- Center of Integrative Medicine, Beijing Ditan Hospital Affiliated to Capital Medical University, Beijing, China
The study aimed to investigate the potential of traditional Chinese medicine (TCM) in reducing the risk of macrovascular invasion (MVI) in Chinese patients with hepatocellular carcinoma (HCC). This retrospective analysis involved 2,267 HCC patients treated at our hospital. Propensity score (PS) matching was used to compare TCM users (n = 485) with non-users (n = 485) in terms of age, Barcelona Clinic Liver Cancer (BCLC) staging, type of treatment, and AFP. The impact of TCM on the hazard ratio (HR) of MVI was evaluated using a Cox multivariate regression model. The efficacy of TCM therapy on MVI was further examined using the log-rank test. The analysis revealed that TCM medication was a significant protective factor for MVI in HCC patients, as evidenced by the Cox analysis (adjusted HR = 0.496, 95% CI: 0.387–0.635, p < 0.001). After PS matching, the Kaplan-Meier curve demonstrated a lower occurrence rate of MVI in TCM users compared to non-users. The study findings suggest that TCM treatment has the potential to decrease the incidence of MVI in HCC patients, irrespective of etiology, BCLC staging, liver function, or treatment type. Notably, as the use of TCM increased, the percentage of MVI in patients showed a gradual decrease, indicating the potential of TCM therapy as a successful strategy for preventing MVI.
Introduction
According to recent international research statistics, the global incidence of hepatocellular carcinoma (HCC) in 2020 was reported to be 905,600, resulting in 830,100 deaths—an increase of 48,500 cases compared to 2018. Notably, China accounted for over half of the new cases and fatalities (Sung et al., 2021). Numerous patients were in middle to late stages of HCC when diagnosed because of the liver’s robust ability to compensate, the absence of early HCC symptoms, and the disease’s quick progression (Zhong et al., 2017). Microvascular invasion and macrovascular invasion (MVI) are two types of vascular invasion. The growth of microvascular invasion over time leads to MVI, which is a sign of advanced HCC, according to BCLC recommendations. Research has shown that patients with HCC had an incidence of MVI up to 62.2%. Median survival times of patients with MVI are only up to 4 months once a tumor thrombus has formed in the portal vein (Forner et al., 2018). As a result, MVI is frequently present in HCC patients and the prognosis once it occurs is very poor.
The field of HCC treatment is characterized by multi-disciplinary participation and the coexistence of multiple treatment methods. The common treatment methods include hepatectomy, liver transplantation, ablation, transarterial chemoembolization (TACE), radiotherapy, systematic anti-tumor therapy and so on. Choosing reasonable treatment methods for patients with different stages of HCC can maximize the curative effect (Omata et al., 2017). TACE, radiofrequency ablation (RFA), and surgical various surgical procedures (transplantation of the liver, resection) and other treatments are available for HCC patients without MVI. RFA and TACE are common treatment approaches that can be used for various tumor stages among them (Llovet et al., 2021a). Vascular endothelial growth factor (VEGF) is a cytokine. After treatment with RFA (Guan et al., 2015; Markezana et al., 2021) and TACE (Petrillo et al., 2018), the concentration of VEGF in HCC patients increases. The growth of tumors and vascular invasion can be accelerated by VEGF by impairing T cell function, increasing the recruitment of T regulatory cells, myelogenous suppressor cells (MDSC), and mast cells, and preventing dendritic cell development and activation (Fukumura et al., 2018; Yang et al., 2018). Interestingly, as an effective multi kinase inhibitor against the VEGF receptor, sorafenib combined with TACE is considered to have synergistic effect in the treatment of HCC. Unfortunately, throughout the past 10 years, a number of multicenter randomised controlled trials (Lencioni et al., 2016; Meyer et al., 2017; Park et al., 2019) failed to show the BCLC and TACE combination’s anticipated synergistic effects as a unique treatment for patients with advanced disease. Brivanib, a humanized monoclonal antibody against VGEF, has become a widely administered choice for anti-angiogenesis therapy (Galanopoulos et al., 2016). Unfortunately, TACE combined with brivanib and tyrosine kinase inhibitors (such as orantinib) also showed negative treatment benefits (Kudo et al., 2014; Kudo et al., 2018). Therefore, the ideal candidate for TACE combined with molecular targeted drugs for the treatment of HCC or biomarkers for predicting MVI remain to be unequivocally identified. In order to reduce the incidence of MVI and weaken the adverse reactions caused by treatment, patients are given supplementary replacement therapy (Shi et al., 2017).
In China, traditional Chinese medicine (TCM) is the predominant alternative therapy for individuals with chronic liver disease. Studies have demonstrated that TCM can prevent tumour escape, progression, and metastasis as well as liver cancer growth in the particular immunological tolerance setting of HCC (Wang et al., 2015; Jia and Wang, 2020). It has been found that TCM can block the mTOR/HIF-1α/VEGF signaling pathway which effectively inhibits the migration, invasion and angiogenesis of colon cancer cells (Peng et al., 2018). So far, TCM derived metabolites have shown great potential in slowing tumor progression by downregulating VEGF-related signal pathways (Zhang C. et al., 2018). There has been, however, only limited clinical research on MVI and anti-tumor Chinese patent medication in patients with HCC. Assessing the influence of TCM adjuvant therapy in combination with conventional chemoradiotherapy on the occurrence of MVI, the research analyzed the impact of TCM treatment on HCC from various causes. The findings of this study offer robust guidelines for the utilization of adjuvant TCM therapy for patients with HCC.
Methods
Patients and follow-up
In our hospital, 2,267 individuals were diagnosed with primary liver cancer between January 2009 and December 2020. The research project received approval from the ethical committee at Capital Medical University’s Beijing Ditan Hospital, along with a waiver of informed consent. All procedures were conducted in accordance with the 2008 Helsinki Declaration and the ethical standards outlined by the relevant national and institutional committees governing human research. Since the observation population was patients without MVI, we also selected patients for inclusion in minimally invasive treatment recommended by the guidelines (Omata et al., 2017). The study’s inclusion criteria comprised: 1) primary liver cancer; 2) absence of vascular invasion; 3) ages ranging from 18 to 75; and 4) history of receiving TACE, RFA, or a combination of both. Exclusion criteria included cholangiocarcinoma (n = 298 patients), subsequent liver cancer (n = 135), co-occurrence with other tumor types (n = 103), lack of follow-up (n = 228 patients), and inadequate clinical records (n = 230 patients). As depicted in Figure 1, a total of 1,273 patients was included in the study and categorized into two groups: those utilizing TCM extensively and those who did not. The groups were matched in a 1:1 ratio based on age, BCLC stage, type of treatment, and AFP levels. The primary outcome of interest was the occurrence of MVI in patients with HCC. The observation period for each patient extended from the commencement of their participation in the study to the development of MVI or the date of 31 December 2021, whichever came first.
