- 1National Regional Chinese Medicine (Lung Disease) Diagnostic and Treatment Centre of the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 2Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases co-constructed by Henan Province and Education Ministry of P.R. China, Henan University of Chinese Medicine, Zhengzhou, China
- 3Henan Key Laboratory of Chinese Medicine for Respiratory Disease, Henan University of Chinese Medicine, Zhengzhou, China
Characterized by sudden onset, accelerated disease progression, and high mortality rates, acute exacerbation (AE) represents the most critical clinical challenge faced by patients with idiopathic pulmonary fibrosis (IPF). The absence of standardized animal models that recapitulate human disease phenotypes remains a significant impediment to the study of AE-IPF. In this work, we conducted a systematic review of experimental protocols for acute exacerbation of pulmonary fibrosis (AE-PF) over the past 20 years relating to aspects such as animal species, drugs, drug doses, drug administration routes, and model characteristics, and summarized research progress on the mechanism underlying this condition. Statistical analysis revealed that bleomycin combined with lipopolysaccharide represents the predominant experimental paradigm for AE-PF, accounting for 26.3% (5/19) of all AE-PF models. Our analysis further showed that the major mechanisms involved in AE-IPF are inflammation, immune imbalance, oxidative stress, endoplasmic reticulum (ER) stress, and apoptosis of alveolar epithelial cells.
1 Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic pulmonary disease of unknown etiology, characterized by progressive fibrosis that leads to continuous, irreversible decline in lung function and progressive respiratory failure (Lederer and Martinez, 2018). The global prevalence of IPF is estimated at 0.33–4.51 cases per 10,000 population (Maher et al., 2021), with a median post-diagnosis survival of only 2–4 years (Richeldi et al., 2017). Most patients die from chronic respiratory failure.
During the disease trajectory, patients with IPF may experience sudden, unexplained respiratory deterioration, clinically termed acute exacerbation of IPF (AE-IPF) (Kim et al., 2015). Current diagnostic criteria include acute worsening (<1 month duration), new bilateral ground-glass opacities on high-resolution computed tomography (HRCT), and exclusion of alternative explanations, including pulmonary embolism, cardiac failure, or volume overload (Collard et al., 2016).
While the definition of AE-IPF was initially restricted to unexplained respiratory deterioration, current criteria categorize it into triggered AE-IPF (e.g., infection, post-procedural/postoperative complications, drug toxicity) and AE-IPF of no known cause (Leuschner and Behr, 2017). The main clinical feature of AE-IPF is the rapid deterioration of respiratory symptoms in a short period, including aggravated dyspnea, cough, sputum, fever, and flu-like symptoms, along with hypoxemia, weight loss, and cyanosis (Luppi et al., 2015). On HRCT, AE-IPF primarily manifests as new peripheral, multifocal, or diffuse ground-glass opacities superimposed on the underlying IPF, with or without partial consolidation (Antoniou and Wells, 2013). Recent guidelines have simplified the imaging features of IPF from the previously defined usual interstitial pneumonia (UIP) pattern to a system comprising UIP, probable UIP, indeterminate UIP, and alternative diagnostic patterns (Raghu et al., 2018). The hallmark imaging feature of UIP is honeycombing, which may occur with or without associated peripheral traction bronchiectasis or bronchiolectasis, predominantly in subpleural and basal regions (Escalon and Lynch, 2018). In contrast, both probable and indeterminate UIPs lack definitive CT imaging features indicative of fibrosis, such as distinct honeycombing. Nevertheless, both patterns are characterized by subpleural ground-glass opacification or reticulation, and a considerable proportion of patients demonstrate a histopathological pattern consistent with UIP/IPF (Gruden et al., 2013). These imaging features, defined by a combination of newly emerging ground-glass opacities and inherent UIP changes, are consistent with acute exacerbation in the context of IPF. The annual incidence of AE-IPF ranges from 10% to 20% (Collard et al., 2016; Kakugawa et al., 2016), and up to 46% of IPF-related deaths are attributable to acute exacerbations each year. Within 6 months after the first acute exacerbation, mortality exceeds 90%, and the median survival time of patients with IPF who have experienced acute exacerbation is only 3–4 months (Collard et al., 2016). The high morbidity and mortality make acute exacerbation the most devastating complication of IPF and a leading cause of IPF-related death (Churg et al., 2011).
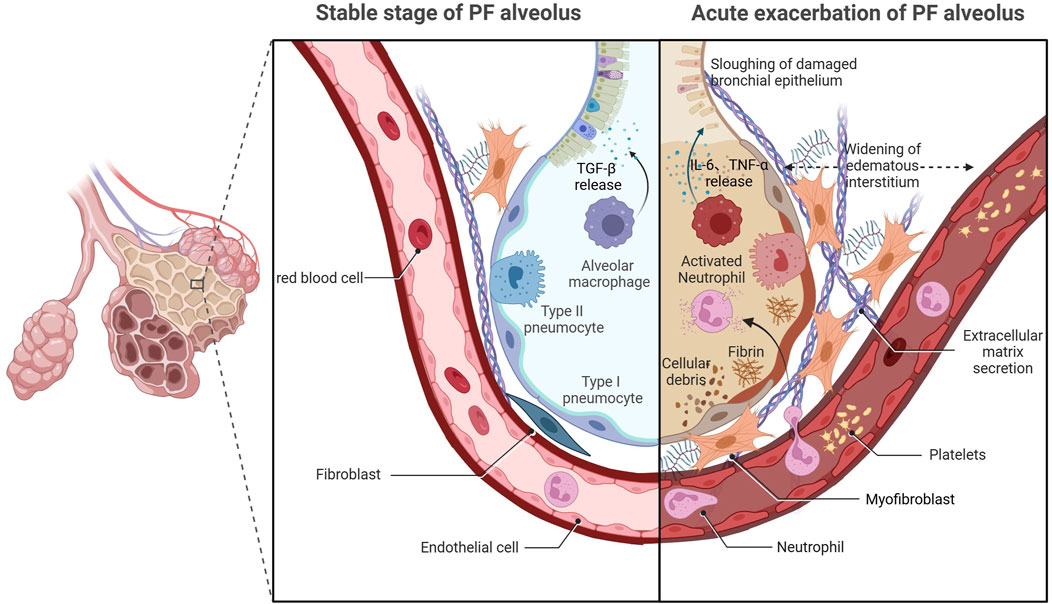
Despite retaining the idiopathic features of IPF, the precise etiopathogenesis of AE-IPF remains contentious compared with that for stable IPF (Ryerson and Collard, 2014). Recent studies have identified multiple triggers for AE-IPF, including drugs, blood transfusion, latent infection, gastroesophageal micro-reflux, invasive mechanical ventilation, inhalation of oxygen at high concentrations, thoracic surgery, and bronchoscopy (Ryerson et al., 2015; Kondoh et al., 2017). Critical knowledge gaps persist regarding the respective contributions of exogenous triggers and endogenous susceptibility factors in AE-IPF pathogenesis, highlighting the need for mechanistic studies to delineate their interplay (Johannson et al., 2014; Qiu et al., 2018). Histopathological analyses have identified two distinct pathological components in AE-IPF—chronic subpleural fibroblastic foci with airway remodeling, and acute diffuse alveolar damage (DAD) characterized by epithelial necrosis and hyaline membrane deposition (Dotan et al., 2020; Oda et al., 2014). The acute-phase histopathological features of AE-IPF show striking parallels to those observed in acute respiratory distress syndrome (ARDS), suggesting that the two diseases have a similar pathogenesis (Parambil et al., 2005). Therefore, the traditional view is that AE-IPF is primarily an inflammatory condition. However, clinical practice has shown that the application of corticosteroids does not reduce mortality among patients with AE-IPF (Hozumi et al., 2019; Farrand et al., 2020), indicating that inflammation alone does not fully explain its onset and progression. Significant gaps remain in the understanding of the mechanism underlying AE-IPF, and further studies are needed to unravel its pathogenesis (Figure 1).
The acute clinical trajectory and elevated mortality rate of AE-IPF pose significant challenges for longitudinal clinical investigations. Establishing animal models that recapitulate the clinicopathological features of human AE-IPF, including the timeline of disease progression and biomarker profiles, is essential for elucidating its underlying pathophysiological mechanisms and developing effective preventive and therapeutic strategies. The bleomycin (BLM)-induced pulmonary fibrosis (PF) model remains the most extensively characterized preclinical system for investigating this condition (Moeller et al., 2008). BLM exerts antineoplastic effects through iron-dependent oxidative cleavage of DNA strands, preferentially targeting rapidly dividing tumor cells (Czaja et al., 2022). The susceptibility of the lung to BLM toxicity stems from the low expression of BLM hydrolase in this tissue, which, coupled with high alveolar oxygen tension, potentiates the generation of reactive oxygen species (ROS) (Tashiro et al., 2017). In the presence of BLM, excessive ROS production directly results in the death of alveolar epithelial cells (AECs) between days 1 and 3 (Degryse and Lawson, 2011), followed by significant infiltration of neutrophils and lymphocytes into lung tissue between days 3 and 9 (Feng et al., 2024; Cheng et al., 2019). This ultimately leads to the activation of fibroblasts into myofibroblasts (Peyser et al., 2019), substantially enhancing extracellular matrix (ECM) deposition and promoting the progression of fibrosis between days 10 and 21 (Tashiro et al., 2017; Kolb et al., 2020). However, IPF is characterized by a dysfunction in epithelial repair mechanisms due to both aging and recurrent injury (Yao et al., 2021). The stemness of type II AECs diminishes, and the apoptosis of these cells triggers inflammatory responses, leading to the release of proinflammatory and profibrotic cytokines such as tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), and platelet-derived growth factor, among others (Knudsen et al., 2017; Hewlett et al., 2018). This cascade induces epithelial-mesenchymal transition (EMT) in AECs, along with fibroblast activation and the subsequent secretion of substantial amounts of ECM (Upagupta et al., 2018), thereby further advancing the progression of fibrosis. Notably, the persistent impairment of epithelial repair observed in IPF contributes to its irreversible and continuous advancement. However, animal BLM models demonstrate compressed fibrotic progression and thus cannot fully replicate the pathophysiological development seen in human IPF (Peng et al., 2013). Despite this limitation, the BLM-induced PF model remains the best-characterized animal model for preclinical testing currently available (Jenkins et al., 2017). Importantly, a mature and recognized method for modeling the acute exacerbation of pulmonary fibrosis is still lacking. In this study, we reviewed 19 studies involving AE-PF animal models prepared using different methods, with three new models added relative to the study of Ye et al. (2023). We compared the characteristics of the different approaches, expanding the discussion to include survival rate, fibrosis quantification, and cytokine profiles. Our analysis provides both novel insights into the mechanisms underlying the progression of AE-IPF and ideas for AE-IPF-related research.
2 Methods
2.1 Research selection
In this work, questions were formulated primarily based on the “P” and “I” components of the PICO method, employing rats or mice as the study populations and a range of chemical or biological stimuli as interventions. The objective was to evaluate reliable animal models and established induction methods, thereby providing a reference for AE-PF research.
2.2 Search strategy
A systematic review was carried out using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria. PubMed, Scopus, and Web of Science were searched using the following search terms: “acute exacerbation of idiopathic pulmonary fibrosis” AND “animal models,” “AE-IPF” AND “animal models,” “acute exacerbation of pulmonary fibrosis” AND “animal models,” “acute exacerbation of interstitial lung diseases” AND “animal models,” “acute exacerbation of idiopathic pulmonary fibrosis” AND “mouse,” “AE-IPF” AND “mouse,” “acute exacerbation of pulmonary fibrosis” AND “mouse,” “acute exacerbation of interstitial lung diseases” AND “mouse,” “acute exacerbation of idiopathic pulmonary fibrosis” AND “rat,” “AE-IPF” AND “rat,” “acute exacerbation of pulmonary fibrosis” AND “rat,” and “acute exacerbation of interstitial lung diseases” AND “rat.”
2.3 Inclusion criteria
Only rat and mouse AE-PF models induced by chemical and biological factors were included. Additionally, only studies published in English were considered.
