- 1Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Medical Biosciences, Faculty of Natural Sciences, University of the Western Cape, Bellville, South Africa
- 3Infectious Diseases Department, O.Bogomolets National Medical University, Kyiv, Ukraine
- 4The Research Institute of Virology, Ministry of Health, Tashkent, Uzbekistan
- 5Hai Phong University of Medicine and Pharmacy, Hai Phong, Vietnam
- 6Department of Vascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Schistosomiasis is a parasitic disease that endangers human health and social development. The granulomatous reaction of Schistosoma eggs in the liver is the main cause of hepatosplenomegaly and fibrotic lesions. Anti liver fibrosis therapy is crucial for patients with chronic schistosomiasis. Although Praziquantel is the only clinical drug used, it is limited in insecticide treatment and has a long-term large-scale use, which is forcing the search for cost-effective alternatives. Previous research has demonstrated that plant metabolites and extracts have effective therapeutic effects on liver fibrosis associated with schistosomiasis. This paper summarizes the mechanisms of action of metabolites and some plant extracts in alleviating schistosomiasis-associated liver fibrosis. The analysis was conducted using databases such as PubMed, Google Scholar, and China National Knowledge Infrastructure (CNKI) databases. Some plant metabolites and extracts ameliorate liver fibrosis by targeting multiple signaling pathways, including reducing inflammatory infiltration, oxidative stress, inhibiting alternate macrophage activation, suppressing hepatic stellate cell activation, and reducing worm egg load. Natural products improve liver fibrosis associated with schistosomiasis, but further research is needed to elucidate the effectiveness of natural products in treating liver fibrosis caused by schistosomiasis, as there is no reported data from clinical trials in the literature.
1 Introduction
Schistosomiasis is a parasitic disease, in which trematodes infections poses a serious threat to human health and social development. Schistosomiasis is found in 78 countries in the tropics and subtropics and is predominantly endemic in sub-Saharan Africa (WHO, 2022). Intestinal schistosomiasis and urogenital schistosomiasis are two primary pathologies caused by Schistosoma infections in humans, with the former being primarily caused by S. japonicum and S. mansoni and the latter by S. haematobium. (McManus et al., 2018). Worms parasitize the veins of human hosts to mate and lay eggs are excreted in feces or urine. These eggs hatch as miracidia and infect intermediate species-specific host snails in fresh water. After 4–6 weeks, the eggs develop into infectious cercariae that can penetrate human skin and cause disease. Acute infections occur mostly in locals, travelers and immigrants and present symptoms of transient urticaria rash, allergic pneumonia, and Katayama syndrome. (Clerinx and Van Gompel, 2011). The progression of these infections can be characterized by chronic abdominal pain, loss of appetite, and liver polyps, and eventually lead to hepatosplenomegaly, portal hypertension, ascites, gastrointestinal varices, and even life-threatening gastrointestinal bleeding (Colley et al., 2014). Currently, the only clinically effective drug for treating schistosomiasis is praziquantel (Kabuyaya et al., 2023). However, due to the incomplete efficacy of praziquantel and the potential for drug resistance, there is an urgent need to find cost-effective alternatives or complementary treatments (Santana et al., 2021). More research is crucial to investigate novel targeting mechanisms of schistosome-induced liver fibrosis in order to discover new drugs or praziquantel analogs that can treat schistosomiasis.
2 Pathogenesis of chronic schistosomiasis
Fibrosis of the liver and portal system is the main pathological manifestation of intestinal schistosomiasis and is the result of an immune response caused by the invasion of schistosome eggs into the liver and blood vessels. The deposition of eggs causes a granulomatous inflammatory response mediated by CD4 T lymphocytes+, which is characterized by a markedly active Th2 immune response, such as increased levels of cytokines and chemokines, and the recruitment of lymphocytes, neutrophils, eosinophils, macrophages, and fibroblasts, followed by extracellular matrix (ECM) and collagen fibril production of liver tissue, with the eggs being trapped in the liver and unable to be excreted, finally resulting in a fibrotic inflammatory infiltrate forming around the eggs (Chiu et al., 2003; Amaral et al., 2017; Ho et al., 2022). The expedited Th2 response during the schistosomiasis infection may be related to alternate activation of macrophages. During the chronic infection phase, alternatively activated macrophages (M2 macrophages) are stimulated by IL-13, IL-33, IL-4, and ROS to regulate the expression of Arg1, IL-10, and TGF-β1, which act directly or indirectly on hepatic stellate cells and contribute to α-SMA and collagen production, leading to liver fibrosis (Peng et al., 2017; Tan et al., 2018; Yu et al., 2021). The soluble egg antigen (SEA) can also activate M2 macrophages via STAT6 and PI3K signaling pathways or directly activate hepatic stellate cells via the P38/JNK MAPK signaling pathway (Liu P. et al., 2013; Tang et al., 2017). In addition, the deposition of eggs significantly reduced the enzymatic activities of O2.- and H2O2 detoxification, by superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSHPx) and increased the levels of hepatic products from lipid peroxidation, which may stimulate the progression of liver fibrosis (Gharib et al., 1999).
3 Natural products against schistosomiasis-associated liver fibrosis
An increasing number of studies have demonstrated that bioactive ingredients of medicinal plants are a promising alternative to current clinical therapy. The current direction of anti-schistosomiasis drug research is focused on the screening of compounds with therapeutic targets and the development of praziquantel analogs. Anti-fibrotic treatment is essential for patients with chronic schistosomiasis as even deworming does not completely stop the progression of liver fibrosis (Bergquist et al., 2017; LoVerde et al., 2021). In schistosomiasis-endemic countries such as China, Brazil, Zimbabwe, and Kenya, the anti-schistosomiasis pharmacological effects of natural products or plant extracts have been extensively studied in an attempt to discover alternative drugs (Molgaard et al., 2001). Currently, the active mechanism of various natural products in the treatment of schistosomiasis-associated liver fibrosis has been reported, which may modulate fibrotic factors such as IL-13, growth stimulation expressed gene 2 (ST2), TGF-β1, TNF-α and anti-fibrotic factors such as IL-10, Tregs, MHC II through intracellular signaling pathways such as NF-κB pathway, PI3K/AKT pathway and TGF-β1/Smad pathway (Liu et al., 2014; Tang et al., 2017; Kamdem et al., 2018; Huang et al., 2020).
3.1 Natural compounds
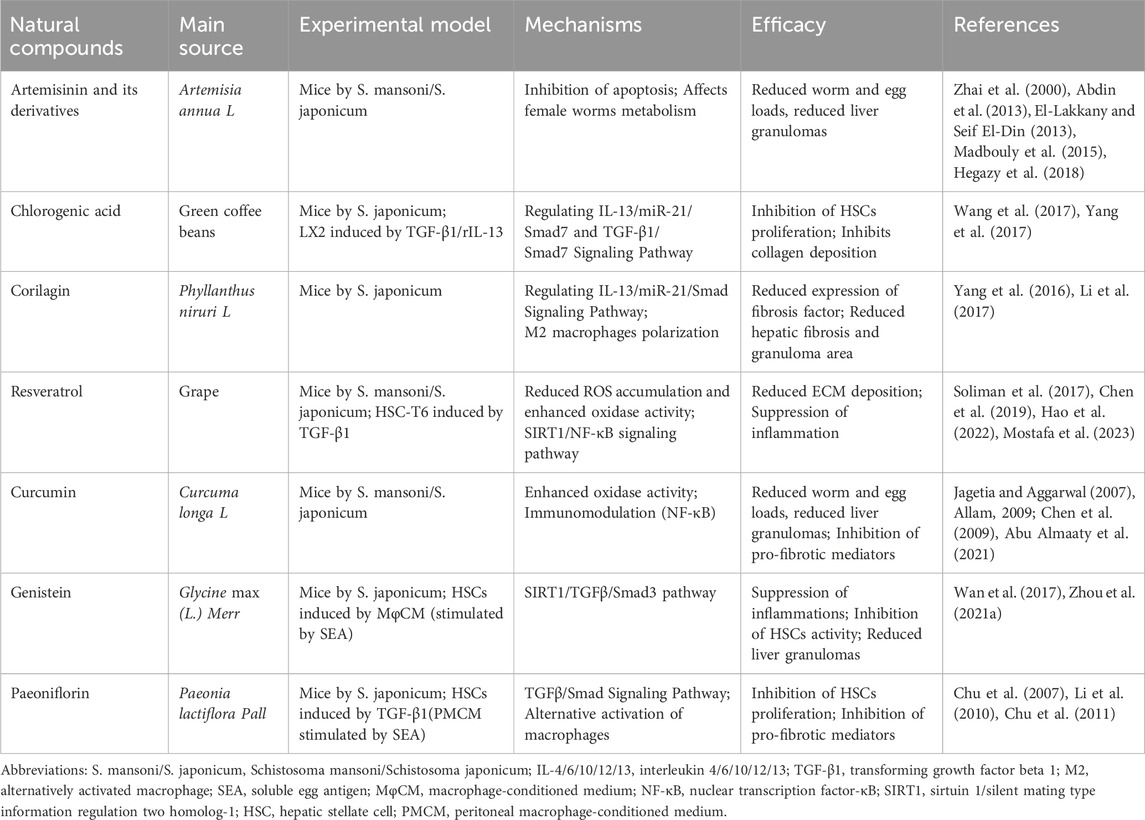
Based on the diversity that biological exploration of natural products provides for drug discovery, the active substances of natural products and their independent pharmacological effects in mixtures have attracted much attention (Phillipson, 2001; Simoben et al., 2018). Traditional medicinal plants are highly diverse and many metabolites have been shown to have therapeutic effects on liver fibrosis in schistosomiasis (Table 1).
