- 1Department of Clinical Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Shiraz Transplant Center, Abu-Ali Sina Hospital, Shiraz University of Medical Sciences, Shiraz, Iran
- 3Department of Pharmaceutics, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
- 4Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran
- 6Shiraz Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Background: Vancomycin is a glycopeptide antibiotic of choice for treating serious Gram-positive bacterial infections, including methicillin-resistant Staphylococcus aureus (MRSA). However, its therapeutic efficacy and risk of nephrotoxicity are closely related to maintaining specific serum concentration levels. Liver transplant recipients (LTRs) require precise therapeutic drug monitoring (TDM) due to altered pharmacokinetics. This study compares the accuracy and precision of two vancomycin measurement methods—chemiluminescent microparticle immunoassay (CMIA) and high-performance liquid chromatography (HPLC) in LTRs.
Methods: The cross-sectional study was conducted over 11 months at the Abu-Ali Sina Solid Organ Transplant Hospital in Shiraz, Iran. The study included 34 adult LTRs on vancomycin treatment, excluding those with hypersensitivity, chronic kidney disease, burn injuries, or receiving phenytoin. Blood samples were collected at different intervals post-vancomycin administration and analyzed using both CMIA and HPLC methods.
Results: HPLC demonstrated superior accuracy and precision in measuring vancomycin concentrations, particularly in identifying patients with vancomycin-induced nephrotoxicity. Significantly higher trough (p-value: 0.026) and intermediate (p-value: 0.49) concentrations were detected by HPLC in patients experiencing nephrotoxicity, whereas CMIA did not show significant differences between groups. Pharmacokinetic variables such as half-life (p-value: 0.024) and AUC (p-value:0.037), measured by HPLC, were significantly different between LTRs with and without nephrotoxicity, which was not observed with CMIA.
Conclusion: HPLC is more sensitive and reliable than CMIA for measuring vancomycin levels in LTRs, which is critical for optimizing vancomycin therapy and preventing adverse effects. The research suggests that HPLC should be the preferred method for vancomycin TDM in LTRs and further multicenter studies are recommended to validate these results.
1 Introduction
Vancomycin is a glycopeptide antibiotic that is commonly used in the treatment of serious infections caused by Gram-positive bacteria. It is a drug of choice in the management of methicillin-resistant Staphylococcus aureus (MRSA) infections, and its therapeutic efficacy is highly dependent on achieving appropriate serum concentrations (Pai et al., 2014). The Chinese Pharmacological Society recommends maintaining a serum trough concentration of vancomycin between 10 and 15 mg/L for adult patients, while a higher range of 10–20 mg/L is advised for serious MRSA infections. Clinical studies indicate that insufficient vancomycin levels can contribute to the emergence of resistance in S. aureus strains. Conversely, it is crucial to recognize that achieving a serum trough concentration of 15 mg/L or higher may increase the risk of nephrotoxicity (Ye et al., 2016). Moreover, trough levels alone may not provide a complete picture of the drug’s efficacy or potential for toxicity. Therefore, according to pharmacokinetic (PK)/pharmacodynamic (PD) theory, the better evaluation indicator for therapeutic drug monitoring (TDM) of vancomycin is the ratio of area under the concentration-time curve (AUC) to minimum inhibitory concentration (MIC), which takes into account the entire drug exposure over a dosing interval. To enhance clinical outcomes in the management of invasive MRSA infections, the latest guidelines from the American Society of Health-System Pharmacists (ASHP), Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) recommended transitioning from trough-based to AUC-guided vancomycin dosing, targeting an AUC/MIC ratio of 400–600 mg h/L based on the MIC of less than 1 mg/L for optimal efficacy against MRSA infections. A loading dose of 20–35 mg/kg is advised, with subsequent dosing adjusted based on renal function (Chen et al., 2022; Rybak et al., 2020; Kaushik et al., 2024). In addition, vancomycin presents a wide interindividual pharmacokinetic variation, a narrow therapeutic index, and the potential for various adverse effects, including nephrotoxicity (Matsumoto et al., 2022). Therefore, TDM of vancomycin and individualized dosing regimens based on patient-specific factors are highly recommended for increasing treatment response while preventing adverse effects (Rybak et al., 2020).
