- 1Department of Pharmacy, Traditional Chinese and Western Medicine Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pharmacy, Wuhan No.1 hospital, Wuhan, China
- 3Department of Oncology, Traditional Chinese and Western Medicine Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Oncology, Wuhan No.1 hospital, Wuhan, China
Background: Inhibitors of the anaplastic lymphoma kinase (ALK) gene mutation are first-line treatments in patients with ALK-positive lung cancer. The FDA label warns of the risk of interstitial lung disease (ILD) in patients receiving ALK TKIs. However, ILD associated with ALK TKIs is not fully understood. The aim of this study was to characterize the features of ALK TKI-related ILD and to explore risk factors for ALK TKI-related ILD.
Methods: FDA’s Adverse Event Reporting System (FAERS) reports from 2011 Q1 to 2023 Q2 were extracted and combined. Standardized MedDRA queries (SMQs) were used to search for AEs at the preferred term (PT) level. Four algorithms were employed to quantify the signals of ILD associated with ALK TKIs. The risk of ILD was further analyzed using logistic regression.
Results: A total of 20,064 reports of ALK TKIs and 640 (3.2%) reports of ILD AEs were extracted. Significant disproportionality was detected in all five ALK TKIs. Interstitial lung disease and pneumonitis were the most common lung toxicities induced by ALK TKIs. Results of further analyses revealed a different spectrum of lung toxicity among the various TKIs. The median time to onset of ILD related to ALK TKIs was 53 days (Q1:12, Q3:209), and more than 70% of AEs occurred within the first 2 months. Logistic regression analysis and risk prediction model both showed that different ALK TKIs and their combination with PPIs, amlodipine, and magnesium oxide were independent risk factors for ILD (p<0.05).
Conclusion: ALK TKIs have different safety profiles regarding lung toxicity, which normally occurs within the first 2 months. Administration in combination with PPIs, amlodipine, and magnesium oxide significantly increases the risk of ILD. These results provide risk prediction for ILD related to ALK TKIs and support pharmacovigilance to promote safe prescribing in oncology.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related deaths globally (Sung et al., 2021). A rearranged anaplastic lymphoma kinase (ALK) gene/fusion is a unique biomarker in NSCLC patients, presenting in approximately 3%–7% of cases (Horn and Pao, 2009). Over the past decade, the emergence of ALK tyrosine kinase inhibitors (TKIs) has significantly transformed the treatment landscape and outcomes for ALK+ NSCLC patients (Solomon et al., 2014; Shaw et al., 2014; Peters et al., 2017; Camidge et al., 2018; Shaw et al., 2020).
In 2011, the Food and Drug Administration (FDA) approved crizotinib as the first ALK TKI for patients with ALK+ NSCLC (Crino et al., 2011; Kim et al., 2012). This multi-targeted TKI demonstrated efficacy against MET (mesenchymal–epithelial transition, a prototypical receptor tyrosine kinase, whose alterations are drivers of human cancer), ALK, and ROS1 (ROS proto-oncogene 1, a receptor tyrosine kinase, which is involved in genetic rearrangement in a variety of human cancers), ALK, and ROS1, and was proven to improve progression-free survival (PFS) compared with traditional chemotherapy (median 10.9 months vs. 7.0 months; HR 0.45; 95% CI 0.35–0.60; p < 0.001) in patients with ALK-positive lung cancer (Shaw et al., 2103). However, crizotinib has been widely replaced by next-generation TKIs in first-line therapy due to its poor intracranial activity and shorter PFS (Gadgeel et al., 2120; Crinò et al., 2016). Currently, second-generation ALK TKIs have been developed, including ceritinib, alectinib, and brigatinib, which address the crizotinib resistance and offer better blood–brain barrier (BBB) penetration in patients with CNS involvement (Mok et al., 2011; Ou et al., 2016; Dong-Wan et al., 2017; D Ross Camidge et al., 2018). Alectinib exhibits longer PFS than crizotinib (median 34.8 months vs. 10.9 months) (Camidge et al., 2019); however, it has also been associated with the problem of drug resistance (Gainor et al., 2016). Lorlatinib, an ATP-competitive macrocyclic small-molecule inhibitor, was developed to resolve the problem of resistant mutations that occur during treatment with the first second-generation ALK TKIs (Abbattista et al., 2019; Zou et al., 2015). To date, the FDA has approved five ALK TKIs (crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib) as the first-line and follow-up treatment drugs for patients with ALK+ NSCLC (Pirker and Filipits, 2019).
Treatment with ALK TKIs is normally well-tolerated. However, the patterns and frequency of side effects differ among ALK TKIs (Omar et al., 2021), which may be a key consideration for physicians when choosing a medication. Interstitial lung disease (ILD) is a heterogeneous group of parenchymal lung diseases with high morbidity and mortality (Antoniou et al., 2014). Several risk factors for ILD also coexist, including anticancer drugs (Spagnolo et al., 2022). A system study found that ALK TKIs showed significant respiratory system toxicity, with pneumonia being the most common serious adverse event with the highest incidence (Hou et al., 2019). Recently, a report characterized interstitial pneumonitis (IP) associated with ALK TKIs in the real world and indicated a fatal risk of IP induced by ALK TKIs (Ma et al., 2023; Zhao et al., 2023). The FDA released a hazard alert on ALK TKIs for ILD; however, a comprehensive evaluation of ILD induced by five ALK TKIs and the risks of ILD was still inadequate.
Therefore, it is important to explore the clinical characteristics and risk factors of ALK TKI-induced ILD to ensure appropriate drug selection. The aim of this study was to comprehensively characterize ILD with ALK TKIs in real-world patterns by investigating the FDA’s Adverse Event Reporting System (FAERS) and to evaluate the risk of ILD. A risk model for predicting ILD with ALK TKIs was constructed using logistic regression, and risk factors were determined for medication decisions.
Materials and methods
Data sources
This observational, retrospective pharmacovigilance analysis was conducted using data from adverse event reports recorded in the FAERS database, which are available at https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html. The data downloaded were limited to the period from 1 January 2011 to June 2023 (the most recent available data), which comprised seven tables named “DEMO,” “DRUG,” “REAC,” “OUTC,” “RPSR,” “THER,” and “INDI”. The “DEMO” tables were used in the missing value imputation and case de-duplication steps. AEs were coded according to the Medical Dictionary for Regulatory Activities Terminology (MedDRA) at the preferred term (PT) level. For signal detection, the drug was considered the primary suspect.
