- Department of Pharmacology and Toxicology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
Background: Alcohol use disorder (AUD) represents a significant global health burden, characterized by high relapse rates and limited treatment options. Valproic acid, primarily used as an anticonvulsant and mood stabilizer, has been suggested as a potential therapeutic agent for AUD, particularly in patients with coexisting psychiatric conditions. This study systematically analyses clinical trials from ClinicalTrials.gov to evaluate the efficacy of valproic acid in treating AUD.
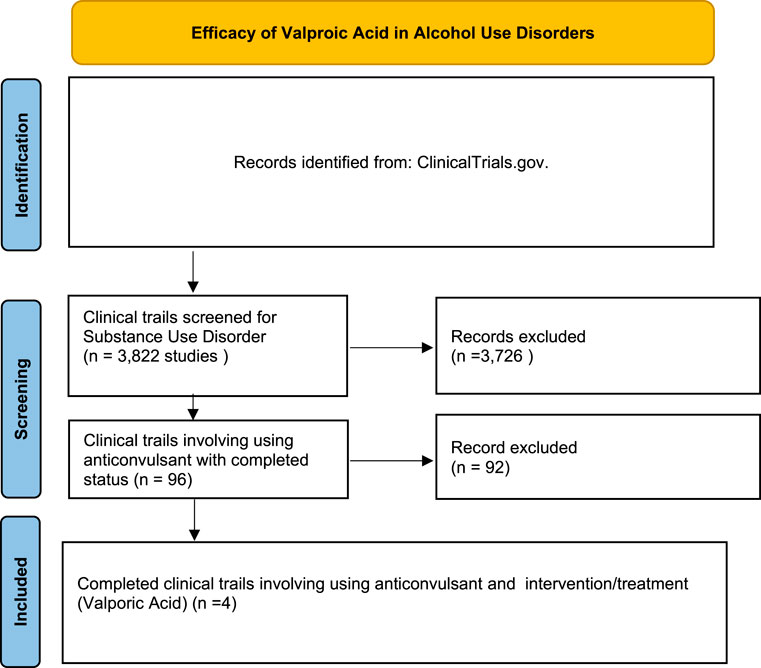
Methods: A systematic search of ClinicalTrials.gov was conducted to identify clinical trials involving valproic acid in the management of substance use disorder (SUD). A total of 3,822 studies related to SUD were initially identified. Screening for anticonvulsant use narrowed this to 96 trials, and four completed studies specifically involving valproic acid and AUD were included in the final analysis. Key outcomes related to relapse rates, substance use reduction, mood stabilization, and withdrawal symptoms were examined.
Results: The included studies focused on various conditions, including alcohol dependence, bipolar disorder with substance abuse, traumatic brain injury with alcohol use, and medication-overuse headache. Valproic acid demonstrated potential benefits in reducing alcohol consumption, stabilizing mood, and managing withdrawal symptoms in specific subpopulations. However, relapse rates remained high in some trials, indicating limited long-term efficacy. Secondary outcomes showed improvements in psychiatric symptoms, though adverse effects such as sedation and gastrointestinal disturbances were noted.
Conclusion: Valproic acid shows potential as a therapeutic option for managing AUD, particularly in individuals with coexisting psychiatric conditions or complex clinical profiles. While the drug showed some efficacy in reducing substance use and stabilizing mood, the overall impact on long-term abstinence remains uncertain. Further research is needed to better define the role of valproic acid in AUD treatment and to identify patient populations that may benefit most from its use.
Introduction
Substance use disorder (SUD) represents a critical global health challenge with important clinical, social, and economic implications (Taylor et al., 2023; Bush et al., 2016). According to the World Drug Report 2021 by UNODC, an estimated 275 million people aged 15–64, or 1 in 18 globally, used substances in 2023, with 13% experiencing SUD (WHO, 2023). SUD is frequently associated with high rates of psychiatric and somatic comorbidities, complicating treatment and reducing the quality of life for affected individuals (Gardvik et al., 2021; Schuckit, 2006; Skarstein et al., 2023). The economic burden is significant, with annual treatment costs for SUD in hospitals in the United States alone reaching US$13.2 billion (Peterson et al., 2021). Despite the significant public health impact, current treatment strategies for SUD remain limited and often ineffective, resulting in poor long-term outcomes and high relapse rates.
