- Department of Nephrology, The Second Hospital of Jilin University, Changchun, Jilin Province, China
Introduction: Acute kidney injury (AKI) is a key clinical condition that has puzzled clinicians for many years since there is currently no efficient drug therapy. Vitamin E is found to exert a vital antioxidant role and can protect the kidney. However, clinical studies that analyze the correlation between vitamin E and AKI are scarce, and no consistent conclusions are reported from current studies. Therefore, this study was performed to evaluate the impact of vitamin E on treating AKI.
Methods: The PubMed, Embase, and Cochrane Library databases were comprehensively searched on 27 December 2023. Qualified studies were selected following the eligibility criteria. The incidence of AKI, serum creatinine, and urea nitrogen levels after vitamin E treatment were evaluated. Then, the data were combined with a fixed- or random-effects model, depending on the heterogeneity test results.
Results: Six eligible randomized controlled trials that used vitamin E for the prevention of kidney injury were included. According to our pooled analysis, vitamin E elevated eGFR levels [MD: 0.36; 95% CI (0.19, 0.53), p = 0.000], reduced serum creatinine levels [MD: −0.32; 95% CI (−0.48, 0.16), p = 0.000], and effectively inhibited the occurrence of AKI [RR: 0.69; 95% CI (0.49, 0.98), p = 0.036].
Conclusion: Vitamin E elevates eGFR levels, reduces serum creatinine levels, and efficiently suppresses AKI occurrence.Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024499597, identifier CRD42024499597
Introduction
Acute kidney injury (AKI) is a common yet severe condition that affects millions of people and causes disability and mortality in many sufferers (Kellum et al., 2013). AKI is defined by the AKI Network by at least one or more conditions: (1) elevation of serum creatinine (Scr) level by 0.3 mg/dL (26.5 mol/L) in 48 h; (2) increase of Scr level to 1.5 folds of baseline over the past week; (3) urine volume <0.5 mL/kg/h during 6 h (Mehta et al., 2007). AKI exhibits a high and growing incidence rate globally (Hoste et al., 2018; Ronco et al., 2019). It has been reported in 7.1% of patients receiving immune checkpoint inhibitor treatment (Ji et al., 2022). In addition, 22.4% of patients develop AKI within 48 h after surgery (Gameiro et al., 2020). The presence of AKI affects acute morbidity and mortality and is related to unfavorable long-time outcomes, including an increased risk of cardiovascular complications, long-term mortality, and development of (or progression to) chronic kidney disease (CKD) (Hoste et al., 2018; Pickkers et al., 2021; James et al., 2020).
Nonetheless, effective interventions for AKI remain limited, highlighting the critical role of therapeutic drug monitoring as a cornerstone strategy in mitigating drug-associated nephrotoxicity (Ostermann et al., 2020). Based on clinical trials, early RRT implementation may not be beneficial for the survival of patients with no obvious urgent indications (Zarbock et al., 2016; Bagshaw, 2020; Gaudry et al., 2020). Implementing care bundles of AKI among inpatients can decrease the risk of moderate to severe AKI (Schaubroeck et al., 2021). AKI may be cured with early detection and treatment (Yue et al., 2023). Until now, there have been no drugs and treatments for specifically preventing or treating AKI in humans (Pickkers et al., 2021; Fang et al., 2021a).
As a fat-soluble vitamin, vitamin E exerts a critical antioxidant impact on the human body. As an essential nutrient, it was first identified by Evans and Bishop (1922) and was initially called “anti-sterility factor” or “factor X,” according to its effect on rat reproduction. It is composed of a variety of compounds, and there are eight isomers, including four tocopherols (α-, β-, γ-, and δ-tocopherol) and four tocotrienols (α-, β-, γ-, and δ-tocotrienol) in the vitamin E family (Miyazawa et al., 2019). Currently, most studies concerning vitamin E metabolism pay more attention to alpha- and gamma-tocopherols because these are bioavailable and can be more easily absorbed from diets than other vitamin E isomers (Wu and Croft, 2007).
Unstable free radical molecules may induce oxidative stress and damage cells and tissues in the body. Vitamin E, containing the chromanol hydroxyl group, can chemically scavenge free radical oxidants. Previous in vitro results indicate that alpha-tocopherol can prevent lipid peroxidation catalyzed by free radicals (Esterbauer et al., 1989). AKI may result from various stimuli, among which reactive oxygen species (ROS) are shown to exert a vital impact on AKI, which can induce injuries to proteins, DNA, and carbohydrates (Salehipour et al., 2010). Nonetheless, vitamin E may mitigate AKI symptoms (Koga et al., 2012; Kataoka et al., 2012; Liu et al., 2015). Additionally, emerging mechanistic studies indicate that vitamin E significantly suppresses ROS and ameliorates AKI (Zhang et al., 2024; Georgiev et al., 2023). Apart from its antioxidant effect, vitamin E can regulate gene expression, resist inflammation, and protect the kidneys (Zaaboul and Liu, 2022).
The conclusions drawn from current studies on the role of vitamin E in protecting AKI are still inconsistent (Cho et al., 2017; Xu et al., 2018; Monami et al., 2021). To our knowledge, there is currently no meta-analysis that explores the effect of vitamin E on drug-induced AKI. Therefore, this study aimed to investigate the correlation between vitamin E and drug-induced AKI in randomized controlled trials (RCTs) performed with humans.
Methods
Search methods
The PubMed, Cochrane Library, and Embase databases were searched from inception to 27 December 2023 to find published RCTs on the use of vitamin E in high-risk AKI patients with available kidney function outcomes. Keywords included “contrast induced kidney injury,” “renal ischemia reperfusion injury,” “acute renal failure,” “nephrotoxicity,” “AKI,” “kidney damage,” “renal function,” “vitamin E,” “tocopherols,” “tocopherylquinone,” “tocotrienols,” and “tocophersolan.” The research was limited to human studies, and only English language manuscripts were included. Abstracts of all articles were reviewed to develop a full reference list. In addition, references in related original and review studies were checked to manually identify qualified RCTs. This study is registered with PROSPERO (number CRD42024499597).
Study selection
In the current meta-analysis, two investigators (Lingfei Meng and Shengmao Liu) independently conducted title- and abstract-screening of eligible publications. To identify whether these studies satisfied our preset eligibility criteria, the full texts of those screened articles were downloaded. Any disagreements between them were settled by mutual negotiation with a third investigator (Wenpeng Cui).
