- 1Department of Orthopaedics, Tianjin First Central Hospital, Tianjin, China
- 2Orthopedic Soft Tissue Research Program, Hospital for Special Surgery, New York, NY, United States
The transition of fibroblasts to myofibroblasts (FMT) represents a pivotal process in wound healing, tissue repair, and fibrotic diseases. This intricate transformation involves dynamic changes in cellular morphology, gene expression, and extracellular matrix remodeling. While extensively studied at the molecular level, recent research has illuminated the regulatory roles of non-coding RNAs (ncRNAs) in orchestrating FMT. This review explores the emerging roles of ncRNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), in regulating this intricate process. NcRNAs interface with key signaling pathways, transcription factors, and epigenetic mechanisms to fine-tune gene expression during FMT. Their functions are critical in maintaining tissue homeostasis, and disruptions in these regulatory networks have been linked to pathological fibrosis across various tissues. Understanding the dynamic roles of ncRNAs in FMT bears therapeutic promise. Targeting specific ncRNAs holds potential to mitigate exaggerated myofibroblast activation and tissue fibrosis. However, challenges in delivery and specificity of ncRNA-based therapies remain. In summary, ncRNAs emerge as integral regulators in the symphony of FMT, orchestrating the balance between quiescent fibroblasts and activated myofibroblasts. As research advances, these ncRNAs appear to be prospects for innovative therapeutic strategies, offering hope in taming the complexities of fibrosis and restoring tissue equilibrium.
1 Introduction
Fibroblast to myofibroblast transition (FMT) is a fundamental process that holds immense significance in various physiological contexts such as wound healing, tissue repair, and the pathogenesis of fibrotic diseases (Zhang et al., 2016; Usher et al., 2019; Blessing et al., 2021; Wang et al., 2023). This intricate transition is characterized by profound changes in cellular phenotype, encompassing alterations in cellular morphology, gene expression profiles, and the synthesis of extracellular matrix components (Michalik et al., 2018). These modifications collectively culminate in substantial tissue remodeling, which is essential for restoring tissue integrity and function following injury or damage (D'Urso and Kurniawan, 2020).
The process of FMT can be broadly divided into four stages (Wynn and Ramalingam, 2012). Initially, quiescent fibroblasts are activated in response to injury or stress signals, leading them to start proliferating. Following this, activated fibroblasts differentiate into myofibroblasts, characterized by the expression of alpha-smooth muscle actin (α-SMA) and increased production of extracellular matrix (ECM) components. Myofibroblasts then play a crucial role in extracellular matrix remodeling, depositing collagen and other ECM proteins to repair tissue. Normally, myofibroblasts undergo apoptosis once the tissue is repaired. However, in pathological conditions, myofibroblasts persist, leading to fibrosis. Several key signaling pathways regulate FMT (Zhang et al., 2023), including the Transforming Growth Factor-beta (TGF-β) pathway, which is a major driver of FMT, promoting myofibroblast differentiation and ECM production. The MAPK pathway is involved in fibroblast activation and differentiation, while the PI3K/Akt pathway plays a role in cell survival and proliferation during FMT.
The exploration of the molecular intricacies governing FMT has been a subject of extensive research, driven by the imperative to comprehend the underlying mechanisms that drive tissue repair and fibrosis (Li et al., 2015). In this context, recent scientific exploration has evaluated the pivotal role of non-coding RNAs (ncRNAs) as indispensable orchestrators of the FMT process (Zhang et al., 2023). Traditionally overlooked due to their lack of protein-coding capacity (Ilieva and Uchida, 2022), ncRNAs are now recognized as key players in shaping the delicate equilibrium between quiescent fibroblasts and their activated myofibroblast counterparts during FMT (Creemers and van Rooij, 2016).
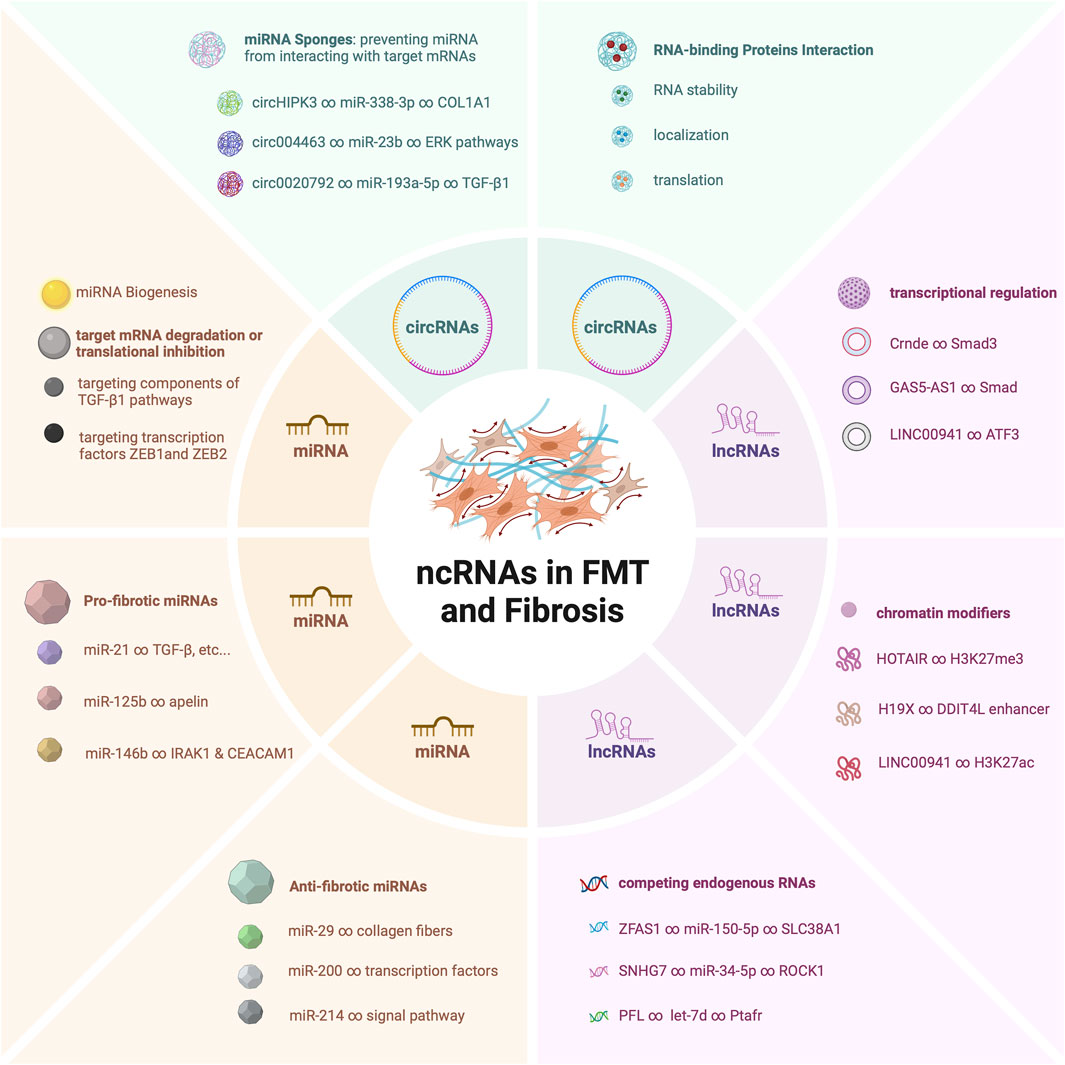
The ensemble of ncRNAs, including microRNAs (miRNAs) (Lu and Rothenberg, 2018), long non-coding RNAs (lncRNAs) (Fernandes et al., 2019), and circular RNAs (circRNAs) (Li et al., 2018a), showcases a multifaceted array of regulatory molecules that converge to finely tune the transition from fibroblasts to myofibroblasts (Wang et al., 2014; Fan et al., 2021; Su et al., 2021). This cascade of molecular events encompasses miRNAs that function as fine-tuners (Miao et al., 2018), lncRNAs that orchestrate complex gene expression networks (Dong et al., 2022), and circRNAs that act as dynamic sponges and orchestrators of intricate interactions (Yang et al., 2022). These ncRNAs are far from being bystanders; rather, they intricately interweave with signaling pathways, transcription factors, and epigenetic modulators to steer the gene expression programs that govern FMT (Zhou et al., 2018; Niu et al., 2022; Hertig et al., 2023).
The pivotal roles of these ncRNAs do not exist in isolation (Figure 1). Rather, they synergistically contribute to a complex regulatory network that dictates the fine balance between fibroblast quiescence and myofibroblast activation (Wang et al., 2020). Dysregulation of these ncRNAs has been found to be a common thread linking to the development of pathological fibrosis across diverse tissues (Tao et al., 2016; Tarbit et al., 2019; Senavirathna et al., 2020). Their dysregulated expression levels or altered interactions can have profound implications, leading to exaggerated myofibroblast activation (Wasson et al., 2020a), excessive extracellular matrix deposition (Zhang et al., 2018), and ultimately tissue fibrosis (Lino Cardenas et al., 2013).
In this review, our primary emphasis will be on elucidating the involvement of ncRNAs in both FMT process and fibrotic diseases, highlighting their significant therapeutic promise. Insights into their roles not only deepen our comprehension of fibrotic processes but also offer potential avenues for therapeutic interventions aimed at mitigating the excessive activation of myofibroblasts and inhibiting the progression of fibrosis.
2 MicroRNAs (miRNAs) in FMT
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules, typically about 22 nucleotides in length, that play crucial roles in post-transcriptional gene regulation. MiRNAs exert their regulatory effects by binding to the 3’untranslated region (UTR) of target messenger RNAs (mRNAs), leading to mRNA degradation or translational repression. In the context of fibrotic diseases, miRNAs are significantly altered (Selman et al., 2016). Emerging evidence highlights the substantial impact of miRNAs in modulating FMT dynamics. MiRNAs play intricate roles in both promoting and inhibiting FMT, making them key regulators of this transition.
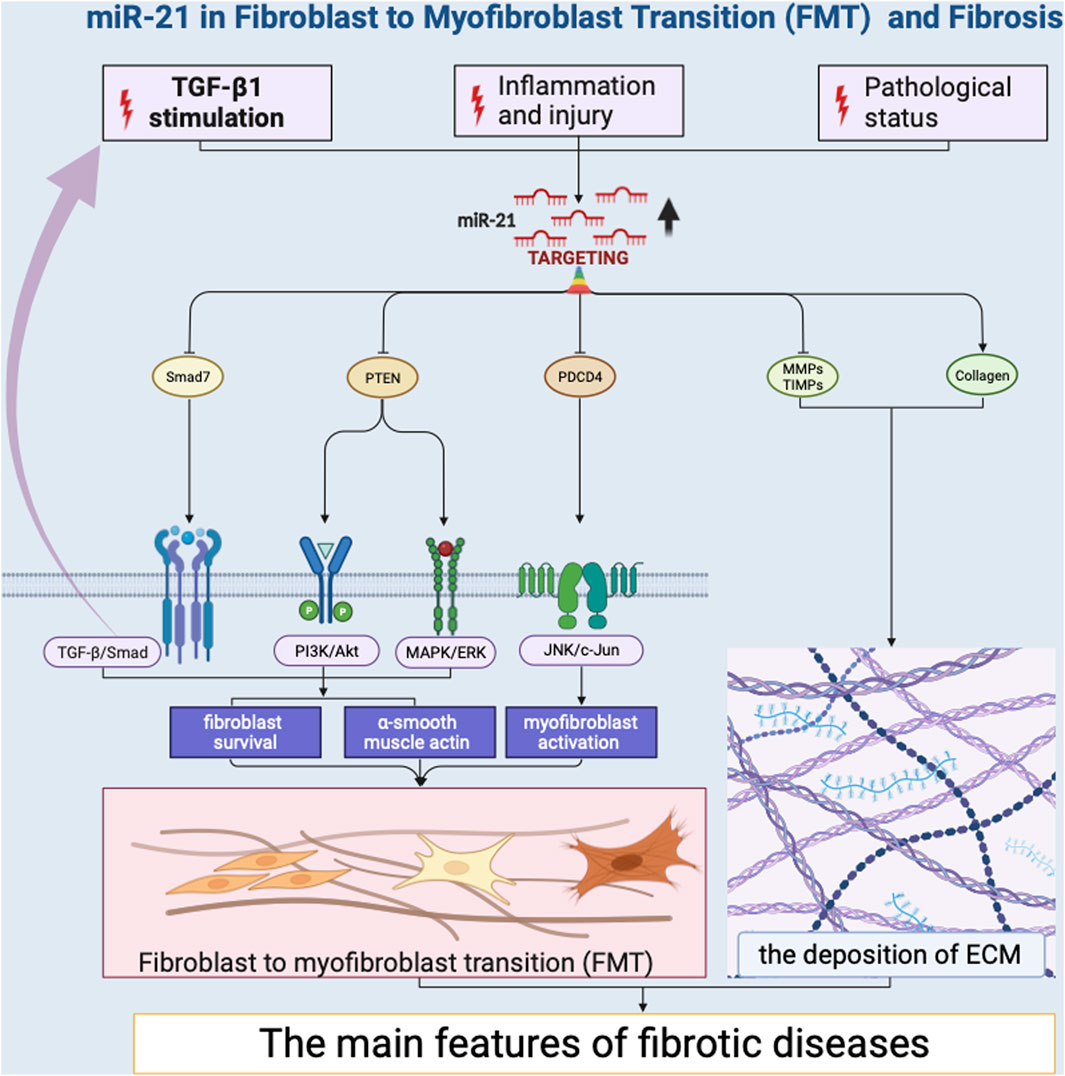
Several miRNAs have been identified as promoters of FMT by targeting key regulators of the transition. Notably, miR-21 has emerged as a potent inducer of FMT (Liu et al., 2010; Yao et al., 2011; Liang et al., 2012; Wang et al., 2012; Bullock et al., 2013; Glowacki et al., 2013; Gong et al., 2014; Hedbäck et al., 2014; Lorenzen et al., 2015; Cui et al., 2018; Xu et al., 2018; Li et al., 2019; Kilari et al., 2019; Schipper et al., 2020; Wang et al., 2021; Nonaka et al., 2021; Ramanujam et al., 2021; Yang et al., 2021; Liao et al., 2022) (Figure 2). Its impact on FMT is primarily mediated through its ability to regulate the transforming growth factor-beta (TGF-β) signaling pathway (Liu et al., 2010; Yao et al., 2011; Liang et al., 2012; Cui et al., 2018; Nonaka et al., 2021; Yang et al., 2021). TGF-β is a pivotal cytokine that plays a central role in fibrotic processes (Fernandez and Eickelberg, 2012; Yousefi et al., 2020). MiR-21 achieves this regulatory effect by targeting TGF-β receptor inhibitors, leading to their downregulation. This downregulation results in an increased responsiveness of fibroblasts to TGF-β signaling, effectively priming them for myofibroblast differentiation. MiR-21 also promotes the expression of various extracellular matrix (ECM) components, such as collagens (Liang et al., 2012; Cui et al., 2018; Nonaka et al., 2021) and fibronectin (Cui et al., 2018), thereby contributing to the phenotypic shift of fibroblasts into myofibroblasts. This induction of ECM components strengthens the fibrotic matrix, leading to tissue remodeling and fibrosis development. Additionally, miR-146b have been shown to facilitate FMT by targeting interleukin 1 receptor-associated kinase 1 (IRAK1) and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) that inhibit myofibroblast activation (Liao et al., 2021). The research revealed that miR-146b led to increased proliferation and migration of fibroblasts, the conversion of fibroblasts into myofibroblasts, and disrupted signaling among macrophages. Likewise, miR-125b contributes to FMT by downregulating apelin that would otherwise repress the activation of fibroblasts into myofibroblasts (Nagpal et al., 2016). This downregulation effectively removes barriers that restrain the transition process, resulting in enhanced myofibroblast formation. Collectively, these miRNAs exemplify the intricate regulatory landscape of FMT. Their effects extend beyond singular pathways, intertwining with the TGF-β, WNT, and PI3K/AKT signaling pathways (Zhang et al., 2023), ultimately driving the progression of fibrosis.
