- 1Affiliated Hospital of Inner Mongolia Medical University, Inner Mongolia Key Laboratory of Medical Cell Biology, Hohhot, Inner Mongolia, China
- 2Department of Gynaecology, Inner Mongolia People’s Hospital, Hohhot, Inner Mongolia, China
- 3Department of Gastrointestinal Surgery, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
Colorectal cancer (CRC) is a significant global health burden, with high morbidity and mortality rates. It is often diagnosed at middle to advanced stage, affecting approximately 35% of patients at the time of diagnosis. Currently, chemotherapy has been used to improve patient prognosis and increase overall survival. However, chemotherapy can also have cytotoxic effects and lead to adverse reactions, such as inhibiting bone marrow hematopoiesis, causing digestive dysfunction, hand-foot syndrome, and even life-threatening conditions. In response to these adverse effects, researchers have proposed using Traditional Chinese Medicine (TCM) as an option to treat cancer. TCM research focuses on prescriptions, herbs, and components, which form essential components of the current research in Chinese medicine. The study and implementation of TCM prescriptions and herbs demonstrate its distinctive holistic approach to therapy, characterized by applying multi-component and multi-target treatment. TMC components have advantages in developing new drugs as they consist of single ingredients, require smaller medication dosages, have a precise measure of pharmacodynamic effects, and have a clear mechanism of action compared to TCM prescriptions and herbs. However, further research is still needed to determine whether TMC components can fully substitute the therapeutic efficacy of TCM prescriptions. This paper presents a comprehensive analysis of the research advancements made in TCM prescriptions, herbs, and components. The findings of this study can serve as a theoretical basis for researchers who are interested in exploring the potential of TCM for the treatment of colorectal cancer.
1 Introduction
Colorectal cancer (CRC) is a prevalent form of cancer, ranking third in terms of occurrence and second in terms of mortality globally. In 2020, there were over 1.9 million new cases and 935,000 deaths, comprising roughly one-tenth of all cancer cases and fatalities (Sung et al., 2021). Notably, in China, the incidence and mortality of CRC are significantly increasing. Its incidence is expected to reach three million by 2024, making it one of the most menacing cancers in terms of lives and wellbeing (Qu et al., 2022; Morgan et al., 2023). CRC is a highly malignant disease characterized by quick disease progression and lymphatic and blood circulation metastasis. Advanced stages of CRC can lead to severe complications such as anemia and acute organ perforation. Thus, exploring efficacious remedies has become a focal point of research.
Currently, CRC treatment relies mainly on surgery, with additional therapies such as chemotherapy and targeted therapy. Surgical resection is a widely used approach for managing stage I and stage II colorectal cancer, demonstrating a promising 5-year survival rate of over 90% for stage I cases. However, the survival rate for advanced CRC is only 14% (Siegel et al., 2023). CRC is identified by its subtle early symptoms, with most patients not diagnosed until the intermediate or late stages of the disease, when symptoms appear and medical attention is sought. Medical advancements have enabled chemotherapy in combination with surgery to treat intermediate and late CRC patients, substantially improving primary tumor control and patient survival rates (Khalil et al., 2022). Chemotherapy has some benefits for patients but also brings various side effects, such as myelosuppression and infections due to impaired immune function. These side effects not only reduce patient compliance but also severely affect their quality of life, leading to the recurrence of tumor metastases and ultimately affecting patients’ long-term survival (Miller et al., 2019). As a result, finding an effective treatment for CRC becomes the focus of research hotspot at home and abroad.
Research has increasingly demonstrated that TCM have potent effects in treating cancer by experimental and clinical models. Therefore, they are being explored as therapeutic agents for CRC. TCM has been extensively researched and used for centuries. These medicines are primarily derived from botanical sources and are essential for open anticancer drugs (Kong et al., 2020). As a valuable treatment for CRC, TCM can have a multi-targeted impact on colorectal cancer, minimizing toxic side effects and extending patient survival periods caused by surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy (Ranjan et al., 2019). Experimental research has demonstrated that TCM and its ingredients can efficiently impede the growth of CRC cells, trigger apoptosis, stimulate cell autophagy, and suppress angiogenesis; it also contributes to treat colorectal cancer when combined with radiotherapy (Chen J-F et al., 2023). TCM has a lengthy history and extensive clinical applications. TCM research usually focuses on prescriptions, herbs, and components (Sun et al., 2021). Prescriptions present notable benefits in inhibiting the proliferation and metastasis of (Wei et al., 2023). Herbs comprise a single medicinal ingredient and are extracted using various methods, resulting in increased CRC efficacy due to their high concentrations of active components and ability to reduce harmful side effects (Xia et al., 2014). Compared with prescriptions and herbal medicines, ingredients have clear chemical structures and pharmacological functions and have become an important part of the research and development of new TCM treatments (Guo T-H et al., 2022). This paper will review the research advancements of prescriptions, herbs, and components in CRC. We will explore their mechanisms of action, avoid subjective evaluations, and provide a theoretical basis for future research on TCM’s effectiveness against CRC (Figure 1).

Figure 1. The parts of TCM contains prescriptions, herbs and components that participate in the treatment of CRC through different mechanisms.
2 Application of prescriptions in CRC
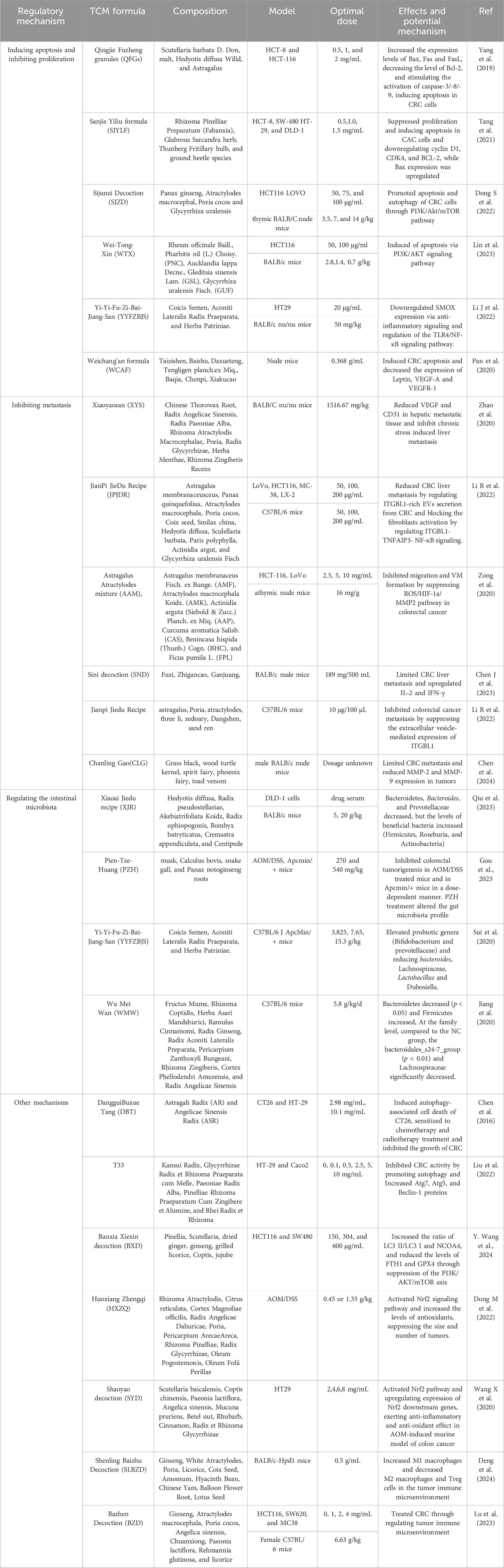
The study and implementation of prescriptions in TCM demonstrate its distinctive holistic approach to therapy, characterized by applying multi-component and multi-target. Similar to the compound presented in this paper, it is categorized based on its primary effects, including inhibition of apoptosis and proliferation, the inhibition of migration, invasion and adhesion, the regulation of gut microbiota and other mechanisms (Table 1).
2.1 Inducing apoptosis and inhibiting proliferation
Traditional Chinese medicine Qingjie Fuzheng granules (QFGs), consisting of malt, Scutellaria barbata D. Don, Hedyotis diffusa Willd, and Astragulus, showed the function of inhibiting proliferation and inducing apoptosis at concentrations of 0.5–2.0 mg/mL. QFGs increased the expression level of Bax, Fas, and Fasl, decreased Bcl-2 levels, and stimulated activation of caspase-3/8/9 in HCT-116 and HCT-8 cell. This study showed that QFGs induced apoptosis via the mitochondria-dependent pathway and the death receptor apoptosis pathway in two types of CRC cells (Yang et al., 2019). Tang et al. conducted an experiment that differed from the Qingjie Fuzheng granule study. The experiment selected four CRC cells and found that the Sanjie Yiliu formula (SJYLF) significantly inhibited the activity of HCT-8, SW480, HT29, and DLD-1 CRC cells. SJYLF components, consisting of Fabanxia, Glabrous Sarcandra herb, Thunberg Fritillary bulb, and ground beetle species, initiated apoptosis through downregulating Bcl-2, cylin D1, and CDK4 protein, as well as increasing Bax expression (Tang et al., 2021). Besides, Sijunzi decoction (SJZD) consistsing of Panax ginseng C.A.Mey., Atractylodes macrocephala Koidz., Poria cocos Wolf., and Glycyrrhiza uralensis Fisch., proportioned at a ratio of 2:2:2:1 and clinically used in treating CRC. Experimentally, SJZD could induce apoptosis and autophagy of CRC cells via PI3K/Akt/mTOR pathway. This article analyzed the function of SJZD through network pharmacology technology and experimental in vivo and vitro (Shang et al., 2023). The PI3k/AKT pathway can regulate the proliferation and cycle of tumor cells, promote tumor angiogenesis, facilitate tumor invasion and metastasis, and regulate apoptosis (Dong S et al., 2022). Zhang and others, being similar to the previous of SJZD experiment, discovered 286 bioactive compounds and 130 potential therapeutic targets in the ethanolic extract of gastric tonic. They demonstrated that Wei-Tong-Xin induces colon cancer cell apoptosis by activating the PI3K/AKT pathway instead of iron death in HCT116 cells. The western blot analysis revealed increased expression of Bax, caspase 3, and caspase 9, and decreased expression of BCL-2. Additionally, Zhang and others conducted in vivo mouse experiments to validate the apoptotic role of Wei-Tong-Xin in colorectal cancer. They also confirmed the apoptotic effect of Wei-Tong-Xin on colorectal cancer through in vivo mouse experiments (Lin et al., 2023). Contrast to the previous western blot analysis, Weichang’an formula (WCAF) plays an important role in inducing CRC apoptosis through TUNEL assay. And the treatment group of WCAF decreases the expression of Leptin, VEGF-A and VEGFR-1 (Pan et al., 2020).