2.4 Exclusion criteria
Only studies published in the last 2 decades (after 2004) were considered for evaluation. Additionally, investigations using animal models of stable PF induced via a single administration of BLM were omitted from the review, as were studies with sample sizes of less than six animals.
2.5 Data collection
Independent data collection methods were used to search literature published between January 2004 and July 2024. Four of the authors (Kai Chen, Zhaoxu Yao, Siyu Tao, and Qian Ma) first reviewed the title and abstract, and then read the full article. Disagreements were resolved by Hailong Zhang. The numbers of included and excluded articles were reported using the PRISMA flowchart.
2.6 Data items
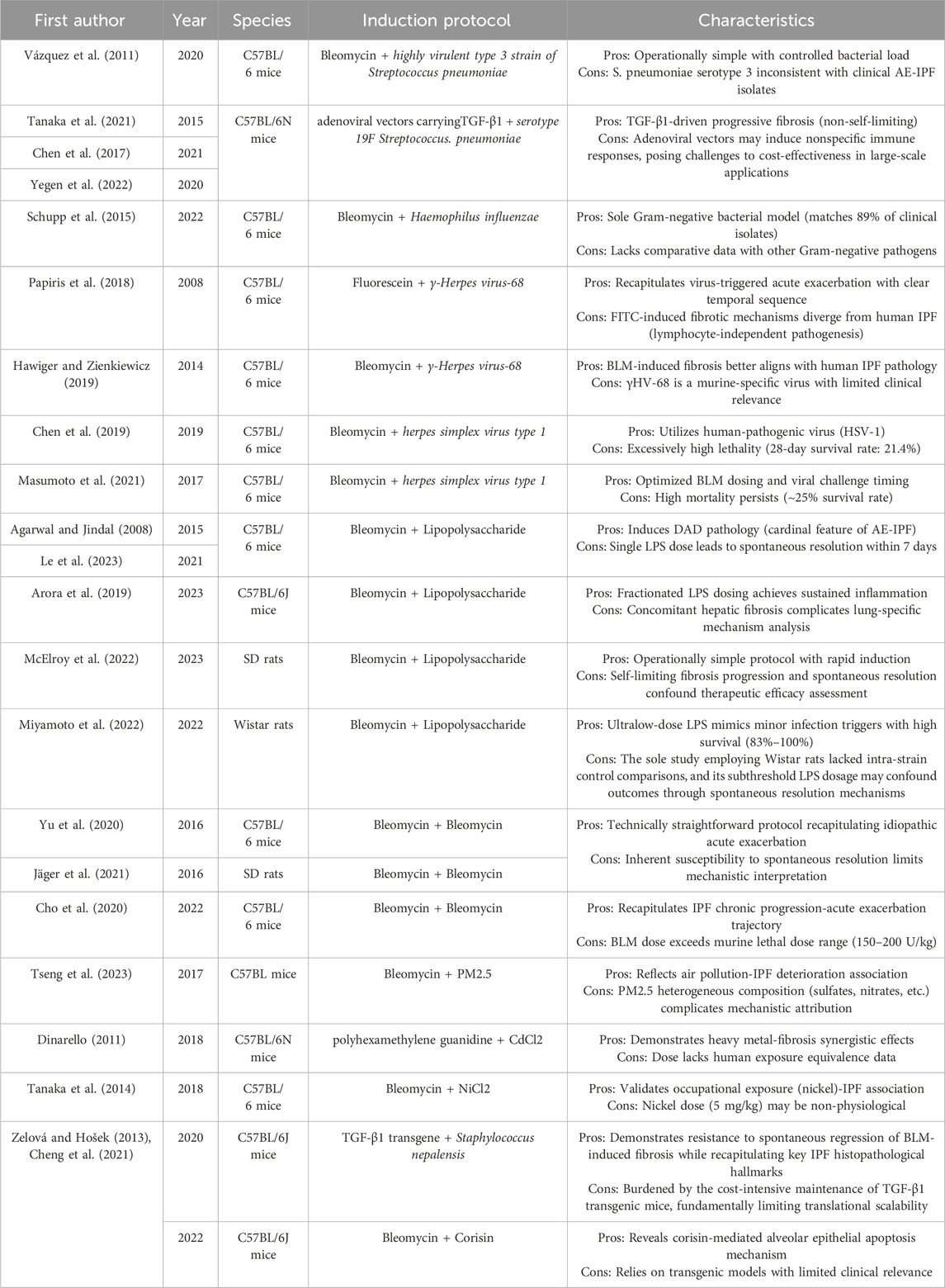
Data relating to the main author, publication year, animal species, modeling method, dose, survival, clinical features, histopathological features, quantitative indexes of fibrosis, and cytokine profiles were extracted (Tables 1, 2).
3 Results
3.1 Study selection
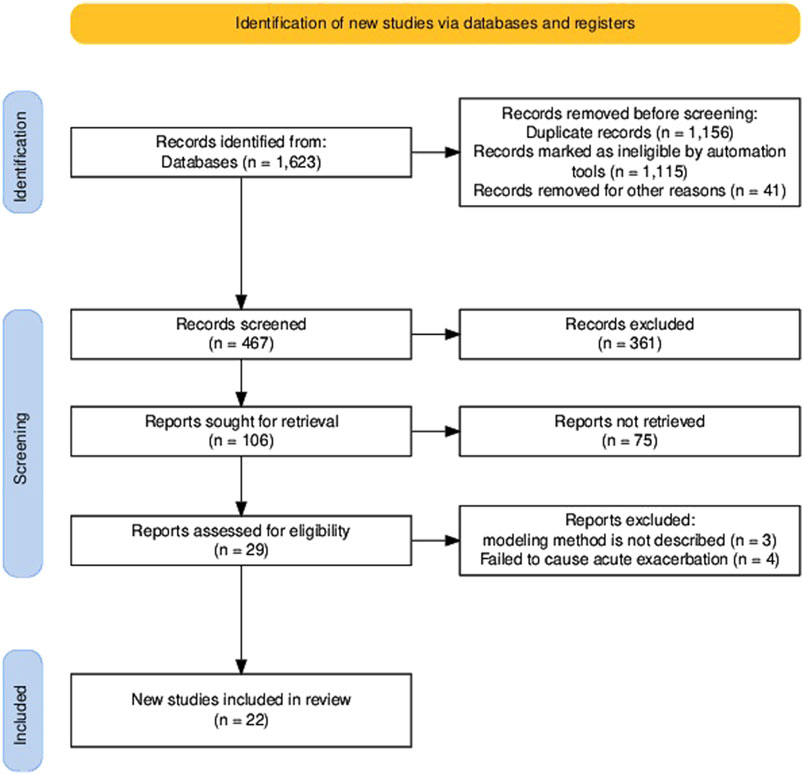
Our systematic search across PubMed, Embase, and Web of Science identified 1,623 candidate articles. Following duplicate (n = 1,156) removal, the remaining 467 records underwent title/abstract screening using predefined criteria (non-English articles, studies using non-mammalian models, and studies involving non-acute exacerbation were excluded). Following a full-text review, 84 of the remaining 106 articles were excluded for protocol non-compliance, yielding 22 studies meeting all inclusion criteria (see the PRISMA flowchart in Figure 2). The 22 articles discussed here provide information on animal models currently available for AE-PF research. To date, only mice and rats have been used to study AE-PF, making it highly challenging to replicate human clinical conditions in animal models. In this review, we systematically evaluated modeling methodologies, survival parameters, and histopathological features to delineate conserved pathophysiological signatures across the AE-PF models and elucidate the molecular mechanisms underlying acute exacerbation transitions.
3.2 Animal characteristics
Murine models predominated the included studies (86.4%, 19/22), with the C57BL/6 strain constituting 89.5% (17/19) of these studies. Of the 19 studies using mouse models, 36.8% (7/19) used male mice, 26.3% (5/19) used female mice, and 36.8% (7/19) did not provide gender information. Research using rats accounted for 13.6% (3/22) of the studies, of which two used the Sprague-Dawley strain and one used the Wistar strain (Table 1).
3.3 Modeling methods
Of the 19 established AE-PF models, 89.5% (17/19) employed sequential induction protocols, namely, initial fibrosis induction followed by acute exacerbation triggers. BLM was utilized in 78.9% (15/19) of PF models, while lipopolysaccharide (LPS) served as the main exacerbation trigger (26.3%, 5/19). Intratracheal instillation was the primary route of administration (84.2%, 16/19), with alternative methods including osmotic pump delivery (10.5%, 2/19) and aerosol inhalation (5.3%, 1/19) (Table 1).
3.4 Survival rate
Reported mortality rates ranged from 21% to 100% across 10 studies. Miyamoto et al. reported dose-dependent survival outcomes in BLM-LPS rat models, with 100% survival observed with 0.05 mg/kg LPS versus 83% survival with 0.15 mg/kg on day 7 post-induction (Miyamoto et al., 2022). Conversely, BLM-herpes virus co-exposure models showed a 21% survival rate on day 21 (Chen et al., 2019) (Table 1).
3.5 Clinical features
Among the included studies, 54.5% (n = 12) documented clinically relevant assessments in animal models, encompassing pulmonary function, radiographic imaging, arterial blood gas analysis, and oxygen saturation monitoring. Regarding pulmonary mechanics, 31.8% (n = 7) demonstrated compromised lung compliance. In radiographic evaluations, 13.6% (n = 3) identified characteristic imaging alterations. Oxygenation parameters showed clinically relevant deviations, with 13.6% (n = 3) revealing decreased arterial oxygen tension and 9.1% (n = 2) exhibiting reduced peripheral oxygen saturation (Table 2).
3.6 Histopathological features
Of the analyzed studies, 81.8% (n = 18) characterized histopathological features in AE-PF model animals. Notably, 77.3% (n = 17) of the studies reported significant neutrophil infiltration within pulmonary parenchyma, while 50% (n = 11) quantified collagen deposition, identifying marked increases indicative of progressive fibrosis. Additionally, 40.9% (n = 9) identified histopathology characteristic of ARDS, including alveolar septal collapse, fibrinous exudates, interstitial edema, intra-alveolar hemorrhage, and extensive hyaline membrane formation. These pathological manifestations showed strong concordance with established clinicopathological criteria (Table 2).
3.7 Quantitative indexes of fibrosis
A total of 63.6% (n = 14) of the studies undertook a quantitative fibrosis analysis, with 54.5% (n = 12) detecting alterations in pulmonary hydroxyproline levels. Notably, 50% (n = 11) of the studies reported that the contents of the collagen-specific biomarker were significantly elevated, while one study found no statistically significant difference in hydroxyproline levels. Concurrently, 9.1% (n = 2) of the evaluated studies reported increases in soluble collagen content (Table 2).
3.8 Cytokine profile
Dysregulated expression of 18 cytokines was systematically documented in bronchoalveolar lavage fluid (BALF) (n = 11), serum samples (n = 1), and lung tissue homogenates (n = 6) across 17 studies. The most frequently detected cytokines were TNF-α (n = 9), interleukin-6 (IL-6) (n = 8), and TGF-β (n = 6) (Table 2).