Conversely, certain miRNAs act as suppressors of FMT. These miRNAs play a crucial role in counteracting the signals and factors that drive fibroblasts towards myofibroblast differentiation, ultimately contributing to the maintenance of tissue homeostasis and preventing excessive fibrosis. The miR-29 family stands out as a group of miRNAs that counteract FMT by targeting collagen synthesis and deposition, essential processes in fibrosis (Kwiecinski et al., 2011; Wang et al., 2021; Yu et al., 2021; Yang et al., 2022; Xi et al., 2023). miR-29 directly targets and downregulates the expression of various collagens, including collagen type I (Yu et al., 2021), III (Wang et al., 2019), and IV (Kwiecinski et al., 2011), as well as other extracellular matrix components (Zhang et al., 2023). This regulatory mechanism orchestrated by miR-29 efficiently dampens the excessive accumulation of collagen fibers, which is a hallmark of fibrotic tissue remodeling. By inhibiting collagen production, miR-29 acts as a protection against the pathological transformation of fibroblasts into myofibroblasts, thus preventing the progression of fibrosis. Moreover, the miR-200 family members counteract FMT by targeting transcription factors ZEB1 (Bhome et al., 2022) and ZEB2 (Liao et al., 2018), which are integral to the epithelial-mesenchymal transition. By inhibiting ZEB1 and ZEB2 expression, miR-200 miRNAs effectively impede the transition of fibroblasts into myofibroblasts, contributing to the maintenance of the fibroblast phenotype and preventing fibrotic tissue remodeling. Similarly, miR-214 plays a role in inhibiting FMT by targeting factors that repress the activation of myofibroblasts (Izawa et al., 2015; Zhu et al., 2016; Yang et al., 2019). By suppressing these inhibitory elements, miR-214 helps tilt the balance in favor of myofibroblast differentiation. In brief, the balanced interplay between miRNAs that promote and those that suppress fibroblast to myofibroblast transition is crucial for maintaining tissue integrity and preventing pathological fibrosis. The opposing actions of these miRNAs create a finely tuned regulatory network that governs the dynamic equilibrium between fibroblasts and myofibroblasts.
In fibrotic conditions, miRNAs undergo various modifications that affect their expression and function. These modifications include changes in miRNA transcription, processing, and stability. Fibrotic signals such as TGF-β can induce or repress the transcription of specific miRNAs (Selman et al., 2016). Additionally, alterations in miRNA processing enzymes, such as Drosha and Dicer, can impact miRNA maturation and stability (Mishra et al., 2009; Cho et al., 2020). Epigenetic modifications, including DNA methylation and histone modifications, also play a role in regulating miRNA expression in fibrotic tissues (Yang et al., 2015). These mechanisms collectively contribute to the dysregulation of miRNAs in fibrosis, influencing their ability to modulate gene expression during FMT.
The regulatory roles of miRNAs in FMT are far from linear, as many miRNAs participate in intricate regulatory networks. MiRNAs often target multiple genes and pathways simultaneously, influencing the balance between pro-fibrotic and anti-fibrotic processes. This phenomenon allows miRNAs to fine-tune the overall outcome of FMT by modulating the expression of various genes that are involved in different stages of the transition. One key feature of miRNA-mediated regulation in FMT is the concurrent targeting of multiple genes within the same or related signaling pathways (Kwiecinski et al., 2011; Gong et al., 2014; Lorenzen et al., 2015; Nagpal et al., 2016; Wang et al., 2021; Medzikovic et al., 2023). This results in a synergistic impact on the cellular processes associated with FMT. This multi-targeting capacity enables miRNAs to exert a more potent and coordinated influence on FMT compared to a linear one-to-one relationship between miRNA and target gene. Cross-talk between miRNAs and other non-coding RNAs, such as lncRNAs(Li et al., 2018b; Wang et al., 2019) and circRNAs(Zhang et al., 2020; Ma et al., 2023), further complicates the regulatory landscape. The interplay between miRNAs, target genes, and other ncRNAs collectively constitutes a systems-level regulatory network that governs FMT. This network-based perspective highlights the interconnectedness and interdependence of various components in shaping the outcome of FMT.
MiRNAs play pivotal roles in orchestrating fibroblast to myofibroblast transition. Their dual nature as promoters and inhibitors of FMT underscores their complex regulatory functions in fibrosis. As our understanding of the roles of miRNAs in FMT continues to evolve, the prospects for innovative therapeutic strategies in fibrotic diseases become increasingly promising. The ability to manipulate miRNAs to finely tune the fibrotic response offers a level of precision that was previously unimaginable.
3 Long non-coding RNAs (lncRNAs) in FMT
Long Non-Coding RNAs (lncRNAs) constitute a diverse group of RNA molecules exceeding 200 nucleotides in length that lack protein-coding capacity but exert critical regulatory roles across various cellular processes. Within the intricate processes of FMT, a recent focus has emerged on lncRNAs as key regulatory elements. These lncRNAs establish their presence within the framework of FMT by orchestrating complex molecular interactions. They serve as regulators, directing the delicate interplay among chromatin modifiers, transcription factors, and a competing endogenous RNA (ceRNA) that govern gene expression patterns critical to FMT. These orchestrated activities assume a crucial role in the transformation of fibroblasts into myofibroblasts, a pivotal event in the development of tissue fibrosis. A diverse group of lncRNAs, including notable examples such as MALAT1 (Wu et al., 2015), H19X (Pachera et al., 2020), ZFAS1 (Yang et al., 2020), and SAFE (Hao et al., 2019), have garnered attention for their role as promoters of myofibroblast differentiation. Their contributions add a novel layer of regulatory intricacy to the evolving narrative surrounding FMT.
lncRNAs have emerged as key regulators of chromatin remodeling in the process of myofibroblast differentiation. These lncRNAs act as guides, directing chromatin modifiers to specific genomic loci that are strategically poised to undergo transformation. Through their interaction with chromatin-modifying complexes, these lncRNAs initiate a cascade of epigenetic changes that play a central role in the activation of genes critical for FMT. For example, HOTAIR and H19X have important effects on chromatin. Their strategic interaction with chromatin modifiers, including histone methyltransferases (Wasson et al., 2020b; Wang et al., 2023) and chromatin accessibility (Pachera et al., 2020), initiates the unwinding of the tightly packed chromatin structure. This allows for increased accessibility of transcription factors, such as GLI2, and other regulatory molecules to the gene promoters that drive myofibroblast differentiation. As chromatin remodeling takes place under the guidance of these lncRNAs, a series of events unfold that culminate in the activation of genes pivotal to FMT. These activated genes include those encoding extracellular matrix components, cytoskeletal proteins, and signaling molecules that are characteristic of the myofibroblast phenotype. The orchestrated chromatin changes initiated by lncRNAs lead to the establishment of a permissive transcriptional environment that favors the expression of genes essential for myofibroblast differentiation.
Through their intricate interplay with transcription factors smad, lncRNAs wield significant influence over the gene expression landscape that guides fibroblasts through the intricate process of myofibroblast differentiation. In zheng’s study (Zheng et al., 2019), Smad3 activated the expression of Crnde, revealing insights into the molecular process. Intriguingly, Crnde also suppressed Smad3’s transcriptional activation of target genes, thus blocking the expression of myofibroblast-specific marker genes in cardiac fibroblasts. Lin’s research demonstrated that GAS5-AS1 levels were significantly reduced in oral submucous fibrosis tissues and fibrotic buccal mucosal fibroblasts (Lin et al., 2018). Furthermore, increasing GAS5-AS1 expression led to inhibition of both p-Smad expression and myofibroblast markers. Their presence ensures the coordination, precision, and fidelity of gene expression programs essential for driving FMT. By functioning as transcriptional regulators, lncRNAs contribute to orchestrating a complex series of molecular events culminating in the acquisition of the myofibroblast phenotype.
In the realm of gene expression regulation, transcription factors assume the role of master regulators, directing the intricate sequence of molecular events that govern cellular differentiation. However, this role is not undertaken in isolation. lncRNAs serve as adept collaborators, guiding the transformative process of FMT. LncRNAs emerge as crucial co-regulators in this complex transcriptional symphony, intricately woven into the regulatory landscape to ensure the precise execution of gene expression programs that steer fibroblasts along the path of myofibroblast differentiation. Through specific interactions with transcription factors, they play a role beyond conventional transcriptional regulation. LINC00941 act as co-regulators, interacting transcription factors ATF3 and histone 3 lysine 27 acetylation to play its pro-fibrotic role (Zhang et al., 2022). This coordinated collaboration guarantees the timely and accurate activation of the genes necessary for driving the transformation of fibroblasts into myofibroblasts.
Furthermore, the co-regulator role of lncRNAs extends beyond mere guidance; LncRNA Airn actively participate in modulating the development of cardiac fibrosis via IMP2-p53 axis in an m6A dependent manner (Peng et al., 2022). Serving as molecular scaffolds, lncRNA H19X create a conducive environment for the assembly of complexes, thereby influencing the accessibility of target gene enhancer (Pachera et al., 2020). This interaction fine-tunes transcriptional activity, either amplifying or attenuating the expression of genes involved in FMT.
The incorporation of lncRNAs into the narrative of FMT introduces a fresh and intricate layer to the multifaceted story of fibrosis. These elusive molecules, previously overshadowed by protein-coding genes, now emerge as critical protagonists, orchestrating the delicate balance between fibroblast quiescence and the transformative process into myofibroblasts. By seamlessly integrating themselves into the complex molecular choreography of FMT, lncRNAs exert their influence in previously unforeseen ways. Their roles as guides, regulators, and network architects has provided new insights into our understanding of fibrosis, suggesting their potential as therapeutic targets for the benefit of patients afflicted with fibrotic conditions.
4 Circular RNAs (circRNAs) in FMT
Circular RNAs (circRNAs) are a class of non-coding RNAs (ncRNAs) characterized by their covalently closed loop structure. Unlike linear RNAs, circRNAs lack 5′caps and 3′polyadenylated tails, making them resistant to exonucleases. This unique structure imparts remarkable stability, allowing circRNAs to persist longer in cells compared to their linear counterparts (Kristensen et al., 2019). These stable molecules are involved in various cellular processes by acting as miRNA sponges, interacting with RNA-binding proteins, and influencing gene expression. In the intricate landscape of FMT, circRNAs have emerged as pivotal players, wielding their regulatory influence through multifaceted mechanisms that are now elucidated by recent studies.
One of the prominent roles that circRNAs play in FMT is that of miRNA sponges, implying that circRNAs have sequences that can bind to and interact with miRNAs, preventing them from carrying out their usual regulatory functions on other messenger RNAs. CircRNAs possess a remarkable ability to sequester miRNAs, small regulatory RNAs that modulate gene expression by binding to mRNA targets and suppressing their translation or promoting their degradation (Patop et al., 2019). By acting as miRNA sponges, circRNAs effectively titrate miRNAs away from their mRNA targets, thus preventing their inhibitory effects. This intricate regulation allows circRNAs to regulate gene expression programs that are crucial for FMT (Zhu et al., 2019; Hu et al., 2022; Zou et al., 2023). Notably, circRNAs like circHIPK3 have been identified as potent regulators of FMT-associated genes (Zhang et al., 2019). By binding to miR-338-3p, circHIPK3 prevents the miR-338-3p from interacting with their intended mRNA targets. As a result, the expression of target gene SOX4 and COL1A1, is spared from miRNA-mediated suppression, leading to the enhancement of fibroblast activation. This mechanism underscores the pivotal role circRNAs play in modulating gene expression patterns that drive the transition of fibroblasts into myofibroblasts.
Beyond their role as miRNA sponges, circRNAs also interact with RNA-binding proteins, adding another layer of complexity to their regulatory functions. For example, Circ-sh3rf3 (circular RNA SH3 domain containing Ring Finger 3) interacts with RNA-binding protein GATA-4 to promote the expression of miR-29a, thereby inhibiting FMT and myocardial fibrosis (Ma et al., 2023). These interactions can impact RNA stability, localization, and translation, further expanding the repertoire of mechanisms through which circRNAs influence FMT. Through their interactions with both miRNAs and RNA-binding proteins, circRNAs wield a dynamic and multifaceted influence on the regulatory networks that govern FMT.
Recent studies have also shed light on circRNAs’ role in modulating signaling pathways critical for FMT. CircTTN, for instance, has been implicated in the PI3K/AKT pathway, a key signaling cascade in myofibroblast differentiation. By sponging miR-432, circTTN regulates the expression of genes like IGF2, thereby influencing the activation of the PI3K/AKT signaling pathway (Wang et al., 2019). This regulation demonstrates how circRNAs can modulate specific signaling pathways, affecting the cellular transitions in fibrosis.
