- 1The Ohio State University College of Medicine, Columbus, OH, United States
- 2Department of Anesthesiology and Pain Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 3Department of Psychiatry, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 4Department of Internal Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, United States
Effective pain management is essential for optimal surgical outcomes; however, it can be challenging in patients with a history of opioid use disorder (OUD). Buprenorphine, a partial opioid agonist, is a valuable treatment option for patients with OUD. Initiating buprenorphine treatment in patients concurrently taking opioids can be complex due to potential adverse outcomes like precipitated withdrawal. Evolving guidelines suggest there are benefits to continuing buprenorphine for surgical patients throughout the perioperative period, however situations do arise when buprenorphine has been discontinued. Typically, in this scenario patients would be restarted on buprenorphine after they have fully recovered from post-surgical pain and no longer require opioids for pain control. Unfortunately, holding MOUD may expose the patient to risks such as opioid induced respiratory depression or addiction relapse. In this case series, we discuss a novel method to restart buprenorphine in small incremental doses, known as micro-dosing, while the patient is still taking opioids for pain. We will present two complex clinical cases when this method was used successfully at a tertiary care hospital system.
Introduction
The opioid epidemic is one of the most pressing public health crises facing the United States today. In the 12 month period ending in December 2022, an estimated 82,310 people died from opioid overdose (CDC, 2023). This statistic marks a worrying continuation in the rise of drug overdoses seen since the onset of the COVID-19 pandemic (Centers for Disease Control and Prevention, 2020). Contributing to the increasing scale of the epidemic is the rise in prevalence of synthetic opioids such as fentanyl (CDC, 2022). The true scale of the opioid epidemic becomes clear through studies that show the true prevalence of people living with OUD is between 6–7 million (Keyes et al., 2022).
Medication treatment of Opioid Use Disorder (MOUD) has been shown to be highly effective in reducing the overall rates of mortality and relapse in patients (Sordo et al., 2017). However, there are certain circumstances when patients on MOUD are at risk for relapse. These include the induction period, the cessation of MOUD due to medical care, and the time period after hospital discharge prior to following up with outpatient addiction clinic (White et al., 2015; Sordo et al., 2017; Kohan et al., 2021). Newer research and guidelines advocate for the continuation of buprenorphine postoperatively given these risks (American Society of Addiction Medicine, 2020; Kohan et al., 2021). Additionally, it has been shown that buprenorphine may improve mortality in hospitalized patients with a history of OUD even in patients who had not been taking buprenorphine prior to hospitalization (Evans et al., 2015; Kohan et al., 2021; Button et al., 2022). Furthermore, buprenorphine treatment likely increases patient quality of life across a range of metrics (Golan et al., 2022). Unfortunately, hurdles to addiction treatment exist as many patients are hesitant to start or continue buprenorphine perioperatively due to fears of precipitated withdrawal, inadequate pain control, or limited time in the hospital (Button et al., 2022).
Historically it was recommended that buprenorphine be discontinued prior to surgery due to fears of inadequate pain control. MOUD would then be restarted once the patient no longer needed opioids for pain control. Unfortunately, perioperative discontinuation of MOUD put these patients at higher risk of OUD relapse as well as other complications such as respiratory depression (Kohan et al., 2021; Komatsu et al., 2021). To avoid these risks, micro-dosing protocols have been attempted as a way to initiate MOUD while continuing opioid medications. These protocols could allow patients to restart MOUD earlier while avoiding withdrawal symptoms, thereby reducing the risks associated with stopping buprenorphine in the perioperative period. Despite the early promise of micro induction, there is still a lack of consensus surrounding ideal dosing, time frame, and patient selection (Ahmed et al., 2021). In this case series we summarize current literature on the micro-induction of buprenorphine in the postoperative period. We also present our institution’s successful experience using patient tailored micro-induction protocols. Here, two patient cases demonstrate how micro-dosing protocols can be best applied and adapted to the individual patient.
