- 1Department of Medical Biotechnology, Institute of Biotechnology, University of Gondar, Gondar, Ethiopia
- 2College of Veterinary Medicine and Animal Sciences, University of Gondar, Gondar, Ethiopia
- 3Unit of Pediatric Hematology Oncology, Department of Pediatrics and Child Health, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 5Unit of Pediatric Hematology Oncology, Department of Pediatrics and Child Health, School of Medicine, College of Medicine and Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 6Department of pathology, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 7Department of Pharmacy, Asrat Woldeyes Health Science Campus, Debre Berhan University, Debre Berhan, Ethiopia
- 8Unit of Molecular Biology and Bioinformatics, Institute of Biotechnology, Addis Ababa University, Addis Ababa, Ethiopia
- 9Medical Laboratory Department, Dilla University College of Medicine and Health Sciences, Dilla, Ethiopia
Background: Knowledge, attitudes, and practices (KAP) regarding the use of thiopurine chemotherapeutic drugs in the treatment of acute lymphoblastic leukemia (ALL) are critical for healthcare professionals. Thiopurines are associated with varying levels of toxicity, including myelosuppression, hepatotoxicity, and gastrointestinal intolerance. Approximately 20% of ALL patients discontinue thiopurine therapy due to toxicity and other adverse events. This study aims to assess the KAP of healthcare providers (HCPs) concerning thiopurine drugs used in the treatment of ALL in hospital wards in Northwestern Ethiopia.
Methods: A hospital-based cross-sectional study was conducted among 161 HCPs at the University of Gondar Comprehensive Specialized Hospital from June 1, 2023, to August 30, 2023. Data were collected using a self-administered questionnaire. The collected data were coded, entered and analyzed using SPSS version 25. Associations between categorical variables were assessed using cross tabulation, and both crude and adjusted odds ratios were calculated with a 95% confidence interval. Variables with p-values less than 0.05 were considered statistically significant.
Results: A total of 161 HCPs participated in the study, comprising 69 (42.9%) females and 92 (57.1%) males. The majority of participants were nurses 105 (65%), followed by physicians 20 (12.4%), pharmacists 19 (11.8%), midwives 10 (6.2%), and health officers 7 (4.3%). More than half of the participants demonstrated inadequate knowledge 94 (58.4%), negative attitudes 82 (50.9%), and poor practices 90 (55.9%) regarding the use of thiopurine drugs for the treatment of ALL. Approximately 50 (31.1%) participants understood the term “thiopurine drugs,” while 89 (55.3%) were aware of their adverse drug reactions. However, the majority 136 (84.5%) had not received training on how to report adverse drug reactions.
Conclusion: The majority of healthcare professionals demonstrated negative attitudes, inadequate knowledge, and poor practices concerning the reporting of adverse drug reactions associated with thiopurine drugs.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer worldwide. Treatment outcomes have significantly improved over the past several decades due to the combined use of multiple chemotherapeutic agents and advanced therapeutic protocols (Duffield et al., 2023). Thiopurines, such as thioguanine, 6-mercaptopurine, and azathioprine, are widely used in clinical practice for the treatment of children with ALL and inflammatory bowel diseases. However, these drugs are associated with severe adverse drug reactions (ADRs), including allergic reactions (25%), liver test abnormalities (34%), nausea and vomiting (6%), bone marrow suppression (7%), hepatotoxicity, gastrointestinal intolerance, myelosuppression, and secondary tumor formation. These serious ADRs pose significant challenges in reducing ALL-related mortality and limit the broader application of thiopurines (Gearry et al., 2004; Chan and Ismail, 2014; Bárcenas-López et al., 2021; Zhu et al., 2018).
Metabolizing enzymes, such as thiopurine S-methyltransferase (TPMT), and drug transporters, including multidrug resistance-associated proteins, have been reported to play a critical role in the metabolism and transportation of thiopurine drugs (Guo et al., 2022). Genetic variations in drug-metabolizing enzymes and drug transport systems can lead to significant interindividual differences in drug exposure, resulting in toxicity in a substantial proportion of patients (Sileshi et al., 2022; Shyamveer and Singh, 2023). Consequently, patients undergoing thiopurine therapy require routine monitoring of blood cell counts. Thiopurines are typically administered daily as an oral dose for up to two and a half years during the maintenance phase of therapy (Toksvang et al., 2022). Pharmacogenetic factors contributing to thiopurine-induced myelotoxicity are partially attributed to genetic variants in the TPMT gene (Franca et al., 2019).
Today, with the use of intensive multi-agent chemotherapy, the majority of children with ALL can be cured, with overall remission rates typically exceeding 90% (Ramadhan et al., 2024). However, childhood cancer survival rates are significantly lower in low-income countries (ranging from 5% to 60%) compared to high-income countries (approximately 90%) (Farrag et al., 2023). This disparity can be attributed to several factors, including death from toxicity, relapse, and non-adherence to treatment. Chemotherapy aims to achieve three primary goals: cure, control, or palliation (Neugut and Prigerson, 2017; El Kamar et al., 2003). Nevertheless, chemotherapy-related side effects can adversely affect a patient’s quality of life and hinder families’ acceptance of and adherence to prescribed medications. Non-adherence to cancer treatment is associated with an increased risk of relapse and reduced survival rates. Healthcare providers (HCPs) perceptions of drug side effects and toxicity can influence their attitudes and management strategies, which in turn may impact patient adherence and treatment outcomes (Handayani et al., 2022).
Globally, thousands of drugs enter the market daily; however, medication safety remains a significant concern for diverse populations due to insufficient knowledge. According to the World Health Organization (WHO), an ADR is defined as any harmful, unintended, or undesired response to a drug that occurs at therapeutic doses used for prevention, diagnosis, or treatment, or for the modification of physiological functions. This definition excludes reactions resulting from accidental or intentional overdoses or medication errors (Seid et al., 2018). Adverse drug reactions can be categorized as predictable and dose-related, unpredictable and non-dose-related, dose- and time-related (delayed reactions), withdrawal reactions, or unexpected reactions due to treatment failure (Kassa Alemu and Biru, 2019). Pharmacovigilance encompasses the science and activities related to detecting, assessing, understanding, and preventing adverse effects or any other potential drug-related problems (Kassa Alemu and Biru, 2019; Chatterjee and Aparasu, 2023; Cervantes-Arellano et al., 2024).
Nearly half of all medicines worldwide are used irrationally, leading to severe consequences such as ADRs, drug toxicity, drug resistance, prolonged illness, and even death (Melku et al., 2021). The underreporting of ADRs associated with thiopurine and other chemotherapy drugs remains a persistent challenge in Ethiopia. This study assessed HCPs knowledge and attitudes toward ALL patients and their chemotherapy treatments. The findings reveal significant gaps in understanding among healthcare professionals, which directly impacts patient care. These insights are valuable for improving the quality of treatment for childhood leukemia. Furthermore, this information can guide the development of more effective patient education strategies and serve as a foundation for future research by HCPs. By identifying specific gaps in knowledge, attitudes, and practices, this study contributes to the broader goal of optimizing chemotherapy management in resource-limited settings.
Methods
Study area, design, and period
A hospital-based cross-sectional study was conducted from June 1, 2023, to August 30, 2023, among healthcare providers (HCPs) working at the UoGCSH. The hospital is located in the Amhara National Regional State (ANRS), Northwestern Ethiopia, approximately 750 km from Addis Ababa. It serves a population of over 7 million people in its catchment areas and a wide range of health services, including pediatric hematology and oncology services. Notably, the pediatric hematology-oncology center at UoGCSH is the only facility of its kind in the ANRS (Taddese et al., 2020; Tesfay et al., 2022).
Study population
The study population included all physicians, pharmacists, nurses, health officers, and midwives who were working in various pediatric units at the UoGCSH during the study period. The units comprised Pediatric Oncology Ward (N = 10), Neonatology Ward (N = 38), Main Pediatric Wards (N = 10), Pediatric Surgical and Emergency Ward (N = 11), Pediatric Emergency Ward (N = 20), Pediatric ICU (N = 8), Maternity Ward (N = 30), Pediatric Ward Pharmacy (N = 20), and Pediatric outpatient department (OPD) (N = 7). Additionally, healthcare providers from the adolescent oncology ward (N = 10) were included in the study.
Inclusion and exclusion criteria
Healthcare providers were eligible for inclusion in the study if they were current employees of the UoGCSH and held a valid hospital identification card. Participants were also required to fall within the age intervals 18–60 years. Furthermore, they needed to have earned a diploma, degree, or master’s qualification from a recognized university accredited by the Ethiopian Ministry of Health and the Ministry of Education including private organization. Healthcare providers who declined to participate, who were on leave, or were absent from work during the data collection period due to illness or other reasons were excluded from the study.