3.9 Mechanisms underlying AE-IPF pathogenesis
3.9.1 Inflammation
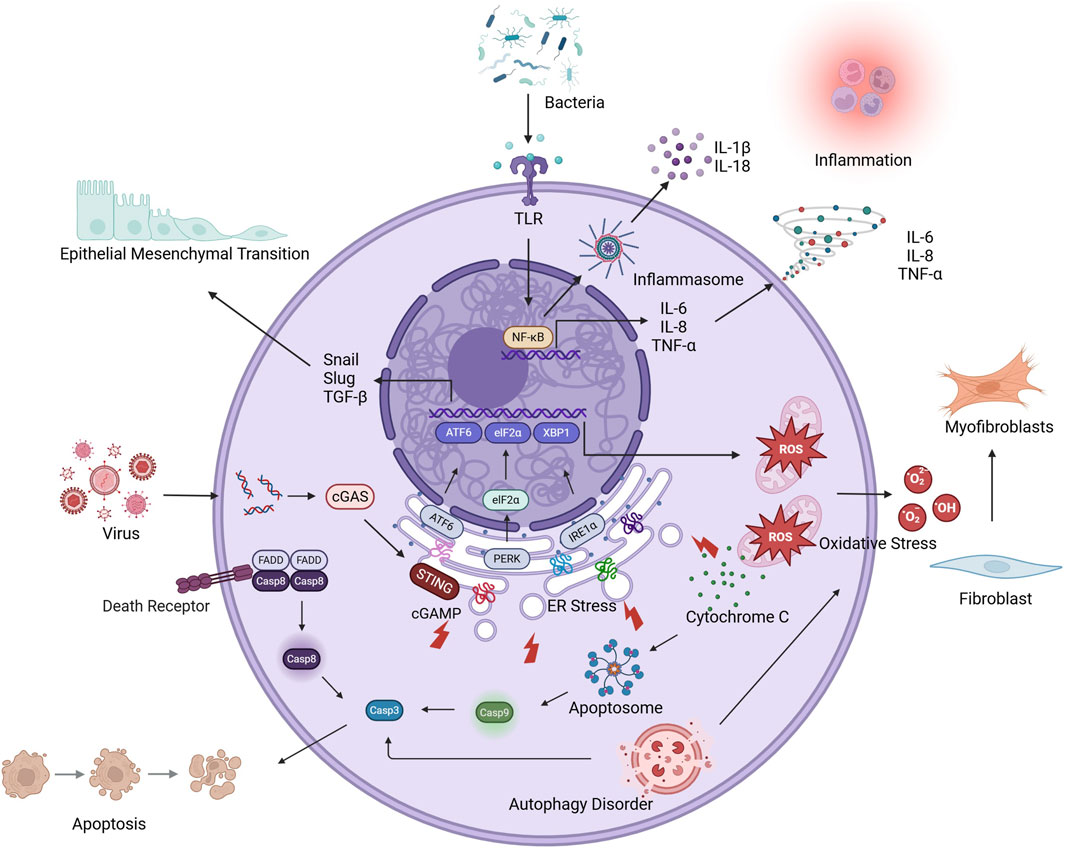
Accumulating evidence has established self-perpetuating inflammatory cascades as pivotal drivers of AE-IPF pathogenesis (Vázquez et al., 2011). Histopathological hallmarks of AE-IPF, including diffuse inflammatory infiltrates, intra-alveolar hemorrhage, proteinaceous edema, and hyaline membrane deposition, are consistently observed in clinical specimens (Tanaka et al., 2021) and experimental models (Chen et al., 2019). Studies investigating the molecular mechanisms revealed that the mRNA levels of nuclear factor-kappa B (NF-κB) were elevated, as were those of TNF-α, CXCL1, IL-1β, IL-6, and IL-8 (Chen et al., 2017; Yegen et al., 2022; Schupp et al., 2015; Papiris et al., 2018), indicative of progressive inflammatory milieu dysregulation. Acute inflammation represents an evolutionarily conserved defense mechanism involving the coordinated activity of the innate and adaptive immune responses to eliminate pathogens and initiate tissue repair (Hawiger and Zienkiewicz, 2019). However, in AE-IPF, this mechanism is dysregulated, primarily due to pattern recognition receptor (PRR) hyperactivation (Masumoto et al., 2021; Agarwal and Jindal, 2008). TLR engagement on AECs, endothelial cells, and macrophages initiates canonical NF-κB signaling via inhibitor of NF-κB (IκB) kinase-mediated phosphorylation (Le et al., 2023; Arora et al., 2019; McElroy et al., 2022). Activated NF-κB, acting as a dimer, is released from cytoplasmic inhibition and translocates to the nucleus, where it drives the transcription of inflammatory factors such as TNF-α, IL-1β, and IL-6 (Yu et al., 2020). Parallel inflammasome activation through the NOD-like receptor protein 3 (NLRP3) (Jäger et al., 2021) and absent in melanoma 2 (AIM2) (Cho et al., 2020) platforms in macrophages induces the caspase-1-dependent cleavage of pro-IL-1β and pro-IL-18, which, coupled with gasdermin D-mediated pyroptosis, amplifies the inflammatory response (Tseng et al., 2023). IL-1β, primarily secreted by monocyte-derived macrophages, initiates inflammatory cascades by activating CD4+ T cells and B cells, leading to the production of IL-6 and TNF-α (Dinarello, 2011). IL-6 rapidly responds to tissue damage, driving T-cell proliferation, antibody secretion by B cells, and inflammation amplification via STAT3 signaling (Tanaka et al., 2014). TNF-α exerts dual proinflammatory effects through TNF receptor-mediated, caspase-8-dependent apoptosis and endothelial vascular cell adhesion molecule-1 (VCAM-1)/ICAM-1 upregulation, thereby facilitating transendothelial neutrophil migration (Zelová and Hošek, 2013). Simultaneously, these cytokines collectively drive the polarization of alveolar macrophages toward a proinflammatory M1 phenotype (Cheng et al., 2021) and recruit circulating neutrophils, which accumulate in pulmonary parenchyma and undertake phagocytic clearance of pathogens and cellular debris (Piccinini and Midwood, 2010). Activated neutrophils and M1 macrophages further amplify the inflammatory response through the sustained secretion of TNF-α, IL-1β, IL-6, and CXCL8, thereby establishing chemotactic gradients that perpetuate monocyte-neutrophil infiltration (Meyer et al., 2021). This self-reinforcing inflammatory cascade propagates through feedforward mechanisms (Karki and Kanneganti, 2021; Marchioni et al., 2018), ultimately precipitating the acute exacerbation of stable IPF via pulmonary microenvironment destabilization (Savin et al., 2022) (Figure 3).
3.9.2 Th17/Treg Imbalance
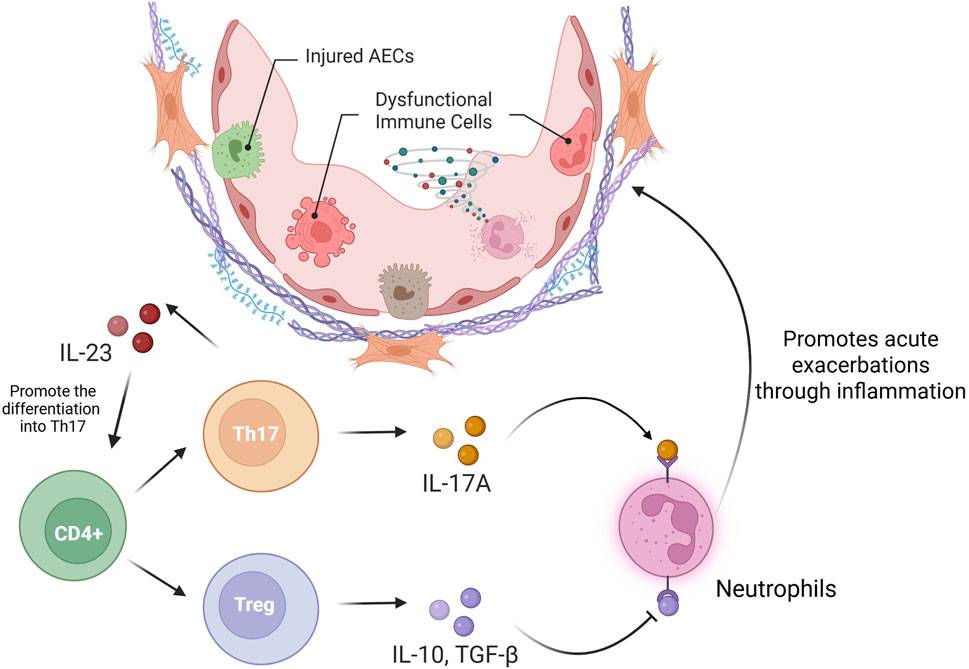
An imbalance between T-helper 17 (Th17) cells and regulatory T cells (Tregs) is a hallmark of immune dysregulation in pulmonary pathologies, including IPF (Thomas et al., 2023). These T-cell types originate from the divergent differentiation of naive CD4+ T cells. Th17 cells predominantly secrete IL-17A, IL-22, and IL-23, thus promoting proinflammatory responses, whereas Tregs counterbalance immune activation through IL-10 and TGF-β-mediated immunosuppression (Lee, 2018). Clinical studies have identified elevated Th17 cell frequencies and IL-17A concentrations in BALF during acute exacerbations (Wei et al., 2016), with murine models supporting these findings, as evidenced by the observed reduction in the number of inflammatory foci and diminished fibrosis in AE-PF mice with IL-17A deficiency relative to their wild-type counterparts (Chen et al., 2022). Mechanistically, IL-17A/IL-17RA axis activation leads to TGF-β release and neutrophil-mediated fibrogenesis, thereby functionally coupling inflammatory and fibrotic pathways in IPF pathogenesis (Huangfu et al., 2023; Lorè et al., 2016). In acute exacerbation, IL-17A promotes the chemotaxis of neutrophils and eosinophils (Chen et al., 2022) while suppressing Treg-mediated immunosuppression (Xu et al., 2023). In contrast, Treg depletion exacerbates inflammatory infiltration and fibroproliferation in experimental models (Moyé et al., 2020). The IL-23/Th17 regulatory axis plays an essential role in disease progression, as evidenced by the attenuation of airway inflammation and the decrease in the number of fibroblast foci in IL-23-deficient mice, alongside the efficacy of IL-23 neutralization in reducing Th17 populations and mitigating acute exacerbations (Gaffen et al., 2014). Collectively, these findings underscore the potential of Th17/Treg rebalancing as a therapeutic paradigm for AE-IPF (Figure 4).
3.9.3 Oxidative stress
Oxidative stress occurs when the equilibrium between ROS/reactive nitrogen species (RNS) production and the antioxidant capacity is disrupted, leading to cellular dysfunction and tissue damage (Jomova et al., 2023). Pulmonary neutrophils and macrophages constitute primary ROS/RNS sources through NADPH oxidase and myeloperoxidase activities (Morris et al., 2022). During acute exacerbations, respiratory bursts lead to the release of superoxide (O2−), hydrogen peroxide (H2O2), nitric oxide (NO), and hypochlorous acid (HOCl) by phagocytic cells into alveolar spaces, which overwhelm endogenous defenses (Glennon-Alty et al., 2018). In addition to direct macromolecular damage, these oxidants promote TGF-β secretion from alveolar epithelium (Cui et al., 2011), triggering myofibroblast differentiation via α-smooth muscle actin (α-SMA) overexpression and excessive ECM deposition (Estornut et al., 2021). ROS accelerate cellular senescence by inducing telomere erosion, DNA damage, and proteotoxic stress, consequently promoting cell cycle arrest and the development of a senescence-associated secretory phenotype (SASP) (Korfei et al., 2020; Ogrodnik, 2021). In patients with IPF, senescent cells in the lung microenvironment displaying this ROS-induced feature secrete a wide range of proinflammatory and profibrotic mediators, such as IL-1β, IL-6, plasminogen activator inhibitor-1 (PAI-1), VCAM-1, matrix metalloproteinase-2 (MMP-2), and TGF-β (Álvarez et al., 2017), which collectively function as a signaling hub that perpetuates tissue injury responses and drives fibrotic progression (Schafer et al., 2017; Waters et al., 2018). While oxidative stress drives stable IPF progression through well-characterized mechanisms such as fibroblast activation and cellular senescence induction, its contribution to acute exacerbation remains ambiguous. Emerging evidence has implicated oxidative stress in AE-IPF. Alveolar macrophages in AE-IPF demonstrate marked upregulation of inducible nitric oxide synthase (iNOS) expression at both protein and mRNA levels (Miyamoto et al., 2022). iNOS catalyzes the oxidation of arginine, generating nitric oxide (NO) and peroxynitrite, the excessive production of which can induce protein nitrosation, thus amplifying oxidative damage (Cinelli et al., 2020). The antioxidant N-acetylcysteine (NAC) reduces mortality in AE-PF murine models (Hu et al., 2017) and inhibits fibroblast proliferation and activation (Yang et al., 2018), implicating oxidative stress as a central driver of AE-IPF pathobiology.
