Abstract
Carbapenem-resistant (CR) Gram-negative bacteria have become a significant public health problem in the last decade. In recent years, the prevalence of CR bacteria has increased. The resistance to carbapenems could result from different mechanisms such as loss of porin, penicillin-binding protein alteration, carbapenemase, efflux pump, and biofilm community. Additionally, genetic variations like insertion, deletion, mutation, and post-transcriptional modification of corresponding coding genes could decrease the susceptibility of bacteria to carbapenems. In this regard, scientists are looking for new approaches to inhibit CR bacteria. Using bacteriophages, natural products, nanoparticles, disulfiram, N-acetylcysteine, and antimicrobial peptides showed promising inhibitory effects against CR bacteria. Additionally, the mentioned compounds could destroy the biofilm community of CR bacteria. Using them in combination with conventional antibiotics increases the efficacy of antibiotics, decreases their dosage and toxicity, and resensitizes CR bacteria to antibiotics. Therefore, in the present review article, we have discussed different aspects of non-antibiotic approaches for managing and inhibiting the CR bacteria and various methods and procedures used as an alternative for carbapenems against these bacteria.
1 Introduction
The spread of multidrug-resistant (MDR) bacteria is one of the most severe challenges to global health, raising questions about managing these pathogens (Moghadam et al., 2020; Tompkins and van Duin, 2021). Carbapenem resistance in Gram-negative pathogens provides a unique therapeutic problem, as carbapenems have long been regarded as the most productive and strong medicines against MDR Gram-negative pathogens (Doi, 2019). Carbapenems are considered the first line of treatment for infections caused by resistant bacteria, such as Klebsiella pneumonia, Acinetobacter baumannii, Escherichia coli, and Pseudomonas aeruginosa (Aurilio et al., 2022). Once regarded as the “last resort” antibiotics in many hospitals, this family is a powerful class of broad-spectrum antibiotics that inhibit penicillin-binding proteins, preventing the formation of cell walls (Papp-Wallace et al., 2011). Carbapenem-resistant (CR) bacteria are defined by the United States Centers for Disease Control and Prevention (CDC) as having in vitro resistance to at least one carbapenem (Tompkins and van Duin, 2021). Compared to drug-susceptible infections, the higher mortality, hospital stay duration, and CR bacteria expense make them a specific health concern (Zilberberg et al., 2017; Martin et al., 2018). Patients most susceptible to CR bacterial infections include those with underlying conditions and those with indwelling catheters or permanent devices (Voor in ‘t holt et al., 2014; Igbinosa et al., 2020). The carbapenem resistance can be mediated via increases in efflux pump expression, alteration of antibiotic binding targets, decreased membrane permeability and mutation or deletion of pore proteins, and finally, carbapenemase enzymes (Ruppé et al., 2015; Tompkins and van Duin, 2021). Carbapenemases are a diverse family of β-lactamases that have the power to hydrolyze and inactive several antibiotics, including carbapenems, cephalosporins, penicillins, and monobactam. (Tompkins and van Duin, 2021).
The conventional antibiotics that still have anti-CR activity, novel β-lactam-β-lactamase inhibitor combinations that have recently entered the market, and novel aminoglycosides, tetracyclines, and cephalosporins are just a few of the treatment classes that are currently available to treat CR infections (Tompkins and van Duin, 2021). Additionally, combinations of CR-active antibiotics with antibiotics with different mechanisms of action or with “repurposed” medicines from other classes have also shown some promise in treating CR infections (Peyclit et al., 2019). For instance, in vitro research demonstrates the effectiveness of mixing colistin with other antibiotics like clarithromycin, rifamycin, or the HIV medication azidothymidine to treat CR bacteria that are also colistin resistant (MacNair et al., 2018). Azidothymidine with tigecycline, pentamidine combined with tigecycline, rifampicin, amikacin, or tobramycin, and polymyxin B combined with sertraline, citalopram, or spironolactone are further combinations that have demonstrated in vitro efficacy against CR bacteria (Cebrero-Cangueiro et al., 2018). However, additional animal studies and clinical trials are required to determine the exact efficacy of these combination treatments in actual clinical infections; consequently, the utility of these combination regimens is currently speculative.
Therefore, antibiotic-based approaches were used in recently published studies to inhibit CR bacteria. However, antibiotics could lead to fatal side effects in patients. For instance, an inherent drawback of the extensive utilization of colistin is the elevated prevalence of toxicity, including renal and neurotoxicity, neuromuscular blockade, and occasionally fatal outcomes (Bialvaei and Samadi Kafil, 2015). Additionally, studies reported the quick spread of resistance to the newer CR-active antibiotics. For instance, a recently published study reported that while the general susceptibility to ceftazidime-avibactam is strong, there have been seen mutations that lead to resistance, particularly in bacteria carrying Klebsiella pneumoniae carbapenemase (KPC)-2 and KPC-3 enzymes (Hernández-García et al., 2021). To this end, researchers have considered non-antibiotic approaches, such as bacteriophages, natural products and compounds, nanoparticles, etc., for managing CR bacteria. In this regard, this review study aims to examine and discuss the use of the mentioned alternative solutions for inhibiting CR bacteria and destroying their biofilm community.
2 Phage therapy
Bacteriophages, or phages, are viruses that can kill bacteria without hurting eukaryotic cells. How bacteria become resistant to phages differs from how they become resistant to antibiotics. Because of this, phages have been used to treat MDR bacteria. Additionally, phage-antibiotic combination therapy may make antibiotic-resistant bacteria susceptible again to conventional antibiotics (Hagens et al., 2006; Chan et al., 2016). Bacteria could be killed more efficiently by a phage cocktail, mixing two or more phages with different host ranges in a single culture (Gu et al., 2012; Jaiswal et al., 2013). Phage cocktails may result in a more effective reduction in bacterial density and an improvement in the activities of the phages. From this perspective, earlier research has demonstrated that phage cocktails significantly reduce bacterial infections (Hall et al., 2012).
Recently published in vitro studies reported promising inhibitory effects for phage against CR Gram-negative bacteria, especially K. pneumoniae, A. baumannii, and P. aeruginosa (Table 1). Notably, phages have unwanted traits, including harboring drug resistance and virulence genes that limit their therapeutic application. In this regard, whole-genome sequencing is required to assess the phage genome for antibiotic resistance, toxin, virulence-associated genes, or lysogen-forming gene clusters. Furthermore, phage activity and stability are significantly influenced by the temperature at which they are stored. As a result, different pH and temperature ranges should be used to investigate the inhibitory action of phages. Moreover, phages’ antibacterial effectiveness in clinical situations is enhanced by their high adsorption rate and huge burst size (Mahichi et al., 2009; Li et al., 2020). Collectively, as mentioned, in addition to genome analysis, different characteristics, such as strong lytic activity, relatively broad host range, and high stability, should be evaluated in vitro phage studies.
TABLE 1
| Year of publication | Phage | CR-bacteria | Outcome | References |
|---|---|---|---|---|
| 2015 | BΦ-R3177 | A. baumannii | This phage showed high stability and lytic activity against host bacteria | Jeon et al. (2015) |
| 2016 | BΦ-C62 | A. baumannii | This phage managed intranasal bacterial challenge in mice and removed bacteria from the lung after 3 days | Jeon et al. (2016) |
| 2017 | ϕBO1E | K. pneumoniae clade II lineage of CG258 | This phage showed strict specificity for targeted bacteria and protected larvae from death following bacterial infection | D'Andrea et al. (2017) |
| 2018 | WCHABP1 and WCHABP12 | A. baumannii | Phage therapy was effective in treating infections in the G. mellonella larvae model | Zhou et al. (2018) |
| 2018 | vB_Kpn_F48 | K. pneumoniae Sequence Type 101 | This phage showed a short latent period, a narrow host range, and a low burst size | Ciacci et al. (2018) |
| 2019 | vB_EaeM_0Eap-3 | Enterobacter aerogenes | This phage showed an inhibitory effect against 18 of the 28 tested bacteria | Zhao et al. (2019) |
| 2019 | Henu1 | K. pneumoniae | This phage-infected bacteria strains with the capsular types K-1, K-2, and K-57 | Teng et al. (2019) |
| 2019 | Phage 117 and phage 31 | K. pneumoniae sequence type 11 | The phage cocktail indicated higher antibacterial function than phage 117 alone in LB culture | Tan et al. (2019) |
| 2020 | P509 | K. pneumoniae | This phage at different MOIs decreased the number of bacteria | Li et al. (2020) |
| 2020 | vB_KpnP_IME337 | K. pneumoniae | This phage exhibited an infection lifetime of approximately 90 min, with a latent period of 10 min. Additionally, it showed a high degree of specificity towards the host strain | Gao et al. (2020) |
| 2020 | kpssk3 | K. pneumoniae | This phage was able to lyse 92.59% of clinically isolated bacteria | Shi et al. (2020) |
| 2020 | vB_KpnS_Kp13 | K. pneumoniae K24 capsular type | This phage was effective against all VIM-producing bacteria | Horváth et al. (2020) |
| 2021 | BUCT556A | K. pneumoniae | This phage showed lytic activity against bacteria | Feng et al. (2021) |
| 2021 | TUN1 | K. pneumoniae | This phage indicated a narrow host range, as it could only lyse K64 K. pneumoniae strains | Eckstein et al. (2021) |
| 2022 | vB-AbaI-TMU2 |
A. baumannii
P. aeruginosa K. pneumoniae |
CR A. baumannii was inhibited by phage therapy, while other bacterial strains were resistant to phages | Esmaeili-Fard-Barzegar et al. (2022) |
| 2022 | P13 | K. pneumoniae | This phage indicated a large lytic plaque after overnight coculture with its host bacteria | Fang and Zong (2022) |
| 2022 | Eight different phages | K. pneumoniae | All of the phages significantly decreased the number of bacteria | Baqer et al. (2022) |
| 2023 | Abp95 | A. baumannii | The phage showed a beneficial effect on wound healing in a diabetic mouse wound infection model by effectively eliminating local infections | Huang et al. (2023a) |
| 2023 | vB_PseuP-SA22 | P. aeruginosa | This phage decreased the number of live bacteria (five logs) in the biofilm community | Teklemariam et al. (2023) |
| 2023 | vB_KpnS_SXFY507 | K. pneumoniae | This phage showed antibacterial activity and increased Galleria mellonella larvae survival rate after infection | Feng et al. (2023) |
| 2023 | vB_KshKPC-M | K. pneumoniae | This phage showed high killing activity against planktonic and biofilm forms of bacteria | Mohammadi et al. (2023) |
The activities of phages against carbapenems-resistant bacteria.
CR: carbapenems-resistant. MOI: Multiplicity of infection. VIM: Verona integron-encoded metallo-β-lactamase. LB: luria broth.
Because the biofilm community is one of the most significant problems in treating CR bacterial infection, phage interactions with biofilm are an essential topic in vitro investigation. Due to the inability of antibiotics to penetrate the complex polysaccharide matrix (glycocalyx) of biofilms, they are 10–1,000 times more resistant to antibiotics than planktonic organisms. To that purpose, recent research found that phages could limit biofilm formation and remove the mature biofilm of CR bacteria (Wu et al., 2019; Santiago et al., 2020; Mulani et al., 2022). Notably, phages can enter the biofilm and obliterate its structure by triggering the production of enzymes such as polysaccharide depolymerase. Besides, this enzyme can specifically destroy the host bacterial envelope’s macromolecule carbohydrates (Yan et al., 2014). The findings of the experiments showed that natural lytic phage can diminish the biofilm community of CR bacteria by phage-induced lysis and exopolysaccharide degradation (Vukotic et al., 2020; Hao et al., 2021; Li J. et al., 2021; Li M. et al., 2021).
Depolymerase pretreatment of the biofilm followed by other antibacterial agents may be a viable alternative for managing bacterial biofilm. In a study, the scientists managed the MDR K. pneumonia biofilm using ciprofloxacin, recombinant phage-encoded enzyme, and lytic phages (producing and non-producing depolymerase). The results demonstrated that ciprofloxacin and depolymerase-producing phage were the most effective antibiofilm combination against bacterial biofilm (Latka and Drulis-Kawa, 2020). Additionally, Wu et al. reported that depolymerase could enhance the polymixin activity against K. pneumoniae biofilms when combined with antibiotics (Wu et al., 2019). Besides, another experiment showed that capsule depolymerase could make CR K. pneumoniae fully susceptible to the killing effect of serum complement (Liu Y. et al., 2020). Therefore, phage depolymerase should be considered for managing CR K. pneumoniae biofilm; however, more confirmatory studies are required.
The combination of phages with antibiotics was also used to manage CR bacteria. For instance, in 2022, a study’s findings demonstrated that combining a two-phage cocktail and imipenem effectively delayed carbapenemase growth-producing K. pneumoniae (Michodigni et al., 2022). Another study reported that combined usage of gentamycin and phage treated the mice with acute pneumonia caused by MDR K. pneumoniae (Wang et al., 2021). Consistent with these findings, using phage in conjunction with colistin was more effective at preventing the growth of CR A. baumannii than either treatment alone (Wintachai et al., 2022).
Although the precise mechanism of the interaction between phages and antibiotics has not yet been determined, recent investigations have indicated various potential pathways. The sensitivity of the chosen antibiotic to the particular bacterial strains following phage activity may cause the synergistic activity between antibiotics and phage cocktails. Phage-resistant bacterial strains are more vulnerable to antibiotics and develop more slowly than wild ones. Phages particularly alter the bacterial surface structures (outer membrane proteins, polysaccharides, etc.), removing obstacles to the entry of various antibiotics, demonstrating that phages have the impact of increasing bacterial antibiotic sensitivity (Wang et al., 2021; Michodigni et al., 2022; Wintachai et al., 2022). Notably, during the last stages of the replication cycle, phages produce endolysins to breach the bacterial cell wall and produce offspring virion. Endolysins demonstrated effective inhibition of Gram-positive bacteria, but their ability to inhibit Gram-negative bacteria was constrained by the existence of the outer membrane (Schmelcher et al., 2012; Baliga et al., 2022). Colistin can enhance endolysins’ capacity to overcome the outer membrane’s impermeability (Baliga et al., 2022).
Phage-encoded depolymerases play a role in the degradation of the host bacterium’s EPS, LPS, and capsular polysaccharides during phage invasion. Hence, phages can dismantle the biofilm architecture and enhance the infiltration of antibiotics into the inner layers of the biofilm by stimulating the production of enzymes like polysaccharide depolymerase. Therefore, antibiotic-phage combination therapy shows potential as a treatment strategy for controlling CR bacteria, including their biofilm population (Hanlon, 2007).
As mentioned in the previous paragraphs, in vitro studies reported different phages for managing CR bacteria. Moreover, other studies evaluated the function of phages against CR bacteria in animal studies and clinical seating. In this regard, intraperitoneally injection of phages, controlled K. pneumoniae infection in mice. Another study also showed that phages distributed more rapidly into the systemic circulation via the intraperitoneal route than the oral route (Dhungana et al., 2021; Shi et al., 2021; Bai et al., 2022; Li et al., 2022b). Liang et al. reported phage therapy leads to a better survival rate in mice with CR K. pneumoniae bacteremia than ceftazidime/avibactam and tigecycline (Liang et al., 2023). Another investigation used intra-rectal and oral therapy with a custom-made phage to treat patients with multi-site colonization of CR K. pneumoniae (Corbellino et al., 2020). In addition to K. pneumoniae, phage therapy was used to manage CR A. baumannii and P. aeruginosa infection in animal models and clinical settings. To this end, phage therapy successfully manages CR A. baumannii acute pneumonia and lung infection in mice (Hua et al., 2017; Jeon et al., 2019).