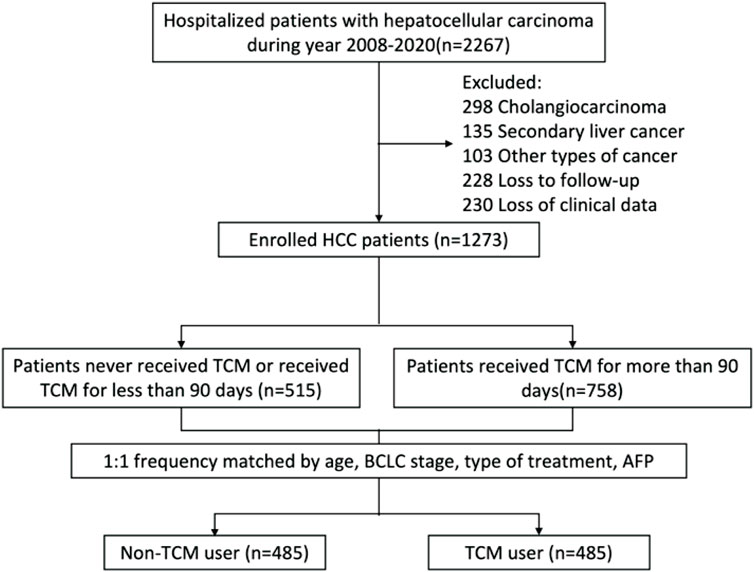
FIGURE 1. An overview of the identification and recruitment procedures for the hepatocellular carcinoma study cohort. TCM, traditional Chinese medicine.
Diagnostic criteria for HCC and MVI
HCC is diagnosed by histopathology at first, and then by clinical diagnosis. Two imaging methods (liver ultrasound, CT, hepatic angiography and MRI) indicate the lesions, or one imaging method indicates the lesions with alpha-fetoprotein (AFP) ≥ 400 ng/mL (Llovet et al., 2021b). Using enhanced CT or enhanced MRI imaging, MVI was diagnosed when filling defects occurred in the portal or hepatic veins, inferior vena cava and when embolus enhancement was similar to that of HCC (Shah et al., 2007).
The treatment of TCM and the composition of each TCM formulation
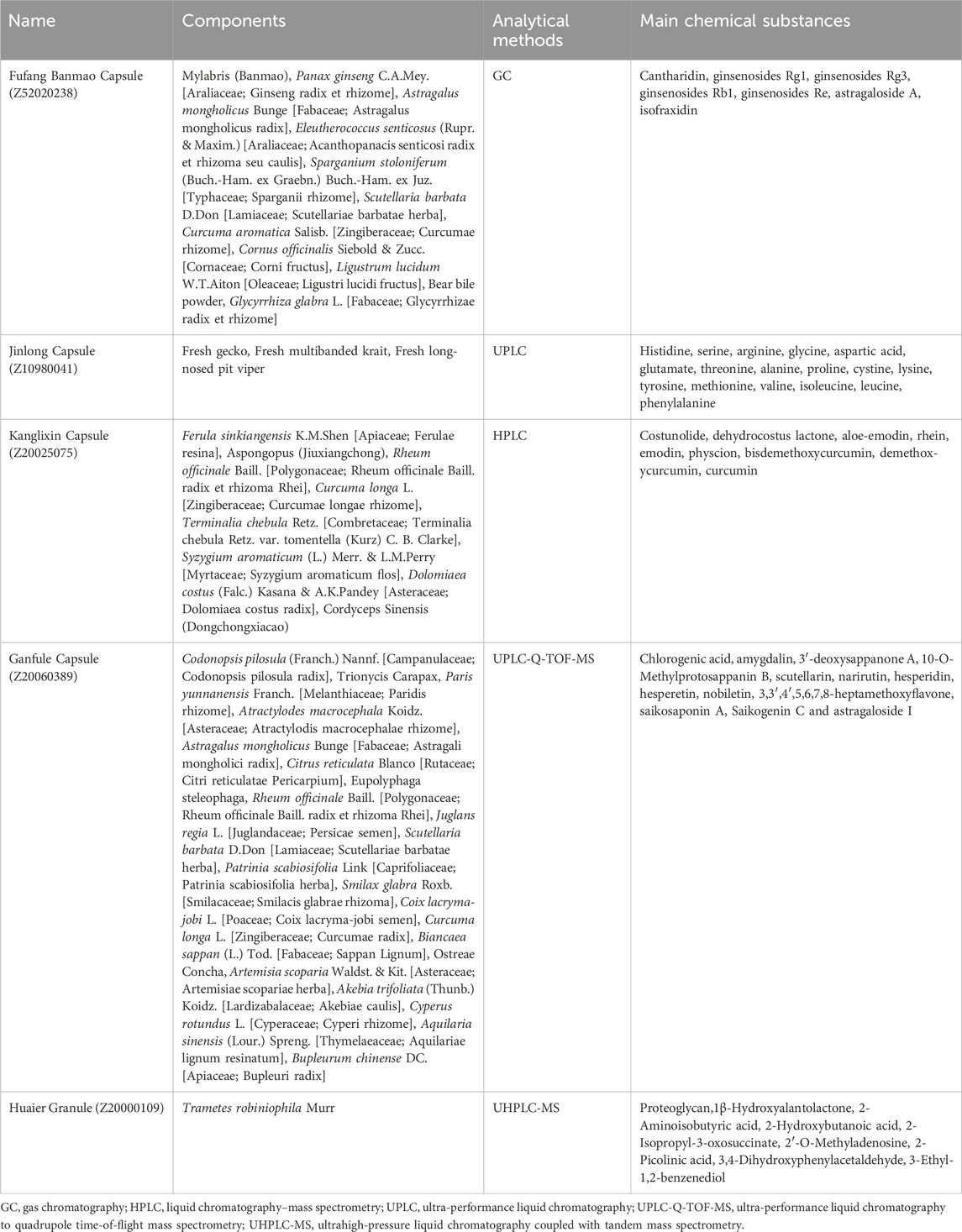
After diagnosing HCC, it is necessary to have a medical record indicating that the patient has undergone a minimum of 3 months of TCM treatment. The TCM treatment primarily consists of Chinese patent medicines sanctioned by the China State Food and Drug Administration (SFDA) and commonly used for treating HCC. These include Fufang Banmao (Z52020238), Jinlong (Z10980041), Kanglixin (Z20025075), Ganfule (Z20060389) capsules, and Huaier Granule (Z20000109)(Li K. et al., 2022). We follow the ConPhyMP statement to ensure the scientific accuracy and credibility of the article (Heinrich et al., 2022). All species in these proprietary Chinese medicines are named according to the rules established by Rivera et al. (Rivera et al., 2014). Their main chemical composition and analytical methods are provided in Table 1. Fufang Banmao Capsule comprises 11 traditional Chinese medicines, with Cantharidin being the primary material present at a minimum concentration of 0.0175 mg/g (Naz et al., 2020). Jinlong Capsule is an animal-derived Chinese patent medicine prepared using modern biochemical separation technology involving low temperature and freezing. It consists of 17 amino acids and a variety of bioactive peptides, with the active principles accounting for 31.2% of the capsule content (Li C. et al., 2022). Kanglixin Capsule, as reported in previous studies, contains 9 traditional Chinese medicines ranging in concentration from 0.562 mg/g to 3.874 mg/g (Li and Kong, 2017). Ganfule Capsule is a compound preparation consisting of 21 traditional Chinese medicines. The active metabolites in Ganfule Capsule account for 25% of its content (Xu et al., 2022). Huaier granules primarily contain Trametes robiniophila Murr, a medicinal fungus rich in various organic metabolites and more than 10 minerals. The active metabolite in Huaier Granule is polysaccharide protein, with a protein content reported to be no less than 71.5 mg/g (Pan et al., 2019).