Dependent and independent variables
The study identified the knowledge, attitudes, and practices (KAP) of HCPs regarding the use of thiopurine drugs for the treatment of ALL as the dependent variables. The independent variables included age, sex, profession, level of education, years of experience, and training on ADR reporting related to chemotherapy.
Operational definition and measurements
• Adverse Drug Reactions (ADRs): ADRs are defined as any noxious, unintended, or undesired effects of a drug that occur at doses typically used in humans for prophylaxis, diagnosis, or therapy (Zimamu et al., 2021).
• Side Effects: Side effects refer to unintended effects that occur at normal doses and are related to the pharmacological properties of the drugs.
• Pharmacovigilance: Pharmacovigilance encompasses the science and activities related to the detection, assessment, understanding, and prevention of ADRs or any other medicine-related problems, aiming to improve the safety of medicines (Zimamu et al., 2021).
• Knowledge: Knowledge refers to the information stored in memory and was assessed based on participants’ understanding of thiopurine drugs and their adverse effects (Zimamu et al., 2021).
• Adequate Knowledge: Participants who correctly answered ≥50% of the 22 knowledge-related questions. Each correct answer was scored 1, and incorrect answers were scored 0.
• Inadequate Knowledge: Participants who answered <50% of the knowledge-related questions about thiopurine ADRs.
• Attitude: Attitude refers to the complex interaction of beliefs, feelings, and values that influence responses to thiopurine ADRs and their adverse effects (Zimamu et al., 2021).
• Good Attitude: Participants who answered ≥50% of the attitude-related questions about thiopurine ADRs correctly.
• Poor Attitude: Participants who answered <50% of the attitude-related questions about thiopurine ADRs in the context of ALL treatment reporting.
• Practice: Practice refers to the actual application or use of an idea, belief, or method, as opposed to theories related to it (Zimamu et al., 2021).
• Good Practice: Participants who answered ≥50% of the practice-related questions about thiopurine ADRs for ALL treatment reporting correctly.
• Poor Practice: Participants who answered <50% of the practice-related questions about thiopurine ADRs for ALL treatment reporting
Sampling technique and sample size determination
The study included all HCPs working in various pediatric units, comprising 178 physicians, nurses, pharmacies, health officers, and midwives who were directly or indirectly involved in the management of thiopurine drug treatments were included in the study. Since the entire study population was included, the sampling technique used was the census methodology.
Data collection tool and data collection process
Data were collected using a structured questionnaire developed through an extensive review of the literature. The self-administered questionnaire was designed to gather relevant information on sociodemographic characteristics and the assessment of KAP. The questionnaire was adapted from tools used in similar studies and aligned with the guidelines provided by the Ethiopian Food and Drug Administration (EFDA) (Seid et al., 2018; Kassa Alemu and Biru, 2019). Prior to the commencement of the study, a pretest was conducted. The questionnaire, along with an attached written consent form, was distributed to the HCPs.
In this survey, the knowledge of HCPs regarding thiopurine ADRs for the treatment of ALL was assessed using 22 questions. Each correct response was assigned a score of one (1), while each incorrect response was scored as zero (0), resulting in a total score ranging from 0 to 22 points. The overall level of knowledge was categorized based on the mean score. Participants who scored equal to or above the mean were classified as having adequate knowledge, whereas those scoring below the mean were classified as having inadequate knowledge.
Participants’ attitudes were evaluated using 14 questions rated on a three-point Likert scale (agree, neutral, and disagree). An “agree” response was assigned a score of 3, a “neutral” response a score of 2, and a “disagree” response a score of 1. A score equal to or greater than 50% indicated a good attitude, while a score below 50% indicated a poor attitude toward thiopurine ADRs in the treatment of ALL.
Healthcare providers were further assessed based on whether they documented and reported ADRs. A correct response required not only providing the precise definition but also demonstrating a general understanding of pharmacovigilance concepts.
Data quality control measures
Following the preparation of the data collection format, a pretest of the tool was conducted with 10 HCPs working at the UoGCSH. This pretest aimed to identify any necessary amendments and ensure the tool’s suitability for actual data collection. The collected data were reviewed and checked daily for completeness and consistency before proceeding with data processing and analysis.
Data analysis
The data were entered into Epi Data version 4.3, cleaned, and subsequently exported to the Statistical Package for Social Sciences version 25 (SPSS-25) for further analysis. A binary logistic regression model was employed to assess the relationship between the outcome variable (knowledge, attitudes, and practices [KAP] status of HCPs and predictor variables (sociodemographic factors). The association between selected categorical variables was examined using cross-tabulation, and Pearson chi-square tests were conducted. Crude and adjusted odds ratios were calculated with 95% confidence intervals. Variables with a p-value of less than 0.05 were considered statistically significant.
Results
Sociodemographic characteristics
A total of 178 healthcare providers were approached for the study. Of these, 161 participants completed the self-administered questionnaire correctly and returned it within the stipulated time, yielding a response rate of 90.4%. The majority of the healthcare providers, 92 (57.1%), were male. In terms of age distribution, 109 (67.7%) participants were between 26 and 35 years old. The largest professional group among the respondents was nurses, comprising 105 (65.2%) of the total, followed by physicians (20, 12.4%), pharmacists (19, 11.8%), health officers (7, 4.3%), and midwives (10, 6.2%). Approximately 132 respondents (82.0%) reported having more than 3 years of professional experience (Table 1).
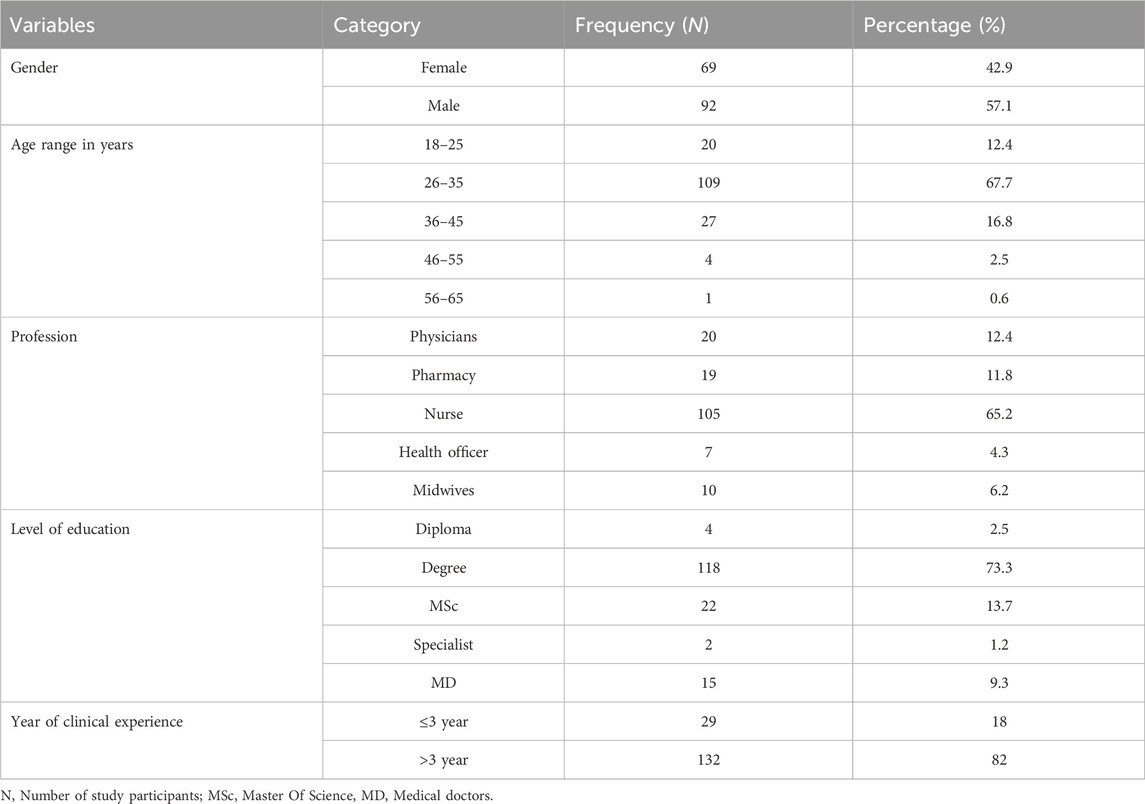
Table 1. Sociodemographic characteristics of healthcare providers at the University Gondar Comprehensive Specialized Hospital Northwestern Ethiopia, 2023 (n = 161).