3.1.1 Artemisinin and its derivatives
Artemisinin is a sesquiterpene lactone derived from Artemisia annua L., and artemisinin-based combination therapies (ACTs) are widely used to treat malaria. Artemisinin including its derivatives such as artesunate, dihydroartemisinin, and artemether has also been shown to have pharmacological effects such as anti-cancer, anti-viral, anti-inflammatory, and anti-parasitic (Ho et al., 2014). Artemisinin and its derivatives have been shown to kill helminths in animal models (rabbits, rats, dogs) with worm reduction rates ranging from 41% to 98%, which is based on its high lethality to larvae and females (You et al., 1992; Xiao et al., 1994; Gold et al., 2017; Correa et al., 2019). In Schistosoma mansoni infected mice, the combination of artesunate (400 mg/kg) and praziquantel (500 mg/kg) significantly decreased hepatic P53 expression and increased Bcl-2 expression (Hegazy et al., 2018). Lower doses of artemether (50 mg/kg), artemether reduced the number of eggs in mice’s feces, whereas higher doses of 400 mg/kg artemether greatly reduced the number and diameter of liver granulomas (Lescano et al., 2004; El-Beshbishi et al., 2013). A possible mechanism for this reduction is the inhibition of the expression of schistosomal metabolic enzymes such as glycolytic key enzymes as well as thioredoxin glutathione reductase (TGR), cytochrome c peroxidase (CcP) and SOD (Zhai et al., 2000; Abdin et al., 2013; El-Lakkany and Seif El-Din, 2013). Furthermore artemether has been shown to mediate a shift from a Th2 to a Th1 response in schistosomes, characterized by an increase in IFN-γ levels and a decrease in IL-4 and IL-10 levels (Madbouly et al., 2015). Interestingly, Keiser et al. found that artemether did not rely on synergy with the immune response for its anti-schistosomal effects, even though immunomodulation was beneficial in suppressing egg-induced hepatotoxicity (Keiser et al., 2010). In summary, artemisinin and its derivatives may be a potential treatment for schistosomiasis liver fibrosis.
3.1.2 Chlorogenic acid
Chlorogenic acid, 5-O-caffeoylquinic acid (5-CQA), a natural polyphenolic compound, is widely found in various fruits, vegetables, and medicinal plants, such as apples, eggplants, coffee beans, Lonicera japonica Thunb. And Eucommia ulmoides Oliv. It is most abundant in green coffee beans. It has significant protective effects on cardiovascular, gastrointestinal, liver, nerve, and metabolism due to its antioxidant, antibacterial, and anticancer biological activities (Naveed et al., 2018; Lu et al., 2020). Chlorogenic acid has been reported to protect against different types of liver fibrosis through NOX/ROS/MAPK, ERK/Nrf2, TLR4, and NF-κB pathways (Shi et al., 2013; Shi et al., 2016; Yuan et al., 2017; Wei et al., 2018). Chlorogenic acid inhibited the elevated expression of TGF-β receptor I, CTGF, and α-SMA after IL-13 treatment of LX2 cells in a dose-dependent manner, ranging from 56 μM to 225 µM. In vivo studies in Schistosoma japonicum-infected mice have shown that the use of chlorogenic acid (5–20 mg/kg for 4 weeks) reduces IL-13 expression and significantly reduces the size of liver granulomas (Wang et al., 2017). Further mechanisms suggest that IL-13 affects miR-21/Smad7 signaling, which in turn affects liver fibrosis. IL-13 is a key factor in the progression of schistosomiasis disease which is consistent with the view of Wynn et al. (2004). Furthermore, 56–225 µM chlorogenic acid also directly interferes with miR-21-regulated TGF-β1/Smad7 signaling, which reduces the expression of CTGF, TIMP and MMP-9 and decreases collagen and ECM deposition (Yang et al., 2017).
3.1.3 Corilagin
Corilagin, β-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-d-glucose, a natural ellagitannin, mainly derived from plants such as Phyllanthus niruri L. and Geranium sibiricum L., has a variety of pharmacological activities including anti-cancer, anti-inflammatory, antioxidant and hepatoprotective (Li et al., 2018; Gupta et al., 2019). Polarization of M2 macrophages induced by IL-4/IL-13 plays an important role in the granuloma response of Schistosoma eggs (Herbert et al., 2004). Corilagin (20, 40, 80 mg/kg/day for 28 days) significantly reduced hepatic fibrosis by decreasing the expression of pro-fibrotic factors (e.g., IL-13, IL-13 receptor α1, IL-4 receptor α), as well as by decreasing the expression of PPARγ, KLF4, SOCS1, and p-STAT6, and by inhibiting the polarization of M2 macrophage in Schistosoma hepatic tissues in mice (Du et al., 2016). Li et al. showed that Corilagin (39–157 μM, 24 h) significantly inhibited downstream fibrotic factors by interfering with the binding of IL-13 to IL-13Rα1 in Ana-1 cells (Li et al., 2017). Yang et al. found that treatment of schistosome mice with 20 mg/kg Corilagin reduced the number of liver eggs and effectively protected against liver fibrosis by inhibiting miR21 regulation of Smad7 and Smad1/2 phosphorylation (Yang et al., 2016). The above studies show that Corilagin is an effective drug in the treatment of schistosomiasis-induced liver fibrosis.
3.1.4 Resveratrol
Resveratrol (3,5,4 0-trihydroxy-trans-stilbene) is a non-flavonoid polyphenol found in over 70 plants such as Veratrum grandiflorum (Maxim. ex Miq.) O. Loes., Polygonum cuspidatum Siebold & Zucc., grapes and peanuts. Resveratrol (RSV) is highly valued for its antioxidant, anti-inflammatory, anti-cancer, anti-diabetic, anti-aging, cardioprotective, and neuroprotective effects (Zhang L. X. et al., 2021). RSV-containing nanocarriers reduced ROS levels, inhibited the growth of activated HSC-T6 cells in vitro (20 µM) and significantly reduced hepatic ECM accumulation in vivo (5 mg) (Hao et al., 2022). The reduction of GSH and SOD expression in livers infected with schistosomes was significantly reversed in 2 weeks of treatment with 20 mg/kg RSV (Soliman et al., 2017). Chen et al. showed that RSV (400 mg/kg for 3 days) increased mitochondrial membrane potential (Δφm) and peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) expression in mouse liver. Interestingly, the improvement of mitochondrial function was not the only factor that affected liver fibrosis amelioration with RSV(Chen et al., 2019). As a Sirt-1 activator, RSV (20 mg/kg or 100 mg/kg for 4 weeks) reduced anti-inflammatory markers and anti-fibrotic markers in schistosome-infected mice via the SIRT1/NF-κB signaling pathway (Mostafa et al., 2023). In addition, RSV (20 mg/kg for 3 weeks) inhibited the development and progression of liver granulomas by regulating Th17/Treg responses (Han et al., 2019). Therefore, RSV may exert its anti-schistosomal liver fibrosis effects through the above mechanisms.