Furthermore, phage therapy indicated promising results in treating patients with CR A. baumannii lung infection (Tan et al., 2021; Wu et al., 2021). Finally, two-phage cocktails formulated as hydrogels inhibited CR P. aeruginosa wound infection in animal models. Combined phages and conventional antibiotics successfully managed patients with empyema caused by this bacterium (Chen et al., 2022).
Therefore, in addition to in vitro studies, animal models and preclinical studies also reported promising effects for phage therapy against CR bacteria. Nevertheless, phages still possess constraints in their actual implementation in clinical settings. Phage formulations exhibit varying in vivo pharmacokinetics and pharmacodynamics compared to antibiotic therapy. Since the preparations of different phages have distinct biological characteristics, there are significant variations in actual clinical applications. There is a lack of established guidelines regarding the optimal dosage, duration, and method of administering phage therapy, and there are no conventional treatment protocols for phage therapy. Furthermore, when phage preparations are made, specific endotoxins are created that could be cytotoxic and immunogenic. In the end, certain bacteria have acquired resistance to phage infection through various mechanisms, including adsorption resistance, spontaneous mutations, receptor and penetration blocking systems, and adaptive immunity linked to CRISPR/Cas systems (Hibstu et al., 2022). Hence, although phage therapy showed promising effects for managing CR bacteria, the mentioned issues should be evaluated in future studies.
3 Natural products
Research has demonstrated that plant-derived substances, such as essential oils (EOs), extracts, and pure chemicals, substantially impact bacteria and their biofilm community. The bioactive constituents derived from various plant components, including roots, leaves, and fruits, possess therapeutic characteristics and exhibit distinct medicinal effects upon modification (Ahmad et al., 2015; Rangel et al., 2018). To this end, recently published studies used different natural products to inhibit CR bacteria and their biofilm community. The exact interaction of natural products and CR bacteria is not elucidated yet, but in this section, we will discuss some of the most important antibacterial mechanisms of natural products.
According to research, eugenol, a phenolic aromatic compound primarily derived from cinnamomum and clove essential oils, destroys the membrane integrity of CR K. pneumoniae by producing reactive oxygen species (ROS), and glutathione depletion, causes the leakage of bacterial cytoplasmic components like protein, β-galactosidase, and DNA. Moreover, when eugenol comes into contact with bacterial biofilm, the entire matrix’s thickness diminishes, and its integrity is lost (Liu et al., 2023). Another investigation also reported that the cell membrane of CR K. pneumoniae was harmed by eugenol, as indicated by a drop in intracellular ATP concentration, a reduction in intracellular pH, cell membrane hyperpolarization, and an increase in membrane permeability. In addition, eugenol disrupted the cellular structure and caused the loss of internal components in CR K. pneumoniae (Qian et al., 2020).
Thymol and carvacrol, the main ingredients of different EOs of various aromatic plants, also showed promising inhibitory effects against CR bacteria. In this regard, the findings of the studies showed that the lipophilic nature of these compounds and their accumulation in cell membranes are related to their antibacterial properties. This interaction inhibits electron transport for energy production and disrupts the proton motive force, synthesis of cellular components, and protein translocation. Cell lysis and death may occur as a result of these physiological changes. Lipopolysaccharide (LPS), a powerful barrier for hydrophobic compounds, including hydrophobic antibiotics, is found in the outer membrane of Gram-negative bacteria. Thymol and carvacrol, lipid-based compounds made from γ-terpinene, can assist in transporting hydrophobic antibiotics inside cells (Nazzaro et al., 2013; Kwiatkowski et al., 2022). In line with these results, De Souza et al., after observation of carvacrol inhibitory effect against carbapenemase (KPC)-producing K. pneumoniae, supposed that the inhibitory effects of carvacrol can be attributed to its interactions with the structural and functional properties of the cytoplasmic membrane. Carvacrol interacts with the lipid bilayer and positions itself between fatty acid chains, causing the expansion and destabilization of the cytoplasmic membrane (de Souza et al., 2021). Additionally, carvacrol and other hydrophobic substances may enter the bacterial cell’s outer membrane pores and the periplasmic region. Carvacrol fits between fatty acid chains because it can bind to hydrogen, letting ions leave the cytoplasm. Cell membrane destabilization makes membranes more fluid and cells more permeable (Amaral et al., 2020).
Phytol, a diterpenes alcohol from chlorophyll widely used as a food additive and in medicinal fields, is also reported as the antibiofilm agent, inhibiting exopolysaccharide production as well as initial cell attachment, hypermucoviscosity, and curli expression in CR K. pneumoniae (Adeosun et al., 2022b). In another experiment, the authors used linalool to inhibit this bacterium. The results demonstrated a notable decrease in the quantity of cytoplasmic and membrane proteins, suggesting that the cells of KPC- K. pneumoniae treated with linalool had damage to their membranes. The presence of oxidative stress was confirmed by the downregulation of proteins sensitive to oxidative stress and the overexpression of proteins that regulate oxidative stress. The zeta potential measurement and outer membrane permeability assay demonstrated that linalool enhances the bacterial surface charge and the membrane’s permeability. Linalool therapy detected intracellular leakage of nucleic acid and proteins (Yang et al., 2021).
Finally, Yang et al. reported that cinnamomum has antimicrobial effects on KPC- K. pneumoniae cells by disrupting their cell membranes. Proteomic profiling reveals that the membrane damage is caused by oxidative stress. Cinnamomum treatment disrupted the production process of the plasma membrane, cell wall, and outer membrane, impairing the structural repair system. The oxidation due to this process damages the bacterial membrane, eventually allowing ROS to enter the cells. Simultaneously, it also causes the leakage of intracellular contents. ROS causes genetic damage and hinders the functioning of DNA and membrane repair mechanisms (Yang S. K. et al., 2019). Recent investigations have collectively demonstrated that natural products induce oxidative stress, damage bacterial membranes, cause cellular leakage, and result in cell death.
It is noteworthy to mention that new antimicrobial agents are required to reduce the toxicity of conventional antibiotics. Moreover, combination therapy could enhance the efficacy of different antibiotics (Pinto et al., 2009; Ahmad et al., 2010). In this concept, the combined use of natural products and other antibiotics was considered to inhibit CR bacteria and their biofilm community. To this end, Yadav et al. reported that a water-soluble curcumin derivative inhibited the AcrAB-TolC efflux system in MDR K. pneumoniae by disrupting the membrane potential and causing depolarization. Combining this compound and meropenem was highly synergistic, reducing drug dose regimes and toxicity (Yadav et al., 2021). In line with these results, a study published in 2020 also reported that a combination of colistin + curcumin showed a remarkable reversal in colistin minimum inhibitory concentration (MIC) in Enterobacteriaceae. Noteworthy, the authors proposed that efflux inhibition is the primary mechanism responsible for curcumin’s synergistic and modulation ability (Sundaramoorthy et al., 2020). It can be concluded that curcumin can shut down the efflux system because almost all efflux pump systems need energy (ATP) for their functions, and the curcumin that inhibits the proton motive force will inhibit ATP generation. Therefore, this natural compound could improve the activity of antibiotics by targeting efflux pumps.
In another investigation, the researchers reinstate the efficacy of carbapenem against CR K. pneumoniae by employing celastrol and thymol. The results of this study indicate that celastrol alone did not have an impact on meropenem-MIC, and when combined with thymol, it only caused a 2-fold drop. However, both celastrol and thymol resulted in a significant decrease of 4–64 folds in meropenem-MIC. Celastrol effectively inhibited carbapenemase activity, but its access to the target was hindered by the outer membranes of Gram-negative bacteria, which act as a barrier to its penetration. However, thymol exerts its effects by disrupting the outer membranes and porins through its lipophilic action. It does this by integrating into the polar head groups of the lipid bilayer, which leads to changes in the permeability of the cell membrane. In summary, thymol enhances the capacity of celastrol and meropenem to pass through the CR K. pneumoniae. Additionally, celastrol suppresses carbapenemase-hydrolytic activity. Hence, thymol-meropenem-celastrol combination therapy can efficiently kill CR bacteria (Abdel-Halim et al., 2022).
Furthermore, a recently published study reported synergistic effects of polymyxin B in combination with Cinnamomum cassia L. EO (CEO) against carbapenemase-producing Serratia marcescens and K. pneumoniae. Notably, the CEO successfully suppressed the germs stated and achieved this suppression by combining with polymyxin B at a lower dosage of antibiotics. It is possible to assume that CEO caused damage to cell membranes, and this damage destabilized the outer membrane of the carbapenemase-producing bacteria, which then allowed polymyxin B to enter the periplasm of the cell. As a result, the outer membrane loses its integrity, leading to the leakage of cellular contents and ultimately causing cell death (Vasconcelos et al., 2020).
In the end, fisetin (1; 3,7,3′,4′-tetrahydroxyflavone) is a type of flavonoid that has been shown to have anticancer, antiangiogenic, antiviral, anti-invasive, and anti-aging effects. These features are due to its property of creating free radicals (Jash and Mondal, 2014). Until now, various experiments have been conducted to measure the effectiveness of fisetin in treating diseases caused by bacterial pathogens. The studies showed that fisetin has activities against the lipopolysaccharide of Gram-negative bacteria and can reduce the activity of pathogens in human cells. In addition, fisetin significantly suppressed the expression of nitric oxide (NO), prostaglandin E2 (PGE2), and cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-), which are pro-inflammatory factors. Fisetin can also inhibit the biofilm formation of bacterial cells such as MDR A. baumannii; however, the exact interaction of fisetin and the biofilm community of this bacterium was not reported (Raorane et al., 2019).
Studies reported that fisetin inhibits CR K. pneumoniae (Adeosun et al., 2022a; Zhang et al., 2022c). One of the main classes of beta-lactamases is OXA-48, which was identified in Turkey in 2001 and spread worldwide. This enzyme makes the organism resistant to carbapenems and penicillins but cannot hydrolyze cephalosporins (Poirel et al., 2004). A study showed that fisetin effectively restored the antibacterial efficacy of piperacillin or imipenem against E. coli producing OXA-48, resulting in a 2–8-fold reduction in minimum inhibitory concentration (MIC) (Zhang et al., 2022b). In line with these findings, Adeosun et al. introduced fisetin as the best anti-CR K. pneumoniae, demonstrating a MIC value of 0.0625 mg/mL. This compound inhibited curli expression, a type of fimbriae composed of proteins called curlins and functional amyloid surface fiber, and reduced hypermucoviscosity (Adeosun et al., 2022a). Finally, a recently published study evaluated fisetin’s effect on NDM-producing E. coli. Molecular dynamics simulations revealed that fisetin successfully inhibits the hydrolytic activity of NDM-1. Notably, the mutation of NDM-1 resulted in a decreased inhibition of NDM-1 activity by fisetin compared with the wild-type protein. To this end, the authors proposed that fisetin is an effective NDM-1 inhibitor, which suggests the combination of this compound with meropenem is a promising strategy for CR bacterial infection (Guo et al., 2022). Therefore, fisetin can be employed as a model in the search for new medications or as an alternative in regulating the pathogenicity of CR K. pneumoniae.
Therefore, as mentioned, natural compounds could inhibit CR bacteria and enhance the performance of antibiotics against these bacteria (Figure 1). Nevertheless, the effectiveness of natural compounds is frequently impeded by their low solubility in water, tendency to evaporate, and vulnerability to degradation by light and oxidative substances. The use of different drug platforms may resolve these limitations. For instance, Tayeb et al. found that the developed nanoemulsion significantly improved the antibacterial effectiveness of meropenem and clove EO. This nanoemulsion showed promise as a carrier for antimicrobial substances (Tayeb et al., 2022). Besides, chitosan-coated nanoemulsion showed potential and effective intranasal formulation against CR A. baumannii and K. pneumoniae (Rinaldi et al., 2020). Therefore, due to their strong physical and chemical properties and ability to kill bacteria, drug delivery systems have the potential to enhance treatment choices for human infections and serve as more effective carriers for drugs with limited bioavailability. Implementing this approach might mitigate drug toxicity and prolong the efficacy of antibacterial treatments that are already on the market. Due to their high cellular absorption and controlled release distribution, nanostructured devices have been devised to encapsulate EO to increase their bioavailability and bioefficacy. However, data about nano-platform usage for enhancing the efficacy of natural products against CR bacteria is limited, and more confirmatory studies are needed in this field.
FIGURE 1
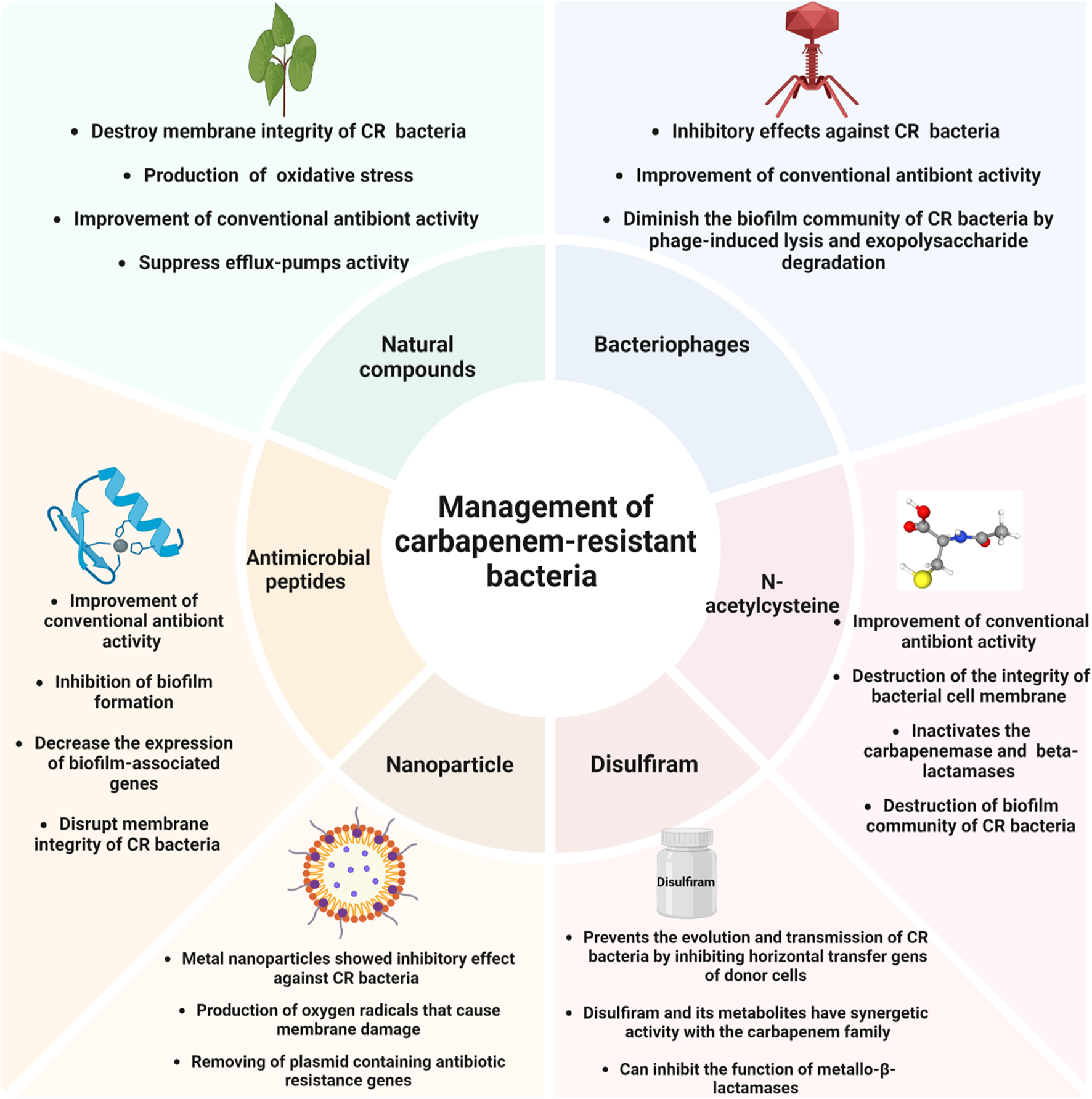
Non-antibiotic approaches for managing carbapenem-resistant bacteria.