Pharmacological interventions are important in the management of SUD, particularly for specific substances such as alcohol and opioids (Han et al., 2021; Ray et al., 2020; Bell and Strang, 2020). For example, medications like naltrexone, acamprosate, and methadone have been widely utilized to manage alcohol and opioid use disorders (Bell and Strang, 2020; Edinoff et al., 2021; Kirchoff et al., 2021; Maisel et al., 2013; Soyka, 2015). However, the efficacy of these treatments differs among individuals, and relapse rates are still high. Therefore, recent studies have shown that medications such as valproic acid could have potential for the treatment of AUDs (Romão et al., 2022; Ahmed et al., 2019; Singh et al., 2021). Valproic acid, a mood stabilizer commonly used in the treatment of epilepsy and bipolar disorder, exerts its effects through multiple mechanisms, including the modulation of gamma-aminobutyric acid (GABA) levels, inhibition of voltage-gated sodium channels, and suppression of histone deacetylases (HDACs) (Rahman et al., 2020; Tomson et al., 2016).
Emerging evidence suggests that valproic acid may offer therapeutic potential for certain subpopulations of individuals with alcohol use disorder (AUD), particularly those with AUD and comorbid psychiatric conditions (Salloum et al., 2005; Weiss et al., 2023; Carli et al., 2023). Clinical trials investigating the efficacy of valproic acid as a treatment for AUD have produced mixed but promising results, highlighting the need for further exploration. For instance, studies have demonstrated that valproic acid may reduce alcohol consumption and improve mood stability in patients with AUD and co-occurring mood disorders, suggesting a dual benefit in this challenging population (Litten et al., 2016). Moreover, valproic acid’s potential to modulate the neural circuits involved in reward processing and impulse control positions it as a candidate for addressing some of the core neurobiological dysfunctions observed in AUDs in animals (Al Ameri et al., 2014; Bass et al., 2020).
This review systematically evaluates the efficacy of valproic acid as a treatment option for AUD, particularly in populations with coexisting psychiatric conditions. Given the high relapse rates and limited effectiveness of existing treatments for AUDs, this analysis aimed to assess the clinical potential of valproic acid by reviewing completed trials from ClinicalTrials.gov. Thus, this review helps to understand valproic acid’s role in managing AUDs and identifies patient subgroups that could benefit most from its therapeutic effects.
Methods
A systematic analysis was conducted to identify clinical trials evaluating the use of valproic acid in SUD. The primary data source was ClinicalTrials.gov, a comprehensive registry of clinical studies. The search strategy was designed to capture all relevant trials involving anticonvulsants in the context of SUD.
Study identification and screening
Records Identified: A total of 3,822 studies were initially identified from ClinicalTrials.gov by searching for trials related to SUD on September 2024. Screening for Anticonvulsant Use: From the identified records, 96 studies involved the use of anticonvulsant medications in the treatment of SUD. Selection of Valproic Acid Trials: Further screening identified four completed clinical trials that specifically investigated the use of valproic acid as an intervention for AUD. These trials met the inclusion criteria of focusing on completed studies with reported outcomes.
Inclusion and exclusion criteria
The inclusion criteria were: (1) studies investigating the use of valproic acid or its derivatives as a primary intervention, (2) trials targeting populations with a diagnosis of SUD or related conditions such as alcohol dependence, bipolar disorder with substance abuse, or withdrawal syndromes, and (3) trials that were completed and had reported primary and/or secondary outcomes. Studies were excluded if they were ongoing, withdrawn, unknown, or did not specifically assess valproic acid as part of the intervention.
Outcome measures and data extraction
Primary outcomes included efficacy measures such as relapse rates, changes in substance use patterns, and improvements in mood or psychiatric symptoms. Secondary outcomes focused on withdrawal symptoms, mood stabilization, and any reported adverse effects associated with valproic acid treatment. Data were extracted from the identified trials, including information on study titles, statuses, conditions treated, specific interventions, and reported outcomes.
Results
A systematic search of ClinicalTrials.gov showed 3,822 clinical trials related to SUD. Of these, 96 studies involved the use of anticonvulsant medications. Further screening identified four completed trials specifically investigating valproic acid as an intervention for AUDs. The included studies varied in populations, interventions, and primary outcomes, focusing on conditions such as alcohol dependence, bipolar disorder with substance abuse, traumatic brain injury (TBI) with alcohol use, and medication-overuse headache. The study selection process is detailed in the PRISMA flow diagram (Figure 1), and the characteristics of each study are summarized in Table 1.