3.1.5 Curcumin
Curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione), is a natural polyphenolic compound, mainly extracted from the rhizome of Curcuma longa L. Curcumin is the key active component of turmeric, showing antioxidant, anti-inflammatory, anti-cancer, anti-microbial, and tissue (heart, nerve, liver) protective effects (Sohn et al., 2021; El-Saadony et al., 2022). The protective effects of curcumin against different types of liver injury are mainly mediated by reducing lipid peroxidation, activating the Nrf2 signal and inhibiting NF-κB activity (Khan et al., 2019). Curcumin (50–200 mg/kg) was found to upregulate MMP-1 and inhibit TIMP-1, resulting in a reduction in liver granuloma volume by up to 79% and collagen content by 38.6% (Li et al., 2007). Low expression of GSH, GST, SOD, and CAT caused by S. mansoni infection increased significantly after 2 weeks of treatment with 40 mg/kg curcumin (Abu Almaaty et al., 2021). A total dose of 400 mg/kg curcumin treatment was found to suppress serum levels of IL-12 and TNF-α in the infected group, possibly related to immune regulation triggered by inhibition of NF-κB activity (Jagetia and Aggarwal, 2007; Allam, 2009). Additionally, it significantly raised the mRNA expression of PPAR while reducing TGF-β1 (Chen et al., 2009). These studies suggest that curcumin may be an effective in the treatment of schistosomiasis-induced liver fibrosis.
3.1.6 Genistein
Genistein (5,7-dihydroxy-3-(4-hydroxyphenyl) chromium-4-one) is the most potent functional component of soy (Glycine max (L.) Merr.) isoflavone products, with anti-cancer, anti-oxidant, anti-atherosclerotic and anti-inflammatory activities (Mukund et al., 2017; Sharifi-Rad et al., 2021). 25, 50 mg/kg genistein significantly inhibited NF-κB signaling in schistosome-infected liver tissues, as evidenced by decreased mRNA levels of MCP1, TNFα, and IL10, and decreased expression of TGF-β1 and α-SMA (Wan et al., 2017). The same concentration of genistein reversed the reduction of STRT1 expression and activity in schistosome liver fibrosis tissues. By suppressing SIRT1 activity, 5–10 and 20 µM doses of genistein significantly decreased HSC-T6 cell activation (Zhou C. et al., 2021). Previous studies have demonstrated that genistein attenuates hepatic fibrosis by inhibiting TGF-β/Smad signaling through downregulation of p-Smad3 (Ganai and Husain, 2017). Interestingly, the knockdown of SIRT1 enhanced TGF-β1-induced Smad3 phosphorylation (Ma et al., 2019). Thus, genistein may also ameliorate schistosomiasis liver fibrosis via the SIRT1/TGFβ/Smad3 pathway. The above studies suggest that genistein may be an effective drug for the treatment of schistosomiasis liver fibrosis.
3.1.7 Paeoniflorin
Paeoniflorin is the main active ingredient of the Paeonia lactiflora Pall., a monoterpene glycoside compound used in the treatment of cancer, depression, diabetes, liver disease, and autoimmune disorders (Ma et al., 2020; Zhang and Wei, 2020). Mouse peritoneal macrophages that are stimulated to produce TGF-β1 by SEA, causing the promotion of the proliferation of HSC and the synthesis of collagen. Chu et al. demonstrated for the first time that 7.5–120 mg/L of paeoniflorin (colchicine, positive control, 1 µM) selectively downregulated the level of Smad3 phosphorylation through TGF-β1 signaling and inhibited the proliferation of HSC(Chu et al., 2007). Another study found that 30 mg/kg paeoniflorin significantly reduced schistosome-induced elevated levels of IL-13 and decreased STAT6 phosphorylation levels and collagen I expression by increasing SOCS-1 expression (Li et al., 2010). Further studies showed that 100 μg/mL paeoniflorin directly or indirectly inhibited alternative activation of Kupffer cells by reducing JAK2 and STAT6 phosphorylation (Chu et al., 2011). Paeoniflorin may be a promising drug for the treatment of fibrosis in schistosomiasis.
3.2 Plant extracts
The roots, stems, leaves, flowers, and fruits of plants are processed using certain technological methods to obtain herbal bioactive ingredients that affect diseases.
3.2.1 Silymarin
Silymarin is a standardized dried extract of the fruit and seeds of Silybum marianum (L.) Gaertn. Silybin, isosilybin, silydianin and silychristin, are the four major flavonoid lignan isomers in silymarin. Silybin is the main active ingredient and is known for its anti-inflammatory, antioxidant, anti-fibrotic, and hepatoprotective effects (Abenavoli et al., 2018; Gillessen and Schmidt, 2020). Mata-Santos et al. found that silymarin (silybin content,47%) reduced the size of liver granulomas and alleviated liver fibrosis by inhibiting the production of pro-inflammatory and fibrotic factors, including IL-13, IL-4, TNF-α and TGF-β1, and HSC proliferation (Mata-Santos et al., 2010; Mata-Santos et al., 2014; El-Sayed et al., 2016). In a study of acute and chronic schistosomiasis liver fibrosis, silymarin (750 mg/kg/day, 5 days/week for 6 weeks) significantly reduced hepatic HYP levels, TGF-β1 and MMP-2 expression and restored GSH levels in both stages, reducing hepatic egg load and regulating granuloma size (El-Lakkany et al., 2012).
3.2.2 Green tea extract
Camellia sinensis (L.) Kuntze is a perennial woody plant whose young leaves and flowers are processed into beverages or medicines (Butt et al., 2015). Green tea has been widely demonstrated to have preventive effects against diabetes, cancer, and cardiovascular disease. Its health properties are attributed to the bioactive polyphenols contained in it, particularly catechins (Xing et al., 2019). Epigallocatechin gallate contains 30%–50% of green tea catechins and is known for its potent antioxidant, anti-obesity, anti-inflammatory, anti-cancer, and other pharmacological activities (Yang et al., 2020). Bin Dajem et al. showed that green tea at a concentration of 3% (w/v) reduced hepatocellular necrosis and perivascular collagen fibers by decreasing lipid peroxidation, but failed to significantly improve liver function (Bin Dajem et al., 2011). Another study found Matcha (a Japanese green tea powder made from finely powdered dried tea leaves) to have lower levels of polyphenols and higher levels of caffeine, quercetin, and rutin than traditional green tea (Ramez et al., 2021). Matcha (3 g/kg b. w) contained more theanine and rutin than other green teas, reducing TNF-α, IFN-γ, and IL-13 levels, increasing IL10 levels, which led to the inhibition of the development of liver granulomas, the restoration of SOD, CAT and GSH-Px activity as well as MDA and TAC levels through antioxidant capacity (Kochman et al., 2020). The natural components of green tea may be a promising complementary treatment therapy for schistosomiasis.
3.2.3 Boswellia serrata resin extract
Frankincense is the resin that exudes from the bark of the Boswellia Roxb. tree, a member of the olive family, and boswellic acid is the most important triterpenoid of frankincense, especially 3-O-acetyl-11-keto-β-boswellic acid (β-AKBA) (Al-Harrasi et al., 2021). Liu et al. combined frankincense oil resin extract with cyclodextrin (BSE-CD) to address hydrophilicity issues. They first found that liver egg granulomas formed by the eggs of S. japonicum contained high levels of leukotriene B4. BSE-CD (280 mg/kg for 3 weeks) significantly reduced the size of liver granulomas, possibly caused by the reduced expression of MMP-9, LTB4, and PGE2 (Liu M. et al., 2013). Further postulated mechanisms suggested that it could reduce the inflammatory response around eggs by inhibiting NF-κB signaling and reducing the expression of VEGF, TNF-α, and MCP-1 in mice (Liu et al., 2014).