4 Disulfiram
Disulfiram (Tetraethylthiuram disulfide), primarily known as “Antabuse,” was first introduced in 1981 as a drug for the treatment of chronic alcoholism patients by inhibiting the function of erythrocyte aldehyde-dehydrogenase enzyme by producing acute sensitivity to alcohol (Kleczkowska et al., 2021). Later, it was introduced to have toxic activity against lower forms of life utilizing copper-containing respiratory enzymes and have inhibitory function against copper-chelating enzymes, especially in fungal and bacterial cells (Tisato et al., 2010; Dubey et al., 2022). The antibacterial activity of disulfiram and its derivatives has been proven in recent years. For example, Thakare et al. reported the antibacterial activity of disulfiram, which could successfully cause the disappearance of Staphylococcus aureus biofilm and its intracellular population (Thakare et al., 2019).
In addition, disulfiram prevents the evolution and transmission of CR bacteria by inhibiting horizontal transfer genes of donor cells through plasmid to another cell and minimizing the spread of meropenem-resistant cells. Moreover, disulfiram and its metabolites have synergetic activity with the carbapenem family, such as meropenem, and have a destructive effect on the biofilm formation of CR bacteria. In one study conducted on CR bacteria, it was shown that not only disulfiram could enhance the antibacterial activity of meropenem and colistin against bacteria but also increase the potent ability of colistin by damaging bacterial cell membranes (Chen et al., 2023). A study by Dubey et al. aimed to investigate the effect of meropenem in combination with disulfiram against CR A. baumannii infections. The results reported that disulfiram has a successful synergetic effect on New-Delhi metallo beta-lactamase (NDM) and IMP-type metallo-β-lactamases. The mechanism is assumed that disulfiram binds to NDM and chelates the Zink active site. It also makes a disulfide bond through its Cys208 residue, which has an inhibition function in the meropenem hydrolysis process (Dubey et al., 2022). Other similar studies were also done to determine the activity of disulfiram on the inhibition of CR bacteria. The results showed that using disulfiram as an adjuvant increases the drug’s efficacy (Chen et al., 2023). Therefore, as mentioned, disulfiram can inhibit the function of metallo-β-lactamases in CR bacteria and improve the function of carbapenems against these bacteria. However, there is limited data about the interactions of disulfiram and CR bacteria; therefore, this compound should be considered for future studies on managing CR bacteria.
5 Metal nanoparticles
Recent advances in nanotechnology have provided the ability to prepare nanoparticles of specific size and shape, which can help develop new antibacterial agents. The advantage of nanomaterials over traditional antibiotics is due to their surface area and chemical reactivity compared to bulk materials (Shariati et al., 2022; Hosseini et al., 2023b; a). Therefore, nanoparticles have attracted a lot of attention in biomedicine. Metal nanoparticles became the main focus of many biomedical applications, including antibacterial agents, due to their tunable shape- and size-dependent properties. Due to the antimicrobial nature of metal nanoparticles such as copper (Cu), silver (Ag), zinc (Zn), iron (Fe), and titanium (Ti), they can be used against MDR bacteria (Huh and Kwon, 2011). The critical point is that biogenic nanoparticles have long-term stability and are biocompatible; therefore, they are used primarily for antimicrobial applications. The mechanisms of antimicrobial action of biogenic nanoparticles include metal ion release, oxidative stress, and non-oxidative stress that can occur simultaneously (Huh and Kwon, 2011; Fernandez-Moure et al., 2017).
The findings showed that combining antibiotics with biogenic metal nanoparticles can be useful for increasing their antimicrobial activity. Additionally, by examining nanoparticles synthesized by biological and chemical methods, it was found that biological nanoparticles have a better antimicrobial effect than nanoparticles synthesized by chemical methods (Singh et al., 2018).
5.1 Effect of silver nanoparticles on carbapenem-resistant bacteria
One of the causes of death due to A. baumannii is resistance to most of the antibiotics used for treatment. Resistance to carbapenem, the most effective beta-lactam antibiotic for Acinetobacter, is a significant challenge and concern. Lung inflammation is one of the important symptoms of pneumonia caused by A. baumannii, which leads to the destruction of the epithelial barrier. The interaction between this bacterium and human lung cells (alveolar epithelial) due to its adhesion and invasion of these cells leads to infection and causes cell death (Lee et al., 2001; Choi et al., 2008). One of the reasons for the pathogenicity of A. baumannii is its ability to survive in human lung cells. Therefore, investigating this bacterium’s interaction with the host cell can be helpful. Silver nanoparticles (AgNPs) have antibacterial properties against different organisms due to their different mechanisms of antimicrobial action. Polyvinylpyrrolidone (PVP) is a stabilizer with minor sensitivity to pH changes and surface charge changes. Recent reports have shown that AgNPs conjugated with PVP are more stable than other AgNPs; it has also been found that PVP-conjugated AgNPs are less toxic to mammalian cells (Tiwari et al., 2017). Studies have reported that AgNPs conjugated with PVP have better antimicrobial activity in vivo than other AgNPs. Hence, they can be used as alternatives to carbapenems (Gnanadhas et al., 2013).
The study of A. baumannii infection in the human lung host cell (A-549) is a good model for surface interaction with bacteria. In a study, AgNPs conjugated with PVP were synthesized using chemical methods, and their effectiveness against CR A. baumannii was investigated (Tiwari et al., 2012). The survey of this cell type showed that during bacterial infection, about 40% of the bacteria adhered to the A-549 cell line, while 20% entered the cell, causing a threefold increase in the production of ROS. In this regard, 30 μM PVP-AgNPs showed antibacterial activity against CR A. baumannii strain, and this concentration had no cytotoxic effect on the human lung cell line. Noteworthy, the results of this study showed that during A. baumannii infection, ROS concentrations increased up to threefold. PVP-AgNP treatment causes a decrease of about 80% in the viability of intracellular bacteria (Tiwari et al., 2012). Therefore, it can be concluded that AgNPs conjugated with PVP can be a suitable alternative to the current antibiotics used against CR A. baumannii.
Metals and their alloys, synthetic and natural polymers, have clear characteristics that make them candidates for biomedical applications. Among these metal nanoparticles, metal oxides, such as zinc oxide (ZnO), have attracted much attention today because they can be stable at low temperatures and in various conditions (Tiwari et al., 2018). One of the remarkable points of ZnO particles is that they have antibacterial activity against Gram-negative and Gram-positive bacteria and also activity against bacterial spores (Hoseinzadeh et al., 2014). It has been pointed out that ZnONPs are low toxicity, biocompatible, and bio-safe. Considering that the mechanism of antimicrobial activity of ZnONPs has not been well explained, several studies have suggested that the production of hydrogen peroxide can be one of the main factors of its antimicrobial activity, and also, the binding of ZnONPs on the surface of bacteria can have an inhibitory effect (Hoseinzadeh et al., 2014). To this end, Vishvanath et al. have investigated the antimicrobial activity of ZnONPs against CR A. baumannii (Tiwari et al., 2018).
ZnONPs can be used as an alternative to carbapenem antibiotics that inhibit the growth of CR A. baumannii by producing oxygen radicals that cause membrane damage. Therefore, ZnONPs can be considered an alternative carbapenem antibiotic drug against CR A. baumannii. The virulence of A. baumannii is influenced by its ability to survive in human lung cells; therefore, it is crucial to study the effect of zinc oxide in the interaction of A. baumannii with human lung host cells. Targeted delivery of nanoparticles to lung cells in an animal model requires further studies to make it a suitable drug against A. baumannii. Also, to identify proteins involved in the mechanism of action of this nanoparticle, an accurate proteomic analysis of A. baumannii in the presence of ZnO is needed (Tiwari et al., 2018).
Drug resistance traits are rapidly spread among bacteria by horizontal gene transfer, especially through plasmids. Pectin-coated platinum nanoparticles (ptNps) at a concentration of less than 20 μM are effective in removing the plasmid containing extended-spectrum beta-lactamase (ESBL) in E. coli (Bharathan et al., 2019). Plasmid curing means plasmid loss from bacterial strain due to treatment with different compounds. Plasmid removal from the host mainly occurs by two mechanisms: 1) inhibition of plasmid replication and 2) interfering with plasmid segregation. So far, many plasmid curing agents are known, including ethidium bromide surfactants such as SDS, glycine, organic heterocyclic compounds, acridine orange, and specific plant metabolites, such as plumbagin. Most of these factors were only in the environment. Either they were toxic in vivo, or their efficacy as in vivo plasmid curing agents has not been investigated previously (Molnar et al., 1977; Crameri et al., 1986; Spengler et al., 2006).
Bharathan et al. developed pectin-capped platinum nanoparticles (PtNPs) to treat fish infected with CR bacteria and rescue fish from infection without additional toxicity (Bharathan et al., 2019). PtNPs controlled the infection in fish and enhanced the adaptive immune response against pathogen re-entry; thus, the fish survived repeated infections. PtNPs can act as a plasmid removal agent in the clinical isolate of MDR E. coli in a fish infection model. Investigations by PCR method showed that the plasmid contains bla-OXA23, blaNDM-5, and bla OXA-48 genes that can encode carbapenem resistance. Various techniques are used to investigate the plasmid curing mechanism, such as membrane permeability, TEM imaging, ROS production, and membrane potential integrity (Buckner et al., 2018). It shows that sub-MIC levels of PtNPs interact with the cell surface and compromise the integrity of the inner membrane. Gyrase inhibition assay showed that treatment of bacteria with PtNP in the presence and absence of gyrase caused DNA cleavage even at concentrations lower than the MIC, which may account for the ability of PtNPs to eliminate plasmid (Bharathan et al., 2019).
PtNPs at a concentration of less than 20 µM caused the formation of colonies with small morphology and decreased the growth of CR in laboratory conditions and the body. The treated strain (lacking plasmid) had less resistance to meropenem and ceftriaxone than the wild type. Also, the treated strain showed a 50% reduction in biofilm formation compared to the wild type. This study demonstrated for the first time that Biogen’s PtNPs induced selective plasmid loss from E. coli strain U3790, leading to a significant decrease in MICs for meropenem and ceftriaxone. The absence of the plasmid leads to the formation of small colony variants, which have slower growth. Importantly, this study showed that nanoparticles are non-toxic and can cause plasmid loss in vivo. Its combined administration with meropenem causes a significant reduction in bacterial load compared to treatment with meropenem alone (Bharathan et al., 2019).
6 N-acetyl cysteine
N-acetyl cysteine (NAC) is the N-acetyl form of the amino acid L-cysteine. While it is not classified as an antibiotic, it does have antibacterial capabilities and the ability to destroy biofilms. Additionally, it has shown promise in removing bacteria already attached to stainless steel surfaces (Olofsson et al., 2003; Zhao and Liu, 2010; Wendorf et al., 2015). Different studies have demonstrated the in vitro efficacy of NAC in inhibiting the growth of bacteria and preventing the formation of biofilms. These effects have been observed in Gram-positive and Gram-negative microorganisms, including Burkholderia cepacia complex, Stenotrophomonas maltophilia, and P. aeruginosa (Parry and Neu, 1977; Roberts and Cole, 1981; Alfredsson et al., 1987; Blasi et al., 2016; Pollini et al., 2018b; Aiyer et al., 2021; Alarfaj et al., 2022).
NAC possesses antioxidant and anti-inflammatory characteristics by enhancing glutathione production, which helps neutralize harmful oxygen radicals and counteract the effects of proinflammatory cytokines (Zafarullah et al., 2003; Tenório et al., 2021). The NAC counteracts the harmful effects of free radicals, diminishes oxidative stress and inflammation, and enhances the functioning of the immune system. In addition, NAC exhibits vasodilatory effects on microcirculation, leading to an improvement in locoregional blood flow (Forman et al., 2009; Chertoff, 2018). The events mentioned above can have significant ramifications in an unregulated host response to infection, characterized by a high release of pro-inflammatory cytokines, ROS, and profound disruption of microcirculation, as observed in septic shock (Ince, 2005; Ait-Oufella et al., 2010; Angus and Poll, 2013; Ince et al., 2016). In this regard, animal studies have shown that NAC improves organ damage caused by endotoxin shock by reducing the formation of free radicals and inflammatory cytokines (Hsu et al., 2006). Therefore, NAC can serve as a valuable adjunctive therapy in infectious disorders, mitigating organ damage and protecting against septic shock. Given that septic shock is distinguished by excessive and unbalanced production of pro-inflammatory cytokines, ROS, and significant disruption of circulation, the use of substances that can counteract these effects is justified in treating this illness (Oliva et al., 2021).
As mentioned, NAC is a substance that has antioxidant and anti-inflammatory properties. It can be utilized alongside antimicrobial therapy to treat severe infections caused by MDR organisms, such as CR K. pneumoniae and CR A. baumannii. To this end, significant changes in the bacterial structure were observed when the K. pneumoniae strain, which is highly resistant to carbapenem and colistin, was exposed to NAC alone or in combination with low concentrations of meropenem. These changes included elongation of the bacteria, disruption of cell integrity, breakdown of the outer cell wall or inner membrane, and forming outer membrane vesicles (OMVs) (De Angelis et al., 2022). Another study also indicated that NAC showed strong antibacterial effects against CR K. pneumoniae and CR A. baumannii in a way that depended on the concentration. Additionally, NAC showed excellent synergy with both meropenem and ampicillin/sulbactam by restoring their susceptibility (Oliva et al., 2023).
De Angelis et al. discovered a strong synergy between NAC and meropenem when used against clinical strains of CR bacteria. Similarly, Pollini et al. observed a significant synergistic effect when combining colistin with NAC against CR A. baumannii (Pollini et al., 2018a; De Angelis et al., 2022). Additionally, the Ceftazidime/avibactam + NAC combination significantly deteriorated the integrity of bacterial cell membranes (Huang Z. et al., 2023). Therefore, as mentioned, NAC can effectively control CR bacteria and enhance the efficacy of traditional antibiotics against these bacteria. The presence of a NAC thiol group can change the redox state of bacterial periplasm. This change deactivates a controlled mechanism and causes proteins to misfold. These misfolded proteins then build up in the cytoplasm and are released through the creation of OMV (Volgers et al., 2017). Periplasmic bacterial enzymes, including carbapenemase and other beta-lactamases, undergo regulation through a sophisticated regulatory system. In the presence of NAC, these enzymes are deactivated due to protein misfolding, resulting in the loss of their function (De Angelis et al., 2022).