Eligibility criteria
Inclusion criteria
The inclusion criteria were (1) RCTs with a crossover or parallel design; (2) studies exploring the role of vitamin E in kidney function in human; (3) studies including patients aged ≥14 years; (4) the use of vitamin E in the treatment group; (5) studies setting a placebo or control group; (6) studies with primary outcomes of Scr, blood urea nitrogen (BUN), eGFR, or AKI; (7) studies published in English.
Types of studies
RCTs evaluating the application of vitamin E in renoprotection in humans were included.
Types of study populations
The study populations included adults with AKI associated with drug application in humans.
Types of interventions
All vitamin E types compared with control or placebo were involved, with no limitation of dosage, dosage form, vitamin E application time, or duration.
Types of outcomes
Primary outcomes were probabilities of AKI, eGFR, Scr, and BUN to evaluate kidney function.
Exclusion criteria
The following exclusion criteria were applied: (1) studies in which vitamin E was accompanied by other interventions, uncontrolled clinical trials, conference abstracts, book chapters, comments, interviews, opinion pieces, position papers, methodological papers, letters, editorials, and animal or cell culture studies; (2) studies irrelevant to drugs related kidney injury; (3) studies not reporting relevant outcomes, mean or median and standard deviation (SD), and the number of AKI episodes; (3) studies with in vitro experiments and animal experiments alone; and (4) non-available articles.
Data collection
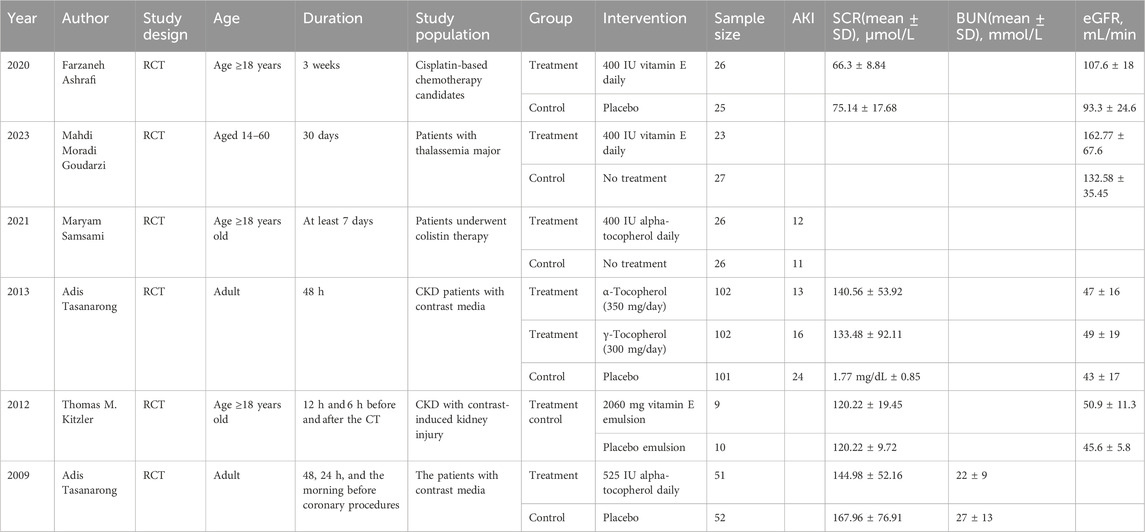
Data were collected from eligible RCTs by two investigators (Lingfei Meng and Shengmao Liu) independently. Any disagreements between them were settled by negotiation with a third investigator (Wenpeng Cui). These relevant data were collected from each study (Table 1): (1) study features (first author, publication year); (2) study population; (3) interventions (grouping, dosage and time, route, timing, and duration of administration); and (4) eGFR, BUN, and Scr data, as well as the number of AKI episodes. In addition, the means, SD, AKI episodes, and population size in each group were collected.
Assessment of risk of bias
To examine the overall study quality, two reviewers (Lingfei Meng and Shengmao Liu) assessed the bias risk using RevMan 5.3. Bias was marked as high, low, or “unclear” (indicating that the bias risk was unknown). Any disagreement was settled by discussion with a third reviewer (Wenpeng Cui).
Statistical analysis
If two or more studies presented the findings of an identical outcome, we summarized data and assessed the outcomes (eGFR, BUN, Scr, and AKI). Initially, we performed meta-analysis of articles comparing vitamin E with control groups, using Review Manager 5.3 software and Stata SE 15 for data analysis. The results presented in the current report were mean difference (MD) of measurements with the same unit or standardized mean difference (SMD) with different units. To evaluate the therapeutic effect of vitamin E, the pooled data were employed. In addition, I2 statistic was used to assess heterogeneity among our enrolled articles. A fixed-effect model should be applied in the case of insignificant heterogeneity (i.e., p > 0.1; I2 ≤ 50%); otherwise, a random-effect model was applied.
Results
Characteristics of eligible studies
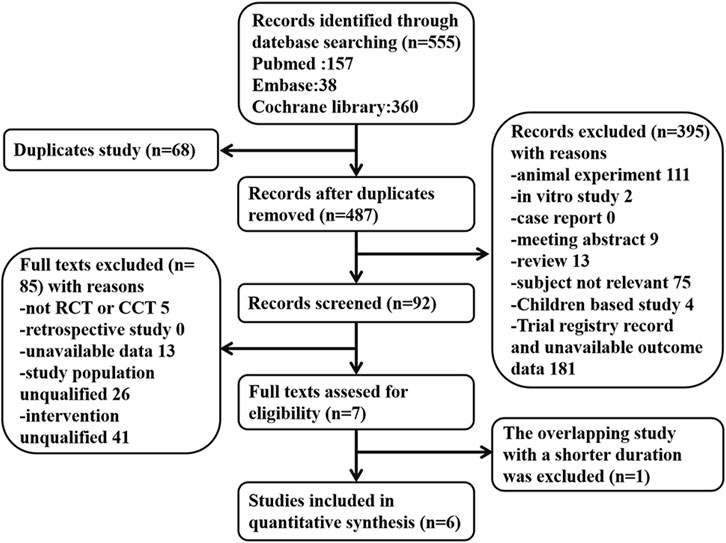
A total of 555 articles were acquired through literature search and later screened following the inclusion and exclusion criteria. If two reports covered overlapping studies, the one involving more samples and/or having a longer study period was chosen. There were six eligible RCTs concerning vitamin E for the prevention of kidney injury. Of these trials, kidney function was applied as the primary or secondary outcome. Contrast media-induced kidney injury was reported in three RCTs. Other drug-induced kidney injuries were reported in three RCTs. AKI was reported in two studies, while eGFR, Scr, and BUN were reported in four, four, and one study, respectively. Figure 1 summarizes the screening process of eligible clinical trials. Finally, six RCTs involving 580 participants were included for analysis. All patients in these studies received vitamin E treatment, and the follow-up time in all studies ranged from 1 day to 2 months. Table 1 presents the characteristics of the studies we included on analyzing kidney protection.
