- 1Core Facilities of West China Hospital, Sichuan University, Chengdu, China
- 2Laboratory of Liver Transplantation, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Respiratory and Critical Care Medicine, Institute of Respiratory Health, Center of Precision Medicine, West China Hospital, Sichuan University, Chengdu, China
Ferroptosis is caused by the accumulation of cellular reactive oxygen species that exceed the antioxidant load that glutathione (GSH) and phospholipid hydroperoxidases with GSH-based substrates can carry When the antioxidant capacity of cells is reduced, lipid reactive oxygen species accumulate, which can cause oxidative death. Ferroptosis, an iron-dependent regulatory necrosis pathway, has emerged as a new modality of cell death that is strongly associated with cancer. Surgery, chemotherapy and radiotherapy are the main methods of cancer treatment. However, resistance to these mainstream anticancer drugs and strong toxic side effects have forced the development of alternative treatments with high efficiency and low toxicity. In recent years, an increasing number of studies have shown that traditional Chinese medicines (TCMs), especially herbs or herbal extracts, can inhibit tumor cell growth and metastasis by inducing ferroptosis, suggesting that they could be promising agents for cancer treatment. This article reviews the current research progress on the antitumor effects of TCMs through the induction of ferroptosis. The aim of these studies was to elucidate the potential mechanisms of targeting ferroptosis in cancer, and the findings could lead to new directions and reference values for developing better cancer treatment strategies.
1 Introduction
In the last decade, the Nomenclature Committee on Cell Death (NCCD) has meticulously examined an extensive body of literature, ultimately formulating a definition for cell death that incorporates considerations of morphology, biochemistry, and function. This definition remains applicable to the present day. In terms of functional characteristics, cell death modalities can be categorized into two types: accidental cell death (ACD) and regulated cell death (RCD). (Galluzzi et al., 2018). Ferroptosis, a novel type of regulated cell death, is morphologically, biochemically, and genetically distinct from apoptosis, necrosis, and pyroptosis, among others. Elevated levels of iron-dependent reactive oxygen species (ROS) result in the buildup of lipid peroxides, disrupting redox homeostasis and causing oxidative damage to cell membranes. This imbalance adversely impacts normal cellular processes, ultimately leading to ferroptosis (Xing et al., 2023).
In 2003, Dolma et al. reported that a new compound, erastin, could induce RAS-mutated human foreskin fibroblasts (BJeLRs) death. However, this pattern of cell death differs from classical apoptosis previously found. During erastin-induced cell death, there were observations of distorted mitochondrial morphology and the disappearance of mitochondrial cristae. (Dolma et al., 2003). However, no classic features of apoptosis, such as mitochondrial cytochrome c release, caspase activation, or chromatin fragmentation, are observed in RSL-treated cells (Yagoda et al., 2007; Yang and Stockwell, 2008). In Nine years later, this new mode of cell death was officially named ferroptosis by Dixon et al. (Dixon et al., 2012). Since then, researchers have continued to explore the relationship between ferroptosis and human disease. Although the specific mechanisms and physiological functions of ferroptosis have not been fully elucidated, increasing evidence indicates that ferroptosis is involved in multiple pathological conditions, such as ischemia‒reperfusion injury (Carlson et al., 2016), stroke mitigation and neurodegeneration (Hambright et al., 2017; Tuo et al., 2017), and cancer treatment (Conrad et al., 2016; Stockwell et al., 2017; Tonnus and Linkermann, 2017; Toyokuni et al., 2017). Several studies have shown that suppressing system xc- or GPX4 inhibits tumor growth and metastasis in various types of cancer (Yang et al., 2014; Zhang et al., 2019a). In addition, multiple tumor suppressors have been found to sensitize cells to ferroptosis. For example, p53 can enhance tumor ferroptosis by suppressing the transcription of the system xc-subunit SLC7A11 (Wang et al., 2016). Malignant mutations in oncogenes usually accelerate metastasis, protect cancer cells from apoptosis and increase resistance to common cancer therapies (Piccolo et al., 2014; Hansen et al., 2015). However, the finding that these same mutations sensitize cancer cells to ferroptosis brings new hope for cancer treatment (Wu et al., 2019).
Traditional Chinese medicine (TCM), a medical theory system gradually formed and developed through long-term medical practice under the guidance of ancient simple materialism and spontaneous dialectics, is one of the oldest medical systems in the world. It has condensed thousands of years of health-preserving concepts and practical experience. TCM and its natural extracts have been widely used to treat various diseases, making significant contributions to medical health worldwide. Traditional Chinese medicine has shown efficacy in treating acute pancreatitis (Ge et al., 2023), neurodegenerative diseases (Lin et al., 2023), and polycystic ovary syndrome (PCOS) (Liu et al., 2022). Myocardial infarction (MI) (Wang et al., 2023a), atherosclerosis (AS) and hyperlipidemia (HLP) (Chen et al., 2021a; Wu et al., 2023)develop through the activation of ferroptosis. Given the recent studies highlighting the association between ferroptosis and the inhibition of tumor cells, there is a growing suggestion that ferroptosis could be a potential target for anticancer therapy. (Chen et al., 2021b). Consequently, it is a novel and meaningful research direction to explore the ferroptosis induced by traditional Chinese medicine.
In this review, we list the traditional Chinese medicines and their natural extracts and prescriptions that can be used to treat different cancers. The mechanism of the ferroptosis pathway was investigated to guide rational clinical application, improve disease prognosis and reduce patient suffering caused by toxic side effects.
2 Main TCMs as ferroptosis regulators in cancer
2.1 Terpenoids
2.1.1 Artemisinin and its derivatives
400 years ago, the famous Chinese herbalist Li Shi Zhen published in the Compendium of Materia Medica that “fever and colds” could be treated with qinghaosu preparations (Klayman, 1985). Artemisinin is indeed a natural sesquiterpene lactone compound. It is derived from the sweet wormwood plant (Artemisia annua). Since it was first isolated from the Asteraceae plant Artemisia annua by the Nobel laureate in Medicine and the Chinese scientist Tu Youyou in 1971, artemisinin has saved countless lives as an antimalarial drug (Bhattacharjee et al., 2018). Recently, the artemisinin and its derivatives not only save lives as antimalarials, but have also been explored as potential anticancer drugs.
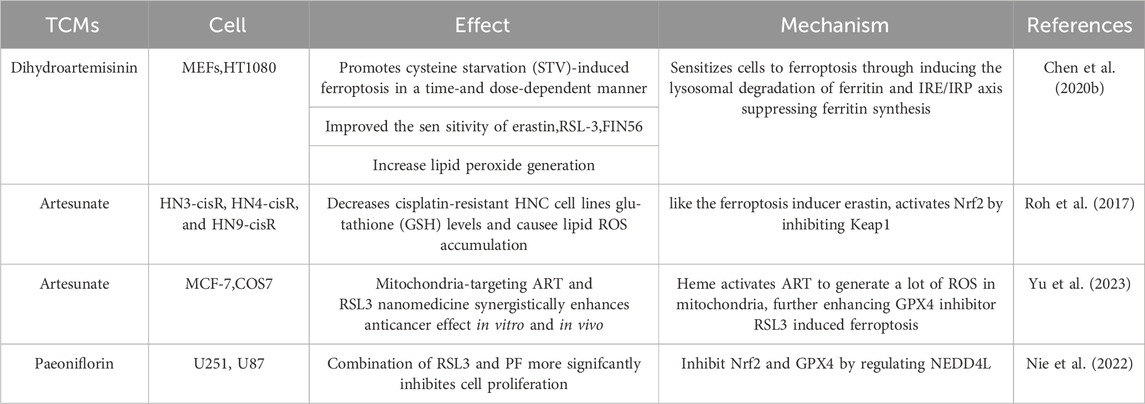
Dihydroartemisinin (DHA), a derivative of artemisinin, can not only inhibit cell proliferation by inducing autophagy (Zou et al., 2019), but also exert its role by inhibiting the PRIM2/SLC7A11 axis and inducing ferroptosis (Yuan et al., 2020). The peroxide bridge structure of DHA triggers a Fenton reaction with the release of iron ions that may contribute to ferroptosis in tumor cells by promoting transferrin receptor expression and inhibits glutathione peroxidase (GPX4) (Greenshields et al., 2017; Yang et al., 2019; Su et al., 2021). DHA can induce ferritin lysosomal degradation and increases cellular free iron levels, increasing cellular susceptibility to ferroptosis. Furthermore, by binding to cellular free iron, thereby stimulating iron-regulatory proteins (IRPs) binding to mRNA molecules containing iron-responsive element (IRE) sequences. After the iron homeostasis controlled by IRP/IRE was broken by DHA, the cellular free iron continued to increase. Ferroptosis was induced in cancer cells by induction of GPX4 knockout in vitro and mouse xenograft models. DHA significantly increased the sensitivity of these cells to RSL3-induced ferroptosis and lipid peroxidation, including the human lung cancer cell line H292; the human colorectal cancer cell lines SW480, HCT116 and HT29; and the human breast cancer cell lines MDA453 and MCF-7 (Chen et al., 2020b).
Artesunate (ART) has exhibited antitumor effects on several nonurologic tumors. In both sunitinib-sensitive and sunitinib-resistant Renal Cell Carcinoma (RCC) cells, ART has been shown to inhibit proliferation and metastasis, and reduce metabolism. Artemisinin (ATS) substantially elevated cytotoxicity and suppressed proliferation in sunitinib-resistant RCC cells. In Caki-1, 786-O, and A-498 cell lines, the inhibition of growth was related to G0/G1 phase arrest and differential regulation of cell cycle-regulating proteins. Artesunate (ART) primarily impacts KTCTL-26 cells through ROS accumulation, ferroptosis, and decreased metabolism, as reported by Markowitsch et al., in 2020 (Markowitsch et al., 2020). Additionally, Roh et al. found that Artesunate (ART) selectively induces ferroptosis in head and neck cancer (HNC) cells while sparing normal tissue cells. Similarly, ART selectively killed cisplatin-resistant HNC cells without harming normal cells. As a pivotal transcription factor that regulates antioxidant stress, Nrf2 plays a crucial role in initiating the body’s antioxidant response. It controls the intricate cellular antioxidant system responsible for generating glutathione (GSH) in cancer cells. Artesunate (ART) has the ability to stimulate the generation of reactive oxygen species (ROS), which can result in ferroptosis, as well as other modes of cell death (Roh et al., 2017). ART can also suppress the proliferation of CA-46 cells in vivo through ferroptosis (Wang et al., 2019b). At the very least, ART has been shown to play a key role in improving the effectiveness of cancer treatment by inducing ferroptosis. It can also be combined with other antioxidants to enhance anticancer effects.
2.1.2 Cucurbitacin B
Cucurbitacins, classified as tetracyclic triterpenoid natural products, are primarily derived from plants within the Cucurbitaceae family. These compounds showcase diverse pharmacological activities, including anti-inflammatory, hepatoprotective, antibacterial, antipyretic, and antitumor properties, achieved through the modulation of multiple signal pathways. Among these, Cucurbitacin B, extracted from Trichosanthes kirilowii Maximowicz, stands out as one of the most abundant and extensively researched derivatives of the cucurbitacin family. In traditional medicine, (Chen et al., 2005; Zhang et al., 2009; Chan et al., 2010a; Chan et al., 2010b; Dakeng et al., 2012). CuB could induce intracellular accumulation of iron ions and depletion of glutathione. A study revealed that CuB treatment of nasopharyngeal cancer cells downregulated GPX4 expression with iron accumulation and glutathione depletion, initiating extensive lipid peroxidation and ultimately leading to ferroptosis. Though, CuB significantly inhibited tumor progression and caused no significant side effects in vivo (Huang et al., 2021).