3.9.4 Endoplasmic reticulum stress
The ER maintains proteostasis through the coordination of protein biosynthesis, folding, and quality control. When biosynthetic demands exceed ER processing capacity, misfolded proteins accumulate, triggering ER stress—a state characterized by functional impairment and arrested protein synthesis (Oakes and Papa, 2015). This initiates the unfolded protein response (UPR) via three transmembrane sensors, namely, inositol-requiring enzyme one alpha (IRE1α), protein kinase R-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Read and Schröder, 2021; Hwang and Qi, 2018). The endoribonuclease domain of IRE1α is rapidly activated, leading to the splicing of X-box binding protein 1 (XBP1) mRNA, and, consequently, the generation of the transcriptionally active XBP1s isoform, which enhances the expression of genes encoding ER chaperones and components of the protein degradation machinery (Langlais et al., 2021; Grandjean et al., 2020). ATF6 undergoes regulated intramembrane proteolysis, following which its cytosolic fragment translocates to the nucleus and upregulates the expression of protein folding machinery-related genes (Lei et al., 2024). PERK demonstrates delayed but sustained activation during chronic stress, phosphorylating eukaryotic initiation factor 2 alpha (eIF2α), thereby reducing global protein synthesis, while simultaneously selectively inducing C/EBP homologous protein (CHOP) expression (Li et al., 2014). Persistent UPR failure increases CHOP-mediated transcription of Bcl-2-associated X protein (BAX), BCL2-antagonist/killer (BAK), and caspase-3, thus committing cells to apoptosis (Shore et al., 2011). In IPF, ER stress can arise through viral infections, oxidative damage, hypoxia, or impaired autophagy (Aghaei et al., 2020). ER stress elicits distinct consequences include the apoptosis or EMT of AECs, defective repair, and the differentiation of fibroblasts into α-SMA-positive myofibroblasts that drive collagen overproduction and fibrotic lesion expansion (Burman et al., 2018). Notably, viral-induced ER stress in IPF promotes the E3 ubiquitin ligase RNF5-mediated degradation of stimulator of interferon genes (STING), compromising antiviral defenses and predisposing to acute exacerbations (Qiu et al., 2017; Zhang et al., 2022). Concurrent CHOP/ATF6 overexpression activates NF-κB-dependent inflammation (Chen et al., 2019), establishing the disruption of ER proteostasis as a key mechanism linking chronic fibrosis progression and acute deterioration in IPF pathogenesis.
3.9.5 Apoptosis
Apoptosis constitutes a pivotal mechanism in IPF pathogenesis, serving as the convergent endpoint of oxidative stress, inflammatory cascades, and ER stress, while also amplifying cytokine storms and fibrogenesis (Phan et al., 2021). This caspase-dependent programmed cell death occurs through both extrinsic and intrinsic pathways (Xu et al., 2019). The extrinsic pathway, activated through death receptors or TNF receptors, initiates caspase-8 activation via death domain adaptors and represents the principal apoptotic mechanism in inflammatory settings (Amaral and Bortoluci, 2020). Meanwhile, the intrinsic pathway involves mitochondrial permeability transition accompanied by cytochrome c and apoptotic protease-activating factor 1 (Apaf-1) release, culminating in caspase-9-mediated caspase-3 activation. The intrinsic pathway is predominantly activated by hypoxia (Suresh et al., 2023), oxidative stress (Liu et al., 2023), and ER stress (Wang et al., 2022). In patients with AE-IPF, the expression of cyclin A2 and α-defensin is upregulated, whereas advanced glycation end-product receptor levels are reduced, leading to marked alveolar epithelial injury and apoptosis (Konishi et al., 2009). Type II AECs, functioning as adult stem cells, activate repair mechanisms post-injury by proliferating and differentiating into Type I AECs (Wang et al., 2024). However, excessive Type II AEC apoptosis in IPF not only impairs epithelial regeneration but also promotes TGF-β secretion by macrophages via apoptotic bodies, thus accelerating fibrosis (Kim KK. et al., 2018). Failed repair induces EMT and fibroblast hyperactivation, which drives pathological ECM deposition (Chambers and Mercer, 2015; Chan and Liu, 2022). Notably, apoptotic susceptibility is cell-type-specific in IPF, with Type II AECs demonstrating enhanced vulnerability while fibroblasts and endothelial cells develop apoptosis resistance (Corteselli et al., 2022), creating a microenvironment favoring epithelial denudation and aberrant repair (Ptasinski et al., 2021). In infection-triggered exacerbations, bacterial proapoptotic peptides (e.g., corisin) promote mitochondrial ROS accumulation and membrane destabilization in AECs, suppressing the levels of the antiapoptotic factors baculoviral IAP repeat-containing (BIRC) 5, BIRC7, and B-cell lymphoma 2 (BCL2), while upregulating those of caspase-3/Apaf-1 (D'Alessandro-Gabazza et al., 2020). HIF-1α-mediated BNIP3/SERPINE1 upregulation is observed in non-infectious AE-PF models (Yegen et al., 2022), with both proteins promoting AECs apoptosis and post-injury fibrosis (Bhandary et al., 2012; Bernard et al., 2018). This suggests that hypoxia-driven apoptosis contributes to spontaneous exacerbations.
4 Discussion
AE-IPF represents a pathobiologic enigma, marked by heterogeneous triggers and host responses, which complicate translational modeling. Experimental paradigms have advanced our understanding of infectious (viral, bacterial) and noninfectious (e.g., LPS, environmental toxins) drivers. However, they have limitations in their capacity to recapitulate human disease, which hinges on fidelity to core features, namely, diffuse alveolar damage, progressive fibrogenesis, and immune dysregulation. Here, we assessed current rodent models, emphasizing their strengths in mirroring specific mechanisms, such as TGF-β1-driven fibrosis, pathogen-induced epithelial apoptosis, or particulate matter-triggered inflammation, while addressing critical limitations, including the temporary nature of the pathology, species-specific pathogen tropism, and mortality. Emerging insights into the roles of bacterial dysbiosis and occupational exposures in AE-IPF further underscore the need for multifactorial models that integrate environmental stressors. These challenges are contextualized in Table 3, which synthesizes key methodologies, delineating their alignment with clinical AE-PF phenotypes and therapeutic discovery requirements. This comparative framework aims to optimize model selection for both mechanistic and interventional studies, bridging the gap between experimental rigor and the complex etiology of human AE-IPF.
4.1 Infection-induced models
Although the initial diagnostic criteria for AE-IPF excluded infectious etiologies (Collard et al., 2007), this perspective has been substantially revised given contemporary evidence (Raghu et al., 2022). Clinical observations revealed elevated serum IgM levels and the presence of neutrophilia in AE-IPF compared to stable IPF (Invernizzi and Molyneaux, 2019), suggestive of microbial involvement. Early research focused on subclinical, latent viral infections—particularly herpesviruses and respiratory viruses—which are difficult to detect clinically (Moghoofei et al., 2022). Molecular analyses have identified viral footprints (rhinovirus, coronavirus, herpes simplex virus [HSV]) in the lungs of patients with AE-IPF (Wootton et al., 2011), with HSV DNA detection rates significantly exceeding those of healthy controls (Kropski et al., 2012). Chronic viral persistence may provide incessant antigenic stimulation, potentially accelerating fibrogenesis (Stoolman et al., 2011) and predisposing to acute exacerbations (Vannella and Moore, 2008). Recent microbiome-related studies have identified a marked expansion of Proteobacteria with concurrent depletion of Campylobacter/Veillonella during exacerbations (Molyneaux et al., 2017), implicating bacterial dysbiosis in AE-IPF pathogenesis. These findings highlight the need for experimental models that incorporate pathogen exposure to accurately reflect human AE-IPF pathobiology.
4.1.1 γ-Herpes Virus-68 infection-induced AE-PF models
A foundational study by McMillan et al. (2008) established an AE-PF model through the sequential intratracheal administration of 28 mg/mL fluorescein isothiocyanate (FITC) followed by intranasal inoculation with 5 × 104 plaque-forming units (PFUs) of murine γ-herpes virus-68 (γHV-68) at 14-day intervals. Although FITC-induced fibrosis displays fibrotic histopathological hallmarks, including chronic inflammation and extracellular matrix deposition, its pathogenesis is lymphocyte-independent (Moore et al., 2001), which substantially diverges from human IPF pathophysiology, leading to its progressive replacement by BLM-based models. Ashley et al. (2014) employed a similar γHV-68 challenge (5 × 104 PFUs intranasal) for AE induction but diverged in primary fibrosis induction, using 0.025 U BLM, administered intratracheally, instead of FITC. Viral challenge on day 14 post-BLM elicited characteristic AE-IPF features within 7 days, corroborating McMillan’s findings. Notably, these exacerbation phenotypes were absent following inoculation with Pseudomonas aeruginosa or the influenza virus in BLM-primed mice.
4.1.2 HSV type 1 infection-induced AE-PF models
Chen et al. (2019) established an AE-PF model through the sequential intratracheal administration of BLM (4 mg/kg, equivalent to 60 U/kg) followed by intranasal challenge with HSV-1 (5 × 105 PFUs) on day 21. This HSV-1-based model, which employed human-pathogenic herpesvirus rather than the species-specific γHV-68, better approximates AE-IPF pathophysiology, although its 28-day survival rate of 21.4% raises translational concerns. Qiu et al. (2017) employed a comparable protocol with modified BLM dosing (5 U/kg intratracheal) and earlier HSV-1 administration (day 14 post-BLM). Although the use of this variant replicated Chen’s histopathological and functional characteristics, it resulted in similarly prohibitive mortality (∼25% survival), further underscoring the need for model refinement.
4.1.3 Streptococcus pneumoniae infection-induced AE-PF models
Among bacterial infection models, the Streptococcus pneumoniae (Spn) challenge paradigm demonstrates relative methodological maturity. Cho et al. (Cho et al., 2020) established AE-PF through the sequential oropharyngeal administration of BLM (0.01 mg/mouse, equivalent to 0.15 U/mouse), followed 14 days later by intranasal Spn serotype 3 instillation (1 × 105 colony-forming units [CFUs]/mouse). In contrast, Knippenberg et al. (2015), Bormann et al. (2021), and Moyé et al. (2020) employed adenovirus-mediated TGF-β1 overexpression combined with Spn infection. Their experimental protocol involved the oropharyngeal administration of TGF-β1-encoding adenoviral vectors (1 × 108 PFUs/mouse) to induce progressive fibrosis, followed by Spn serotype 19 challenge (1 × 107 CFUs/mouse) via the same route during disease stabilization. TGF-β1, recognized as the principal fibrogenic mediator in IPF pathogenesis (Caja et al., 2018), induces spontaneous extracellular matrix remodeling in these adenoviral overexpression models. Histopathological evaluation revealed characteristic IPF features, including diffuse collagen deposition, honeycombing, and the development of fibroblastic foci (D'Alessandro-Gabazza et al., 2018). Unlike the self-limiting fibrosis observed in BLM-induced models post-treatment cessation, TGF-β1-overexpressing systems demonstrate progressive fibrotic deterioration that better mirrors the relentless disease progression observed in human IPF.
4.1.4 Staphylococcus nepalensis infection-induced AE-PF models
D’Alessandro-Gabazza et al. (D'Alessandro-Gabazza et al., 2020) induced acute exacerbation in TGF-β1 transgenic PF models through the intratracheal administration of Staphylococcus nepalensis (1 × 108 CFUs/mouse). This bacterial challenge occasioned pronounced macrophage/neutrophil infiltration, exacerbated collagen deposition, and enhanced alveolar epithelial cell apoptosis, a central pathogenic mechanism in IPF progression (Sauler et al., 2019). Mechanistic studies revealed that S. nepalensis secretes the proapoptotic peptide corisin under hypertonic microenvironmental conditions, directly driving pan-alveolar epithelial apoptosis. Building on these findings, the same research group (D'Alessandro-Gabazza et al., 2022) developed an optimized AE-PF model combining BLM exposure with corisin challenge.