Data processing
Data processing included missing value imputation, case de-duplication, and standardization (Supplementary Figure S1), with reference to the study by Nigam H. Shah et al. (Banda et al., 2016). First, a single missing value imputation was performed for four fields, including event date, age, sex, and reporter country. For other versions of the same case, the maximum demographic values from the fully populated case versions were employed to fill in single missing values. Then, a two-step de-duplication was performed. If all available cases shared identical values for case ID, initial/follow-up code, case event date, age, sex, reporter country, drug names, and outcomes, the most recent case version was retained. Based on four demographic data fields (event date, age, sex, and reporter country), data from the DEMOyyQq tables were de-duplicated and linked to DRUG, INDI, and REAC, respectively, by primaryid,. The adverse events in which ALK inhibitors were the primary suspect were included, while the cases with ages less than 18 years were excluded. Finally, data from DRUG, INDI, and REAC were standardized by using the Observational Health Data Sciences and Informatics (OHDSI) Vocabulary 5.0 and the Medical Dictionary for Regulatory Activities (MedDRA). We screened available standardized MedDRA queries (SMQs) for “Interstitial lung disease (20000042).” Associated PTs were acute interstitial pneumonitis (10066728), idiopathic interstitial pneumonia (10078268), interstitial lung abnormality (10087834), interstitial lung disease (10022611), pneumonitis (10035742), pulmonary fibrosis (10037383), etc. Supplementary Table S1 shows the full list of PTs within the ILD SMQs.
Statistical analyses
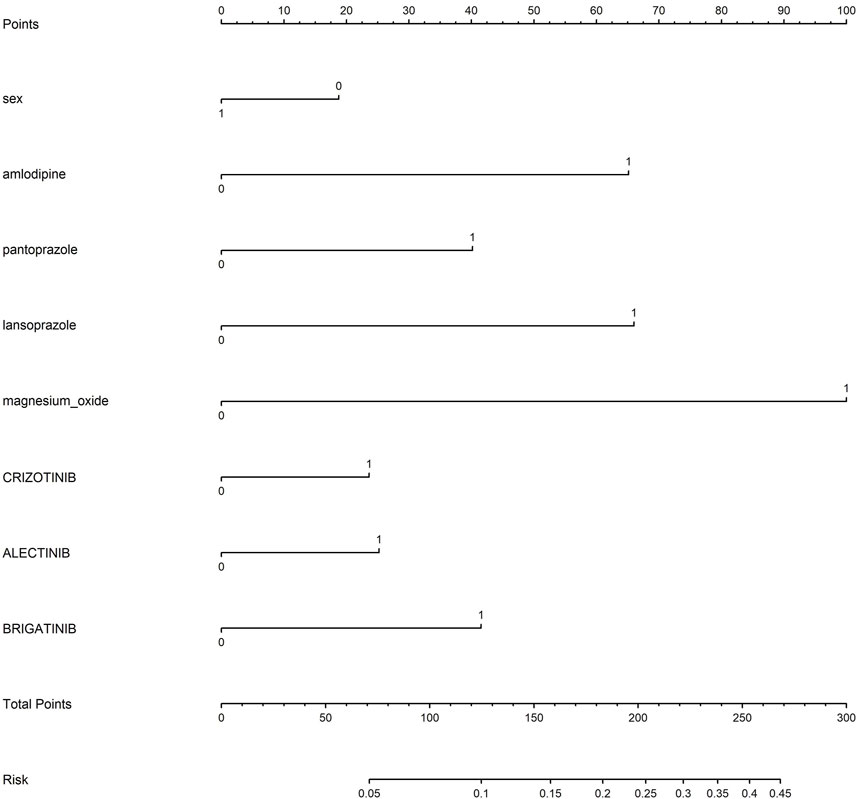
The measurement data were characterized by median and interquartile ranges, while enumeration data were presented as numbers and percentages. The correlation between an AE and the drug was investigated by disproportionality analysis, including the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the information component (IC), and the empirical Bayes geometric mean (EBGM). Odds ratios (ORs) were calculated using a logistic regression model to assess the association between potential risk factors and ILD in patients receiving ALK TKIs. Additionally, a nomogram was constructed to estimate the probability of developing ILD in NSCLC patients receiving different ALK TKIs. Each variable corresponds to a line segment with scales marked, which represents the range of values that the variable can take, and the length of the line segment reflects the contribution of that factor to the occurrence of ILD.
Results
Descriptive analysis
From January 2011 to June 2023, 15,656,531 reports were recorded in FAERS. After excluding duplicate cases (2,126,495) and aberrant cases with age less than 18 years (439,205), 13,090,831 reports were included in the present analysis. Of these, 20,064 AE reports were related to ALK TKIs (0.15%), including 9,130 for crizotinib, 1,929 for ceritinib, 4,673 for alectinib, 2,138 for brigatinib and 2,492 for lorlatinib (Supplementary Table S2).