Quality assessment
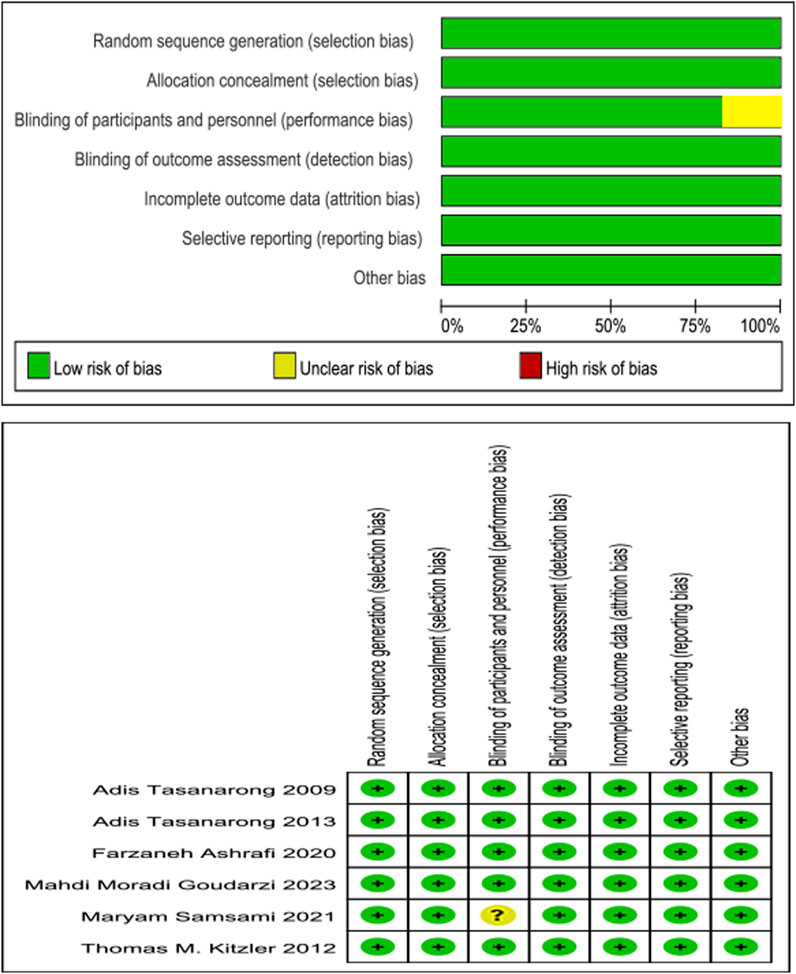
The methodological features most associated with the risk of bias below were assessed, including randomization, allocation concealment, treatment allocation blinding, outcome assessment blinding, incomplete outcome data and selective reporting, and other biases. With study quality, five of these six enrolled RCTs met seven of the above criteria, and one of these six enrolled RCTs met six (Figure 2).
Effects of interventions
Primary outcome: AKI
Two RCTs (Tasanarong et al., 2013; Samsami et al., 2021) satisfied our inclusion criteria. One study included both α- and γ-tocopherol in the treatment group, and thus study data were extracted twice. Due to the absence of significant heterogeneity, a fixed-effects model (p = 0.244, I2 = 29.1%) was used. After combined analysis of these two studies, the AKI incidence of the vitamin E group significantly decreased compared to the control group [RR: 0.69; 95% CI (0.49, 0.98), p = 0.036] (Figure 3A). The galbr plot exhibited little heterogeneity between the three outcomes of the two studies (Figure 3B). Based on sensitivity analysis, no study exerted a disproportionate impact on the outcomes (Figure 3C).
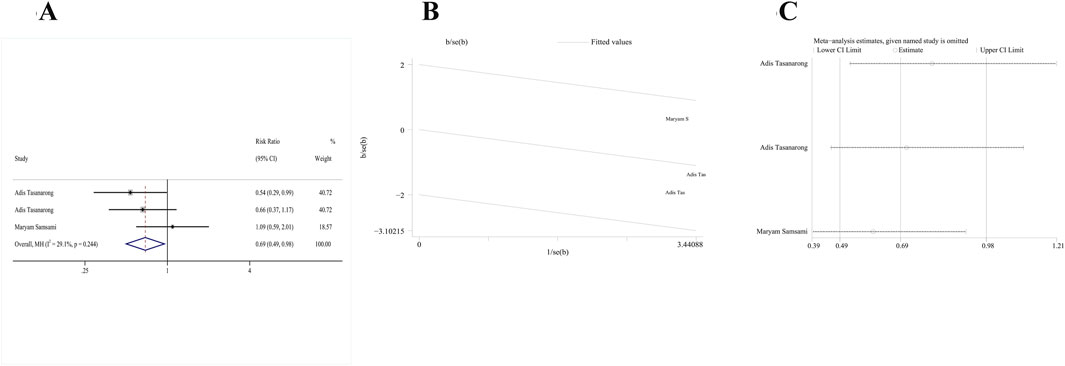
Figure 3. Comparison of efficacy between vitamin E and placebo in AKI. (A) Forest map. (B) Test of heterogeneity by the galbr plot. (C) Sensitivity analysis.
Primary outcome: eGFR
Four RCTs (Tasanarong et al., 2013; Goudarzi et al., 2023; Ashrafi et al., 2020; Kitzler et al., 2012) met our inclusion criteria. One study included both α- and γ-tocopherol in the treatment group; thus, study data were extracted twice. A fixed-effects model was employed since no significant heterogeneity was detected (p = 0.627, I2 = 0%). According to pooled results from these four RCTs, the change of serum eGFR from baseline to follow-up of vitamin E group significantly decreased compared with the control group [MD: 0.36; 95% CI (0.19, 0.53), p = 0.000] (Figure 4A). In addition, the galbr plot showed little heterogeneity between the included studies (Figure 4B). According to sensitivity analysis, no study exerted a disproportionate effect on the outcome (Figure 4C).