4.1.5 Haemophilus influenzae infection-induced AE-PF models
Chen et al. (2022) established a novel Gram-negative bacteria-induced AE-PF model through the sequential intranasal administration of BLM (30 μg/mouse, equivalent to 0.45 U/mouse), followed by H. influenzae administration (1 × 107 CFUs/mouse) at 7-day intervals. This is the sole reported Gram-negative bacterial model of AE-PF. This study systematically compared BLM and Haemophilus influenzae dosing regimens (BLM: 15, 30, and 60 μg/mouse, equivalent to 0.225, 0.45, and 0.9 U/mouse; H. influenzae: 1 × 106–1 × 108 CFUs/mouse), and reported that the 30 μg BLM dose optimally induced pulmonary fibrosis without elevating mortality, while a bacterial load of 1 × 107 CFUs induced significant weight loss with sustained pulmonary colonization. Mechanistically, chronic IL-6/IL-8-mediated neutrophilic airway inflammation drives immunopathology and functional decline in chronic lung diseases (Saliu et al., 2021). Clinically, 89% of bacterial isolates from the airways of patients with AE-IPF are Gram-negative (Weng et al., 2019). Compared with existing viral and Gram-positive bacterial models, this Gram-negative model demonstrated superior survival rates while maintaining pathophysiological fidelity.
4.2 Noninfectious factor-induced models
AE-IPF represents a multifactorial clinical crisis superimposed on chronic fibrotic lung remodeling. Current pathophysiological paradigms implicate synergistic interactions between endogenous fibrogenic pathways and exogenously triggered acute insults in driving AE-IPF initiation and progression. However, integrative multi-omics investigations incorporating clinical phenotyping, BALF profiling, and molecular histopathology have failed to identify unifying pathomechanisms (Kakugawa et al., 2016; Oda et al., 2014). This pathobiological complexity has hindered faithful animal model recapitulation, constituting a major translational bottleneck in AE-IPF research. While infectious triggers are recognized contributors, a substantial proportion of exacerbations lack definitive microbiological evidence. The mechanistic dissection of noninfectious AE-IPF subtypes has emerged as a critical research priority, with the potential to unravel disease-specific drivers and accelerate the development of targeted therapy.
4.2.1 Lipopolysaccharide-induced AE-PF models
Contemporary histopathological analyses of lung biopsies and autopsies have confirmed that DAD is the cardinal feature of AE-IPF (Dotan et al., 2020; Kawaguchi et al., 2022; Kishaba et al., 2020). LPS, a potent experimental inflammogen, reliably induces DAD pathology (Domscheit et al., 2020) and acute exacerbation of lung disease (Mohamed et al., 2019), providing a translational platform bridging preclinical models and human pathophysiology.
Kimura et al. (2015) and Senoo et al. (2021) established a model employing sequential BLM-induced fibrosis (1 mg/kg equivalent to 15 U/kg; intratracheal) followed by LPS challenge (0.5 mg/kg) on day 7, eliciting hallmark clinical features, including acute hypoxemia (PaO2 <60 mmHg within 24 h) and diffuse ground-glass opacities on CT. However, single-dose LPS models exhibit transient inflammation with spontaneous resolution within 7 days. To circumvent this limitation, Jia et al. (2023) implemented fractionated LPS dosing (1 mg/kg on days 5, 7, and 9 post-BLM), achieving sustained exacerbation concomitant with hepatic inflammation and collagen deposition, which mirrored the subclinical liver fibrosis observed in >30% of patients with IPF (Cocconcelli et al., 2021).
Kurniawan et al. (2023) established an AE-PF model that combined intratracheal BLM administration (5 mg/kg, equivalent to 75 U/kg) with intraperitoneal LPS challenge (7.5 mg/kg), and identified distinct pathophysiological phases—a neutrophilic BALF peak on day 1 (inflammatory phase), maximal fibrosis expansion on day 7 (proliferative phase), and partial resolution by day 14 (remodeling phase)—thus establishing a critical therapeutic window within 7 days post-exacerbation.
Miyamoto et al. (2022) demonstrated that ultralow-dose LPS (0.05–0.15 mg/kg) triggers AE-PF in BLM-primed Wistar rats (3 mg/kg [45 U/kg] daily for 7 consecutive days), replicating clinical exacerbation patterns after minor insults while preserving survival rates (83%–100% on day 7). This paradigm employs LPS doses 10-fold lower than conventional acute lung injury models, thus enhancing clinical relevance while maintaining experimental feasibility.
4.2.2 BLM-induced AE-PF models
Chen et al. (2017), Wei et al. (2016) developed AE-PF models through sequential intratracheal BLM challenge. This dual-administration protocol demonstrates operational simplicity and high reproducibility while effectively mimicking acute lung injury dynamics during exacerbations, although limited by spontaneous fibrotic regression. In contrast, Yegen et al. (2022) implemented a chronic fibrotic priming strategy with biweekly intratracheal BLM (0.8 U/g twice weekly for 4 weeks) followed by double-dose exacerbation triggers (1.6 U/g twice weekly for 2 weeks). This extended protocol better recapitulates the progressive fibrotic trajectory of IPF, circumventing the issue of self-limiting fibrosis observed in acute models. However, the prolonged experimental timeline (6-week duration) and technical complexity present practical implementation challenges. Notably, the applied dose of 0.8 U/g (equivalent to 800 U/kg) substantially exceeds reported lethal dose ranges for BLM in murine models (typically 150–200 U/kg), raising critical concerns regarding experimental mortality and translational relevance.
4.2.3 Environmental factor-induced AE-PF models
Hu et al. (2017) investigated acute exacerbation mechanisms using a model combining BLM and exposure to straw combustion-derived particulate matter with an aerodynamic diameter ≤5 µm (PM2.5). Following 7-day intratracheal BLM priming (0.2 mg/mouse, equivalent to 3 U/mouse), mice underwent twice-daily PM2.5 inhalation (30 min/session), eliciting progressive macrophage infiltration with characteristic black particulate deposition and time-dependent collagen accumulation. This experimental paradigm aligns with clinical evidence linking air pollution exposure to accelerated IPF progression, including functional decline and radiological deterioration (Ko and Kyung, 2022; Mariscal-Aguilar et al., 2023). PM2.5, which predominantly comprises secondary sulfates, nitrates, and biomass combustion byproducts (Yao et al., 2016), demonstrates dose-dependent associations with fibrotic interstitial lung disease mortality and progression (Goobie et al., 2022), highlighting its emerging role in AE-IPF pathogenesis.
Kim MS. et al. (2018) developed a polyhexamethylene guanidine (PHMG)-cadmium co-exposure model, leveraging cadmium’s established pulmonary toxicity from cigarette smoke-mediated deposition and its mechanistic links to COPD/IPF overlap syndromes (Ganguly et al., 2018). Complementary studies by Yang et al. (2018) demonstrated that nickel chloride (NiCl2, 5 mg/kg) can exacerbate BLM-induced fibrosis (2.5 mg/kg, equivalent to 37.5 U/kg) when co-administered intratracheally. These findings emphasize the underappreciated impact of occupational metallurgical exposures, which not only elevate IPF incidence among industrial workers but also exhibit dose-responsive mortality correlations in established IPF cohorts (Gandhi et al., 2023; Trethewey and Walters, 2018).
5 Conclusion
AE-IPF remains the most critical clinical challenge in the management of fibrotic lung disease. The elusive pathogenesis underlying AE-IPF onset and progression has severely hindered therapeutic breakthroughs, compounded by inherent difficulties in conducting human trials due to fulminant disease courses and excess mortality rates. These challenges accentuate the importance of developing animal models that faithfully replicate human AE-IPF clinicopathological characteristics. Current experimental paradigms exhibit substantial heterogeneity in pharmacologic agents, dosing regimens, administration routes, and temporal sequences, complicating cross-study comparisons. Among existing approaches, sequential intratracheal BLM-induced fibrosis followed by LPS challenge on day 7 emerges as the most validated methodology, balancing pathophysiological fidelity with acceptable survival profiles. Nevertheless, critical limitations persist, such as the absence of standardized histopathological criteria, suboptimal survival rates (frequently unreported), and an incomplete mechanistic understanding of exacerbation triggers. This systematic synthesis identified alveolar epithelial cell apoptosis and dysregulated inflammation as central drivers of acute exacerbations, with oxidative stress, ER stress, and immune dyshomeostasis constituting key contributory pathways. To unravel the pathobiological complexity of AE-IPF, future efforts must prioritize 1) the establishment of standardized, pathologically relevant animal models through multicenter validation studies; 2) mechanistic dissection combining these experimental platforms with multi-omics; and 3) translating preclinical mechanistic insights into evidence-based therapeutic innovations, thereby establishing novel clinical paradigms to reduce acute exacerbation-related mortality and improve long-term prognosis in patients with IPF.
Author contributions
KC: Conceptualization, Investigation, Methodology, Writing – original draft. HZ: Conceptualization, Methodology, Supervision, Writing – review and editing. ZY: Data curation, Investigation, Writing – original draft. ST: Data curation, Investigation, Writing – original draft. QM: Data curation, Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research presented in this paper is supported by the Joint funds of the Research and Development Program of Science and Technology of Henan province of China (Type of the Cultivation of advantageous disciplines) (No. 232301420091) and the Medical Science and Technology Tackling program of Henan province of China (No. LHGJ20230682).