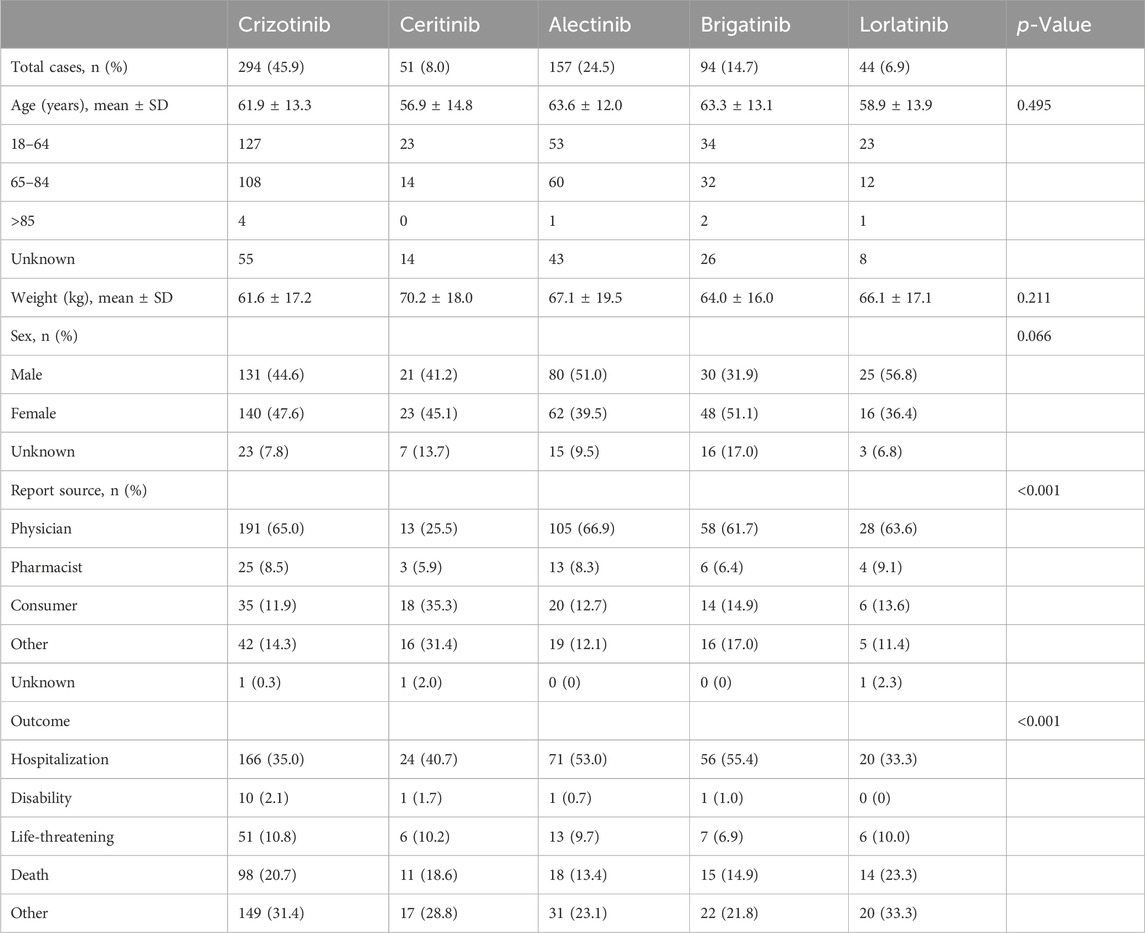
AE reports of ILD among ALK TKI users accounted for 3.2% (640/20,064) of the total AEs related to ALK TKIs; of these, 45.9% were related to crizotinib, 8.0% to ceritinib, 24.5% to alectinib, 14.7% to brigatinib, and 6.9% to lorlatinib. The average age of ALK TKI users differed: for alectinib and brigatinib, it was 63 years, and the lowest mean age for ceritinib was 56 years. Comparable percentages of male and female patients were observed for all five drugs. Of note, the majority of reports were submitted by physicians with the highest percentage of reports for crizotinib (65.0%), alectinib (66.9%), brigatinib (61.7%), and lorlatinib (63.6%), or by consumers with the highest percentage of reports for ceritinib (35.3%). Hospitalization and death were recorded in 40.7% and 18.8% of cases, respectively (Table 1).
Disproportionality analysis
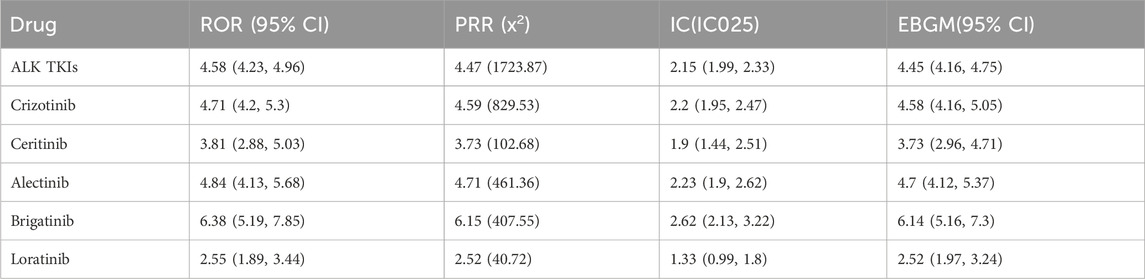
As shown in Table 2, the signal of ILD was detected in all ALK TKIs. Treatment with ALK TKIs was significantly associated with ILD, with ROR (4.58, 95% CI 4.23–4.96), PRR (4.47), IC (2.15, IC025 1.99–2.33), and EBGM (4.45, 95% CI 4.16–4.75). Brigatinib reported the highest ROR for ILD (6.38, 95%CI 5.19–7.85) with alectinib (4.84, 95% CI 4.13–5.68) and crizotinib (4.71, 95%CI 4.2–5.3) following.