Demographic and clinical data
Data retrieved from medical records were: patient ages; gender; family HCC history; smoking or alcohol use; hypertension; diabetes; hyperlipidemia; coronary disease; cirrhosis, etiology; model for end-stage liver disease (MELD) scores; Child-Pugh stage; types of treatment; and hepatitis B virus related indicators such as HBeAg and HBV-DNA. Tumor-related indicators included: BCLC staging; multiple tumors and their sizes. White blood cell counts (WBC), hemoglobin concentration (HGB), platelet counts (PLT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), γ-glutamyl transferase (γ-GGT), prothrombin activity (PTA), alanine aminotransferase (ALT), international normalised ratio (INR), AFP and C-reactive protein (CRP) tests were carried out in the hospital laboratory.
Statistical analysis
Statistical analyses were carried out using R ver. 4.0.1. To check for normalcy, the Kolmogorov-Smirnov method was employed. A t-test was employed to evaluate quantitative data that was normally distributed with the results presented as means ± SDs. The Mann-Whitney U test was employed to make comparisons between non-normal distributions using median (m) and quartile range (QR) (m, QR). Qualitative data differences were evaluated using a χ2 test, and the results are expressed as frequencies/numbers. In order to examine the independent risk variables for vascular invasion in HCC patients, univariate and multivariate Cox analyses were utilised. Kaplan-Meier was utilized to produce the patient occurrence curve, and a log-rank test to compare the occurrence rates of MVI in TCM users and non-TCM users. Forest plots were built to compare the 2 groups for the 1-year hazard ratio (HR) of vascular invasion for each aetiology, BCLC stage, Child-Pugh staging, method of treatment, and varied AFP levels. Sankey plots were created using networkD3 software to demonstrate the MVI outcomes for patients who used TCM at various intervals. A p-value below 0.05 indicates statistical significance.
Propensity score matching
We employed propensity score matching to adjust variables and utilized a logistic regression model to calculate the propensity score, which indicates the likelihood of each patient receiving TCM treatment. The matching propensity score model incorporated key variables including age, BCLC stage, type of treatment, and AFP. Utilizing the nearest neighbor method with a 1:1 ratio, a caliper width of 0.05 was applied without replacement. The standardized mean difference (SMD) was used to assess the balance of variables between groups before and after matching, with values below 0.10 indicating balance.
Results
Baseline characteristics of study participants
Out of a total of 1,273 HCC patients, 758 (59.54%) received TCM treatment for >90 days after their diagnosis and 515 (40.46%) did not. Prior to PS matching, patients with HBV-DNA levels under 500 IU/mL were more likely to take TCM. TCM users were more prevalent in BCLC stage 0-A than non-users were, although fewer patients were in stage B and there were more single tumors with diameters <5 cm (p < 0.001). Additionally, PLT, AST and γ-GGT levels were lower. The proportion of patients an AFP concentration less than 400 ng/mL was greater (p < 0.05), as was the albumin level. The number of TCM users who had received TACE was lower, and they were given combination TACE + RFA treatment (p < 0.001). The clinical measures of WBC, ALT, PTA, and INR were all within the normal range, despite statistical variations being found between TCM and non-TCM users. (Table 2). PS matching was carried out as a result of the patients’ baseline heterogeneity between those who used TCM and those who did not. Following the analysis, tumor features and available treatments were virtually identical for TCM and non-TCM users (Supplementary Table S1).
Univariate and multivariate statistical analyses
The impact of baseline indicators on the incidence of 1-year MVI was evaluated by employing a multivariate Cox proportional hazards model. Independent protective factor for the 1-year incidence of MVI in HCC patients were taking TCM (adjusted HR = 0.4941, 95% CI: 0.3853–0.6336, p < 0.001) and high PTA (adjusted HR = 0.9893, 95% CI: 0.9807–0.9980, p = 0.016). Compared to the combination therapy of TACE and RFA, both only TACE and only RFA were independent risk factors, TACE (adjusted HR = 1.5771, 95% CI: 1.2238–2.0324, p < 0.001), RFA (adjusted HR = 2.1287, 95% CI: 1.4239–3.1824, p < 0.001). In addition, Cirrhosis (adjusted HR = 1.8541, 95% CI: 1.1379–3.0213, p = 0.013), size of tumor ≥5 cm (adjusted HR = 1.9713, 95% CI: 1.5209–2.5550, p < 0.001), AFP ≥400 ng mL (adjusted HR = 1.8929, 95% CI: 1.4668–2.4428, p < 0.001), and a high serum CRP concentration (adjusted HR = 1.0053, 95% CI: 1.0024–1.0083, p < 0.001) were independent risk factors (Table 3).
Survival analysis
Kaplan-Meier curve showed that before matching, the number of 1-year MVI occurrence in TCM-users group and non TCM-users group was 114 (15.0%) and 174 (33.8%) respectively, and after PS matching was 87 (17.9%) and 160 (32.9%), respectively. Regardless of PS matching or not, the 1-year MVI incidence in TCM-users was less than for non-users (Figures 2A,B).