Yi-Yi-Fu-Zi-Bai-Jiang-San (YYFZBJS) comprises Coix lacryma, Radix et Rhizoma Pinelliae and Radix et Rhizoma Bianchi, three Chinese herbs combined in a 30:6:15 ratio. It is commonly administered for the treatment of gastrointestinal tumours. YYFZBJS, at concentrations of 30 µg/mL, 60 µg/mL, and 90 µg/mL, significantly decreased the expression of CDK1, p-AKT, and p-PI3K proteins in HCT116 and SW480 cells. This finding proves that by regulating the CDK1/PI3K/AKT pathway, inducing apoptosis and blocking the cell cycle, YYFZBJS effectively inhibits the proliferation of CRC. The efficacy of YYFZBJS on proliferation was validated by establishing the AOM/DSS mouse model (Li J et al., 2022). In an identical study, Xiang et al. conducted a network pharmacological analysis to screen four active ingredients from the YYFZBJS recipe. These four active ingredients were identified using high-performance liquid chromatography and were included in the HT-29 cell culture medium. The study found that the four active ingredients efficiently inhibited the growth and induced apoptosis of CRC cells by regulating TLR4/NFBJS (Xiang et al., 2022).
2.2 Inhibiting migration, invasion and adhesion
Metastatic cases of CRC can be detected at initial diagnosis in 20%–25% of CRC patients (Piawah and Venook, 2019). CRC patients drops to 14% after metastasis for 5-year survival rate (Siegel et al., 2023). Metastasis of tumors poses a significant challenge in the current treatment of CRC. Studies have confirmed that angiogenesis is a key factor in tumor metastasis, and vascular endothelial growth factor (VEGF) plays a crucial role in angiogenesis (Sakata and Larson, 2022). Lu Zhao et al. administered a safe clinical Chinese medicine, Xiaoyaosan, to C57BL/6J mice in an aqueous solution of 1516.67 mg/kg through gavage for 7 days. HT-29 colon cancer cells were then injected into the spleen of mice in order to establish a liver metastasis model of C57BL/6J colon cancer, and they continued to receive the treatment for 21 days. The study found that the Xiaoyaosan group had a significant inhibitory effect on liver metastasis of colon cancer by reducing the expression of VEGR and CD31 in liver metastatic tissues (Zhao et al., 2020). In addition, tumor cells can interact with the extracellular matrix, creating a pipeline system that transports blood, also known as angiogenic mimicry. This process leads to remodeling of the tumor microenvironment and is related to metastasis and prognosis (Wang et al., 2017). Zong et al. established a nude mouse model of lung metastasis by administering an Astragalus Atractylodes mixture, 16 mg/g, by gavage for 50 days. Zong found that the number of instances of lung metastasis in the Astragalus Atractylodes mixture group was significantly lower. The result showed that the mixture of Astragalus Atractylodes effectively inhibited CRC angiogenesis mimicry and migration of HCT116 and LOVO cells (Zong et al., 2020). Sini decoction (SND) consists of Fuzi, Zhigancao and Ganjuang, limiting CRC liver metastasis and upregulating IL-2 and IFN-γ. The effective of SND is associated with PI3K-Akt, EGFR and HIF-1 signaling pathway (Chen J et al., 2023).
The Jianpi Jiedu Recipe is a traditional Chinese medicinal compound derived from clinical practice that has found extensive use in treating gastrointestinal tumors. C57BL/6 mice were injected with 10 µg/100 µL Jianpi Jiedu Recipe into the tail vein every other day for 3 weeks, followed by an intra-splenic injection of MC38 colorectal cancer cells. The study revealed that Jianpi Jiedu Recipe effectively inhibits colorectal cancer metastasis by suppressing the extracellular vesicle-mediated expression of ITGBL1, inhibiting the TNFAIP3-NF-kB pathway activity, and subsequently reducing the activation of CAFs (Li R et al., 2022). Compared to regulate TNFAIP3-NF-kB pathway activity, a novel research shows that chanling Gao (CLG), a Chinese medicine formula, can limit CRC metastasis and reduce MMP-2 and MMP-9 expression in tumors. The result indicate that CLG regulate the PI3K/Akt/mTOR signaling pathway to inhibit metastasis of CRC (Chen et al., 2024).
2.3 Regulating the gut microbiota
The gut microbiota is a highly complex system that regulates innate and adaptive immunity. Disruption of gut microbiota can result in the procession of colorectal cancer (Jain et al., 2021). Patients with CRC often have dominant gut microbiota consisting of certain germs such as Escherichia coli, Bacillus fragilis, and Clostridium nucleatum. These pathogenic bacteria interfere with the immune surveillance mechanism by impairing intestinal mucosal immunity, promoting CRC development (Clay et al., 2022). Pien-Tze-Huang (PZH) can deplete pathogenic bacteria Peptoniphilus harei, Campylobacter jejuni, Collinsella aerofaciens and Aeromonas veronii in AOM/DSS mice and Apcmin/+ mice. At the same time, PZH inhibited tumorigenesis of CRC through increasing the abundance of probiotics Eubacterium limosum and Pseudobutyrivibrio xylanivorans (Gou et al., 2023).
Xiaoai Jiedu Recipe (XJR) is a kind of traditional Chinese medicine prescription for the treatment of colorectal cancer. 5 g/kg and 20 g/kg of XJR was used to treat CRC in xenograft model of mice by gavage for 14 consecutive days. Using 16s rRNA gene sequencing, the XJR dosing group decreased the abundance of Prevotellaceae, Bacteroides and Bacteroidetes. Studies demonstrated that XJR can inhibit the development of CRC in mice by modulating gut microbiota (Qiu et al., 2023).
Compared to the xenograft model of mice, Sui Hua et al. chose C57BL/6J-APCmin/+ mice to investigate the role of Yi-Yi-Fu-Zi-Bai-Jiang-San (YYFZBJS). This study used healthy controls and feces from volunteers receiving YYFZBJS to gavage APCmin/+ mice for 12 weeks. Contrasted to the healthy control group, mice receiving feces from volunteers receiving the drug had a reduced number of intestinal tumors, and gut microbiota was significantly regulated, as evidenced by an increase in the fractionation of Bifidobacterium and Prevotellaceae and a decrease in the abundance of Bacteroides, Lachnospiraceae, and Dubosiella. The altered gut microbiota mediated by YYFZBJS repressed CRC cell growth (Sui et al., 2020).
Feng et al. chose the AOM/DSS mouse model to conduct their study to explore Wu Mei Wan’s mechanism. Wu Mei Wan (WMW) was derived from the Treatise on Typhoid Fever and can treat abdominal pain and dysentery. The results indicated that after WMW intervention, the abundance of Bacteroidetes decreased, and that of Firmicutes increased at the phylum level. Additionally, the abundance of Bacteroidales_s24-7_group decreased, while that of Lachnospiraceae increased at the family level. WMW regulated NF-kB/IL-6/STAT3 pathway to balance between tumor-promoting and tumour-suppressing bacteria, thereby attenuating CAC (Jiang et al., 2020). Contrast to NF-kB/IL-6/STAT3 pathway, Anchang Yuyang Decoction (AYD) can regulate PPAR signaling pathway in CRC. AYD treatment group showed that the relative abundance of genera decreaesed, including Romboutsia, Monoglobus, norank_f_Oscillospiraceae, norank_f_ruminococcaceae, and other generas upregulated, such as norank_f_Muribaculaceae, Bacteroides, unclassified_f_Prevotellaceae, and Alistipes (Wei et al., 2024). Above all, there is a great importance to regulate the intestinal flora. The balance of intestinal flora is related to CRC. The application of prescriptions in CRC has benefit on the balance of intestinal flora, so that it can effectively treat CRC.
2.4 Other mechanisms
Autophagy is a free cellular mechanism of action to maintain homeostasis in response to various external stimuli, and in the case of tumours, excessive autophagy leads to autophagic cell death by degrading the cytoplasm beyond recovery (Mariño et al., 2014). The polysaccharide-depleted fraction of DangguiBuxue Tang (DBT) induced autophagy-associated cell death of CT26, sensitizing to chemotherapy and radiotherapy treatment and inhibiting the growth of CRC (Chen et al., 2016). What’s more, T33 is composed of five traditional Chinese herbs, namely, Kansui Radix, Glycyrrhizae Radix et Rhizoma Praeparata cum Melle, Paeoniae Radix Alba, Pinelliae Rhizoma Praeparatum Cum Zingibere et Alumine, and Rhei Radix et Rhizoma. T33 inhibits CRC activity by promoting autophagy, increasing Atg7, Atg5, and Beclin-1 proteins in HT-29 and Caco2 cells (Liu et al., 2022). Banxia Xiexin decoction (BXD) promoted ferritinophagy in CRC cells. BXD increased the ratio of LC3 II/LC3 I and NCOA4, and reduced the levels of FTH1 and GPX4 through suppression of the PI3K/AKT/mTOR axis (Wang et al., 2024).
Besides, inflammation and oxidative stress-induced carcinogenesis play significant part in the progression of CRC (Balmus et al., 2016). Jianpi Yiqi decoction is a commonly used treatment for gastrointestinal ailments like gastritis and colitis. Research found a significant decrease in IL-6 and TNF-a in venous blood, indicating that Jianpi Yiqi decoction has excellent anti-inflammatory properties and significantly reduces inflammatory responses. This result shows that good clinical efficacy was reflected in treating CRC patients through using Jianpi Yiqi prescription (Yang et al., 2023). It’s related to inflammatory in the next research which includes Huoxiang Zhengqi (HXZQ) significantly to reduce inflammation and oxidative stress in colitis-associated cancer by regulating Nrf2/NF-kB/NLRP3 pathway (Dong M et al., 2022). Compared with the study of HXZQ, ShaoYao decoction (SYD) also can activate the Nrf2 pathway, upregulating the expression of downstream Nrf2 genes and attenuating oxidative stress in AOM/DSS model mice. SYD can prevent and treat the ulcerrelated colorectal cancer (Wang X et al., 2020).