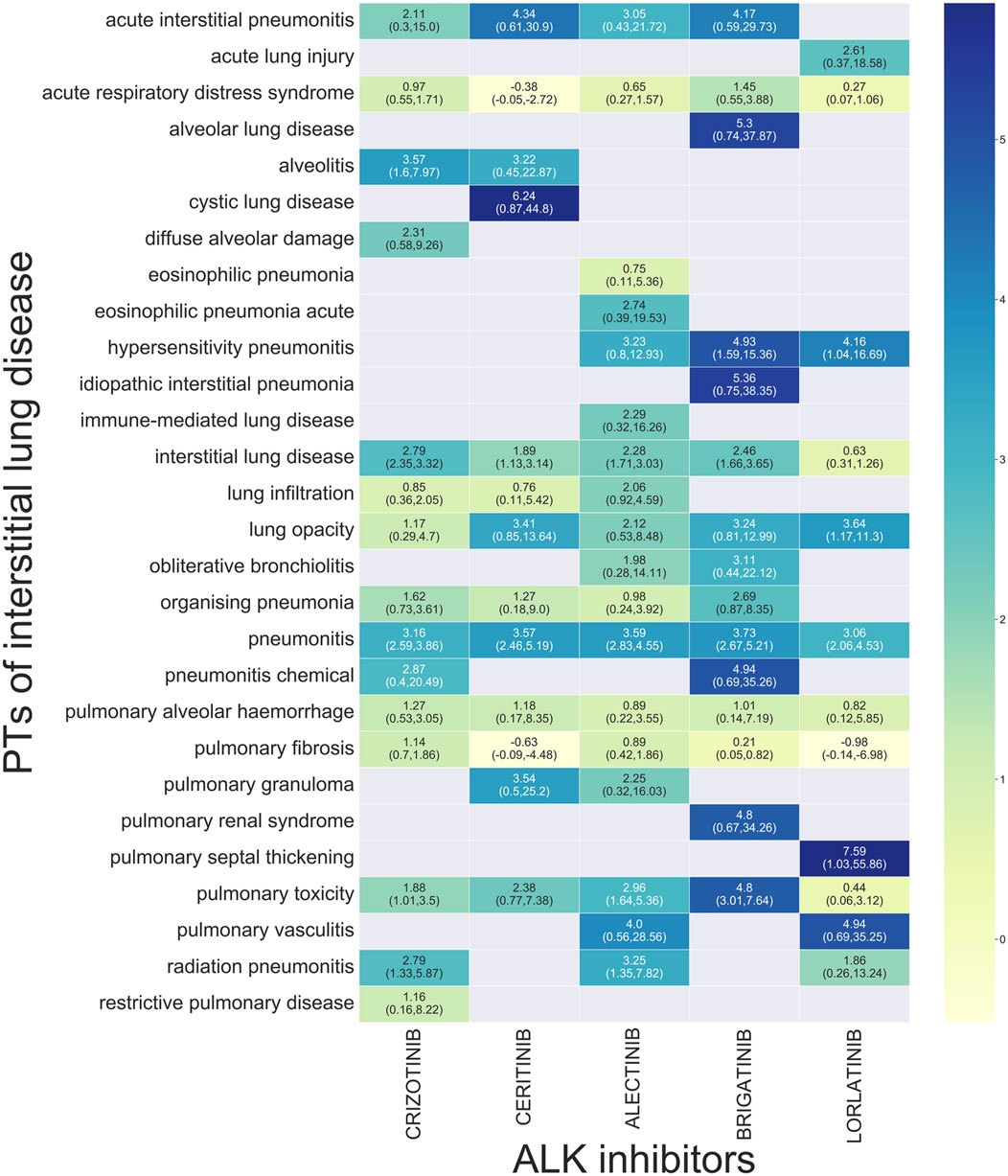
Disproportionality analysis for subgroups of ILD showed that 28 preferred terms (PTs) were significantly associated with ALK TKIs overall. Twelve PTs were significantly associated with crizotinib and ceritinib, and seven PTs were associated with alectinib and brigatinib. In contrast, only five PTs were associated with lorlatinib. Our analysis found a significant signal for pneumonitis for all analyzed ALK TKIs. Concerning acute interstitial pneumonitis, a significant signal was found for all ALK TKIs, with the exception of lorlatinib. Only crizotinib was associated with acute eosinophilic pneumonia and immune-mediated lung disease. Signals of alveolar lung disease, idiopathic interstitial pneumonia, and pulmonary-renal syndrome were only found for ceritinib. Only brigatinib was associated with diffuse alveolar damage. Only lorlatinib was associated with a significant ROR for acute lung injury and pulmonary septal thickening (Figure 1).
Time of onset and cumulative dose of ALK TKI-associated ILD
Of the patients who developed ILD, 56.8% developed ILD within 4 weeks of initiating ALK TKI therapy, and 70.4% developed it within 8 weeks. For crizotinib, 56.8% and 72.3% of cases developed ILD within 4 and 8 weeks, respectively. For ceritinib, 59.1% of cases developed ILD within 8 weeks. For alectinib, 44.4% and 61.1% of cases developed ILD within 4 and 8 weeks, respectively. For brigatinib, 77.6% and 85.7% of cases developed ILD within 4 and 8 weeks, respectively. For lorlatinib, 47.1% and 64.7% of cases developed ILD within 4 and 8 weeks, respectively (Supplementary Table S3).
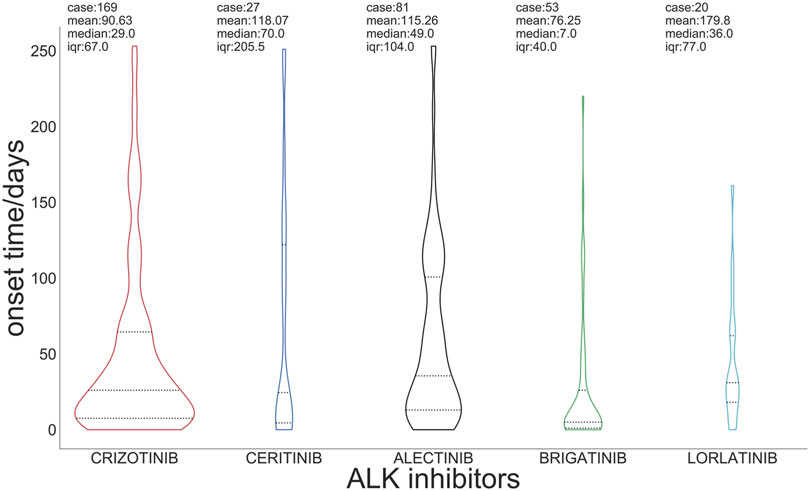
Figure 2 presents the box plots and medians of the onset time of ILD with regard to ALK TKIs. The median onset time of ILD related to total ALK TKIs was 53 days (Q1:12, Q3:209). The median time of onset was 29 days for crizotinib, 70 days for ceritinib, 49 days for alectinib, 7 days for brigatinib, and 36 days for lorlatinib (Supplementary Table S3).