Current state of perioperative buprenorphine management
Buprenorphine is a highly effective drug treatment of OUD but concerns about its initiation (also known as induction) have traditionally limited its use in practice. Buprenorphine binds the μ-opioid receptor with a high affinity and thus outcompetes other opioids at the opioid receptor. When a patient has opioids in their system and has developed a tolerance to opioids, buprenorphine induction could lead to “precipitated withdrawal.” Precipitated withdrawal is the development of intense and sudden withdrawal symptoms. To avoid this, buprenorphine is typically initiated while patients are already in clinical withdrawal and have abstained from opioids (American Society of Addiction Medicine, 2020).
Though guidelines have evolved to now recommend continuation of buprenorphine in the perioperative period, variations in clinical practice still exist (Acampora et al., 2020; Wyse et al., 2022). One retrospective cohort study using Veterans Affairs Corporate Data Warehouse found that 66% of patients experienced a perioperative buprenorphine dose hold and that a year after surgery, 33% of patients lacked an active buprenorphine prescription (Wyse et al., 2022).
Patients with OUD may face more challenges with their postoperative care, like increased severity of pain following surgery and may require higher doses of opioids for adequate pain control (Cleary and Rood, 2022). In addition, nearly half of patients on MOUD may also suffer from chronic pain. (Delorme et al., 2023). Pain control perioperatively and prompt initiation of MOUD is of particular concern in this patient population. Studies have found that for patients taking MOUD, momentary pain can create cravings and may be linked to relapse (Mun et al., 2021).
Micro-induction techniques can help patients start buprenorphine perioperatively while minimizing the risks of precipitated withdrawal and effects on pain control. Such protocols are used to decrease the risk of precipitated withdrawal and allow for timely treatment of acute pain (Ahmed et al., 2021). Low doses of buprenorphine activate a few mu opioid receptors at a time minimizing symptoms of precipitated withdrawal. Furthermore, introducing small doses of buprenorphine enables clinicians to start or re-start OUD treatment earlier, therefore preventing relapse in patients whose MOUD was stopped for surgery. However, given the multifactorial nature of OUD and the lack of consensus regarding MOUD micro-induction, it is important to work with patients and tailor induction to their unique situations. This is echoed by the 2020 ASAM National Practice Guideline for the Treatment of Opioid Use Disorder which recommends that “decisions related to discontinuing or adjusting the dose of buprenorphine prior to a planned surgery should be made on an individual basis, through consultation between the surgical and anesthesia teams and the addiction treatment provider when possible” (American Society of Addiction Medicine, 2020).
Current micro-induction strategies
The micro-induction technique is versatile and can be used to start buprenorphine for the first time, to convert MOUD from methadone to buprenophine, and in cliical scenarios with an increased risk of precipitated withdrawal. De Aquino, et al. describes success in the outpatient setting using buprenorphine transdermal patches for rapid micro-induction (De Aquino et al., 2020). Similar success was described by Silva, et al. in using the FOOT STEP protocol for outpatient micro-induction (Jasmine Silva et al., 2022). Of note, considerable variation existed among all these protocols further emphasizing the importance of adapting treatments to individual patients. Hammig, et al. describes multiple micro-dosing protocols for the re-induction of buprenorphine (Hämmig et al., 2016). Similar protocols have since been adapted in several other case studies to successfully induce and transition patients on methadone to buprenorphine (De Aquino et al., 2020; Jasmine Silva et al., 2022).
More recent studies have also explored micro-induction in the inpatient setting. DeWeese et al., reported a successful experience using an accelerated schedule in hospitalized patients. They were able to administer sizable doses over the course of 3 days and even a fully therapeutic dose of 8 mg TID over the subsequent 6 days (DeWeese et al., 2021). Another retrospective cohort study described three different micro-induction protocols. The most common method utilized in that study started buprenorphine at 0.5 mg and titrated to 4 mg BID over 6 days. A rapid-micro induction technique was also trialed which started at 0.5 mg q3-q4h. It was found that most of the patients who elected to discontinue buprenorphine initiation due to side effects were undergoing rapid-micro induction (Nunn et al., 2023). This suggests that hospital based micro-induction is feasible and effective, however careful attention to side effects is required. One characteristic that was shared by these studies was that the majority of patients on micro-induction of buprenorphine were titrated to a lower dose of 8 mg daily while on full-agonist opioids and then increased to doses between 12 and 16 mg daily once opioids were discontinued (DeWeese et al., 2021; Kohan et al., 2021).