Additionally, we can’t aviod to mention the importance of the tumor microenvironment. A lot of researches found tirelizumab (TLzmab) resulted in imbalance of tumor immune microenvironment during treating CRC. Shenling Baizhu Decoction (SLBZD) can increase M1 macrophages and decrease M2 macrophages and Treg cells in the tumor immune microenvironment. Thus SLBZD has exerted the synergistic effect of TLzmab for maintaining the balance of microenvironment (Deng et al., 2024). Compared to regulate the macrophages in the microenvironment, Bazhen Decoction (BZD) can increase the ratio of CD4+T cells to CD8+T cells in the spleen and tumor tissues, downregulate the PD-1 expression on T cell surfaces. The study indicated BZD treated CRC through regulating tumor immune microenvironment (Lu et al., 2023). Thus, the prescriptions of TCM can also treat CRC through diversity mechanisms, which are potential targets to explore.
3 Application of herbs in CRC
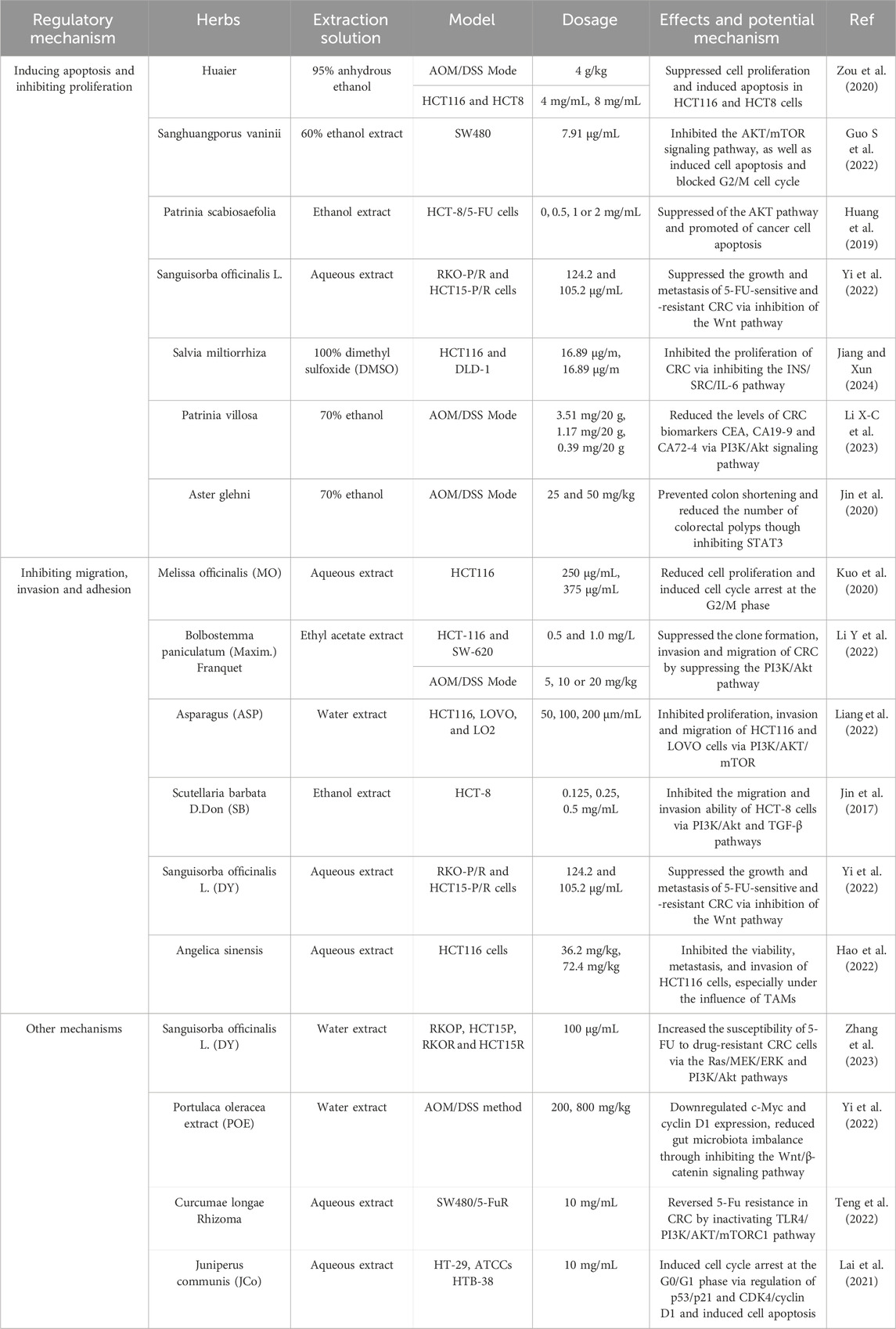
In recent years, numerous herbs and extracts have demonstrated remarkable therapeutic effects in treating CRC (Xia et al., 2014). As the development of TCM, natural products have been widely applied in the treatment cancer. Natural products included traditional and herb medicines, abundant of researches will reveal their biofunctions and applications in cancer therapy (Newman and Cragg, 2016). These are commonly categorized by their extraction solution, including aqueous extract, ethanol extracts, and ethyl acetate extracts in traditional and herb medicines. Notably, varying extraction techniques of the same herb yield different pharmaceutical activity.
3.1 Inducing apoptosis and inhibiting proliferation
The trametes robiniophila murr (Huaier) were extracted with 95% anhydrous ethanol. Huaier extract improved the severity of tumorigenesis of CRC, reducing tumor number, size and load. After using Huaier, the apoptosis-associated protein levels, such as P53, Bax, and Bcl-2, showed significant differences. The results demonstrated that huaier extract suppressed cell proliferation and induced apoptosis in HCT116 and HCT8 cells (Zou et al., 2020). Compared with 95% ethanol and water extracts, the 60% ethanol extract of Sanghuangporus vaninii significantly inhibited the AKT/mTOR signaling pathway, as well as induced cell apoptosis and blocked G2/M cell cycle (Guo S et al., 2022). Patrinia scabiosaefolia also regulates the AKT pathway. The ethanol extract of Patrinia scabiosaefolia significantly reduced HCT-8/5-FU cell number and apoptosis (Huang et al., 2019). Additionally, Sanguisorba officinalis L. (DY) was extracted by aqueous. The aqueous extract of DY can suppress cell proliferation and apoptosis via increasing the expression of Bax, cleaved-caspase3 and cleaved-PARP proteins and reducing Bcl-2 expression (Zhang W et al., 2022). Salvia miltiorrhiza belongs to the Salvia genus. Salvia miltiorrhiza was dissolved in 100% dimethyl sulfoxide (DMSO). This study was based on network pharmacology and molecular docking technology, showing that Salvia miltiorrhiza was related to three key targets: SRC, IL-6, and INS. In vitro experiments, Salvia miltiorrhiza inhibited the proliferation of CRC via inhibiting the INS/SRC/IL-6 pathway (Jiang and Xun, 2024).
What’s more, some researchers had different view about using herbs to treat CRC. Patrinia villosa Juss. (P.V) can reduce the levels of CRC biomarkers CEA, CA19-9 and CA72-4 via PI3K/Akt signaling pathway (Li X-C et al., 2023). Besides, many studies though that a hig-fat diet (HFD) accelerates the risk of CRC. Jin found that Aster glehni (AG) had anti-adipogenic effects in mice model. AG inhibited colitis-associated colon carcinogenesis in mice via preventing colon shortening and reducing the number of colorectal polyps though inhibiting STAT3 (Jin et al., 2020). Above all, numerous herbs and extracts of TCM have remarkable therapeutic effects in inducing apoptosis and inhibiting proliferation of CRC.
3.2 Inhibiting migration, invasion and adhesion
The hot water extract of Melissa officinalis (MO) has more effective anti-CRC activity. By modulating the epithelial-mesenchymal transition (EMT), MO can inhibit migration, proliferation, and trigger apoptosis of CRC (Kuo et al., 2020). Compared with the hot water extract, the ethyl acetate extract 3 (EA3) of Bolbostemma paniculatum (Maxim.) Franquet can effectively suppress the clone formation, invasion and migration of CRC by suppressing the PI3K/Akt pathway (Li Y et al., 2022). Besides, Asparagus (ASP) can regulate the PI3K/AKT/mTOR signaling pathway, inhibiting proliferation, invasion and migration of HCT116 and LOVO cells (Liang et al., 2022). A similar result showed that Scutellaria barbata D.Don (SB) can effectively inhibit the migration and invasion ability of HCT-8 cells in a dose-dependent manner via PI3K/Akt and TGF-β pathways. The ethanol extract of SB can reduce the expression of MMP-1, MMP2, MMP-3/10, MMP-9, and MMP-13. And E-cadherin and N-cadherin had no significantly difference in using the ethanol extract of SB (Jin et al., 2017). Beside, Sanguisorba officinalis Linn. (DY) can reverse EMT procession, so that inhibition cell metastasis. After DY treatment, the results showed that DY can reduce the expression of N-cadherin, vimentin and snail proteins, and upregulate E-cadherin expression via inhibition of the Wnt pathway (Zhang W et al., 2022).
Furthermore, when Angelica sinensis and OXA act in combination on HCT116 cells, the combinations show synergistic or additive effects. The expression levels of Ki67, MMP9, and CD206 in the Angelica sinensis group combined with OXA group were lower than those in the OXA group. The results suggest that Angelica sinensis can be used as an auxiliary drug in the treatment of colorectal cancer (Hao et al., 2022). More detailed information concerning anti-CRC of herbs is depicted in Table 2.
3.3 Other mechanisms
As we all known, 5-fluorouracil (5-FU) was the first-line cure of medicine in treatment CRC. But, the acquisition of chemotherapy drug resistance always caused of cancer treatment failure. Sanguisorba officinalis L. (DY) increased the susceptibility of 5-FU to drug-resistant CRC cells via the Ras/MEK/ERK and PI3K/Akt pathways (Zhang et al., 2023). Coupled with Sanguisorba officinalis L., Portulaca oleracea extract (POE) downregulate c-Myc and cyclin D1 expression, reducing gut microbiota imbalance through inhibiting the Wnt/β-catenin signaling pathway (Yi et al., 2022). What’s more, Curcumae longae Rhizoma can reverse CRC 5-Fu resistance by inactivating the TLR4/PI3K/AKT/mTORC1 pathway. Curcumae longae Rhizoma combined with 5-Fu can induce cell apoptosis by inhibiting bcl-2 and activating caspase-3 and Bax, thereby reversing 5-FU resistance (Teng et al., 2022). Additionally, Juniperus communis (JCo) is a well-known plant to treat cancer in traditional herbal medicine. The results showed that JCo, which was extracted by steam distillation, had a synergistic effect with 5-FU in CRC cells. In fact, the cell cycle played an important role in treating CRC. Jco extract can reduced cell cycle arrest to inhibit CRC growth (Lai et al., 2021).