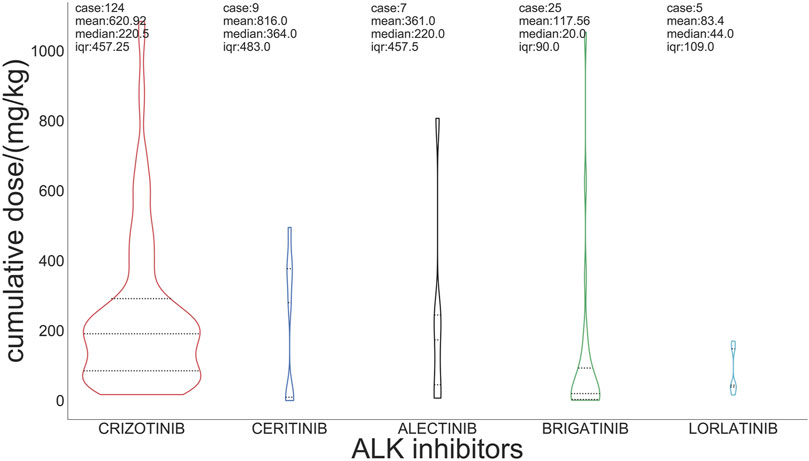
For the incidence of ILD, the median cumulative dose of crizotinib was 220 mg/kg, and the doses of ceritinib, alectinib, brigatinib, and lorlatinib were 364 mg/kg, 220 mg/kg, 20 mg/kg, and 44 mg/kg, respectively (Figure 3).
Risk factors for developed ILD and nomogram
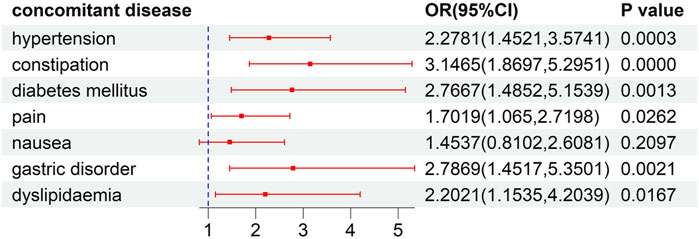
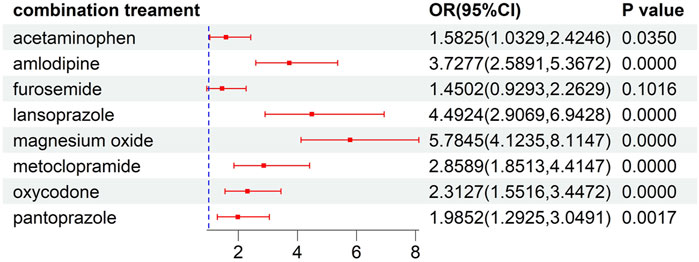
This analysis of risk factors for developed ILD was conducted on patients receiving ALK TKI therapy. The results of univariate logistic regression, as illustrated in Supplementary Table S4, indicated that female gender, concomitant disease, and concomitant drug significantly increased the risk of the developed ILD (P<0.05). To further explore the effects of concomitant factors on the developed ILD, we observed that patients with gastric disorder, pain, diabetes mellitus, hypertension, dyslipidemia, and constipation might be at a higher risk than those without concomitant diseases (Figure 4). Meanwhile, the risks of ILD associated with ALK inhibitors in combination with acetaminophen, amlodipine, lansoprazole, magnesium oxide, metoclopramide, oxycodone, and pantoprazole were higher than the risks of ALK inhibitor monotherapy (Figure 5).
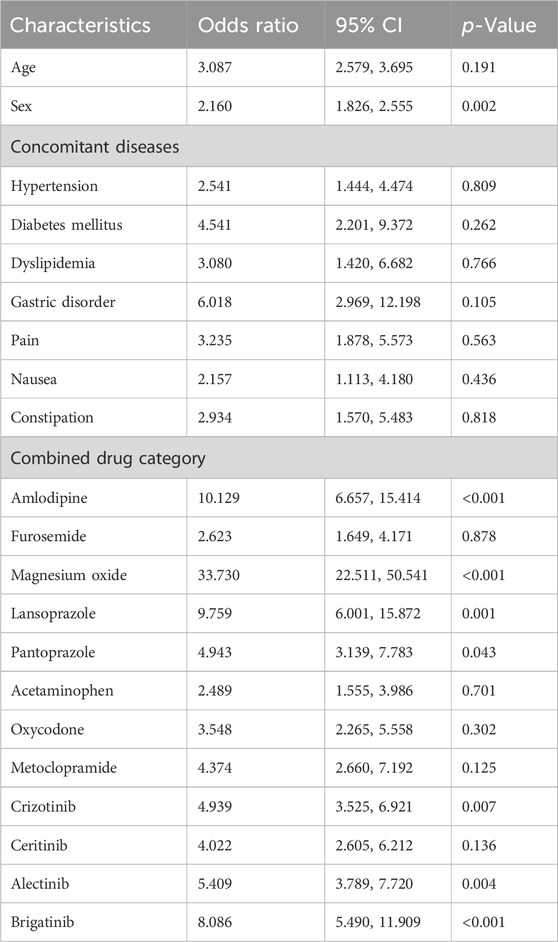
According to multivariate logistic regression analysis with adjustment for confounding variables, as shown in Table 3, amlodipine, magnesium oxide, lansoprazole, and pantoprazole had a significant effect on the development of ILD in patients receiving ALK TKIs (P<0.05).
Based on the results of univariate and multivariate logistic regression, sex, concomitant diseases, and concomitant drugs were included in the nomogram model. Figure 6 represents the nomogram for predicting the risk of developing ILD in patients receiving ALK TKIs. The results suggested that women were at higher risk than men. Between the two PPIs, lansoprazole posed a greater risk of ILD than pantoprazole. Furthermore, brigatinib caused the highest risk among five ALK TKIs, whereas ceritinib and loratinib did not significantly contribute to the risk of ILD.
Discussion
Pulmonary toxicities induced by targeted anticancer therapy are overall infrequent but potentially life-threatening (Teuwen et al., 2015). Especially combined with risk factors, the management of pulmonary toxicities can become a substantial therapeutic challenge and significantly influence the overall prognosis of cancer patients.
ALK TKI has become the standard treatment for NSCLC. However, the safety profile of each ALK TKI is different with regard to pulmonary toxicity. The retrospective studies indicated that the median age of diagnosis for ALK+ NSCLC was approximately 60 years (Auliac et al., 2017; Lim et al., 2017), and these older patients have comorbidities and polypharmacy (Decoster and Schallier, 2019), which may increase TKI-mediated toxicities during long-term treatment. Although the present study characterizes a different toxicity profile of ALK TKIs regarding ILD (Zhao et al., 2023), a comprehensive risk evaluation of ILD induced by ALK TKIs was still inadequate. Therefore, our pharmacovigilance analysis further illuminated the complex safety profile of ALK TKIs and independent risk factors by characterizing global data from the FAERS database.