Furthermore, circRNAs like circ004463 have been found to interact with AKT/ERK pathways. Circ004463 sponges miR-23b, which targets the mRNA of AKT and ERK. By regulating CADM3 and MAP4K4 expression, circ004463 plays a significant role in promoting fibroblast proliferation and collagen type I synthesis (Zou et al., 2023). Another notable circRNA is hsa_circ_0020792, which acts as a sponge for miR-193a-5p, thereby regulating the expression of pro-fibrotic genes such as TGF-β1 (Hu et al., 2022). This interaction is crucial in the context of fibrosis, as TGF-β1 is a key cytokine driving fibrogenesis, and collagen type I is a major component of the extracellular matrix.
The intricate regulatory function of circRNAs within the context of FMT suggests their significance in shaping cell fate. Their capacity to sponge miRNAs and interact with RNA-binding proteins underscores their ability to modulate gene expression programs, thus determining whether fibroblasts remain in their quiescent state or transition into myofibroblasts. As ongoing research unravels the intricacies of these regulatory mechanisms, circRNAs hold the promise of becoming not only diagnostic markers but also potential therapeutic targets for mitigating the progression of fibrotic diseases.
5 Role of non-coding RNAs (ncRNAs) in fibrotic diseases
Recent research reveals a substantial exploration into the contribution of dysregulated ncRNAs to the intricate landscape of pathological fibrosis. These ncRNAs have emerged as critical players in driving the development and progression of fibrotic diseases across diverse tissues. MiRNAs exhibit a multifaceted role in pathological fibrosis. Pro-fibrotic miRNAs, exemplified by miR-21, facilitate fibrosis by augmenting fibroblast responsiveness to profibrotic stimuli and promoting extracellular matrix deposition. Conversely, anti-fibrotic miRNAs like miR-133a (Wei et al., 2019), counteract fibrosis by targeting multiple components of TGF-β1 profibrogenic pathways. LncRNAs exert significant influence on pathological fibrosis. Pro-fibrotic lncRNAs such as HOTAIR and H19X contribute to myofibroblast differentiation by engaging with chromatin modifiers, transcription factors, and regulatory molecules. This interaction modulates gene expression profiles and drives fibroblasts towards the myofibroblast phenotype. In contrast, certain lncRNAs such as PFI (Sun et al., 2021) and LOC344887 (Liu et al., 2021) act as suppressors of fibrosis, impeding myofibroblast activation and promoting tissue equilibrium. CircRNAs, with their circular structure, introduce an additional layer of complexity to the fibrotic scenario. Operating as miRNA sponges and interacting with RNA-binding proteins, circRNAs regulate gene expression patterns with precision. CircRNAs like circHIPK3 exemplify this role by sequestering miRNAs targeting key genes involved in fibrotic processes, thereby modulating gene expression profiles that underpin fibrosis. In summary, prior studies underscore the integral roles of dysregulated ncRNAs in driving pathological fibrosis. These ncRNAs impact the equilibrium between fibroblast activation and tissue health.
The formation and expression of ncRNAs are tightly regulated processes that are often altered during disease conditions. ncRNAs are transcribed by RNA polymerase II and III, and their maturation involves complex processing steps, including splicing, editing, and modifications. For example, primary miRNAs (pri-miRNAs) are processed by Drosha and Dicer enzymes to generate mature miRNAs that can bind to target mRNAs (Herrera et al., 2018; Cho et al., 2020). Similarly, lncRNAs undergo splicing and modifications that influence their stability and function (Hao et al., 2019). The expression of ncRNAs is tightly regulated under normal conditions but can become dysregulated during fibrosis. This dysregulation plays a crucial role in the pathological progression of fibrosis by affecting the balance between fibroblast quiescence and myofibroblast activation. In kidney fibrosis, the upregulation of miR-21 correlates with increased kidney stiffness and fibrosis severity, indicating its role in disease progression (Glowacki et al., 2013). Similarly, reduced levels of miR-449a are observed in fibrotic lung tissues and correlate with the severity of lung lesions induced by silica, suggesting its involvement in the Silicosis (Han et al., 2016). Understanding the correlation between ncRNA expression and fibrosis progression provides valuable insights into the molecular mechanisms underlying fibrotic diseases. These insights highlight the potential of ncRNAs as biomarkers for disease diagnosis and prognosis and as therapeutic targets for modulating fibrotic processes and restoring tissue homeostasis.
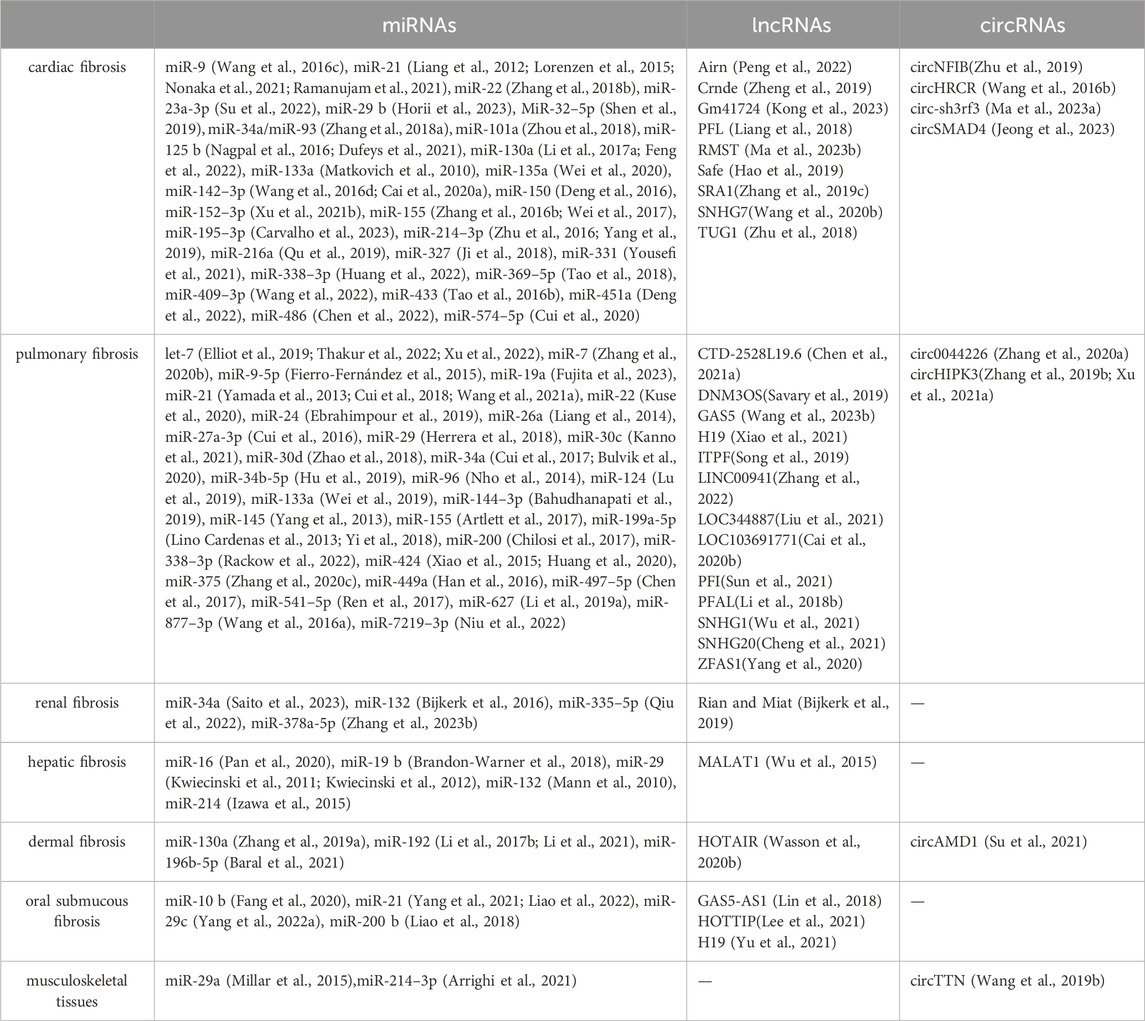
Notably, ncRNAs exhibit their multifaceted roles across a diverse spectrum of fibrotic conditions, ranging from cardiac fibrosis, hepatic fibrosis, pulmonary fibrosis, renal fibrosis, dermal fibrosis, and musculoskeletal tissues (Table 1). This broad influence underscores the significance of ncRNAs as central regulators of fibrotic processes across diverse tissues and organs. In particular, arthrofibrosis is a common and debilitating complication that can occur following knee surgery (Lee et al., 2022). Abdel et al. have identified differentially expressed genes associated with arthrofibrosis by comparing tissue samples from fibrotic and non-fibrotic human knee joints using RNA sequencing (Bayram et al., 2020). Further, Chen et al. carried out further bioinformatics analysis and reported new biomarkers for diagnosing arthrofibrosis, shedding light on the role of transforming growth factor-beta receptor 1 (TGFBR1) (Chen et al., 2021). These data provide further insight into the role of ncRNAs in the regulation of joint fibrosis.
ncRNAs exhibit both ubiquitous and tissue-specific functions, which together shape the initiation and progression of fibrosis. Ubiquitous ncRNAs, such as miR-21, are widely expressed across different tissues and play a central role in fibrosis by modulating common fibrogenic pathways. miR-21 enhances fibroblast activation and extracellular matrix deposition by targeting multiple genes involved in the TGF-β signaling pathway, including SMAD7 and PTEN, thus promoting fibrosis in various organs (Glowacki et al., 2013; Li et al., 2019; Wang et al., 2021; Nonaka et al., 2021; Liao et al., 2022). In contrast, tissue-specific ncRNAs are expressed in particular organs and contribute to localized fibrotic processes. For instance, lncRNA MALAT1 is prominently expressed in the liver and contributes to hepatic fibrosis by interacting with the silent information regulator 1(SIRT1) and promoting the expression of pro-fibrotic genes such as COL1A1 and α-SMA (Wu et al., 2015). Similarly, circNFIB is predominantly expressed in the heart and, where it activates the TGF-β–Smad3 signaling pathway and is crucial in cardiac fibrosis (Zhu et al., 2019).
ncRNAs can exert paracrine effects, influencing cells beyond their origin and contributing to multi-organ fibrosis. These ncRNAs can be secreted into the extracellular environment and transported to distant cells and tissues through extracellular vesicles (EVs), such as exosomes and microvesicles. This capability allows ncRNAs to participate in intercellular communication and influence various physiological and pathological processes across different organs.
In the context of fibrosis, ncRNAs can be secreted by fibroblasts or other cell types and taken up by neighboring cells, thereby modulating their behavior. For example, miR-21, a well-known pro-fibrotic miRNA, can be packaged into EVs and transferred from myofibroblasts to adjacent endothelial cells. This transfer can induce a pro-angiogenic process of endothelial cells, a process contributing to the fibrotic response (Li et al., 2019). Similarly, miR-200, another miRNA implicated in fibrosis, can be secreted by endothelial cells and taken up by fibroblasts, influencing fibroblast heterogeneity in colorectal cancer (Bhome et al., 2022).
NcRNAs can enter the systemic circulation, allowing them to travel to distant organs and exert their effects. Circulating miRNAs, for instance, have been detected in blood, urine, and other body fluids, serving as biomarkers for various diseases (De Guire et al., 2013). These circulating ncRNAs extend their impact beyond the local tissue environment, affecting distant organs and contributing to the pathology of multi-organ diseases. For instance, miR-29, which regulates extracellular matrix production, is involved in cutaneous, prostate, cardiac and oral submucous fibrosis. Its dysregulation in one organ can have implications for fibrotic processes in others.
LncRNAs also exhibit multi-organ effects. LncRNA H19, known for its role in pulmonary fibrosis, can influence fibrotic buccal mucosal myofibroblast activities, such as collagen gel contractility and migration ability when dysregulated, highlighting its potential impact on both pulmonary and oral submucous tissues. Similarly, the lncRNA GAS5, which modulates fibrotic pathways in the skin, can have systemic effects, potentially affecting other fibrotic conditions in organs like the lung.
Understanding the paracrine and multi-organ effects of ncRNAs is crucial for developing therapeutic strategies targeting fibrotic diseases. Therapies designed to modulate ncRNA levels in one organ might have beneficial effects on fibrosis in other organs, offering a systemic approach to treating multi-organ fibrotic conditions. For example, therapeutic inhibition of miR-21 has shown promise in reducing fibrosis in both the heart and lung, demonstrating the potential of ncRNA-targeted therapies to address multi-organ fibrosis.
ncRNAs may have distinct impacts on acute versus chronic diseases, reflecting their roles in immediate injury responses versus long-term maladaptive processes. During acute injury, the rapid and transient changes in ncRNA expression are crucial for the immediate response to cellular damage and stress. For instance, miR-101a is rapidly upregulated following myocardial infarction (MI) and plays a critical role in promoting cardiac fibroblast activation and fibrosis to stabilize the injured tissue (Zhou et al., 2018). In contrast, chronic conditions and aging involve sustained ncRNA dysregulation, contributing to persistent fibrosis and organ dysfunction. For example, miR-34A is consistently dysregulated in chronic liver and renal fibrosis, leading to sustained extracellular matrix production and fibrogenesis (Cui et al., 2017; Saito et al., 2023). Understanding the distinct roles of ncRNAs in acute and chronic conditions can inform the development of targeted therapies. In acute injury, therapeutic strategies may aim to modulate ncRNAs to enhance tissue repair and limit damage. In chronic diseases and aging, ncRNA-based therapies could focus on reversing maladaptive gene expression patterns and reducing fibrosis and inflammation.
In short, the regulatory influence of various ncRNAs extends across diverse fibrotic diseases. The pervasive presence of these ncRNAs within the fibrotic milieu underscores the need for a comprehensive understanding of their intricate functions. Unraveling the precise molecular mechanisms through which ncRNAs exert their regulatory effects could pave the way for the development of targeted therapeutic strategies. By targeting these ncRNAs or modulating their interactions with key regulatory molecules, it might be possible to attenuate fibrosis progression and restore tissue homeostasis in a range of fibrotic diseases.