Despite the success of micro-inductions in the literature, there remains significant variability in accepted protocol. Hjelmstrom et al.’s review of buprenorphine efficacy data could not reach any firm conclusions on dosage, or protocol, from the existing data. They recommended buprenorphine should be largely “individualized based on a continuous benefit-risk assessment” (Hjelmström et al., 2020). Together these studies show that micro-induction can be an effective tool despite the lack of consensus on protocol.
Patient selection
Patient selection is important for successful induction of buprenorphine using micro-dosing protocol. Patients who can undergo micro-induction are those who have used illicit opioids in the preceding 5 days. Another group includes patients with OUD who are hospitalized with injury or infection causing acute pain and are receiving short acting opioids to manage pain. Using a micro-induction protocol in this circumstance decreases the 12-h waiting period after the last dose of opioid and enables quicker pain treatment. Additionally, patients who are currently on buprenorphine which has been discontinued or held for longer than 24 h over the course of treatment are also good candidates.
Micro-induction of buprenorphine is not recommended for patients who necessitate standard induction protocol. These include patients whose last illicit opioid use was more than 5 days ago and those in severe withdrawal. Patients must consent to any form of MOUD induction, and patients who have an allergy to buprenorphine buccal film or patch are also not candidates for micro-induction.
Variable dosing schedules
The Ohio State University Medical Center (OSUMC) addiction service has utilized various micro-dosing schedules. Patient can be initially started on buprenorphine buccal film (Belbuca™) and then transitioned to sublingual buprenorphine formulations (Suboxone™, Subutex™). Alternatively, patients can be started on a buprenorphine transdermal patch (Butrans™), then transitioned to buprenorphine buccal and then ultimately switched to sublingual buprenorphine formulations. Examples of the most commonly used dosing schedules are outlined in Figure 1.
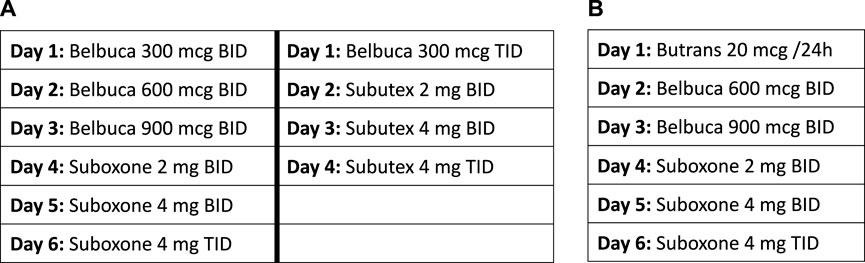
FIGURE 1. The Ohio State University variable dosing schedules. (A) Example dosing schedule utilizing buccal film. (B) Example dosing schedule utilizing buccal film and transdermal patch.