4 Application of components in CRC
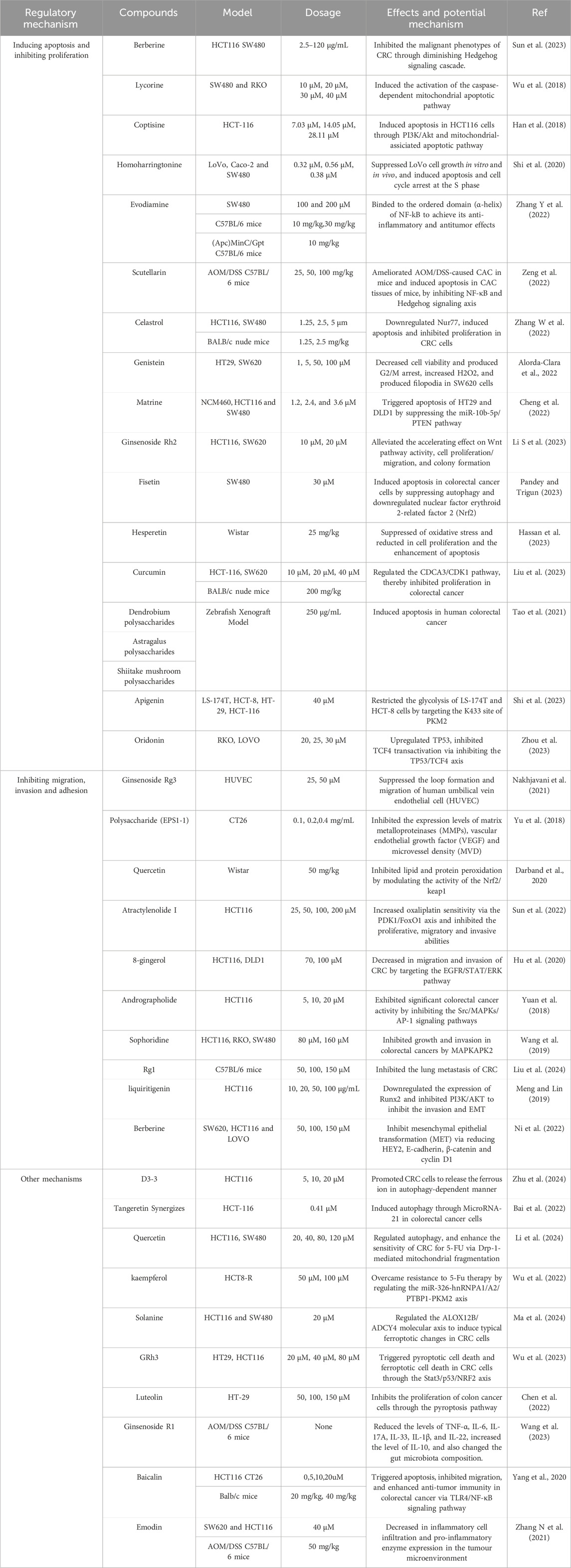
Compared with classical Chinese medicine prescriptions and the previous clinically applied herbs for CRC, TMC components have the benefit of being single, administered in small dosages, presenting clear effectiveness indicators, and a precisely defined mechanism of action (Guo T-H et al., 2022). According to their chemical structure, components comprise alkaloids, flavone, glycosides, and other components. Extensive research has made considerable progress in exploring the properties of components for treatment CRC (Guo T-H et al., 2022). Their mechanism of action has become more apparent, promoting the precise treatment of CRC by components. Details of the anti-CRC activity of the TMC components are shown in Table 3.
4.1 Inducing apoptosis and inhibiting proliferation
Sun et al. found that berberine induced apoptosis and blocked the cell cycle at phase G0/G1 in HCT116 and SW480 with a dampened hedgehog pathway (Sun et al., 2023). As showed by the increase of the ratio of Bax/Bcl-2 and mitochondrial depolarization, Lycorine induced mitochondrial apoptosis by targeting the STAT3 pathway (Wu et al., 2018). Besides, Coptisine also activated mitochondrial apoptosis of HCT-116 by down-regulating pro-caspase 3, Bcl-2 and upregulating Bax, cytochrome c and cleaved caspase-3 expression (Han et al., 2018). Homoharringtonine regulated cyclinA2 and CDC2 in the Bcl-2 apoptosis pathway by inhibiting the PI3K/AKT pathway of Lovo cells. The study showed that Homoharringtonine significantly suppressed LoVo cell growth in vitro and in vivo (Shi et al., 2020).
Evodiamine inhibited the NF-κB pathway by binding to the α-helix of NF-κB, inhibiting colon cancer proliferation (Zhang Y et al., 2022). Scutellarin significantly ameiorated tissue apoptosis in the AOM/dss mouse model by inhibiting NF-κB and Hedgehog signaling axis (Zeng et al., 2022). Celastrol can also regulate the NF-kB/COX-2 signaling pathway, activate cysteine-dependent apoptosis, and promote G1 cell cycle arrest, thereby inhibiting the proliferation and inducing apoptosis of CRC (Zhang H et al., 2022). Genistein could effectively decrease the viability of HT29 and SW620 cells and found that intracellular NF-KB was translocated from the cytoplasm to the nucleus, which proved that genistein could decrease cell viability of colon cancer cells and inhibit the proliferation by increasing the oxidative stress and inflammatory response of colon cancer cells (Alorda-Clara et al., 2022).
Compared to inhibiting the NF-κB pathway, Chen et al. discovered that Matrine triggered apoptosis of HT29 and DLD1 by suppressing the miR-10b-5p/PTEN pathway (Cheng et al., 2022). Ginsenoside Rh2 inhibited Wnt pathway activity and inhibits cell proliferation/migration and colony formation (Li S et al., 2023). Besides, Fisetin induces apoptosis by down-regulating nuclear factor erythroid 2-related factor 2 (Nrf2) in CRC (Pandey and Trigun, 2023). In the same animal model, the study found that Hesperetin reduced the occurrence of CRC induced by 1,2-dimethylhydrazine in Wistar rats by inhibiting oxidative stress, enhancing antioxidant, anti-inflammatory and apoptosis effects (Hassan et al., 2023). Liu F et al. reported that the administration of curcumin significantly suppressed the size of xenograft tumors. Mechanistic exploration determined that curcumin can target miR-134-5p expression and regulate the CDCA3/CDK1 pathway, thereby inhibiting proliferation in CRC (Liu et al., 2023). Additionally, Other authors have established a zebrafish transplantation model and demonstrated that dendrobium polysaccharides, astragalus polysaccharides, and shiitake mushroom polysaccharides can effectively inhibit the growth of HT29 cells. Their mechanism of action may involve immune modulation and the induction of apoptosis in tumor cells (Tao et al., 2021).
Besides, Apigenin was positively correlated with pyruvate kinase M2 (PKM2) expression in LS-174T cells and HCT-8 cells. The characterized of Apigenin suppressed cell proliferation and increased of apoptotic effects (Shi et al., 2023). What’s more, Oridonin, a diterpenoid compound extracted from Rabdosia rubescens, has been indicated to inhibit the proliferation of CRC. Oridonin promoted CRC cell death, upregulating TP53, inhibiting TCF4 transactivation via inhibiting the TP53/TCF4 axis (Zhou et al., 2023).
4.2 Inhibiting migration, invasion and adhesion
Metastasis of colorectal cancer is a complex pathophysiological process that involves multiple factors and steps. One crucial factor is angiogenesis, which is necessary for primary tumour metastasis and is regulated by both pro-angiogenic and anti-angiogenic factors. Ginsenoside Rg3 (Rg3) has stereoselective activities to decrease the expression of vascular endothelial growth factor receptor 2(VEGFR2) and aquaporin1. Through response surface methodology, Rg3 can significantly suppress the loop formation and migration of human umbilical vein endothelial cell (HUVEC) (Nakhjavani et al., 2021). Polysaccharide (EPS1-1) dose-dependently suppressed the migration, invasion and adhesion abilities of CT26 cells. EPS1-1 dramatically inhibited the expression levels of matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF) and microvessel density (MVD) in CT26 cells (Yu et al., 2018).
The components of TCM can effectively exert the inhibition of migration of CRC via multi-pathway. Quercetin can effectively suppress the migration and invasion of RKO cells through modulation of the JNK pathway (Trinh et al., 2022). Besides, Atractylenolide can affect PDK1/FoxO1, AKT/mTOR, and JAK/STAT3 pathways, inhibiting cancer cells’ proliferation, migration, and invasive ability (Li et al., 2020; Wang K et al., 2020; Sun et al., 2022). 8-gingerol, which is extracted from ginger, resulted in dose-dependent decrease in migration and invasion of CRC by targeting the EGFR/STAT/ERK pathway (Hu et al., 2020). Andrographolide has exhibited significant colorectal cancer activity by inhibiting the Src/MAPKs/AP-1 signaling pathways in a concentration-dependent manner (Yuan et al., 2018). Through cell heat shift experiments and drug affinity response target stability experiments, MAPK/APK2 plays a crucial role in Sophoridine inhibiting the growth and invasion of HCT116, SW480, and RKO(Wang et al., 2019). Bufalin, as the main active monomer of huachanshu, induced M2-type polarization and inhibited CRC metastasis via the SRC-3/IL-6 pathway (Tang et al., 2024). Rg1 also inhibited migration of CRC. Liu et al. found that the combination of rosmarinic acid (RA) and Rg1 can have anti-metastatic effects against CRC in regulating of PD-1/PD-L1 in CRC. Thus, Rg1 can inhibit the lung metastasis of CRC (Liu et al., 2024).
Besides, EMT was related to invasion and metastasis of tumor cells via inducing loss of cell-cell junctions and apicobasolateral polarity (Zhang N et al., 2021). Meng et al. found that liquiritigenin, a flavonoid extracted from the roots of Glycyrrhiza uralensis Fisch, downregulated the expression of Runx2 and inhibited PI3K/AKT to inhibit the invasion and EMT in HCT116 cell (Meng and Lin, 2019). What’s more, berberine treatment can inhibit mesenchymal epithelial transformation (MET) via reducing HEY2, E-cadherin, β-catenin and cyclin D1 (Ni et al., 2022). Besides, peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), being a regulator of mitochondrial function, can promote ABCA1 expression to promote CRC metastasis through EMT. Chen et al. found that the natural compound Isoliquiritigenin (ISL), as an inhibitor of PGC-1α, targeted ABCA1 and reduced CRC metastasis by inhibiting EMT (W. Chen W et al., 2023). All in all, the components of TCM can effectively regulate multiple factors and steps of CRC to inhibiting migration, invasion and adhesion.