6 Therapeutic implications
The intricate involvement of ncRNAs in FMT has opened new avenues for therapeutic interventions in fibrotic diseases. These regulatory molecules have been identified as critical players in fine-tuning gene expression programs that govern the delicate balance between fibroblast quiescence and myofibroblast activation. By deciphering the precise roles of ncRNAs in regulating this transition, researchers have uncovered potential targets that could be manipulated to mitigate the excessive activation of myofibroblasts and slow the progression of fibrosis.
Therapeutic strategies involving miRNAs typically include miRNA mimics to restore the function of downregulated miRNAs or miRNA antagonists (antagomirs) to inhibit the function of upregulated miRNAs. For instance, MiR-29 family mimics exhibit antifibrotic effects across various tissues by targeting collagen synthesis and extracellular matrix remodeling. A completed open-label phase 2 RCT clinical trial (Clinical Trial Number: NCT03601052) has defined the efficacy, safety, and tolerability of Remlarsen (MRG-201), which is designed to mimic the activity of miR-29 that may be an effective therapeutic to prevent cutaneous fibrosis. This study demonstrated that administering high doses of this miR-29 mimic could effectively decrease fibrosis (Gallant-Behm et al., 2019). It is worth noting that the dosage utilized in this research was excessively high for practical use in human patients. Nevertheless, these findings provided encouraging evidence for investigators working towards the development of microRNA mimics as potential therapeutics for fibrosis. Anti-miR oligonucleotides, designed to inhibit the function of pro-fibrotic miRNAs, also show potential; for example, targeting miR-21, a pro-fibrotic miRNA, has shown promise in reducing fibrosis in preclinical models. Anti-miR-21 therapies aim to decrease fibroblast responsiveness to pro-fibrotic stimuli and reduce extracellular matrix deposition.
LncRNA-based therapeutics involve targeting pro-fibrotic lncRNAs, such as ASLNCS5088 (Chen et al., 2019) and Gm41724 (Kong et al., 2023), to mitigate fibrosis by disrupting their interactions with RNA-binding proteins, and M2 macrophage modulation. By preventing these interactions, it is possible to modulate gene expression profiles that drive fibroblast activation and myofibroblast differentiation. Additionally, boosting the expression of anti-fibrotic lncRNAs like GAS5 can help inhibit myofibroblast activation and fibrogenesis through suppressing TGF-β/Smad3 signaling (Tang et al., 2020). Therapeutic strategies may involve gene therapy approaches to deliver these lncRNAs or small molecules that enhance their endogenous expression.
CircRNA-based therapeutics focus on the unique abilities of circRNAs to act as miRNA sponges or interact with RNA-binding proteins. CircHIPK3 serves as a prime example, as it influences myofibroblast differentiation by sponging miR-338-3p that target SOX4 and COL1A1 (Zhang et al., 2019). Designing synthetic circRNA sponges can regulate miRNA activity and modulate gene expression patterns involved in fibrosis. Additionally, modulating circRNA-protein interactions can impact the regulatory networks driving fibrosis. For example, Circ-sh3rf3 can bind to GATA-4 proteins and decrease their expression, which prevents GATA-4 from suppressing miR-29a expression. As a result, miR-29a expression is increased, leading to the inhibition of fibroblast-to-myofibroblast differentiation and myocardial fibrosis. Targeting these ncRNAs might offer a means to disrupt the regulatory networks that drive fibroblast activation. Such precision-based approaches could revolutionize the treatment landscape for fibrotic diseases, allowing for tailored interventions that target the underlying molecular mechanisms.
While the potential of ncRNA-based therapies for fibrosis is exciting, several challenges must be navigated for successful translation into clinical applications. One significant hurdle is the delivery of ncRNA-based therapeutics to target tissues. Ensuring efficient and specific delivery remains a key obstacle. Strategies such as viral vectors (Tang et al., 2020), nanoparticle-mediated delivery (Zahir-Jouzdani et al., 2018), or organ-targeted liposomes (Yan et al., 2023) are being explored to address this challenge. Additionally, the specificity of ncRNA-targeting therapies is crucial to avoid off-target effects and unintended consequences (Yan et al., 2023). Ensuring that therapies selectively target the dysregulated ncRNAs while preserving the physiological functions of others is essential for clinical success. The stability and bioavailability of ncRNA-based therapeutics are critical factors for their effectiveness. Chemical modifications, such as locked nucleic acids (LNAs) and phosphorothioate backbones, can enhance the stability and resistance of ncRNA-based therapeutics to degradation. These modifications improve the pharmacokinetic properties and therapeutic efficacy of ncRNA-based treatments (Ali Zaidi et al., 2023). ncRNA-based therapeutics, particularly those involving viral vectors, may elicit immune responses. Strategies to minimize immunogenicity include optimizing vector design, using tissue-specific promoters, and developing non-viral delivery systems (Awan et al., 2017). Furthermore, the complex regulatory networks involving ncRNAs add another layer of complexity. Many ncRNAs participate in intricate crosstalk with other regulatory molecules, such as transcription factors and signaling pathways, leading to a network of interdependencies. Designing therapies that effectively modulate these networks requires a deep understanding of the molecular interactions and their consequences.
Future directions and prospects in ncRNA-based therapies for fibrotic diseases include combination therapies, personalized medicine, advancements in delivery systems, and robust translational research efforts. Combining ncRNA-based therapies with existing antifibrotic drugs or other therapeutic modalities may enhance efficacy and overcome resistance mechanisms. Personalized approaches tailored to individual patients’ specific ncRNA expression profiles can improve treatment outcomes and minimize adverse effects. Ongoing advancements in delivery systems, such as exosome-based delivery and tissue-specific nanoparticles, hold promise for improving the targeted delivery of ncRNA-based therapeutics. Collaborative efforts between academia, industry, and regulatory agencies can accelerate the development and approval of ncRNA-based therapies.
The emerging roles of ncRNAs in FMT offer novel avenues for therapeutic intervention in fibrotic diseases. By targeting specific ncRNAs, it is possible to intervene in the processes that drive myofibroblast activation and tissue fibrosis. However, the journey from bench to bedside requires the successful resolution of delivery challenges, the mitigation of off-target effects, and in depth understanding of the complex regulatory networks involved. As research in this field advances, the development of effective and precise therapies holds the promise of transforming the landscape of fibrotic disease treatment.
7 Conclusion
In summary, recent research highlights the crucial involvement of ncRNAs in the complex process of FMT. These ncRNAs, including miRNAs, lncRNAs, and circRNAs, collectively constitute a regulatory ensemble that finely modulates the equilibrium between quiescent fibroblasts and their activated myofibroblast counterparts. Recent studies have meticulously unraveled the multifaceted mechanisms by which these ncRNAs exert their influence.
The narrative begins with miRNAs, which play a central role by targeting key regulators of FMT. MiR-21 assumes a prominent position as a potent inducer of FMT, primarily by inhibiting TGF-β receptor inhibitors. This action sensitizes fibroblasts to TGF-β signaling, thereby promoting myofibroblast differentiation and subsequent fibrosis. MiR-146b and miR-125b also contribute to FMT by targeting factors that otherwise restrain myofibroblast activation. Conversely, miRNAs such as miR-29 and the miR-200 family act as suppressors of FMT, counteracting excessive collagen synthesis and inhibiting myofibroblast differentiation through their targeting of related genes.
LncRNAs act as pivotal regulators of FMT. Notable lncRNAs like H19X and GAS5 emerge as regulators in the FMT process. They engage with chromatin modifiers, transcription factors, and regulatory molecules, facilitating chromatin remodeling, reprogramming of gene expression, and the orchestration of transcriptional forces that guide fibroblasts toward the myofibroblast lineage. LncRNAs further their influence by fostering crosstalk among regulatory molecules, perpetuating essential signaling cascades crucial for FMT progression.
Simultaneously, circRNAs embrace their role as miRNA sponges, intricately fine-tuning gene expression during FMT. Notable circRNAs like circHIPK3 demonstrate their ability to sequester miRNAs targeting genes associated with FMT. In doing so, these circRNAs release these genes from miRNA-mediated suppression, ultimately enhancing the differentiation of fibroblasts into myofibroblasts. Moreover, the intricate interactions of circRNAs with RNA-binding proteins add an additional layer of complexity to their regulatory repertoire.
In a broader context, these ncRNAs collaboratively interweave their actions, constructing a complex network of regulatory interactions that modulate the transformation of fibroblasts into myofibroblasts. Their contributions extend beyond individual roles, creating a dynamic interplay that profoundly influences the delicate equilibrium between fibroblast quiescence and myofibroblast activation. Dysregulation of these ncRNAs has been closely linked to the development of pathological fibrosis in various tissues, underscoring their significance as potential therapeutic targets.
As we stand on the cusp of a new era in the treatment of fibrotic diseases, the emerging roles of ncRNAs in FMT offer substantial therapeutic promise. By deciphering the intricacies of ncRNA-mediated regulatory networks, researchers could uncover innovative therapeutic avenues that could effectively counteract the progression of fibrotic diseases. However, translating these insights into clinical applications presents challenges such as efficient delivery methods, specificity, and the potential for off-target effects. As the journey continues, the potential to harness the power of ncRNAs may illuminate a path toward restoring tissue health and function, offering renewed hope to those affected by these debilitating conditions.
In conclusion, the process of FMT occupies a central role in tissue repair and the pathogenesis of fibrotic diseases. The intricate interplay of cellular morphological changes, altered gene expression profiles, and extracellular matrix remodeling underscores its significance. With recent discoveries revealing the pivotal roles of ncRNAs, including miRNAs, lncRNAs, and circRNAs, in orchestrating FMT, a new chapter has opened in our understanding of tissue remodeling. These ncRNAs act as master regulators, shaping the symphony of FMT by influencing a diverse array of molecular players. Their regulatory capabilities extend across signaling cascades, transcriptional programs, and intricate interactions, and their dysregulation can lead to pathological fibrosis. As research continues to elucidate the precise mechanisms by which ncRNAs guide FMT, their therapeutic potential emerges as a promising frontier, offering novel strategies to combat fibrotic diseases and restore tissue health.
Author contributions
XX: Writing–original draft. SR: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by by grants from Tianjin biomedical science and technology major special project (No. 21ZXJBSY00070), Tianjin Applied basic Research project (No. 21JCYBJC00130).