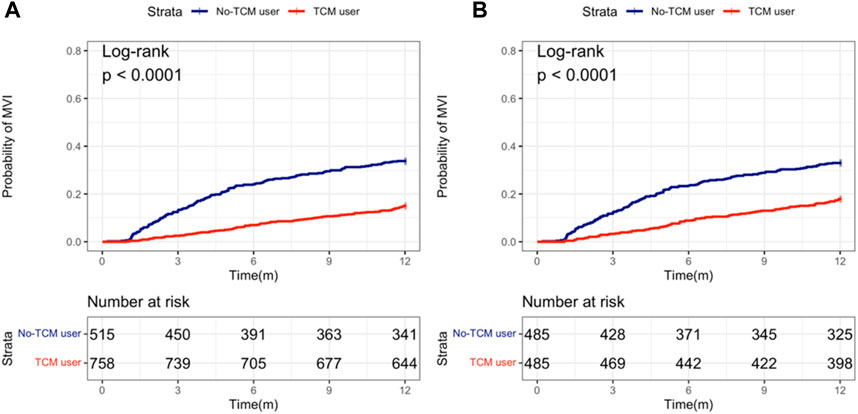
FIGURE 2. Probability of MVI occurring between TCM and non-TCM users in patients with hepatocellular carcinoma. (A) Before propensity score (PS) matching; (B) After PS matching.
In addition, we also analyzed the effects of taking TCM on the 1-year MVI incidence of HCC patients with different etiology, tumor stage, serum AFP level, liver functions and treatment type. The forest map revealed that the incidence of MVI in TCM users was less than in non-users, irrespective of the stage of HCC, good or poor level of liver function, high or low level of AFP. In addition, the incidence of MVI was significantly reduced for TCM users with HBV infection (HR = 0.446, 95% CI: 0.334–0.594) and TACE + RFA combination treatment (HR = 0.301, 95% CI: 0.194–0.469) (Figure 3).
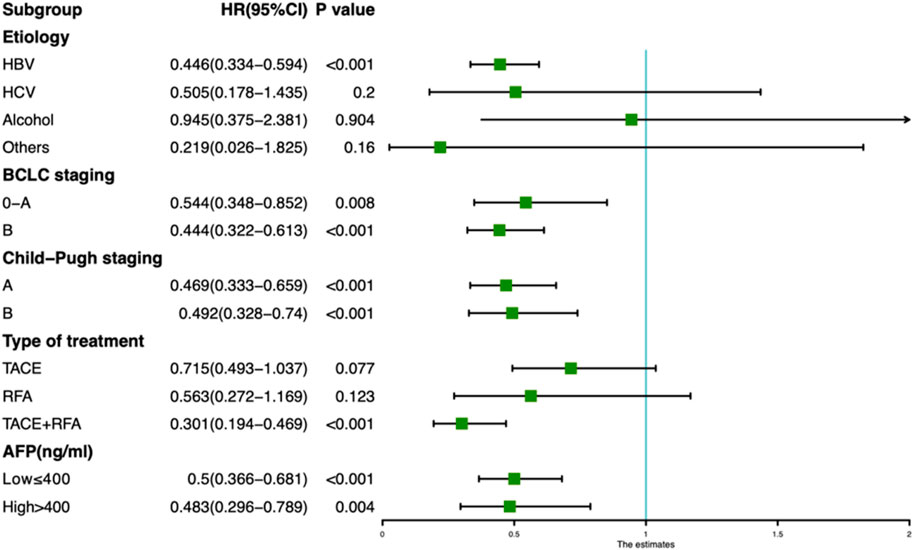
FIGURE 3. Forest plot showing the risk of developing MVI for TCM and non-TCM users in different subgroups of hepatocellular carcinoma patients.
Comparison of mortality risks in individuals treated at various points in time and with various TCM modalities
Users of TCM were split into three categories based on how long they have been using Chinese medicine: 3 to 6, 6 to 9 and 9–12 months groups. Kaplan-Meier analysis demonstrated that the rate of occurrence of MVI was independent of PS matching and considerably lower in patients who were prescribed long-term TCM compared to non-users (Figure 4). The Cox model showed that, following PS matching, the probability of MVI emerging in the subgroup of patients taking TCM for 9–12 months was considerably lower than that in those not taking TCM. The Cox model also demonstrated that, as TCM use increased, the incidence of MVI decreased (Table 4).
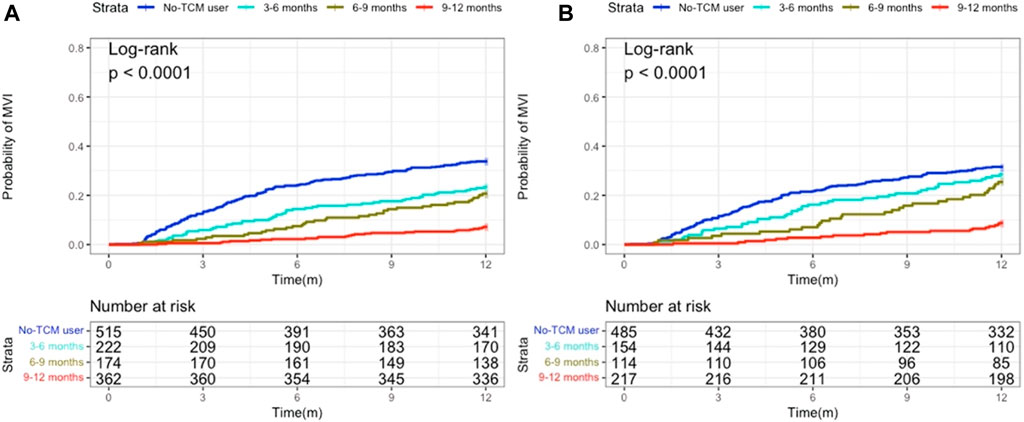
FIGURE 4. Kaplan-Meier analysis revealing the probability of MVI developing according to administration periods of TCM. (A) Before propensity score (PS) matching; (B) After PS matching.
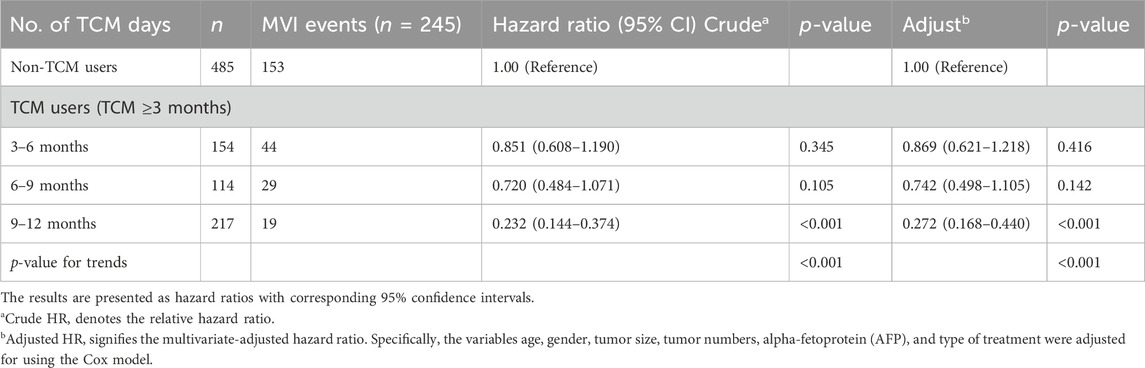
TABLE 4. Risk of developing MVI according to the cumulative use (in months) of TCM among patients with hepatocellular carcinoma.