ILD is a fatal but less frequently occurring category of adverse reactions. The FAERS database showed that ILD occurred in 3.2% (1.8%–4.4%) of the reports treated with ALK TKIs from 2011 to 2023. The proportion of ILD was the highest in brigatinib reports (4.4%) and lowest in lorlatinib reports (1.8%), which is consistent with the ALTA-1L clinical trials (Camidge et al., 2018) (D Ross Camidge et al., 2020) and FDA labeling (Fdalabel, 2023).
All five ALK TKIs could induce pulmonary toxicity; however, the adverse event profiles at the PT level were different between different ALK TKIs. Brigatinib was associated with the strongest and most robust disproportionality signal of ILD, which was consistent with the evidence from a systematic study (Pellegrino et al., 2018). All ALK TKIs present pneumonitis and lung opacities, but alectinib was associated with more PTs than acute eosinophilic pneumonia, and lung infiltration was not shown with other inhibitors. The mechanism of ILD induced by ALK TKI is not fully understood. Research has demonstrated that cross-reactivity with other kinases, such as EGFR, MET, and ROS1, may significantly contribute to the inhibition of normal signaling and impairment of the lung epithelium (Hwang et al., 2018; Harada et al., 2011). Gurule et al. speculated that TKI may be related to the disruption of normal epithelial tissue homeostasis and the induction of an innate inflammatory immune response (Gurule and Heasley, 2018). The mechanism underlying superimposed lung damage may be complex and must be further explored.
More than 70% of cases of ALK TKI-related ILD occurred within the first 2 months of initiating ALK TKI treatments. However, the median time to onset of ILD varied among ALK TKIs, with ceritinib exhibiting a notably longer delay, whereas brigatinib displayed the shortest time to onset. In clinical trials, early-onset pulmonary events were observed in the brigatinib group, especially within the first 3–8 days of treatment (Camidge et al., 2018). This indicates that early monitoring for pulmonary toxicity associated with brigatinib is necessary, especially for the prompt diagnosis of signs and typical symptoms such as dyspnea, hypoxia, and dry cough.
In a further investigation of factors affecting ALK TKI-related ILD, we observed a higher risk of ALK TKI-related ILD in the female group. A retrospective study showed that sex has no impact on lung toxicity in NSCLC patients exposed to ALK TKIs (Hwang et al., 2019), but another study suggested that ILD onset in patients receiving crizotinib was affected by sex in the univariate model (Gemma et al., 2019). The controversy regarding the effect of sex on ILD in ALK TKIs may be due to a variety of reasons, including variations in sampling, disparities between study cohorts, and different adjustments for confounding variables.
To date, no study has examined how coadministration with other medications influences the development of ILD with ALK TKIs. We observed that amlodipine, PPIs, and magnesium oxide were significantly associated with an increased risk of ALK TKI-related ILD. There was evidence indicating an increased risk of ILD in patients receiving lansoprazole (Kambara Kh et al., 2020; Atkins et al., 2014). According to the report by Kawamura et al., patients receiving a concomitant PPI demonstrated a higher incidence and risk of acute exacerbation of interstitial pneumonia (Kawamura et al., 2019). These studies indicate a strong correlation between PPI use and the occurrence of ILD. There was little evidence of a relationship between amlodipine, magnesium oxide, and ILD. However, amlodipine has been reported to improve the anticancer effects of gefitinib and regorafenib (Fu et al., 2022; Alandağ et al., 2022). The mechanism of the synergistic anticancer effect was that amlodipine can enhance the intracellular uptake of anticancer drugs (Li et al., 2006) as a Ca2+ channel blocker. Therefore, we speculated that the cytotoxicity of the targeted drug was probably potentiated by amlodipine, which resulted in an increased risk of ADR with concomitant use of amlodipine in patients receiving targeted therapy. These findings underscore the need for ongoing epidemiologic monitoring and urgent clarification through large-scale, population-based studies in addition to specialized RCTs.
A model to predict the risk of ILD after administration of ALK TKIs in NSCLC patients was developed based on univariate and multivariable regression. The risk of ILD caused by ALK inhibitors is approximately 3%, but we could observe that the risk of developing ILD increased to approximately 10% in a female lung cancer patient who was receiving treatment with brigatinib and concurrently taking amlodipine, as determined by the accumulation of individual risk factors. The results indicate that patients, particularly women receiving ALK inhibitors, need to avoid or cautiously combine the use of drugs with a higher risk of developing ILD. The predictors used in our study are available in the clinic, including sex and concomitant drugs, such as amlodipine prescribed to patients with hypertension and PPIs used by patients with gastric ulcers. The model allows physicians to assess the risk of ILD in cancer patients with concomitant drugs and make treatment decisions.