The biofilm community of CR bacteria is also a critical factor for antibiotic resistance in clinical settings. The mucolytic effects of NAC are attributed to its free sulfhydryl group. This group is responsible for breaking the disulfide bonds present in mucus, decreasing its viscosity (Olofsson et al., 2003; Zhao and Liu, 2010; Wendorf et al., 2015). The functional group -SH can disrupt the disulfide bridges of proteins found in the bacteria, causing a loss of their three-dimensional structure and ultimately rendering them inactive. According to these results, it may be inferred that NAC acts by chemically altering the structure of the biofilm and could potentially be a significant agent for combating bacterial biofilms (Samuni et al., 2013; Temel and Erac, 2022). In this regard, Feng et al. conducted a study to examine the impact of NAC, both alone and in combination with tigecycline, on A. baumannii biofilms. They found that the presence of NAC alone and NAC + tigecycline combinations at low concentrations dramatically reduced the creation of biofilms by the isolates (Feng et al., 2018). In line with these results, another study also reported that NAC + tigecycline combinations could significantly reduce the biofilm formation of CR A. baumannii strains (Temel and Erac, 2022). The study also examined the impact of combining NAC and tigecycline on the expression of A. baumannii biofilm-related genes, including bap (Biofilm associated cell surface protein) and csuE (Pilus formation). Following exposure to drug combinations, notable decreases were found in the expressions of the bap and csuE genes in the strains. The decrease in expression levels may be attributed to the direct impact of the drugs on transcription factors associated with the relevant genes, or it could result from overall inhibition in the quorum-sensing process (January 2017). Therefore, NAC can inhibit the growth of CR bacteria, improve the activity of conventional antibiotics, and destroy the biofilm community of these bacteria. However, data about NAC interaction with CR bacteria are limited, and more confirmatory studies are needed.
7 Antimicrobial peptides
Antimicrobial peptides (AMPs) are a group of basic polypeptides consisting of 12–50 amino acid residues. They have significant functions in both innate and adaptive immunity (Takahashi and Yamasaki, 2020; Moghadam et al., 2022). Natural AMPs are found in vertebrates, plants, and small organisms such as bacteria and fungi (Wang et al., 2016). Hence, AMPs are a group of molecules found in the innate immune system that can kill microorganisms and regulate the immune response. They are the initial defense mechanism against invading pathogens (Falanga and Galdiero, 2017).
Studies have shown that AMPs can successfully kill drug-resistant bacteria (Magiorakos et al., 2012; Choi et al., 2021). Noteworthy, AMPs can penetrate or interact with the biofilms created by drug-resistant bacteria, or they can enhance the effectiveness of conventional antibiotics through a synergistic effect (Martinez et al., 2019a). A study showed that combining PapMA-3 (a new PapMA analog) with vancomycin, rifampin, and erythromycin effectively produced synergistic effects against CR A. baumannii. Furthermore, PapMA-3 has the potential to enhance the permeability of the bacterium membrane to imipenem and meropenem. PapMA-3 exhibited the ability to inhibit biofilm development at its MIC. Furthermore, it demonstrated the ability to effectively suppress biofilm formation at lower doses when used with antibiotics. PapMA3 disrupted the structure of the bacterial membrane, even at concentrations lower than the MIC (Choi et al., 2021). Besides, the findings of a study on the impact of WAM-1, derived from the mammary gland of the Tammar wallaby, indicate that WAM-1 exhibits potential as a therapeutic agent for treating infections caused by CR K. pneumoniae. Furthermore, this AMP also exhibited anti-inflammatory properties (Zhang X. et al., 2022).
Furthermore, P5, a newly created AMP, showed notable synergistic effects when combined with meropenem. It also demonstrated the ability to dissolve biofilms and effectively kill bacteria associated with biofilms, specifically against a CR P. aeruginosa (Martinez et al., 2019a). Cec4 was another AMP with an inhibitory effect against CR bacteria. Cec4 is an AMP consisting of 41 amino acids. It has demonstrated inhibitory action against CR A. baumannii. Furthermore, Cec4 can remarkably eliminate biofilm formation by this particular bacterium. Significantly, following the administration of Cec4, there were distinct variations in the expression of membrane proteins, bacterial resistance, and pilus-related genes. Cec4 significantly influences the expression of genes that play a role in developing A. baumannii biofilms, including CsuE, BfmR, BfmS, AbaI, and Bap (Liu W. et al., 2020). In the end, WLBU2, a cationic synthetic peptide, indicated good activity against Gram-positive and Gram-negative bacteria (Lin et al., 2018). A study was conducted to explore the antibiofilm impact of WLBU2 against CR P. aeruginosa. The results indicated that the WLBU2 peptide exhibits potent inhibitory and eradication effects on the P. aeruginosa biofilm. The WLBU2 peptide decreased gene expression levels associated with biofilm growth and maturation (Masihzadeh et al., 2023). Noteworthy, other AMPs with inhibitory effects against CR bacteria are presented in Table 2.
TABLE 2
| Year of publication | AMPs | AMP’s description | Bacteria | Outcome | References |
|---|---|---|---|---|---|
| 2020 | DRGN-6,-7,-8 | These peptides are artificially created from the cathelicidin found in Komodo dragons | CR K. pneumoniae | These AMPs caused significant increases in the permeability of the cells and considerable depolarization | Hitt et al. (2020) |
| 2019 | Cathelicidin-BF15-a4 (ZY4) | The peptide was synthesized by amino acid substitutions based on cathelicidin-BF15 | MDR P. aeruginosa and A. baumannii | ZY4 killed bacteria and persister cells and inhibited the biofilm community. The AMP decreased susceptibility to P. aeruginosa lung infection and suppressed dissemination of bacteria to target organs in a mouse septicemia infection model | Mwangi et al. (2019) |
| 2019 | CM15 and its ATCUN variants (GGH-CM15 and VIH-CM15) | CM15 is a chimeric peptide from melittin and cecropin-A. ATCUN motifs were designed by adding the tripeptide motifs Gly-GlyHis (GGH) or Val-Ile-His (VIH) to CM15 | CR K. pneumoniae and Escherichia coli | AMPs, when combined with meropenem, streptomycin, or chloramphenicol, showed synergistic effects against biofilms | Agbale et al. (2019) |
| 2017 | NN2_0050 and NN2_ 0018 | AMPs are designed by LSTM algorithms | MDR E. coli, A. baumannii, K. pneumoniae, P. aeruginosa, Staphylococcus aureus, and coagulase-negative staphylococci | These designed peptides selectively interacted with and disrupted bacterial cell membranes and caused secondary gene regulatory effects | Nagarajan et al. (2018) |
| 2023 | GAN-pep 3 and GAN-pep 8 | New AMPs generated based on WGAN-GP. | MR S. aureus and CR P. aeruginosa | Inhibitory effects against both bacteria | Lin et al. (2023) |
| 2022 | Epi-1 and hBD-3 | Human beta-defensin-3 (hBD-3) is produced by epithelial cells, and Epinecidin-1 (Epi-1) is an AMP derived from the orange-spotted grouper (Epinephelus coioides) | CR K. pneumoniae, Klebsiella aerogenes, P. aeruginosa and A. baumannii | Antibacterial activity against all studied clinical isolates. In experimental mouse sepsis models with K. pneumoniae and P. aeruginosa, increased survival rates were observed with hBD-3 monotherapy, hBD-3 + meropenem, and hBD-3 + Epi-1 | Bolatchiev (2022) |
| 2021 | LL-37 | LL-37 is a synthetic peptide derived from the C-terminal region of the human cationic antimicrobial protein (hCAP) | MDR E. coli | Inhibited mcr-1 carrying, carbapenemase, and ESBL-producing E. coli | Morroni et al. (2021) |
| 2022 | DGL13K | The D-enantiomers of antimicrobial peptide GL13K, which derived from the salivary protein BPIFA2 | CR K. pneumoniae, MDR and XDR P. aeruginosa and XDR A. baumannii | Inhibitory effect against all bacteria | Gorr et al. (2022) |
| 2022 | PEP-38 and PEP-137 | The new peptides are designed by LSTM RNN. | CR K. pneumoniae and K. aerogenes | Inhibitory effect against bacteria. PEP-137 showed a survival rate of 50%, while PEP-38 was ineffective in the experimental murine model of K. pneumoniae-induced sepsis | Bolatchiev et al. (2022) |
| 2021 | MSI-78 | The MSI-78, also named pexiganan, is a synthetic analog of maganin-2 | CR K. pneumoniae | It showed an inhibitory effect, and this antibacterial effect is thought to result from irreversible membrane-disruptive damage | Denardi et al. (2022) |
| 2023 | 11pep and D −11pep | Two novel antibiotic peptides were designed and synthesized that polymerized the β1, β9, β15, and β16 chains of BamA (BamA, a major component of the outer membrane protein family) | CR E. coli, P. aeruginosa and MDR A. baumannii | Both peptides disrupted the bacterial outer membrane and showed broad-spectrum antibacterial activity. D-11pep effectively inhibited the initial attachment of CR E. coli for biofilm formation | Yang et al. (2023) |
| 2024 | Osmin | Osmin comprises 17 amino acids and is isolated from solitary bee (Osmia rufa) venom | drug-resistant K. pneumoniae | Reduced bacterial growth and the expression of pro-inflammatory cytokines and fibrosis-related genes in mice with CR K. pneumoniae sepsis | Jeon et al. (2024) |
| 2012 | LLKKLLKKC ((LLKK)2C) and CLLKKLLKKC (C(LLKK)2C) | The cationic amphiphilic alpha-helical peptides | CR A. baumannii | These peptides showed excellent potency in mouse models of peritonitis and pneumonia infections caused by CR A. baumannii | Huang et al. (2012) |
| 2021 | LyeTx I-b and PEGylated LyeTx I-b (LyeTx I-bPEG) | LyeTx I-b is a synthetic peptide derived from native LyeTx I, originally isolated from Lycosa erythrognatha spider venom | CR A. baumannii | LyeTx I-b was active against A. baumannii. LyeTx I-bPEG, was slightly less active than its analogue. PEGylation improved the anti-biofilm activity of LyeTx I-b | César Moreira Brito et al. (2021) |
| 2021 | 1B and C | Two derivatives of the Temporin L from Rana temporaria | CR K. pneumoniae | Both peptides were able to inhibit the growth of carbapenemase-producing strains effectively | Roscetto et al. (2021) |
| 2018 | PaDBS1R1 | It is a novel cationic antimicrobial peptide engineered by ribosomal protein L39E from the hyperthermophilic archaeon Pyrobaculum aerophilum | CR K. pneumoniae | Induced permeabilization and depolarization of the cytoplasmic bacterial membrane, leading to leakage of the intracellular content and finally cell death | Irazazabal et al. (2019) |
| 2020 | sDq-3162 | It’s a 28-residue ponericin G-like dinoponeratoxin from the giant ant Dinoponera quadriceps venom | CR bacteria (i.e., A. baumannii, K. pneumoniae, P. aeruginosa and E. coli) | Displayed a significant bacteriostatic and bactericidal effect | Dodou Lima et al. (2020) |
| 2024 | AS-12W | Cathelicidin AS-12W Derived from the Alligator sinensis | CR P. aeruginosa | Demonstrated broad-spectrum antibacterial activity and removed CR P. aeruginosa from blood and organs. This peptide could neutralize the negative charge on the surface of the bacteria and disrupt the integrity of the bacterial cell membrane. The peptide can bind to the genomic DNA of bacteria and stimulate the production of ROS within bacteria | Zhang et al. (2024) |
| 2017 | AM-CATH36, AM-CATH28, AM-CATH21 | These peptides are cathelcidin and two shorter fragments from Alligator mississippiensis (American alligator) | MDR A. baumannii and CR K. pneumoniae | Strong activity against bacteria. These peptides permeabilize the bacterial membrane | Barksdale et al. (2017) |
| 2024 | Gy-CATH | A novel anionic antimicrobial peptide identified from the skin of the frog Glyphoglossus yunnanensis | CR E. coli | Preventive and therapeutic capacities in mice that are infected with bacteria | He et al. (2024) |
| 2021 | Octopromycin | A novel peptide derived from Octopus minor | MDR A. baumannii | Increased ROS production inhibited the biofilm formation and showed biofilm eradication activity. In vivo study results revealed that the A. baumannii-infected fish treated with this peptide exhibited a significantly higher relative percent survival (37.5%) than the infected mock-treated fish with PBS (16.6%) | Rajapaksha et al. (2021) |
| 2015 | Tilapia piscidin 3 (TP3) and tilapia piscidin 4 (TP4) | Synthetic antimicrobial peptides from an aquatic organism Oreochromis niloticus | MDR A. baumannii and CR K. pneumoniae | Showed antibacterial effects and administration of these peptides 30 min after infection with bacteria significantly increased survival in mice | Pan et al. (2015) |
| 2022 | RaCa-1, RaCa-2, RaCa-3 and RaCa-7 | Novel AMPs were identified using amplify derived from the Rana [Lithobates] catesbeiana genome | CR E. coli | These peptides were active against CR strain with MIC = 2–44 μM | Li et al. (2022b) |
| 2018 | IARR-Anal10 | The analog derived from the antimicrobial peptide mBjAMP1 isolated from Branchiostoma japonicum | MDR K. pneumoniae | Suppressed the virulence of K. pneumoniae to a degree similar to tigecycline and did not induce development of resistance by this bacterium | Park et al. (2018) |
| 2019 | Pen-BR, Pen-RRR and Cecropin P1 (CECP1), Cap11-1–18 m2 (CapM2) | Pen-BR, Pen-RRR were generated by fusing HEXIM1 BR and BR-RRR12 peptides with a cell-penetrating peptide, Pen. CECP1 is an AMP from Ascaris suum. CapM2 is a derivative of guinea pig cathelicidin CAP11 | CR E. coli and P. aeruginosa | Showed improved and potent bacterial inhibitory and killing activities | Ho et al. (2019) |
Antibacterial and antibiofilm activity of AMPs against CR bacteria.
AMPs: antimicrobial peptides. ATCUN: amino terminal Cu(II) and Ni(II). CR: carbapenem-resistant. LSTM: long short-term memory. MR: Methicillin-resistant. MDR: multidrug-resistant. PBS: phosphate-buffered saline. ROS: reactive oxygen species. RNN: recurrent neural network. XDR: extensively drug-resistant. WGAN-GP: Wasserstein generative adversarial network with gradient penalty.
As mentioned, AMPs can decrease the expression of genes related to biofilms, hinder the initial attachment of bacteria to a surface, target bacteria before they can form a biofilm, eliminate bacteria already embedded in biofilms, or eradicate developed biofilms. These actions effectively inhibit or eliminate biofilms (Liu W. et al., 2020). Additionally, they demonstrated promise when combined with conventional antibiotics in combination therapy. AMPs can be readily modified by substituting their amino acid residues to combat drug resistance, thereby creating new and highly effective AMPs (Choi et al., 2021).