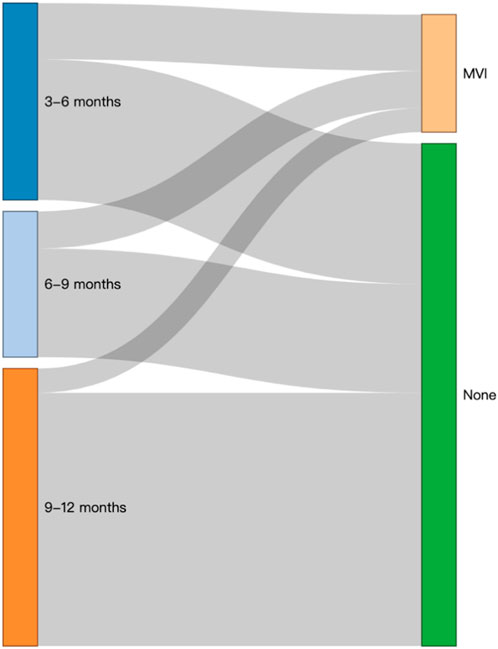
In addition, the Sankey diagram intuitively showed the outcome and flow direction of MVI in patients who underwent different lengths of TCM treatment (Figure 5).
The analysis of different Chinese patent medicines taken in this study shows that Huier granule, Kanglixin capsule, Ganfule capsule, Jinlong capsule and Fufang Banmao capsule have significant protective effects on the rate of occurrence of MVI in HCC patients. The adjusted risk of MVI in 1 year is 0.402 (0.250–0.647), 0.402 (0.205–0.788), 0.541 (0.305–0.957), respectively 0.543 (0.325–0.907) and 0.594 (0.412–0.857) (Table 5).
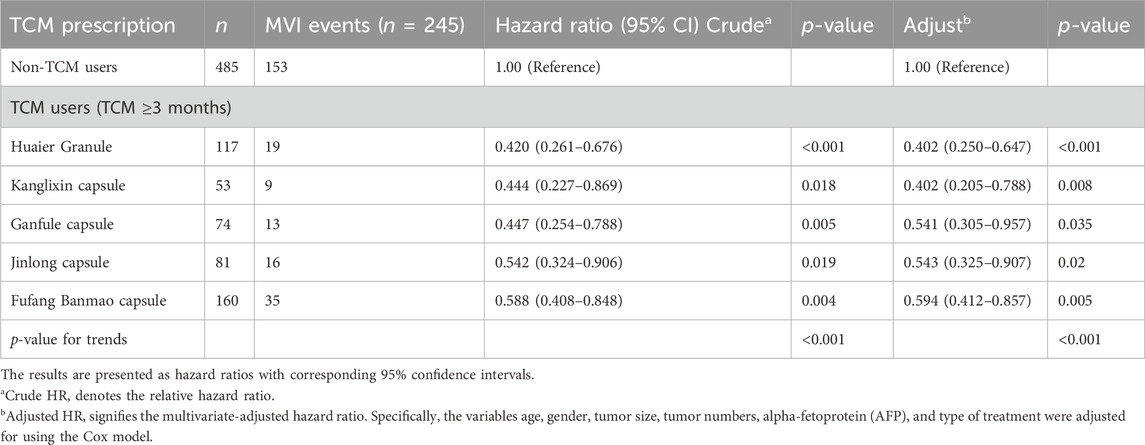
TABLE 5. Risk of developing MVI according to the use of most common TCM among patients with hepatocellular carcinoma.
Discussion
So far, TCM has shown potential in slowing down tumor progression by downregulating VEGF-related signaling pathways. A number of studies have shown that the metabolite of TCM has significant therapeutic uses to suppress tumor angiogenesis in breast cancer, colon cancer, prostate cancer, glioblastoma and so on (Jiang et al., 2010; Yu et al., 2017; Zhu et al., 2017; Wang et al., 2018). The present study demonstrated that TCM can be used as a potential chemoprophylaxis to provide a lasting and significant inhibitory effect on tumor angiogenesis. Most studies focus on metabolites derived from TCM, rather than formulations, targeting tumor angiogenesis. It is worth noting that in the theory of TCM, the formula containing a variety of botanical drug combinations is the main form and are more commonly used as a treatment for cancer. Therefore, more research on the anti-tumor neovascularization of TCM should be conducted in order to explore further potential TCM therapeutic effects. Although one study has reported that a TCM metabolite can inhibit HCC angiogenesis by blocking the migration and invasion of vascular endothelial cells derived from tumor (Wei et al., 2017), there is a paucity of information on the potential effects of proprietary Chinese medicine at different tumor stages. This study analyzed the effect of proprietary Chinese medicine on MVI in patients diagnosed with HCC according to the clinical stage. The administration of proprietary Chinese medicine was shown to be a protective factor in reducing the rate of occurrence of 1-year MVI in patients with HCC, and any treatment of clinical tumor stages with TCM is related to the lower incidence of MVI.
In the early stage of tumor development, TCM can be used as a treatment to limit tumor progression. This study found that TCM significantly reduced the risk of developing MVI for patients treated with TACE combined with RFA. A randomized controlled trial reported that TCM combined with TACE could increase OS and PFS in patients with HCC, especially those with early HCC. TCM can effectively inhibit the proliferation and migration of HCC cells (Yang et al., 2021). In addition, a randomized, double-blind clinical trial showed that TCM can reduce the risk of recurrence of HCC after TACE and alleviate the postembolization syndrome (Xu et al., 2016). The curative effect of TCM is dependent on the treatment duration. Research has found that patients with HCC who have taken TCM for >36 months had a 90% lower risk of mortality than those who have not (Liu et al., 2019), findings in good agreement with our results, indicating that taking TCM for a long time will produce a stronger protective effect.