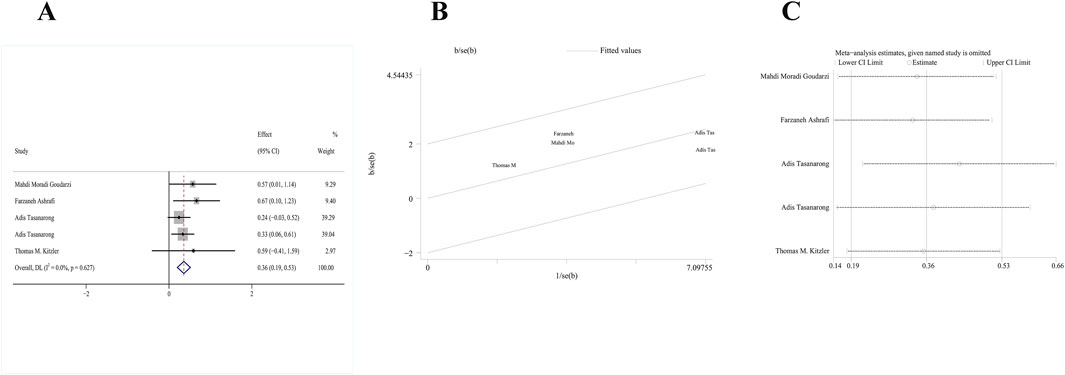
Figure 4. Comparison of efficacy between vitamin E and placebo in eGFR. (A) Forest map. (B) Test of heterogeneity by the galbr plot. (C) Sensitivity analysis.
Primary outcome: Scr
Four RCTs (Tasanarong et al., 2013; Ashrafi et al., 2020; Kitzler et al., 2012; Tasanarong et al., 2009) mentioning the role of vitamin E in Scr as the primary outcome satisfied our inclusion criteria. The serum creatinine follow-up data were extracted. A fix-effects model was used owing to the absence of heterogeneity (p = 0.731, I2 = 0.00%). The pooled analysis of these studies demonstrated that the change of serum creatinine level in the treatment group between baseline and follow-up significantly decreased relative to the control group [MD: −0.32; 95% CI (−0.48, 0.16), p = 0.000] (Figure 5A). The galbr plot exhibited little heterogeneity between the included studies (Figure 5B). Sensitivity analysis indicated that the serum creatinine data in the included RCTs remained stable (Figure 5C).
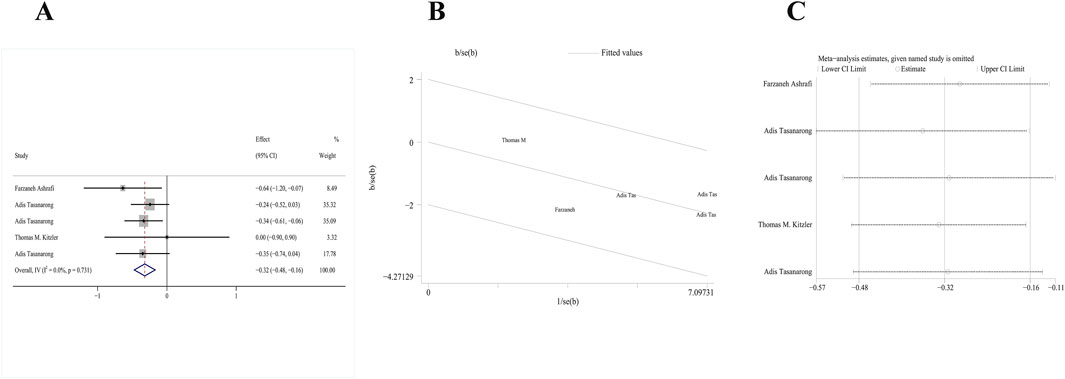
Figure 5. Comparison of efficacy between vitamin E and placebo in Scr. (A) Forest map. (B) Test of heterogeneity by the galbr plot. (C) Sensitivity analysis.
Primary outcome: BUN
One RCT (Tasanarong et al., 2009) mentioned the role of vitamin E in serum BUN as the primary outcome. No data extraction or analysis was performed.
Discussion
This meta-analysis enrolled six RCTs about vitamin E for AKI. The designs of these studies were reasonable, and they were of high quality. Our pooled analysis indicated that vitamin E effectively inhibited the occurrence of AKI, increased the eGFR level, and reduced the Scr level in drug-induced kidney injury.
AKI has puzzled clinicians as its pathophysiology has not been completely understood. There are multiple mechanisms involved in its process, including oxidative stress, inflammation, RAAS activation, and DNA damage (De Chiara et al., 2022; Schefold et al., 2016). Among these, oxidative stress exerts a vital role in AKI. According to related basic and clinical study results, vitamin E makes an anti-oxidative stress impact on AKI. It also shows a protective effect against nephrotoxicity induced by drugs like cisplatin or other nephrotoxic medicines.
Vitamin E prevents drug-mediated kidney damage. Numerous animal experiments regarding the therapeutic efficacy of vitamin E in cisplatin-related toxicities have been published (Abdel-Daim et al., 2019; Abo-Elmaaty et al., 2020; Busari et al., 2018). Cisplatin-related toxicities may mostly result from free radical generation, and the latter can induce oxidative organ injury (Hakiminia et al., 2019). ROS production in mitochondria is a major mechanism related to nephrotoxicity. ROS production and proapoptotic protein activation within an intrinsic pathway can lead to increased mitochondrial membrane permeability (Servais et al., 2008) which reduces the mitochondrial membrane potential, causes calcium homeostasis imbalance, decreases adenosine triphosphate (ATP) generation, and leads to mitochondrial respiratory chain impairment (dos Santos et al., 2012). Vitamin E can increase ATP levels in every tissue except muscle and improve drug-induced mitochondrial dysfunctions in rats (Eser Faki et al., 2020).
Vitamin E also has a protective impact on kidney damage induced by other drugs. Its use can improve oxidative stress indicators, inflammatory markers, histopathological results, and kidney functions in rats with toxic acetamiprid (Erdemli et al., 2020). Vitamin E supplementation can mitigate cadmium-mediated oxidative stress and injury in rat kidneys by suppressing renal cell apoptosis and promoting the antioxidant defense system (Fang et al., 2021b). Supplementation with vitamins E alone in rats applied with amphotericin B can improve kidney tissue structures, functional parameters, and oxidative stress status (Salehzadeh et al., 2020). Vitamin E can regulate apoptosis and autophagy to prevent HgCl2-mediated kidney injury (Alhusaini et al., 2022). Furthermore, vitamin E can be used to mitigate kidney impairments induced by chronic gabapentin use, and its possible mechanism is associated with the inhibition of tissue injury and apoptosis biomarkers (Welson et al., 2021).