4.3 Other mechanisms
There are some different mechanisms, such as autophagy and ferroptosis, being implicated in the cell death of cancer cells (Gao et al., 2022). D3-3 stemming from sinomenine, is a new compound through synthesis and design. D3-3 apparently promote CRC cells to release the ferrous ion in autophagy-dependent manner (Zhu et al., 2024). On the other hand, Tangeretin regulated miRNA-21 to induce autophagy by synergizing with 5-Fluorouracil in CRC (Bai et al., 2022). As the same mechanism, quercetin also could regulate autophagy, and enhance the sensitivity of CRC for 5-FU via Drp-1-mediated mitochondrial fragmentation (Li et al., 2024). Kaempferol regulated the miR-326-hnRNPA1/A2/PTBP1-PKM2 axis to overcome resistance to 5-Fu therapy (Wu et al., 2022). Solanine regulated the ALOX12B/ADCY4 molecular axis to induce typical ferropto in CRC cells. Simutaneously, solanine-induced ferroptosis is suppressed by silencing ALOX12B (Ma et al., 2024). Ginsenoside Rh3 triggered pyroptotic and ferroptotic cell death in CRC cells through the Stat3/p53/NRF2 axis while causing minimal damage to normal cells. These findings demonstrate remarkable anticancer potential for GRh3 (Wu et al., 2023). Luteolin experiments confirmed that it inhibits the proliferation of colon cancer cells through the pyroptosis pathway. Luteolin treatment increased the expression of Caspase1 and Gasdermin D. And we observed through immunofluorescence co-localization that NLRP3/Gasdermin D combined and inhibited CRC (Chen et al., 2022).
Additionally, the component of TCM was related to the tumor microenvironment. Ginsenoside R1 significantly decreased intestinal inflammatory factors TNF-a, IL-6, IL-1β, and IL-22. It also altered the composition of gut microbiota, effectively alleviating chronic inflammation and repairing the intestinal microenvironment in the AOM/DSS model (Wang et al., 2023). Baicalin could prompt apoptosis in both HCT116 and CT-26 by activating the TLR4/NF-kB pathway, significantly reducing the proliferation of colon cancer cells. Alongside this, baicalin could improve the anti-tumor immune function, down-regulating PD-L1 expression and upregulating the CD4+ and CD8+ T cell ratio, thereby improving the tumor immune microenvironmen (Yang et al., 2020). What’s more, it has been reported that using Emodin on the AOM/DSS mouse model decreased inflammatory cell infiltration and pro-inflammatory enzyme expression in the tumor microenvironment while increasing CD3 (+) T-lymphocyte levels. Moreover, it effectively reduced the cell viability of SW620 and HCT116 cells in in vitro experiments (Zhang Y et al., 2021).
5 Discussion
Colorectal cancer represents a significant global health burden, with high morbidity and mortality rates (Siegel et al., 2023). It is frequently diagnosed and approximately 35% of patients are found to have intermediate to advanced stage cancer at initial diagnosis. According to clinical practice guidelines developed by the National Comprehensive Cancer Network and the European Society for Medical Oncology, adjuvant chemotherapy with the FoLFox regimen is the standard of care for patients with intermediate to advanced colorectal cancer. This regimen has also been demonstrated to significantly enhance patient prognosis and increase overall survival (Guo et al., 2016). However, chemotherapy also has cytotoxic effects and is prone to causing adverse reactions, such as the inhibition of bone marrow haematopoiesis, digestive dysfunction, hand-foot syndrome, and even life-threatening conditions (Guo et al., 2016). TCM anti-tumour treatment options have been proposed by researchers as a response to these adverse effects.
TCM has a distinct theoretical framework with holism and dialectics at its core. It is a medical science developed through the practical experiences of Chinese people from all ethnic backgrounds in treating various diseases and has gained extensive clinical knowledge. TCM have focused on reducing adverse reactions and preventing tumor recurrence and metastasis. Research has shown that TCM can lower the tumor recurrence and metastasis rate in patients with CRC, as well as reduce the occurrence of complications.
This paper presents a detailed analysis of prescriptions, herbs, and components. The study and implementation of prescriptions in TCM demonstrate its distinctive holistic approach to therapy, characterized by applying multi-component and multi-target strategies. Similar to the compound presented in this paper, it is categorized based on its primary effects, including inhibition of apoptosis and proliferation, inhibition of metastasis, the regulation of gut microbiota and other mechanisms (Table 1).
The use of TCM in the treatment of CRC is becoming increasingly widespread. It is often used in conjunction with conventional Western medicine or as a standalone treatment. Despite the considerable progress made in TCM research on CRC, with a wide range of research topics and directions, there are still some outstanding issues. These mainly include: first and foremost, in the research of TCM against CRC, most studies focus on herb or compound of TCM, with fewer studies on TCM prescriptions. In reality, TCM prescriptions have multiple targets and roles. For example, Gegen Qinlian Decoction can block PD-1 by reshaping the gut microbiota and tumor microenvironment in CRC (Lv et al., 2019). Meanwhile, Gegen Qinlian decoction can increase the activity of Nrf2/ARE signaling and enhance the effect of antioxidant stress (Lin et al., 2022). Second, the observation indexes are relatively broad in clarifying Chinese medicine’s clinical treatment of CRC. The study of TCM in the treatment of CRC lacks precise observation indexes, which undermines its ability to convincingly elucidate therapeutic efficacy. Third, Currently, the multi-component and multi-target nature has also limited research related to TCM prescriptions. Clarifying the material basis, targets, and molecular biological mechanisms is challenging. Finally, The TCM theory emphasizes a holistic approach (Chen J-F et al., 2023). An identified compound represents only one constituent among the many ingredients found in TCM prescriptions. The diverse biological impacts resulting from the interdependence of the numerous ingredients in TCM still need to be fully comprehended. Hence, there is a pressing requirement for further excavation techniques and methods to investigate TCM and uncover its role in treating colorectal cancer and its mechanism of action. This paper covers a comprehensive analysis of the research advancements made in TCM prescriptions, herbs, and components, offering a specific theoretical basis for researchers exploring the treatment of CRC with TCM.
6 Summary
TCM is often utilised for anti-tumour purposes and has showcased encouraging anti-tumour efficacy in research studies. As science and technology progress, there is an expectation that research on the anticancer mechanism of traditional Chinese medicine will advance and improve. Cutting-edge medical research technology enables researchers to identify disease targets and apply multi-component, multi-pathway, and multi-target treatment of TCM to treat CRC. This approach is also an important avenue for studying TCM treatment of CRC in the future. Currently, there are still some shortcomings in the research of TCM for the treatment of CRC. However, it is believed that with the continued development of medical science and technology, the field of Chinese medicine’s anti-tumour properties will deepen, leading to more abundant results in the research of colorectal cancer.
Author contributions
JS: Writing–original draft. YW: Writing–review and editing. JW: Writing–review and editing. MH: Writing–review and editing. LS: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the research project of Inner Mongolia Medical University Affiliated Hospital (2023NYFYGG015). The Natural Science Foundation of Inner Mongolia (2021BS08003). University Innovation Team of Inner Mongolia Autonomous Region Education Department (NMGIRT2225).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRC, Colorectal cancer; TCM, Traditional Chinese medicines; VEGF, endothelial growth factor; QFGs, Qingjie Fuzheng granules; SJYLF, Sanjie Yiliu formula; SJZD, Sijunzi Decoction; WTX, Wei-Tong-Xin; YYFZBJS, Yi-Yi-Fu-Zi-Bai-Jiang-San; XYS, Xiaoyaosan; JPJDR, JianPi JieDu Recipe; AAM, Astragalus Atractylodes mixture; GQ, Gegen Qinlian decoction; SYD, Shaoyao decoction; HXZQ, Huoxiang Zhengqi; XJR, Xiaoai Jiedu recipe; PZH, Pien-Tze-Huang; WMW, Wu Mei Wan.
References
Alorda-Clara, M., Torrens-Mas, M., Morla-Barcelo, P. M., Roca, P., Sastre-Serra, J., and Pons, D. G. (2022). High Concentrations of Genistein Decrease Cell Viability Depending on Oxidative Stress and Inflammation in Colon Cancer Cell Lines. Int J Mol Sci. 23 (14), 7526. doi:10.3390/ijms23147526
Bai, Y., Xiong, Y., Zhang, Y.-Y., Cheng, L., Liu, H., Xu, K., et al. (2022). Tangeretin synergizes with 5-fluorouracil to induce autophagy through MicroRNA-21 in colorectal cancer cells. Am. J. Chin. Med. 50 (06), 1681–1701. doi:10.1142/S0192415X22500719
Balmus, I. M., Ciobica, A., Trifan, A., and Stanciu, C. (2016). The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: clinical aspects and animal models. Saudi J. gastroenterology official J. Saudi Gastroenterology Assoc. 22 (1), 3–17. doi:10.4103/1319-3767.173753
Chen, G., Tian, T.-T., Wang, F.-Q., Pan, C.-S., Sun, K., Wang, X.-Y., et al. (2024). Chanling Gao suppresses colorectal cancer via PI3K/Akt/mTOR pathway modulation and enhances quality of survival. Environ. Toxicol. 39 (3), 1107–1118. doi:10.1002/tox.23994
Chen, S.-T., Lee, T.-Y., Tsai, T.-H., Lin, Y.-C., Lin, C.-P., Shieh, H.-R., et al. (2016). The traditional Chinese medicine DangguiBuxue Tang sensitizes colorectal cancer cells to chemoradiotherapy. Mol. (Basel, Switz.) 21 (12), 1677. doi:10.3390/molecules21121677
Chen, Y., Ma, S., Pi, D., Wu, Y., Zuo, Q., Li, C., et al. (2022). Luteolin induces pyroptosis in HT-29 cells by activating the Caspase1/Gasdermin D signalling pathway. Front. Pharmacol. 13, 952587. doi:10.3389/fphar.2022.952587
Cheng, Y., Yu, C., Li, W., He, Y., and Bao, Y. (2022). Matrine inhibits proliferation, invasion, and migration and induces apoptosis of colorectal cancer cells via miR-10b/PTEN pathway. Cancer Biotherapy Radiopharm. 37 (10), 871–881. doi:10.1089/cbr.2020.3800
Chen J-F, J.-F., Wu, S.-W., Shi, Z.-M., and Hu, B. (2023). Traditional Chinese medicine for colorectal cancer treatment: potential targets and mechanisms of action. Chin. Med. 18 (1), 14–44. doi:10.1186/s13020-023-00719-7
Chen J, J., Zheng, X., Xu, G., Wang, B., Hu, L., Mao, J., et al. (2023). Sini decoction inhibits tumor progression and enhances the anti-tumor immune response in a murine model of colon cancer. Comb. Chem. High Throughput Screen. 26 (14), 2517–2526. doi:10.2174/1386207326666230320103437
Chen W, W., Zhang, Q., Dai, X., Chen, X., Zhang, C., Bai, R., et al. (2023). PGC-1α promotes colorectal carcinoma metastasis through regulating ABCA1 transcription. Oncogene 42 (32), 2456–2470. doi:10.1038/s41388-023-02762-y
Clay, S. L., Fonseca-Pereira, D., and Garrett, W. S. (2022). Colorectal cancer: the facts in the case of the microbiota. J. Clin. investigation 132 (4), e155101. doi:10.1172/JCI155101
Darband, S. G., Sadighparvar, S., Yousefi, B., Kaviani, M., Ghaderi-Pakdel, F., Mihanfar, A., et al. (2020). Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. doi:10.1016/j.lfs.2020.117584
Deng, X., Zhang, C., Yang, Y., Wang, J., Ye, X., Gu, J., et al. (2024). Shenling Baizhu Decoction (SLBZD) may play a synergistic role of tirelizumab in the treatment of colorectal cancer by influencing the imbalance of colon flora and Tumor microenvironment. J. Cancer 15 (1), 30–40. doi:10.7150/jca.88854
Dong M, M., Liu, H., Cao, T., Li, L., Sun, Z., Qiu, Y., et al. (2022). Huoxiang Zhengqi alleviates azoxymethane/dextran sulfate sodium-induced colitis-associated cancer by regulating Nrf2/NF-κB/NLRP3 signaling. Front. Pharmacol. 13, 1002269. doi:10.3389/fphar.2022.1002269
Dong S, S., Liang, S., Cheng, Z., Zhang, X., Luo, L., Li, L., et al. (2022). ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 41 (1), 15–27. doi:10.1186/s13046-021-02229-6
Gao, W., Wang, X., Zhou, Y., Wang, X., and Yu, Y. (2022). Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 7 (1), 196. doi:10.1038/s41392-022-01046-3
Gou, H., Su, H., Liu, D., Wong, C. C., Shang, H., Fang, Y., et al. (2023). Traditional medicine pien tze huang suppresses colorectal tumorigenesis through restoring gut microbiota and metabolites. Gastroenterology S0016-5085 (12), 04982–X.