3.2.4 Ampelopsis grossedentata extract
Ampelopsis grossedentata (Hand. -Mazz.) W.T. Wang, also known as vine tea, is a plant of the genus Ampelopsis in the family Vitaceae, mainly distributed in southern China. The pharmacological effects of vine tea are mainly summarized as anti-inflammatory and analgesic, hepatoprotective, hypotensive, hypolipidemic, antitumor, and anti-aging (Xie et al., 2019; Tong et al., 2020; Wang et al., 2023). Flavonoids are the main efficacy components of vine tea, which includes dihydromyricetin, myricitrin, myricetin, quercetin, rutin, and kaempferol (Xu et al., 2012). Dihydromyricetin has the largest content with a mass fraction of 34% and is considered to be foundational for the health benefits of vine tea (Feng et al., 2018; Zhang Q. et al., 2021). The total flavonoids of vine tea have been proven to have anti-liver fibrosis effects (Li et al., 2022). Ampelopsis grossedentata extract containing 90% dihydromyricetin (150 mg/kg for 8 weeks) significantly ameliorated hepatic fibrosis in S. japonicum-infected mice, which was superior to praziquantel alone. (Fang et al., 2010). 30 μM Dihydromyricetin significantly inhibited the activation of HSC-T6 cells in vitro, mediated through the promotion of AMPK phosphorylation and inhibition of the TGF-β1/Smad signaling pathway (Zhang et al., 2018). In vivo, it (100, 200, 400 mg/kg) downregulated TGF-β1/Smad signaling, improved liver function and reduced ECM deposition. Colchicine was used as a positive control at a dose of 0.2 mg/kg (Liang et al., 2019). In addition, another active ingredient, myricetin was shown to have a toxic effect on S. japonicum worms through induction of apoptosis, with an LC50 of 600 μM at 24 h. Interestingly, it (at 250 mg/kg) reduces the number of worms and eggs as well as the size of liver granulomas by modulating the immune response (lowering the ratio of Th2 and Th17 cells) (Huang et al., 2020).
3.2.5 Ginger extract
Ginger (Zingiber officinale Roscoe), a perennial herb of the ginger family, is a medicinal plant with the same origin as food, and has the effect of promoting sweating and relieving symptoms, causing a warming sensation of the body, and suppresses vomiting (Zhang M. et al., 2021). Ginger crude aqueous extract (500 mg/kg) slowed the development of granulomatous inflammatory infiltrates and reduced hepatic egg load after schistosome infection, which was more pronounced after treatment with ginger-derived nanoparticles (Mostafa et al., 2011; Abd El Wahab et al., 2021). This may be related to the powerful antioxidant effect of ginger extract and its ability to scavenge free radicals, as evidenced by the restoration of CAT activity and MDA levels. Another study showed that ethanolic extract of ginger also inhibited oxidative stress and inflammatory mediators to improve schistosomiasis-associated liver fibrosis (Aly and Mantawy, 2013). Interestingly, Sanderson et al. suggested that the ethyl acetate extract of ginger (150 mg/kg) did not kill the egg load and helminth load of schistosome-infected mice, which was attributed to the alternative extraction solvent and the varying treatment doses of the extracts (Sanderson et al., 2002). Given the lack of data on the role of ginger extract as a treatment for schistosomiasis-associated liver fibrosis, further study is required.
3.2.6 Other extracts
Ziziphus spina-christi leaf extract (ZLE) is extracted from Z. spina-christi (L.) Willd, alkaloids and flavonoids are the main constituent classes. The pharmacological effects of it include antibacterial, anti-inflammatory, antiparasitic, and anticancer (Abdulrahman et al., 2022). 600 mg/kg Ziziphus spina-christi showed granuloma reduction and anti-hepatic fibrosis in mice infected with S. haematobium (Alghamdi et al., 2023). 400 mg/kg ZLE treatment reduces hepatic granuloma area in mice infected with S. mansoni and reduces hepatic fibrosis by inhibiting the expression of TGF-β1, VEGF, α-SMA, TIMP-1, and MMP-9, as well as inhibiting oxidative stress and inflammation by upregulating Nrf2 (Almeer et al., 2018). An aqueous extract of Moringa Oleifera Lam. Leaves (150 mg/kg for 15 days) significantly reduced NF-κB expression and thereby ameliorated schistosome-induced hepatic fibrosis (Saad El-Din et al., 2023). Ceratonia siliqua pod extract (Ceratonia siliqua L.) at doses of 300 mg/kg or 600 mg/kg reduced the area of granulomas and fibrosis by counteracting oxidative stress and decreasing TIMP-2 expression (Al-Olayan et al., 2016). 1.5 g/kg Artichoke leaf extract (Cynara scolymus L.) reduces granuloma size by increasing HSC recruitment within the granuloma (Sharaf El-Deen et al., 2017).
4 Conclusion
The main objective of this paper is to summarize the mechanistic studies of selected metabolites and plant extracts for the treatment of schistosomiasis-associated liver fibrosis. Some metabolites or plant extracts that did not address pharmacological mechanisms or were partially uncommon were excluded from the review. In addition, in the literature reviewed reported inconsistent ranges in dosage and varying assessment criteria for fibrosis.
Praziquantel is currently the most commonly used medication for schistosome prophylaxis and treatment. Due to praziquantel’s low toxicity to eggs, it is not effective in preventing the progression of liver fibrosis caused by schistosomiasis infection According to pharmacological monographs, current literature, and experimental studies, metabolites or plant extracts have the potential to treat schistosome-induced liver fibrosis. Their therapeutic mechanisms are characterized by 1) reduction of inflammation, 2) reduction of the granulomatous response, 3) reduction of oxidative stress, and 4) reduction of egg loading (Figure 1). There is considerable evidence that therapeutic efficiency is improved by combining metabolites/plant extracts with praziquantel, or with nanocarriers, or by using liposomes. Previous basic and clinical studies have shown good safety and tolerability for metabolic substances like resveratrol and chlorogenic acid, but adverse effects cannot be excluded due to experimental variability, inter-individual variability, and the lack of clinical trial reports. Furthermore, studies on the above metabolites and botanicals for the treatment of hepatic fibrosis due to schistosomiasis have been limited to basic research and no clinical studies have been reported. In conclusion, the protective role of natural products in the treatment of liver fibrosis in schistosomiasis needs to be confirmed by more standardized cellular studies, and supported by in vivo data from animal studies, which if promising should be escalated to randomized controlled clinical trials in humans.
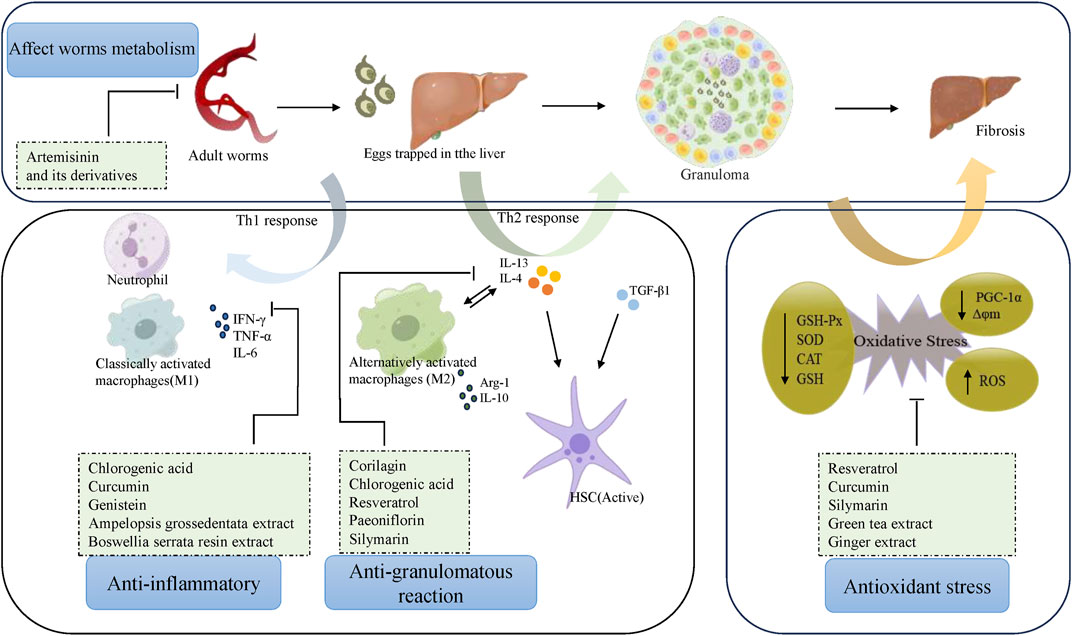
Figure 1. Pharmacologic mechanisms of natural products against schistosomiasis-associated liver fibrosis.