AMPs can be categorized into two primary groups based on their mechanisms: (1) direct eradication by altering the integrity of the cell membrane or affecting the production of internal components such as nucleic acids and proteins, and (2) regulating the immune response to eliminate harmful infections (Beaumont et al., 2014; Yang M. et al., 2019). In addition, AMPs exhibit several additional activities, such as inducing membrane depolarization and destabilization (Irazazabal et al., 2019; Hitt et al., 2020). AMPs can also induce cell apoptosis by regulating the production of ROS (Hwang et al., 2011). Besides, AMPs work as immunomodulators by attracting and stimulating immune cells such as neutrophils, mast cells, macrophages, and T cells. Consequently, this affects the roles of neutrophils in generating chemokines (Di Nardo et al., 2008; Pundir et al., 2014).
Therefore, unlike empirical antibiotics that target single or specific bacterial processes, AMPs exhibit multifunctional bacterial killing effects (Hurdle et al., 2011; Gee et al., 2013). To this end, the likelihood of antimicrobial peptides developing resistance is low, as microorganisms would need to undergo substantial changes to their gene sequences, membrane structure, and lipid composition to evade the peptides (Zasloff, 2002; Hein-Kristensen et al., 2013; Ravensdale et al., 2016). For this reason, due to their wide-ranging effectiveness and minimal harm to the host, antimicrobial peptides have garnered increased interest as potential therapeutic agents against drug-resistant bacteria (Ho et al., 2019).
Collectively, AMPs show promise in controlling MDR bacteria and associated biofilm communities. However, these biomacromolecules have some limitations, including their susceptibility to degradation, limited absorption, distribution, metabolism, and excretion (ADME) capabilities, transport mechanism, target delivery, and potential toxicity (Di, 2015; Khara et al., 2015; Wagner et al., 2018; Wang et al., 2019). To this end, recently published studies have been conducted to enhance the therapeutic effectiveness of these biomolecules and mitigate their negative side effects. There are two methods for this objective. One approach involves modifying peptides that have already been altered to enhance their effectiveness against pathogens. This modified peptide can be achieved by changing, removing, adding, or substituting amino acids in the original sequence. Additionally, modifications can be made to the N or C terminal parts of the peptides, such as cyclizing or conjugating them with antibiotics or other molecules (Radzishevsky et al., 2007; Costa et al., 2015; Liu et al., 2017). Another method entails utilizing nanotechnology via nanoparticles to inhibit peptide breakdown, enhance antimicrobial effectiveness and bioavailability, amplify selectivity, and regulate the administered dosage according to physicochemical factors such as time, temperature, and pH (Roque-Borda et al., 2022). Therefore, AMPs showed potential for managing CR bacteria; however, some drawbacks limited their clinical usage. In this regard, scientists should consider using the abovementioned approaches to improve AMPs’ performance against CR bacteria.
8 Conclusion
Antibiotic resistance and MDR bacteria are challenging and threatening to the global community. One of the main reasons is the indiscriminate use of antibiotics, which causes the creation of new antibiotic-resistant strains at a high rate. One of the most important causes of death in the world is infections caused by antibiotic-resistant bacteria. Therefore, the synthesis of new and effective antimicrobial agents is critical. Evidence from scientific investigations indicates that the progress in creating antibiotics is not keeping pace with the rise of antibacterial resistance patterns, particularly for significant bacterial infections. Multiple antibacterial resistance profiles, such as the CR bacterium, have been recently identified. Recent studies have documented the potential of phages, nanoparticles (drug platforms), and natural substances for managing these bacteria. Moreover, various management approaches have been employed to address the issue of resistant pathogens. These include (a) gaining a thorough understanding of resistance at the molecular level, as well as its evolution and spread; (b) discovering novel chemical agents with antibiotic properties; and (c) improving the effectiveness of antibiotics through innovative methods like combination therapy. It is noteworthy to mention that there is a growing prevalence of bacteria that are becoming increasingly resistant. Hence, it is imperative to employ other strategies to manage resistant infections, given that antibiotic resistance poses a significant challenge in clinical environments. However, there is a lack of extensive clinical data and in vitro studies in this area. Therefore, further research is needed to determine the most effective non-antibiotic approaches that cause minimal harm to humans, enhance their impact on bacterial pathogens, identify the optimal timing for treatment, and establish the appropriate route and administration dosage. However, non-antibiotic methods could soon be implemented as a viable antibiotic substitute.
Statements
Author contributions
AS: Conceptualization, Writing–original draft, Writing–review and editing. MK: Investigation, Writing–original draft. ZC: Conceptualization, Writing–original draft. SH: Investigation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We greatly appreciate the input from the BioRender team (BioRender.com) for their collaboration with us in figure design.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdel-Halim M. S. Askoura M. Mansour B. Yahya G. El-Ganiny A. M. (2022). In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates. J. Antibiot. (Tokyo)75 (12), 679–690. 10.1038/s41429-022-00566-y
2
Adeosun I. J. Baloyi I. T. Cosa S. (2022a). Anti-biofilm and associated anti-virulence activities of selected phytochemical compounds against Klebsiella pneumoniae. Plants11 (11), 1429. 10.3390/plants11111429
3
Adeosun I. J. Baloyi I. T. Cosa S. (2022b). Anti-biofilm and associated anti-virulence activities of selected phytochemical compounds against Klebsiella pneumoniae. Plants (Basel)11 (11), 1429. 10.3390/plants11111429
4
Agbale C. M. Sarfo J. K. Galyuon I. K. Juliano S. A. Silva G. G. O. Buccini D. F. et al (2019). Antimicrobial and antibiofilm activities of helical antimicrobial peptide sequences incorporating metal-binding motifs. Biochemistry58 (36), 3802–3812. 10.1021/acs.biochem.9b00440
5
Ahmad A. Khan A. Khan L. A. Manzoor N. (2010). In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol.59 (10), 1178–1184. 10.1099/jmm.0.020693-0
6
Ahmad A. Wani M. Y. Khan A. Manzoor N. Molepo J. (2015). Synergistic interactions of eugenol-tosylate and its congeners with fluconazole against Candida albicans. Plos one10 (12), e0145053. 10.1371/journal.pone.0145053
7
Ait-Oufella H. Maury E. Lehoux S. Guidet B. Offenstadt G. (2010). The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med.36 (8), 1286–1298. 10.1007/s00134-010-1893-6
8
Aiyer A. Manoharan A. Paino D. Farrell J. Whiteley G. S. Kriel F. H. et al (2021). Disruption of biofilms and killing of Burkholderia cenocepacia from cystic fibrosis lung using an antioxidant-antibiotic combination therapy. Int. J. Antimicrob. Agents58 (2), 106372. 10.1016/j.ijantimicag.2021.106372
9
Alarfaj R. E. Alkhulaifi M. M. Al-Fahad A. J. Aljihani S. Yassin A. E. B. Alghoribi M. F. et al (2022). Antibacterial efficacy of liposomal formulations containing tobramycin and N-acetylcysteine against tobramycin-resistant Escherichia coli, Klebsiella pneumoniae, and acinetobacter baumannii. Pharmaceutics14 (1), 130. 10.3390/pharmaceutics14010130
10
Alfredsson H. Malmborg A. S. Strandvik B. (1987). N-acetylcysteine and 2-mercaptoethane sulphonate inhibit anti-pseudomonas activity of antibiotics in vitro. Eur. J. Respir. Dis.70 (4), 213–217.
11
Amaral S. C. Pruski B. B. De Freitas S. B. Allend S. O. Ferreira M. R. A. Moreira C. Jr. et al (2020). Origanum vulgare essential oil: antibacterial activities and synergistic effect with polymyxin B against multidrug-resistant Acinetobacter baumannii. Mol. Biol. Rep.47 (12), 9615–9625. 10.1007/s11033-020-05989-0
12
Angus D. C. Poll T. V. D. (2013). Severe sepsis and septic shock. 369(9),840–851. 10.1056/NEJMra1208623
13
Aurilio C. Sansone P. Barbarisi M. Pota V. Giaccari L. G. Coppolino F. et al (2022). Mechanisms of action of carbapenem resistance. Antibiotics11 (3), 421. 10.3390/antibiotics11030421
14
Bai J. Zhang F. Liang S. Chen Q. Wang W. Wang Y. et al (2022). Isolation and characterization of vB_kpnM_17-11, a novel phage efficient against carbapenem-resistant Klebsiella pneumoniae. Front. Cell Infect. Microbiol.12, 897531. 10.3389/fcimb.2022.897531
15
Baliga P. Goolappa P. T. Shekar M. Kallappa G. S. (2022). Cloning, characterization, and antibacterial properties of endolysin LysE against planktonic cells and biofilms of aeromonas hydrophila. Probiotics Antimicrob. Proteins15, 646–654. 10.1007/s12602-021-09880-7
16
Baqer A. A. Fang K. Mohd-Assaad N. Adnan S. N. A. Md Nor N. S. (2022). In vitro activity, stability and molecular characterization of eight potent bacteriophages infecting carbapenem-resistant Klebsiella pneumoniae. Viruses15 (1), 117. 10.3390/v15010117
17
Barksdale S. M. Hrifko E. J. Van Hoek M. L. (2017). Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumoniae. Dev. Comp. Immunol.70, 135–144. 10.1016/j.dci.2017.01.011
18
Beaumont P. E. Mchugh B. Gwyer Findlay E. Mackellar A. Mackenzie K. J. Gallo R. L. et al (2014). Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS One9 (6), e99029. 10.1371/journal.pone.0099029
19
Bharathan S. Sundaramoorthy N. S. Chandrasekaran H. Rangappa G. Arunkumar G. Subramaniyan S. B. et al (2019). Sub lethal levels of platinum nanoparticle cures plasmid and in combination with carbapenem, curtails carbapenem resistant Escherichia coli. Sci. Rep.9 (1), 5305. 10.1038/s41598-019-41489-3
20
Bialvaei A. Z. Samadi Kafil H. (2015). Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin.31 (4), 707–721. 10.1185/03007995.2015.1018989
21
Blasi F. Page C. Rossolini G. M. Pallecchi L. Matera M. G. Rogliani P. et al (2016). The effect of N-acetylcysteine on biofilms: implications for the treatment of respiratory tract infections. Respir. Med.117, 190–197. 10.1016/j.rmed.2016.06.015
22
Bolatchiev A. (2022). Antimicrobial peptides epinecidin-1 and beta-defesin-3 are effective against a broad spectrum of antibiotic-resistant bacterial isolates and increase survival rate in experimental sepsis. Antibiot. (Basel)11 (1), 76. 10.3390/antibiotics11010076
23
Bolatchiev A. Baturin V. Shchetinin E. Bolatchieva E. (2022). Novel antimicrobial peptides designed using a recurrent neural network reduce mortality in experimental sepsis. Antibiot. (Basel)11 (3), 411. 10.3390/antibiotics11030411
24
Buckner M. M. Ciusa M. L. Piddock L. J. (2018). Strategies to combat antimicrobial resistance: anti-plasmid and plasmid curing. FEMS Microbiol. Rev.42 (6), 781–804. 10.1093/femsre/fuy031
25
Cebrero-Cangueiro T. Álvarez-Marín R. Labrador-Herrera G. Smani Y. Cordero-Matía E. Pachón J. et al (2018). In vitro activity of pentamidine alone and in combination with aminoglycosides, tigecycline, rifampicin, and doripenem against clinical strains of carbapenemase-producing and/or colistin-resistant Enterobacteriaceae. Front. Cell. Infect. Microbiol.8, 363. 10.3389/fcimb.2018.00363
26
César Moreira Brito J. Gustavo Lima W. Magalhães Resende J. Cristina Sampaio De Assis D. Boff D. Nascimento Cardoso V. et al (2021). Pegylated LyeTx I-b peptide is effective against carbapenem-resistant Acinetobacter baumannii in an in vivo model of pneumonia and shows reduced toxicity. Int. J. Pharm.609, 121156. 10.1016/j.ijpharm.2021.121156
27
Chan B. K. Sistrom M. Wertz J. E. Kortright K. E. Narayan D. Turner P. E. (2016). Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep.6, 26717. 10.1038/srep26717
28
Chen C. Cai J. Shi J. Wang Z. Liu Y. (2023). Resensitizing multidrug-resistant Gram-negative bacteria to carbapenems and colistin using disulfiram. Commun. Biol.6 (1), 810. 10.1038/s42003-023-05173-7
29
Chen P. Liu Z. Tan X. Wang H. Liang Y. Kong Y. et al (2022). Bacteriophage therapy for empyema caused by carbapenem-resistant Pseudomonas aeruginosa. Biosci. Trends16 (2), 158–162. 10.5582/bst.2022.01147
30
Chertoff J. (2018). N-Acetylcysteine’s role in sepsis and potential benefit in patients with microcirculatory derangements. J. Intensive Care Med.33 (2), 87–96. 10.1177/0885066617696850
31
Choi C. H. Lee J. S. Lee Y. C. Park T. I. Lee J. C. (2008). Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol.8, 216. 10.1186/1471-2180-8-216
32
Choi J. Jang A. Yoon Y. K. Kim Y. (2021). Development of novel peptides for the antimicrobial combination therapy against carbapenem-resistant acinetobacter baumannii infection. Pharmaceutics13 (11), 1800. 10.3390/pharmaceutics13111800
33
Ciacci N. D'andrea M. M. Marmo P. Demattè E. Amisano F. Di Pilato V. et al (2018). Characterization of vB_Kpn_F48, a newly discovered lytic bacteriophage for Klebsiella pneumoniae of sequence type 101. Viruses10 (9), 482. 10.3390/v10090482
34
Corbellino M. Kieffer N. Kutateladze M. Balarjishvili N. Leshkasheli L. Askilashvili L. et al (2020). Eradication of a multidrug-resistant, carbapenemase-producing Klebsiella pneumoniae isolate following oral and intra-rectal therapy with a custom made, lytic bacteriophage preparation. Clin. Infect. Dis.70 (9), 1998–2001. 10.1093/cid/ciz782
35
Costa F. M. T. A. Maia S. R. Gomes P. a.C. Martins M. C. L. (2015). Dhvar5 antimicrobial peptide (AMP) chemoselective covalent immobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials52, 531–538. 10.1016/j.biomaterials.2015.02.049
36
Crameri R. Davies J. E. Hütter R. (1986). Plasmid curing and generation of mutations induced with ethidium bromide in streptomycetes. Microbiology132 (3), 819–824. 10.1099/00221287-132-3-819
37
D'andrea M. M. Marmo P. Henrici De Angelis L. Palmieri M. Ciacci N. Di Lallo G. et al (2017). φBO1E, a newly discovered lytic bacteriophage targeting carbapenemase-producing Klebsiella pneumoniae of the pandemic Clonal Group 258 clade II lineage. Sci. Rep.7 (1), 2614. 10.1038/s41598-017-02788-9
38
De Angelis M. Mascellino M. T. Miele M. C. Al Ismail D. Colone M. Stringaro A. et al (2022). High activity of N-acetylcysteine in combination with beta-lactams against carbapenem-resistant Klebsiella pneumoniae and acinetobacter baumannii. Antibiot. (Basel)11 (2), 225. 10.3390/antibiotics11020225
39
Denardi L. B. De Arruda Trindade P. Weiblen C. Ianiski L. B. Stibbe P. C. Pinto S. C. et al (2022). In vitro activity of the antimicrobial peptides h-Lf1-11, MSI-78, LL-37, fengycin 2B, and magainin-2 against clinically important bacteria. Braz J. Microbiol.53 (1), 171–177. 10.1007/s42770-021-00645-6
40
De Souza G. H. A. Dos Santos Radai J. A. Mattos Vaz M. S. Esther Da Silva K. Fraga T. L. Barbosa L. S. et al (2021). In vitro and in vivo antibacterial activity assays of carvacrol: a candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae. PLoS One16 (2), e0246003. 10.1371/journal.pone.0246003
41
Dhungana G. Nepal R. Regmi M. Malla R. (2021). Pharmacokinetics and pharmacodynamics of a novel virulent Klebsiella phage Kp_Pokalde_002 in a mouse model. Front. Cell Infect. Microbiol.11, 684704. 10.3389/fcimb.2021.684704
42
Di L. (2015). Strategic approaches to optimizing peptide ADME properties. AAPS J.17 (1), 134–143. 10.1208/s12248-014-9687-3
43
Di Nardo A. Yamasaki K. Dorschner R. A. Lai Y. Gallo R. L. (2008). Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J. Immunol.180 (11), 7565–7573. 10.4049/jimmunol.180.11.7565
44
Dodou Lima H. V. Sidrim De Paula Cavalcante C. Rádis-Baptista G. (2020). Antimicrobial activity of synthetic Dq-3162, a 28-residue ponericin G-like dinoponeratoxin from the giant ant Dinoponera quadriceps venom, against carbapenem-resistant bacteria. Toxicon187, 19–28. 10.1016/j.toxicon.2020.08.015
45
Doi Y. (2019). Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin. Infect. Dis.69 (Suppl. ment_7), S565–S575. 10.1093/cid/ciz830
46
Dubey V. Devnath K. Gupta V. K. Kalyan G. Singh M. Kothari A. et al (2022). Disulfiram enhances meropenem activity against NDM-and IMP-producing carbapenem-resistant Acinetobacter baumannii infections. J. Antimicrob. Chemother.77 (5), 1313–1323. 10.1093/jac/dkac057
47
Eckstein S. Stender J. Mzoughi S. Vogele K. Kühn J. Friese D. et al (2021). Isolation and characterization of lytic phage TUN1 specific for Klebsiella pneumoniae K64 clinical isolates from Tunisia. BMC Microbiol.21 (1), 186. 10.1186/s12866-021-02251-w
48
Esmaeili-Fard-Barzegar K. Najar-Peerayeh S. Mazaheri Nezhad Fard R. Bakhshi B. (2022). Lytic potential of a filamentous bacteriophage isolated from sewage water in Tehran on clinical carbapenem-resistant strains. Iran. J. Microbiol.14 (5), 705–711. 10.18502/ijm.v14i5.10966
49
Falanga A. Galdiero S. (2017). “Emerging therapeutic agents on the basis of naturally occurring antimicrobial peptides,” in Amino acids, peptides and proteins: 42, eds. RyadnovM.HudeczF.The Royal Society of Chemistry.