2.2 Phenols
2.2.1 Curcumin
Curcumin, extracted from Curcuma longa L., has antioxidant properties and is now commonly used as a food additive. (Gao et al., 2022). A large number of studies have shown that curcumin inhibits the progression of non-small cell lung cancer (NSCLC) (Tang et al., 2021a), colorectal cancer (Miyazaki et al., 2023), follicular thyroid cancer (Chen et al., 2023b), clear cell renal cell carcinoma (ccRCC) (Xu et al., 2021a), and breast cancer (Li et al., 2020; Cao et al., 2022) by inducing ferroptosis. EF24, a synthetic analog of curcumin, induces ferroptosis through upregulating HMOX1 in osteosarcoma cells (Lin et al., 2021a). While Cao et al.proved that curcumin exerts anti-breast cancer activity by upregulating SLAC1A5 to induce lipid peroxidation and MDA accumulation (Cao et al., 2022).
Curdione, the predominant sesquiterpene in Curcumae Rhizoma (Xia et al., 2012), enhanced the expression of METTL14, a methylation transferase, and YTHDF2, a reader protein associated with m6A, through N6-methyladenosine. This leads to enhanced methylation of SLC7A11 mRNA and HOXA13 mRNA. Consequently, the expression of HOXA13 is reduced, resulting in a decrease in SLC3A2 expression. This intricate molecular modulation activates ferroptosis in colorectal cancer (Wang et al., 2023b). System Xc-, cystine/glutamic acid inverse transporter, can take cystine, excrete glutamic acid, both for intracellular glutathione synthesis to provide raw materials. SLC7A11 and SLC3A2 as transporter proteins play a key role in ferroptosis activated by GSH depletion. Inhibition of SLC7A11 expression serves as a trigger for inducing ferroptosis. Notably, HOXA13, functioning as a transcription factor, promotes the transcription of SLC3A2 and contributes to the promotion, growth, and therapeutic resistance observed in various malignancies (Gu et al., 2009; Quagliata et al., 2018; Ma et al., 2021).
2.2.2 Erianin
Dendrobium was first recorded in the earliest Chinese pharmaceutical classic Shennong Bencao Jing. Erianin is a bibenzyl natural product extracted from Dendrobium chrysotoxum that have been reported to inhibit growth of cancer cells nowaday (Su et al., 2017; Ouyang et al., 2018; Zhang et al., 2019b). Chen, P., et al. reported After treatment of lung cancer cells with erianin, mitochondrial matrix coagulation and enlarged cristae of cancer cells were observed under electron microscope. In addition, HO-1 and TRF expression increased significantly, whereas GPX4, CHAC2, SLC40A1, SLC7A11 and glutaminase expression decreased significantly. This is the first demonstration that lanolin induces ROS accumulation, lipid peroxidation and GSH depletion in lung cancer cells, ultimately triggering ferroptosis (Chen et al., 2020b). Shen et al. confirmed that erianin significantly hampered the proliferation, invasion in Human renal cancer stem cells (HuRCSCs) while inducing oxidative stress injury and iron ion accumulation. Erianin’s mechanism of inducing ferroptosis in renal cancer stem cells involves inhibiting the expression of GPX4 and promoting the N6-methyladenosine modification of ALOX12/P53 mRNA. Ultimately, this process contributes to slowing down the development of renal cancer (Shen et al., 2023).
2.2.3 Gallic acid
The process of obtaining gallic acid from gallnuts by fermentation was described in Li Ting’s Introduction to Medicine (1575) of the Ming Dynasty. Gallic acid (3,4,5-trihydroxybenzoic acid), a polyhydroxyphenolic compound, is the earliest documented organic acid (Hsu et al., 2011) that has inflammatory, antioxidant, antiviral, antianxiety and antineoplastic effects (Hsu et al., 2011; Mori et al., 2020), especially in liver cancer (Sun et al., 2016). Xie, J., et al. reported that GA could downregulate the expression of amino acid transporter SLC7A11 and ferroptosis signaling protein GPX4 in hepatocellular carcinoma cells by blocking β-catenin transport from the nucleus to the cytoplasm, thus inducing HepG2 ferroptosis (Xie et al., 2023).
2.3 Quinones
2.3.1 Salvia miltiorrhiza bunge
Salvia miltiorrhiza Bunge, belonging to the Dicotyledonaceae family, is a perennial upright herb commonly known as Sage. In traditional Chinese medicine (TCM), the dried root and rhizome of salvia are utilized. This medicinal herb is known for its properties in activating blood circulation, removing stasis, dredging channels, relieving pain, and eliminating carbuncles (Song et al., 2013). Guan et al. documented that tanshinone IIA, extracted from Salvia miltiorrhiza, has the capacity to inhibit the proliferation of gastric cancer cells. This inhibitory effect is achieved through the induction of p53 upregulation-mediated ferroptosis (Guan et al., 2020). Haiwei Ni et al. demonstrated that tanshinone IIA suppresses the stemness of gastric cancer cells by triggering the activation of ferroptosis (Ni et al., 2022a). Lin et al. reported that dihydroisotanshinone I (DT), one of the main effective components of Salvia miltiorrhiza Bunge, has many biological activities and can inhibit the growth of breast carcinoma cells such as MCF-7 cells and MDA-MB-231 cells. In addition, DT treatment also significantly inhibited tumor proliferation in xenograft nude mice models in vivo without side effects. DT anti-tumor mechanism is associated with ferroptosis induced by down-regulating GPX4 protein expression (Lin et al., 2019).
A parallel mechanism is observed in another Danshen extract, cryptotanshinone (CTS), which exhibits the dual capability of activating caspase-3 to promote apoptosis and triggering ferroptosis in lung cancer by inhibiting GPX4 activity (Cao et al., 2021).
2.4 Saponins
2.4.1 Ginsenoside
Ginsenoside is a steroidal compound found only in plants of the genus Ginseng and has a wide range of biological activities, including immunoregulatory (Shin et al., 2020), anti-inflammatory (Wang et al., 2018; Im, 2020), and antioxidative (Li et al., 2009) activities; in addition to its antitumor effects (Zhu et al., 2021). Rh4 inhibits colorectal cancer (CRC) cell proliferation by activating ROS/p53 signaling pathway, up-regulating p53 expression activates autophagy, downregulates GPX4, SLC7A11, and induces ferroptosis (Wu et al., 2022).
2.4.2 Timosaponin (Tim-AIII)
Timosaponin AIII (Tim-AIII), classified as a steroid saponin, stands out as the primary active ingredient derived from Anemarrhena asphodeloides Bunge. Timosaponin AIII also has strong anticancer effects on liver cancer (Wang et al., 2013; Nho et al., 2016) and breast cancer (Sy et al., 2008; King et al., 2009), especially lung cancer (Zhou et al., 2023a). Zhou et al. disclosed that Timosaponin AIII (Tim-AIII) binds to HSP90, forming a complex that subsequently targets the degradation of GPX4 and promotes the ubiquitination of GPX4. Furthermore, Tim-AIII induces the accumulation of reactive oxygen species (ROS) and iron ions, as well as the production of malondialdehyde (MDA) and depletion of glutathione (GSH). These combined effects ultimately lead to therapeutic outcomes by inhibiting cell growth and reducing tumor volume through the induction of ferroptosis in non-small cell lung cancer (NSCLC) cell lines (Zhou et al., 2023b).
2.4.3 Ophiopogon in B (OP-B)
Ophiopogon in B (OP-B) is extracted from Radix Ophiopogon japonicus and has been reported to exert anticancer effects on different types of cancer, such as nasopharyngeal carcinoma (Dong et al., 2021), hepatocellular carcinoma (Yuan et al., 2022), lung cancer (Cheng et al., 2022). Previous findings have shown that OP-B has anticancer effects through the noniron ferroptosis pathway (Chen et al., 2016; Zhang et al., 2022). OP-B has been observed to significantly degrade glutathione peroxidase 4 (GPX4) and solute carrier family seven member 11 (SLC7A11) following treatment of gastric cancer cells in vitro. Morever, the tumor volume and weight of AGS decreased after OP-B administration in vivo. It suggested that OP-B may induce ferroptosis in gastric cancer cells by inhibiting GPX4/Xc−, inducing ROS accumulation and glutathione deficiency (Zhang et al., 2022).
2.4.4 Saikosaponin a (SsA)
Radix Bupleuri (RB) is derived from the dried roots of Bupleurum chinense or Bupleurum scorzonerifolium Willd. These plants are commonly found in sandy grasslands and dune meadows.
Saikosaponin A (SsA), a natural bioactive triterpenoid saponin extracted from RB (Wang et al., 2023a), has demonstrated potent antitumor activity against various types of tumors, including breast cancer (Zhang et al., 2021), cervical cancer (Du et al., 2021), pancreatic cancer (Shi et al., 2023), bladder cancer (Zhou et al., 2022), and colon cancer (Kang et al., 2017). Lan et al. reported that SsA induces ferroptosis in hepatocellular carcinoma (HCC) cells. This is achieved by activating endoplasmic reticulum (ER) stress-induced ATF3 upregulation and concurrently inhibiting the expression of the cystine transporter solute carrier family seven member 11 (SLC7A11) (Lan et al., 2023). (Figure 1)
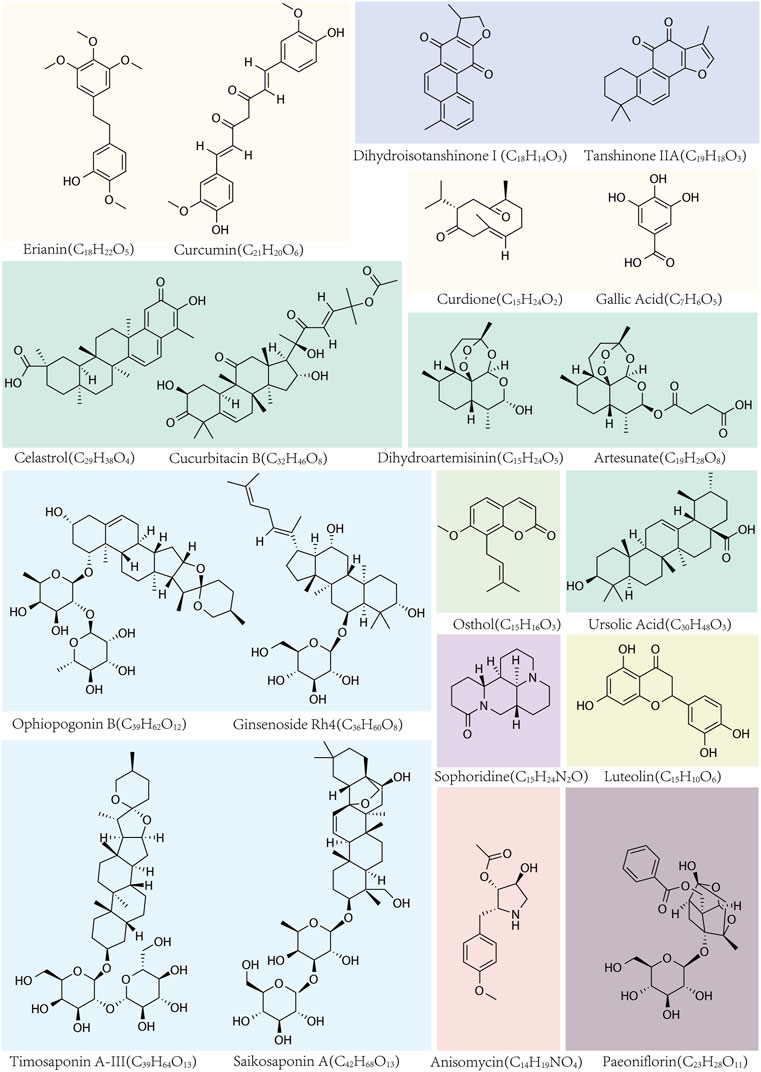
Figure 1. The names, structural formulas, and molecular formulas of TCMs that target ferroptosis for cancer treatment.
2.5 Prescriptions
2.5.1 Fu Fang Ku Shen injection (FKI)
Fu Fang Ku Shen injection has been widely used in adjuvant cancer therapy (Zhu et al., 2011; Zhao et al., 2014; Liu et al., 2017). Compared with single drugs, the combination of Fu Fang Ku Shen and chemotherapy drugs can improve leukaemia and relieve adverse reactions of digestive system gastric cancer (Yang et al., 2013; Lu et al., 2021a). Sophoridine is a natural plant monomer alkaloid obtained from Sophora alopecuroides and the main active compound of the Chinese traditional medicine Fu Fang Ku Shen (Qi et al., 2013). Sophoridine derivative 6j upregulates ATF3 expression through endoplasmic reticulum stress, promoting intracellular accumulation of iron ion, lipid oxygen species (ROS) and MDA, and activates ferroptosis in hepatocellular carcinoma cells (Tian et al., 2023).