Author contributions
CL: Writing–original draft, Conceptualization. DF: Writing–review and editing. KP: Writing–review and editing. EM: Writing–review and editing, Visualization. NT: Writing–review and editing, Visualization. YD: Writing–review and editing, Supervision. LZ: Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by grants from the National Natural Science Foundation of China (No. 81974530) and Hubei International Scientific and Technological Cooperation Project (Nos. 2022EHB039, 2023EHA057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El Wahab, W. M., El-Badry, A. A., Mahmoud, S. S., El-Badry, Y. A., El-Badry, M. A., and Hamdy, D. A. (2021). Ginger (Zingiber Officinale)-derived nanoparticles in Schistosoma mansoni infected mice: hepatoprotective and enhancer of etiological treatment. PLoS Negl. Trop. Dis. 15 (5), e0009423. doi:10.1371/journal.pntd.0009423
Abdin, A. A., Ashour, D. S., and Shoheib, Z. S. (2013). Artesunate effect on schistosome thioredoxin glutathione reductase and cytochrome c peroxidase as new molecular targets in schistosoma mansoni-infected mice. Biomed. Environ. Sci. 26 (12), 953–961. doi:10.3967/bes2013.030
Abdulrahman, M. D., Zakariya, A. M., Hama, H. A., Hamad, S. W., Al-Rawi, S. S., Bradosty, S. W., et al. (2022). Ethnopharmacology, biological evaluation, and chemical composition of ziziphus spina-christi (L.) desf.: a review. Adv. Pharmacol. Pharm. Sci. 2022, 4495688. doi:10.1155/2022/4495688
Abenavoli, L., Izzo, A. A., Milic, N., Cicala, C., Santini, A., and Capasso, R. (2018). Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 32 (11), 2202–2213. doi:10.1002/ptr.6171
Abu Almaaty, A. H., Rashed, H. A. E., Soliman, M. F. M., Fayad, E., Althobaiti, F., and El-Shenawy, N. S. (2021). Parasitological and biochemical efficacy of the active ingredients of allium sativum and Curcuma longa in schistosoma mansoni infected mice. Molecules 26 (15), 4542. doi:10.3390/molecules26154542
Alghamdi, T., Salem, D. A., and El-Refaei, M. F. (2023). Anti-angiogenic and anti-proliferative activity of ziziphus leaf extract as a novel potential therapeutic agent for reducing hepatic injury in experimental hamster schistosomiasis. PLoS Negl. Trop. Dis. 17 (6), e0011426. doi:10.1371/journal.pntd.0011426
Al-Harrasi, A., Khan, A. L., Rehman, N. U., and Csuk, R. (2021). Biosynthetic diversity in triterpene cyclization within the Boswellia genus. Phytochemistry 184, 112660. doi:10.1016/j.phytochem.2021.112660
Allam, G. (2009). Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 214 (8), 712–727. doi:10.1016/j.imbio.2008.11.017
Almeer, R. S., El-Khadragy, M. F., Abdelhabib, S., and Abdel Moneim, A. E. (2018). Ziziphus spina-christi leaf extract ameliorates schistosomiasis liver granuloma, fibrosis, and oxidative stress through downregulation of fibrinogenic signaling in mice. PLoS One 13 (10), e0204923. doi:10.1371/journal.pone.0204923
Al-Olayan, E. M., El-Khadragy, M. F., Alajmi, R. A., Othman, M. S., Bauomy, A. A., Ibrahim, S. R., et al. (2016). Ceratonia siliqua pod extract ameliorates Schistosoma mansoni-induced liver fibrosis and oxidative stress. BMC Complement. Altern. Med. 16 (1), 434. doi:10.1186/s12906-016-1389-1
Aly, H. F., and Mantawy, M. M. (2013). Efficiency of ginger (Zingbar officinale) against Schistosoma mansoni infection during host-parasite association. Parasitol. Int. 62 (4), 380–389. doi:10.1016/j.parint.2013.04.002
Amaral, K. B., Silva, T. P., Dias, F. F., Malta, K. K., Rosa, F. M., Costa-Neto, S. F., et al. (2017). Histological assessment of granulomas in natural and experimental Schistosoma mansoni infections using whole slide imaging. PLoS One 12 (9), e0184696. doi:10.1371/journal.pone.0184696
Bergquist, R., Utzinger, J., and Keiser, J. (2017). Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infect. Dis. Poverty 6 (1), 74. doi:10.1186/s40249-017-0286-2
Bin Dajem, S. M., Shati, A. A., Adly, M. A., Ahmed, O. M., Ibrahim, E. H., and Mostafa, O. M. (2011). Green tea (Camellia sinesis) ameliorates female Schistosoma mansoni-induced changes in the liver of Balb/C mice. Saudi J. Biol. Sci. 18 (4), 361–368. doi:10.1016/j.sjbs.2011.06.003
Butt, M. S., Ahmad, R. S., Sultan, M. T., Qayyum, M. M., and Naz, A. (2015). Green tea and anticancer perspectives: updates from last decade. Crit. Rev. Food Sci. Nutr. 55 (6), 792–805. doi:10.1080/10408398.2012.680205
Chen, H., Zhang, J., and Liu, W. (2009). Jiang Huang su kang xue xi chong bing Gan xian Wei hua ji qi ji zhi de Shi yan yan jiu. Chin. Traditional Herb. Drugs 40 (08), 1274–1277. doi:10.3321/j.issn:0253-2670.2009.08.032
Chen, T. T., Peng, S., Wang, Y., Hu, Y., Shen, Y., Xu, Y., et al. (2019). Improvement of mitochondrial activity and fibrosis by resveratrol treatment in mice with schistosoma japonicum infection. Biomolecules 9 (11), 658. doi:10.3390/biom9110658
Chiu, B. C., Freeman, C. M., Stolberg, V. R., Komuniecki, E., Lincoln, P. M., Kunkel, S. L., et al. (2003). Cytokine-chemokine networks in experimental mycobacterial and schistosomal pulmonary granuloma formation. Am. J. Respir. Cell Mol. Biol. 29 (1), 106–116. doi:10.1165/rcmb.2002-0241OC
Chu, D., Du, M., Hu, X., Wu, Q., and Shen, J. (2011). Paeoniflorin attenuates schistosomiasis japonica-associated liver fibrosis through inhibiting alternative activation of macrophages. Parasitology 138 (10), 1259–1271. doi:10.1017/S0031182011001065
Chu, D., Luo, Q., Li, C., Gao, Y., Yu, L., Wei, W., et al. (2007). Paeoniflorin inhibits TGF-beta1-mediated collagen production by Schistosoma japonicum soluble egg antigen in vitro. Parasitology 134 (11), 1611–1621. doi:10.1017/S0031182007002946
Clerinx, J., and Van Gompel, A. (2011). Schistosomiasis in travellers and migrants. Travel Med. Infect. Dis. 9 (1), 6–24. doi:10.1016/j.tmaid.2010.11.002
Colley, D. G., Bustinduy, A. L., Secor, W. E., and King, C. H. (2014). Human schistosomiasis. Lancet 383 (9936), 2253–2264. doi:10.1016/S0140-6736(13)61949-2
Correa, S. A. P., de Oliveira, R. N., Mendes, T. M. F., Dos Santos, K. R., Boaventura, S., Garcia, V. L., et al. (2019). In vitro and in vivo evaluation of six artemisinin derivatives against Schistosoma mansoni. Parasitol. Res. 118 (2), 505–516. doi:10.1007/s00436-018-6188-9
Du, P., Ma, Q., Zhu, Z. D., Li, G., Wang, Y., Li, Q. Q., et al. (2016). Mechanism of Corilagin interference with IL-13/STAT6 signaling pathways in hepatic alternative activation macrophages in schistosomiasis-induced liver fibrosis in mouse model. Eur. J. Pharmacol. 793, 119–126. doi:10.1016/j.ejphar.2016.11.018
El-Beshbishi, S. N., Taman, A., El-Malky, M., Azab, M. S., El-Hawary, A. K., and El-Tantawy, D. A. (2013). In vivo effect of single oral dose of artemether against early juvenile stages of Schistosoma mansoni Egyptian strain. Exp. Parasitol. 135 (2), 240–245. doi:10.1016/j.exppara.2013.07.006
El-Lakkany, N. M., Hammam, O. A., El-Maadawy, W. H., Badawy, A. A., Ain-Shoka, A. A., and Ebeid, F. A. (2012). Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis. Parasit. Vectors 5, 9. doi:10.1186/1756-3305-5-9
El-Lakkany, N. M., and Seif El-Din, S. H. (2013). Haemin enhances the in vivo efficacy of artemether against juvenile and adult Schistosoma mansoni in mice. Parasitol. Res. 112 (5), 2005–2015. doi:10.1007/s00436-013-3358-7
El-Saadony, M. T., Yang, T., Korma, S. A., Sitohy, M., Abd El-Mageed, T. A., Selim, S., et al. (2022). Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: a comprehensive review. Front. Nutr. 9, 1040259. doi:10.3389/fnut.2022.1040259
El-Sayed, N. M., Fathy, G. M., Abdel-Rahman, S. A., and El-Shafei, M. A. (2016). Cytokine patterns in experimental schistosomiasis mansoni infected mice treated with silymarin. J. Parasit. Dis. 40 (3), 922–929. doi:10.1007/s12639-014-0606-4
Fang, H., Wang, J., Chen, M., Jia, L., and Li, C. (2010). Therapeutic effects of extract from caulis Ampelopsis grossdentatae on murine SchistosomiasisHepatic fibrosis. Chin. General Pract. 13 (18), 2004–2006.