50
Fang Q. Zong Z. (2022). Lytic phages against ST11 K47 carbapenem-resistant Klebsiella pneumoniae and the corresponding phage resistance mechanisms. mSphere7 (2), e0008022. 10.1128/msphere.00080-22
51
Feng J. Gao L. Li L. Zhang Z. Wu C. Li F. et al (2021). Characterization and genome analysis of novel Klebsiella phage BUCT556A with lytic activity against carbapenemase-producing Klebsiella pneumoniae. Virus Res.303, 198506. 10.1016/j.virusres.2021.198506
52
Feng J. Li F. Sun L. Dong L. Gao L. Wang H. et al (2023). Characterization and genome analysis of phage vB_KpnS_SXFY507 against Klebsiella pneumoniae and efficacy assessment in Galleria mellonella larvae. Front. Microbiol.14, 1081715. 10.3389/fmicb.2023.1081715
53
Feng J. Liu B. Xu J. Wang Q. Huang L. Ou W. et al (2018). In vitro effects of N-acetylcysteine alone and combined with tigecycline on planktonic cells and biofilms of Acinetobacter baumannii. J. Thorac. Dis.10 (1), 212–218. 10.21037/jtd.2017.11.130
54
Fernandez-Moure J. S. Evangelopoulos M. Colvill K. Van Eps J. L. Tasciotti E. (2017). Nanoantibiotics: a new paradigm for the treatment of surgical infection. Nanomedicine12 (11), 1319–1334. 10.2217/nnm-2017-0401
55
Forman H. J. Zhang H. Rinna A. (2009). Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med.30 (1), 1–12. 10.1016/j.mam.2008.08.006
56
Gao M. Wang C. Qiang X. Liu H. Li P. Pei G. et al (2020). Isolation and characterization of a novel bacteriophage infecting carbapenem-resistant Klebsiella pneumoniae. Curr. Microbiol.77 (5), 722–729. 10.1007/s00284-019-01849-8
57
Gee M. L. Burton M. Grevis-James A. Hossain M. A. Mcarthur S. Palombo E. A. et al (2013). Imaging the action of antimicrobial peptides on living bacterial cells. Sci. Rep.3, 1557. 10.1038/srep01557
58
Gnanadhas D. P. Ben Thomas M. Thomas R. Raichur A. M. Chakravortty D. (2013). Interaction of silver nanoparticles with serum proteins affects their antimicrobial activity in vivo. Antimicrob. agents Chemother.57 (10), 4945–4955. 10.1128/AAC.00152-13
59
Gorr S. U. Brigman H. V. Anderson J. C. Hirsch E. B. (2022). The antimicrobial peptide DGL13K is active against drug-resistant gram-negative bacteria and sub-inhibitory concentrations stimulate bacterial growth without causing resistance. PLoS One17 (8), e0273504. 10.1371/journal.pone.0273504
60
Gu J. Liu X. Li Y. Han W. Lei L. Yang Y. et al (2012). A method for generation phage cocktail with great therapeutic potential. PLoS One7 (3), e31698. 10.1371/journal.pone.0031698
61
Guo Y. Yang Y. Xu X. Li L. Zhou Y. Jia G. et al (2022). Metallo-β-lactamases inhibitor fisetin attenuates meropenem resistance in NDM-1-producing Escherichia coli. Eur. J. Med. Chem.231, 114108. 10.1016/j.ejmech.2022.114108
62
Hagens S. Habel A. Bläsi U. (2006). Augmentation of the antimicrobial efficacy of antibiotics by filamentous phage. Microb. Drug Resist12 (3), 164–168. Augmentation of the antimicrobial efficacy of antibiotics by filamentous phage. 10.1089/mdr.2006.12.164
63
Hall A. R. De Vos D. Friman V. P. Pirnay J. P. Buckling A. (2012). Effects of sequential and simultaneous applications of bacteriophages on populations of Pseudomonas aeruginosa in vitro and in wax moth larvae. Appl. Environ. Microbiol.78 (16), 5646–5652. 10.1128/aem.00757-12
64
Hanlon G. W. (2007). Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agents30 (2), 118–128. 10.1016/j.ijantimicag.2007.04.006
65
Hao G. Shu R. Ding L. Chen X. Miao Y. Wu J. et al (2021). Bacteriophage SRD2021 recognizing capsular polysaccharide shows therapeutic potential in serotype K47 Klebsiella pneumoniae infections. Antibiot. (Basel)10 (8), 894. 10.3390/antibiotics10080894
66
He Y. Ruan S. Liang G. Hao J. Zhou X. Li Z. et al (2024). A nonbactericidal anionic antimicrobial peptide provides prophylactic and therapeutic efficacies against bacterial infections in mice by immunomodulatory-antithrombotic duality. J. Med. Chem.67 (9), 7487–7503. 10.1021/acs.jmedchem.4c00342
67
Hein-Kristensen L. Franzyk H. Holch A. Gram L. (2013). Adaptive evolution of Escherichia coli to an α-peptide/β-peptoid peptidomimetic induces stable resistance. PLOS ONE8 (9), e73620. 10.1371/journal.pone.0073620
68
Hernández-García M. Sánchez-López J. Martínez-García L. Becerra-Aparicio F. Morosini M. I. Ruiz-Garbajosa P. et al (2021). Emergence of the new KPC-49 variant conferring an ESBL phenotype with resistance to ceftazidime-avibactam in the ST131-H30R1 Escherichia coli high-risk clone. Pathogens10 (1), 67. 10.3390/pathogens10010067
69
Hibstu Z. Belew H. Akelew Y. Mengist H. M. (2022). Phage therapy: a different approach to fight bacterial infections. Biologics16, 173–186. 10.2147/btt.s381237
70
Hitt S. J. Bishop B. M. Van Hoek M. L. (2020). Komodo-dragon cathelicidin-inspired peptides are antibacterial against carbapenem-resistant Klebsiella pneumoniae. J. Med. Microbiol.69 (11), 1262–1272. 10.1099/jmm.0.001260
71
Ho P. L. Ong H. K. Teo J. Ow D. S. Chao S. H. (2019). HEXIM1 peptide exhibits antimicrobial activity against antibiotic resistant bacteria through guidance of cell penetrating peptide. Front. Microbiol.10, 203. 10.3389/fmicb.2019.00203
72
Horváth M. Kovács T. Koderivalappil S. Ábrahám H. Rákhely G. Schneider G. (2020). Identification of a newly isolated lytic bacteriophage against K24 capsular type, carbapenem resistant Klebsiella pneumoniae isolates. Sci. Rep.10 (1), 5891. 10.1038/s41598-020-62691-8
73
Hoseinzadeh E. Alikhani M.-Y. Samarghandi M.-R. Shirzad-Siboni M. (2014). Antimicrobial potential of synthesized zinc oxide nanoparticles against gram positive and gram negative bacteria. Desalination Water Treat.52 (25-27), 4969–4976. 10.1080/19443994.2013.810356
74
Hosseini S. Morovati Moez N. Arabestani M. Antibacterial and antifungal materials
75
Hsu B.-G. Lee R.-P. Yang F.-L. Harn H.-J. Chen H. I. (2006). Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci.79 (21), 2010–2016. 10.1016/j.lfs.2006.06.040
76
Hua Y. Luo T. Yang Y. Dong D. Wang R. Wang Y. et al (2017). Phage therapy as a promising new treatment for lung infection caused by carbapenem-resistant acinetobacter baumannii in mice. Front. Microbiol.8 (2659), 2659. 10.3389/fmicb.2017.02659
77
Huang L. Huang S. Jiang L. Tan J. Yan X. Gou C. et al (2023a). Characterisation and sequencing of the novel phage Abp95, which is effective against multi-genotypes of carbapenem-resistant Acinetobacter baumannii. Sci. Rep.13 (1), 188. 10.1038/s41598-022-26696-9
78
Huang Y. Wiradharma N. Xu K. Ji Z. Bi S. Li L. et al (2012). Cationic amphiphilic alpha-helical peptides for the treatment of carbapenem-resistant Acinetobacter baumannii infection. Biomaterials33 (34), 8841–8847. 10.1016/j.biomaterials.2012.08.026
79
Huang Z. Han Y. Zhang X. Sun Y. Lin Y. Feng L. et al (2023b). Acetylcysteine increases sensitivity of ceftazidime-avibactam-resistant enterobacterales with different enzymatic resistance to ceftazidime-avibactam in vitro and in vivo. BMC Microbiol.23 (1), 321. 10.1186/s12866-023-03068-5
80
Huh A. J. Kown Y. J. (2011). “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. release156 (2), 128–145. 10.1016/j.jconrel.2011.07.002
81
Hurdle J. G. O'neill A. J. Chopra I. Lee R. E. (2011). Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol.9 (1), 62–75. 10.1038/nrmicro2474
82
Hwang B. Hwang J.-S. Lee J. Lee D. G. (2011). The antimicrobial peptide, psacotheasin induces reactive oxygen species and triggers apoptosis in Candida albicans. Biochem. Biophysical Res. Commun.405 (2), 267–271. 10.1016/j.bbrc.2011.01.026
83
Igbinosa O. Dogho P. Osadiaye N. (2020). Carbapenem-resistant Enterobacteriaceae: a retrospective review of treatment and outcomes in a long-term acute care hospital. Am. J. Infect. Control48 (1), 7–12. 10.1016/j.ajic.2019.07.006
84
Ince C. (2005). The microcirculation is the motor of sepsis. Crit. Care9 (4), S13–S19. 10.1186/cc3753
85
Ince C. Mayeux P. R. Nguyen T. Gomez H. Kellum J. A. Ospina-Tascón G. A. et al (2016). The endothelium in sepsis. Shock45, 259–270. 10.1097/SHK.0000000000000473
86
Irazazabal L. N. Porto W. F. Fensterseifer I. C. M. Alves E. S. F. Matos C. O. Menezes A. C. S. et al (2019). Fast and potent bactericidal membrane lytic activity of PaDBS1R1, a novel cationic antimicrobial peptide. Biochim. Biophys. Acta Biomembr.1861 (1), 178–190. 10.1016/j.bbamem.2018.08.001
87
Jaiswal A. Koley H. Ghosh A. Palit A. Sarkar B. (2013). Efficacy of cocktail phage therapy in treating Vibrio cholerae infection in rabbit model. Microbes Infect.15 (2), 152–156. 10.1016/j.micinf.2012.11.002
88
Jan A. T. (2017). Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front. Microbiol., Outer Membr. Vesicles (OMVs) Gram-negative Bact. A Perspective Update8, 1053. 10.3389/fmicb.2017.01053
89
Jash S. K. Mondal S. (2014). Bioactive flavonoid fisetin–A molecule of pharmacological interest. Cardiovasc. Dis.5 (6).