Recent research has shown that Fufang Banmao Capsule, a popular TCM, can be used as an adjuvant therapy for patients with HCC. It contains several active metabolites, including cantharidin, ginsenosides Rg1, Rg3, Rb1, Re, astragaloside A, and isofraxidin. Cantharidin is the main substance present in a concentration of at least 0.0175 mg/g (Naz et al., 2020). Studies have demonstrated that its active metabolite, astragaloside A effectively inhibits hepatocarcinogenesis by interfering with the NRF2/HO-1 signal pathway (Fang Gong et al., 2023). Another active metabolite of Fufang Banmao Capsule called Ginsenoside Rg3 has been found to inhibit tumor progression and metastasis in an orthotopic HCC transplantation mouse model by reducing VEGF levels and downregulating VEGF-R2 (Zhou et al., 2014). The results of studies on Fufang Banmao Capsule suggest that it is a preferred choice for adjuvant cancer therapy due to minimal adverse reactions and independent prediction of OS(Liu et al., 2020). Jinlong Capsule is a Chinese patent medicine derived from animal sources, commonly used in TCM for treating HCC. The capsule contains 17 amino acids and various bioactive peptides, including histidine, serine, arginine, glycine, aspartic acid, glutamate, threonine, alanine, proline, cystine, lysine, tyrosine, methionine, valine, isoleucine, leucine, and phenylalanin (Li C. et al., 2022). A meta-analysis conducted on the Chinese population showed that combining TACE with Jinlong Capsule significantly prolonged patients’ OS times and improved liver functions compared to TACE alone. This study also found that proprietary Chinese medicine had a protective effect on patients with compromised liver functions (Jia et al., 2020). Another meta-analysis evaluated the efficacy and safety of Jinlong Capsule as an adjuvant treatment for HCC, demonstrating its effectiveness in reducing adverse reactions such as leukopenia, gastrointestinal events, hepatotoxicity, and bone marrow suppression (Xu et al., 2020). Previous studies have shown that Kanglixin Capsule contains various metabolites, including costunolide, dehydrocostus lactone, aloe-emodin, rhein, emodin, physcion, bisdemethoxycurcumin, demethox-ycurcumin, and curcumin (Li and Kong, 2017). Curcumin is particularly noteworthy as it possesses strong anti-inflammatory and antioxidant properties (Lim et al., 2010). Research has indicated that when combined with metformin, curcumin can activate the mitochondrial pathway to induce apoptosis in cancer cells. Additionally, this combination treatment enhances the metastasis and invasion capabilities of HCC cells while promoting angiogenesis in HUVEC cells (Zhang H.-H. et al., 2018). UPLC-QTOF-MS analysis Ganfule Capsule showed that it contained 13 key metabolites. These metabolites include akebia saponin D, chlorogenic acid, amygdalin, 3′-deoxysappanone A, 10-O-Methylprotosappanin B, saikosaponin C and astragaloside I (Xu et al., 2022). Studies have shown that akebia saponin D inhibits the survival, proliferation, adhesion, migration and invasion of HCC cells in a concentration-dependent manner (Lu et al., 2019). The metabolites of Huaier Granule include proteoglycan, 2-Hydroxybutanoic acid, 2-Isopropyl-3-oxosuccinate, 2′-O-Methyladenosine, 3-Ethyl-1,2-benzenediol, and others. A multicenter study involving 1,044 patients demonstrated that Huaier Granule significantly improved postoperative intrahepatic metastasis (Chen et al., 2018). Additionally, Huaier Granule has shown promise as a prognostic tool for early-stage HCC patients treated with thermal ablation by increasing the PFS rate and reducing the rate of extrahepatic metastasis without notable side effects (Wang et al., 2021).
This study’s research methodology and data sources had several drawbacks including its retrospective nature. The clinical features of TCM and non-TCM users varied to some extent. There were still some biochemical discrepancies even after the patient groups were PS score matched to remove various confounding factors. Therefore, a prospective randomised controlled clinical trial should be conducted to validate the impact of TCM on MVI in HCC patients. The study excluded those individuals who were treated with decoction and only included patients who received proprietary Chinese medication. This study did not perform a thorough investigation because the majority of patients who consumed the decoction went to the clinic and only a tiny percentage of patients consumed it for longer than 3 months. Because there is no effective drug to treat HCC, there was no positive control drug. Most patients were in BCLC stage B and sorafenib should be considered as first-line treatment for advanced HCC patients. The patients in our study were not suitable for this treatment.
Conclusion
In our retrospective cohort study, we found that adjuvant TCM treatment reduced the occurrence of MVI in patients with HCC. The use of TCM over an extended period showed a gradual decrease in the proportion of MVI, indicating its potential in preventing MVI in HCC patients. These findings suggest that TCM therapy is effective in preventing MVI in HCC patients. However, it is important to acknowledge the study limitations, including its retrospective design and reliance on observational data. To validate the efficacy and safety of TCM therapy in HCC treatment, future research should conduct prospective randomized controlled trials (RCTs) with robust experimental designs. Additionally, further investigations are needed to understand the mechanisms of action and identify optimal treatment protocols.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Beijing Ditan Hospital’s ethical committee at Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the data are anonymous, and the requirement for informed consent was therefore waived.
Author contributions
HY: Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing–original draft, Writing–review and editing, Conceptualization, Resources. XW: Project administration, Software, Supervision, Validation, Visualization, Writing–review and editing. LY: Project administration, Validation, Writing–review and editing. XL: Project administration, Validation, Writing–review and editing. FY: Supervision, Validation, Writing–review and editing. YX: Software, Writing–review and editing. QP: Software, Writing–review and editing. ZY: Conceptualization, Funding acquisition, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this research was made possible thanks to grants from the National Natural Science Foundation of China (grant no. 82274479), the Special Fund of Capital Health Research and Development (grant no. 2020-2-2173), High-level Public Health Technical Personnel Construction Project(Subject leaders-02-16), the Beijing Hospitals Authority Clinical Medicine Development of Special Funding (grant no. ZYLX202127), and the Dengfeng Talent Support Program of Beijing Municipal Administration of Hospitals (grant no. DFL20191803).
Acknowledgments
Peng Wang and Dongdong Zhou were helpful in the follow-up of MVI data in HCC patients, and we appreciate their help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1353720/full#supplementary-material
Abbreviations
AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver cancer; CRP, C-reactive protein; γ-GGT, γ-glutamyl transferase; HBeAg, hepatitis B e Antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HGB, hemoglobin; HR, hazard ratio; INR, international normalized ratio; MELD, model for end-stage liver disease; PLT, platelet counts; PTA, prothrombin activity; WBC, white blood cell counts.