Knowledge of healthcare providers in the treatment of acute lymphoblastic leukemia
Twenty-two questions were used to assess HCPs knowledge of the treatment of ALL patients. More than half of the respondents, 81 (50.3%), correctly identified ALL as the most prevalent childhood blood cancer, as opposed to acute myeloid leukemia (AML) (Table 2). The majority of HCPs, 116 (72.0%), correctly identified ALL as a cancer of the white blood cells (WBC). However, less than half of the respondents, 68 (42.2%), were aware that ALL is more common in children than in adults. Additionally, only a small proportion of HCPs, 25 (15.5%), knew that ALL has a cure rate of 98%. Conversely, a significantly higher number of respondents, 125 (79.5%), incorrectly believed that chemotherapy only makes children sicker and cannot cure cancer. Furthermore, 15 respondents (9.3%) incorrectly believed that blood cancer can be prevented by vaccination.

Table 2. Knowledge of HCPs on the treatment of acute lymphoblastic leukemia at the UoGCSH, Northwestern Ethiopia, 2023 (n = 161).
A considerable proportion of HCPs were unfamiliar with key terms related to thiopurine drugs and their mechanisms: 111 (68.9%) were unaware of “thiopurine drugs,” 139 (86.3%) were unfamiliar with the TPMT gene, 76 (47.2%) did not know about TPMT enzyme deficiency, and 69 (42.9%) were unaware of “testing strategies to assess TPMT activity.” Nevertheless, a majority of respondents, 89 (55.3%), correctly recognized thiopurine ADRs, although only 34 (21.1%) knew that these ADRs include myelosuppression, leukopenia, or hepatotoxicity. Additionally, 63 (39.1%) of the HCPs correctly identified mercaptopurine (6-MP) as the drug of choice for ALL. In contrast, 47 (29.2%) believed azathioprine and 24 (21.1%) thought thioguanine were the drugs of choice for ALL, indicating some misconceptions among respondents.
Among the 161 study participants, less than half, 63 (39.1%), knew that ADR reporting is a professional obligation. Most respondents, 136 (84.5%), had not received training on ADR reporting. Additionally, only 33 (20.5%) of HCPs were able to identify the responsible body for monitoring ADRs in Ethiopia. The majority of respondents, 124 (77.0%), were unfamiliar with pharmacovigilance and could not accurately define it. Physicians, compared to other healthcare professionals, demonstrated significantly higher knowledge about pharmacovigilance (65.0%, p < 0.05).
In the overall assessment of knowledge, most participants answered questions incorrectly regarding ADR types and treatments for ALL, with an error rate exceeding 94 (58.4%). Among the 161 respondents, physicians (60.0%, p < 0.05) and pharmacists (36.8%, p < 0.05) were significantly more aware of the national ADR reporting system in Ethiopia. Physicians (76.45%, p < 0.05) also demonstrated significantly greater awareness of the availability of ADR reporting forms compared to other professionals. However, 129 respondents (80.1%) were unaware of the existence of the national ADR reporting form, which hindered their ability to report ADRs. Similarly, 136 (84.5%) respondents were uncertain about how to report ADRs, further impeding their reporting practices. Additionally, 128 (79.5%) healthcare professionals refrained from reporting ADRs due to the absence of a responsible body monitoring ADRs in Ethiopia (Table 2).
Regarding methods for obtaining information on thiopurine drug reporting, respondents indicated awareness of at least one method, such as reporting via the internet (39.1%), through colleagues (41.6%), or via formal education (19.3%). However, 127 participants (78.9%) did not know any methods for ADR reporting. Some participants also selected multiple answers regarding methods used for ADR reporting (Table 2).
Association of years of experience with knowledge of ADR reporting
According to the findings of this study, healthcare professionals with more than 3 years of experience demonstrated significantly higher awareness of the national ADR reporting system (80%, p < 0.05). In relation to the availability of the ADR reporting form, healthcare professionals with less than 3 years of experience showed a lower awareness level at 12.5% (p = 0.335), whereas those with more than 3 years of experience exhibited a significantly higher awareness level at 87.5% (p < 0.05).
Knowledge and general awareness of ADR reporting among healthcare providers
The general awareness of respondents regarding ADR reporting, more than half of the respondents, 80 (49.7%), indicated that they obtained information about ADRs from the National Drug Formulary and Standard Treatment Guidelines (STGs), followed by standard textbooks, 18 (11.2%). Regarding ADR reporting practices, responses varied significantly: 43 (26.7%) mentioned reporting to manufacturers, 37 (23.9%) to the Ministry of Health (MOH), 21 (13.9%) to pharmacy departments, 13 (8.1%) to the Ethiopian Pharmaceutical Association (EPA), 15 (9.3%) to the Ethiopian Food and Drug Administration (EFDA), and 16 (9.9%) to the Drug and Therapeutic Committee (DTC) of their respective health facilities.
Factors reported to increase the likelihood of ADRs among patients included overdose, prescription errors, dispensing errors, patient lifestyle, and non-adherence. These factors were reported by 52 (32.3%), 27 (16.8%), 22 (13.7%), 22 (13.7%), and 8 (5.0%) of respondents, respectively. Notably, 26 (16.5%) identified both prescribing and dispensing errors as predisposing factors. However, only a few respondents, 4 (2.5%), correctly identified all these factors as contributing to ADRs (Table 3).
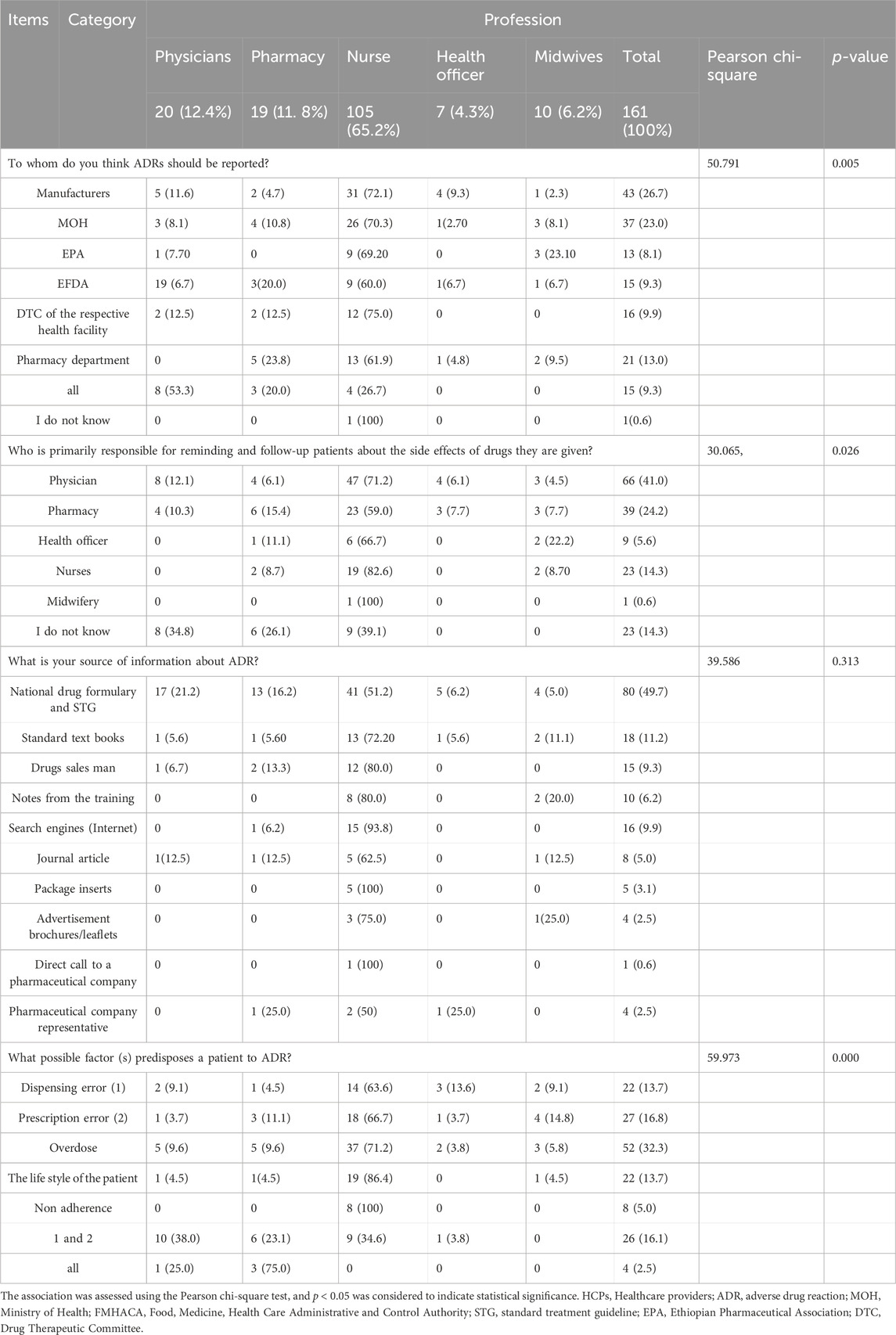
Table 3. General awareness of HCPs about ADR reporting at the UoGCSH, Northwestern Ethiopia, 2023 (n = 161).