Our study has certain limitations. First, as a spontaneous reporting system, the FAERS database often contains incomplete or missing information and lacks data on population exposure. This precluded us from providing incidence rates of ADR (Bate and Evans, 2009). Furthermore, insufficient clinical features (such as body mass and patient history) and instrumental assessments (e.g., CT imaging and laboratory parameters) to support disease diagnosis, severity assessment, and prediction of potential ILD risk limit in-depth analysis and understanding of adverse events. Finally, the contribution of ALK TKIs to ILD-related deaths cannot be determined but is worth confirmation in a large-scale prospective study. Nonetheless, the results of our study highlight an increased risk of ILD associated with ALK TKIs for the treatment of NSCLC and provide a better basis for understanding potential ILD associated with ALK TKIs, which helps clinicians pay attention to risk management.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
JD: conceptualization, writing–original draft, and writing–review and editing. LL: writing–original draft. TD: writing–original draft. HS: writing–review and editing. SZ: project administration and writing–review and editing. MZ: writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Wuhan Pharmaceutical Association. (Project No: WHPA202305016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1361443/full#supplementary-material
References
Abbattista, H. T., Satouchi, M., Ross Camidge, D., Kao, S., Chiari, R., Gadgeel, S. M., et al. (2019). ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J. Clin. Oncol. 37, 1370–1379. doi:10.1200/JCO.18.02236
Alandağ, C., Karaman, E., and Yüce, E. (2022). Amlodipine improves the outcomes of regorafenib in metastatic colorectal cancer. Anti-Cancer Drugs 33, 389–393. doi:10.1097/CAD.0000000000001273
Antoniou, K. M., Margaritopoulos, G. A., Tomassetti, S., Bonella, F., Costabel, U., and Poletti, V. (2014). Interstitial lung disease. Eur. Respir. Rev. 23, 40–54. doi:10.1183/09059180.00009113
Atkins, C., Maheswaran, T., Rushbrook, S., and Kamath, A. (2014). Lansoprazole-induced acute lung and liver injury: a case report. Int. J. Clin. Pharmacol. Ther. 52, 1102–1104. doi:10.5414/CP202110
Auliac, J.-B., Monnet, I., Dubos-Arvis, C., Chiappa, A. M., Baize, N., Bota, S., et al. (2017). Non-small-cell lung cancer (NSCLC) harboring ALK translocations: clinical characteristics and management in a real-life setting: a French retrospective analysis (GFPC 02–14 study). Target. Oncol. 12, 833–838. doi:10.1007/s11523-017-0520-7
Banda, J. M., Evans, L., Vanguri, R. S., Tatonetti, N. P., Ryan, P. B., and Shah, N. H. (2016). A curated and standardized adverse drug event resource to accelerate drug safety research. Sci. Data 3, 160026. doi:10.1038/sdata.2016.26
Bate, A., and Evans, S. J. W. (2009). Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 18, 427–436. doi:10.1002/pds.1742
Camidge, D. R., Dziadziuszko, R., Peters, S., Mok, T., Noe, J., Nowicka, M., et al. (2019). Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 14, 1233–1243. doi:10.1016/j.jtho.2019.03.007
Camidge, D. R., Kim, H. R., Ahn, M. J., Yang, J. C. H., Han, J. Y., Lee, J. S., et al. (2018). Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 379, 2027–2039. doi:10.1056/NEJMoa1810171
Crinò, L., Ahn, M.-J., De, M. F., Groen, H. J., Wakelee, H., Hida, T., et al. (2016). Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non–small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J. Clin. Oncol. 34, 2866–2873. doi:10.1200/jco.2015.65.5936
Crino, L., Kim, D., and GjjjoCO, R. (2011). Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J. Clin. Oncol. 29, 7514. doi:10.1200/jco.2011.29.15_suppl.7514
Decoster, L., and Schallier, D. (2019). Treatment of older patients with advanced non-small cell lung cancer: a challenge. J. Geriatric Oncol. 10, 528–533. doi:10.1016/j.jgo.2018.09.008
Dong-Wan, K. M. T., Ahn, M.-Ju, Reckamp, K. L., Hansen, K. H., Kim, S.-We, Huber, R. M., et al. (2017). Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J. Clin. Oncol. 35, 2490–2498. doi:10.1200/JCO.2016.71.5904
D Ross Camidge, D.-W. K., Tiseo, M., Tiseo, M., Langer, C. J., Ahn, M. J., Shaw, A. T., et al. (2018). Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J. Clin. Oncol. 36, 2693–2701. doi:10.1200/JCO.2017.77.5841
D Ross Camidge, H. R. K., Ahn, M.-Ju, Ahn, M. J., Yang, J. C. H., Han, J. Y., Hochmair, M. J., et al. (2020). Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J. Clin. Oncol. 38, 3592–3603. doi:10.1200/JCO.20.00505
Fdalabel (2023). Lorlatinib. Available at: https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/380270.
Fu, B., Dou, X., Zou, M., Lu, H., Wang, K., Liu, Q., et al. (2022). Anticancer effects of amlodipine alone or in combination with gefitinib in non-small cell lung cancer. Front. Pharmacol. 13, 902305. doi:10.3389/fphar.2022.902305
Gadgeel, S. P. S., Mok, T., Shaw, A. T., Kim, D. W., Ou, S. I., Pérol, M., et al. (2120). Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study2018. Ann. Oncol. doi:10.1093/annonc/mdy405
Gainor, J. F., Dardaei, L., Yoda, S., Friboulet, L., Leshchiner, I., Katayama, R., et al. (2016). Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 6, 1118–1133. doi:10.1158/2159-8290.CD-16-0596
Gemma, A., Kusumoto, M., Kurihara, Y., Masuda, N., Banno, S., Endo, Y., et al. (2019). Interstitial lung disease onset and its risk factors in Japanese patients with ALK-positive NSCLC after treatment with crizotinib. J. Thorac. Oncol. 14, 672–682. doi:10.1016/j.jtho.2018.11.022
Gurule, N. J., and Heasley, L. E. (2018). Linking tyrosine kinase inhibitor-mediated inflammation with normal epithelial cell homeostasis and tumor therapeutic responses. Cancer Drug Resist. 1, 118–125. doi:10.20517/cdr.2018.12
Harada, C., Kawaguchi, T., Ogata-Suetsugu, S., Yamada, M., Hamada, N., Maeyama, T., et al. (2011). EGFR tyrosine kinase inhibition worsens acute lung injury in mice with repairing airway epithelium. Am. J. Respir. Crit. Care Med. 183, 743–751. doi:10.1164/rccm.201002-0188OC
Horn, L., and Pao, W. (2009). EML4-ALK: honing in on a new target in non-small-cell lung cancer. J. Clin. Oncol. 27, 4232–4235. doi:10.1200/JCO.2009.23.6661
Hou, H., Sun, D., Liu, K., Jiang, M., Liu, D., Zhu, J., et al. (2019). The safety and serious adverse events of approved ALK inhibitors in malignancies: a meta-analysis. Cancer Manag. Res. 11, 4109–4118. doi:10.2147/CMAR.S190098
Hwang, A., Iskandar, A., and Dasanu, C. A. (2018). Successful re-introduction of alectinib after inducing interstitial lung disease in a patient with lung cancer. J. Oncol. Pharm. Pract. 25, 1531–1533. doi:10.1177/1078155218820580
Hwang, H. J., Kim, M. Y., Choi, C.-M., and Lee, J. C. (2019). Anaplastic lymphoma kinase inhibitor related pneumonitis in patients with non-small cell lung cancer: clinical and radiologic characteristics and risk factors. Medicine 98, e18131. doi:10.1097/MD.0000000000018131
Kambara Kh, H., Nakatsuji, T., Ueno, S., Oyama, S., Inada, A., Niinomi, I., et al. (2020). Safety profile of vonoprazan compared with proton pump inhibitors: insight from a pharmacovigilance study2020. Pharmazie., 75, 527–530.