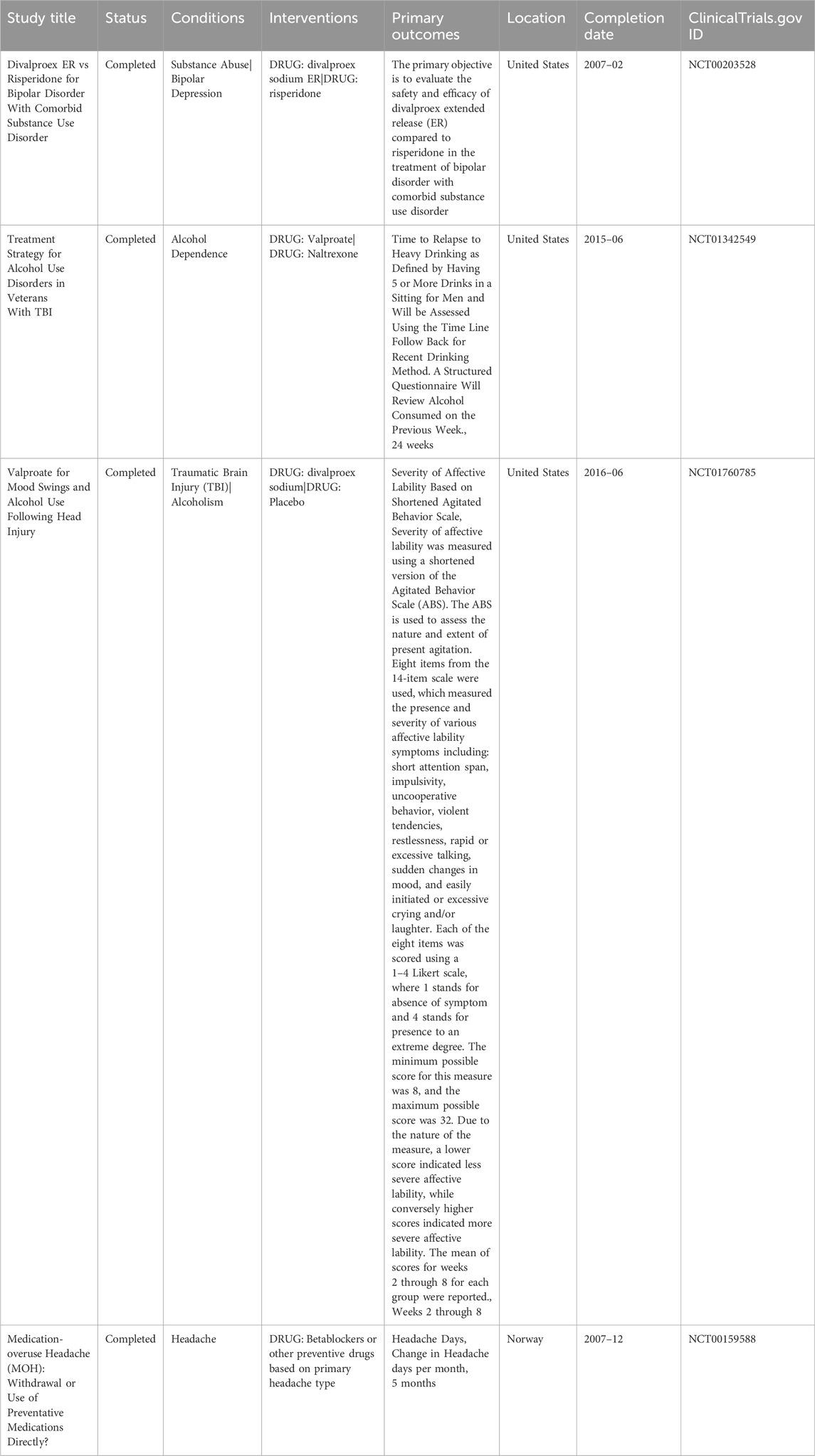
Table 1. Summarizing the key characteristics of the clinical trials, including study titles, status, conditions, interventions, primary outcomes, and completion dates.