Attitudes of healthcare providers in the treatment of acute lymphoblastic leukemia
Regarding the attitudes of HCPs toward ALL treatment and ADR reporting, 108 (67.1%) respondents agreed that ADR reporting could benefit public health, while 76 (47.2%) stated that ADR reporting should be compulsory. Conversely, 33 (20.5%) and 34 (21.1%) respondents believed that ADR reporting is time-consuming with no significant outcomes and that awareness of parental socioeconomic status (SES) is crucial for ALL treatment outcomes, respectively.
A total of 57 (35.4%) HCPs agreed that thiopurine chemotherapy drugs reduce red blood cell, white blood cell, and platelet counts. Additionally, 49 (30.4%), 47 (29.2%), 67 (41.6%), and 55 (34.2%) respondents expressed beliefs that blood cancer is hereditary, TPMT variants alone do not explain all thiopurine toxicity, severe ADRs related to TPMT genetic variants have been identified, and there are ethnic differences in the TPMT gene, respectively. Furthermore, a minority of respondents, 19 (11.8%), correctly disagreed that thiopurine drugs are safe for pregnant women (Table 4).
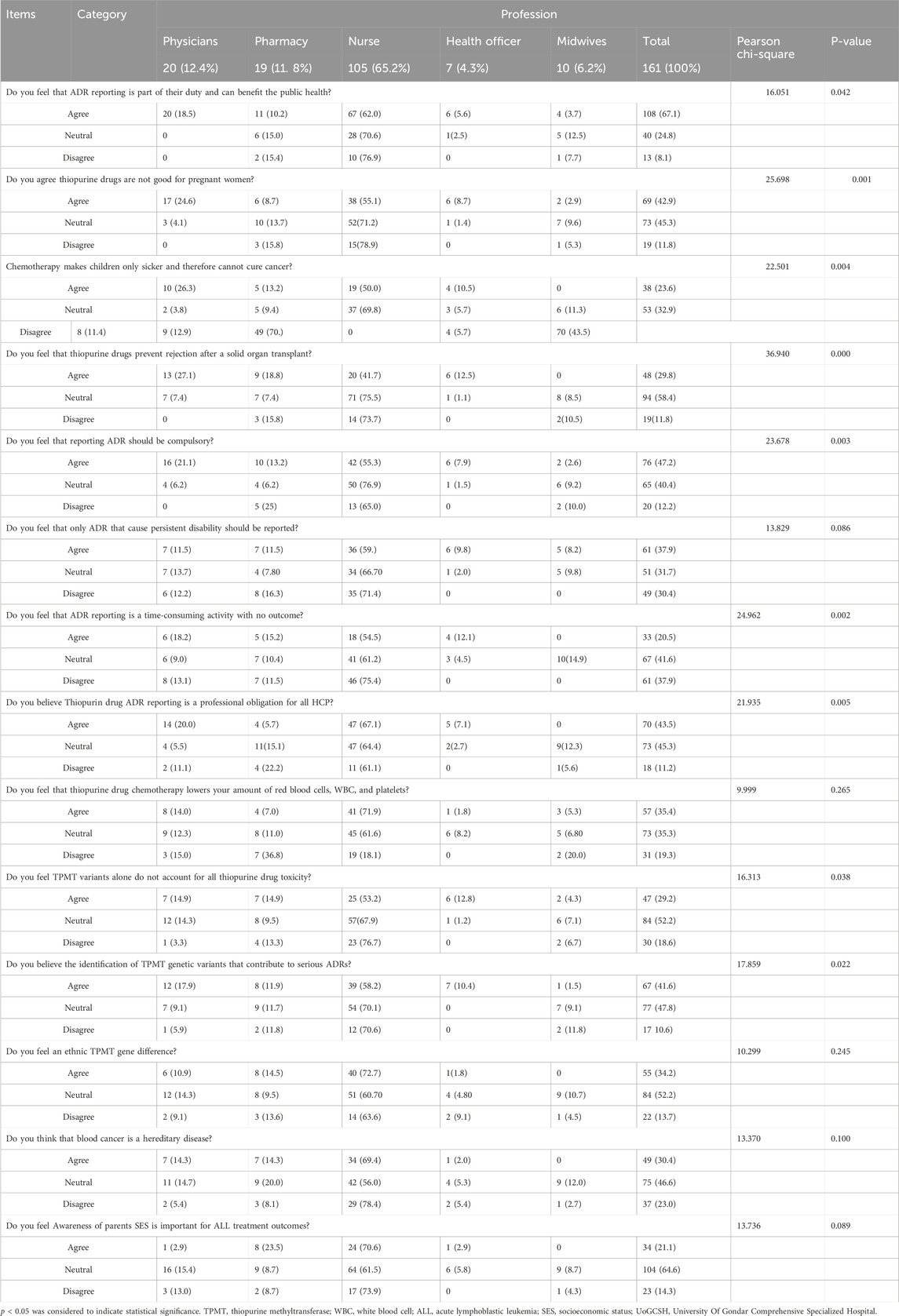
Table 4. Attitudes toward adverse drug reactions (ADRs) associated with thiopurine use for the treatment of ALL among healthcare providers at the UoGCSH, Northwestern Ethiopia, 2023 (n = 161).
Practice of healthcare providers in the treatment of ALL and reporting ADRs
The present study revealed that only 13 (8.1%) patients experienced at least one thiopurine ADR in the past 12 months of clinical practice. Approximately 32 (19.9%), 50 (31.1%), and 58 (36.0%) HCPs reported that they always, usually, and sometimes provided their patients with appropriate advice on potential drug adverse effects, respectively. In contrast, 21 (13.0%) admitted they never advised their patients adequately regarding ADRs. Most respondents, 135 (85.7%), reported a lack of available ADR reporting forms. However, the majority of respondents, 109 (67.7%), expressed readiness to report any ADRs they might encounter in their future practice.
Few respondents, 44 (27.3%), believed that thiopurine medications induce remission in 50% of ALL cases. Additionally, 108 (67.1%) of the respondents were unsure whether TPMT genotype or TPMT enzyme activity testing was available in Ethiopia (Table 5).
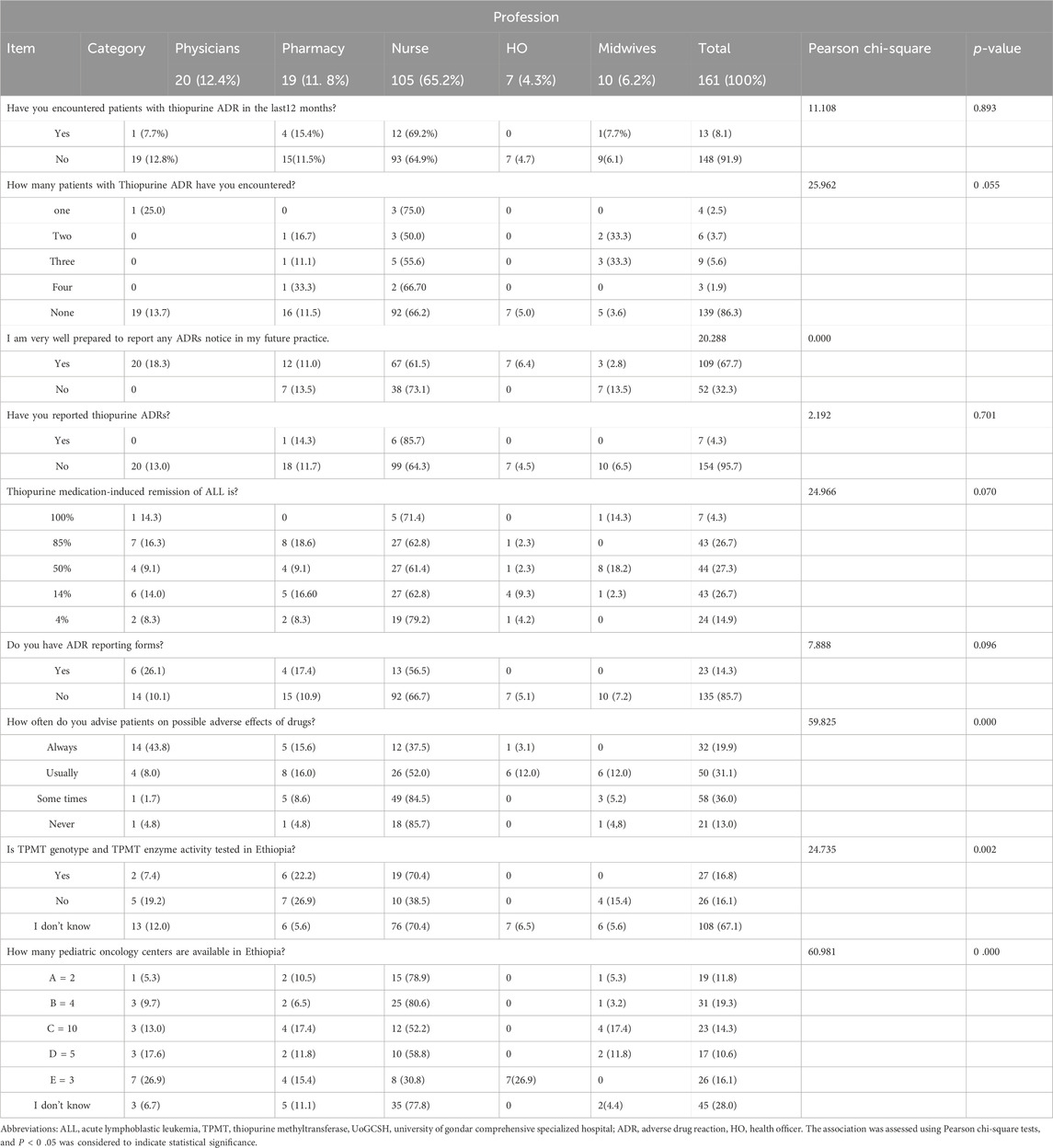
Table 5. Thiopurine ADR reporting practices for ALL treatment among HCPs at the UoGCSH, Northwestern Ethiopia, 2023 (n = 161).