Case series
Case 1
Patient information and clinical findings
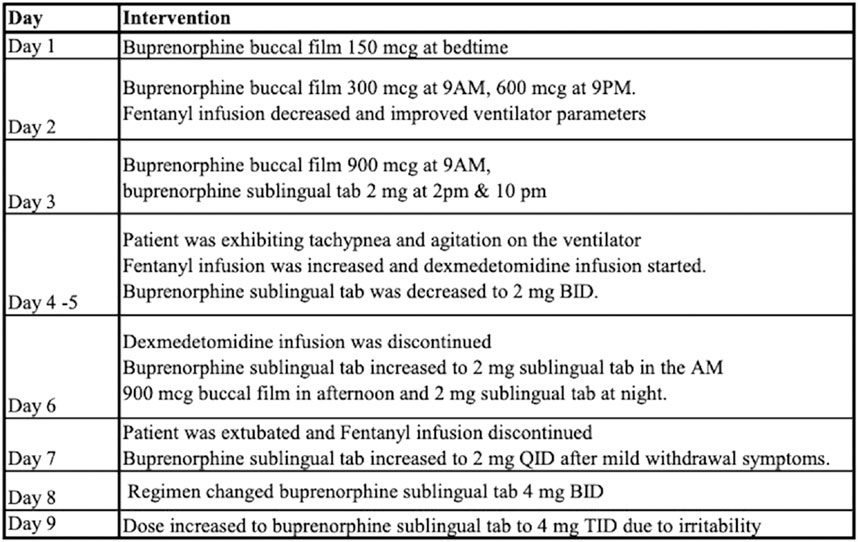
Patient 1 is a critically ill female in her thirties transferred to our institution from an outside facility for management of sepsis and respiratory failure due to pneumonia. Upon arrival she was mechanically ventilated and in septic shock requiring vasopressor support and broad-spectrum antibiotics. Pertinent medical history includes OUD treated with maintenance buprenorphine-naloxone which was discontinued. She was sedated with fentanyl and midazolam infusions. Later in the hospitalization, the addiction service was consulted for recommendations on restarting MOUD. Review of her prescription drug monitoring program history revealed that she had consistently been on buprenorphine-naloxone 8–2 mg daily. After discussion with immediate family, it was determined that restarting MOUD was in line with the patient’s treatment goals. A micro-dosing protocol was tailored for this specific clinical circumstances and is outlined in Figure 2. While the patient was on the micro-dosing protocol, breakthrough pain was controlled with short acting full agonist opioids (ex. hydromorphone) and medications for the treatment of opioid withdrawal were administered when needed.
Outcomes and follow-up
Patient was discharged home on HD17 on buprenorphine sublingual tab 4 mg TID with outpatient follow up schedule with addiction clinic.
Case 2
Patient information and clinical findings
Patient 2 is a male in his late thirties, with unknown past medical history upon admission to an outside hospital sustaining injuries after a motor vehicle accident. The patient presented obtunded with a Glasgow Coma Scale 5 and was intubated. Workup revealed facial, ankle and rib fractures, a left sided pneumothorax, and a subarachnoid hemorrhage. In addition, he sustained injury to the right iliac artery and was transferred to our institution for further management.
He underwent multiple procedures including:
HD1: IR embolization of iliac artery. External fixation of left ankle and closed reduction of right hip with traction pinning.
HD6: Tracheostomy, ORIF of Le Fort facial fractures, dental extractions, and PEG tube placement.
HD7: Hip acetabular fracture repair.
HD15: Exploratory laparotomy and splenectomy after development of acute abdomen and hemorrhagic shock.
HD16: Abdominal wound closure.
HD18: ORIF ankle fracture.
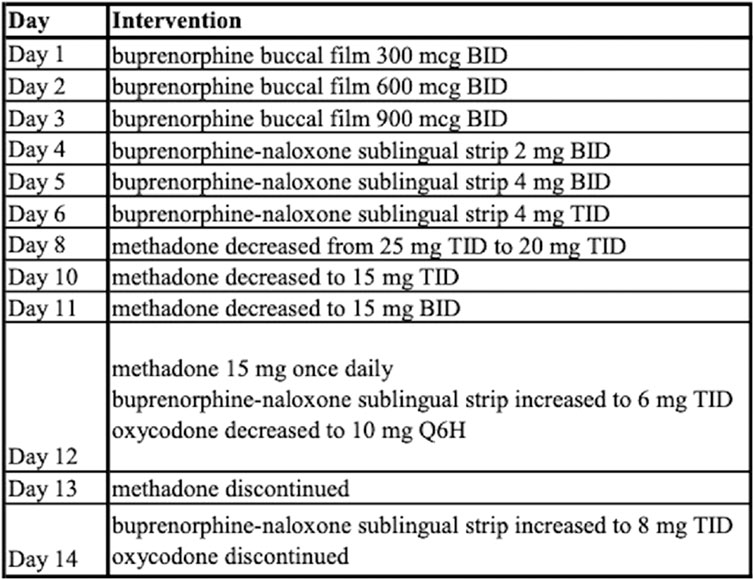
The addiction service was consulted on day 24 and the patient discussed history of substance use disorder and consented to buprenorphine initiation. A buprenorphine micro-dosing schedule was initiated as outlined in Figure 3. The patient’s pain was managed initially with hydromorphone and ketamine infusions along with oral methadone 25 mg TID. He was then transitioned to intermittent doses of intravenous hydromorphone and scheduled oral oxycodone which was tapered and discontinued prior to discharge.