Acknowledgments
Thanks to the researchers who published relevant papers. We would like to thank the academic editor and reviewers for their important contributions that improved the quality of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali Zaidi, S. S., Fatima, F., Ali Zaidi, S. A., Zhou, D., Deng, W., and Liu, S. (2023). Engineering siRNA therapeutics: challenges and strategies. J. Nanobiotechnology 21 (1), 381. doi:10.1186/s12951-023-02147-z
Arrighi, N., Moratal, C., Savary, G., Fassy, J., Nottet, N., Pons, N., et al. (2021). The FibromiR miR-214-3p is upregulated in duchenne muscular dystrophy and promotes differentiation of human fibro-adipogenic muscle progenitors. Cells 10 (7), 1832. doi:10.3390/cells10071832
Artlett, C. M., Sassi-Gaha, S., Hope, J. L., Feghali-Bostwick, C. A., and Katsikis, P. D. (2017). Mir-155 is overexpressed in systemic sclerosis fibroblasts and is required for NLRP3 inflammasome-mediated collagen synthesis during fibrosis. Arthritis Res. Ther. 19 (1), 144. doi:10.1186/s13075-017-1331-z
Awan, H. M., Shah, A., Rashid, F., and Shan, G. (2017). Primate-specific long non-coding RNAs and MicroRNAs. Genomics Proteomics Bioinforma. 15 (3), 187–195. doi:10.1016/j.gpb.2017.04.002
Bahudhanapati, H., Tan, J., Dutta, J. A., Strock, S. B., Sembrat, J., Àlvarez, D., et al. (2019). MicroRNA-144-3p targets relaxin/insulin-like family peptide receptor 1 (RXFP1) expression in lung fibroblasts from patients with idiopathic pulmonary fibrosis. J. Biol. Chem. 294 (13), 5008–5022. doi:10.1074/jbc.RA118.004910
Baral, H., Uchiyama, A., Yokoyama, Y., Sekiguchi, A., Yamazaki, S., Amalia, S. N., et al. (2021). Antifibrotic effects and mechanisms of mesenchymal stem cell-derived exosomes in a systemic sclerosis mouse model: possible contribution of miR-196b-5p. J. Dermatol Sci. 104 (1), 39–47. doi:10.1016/j.jdermsci.2021.08.006
Bayram, B., Limberg, A. K., Salib, C. G., Bettencourt, J. W., Trousdale, W. H., Lewallen, E. A., et al. (2020). Molecular pathology of human knee arthrofibrosis defined by RNA sequencing. Genomics 112 (4), 2703–2712. doi:10.1016/j.ygeno.2020.03.004
Bhome, R., Emaduddin, M., James, V., House, L. M., Thirdborough, S. M., Mellone, M., et al. (2022). Epithelial to mesenchymal transition influences fibroblast phenotype in colorectal cancer by altering miR-200 levels in extracellular vesicles. J. Extracell. Vesicles 11 (5), e12226. doi:10.1002/jev2.12226
Bijkerk, R., Au, Y. W., Stam, W., Duijs, J., Koudijs, A., Lievers, E., et al. (2019). Long non-coding RNAs rian and miat mediate myofibroblast formation in kidney fibrosis. Front. Pharmacol. 10, 215. doi:10.3389/fphar.2019.00215
Bijkerk, R., De Bruin, R. G., Van Solingen, C., Van Gils, J. M., Duijs, J. M., Van Der Veer, E. P., et al. (2016). Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int. 89 (6), 1268–1280. doi:10.1016/j.kint.2016.01.029
Blessing, W. A., Williamson, A. K., Kirsch, J. R., and Grinstaff, M. W. (2021). The prognosis of arthrofibroses: prevalence, clinical shortcomings, and future prospects. Trends Pharmacol. Sci. 42 (5), 398–415. doi:10.1016/j.tips.2021.02.007
Brandon-Warner, E., Benbow, J. H., Swet, J. H., Feilen, N. A., Culberson, C. R., Mckillop, I. H., et al. (2018). Adeno-associated virus serotype 2 vector-mediated reintroduction of microRNA-19b attenuates hepatic fibrosis. Hum. Gene Ther. 29 (6), 674–686. doi:10.1089/hum.2017.035
Bullock, M. D., Pickard, K. M., Nielsen, B. S., Sayan, A. E., Jenei, V., Mellone, M., et al. (2013). Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. 4 (6), e684. doi:10.1038/cddis.2013.213
Bulvik, R., Biton, M., Berkman, N., Breuer, R., and Wallach-Dayan, S. B. (2020). Forefront: MiR-34a-knockout mice with wild type hematopoietic cells, retain persistent fibrosis following lung injury. Int. J. Mol. Sci. 21 (6), 2228. doi:10.3390/ijms21062228
Cai, L., Chao, G., Li, W., Zhu, J., Li, F., Qi, B., et al. (2020a). Activated CD4(+) T cells-derived exosomal miR-142-3p boosts post-ischemic ventricular remodeling by activating myofibroblast. Aging (Albany NY) 12 (8), 7380–7396. doi:10.18632/aging.103084
Cai, W., Xu, H., Zhang, B., Gao, X., Li, S., Wei, Z., et al. (2020b). Differential expression of lncRNAs during silicosis and the role of LOC103691771 in myofibroblast differentiation induced by TGF-β1. Biomed. Pharmacother. 125, 109980. doi:10.1016/j.biopha.2020.109980
Carvalho, A., Ji, Z., Zhang, R., Zuo, W., Qu, Y., Chen, X., et al. (2023). Inhibition of miR-195-3p protects against cardiac dysfunction and fibrosis after myocardial infarction. Int. J. Cardiol. 387, 131128. doi:10.1016/j.ijcard.2023.131128
Chen, H., Lv, L., Liang, R., Guo, W., Liao, Z., Chen, Y., et al. (2022). miR-486 improves fibrotic activity in myocardial infarction by targeting SRSF3/p21-Mediated cardiac myofibroblast senescence. J. Cell Mol. Med. 26 (20), 5135–5149. doi:10.1111/jcmm.17539
Chen, J., Zhou, R., Liang, Y., Fu, X., Wang, D., and Wang, C. (2019). Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. Faseb J. 33 (11), 12200–12212. doi:10.1096/fj.201901610
Chen, T., Guo, Y., Wang, J., Ai, L., Ma, L., He, W., et al. (2021a). LncRNA CTD-2528L19.6 prevents the progression of IPF by alleviating fibroblast activation. Cell Death Dis. 12 (6), 600. doi:10.1038/s41419-021-03884-5
Chen, X., Shi, C., Wang, C., Liu, W., Chu, Y., Xiang, Z., et al. (2017). The role of miR-497-5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Sci. Rep. 7, 40958. doi:10.1038/srep40958
Chen, X., Wang, Z., Huang, Y., Deng, W., Zhou, Y., and Chu, M. (2021b). Identification of novel biomarkers for arthrofibrosis after total knee arthroplasty in animal models and clinical patients. EBioMedicine 70, 103486. doi:10.1016/j.ebiom.2021.103486
Cheng, D., Xu, Q., Liu, Y., Li, G., Sun, W., Ma, D., et al. (2021). Long noncoding RNA-SNHG20 promotes silica-induced pulmonary fibrosis by miR-490-3p/TGFBR1 axis. Toxicology 451, 152683. doi:10.1016/j.tox.2021.152683
Chilosi, M., Caliò, A., Rossi, A., Gilioli, E., Pedica, F., Montagna, L., et al. (2017). Epithelial to mesenchymal transition-related proteins ZEB1, β-catenin, and β-tubulin-III in idiopathic pulmonary fibrosis. Mod. Pathol. 30 (1), 26–38. doi:10.1038/modpathol.2016.147
Cho, S. J., Lee, M., Stout-Delgado, H. W., and Moon, J. S. (2020). DROSHA-dependent miRNA and AIM2 inflammasome activation in idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 21 (5), 1668. doi:10.3390/ijms21051668
Creemers, E. E., and Van Rooij, E. (2016). Function and therapeutic potential of noncoding RNAs in cardiac fibrosis. Circ. Res. 118 (1), 108–118. doi:10.1161/circresaha.115.305242
Cui, H., Banerjee, S., Xie, N., Ge, J., Liu, R. M., Matalon, S., et al. (2016). MicroRNA-27a-3p is a negative regulator of lung fibrosis by targeting myofibroblast differentiation. Am. J. Respir. Cell Mol. Biol. 54 (6), 843–852. doi:10.1165/rcmb.2015-0205OC
Cui, H., Ge, J., Xie, N., Banerjee, S., Zhou, Y., Antony, V. B., et al. (2017). miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am. J. Respir. Cell Mol. Biol. 56 (2), 168–178. doi:10.1165/rcmb.2016-0163OC
Cui, J., Qi, S., Liao, R., Su, D., Wang, Y., and Xue, S. (2020). MiR-574-5p promotes the differentiation of human cardiac fibroblasts via regulating ARID3A. Biochem. Biophys. Res. Commun. 521 (2), 427–433. doi:10.1016/j.bbrc.2019.09.107
Cui, X., Sun, X., Lu, F., and Jiang, X. (2018). Baicalein represses TGF-β1-induced fibroblast differentiation through the inhibition of miR-21. Toxicol. Appl. Pharmacol. 358, 35–42. doi:10.1016/j.taap.2018.09.007
De Guire, V., Robitaille, R., Tétreault, N., Guérin, R., Ménard, C., Bambace, N., et al. (2013). Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: promises and challenges. Clin. Biochem. 46 (10-11), 846–860. doi:10.1016/j.clinbiochem.2013.03.015
Deng, H. Y., He, Z. Y., Dong, Z. C., Zhang, Y. L., Han, X., and Li, H. H. (2022). MicroRNA-451a attenuates angiotensin II-induced cardiac fibrosis and inflammation by directly targeting T-box1. J. Physiol. Biochem. 78 (1), 257–269. doi:10.1007/s13105-021-00861-6
Deng, P., Chen, L., Liu, Z., Ye, P., Wang, S., Wu, J., et al. (2016). MicroRNA-150 inhibits the activation of cardiac fibroblasts by regulating c-myb. Cell Physiol. Biochem. 38 (6), 2103–2122. doi:10.1159/000445568
Dong, Y., Peng, N., Dong, L., Tan, S., and Zhang, X. (2022). Non-coding RNAs: important participants in cardiac fibrosis. Front. Cardiovasc Med. 9, 937995. doi:10.3389/fcvm.2022.937995
Dufeys, C., Daskalopoulos, E. P., Castanares-Zapatero, D., Conway, S. J., Ginion, A., Bouzin, C., et al. (2021). AMPKα1 deletion in myofibroblasts exacerbates post-myocardial infarction fibrosis by a connexin 43 mechanism. Basic Res. Cardiol. 116 (1), 10. doi:10.1007/s00395-021-00846-y
D'urso, M., and Kurniawan, N. A. (2020). Mechanical and physical regulation of fibroblast-myofibroblast transition: from cellular mechanoresponse to tissue pathology. Front. Bioeng. Biotechnol. 8, 609653. doi:10.3389/fbioe.2020.609653
Ebrahimpour, A., Shrestha, S., Bonnen, M. D., Eissa, N. T., Raghu, G., and Ghebre, Y. T. (2019). Nicotine modulates growth factors and MicroRNA to promote inflammatory and fibrotic processes. J. Pharmacol. Exp. Ther. 368 (2), 169–178. doi:10.1124/jpet.118.252650
Elliot, S., Periera-Simon, S., Xia, X., Catanuto, P., Rubio, G., Shahzeidi, S., et al. (2019). MicroRNA let-7 downregulates ligand-independent estrogen receptor-mediated male-predominant pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 200 (10), 1246–1257. doi:10.1164/rccm.201903-0508OC
Fan, P., Wang, Y., Li, J., and Fang, M. (2021). lncRNA PAPPA-AS1 induces the development of hypertrophic scar by upregulating TLR4 through interacting with TAF15. Mediat. Inflamm. 2021, 3170261. doi:10.1155/2021/3170261
Fang, C. Y., Yu, C. C., Liao, Y. W., Hsieh, P. L., Ohiro, Y., Chu, P. M., et al. (2020). miR-10b regulated by Twist maintains myofibroblasts activities in oral submucous fibrosis. J. Formos. Med. Assoc. 119 (7), 1167–1173. doi:10.1016/j.jfma.2020.03.005
Feng, Y., Bao, Y., Ding, J., Li, H., Liu, W., Wang, X., et al. (2022). MicroRNA-130a attenuates cardiac fibrosis after myocardial infarction through TGF-β/Smad signaling by directly targeting TGF-β receptor 1. Bioengineered 13 (3), 5779–5791. doi:10.1080/21655979.2022.2033380
Fernandes, J. C. R., Acuña, S. M., Aoki, J. I., Floeter-Winter, L. M., and Muxel, S. M. (2019). Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA 5 (1), 17. doi:10.3390/ncrna5010017
Fernandez, I. E., and Eickelberg, O. (2012). The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc. 9 (3), 111–116. doi:10.1513/pats.201203-023AW
Fierro-Fernández, M., Busnadiego, Ó., Sandoval, P., Espinosa-Díez, C., Blanco-Ruiz, E., Rodríguez, M., et al. (2015). miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX4 and TGFBR2. EMBO Rep. 16 (10), 1358–1377. doi:10.15252/embr.201540750
Fujita, Y., Fujimoto, S., Miyamoto, A., Kaneko, R., Kadota, T., Watanabe, N., et al. (2023). Fibroblast-derived extracellular vesicles induce lung cancer progression in the idiopathic pulmonary fibrosis microenvironment. Am. J. Respir. Cell Mol. Biol. 69 (1), 34–44. doi:10.1165/rcmb.2022-0253OC
Gallant-Behm, C. L., Piper, J., Lynch, J. M., Seto, A. G., Hong, S. J., Mustoe, T. A., et al. (2019). A MicroRNA-29 mimic (remlarsen) represses extracellular matrix expression and fibroplasia in the skin. J. Invest. Dermatol 139 (5), 1073–1081. doi:10.1016/j.jid.2018.11.007
Glowacki, F., Savary, G., Gnemmi, V., Buob, D., Van Der Hauwaert, C., Lo-Guidice, J. M., et al. (2013). Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One 8 (2), e58014. doi:10.1371/journal.pone.0058014
Gong, C., Nie, Y., Qu, S., Liao, J. Y., Cui, X., Yao, H., et al. (2014). miR-21 induces myofibroblast differentiation and promotes the malignant progression of breast phyllodes tumors. Cancer Res. 74 (16), 4341–4352. doi:10.1158/0008-5472.Can-14-0125