90
Jeon E. Kim M. K. Park Y. (2024). Efficacy of the bee-venom antimicrobial peptide Osmin against sensitive and carbapenem-resistant Klebsiella pneumoniae strains. Int. J. Antimicrob. Agents63 (2), 107054. 10.1016/j.ijantimicag.2023.107054
91
Jeon J. D'souza R. Pinto N. Ryu C. M. Park J. H. Yong D. et al (2015). Complete genome sequence of the siphoviral bacteriophage Βϕ-R3177, which lyses an OXA-66-producing carbapenem-resistant Acinetobacter baumannii isolate. Arch. Virol.160 (12), 3157–3160. 10.1007/s00705-015-2604-y
92
Jeon J. Park J. H. Yong D. (2019). Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol.19 (1), 70. 10.1186/s12866-019-1443-5
93
Jeon J. Ryu C. M. Lee J. Y. Park J. H. Yong D. Lee K. (2016). In vivo application of bacteriophage as a potential therapeutic agent to control OXA-66-like carbapenemase-producing acinetobacter baumannii strains belonging to sequence type 357. Appl. Environ. Microbiol.82 (14), 4200–4208. 10.1128/aem.00526-16
94
Khara J. S. Lim F. K. Wang Y. Ke X.-Y. Voo Z. X. Yang Y. Y. et al (2015). Designing α-helical peptides with enhanced synergism and selectivity against Mycobacterium smegmatis: discerning the role of hydrophobicity and helicity. Acta Biomater.28, 99–108. 10.1016/j.actbio.2015.09.015
95
Kleczkowska P. Sulejczak D. Zaremba M. (2021). Advantages and disadvantages of disulfiram coadministered with popular addictive substances. Eur. J. Pharmacol.904, 174143. 10.1016/j.ejphar.2021.174143
96
Kwiatkowski P. Sienkiewicz M. Pruss A. Łopusiewicz Ł. Arszyńska N. Wojciechowska-Koszko I. et al (2022). Antibacterial and anti-biofilm activities of essential oil compounds against New Delhi metallo-β-lactamase-1-producing uropathogenic Klebsiella pneumoniae strains. Antibiot. (Basel)11 (2), 147. 10.3390/antibiotics11020147
97
Latka A. Drulis-Kawa Z. (2020). Advantages and limitations of microtiter biofilm assays in the model of antibiofilm activity of Klebsiella phage KP34 and its depolymerase. Sci. Rep.10 (1), 20338. 10.1038/s41598-020-77198-5
98
Lee J. Oh J. Kim K. Jeong Y. Park J. Cho J. (2001). Apoptotic cell death induced by Acinetobacter baumannii in epithelial cells through caspase-3 activation. Apmis109 (10), 679–684. 10.1034/j.1600-0463.2001.d01-132.x
99
Li C. Sutherland D. Hammond S. A. Yang C. Taho F. Bergman L. et al (2001). AMPlify: attentive deep learning model for discovery of novel antimicrobial peptides effective against WHO priority pathogens. BMC Genomics23 (1), 77. 10.1186/s12864-022-08310-4
100
Li J. Sheng Y. Ma R. Xu M. Liu F. Qin R. et al (2021a). Identification of a depolymerase specific for K64-serotype Klebsiella pneumoniae: potential applications in capsular typing and treatment. Antibiot. (Basel)10 (2), 144. 10.3390/antibiotics10020144
101
Li M. Li P. Chen L. Guo G. Xiao Y. Chen L. et al (2021b). Identification of a phage-derived depolymerase specific for KL64 capsule of Klebsiella pneumoniae and its anti-biofilm effect. Virus Genes57 (5), 434–442. 10.1007/s11262-021-01847-8
102
Li M. Wang H. Chen L. Guo G. Li P. Ma J. et al (2022b). Identification of a phage-derived depolymerase specific for KL47 capsule of Klebsiella pneumoniae and its therapeutic potential in mice. Virol. Sin.37 (4), 538–546. 10.1016/j.virs.2022.04.005
103
Li M. Xiao Y. Li P. Wang Z. Qi W. Qi Z. et al (2020). Characterization and genome analysis of Klebsiella phage P509, with lytic activity against clinical carbapenem-resistant Klebsiella pneumoniae of the KL64 capsular type. Arch. Virol.165 (12), 2799–2806. 10.1007/s00705-020-04822-0
104
Liang Z. Shi Y. L. Peng Y. Xu C. Zhang C. Chen Y. et al (2023). BL02, a phage against carbapenem- and polymyxin-B resistant Klebsiella pneumoniae, isolated from sewage: a preclinical study. Virus Res.331, 199126. 10.1016/j.virusres.2023.199126
105
Lin Q. Deslouches B. Montelaro R. C. Di Y. P. (2018). Prevention of ESKAPE pathogen biofilm formation by antimicrobial peptides WLBU2 and LL37. Int. J. Antimicrob. Agents52 (5), 667–672. 10.1016/j.ijantimicag.2018.04.019
106
Lin T. T. Yang L. Y. Lin C. Y. Wang C. T. Lai C. W. Ko C. F. et al (2023). Intelligent de novo design of novel antimicrobial peptides against antibiotic-resistant bacteria strains. Int. J. Mol. Sci.24 (7), 6788. 10.3390/ijms24076788
107
Liu B. Zhang W. Gou S. Huang H. Yao J. Yang Z. et al (2017). Intramolecular cyclization of the antimicrobial peptide Polybia-MPI with triazole stapling: influence on stability and bioactivity. J. Pept. Sci.23 (11), 824–832. 10.1002/psc.3031
108
Liu W. Chen G. Dou K. Yi B. Wang D. Zhou Q. et al (2023). Eugenol eliminates carbapenem-resistant Klebsiella pneumoniae via reactive oxygen species mechanism. Front. Microbiol.14, 1090787. 10.3389/fmicb.2023.1090787
109
Liu W. Wu Z. Mao C. Guo G. Zeng Z. Fei Y. et al (2020a). Antimicrobial peptide Cec4 eradicates the bacteria of clinical carbapenem-resistant acinetobacter baumannii biofilm. Biofilm11, 1532. 10.3389/fmicb.2020.01532
110
Liu Y. Leung S. S. Y. Huang Y. Guo Y. Jiang N. Li P. et al (2020b). Identification of two depolymerases from phage IME205 and their antivirulent functions on K47 capsule of Klebsiella pneumoniae. Front. Microbiol.11, 218. 10.3389/fmicb.2020.00218
111
Macnair C. R. Stokes J. M. Carfrae L. A. Fiebig-Comyn A. A. Coombes B. K. Mulvey M. R. et al (2018). Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun.9 (1), 458. 10.1038/s41467-018-02875-z
112
Magiorakos A. P. Srinivasan A. Carey R. B. Carmeli Y. Falagas M. E. Giske C. G. et al (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect.18 (3), 268–281. 10.1111/j.1469-0691.2011.03570.x
113
Mahichi F. Synnott A. J. Yamamichi K. Osada T. Tanji Y. (2009). Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol. Lett.295 (2), 211–217. 10.1111/j.1574-6968.2009.01588.x
114
Martin A. Fahrbach K. Zhao Q. Lodise T. (2018). “Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis,” in Open forum infectious diseases (Oxford University Press US).ofy150
115
Martinez M. Gonçalves S. Felício M. R. Maturana P. Santos N. C. Semorile L. et al (2019a). Synergistic and antibiofilm activity of the antimicrobial peptide P5 against carbapenem-resistant Pseudomonas aeruginosa. Biochimica Biophysica Acta (BBA)-Biomembranes1861 (7), 1329–1337. 10.1016/j.bbamem.2019.05.008
116
Masihzadeh S. Amin M. Farshadzadeh Z. (2023). In vitro and in vivo antibiofilm activity of the synthetic antimicrobial peptide WLBU2 against multiple drug resistant Pseudomonas aeruginosa strains. BMC Microbiol.23 (1), 131. 10.1186/s12866-023-02886-x
117
Michodigni N. F. Nyachieo A. Akhwale J. K. Magoma G. Ouédraogo A. S. Kimang'a A. N. (2022). Formulation of phage cocktails and evaluation of their interaction with antibiotics in inhibiting carbapenemase-producing Klebsiella pneumoniae in vitro in Kenya. Afr. J. Lab. Med.11 (1), 1803. 10.4102/ajlm.v11i1.1803
118
Moghadam M. T. Mojtahedi A. Moghaddam M. M. Fasihi-Ramandi M. Mirnejad R. (2022). Rescuing humanity by antimicrobial peptides against colistin-resistant bacteria. Appl. Microbiol. Biotechnol.106 (11), 3879–3893. 10.1007/s00253-022-11940-z
119
Moghadam M. T. Shariati A. Mirkalantari S. Karmostaji A. (2020). The complex genetic region conferring transferable antibiotic resistance in multidrug-resistant and extremely drug-resistant Klebsiella pneumoniae clinical isolates. New Microbes New Infect.36, 100693. 10.1016/j.nmni.2020.100693
120
Mohammadi M. Saffari M. Siadat S. D. Hejazi S. H. Shayestehpour M. Motallebi M. et al (2023). Isolation, characterization, therapeutic potency, and genomic analysis of a novel bacteriophage vB_KshKPC-M against carbapenemase-producing Klebsiella pneumoniae strains (CRKP) isolated from Ventilator-associated pneumoniae (VAP) infection of COVID-19 patients. Ann. Clin. Microbiol. Antimicrob.22 (1), 18. 10.1186/s12941-023-00567-1
121
Molnar J. Mandi Y. Holland I. Schneider G. (1977). Antibacterial effect, plasmid curing activity and chemical structure of some tricyclic compounds. Acta Microbiol. Acad. Sci. Hung.24 (1), 1–6.
122
Morroni G. Sante L. D. Simonetti O. Brescini L. Kamysz W. Kamysz E. et al (2021). Synergistic effect of antimicrobial peptide LL-37 and colistin combination against multidrug-resistant Escherichia coli isolates. Future Microbiol.16, 221–227. 10.2217/fmb-2020-0204
123
Mulani M. S. Kumkar S. N. Pardesi K. R. (2022). Characterization of novel Klebsiella phage PG14 and its antibiofilm efficacy. Microbiol. Spectr.10 (6), e0199422. 10.1128/spectrum.01994-22
124
Mwangi J. Yin Y. Wang G. Yang M. Li Y. Zhang Z. et al (2019). The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. U S A116 (52), 26516–26522. 10.1073/pnas.1909585117
125
Nagarajan D. Nagarajan T. Roy N. Kulkarni O. Ravichandran S. Mishra M. et al (2018). Computational antimicrobial peptide design and evaluation against multidrug-resistant clinical isolates of bacteria. J. Biol. Chem.293 (10), 3492–3509. 10.1074/jbc.M117.805499
126
Nazzaro F. Fratianni F. De Martino L. Coppola R. De Feo V. (2013). Effect of essential oils on pathogenic bacteria. Pharm. (Basel)6 (12), 1451–1474. 10.3390/ph6121451
127
Oliva A. Bianchi A. Russo A. Ceccarelli G. Cancelli F. Aloj F. et al (2021). Effect of N-acetylcysteine administration on 30-day mortality in critically ill patients with septic shock caused by carbapenem-resistant Klebsiella pneumoniae and acinetobacter baumannii: a retrospective case-control study. Antibiot. (Basel)10 (3), 271. 10.3390/antibiotics10030271
128
Oliva A. Pallecchi L. Rossolini G. M. Travaglino F. Zanatta P. (2023). Rationale and evidence for the adjunctive use of N-acetylcysteine in multidrug-resistant infections. Eur. Rev. Med. Pharmacol. Sci.27 (9), 4316–4325. 10.26355/eurrev_202305_32342
129
Olofsson A. C. Hermansson M. Elwing H. (2003). N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl. Environ. Microbiol.69 (8), 4814–4822. 10.1128/aem.69.8.4814-4822.2003
130
Pan C. Y. Chen J. C. Chen T. L. Wu J. L. Hui C. F. Chen J. Y. (2015). Piscidin is highly active against carbapenem-resistant Acinetobacter baumannii and NDM-1-producing Klebsiella pneumonia in a systemic Septicaemia infection mouse model. Mar. Drugs13 (4), 2287–2305. 10.3390/md13042287
131
Papp-Wallace K. M. Endimiani A. Taracila M. A. Bonomo R. A. (2011). Carbapenems: past, present, and future. Antimicrob. agents Chemother.55 (11), 4943–4960. 10.1128/AAC.00296-11
132
Park J. Kang H. K. Choi M. C. Chae J. D. Son B. K. Chong Y. P. et al (2018). Antibacterial activity and mechanism of action of analogues derived from the antimicrobial peptide mBjAMP1 isolated from Branchiostoma japonicum. J. Antimicrob. Chemother.73 (8), 2054–2063. 10.1093/jac/dky144
133
Parry M. F. Neu H. C. (1977). Effect of N-acetylcysteine on antibiotic activity and bacterial growth in vitro. J. Clin. Microbiol.5 (1), 58–61. 10.1128/jcm.5.1.58-61.1977
134
Peyclit L. Baron S. A. Rolain J.-M. (2019). Drug repurposing to fight colistin and carbapenem-resistant bacteria. Front. Cell. Infect. Microbiol.9, 193. 10.3389/fcimb.2019.00193
135
Pinto E. Vale-Silva L. Cavaleiro C. Salgueiro L. (2009). Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol.58 (11), 1454–1462. 10.1099/jmm.0.010538-0
136
Poirel L. Héritier C. Tolün V. Nordmann P. (2004). Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother.48 (1), 15–22. 10.1128/aac.48.1.15-22.2004
137
Pollini S. Boncompagni S. Di Maggio T. Di Pilato V. Spanu T. Fiori B. et al (2018a). In vitro synergism of colistin in combination with N-acetylcysteine against Acinetobacter baumannii grown in planktonic phase and in biofilms. J. Antimicrob. Chemother.73 (9), 2388–2395. 10.1093/jac/dky185
138
Pollini S. Di Pilato V. Landini G. Di Maggio T. Cannatelli A. Sottotetti S. et al (2018b). In vitro activity of N-acetylcysteine against Stenotrophomonas maltophilia and Burkholderia cepacia complex grown in planktonic phase and biofilm. PLOS ONE13 (10), e0203941. 10.1371/journal.pone.0203941
139
Pundir P. Catalli A. Leggiadro C. Douglas S. E. Kulka M. (2014). Pleurocidin, a novel antimicrobial peptide, induces human mast cell activation through the FPRL1 receptor. Mucosal Immunol.7 (1), 177–187. 10.1038/mi.2013.37
140
Qian W. Sun Z. Wang T. Yang M. Liu M. Zhang J. et al (2020). Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog.139, 103924. 10.1016/j.micpath.2019.103924
141
Radzishevsky I. S. Rotem S. Bourdetsky D. Navon-Venezia S. Carmeli Y. Mor A. (2007). Improved antimicrobial peptides based on acyl-lysine oligomers. Nat. Biotechnol.25 (6), 657–659. 10.1038/nbt1309
142
Rajapaksha D. C. Jayathilaka E. Edirisinghe S. L. Nikapitiya C. Lee J. Whang I. et al (2021). Octopromycin: antibacterial and antibiofilm functions of a novel peptide derived from Octopus minor against multidrug-resistant Acinetobacter baumannii. Fish. Shellfish Immunol.117, 82–94. 10.1016/j.fsi.2021.07.019
143
Rangel M. D. L. Aquino S. G. D. Lima J. M. D. Castellano L. R. Castro R. D. D. (2018). In vitro effect of Cinnamomum zeylanicum Blume essential oil on Candida spp. involved in oral infections. Evidence-Based Complementary Altern. Med.2018, 4045013. 10.1155/2018/4045013
144
Raorane C. J. Lee J.-H. Kim Y.-G. Rajasekharan S. K. García-Contreras R. Lee J. (2019). Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front. Microbiol.10, 990. 10.3389/fmicb.2019.00990
145
Ravensdale J. Wong Z. O’brien F. Gregg K. (2016). Efficacy of antibacterial peptides against peptide-resistant MRSA is restored by permeabilization of bacteria membranes. Efficacy Antibact. Peptides Against Peptide-Resistant MRSA Is Restored by Permeabilization Bact. Membr.7, 1745. 10.3389/fmicb.2016.01745