Outcomes and follow-up
Patient discharged home on day 43 with prescription for buprenorphine-naloxone sublingual strip 8 mg TID with follow up planned at a local addiction clinic.
Discussion/conclusion
The current literature supports continuing buprenorphine during episodes of acute pain as it offers distinct advantages (Kohan et al., 2021). By capitalizing on buprenorphine’s unique pharmacological properties, clinicians can achieve effective pain control while minimizing the risk of opioid withdrawal, and potentially use buprenorphine as a bridge between analgesia and addiction management (Ahmed et al., 2021; Edinoff et al., 2023). This approach aligns with the overarching shift towards individualized pain management paradigms and reinforces the importance of personalized care plans.
Specific to micro-induction, the existing body of literature is conflicted on its utility. A recent systematic review of buprenorphine micro-induction by Spreen et al., concluded that micro-induction protocols were comparable to traditional initiation protocols, and effectively reduce withdrawal symptoms (Spreen et al., 2022). However, a separate review and pharmacological model suggests that micro-induction protocols may have limited use, and that traditional induction may be a more effective method of induction in many settings (Greenwald et al., 2022). Given the ongoing controversy in the field, case series like this one offer additional value in contributing to this rapidly evolving literature.
Our case series provides a tangible example of the successful micro-dosing of buprenorphine in patients on concurrent full opioid agonists. The seamless integration of buprenorphine within a multimodal analgesic regimen resulted in optimal pain relief, mitigated opioid cravings, and facilitated a smoother recovery process. However, the case also highlights the significance of meticulous patient selection, interdisciplinary collaboration, and judicious dose adjustment to ensure patient safety and favorable outcomes. This is consistent with literature reviews, which emphasize the importance of protocol flexibility to treat patients most effectively across wide ranges of settings (Ahmed et al., 2021).
While the reviewed evidence and case studies underscore the potential benefits, it is crucial to acknowledge the complexities associated with continuing buprenorphine in the setting of acute pain. Challenges such as individual variability in response, potential drug interactions, and the need for clear communication among clinicians is paramount (Spreen et al., 2022; Edinoff et al., 2023). Further research is warranted to delve into the nuances of dosing strategies, patient selection criteria, and the long-term impact of this approach on pain trajectories and addiction management. As the medical community continues to advance in its understanding addiction treatment, collaborative efforts will play a pivotal role in shaping its integration strategies like micro-induction, thereby optimizing outcomes for patients facing the intersection of acute pain and opioid use disorder.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TS: Writing–original draft. SN: Conceptualization, Supervision, Writing–original draft, Writing–review and editing. OH: Writing–review and editing, Methodology. MW: Data curation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acampora, G. A., Nisavic, M., and Zhang, Y. (2020). Perioperative buprenorphine continuous maintenance and administration simultaneous with full opioid agonist: patient priority at the interface between medical disciplines. J. Clin. Psychiatry 81 (1), 19com12810. doi:10.4088/JCP.19com12810
Ahmed, S., Bhivandkar, S., Lonergan, B. B., and Suzuki, J. (2021). Microinduction of buprenorphine/naloxone: a review of the literature. Am. J. Addict. 30 (4), 305–315. doi:10.1111/ajad.13135
American Society of Addiction Medicine (2020). The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J. Addict. Med. 14 (2S), 1–91. doi:10.1097/ADM.0000000000000633
Button, D., Hartley, J., Robbins, J., Levander, X. A., Smith, N. J., and Englander, H. (2022). Low-dose buprenorphine initiation in hospitalized adults with opioid use disorder: a retrospective cohort analysis. J. Addict. Med. 16 (2), e105–e111. doi:10.1097/ADM.0000000000000864
CDC (2022). Understanding the opioid overdose epidemic, opioids. Available from: https://www.cdc.gov/opioids/basics/epidemic.html (Accessed July 8, 2023).