The overall knowledge of HCPs
The data presented in the chart strongly support the statement that, in general, the overall knowledge is inadequate. A significant 94% of respondents’ demonstrated inadequate knowledge, 82% exhibited a poor attitude, and 90% engaged in unfavorable or poor practices (Figure 1).
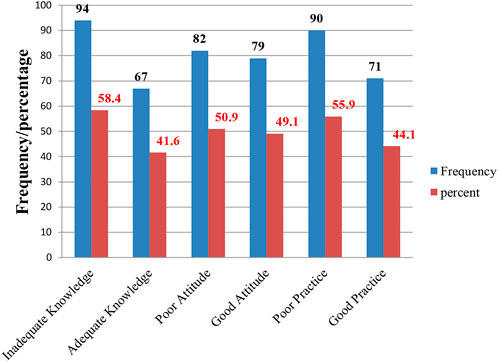
Figure 1. The overall knowledge, attitudes, and practices of HCPs toward ADR reporting regarding thiopurine drugs for the treatment of ALL at the UoGCSH, Northwest Ethiopia, in 2023 (n = 161).
As shown in Table 6, following bivariate logistic regression analysis and multivariate binary logistic regression screening, only seven categorical variables were included in the final model. According to the final multivariable logistic regression model, the curability status of ALL was correlated with education level, clinical experience, profession, and gender. Additionally, comprehensive professional knowledge, chemotherapy knowledge, and training were found to be significantly associated with ADR reporting knowledge.
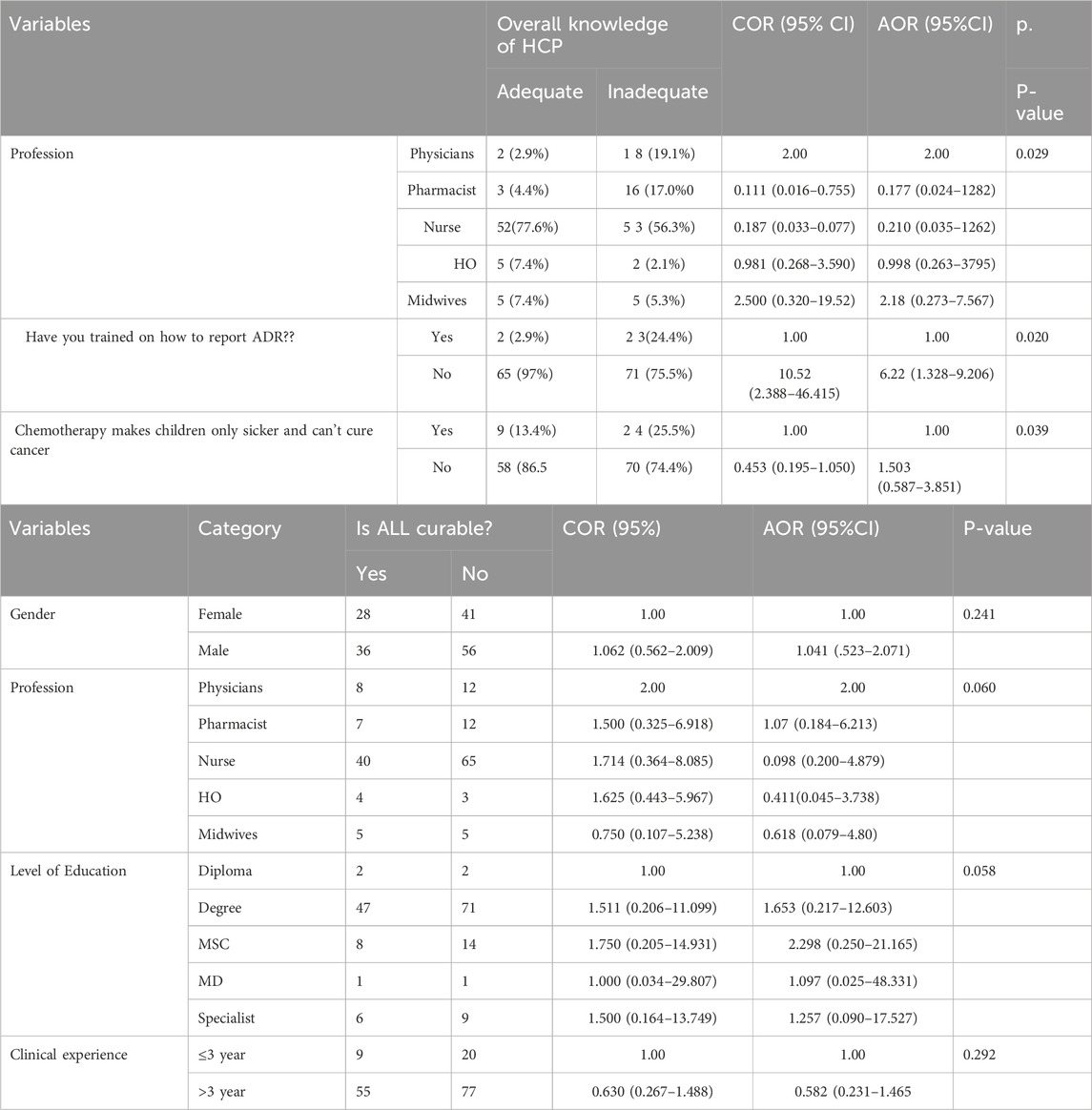
Table 6. Bivariate and multivariate logistic regression analysis of KAP at the University Of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2023 (n = 161).
As illustrated in Table 6, bivariate logistic regression analysis was conducted, where each independent variable was tested against the dependent variable, and variables with p-values less than or equal to 0.2 were included in the multiple logistic regression for further analysis. A p-value of less than 0.05 was considered statistically significant. According to the multiple logistic regression results, profession, training, and overall chemotherapy knowledge showed statistically significant associations with the overall knowledge of healthcare professionals (HCPs). Educational status also demonstrated a statistically significant association with the perceived curability of ALL.
Compared to physicians, pharmacists, nurses, and health officers, midwives were approximately 2.189 times more likely to possess overall knowledge of thiopurine drugs for the treatment of ALL-related ADRs [adjusted odds ratio (AOR): 2.189, 95% confidence interval (CI): 0.2731–7.567]. Healthcare professionals who had received prior ADR training were 6.227 times more likely to demonstrate adequate overall knowledge compared to those without any ADR training [AOR: 6.227, 95% CI: 1.328–29.206]. Furthermore, healthcare professionals with master’s degrees or specialisit were 2.298 and 1.257 times more likely, respectively, to exhibit good knowledge regarding the curability of ALL compared to those with diplomas or lower qualifications [AOR: 2.298, 95% CI: 0.250–21.165] and [AOR: 1.257, 95% CI: 0.090–17.527].