Feng, C., Zhang, N., zhou, D., Jiao, S., Wu, S., Zou, R., et al. (2018). Determination of the content of 4 flavonoids in Ampelopsis grossedentatas leaves by HPLC. Sci. Technol. Food Industry 39 (24), 240–245. doi:10.13386/j.issn1002-0306.2018.24.041
Ganai, A. A., and Husain, M. (2017). Genistein attenuates D-GalN induced liver fibrosis/chronic liver damage in rats by blocking the TGF-β/Smad signaling pathways. Chem. Biol. Interact. 261, 80–85. doi:10.1016/j.cbi.2016.11.022
Gharib, B., Abdallahi, O. M., Dessein, H., and De Reggi, M. (1999). Development of eosinophil peroxidase activity and concomitant alteration of the antioxidant defenses in the liver of mice infected with Schistosoma mansoni. J. Hepatol. 30 (4), 594–602. doi:10.1016/s0168-8278(99)80189-5
Gillessen, A., and Schmidt, H. H. (2020). Silymarin as supportive treatment in liver diseases: a narrative review. Adv. Ther. 37 (4), 1279–1301. doi:10.1007/s12325-020-01251-y
Gold, D., Alian, M., Domb, A., Karawani, Y., Jbarien, M., Chollet, J., et al. (2017). Elimination of Schistosoma mansoni in infected mice by slow release of artemisone. Int. J. Parasitol. Drugs Drug Resist 7 (2), 241–247. doi:10.1016/j.ijpddr.2017.05.002
Gupta, A., Singh, A. K., Kumar, R., Ganguly, R., Rana, H. K., Pandey, P. K., et al. (2019). Corilagin in cancer: a critical evaluation of anticancer activities and molecular mechanisms. Molecules 24 (18), 3399. doi:10.3390/molecules24183399
Han, Q., Zhu, J., Lv, N., Tong, S., and Zhang, W. (2019). Resveratrol inhibited hepatic granulama in mice with schistosomiasis by modulating Th17 and Treg responses. Chin. Pharmacol. Bull. 35 (01), 132–138.
Hao, Y., Song, K., Tan, X., Ren, L., Guo, X., Zhou, C., et al. (2022). Reactive oxygen species-responsive polypeptide drug delivery system targeted activated hepatic stellate cells to ameliorate liver fibrosis. ACS Nano 16 (12), 20739–20757. doi:10.1021/acsnano.2c07796
Hegazy, L. A. M., Motiam, M. H. A., Abd El-Aal, N. F., Ibrahim, S. M., and Mohamed, H. K. (2018). Evaluation of artesunate and praziquantel combination therapy in murine schistosomiasis mansoni. Iran. J. Parasitol. 13 (2), 193–203.
Herbert, D. R., Holscher, C., Mohrs, M., Arendse, B., Schwegmann, A., Radwanska, M., et al. (2004). Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20 (5), 623–635. doi:10.1016/s1074-7613(04)00107-4
Ho, C. H., Cheng, C. H., Huang, T. W., Peng, S. Y., Lee, K. M., and Cheng, P. C. (2022). Switched phenotypes of macrophages during the different stages of Schistosoma japonicum infection influenced the subsequent trends of immune responses. J. Microbiol. Immunol. Infect. 55 (3), 503–526. doi:10.1016/j.jmii.2021.06.005
Ho, W. E., Peh, H. Y., Chan, T. K., and Wong, W. S. (2014). Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol. Ther. 142 (1), 126–139. doi:10.1016/j.pharmthera.2013.12.001
Huang, P., Zhou, M., Cheng, S., Hu, Y., Gao, M., Ma, Y., et al. (2020). Myricetin possesses anthelmintic activity and attenuates hepatic fibrosis via modulating TGFβ1 and akt signaling and shifting Th1/Th2 balance in schistosoma japonicum-infected mice. Front. Immunol. 11, 593. doi:10.3389/fimmu.2020.00593
Jagetia, G. C., and Aggarwal, B. B. (2007). Spicing up" of the immune system by curcumin. J. Clin. Immunol. 27 (1), 19–35. doi:10.1007/s10875-006-9066-7
Kabuyaya, M., Chimbari, M. J., and Mukaratirwa, S. (2023). Correction: efficacy of praziquantel treatment regimens in pre-school and school aged children infected with schistosomes in sub-Saharan Africa: a systematic review. Infect. Dis. Poverty 12 (1), 13. doi:10.1186/s40249-023-01064-5
Kamdem, S. D., Moyou-Somo, R., Brombacher, F., and Nono, J. K. (2018). Host regulators of liver fibrosis during human schistosomiasis. Front. Immunol. 9, 2781. doi:10.3389/fimmu.2018.02781
Keiser, J., Vargas, M., and Doenhoff, M. J. (2010). Activity of artemether and mefloquine against juvenile and adult Schistosoma mansoni in athymic and immunocompetent NMRI mice. Am. J. Trop. Med. Hyg. 82 (1), 112–114. doi:10.4269/ajtmh.2010.09-0461
Khan, H., Ullah, H., and Nabavi, S. M. (2019). Mechanistic insights of hepatoprotective effects of curcumin: therapeutic updates and future prospects. Food Chem. Toxicol. 124, 182–191. doi:10.1016/j.fct.2018.12.002
Kochman, J., Jakubczyk, K., Antoniewicz, J., Mruk, H., and Janda, K. (2020). Health benefits and chemical composition of Matcha green tea: a review. Molecules 26 (1), 85. doi:10.3390/molecules26010085
Lescano, S. Z., Chieffi, P. P., Canhassi, R. R., Boulos, M., and Amato Neto, V. (2004). Antischistosomal activity of artemether in experimental Schistosomiasis mansoni. Rev. Saude Publica 38 (1), 71–75. doi:10.1590/s0034-89102004000100010
Li, K., Zhang, L., Fan, Z., and Li, W. (2007). The mechanism of curcumin on inhibiting schistosomiasis liver fibrosis in mice. Chin. J. Endemiology 26 (06), 643–645.