CDC (2023). Provisional data shows U.S. Drug overdose deaths top 100,000 in 2022, blogs. Available from: https://blogs.cdc.gov/nchs/2023/05/18/7365/ (Accessed July 8, 2023).
Centers for Disease Control and Prevention (2020). Coronavirus Disease 2019. Available from: https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html (Accessed June 26, 2023).
Cleary, E. M., and Rood, K. M. (2022). Postoperative cesarean pain management and opioid use disorder: anticipate the need for higher opioid doses and communicate expectations with patients and the obstetric team. J. Addict. Med. 16 (5), 495–498. doi:10.1097/ADM.0000000000000963
De Aquino, J. P., Fairgrieve, C., Klaire, S., and Garcia-Vassallo, G. (2020). Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J. Addict. Med. 14 (5), e271–e273. doi:10.1097/ADM.0000000000000618
Delorme, J., Kerckhove, N., Authier, N., Pereira, B., Bertin, C., and Chenaf, C. (2023). Systematic review and meta-analysis of the prevalence of chronic pain among patients with opioid use disorder and receiving opioid substitution therapy. J. Pain 24 (2), 192–203. doi:10.1016/j.jpain.2022.08.008
DeWeese, J. P., Krenz, J. R., Wakeman, S. E., and Peckham, A. M. (2021). Rapid buprenorphine microdosing for opioid use disorder in a hospitalized patient receiving very high doses of full agonist opioids for acute pain management: titration, implementation barriers, and strategies to overcomes. Subst. Abuse 42 (4), 506–511. doi:10.1080/08897077.2021.1915914
Edinoff, A. N., Fahmy, O. H., Spillers, N. J., Zaheri, A. R., Jackson, E. D., De Witt, A. J., et al. (2023). Low-dose initiation of buprenorphine: a narrative review. Curr. Pain Headache Rep. 27 (7), 175–181. doi:10.1007/s11916-023-01116-3
Evans, E., Li, L., Min, J., Huang, D., Urada, D., Liu, L., et al. (2015). Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addict. Abingdon Engl. 110 (6), 996–1005. doi:10.1111/add.12863
Golan, O. K., Totaram, R., Perry, E., Fortson, K., Rivera-Atilano, R., Entress, R., et al. (2022). Systematic review and meta-analysis of changes in quality of life following initiation of buprenorphine for opioid use disorder. Drug Alcohol Depend. 235, 109445. doi:10.1016/j.drugalcdep.2022.109445
Greenwald, M. K., Herring, A. A., Perrone, J., Nelson, L. S., and Azar, P. (2022). A neuropharmacological model to explain buprenorphine induction challenges. Ann. Emerg. Med. 80 (6), 509–524. doi:10.1016/j.annemergmed.2022.05.032
Hämmig, R., Kemter, A., Strasser, J., Bardeleben, U., Gugger, B., Walter, M., et al. (2016). Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the “Bernese method”. Subst. Abuse Rehabil. 7, 99–105. doi:10.2147/SAR.S109919
Hjelmström, P., Banke Nordbeck, E., and Tiberg, F. (2020). Optimal dose of buprenorphine in opioid use disorder treatment: a review of pharmacodynamic and efficacy data. Drug Dev. Ind. Pharm. 46 (1), 1–7. doi:10.1080/03639045.2019.1706552
Jasmine Silva, M., Coffee, Z., Goza, J., and Rumrill, K. (2022). Microinduction to buprenorphine from methadone for chronic pain: outpatient protocol with case examples. J. Pain Palliat. Care Pharmacother. 36 (1), 40–48. doi:10.1080/15360288.2022.2049422