The use of vitamin E in treating AKI thus has a profound pathophysiological basis. Human studies on the application of vitamin E in treating AKI are limited, and no consistent conclusion has been drawn (Samsami et al., 2021; Goudarzi et al., 2023; Kitzler et al., 2012; Zhao et al., 2018; Koay et al., 2021). Although some systematic reviews on contrast-induced AKI (CIAKI) have been performed, the conclusions are also inconsistent (Cho et al., 2017; Xu et al., 2018; Monami et al., 2021; Cho et al., 2017). One meta-analysis showed that vitamin E significantly decreased the CIAKI risk ratio by 62% and reduced Scr elevation following contrast application; however, no differences were found in GFR (Cho et al., 2017). Another meta-analysis showed that α-tocopherol pretreatment prior to contrast medium-requiring imaging examinations significantly reduced contrast-induced nephropathy risk, while it exerted no impact on Scr or eGFR (Monami et al., 2021). The meta-analysis performed by Xu et al. (2018) found that vitamin E was beneficial for decreasing CIAKI risk. These studies’ conclusions are probably inconsistent due to their heterogeneous timing and inclusion criteria and their outcome definitions. Our meta-analysis offers some strengths as the sample size increases as the study population focuses on patients with drug-induced AKI.
Four studies regarding the impact of vitamin E on Scr were included in our meta-analysis (Kitzler et al., 2012; Tasanarong et al., 2009; Schefold et al., 2016). In other clinical trials, one study showed that vitamin E, compared to placebo, significantly decreased Scr levels in patients with diabetic nephropathy (Tan et al., 2018), supporting our conclusion. Another study found that vitamin E and allopurinol did not significantly decrease Scr levels among patients developing pre-existing kidney failure who received coronary artery bypass grafting (CABG) surgery (Nouri-Majalan et al., 2009). This seemed inconsistent with our conclusion. The inconsistency in the findings may be related to the inclusion of interventions other than vitamin E alone and difference in the study population. Our meta-analysis only included vitamin E not accompanied by other treatments, which is more beneficial.
As was concluded in four RCTs about the function of vitamin E in eGFR, vitamin E effectively increased eGFR levels. Patients with drug-induced AKI were studied in this meta-analysis. However, most clinical trials have focused on vitamin E and eGFR in diabetic kidney disease. One RCT showed that compared with placebo, vitamin E did not influence eGFR in patients with diabetic nephropathy at 2 months (Tan et al., 2018). Another study indicated that vitamin E application led to improved kidney function at 8 and 12 months, as evaluated through eGFR in diabetic kidney disease (Koay et al., 2021). The course of vitamin E was different in the above two studies. A longer-term application of vitamin E had better renoprotection. Nevertheless, vitamin E may have an inconsistent function in people with different genotypes of diabetes. Based on Dalan et al. (2020), vitamin E application resulted in increased eGFR levels in patients with haptoglobin 2-2 (Hp2-2) genotype diabetes compared with the non-Hp2-2 group. A newly published meta-analysis reported that eGFR of the vitamin E group did not exhibit any statistical significance in diabetic nephropathy (Jin et al., 2024). Furthermore, the pathogenesis of diabetic nephropathy involves multiple interconnected pathways, such as hemodynamic disturbances, oxidative stress, cellular apoptosis, and genetic/epigenetic regulation, with glomerular lesions representing the most prominent pathological feature (Wang et al., 2023). In contrast, drug-induced kidney injury, although it also involves oxidative stress and other mechanisms, primarily manifests as tubulointerstitial damage, a leading contributor to AKI (Perazella and Rosner, 2022). These distinct pathogenic mechanisms, combined with the heterogeneity of study populations, may explain the differential effects of vitamin E on eGFR.
In two RCTs included in this study, the vitamin E group showed a significantly reduced AKI risk, although no significant difference was found in AKI prevalence and duration between vitamin E and control groups in Samsami et al. (2021). Currently, vitamin E combined with additional drugs attracts more attention. Guo and Wang (2018) suggested that vitamin E combined with umbilical cord mesenchymal stem cells (UC-MSC) apparently suppressed the renal inflammatory response by regulating inflammatory cytokines within the kidney microenvironment in AKI rats. They also found that vitamin E combined with UC-MSC has superior efficacy in treating AKI to vitamin E or UC-MSC monotherapy. Truksa et al. (2015) reported that vitamin E analogs targeting mitochondria represent a new mitocan class, which can cause apoptosis and inhibit proliferation, transcription and normal mitochondrial activity. Regulating mitochondrial activity with the mitochondria-targeting antioxidant is the candidate way to mitigate injury of proximal tubular epithelial cells (Sakamoto et al., 2017). Site-targeting vitamin E is also a vital direction in the future of AKI. Due to the limited number of studies included, caution is warranted when generalizing the findings regarding the therapeutic effect of vitamin E on AKI. Further large-scale studies are needed to validate our conclusions.
Nevertheless, as reported in some clinical studies, high-dose α-tocopherol supplementation induces unfavorable effects (Brown et al., 2001; Ward et al., 2007), which is also supported by a meta-analysis (Miller et al., 2005). The dosage of vitamin E therapy is an important issue. Clearly, the mean dietary source of α-tocopherol is approximately 1–10 mg68. Oral vitamin E can be absorbed by the body and catabolized in the liver and intestine. In an intervention with 40% fat, it can be estimated that most metabolite excreted in urine is derived from the liver. In the fasting period, nearly half of metabolite is derived from the liver, while the rest is from the intestine (Traber et al., 2021). Nevertheless, research has not yet identified the clinically effective blood concentration of biologically active vitamin E for ameliorating AKI. Concerning serum levels, 13% of all recruited data points worldwide are below 12 μmol/L, which is the threshold of functional deficiency and is mainly for neonates and children. Based on some prospective observational studies, the α-tocopherol content ≥30 μmol/L in serum benefits human health (Péter et al., 2015). The excessive use of vitamin supplements containing high-dose vitamins A, D, and E may lead to AKI (De Francesco Daher et al., 2017). Both the dose of vitamin E therapy and target serum concentration will be directions of future studies.
The strength of our study is that all the included studies were high-quality RCTs with a relatively large population. As far as we know, this is the first meta-analysis exploring the effect of vitamin E on drug-induced AKI in RCTs performed in humans. Nonetheless, this study does have the following limitations: differences in the agents causing AKI, the inclusion of patients with varying underlying conditions, variations in dosing regimens, interventions in the control groups, and follow-up periods that may have contributed to heterogeneity. Due to the limiting data from the included RCTs, the side effects of vitamin E were not analyzed systematically. Therefore, more RCTs are needed to study the therapeutic effects of different vitamin E doses, the effect of vitamin E on markers of oxidative stress, and the side effects of vitamin E.