Gou, H., Su, H., Liu, D., Wong, C. C., Shang, H., Fang, Y., et al. (2023). Traditional Medicine Pien Tze Huang Suppresses Colorectal Tumorigenesis Through Restoring Gut Microbiota and Metabolites. Gastroenterology 165 (6), 1404–1419. doi:10.1053/j.gastro.2023.08.052
Guo S, S., Duan, W., Wang, Y., Chen, L., Yang, C., Gu, X., et al. (2022). Component analysis and anti-colorectal cancer mechanism via AKT/mTOR signalling pathway of Sanghuangporus vaninii extracts. Molecules 27 (4), 1153. doi:10.3390/molecules27041153
Guo T-H, T.-h., Li, Y.-y., Hong, S.-w., Cao, Q.-y., Chen, H., Xu, Y., et al. (2022). Evidence for anticancer effects of Chinese medicine monomers on colorectal cancer. Chin. J. Integr. Med. 28 (10), 939–952. doi:10.1007/s11655-022-3466-2
Han, B., Jiang, P., Li, Z., Yu, Y., Huang, T., Ye, X., et al. (2018). Coptisine-induced apoptosis in human colon cancer cells (HCT-116) is mediated by PI3K/Akt and mitochondrial-associated apoptotic pathway. Phytomedicine 48, 152–160. doi:10.1016/j.phymed.2017.12.027
Hao, D., Liu, J., Guo, Z., Chen, J., Li, T., Li, X., et al. (2022). Supercritical fluid extract of Angelica sinensis promotes the anti-colorectal cancer effect of oxaliplatin. Front. Pharmacol. 13, 1007623. doi:10.3389/fphar.2022.1007623
Hassan, A. K., El-Kalaawy, A. M., Abd El-Twab, S. M., Alblihed, M. A., and Ahmed, O. M. (2023). Hesperetin and capecitabine abate 1, 2 dimethylhydrazine-induced colon carcinogenesis in wistar rats via suppressing oxidative stress and enhancing antioxidant, anti-inflammatory and apoptotic actions. Life 13 (4), 984. doi:10.3390/life13040984
Hu, S.-M., Yao, X.-H., Hao, Y.-H., Pan, A.-H., and Zhou, X.-W. (2020). 8-Gingerol regulates colorectal cancer cell proliferation and migration through the EGFR/STAT/ERK pathway. Int. J. Oncol. 56 (1), 390–397. doi:10.3892/ijo.2019.4934
Huang, S.-z., Liu, W.-y., Huang, Y., Shen, A.-l., Liu, L.-y., and Peng, J. (2019). Patrinia scabiosaefolia inhibits growth of 5-FU-resistant colorectal carcinoma cells via induction of apoptosis and suppression of AKT pathway. Chin. J. Integr. Med. 25, 116–121. doi:10.1007/s11655-018-3002-6
Jain, T., Sharma, P., Are, A. C., Vickers, S. M., and Dudeja, V. (2021). New insights into the cancer–microbiome–immune axis: decrypting a decade of discoveries. Front. Immunol. 12, 622064. doi:10.3389/fimmu.2021.622064
Jiang, F., Liu, M., Wang, H., Shi, G., Chen, B., Chen, T., et al. (2020). Wu Mei Wan attenuates CAC by regulating gut microbiota and the NF-kB/IL6-STAT3 signaling pathway. Biomed. Pharmacother. 125, 109982. doi:10.1016/j.biopha.2020.109982
Jiang, Y.-L., and Xun, Y. (2024). Molecular mechanism of Salvia miltiorrhiza in the treatment of colorectal cancer based on network pharmacology and molecular docking technology. Drug Des. Dev. Ther. 18, 425–441. doi:10.2147/DDDT.S443102
Jin, B.-R., Chung, K.-S., Lee, M., and An, H.-J. (2020). High-fat diet propelled AOM/DSS-Induced colitis-associated colon cancer alleviated by administration of aster glehni via STAT3 signaling pathway. Biology 9 (2), 24. doi:10.3390/biology9020024
Jin, Y., Chen, W., Yang, H., Yan, Z., Lai, Z., Feng, J., et al. (2017). Scutellaria barbata D. Don inhibits migration and invasion of colorectal cancer cells via suppression of PI3K/AKT and TGF-β/Smad signaling pathways. Exp. Ther. Med. 14 (6), 5527–5534. doi:10.3892/etm.2017.5242
Khalil, L., Gao, X., Switchenko, J. M., Alese, O. B., Akce, M., Wu, C., et al. (2022). Survival outcomes of adjuvant chemotherapy in elderly patients with stage III colon cancer. Oncol. 27 (9), 740–750. doi:10.1093/oncolo/oyac082
Kong, M.-y., Li, L.-y., Lou, Y.-m., Chi, H.-y., and Wu, J.-j. (2020). Chinese herbal medicines for prevention and treatment of colorectal cancer: from molecular mechanisms to potential clinical applications. J. Integr. Med. 18 (5), 369–384. doi:10.1016/j.joim.2020.07.005
Kuo, T.-T., Chang, H.-Y., Chen, T.-Y., Liu, B.-C., Chen, H.-Y., Hsiung, Y.-C., et al. (2020). Melissa officinalis extract induces apoptosis and inhibits migration in human colorectal cancer cells. ACS Omega 5 (49), 31792–31800. doi:10.1021/acsomega.0c04489
Lai, W.-L., Lee, S.-C., Chang, K.-F., Huang, X.-F., Li, C.-Y., Lee, C.-J., et al. (2021). Juniperus communis extract induces cell cycle arrest and apoptosis of colorectal adenocarcinoma in vitro and in vivo. Braz. J. Med. Biol. Res. 54 (10), e10891. doi:10.1590/1414-431X2020e10891
Li, M., Fan, J., Hu, M., Xu, J., He, Z., and Zeng, J. (2024). Quercetin enhances 5-fluorouracil sensitivity by regulating the autophagic flux and inducing drp-1 mediated mitochondrial fragmentation in colorectal cancer cells. Curr. Mol. Pharmacol. 17. doi:10.2174/0118761429283717231222104730
Li, Y., Wang, Y., Liu, Z., Guo, X., Miao, Z., and Ma, S. (2020). Atractylenolide I induces apoptosis and suppresses glycolysis by blocking the JAK2/STAT3 signaling pathway in colorectal cancer cells. Front. Pharmacol. 11, 273. doi:10.3389/fphar.2020.00273
Liang, H., Li, Y., Wang, F., Zhao, J., Yang, X., Wu, D., et al. (2022). Combining network pharmacology and experimental validation to study the action and mechanism of water extract of Asparagus against colorectal cancer. Front. Pharmacol. 13, 862966. doi:10.3389/fphar.2022.862966
Li J, J., Zhou, F., Shang, L., Liu, N., Liu, Y., Zhang, M., et al. (2022). Integrated network pharmacology and experimental verification to investigate the mechanisms of YYFZBJS against colorectal cancer via CDK1/PI3K/Akt signaling. Front. Oncol. 12, 961653. doi:10.3389/fonc.2022.961653
Lin, C., Zhou, Z., Zhang, L., Wang, H., Lu, J., Wang, X., et al. (2022). Gegen qinlian decoction relieves ulcerative colitis via adjusting dysregulated Nrf2/ARE signaling. Evidence-Based Complementary Altern. Med. 2022, 2934552. doi:10.1155/2022/2934552
Lin, F., Zhang, G., Yang, X., Wang, M., Wang, R., Wan, M., et al. (2023). A network pharmacology approach and experimental validation to investigate the anticancer mechanism and potential active targets of ethanol extract of Wei-Tong-Xin against colorectal cancer through induction of apoptosis via PI3K/AKT signaling pathway. J. Ethnopharmacol. 303, 115933. doi:10.1016/j.jep.2022.115933
Li R, R., Zhou, J., Wu, X., Li, H., Pu, Y., Liu, N., et al. (2022). Jianpi Jiedu Recipe inhibits colorectal cancer liver metastasis via regulating ITGBL1-rich extracellular vesicles mediated activation of cancer-associated fibroblasts. Phytomedicine 100, 154082. doi:10.1016/j.phymed.2022.154082
Li S, S., Han, W., He, Q., Wang, Y., Jin, G., and Zhang, Y. (2023). Ginsenoside Rh2 suppresses colon cancer growth by targeting the miR-150-3p/SRCIN1/Wnt axis. Acta Biochimica Biophysica Sinica(Shanghai) 55 (4), 633–648. doi:10.3724/abbs.2023032
Liu, F., Zhu, C., Ma, H., and Yang, Q. (2023). Curcumin targets miR-134-5p to suppress the progression of colorectal cancer through regulating the CDCA3/CDK1 pathway. Naunyn Schmiedeb. Arch. Pharmacol. 7, 109–122. doi:10.1007/s00210-023-02584-5
Liu, H., Deng, R., Zhu, C.-W., Han, H.-K., Zong, G.-F., Ren, L., et al. (2024). Rosmarinic acid in combination with ginsenoside Rg1 suppresses colon cancer metastasis via co-inhition of COX-2 and PD1/PD-L1 signaling axis. Acta Pharmacol. Sin. 45 (1), 193–208. doi:10.1038/s41401-023-01158-8
Liu, Y.-T., Tzang, B.-S., Yow, J., Chiang, Y.-H., Huang, C.-Y., and Hsu, T.-C. (2022). Traditional Chinese medicine formula T33 inhibits the proliferation of human colorectal cancer cells by inducing autophagy. Environ. Toxicol. 37 (5), 1007–1017. doi:10.1002/tox.23460
Li X-C, X.-C., Wang, S., Yang, X.-X., Li, T.-J., Gu, J.-X., Zhao, L., et al. (2023). Patrinia villosa treat colorectal cancer by activating PI3K/Akt signaling pathway. J. Ethnopharmacol. 309, 116264. doi:10.1016/j.jep.2023.116264