Keyes, K. M., Rutherford, C., Hamilton, A., Barocas, J. A., Gelberg, K. H., Mueller, P. P., et al. (2022). What is the prevalence of and trend in opioid use disorder in the United States from 2010 to 2019? Using multiplier approaches to estimate prevalence for an unknown population size. Drug Alcohol Depend. Rep. 3, 100052. doi:10.1016/j.dadr.2022.100052
Kohan, L., Potru, S., Barreveld, A. M., Sprintz, M., Lane, O., Aryal, A., et al. (2021). Buprenorphine management in the perioperative period: educational review and recommendations from a multisociety expert panel. Reg. Anesth. Pain Med. 46 (10), 840–859. doi:10.1136/rapm-2021-103007
Komatsu, R., Nash, M., Peperzak, K. A., Wu, J., Dinges, E. M., and Bollag, L. A. (2021). Postoperative pain and opioid dose requirements in patients on sublingual buprenorphine: a retrospective cohort study for comparison between postoperative continuation and discontinuation of buprenorphine. Clin. J. Pain 38 (2), 108–113. doi:10.1097/AJP.0000000000000996
Mun, C. J., Finan, P. H., Epstein, D. H., Kowalczyk, W. J., Agage, D., Letzen, J. E., et al. (2021). Craving mediates the association between momentary pain and illicit opioid use during treatment for opioid-use disorder: an ecological momentary assessment study. Addiction 116 (7), 1794–1804. doi:10.1111/add.15344
Nunn, R., Sylvestre, A., Sequeira, K., and Tanzini, R. M. (2023). Buprenorphine/naloxone micro-induction in a tertiary care hospital: a retrospective cohort analysis. J. Addict. Dis. 1–7, 1–7. doi:10.1080/10550887.2023.2229609
Sordo, L., Barrio, G., Bravo, M. J., Indave, B. I., Degenhardt, L., Wiessing, L., et al. (2017). Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 357, j1550. doi:10.1136/bmj.j1550
Spreen, L. A., Dittmar, E. N., Quirk, K. C., and Smith, M. A. (2022). Buprenorphine initiation strategies for opioid use disorder and pain management: a systematic review. Pharmacotherapy 42 (5), 411–427. doi:10.1002/phar.2676
White, S. R., Bird, S. M., Merrall, E. L. C., and Hutchinson, S. J. (2015). Drugs-related death soon after hospital-discharge among drug treatment clients in scotland: record linkage, validation, and investigation of risk-factors. PLoS ONE 10 (11), e0141073. doi:10.1371/journal.pone.0141073
Keywords: MOUD, OUD, micro-dosing, Belbuca, buprenophine
Citation: Shelton T, Nama S, Hall O and Williams M (2024) Case report: Successful induction of buprenorphine in medically complex patients concurrently on opioids: a case series at a tertiary care center. Front. Pharmacol. 15:1335345. doi: 10.3389/fphar.2024.1335345
Received: 08 November 2023; Accepted: 08 February 2024;
Published: 08 March 2024.
Edited by:
Sergio Daniel Bergese, Stony Brook University, United StatesReviewed by:
Vinod Narla, University of California, San Diego, United StatesRohini Kotha, Moffitt Cancer Center, United States
Copyright © 2024 Shelton, Nama, Hall and Williams. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharanya Nama, c2hhcmFueWEubmFtYUBvc3VtYy5lZHU=
 Thomas Shelton1
Thomas Shelton1 Sharanya Nama
Sharanya Nama Margaret Williams
Margaret Williams