The primary outcomes assessed across the trials included relapse rates, reductions in substance use, and improvements in mood or psychiatric symptoms. One trial compared divalproex extended release (ER) with risperidone in patients with bipolar disorder and substance abuse, reporting reductions in mood instability and substance use. Another study examined valproate in veterans with alcohol dependence and comorbid psychiatric conditions, which reported a decrease in episodes of heavy drinking and improvements in mood stability. A separate trial investigated valproate use in individuals with TBI and alcohol use, reporting reductions in mood swings and alcohol consumption. The medication-overuse headache (MOH) trial primarily investigated headache frequency but also assessed aspects of withdrawal symptom management.
Secondary outcomes across the trials included additional assessments of psychiatric symptoms, withdrawal severity, and adverse effects. Reported adverse effects included weight gain, sedation, and gastrointestinal symptoms. The risk of bias was assessed as low to moderate, with most studies reporting adequate randomization and blinding procedures. However, some trials had limitations related to allocation concealment and participant withdraw, which could influence the reliability of findings. These results provide insights into the efficacy and safety of valproic acid across different AUD subpopulations, though variations in study design and outcome measures limit direct comparisons.
Clinical trials outcomes
The clinical trial (NCT00203528) compared divalproex ER to risperidone in 30 adults with bipolar disorder and comorbid substance use disorder in a 12-week, randomized, double-blind Phase 4 study. Participants underwent a washout period before randomization and were monitored biweekly using the CGI, GAF, and substance use assessments. Divalproex was titrated to therapeutic serum levels (80–100 mcg/mL). Although formal results were not posted, the study reported improvements in mood stability and reductions in substance use in the divalproex group, indicating potential dual benefits in this population (Le Fauve et al., 2004).
Another clinical trial (NCT01342549) was a randomized, triple-masked Phase 3 trial assessing the effectiveness of valproate versus naltrexone in 62 veterans with alcohol dependence and comorbid TBI. Conducted over 24 weeks, the trial measured time to relapse to heavy drinking, defined as consuming five or more drinks in one sitting, using the Time Line Follow Back method. Participants received either valproate (titrated up to 60 mg/kg/day) or naltrexone (50 mg/day). Results showed that valproate contributed to delayed relapse and improved mood stabilization, supporting its potential use in complex cases of AUD with psychiatric and neurological comorbidities.
The third clinical trial (NCT01760785) evaluated the effects of divalproex sodium on affective lability and alcohol use in 50 adults with a history of TBI and co-occurring alcohol misuse. This randomized, quadruple-blind trial compared divalproex sodium (750–1,250 mg/day) to placebo over an 8-week period. The primary outcome was the severity of affective lability, measured using a shortened version of the Agitated Behavior Scale (ABS). Secondary outcomes included frequency of alcohol use, assessed weekly via the Timeline Follow back method and breath alcohol testing. Results showed that valproate significantly reduced affective lability scores, and many participants also demonstrated a decrease in alcohol consumption, highlighting valproate’s potential in treating neuropsychiatric symptoms that contribute to AUD following TBI.
The fourth clinical trial (NCT00159588) was a randomized, open-label, multicenter study conducted in Norway, assessing different treatment strategies for medication-overuse headache (MOH) in 64 patients. Participants were assigned to one of three arms: abrupt withdrawal of overused medications, immediate initiation of preventive medications (including valproate), or no specific treatment (control). The primary outcome was change in headache days per month over a 5-month period. Valproate was among the preventive agents used, depending on the patient’s headache type. Although not specific to AUD, the study included valproate’s role in withdrawal management, offering indirect insights into its potential for treating alcohol-related withdrawal symptoms (Hagen et al., 2009; Hagen and Stovner, 2011).
Discussion
The systematic analysis of clinical trials from ClinicalTrials.gov highlights the potential role of valproic acid as a therapeutic agent in managing AUD. The findings from the five included trials provide excellent understanding of the drug’s efficacy across different AUD populations, offering valuable insights into its potential benefits and limitations. One of the key findings from this analysis is that valproic acid may be particularly effective in subpopulations with coexisting psychiatric conditions, such as bipolar disorder or TBI. In these contexts, valproic acid’s mood-stabilizing properties can offer dual benefits, simultaneously addressing mood instability and reducing substance use (Salloum et al., 2005; Perugi et al., 2010). For instance, the trial comparing divalproex ER with risperidone in patients with bipolar disorder and substance abuse demonstrated that valproic acid effectively reduced mood instability and substance consumption. This suggests that the drug’s effects on GABAergic modulation.