2.5.2 Qing Re Huo Xue Formula (QRHXF)
The main ingredient of Qing Re Huo Xue Formula are Radix Paeoniae Rubra and Scutellaria baicalensis (Xu et al., 2017). The majority of these chemical components play a role in regulating the biological process of oxidative stress and have an impact on the balance of antioxidants (Lin et al., 2021b; Han et al., 2022b). Studies have confirmed that Qing Re Huo Xue Fang has therapeutic effects on lung diseases including pulmonary fibrosis (Yang et al., 2023), chronic obstructive pulmonary disease (Lin et al., 2016) and lung cancer (Xu et al., 2017). Qing Re Huo Xue Formula significantly improved the accumulation of lipid ROS, iron ion and MDA while reducing GSH levels and strongly suppressed SLC7A11 and GPX4 protein levels. Qing Re Huo Xue Formula (QRHXF) activates ferroptosis to impede the progression of non-small cell lung cancer (NSCLC) cells through the involvement of the p53 signaling pathway (Xu et al., 2023).
2.6 Others
Red ginseng polysaccharide, an active ingredient of the herb Panax ginseng C. A. Meyer (Araliaceae), exhibits anticancer effects on human lung cancer and breast cancer. It achieves this by inducing ferroptosis, primarily through the targeting of GPX4 (Zhai et al., 2022).
Luteolin, a flavonoid, is naturally found in various medicinal herbs (Franza et al., 2021). It possesses a wide range of activities, including antioxidant (Ahmadi et al., 2020), anti-inflammatory (Wang et al., 2020a; Gendrisch et al., 2021), antifibrotic (Li et al., 2015), and anticancer properties (Prasher et al., 2022; Matić et al., 2023). Luteolin has been shown to induce ferroptosis in prostate cancer by promoting the nuclear translocation of transcription factor EB (TFEB) and enhancing ferritinophagy (Fu et al., 2023). Heme oxygenase-1 (HMOX1), an inducible enzyme, is considered a measurable indicator of oxidative stress (Maines, 1997). Previous reports have shown that targeting heme oxygenase-1 (HMOX1) can induce ferroptosis in liver cancer cells (Zheng et al., 2023) and ovarian cancer (Ni et al., 2023). Han et al. reported that luteolin exhibits an anticancer effect on clear renal cell carcinoma by upregulating heme oxygenase-1 (HO-1) expression, thereby triggering ferroptosis. It was demonstrated that luteolin exerted potent antitumor activity both in vivo and in vitro. (Han et al., 2022a). (Table 1)
3 TCMs in combination target ferroptosis
TCM can also be combined with other drugs to exert anticancer effects synergistically via ferroptosis (Table 3). DHA promotes lipid peroxidation and ROS accumulation through nanocarriers combined with tetrandrine (TET), and synergistically inhibits DOX-resistant breast cancer cell growth (Zhang et al., 2023). ART, another artemisinin derivative, in combination with Nrf2 inhibitors promotes ferroptosis in tumor cells, achieving a more potent anticancer effect without damaging normal cells. RSL3 or Artesunate alone in the mitochondria could inhibite GPX4 activity to trigger ferroptosis. While the mitochondria-targeting artemisinin/RSL3 nanomedicine, A/R-PLGA/CPT/DSSP, treatment resulted in the strongest GPX4 inhibition compared with RSL3-PLGA/CPT/DSSP or ART-PLGA/CPT/DSSP. Carbon centered free radicals and ROS are produced in mitochondria to induce ferroptosis (Yu et al., 2023). β-Elemene, derived and purified from the roots and stems of the traditional Chinese medicine turmeric, is classified as a second-class anticancer drug. It has been used to participate in the treatment of some cancers (Wang et al., 2012; Wang et al., 2019a; Xiaomeng et al., 2020; Chen et al., 2021a). β-Elemene as a complementary drug in combination with cetuximab inhibites tumor growth and migration of KRAS mutant CRC cells by inducing ferroptosis. (Chen et al., 2020c).
Ursolic acid (UA) is a naturally occurring triterpenoid that is widely found in common fruits and herbs. Previous studies have shown that UA can inhibit the proliferation of prostate, lung, pancreatic and other tumor cells by inducing apoptosis (Chen et al., 2019; Lin et al., 2020; Kornel et al., 2023). Ursolic acid not only synergized with low doses of cisplatin in a mouse osteosarcoma xenograft model, significantly reducing tumor growth, but also reduced cisplatin-induced weight loss in mice. Detailed molecular studies have shown that ursolic acid activates autophagy first, then degrades ferritin, intracellular ferrous ion overload, and triggers ferroptosis ultimately. In addition, ursolic acid elevated the ability of cisplatin to destroy DNA damage in osteosarcoma cells (Tang et al., 2021b). Notably, In another study, ursolic acid combined with sorafenib treatment of HCT116 resulted in accumulation of lipid ROS, and downregulation of the apoptosis-related proteins Mcl-1 and ferroptosis -associated protein SLC7A11. Therefore, these results suggest that the synergistic antitumor mechanism of sorafenib/UA may also trigger ferroptosis by inducing apoptosis. (Li et al., 2022).
Osthole, chemically known as 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one, is a natural coumarin extracted from Cnidium spp. and various other plants belonging to the Apiaceae family (Sun et al., 2021). An increasing number of studies have demonstrated that osthole has anticancer effects on a variety of cancers (Shokoohinia et al., 2018), including glioma (Huangfu et al., 2021), endometrial cancer (Liang et al., 2021), and colorectal cancer (Huang et al., 2014; Zhou et al., 2021). Zhou et al. revealed that osthole can reduce the phosphorylation of AMPK, Akt and mTOR in HCT116 and SW480 cells and induce Ferroptosis by inhibiting the AMPK/Akt/mTOR signaling pathway. Combinative treatment of β-elemene and cetuximab enhance anticancer effect of cetuximab. Thus osthole plays an antitumor role in colorectal cancer cells with KRAS mutations (Zhou et al., 2023a).
Sheng Mai Yin(SMY), as a decoction of traditional Chinese medicine, comes from the Jin Dynasty Chinese medicine classic “Medical Qiyuan,” which is mainly composed of Radix ginseng, Ophiopogon and Schisandra. According to the testing of researchers, it was found that the active ingredients of Sheng Mai Yin mainly include triterpenoid saponins, steroidal saponins, lignans, etc. (Zheng et al., 2009; Liu et al., 2016; Wang et al., 2020b; Xu et al., 2021b). Sheng Mai Yin has previously been reported to have therapeutic effects on acute lymphoblastic leukemia (Guo et al., 2023), cardiac hypertrophy (Ming et al., 2022), heart failure (Kan et al., 2022), type 2 diabetes mellitus (T2DM) (Li et al., 2019). In addition, Sheng Mai Yin has been shown to inhibit the toxic side effects of doxorubicin and induced ferroptosis by modulating HMOX1, assisting cancer treatment and reducing patient complications (Meng et al., 2023).
4 Summary and outlook
Since ferroptosis was first defined in 2012, it has become a hot topic in the field of various diseases. The relationship between traditional Chinese medicine and ferroptosis and the corresponding regulatory mechanism in cancer are new research directions. Although several articles have reported the anticancer effects of Traditional Chinese Medicines (TCMs) by regulating ferroptosis, there is a limited summary of the underlying mechanisms. Combining these research results, the anticancer effects of TCMs through ferroptosis can be broadly categorized into four pathways: (1) inhibiting GPX4, (2) inhibiting system Xc−, (3) activating endoplasmic reticulum stress, and (4) imbalance of iron ion homeostasis (Figure 2).
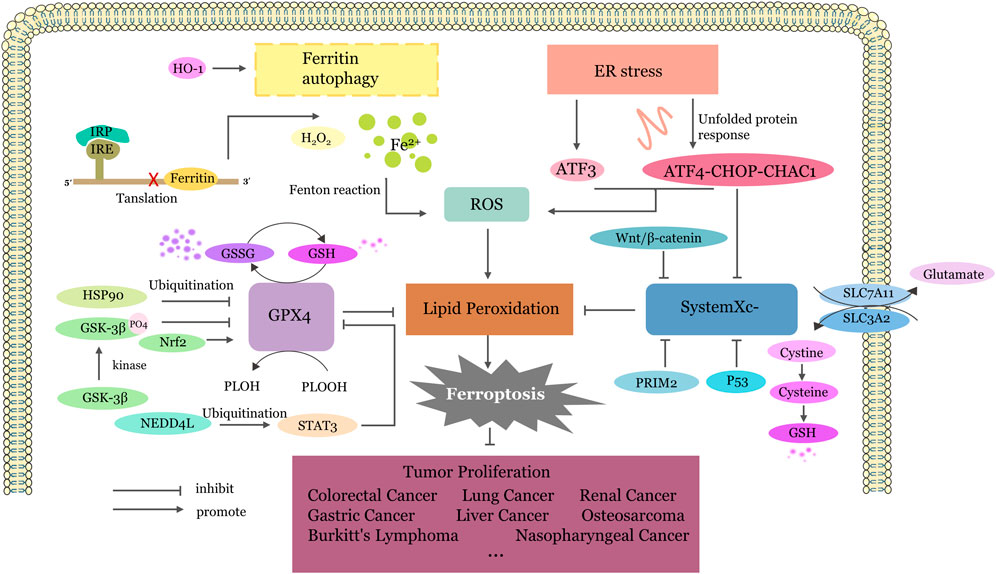
Figure 2. The Four Main Pathways of TCM Targeted Ferroptosis. Inhibiting GPX4:GPX4 undergoes ubiquitination degradation or translation inhibition mediated by different factors, thereby promoting the conversion of GSH to GSSG, reducing the reduction of phospholipid peroxides to phospholipid alcohols and increase lipid peroxidation. Inhibiting system Xc−: SLC7A11 and SLC3A2, as two components of the system Xc−, output glutamic acid and take up cysteine. Cysteine further participates in the synthesis of glutathione. When the system Xc− is inhibited, insufficient GSH conversion leads to the accumulation of lipid peroxidation products and ferroptosis. Activating endoplasmic reticulum stress: Upregulation of ATF3 expression or activation of the ATF4-CHOP-CHAC1 pathway by endoplasmic reticulum stress promotes the production of lipid ROS and inhibits system Xc−. Imbalance of iron ion homeostasis: Most Fe2+is stored in the form of an labile iron pool in ferritin. When ferritin autophagy occurs, excessive free iron is released, driving the Fenton reaction. The iron homeostasis regulation system IRP/IRE can be disrupted, receiving low iron signals, causing IRP to bind to the 5′ IRE, blocking ferritin synthesis, and promoting excessive free iron generation. ER, endoplasmic reticulum; ATF3, Activating Transcription Factor 3; ATF4, Activating Transcription Factor 4; GPX4, glutathione peroxidase 4; System Xc−,cystine/glutamate antiporter system; ROS, reactive oxygen species; CHAC1, Glutathione-specific gamma-glutamylcyclotransferase 1; PRIM2, DNA primase subunit 2; HSP90, Heat Shock Protein90; GSK3β, Glycogen synthase kinase 3β; GSH, Glutathione; GSSG, Glutathione disulfide; HO-1, heme oxygenase-1; IRP, Iron regulatory protein; IRE, iron-responsive element; HO-1, Heme oxygenase1; NEDD4L, Neural precursor cell expressed developmentally downregulated gene 4-like; STAT3, signal transducer and activator of transcription 3; Nrf2, nuclear factor erythroid 2-related factor 2; PLOH, phospholipid alcohol; PLOOH, phospholipid hydroperoxides; SLC7A11, Solute Carrier Family 7 Member 11; SLC3A2, Solute Carrier Family 3 Member two.