146
Rinaldi F. Oliva A. Sabatino M. Imbriano A. Hanieh P. N. Garzoli S. et al (2020). Antimicrobial essential oil formulation: chitosan coated nanoemulsions for nose to brain delivery. Pharmaceutics12 (7), 678. 10.3390/pharmaceutics12070678
147
Roberts D. Cole P. (1981). N-acetylcysteine potentiates the anti-pseudomonas activity of carbenicillin in vitro. J. Infect.3 (4), 353–359. 10.1016/S0163-4453(81)91958-7
148
Roque-Borda C. A. Bento Da Silva P. Rodrigues M. C. Di Filippo L. D. Duarte J. L. Chorilli M. et al (2022). Pharmaceutical nanotechnology: antimicrobial peptides as potential new drugs against WHO list of critical, high, and medium priority bacteria. Eur. J. Med. Chem.241, 114640. 10.1016/j.ejmech.2022.114640
149
Roscetto E. Bellavita R. Paolillo R. Merlino F. Molfetta N. Grieco P. et al (2021). Antimicrobial activity of a lipidated temporin L analogue against carbapenemase-producing Klebsiella pneumoniae clinical isolates. Antibiot. (Basel)10 (11), 1312. 10.3390/antibiotics10111312
150
Ruppé É. Woerther P.-L. Barbier F. (2015). Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann. intensive care5, 61. 10.1186/s13613-015-0061-0
151
Samuni Y. Goldstein S. Dean O. M. Berk M. (2013). The chemistry and biological activities of N-acetylcysteine. Biochimica Biophysica Acta (BBA) - General Subj.1830 (8), 4117–4129. 10.1016/j.bbagen.2013.04.016
152
Santiago A. J. Burgos-Garay M. L. Kartforosh L. Mazher M. Donlan R. M. (2020). Bacteriophage treatment of carbapenemase-producing Klebsiella pneumoniae in a multispecies biofilm: a potential biocontrol strategy for healthcare facilities. AIMS Microbiol.6 (1), 43–63. 10.3934/microbiol.2020003
153
Schmelcher M. Donovan D. M. Loessner M. J. (2012). Bacteriophage endolysins as novel antimicrobials. Future Microbiol.7 (10), 1147–1171. 10.2217/fmb.12.97
154
Shariati A. Chegini Z. Ghaznavi-Rad E. Zare E. N. Hosseini S. M. (2022). PLGA-based nanoplatforms in drug delivery for inhibition and destruction of microbial biofilm. Front. Cell. Infect. Microbiol.12, 926363. 10.3389/fcimb.2022.926363
155
Shi Y. Chen Y. Yang Z. Zhang Y. You B. Liu X. et al (2020). Characterization and genome sequencing of a novel T7-like lytic phage, kpssk3, infecting carbapenem-resistant Klebsiella pneumoniae. Arch. Virol.165 (1), 97–104. 10.1007/s00705-019-04447-y
156
Shi Y. Peng Y. Zhang Y. Chen Y. Zhang C. Luo X. et al (2021). Safety and efficacy of a phage, kpssk3, in an in vivo model of carbapenem-resistant hypermucoviscous Klebsiella pneumoniae bacteremia. Front. Microbiol.12, 613356. 10.3389/fmicb.2021.613356
157
Singh P. Garg A. Pandit S. Mokkapati V. Mijakovic I. (2018). Antimicrobial effects of biogenic nanoparticles. Nanomaterials8 (12), 1009. 10.3390/nano8121009
158
Spengler G. Molnár A. Schelz Z. Amaral L. Sharples D. Molnár J. (2006). The mechanism of plasmid curing in bacteria. Curr. drug targets7 (7), 823–841. 10.2174/138945006777709601
159
Sundaramoorthy N. S. Sivasubramanian A. Nagarajan S. (2020). Simultaneous inhibition of MarR by salicylate and efflux pumps by curcumin sensitizes colistin resistant clinical isolates of Enterobacteriaceae. Microb. Pathog.148, 104445. 10.1016/j.micpath.2020.104445
160
Takahashi T. Yamasaki K. (2020). Psoriasis and antimicrobial peptides. Int. J. Mol. Sci.21 (18), 6791. 10.3390/ijms21186791
161
Tan D. Zhang Y. Cheng M. Le S. Gu J. Bao J. et al (2019). Characterization of Klebsiella pneumoniae ST11 isolates and their interactions with lytic phages. Viruses11 (11), 1080. 10.3390/v11111080
162
Tan X. Chen H. Zhang M. Zhao Y. Jiang Y. Liu X. et al (2021). Clinical experience of personalized phage therapy against carbapenem-resistant acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front. Cell Infect. Microbiol.11, 631585. 10.3389/fcimb.2021.631585
163
Tayeb H. H. Moqaddam S. A. Hasaballah N. H. Felimban R. I. (2022). Development of nanoemulsions for the delivery of hydrophobic and hydrophilic compounds against carbapenem-resistant Klebsiella pneumoniae. RSC Adv.12 (40), 26455–26462. 10.1039/d2ra03925g
164
Teklemariam A. D. Al-Hindi R. R. Alharbi M. G. Alotibi I. Azhari S. A. Qadri I. et al (2023). Isolation and characterization of a novel lytic phage, vB_PseuP-SA22, and its efficacy against carbapenem-resistant Pseudomonas aeruginosa. Antibiot. (Basel)12 (3), 497. 10.3390/antibiotics12030497
165
Temel A. Erac B. (2022). Investigating biofilm formation and antibiofilm activity using real time cell analysis method in carbapenem resistant acinetobacter baumannii strains. Curr. Microbiol.79 (9), 256. 10.1007/s00284-022-02943-0
166
Teng T. Li Q. Liu Z. Li X. Liu Z. Liu H. et al (2019). Characterization and genome analysis of novel Klebsiella phage Henu1 with lytic activity against clinical strains of Klebsiella pneumoniae. Arch. Virol.164 (9), 2389–2393. 10.1007/s00705-019-04321-x
167
Tenório M. Graciliano N. G. Moura F. A. Oliveira A. C. M. Goulart M. O. F. (2021). N-acetylcysteine (NAC): impacts on human health. Antioxidants (Basel)10 (6), 967. 10.3390/antiox10060967
168
Thakare R. Shukla M. Kaul G. Dasgupta A. Chopra S. (2019). Repurposing disulfiram for treatment of Staphylococcus aureus infections. Int. J. Antimicrob. agents53 (6), 709–715. 10.1016/j.ijantimicag.2019.03.024
169
Tisato F. Marzano C. Porchia M. Pellei M. Santini C. (2010). Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev.30 (4), 708–749. 10.1002/med.20174
170
Tiwari V. Kapil A. Moganty R. R. (2012). Carbapenem-hydrolyzing oxacillinase in high resistant strains of Acinetobacter baumannii isolated from India. Microb. Pathog.53 (2), 81–86. 10.1016/j.micpath.2012.05.004
171
Tiwari V. Mishra N. Gadani K. Solanki P. Shah N. Tiwari M. (2018). Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol.9, 1218. 10.3389/fmicb.2018.01218
172
Tiwari V. Tiwari M. Solanki V. (2017). Polyvinylpyrrolidone-capped silver nanoparticle inhibits infection of carbapenem-resistant strain of Acinetobacter baumannii in the human pulmonary epithelial cell. Front. Immunol.8, 973. 10.3389/fimmu.2017.00973
173
Tompkins K. Van Duin D. (2021). Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur. J. Clin. Microbiol. & Infect. Dis.40 (10), 2053–2068. 10.1007/s10096-021-04296-1
174
Vasconcelos N. G. Queiroz J. Silva K. E. D. Vasconcelos P. C. P. Croda J. Simionatto S. (2020). Synergistic effects of Cinnamomum cassia L. essential oil in combination with polymyxin B against carbapenemase-producing Klebsiella pneumoniae and Serratia marcescens. PLoS One15 (7), e0236505. 10.1371/journal.pone.0236505
175
Volgers C. Benedikter B. J. Grauls G. E. Hellebrand P. H. M. Savelkoul P. H. M. Stassen F. R. M. (2017). Effects of N-acetyl-L-cysteine on the membrane vesicle release and growth of respiratory pathogens. FEMS Microbiol. Lett.364 (9), fnx087. 10.1093/femsle/fnx087
176
Voor in ‘T Holt A. F. Severin J. A. Lesaffre E. M. Vos M. C. (2014). A systematic review and meta-analyses show that carbapenem use and medical devices are the leading risk factors for carbapenem-resistant Pseudomonas aeruginosa. Antimicrob. agents Chemother.58 (5), 2626–2637. 10.1128/AAC.01758-13
177
Vukotic G. Obradovic M. Novovic K. Di Luca M. Jovcic B. Fira D. et al (2020). Characterization, antibiofilm, and depolymerizing activity of two phages active on carbapenem-resistant acinetobacter baumannii. Front. Med. (Lausanne)7, 426. 10.3389/fmed.2020.00426
178
Wagner A. M. Gran M. P. Peppas N. A. (2018). Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm. Sin. B8 (2), 147–164. 10.1016/j.apsb.2018.01.013
179
Wang B. Xie N. Li B. (2019). Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: a review. J. Food Biochem.43 (1), e12571. 10.1111/jfbc.12571
180
Wang G. Li X. Wang Z. (2016). APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res.44 (D1), D1087–D1093. 10.1093/nar/gkv1278
181
Wang Z. Cai R. Wang G. Guo Z. Liu X. Guan Y. et al (2021). Combination therapy of phage vB_KpnM_P-KP2 and gentamicin combats acute pneumonia caused by K47 serotype Klebsiella pneumoniae. Front. Microbiol.12, 674068. 10.3389/fmicb.2021.674068
182
Wendorf K. A. Kay M. Baliga C. Weissman S. J. Gluck M. Verma P. et al (2015). Endoscopic retrograde cholangiopancreatography-associated AmpC Escherichia coli outbreak. Infect. Control Hosp. Epidemiol.36 (6), 634–642. 10.1017/ice.2015.66
183
Wintachai P. Phaonakrop N. Roytrakul S. Naknaen A. Pomwised R. Voravuthikunchai S. P. et al (2022). Enhanced antibacterial effect of a novel Friunavirus phage vWU2001 in combination with colistin against carbapenem-resistant Acinetobacter baumannii. Sci. Rep.12 (1), 2633. 10.1038/s41598-022-06582-0
184
Wu N. Dai J. Guo M. Li J. Zhou X. Li F. et al (2021). Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg. Microbes Infect.10 (1), 612–618. 10.1080/22221751.2021.1902754
185
Wu Y. Wang R. Xu M. Liu Y. Zhu X. Qiu J. et al (2019). A novel polysaccharide depolymerase encoded by the phage SH-KP152226 confers specific activity against multidrug-resistant Klebsiella pneumoniae via biofilm degradation. Front. Microbiol.10, 2768. 10.3389/fmicb.2019.02768
186
Yadav S. Singh A. K. Agrahari A. K. Pandey A. K. Gupta M. K. Chakravortty D. et al (2021). Galactose-clicked curcumin-mediated reversal of meropenem resistance among Klebsiella pneumoniae by targeting its carbapenemases and the AcrAB-TolC efflux system. Antibiot. (Basel)10 (4), 388. 10.3390/antibiotics10040388
187
Yan J. Mao J. Xie J. (2014). Bacteriophage polysaccharide depolymerases and biomedical applications. BioDrugs28 (3), 265–274. 10.1007/s40259-013-0081-y
188
Yang L. Luo M. Liu Z. Li Y. Lin Z. Geng S. et al (2023). BamA-targeted antimicrobial peptide design for enhanced efficacy and reduced toxicity. Amino Acids55 (10), 1317–1331. 10.1007/s00726-023-03307-z
189
Yang M. Zhang C. Hansen S. A. Mitchell W. J. Zhang M. Z. Zhang S. (2019a). Antimicrobial efficacy and toxicity of novel CAMPs against P. aeruginosa infection in a murine skin wound infection model. BMC Microbiol.19 (1), 293. 10.1186/s12866-019-1657-6
190
Yang S. K. Yusoff K. Ajat M. Thomas W. Abushelaibi A. Akseer R. et al (2019b). Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PLoS One14 (4), e0214326. 10.1371/journal.pone.0214326
191
Yang S. K. Yusoff K. Ajat M. Wee C. Y. Yap P. S. Lim S. H. et al (2021). Combinatorial antimicrobial efficacy and mechanism of linalool against clinically relevant Klebsiella pneumoniae. Front. Microbiol.12, 635016. 10.3389/fmicb.2021.635016
192
Zafarullah M. Li W. Q. Sylvester J. Ahmad M. (2003). Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. CMLS60 (1), 6–20. 10.1007/s000180300001
193
Zasloff M. (2002). Antimicrobial peptides of multicellular organisms. Nature415 (6870), 389–395. 10.1038/415389a
194
Zhang M. Wang J. Li C. Wu S. Liu W. Zhou C. et al (2024). Cathelicidin AS-12W derived from the Alligator sinensis and its antimicrobial activity against drug-resistant gram-negative bacteria in vitro and in vivo. Probiotics Antimicrob. Proteins. 10.1007/s12602-024-10250-2
195
Zhang X. Shi S. Yao Z. Zheng X. Li W. Zhang Y. et al (2022a). Antimicrobial peptide WAM-1: a promising antibacterial and anti-inflammatory drug against carbapenem-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother.77 (7), 1903–1911. 10.1093/jac/dkac128
196
Zhang Y. Chen C. Cheng B. Gao L. Qin C. Zhang L. et al (2022b). Discovery of quercetin and its analogs as potent OXA-48 beta-lactamase inhibitors. Front. Pharmacol.13, 926104. 10.3389/fphar.2022.926104
197
Zhang Y. Chen C. Cheng B. Gao L. Qin C. Zhang L. et al (2022c). Discovery of quercetin and its analogs as potent OXA-48 beta-lactamase inhibitors. Front. Pharmacol.13, 926104. 10.3389/fphar.2022.926104
198
Zhao J. Zhang Z. Tian C. Chen X. Hu L. Wei X. et al (2019). Characterizing the biology of lytic bacteriophage vB_EaeM_φEap-3 infecting multidrug-resistant Enterobacter aerogenes. Front. Microbiol.10, 420. 10.3389/fmicb.2019.00420
199
Zhao T. Liu Y. (2010). N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol.10 (1), 140. 10.1186/1471-2180-10-140
200
Zhou W. Feng Y. Zong Z. (2018). Two new lytic bacteriophages of the myoviridae family against carbapenem-resistant acinetobacter baumannii. Front. Microbiol.9, 850. 10.3389/fmicb.2018.00850
201
Zilberberg M. D. Nathanson B. H. Sulham K. Fan W. Shorr A. F. (2017). Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect. Dis.17 (1), 279. 10.1186/s12879-017-2383-z
Summary
Keywords
carbapenems-resistant, natural compounds, bacteriophages, nanoparticle, N-acetylcysteine, antimicrobial peptides
Citation
Shariati A, Kashi M, Chegini Z and Hosseini SM (2024) Antibiotics-free compounds for managing carbapenem-resistant bacteria; a narrative review. Front. Pharmacol. 15:1467086. doi: 10.3389/fphar.2024.1467086
Received
19 July 2024
Accepted
04 September 2024
Published
17 September 2024
Volume
15 - 2024
Edited by
Hong Zhou, Zunyi Medical University, China
Reviewed by
Gabriela Cristina Fernandez, National Scientific and Technical Research Council (CONICET), Argentina
Ya-Si Huang, Zunyi Medical University, China
Updates
Copyright
© 2024 Shariati, Kashi, Chegini and Hosseini.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aref Shariati, arefshariati0111@gmail.com; Seyed Mostafa Hosseini, smhoseiny88@yahoo.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.