Kawamura, K., Ichikado, K., Ichiyasu, H., Anan, K., Yasuda, Y., Suga, M., et al. (2019). Acute exacerbation of chronic fibrosing interstitial pneumonia in patients receiving antifibrotic agents: incidence and risk factors from real-world experience. BMC Pulm. Med. 19, 113. doi:10.1186/s12890-019-0880-0
Kim, D. W., Ahn, M. J., Shi, Y., Pas, T. M. D., and Shaw, A. T. (2012). Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer NSCLC. J. Clin. Oncol. 30 (15_suppl), 7533–7533.
Li, X., Ruan, G.-R., Lu, W.-L., Hong, H. Y., Liang, G. W., Zhang, Y. T., et al. (2006). A novel stealth liposomal topotecan with amlodipine: apoptotic effect is associated with deletion of intracellular Ca2+ by amlodipine thus leading to an enhanced antitumor activity in leukemia. J. Control. Release 112, 186–198. doi:10.1016/j.jconrel.2006.01.007
Lim, S. H., Yoh, K. A., Lee, J. S., Ahn, M. J., Kim, Y. J., Kim, S. H., et al. (2017). Characteristics and outcomes of ALK+ non-small cell lung cancer patients in Korea. Asia-Pacific J. Clin. Oncol. 13, e239–e245. doi:10.1111/ajco.12645
Ma, Z., Pei, J., Zhang, Y., Li, H., Sun, D., Zhang, Y., et al. (2023). Interstitial pneumonitis associated with EGFR/ALK tyrosine kinase inhibitors used in non-small cell lung cancer: an observational, retrospective, pharmacovigilance study. Expert Opin. Drug Saf. 22, 237–242. doi:10.1080/14740338.2022.2110235
Mok, T. S., Spigel, D. R., and Felip, E. (2011). ASCEND-2: a single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ).
Omar, N. E., Fahmy Soliman, A. I., Eshra, M., Saeed, T., Hamad, A., and Abou-Ali, A. (2021). Postmarketing safety of anaplastic lymphoma kinase (ALK) inhibitors: an analysis of the FDA Adverse Event Reporting System (FAERS). ESMO Open 6, 100315. doi:10.1016/j.esmoop.2021.100315
Ou, S. H., Ahn, J. S., De Petris, L., Govindan, R., Yang, J. C. H., Hughes, B., et al. (2016). Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J. Clin. Oncol. 34, 661–668. doi:10.1200/jco.2015.63.9443
Pellegrino, B., Facchinetti, F., Bordi, P., Silva, M., Gnetti, L., and Tiseo, M. (2018). Lung toxicity in non–small-cell lung cancer patients exposed to ALK inhibitors: report of a peculiar case and systematic review of the literature. Clin. Lung Cancer 19, e151–e161. doi:10.1016/j.cllc.2017.10.008
Peters, S., Camidge, D. R., Shaw, A. T., Gadgeel, S., Ahn, J. S., Kim, D. W., et al. (2017). Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377, 829–838. doi:10.1056/NEJMoa1704795
Pirker, R., and Filipits, M. (2019). From crizotinib to lorlatinib: continuous improvement in precision treatment of ALK-positive non-small cell lung cancer. ESMO Open 4, e000548. doi:10.1136/esmoopen-2019-000548
Shaw, A. T., Bauer, T. M., de Marinis, F., Felip, E., Goto, Y., Liu, G., et al. (2020). First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N. Engl. J. Med. 383, 2018–2029. doi:10.1056/NEJMoa2027187
Shaw, A. T., Kim, D. W., Mehra, R., Tan, D. S. W., Felip, E., Chow, L. Q. M., et al. (2014). Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 370, 1189–1197. doi:10.1056/NEJMoa1311107
Shaw, A. T. K. D., Nakagawa, K., Seto, T., Crinó, L., Ahn, M. J., De Pas, T., et al. (2103). Crizotinib versus chemotherapy in advanced ALK-positive lung cancer.
Solomon, B. J., Mok, T., Kim, D. W., Wu, Y. L., Nakagawa, K., Mekhail, T., et al. (2014). First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177. doi:10.1056/NEJMoa1408440
Spagnolo, P., Bonniaud, P., Rossi, G., Sverzellati, N., and Cottin, V. (2022). Drug-induced interstitial lung disease. Eur. Respir. J. 60, 2102776. doi:10.1183/13993003.02776-2021
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Teuwen, L.-A., Van den Mooter, T., and Dirix, L. (2015). Management of pulmonary toxicity associated with targeted anticancer therapies. Expert Opin. Drug Metabolism & Toxicol. 11, 1695–1707. doi:10.1517/17425255.2015.1080687
Zhao, M., Liu, S., Xie, R., Zhang, J., and Li, J. (2023). Interstitial lung disease risk of anaplastic lymphoma kinase tyrosine kinase inhibitor treatment of non-small cell lung cancer: a real-world pharmacovigilance study. Expert Opin. Drug Saf. 22, 1309–1316. doi:10.1080/14740338.2023.2245324
Keywords: FDA adverse event reporting system, ALK TKIs, interstitial lung disease, pharmacovigilance, adverse events
Citation: Dong J, Li L, Deng T, Song H, Zhang S and Zhong M (2024) Interstitial lung disease associated with ALK inhibitors and risk factors: an updated comparative pharmacovigilance analysis. Front. Pharmacol. 15:1361443. doi: 10.3389/fphar.2024.1361443
Received: 26 December 2023; Accepted: 02 September 2024;
Published: 27 September 2024.
Edited by:
Patricia Moriel, State University of Campinas, BrazilReviewed by:
Magesh Muthu, Wayne State University, United StatesCarolina Dagli Hernandez, State University of Campinas, Brazil
Copyright © 2024 Dong, Li, Deng, Song, Zhang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohui Zhang, enNodGptdUBob3RtYWlsLmNvbQ==; Minyu Zhong, em15NDA1NjQ1MDczQDE2My5jb20=
†These authors have contributed equally to this work
 Junli Dong
Junli Dong Lulu Li1,2
Lulu Li1,2