Valproic acid’s potential therapeutic effects in patients with AUD are attributed to its multifaceted mechanism of action, which targets several neurobiological pathways implicated in addiction (Tursunov et al., 2023; Koijam et al., 2024; Lum et al., 2006). The primary pharmacological actions of valproic acid include enhancing gamma-aminobutyric acid (GABA) neurotransmission, inhibiting voltage-gated sodium channels, and modulating histone deacetylase (HDAC) activity (Mishra et al., 2021). These actions collectively contribute to its mood-stabilizing, neuroprotective, and anti-seizure properties, which may also play a role in reducing substance use and managing withdrawal symptoms (Farrokh et al., 2021; Guirguis et al., 2017). One of the key mechanisms through which valproic acid could assist individuals with AUD is through the enhancement of GABAergic activity. GABA is the primary inhibitory neurotransmitter in the central nervous system and plays a critical role in regulating neuronal excitability, stress responses, and reward pathways (Rudolph, 2022). By increasing GABA levels in the brain, valproic acid helps to dampen hyperactivity in the neural circuits associated with craving and impulsivity, which are core features of AUD (Dharavath et al., 2023). This modulation of the GABAergic system may help to reduce the reinforcing effects of addictive substances and alleviate withdrawal symptoms, thus supporting abstinence.
Valproic acid also exhibits epigenetic effects through its inhibition of histone deacetylases (HDACs), enzymes that regulate gene expression by altering chromatin structure (Sixto-Lopez et al., 2020). HDAC inhibition by valproic acid can lead to changes in the expression of genes involved in neural plasticity, stress responses, and reward processing, which are often dysregulated in AUD (Al Ameri et al., 2014). This epigenetic modulation may help to reverse some of the long-term neurobiological changes induced by alcohol, potentially restoring more normal functioning of the brain’s reward system and reducing the propensity for relapse. Furthermore, valproic acid’s impact on dopamine and glutamate neurotransmission, two key systems involved in addiction, may contribute to its therapeutic potential in AUD (Chen et al., 2006; Ueda and Willmore, 2000). By modulating these neurotransmitter systems, valproic acid can attenuate the dysregulated reward and stress circuits that drive compulsive alcohol use. This multi-target approach may make valproic acid particularly useful in managing AUD where traditional monotherapies have limited efficacy.
The use of valproic acid in the treatment of AUD and SUD requires a patient-centred approach (Celik et al., 2024). Patients with AUD frequently present with pre-existing hepatic dysfunction, necessitating baseline and routine liver function tests to monitor potential hepatotoxicity (Caputo et al., 2019). Additionally, pre-treatment screening for pancreatitis and metabolic disturbances is essential, as valproic acid has been associated with an increased risk of these conditions (Nanau and Neuman, 2013; Chapman et al., 2001). Ongoing monitoring is also required for neurological, cognitive, gastrointestinal, cardiovascular, and endocrine-related adverse effects, which have been reported in patients receiving valproic acid therapy (Singh et al., 2021). Given the complexity of valproic acid metabolism, drug interactions via CYP450 and UGT pathways should be carefully considered, particularly in patients with concurrent medication use (Shnayder et al., 2023).
This study has several limitations. The reliance on ClinicalTrials.gov only may have excluded unregistered trials, particularly those outside the United States, limiting the generalizability of the findings. While one international study (Norway) was included, most trials were U.S.-based, and the impact of unpublished data remains unknown. The heterogeneity in study populations and primary outcomes, ranging from withdrawal management to mood stabilization, restricted meta-analysis and effect size calculations, requiring a narrative synthesis instead. Variations in study design, follow-up duration, and outcome reporting may also affect reliability. While most trials used randomization and blinding, some had limitations in allocation concealment and participant withdrawal, introducing potential bias. Future research should expand on registered trials to improve external validity.