Acknowledgments
Figures 1, 3, and 4 were created with BioRender (https://www.biorender.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, R., and Jindal, S. K. (2008). Acute exacerbation of idiopathic pulmonary fibrosis: a systematic review. Eur. J. Intern Med. 19 (4), 227–235. doi:10.1016/j.ejim.2007.04.024
Aghaei, M., Dastghaib, S., Aftabi, S., Aghanoori, M. R., Alizadeh, J., Mokarram, P., et al. (2020). The ER stress/UPR Axis in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Life (Basel) 11 (1), 1. doi:10.3390/life11010001
Álvarez, D., Cárdenes, N., Sellarés, J., Bueno, M., Corey, C., Hanumanthu, V. S., et al. (2017). IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell Mol. Physiol. 313 (6), L1164–L1173. doi:10.1152/ajplung.00220.2017
Amaral, M. P., and Bortoluci, K. R. (2020). Caspase-8 and FADD: where cell death and inflammation collide. Immunity 52 (6), 890–892. doi:10.1016/j.immuni.2020.05.008
Antoniou, K. M., and Wells, A. U. (2013). Acute exacerbations of idiopathic pulmonary fibrosis. Respiration 86 (4), 265–274. doi:10.1159/000355485
Arora, S., Ahmad, S., Irshad, R., Goyal, Y., Rafat, S., Siddiqui, N., et al. (2019). TLRs in pulmonary diseases. Life Sci. 233, 116671. doi:10.1016/j.lfs.2019.116671
Ashley, S. L., Jegal, Y., Moore, T. A., van Dyk, L. F., Laouar, Y., and Moore, B. B. (2014). γ-Herpes virus-68, but not Pseudomonas aeruginosa or influenza A (H1N1), exacerbates established murine lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 307 (3), L219–L230. doi:10.1152/ajplung.00300.2013
Bernard, O., Jeny, F., Uzunhan, Y., Dondi, E., Terfous, R., Label, R., et al. (2018). Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 314 (3), L360-–L371. doi:10.1152/ajplung.00153.2017
Bhandary, Y. P., Shetty, S. K., Marudamuthu, A. S., Gyetko, M. R., Idell, S., Gharaee-Kermani, M., et al. (2012). Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. Am. J. Physiol. Lung Cell Mol. Physiol. 302 (5), L463–L473. doi:10.1152/ajplung.00099.2011
Bormann, T., Maus, R., Stolper, J., Jonigk, D., Welte, T., Gauldie, J., et al. (2021). Role of the COX2-PGE(2) axis in S. pneumoniae-induced exacerbation of experimental fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 320 (3), L377–L392. doi:10.1152/ajplung.00024.2020
Burman, A., Tanjore, H., and Blackwell, T. S. (2018). Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. J. Int. Soc. Matrix Biol. 68-69, 355–365. doi:10.1016/j.matbio.2018.03.015
Caja, L., Dituri, F., Mancarella, S., Caballero-Diaz, D., Moustakas, A., Giannelli, G., et al. (2018). TGF-Β and the tissue microenvironment: relevance in fibrosis and cancer. Int. J. Mol. Sci. 19 (5), 1294. doi:10.3390/ijms19051294
Chambers, R. C., and Mercer, P. F. (2015). Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann. Am. Thorac. Soc. 12 (Suppl. 1), S16–S20. doi:10.1513/AnnalsATS.201410-448MG
Chan, M., and Liu, Y. (2022). Function of epithelial stem cell in the repair of alveolar injury. Stem Cell Res. Ther. 13 (1), 170. doi:10.1186/s13287-022-02847-7
Chen, S., Zhang, X., Yang, C., Wang, S., and Shen, H. (2022). Essential role of IL-17 in acute exacerbation of pulmonary fibrosis induced by non-typeable Haemophilus influenzae. Theranostics 12 (11), 5125–5137. doi:10.7150/thno.74809
Chen, S. S., Yin, Z. F., Chen, T., Qiu, H., Wei, Y. R., Du, S. S., et al. (2017). Development of a non-infectious rat model of acute exacerbation of idiopathic pulmonary fibrosis. J. Thorac. Dis. 9 (1), 96–105. doi:10.21037/jtd.2017.01.22
Chen, T., Qiu, H., Zhao, M. M., Chen, S. S., Wu, Q., Zhou, N. Y., et al. (2019). IL-17A contributes to HSV1 infection-induced acute lung injury in a mouse model of pulmonary fibrosis. J. Cell Mol. Med. 23 (2), 908–919. doi:10.1111/jcmm.13992
Cheng, I. Y., Liu, C. C., Lin, J. H., Hsu, T. W., Hsu, J. W., Li, A. F., et al. (2019). Particulate matter increases the severity of bleomycin-induced pulmonary fibrosis through KC-mediated neutrophil chemotaxis. Int. J. Mol. Sci. 21 (1), 227. doi:10.3390/ijms21010227
Cheng, P., Li, S., and Chen, H. (2021). Macrophages in lung injury, repair, and fibrosis. Cells 10 (2), 436. doi:10.3390/cells10020436
Cho, S. J., Moon, J. S., Nikahira, K., Yun, H. S., Harris, R., Hong, K. S., et al. (2020). GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation. Thorax 75 (3), 227–236. doi:10.1136/thoraxjnl-2019-213571
Churg, A., Wright, J. L., and Tazelaar, H. D. (2011). Acute exacerbations of fibrotic interstitial lung disease. Histopathology 58 (4), 525–530. doi:10.1111/j.1365-2559.2010.03650.x
Cinelli, M. A., Do, H. T., Miley, G. P., and Silverman, R. B. (2020). Inducible nitric oxide synthase: regulation, structure, and inhibition. Med. Res. Rev. 40 (1), 158–189. doi:10.1002/med.21599
Cocconcelli, E., Tonelli, R., Abbati, G., Marchioni, A., Castaniere, I., Pelizzaro, F., et al. (2021). Subclinical liver fibrosis in patients with idiopathic pulmonary fibrosis. Intern Emerg. Med. 16 (2), 349–357. doi:10.1007/s11739-020-02376-2
Collard, H. R., Moore, B. B., Flaherty, K. R., Brown, K. K., Kaner, R. J., King, T. E., et al. (2007). Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. care Med. 176 (7), 636–643. doi:10.1164/rccm.200703-463PP
Collard, H. R., Ryerson, C. J., Corte, T. J., Jenkins, G., Kondoh, Y., Lederer, D. J., et al. (2016). Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am. J. Respir. Crit. care Med. 194 (3), 265–275. doi:10.1164/rccm.201604-0801CI
Corteselli, E., Aboushousha, R., and Janssen-Heininger, Y. (2022). S-Glutathionylation-Controlled apoptosis of lung epithelial cells; potential implications for lung fibrosis. Antioxidants (Basel) 11 (9), 1789. doi:10.3390/antiox11091789
Cui, Y., Robertson, J., Maharaj, S., Waldhauser, L., Niu, J., Wang, J., et al. (2011). Oxidative stress contributes to the induction and persistence of TGF-β1 induced pulmonary fibrosis. Int. J. Biochem. Cell Biol. 43 (8), 1122–1133. doi:10.1016/j.biocel.2011.04.005
Czaja, M., Skirlińska-Nosek, K., Adamczyk, O., Sofińska, K., Wilkosz, N., Rajfur, Z., et al. (2022). Raman research on bleomycin-induced DNA strand breaks and repair processes in living cells. Int. J. Mol. Sci. 23 (7), 3524. doi:10.3390/ijms23073524
D'Alessandro-Gabazza, C. N., Kobayashi, T., Yasuma, T., Toda, M., Kim, H., Fujimoto, H., et al. (2020). A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis. Nat. Commun. 11 (1), 1539. doi:10.1038/s41467-020-15344-3
D'Alessandro-Gabazza, C. N., Méndez-García, C., Hataji, O., Westergaard, S., Watanabe, F., Yasuma, T., et al. (2018). Identification of halophilic microbes in lung fibrotic tissue by oligotyping. Front. Microbiol. 9, 1892. doi:10.3389/fmicb.2018.01892
D'Alessandro-Gabazza, C. N., Yasuma, T., Kobayashi, T., Toda, M., Abdel-Hamid, A. M., Fujimoto, H., et al. (2022). Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis. Nat. Commun. 13 (1), 1558. doi:10.1038/s41467-022-29064-3
Degryse, A. L., and Lawson, W. E. (2011). Progress toward improving animal models for idiopathic pulmonary fibrosis. Am. J. Med. Sci. 341 (6), 444–449. doi:10.1097/MAJ.0b013e31821aa000
Dinarello, C. A. (2011). A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 41 (5), 1203–1217. doi:10.1002/eji.201141550
Domscheit, H., Hegeman, M. A., Carvalho, N., and Spieth, P. M. (2020). Molecular dynamics of lipopolysaccharide-induced lung injury in rodents. Front. Physiol. 11, 36. doi:10.3389/fphys.2020.00036
Dotan, Y., Shapiro, W. B., Male, E., Dominguez, E. C., Aneja, A., Huaqing, Z., et al. (2020). Clinical predictors and explant lung pathology of acute exacerbation of idiopathic pulmonary fibrosis. ERJ Open Res. 6 (4). doi:10.1183/23120541.00261-2019
Escalon, J. G., and Lynch, D. A. (2018). Imaging features of typical usual interstitial pneumonia (UIP). Qjm 111 (9), 653–655. doi:10.1093/qjmed/hcy098
Estornut, C., Milara, J., Bayarri, M. A., Belhadj, N., and Cortijo, J. (2021). Targeting oxidative stress as a therapeutic approach for idiopathic pulmonary fibrosis. Front. Pharmacol. 12, 794997. doi:10.3389/fphar.2021.794997
Farrand, E., Vittinghoff, E., Ley, B., Butte, A. J., and Collard, H. R. (2020). Corticosteroid use is not associated with improved outcomes in acute exacerbation of IPF. Respirology 25 (6), 629–635. doi:10.1111/resp.13753
Feng, X., Yu, F., He, X. L., Cheng, P. P., Niu, Q., Zhao, L. Q., et al. (2024). CD8(+) tissue-resident memory T cells are essential in bleomycin-induced pulmonary fibrosis. Am. J. physiology Cell physiology 327 (5), C1178–C1191. doi:10.1152/ajpcell.00368.2024
Gaffen, S. L., Jain, R., Garg, A. V., and Cua, D. J. (2014). The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14 (9), 585–600. doi:10.1038/nri3707
Gandhi, S., Tonelli, R., Murray, M., Samarelli, A. V., and Spagnolo, P. (2023). Environmental causes of idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 24 (22), 16481. doi:10.3390/ijms242216481
Ganguly, K., Levänen, B., Palmberg, L., Åkesson, A., and Lindén, A. (2018). Cadmium in tobacco smokers: a neglected link to lung disease? Eur. Respir. Rev. official J. Eur. Respir. Soc. 27 (147), 170122. doi:10.1183/16000617.0122-2017
Glennon-Alty, L., Hackett, A. P., Chapman, E. A., and Wright, H. L. (2018). Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 125, 25–35. doi:10.1016/j.freeradbiomed.2018.03.049
Goobie, G. C., Carlsten, C., Johannson, K. A., Khalil, N., Marcoux, V., Assayag, D., et al. (2022). Association of particulate matter exposure with lung function and mortality among patients with fibrotic interstitial lung disease. JAMA Intern Med. 182 (12), 1248–1259. doi:10.1001/jamainternmed.2022.4696
Grandjean, J. M. D., Madhavan, A., Cech, L., Seguinot, B. O., Paxman, R. J., Smith, E., et al. (2020). Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming. Nat. Chem. Biol. 16 (10), 1052–1061. doi:10.1038/s41589-020-0584-z
Gruden, J. F., Panse, P. M., Leslie, K. O., Tazelaar, H. D., and Colby, T. V. (2013). UIP diagnosed at surgical lung biopsy, 2000-2009: HRCT patterns and proposed classification system. AJR Am. J. Roentgenol. 200 (5), W458–W467. doi:10.2214/AJR.12.9437
Hawiger, J., and Zienkiewicz, J. (2019). Decoding inflammation, its causes, genomic responses, and emerging countermeasures. Scand. J. Immunol. 90 (6), e12812. doi:10.1111/sji.12812
Hewlett, J. C., Kropski, J. A., and Blackwell, T. S. (2018). Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. J. Int. Soc. Matrix Biol. 71-72, 112–127. doi:10.1016/j.matbio.2018.03.021
Hozumi, H., Hasegawa, H., Miyashita, K., Yasui, H., Suzuki, Y., Kono, M., et al. (2019). Efficacy of corticosteroid and intravenous cyclophosphamide in acute exacerbation of idiopathic pulmonary fibrosis: a propensity score-matched analysis. Respirology 24 (8), 792–798. doi:10.1111/resp.13506
Hu, Y., Wang, L. S., Li, Y., Li, Q. H., Li, C. L., Chen, J. M., et al. (2017). Effects of particulate matter from straw burning on lung fibrosis in mice. Environ. Toxicol. Pharmacol. 56, 249–258. doi:10.1016/j.etap.2017.10.001