Glutathione peroxidase 4 (GPX4), an antioxidant enzyme belonging to the GPX protein family, is a critical aspect in defending against ferroptosis. GPX4, a selenium-containing cysteine enzyme, serves as a key defense mechanism against this form of cell death. Both GSH and GPX4 are important molecules in regulating cellular oxidative environment. GSH is also an essential cofactor of GPX4, so GPX4 is inhibited when GSH is depleted. (Cao and Dixon, 2016). It uses reduced GSH to convert toxic phospholipid hydroperoxides (PLOOHs) into nontoxic phospholipid alcohols (PLOHs) (Jiang et al., 2021). When GPX4 decreases, with an increase in toxic PLOOH, the membrane structure is destroyed to stimulate ferroptosis. (Stockwell et al., 2020). The Tim-AIII-HSP90 complex triggers ferroptosis in non-small cell lung cancer (NSCLC) through targeting ubiquitination and degradation of GPX4 (Zhou et al., 2023b). Anomanolide C (AC) reduces the expression of GPX4 through ubiquitination and inhibits triple-negative breast cancer (TNBC) proliferation and metastasis both in vitro and in vivo (Chen et al., 2023a). Paeoniflorin suppressd Nrl2 and GPX4 via upregulation of NEDD4L and ubiquitination of STAT3 (Nie et al., 2022). Dihydroisotanshinone I induces ferroptosis in Breast cancer cells by inhibiting GPX4, leading to depletion of intracellular glutathione and a sharp increase in GSSG(Ahmadi et al., 2020). Similarly,OP-B (Zhang et al., 2022) and cucurbitacin B (Huang et al., 2021)induce ferroptosis by inhibiting GPX4.
The cystine/glutamate reverse transport system (system xc -) exports glutamate out of the cell and imports cystine into the cell in equivalent proportions, which plays a key role in the synthesis of glutathione (GSH), an important antioxidant (Niu et al., 2021). Upon entering the cell, cystine undergoes rapid conversion to L-cysteine, playing a pivotal role as a key constituent in the synthesis of intracellular glutathione (GSH). Glutathione is a substance that contains γ- Tripeptides with amide bonds and thiol groups, as antioxidants, are present in almost every cell of the mammalian body. It serves to restore intracellular reduction‒oxidation (REDOX) balance subsequent to reactive oxygen species (ROS) production, thus preventing cellular damage from free radicals, peroxides, and lipid peroxides. Additionally, it acts as an essential substrate for the enzymatic activity of GPX4 (Niu et al., 2021). Inhibiting the Xc− system indirectly results in GSH depletion, disrupting endogenous antioxidant mechanisms and leading to a substantial accumulation of ROS, ultimately triggering ferroptosis (Yu et al., 2017). Iron overload, accumulation of reactive oxygen species (ROS) and phospholipid hydroperoxides (PLOOH), initiation of Fenton reaction and further release of PLOOH are markers of Ferroptosis (Conrad and Pratt, 2019; Liang et al., 2022). SLC7A11, an amino acid transporter, regulates cystine uptake and the biosynthesis of glutathione and promotes the establishment of the antioxidant defense system. Targeted inhibition of SLC7A11 promotes intracellular lipid peroxide accumulation, induce tumor cell ferroptosis, and enhances sensitivity to immunotherapy, radiotherapy, and chemotherapy (Koppula et al., 2021; Yan et al., 2023). Dihydroartemisinin downregulate the level of PRIM2/SLC7A11 axis so that induce ferroptosis and inhibits the proliferation of lung cancer cell (Yuan et al., 2020). Gallic acid inhibit SLC7A11 and Wnt/β-catenin signaling to promote hepatocellular carcinoma ferroptosis (Xie et al., 2023). Qingrehuoxue Formula activated GSK-3β phosphorylation while reduced GSH and SLC7A11 level. Ferroptosis in NSCLC is subsequently activated with the accumulation of ROS and MDA (Xu et al., 2023). Curcumin suppresses breast cancer by elevating solute carrier family one member 5 (SLC1A5) and enhanced glutamine uptake (Cao et al., 2022).
Endoplasmic reticulum (ER) stressis a cellular response triggered by protein misfolding, accumulation of unfolded proteins, and disturbances in calcium homeostasis within the ER lumen. This activates unfolded protein responses, ER overload responses, and, in severe cases, apoptosis (Wei et al., 2021). There is increasing evidence suggesting a close relationship between ferroptosis and endoplasmic reticulum (ER) stress (Dixon et al., 2014). Moreover, the mechanism by which certain natural products induce ferroptosis is closely related to endoplasmic reticulum (ER) stress (Lu et al., 2021b). Saikosaponin A (SsA) (Lan et al., 2023) and the sophoridine derivative 6j (Tian et al., 2023) induced ferroptosis in hepatocellular carcinoma (HCC) cells by activating ER stress. Artesunate (ART) enhances ferroptosis in Burkitt’s lymphoma cell lines by activating the ATF4/CHOP/CHAC1 pathway, an endoplasmic reticulum stress response (Wang et al., 2019b).
Some studies have suggested that ferroptosis is an autophagy-dependent form of cell death. Excessive autophagy can activate ferroptosis through the accumulation of iron ions or lipid reactive oxygen species (ROS). Ferritin autophagy, a process in which ferritin is degraded through autophagy, has been identified as triggering ferroptosis in various cancer cells (Gao et al., 2016; Hou et al., 2016). Luteolin (Fu et al., 2023), Rh4 (Wu et al., 2022) and ursolic acid (Tang et al., 2021a)promote ferroptosis by increasing autophagy. Ferritin is a cytoplasmic iron storage protein, negative feedback protein of Ferroptosis. It consists of two subunits, ferritin heavy chain 1 (FTH1) and ferritin light chain (FTL) (Xing et al., 2023). When ferritin autophagy degrades, it releases large amounts of free ferrous ions that combine with hydrogen peroxide to trigger the Fenton reaction that activates Ferroptosis (Dixon et al., 2012). Therefore, the death mode of ferritinophagy is essentially an iron metabolism disorder in cells, and iron homeostasis is unbalanced. Luteolin demonstrates an anticancer effect on clear renal cell carcinoma by upregulating heme oxygenase-1 (HO-1) expression and activating labile iron pool (LIP)directly, thus triggering ferroptosis (Han et al., 2022b).
In addition to the four main pathways mentioned above earlier, there are other ways of regulation (Figure 3). Erianin has the capacity to induce ferroptosis in renal cancer stem cells by promoting the N6-methyladenosine modification of ALOX12/P53 mRNA (Shen et al., 2023). Curdione can activate ferroptosis by enhancing the methylation of SLC7A11 mRNA and HOXA13 mRNA (Wang et al., 2023b). Erianin triggered ferroptosis in lung cancer cells by activating Ca2+/CaM signaling (Chen et al., 2020c). Heme activates artemisinin to generate alkyl radicals and/or ROS in mitochondria of cancer cells. RSL-3 inhibits GPX4 and further induces mitochondrial lipid peroxidation (Yu et al., 2023). AMP-activated protein kinase (AMPK) has been shown to inhibit tumor growth by resisting ferroptosis in some studies (Lee et al., 2020). Yi et al. have suggested that upregulation of PI3K-AKT-mTOR signaling inhibit ferroptosis though SREBP-mediated lipogenesis (Yi et al., 2020). Activation of PI3K/AKT/Nrf2 was shown to improve cognitive impairment after cerebral ischemia by up-regulating GPX4 to inhibit ferroptosis (Fu et al., 2022). Similarly, osthole decreases AMPK phosphorylation and exactly promotes ferroptosis in KRAS-mutant colorectal cancer cells (Zhou et al., 2023a). These regulatory pathways involving key physiological and biochemical targets shed light on whether we might be able to use related factor inhibitors to aid in cancer prevention in the future. Certainly, this needs to be studied more thoroughly and in conjunction with clinical trials.
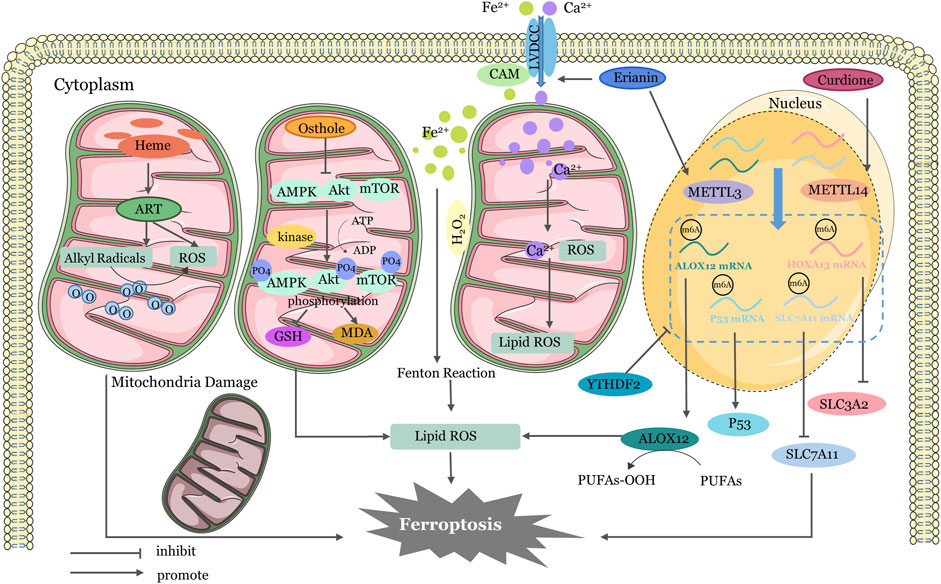
Figure 3. Mechanisms of Four Other Targeted Ferroptosis Pathways in TCMs. Activation of ART by heme produces ROS and alkyl radicals in cancer cell mitochondria, which damage membrane structures and damage mitochondria. Osthole supresses GSH generation and lipid ROS accumulation by activating AMPK/Akt/mTOR pathway phosphorylation. Erianin-activated calmodulin regulates LVDCC, increasing absorption of Fe2+ and Ca2+. Excessive calcium and iron ions induce lipid peroxidation. Erianin upregulates METTL3 to promote ALOX12 and P53mRNA methylation and increases ROS accumulation. Curdione upregulates METTL14 to promote SLC7A11mRNA and HOXA13mRNA methylation, reduce YTHDF2 stability, and inhibit expression of SLC7A11 and SLC3A2. ER, endoplasmic reticulum; ATF3, Activating Transcription Factor 3; ATF4, Activating Transcription Factor 4; GPX4, glutathione peroxidase 4; System Xc−,cystine/glutamate antiporter system; ROS, reactive oxygen species; CHAC1, Glutathione-specific gamma-glutamylcyclotransferase 1; PRIM2, DNA primase subunit 2; HSP90, Heat Shock Protein90; GSK3β, Glycogen synthase kinase 3β; GSH, Glutathione; GSSG, Glutathione disulfide; HO-1, heme oxygenase-1; IRP, Iron regulatory protein; IRE, iron-responsive element; HO-1, Heme oxygenase1; NEDD4L, Neural precursor cell expressed developmentally downregulated gene 4-like; STAT3, signal transducer and activator of transcription 3; Nrf2, nuclear factor erythroid 2-related factor 2; PLOH, phospholipid alcohol; PLOOH, phospholipid hydroperoxides; SLC7A11, Solute Carrier Family 7 Member 11; SLC3A2, Solute Carrier Family 3 Member two.
Indeed, several researchers have employed nanoparticles as tools to induce ferroptosis in tumor cells. The unique properties of nanoparticles make them promising agents for targeted therapeutic strategies, including the induction of ferroptosis in cancer cells (Jiang et al., 2021). For example, Ni et al. used atranorin complexes comprising superparamagnetic iron oxide nanoparticles (SPIONs) (Atranorin@SPION) to induce ferroptosis in GCSCs by decreasing the expression level of the Xc−/GPX4 axis and enhancing the 5-hydroxymethylcytosine modification of mRNAs in the pathway, thereby achieving therapeutic effects on gastric cancer (Ni et al., 2022b). Additionally, Yu et al. first demonstrated that artemisinin, a mitochondrial targeting agent, was much more toxic in vitro and in vivo than free artemisinin and non-targeted artemisinin nanodrugs against a variety of cancer cells, including MCF-7, HeLa, HepG2 and C6 cells. Mitochondrial artemisinin toxicity is mainly caused by free radicals and/or ROS-related apoptosis associated with artemisinin induced ferroptosis (Yu et al., 2023).