Conclusion
Valproic acid shows potential as a treatment option for AUD, particularly in individuals with coexisting psychiatric conditions, but its role remains adjunctive rather than being prescribed as a monotherapy. The drug’s mood-stabilizing and neuroprotective properties offer a valuable addition to the existing treatment for AUD. However, its efficacy appears contingent on individual patient factors and the context of use. The findings underscore the need for personalized treatment strategies and highlight the importance of further research to explain the mechanisms through which valproic acid may benefit specific AUD subgroups. Future studies should aim to optimize its therapeutic use, assess its long-term outcomes, and explore potential combination therapies that could enhance its effectiveness in managing AUD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
FA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1503035/full#supplementary-material
References
Ahmed, S., Bachu, R., Kotapati, P., Adnan, M., Ahmed, R., Farooq, U., et al. (2019). Use of gabapentin in the treatment of substance use and psychiatric disorders: a systematic review. Front. Psychiatry 10, 228. doi:10.3389/fpsyt.2019.00228
Al Ameri, M., Al Mansouri, S., Al Maamari, A., and Bahi, A. (2014). The histone deacetylase (HDAC) inhibitor valproic acid reduces ethanol consumption and ethanol-conditioned place preference in rats. Brain Res. 1583, 122–131. doi:10.1016/j.brainres.2014.07.051
Bass, J. S., Tuo, A. H., Ton, L. T., Jankovic, M. J., Kapadia, P. K., Schirmer, C., et al. (2020). On the digital psychopharmacology of valproic acid in mice. Front. Neurosci. 14, 594612. doi:10.3389/fnins.2020.594612
Bell, J., and Strang, J. (2020). Medication treatment of opioid use disorder. Biol. Psychiatry 87 (1), 82–88. doi:10.1016/j.biopsych.2019.06.020
Caputo, F., Domenicali, M., and Bernardi, M. (2019). Diagnosis and treatment of alcohol use disorder in patients with end-stage alcoholic liver disease. Hepatology 70 (1), 410–417. doi:10.1002/hep.30358
Carli, M., Weiss, F., Grenno, G., Ponzini, S., Kolachalam, S., Vaglini, F., et al. (2023). Pharmacological strategies for bipolar disorders in acute phases and chronic management with a special focus on lithium, valproic acid, and atypical antipsychotics. Curr. Neuropharmacol. 21 (4), 935–950. doi:10.2174/1570159X21666230224102318
Celik, M., Gold, M. S., and Fuehrlein, B. (2024). A narrative review of current and emerging trends in the treatment of alcohol use disorder. Brain Sci. 14 (3), 294. doi:10.3390/brainsci14030294
Chapman, S. A., Wacksman, G. P., and Patterson, B. D. (2001). Pancreatitis associated with valproic acid: a review of the literature. Pharmacotherapy 21 (12), 1549–1560. doi:10.1592/phco.21.20.1549.34480
Chen, P. S., Peng, G. S., Yang, S., Wu, X., Wang, C. C., Wilson, B., et al. (2006). Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychiatry 11 (12), 1116–1125. doi:10.1038/sj.mp.4001893
Dharavath, R. N., Pina-Leblanc, C., Tang, V. M., Sloan, M. E., Nikolova, Y. S., Pangarov, P., et al. (2023). GABAergic signaling in alcohol use disorder and withdrawal: pathological involvement and therapeutic potential. Front. Neural Circuits 17, 1218737. doi:10.3389/fncir.2023.1218737
Edinoff, A. N., Nix, C. A., Orellana, C. V., StPierre, S. M., Crane, E. A., Bulloch, B. T., et al. (2021). Naltrexone implant for opioid use disorder. Neurol. Int. 14 (1), 49–61. doi:10.3390/neurolint14010004
Farrokh, S., Roels, C., Owusu, K. A., Nelson, S. E., and Cook, A. M. (2021). Alcohol withdrawal syndrome in neurocritical care unit: assessment and treatment challenges. Neurocrit Care 34 (2), 593–607. doi:10.1007/s12028-020-01061-8
Gardvik, K. S., Rygg, M., Torgersen, T., Lydersen, S., and Indredavik, M. S. (2021). Psychiatric morbidity, somatic comorbidity and substance use in an adolescent psychiatric population at 3-year follow-up. Eur. Child. Adolesc. Psychiatry 30 (7), 1095–1112. doi:10.1007/s00787-020-01602-8