Conclusion
Vitamin E can effectively inhibit the occurrence of AKI, increase eGFR levels, and decrease Scr levels. Further well-designed RCTs should be performed to verify the protection, side effects, and appropriate dosing of vitamin E.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
LM: writing–original draft and writing–review and editing. SL: data curation and writing–review and editing. WC: conceptualization and writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Daim, M. M., Aleya, L., El-Bialy, B. E., Abushouk, A. I., Alkahtani, S., Alarifi, S., et al. (2019). The ameliorative effects of ceftriaxone and vitamin E against cisplatin-induced nephrotoxicity. Environ. Sci. Pollut. Res. Int. 26, 15248–15254. doi:10.1007/s11356-019-04801-2
Abo-Elmaaty, A. M. A., Behairy, A., El-Naseery, N. I., and Abdel-Daim, M. M. (2020). The protective efficacy of vitamin E and cod liver oil against cisplatin-induced acute kidney injury in rats. Environ. Sci. Pollut. Res. Int. 27, 44412–44426. doi:10.1007/s11356-020-10351-9
Alhusaini, A. M., Alhumaidan, S. A., Alharbi, G. M., Alzahrani, E. A., Sarawi, W. S., Alomar, H. A., et al. (2022). Cross-regulation between autophagy and apoptosis induced by vitamin E and lactobacillus plantarum through beclin-1 Network. Int. J. Mol. Sci. 23, 15305. doi:10.3390/ijms232315305
Ashrafi, F., Tabiei, M. N., Mousavi, S., Nematbakhsh, M., Sotoodehnasab, P., and Janbabaei, G. (2020). Does vitamin E mitigate cisplatin-induced nephrotoxicity in cancer patients: results from a randomized placebo-controlled clinical trial. Middle East J. cancer 11, 174–184. doi:10.30476/mejc.2019.78710.0
Bagshaw, S. M. (2020). Timing of initiation of renal-replacement therapy in acute kidney injury. Injury 383, 240–251. doi:10.1056/NEJMoa2000741
Brown, B. G., Zhao, X. Q., Chait, A., Fisher, L. D., Cheung, M. C., Morse, J. S., et al. (2001). Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345, 1583–1592. doi:10.1056/NEJMoa011090
Busari, A. A., Adejare, A. A., Shodipe, A. F., Oduniyi, O. A., Ismail-Badmus, K. B., and Oreagba, I. A. (2018). Protective but non-synergistic effects of nigella sativa and vitamin E against cisplatin-induced renal toxicity and oxidative stress in wistar rats. Animals open access J. MDPI 68, 696–703. doi:10.1055/a-0626-7003
Cho, M., Kim, S., Park, H., Chung, S., and Kim, K. (2017). Could vitamin E prevent contrast-induced acute kidney injury? A systematic review and meta-analysis. J. Korean Med. Sci. 32, 1468–1473. doi:10.3346/jkms.2017.32.9.1468
Dalan, R., Goh, L. L., Lim, C. J., Seneviratna, A., Liew, H., Seow, C. J., et al. (2020). Impact of Vitamin E supplementation on vascular function in haptoglobin genotype stratified diabetes patients (EVAS Trial): a randomised controlled trial. Nutr. Diabetes 10, 13. doi:10.1038/s41387-020-0116-7
De Chiara, L., Lugli, G., Villa, G., Raglianti, V., Husain-Syed, F., Ravaglia, F., et al. (2022). Molecular mechanisms and biomarkers associated with chemotherapy-induced AKI. Int. J. Mol. Sci. 23, 2638. doi:10.3390/ijms23052638
De Francesco Daher, E., Mesquita Martiniano, L. V., Lopes Lima, L. L., Viana Leite Filho, N. C., de Oliveira Souza, L. E., Duarte Fernandes, P. H. P., et al. (2017). Acute kidney injury due to excessive and prolonged intramuscular injection of veterinary supplements containing vitamins A, D and E: a series of 16 cases. Nefrol. publicacion Of. Soc. Espanola Nefrol. 37, 61–67. doi:10.1016/j.nefro.2016.05.017
dos Santos, N. A., Carvalho Rodrigues, M. A., Martins, N. M., and dos Santos, A. C. (2012). Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Archives Toxicol. 86, 1233–1250. doi:10.1007/s00204-012-0821-7
Erdemli, M., Zayman, E., Erdemli, Z., Gul, M., Gul, S., and Gozukara Bag, H. (2020). Protective effects of melatonin and vitamin E in acetamiprid-induced nephrotoxicity. Environ. Sci. Pollut. Res. Int. 27, 9202–9213. doi:10.1007/s11356-019-06754-y
Eser Faki, H., Tras, B., and Uney, K. (2020). Alpha lipoic acid and vitamin E improve atorvastatin-induced mitochondrial dysfunctions in rats. Mitochondrion 52, 83–88. doi:10.1016/j.mito.2020.02.011
Esterbauer, H., Striegl, G., Puhl, H., and Rotheneder, M. (1989). Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic. Res. Commun. 6, 67–75. doi:10.3109/10715768909073429
Evans, H. M., and Bishop, K. S. (1922). On the existence of a hitherto unrecognized dietary factor essential for reproduction. Sci. (New York, N.Y.) 56, 650–651. doi:10.1126/science.56.1458.650
Fang, C. Y., Lou, D. Y., Zhou, L. Q., Wang, J. C., Yang, B., He, Q. J., et al. (2021a). Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 42, 1951–1969. doi:10.1038/s41401-021-00620-9
Fang, J., Xie, S., Chen, Z., Wang, F., Chen, K., Zuo, Z., et al. (2021b). Protective effect of vitamin E on cadmium-induced renal oxidative damage and apoptosis in rats. Biol. Trace Elem. Res. 199, 4675–4687. doi:10.1007/s12011-021-02606-4
Gameiro, J., Duarte, I., Marques, F., Fonseca, J. A., Jorge, S., Rosa, R., et al. (2020). Transient and persistent AKI and outcomes in patients undergoing major abdominal surgery. Nephron 144, 236–244. doi:10.1159/000506397
Gaudry, S., Hajage, D., Benichou, N., Chaïbi, K., Barbar, S., Zarbock, A., et al. (2020). Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet London, Engl. 395, 1506–1515. doi:10.1016/s0140-6736(20)30531-6
Georgiev, T., Nikolova, G., Dyakova, V., Karamalakova, Y., Georgieva, E., Ananiev, J., et al. (2023). Vitamin E and silymarin reduce oxidative tissue damage during gentamycin-induced nephrotoxicity. Pharm. (Basel). 16, 1365. doi:10.3390/ph16101365
Goudarzi, M. M., Falahati, V., Yousefichaijan, P., and Tajerian, A. (2023). Efficacy of vitamin E on renal function and preventing proximal tubulopathy caused by iron chelation therapy in thalassemia major patients: a randomized controlled clinical trial. Nephro-urology Mon. 15. doi:10.5812/numonthly-139443
Guo, Q., and Wang, J. (2018). Effect of combination of vitamin E and umbilical cord-derived mesenchymal stem cells on inflammation in mice with acute kidney injury. Immunopharmacol. Immunotoxicol. 40, 168–172. doi:10.1080/08923973.2018.1424898
Hakiminia, B., Goudarzi, A., and Moghaddas, A. (2019). Has vitamin E any shreds of evidence in cisplatin-induced toxicity. J. Biochem. Mol. Toxicol. 33, e22349. doi:10.1002/jbt.22349
Hoste, E. A. J., Kellum, J. A., Selby, N. M., Zarbock, A., Palevsky, P. M., Bagshaw, S. M., et al. (2018). Global epidemiology and outcomes of acute kidney injury. injury 14, 607–625. doi:10.1038/s41581-018-0052-0
James, M., Bhatt, M., Pannu, N., and Tonelli, M. (2020). Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. 16, 193–205. doi:10.1038/s41581-019-0247-z
Ji, M., Wu, R., Feng, Z., Wang, Y. D., Wang, Y., Zhang, L., et al. (2022). Incidence, risk factors and prognosis of acute kidney injury in patients treated with immune checkpoint inhibitors: a retrospective study. Sci. Rep. 12, 18752. doi:10.1038/s41598-022-21912-y
Jin, Z., Sun, J., and Zhang, W. (2024). Effect of vitamin E on diabetic nephropathy: a meta-analysis. Altern. Ther. health Med. 30, 344–349.