Huangfu, L., Li, R., Huang, Y., and Wang, S. (2023). The IL-17 family in diseases: from bench to bedside. Signal Transduct. Target Ther. 8 (1), 402. doi:10.1038/s41392-023-01620-3
Hwang, J., and Qi, L. (2018). Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 43 (8), 593–605. doi:10.1016/j.tibs.2018.06.005
Invernizzi, R., and Molyneaux, P. L. (2019). The contribution of infection and the respiratory microbiome in acute exacerbations of idiopathic pulmonary fibrosis. Eur. Respir. Rev. official J. Eur. Respir. Soc. 28 (152), 190045. doi:10.1183/16000617.0045-2019
Jäger, B., Seeliger, B., Terwolbeck, O., Warnecke, G., Welte, T., Müller, M., et al. (2021). The NLRP3-inflammasome-caspase-1 pathway is upregulated in idiopathic pulmonary fibrosis and acute exacerbations and is inducible by apoptotic A549 cells. Front. Immunol. 12, 642855. doi:10.3389/fimmu.2021.642855
Jenkins, R. G., Moore, B. B., Chambers, R. C., Eickelberg, O., Königshoff, M., Kolb, M., et al. (2017). An official American thoracic society workshop report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am. J. Respir. cell Mol. Biol. 56 (5), 667–679. doi:10.1165/rcmb.2017-0096ST
Jia, K., Wu, J., Li, Y., Liu, J., Liu, R., Cai, Y., et al. (2023). A novel pulmonary fibrosis murine model with immune-related liver injury. Anim. Model Exp. Med. 6 (3), 274–282. doi:10.1002/ame2.12263
Johannson, K. A., Vittinghoff, E., Lee, K., Balmes, J. R., Ji, W., Kaplan, G. G., et al. (2014). Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur. Respir. J. 43 (4), 1124–1131. doi:10.1183/09031936.00122213
Jomova, K., Raptova, R., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., et al. (2023). Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97 (10), 2499–2574. doi:10.1007/s00204-023-03562-9
Kakugawa, T., Sakamoto, N., Sato, S., Yura, H., Harada, T., Nakashima, S., et al. (2016). Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir. Res. 17 (1), 79. doi:10.1186/s12931-016-0400-1
Karki, R., and Kanneganti, T. D. (2021). The 'cytokine storm': molecular mechanisms and therapeutic prospects. Trends Immunol. 42 (8), 681–705. doi:10.1016/j.it.2021.06.001
Kawaguchi, T., Tachiwada, T., Yamasaki, K., Nakamura, K., Katafuchi, E., Tahara, M., et al. (2022). An autopsy case of an acute exacerbation of idiopathic pulmonary fibrosis triggered by the inhalation of a waterproofing spray. Intern Med. 61 (12), 1881–1886. doi:10.2169/internalmedicine.8330-21
Kim, H. J., Perlman, D., and Tomic, R. (2015). Natural history of idiopathic pulmonary fibrosis. Respir. Med. 109 (6), 661–670. doi:10.1016/j.rmed.2015.02.002
Kim, K. K., Dotson, M. R., Agarwal, M., Yang, J., Bradley, P. B., Subbotina, N., et al. (2018a). Efferocytosis of apoptotic alveolar epithelial cells is sufficient to initiate lung fibrosis. Cell Death Dis. 9 (11), 1056. doi:10.1038/s41419-018-1074-z
Kim, M. S., Kim, S. H., Jeon, D., Kim, H. Y., Han, J. Y., Kim, B., et al. (2018b). Low-dose cadmium exposure exacerbates polyhexamethylene guanidine-induced lung fibrosis in mice. J. Toxicol. Environ. Health A 81 (11), 384–396. doi:10.1080/15287394.2018.1451177
Kimura, T., Nojiri, T., Hosoda, H., Shintani, Y., Inoue, M., Miyazato, M., et al. (2015). Exacerbation of bleomycin-induced injury by lipopolysaccharide in mice: establishment of a mouse model for acute exacerbation of interstitial lung diseases. Eur. J. Cardiothorac. Surg. 48 (4), e85–e91. doi:10.1093/ejcts/ezv261
Kishaba, T., Hozumi, H., Fujisawa, T., Nei, Y., Enomoto, N., Sugiura, H., et al. (2020). Predictors of acute exacerbation in biopsy-proven idiopathic pulmonary fibrosis. Respir. Investig. 58 (3), 177–184. doi:10.1016/j.resinv.2020.02.004
Knippenberg, S., Ueberberg, B., Maus, R., Bohling, J., Ding, N., Tort, T. M., et al. (2015). Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin. Thorax 70 (7), 636–646. doi:10.1136/thoraxjnl-2014-206420
Knudsen, L., Ruppert, C., and Ochs, M. (2017). Tissue remodelling in pulmonary fibrosis. Cell tissue Res. 367 (3), 607–626. doi:10.1007/s00441-016-2543-2
Ko, U. W., and Kyung, S. Y. (2022). Adverse effects of air pollution on pulmonary diseases. Tuberc. Respir. Dis. Seoul. 85 (4), 313–319. doi:10.4046/trd.2022.0116
Kolb, P., Upagupta, C., Vierhout, M., Ayaub, E., Bellaye, P. S., Gauldie, J., et al. (2020). The importance of interventional timing in the bleomycin model of pulmonary fibrosis. Eur. Respir. J. 55 (6), 1901105. doi:10.1183/13993003.01105-2019
Kondoh, Y., Cottin, V., and Brown, K. K. (2017). Recent lessons learned in the management of acute exacerbation of idiopathic pulmonary fibrosis. Eur. Respir. Rev. official J. Eur. Respir. Soc. 26 (145), 170050. doi:10.1183/16000617.0050-2017
Konishi, K., Gibson, K. F., Lindell, K. O., Richards, T. J., Zhang, Y., Dhir, R., et al. (2009). Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. care Med. 180 (2), 167–175. doi:10.1164/rccm.200810-1596OC
Korfei, M., MacKenzie, B., and Meiners, S. (2020). The ageing lung under stress. Eur. Respir. Rev. official J. Eur. Respir. Soc. 29 (156), 200126. doi:10.1183/16000617.0126-2020
Kropski, J. A., Lawson, W. E., and Blackwell, T. S. (2012). Right place, right time: the evolving role of herpesvirus infection as a “second hit” in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 302 (5), L441–L444. doi:10.1152/ajplung.00335.2011
Kurniawan, S. V., Louisa, M., Zaini, J., Surini, S., Soetikno, V., Wuyung, P. E., et al. (2023). Acute exacerbation of idiopathic pulmonary fibrosis model in the rats using bleomycin and lipopolysaccharides. J. Adv. Vet. Anim. Res. 10 (2), 196–204. doi:10.5455/javar.2023.j669
Langlais, T., Pelizzari-Raymundo, D., Mahdizadeh, S. J., Gouault, N., Carreaux, F., Chevet, E., et al. (2021). Structural and molecular bases to IRE1 activity modulation. Biochem. J. 478 (15), 2953–2975. doi:10.1042/BCJ20200919
Le, J., Kulatheepan, Y., and Jeyaseelan, S. (2023). Role of toll-like receptors and nod-like receptors in acute lung infection. Front. Immunol. 14, 1249098. doi:10.3389/fimmu.2023.1249098
Lederer, D. J., and Martinez, F. J. (2018). Idiopathic pulmonary fibrosis. N. Engl. J. Med. 378 (19), 1811–1823. doi:10.1056/NEJMra1705751
Lee, G. R. (2018). The balance of Th17 versus Treg cells in autoimmunity. Int. J. Mol. Sci. 19 (3), 730. doi:10.3390/ijms19030730
Lei, Y., Yu, H., Ding, S., Liu, H., Liu, C., and Fu, R. (2024). Molecular mechanism of ATF6 in unfolded protein response and its role in disease. Heliyon 10 (5), e25937. doi:10.1016/j.heliyon.2024.e25937
Leuschner, G., and Behr, J. (2017). Acute exacerbation in interstitial lung disease. Front. Med. 4, 176. doi:10.3389/fmed.2017.00176
Li, Y., Guo, Y., Tang, J., Jiang, J., and Chen, Z. (2014). New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. (Shanghai) 46 (8), 629–640. doi:10.1093/abbs/gmu048
Liu, L., Zhang, X., Zhang, R., Wang, L., Zhi, S., Feng, X., et al. (2023). Sohlh2 promotes pulmonary fibrosis via repression of p62/Keap1/Nrf2 mediated anti-oxidative signaling pathway. Cell Death Dis. 14 (10), 698. doi:10.1038/s41419-023-06179-z
Lorè, N. I., Bragonzi, A., and Cigana, C. (2016). The IL-17A/IL-17RA axis in pulmonary defence and immunopathology. Cytokine Growth Factor Rev. 30, 19–27. doi:10.1016/j.cytogfr.2016.03.009
Luppi, F., Cerri, S., Taddei, S., Ferrara, G., and Cottin, V. (2015). Acute exacerbation of idiopathic pulmonary fibrosis: a clinical review. Intern Emerg. Med. 10 (4), 401–411. doi:10.1007/s11739-015-1204-x
Maher, T. M., Bendstrup, E., Dron, L., Langley, J., Smith, G., Khalid, J. M., et al. (2021). Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 22 (1), 197. doi:10.1186/s12931-021-01791-z
Marchioni, A., Tonelli, R., Ball, L., Fantini, R., Castaniere, I., Cerri, S., et al. (2018). Acute exacerbation of idiopathic pulmonary fibrosis: lessons learned from acute respiratory distress syndrome? Crit. Care 22 (1), 80. doi:10.1186/s13054-018-2002-4
Mariscal-Aguilar, P., Gómez-Carrera, L., Bonilla, G., Díaz-Almirón, M., Gayá, F., Carpio, C., et al. (2023). Air pollution exposure and its effects on idiopathic pulmonary fibrosis: clinical worsening, lung function decline, and radiological deterioration. Front. Public Health 11, 1331134. doi:10.3389/fpubh.2023.1331134
Masumoto, J., Zhou, W., Morikawa, S., Hosokawa, S., Taguchi, H., Yamamoto, T., et al. (2021). Molecular biology of autoinflammatory diseases. Inflamm. Regen. 41 (1), 33. doi:10.1186/s41232-021-00181-8
McElroy, A. N., Invernizzi, R., Laskowska, J. W., O'Neill, A., Doroudian, M., Moghoofei, M., et al. (2022). Candidate role for toll-like receptor 3 L412F polymorphism and infection in acute exacerbation of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. care Med. 205 (5), 550–562. doi:10.1164/rccm.202010-3880OC
McMillan, T. R., Moore, B. B., Weinberg, J. B., Vannella, K. M., Fields, W. B., Christensen, P. J., et al. (2008). Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am. J. Respir. Crit. care Med. 177 (7), 771–780. doi:10.1164/rccm.200708-1184OC
Meyer, N. J., Gattinoni, L., and Calfee, C. S. (2021). Acute respiratory distress syndrome. Lancet. 398 (10300), 622–637. doi:10.1016/S0140-6736(21)00439-6
Miyamoto, H., Takemura, S., Minamiyama, Y., Tsukioka, T., Toda, M., Nishiyama, N., et al. (2022). Acute exacerbation of idiopathic pulmonary fibrosis model by small amount of lipopolysaccharide in rats. J. Clin. Biochem. Nutr. 70 (2), 129–139. doi:10.3164/jcbn.21-7
Moeller, A., Ask, K., Warburton, D., Gauldie, J., and Kolb, M. (2008). The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 40 (3), 362–382. doi:10.1016/j.biocel.2007.08.011
Moghoofei, M., Mostafaei, S., Kondori, N., Armstrong, M. E., and Babaei, F. (2022). Bacterial and viral coinfection in idiopathic pulmonary fibrosis patients: the prevalence and possible role in disease progression. BMC Pulm. Med. 22 (1), 60. doi:10.1186/s12890-022-01853-y
Mohamed, H. A., Elbastawisy, Y. M., and Elsaed, W. M. (2019). Attenuation of lipopolysaccharide-induced lung inflammation by ascorbic acid in rats: histopathological and ultrastructural study. SAGE Open Med. 7, 2050312119828260. doi:10.1177/2050312119828260
Molyneaux, P. L., Cox, M. J., Wells, A. U., Kim, H. C., Ji, W., Cookson, W. O., et al. (2017). Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir. Res. 18 (1), 29. doi:10.1186/s12931-017-0511-3
Moore, B. B., Paine, R., Christensen, P. J., Moore, T. A., Sitterding, S., Ngan, R., et al. (2001). Protection from pulmonary fibrosis in the absence of CCR2 signaling. J. Immunol. 167 (8), 4368–4377. doi:10.4049/jimmunol.167.8.4368
Morris, G., Gevezova, M., Sarafian, V., and Maes, M. (2022). Redox regulation of the immune response. Cell Mol. Immunol. 19 (10), 1079–1101. doi:10.1038/s41423-022-00902-0
Moyé, S., Bormann, T., Maus, R., Sparwasser, T., Sandrock, I., Prinz, I., et al. (2020). Regulatory T cells limit pneumococcus-induced exacerbation of lung fibrosis in mice. J. Immunol. 204 (9), 2429–2438. doi:10.4049/jimmunol.1900980
Oakes, S. A., and Papa, F. R. (2015). The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 10, 173–194. doi:10.1146/annurev-pathol-012513-104649