Guirguis, E., Richardson, J., Kuhn, T., and Fahmy, A. (2017). Treatment of severe alcohol withdrawal: a focus on adjunctive agents. J. Pharm. Technol. 33 (5), 204–212. doi:10.1177/8755122517714491
Hagen, K., Albretsen, C., Vilming, S. T., Salvesen, R., Grønning, M., Helde, G., et al. (2009). Management of medication overuse headache: 1-year randomized multicentre open-label trial. Cephalalgia 29 (2), 221–232. doi:10.1111/j.1468-2982.2008.01711.x
Hagen, K., and Stovner, L. J. (2011). A randomized controlled trial on medication-overuse headache: outcome after 1 and 4 years. Acta Neurol. Scand. Suppl. 124 (191), 38–43. doi:10.1111/j.1600-0404.2011.01542.x
Han, B., Jones, C. M., Einstein, E. B., Powell, P. A., and Compton, W. M. (2021). Use of medications for alcohol use disorder in the US: results from the 2019 national survey on drug use and health. JAMA Psychiatry 78 (8), 922–924. doi:10.1001/jamapsychiatry.2021.1271
Kirchoff, R. W., Mohammed, N. M., McHugh, J., Markota, M., Kingsley, T., Leung, J., et al. (2021). Naltrexone initiation in the inpatient setting for alcohol use disorder: a systematic review of clinical outcomes. Mayo Clin. Proc. Innov. Qual. Outcomes 5 (2), 495–501. doi:10.1016/j.mayocpiqo.2021.01.013
Koijam, A. S., Singh, K. D., Nameirakpam, B. S., Haobam, R., and Rajashekar, Y. (2024). Drug addiction and treatment: an epigenetic perspective. Biomed. Pharmacother. 170, 115951. doi:10.1016/j.biopha.2023.115951
Le Fauve, C. E., Litten, R. Z., Randall, C. L., Moak, D. H., Salloum, I. M., and Green, A. I. (2004). Pharmacological treatment of alcohol abuse/dependence with psychiatric comorbidity. Alcohol Clin. Exp. Res. 28 (2), 302–312. doi:10.1097/01.alc.0000113413.37910.d7
Litten, R. Z., Wilford, B. B., Falk, D. E., Ryan, M. L., and Fertig, J. B. (2016). Potential medications for the treatment of alcohol use disorder: an evaluation of clinical efficacy and safety. Subst. Abus 37 (2), 286–298. doi:10.1080/08897077.2015.1133472
Lum, E., Gorman, S. K., and Slavik, R. S. (2006). Valproic acid management of acute alcohol withdrawal. Ann. Pharmacother. 40 (3), 441–448. doi:10.1345/aph.1G243
Maisel, N. C., Blodgett, J. C., Wilbourne, P. L., Humphreys, K., and Finney, J. W. (2013). Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108 (2), 275–293. doi:10.1111/j.1360-0443.2012.04054.x
Mishra, M. K., Kukal, S., Paul, P. R., Bora, S., Singh, A., Kukreti, S., et al. (2021). Insights into structural modifications of valproic acid and their pharmacological profile. Molecules 27 (1), 104. doi:10.3390/molecules27010104
Nanau, R. M., and Neuman, M. G. (2013). Adverse drug reactions induced by valproic acid. Clin. Biochem. 46 (15), 1323–1338. doi:10.1016/j.clinbiochem.2013.06.012
Perugi, G., Pacini, M., Lamanna, F., Pani, P. P., Deltito, J., Salloum, I. M., et al. (2010). Mood stabilizers in the treatment of substance use disorders. CNS Spectr. 15 (2), 95–109. doi:10.1017/s1092852900027346
Peterson, C., Li, M., Xu, L., Mikosz, C. A., and Luo, F. (2021). Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw. Open 4 (3), e210242. doi:10.1001/jamanetworkopen.2021.0242
Ray, L. A., Meredith, L. R., Kiluk, B. D., Walthers, J., Carroll, K. M., and Magill, M. (2020). Combined pharmacotherapy and cognitive behavioral therapy for adults with alcohol or substance use disorders: a systematic review and meta-analysis. JAMA Netw. Open 3 (6), e208279. doi:10.1001/jamanetworkopen.2020.8279
Romão, J., Gonçalves, M., Ribeiro, M., André, R., Saraiva, R., and Abreu, M. (2022). Growing use of valproic acid in substance use disorders. Eur. Psychiatry 65 (S1), S243–S244. doi:10.1192/j.eurpsy.2022.628
Rudolph, U. (2022). “GABAergic system,” in Encyclopedia of molecular pharmacology (Springer), 679–684.