Reasons for not reporting adverse drug reactions
Among the 161 study participants, 129 (80.1%) did not report ADRs, primarily due to the unavailability of reporting forms. Similarly, 136 (84.5%) respondents were uncertain about how to report ADRs, which further hindered their reporting practices. Additionally, 128 (79.5%) healthcare professionals refrained from reporting ADRs because of the absence of a responsible body monitoring ADRs in Ethiopia. Several participants cited multiple reasons for their failure to report ADRs (Table 2).
Discussion
Thiopurines drugs are one of the cornerstones of current acute lymphoblastic leukemia (ALL) treatment. However, ADRs and drug toxicity can severely impact patient health, increasing morbidity, mortality, and hospitalization rates, thereby leading to unnecessary healthcare expenditures (Ray et al., 2022). To our knowledge, this is the first study to report and analyze the KAP of HCPs toward thiopurine drugs for the treatment of ALL in Ethiopia. Our study primarily targeted physicians, pharmacists, nurses, health officers, and midwives who closely interact with patients. Notably, nurses constitute the largest segment of this group, followed by physicians and pharmacists, which aligns with findings from a study conducted at Hiwot Fana Specialized University Hospital in Harar (Shanko and Abdela, 2018).
The findings of this study indicated that approximately 58.4%, 55.9%, and 50.9% of respondents demonstrated inadequate knowledge, unfavorable practices, and poor attitudes, respectively, toward thiopurine ADR treatments for ALL. However, thiopurine maintenance therapies are widely used globally (Toksvang et al., 2022), despite concerns such as myelotoxicity and hepatotoxicity limiting their utility (Guo et al., 2022). While genetic testing and TPMT enzymatic activity testing are available in developed countries for several TPMT variant alleles (Dean and Kane, 2020), these remain inaccessible in Ethiopia, with 65.1% of HCPs uncertain about their implementation.
Our study revealed that only a minority of respondents were familiar with terms such as “thiopurine drugs” 50 (31.1%), “TPMT gene” 22 (13.7%), “TPMT enzyme deficiency” 22 (13.7%), and “reporting.” Additionally, only 88 (54.7%) provided satisfactory responses regarding whom to prescribe thiopurine drugs, and 89 (55.3%) correctly identified thiopurine drug adverse reactions. Fifty point three percent (50.3%) of HCPs correctly identified ALL as the more prevalent condition, whereas 49.7% identified AML. Most studies indicate that ALL is the most prevalent hematological malignancy in children, accounting for approximately 86% of cases, with 91% of childhood leukemias being ALL and 9% being AML (Mostert et al., 2013; Kassahun et al., 2020).
Most studies report a high curability rate (98%) for ALL (Kassahun et al., 2020); however, our study found a widespread belief among HCPs that ALL is incurable. Awareness of curability rates varied: 15.1% knew the 98% curability rate, 28.9% believed it was 50%, 18.7% thought it was 25%, and 11.4% believed it was 4%, while 22.9% were unsure. This suggests inadequate training among HCPs in managing ALL with thiopurine drugs.
Thiopurine drugs, such as 6-mercaptopurine, exhibit variable toxicity due to polymorphisms in TPMT genes across ethnic groups (Rudin et al., 2017). However, our study contradicts this, as 50.6% of HCPs believed there were no ethnic differences in TPMT gene activity. Regarding thiopurine chemotherapeutic treatment, our study revealed that the knowledge levels of participants were very low (15.1%). These findings are consistent with a study conducted in India, where approximately 30% of participants were knowledgeable about chemotherapy (Yohannes, 2017).
Parental socioeconomic status (SES) significantly influences the decision to initiate childhood ALL treatment (86%), with better adherence observed among affluent parents (67%) (Mostert et al., 2013; Kassahun et al., 2020; Rudin et al., 2017; Yohannes, 2017; Mostert et al., 2008; Totadri et al., 2019). However, HCP awareness of SES’s impact on treatment outcomes was low, with only 20.5% demonstrating positive attitudes. Moreover, our study highlighted inadequate knowledge among HCPs regarding their professional obligation to report ADRs (39.1%), consistent with findings in Addis Ababa (21.1%) (Kefale et al., 2017).
Furthermore, a small proportion of respondents (23.0%) in our study were familiar with pharmacovigilance, similar to findings in the Jimma zone (19.5%) (Angamo et al., 2012; Adisa and Omitogun, 2019). Awareness of the national ADR reporting system (21.1%) and reporting forms (19.9%) was lower than reported in Lagos, Nigeria (40.4%) (Oshikoya and Awobusuyi, 2009). Additionally, only 20.5% and 39.1% of respondents knew that all HCPs are mandated to monitor ADRs in Ethiopia, likely due to limited training (15.5%) on ADRs and reporting.
Most HCPs (67.1%) agreed that ADR reporting is crucial for public health, aligning with findings in Addis Ababa (84%) (Kefale et al., 2017) but higher than in Jimma (57.31%) (Angamo et al., 2012). However, 20.5% perceived ADR reporting as time-consuming, potentially impacting their motivation (47.2%) to report ADRs in clinical practice.
Factors influencing ADR reporting knowledge included midwives, who were 2.189 times more likely to possess overall adequate knowledge compared to physicians, pharmacists, nurses, and health officers [adjusted odds ratio (AOR): 2.189, 95% confidence interval (CI): 0.2731–7.567]. Health professionals with previous ADR training were 6.227 times more likely to demonstrate overall adequate knowledge than those without [AOR: 6.227, 95% CI: 1.328–29.206]. Similarly, in one study, nurses (p = 0.002) and health officers (p = 0.018) had inadequate knowledge compared to physicians and pharmacists (Seid et al., 2018). In contrast, studies in the Philippines showed that nurses (86%), physicians (72%), and pharmacists (61%) had adequate ADR reporting knowledge (Carandang et al., 2015).
The table highlights a clear association between the healthcare profession and the level of knowledge regarding ADR reporting and related pharmacogenomic and pharmacovigilance concepts among healthcare providers at UoGCSH. Physicians and Pharmacy professionals generally possess significantly greater knowledge in these areas compared to Nurses, Health Officers, and Midwives. The statistically significant p-values for most knowledge-based questions indicate that these differences are unlikely to be due to chance. This suggests a need for targeted educational interventions and training programs tailored to the specific knowledge gaps identified within each professional group to improve ADR reporting practices and overall patient safety at the institution (Table 7).
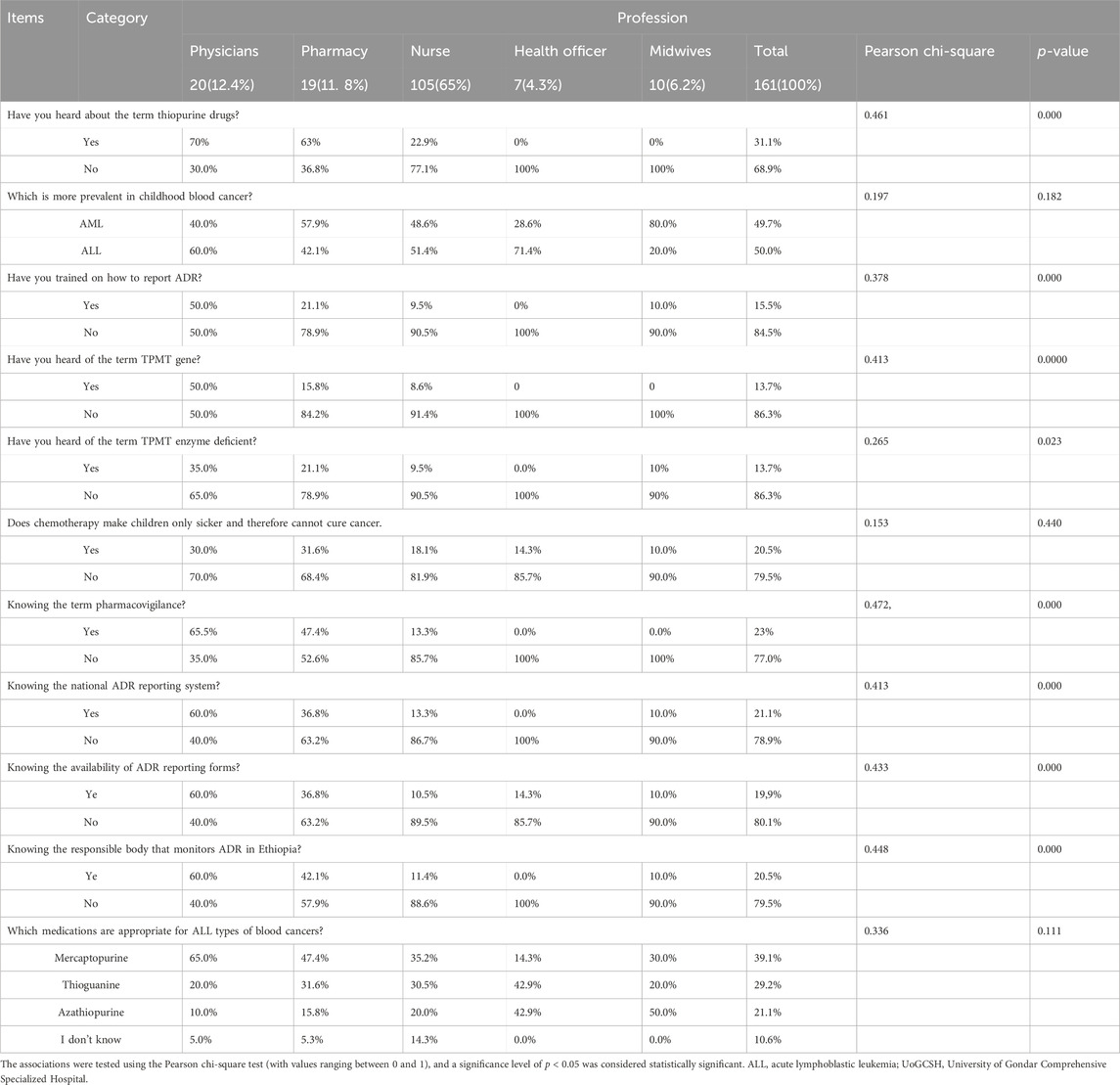
Table 7. Association within the profession and knowledge of ADR reporting among healthcare providers at UoGCSH, Northwest Ethiopia, 2023 (n = 161).