Some studies have also compared the effects of TCMs with classical ferroptosis inducers RSL-3 or erastin, which tend to increase the sensitivity of tumor cells to ferroptosis, resulting in mutual promotion (Table 2). Additionally, TCMs not only promote anticancer effects alone but also produce additive or synergistic effects when combined with Western medicines (Table 3). On the one hand, TCMs enhance the inhibition of tumor growth and metastasis by targeting iron death, and on the other hand, they alleviate the toxic side effects caused by chemotherapy drugs. After all, drug toxicity is an important consideration in terminal treatment options for cancer patients. These advantages make TCMs promising antitumor drugs.
Despite rapid and promising advances in research into the role of TCMs in iron death in cancer therapy, implementation into clinical use remains challenging. Activating ferroptosis can kill cancer cells, but whether normal tissues are damaged at the same time raises concerns about potential complications associated with the use of iron death inducers (Sui et al., 2019). Moreover, there are still doubts about the nonstandard compatibility, inaccurate dosage and nonsingle extract of traditional Chinese medicine. While Traditional Chinese Medicines (TCMs) and their active ingredients have been reported to have regulatory effects on ferroptosis, encompassing additional targets, stable structures, high safety, low cost, and easy availability, there is a notable insufficiency in the accumulation of related studies. Clinical trials are also lacking, and for many drugs, the mechanisms of action have not been fully elucidated. Therefore, to apply TCMs more rigorously and scientifically in the clinical treatment of cancer, future research should include additional large sample multicenter double-blind randomized controlled trials and related molecular cell biology experiments to further examine the mechanism of action, effectiveness and safety of TCMs. We hope that eventually TCMs can really slow down the progression of the disease, alleviate the suffering of patients, and improve the quality of life.
Author contributions
LQ: Writing–original draft. YZ: Writing–original draft. YL: Formal Analysis, Writing–review and editing. YY: Funding acquisition, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82200078, 92159302), the Interdisciplinary Innovation Project Fund of West China Hospital of Sichuan University (ZYJC21054) and the Science and Technology Project of Sichuan (2022ZDZX0018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmadi, S. M., Farhoosh, R., Sharif, A., and Rezaie, M. (2020). Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 85 (2), 298–305. doi:10.1111/1750-3841.14994
Bhattacharjee, S., Coppens, I., Mbengue, A., Suresh, N., Ghorbal, M., Slouka, Z., et al. (2018). Remodeling of the malaria parasite and host human red cell by vesicle amplification that induces artemisinin resistance. Blood 131 (11), 1234–1247. doi:10.1182/blood-2017-11-814665
Cao, J. Y., and Dixon, S. J. (2016). Mechanisms of ferroptosis. Cell Mol. Life Sci. 73 (11-12), 2195–2209. doi:10.1007/s00018-016-2194-1
Cao, L., Hong, W., Cai, P., Xu, C., Bai, X., Zhao, Z., et al. (2021). Cryptotanshinone strengthens the effect of gefitinib against non-small cell lung cancer through inhibiting transketolase. Eur. J. Pharmacol. 890, 173647. doi:10.1016/j.ejphar.2020.173647
Cao, X., Li, Y., Wang, Y., Yu, T., Zhu, C., Zhang, X., et al. (2022). Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS One 17 (1), e0261370. doi:10.1371/journal.pone.0261370
Carlson, B. A., Tobe, R., Yefremova, E., Tsuji, P. A., Hoffmann, V. J., Schweizer, U., et al. (2016). Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 9, 22–31. doi:10.1016/j.redox.2016.05.003
Chan, K. T., Li, K., Liu, S. L., Chu, K. H., Toh, M., and Xie, W. D. (2010a). Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 289 (1), 46–52. doi:10.1016/j.canlet.2009.07.015
Chan, K. T., Meng, F. Y., Li, Q., Ho, C. Y., Lam, T. S., To, Y., et al. (2010b). Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 294 (1), 118–124. doi:10.1016/j.canlet.2010.01.029
Chen, C. J., Shih, Y. L., Yeh, M. Y., Liao, N. C., Chung, H. Y., Liu, K. L., et al. (2019). Ursolic acid induces apoptotic cell death through AIF and endo G release through a mitochondria-dependent pathway in NCI-H292 human lung cancer cells in vitro. Vivo 33 (2), 383–391. doi:10.21873/invivo.11485
Chen, G. Q., Benthani, F. A., Wu, J., Liang, D., Bian, Z. X., and Jiang, X. (2020c). Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 27 (1), 242–254. doi:10.1038/s41418-019-0352-3
Chen, H., Li, Z., Xu, J., Zhang, N., Chen, J., Wang, G., et al. (2023b). Curcumin induces ferroptosis in follicular thyroid cancer by upregulating HO-1 expression. Oxid. Med. Cell Longev. 2023, 6896790. doi:10.1155/2023/6896790
Chen, J. C., Chiu, M. H., Nie, R. L., Cordell, G. A., and Qiu, S. X. (2005). Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat. Prod. Rep. 22 (3), 386–399. doi:10.1039/b418841c
Chen, M., Guo, Y., Zhao, R., Wang, X., Jiang, M., Fu, H., et al. (2016). Ophiopogonin B induces apoptosis, mitotic catastrophe and autophagy in A549 cells. Int. J. Oncol. 49 (1), 316–324. doi:10.3892/ijo.2016.3514
Chen, P., Li, X., Zhang, R., Liu, S., Xiang, Y., Zhang, M., et al. (2020a). Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 10 (11), 5107–5119. doi:10.7150/thno.44705
Chen, P., Wu, Q., Feng, J., Yan, L., Sun, Y., Liu, S., et al. (2020b). Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target Ther. 5 (1), 51. doi:10.1038/s41392-020-0149-3
Chen, X., Kang, R., Kroemer, G., and Tang, D. (2021a). Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18 (5), 280–296. doi:10.1038/s41571-020-00462-0
Chen, Y., Zhu, Z., Chen, J., Zheng, Y., Limsila, B., Lu, M., et al. (2021b). Terpenoids from Curcumae Rhizoma: their anticancer effects and clinical uses on combination and versus drug therapies. Biomed. Pharmacother. 138, 111350. doi:10.1016/j.biopha.2021.111350
Chen, Y. M., Xu, W., Liu, Y., Zhang, J. H., Yang, Y. Y., Wang, Z. W., et al. (2023a). Anomanolide C suppresses tumor progression and metastasis by ubiquitinating GPX4-driven autophagy-dependent ferroptosis in triple negative breast cancer. Int. J. Biol. Sci. 19 (8), 2531–2550. doi:10.7150/ijbs.82120
Cheng, Z., Li, Z., Gu, L., Li, L., Gao, Q., Zhang, X., et al. (2022). Ophiopogonin B alleviates cisplatin resistance of lung cancer cells by inducing Caspase-1/GSDMD dependent pyroptosis. J. Cancer 13 (2), 715–727. doi:10.7150/jca.66432
Conrad, M., Angeli, J. P., Vandenabeele, P., and Stockwell, B. R. (2016). Regulated necrosis: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 15 (5), 348–366. doi:10.1038/nrd.2015.6
Conrad, M., and Pratt, D. A. (2019). The chemical basis of ferroptosis. Nat. Chem. Biol. 15 (12), 1137–1147. doi:10.1038/s41589-019-0408-1
Dakeng, S., Duangmano, S., Jiratchariyakul, W., Bögler, O., and Patmasiriwat, P. (2012). Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus. J. Cell Biochem. 113 (1), 49–60. doi:10.1002/jcb.23326
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Dixon, S. J., Patel, D. N., Welsch, M., Skouta, R., Lee, E. D., Hayano, M., et al. (2014). Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523. doi:10.7554/eLife.02523
Dolma, S., Lessnick, S. L., Hahn, W. C., and Stockwell, B. R. (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3 (3), 285–296. doi:10.1016/s1535-6108(03)00050-3
Dong, W., Dong, Q., and Ding, H. (2021). Ophiopogonin B induces reactive oxygen species-dependent apoptosis through the Hippo pathway in nasopharyngeal carcinoma. Mol. Med. Rep. 24 (1), 534. doi:10.3892/mmr.2021.12173
Du, J., Song, D., Cao, T., Li, Y., Liu, J., Li, B., et al. (2021). Saikosaponin-A induces apoptosis of cervical cancer through mitochondria- and endoplasmic reticulum stress-dependent pathway in vitro and in vivo: involvement of PI3K/AKT signaling pathway. Cell Cycle 20 (21), 2221–2232. doi:10.1080/15384101.2021.1974791
Franza, L., Carusi, V., Nucera, E., and Pandolfi, F. (2021). Luteolin, inflammation and cancer: special emphasis on gut microbiota. Biofactors 47 (2), 181–189. doi:10.1002/biof.1710
Fu, C., Wu, Y., Liu, S., Luo, C., Lu, Y., Liu, M., et al. (2022). Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 289, 115021. doi:10.1016/j.jep.2022.115021
Fu, W., Xu, L., Chen, Y., Zhang, Z., Chen, S., Li, Q., et al. (2023). Luteolin induces ferroptosis in prostate cancer cells by promoting TFEB nuclear translocation and increasing ferritinophagy. Prostate 84, 223–236. doi:10.1002/pros.24642
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25 (3), 486–541. doi:10.1038/s41418-017-0012-4
Gao, M., Monian, P., Pan, Q., Zhang, W., Xiang, J., and Jiang, X. (2016). Ferroptosis is an autophagic cell death process. Cell Res. 26 (9), 1021–1032. doi:10.1038/cr.2016.95
Gao, Q., Yin, X. D., Zhang, F., Zhu, Y. Z., and Li, Z. L. (2022). The regulatory effects of traditional Chinese medicine on ferroptosis. Oxid. Med. Cell Longev. 2022, 4578381. doi:10.1155/2022/4578381
Ge, P., Luo, Y., Yang, Q., Wen, H., Liu, J., Zhang, Y., et al. (2023). Ferroptosis in rat lung tissue during severe acute pancreatitis-associated acute lung injury: protection of qingyi decoction. Oxid. Med. Cell Longev. 2023, 5827613. doi:10.1155/2023/5827613
Gendrisch, F., Esser, P. R., Schempp, C. M., and Wölfle, U. (2021). Luteolin as a modulator of skin aging and inflammation. Biofactors 47 (2), 170–180. doi:10.1002/biof.1699
Greenshields, A. L., Shepherd, T. G., and Hoskin, D. W. (2017). Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol. Carcinog. 56 (1), 75–93. doi:10.1002/mc.22474
Gu, Z. D., Shen, L. Y., Wang, H., Chen, X. M., Li, Y., Ning, T., et al. (2009). HOXA13 promotes cancer cell growth and predicts poor survival of patients with esophageal squamous cell carcinoma. Cancer Res. 69 (12), 4969–4973. doi:10.1158/0008-5472.Can-08-4546
Guan, Z., Chen, J., Li, X., and Dong, N. (2020). Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci. Rep. 40 (8). doi:10.1042/bsr20201807