References
Chen, Q., Shu, C., Laurence, A. D., Chen, Y., Peng, B.-G., Zhen, Z.-J., et al. (2018). Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut 67, 2006–2016. doi:10.1136/gutjnl-2018-315983
Fang Gong, Y., Hou, S., Xu, J.-C., Chen, Y., Zhu, L.-L., Xu, Y.-Y., et al. (2023). Amelioratory effects of astragaloside IV on hepatocarcinogenesis via Nrf2-mediated pSmad3C/3L transformation. Phytomedicine 117, 154903. doi:10.1016/j.phymed.2023.154903
Forner, A., Reig, M., and Bruix, J. (2018). Hepatocellular carcinoma. Lancet 391, 1301–1314. doi:10.1016/S0140-6736(18)30010-2
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G., and Jain, R. K. (2018). Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340. doi:10.1038/nrclinonc.2018.29
Galanopoulos, M., Ladias, S., Tzannetakou, X., Tsigaridas, A., Sarafis, A., Liatsos, C., et al. (2016). A rectovaginal fistula after treatment with bevacizumab. A dangerous side effect needing emergency treatment. Clin. Case Rep. 4, 449–450. doi:10.1002/ccr3.523
Guan, Q., Gu, J., Zhang, H., Ren, W., Ji, W., and Fan, Y. (2015). Correlation between vascular endothelial growth factor levels and prognosis of hepatocellular carcinoma patients receiving radiofrequency ablation. Biotechnol. Biotechnol. Equip. 29, 119–123. doi:10.1080/13102818.2014.981776
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research-The ConPhyMP-Guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Jia, S., Fu, Y., and Tao, H. (2020). Trans-arterial chemoembolization combined with Jinlong capsule for advanced hepatocellular carcinoma: a PRISMA-compliant meta-analysis in a Chinese population. Pharm. Biol. 58, 771–784. doi:10.1080/13880209.2020.1799040
Jia, W., and Wang, L. (2020). Using traditional Chinese medicine to treat hepatocellular carcinoma by targeting tumor immunity. Evid. Based Complement. Altern. Med. 2020, 9843486. doi:10.1155/2020/9843486
Jiang, Y., Zhang, Y., Luan, J., Duan, H., Zhang, F., Yagasaki, K., et al. (2010). Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology 62, 573–583. doi:10.1007/s10616-010-9310-0
Kudo, M., Cheng, A. L., Park, J. W., Park, J. H., Liang, P. C., Hidaka, H., et al. (2018). Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol. Hepatol. 3, 37–46. doi:10.1016/S2468-1253(17)30290-X
Kudo, M., Han, G., Finn, R. S., Poon, R. T. P., Blanc, J.-F., Yan, L., et al. (2014). Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology 60, 1697–1707. doi:10.1002/hep.27290
Lencioni, R., Llovet, J. M., Han, G., Tak, W. Y., Yang, J., Guglielmi, A., et al. (2016). Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J. Hepatol. 64, 1090–1098. doi:10.1016/j.jhep.2016.01.012
Li, C., Li, F., Ye, H., Xie, X., Liang, Y., Tian, E., et al. (2022a). Molecular quantification, a new strategy for quality control of Chinese patent medicine containing animal-derived crude drug: qi She in Jinlong capsule as an example. J. Pharm. Biomed. Anal. 207, 114428. doi:10.1016/j.jpba.2021.114428
Li, K., Xiao, K., Zhu, S., Wang, Y., and Wang, W. (2022b). Chinese herbal medicine for primary liver cancer therapy: perspectives and challenges. Front. Pharmacol. 13, 889799. doi:10.3389/fphar.2022.889799
Li, S., and Kong, Q. (2017). Simultaneous determination of nine components in Kanglixin Jiaonang by HPLC wavelength switching combined gradient elution method. J. Int. Pharm. Res. 44, 817–822. doi:10.13220/j.cnki.jipr.2017.08.013
Lim, C.-B., Ky, N., Ng, H.-M., Hamza, M. S., and Zhao, Y. (2010). Curcuma wenyujin extract induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo. Integr. Cancer Ther. 9, 36–49. doi:10.1177/1534735409359773
Liu, X., Li, M., Wang, X., Dang, Z., Yu, L., Wang, X., et al. (2019). Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine 62, 152930. doi:10.1016/j.phymed.2019.152930
Liu, Y., Li, Y., Wang, X., Huang, Y., Zhang, Q., Shi, K., et al. (2020). Fufang Banmao capsule, a traditional Chinese medicinal formulation, enhances the survival of patients with hepatocellular carcinoma and vp3–4 portal vein tumor thrombosis undergoing supportive treatment. J. Altern. Complement. Med. 26, 956–965. doi:10.1089/acm.2019.0334
Llovet, J. M., De Baere, T., Kulik, L., Haber, P. K., Greten, T. F., Meyer, T., et al. (2021a). Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 293–313. doi:10.1038/s41575-020-00395-0
Llovet, J. M., Kelley, R. K., Villanueva, A., Singal, A. G., Pikarsky, E., Roayaie, S., et al. (2021b). Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7, 6. doi:10.1038/s41572-020-00240-3
Lu, W.-L., Yang, T., Song, Q.-J., Fang, Z.-Q., Pan, Z.-Q., Liang, C., et al. (2019). Akebia trifoliata (Thunb.) Koidz Seed Extract inhibits human hepatocellular carcinoma cell migration and invasion in vitro. J. Ethnopharmacol. 234, 204–215. doi:10.1016/j.jep.2018.11.044
Markezana, A., Goldberg, S. N., Kumar, G., Zorde-Khvalevsky, E., Gourevtich, S., Rozenblum, N., et al. (2021). Incomplete thermal ablation of tumors promotes increased tumorigenesis. Int. J. Hyperth. 38, 263–272. doi:10.1080/02656736.2021.1887942
Meyer, T., Fox, R., Ma, Y. T., Ross, P. J., James, M. W., Sturgess, R., et al. (2017). Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2, 565–575. doi:10.1016/S2468-1253(17)30156-5
Naz, F., Wu, Y., Zhang, N., Yang, Z., and Yu, C. (2020). Anticancer attributes of cantharidin: involved molecular mechanisms and pathways. Molecules 25, 3279. doi:10.3390/molecules25143279
Omata, M., Cheng, A.-L., Kokudo, N., Kudo, M., Lee, J. M., Jia, J., et al. (2017). Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370. doi:10.1007/s12072-017-9799-9
Pan, J., Yang, C., Jiang, Z., and Huang, J. (2019). Trametes robiniophila Murr: a traditional Chinese medicine with potent anti-tumor effects. Cancer Manag. Res. 11, 1541–1549. doi:10.2147/CMAR.S193174