Limitation of the study
This study has some inherent limitations. As a cross-sectional study, it does not establish causal relationships between healthcare professionals’ knowledge, attitude, and practice regarding adverse drug reaction reporting for thiopurine drugs. The use of self-administered questionnaires may have introduced recall and social desirability bias, potentially affecting the accuracy of responses. The study was conducted at a single center, the UoGCSH, which may limit the generalizability of the findings to other healthcare settings in Ethiopia or beyond. Future multi-center studies with a longitudinal design are recommended to address these limitations.
Conclusion and recommendations
The majority of study participants demonstrated inadequate knowledge, unfavorable practices, and limited attitudes regarding thiopurine drugs, the ADR reporting system, and treatments for ALL. Additionally, most participants were unclear about the responsible body in Ethiopia for addressing ADR-related issues. Healthcare providers need to update their knowledge, improve communication skills, and provide accurate information to patients and caregivers.
To address these gaps, we recommend providing technical support and in-service training for HCPs, alongside implementing a robust reporting system. Such a system is fundamental to strengthening pharmacovigilance and enhancing both spontaneous and professional ADR reporting. Further studies are also needed to raise awareness among HCPs regarding childhood ALL curability, TPMT-related drug toxicity, ADRs, and the impact of parental socioeconomic status (SES) on treatment adherence. These efforts may ultimately improve adherence to childhood ALL treatment and enhance treatment outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Review Board of the University of Gondar. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. MB: Formal analysis, Project administration, Resources, and Writing – review & editing. MA: Conceptualization, Methodology, Data curation, Validation, Writing – original draft and Writing review and editing. AG: Formal Analysis, Writing – review & editing. AMG: Conceptualization, Data curation, and Writing – review & editing. DT: Investigation, validation, and Writing – review & editing. AM: Conceptualization, Data curation, and Writing – review & editing. ET: Investigation, and Writing – review & editing. AsA: Supervision, validation, investigation, and Writing – review & editing. AlA: Methodology, and Writing – review & editing. NB: Conceptualization, project administration, methodology, supervision, validation, Writing – original draft and Writing review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are thankful to the University of Gondar Comprehensive Specialized Hospital, for their kind cooperation in facilitating this study. The authors are also grateful to the data collectors and study participants for their participation and contribution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADR, adverse drug reaction; ALL, acute lymphoblastic leukemia; DIC, Drug Information Center; DTC, Drug and Therapeutic Committee; EFDA, Ethiopian Food and Drug Administration; HCPs, Healthcare professionals; KAP, Knowledge, Attitude, and Practice; MOH, Ministry of Health; SPSS, Statistical Package for Social Sciences; STG, Standard Treatment Guidelines; TPM, Thiopurine mythyltransferase; UoGCSH, University of Gondar Comprehensive Specialized Hospital; WHO, World Health Organization.
References
Adisa, R., and Omitogun, T. I. (2019). Awareness, knowledge, attitude and practice of adverse drug reaction reporting among health workers and patients in selected primary healthcare centres in Ibadan, southwestern Nigeria. BMC health Serv. Res. 19, 926–1014. doi:10.1186/s12913-019-4775-9
Angamo, M. T., Adugna Tesfa, A. T., and Nasir Tajure Wabe, N. T. W. (2012). Knowledge, attitude and practice of adverse drug reaction reporting among health professionals in southwest Ethiopia. TAF Prev. Med. Bull. 11, 397. doi:10.5455/pmb.1-1313732484
Bárcenas-López, D. A., Mendiola-Soto, D. K., Núñez-Enríquez, J. C., Mejía-Aranguré, J. M., Hidalgo- Miranda, A., and Jiménez-Morales, S. (2021). Promising genes and variants to reduce chemotherapy adverse effects in acute lymphoblastic leukemia. Transl. Oncol. 14 (1), 100978. doi:10.1016/j.tranon.2020.100978
Carandang, R. R., Cao, K., Jose, N. B., Almonte, F. D., and Tinio, R. M. (2015). Research article knowledge and attitudes on adverse drug reaction reporting of selected hospital-based health practitioners in Manila, Philippines. Scholars Acad. J. Pharm. 4, 301–307.
Cervantes-Arellano, M. J., Castelán-Martínez, O. D., Marín-Campos, Y., Chávez-Pacheco, J. L., Morales-Ríos, O., and Ubaldo-Reyes, L. M. (2024). Educational interventions in pharmacovigilance to improve the knowledge, attitude and the report of adverse drug reactions in healthcare professionals: systematic Review and Meta-analysis. DARU J. Pharm. Sci., 1–14. doi:10.1007/s40199-024-00508-z
Chan, H.-K., and Ismail, S. (2014). Side effects of chemotherapy among cancer patients in a Malaysian General Hospital: experiences, perceptions and informational needs from clinical pharmacists. Asian Pac. J. Cancer Prev. 15 (13), 5305–5309. doi:10.7314/apjcp.2014.15.13.5305
Chatterjee, S., and Aparasu, R. R. (2023). Pharmacovigilance to inform drug safety: challenges and opportunities, in Encyclopedia of Evidence in Pharmaceutical Public Health and Health Services Research in Pharmacy, 1–12.