Han, R., Ji, X., Rong, R., Li, Y., Yao, W., Yuan, J., et al. (2016). MiR-449a regulates autophagy to inhibit silica-induced pulmonary fibrosis through targeting Bcl2. J. Mol. Med. Berl. 94 (11), 1267–1279. doi:10.1007/s00109-016-1441-0
Hao, K., Lei, W., Wu, H., Wu, J., Yang, Z., Yan, S., et al. (2019). LncRNA-Safe contributes to cardiac fibrosis through Safe-Sfrp2-HuR complex in mouse myocardial infarction. Theranostics 9 (24), 7282–7297. doi:10.7150/thno.33920
Hedbäck, N., Jensen, D. H., Specht, L., Fiehn, A. M., Therkildsen, M. H., Friis-Hansen, L., et al. (2014). MiR-21 expression in the tumor stroma of oral squamous cell carcinoma: an independent biomarker of disease free survival. PLoS One 9 (4), e95193. doi:10.1371/journal.pone.0095193
Herrera, J., Beisang, D. J., Peterson, M., Forster, C., Gilbertsen, A., Benyumov, A., et al. (2018). Dicer1 deficiency in the idiopathic pulmonary fibrosis fibroblastic focus promotes fibrosis by suppressing MicroRNA biogenesis. Am. J. Respir. Crit. Care Med. 198 (4), 486–496. doi:10.1164/rccm.201709-1823OC
Hertig, V., Villeneuve, L., and Calderone, A. (2023). Nestin identifies a subpopulation of rat ventricular fibroblasts and participates in cell migration. Am. J. Physiol. Cell Physiol. 325 (2), C496–c508. doi:10.1152/ajpcell.00161.2023
Horii, Y., Matsuda, S., Toyota, C., Morinaga, T., Nakaya, T., Tsuchiya, S., et al. (2023). VGLL3 is a mechanosensitive protein that promotes cardiac fibrosis through liquid-liquid phase separation. Nat. Commun. 14 (1), 550. doi:10.1038/s41467-023-36189-6
Hu, H., Mao, G., Zheng, J., and Guo, F. (2022). Keloid patient plasma-derived exosomal hsa_circ_0020792 promotes normal skin fibroblasts proliferation, migration, and fibrogenesis via modulating miR-193a-5p and activating TGF-β1/smad2/3 signaling. Drug Des. Devel Ther. 16, 4223–4234. doi:10.2147/dddt.S386786
Hu, R. P., Lu, Y. Y., and Zhang, X. J. (2019). MiR-34b-5p knockdown attenuates bleomycin-induced pulmonary fibrosis by targeting tissue inhibitor of metalloproteinase 3 (TIMP3). Eur. Rev. Med. Pharmacol. Sci. 23 (5), 2273–2279. doi:10.26355/eurrev_201903_17276
Huang, C., Wang, R., Lu, J., He, Y., Wu, Y., Ma, W., et al. (2022). MicroRNA-338-3p as a therapeutic target in cardiac fibrosis through FGFR2 suppression. J. Clin. Lab. Anal. 36 (8), e24584. doi:10.1002/jcla.24584
Huang, Y., Xie, Y., Abel, P. W., Wei, P., Plowman, J., Toews, M. L., et al. (2020). TGF-β1-induced miR-424 promotes pulmonary myofibroblast differentiation by targeting Slit2 protein expression. Biochem. Pharmacol. 180, 114172. doi:10.1016/j.bcp.2020.114172
Ilieva, M., and Uchida, S. (2022). Long non-coding RNAs in cardiac and pulmonary fibroblasts and fibrosis. Noncoding RNA 8 (4), 53. doi:10.3390/ncrna8040053
Izawa, T., Horiuchi, T., Atarashi, M., Kuwamura, M., and Yamate, J. (2015). Anti-fibrotic role of miR-214 in thioacetamide-induced liver cirrhosis in rats. Toxicol. Pathol. 43 (6), 844–851. doi:10.1177/0192623315573587
Jeong, A., Lim, Y., Kook, T., Kwon, D. H., Cho, Y. K., Ryu, J., et al. (2023). Circular RNA circSMAD4 regulates cardiac fibrosis by targeting miR-671-5p and FGFR2 in cardiac fibroblasts. Mol. Ther. Nucleic Acids 34, 102071. doi:10.1016/j.omtn.2023.102071
Ji, Y., Qiu, M., Shen, Y., Gao, L., Wang, Y., Sun, W., et al. (2018). MicroRNA-327 regulates cardiac hypertrophy and fibrosis induced by pressure overload. Int. J. Mol. Med. 41 (4), 1909–1916. doi:10.3892/ijmm.2018.3428
Kanno, Y., Shu, E., Niwa, H., Seishima, M., and Ozaki, K. I. (2021). MicroRNA-30c attenuates fibrosis progression and vascular dysfunction in systemic sclerosis model mice. Mol. Biol. Rep. 48 (4), 3431–3437. doi:10.1007/s11033-021-06368-z
Kilari, S., Cai, C., Zhao, C., Sharma, A., Chernogubova, E., Simeon, M., et al. (2019). The role of MicroRNA-21 in venous neointimal hyperplasia: implications for targeting miR-21 for VNH treatment. Mol. Ther. 27 (9), 1681–1693. doi:10.1016/j.ymthe.2019.06.011
Kong, Q., Zhou, J., Ma, C., Wei, Z., Chen, Y., Cheng, Y., et al. (2023). Inhibition of long noncoding RNA Gm41724 alleviates pressure overload-induced cardiac fibrosis by regulating lamina-associated polypeptide 2α. Pharmacol. Res. 188, 106677. doi:10.1016/j.phrs.2023.106677
Kristensen, L. S., Andersen, M. S., Stagsted, L. V. W., Ebbesen, K. K., Hansen, T. B., and Kjems, J. (2019). The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691. doi:10.1038/s41576-019-0158-7
Kuse, N., Kamio, K., Azuma, A., Matsuda, K., Inomata, M., Usuki, J., et al. (2020). Exosome-Derived microRNA-22 ameliorates pulmonary fibrosis by regulating fibroblast-to-myofibroblast differentiation in vitro and in vivo. J. Nippon. Med. Sch. 87 (3), 118–128. doi:10.1272/jnms.JNMS.2020_87-302
Kwiecinski, M., Elfimova, N., Noetel, A., Töx, U., Steffen, H. M., Hacker, U., et al. (2012). Expression of platelet-derived growth factor-C and insulin-like growth factor I in hepatic stellate cells is inhibited by miR-29. Lab. Invest. 92 (7), 978–987. doi:10.1038/labinvest.2012.70
Kwiecinski, M., Noetel, A., Elfimova, N., Trebicka, J., Schievenbusch, S., Strack, I., et al. (2011). Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction. PLoS One 6 (9), e24568. doi:10.1371/journal.pone.0024568
Lee, D. R., Therrien, E., Song, B. M., Camp, C. L., Krych, A. J., Stuart, M. J., et al. (2022). Arthrofibrosis nightmares: prevention and management strategies. Sports Med. Arthrosc. Rev. 30 (1), 29–41. doi:10.1097/jsa.0000000000000324
Lee, Y. H., Yu, C. C., Hsieh, P. L., Liao, Y. W., Yu, C. H., and Su, T. R. (2021). Inhibition of lncRNA HOTTIP ameliorated myofibroblast activities and inflammatory cytokines in oral submucous fibrosis. J. Formos. Med. Assoc. 120 (5), 1188–1193. doi:10.1016/j.jfma.2020.11.013
Li, F., Zhang, A., Shi, Y., Ma, Y., and Du, Y. (2015). 1α,25-Dihydroxyvitamin D3 prevents the differentiation of human lung fibroblasts via microRNA-27b targeting the vitamin D receptor. Int. J. Mol. Med. 36 (4), 967–974. doi:10.3892/ijmm.2015.2318
Li, J., Kong, X., Jiang, S., Liao, W., Zhang, Z., Song, J., et al. (2019a). miR-627/HMGB1/NF-κB regulatory loop modulates TGF-β1-induced pulmonary fibrosis. J. Cell Biochem. 120 (3), 2983–2993. doi:10.1002/jcb.27038
Li, L., Bounds, K. R., Chatterjee, P., and Gupta, S. (2017a). MicroRNA-130a, a potential antifibrotic target in cardiac fibrosis. J. Am. Heart Assoc. 6 (11), e006763. doi:10.1161/jaha.117.006763
Li, Q., Zhao, H., Chen, W., Huang, P., and Bi, J. (2019b). Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int. J. Biochem. Cell Biol. 114, 105570. doi:10.1016/j.biocel.2019.105570
Li, X., Yang, L., and Chen, L. L. (2018a). The biogenesis, functions, and challenges of circular RNAs. Mol. Cell 71 (3), 428–442. doi:10.1016/j.molcel.2018.06.034
Li, X., Yu, T., Shan, H., Jiang, H., Sun, J., Zhao, X., et al. (2018b). lncRNA PFAL promotes lung fibrosis through CTGF by competitively binding miR-18a. Faseb J. 32 (10), 5285–5297. doi:10.1096/fj.201800055R
Li, Y., Zhang, J., Shi, J., Liu, K., Wang, X., Jia, Y., et al. (2021). Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 12 (1), 221. doi:10.1186/s13287-021-02290-0
Li, Y., Zhang, J., Zhang, W., Liu, Y., Li, Y., Wang, K., et al. (2017b). MicroRNA-192 regulates hypertrophic scar fibrosis by targeting SIP1. J. Mol. Histol. 48 (5-6), 357–366. doi:10.1007/s10735-017-9734-3
Liang, H., Gu, Y., Li, T., Zhang, Y., Huangfu, L., Hu, M., et al. (2014). Integrated analyses identify the involvement of microRNA-26a in epithelial-mesenchymal transition during idiopathic pulmonary fibrosis. Cell Death Dis. 5 (5), e1238. doi:10.1038/cddis.2014.207
Liang, H., Pan, Z., Zhao, X., Liu, L., Sun, J., Su, X., et al. (2018). LncRNA PFL contributes to cardiac fibrosis by acting as a competing endogenous RNA of let-7d. Theranostics 8 (4), 1180–1194. doi:10.7150/thno.20846
Liang, H., Zhang, C., Ban, T., Liu, Y., Mei, L., Piao, X., et al. (2012). A novel reciprocal loop between microRNA-21 and TGFβRIII is involved in cardiac fibrosis. Int. J. Biochem. Cell Biol. 44 (12), 2152–2160. doi:10.1016/j.biocel.2012.08.019
Liao, Y., Li, H., Cao, H., Dong, Y., Gao, L., Liu, Z., et al. (2021). Therapeutic silencing miR-146b-5p improves cardiac remodeling in a porcine model of myocardial infarction by modulating the wound reparative phenotype. Protein Cell 12 (3), 194–212. doi:10.1007/s13238-020-00750-6
Liao, Y. W., Tsai, L. L., Lee, Y. H., Hsieh, P. L., Yu, C. C., and Lu, M. Y. (2022). miR-21 promotes the fibrotic properties in oral mucosa through targeting PDCD4. J. Dent. Sci. 17 (2), 677–682. doi:10.1016/j.jds.2021.09.004
Liao, Y. W., Yu, C. C., Hsieh, P. L., and Chang, Y. C. (2018). miR-200b ameliorates myofibroblast transdifferentiation in precancerous oral submucous fibrosis through targeting ZEB2. J. Cell Mol. Med. 22 (9), 4130–4138. doi:10.1111/jcmm.13690
Lin, C. Y., Liao, Y. W., Hsieh, P. L., Lu, M. Y., Peng, C. Y., Chu, P. M., et al. (2018). LncRNA GAS5-AS1 inhibits myofibroblasts activities in oral submucous fibrosis. J. Formos. Med. Assoc. 117 (8), 727–733. doi:10.1016/j.jfma.2017.09.012
Lino Cardenas, C. L., Henaoui, I. S., Courcot, E., Roderburg, C., Cauffiez, C., Aubert, S., et al. (2013). miR-199a-5p Is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet. 9 (2), e1003291. doi:10.1371/journal.pgen.1003291
Liu, G., Friggeri, A., Yang, Y., Milosevic, J., Ding, Q., Thannickal, V. J., et al. (2010). miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207 (8), 1589–1597. doi:10.1084/jem.20100035
Liu, P., Luo, G., Dodson, M., Schmidlin, C. J., Wei, Y., Kerimoglu, B., et al. (2021). The NRF2-LOC344887 signaling axis suppresses pulmonary fibrosis. Redox Biol. 38, 101766. doi:10.1016/j.redox.2020.101766
Lorenzen, J. M., Schauerte, C., Hübner, A., Kölling, M., Martino, F., Scherf, K., et al. (2015). Osteopontin is indispensable for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur. Heart J. 36 (32), 2184–2196. doi:10.1093/eurheartj/ehv109
Lu, T. X., and Rothenberg, M. E. (2018). MicroRNA. J. Allergy Clin. Immunol. 141 (4), 1202–1207. doi:10.1016/j.jaci.2017.08.034
Lu, Y., Zhang, T., Shan, S., Wang, S., Bian, W., Ren, T., et al. (2019). MiR-124 regulates transforming growth factor-β1 induced differentiation of lung resident mesenchymal stem cells to myofibroblast by repressing Wnt/β-catenin signaling. Dev. Biol. 449 (2), 115–121. doi:10.1016/j.ydbio.2019.02.010
Ma, C. X., Wei, Z. R., Sun, T., Yang, M. H., Sun, Y. Q., Kai, K. L., et al. (2023a). Circ-sh3rf3/GATA-4/miR-29a regulatory axis in fibroblast-myofibroblast differentiation and myocardial fibrosis. Cell Mol. Life Sci. 80 (2), 50. doi:10.1007/s00018-023-04699-7
Ma, T., Qiu, F., Gong, Y., Cao, H., Dai, G., Sun, D., et al. (2023b). Therapeutic silencing of lncRNA RMST alleviates cardiac fibrosis and improves heart function after myocardial infarction in mice and swine. Theranostics 13 (11), 3826–3843. doi:10.7150/thno.82543
Mann, J., Chu, D. C., Maxwell, A., Oakley, F., Zhu, N. L., Tsukamoto, H., et al. (2010). MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 138 (2), 705–714. doi:10.1053/j.gastro.2009.10.002
Matkovich, S. J., Wang, W., Tu, Y., Eschenbacher, W. H., Dorn, L. E., Condorelli, G., et al. (2010). MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ. Res. 106 (1), 166–175. doi:10.1161/circresaha.109.202176
Medzikovic, L., Aryan, L., Ruffenach, G., Li, M., Savalli, N., Sun, W., et al. (2023). Myocardial fibrosis and calcification are attenuated by microRNA-129-5p targeting Asporin and Sox9 in cardiac fibroblasts. JCI Insight 8 (9), e168655. doi:10.1172/jci.insight.168655
Miao, C., Xiong, Y., Zhang, G., and Chang, J. (2018). MicroRNAs in idiopathic pulmonary fibrosis, new research progress and their pathophysiological implication. Exp. Lung Res. 44 (3), 178–190. doi:10.1080/01902148.2018.1455927
Michalik, M., Wójcik-Pszczoła, K., Paw, M., Wnuk, D., Koczurkiewicz, P., Sanak, M., et al. (2018). Fibroblast-to-myofibroblast transition in bronchial asthma. Cell Mol. Life Sci. 75 (21), 3943–3961. doi:10.1007/s00018-018-2899-4
Millar, N. L., Gilchrist, D. S., Akbar, M., Reilly, J. H., Kerr, S. C., Campbell, A. L., et al. (2015). MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun. 6, 6774. doi:10.1038/ncomms7774