Park, J.-W., Kim, Y. J., Kim, D. Y., Bae, S.-H., Paik, S. W., Lee, Y.-J., et al. (2019). Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J. Hepatol. 70, 684–691. doi:10.1016/j.jhep.2018.11.029
Peng, W., Zhang, S., Zhang, Z., Xu, P., Mao, D., Huang, S., et al. (2018). Jianpi Jiedu decoction, a traditional Chinese medicine formula, inhibits tumorigenesis, metastasis, and angiogenesis through the mTOR/HIF-1α/VEGF pathway. J. Ethnopharmacol. 224, 140–148. doi:10.1016/j.jep.2018.05.039
Petrillo, M., Patella, F., Pesapane, F., Suter, M. B., Ierardi, A. M., Angileri, S. A., et al. (2018). Hypoxia and tumor angiogenesis in the era of hepatocellular carcinoma transarterial loco-regional treatments. Future Oncol. 14, 2957–2967. doi:10.2217/fon-2017-0739
Rivera, D., Allkin, R., Obón, C., Alcaraz, F., Verpoorte, R., and Heinrich, M. (2014). What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 152, 393–402. doi:10.1016/j.jep.2013.12.022
Shah, Z. K., McKernan, M. G., Hahn, P. F., and Sahani, D. V. (2007). Enhancing and expansile portal vein thrombosis: value in the diagnosis of hepatocellular carcinoma in patients with multiple hepatic lesions. AJR Am. J. Roentgenol. 188, 1320–1323. doi:10.2214/AJR.06.0134
Shi, Z., Song, T., Wan, Y., Xie, J., Yan, Y., Shi, K., et al. (2017). A systematic review and meta-analysis of traditional insect Chinese medicines combined chemotherapy for non-surgical hepatocellular carcinoma therapy. Sci. Rep. 7, 4355. doi:10.1038/s41598-017-04351-y
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Wang, T., Chen, W., and Wu, J. (2018). H2-P, a honokiol derivative, exerts anti-angiogenesis effects via c-MYC signaling pathway in glioblastoma. J. Cell Biochem. 119, 3142–3148. doi:10.1002/jcb.26462
Wang, X., Wang, N., Cheung, F., Lao, L., Li, C., and Feng, Y. (2015). Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J. Integr. Med. 13, 142–164. doi:10.1016/S2095-4964(15)60171-6
Wang, Z., Yu, X., Zhang, J., Cheng, Z., Han, Z., Liu, F., et al. (2021). Huaier granule prevents the recurrence of early-stage hepatocellular carcinoma after thermal ablation: a cohort study. J. Ethnopharmacol. 281, 114539. doi:10.1016/j.jep.2021.114539
Wei, Y., Yang, Q., Zhang, Y., Zhao, T., Liu, X., Zhong, J., et al. (2017). Plumbagin restrains hepatocellular carcinoma angiogenesis by suppressing the migration and invasion of tumor-derived vascular endothelial cells. Oncotarget 8, 15230–15241. doi:10.18632/oncotarget.14774
Xu, F., Li, H., Pan, Y., Zeng, Y., Li, J., and Li, S. (2022). Effects of Ganfule capsule on microbial and metabolic profiles in anti-hepatocellular carcinoma. J. Appl. Microbiol. 132, 2280–2292. doi:10.1111/jam.15307
Xu, H., Wei, W., Mu, Y., and Dong, C. (2020). Efficacy and safety of Chinese patent medicine (Jinlong capsule) in the treatment of advanced hepatocellular carcinoma: a meta-analysis. Biosci. Rep. 40, BSR20194019. doi:10.1042/BSR20194019
Xu, L., Wang, S., Zhuang, L., Lin, J., Chen, H., Zhu, X., et al. (2016). Jian pi Li qi decoction alleviated postembolization syndrome following transcatheter arterial chemoembolization for hepatocellular carcinoma: a randomized, double-blind, placebo-controlled trial. Integr. Cancer Ther. 15, 349–357. doi:10.1177/1534735415617020
Yang, J., Yan, J., and Liu, B. (2018). Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 9, 978. doi:10.3389/fimmu.2018.00978
Yang, X., Feng, Y., Liu, Y., Ye, X., Ji, X., Sun, L., et al. (2021). Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine 87, 153575. doi:10.1016/j.phymed.2021.153575
Yu, X., Li, W., Deng, Q., You, S., Liu, H., Peng, S., et al. (2017). Neoalbaconol inhibits angiogenesis and tumor growth by suppressing EGFR-mediated VEGF production. Mol. Carcinog. 56, 1414–1426. doi:10.1002/mc.22602
Zhang, C., Wang, N., Tan, H.-Y., Guo, W., Li, S., and Feng, Y. (2018a). Targeting VEGF/VEGFRs pathway in the antiangiogenic treatment of human cancers by traditional Chinese medicine. Integr. Cancer Ther. 17, 582–601. doi:10.1177/1534735418775828
Zhang, H.-H., Zhang, Y., Cheng, Y.-N., Gong, F.-L., Cao, Z.-Q., Yu, L.-G., et al. (2018b). Metformin incombination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol. Carcinog. 57, 44–56. doi:10.1002/mc.22718
Zhong, J. H., Peng, N. F., You, X. M., Ma, L., Xiang, X., Wang, Y. Y., et al. (2017). Tumor stage and primary treatment of hepatocellular carcinoma at a large tertiary hospital in China: a real-world study. Oncotarget 8, 18296–18302. doi:10.18632/oncotarget.15433
Zhou, B., Wang, J., and Yan, Z. (2014). Ginsenoside Rg3 attenuates hepatoma VEGF overexpression after hepatic artery embolization in an orthotopic transplantation hepatocellular carcinoma rat model. Onco Targets Ther. 7, 1945–1954. doi:10.2147/OTT.S69830
Keywords: anticancer Chinese pattern medicine, complementary alternative medicine, hepatocellular carcinoma, macrovascular invasion, traditional Chinese medicine
Citation: Yan H, Wang X, Yu L, Liu X, Yan F, Xie Y, Pu Q and Yang Z (2024) Effectiveness of adjuvant traditional Chinese medicine on macrovascular invasion in patients with hepatocellular carcinoma: a real-world propensity score-matched study. Front. Pharmacol. 15:1353720. doi: 10.3389/fphar.2024.1353720
Received: 13 December 2023; Accepted: 07 February 2024;
Published: 23 February 2024.
Edited by:
Dâmaris Silveira, University of Brasilia, BrazilReviewed by:
Shanshan Liang, Affiliated Zhongshan Hospital of Dalian University, ChinaPriyanka Banerjee, Texas A&M Health Science Center, United States
Copyright © 2024 Yan, Wang, Yu, Liu, Yan, Xie, Pu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyun Yang, eWFuZ3poaXl1bjIwMTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Huiwen Yan
Huiwen Yan Xinhui Wang
Xinhui Wang Lihua Yu
Lihua Yu Xiaoli Liu
Xiaoli Liu Fengna Yan
Fengna Yan Qing Pu
Qing Pu