Li, M., Wang, L., and Li, W. (2022). Improving effect of tengeha flavonoids in mice with liver fibrosis. J. Guangzhou Univ. Traditional Chin. Med. 39 (03), 625–630. doi:10.13359/j.cnki.gzxbtcm.2022.03.027
Li, X., Deng, Y., Zheng, Z., Huang, W., Chen, L., Tong, Q., et al. (2018). Corilagin, a promising medicinal herbal agent. Biomed. Pharmacother. 99, 43–50. doi:10.1016/j.biopha.2018.01.030
Li, X., Shen, J., Zhong, Z., Peng, J., Wen, H., Li, J., et al. (2010). Paeoniflorin ameliorates schistosomiasis liver fibrosis through regulating IL-13 and its signalling molecules in mice. Parasitology 137 (8), 1213–1225. doi:10.1017/S003118201000003X
Li, Y. Q., Chen, Y. F., Dang, Y. P., Wang, Y., Shang, Z. Z., Ma, Q., et al. (2017). Corilagin counteracts IL-13Rα1 signaling pathway in macrophages to mitigate schistosome egg-induced hepatic fibrosis. Front. Cell Infect. Microbiol. 7, 443. doi:10.3389/fcimb.2017.00443
Liang, B., Zheng, Z., Gan, C., and Tang, Y. (2019). Effects of dihydromyricetin from Ampelopsis grossedntata on TGF-β1/smad signaling pathwayin hepatic fibrosis mice. J. Chin. Med. Mater. 42 (12), 2922–2928. doi:10.13863/j.issn1001-4454.2019.12.034
Liu, M., Chen, P., Buchele, B., Dong, S., Huang, D., Ren, C., et al. (2013a). A boswellic acid-containing extract attenuates hepatic granuloma in C57BL/6 mice infected with Schistosoma japonicum. Parasitol. Res. 112 (3), 1105–1111. doi:10.1007/s00436-012-3237-7
Liu, M., Wu, Q., Chen, P., Buchele, B., Bian, M., Dong, S., et al. (2014). A boswellic acid-containing extract ameliorates schistosomiasis liver granuloma and fibrosis through regulating NF-κB signaling in mice. PLoS One 9 (6), e100129. doi:10.1371/journal.pone.0100129
Liu, P., Wang, M., Lu, X. D., Zhang, S. J., and Tang, W. X. (2013b). Schistosoma japonicum egg antigen up-regulates fibrogenesis and inhibits proliferation in primary hepatic stellate cells in a concentration-dependent manner. World J. Gastroenterol. 19 (8), 1230–1238. doi:10.3748/wjg.v19.i8.1230
LoVerde, P. T., Alwan, S. N., Taylor, A. B., Rhodes, J., Chevalier, F. D., Anderson, T. J., et al. (2021). Rational approach to drug discovery for human schistosomiasis. Int. J. Parasitol. Drugs Drug Resist 16, 140–147. doi:10.1016/j.ijpddr.2021.05.002
Lu, H., Tian, Z., Cui, Y., Liu, Z., and Ma, X. (2020). Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 19 (6), 3130–3158. doi:10.1111/1541-4337.12620
Ma, J. Q., Sun, Y. Z., Ming, Q. L., Tian, Z. K., Yang, H. X., and Liu, C. M. (2019). Ampelopsin attenuates carbon tetrachloride-induced mouse liver fibrosis and hepatic stellate cell activation associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int. Immunopharmacol. 77, 105984. doi:10.1016/j.intimp.2019.105984
Ma, X., Zhang, W., Jiang, Y., Wen, J., Wei, S., and Zhao, Y. (2020). Paeoniflorin, a natural product with multiple targets in liver diseases-A mini review. Front. Pharmacol. 11, 531. doi:10.3389/fphar.2020.00531
Madbouly, N. A., Shalash, I. R., El Deeb, S. O., and El Amir, A. M. (2015). Effect of artemether on cytokine profile and egg induced pathology in murine schistosomiasis mansoni. J. Adv. Res. 6 (6), 851–857. doi:10.1016/j.jare.2014.07.003
Mata-Santos, H. A., Dutra, F. F., Rocha, C. C., Lino, F. G., Xavier, F. R., Chinalia, L. A., et al. (2014). Silymarin reduces profibrogenic cytokines and reverses hepatic fibrosis in chronic murine schistosomiasis. Antimicrob. Agents Chemother. 58 (4), 2076–2083. doi:10.1128/AAC.01936-13
Mata-Santos, H. A., Lino, F. G., Rocha, C. C., Paiva, C. N., Castelo Branco, M. T., and Pyrrho Ados, S. (2010). Silymarin treatment reduces granuloma and hepatic fibrosis in experimental schistosomiasis. Parasitol. Res. 107 (6), 1429–1434. doi:10.1007/s00436-010-2014-8
McManus, D. P., Dunne, D. W., Sacko, M., Utzinger, J., Vennervald, B. J., and Zhou, X. N. (2018). Schistosomiasis. Nat. Rev. Dis. Prim. 4 (1), 13. doi:10.1038/s41572-018-0013-8
Molgaard, P., Nielsen, S. B., Rasmussen, D. E., Drummond, R. B., Makaza, N., and Andreassen, J. (2001). Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J. Ethnopharmacol. 74 (3), 257–264. doi:10.1016/s0378-8741(00)00377-9
Mostafa, D. K., Eissa, M. M., Ghareeb, D. A., Abdulmalek, S., and Hewedy, W. A. (2023). Resveratrol protects against Schistosoma mansoni-induced liver fibrosis by targeting the Sirt-1/NF-κB axis. Inflammopharmacology 32, 763–775. doi:10.1007/s10787-023-01382-y
Mostafa, O. M., Eid, R. A., and Adly, M. A. (2011). Antischistosomal activity of ginger (Zingiber officinale) against Schistosoma mansoni harbored in C57 mice. Parasitol. Res. 109 (2), 395–403. doi:10.1007/s00436-011-2267-x
Mukund, V., Mukund, D., Sharma, V., Mannarapu, M., and Alam, A. (2017). Genistein: its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 119, 13–22. doi:10.1016/j.critrevonc.2017.09.004
Naveed, M., Hejazi, V., Abbas, M., Kamboh, A. A., Khan, G. J., Shumzaid, M., et al. (2018). Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother. 97, 67–74. doi:10.1016/j.biopha.2017.10.064
Peng, H., Zhang, Q., Li, X., Liu, Z., Shen, J., Sun, R., et al. (2017). Erratum: IL-33 contributes to schistosoma japonicum-induced hepatic pathology through induction of M2 macrophages. Sci. Rep. 7, 39568. doi:10.1038/srep39568
Phillipson, J. D. (2001). Phytochemistry and medicinal plants. Phytochemistry 56 (3), 237–243. doi:10.1016/s0031-9422(00)00456-8
Ramez, A. M., Elmahallawy, E. K., Elshopakey, G. E., Saleh, A. A., Moustafa, S. M., Al-Brakati, A., et al. (2021). Hepatosplenic protective actions of spirulina platensis and Matcha green tea against schistosoma mansoni infection in mice via antioxidative and anti-inflammatory mechanisms. Front. Vet. Sci. 8, 650531. doi:10.3389/fvets.2021.650531
Saad El-Din, M. I., Gad El-Hak, H. N., Ghobashy, M. A., and Elrayess, R. A. (2023). Parasitological and histopathological studies to the effect of aqueous extract of Moringa oleifera Lam. leaves combined with praziquantel therapy in modulating the liver and spleen damage induced by Schistosoma mansoni to male mice. Environ. Sci. Pollut. Res. Int. 30 (6), 15548–15560. doi:10.1007/s11356-022-23098-2
Sanderson, L., Bartlett, A., and Whitfield, P. J. (2002). In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. J. Helminthol. 76 (3), 241–247. doi:10.1079/JOH2002116
Santana, J. B., de Almeida, T., Lopes, D. M., Page, B., Oliveira, S. C., Souza, I., et al. (2021). Phenotypic characterization of CD4(+) T lymphocytes in periportal fibrosis secondary to schistosomiasis. Front. Immunol. 12, 605235. doi:10.3389/fimmu.2021.605235
Sharaf El-Deen, S. A., Brakat, R. M., and Mohamed, A. (2017). Artichoke leaf extract protects liver of Schistosoma mansoni infected mice through modulation of hepatic stellate cells recruitment. Exp. Parasitol. 178, 51–59. doi:10.1016/j.exppara.2017.05.005
Sharifi-Rad, J., Quispe, C., Imran, M., Rauf, A., Nadeem, M., Gondal, T. A., et al. (2021). Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid. Med. Cell Longev. 2021, 3268136. doi:10.1155/2021/3268136
Shi, H., Dong, L., Jiang, J., Zhao, J., Zhao, G., Dang, X., et al. (2013). Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 303, 107–114. doi:10.1016/j.tox.2012.10.025
Shi, H., Shi, A., Dong, L., Lu, X., Wang, Y., Zhao, J., et al. (2016). Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 35 (6), 1366–1373. doi:10.1016/j.clnu.2016.03.002
