et al (2023). Real-World evidence assessing psoriatic arthritis by disease domain: an evaluation of the CorEvitas psoriatic arthritis/spondyloarthritis registry. ACR Open Rheumatol.5 (8), 388–398. 10.1002/acr2.11556
37
Mease P. J. Smolen J. S. Behrens F. Nash P. Liu Leage S. Li L. et al (2020). A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann. Rheum. Dis.79 (1), 123–131. 10.1136/annrheumdis-2019-215386
38
Migliore A. Gigliucci G. Birra D. Saporito R. Cicerchia L. Massafra U. et al (2021). Biologics for psoriatic arthritis: network meta-analysis in review. Eur. Rev. Med. Pharmacol. Sci.25 (18), 5755–5765. 10.26355/eurrev_202109_26793
39
Nassim D. Alajmi A. Jfri A. Pehr K. (2020). Apremilast in dermatology: a review of literature. Dermatol Ther.33 (6), e14261. 10.1111/dth.14261
40
Ocampo D. V. Gladman D. (2019). Psoriatic arthritis. F1000Res 88, F1000 Faculty Rev-1665. 10.12688/f1000research.19144.1
41
Philippoteaux C. Deprez V. Nottez A. Cailliau E. Houvenagel E. Deprez X. et al (2022). Characteristics of patients treated with JAK inhibitors in rheumatoid arthritis before versus after VTE risk warnings. J. Clin. Med.12 (1), 207. 10.3390/jcm12010207
42
Picchianti-Diamanti A. Spinelli F. R. Rosado M. M. Conti F. Laganà B. (2021). Inhibition of phosphodiesterase-4 in psoriatic arthritis and inflammatory bowel diseases. Int. J. Mol. Sci.22 (5), 2638. 10.3390/ijms22052638
43
Pina Vegas L. Le Corvoisier P. Penso L. Paul M. Sbidian E. Claudepierre P. (2022). Epidemiologic study of patients with psoriatic arthritis in a real-world analysis: a cohort study of the French health insurance database. Rheumatol. Oxf.61 (4), 1243–1251. 10.1093/rheumatology/keaa448
44
Qiu M. Xu Z. Gao W. Xiong M. Wen X. Zhu W. et al (2020). Fourteen small molecule and biological agents for psoriatic arthritis: a network meta-analysis of randomized controlled trials. Med. Baltim.99 (31), e21447. 10.1097/md.0000000000021447
45
Qu X. Zhang S. Tao L. Song Y. (2016). A meta-analysis of apremilast on psoriatic arthritis long-term assessment of clinical efficacy (PALACE). Expert Rev. Clin. Pharmacol.9 (6), 799–805. 10.1586/17512433.2016.1159130
46
Raychaudhuri S. P. Wilken R. Sukhov A. C. Raychaudhuri S. K. Maverakis E. (2017). Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J. Autoimmun.76, 21–37. 10.1016/j.jaut.2016.10.009
47
Reich K. Kristensen L. E. Smith S. D. Rich P. Sapin C. Leage S. L. et al (2022). Efficacy and safety of ixekizumab versus adalimumab in biologic-naïve patients with active psoriatic arthritis and moderate-to-severe psoriasis: 52-week results from the randomized SPIRIT-H2H trial. Dermatol Pract. Concept12 (2), e2022104. 10.5826/dpc.1202a104
48
Ruyssen-Witrand A. Perry R. Watkins C. Braileanu G. Kumar G. Kiri S. et al (2020). Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open6 (1), e001117. 10.1136/rmdopen-2019-001117
49
Silva M. Santos J. Kakehasi A. M. Almeida A. M. Pimenta P. R. K. Alvares-Teodoro J. et al (2022). First-line biologic therapy with tumor necrosis factor inhibitors for psoriatic arthritis: a prospective observational study. Sao Paulo Med. J.140 (6), 787–797. 10.1590/1516-3180.2021.0434.R1.22022022
50
Singh J. A. Guyatt G. Ogdie A. Gladman D. D. Deal C. Deodhar A. et al (2019). Special article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol.71 (1), 5–32. 10.1002/art.40726
51
Smolen J. S. Mease P. Tahir H. Schulze-Koops H. de la Torre I. Li L. et al (2020). Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann. Rheum. Dis.79 (10), 1310–1319. 10.1136/annrheumdis-2020-217372
52
Strand V. McInnes I. Mease P. Nash P. Thom H. Kalyvas C. et al (2019). Matching-adjusted indirect comparison: secukinumab versus infliximab in biologic-naive patients with psoriatic arthritis. J. Comp. Eff. Res.8 (7), 497–510. 10.2217/cer-2018-0141
53
Toussirot E. (2018). Ixekizumab: an anti- IL-17A monoclonal antibody for the treatment of psoriatic arthritis. Expert Opin. Biol. Ther.18 (1), 101–107. 10.1080/14712598.2018.1410133
54
Wells G. A. Shea B. J. O'Connell D. Peterson J. Tugwell P. (2000). The newcastle–ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Summary
Keywords
biologics, biological-naïve, psoriatic arthritis, efficacy, safety, network meta-analysis
Citation
Lin J and Ren Y (2024) Different biologics for biological-naïve patients with psoriatic arthritis: a systematic review and network meta-analysis. Front. Pharmacol. 15:1279525. doi: 10.3389/fphar.2024.1279525
Received
18 August 2023
Accepted
29 January 2024
Published
13 March 2024
Volume
15 - 2024
Edited by
Pablo Andres Evelson, University of Buenos Aires, Argentina
Reviewed by
Marc Henri De Longueville, UCB Pharma, Belgium
Daniel González Maglio, University of Buenos Aires, Argentina
Updates
Copyright
© 2024 Lin and Ren.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yougang Ren, ygangren_dr@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.