Guo, D., Gu, M., Du, F., Zhao, Y., Gao, M., and Hao, J. (2023). Review of molecular biological studies on acute lymphoblastic leukemia treated by modified shengmaiyin. Med. Baltim. 102 (23), e34013. doi:10.1097/md.0000000000034013
Hambright, W. S., Fonseca, R. S., Chen, L., Na, R., and Ran, Q. (2017). Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 12, 8–17. doi:10.1016/j.redox.2017.01.021
Han, S., Lin, F., Qi, Y., Liu, C., Zhou, L., Xia, Y., et al. (2022a). HO-1 contributes to luteolin-triggered ferroptosis in clear cell renal cell carcinoma via increasing the labile iron pool and promoting lipid peroxidation. Oxid. Med. Cell Longev. 2022, 3846217. doi:10.1155/2022/3846217
Han, X., Hu, S., Yang, Q., Sang, X., Tang, D., Cao, G., et al. (2022b). Paeoniflorin ameliorates airway inflammation and immune response in ovalbumin induced asthmatic mice: from oxidative stress to autophagy. Phytomedicine 96, 153835. doi:10.1016/j.phymed.2021.153835
Hansen, C. G., Moroishi, T., and Guan, K. L. (2015). YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25 (9), 499–513. doi:10.1016/j.tcb.2015.05.002
Hou, W., Xie, Y., Song, X., Sun, X., Lotze, M. T., Zeh, H. J., et al. (2016). Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12 (8), 1425–1428. doi:10.1080/15548627.2016.1187366
Hsu, J. D., Kao, S. H., Ou, T. T., Chen, Y. J., Li, Y. J., and Wang, C. J. (2011). Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27(Kip1) attributed to disruption of p27(Kip1)/Skp2 complex. J. Agric. Food Chem. 59 (5), 1996–2003. doi:10.1021/jf103656v
Huang, S., Cao, B., Zhang, J., Feng, Y., Wang, L., Chen, X., et al. (2021). Induction of ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B: molecular mechanism and therapeutic potential. Cell Death Dis. 12 (3), 237. doi:10.1038/s41419-021-03516-y
Huang, S. M., Tsai, C. F., Chen, D. R., Wang, M. Y., and Yeh, W. L. (2014). p53 is a key regulator for osthole-triggered cancer pathogenesis. Biomed. Res. Int. 2014, 175247. doi:10.1155/2014/175247
Huangfu, M., Wei, R., Wang, J., Qin, J., Yu, D., Guan, X., et al. (2021). Osthole induces necroptosis via ROS overproduction in glioma cells. FEBS Open Bio 11 (2), 456–467. doi:10.1002/2211-5463.13069
Im, D. S. (2020). Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of Panax ginseng. Biomolecules 10 (3), 444. doi:10.3390/biom10030444
Jiang, X., Stockwell, B. R., and Conrad, M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22 (4), 266–282. doi:10.1038/s41580-020-00324-8
Kan, M., Wang, J., Ming, S., Sui, X., Zhang, Z., Yang, Q., et al. (2022). Investigating the mechanism of shengmaiyin (codonopsis pilosula) in the treatment of heart failure based on network pharmacology. Comb. Chem. High. Throughput Screen 25 (13), 2191–2202. doi:10.2174/1386207325666220221093415
Kang, S. J., Lee, Y. J., Kang, S. G., Cho, S., Yoon, W., Lim, J. H., et al. (2017). Caspase-4 is essential for saikosaponin a-induced apoptosis acting upstream of caspase-2 and γ-H2AX in colon cancer cells. Oncotarget 8 (59), 100433–100448. doi:10.18632/oncotarget.22247
King, F. W., Fong, S., Griffin, C., Shoemaker, M., Staub, R., Zhang, Y. L., et al. (2009). Timosaponin AIII is preferentially cytotoxic to tumor cells through inhibition of mTOR and induction of ER stress. PLoS One 4 (9), e7283. doi:10.1371/journal.pone.0007283
Klayman, D. L. (1985). Qinghaosu (artemisinin): an antimalarial drug from China. Science 228 (4703), 1049–1055. doi:10.1126/science.3887571
Koppula, P., Zhuang, L., and Gan, B. (2021). Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12 (8), 599–620. doi:10.1007/s13238-020-00789-5
Kornel, A., Nadile, M., Retsidou, M. I., Sakellakis, M., Gioti, K., Beloukas, A., et al. (2023). Ursolic acid against prostate and urogenital cancers: a review of in vitro and in vivo studies. Int. J. Mol. Sci. 24 (8), 7414. doi:10.3390/ijms24087414
Lan, T., Wang, W., Zeng, X. X., Tong, Y. H., Mao, Z. J., and Wang, S. W. (2023). Saikosaponin A triggers cell ferroptosis in hepatocellular carcinoma by inducing endoplasmic reticulum stress-stimulated ATF3 expression. Biochem. Biophys. Res. Commun. 674, 10–18. doi:10.1016/j.bbrc.2023.06.086
Lee, H., Zandkarimi, F., Zhang, Y., Meena, J. K., Kim, J., Zhuang, L., et al. (2020). Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22 (2), 225–234. doi:10.1038/s41556-020-0461-8
Li, H., Yu, Y., Liu, Y., Luo, Z., Law, B. Y. K., Zheng, Y., et al. (2022). Ursolic acid enhances the antitumor effects of sorafenib associated with Mcl-1-related apoptosis and SLC7A11-dependent ferroptosis in human cancer. Pharmacol. Res. 182, 106306. doi:10.1016/j.phrs.2022.106306
Li, J., Li, X., Xu, W., Wang, S., Hu, Z., Zhang, Q., et al. (2015). Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver Int. 35 (4), 1222–1233. doi:10.1111/liv.12638
Li, R., Zhang, J., Zhou, Y., Gao, Q., Wang, R., Fu, Y., et al. (2020). Transcriptome investigation and in vitro verification of curcumin-induced HO-1 as a feature of ferroptosis in breast cancer cells. Oxid. Med. Cell Longev. 2020, 3469840. doi:10.1155/2020/3469840
Li, S., Qian, Y., Xie, R., Li, Y., Jia, Z., Zhang, Z., et al. (2019). Exploring the protective effect of ShengMai-Yin and Ganmaidazao decoction combination against type 2 diabetes mellitus with nonalcoholic fatty liver disease by network pharmacology and validation in KKAy mice. J. Ethnopharmacol. 242, 112029. doi:10.1016/j.jep.2019.112029
Li, X. T., Chen, R., Jin, L. M., and Chen, H. Y. (2009). Regulation on energy metabolism and protection on mitochondria of Panax ginseng polysaccharide. Am. J. Chin. Med. 37 (6), 1139–1152. doi:10.1142/s0192415x09007454
Liang, D., Minikes, A. M., and Jiang, X. (2022). Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 82 (12), 2215–2227. doi:10.1016/j.molcel.2022.03.022
Liang, L., Yang, B., Wu, Y., and Sun, L. (2021). Osthole suppresses the proliferation and induces apoptosis via inhibiting the PI3K/AKT signaling pathway of endometrial cancer JEC cells. Exp. Ther. Med. 22 (4), 1171. doi:10.3892/etm.2021.10605
Lin, H., Chen, X., Zhang, C., Yang, T., Deng, Z., Song, Y., et al. (2021b). EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed. Pharmacother. 136, 111202. doi:10.1016/j.biopha.2020.111202
Lin, J., Xu, F., Wang, G., Kong, L., Luo, Q., Lv, Y., et al. (2016). Paeoniflorin attenuated oxidative stress in rat COPD model induced by cigarette smoke. Evid. Based Complement. Altern. Med. 2016, 1698379. doi:10.1155/2016/1698379
Lin, J. H., Chen, S. Y., Lu, C. C., Lin, J. A., and Yen, G. C. (2020). Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in gemcitabine-resistant human pancreatic cancer cells. Phytother. Res. 34 (8), 2053–2066. doi:10.1002/ptr.6669
Lin, M. Y., Cheng, W. T., Cheng, H. C., Chou, W. C., Chen, H. I., Ou, H. C., et al. (2021a). Baicalin enhances chemosensitivity to doxorubicin in breast cancer cells via upregulation of oxidative stress-mediated mitochondria-dependent apoptosis. Antioxidants (Basel) 10 (10), 1506. doi:10.3390/antiox10101506
Lin, X. M., Pan, M. H., Sun, J., Wang, M., Huang, Z. H., Wang, G., et al. (2023). Membrane phospholipid peroxidation promotes loss of dopaminergic neurons in psychological stress-induced Parkinson's disease susceptibility. Aging Cell 22 (10), e13970. doi:10.1111/acel.13970
Lin, Y. S., Shen, Y. C., Wu, C. Y., Tsai, Y. Y., Yang, Y. H., Lin, Y. Y., et al. (2019). Danshen improves survival of patients with breast cancer and dihydroisotanshinone I induces ferroptosis and apoptosis of breast cancer cells. Front. Pharmacol. 10, 1226. doi:10.3389/fphar.2019.01226
Liu, C., Ju, A., Zhou, D., Li, D., Kou, J., Yu, B., et al. (2016). Simultaneous qualitative and quantitative analysis of multiple chemical constituents in YiQiFuMai injection by ultra-fast liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Molecules 21 (5), 640. doi:10.3390/molecules21050640
Liu, H., Xie, J., Fan, L., Xia, Y., Peng, X., Zhou, J., et al. (2022). Cryptotanshinone protects against PCOS-induced damage of ovarian tissue via regulating oxidative stress, mitochondrial membrane potential, inflammation, and apoptosis via regulating ferroptosis. Oxid. Med. Cell Longev. 2022, 8011850. doi:10.1155/2022/8011850
Liu, Y. X., Wang, Z. F., Xie, Y. M., Zhang, Y., Chen, C., and Zhuang, Y. (2017). Clinical application features of Fufang Kushen injection in treating malignant esophageal tumor: real world study based on hospital information system. Zhongguo Zhong Yao Za Zhi 42 (15), 2877–2882. doi:10.19540/j.cnki.cjcmm.20170705.005
Lu, C., Ke, L., Li, J., Wu, S., Feng, L., Wang, Y., et al. (2021a). Chinese medicine as an adjunctive treatment for gastric cancer: methodological investigation of meta-analyses and evidence map. Front. Pharmacol. 12, 797753. doi:10.3389/fphar.2021.797753
Lu, S., Wang, X. Z., He, C., Wang, L., Liang, S. P., Wang, C. C., et al. (2021b). ATF3 contributes to brucine-triggered glioma cell ferroptosis via promotion of hydrogen peroxide and iron. Acta Pharmacol. Sin. 42 (10), 1690–1702. doi:10.1038/s41401-021-00700-w
Ma, L., Zhang, X., Yu, K., Xu, X., Chen, T., Shi, Y., et al. (2021). Targeting SLC3A2 subunit of system X(C)(-) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic. Biol. Med. 168, 25–43. doi:10.1016/j.freeradbiomed.2021.03.023
Maines, M. D. (1997). The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37, 517–554. doi:10.1146/annurev.pharmtox.37.1.517
Markowitsch, S. D., Schupp, P., Lauckner, J., Vakhrusheva, O., Slade, K. S., Mager, R., et al. (2020). Artesunate inhibits growth of sunitinib-resistant renal cell carcinoma cells through cell cycle arrest and induction of ferroptosis. Cancers (Basel) 12 (11), 3150. doi:10.3390/cancers12113150
Matić, I. Z., Mraković, A., Rakočević, Z., Stoiljković, M., Pavlović, V. B., and Momić, T. (2023). Anticancer effect of novel luteolin capped gold nanoparticles selectively cytotoxic towards human cervical adenocarcinoma HeLa cells: an in vitro approach. J. Trace Elem. Med. Biol. 80, 127286. doi:10.1016/j.jtemb.2023.127286
Meng, P., Chen, Z., Sun, T., Wu, L., Wang, Y., Guo, T., et al. (2023). Sheng-Mai-Yin inhibits doxorubicin-induced ferroptosis and cardiotoxicity through regulation of Hmox1. Aging (Albany NY) 15 (19), 10133–10145. doi:10.18632/aging.205062