Liver transplant-receiving (LTR) patients are a special population that may require vancomycin therapy due to their immunosuppressed state and increased risk of infections (Morales Junior et al., 2023). However, they also have altered the pharmacokinetics and pharmacodynamics of drugs due to their liver dysfunction, post-operative complications, and polypharmacy (Morales Junior et al., 2023; Righi, 2018). Therefore, accurate TDM of vancomycin is crucial in this population to achieve optimal therapeutic outcomes and avoid toxicity. Studies have shown that TDM of vancomycin in LTRs can improve clinical outcomes, including reducing the incidence of nephrotoxicity and improving bacterial clearance. Additionally, TDM can help prevent the development of antibiotic resistance, being a global challenge (Shafiekhani et al., 2023), by ensuring the appropriate drug use.
Since the primary use of this drug, several methods have been developed and evaluated for the quantification of vancomycin levels in biological fluids, including immunoassays (Yeo et al., 1989; Filburn et al., 1983), high-performance liquid chromatography (HPLC) using either UV detection (Hagihara et al., 2013), fluorescence detection (Abu-Shandi, 2009), or photodiode array detection (Cao et al., 2014), and also liquid chromatography-mass spectrometry (LC-MS) (Oyaert et al., 2015). Up to now, HPLC has been considered the one of gold standards due to its high sensitivity, specificity, and accuracy, wide linear range of detection, low sample requirement, and ability to detect multiple analytes (Cheng et al., 2022; Ningrum et al., 2024). However, it typically involves sample preparation steps, such as protein precipitation or solid-phase extraction, which can be time-consuming and require skilled personnel (Aboelezz et al., 2025).
In recent years, a new method for vancomycin measurement has emerged, known as chemiluminescent microparticle immunoassay (CMIA). This solid-phase immunoassay quantifies vancomycin in serum by detecting the chemiluminescent reaction between the drug and a labeled antibody. CMIA offers several advantages over traditional high-performance liquid chromatography (HPLC), including faster processing times, ease of use, lower costs, and the availability of various commercial kits.
Recent studies have highlighted the effectiveness of CMIA for measuring vancomycin levels, particularly in patients undergoing hemodialysis. For example, a study conducted in Brazil compared CMIA with liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) and found a strong correlation between the two methods. The mean difference indicated that CMIA provides reliable measurements without cross-reactivity in hemodialysis patients (Scribel et al., 2024). In another advancement, Fan et al. developed an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) technique to reduce cross-interaction in vancomycin assays. This innovative approach was evaluated against UPLC-UV and CMIA under normal, dialysis, and hemolytic conditions. The results demonstrated a moderate correlation between UPLC-UV and UPLC-MS/MS for samples from dialysis patients; however, some hemolytic samples showed overestimated results when analyzed with CMIA (Fan et al., 2019). The presence of cross-reacting substances, such as the crystalline degradation product of vancomycin, can lead to falsely elevated results in different immunoassay methods. This is particularly concerning in populations with renal impairment, where the accumulation of such metabolites is more likely (Chen et al., 2020).
Comparative studies have assessed CMIA against HPLC for other drugs as well. Guerrero Garduño et al. conducted a comparative analysis of HPLC and CMIA for quantifying carbamazepine and found comparable results, with a correlation coefficient of r ≈ 0.999, indicating that CMIA can reliably measure carbamazepine levels (Guerrero Garduño et al., 2016). Conversely, another study comparing HPLC and CMIA for valproic acid analysis indicated that HPLC offered greater precision than the immunoassay method (Zhao et al., 2016). Notably, highly metabolized drugs like cyclosporine A and tacrolimus were found to have significantly overestimated concentrations when measured by immunoassays compared to the UHPLC-MS method (Mei et al., 2018; Han et al., 2025).
Both CMIA and HPLC have been validated across various populations and for different drugs. Nonetheless, the potential for interference in CMIA necessitates careful interpretation of results, particularly in patients exhibiting altered pharmacokinetics.
Currently, there is a lack of data comparing the performance of CMIA with HPLC specifically for vancomycin, particularly in LTR patients. Therefore, this study aims to compare the accuracy and precision of CMIA and HPLC in measuring vancomycin levels in serum in LTRs, a topic that remains unexplored in the existing literature.
2 Methods
2.1 Study design, setting, and population
This cross-sectional study was done for 11 months, from January 2023 to November 2023, at the Abu-Ali Sina Solid Organ Transplant Hospital, affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. The study was approved by the ethics committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1400.021). All the study protocols were according to the ethical guidelines of the 1975 Helsinki Declaration. Informed consent was obtained from all study participants.