Oda, K., Ishimoto, H., Yamada, S., Kushima, H., Ishii, H., Imanaga, T., et al. (2014). Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir. Res. 15 (1), 109. doi:10.1186/s12931-014-0109-y
Ogrodnik, M. (2021). Cellular aging beyond cellular senescence: markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell 20 (4), e13338. doi:10.1111/acel.13338
Papiris, S. A., Tomos, I. P., Karakatsani, A., Spathis, A., Korbila, I., Analitis, A., et al. (2018). High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine 102, 168–172. doi:10.1016/j.cyto.2017.08.019
Parambil, J. G., Myers, J. L., and Ryu, J. H. (2005). Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 128 (5), 3310–3315. doi:10.1378/chest.128.5.3310
Peng, R., Sridhar, S., Tyagi, G., Phillips, J. E., Garrido, R., Harris, P., et al. (2013). Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for “active” disease. PloS one 8 (4), e59348. doi:10.1371/journal.pone.0059348
Peyser, R., MacDonnell, S., Gao, Y., Cheng, L., Kim, Y., Kaplan, T., et al. (2019). Defining the activated fibroblast population in lung fibrosis using single-cell sequencing. Am. J. Respir. cell Mol. Biol. 61 (1), 74–85. doi:10.1165/rcmb.2018-0313OC
Phan, T. H. G., Paliogiannis, P., Nasrallah, G. K., Giordo, R., Eid, A. H., Fois, A. G., et al. (2021). Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell Mol. Life Sci. 78 (5), 2031–2057. doi:10.1007/s00018-020-03693-7
Piccinini, A. M., and Midwood, K. S. (2010). DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 672395. doi:10.1155/2010/672395
Ptasinski, V. A., Stegmayr, J., Belvisi, M. G., Wagner, D. E., and Murray, L. A. (2021). Targeting alveolar repair in idiopathic pulmonary fibrosis. Am. J. Respir. cell Mol. Biol. 65 (4), 347–365. doi:10.1165/rcmb.2020-0476TR
Qiu, H., Weng, D., Chen, T., Shen, L., Chen, S. S., Wei, Y. R., et al. (2017). Stimulator of interferon genes deficiency in acute exacerbation of idiopathic pulmonary fibrosis. Front. Immunol. 8, 1756. doi:10.3389/fimmu.2017.01756
Qiu, M., Chen, Y., and Ye, Q. (2018). Risk factors for acute exacerbation of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Clin. Respir. J. 12 (3), 1084–1092. doi:10.1111/crj.12631
Raghu, G., Remy-Jardin, M., Myers, J. L., Richeldi, L., Ryerson, C. J., Lederer, D. J., et al. (2018). Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. care Med. 198 (5), e44–e68. doi:10.1164/rccm.201807-1255ST
Raghu, G., Remy-Jardin, M., Richeldi, L., Thomson, C. C., Inoue, Y., Johkoh, T., et al. (2022). Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. care Med. 205 (9), e18–e47. doi:10.1164/rccm.202202-0399ST
Read, A., and Schröder, M. (2021). The unfolded protein response: an overview. Biol. (Basel). 10 (5), 384. doi:10.3390/biology10050384
Richeldi, L., Collard, H. R., and Jones, M. G. (2017). Idiopathic pulmonary fibrosis. Lancet 389 (10082), 1941–1952. doi:10.1016/S0140-6736(17)30866-8
Ryerson, C. J., and Collard, H. R. (2014). Acute exacerbations complicating interstitial lung disease. Curr. Opin. Pulm. Med. 20 (5), 436–441. doi:10.1097/MCP.0000000000000073
Ryerson, C. J., Cottin, V., Brown, K. K., and Collard, H. R. (2015). Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur. Respir. J. 46 (2), 512–520. doi:10.1183/13993003.00419-2015
Saliu, F., Rizzo, G., Bragonzi, A., Cariani, L., Cirillo, D. M., Colombo, C., et al. (2021). Chronic infection by nontypeable Haemophilus influenzae fuels airway inflammation. ERJ Open Res. 7 (1). doi:10.1183/23120541.00614-2020
Sauler, M., Bazan, I. S., and Lee, P. J. (2019). Cell death in the lung: the apoptosis-necroptosis Axis. Annu. Rev. Physiol. 81, 375–402. doi:10.1146/annurev-physiol-020518-114320
Savin, I. A., Zenkova, M. A., and Sen'kova, A. V. (2022). Pulmonary fibrosis as a result of acute lung inflammation: molecular mechanisms, relevant in vivo models, prognostic and therapeutic approaches. Int. J. Mol. Sci. 23 (23), 14959. doi:10.3390/ijms232314959
Schafer, M. J., White, T. A., Iijima, K., Haak, A. J., Ligresti, G., Atkinson, E. J., et al. (2017). Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 8, 14532. doi:10.1038/ncomms14532
Schupp, J. C., Binder, H., Jäger, B., Cillis, G., Zissel, G., Müller-Quernheim, J., et al. (2015). Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PloS one 10 (1), e0116775. doi:10.1371/journal.pone.0116775
Senoo, S., Taniguchi, A., Itano, J., Oda, N., Morichika, D., Fujii, U., et al. (2021). Essential role of IL-23 in the development of acute exacerbation of pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 321 (5), L925–L940. doi:10.1152/ajplung.00582.2020
Shore, G. C., Papa, F. R., and Oakes, S. A. (2011). Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 23 (2), 143–149. doi:10.1016/j.ceb.2010.11.003
Stoolman, J. S., Vannella, K. M., Coomes, S. M., Wilke, C. A., Sisson, T. H., Toews, G. B., et al. (2011). Latent infection by γherpesvirus stimulates profibrotic mediator release from multiple cell types. Am. J. Physiol. Lung Cell Mol. Physiol. 300 (2), L274–L285. doi:10.1152/ajplung.00028.2010
Suresh, M. V., Balijepalli, S., Solanki, S., Aktay, S., Choudhary, K., Shah, Y. M., et al. (2023). Hypoxia-inducible factor 1α and its role in lung injury: adaptive or maladaptive. Inflammation 46 (2), 491–508. doi:10.1007/s10753-022-01769-z
Tanaka, K., Enomoto, N., Hozumi, H., Isayama, T., Naoi, H., Aono, Y., et al. (2021). Serum S100A8 and S100A9 as prognostic biomarkers in acute exacerbation of idiopathic pulmonary fibrosis. Respir. Investig. 59 (6), 827–836. doi:10.1016/j.resinv.2021.05.008
Tanaka, T., Narazaki, M., and Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6 (10), a016295. doi:10.1101/cshperspect.a016295
Tashiro, J., Rubio, G. A., Limper, A. H., Williams, K., Elliot, S. J., Ninou, I., et al. (2017). Exploring animal models that resemble idiopathic pulmonary fibrosis. Front. Med. 4, 118. doi:10.3389/fmed.2017.00118
Thomas, R., Qiao, S., and Yang, X. (2023). Th17/Treg imbalance: implications in lung inflammatory diseases. Int. J. Mol. Sci. 24 (5), 4865. doi:10.3390/ijms24054865
Trethewey, S. P., and Walters, G. I. (2018). The role of occupational and environmental exposures in the pathogenesis of idiopathic pulmonary fibrosis: a narrative literature review. Med. Kaunas. 54 (6), 108. doi:10.3390/medicina54060108
Tseng, Y. H., Chen, I. C., Li, W. C., and Hsu, J. H. (2023). Regulatory cues in pulmonary fibrosis-with emphasis on the AIM2 inflammasome. Int. J. Mol. Sci. 24 (13), 10876. doi:10.3390/ijms241310876
Upagupta, C., Shimbori, C., Alsilmi, R., and Kolb, M. (2018). Matrix abnormalities in pulmonary fibrosis. Eur. Respir. Rev. official J. Eur. Respir. Soc. 27 (148), 180033. doi:10.1183/16000617.0033-2018
Vannella, K. M., and Moore, B. B. (2008). Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogenes. Tissue Repair 1 (1), 2. doi:10.1186/1755-1536-1-2
Vázquez, J. M., Dobaño, J. M., Barcala, F. J., and Cuadrado, L. V. (2011). Acute exacerbation of idiopathic pulmonary fibrosis. Med. Clin. Barc. 136 (9), 403–407. doi:10.1016/j.medcli.2009.12.015
Wang, F., Ma, J., Wang, J., Chen, M., Xia, H., Yao, S., et al. (2022). SIRT1 ameliorated septic associated-lung injury and macrophages apoptosis via inhibiting endoplasmic reticulum stress. Cell Signal 97, 110398. doi:10.1016/j.cellsig.2022.110398
Wang, Y., Wang, L., Ma, S., Cheng, L., and Yu, G. (2024). Repair and regeneration of the alveolar epithelium in lung injury. Faseb J. 38 (8), e23612. doi:10.1096/fj.202400088R
Waters, D. W., Blokland, K. E. C., Pathinayake, P. S., Burgess, J. K., Mutsaers, S. E., Prele, C. M., et al. (2018). Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 315 (2), L162–L172. doi:10.1152/ajplung.00037.2018
Wei, Y. R., Qiu, H., Wu, Q., Du, Y. K., Yin, Z. F., Chen, S. S., et al. (2016). Establishment of the mouse model of acute exacerbation of idiopathic pulmonary fibrosis. Exp. Lung Res. 42 (2), 75–86. doi:10.3109/01902148.2016.1144835
Weng, D., Chen, X. Q., Qiu, H., Zhang, Y., Li, Q. H., Zhao, M. M., et al. (2019). The role of infection in acute exacerbation of idiopathic pulmonary fibrosis. Mediat. Inflamm. 2019, 5160694. doi:10.1155/2019/5160694
Wootton, S. C., Kim, D. S., Kondoh, Y., Chen, E., Lee, J. S., Song, J. W., et al. (2011). Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. care Med. 183 (12), 1698–1702. doi:10.1164/rccm.201010-1752OC
Xu, X., Lai, Y., and Hua, Z. C. (2019). Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 39 (1). doi:10.1042/BSR20180992
Xu, Y., Lan, P., and Wang, T. (2023). The role of immune cells in the pathogenesis of idiopathic pulmonary fibrosis. Med. Kaunas. 59 (11), 1984. doi:10.3390/medicina59111984
Yang, L., Lin, Z., Wang, Y., Li, C., Xu, W., Li, Q., et al. (2018). Nickle(II) ions exacerbate bleomycin-induced pulmonary inflammation and fibrosis by activating the ROS/Akt signaling pathway. Environ. Sci. Pollut. Res. Int. 25 (5), 4406–4418. doi:10.1007/s11356-017-0525-x
Yao, C., Guan, X., Carraro, G., Parimon, T., Liu, X., Huang, G., et al. (2021). Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am. J. Respir. Crit. care Med. 203 (6), 707–717. doi:10.1164/rccm.202004-1274OC
Yao, L., Yang, L., Yuan, Q., Yan, C., Dong, C., Meng, C., et al. (2016). Sources apportionment of PM2.5 in a background site in the North China Plain. Sci. Total Environ. 541, 590–598. doi:10.1016/j.scitotenv.2015.09.123
Ye, X., Zhang, M., Gu, H., Liu, M., Zhao, Y., Shi, Y., et al. (2023). Animal models of acute exacerbation of pulmonary fibrosis. Respir. Res. 24 (1), 296. doi:10.1186/s12931-023-02595-z
Yegen, C. H., Haine, L., Da Costa Ferreira, K., Marchant, D., Bernaudin, J. F., Planès, C., et al. (2022). A new model of acute exacerbation of experimental pulmonary fibrosis in mice. Cells 11 (21), 3379. doi:10.3390/cells11213379
Yu, H., Lin, L., Zhang, Z., Zhang, H., and Hu, H. (2020). Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target Ther. 5 (1), 209. doi:10.1038/s41392-020-00312-6
Zelová, H., and Hošek, J. (2013). TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res. 62 (7), 641–651. doi:10.1007/s00011-013-0633-0
Keywords: animal model, pulmonary fibrosis, acute exacerbation, mechanisms, systematic review
Citation: Chen K, Zhang H, Yao Z, Tao S and Ma Q (2025) Current advancements in the mechanisms and animal models of acute exacerbation of pulmonary fibrosis: a systematic review. Front. Pharmacol. 16:1501085. doi: 10.3389/fphar.2025.1501085
Received: 24 September 2024; Accepted: 23 May 2025;
Published: 30 May 2025.
Edited by:
Jacobo Sellares Torres, Pneumology and Respiratory Allergy Service, Hospital Clinic of Barcelona, SpainReviewed by:
Benoit Allard, Université de la Réunion, FranceMarina A. Zenkova, Institute of Chemical Biology and Fundamental Medicine (RAS), Russia
Copyright © 2025 Chen, Zhang, Yao, Tao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailong Zhang, emhhbmdoYWlsb25nNkAxMjYuY29t
 Kai Chen
Kai Chen Hailong Zhang
Hailong Zhang Zhaoxu Yao
Zhaoxu Yao Siyu Tao1
Siyu Tao1