Ming, S., Kan, M., Liu, L., Zhang, Z., Liu, X., Liu, Y., et al. (2022). Protective effect of shengmaiyin in myocardial hypertrophy-induced rats: a genomic analysis by 16S rDNA. Evid. Based Complement. Altern. Med. 2022, 3188292. doi:10.1155/2022/3188292
Miyazaki, K., Xu, C., Shimada, M., and Goel, A. (2023). Curcumin and andrographis exhibit anti-tumor effects in colorectal cancer via activation of ferroptosis and dual suppression of glutathione peroxidase-4 and ferroptosis suppressor protein-1. Pharm. (Basel) 16 (3), 383. doi:10.3390/ph16030383
Mori, T., Koyama, N., Yokoo, T., Segawa, T., Maeda, M., Sawmiller, D., et al. (2020). Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 295 (48), 16251–16266. doi:10.1074/jbc.RA119.012330
Nho, K. J., Chun, J. M., and Kim, H. K. (2016). Induction of mitochondria-dependent apoptosis in HepG2 human hepatocellular carcinoma cells by timosaponin A-III. Environ. Toxicol. Pharmacol. 45, 295–301. doi:10.1016/j.etap.2016.06.012
Ni, H., Ruan, G., Sun, C., Yang, X., Miao, Z., Li, J., et al. (2022a). Tanshinone IIA inhibits gastric cancer cell stemness through inducing ferroptosis. Environ. Toxicol. 37 (2), 192–200. doi:10.1002/tox.23388
Ni, M., Zhou, J., Zhu, Z., Xu, Q., Yin, Z., Wang, Y., et al. (2023). Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of HMOX1 to promote Fe(2+) accumulation. Phytomedicine 112, 154701. doi:10.1016/j.phymed.2023.154701
Ni, Z., Nie, X., Zhang, H., Wang, L., Geng, Z., Du, X., et al. (2022b). Atranorin driven by nano materials SPION lead to ferroptosis of gastric cancer stem cells by weakening the mRNA 5-hydroxymethylcytidine modification of the Xc-/GPX4 axis and its expression. Int. J. Med. Sci. 19 (11), 1680–1694. doi:10.7150/ijms.73701
Nie, X. H., Qiu, S., Xing, Y., Xu, J., Lu, B., Zhao, S. F., et al. (2022). Paeoniflorin regulates nedd4l/STAT3 pathway to induce ferroptosis in human glioma cells. J. Oncol. 2022, 6093216. doi:10.1155/2022/6093216
Niu, B., Liao, K., Zhou, Y., Wen, T., Quan, G., Pan, X., et al. (2021). Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 277, 121110. doi:10.1016/j.biomaterials.2021.121110
Ouyang, P., He, X., Yuan, Z. W., Yin, Z. Q., Fu, H., Lin, J., et al. (2018). Erianin against Staphylococcus aureus infection via inhibiting sortase A. Toxins (Basel) 10 (10), 385. doi:10.3390/toxins10100385
Piccolo, S., Dupont, S., and Cordenonsi, M. (2014). The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94 (4), 1287–1312. doi:10.1152/physrev.00005.2014
Prasher, P., Sharma, M., Singh, S. K., Gulati, M., Chellappan, D. K., Zacconi, F., et al. (2022). Luteolin: a flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 22 (1), 386. doi:10.1186/s12935-022-02808-3
Qi, L., Zhang, J., and Zhang, Z. (2013). Determination of four alkaloids in compound Kushen Injection by high performance liquid chromatography with ionic liquid as mobile phase additive. Se Pu 31 (3), 249–253. doi:10.3724/sp.j.1123.2012.10039
Quagliata, L., Quintavalle, C., Lanzafame, M., Matter, M. S., Novello, C., di Tommaso, L., et al. (2018). High expression of HOXA13 correlates with poorly differentiated hepatocellular carcinomas and modulates sorafenib response in in vitro models. Lab. Invest. 98 (1), 95–105. doi:10.1038/labinvest.2017.107
Roh, J. L., Kim, E. H., Jang, H., and Shin, D. (2017). Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 11, 254–262. doi:10.1016/j.redox.2016.12.010
Shen, H., Geng, Z., Nie, X., and Liu, T. (2023). Erianin induces ferroptosis of renal cancer stem cells via promoting ALOX12/P53 mRNA N6-methyladenosine modification. J. Cancer 14 (3), 367–378. doi:10.7150/jca.81027
Shi, C., Sun, L., Fang, R., Zheng, S., Yu, M., and Li, Q. (2023). Saikosaponin-A exhibits antipancreatic cancer activity by targeting the EGFR/PI3K/Akt pathway. Curr. Pharm. Biotechnol. 24 (4), 579–588. doi:10.2174/1389201023666220610113514
Shin, K. K., Yi, Y. S., Kim, J. K., Kim, H., Hossain, M. A., Kim, J. H., et al. (2020). Korean red ginseng plays an anti-aging role by modulating expression of aging-related genes and immune cell subsets. Molecules 25 (7), 1492. doi:10.3390/molecules25071492
Shokoohinia, Y., Jafari, F., Mohammadi, Z., Bazvandi, L., Hosseinzadeh, L., Chow, N., et al. (2018). Potential anticancer properties of osthol: a comprehensive mechanistic review. Nutrients 10 (1), 36. doi:10.3390/nu10010036
Song, J. Y., Luo, H. M., Li, C. F., Sun, C., Xu, J., and Chen, S. L. (2013). Salvia miltiorrhiza as medicinal model plant. Yao Xue Xue Bao 48 (7), 1099–1106. doi:10.16438/j.0513-4870.2013.07.008
Stockwell, B. R., Friedmann Angeli, J. P., Bayir, H., Bush, A. I., Conrad, M., Dixon, S. J., et al. (2017). Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171 (2), 273–285. doi:10.1016/j.cell.2017.09.021
Stockwell, B. R., Jiang, X., and Gu, W. (2020). Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 30 (6), 478–490. doi:10.1016/j.tcb.2020.02.009
Su, C., Zhang, P., Liu, J., and Cao, Y. (2017). Erianin inhibits indoleamine 2, 3-dioxygenase -induced tumor angiogenesis. Biomed. Pharmacother. 88, 521–528. doi:10.1016/j.biopha.2017.01.090
Su, Y., Zhao, D., Jin, C., Li, Z., Sun, S., Xia, S., et al. (2021). Dihydroartemisinin induces ferroptosis in HCC by promoting the formation of PEBP1/15-LO. Oxid. Med. Cell Longev. 2021, 3456725. doi:10.1155/2021/3456725
Sui, S., Zhang, J., Xu, S., Wang, Q., Wang, P., and Pang, D. (2019). Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 10 (5), 331. doi:10.1038/s41419-019-1564-7
Sun, G., Zhang, S., Xie, Y., Zhang, Z., and Zhao, W. (2016). Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol. Lett. 11 (1), 150–158. doi:10.3892/ol.2015.3845
Sun, M., Sun, M., and Zhang, J. (2021). Osthole: an overview of its sources, biological activities, and modification development. Med. Chem. Res. 30 (10), 1767–1794. doi:10.1007/s00044-021-02775-w
Sy, L. K., Yan, S. C., Lok, C. N., Man, R. Y., and Che, C. M. (2008). Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in HeLa cancer cells. Cancer Res. 68 (24), 10229–10237. doi:10.1158/0008-5472.Can-08-1983
Tang, X., Ding, H., Liang, M., Chen, X., Yan, Y., Wan, N., et al. (2021a). Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 12 (8), 1219–1230. doi:10.1111/1759-7714.13904
Tang, Z., Dong, H., Li, T., Wang, N., Wei, X., Wu, H., et al. (2021b). The synergistic reducing drug resistance effect of cisplatin and ursolic acid on osteosarcoma through a multistep mechanism involving ferritinophagy. Oxid. Med. Cell Longev. 2021, 5192271. doi:10.1155/2021/5192271
Tian, K., Wei, J., Wang, R., Wei, M., Hou, F., and Wu, L. (2023). Sophoridine derivative 6j inhibits liver cancer cell proliferation via ATF3 mediated ferroptosis. Cell Death Discov. 9 (1), 296. doi:10.1038/s41420-023-01597-6
Tonnus, W., and Linkermann, A. (2017). The in vivo evidence for regulated necrosis. Immunol. Rev. 277 (1), 128–149. doi:10.1111/imr.12551
Toyokuni, S., Ito, F., Yamashita, K., Okazaki, Y., and Akatsuka, S. (2017). Iron and thiol redox signaling in cancer: an exquisite balance to escape ferroptosis. Free Radic. Biol. Med. 108, 610–626. doi:10.1016/j.freeradbiomed.2017.04.024
Tuo, Q. Z., Lei, P., Jackman, K. A., Li, X. L., Xiong, H., Li, X. L., et al. (2017). Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 22 (11), 1520–1530. doi:10.1038/mp.2017.171
Wang, B., Peng, X. X., Sun, R., Li, J., Zhan, X. R., Wu, L. J., et al. (2012). Systematic review of β-elemene injection as adjunctive treatment for lung cancer. Chin. J. Integr. Med. 18 (11), 813–823. doi:10.1007/s11655-012-1271-9
Wang, F., Sun, Z., Zhang, Q., Yang, H., Yang, G., Yang, Q., et al. (2023a). Curdione induces ferroptosis mediated by m6A methylation via METTL14 and YTHDF2 in colorectal cancer. Chin. Med. 18 (1), 122. doi:10.1186/s13020-023-00820-x
Wang, H., Xie, B., Shi, S., Zhang, R., Liang, Q., Liu, Z., et al. (2023b). Curdione inhibits ferroptosis in isoprenaline-induced myocardial infarction via regulating Keap1/Trx1/GPX4 signaling pathway. Phytother. Res. 37 (11), 5328–5340. doi:10.1002/ptr.7964
Wang, J., Song, Y., Chen, Z., and Leng, S. X. (2018). Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid. Med. Cell Longev. 2018, 1972714. doi:10.1155/2018/1972714
Wang, N., Feng, Y., Zhu, M., Siu, F. M., Ng, K. M., and Che, C. M. (2013). A novel mechanism of XIAP degradation induced by timosaponin AIII in hepatocellular carcinoma. Biochim. Biophys. Acta 1833 (12), 2890–2899. doi:10.1016/j.bbamcr.2013.07.018
Wang, N., Zeng, G. Z., Yin, J. L., and Bian, Z. X. (2019b). Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt's Lymphoma. Biochem. Biophys. Res. Commun. 519 (3), 533–539. doi:10.1016/j.bbrc.2019.09.023
Wang, R. L., Wang, H. Q., Fan, X. H., Duan, M. X., Li, H. Y., and Wang, S. F. (2020a). Analysis of chemical constituents in shenmai injection by LC-Q-TOF-MS and LC-IT-MS. Zhongguo Zhong Yao Za Zhi 45 (3), 555–564. doi:10.19540/j.cnki.cjcmm.20191002.308
Wang, S., Cao, M., Xu, S., Shi, J., Mao, X., Yao, X., et al. (2020b). Luteolin alters macrophage polarization to inhibit inflammation. Inflammation 43 (1), 95–108. doi:10.1007/s10753-019-01099-7
Wang, S. J., Li, D., Ou, Y., Jiang, L., Chen, Y., Zhao, Y., et al. (2016). Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Rep. 17 (2), 366–373. doi:10.1016/j.celrep.2016.09.022
Wang, X., Liu, Z., Sui, X., Wu, Q., Wang, J., and Xu, C. (2019a). Elemene injection as adjunctive treatment to platinum-based chemotherapy in patients with stage III/IV non-small cell lung cancer: a meta-analysis following the PRISMA guidelines. Phytomedicine 59, 152787. doi:10.1016/j.phymed.2018.12.010
Wang, Y. F., Ma, R. X., Zou, B., Li, J., Yao, Y., and Li, J. (2023c). Endoplasmic reticulum stress regulates autophagic response that is involved in Saikosaponin a-induced liver cell damage. Toxicol Vitro 88, 105534. doi:10.1016/j.tiv.2022.105534
Wei, R., Zhao, Y., Wang, J., Yang, X., Li, S., Wang, Y., et al. (2021). Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 17 (11), 2703–2717. doi:10.7150/ijbs.59404