Simoben, C. V., Ntie-Kang, F., Akone, S. H., and Sippl, W. (2018). Compounds from african medicinal plants with activities against selected parasitic diseases: schistosomiasis, trypanosomiasis and leishmaniasis. Nat. Prod. Bioprospect 8 (3), 151–169. doi:10.1007/s13659-018-0165-y
Sohn, S. I., Priya, A., Balasubramaniam, B., Muthuramalingam, P., Sivasankar, C., Selvaraj, A., et al. (2021). Biomedical applications and bioavailability of curcumin-an updated overview. Pharmaceutics 13 (12), 2102. doi:10.3390/pharmaceutics13122102
Soliman, R. H., Ismail, O. A., Badr, M. S., and Nasr, S. M. (2017). Resveratrol ameliorates oxidative stress and organ dysfunction in Schistosoma mansoni infected mice. Exp. Parasitol. 174, 52–58. doi:10.1016/j.exppara.2017.02.008
Tan, Z., Liu, Q., Jiang, R., Lv, L., Shoto, S. S., Maillet, I., et al. (2018). Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell Mol. Immunol. 15 (4), 388–398. doi:10.1038/cmi.2016.63
Tang, H., Liang, Y. B., Chen, Z. B., Du, L. L., Zeng, L. J., Wu, J. G., et al. (2017). Soluble egg antigen activates M2 macrophages via the STAT6 and PI3K pathways, and schistosoma japonicum alternatively activates macrophage polarization to improve the survival rate of septic mice. J. Cell Biochem. 118 (12), 4230–4239. doi:10.1002/jcb.26073
Tong, H., Zhang, X., Tan, L., Jin, R., Huang, S., and Li, X. (2020). Multitarget and promising role of dihydromyricetin in the treatment of metabolic diseases. Eur. J. Pharmacol. 870, 172888. doi:10.1016/j.ejphar.2019.172888
Wan, C., Jin, F., Du, Y., Yang, K., Yao, L., Mei, Z., et al. (2017). Genistein improves schistosomiasis liver granuloma and fibrosis via dampening NF-kB signaling in mice. Parasitol. Res. 116 (4), 1165–1174. doi:10.1007/s00436-017-5392-3
Wang, Y., Yang, F., Xue, J., Zhou, X., Luo, L., Ma, Q., et al. (2017). Antischistosomiasis liver fibrosis effects of chlorogenic acid through IL-13/miR-21/smad7 signaling interactions in vivo and in vitro. Antimicrob. Agents Chemother. 61 (2), e01347. doi:10.1128/AAC.01347-16
Wang, Z., Jiang, Q., Li, P., Shi, P., Liu, C., Wang, W., et al. (2023). The water extract of Ampelopsis grossedentata alleviates oxidative stress and intestinal inflammation. Antioxidants (Basel) 12 (3), 547. doi:10.3390/antiox12030547
Wei, M., Zheng, Z., Shi, L., Jin, Y., and Ji, L. (2018). Natural polyphenol chlorogenic acid protects against acetaminophen-induced hepatotoxicity by activating ERK/Nrf2 antioxidative pathway. Toxicol. Sci. 162 (1), 99–112. doi:10.1093/toxsci/kfx230
WHO (2022). Schistosomiasis and soil-transmitted helminthiases: progress report, 2021. Weekly epidemiological record, No. 48, 97, 621–632. World Health Organization. Available at: https://www.who.int/publications/i/item/who-wer9748-621-632 (Accessed June 7, 2023).
Wynn, T. A., Thompson, R. W., Cheever, A. W., and Mentink-Kane, M. M. (2004). Immunopathogenesis of schistosomiasis. Immunol. Rev. 201, 156–167. doi:10.1111/j.0105-2896.2004.00176.x
Xiao, S. H., You, J. Q., Jiao, P. Y., and Mei, J. Y. (1994). Effect of early treatment of artemether against schistosomiasis in mice. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 12 (1), 7–12.
Xie, K., He, X., Chen, K., Chen, J., Sakao, K., and Hou, D. X. (2019). Antioxidant properties of a traditional vine tea, Ampelopsis grossedentata. Antioxidants (Basel) 8 (8), 295. doi:10.3390/antiox8080295
Xing, L., Zhang, H., Qi, R., Tsao, R., and Mine, Y. (2019). Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 67 (4), 1029–1043. doi:10.1021/acs.jafc.8b06146
Xu, L., Ma, P., Xiao, W., Peng, Y., He, C., and Xiao, P. (2012). Preliminary lnvestigation into the ancient and modern application of vine tea. Mod. Chin. Med. 14 (04), 62–66. doi:10.13313/j.issn.1673-4890.2012.04.019
Yang, F., Luo, L., Zhu, Z. D., Zhou, X., Wang, Y., Xue, J., et al. (2017). Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/smad7 signaling pathway in vitro and in vivo. Front. Pharmacol. 8, 929. doi:10.3389/fphar.2017.00929
Yang, F., Wang, Y., Xue, J., Ma, Q., Zhang, J., Chen, Y. F., et al. (2016). Effect of Corilagin on the miR-21/smad7/ERK signaling pathway in a schistosomiasis-induced hepatic fibrosis mouse model. Parasitol. Int. 65 (4), 308–315. doi:10.1016/j.parint.2016.03.001
Yang, Q. Q., Wei, X. L., Fang, Y. P., Gan, R. Y., Wang, M., Ge, Y. Y., et al. (2020). Nanochemoprevention with therapeutic benefits: an updated review focused on epigallocatechin gallate delivery. Crit. Rev. Food Sci. Nutr. 60 (8), 1243–1264. doi:10.1080/10408398.2019.1565490
You, J. Q., Mei, J. Y., and Xiao, S. H. (1992). Effect of artemether against Schistosoma japonicum. Zhongguo Yao Li Xue Bao 13 (3), 280–284.
Yu, Y., Wang, J., Wang, X., Gu, P., Lei, Z., Tang, R., et al. (2021). Schistosome eggs stimulate reactive oxygen species production to enhance M2 macrophage differentiation and promote hepatic pathology in schistosomiasis. PLoS Negl. Trop. Dis. 15 (8), e0009696. doi:10.1371/journal.pntd.0009696
Yuan, Y., Gong, X., Zhang, L., Jiang, R., Yang, J., Wang, B., et al. (2017). Chlorogenic acid ameliorated concanavalin A-induced hepatitis by suppression of Toll-like receptor 4 signaling in mice. Int. Immunopharmacol. 44, 97–104. doi:10.1016/j.intimp.2017.01.017
Zhai, Z. L., Zhang, Y., Liu, H. X., Feng, T., and Xiao, S. H. (2000). Effect of artemether on enzymes involved in carbohydrate metabolism of Schistosoma japonicum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 18 (3), 162–164.
Zhang, L., and Wei, W. (2020). Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 207, 107452. doi:10.1016/j.pharmthera.2019.107452
Zhang, L. X., Li, C. X., Kakar, M. U., Khan, M. S., Wu, P. F., Amir, R. M., et al. (2021a). Resveratrol (RV): a pharmacological review and call for further research. Biomed. Pharmacother. 143, 112164. doi:10.1016/j.biopha.2021.112164
Zhang, M., Zhao, R., Wang, D., Wang, L., Zhang, Q., Wei, S., et al. (2021b). Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 35 (2), 711–742. doi:10.1002/ptr.6858
Zhang, Q., Zhao, Y., Zhang, M., Zhang, Y., Ji, H., and Shen, L. (2021c). Recent advances in research on vine tea, a potential and functional herbal tea with dihydromyricetin and myricetin as major bioactive compounds. J. Pharm. Anal. 11 (5), 555–563. doi:10.1016/j.jpha.2020.10.002
Zhang, Y., Zhou, X., Yi, L., Peng, S., Zhang, Q., and Mi, M. (2018). Dihydromyricetin attenuates activation of hepatic stellate cells through TGF-B1/Smad signaling pathway. J. Army Med. Univ. 40 (04), 282–289. doi:10.16016/j.1000-5404.201709210
Keywords: natural products, plant extracts, schistosomiasis, liver disease, fibrosis
Citation: Liu C, Fisher D, Pronyuk K, Musabaev E, Thu Hien NT, Dang Y and Zhao L (2024) Therapeutic potential of natural products in schistosomiasis-associated liver fibrosis. Front. Pharmacol. 15:1332027. doi: 10.3389/fphar.2024.1332027
Received: 02 November 2023; Accepted: 10 April 2024;
Published: 06 May 2024.
Edited by:
Chunlei Zhang, China Pharmaceutical University, ChinaReviewed by:
Liang Shan, Anhui Medical University, ChinaCopyright © 2024 Liu, Fisher, Pronyuk, Musabaev, Thu Hien, Dang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhao, bGVpemhhb0BodXN0LmVkdS5jbg==; Yiping Dang, MjQ0OTI3MTYwQHFxLmNvbQ==
 Cuiling Liu
Cuiling Liu David Fisher
David Fisher Khrystyna Pronyuk3
Khrystyna Pronyuk3 Lei Zhao
Lei Zhao