Li Y, Y., Sun, Y., Zhang, Q., Liu, Y., Niu, Y., Li, X., et al. (2022). Bolbostemma paniculatum (Maxim.) Franquet extract suppresses the development of colorectal cancer through downregulation of PI3K/Akt pathway. J. Ethnopharmacol. 287, 114937. doi:10.1016/j.jep.2021.114937
Lu, S., Sun, X., Zhou, Z., Tang, H., Xiao, R., Lv, Q., et al. (2023). Mechanism of Bazhen decoction in the treatment of colorectal cancer based on network pharmacology, molecular docking, and experimental validation. Front. Immunol. 14, 1235575. doi:10.3389/fimmu.2023.1235575
Lv, J., Jia, Y., Li, J., Kuai, W., Li, Y., Guo, F., et al. (2019). Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 10 (6), 415. doi:10.1038/s41419-019-1638-6
Ma, X., Li, Y., Liang, D., Jiang, F., Zhang, L., Song, W., et al. (2024). Solanine induces ferroptosis in colorectal cancer cells through ALOX12B/ADCY4 molecular axis. J. Pharm. Pharmacol. 76 (3), 224–235. doi:10.1093/jpp/rgad122
Mariño, G., Niso-Santano, M., Baehrecke, E. H., and Kroemer, G. (2014). Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15 (2), 81–94. doi:10.1038/nrm3735
Meng, F.-C., and Lin, J.-K. (2019). Liquiritigenin inhibits colorectal cancer proliferation, invasion, and epithelial-to-mesenchymal transition by decreasing expression of runt-related transcription factor 2. Oncol. Res. 27 (2), 139–146. doi:10.3727/096504018X15185747911701
Miller, K. D., Nogueira, L., Mariotto, A. B., Rowland, J. H., Yabroff, K. R., Alfano, C. M., et al. (2019). Cancer treatment and survivorship statistics, 2019. CA a cancer J. Clin. 69 (5), 363–385. doi:10.3322/caac.21565
Morgan, E., Arnold, M., Gini, A., Lorenzoni, V., Cabasag, C., Laversanne, M., et al. (2023). Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72 (2), 338–344. doi:10.1136/gutjnl-2022-327736
Nakhjavani, M., Smith, E., Yeo, K., Palethorpe, H. M., Tomita, Y., Price, T. J., et al. (2021). Anti-angiogenic properties of ginsenoside Rg3 epimers: in vitro assessment of single and combination treatments. Cancers 13 (9), 2223. doi:10.3390/cancers13092223
Newman, D. J., and Cragg, G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79 (3), 629–661. doi:10.1021/acs.jnatprod.5b01055
Ni, L., Sun, P., Ai, M., Kong, L., Xu, R., and Li, J. (2022). Berberine inhibited the formation of metastasis by intervening the secondary homing of colorectal cancer cells in the blood circulation to the lung and liver through HEY2. Phytomedicine Int. J. Phytotherapy Phytopharm. 104, 154303. doi:10.1016/j.phymed.2022.154303
Pan, C.-F., Zhang, X., Wang, J.-W., Yang, T., Zhong, L. L. D., and Shen, K.-P. (2020). Weichang'an formula inhibits tumor growth in combination with bevacizumab in a murine model of colon cancer-making up for the deficiency of bevacizumab by inhibiting VEGFR-1. Front. Pharmacol. 11, 512598. doi:10.3389/fphar.2020.512598
Pandey, A., and Trigun, S. K. (2023). Fisetin induces apoptosis in colorectal cancer cells by suppressing autophagy and down-regulating nuclear factor erythroid 2-related factor 2 (Nrf2). J. Cell. Biochem. 124 (9), 1289–1308. doi:10.1002/jcb.30447
Piawah, s., and Venook, A. P. (2019). Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 125 (23), 4139–4147. doi:10.1002/cncr.32163
Qiu, W., Xie, H., Chen, H., Zhou, H., Wang, Z., and Zhou, H. (2023). Integrated gut microbiota and metabolome analysis reveals the mechanism of Xiaoai Jiedu recipe in ameliorating colorectal cancer. Front. Oncol. 13, 1184786. doi:10.3389/fonc.2023.1184786
Qu, R., Ma, Y., Zhang, Z., and Fu, W. (2022). Increasing burden of colorectal cancer in China. Lancet Gastroenterology Hepatology 7 (8), 700. doi:10.1016/S2468-1253(22)00156-X
Ranjan, A., Ramachandran, S., Gupta, N., Kaushik, I., Wright, S., Srivastava, S., et al. (2019). Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 20 (20), 4981. doi:10.3390/ijms20204981
Sakata, S., and Larson, D. W. (2022). Targeted therapy for colorectal cancer. Surg. Oncol. Clin. 31 (2), 255–264. doi:10.1016/j.soc.2021.11.006
Shang, L., Wang, Y., Li, J., Zhou, F., Xiao, K., Liu, Y., et al. (2023). Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J. Ethnopharmacol. 302 (Pt A), 115876. doi:10.1016/j.jep.2022.115876
Shi, J., Ji, X., Shan, S., Zhao, M., Bi, C., and Li, Z. (2023). The interaction between Apigenin and PKM2 restrains progression of colorectal cancer. J. Nutr. Biochem. 121, 109430. doi:10.1016/j.jnutbio.2023.109430
Shi, X., Zhu, M., Gong, Z., Yang, T., Yu, R., Wang, J., et al. (2020). Homoharringtonine suppresses LoVo cell growth by inhibiting EphB4 and the PI3K/AKT and MAPK/EKR1/2 signaling pathways. Food Chem. Toxicol. 136, 110960. doi:10.1016/j.fct.2019.110960
Siegel, R. A.-O., Wagle, N. A.-O. X., Cercek, A., Smith, R. A.-O., and Jemal, A. (2023). Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73 (3), 233–254. doi:10.3322/caac.21772
Sui, H., Zhang, L., Gu, K., Chai, N., Ji, Q., Zhou, L., et al. (2020). YYFZBJS ameliorates colorectal cancer progression in ApcMin/+ mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun. Signal. 18 (1), 113–117. doi:10.1186/s12964-020-00596-9
Sun, Q., He, M., Zhang, M., Zeng, S., Chen, L., Zhao, H., et al. (2021). Traditional Chinese medicine and colorectal cancer: implications for drug discovery. Front. Pharmacol. 12 (1), 685002. doi:10.3389/fphar.2021.685002
Sun, Q., Tao, Q., Ming, T., Tang, S., Zhao, H., Liu, M., et al. (2023). Berberine is a suppressor of Hedgehog signaling cascade in colorectal cancer. Phytomedicine 114, 154792–154826. doi:10.1016/j.phymed.2023.154792
Sun, Y., Liu, Y., Cai, Y., Han, P., Hu, S., and Cao, L. (2022). Atractylenolide I inhibited the development of malignant colorectal cancer cells and enhanced oxaliplatin sensitivity through the PDK1-FoxO1 axis. J. Gastrointest. Oncol. 13 (5), 2382–2392. doi:10.21037/jgo-22-910
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, D., Wang, H., Deng, W., Wang, J., Shen, D., Wang, L., et al. (2024). Mechanism of bufalin inhibition of colon cancer liver metastasis by regulating M2-type polarization of Kupffer cells induced by highly metastatic colon cancer cells. Apoptosis Int. J. Program. Cell Death. doi:10.1007/s10495-023-01930-5
Tang, R. Z., Li, Z. Z., Hu, D., Kanwal, F., Yuan, C. B., Mustaqeem, M., et al. (2021). Sanjie Yiliu formula inhibits colorectal cancer growth by suppression of proliferation and induction of apoptosis. ACS Omega 6 (11), 7761–7770. doi:10.1021/acsomega.0c05565
Tao, S., Ren, Z., Yang, Z., Duan, S., Wan, Z., Huang, J., et al. (2021). Effects of different molecular weight polysaccharides from Dendrobium officinale kimura and migo on human colorectal cancer and transcriptome analysis of differentially expressed genes. Front. Pharmacol. 12, 704486. doi:10.3389/fphar.2021.704486
Teng, Z., Sun, X., Guo, Y., Zhang, M., Liu, Y., and Xu, M. (2022). Curcumae longae Rhizoma (Jianghuang) extract reverses the 5-Fluoruracil resistance in colorectal cancer cells via TLR4/PI3K/Akt/mTOR pathway. Clin. Res. Hepatology Gastroenterology 46 (9), 101976. doi:10.1016/j.clinre.2022.101976
Trinh, N.-T., Nguyen, T. M. N., Yook, J.-I., Ahn, S.-G., and Kim, S.-A. (2022). Quercetin and quercitrin from Agrimonia pilosa Ledeb inhibit the migration and invasion of colon cancer cells through the JNK signaling pathway. Pharmaceuticals 15 (3), 364. doi:10.3390/ph15030364
Wang, L., Zhang, Q.-Q., Xu, Y.-Y., Zhang, R., Zhao, Q., Zhang, Y.-Q., et al. (2023). Ginsenoside Rb1 suppresses AOM/DSS-induced colon carcinogenesis. Anticancer Agents Med Chem. 23 (9), 1067–1073. doi:10.2174/1871520623666230119092735
Wang, M., Zhao, X., Zhu, D., Liu, T., Liang, X., Liu, F., et al. (2017). HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J. Exp. Clin. Cancer Res. 36 (1), 60–14. doi:10.1186/s13046-017-0533-1
Wang, R., Liu, H., Shao, Y., Wang, K., Yin, S., Qiu, Y., et al. (2019). Sophoridine inhibits human colorectal cancer progression via targeting MAPKAPK2. Mol. Cancer Res. 17 (12), 2469–2479. doi:10.1158/1541-7786.MCR-19-0553