Mishra, P. K., Tyagi, N., Kumar, M., and Tyagi, S. C. (2009). MicroRNAs as a therapeutic target for cardiovascular diseases. J. Cell Mol. Med. 13 (4), 778–789. doi:10.1111/j.1582-4934.2009.00744.x
Nagpal, V., Rai, R., Place, A. T., Murphy, S. B., Verma, S. K., Ghosh, A. K., et al. (2016). MiR-125b is critical for fibroblast-to-myofibroblast transition and cardiac fibrosis. Circulation 133 (3), 291–301. doi:10.1161/circulationaha.115.018174
Nho, R. S., Im, J., Ho, Y. Y., and Hergert, P. (2014). MicroRNA-96 inhibits FoxO3a function in IPF fibroblasts on type I collagen matrix. Am. J. Physiol. Lung Cell Mol. Physiol. 307 (8), L632–L642. doi:10.1152/ajplung.00127.2014
Niu, Z., Wang, L., Qin, X., Ye, Z., Xie, B., and Hu, Y. (2022). Macrophage derived miR-7219-3p-containing exosomes mediate fibroblast trans-differentiation by targeting SPRY1 in silicosis. Toxicology 479, 153310. doi:10.1016/j.tox.2022.153310
Nonaka, C. K. V., Sampaio, G. L., Silva, K. N., Khouri, R., Macedo, C. T., Chagas Translational Research, C., et al. (2021). Therapeutic miR-21 silencing reduces cardiac fibrosis and modulates inflammatory response in chronic chagas disease. Int. J. Mol. Sci. 22 (7), 3307. doi:10.3390/ijms22073307
Pachera, E., Assassi, S., Salazar, G. A., Stellato, M., Renoux, F., Wunderlin, A., et al. (2020). Long noncoding RNA H19X is a key mediator of TGF-β-driven fibrosis. J. Clin. Invest. 130 (9), 4888–4905. doi:10.1172/jci135439
Pan, Q., Guo, C. J., Xu, Q. Y., Wang, J. Z., Li, H., and Fang, C. H. (2020). miR-16 integrates signal pathways in myofibroblasts: determinant of cell fate necessary for fibrosis resolution. Cell Death Dis. 11 (8), 639. doi:10.1038/s41419-020-02832-z
Patop, I. L., Wust, S., and Kadener, S. (2019). Past, present, and future of circRNAs. Embo J. 38 (16), e100836. doi:10.15252/embj.2018100836
Peng, T., Liu, M., Hu, L., Guo, D., Wang, D., Qi, B., et al. (2022). LncRNA Airn alleviates diabetic cardiac fibrosis by inhibiting activation of cardiac fibroblasts via a m6A-IMP2-p53 axis. Biol. Direct. 17 (1), 32. doi:10.1186/s13062-022-00346-6
Qiu, Z., Zhong, Z., Zhang, Y., Tan, H., Deng, B., and Meng, G. (2022). Human umbilical cord mesenchymal stem cell-derived exosomal miR-335-5p attenuates the inflammation and tubular epithelial-myofibroblast transdifferentiation of renal tubular epithelial cells by reducing ADAM19 protein levels. Stem Cell Res. Ther. 13 (1), 373. doi:10.1186/s13287-022-03071-z
Qu, C., Liu, X., Ye, T., Wang, L., Liu, S., Zhou, X., et al. (2019). miR‑216a exacerbates TGF‑β‑induced myofibroblast transdifferentiation via PTEN/AKT signaling. Mol. Med. Rep. 19 (6), 5345–5352. doi:10.3892/mmr.2019.10200
Rackow, A. R., Judge, J. L., Woeller, C. F., Sime, P. J., and Kottmann, R. M. (2022). miR-338-3p blocks TGFβ-induced myofibroblast differentiation through the induction of PTEN. Am. J. Physiol. Lung Cell Mol. Physiol. 322 (3), L385–l400. doi:10.1152/ajplung.00251.2021
Ramanujam, D., Schön, A. P., Beck, C., Vaccarello, P., Felician, G., Dueck, A., et al. (2021). MicroRNA-21-Dependent macrophage-to-fibroblast signaling determines the cardiac response to pressure overload. Circulation 143 (15), 1513–1525. doi:10.1161/circulationaha.120.050682
Ren, L., Yang, C., Dou, Y., Zhan, R., Sun, Y., and Yu, Y. (2017). MiR-541-5p regulates lung fibrosis by targeting cyclic nucleotide phosphodiesterase 1A. Exp. Lung Res. 43 (6-7), 249–258. doi:10.1080/01902148.2017.1349210
Saito, S., Ohno, S. I., Harada, Y., Kanno, Y., and Kuroda, M. (2023). MiR-34a induces myofibroblast differentiation from renal fibroblasts. Clin. Exp. Nephrol. 27 (5), 411–418. doi:10.1007/s10157-023-02329-x
Savary, G., Dewaeles, E., Diazzi, S., Buscot, M., Nottet, N., Fassy, J., et al. (2019). The long noncoding RNA DNM3OS is a reservoir of FibromiRs with major functions in lung fibroblast response to TGF-β and pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 200 (2), 184–198. doi:10.1164/rccm.201807-1237OC
Schipper, J., Westerhuis, J. J., Beddows, I., Madaj, Z., Monsma, D., Hostetter, G., et al. (2020). Loss of microRNA-21 leads to profound stromal remodeling and short survival in K-Ras-driven mouse models of pancreatic cancer. Int. J. Cancer 147 (8), 2265–2278. doi:10.1002/ijc.33041
Selman, M., López-Otín, C., and Pardo, A. (2016). Age-driven developmental drift in the pathogenesis of idiopathic pulmonary fibrosis. Eur. Respir. J. 48 (2), 538–552. doi:10.1183/13993003.00398-2016
Senavirathna, L. K., Huang, C., Pushparaj, S., Xu, D., and Liu, L. (2020). Hypoxia and transforming growth factor β1 regulation of long non-coding RNA transcriptomes in human pulmonary fibroblasts. Physiol. Rep. 8 (1), e14343. doi:10.14814/phy2.14343
Shen, J., Xing, W., Liu, R., Zhang, Y., Xie, C., and Gong, F. (2019). MiR-32-5p influences high glucose-induced cardiac fibroblast proliferation and phenotypic alteration by inhibiting DUSP1. BMC Mol. Biol. 20 (1), 21. doi:10.1186/s12867-019-0135-x
Song, X., Xu, P., Meng, C., Song, C., Blackwell, T. S., Li, R., et al. (2019). lncITPF promotes pulmonary fibrosis by targeting hnRNP-L depending on its host gene ITGBL1. Mol. Ther. 27 (2), 380–393. doi:10.1016/j.ymthe.2018.08.026
Su, M., Li, W., Yuan, Y., Liu, S., Liang, C., Liu, H. E., et al. (2022). Epididymal white adipose tissue promotes angiotensin II-induced cardiac fibrosis in an exosome-dependent manner. Transl. Res. 248, 51–67. doi:10.1016/j.trsl.2022.05.004
Su, P., Qiao, Q., Ji, G., and Zhang, Z. (2021). CircAMD1 regulates proliferation and collagen synthesis via sponging miR-27a-3p in P63-mutant human dermal fibroblasts. Differentiation 119, 10–18. doi:10.1016/j.diff.2021.04.002
Sun, J., Jin, T., Su, W., Guo, Y., Niu, Z., Guo, J., et al. (2021). The long non-coding RNA PFI protects against pulmonary fibrosis by interacting with splicing regulator SRSF1. Cell Death Differ. 28 (10), 2916–2930. doi:10.1038/s41418-021-00792-1
Tang, R., Wang, Y. C., Mei, X., Shi, N., Sun, C., Ran, R., et al. (2020). LncRNA GAS5 attenuates fibroblast activation through inhibiting Smad3 signaling. Am. J. Physiol. Cell Physiol. 319 (1), C105–c115. doi:10.1152/ajpcell.00059.2020
Tao, H., Dai, C., Ding, J. F., Yang, J. J., Ding, X. S., Xu, S. S., et al. (2018). Epigenetic aberrations of miR-369-5p and DNMT3A control Patched1 signal pathway in cardiac fibrosis. Toxicology 410, 182–192. doi:10.1016/j.tox.2018.08.004
Tao, H., Yang, J. J., Hu, W., Shi, K. H., Deng, Z. Y., and Li, J. (2016a). Noncoding RNA as regulators of cardiac fibrosis: current insight and the road ahead. Pflugers Arch. 468 (6), 1103–1111. doi:10.1007/s00424-016-1792-y
Tao, L., Bei, Y., Chen, P., Lei, Z., Fu, S., Zhang, H., et al. (2016b). Crucial role of miR-433 in regulating cardiac fibrosis. Theranostics 6 (12), 2068–2083. doi:10.7150/thno.15007
Tarbit, E., Singh, I., Peart, J. N., and Rose'meyer, R. B. (2019). Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail Rev. 24 (1), 1–15. doi:10.1007/s10741-018-9720-1
Thakur, D., Taliaferro, O., Atkinson, M., Stoffel, R., Guleria, R. S., and Gupta, S. (2022). Inhibition of nuclear factor κB in the lungs protect bleomycin-induced lung fibrosis in mice. Mol. Biol. Rep. 49 (5), 3481–3490. doi:10.1007/s11033-022-07185-8
Usher, K. M., Zhu, S., Mavropalias, G., Carrino, J. A., Zhao, J., and Xu, J. (2019). Pathological mechanisms and therapeutic outlooks for arthrofibrosis. Bone Res. 7, 9. doi:10.1038/s41413-019-0047-x
Wang, C., Gu, S., Cao, H., Li, Z., Xiang, Z., Hu, K., et al. (2016a). miR-877-3p targets Smad7 and is associated with myofibroblast differentiation and bleomycin-induced lung fibrosis. Sci. Rep. 6, 30122. doi:10.1038/srep30122
Wang, C., Yin, S., Wang, Q., Jiang, M., Li, S., Zhen, W., et al. (2022). miR-409-3p regulated by GATA2 promotes cardiac fibrosis through targeting Gpd1. Oxid. Med. Cell Longev. 2022, 8922246. doi:10.1155/2022/8922246
Wang, D., Hao, C., Zhang, L., Zhang, J., Liu, S., Li, Y., et al. (2020a). Exosomal miR-125a-5p derived from silica-exposed macrophages induces fibroblast transdifferentiation. Ecotoxicol. Environ. Saf. 192, 110253. doi:10.1016/j.ecoenv.2020.110253
Wang, F., Zhao, X. Q., Liu, J. N., Wang, Z. H., Wang, X. L., Hou, X. Y., et al. (2012). Antagonist of microRNA-21 improves balloon injury-induced rat iliac artery remodeling by regulating proliferation and apoptosis of adventitial fibroblasts and myofibroblasts. J. Cell Biochem. 113 (9), 2989–3001. doi:10.1002/jcb.24176
Wang, J., Zhang, S., Li, X., and Gong, M. (2020b). LncRNA SNHG7 promotes cardiac remodeling by upregulating ROCK1 via sponging miR-34-5p. Aging (Albany NY) 12 (11), 10441–10456. doi:10.18632/aging.103269
Wang, K., Long, B., Liu, F., Wang, J. X., Liu, C. Y., Zhao, B., et al. (2016b). A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 37 (33), 2602–2611. doi:10.1093/eurheartj/ehv713
Wang, L., Ma, L., Fan, H., Yang, Z., Li, L., and Wang, H. (2016c). MicroRNA-9 regulates cardiac fibrosis by targeting PDGFR-β in rats. J. Physiol. Biochem. 72 (2), 213–223. doi:10.1007/s13105-016-0471-y
Wang, P., Xiao, T., Li, J., Wang, D., Sun, J., Cheng, C., et al. (2021a). miR-21 in EVs from pulmonary epithelial cells promotes myofibroblast differentiation via glycolysis in arsenic-induced pulmonary fibrosis. Environ. Pollut. 286, 117259. doi:10.1016/j.envpol.2021.117259
Wang, R., Peng, L., Lv, D., Shang, F., Yan, J., Li, G., et al. (2021b). Leonurine attenuates myocardial fibrosis through upregulation of miR-29a-3p in mice post-myocardial infarction. J. Cardiovasc Pharmacol. 77 (2), 189–199. doi:10.1097/fjc.0000000000000957
Wang, R., Zhang, M., Ou, Z., He, W., Chen, L., Zhang, J., et al. (2019a). Long noncoding RNA DNM3OS promotes prostate stromal cells transformation via the miR-29a/29b/COL3A1 and miR-361/TGFβ1 axes. Aging (Albany NY) 11 (21), 9442–9460. doi:10.18632/aging.102395
Wang, X., Cao, X., Dong, D., Shen, X., Cheng, J., Jiang, R., et al. (2019b). Circular RNA TTN acts as a miR-432 sponge to facilitate proliferation and differentiation of myoblasts via the IGF2/PI3K/AKT signaling pathway. Mol. Ther. Nucleic Acids 18, 966–980. doi:10.1016/j.omtn.2019.10.019
Wang, X. C., Song, K., Tu, B., Sun, H., Zhou, Y., Xu, S. S., et al. (2023a). New aspects of the epigenetic regulation of EMT related to pulmonary fibrosis. Eur. J. Pharmacol. 956, 175959. doi:10.1016/j.ejphar.2023.175959
Wang, Y., Chen, D., Xie, H., Zhou, S., Jia, M., He, X., et al. (2023b). LncRNA GAS5 suppresses TGF-β1-induced transformation of pulmonary pericytes into myofibroblasts by recruiting KDM5B and promoting H3K4me2/3 demethylation of the PDGFRα/β promoter. Mol. Med. 29 (1), 32. doi:10.1186/s10020-023-00620-x
Wang, Y., Ouyang, M., Wang, Q., and Jian, Z. (2016d). MicroRNA-142-3p inhibits hypoxia/reoxygenation-induced apoptosis and fibrosis of cardiomyocytes by targeting high mobility group box 1. Int. J. Mol. Med. 38 (5), 1377–1386. doi:10.3892/ijmm.2016.2756
Wang, Y. S., Li, S. H., Guo, J., Mihic, A., Wu, J., Sun, L., et al. (2014). Role of miR-145 in cardiac myofibroblast differentiation. J. Mol. Cell Cardiol. 66, 94–105. doi:10.1016/j.yjmcc.2013.08.007
Wasson, C. W., Abignano, G., Hermes, H., Malaab, M., Ross, R. L., Jimenez, S. A., et al. (2020a). Long non-coding RNA HOTAIR drives EZH2-dependent myofibroblast activation in systemic sclerosis through miRNA 34a-dependent activation of NOTCH. Ann. Rheum. Dis. 79 (4), 507–517. doi:10.1136/annrheumdis-2019-216542
Wasson, C. W., Ross, R. L., Wells, R., Corinaldesi, C., Georgiou, I. C., Riobo-Del Galdo, N. A., et al. (2020b). Long non-coding RNA HOTAIR induces GLI2 expression through Notch signalling in systemic sclerosis dermal fibroblasts. Arthritis Res. Ther. 22 (1), 286. doi:10.1186/s13075-020-02376-9
Wei, P., Xie, Y., Abel, P. W., Huang, Y., Ma, Q., Li, L., et al. (2019). Transforming growth factor (TGF)-β1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death Dis. 10 (9), 670. doi:10.1038/s41419-019-1873-x