The studied population was patients having undergone liver transplantation and been under vancomycin treatment. Inclusion criteria required participants to be over 18 years old and to have received at least four doses of vancomycin. Excluded from the study were individuals with hypersensitivity to vancomycin, chronic kidney disease or on dialysis, burn injuries, those taking phenytoin, and pregnant women.
A combination of a calcineurin inhibitor (CNI) with sodium mycophenolate and prednisolone was prescribed for all patients as the immunosuppressive regimen. Dose adjustment was performed based on serum drug levels and the patient’s clinical response.
2.2 Data gathering
All necessary data was gathered through patients’ medical records. They included demographic and anthropometric characteristics (age, sex, height, Actual body weight (ABW), Ideal body weight (IBW), and Body mass index (BMI)), time since liver transplant, history of previous ICU admission, positive microbiologic cultures, type of infection, indication for transplantation, comorbidities, laboratory parameters (including, Blood urea nitrogen (BUN), Creatinine (Cr), Albumin (Alb), Total Bilirubin (T.Bili), Direct Bilirubin (D.Bili), Aspartate aminotransferase (AST), Alanine transaminase (ALT), Magnesium (mg), and concomitant antibiotics.
2.3 Vancomycin dosage and administration
The study protocol involved the administration of vancomycin to LTRs using a dosing regimen that included a loading dose of 20–35 mg/kg and a maintenance dose of 15–20 mg/kg, given every 12 h for at least four doses (Rybak et al., 2020). Infectious disease specialists were responsible for deciding whether to prescribe vancomycin empirically or definitively. The vancomycin infusion was given over a period of 60–90 min as per the study protocol, which aimed to evaluate the pharmacokinetics and outcomes associated with vancomycin use in LTRs.
2.4 Sampling
To ensure reaching the steady-state plasma concentrations, TDM should be started 48 h after initiation of vancomycin therapy. Four blood samples were taken from each participant at different time intervals, including 1) trough 1 (just before the fifth dose administration), 2) peak (5 min after the termination of the fifth dose infusion) 3) intermediate (6 h after the fifth dose administration), and 4) trough 2 (12 h after the fifth dose or just before the sixth dose administration).
2.5 Sample preparation and vancomycin CMIA analysis
At four mentioned times (0, 1, 6, and 12 h), a 4 mL sample was collected and decanted into K2EDTA tubes to prevent clotting. The plasma was separated from the whole blood by centrifuging the samples at 4,000 rpm for 3 min. The resulting plasma was then equally aliquoted for both CMIA and HPLC analysis.
According to the manufacturer’s instructions, vancomycin concentration in plasma was analyzed through the Vancomycin kit (The vancomycin assay on Architect i2000SR, Abbott®, North Chicago, 229 Illinois, United States). It was based on a reaction between plasma vancomycin and vancomycin kit reagents, reading the absorbance via an architect CMIA analyzer, and calculating the vancomycin concentration using the calibration curve provided with the Abbott® kit. The detection range was 3.0–100.0.
2.6 Sample preparation and vancomycin HPLC analysis
A 50 µL solution of theophylline standard (with a concentration of 8 mg/L) was mixed with 950 µL of the plasma using a vortex at 2,000 rpm for 1 min. To extract vancomycin from the plasma and precipitate plasma proteins, 1,000 µL of methanol was added to the mixture. The mixture was then centrifuged at 12,000 rpm for 15 min, and the supernatant was subsequently analyzed using a validated HPLC method.
2.7 HPLC apparatus and conditions
The high-performance liquid chromatography (HPLC) system consists of a pump-controller unit and a UV detector was used for sample analysis (Knauer, Germany). Separation was fulfilled by isocratic elution with a mobile phase of phosphate buffer (pH of 2.2, 0.03 M) and acetonitrile (86:14 %v/v ratio), transferred at a flow rate of 0.72 mL/min through a C18 column (250 mm length × 4.6 mm I.D.; 5 μm pore size, Knauer, Germany) as the stationary phase. The chromatographic pattern was recorded at the wavelength of 205 nm and column temperature of 25°C.
2.8 Vancomycin-induced nephrotoxicity detection
According to the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, vancomycin-induced nephrotoxicity is diagnosed based on an increase in serum creatinine by ≥ 0.3 mg/dL within 48 h after vancomycin initiation or an increase in serum creatinine by ≥ 1.5 times baseline within 7 days after starting vancomycin therapy or a urine output <0.5 mL/kg/h for 6 h after vancomycin initiation (Roy et al., 2013).