Kataoka, T., Yamato, K., Nishiyama, Y., Morii, Y., Etani, R., Takata, Y., et al. (2012). Comparative study on the inhibitory effects of α-tocopherol and radon on carbon tetrachloride-induced renal damage. Ren. Fail. 34, 1181–1187. doi:10.3109/0886022x.2012.717496
Kellum, J. A., and Lameire, N.KDIGO AKI Guideline Work Group (2013). Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit. care London, Engl. 17, 204. doi:10.1186/cc11454
Kitzler, T. M., Jaberi, A., Sendlhofer, G., Rehak, P., Binder, C., Petnehazy, E., et al. (2012). Efficacy of vitamin E and N-acetylcysteine in the prevention of contrast induced kidney injury in patients with chronic kidney disease: a double blind, randomized controlled trial. Wien. Klin. Wochenschr. 124, 312–319. doi:10.1007/s00508-012-0169-2
Koay, Y. Y., Tan, G. C. J., Phang, S. C. W., Ho, J. I., Chuar, P. F., Ho, L. S., et al. (2021). A phase IIb randomized controlled trial investigating the effects of tocotrienol-rich vitamin E on diabetic kidney disease. Nutrients 13, 258. doi:10.3390/nu13010258
Koga, H., Hagiwara, S., Mei, H., Hiraoka, N., Kusaka, J., Goto, K., et al. (2012). The vitamin E derivative, ESeroS-GS, attenuates renal ischemia-reperfusion injury in rats. J. Surg. Res. 176, 220–225. doi:10.1016/j.jss.2011.07.039
Liu, P., Feng, Y., Wang, Y., Zhou, Y., and Zhao, L. (2015). Protective effect of vitamin E against acute kidney injury. Bio-medical Mater. Eng. 26 (Suppl. 1), S2133–S2144. doi:10.3233/bme-151519
Mehta, R. L., Kellum, J. A., Shah, S. V., Molitoris, B. A., Ronco, C., Warnock, D. G., et al. (2007). Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. care London, Engl. 11, R31. doi:10.1186/cc5713
Miller, E. R., Pastor-Barriuso, R., Dalal, D., Riemersma, R. A., Appel, L. J., and Guallar, E. (2005). Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 142, 37–46. doi:10.7326/0003-4819-142-1-200501040-00110
Miyazawa, T., Burdeos, G., Itaya, M., Nakagawa, K., and Miyazawa, T. (2019). Vitamin E: regulatory redox interactions. IUBMB life 71, 430–441. doi:10.1002/iub.2008
Monami, M., Cignarelli, A., Pinto, S., D'Onofrio, L., Milluzzo, A., Miccoli, R., et al. (2021). Alpha-tocopherol and contrast-induced nephropathy: a meta-analysis of randomized controlled trials. Int. J. Vitam. Nutr. Res. 91, 188–196. doi:10.1024/0300-9831/a000573
Nouri-Majalan, N., Ardakani, E. F., Forouzannia, K., and Moshtaghian, H. (2009). Effects of allopurinol and vitamin E on renal function in patients with cardiac coronary artery bypass grafts. Vasc. Health Risk Manag. 5, 489–494. doi:10.2147/vhrm.s5761
Ostermann, M., Bellomo, R., Burdmann, E. A., Doi, K., Endre, Z. H., Goldstein, S. L., et al. (2020). Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. 98, 294–309. doi:10.1016/j.kint.2020.04.020
Perazella, M. A., and Rosner, M. H. (2022). Drug-induced acute kidney injury. Clin. J. Am. Soc. Nephrol. CJASN 17, 1220–1233. doi:10.2215/cjn.11290821
Péter, S., Friedel, A., Roos, F. F., Wyss, A., Eggersdorfer, M., Hoffmann, K., et al. (2015). A systematic review of global alpha-tocopherol status as assessed by nutritional intake levels and blood serum concentrations. Int. J. Vitam. Nutr. Res. 85, 261–281. doi:10.1024/0300-9831/a000281
Pickkers, P., Darmon, M., Hoste, E., Joannidis, M., Legrand, M., Ostermann, M., et al. (2021). Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive care Med. 47, 835–850. doi:10.1007/s00134-021-06454-7
Ronco, C., Bellomo, R., and Kellum, J. A. (2019). Acute kidney injury. Lancet 394, 1949–1964. doi:10.1016/s0140-6736(19)32563-2
Sakamoto, Y., Yano, T., Hanada, Y., Takeshita, A., Inagaki, F., Masuda, S., et al. (2017). Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur. J. Pharmacol. 800, 48–56. doi:10.1016/j.ejphar.2017.02.025
Salehipour, M., Monabbati, A., Salahi, H., Nikeghbalian, S., Bahador, A., Marvasti, V. E., and Abbasalipourkab, R. (2010). Protective effect of parenteral vitamin E on ischemia-reperfusion injury of rabbit kidney. Urology 75, 858–861. doi:10.1016/j.urology.2009.04.062
Salehzadeh, A., Salehzadeh, A., Maghsood, A.-H., Heidarisasan, S., Taheri-Azandaryan, M., Ghafourikhosroshahi, A., et al. (2020). Effects of vitamin A and vitamin E on attenuation of amphotericin B-induced side effects on kidney and liver of male Wistar rats. Environ. Sci. Pollut. Res. Int. 27, 32594–32602. doi:10.1007/s11356-020-09547-w
Samsami, M., Shabani, M., Hajiesmaeili, M., Tavakoli-Ardakani, M., Ardehali, S. H., Fatemi, A., et al. (2021). The effects of vitamin E on colistin-induced nephrotoxicity in treatment of drug-resistant gram-negative bacterial infections: a randomized clinical trial. J. Infect. Chemother. 27, 1181–1185. doi:10.1016/j.jiac.2021.03.013