Wu, J., Minikes, A. M., Gao, M., Bian, H., Li, Y., Stockwell, B. R., et al. (2019). Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572 (7769), 402–406. doi:10.1038/s41586-019-1426-6
Wu, X., Pan, J., Yu, J. J., Kang, J., Hou, S., Cheng, M., et al. (2023). DiDang decoction improves mitochondrial function and lipid metabolism via the HIF-1 signaling pathway to treat atherosclerosis and hyperlipidemia. J. Ethnopharmacol. 308, 116289. doi:10.1016/j.jep.2023.116289
Wu, Y., Pi, D., Chen, Y., Zuo, Q., Zhou, S., and Ouyang, M. (2022). Ginsenoside Rh4 inhibits colorectal cancer cell proliferation by inducing ferroptosis via autophagy activation. Evid. Based Complement. Altern. Med. 2022, 6177553. doi:10.1155/2022/6177553
Xia, Q., Wang, X., Xu, D. J., Chen, X. H., and Chen, F. H. (2012). Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil. Thromb. Res. 130 (3), 409–414. doi:10.1016/j.thromres.2012.04.005
Xiaomeng, F., Lei, L., Jinghong, A., Juan, J., Qi, Y., and Dandan, Y. (2020). Treatment with β-elemene combined with paclitaxel inhibits growth, migration, and invasion and induces apoptosis of ovarian cancer cells by activation of STAT-NF-κB pathway. Braz J. Med. Biol. Res. 53 (6), e8885. doi:10.1590/1414-431x20208885
Xie, J., Wang, H., Xie, W., Liu, Y., and Chen, Y. (2023). Gallic acid promotes ferroptosis in hepatocellular carcinoma via inactivating Wnt/β-catenin signaling pathway. Naunyn Schmiedeb. Arch. Pharmacol. 397, 2437–2445. doi:10.1007/s00210-023-02770-5
Xing, N., Du, Q., Guo, S., Xiang, G., Zhang, Y., Meng, X., et al. (2023). Ferroptosis in lung cancer: a novel pathway regulating cell death and a promising target for drug therapy. Cell Death Discov. 9 (1), 110. doi:10.1038/s41420-023-01407-z
Xu, B., Zhu, W. J., Peng, Y. J., and Cheng, S. D. (2021b). Curcumin reverses the sunitinib resistance in clear cell renal cell carcinoma (ccRCC) through the induction of ferroptosis via the ADAMTS18 gene. Transl. Cancer Res. 10 (7), 3158–3167. doi:10.21037/tcr-21-227
Xu, F., Cui, W., Zhao, Z., Gong, W., Wei, Y., Liu, J., et al. (2017). Targeting tumor microenvironment: effects of Chinese herbal formulae on macrophage-mediated lung cancer in mice. Evid. Based Complement. Altern. Med. 2017, 7187168. doi:10.1155/2017/7187168
Xu, F., Zhang, J., Ji, L., Cui, W., Cui, J., Tang, Z., et al. (2023). Inhibition of non-small cell lung cancer by ferroptosis and apoptosis induction through P53 and GSK-3β/nrf2 signal pathways using qingrehuoxue formula. J. Cancer 14 (3), 336–349. doi:10.7150/jca.79465
Xu, L., Shang, Z., Tian, Y., Xiong, M., Nijat, D., Wang, Y., et al. (2021a). Chemical variations among shengmaisan-based TCM patent drugs by ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry. Molecules 26 (13), 4000. doi:10.3390/molecules26134000
Yagoda, N., von Rechenberg, M., Zaganjor, E., Bauer, A. J., Yang, W. S., Fridman, D. J., et al. (2007). RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447 (7146), 864–868. doi:10.1038/nature05859
Yan, X., Liu, Y., Li, C., Mao, X., Xu, T., Hu, Z., et al. (2023). Pien-Tze-Huang prevents hepatocellular carcinoma by inducing ferroptosis via inhibiting SLC7A11-GSH-GPX4 axis. Cancer Cell Int. 23 (1), 109. doi:10.1186/s12935-023-02946-2
Yang, F., Du, W., Tang, Z., Wei, Y., and Dong, J. (2023). Protective effects of Qing-Re-Huo-Xue formula on bleomycin-induced pulmonary fibrosis through the p53/IGFBP3 pathway. Chin. Med. 18 (1), 33. doi:10.1186/s13020-023-00730-y
Yang, J., Zhu, L., Wu, Z., and Wang, Y. (2013). Chinese herbal medicines for induction of remission in advanced or late gastric cancer. Cochrane Database Syst. Rev. (4), Cd005096. doi:10.1002/14651858.CD005096.pub4
Yang, S., Zhang, D., Shen, N., Wang, G., Tang, Z., and Chen, X. (2019). Dihydroartemisinin increases gemcitabine therapeutic efficacy in ovarian cancer by inducing reactive oxygen species. J. Cell Biochem. 120 (1), 634–644. doi:10.1002/jcb.27421
Yang, W. S., SriRamaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156 (1-2), 317–331. doi:10.1016/j.cell.2013.12.010
Yang, W. S., and Stockwell, B. R. (2008). Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15 (3), 234–245. doi:10.1016/j.chembiol.2008.02.010
Yi, J., Zhu, J., Wu, J., Thompson, C. B., and Jiang, X. (2020). Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. U. S. A. 117 (49), 31189–31197. doi:10.1073/pnas.2017152117
Yu, H., Guo, P., Xie, X., Wang, Y., and Chen, G. (2017). Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell Mol. Med. 21 (4), 648–657. doi:10.1111/jcmm.13008
Yu, H., Li, J. M., Deng, K., Zhou, W., Li, K. H., Wang, C. X., et al. (2023). GPX4 inhibition synergistically boosts mitochondria targeting nanoartemisinin-induced apoptosis/ferroptosis combination cancer therapy. Biomater. Sci. 11 (17), 5831–5845. doi:10.1039/d3bm00601h
Yuan, B., Liao, F., Shi, Z. Z., Ren, Y., Deng, X. L., Yang, T. T., et al. (2020). Dihydroartemisinin inhibits the proliferation, colony formation and induces ferroptosis of lung cancer cells by inhibiting PRIM2/slc7a11 Axis. Onco Targets Ther. 13, 10829–10840. doi:10.2147/ott.S248492
Yuan, F., Gao, Q., Tang, H., Shi, J., and Zhou, Y. (2022). Ophiopogonin-B targets PTP1B to inhibit the malignant progression of hepatocellular carcinoma by regulating the PI3K/AKT and AMPK signaling pathways. Mol. Med. Rep. 25 (4), 122. doi:10.3892/mmr.2022.12638
Zhai, F. G., Liang, Q. C., Wu, Y. Y., Liu, J. Q., and Liu, J. W. (2022). Red ginseng polysaccharide exhibits anticancer activity through GPX4 downregulation-induced ferroptosis. Pharm. Biol. 60 (1), 909–914. doi:10.1080/13880209.2022.2066139
Zhang, L., Li, C., Zhang, Y., Zhang, J., and Yang, X. (2022). Ophiopogonin B induces gastric cancer cell death by blocking the GPX4/xCT-dependent ferroptosis pathway. Oncol. Lett. 23 (3), 104. doi:10.3892/ol.2022.13224
Zhang, M., Zhang, H., Sun, C., Shan, X., Yang, X., Li-Ling, J., et al. (2009). Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother. Pharmacol. 63 (4), 635–642. doi:10.1007/s00280-008-0780-0
Zhang, P., Lai, X., Zhu, M. H., Long, M., Liu, X. L., Wang, Z. X., et al. (2021). Saikosaponin A, a triterpene saponin, suppresses angiogenesis and tumor growth by blocking VEGFR2-mediated signaling pathway. Front. Pharmacol. 12, 713200. doi:10.3389/fphar.2021.713200
Zhang, T., Ouyang, H., Mei, X., Lu, B., Yu, Z., Chen, K., et al. (2019b). Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. Faseb J. 33 (11), 11776–11790. doi:10.1096/fj.201802614RRR
Zhang, X., Liu, H., Li, N., Li, J., Wang, M., and Ren, X. (2023). A (traditional Chinese medicine) TCM-inspired doxorubicin resistance reversing strategy: preparation, characterization, and application of a Co-loaded pH-sensitive liposome. AAPS PharmSciTech 24 (7), 181. doi:10.1208/s12249-023-02630-8
Zhang, Y., Tan, H., Daniels, J. D., Zandkarimi, F., Liu, H., Brown, L. M., et al. (2019a). Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem. Biol. 26 (5), 623–633. doi:10.1016/j.chembiol.2019.01.008
Zhao, Z., Fan, H., Higgins, T., Qi, J., Haines, D., Trivett, A., et al. (2014). Fufang Kushen injection inhibits sarcoma growth and tumor-induced hyperalgesia via TRPV1 signaling pathways. Cancer Lett. 355 (2), 232–241. doi:10.1016/j.canlet.2014.08.037
Zheng, C., Hao, H., Wang, X., Wu, X., Wang, G., Sang, G., et al. (2009). Diagnostic fragment-ion-based extension strategy for rapid screening and identification of serial components of homologous families contained in traditional Chinese medicine prescription using high-resolution LC-ESI- IT-TOF/MS: shengmai injection as an example. J. Mass Spectrom. 44 (2), 230–244. doi:10.1002/jms.1502
Zheng, C., Zhang, B., Li, Y., Liu, K., Wei, W., Liang, S., et al. (2023). Donafenib and GSK-J4 synergistically induce ferroptosis in liver cancer by upregulating HMOX1 expression. Adv. Sci. (Weinh) 10 (22), e2206798. doi:10.1002/advs.202206798
Zhou, C., Yu, T., Zhu, R., Lu, J., Ouyang, X., Zhang, Z., et al. (2023a). Timosaponin AIII promotes non-small-cell lung cancer ferroptosis through targeting and facilitating HSP90 mediated GPX4 ubiquitination and degradation. Int. J. Biol. Sci. 19 (5), 1471–1489. doi:10.7150/ijbs.77979
Zhou, Q., Wu, W. W., Yu, C. L., Wang, P., Wen, X. Q., Chen, B. L., et al. (2022). Saikosaponin A inhibits growth of human bladder carcinoma T24 and 5637 cells both in vitro and in vivo. Biol. Pharm. Bull. 45 (7), 863–871. doi:10.1248/bpb.b21-01025
Zhou, X., Kang, J., Zhang, L., and Cheng, Y. (2023b). Osthole inhibits malignant phenotypes and induces ferroptosis in KRAS-mutant colorectal cancer cells via suppressing AMPK/Akt signaling. Cancer Chemother. Pharmacol. 92 (2), 119–134. doi:10.1007/s00280-023-04549-0
Zhou, X. H., Kang, J., Zhong, Z. D., and Cheng, Y. (2021). Osthole induces apoptosis of the HT-29 cells via endoplasmic reticulum stress and autophagy. Oncol. Lett. 22 (4), 726. doi:10.3892/ol.2021.12987
Zhu, A. L., Wang, F., Fan, Q. X., He, W., Wang, L. X., and Zhao, P. R. (2011). Apoptosis and growth arrest of human esophageal squamous cell carcinoma cell EC9706 induced by Fufangkushen injection. Zhonghua Yi Xue Za Zhi 91 (39), 2797–2800. doi:10.3760/cma.j.issn.0376-2491.2011.39.018
Zhu, H., He, Y. S., Ma, J., Zhou, J., Kong, M., Wu, C. Y., et al. (2021). The dual roles of ginsenosides in improving the anti-tumor efficiency of cyclophosphamide in mammary carcinoma mice. J. Ethnopharmacol. 265, 113271. doi:10.1016/j.jep.2020.113271
Keywords: ferroptosis, traditional Chinese medicine, cancer treatment, the role of ferroptosis, cancer
Citation: Qin L, Zhong Y, Li Y and Yang Y (2024) TCM targets ferroptosis: potential treatments for cancer. Front. Pharmacol. 15:1360030. doi: 10.3389/fphar.2024.1360030
Received: 22 December 2023; Accepted: 08 April 2024;
Published: 22 April 2024.
Edited by:
Xiao Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Koraljka Gall Troselj, Rudjer Boskovic Institute, CroatiaZhi-Bin Wang, Central South University, China
Copyright © 2024 Qin, Zhong, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongfeng Yang, eWFuZ3lvbmdmZW5nMDUyM0AxNjMuY29t
 Liwen Qin
Liwen Qin Yuhan Zhong
Yuhan Zhong Yi Li
Yi Li Yongfeng Yang
Yongfeng Yang