Duffield, A. S., Mullighan, C. G., and Borowitz, M. J. (2023). International Consensus Classification of acute lymphoblastic leukemia/lymphoma. Virchows Arch. 482 (1), 11–26. doi:10.1007/s00428-022-03448-8
El Kamar, F. G., Grossbard, M. L., and Kozuch, P. S. (2003). Metastatic pancreatic cancer: emerging strategies in chemotherapy and palliative care. Oncol. 8 (1), 18–34. doi:10.1634/theoncologist.8-1-18
Farrag, A., Ghazaly, M. H., Mohammed, K., Volland, R., Hero, B., and Berthold, F. (2023). Comparing presentations and outcomes of children with cancer: a study between a lower-middle-income country and a high-income country. BMC Pediatr. 23 (1), 443. doi:10.1186/s12887-023-04214-8
Franca, R., Zudeh, G., Pagarin, S., Rabusin, M., Lucafò, M., Stocco, G., et al. (2019). Pharmacogenetics of thiopurines. Cancer Drug Resist. 2 (2), 256–270. doi:10.20517/cdr.2019.004
Gearry, R. B., Barclay, M. L., Burt, M. J., Collett, J. A., and Chapman, B. A. (2004). Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol. drug Saf. 13 (8), 563–567. doi:10.1002/pds.926
Guo, H.-L., Zhao, Y.-T., Wang, W.-J., Hu, Y.-H., Chen, F., Zhang, Y. Y., et al. (2022). Optimizing thiopurine therapy in children with acute lymphoblastic leukemia: a promising “MINT” sequencing strategy and therapeutic “DNA-TG” monitoring. Front. Pharmacol. 13, 941182. doi:10.3389/fphar.2022.941182
Handayani, K., Susilawati, D., Mulatsih, S., Kaspers, G. J., Mostert, S., Sitaresmi, M., et al. (2022). Health-care providers’ perception and communication about traditional and complementary medicine in childhood cancer in Indonesia. Pediatr. Hematol. Oncol. J. 7 (1), 4–9. doi:10.1016/j.phoj.2022.01.003
Jin, F., Tian, W., Xia, L., Yang, M., Chen, Y., Li, J., et al. (2024). Knowledge, attitude, and practice toward leukemia in the general population and among family members of patients with leukemia: a cross-sectional study. Heliyon 10, e26276. doi:10.1016/j.heliyon.2024.e26276
Kassa Alemu, B., and Biru, T. T. (2019). Health care professionals’ knowledge, attitude, and practice towards adverse drug reaction reporting and associated factors at selected public hospitals in Northeast Ethiopia: a cross-sectional study. BioMed Res. Int. 2019, 8690546. doi:10.1155/2019/8690546
Kassahun, W., Tesfaye, G., Bimerew, L. G., Fufa, D., Adissu, W., and Yemane, T. (2020). Prevalence of leukemia and associated factors among patients with abnormal hematological Parameters in Jimma Medical Center, Southwest Ethiopia: a cross-sectional study and associated factors among patients with abnormal hematological parameters in Jimma Medical Center, Southwest Ethiopia: a cross-sectional study. Adv. Hematol. 2020, 1–7. doi:10.1155/2020/2014152
Kefale, A. T., Tefera, B. D., and Biru, T. T. (2017). Knowledge, attitude and practice of healthcare professionals towards adverse drug reaction reporting at inpatient wards of tertiary hospital, Ethiopia. J. Drug Deliv. Ther. 7 (4), 97–102. doi:10.22270/jddt.v7i4.1483
Melku, L., Wubetu, M., and Dessie, B. (2021). Irrational drug use and its associated factors at Debre Markos Referral Hospital’s outpatient pharmacy in East Gojjam, northwest Ethiopia. Northwest Ethiop. SAGE open Med. 9, 20503121211025146. doi:10.1177/20503121211025146
Mostert, S., Gunawan, S., Van Dongen, J., Van De Ven, P., Sitaresmi, M., Wolters, E., et al. (2013). Health-care providers' perspectives on childhood cancer treatment in Manado, Indonesia. Psycho-Oncology 22 (11), 2522–2528. doi:10.1002/pon.3314
Mostert, S., Sitaresmi, M. N., Gundy, C. M., and Sutaryo, V. A. J. (2008). Attitude of health-care providers toward childhood leukemia patients with different socio-economic status. Pediatr. blood and cancer 50 (5), 1001–1005. doi:10.1002/pbc.21324
Neugut, A. I., and Prigerson, H. G. (2017). Curative, life-Extending, and palliative chemotherapy: new outcomes need new names. Oncol. 22 (8), 883–885. doi:10.1634/theoncologist.2017-0041
Oshikoya, K. A., and Awobusuyi, J. O. (2009). Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin. Pharmacol. 9, 14–18. doi:10.1186/1472-6904-9-14
Ramadhan, M. H., Sari, N. M., Peryoga, S. U., and Susanah, S. (2024). Survival and treatment outcomes of childhood acute lymphoblastic leukemia in a low-middle income country: a single-center experience in west Java, Indonesia. J. Blood Med. 15, 77–85. doi:10.2147/JBM.S438042
Ray, S. D., Luzum, J. A., Gray, J. P., and Stohs, S. J. (2022). Focus on pharmacogenomics, phytonutrient–drug interactions and COVID-19 vaccines: Perspectives on ADRs, ADEs, and SEDs. Elsevier. xxv-lii.
Rudin, S., Marable, M., and Huang, R. S. (2017). The promise of pharmacogenomics in reducing toxicity during acute lymphoblastic leukemia maintenance treatment. Genomics, Proteomics Bioinforma. 15 (2), 82–93. doi:10.1016/j.gpb.2016.11.003
Seid, M. A., Kasahun, A. E., Mante, B. M., and Gebremariam, S. N. (2018). Healthcare professionals’ knowledge, attitude and practice towards adverse drug reaction (ADR) reporting at the health center level in Ethiopia. Int. J. Clin. Pharm. 40, 895–902. doi:10.1007/s11096-018-0682-0
Shanko, H., and Abdela, J. (2018). Knowledge, attitudes, and practices of health care professionals toward adverse drug reaction reporting in Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia: a cross-sectional study. Hosp. Pharm. 53 (3), 177–187. doi:10.1177/0018578717737430
Shyamveer, K. A. A., and Singh, H. (2023). Effect of genetic variations in drug transporters, metabolizing enzyme and regulatory genes on the development of HIV-associated lipodystrophy. J. Gene Med. 25 (6), e3493. doi:10.1002/jgm.3493
Sileshi, T., Mekonen, G., Makonnen, E., and Aklillu, E. (2022). Effect of genetic variations in drug-metabolizing enzymes and drug transporters on the pharmacokinetics of rifamycins: a systematic review. Pharmacogenomics Personalized Med. 15, 561–571. doi:10.2147/PGPM.S363058
Taddese, A. A., Gashaye, K. T., Dagne, H., and Andualem, Z. (2020). Maternal and partner’s level of satisfaction on the delivery room service in University of Gondar Referral Hospital, northwest, Ethiopia: a comparative cross-sectional study. BMC health Serv. Res. 20, 233–238. doi:10.1186/s12913-020-05079-8
Tesfay, F. H., Backholer, K., Zorbas, C., Bowe, S. J., Alston, L., and Bennett, C. M. (2022). The magnitude of NCD risk factors in Ethiopia: meta-analysis and systematic review of evidence. Int. J. Environ. Res. Public Health 19 (9), 5316. doi:10.3390/ijerph19095316
Toksvang, L. N., Als-Nielsen, B., Bacon, C., Bertasiute, R., Duarte, X., Escherich, G., et al. (2022). Thiopurine Enhanced ALL Maintenance (TEAM): study protocol for a randomized study to evaluate the improvement in disease-free survival by adding very low dose 6-thioguanine to 6- mercaptopurine/methotrexate-based maintenance therapy in pediatric and adult patients (0–45 years) with newly diagnosed B-cell precursor or T-cell acute lymphoblastic leukemia treated according to the intermediate risk-high group of the ALLTogether1 protocol. BMC cancer 22 (1), 483. doi:10.1186/s12885-022-09522-3
Totadri, S., Trehan, A., Kaur, A., and Bansal, D. (2019). Effect of socio-economic status and proximity of patient residence to hospital on survival in childhood acute lymphoblastic leukaemia. Indian J. Med. Res. 149 (1), 26–33. doi:10.4103/ijmr.IJMR_579_17
Yohannes, E. (2017). The knowledge and attitude of patient on chemotherapy and it’s associated factors in oncology department in Tikur Anbessa Specilaized Hospital, Addis Ababa, Ethiopia. Doctoral dissertation (Addis Ababa University).
Zhu, Y., Yin, D., Su, Y., Xia, X., Moriyama, T., Nishii, R., et al. (2018). Combination of common and novel rare NUDT15 variants improves predictive sensitivity of thiopurine-induced leukopenia in children with acute lymphoblastic leukemia. haematologica 103 (7), e293–e295. doi:10.3324/haematol.2018.187658
Keywords: knowledge, attitude, practice, acute lymphoblastic leukemia, thiopurine drugs, adverse drug reactions, thiopurine methyltransferase gene
Citation: Kassa E, Birhan M, Ayalew M, Gelaw A, Gidey AM, Tilahun Worku D, Mekonen A, Teklehaimanot E, Asnakew A, Amare A and Berhane Tessemma N (2025) Knowledge, attitudes, and practices of healthcare providers toward ADR reporting regarding thiopurine drugs for the treatment of acute lymphoblastic leukemia at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Front. Pharmacol. 16:1486415. doi: 10.3389/fphar.2025.1486415
Received: 26 August 2024; Accepted: 27 March 2025;
Published: 19 May 2025.
Edited by:
Olivier Feron, Université catholique de Louvain, BelgiumReviewed by:
Wen Hu, Zhongnan Hospital, Wuhan University, ChinaSharaf Ezzat Sharaf, Umm Al-Qura University, Saudi Arabia
Copyright © 2025 Kassa, Birhan, Ayalew, Gelaw, Gidey, Tilahun Worku, Mekonen, Teklehaimanot, Asnakew, Amare and Berhane Tessemma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eyuel Kassa, ZXl1ZWwyMDAwQGdtYWlsLmNvbQ==
 Eyuel Kassa
Eyuel Kassa Mastewal Birhan
Mastewal Birhan Mulugeta Ayalew3
Mulugeta Ayalew3 Aschalew Gelaw
Aschalew Gelaw Degalem Tilahun Worku
Degalem Tilahun Worku Ermias Teklehaimanot
Ermias Teklehaimanot