Salloum, I. M., Cornelius, J. R., Daley, D. C., Kirisci, L., Himmelhoch, J. M., and Thase, M. E. (2005). Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch. Gen. Psychiatry 62 (1), 37–45. doi:10.1001/archpsyc.62.1.37
Schuckit, M. A. (2006). Comorbidity between substance use disorders and psychiatric conditions. Addiction 101 (Suppl. 1), 76–88. doi:10.1111/j.1360-0443.2006.01592.x
Shnayder, N. A., Grechkina, V. V., Khasanova, A. K., Bochanova, E. N., Dontceva, E. A., Petrova, M. M., et al. (2023). Therapeutic and toxic effects of valproic acid metabolites. Metabolites 13 (1), 134. doi:10.3390/metabo13010134
Singh, D., Gupta, S., Verma, I., Morsy, M. A., Nair, A. B., and Ahmed, A. S. F. (2021). Hidden pharmacological activities of valproic acid: a new insight. Biomed. Pharmacother. 142, 112021. doi:10.1016/j.biopha.2021.112021
Sixto-Lopez, Y., Bello, M., and Correa-Basurto, J. (2020). Exploring the inhibitory activity of valproic acid against the HDAC family using an MMGBSA approach. J. Comput. Aided Mol. Des. 34 (8), 857–878. doi:10.1007/s10822-020-00304-2
Skarstein, S., Lien, L., and Abebe, D. S. (2023). The burden of somatic diseases among people with alcohol- and drug use disorders are influenced by mental illness and low socioeconomic status. A registry-based cohort study in Norway. J. Psychosom. Res. 165, 111137. doi:10.1016/j.jpsychores.2022.111137
Soyka, M. (2015). Alcohol use disorders in opioid maintenance therapy: prevalence, clinical correlates and treatment. Eur. Addict. Res. 21 (2), 78–87. doi:10.1159/000363232
Taylor, J. L., Wakeman, S. E., Walley, A. Y., and Kehoe, L. G. (2023). Substance use disorder bridge clinics: models, evidence, and future directions. Addict. Sci. Clin. Pract. 18 (1), 23. doi:10.1186/s13722-023-00365-2
Tomson, T., Battino, D., and Perucca, E. (2016). The remarkable story of valproic acid. Lancet Neurol. 15 (2), 141. doi:10.1016/S1474-4422(15)00398-1
Tursunov, A., Vasilyev, D., and Nalivaeva, N. (2023). Molecular mechanisms of valproic acid action on signalling systems and brain functions. J. Evol. Biochem. Physiology 59 (5), 1740–1755. doi:10.1134/s0022093023050228
Ueda, Y., and Willmore, L. J. (2000). Molecular regulation of glutamate and GABA transporter proteins by valproic acid in rat hippocampus during epileptogenesis. Exp. Brain Res. 133 (3), 334–339. doi:10.1007/s002210000443
Weiss, F., Tidona, S., Carli, M., Perugi, G., and Scarselli, M. (2023). Triple diagnosis of attention-deficit/hyperactivity disorder with co-existing bipolar and alcohol use disorders: clinical aspects and pharmacological treatments. Curr. Neuropharmacol. 21 (7), 1467–1476. doi:10.2174/1570159X20666220830154002
Keywords: alcohol use disorders, valproic acid, clinical trials, systematic analysis, alcohol dependence, anticonvulsant therapy
Citation: Alshehri FS (2025) Evaluating the efficacy of valproic acid in alcohol use disorder: a systematic analysis of clinical trials from ClinicalTrials.gov. Front. Pharmacol. 16:1503035. doi: 10.3389/fphar.2025.1503035
Received: 27 September 2024; Accepted: 07 April 2025;
Published: 25 April 2025.
Edited by:
Ilya Blokhin, University of Pittsburgh, United StatesReviewed by:
Robert L. Barkin, Rush University Medical Center, United StatesLoay Alrojolah, Yale University, United States
Copyright © 2025 Alshehri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahad S. Alshehri, ZnNzaGVocmlAdXF1LmVkdS5zYQ==
 Fahad S. Alshehri
Fahad S. Alshehri