Wang, Y., Zhao, T., Huang, C., Liu, F., Zhang, Y., Kong, D., et al. (2024). Effect and mechanism of Banxia Xiexin decoction in colorectal cancer: a network pharmacology approach. Phytomedicine Int. J. Phytotherapy Phytopharm. 123, 155174. doi:10.1016/j.phymed.2023.155174
Wang K, K., Huang, W., Sang, X., Wu, X., Shan, Q., Tang, D., et al. (2020). Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine 68, 153191. doi:10.1016/j.phymed.2020.153191
Wang X, X., Saud, S. M., Wang, F., He, S., Zhang, X., Hua, B., et al. (2020). Protective effect of ShaoYao decoction on colitis-associated colorectal cancer by inducing Nrf2 signaling pathway. J. Ethnopharmacol. 252, 112600. doi:10.1016/j.jep.2020.112600
Wei, X., Leng, X., Li, G., Wang, R., Chi, L., and Sun, D. (2023). Advances in research on the effectiveness and mechanism of Traditional Chinese Medicine formulas for colitis-associated colorectal cancer. Front. Pharmacol. 2 (14), 1120672. doi:10.3389/fphar.2023.1120672
Wei, X., Liang, J., Liu, J., Dai, Y., Leng, X., Cheng, Y., et al. (2024). Anchang Yuyang Decoction inhibits experimental colitis-related carcinogenesis by regulating PPAR signaling pathway and affecting metabolic homeostasis of host and microbiota. J. Ethnopharmacol. 326, 117995. doi:10.1016/j.jep.2024.117995
Wu, H., Du, J. e., Li, C., Li, H., Guo, H., and Li, Z. (2022). Kaempferol can reverse the 5-Fu resistance of colorectal cancer cells by inhibiting PKM2-mediated glycolysis. Int. J. Mol. Sci. 23 (7), 3544. doi:10.3390/ijms23073544
Wu, S., Qiu, Y., Shao, Y., Yin, S., Wang, R., Pang, X., et al. (2018). Lycorine displays potent antitumor efficacy in colon carcinoma by targeting STAT3. Front. Pharmacol. 9, 881. doi:10.3389/fphar.2018.00881
Wu, Y., Pi, D., Zhou, S., Yi, Z., Dong, Y., Wang, W., et al. (2023). Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells: ginsenoside Rh3 has anti-colorectal cancer properties. Acta Biochimica Biophysica Sinica 55 (4), 587–600. doi:10.3724/abbs.2023068
Xia, J., Chen, J., Zhang, Z., Song, P., Tang, W., and Kokudo, N. (2014). A map describing the association between effective components of traditional Chinese medicine and signaling pathways in cancer cells in vitro and in vivo. Drug Discov. Ther. 8 (4), 139–153. doi:10.5582/ddt.2014.01032
Xiang, B., Geng, R., Zhang, Z., Ji, X., Zou, J., Chen, L., et al. (2022). Identification of the effect and mechanism of Yiyi Fuzi Baijiang powder against colorectal cancer using network pharmacology and experimental validation. Front. Pharmacol. 13, 929836. doi:10.3389/fphar.2022.929836
Yang, B., Bai, H., Sa, Y., Zhu, P., and Liu, P. (2020). Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. J Cancer. 11 (8), 2303–2317. doi:10.7150/jca.37242
Yang, H., Liu, J.-X., Shang, H.-X., Lin, S., Zhao, J.-Y., and Lin, J.-M. (2019). Qingjie Fuzheng granules inhibit colorectal cancer cell growth by the PI3K/AKT and ERK pathways. World J. Gastrointest. Oncol. 11 (5), 377–392. doi:10.4251/wjgo.v11.i5.377
Yang, Z., Yao, Y., and Qian, C. (2023). Study on the effect of Jianpi Yiqi decoction on clinical symptoms, inflammation, oxidative stress, efficacy and adverse reactions in sufferers with colorectal cancer. Biotechnol. Genet. Eng. Rev. 21, 1–16. doi:10.1080/02648725.2023.2203004
Yi, S., Jin, X., Liu, B., Wu, P., Xiao, W., and Chen, W. (2022). Portulaca oleracea extract reduces gut microbiota imbalance and inhibits colorectal cancer progression via inactivation of the Wnt/β-catenin signaling pathway. Phytomedicine 105, 154279. doi:10.1016/j.phymed.2022.154279
Yu, Z., Sun, Q., Liu, J., Zhang, X., Song, G., Wang, G., et al. (2018). Polysaccharide from Rhizopus nigricans inhibits the invasion and metastasis of colorectal cancer. Biomed. Pharmacother. = Biomedecine Pharmacother. 103, 738–745. doi:10.1016/j.biopha.2018.04.093
Yuan, M., Meng, W., Liao, W., and Lian, S. (2018). Andrographolide antagonizes TNF-α-induced IL-8 via inhibition of NADPH oxidase/ROS/NF-κB and Src/MAPKs/AP-1 axis in human colorectal cancer HCT116 cells. J. Agric. food Chem. 66 (20), 5139–5148. doi:10.1021/acs.jafc.8b00810
Zeng, S., Tan, L., Sun, Q., Chen, L., Zhao, H., Liu, M., et al. (2022). Suppression of colitis-associated colorectal cancer by scutellarin through inhibiting Hedgehog signaling pathway activity. Phytomedicine 98, 153972. doi:10.1016/j.phymed.2022.153972
Zhang, W., Ou, L., Peng, C., Sang, S., Feng, Z., Zou, Y., et al. (2023). Sanguisorba officinalis L. enhances the 5-fluorouracil sensitivity and overcomes chemoresistance in 5-fluorouracil-resistant colorectal cancer cells via Ras/MEK/ERK and PI3K/Akt pathways. Heliyon 9 (6), e16798. doi:10.1016/j.heliyon.2023.e16798
Zhang H, H., Zhao, X., Shang, F., Sun, H., Zheng, X., and Zhu, J. (2022). Celastrol inhibits the proliferation and induces apoptosis of colorectal cancer cells via downregulating NF-κB/COX-2 signaling pathways. Anticancer Agents Med Chem. 22 (10), 1921–1932. doi:10.2174/1871520621666211103103530
Zhang N, N., Ng, A. S., Cai, S., Li, Q., Yang, L., and Kerr, D. (2021). Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet. Oncol. 22 (8), e358–e368. doi:10.1016/S1470-2045(21)00343-0
Zhang W, W., Peng, C., Yan, J., Chen, P., Jiang, C., Sang, S., et al. (2022). Sanguisorba officinalis L. suppresses 5-fluorouracil-sensitive and-resistant colorectal cancer growth and metastasis via inhibition of the Wnt/β-catenin pathway. Phytomedicine 94, 153844. doi:10.1016/j.phymed.2021.153844
Zhang Y, Y., Pu, W., Bousquenaud, M., Cattin, S., Zaric, J., Sun, L.-k., et al. (2021). Emodin inhibits inflammation, carcinogenesis, and cancer progression in the AOM/DSS model of colitis-associated intestinal tumorigenesis. Front. Oncol. 10, 564674. doi:10.3389/fonc.2020.564674
Zhang Y, Y., Zhang, Y., Zhao, Y., Wu, W., Meng, W., Zhou, Y., et al. (2022). Protection against ulcerative colitis and colorectal cancer by evodiamine via anti-inflammatory effects. Mol. Med. Rep. 25 (5), 188–214. doi:10.3892/mmr.2022.12704
Zhao, L., Zhu, X., Ni, Y., You, J., and Li, A. (2020). Xiaoyaosan, a traditional Chinese medicine, inhibits the chronic restraint stress-induced liver metastasis of colon cancer in vivo. Pharm. Biol. 58 (1), 1085–1091. doi:10.1080/13880209.2020.1839513
Zhou, F., Gao, H., Shang, L., Li, J., Zhang, M., Wang, S., et al. (2023). Oridonin promotes endoplasmic reticulum stress via TP53-repressed TCF4 transactivation in colorectal cancer. J. Exp. Clin. Cancer Res. CR 42 (1), 150. doi:10.1186/s13046-023-02702-4
Zhu, L., Chen, C., Cai, Y., Li, Y., Gong, L., Zhu, T., et al. (2024). Identification of a ferritinophagy inducer via sinomenine modification for the treatment of colorectal cancer. Eur. J. Med. Chem. 268, 116250. doi:10.1016/j.ejmech.2024.116250
Zong, S., Tang, Y., Li, W., Han, S., Shi, Q., Ruan, X., et al. (2020). A Chinese herbal formula suppresses colorectal cancer migration and vasculogenic mimicry through ROS/HIF-1α/MMP2 pathway in hypoxic microenvironment. Front. Pharmacol. 11, 705. doi:10.3389/fphar.2020.00705
Keywords: traditional Chinese medicine, colorectal cancer, prescriptions, herbs, components
Citation: Sun J, Wei Y, Wang J, Hou M and Su L (2024) Treatment of colorectal cancer by traditional Chinese medicine: prevention and treatment mechanisms. Front. Pharmacol. 15:1377592. doi: 10.3389/fphar.2024.1377592
Received: 05 February 2024; Accepted: 15 April 2024;
Published: 09 May 2024.
Edited by:
S. Paul Gao, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Lihong Zhou, Shanghai University of Traditional Chinese Medicine, ChinaJiaqian Luo, Cornell University, United States
Copyright © 2024 Sun, Wei, Wang, Hou and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liya Su, c3VsaXlhMjMwN0Bob3RtYWlsLmNvbQ==
 Jiaxin Sun
Jiaxin Sun Ying Wei
Ying Wei Jia Wang2
Jia Wang2 Liya Su
Liya Su