Wei, Y., Wu, Y., Feng, K., Zhao, Y., Tao, R., Xu, H., et al. (2020). Astragaloside IV inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-β/Smads pathway. J. Ethnopharmacol. 249, 112404. doi:10.1016/j.jep.2019.112404
Wei, Y., Yan, X., Yan, L., Hu, F., Ma, W., Wang, Y., et al. (2017). Inhibition of microRNA-155 ameliorates cardiac fibrosis in the process of angiotensin II-induced cardiac remodeling. Mol. Med. Rep. 16 (5), 7287–7296. doi:10.3892/mmr.2017.7584
Wu, Q., Jiao, B., Gui, W., Zhang, Q., Wang, F., and Han, L. (2021). Long non-coding RNA SNHG1 promotes fibroblast-to-myofibroblast transition during the development of pulmonary fibrosis induced by silica particles exposure. Ecotoxicol. Environ. Saf. 228, 112938. doi:10.1016/j.ecoenv.2021.112938
Wu, Y., Liu, X., Zhou, Q., Huang, C., Meng, X., Xu, F., et al. (2015). Silent information regulator 1 (SIRT1) ameliorates liver fibrosis via promoting activated stellate cell apoptosis and reversion. Toxicol. Appl. Pharmacol. 289 (2), 163–176. doi:10.1016/j.taap.2015.09.028
Wynn, T. A., and Ramalingam, T. R. (2012). Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18 (7), 1028–1040. doi:10.1038/nm.2807
Xi, T., Wang, R., Pi, D., Ouyang, J., and Yang, J. (2023). The p53/miR-29a-3p axis mediates the antifibrotic effect of leonurine on angiotensin II-stimulated rat cardiac fibroblasts. Exp. Cell Res. 426 (1), 113556. doi:10.1016/j.yexcr.2023.113556
Xiao, T., Zou, Z., Xue, J., Syed, B. M., Sun, J., Dai, X., et al. (2021). LncRNA H19-mediated M2 polarization of macrophages promotes myofibroblast differentiation in pulmonary fibrosis induced by arsenic exposure. Environ. Pollut. 268 (Pt A), 115810. doi:10.1016/j.envpol.2020.115810
Xiao, X., Huang, C., Zhao, C., Gou, X., Senavirathna, L. K., Hinsdale, M., et al. (2015). Regulation of myofibroblast differentiation by miR-424 during epithelial-to-mesenchymal transition. Arch. Biochem. Biophys. 566, 49–57. doi:10.1016/j.abb.2014.12.007
Xu, C., Hou, L., Zhao, J., Wang, Y., Jiang, F., Jiang, Q., et al. (2022). Exosomal let-7i-5p from three-dimensional cultured human umbilical cord mesenchymal stem cells inhibits fibroblast activation in silicosis through targeting TGFBR1. Ecotoxicol. Environ. Saf. 233, 113302. doi:10.1016/j.ecoenv.2022.113302
Xu, H., Ling, M., Xue, J., Dai, X., Sun, Q., Chen, C., et al. (2018). Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics 8 (19), 5419–5433. doi:10.7150/thno.27876
Xu, Q., Cheng, D., Li, G., Liu, Y., Li, P., Sun, W., et al. (2021a). CircHIPK3 regulates pulmonary fibrosis by facilitating glycolysis in miR-30a-3p/FOXK2-dependent manner. Int. J. Biol. Sci. 17 (9), 2294–2307. doi:10.7150/ijbs.57915
Xu, S. S., Ding, J. F., Shi, P., Shi, K. H., and Tao, H. (2021b). DNMT1-Induced miR-152-3p suppression facilitates cardiac fibroblast activation in cardiac fibrosis. Cardiovasc Toxicol. 21 (12), 984–999. doi:10.1007/s12012-021-09690-x
Yamada, M., Kubo, H., Ota, C., Takahashi, T., Tando, Y., Suzuki, T., et al. (2013). The increase of microRNA-21 during lung fibrosis and its contribution to epithelial-mesenchymal transition in pulmonary epithelial cells. Respir. Res. 14 (1), 95. doi:10.1186/1465-9921-14-95
Yan, L., Su, Y., Hsia, I., Xu, Y., Vincent-Chong, V. K., Mojica, W., et al. (2023). Delivery of anti-microRNA-21 by lung-targeted liposomes for pulmonary fibrosis treatment. Mol. Ther. Nucleic Acids 32, 36–47. doi:10.1016/j.omtn.2023.02.031
Yang, H. W., Yu, C. C., Hsieh, P. L., Liao, Y. W., Chu, P. M., Yu, C. H., et al. (2021). Arecoline enhances miR-21 to promote buccal mucosal fibroblasts activation. J. Formos. Med. Assoc. 120 (4), 1108–1113. doi:10.1016/j.jfma.2020.10.019
Yang, J. J., Tao, H., Deng, Z. Y., Lu, C., and Li, J. (2015). Non-coding RNA-mediated epigenetic regulation of liver fibrosis. Metabolism 64 (11), 1386–1394. doi:10.1016/j.metabol.2015.08.004
Yang, K., Shi, J., Hu, Z., and Hu, X. (2019). The deficiency of miR-214-3p exacerbates cardiac fibrosis via miR-214-3p/NLRC5 axis. Clin. Sci. (Lond). 133 (17), 1845–1856. doi:10.1042/cs20190203
Yang, P. Y., Ho, D. C., Chen, S. H., Hsieh, P. L., Liao, Y. W., Tsai, L. L., et al. (2022a). Down-regulation of miR-29c promotes the progression of oral submucous fibrosis through targeting tropomyosin-1. J. Formos. Med. Assoc. 121 (6), 1117–1122. doi:10.1016/j.jfma.2021.10.006
Yang, S., Cui, H., Xie, N., Icyuz, M., Banerjee, S., Antony, V. B., et al. (2013). miR-145 regulates myofibroblast differentiation and lung fibrosis. Faseb J. 27 (6), 2382–2391. doi:10.1096/fj.12-219493
Yang, X., Jiang, Z., Li, Y., Zhang, Y., Han, Y., and Gao, L. (2022b). Non-coding RNAs regulating epithelial-mesenchymal transition: research progress in liver disease. Biomed. Pharmacother. 150, 112972. doi:10.1016/j.biopha.2022.112972
Yang, Y., Tai, W., Lu, N., Li, T., Liu, Y., Wu, W., et al. (2020). lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging (Albany NY). 12 (10), 9085–9102. doi:10.18632/aging.103176
Yao, Q., Cao, S., Li, C., Mengesha, A., Kong, B., and Wei, M. (2011). Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int. J. Cancer 128 (8), 1783–1792. doi:10.1002/ijc.25506
Yi, M., Liu, B., Tang, Y., Li, F., Qin, W., and Yuan, X. (2018). Irradiated human umbilical vein endothelial cells undergo endothelial-mesenchymal transition via the snail/miR-199a-5p Axis to promote the differentiation of fibroblasts into myofibroblasts. Biomed. Res. Int. 2018, 4135806. doi:10.1155/2018/4135806
Yousefi, F., Shabaninejad, Z., Vakili, S., Derakhshan, M., Movahedpour, A., Dabiri, H., et al. (2020). TGF-β and WNT signaling pathways in cardiac fibrosis: non-coding RNAs come into focus. Cell Commun. Signal 18 (1), 87. doi:10.1186/s12964-020-00555-4
Yousefi, F., Soltani, B. M., and Rabbani, S. (2021). MicroRNA-331 inhibits isoproterenol-induced expression of profibrotic genes in cardiac myofibroblasts via the TGFβ/smad3 signaling pathway. Sci. Rep. 11 (1), 2548. doi:10.1038/s41598-021-82226-z
Yu, C. C., Liao, Y. W., Hsieh, P. L., and Chang, Y. C. (2021). Targeting lncRNA H19/miR-29b/COL1A1 Axis impedes myofibroblast activities of precancerous oral submucous fibrosis. Int. J. Mol. Sci. 22 (4), 2216. doi:10.3390/ijms22042216
Zahir-Jouzdani, F., Soleimani, M., Mahbod, M., Mottaghitalab, F., Vakhshite, F., Arefian, E., et al. (2018). Corneal chemical burn treatment through a delivery system consisting of TGF-β1 siRNA: in vitro and in vivo. Drug Deliv. Transl. Res. 8 (5), 1127–1138. doi:10.1007/s13346-018-0546-0
Zhang, C., Zhang, Y., Zhu, H., Hu, J., and Xie, Z. (2018a). MiR-34a/miR-93 target c-Ski to modulate the proliferaton of rat cardiac fibroblasts and extracellular matrix deposition in vivo and in vitro. Cell Signal 46, 145–153. doi:10.1016/j.cellsig.2018.03.005
Zhang, C. Y., Yuan, W. G., He, P., Lei, J. H., and Wang, C. X. (2016a). Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 22 (48), 10512–10522. doi:10.3748/wjg.v22.i48.10512
Zhang, D., Cui, Y., Li, B., Luo, X., Li, B., and Tang, Y. (2016b). miR-155 regulates high glucose-induced cardiac fibrosis via the TGF-β signaling pathway. Mol. Biosyst. 13 (1), 215–224. doi:10.1039/c6mb00649c
Zhang, H., Zhou, Y., Wen, D., and Wang, J. (2023a). Noncoding RNAs: master regulator of fibroblast to myofibroblast transition in fibrosis. Int. J. Mol. Sci. 24 (2), 1801. doi:10.3390/ijms24021801
Zhang, J., Wang, H., Chen, H., Li, H., Xu, P., Liu, B., et al. (2022). ATF3 -activated accelerating effect of LINC00941/lncIAPF on fibroblast-to-myofibroblast differentiation by blocking autophagy depending on ELAVL1/HuR in pulmonary fibrosis. Autophagy 18 (11), 2636–2655. doi:10.1080/15548627.2022.2046448
Zhang, J., Zhou, Q., Wang, H., Huang, M., Shi, J., Han, F., et al. (2019a). MicroRNA-130a has pro-fibroproliferative potential in hypertrophic scar by targeting CYLD. Arch. Biochem. Biophys. 671, 152–161. doi:10.1016/j.abb.2019.07.003
Zhang, J. X., Lu, J., Xie, H., Wang, D. P., Ni, H. E., Zhu, Y., et al. (2019b). circHIPK3 regulates lung fibroblast-to-myofibroblast transition by functioning as a competing endogenous RNA. Cell Death Dis. 10 (3), 182. doi:10.1038/s41419-019-1430-7
Zhang, L., Chi, X., Luo, W., Yu, S., Zhang, J., Guo, Y., et al. (2020a). Lung myofibroblast transition and fibrosis is regulated by circ0044226. Int. J. Biochem. Cell Biol. 118, 105660. doi:10.1016/j.biocel.2019.105660
Zhang, L., Wang, X., He, S., Zhang, F., and Li, Y. (2023b). Gypenosides suppress fibrosis of the renal NRK-49F cells by targeting miR-378a-5p through the PI3K/AKT signaling pathway. J. Ethnopharmacol. 311, 116466. doi:10.1016/j.jep.2023.116466
Zhang, L., Yin, H., Jiao, L., Liu, T., Gao, Y., Shao, Y., et al. (2018b). Abnormal downregulation of caveolin-3 mediates the pro-fibrotic action of MicroRNA-22 in a model of myocardial infarction. Cell Physiol. Biochem. 45 (4), 1641–1653. doi:10.1159/000487732
Zhang, S., Gao, S., Wang, Y., Jin, P., and Lu, F. (2019c). lncRNA SRA1 promotes the activation of cardiac myofibroblasts through negative regulation of miR-148b. DNA Cell Biol. 38 (4), 385–394. doi:10.1089/dna.2018.4358
Zhang, S., Jia, X., Zhang, Q., Zhang, L., Yang, J., Hu, C., et al. (2020b). Neutrophil extracellular traps activate lung fibroblast to induce polymyositis-related interstitial lung diseases via TLR9-miR-7-Smad2 pathway. J. Cell Mol. Med. 24 (2), 1658–1669. doi:10.1111/jcmm.14858
Zhang, X., Chen, Q., Song, H., Jiang, W., Xie, S., Huang, J., et al. (2020c). MicroRNA‑375 prevents TGF‑β‑dependent transdifferentiation of lung fibroblasts via the MAP2K6/P38 pathway. Mol. Med. Rep. 22 (3), 1803–1810. doi:10.3892/mmr.2020.11261
Zhao, S., Xiao, X., Sun, S., Li, D., Wang, W., Fu, Y., et al. (2018). MicroRNA-30d/JAG1 axis modulates pulmonary fibrosis through Notch signaling pathway. Pathol. Res. Pract. 214 (9), 1315–1323. doi:10.1016/j.prp.2018.02.014
Zheng, D., Zhang, Y., Hu, Y., Guan, J., Xu, L., Xiao, W., et al. (2019). Long noncoding RNA Crnde attenuates cardiac fibrosis via Smad3-Crnde negative feedback in diabetic cardiomyopathy. Febs J. 286 (9), 1645–1655. doi:10.1111/febs.14780
Zhou, Y., Shiok, T. C., Richards, A. M., and Wang, P. (2018). MicroRNA-101a suppresses fibrotic programming in isolated cardiac fibroblasts and in vivo fibrosis following trans-aortic constriction. J. Mol. Cell Cardiol. 121, 266–276. doi:10.1016/j.yjmcc.2018.07.251
Zhu, W. S., Tang, C. M., Xiao, Z., Zhu, J. N., Lin, Q. X., Fu, Y. H., et al. (2016). Targeting EZH1 and EZH2 contributes to the suppression of fibrosis-associated genes by miR-214-3p in cardiac myofibroblasts. Oncotarget 7 (48), 78331–78342. doi:10.18632/oncotarget.13048
Zhu, Y., Feng, Z., Jian, Z., and Xiao, Y. (2018). Long noncoding RNA TUG1 promotes cardiac fibroblast transformation to myofibroblasts via miR-29c in chronic hypoxia. Mol. Med. Rep. 18 (3), 3451–3460. doi:10.3892/mmr.2018.9327
Zhu, Y., Pan, W., Yang, T., Meng, X., Jiang, Z., Tao, L., et al. (2019). Upregulation of circular RNA CircNFIB attenuates cardiac fibrosis by sponging miR-433. Front. Genet. 10, 564. doi:10.3389/fgene.2019.00564
Zou, Q., Wang, X., Yuan, R., Gong, Z., Luo, C., Xiong, Y., et al. (2023). Circ004463 promotes fibroblast proliferation and collagen I synthesis by sponging miR-23b and regulating CADM3/MAP4K4 via activation of AKT/ERK pathways. Int. J. Biol. Macromol. 226, 357–367. doi:10.1016/j.ijbiomac.2022.12.029
Keywords: non-coding RNAs, fibroblast, myofibroblast, fibrosis, therapies
Citation: Xing X and Rodeo SA (2024) Emerging roles of non-coding RNAs in fibroblast to myofibroblast transition and fibrotic diseases. Front. Pharmacol. 15:1423045. doi: 10.3389/fphar.2024.1423045
Received: 25 April 2024; Accepted: 01 July 2024;
Published: 24 July 2024.
Edited by:
Yaying Sun, Fudan University, ChinaReviewed by:
Aude Angelini, Baylor College of Medicine, United StatesAyyanar Sivanantham, Versiti Blood Research Institute, United States
Copyright © 2024 Xing and Rodeo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott A. Rodeo, cm9kZW9zQGhzcy5lZHU=
 Xuewu Xing
Xuewu Xing Scott A. Rodeo
Scott A. Rodeo