Schaubroeck, H., Vargas, D., Vandenberghe, W., and Hoste, E. (2021). Impact of AKI care bundles on kidney and patient outcomes in hospitalized patients: a systematic review and meta-analysis. BMC Nephrol. 22, 335. doi:10.1186/s12882-021-02534-4
Schefold, J. C., Filippatos, G., Hasenfuss, G., Anker, S. D., and von Haehling, S. (2016). Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat. Rev. Nephrol. 12, 610–623. doi:10.1038/nrneph.2016.113
Servais, H., Ortiz, A., Devuyst, O., Denamur, S., Tulkens, P. M., and Mingeot-Leclercq, M. P. (2008). Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis Int. J. Program. Cell death 13, 11–32. doi:10.1007/s10495-007-0151-z
Tan, S., Chiew, Y., Ahmad, B., and Kadir, K. (2018). Tocotrienol-rich vitamin E from palm oil (tocovid) and its effects in diabetes and diabetic nephropathy: a pilot phase II clinical trial. Nutrients 10, 1315. doi:10.3390/nu10091315
Tasanarong, A., Piyayotai, D., and Thitiarchakul, S. (2009). Protection of radiocontrast induced nephropathy by vitamin E (alpha tocopherol): a randomized controlled pilot study. Chotmaihet thangphaet J. Med. Assoc. Thail. 92, 1273–1281.
Tasanarong, A., Vohakiat, A., Hutayanon, P., and Piyayotai, D. (2013). New strategy of α- and γ-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrol. Dial. Transplant. 28, 337–344. doi:10.1093/ndt/gfs525
Traber, M. G., Leonard, S. W., Ebenuwa, I., Violet, P. C., Niyyati, M., Padayatty, S., et al. (2021). Vitamin E catabolism in women, as modulated by food and by fat, studied using 2 deuterium-labeled α-tocopherols in a 3-phase, nonrandomized crossover study. Am. J. Clin. Nutr. 113, 92–103. doi:10.1093/ajcn/nqaa298
Truksa, J., Dong, L. F., Rohlena, J., Stursa, J., Vondrusova, M., Goodwin, J., et al. (2015). Mitochondrially targeted vitamin E succinate modulates expression of mitochondrial DNA transcripts and mitochondrial biogenesis. Antioxidants and redox Signal. 22, 883–900. doi:10.1089/ars.2013.5594
Wang, H., Liu, D., Zheng, B., Yang, Y., Qiao, Y., Li, S., et al. (2023). Emerging role of ferroptosis in diabetic kidney disease: molecular mechanisms and therapeutic opportunities. Int. J. Biol. Sci. 19, 2678–2694. doi:10.7150/ijbs.81892
Ward, N. C., Wu, J. H. Y., Clarke, M. W., Puddey, I. B., Burke, V., Croft, K. D., et al. (2007). The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J. Hypertens. 25, 227–234. doi:10.1097/01.hjh.0000254373.96111.43
Welson, N. N., Rofaeil, R. R., Ahmed, S. M., Gaber, S. S., Batiha, G. E. S., and Shahataa, M. G. (2021). Vitamin E protects against gabapentin-induced chronic hepatic and renal damage associated with the inhibition of apoptosis and tissue injury in rats. Life Sci. 267, 118940. doi:10.1016/j.lfs.2020.118940
Wu, J., and Croft, K. (2007). Vitamin E metabolism. Mol. aspects Med. 28, 437–452. doi:10.1016/j.mam.2006.12.007
Xu, Y., Zheng, X., Liang, B., Gao, J., and Gu, Z. (2018). Vitamins for prevention of contrast-induced acute kidney injury: a systematic review and trial sequential analysis. Am. J. Cardiovasc. Drugs 18, 373–386. doi:10.1007/s40256-018-0274-3
Yue, J., Bao, X., and Meng, L. F. (2023). Protective role of melatonin for acute kidney injury: a systematic review and meta-analysis. Shock (Augusta, Ga.) 61, 167–174. doi:10.1097/shk.0000000000002278
Zaaboul, F., and Liu, Y. (2022). Vitamin E in foodstuff: nutritional, analytical, and food technology aspects. Compr. Rev. food Sci. food Saf. 21, 964–998. doi:10.1111/1541-4337.12924
Zarbock, A., Kellum, J. A., Schmidt, C., Van Aken, H., Wempe, C., Pavenstädt, H., et al. (2016). Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. Jama 315, 2190–2199. doi:10.1001/jama.2016.5828
Zhang, J., Ren, X., Nie, Z., You, Y., Zhu, Y., Chen, H., et al. (2024). Dual-responsive renal injury cells targeting nanoparticles for vitamin E delivery to treat ischemia reperfusion-induced acute kidney injury. J. nanobiotechnology 22, 626. doi:10.1186/s12951-024-02894-7
Keywords: vitamin E, nephrotoxin, acute kidney injury, anti-oxidation, meta
Citation: Meng L, Liu S and Cui W (2025) Renal protective effects of vitamin E for drug-induced kidney injury: a meta-analysis. Front. Pharmacol. 16:1461792. doi: 10.3389/fphar.2025.1461792
Received: 09 July 2024; Accepted: 28 February 2025;
Published: 14 April 2025.
Edited by:
Swayam Prakash Srivastava, University of Michigan, United StatesReviewed by:
Osama Ashry Gheith, Mansoura University, EgyptFang-Fang He, Huazhong University of Science and Technology, China
Copyright © 2025 Meng, Liu, and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenpeng Cui, d2VucGVuZ2N1aUAxNjMuY29t
 Lingfei Meng
Lingfei Meng Shengmao Liu
Shengmao Liu Wenpeng Cui
Wenpeng Cui