Serum creatinine levels were measured before vancomycin administration, and daily monitoring of serum creatinine levels and creatinine clearance was conducted until hospital discharge to detect changes in kidney function and diagnose vancomycin-induced nephrotoxicity regarding the KDIGO criteria.
2.9 Pharmacokinetic variables
To determine pharmacokinetic parameters for each patient, four equations were used.
Equation 1 was applied to calculate systemic clearance of the vancomycin.
Where Cl stands for clearance in L/h, X0 is the symbol of the administered dose in mg in each dosing interval, and AUCτ is the AUC of intervals in mg.h/L. AUCτ was computed through the trapezoidal method, which estimates the area by dividing it into trapezoidal sections, using four blood samples collected from each participant.
To align with recent guidelines (He et al., 2020), we calculated the AUC24 h for these patients by doubling the AUC12. This approach was adopted based on the absence of significant differences between the first and second trough concentrations in patients receiving vancomycin, indicating that drug accumulation was unlikely during the assessment period.
Equation 2 was applied to measure the volume of drug distribution within the body.
Where Vd stands for the volume of distribution in L, X0 is the symbol of the administered dose in mg in each dosing interval, and Cmax and Cmin show the peak and trough concentration in mg/L, respectively.
Equation 3 was applied to compute the elimination constant of vancomycin.
Where k is the symbol of the elimination constant in h−1, Cl stands for clearance in L/h, and Vd is the volume of distribution in L.
Equation 4 was applied to account for the elimination half-life of vancomycin.
Where t1/2 stands for vancomycin half-life in h and k is the elimination constant in h−1.
After each patient sample analysis, the doses were adjusted based on Equations 5, 6.
Where k0 is the symbol of the administration rate in mg/h, Cmin stands for the targeted trough concentration in mg/L, Vd shows the volume of distribution in L, k is the elimination constant in h−1, and τ is the dosing interval in h. (Assuming a one-compartment distribution model).
Where AUC2 is the target AUC, AUC1 is the current AUC, Dose2 is the new required dose to obtain the target AUC, and Dose1 is the current dose. (Assuming linear pharmacokinetic).
The seven following Equations 7–13 were applied to calculate trough and peak concentrations, the area under the curve, the volume of distribution, creatinine clearance, and the elimination rate constant.
Where Cmin and Cmax are trough and peak concentrations at the steady state, respectively.
Where Dose24h stands for a total daily dose of vancomycin.
Where Clcr stands for creatinine clearance in mL/min being a measure of kidney function and Vd is the volume of distribution in L which is dependent on Clcr, itself.
Where Clv stands for vancomycin clearance.
Where k is the elimination rate constant in
2.10 Statistical analysis
All statistical analyses were accomplished using IBM Statistical Package for Social Sciences software, version 25 (IBM Corporation, NY, United States). Continuous variables were reported as mean ± SD or median ± IQR and categorical parameters were expressed as percentages. The normality of continuous variables was examined via the Kolmogorov-Smirnov test. To determine the association between concentration obtained from HPLC and CMIA analysis the univariate regression model was used. The assessment of the correlation between data of HPLC and CMIA was carried out through the Pearson correlation test, as well. A p-value of less than 0.05 was considered statistically significant for all the above analyses.
3 Results
3.1 Demographic characteristics
With consideration of eligible criteria, 34 adult liver transplant recipients were included. More than half of the patients (61.8%) were male. The mean ± SD age and weight of participants were 43.3 ± 2.05 years and 69.9 ± 2.32 kg, respectively. The leading causes of liver failure in these participants were autoimmune hepatitis, followed by viral hepatitis, primary sclerosing cholangitis (PSC), and non-alcoholic steatohepatitis (NASH), respectively. The median (median ± IQR) serum creatinine of patients before vancomycin initiation was 0.9 ± 0.52 mg/dL. Most (88.2%) patients received vancomycin during the first month after transplantation. The mean loading and maintenance dose of vancomycin were 1030.9 ± 25.15 mg and 714.7 ± 21.16 mg, respectively. The median time of vancomycin receiving was 5 ± 3 days. Fifteen patients (44.1%) experienced nephrotoxicity during the study period within vancomycin treatment. Table 1 summarizes the demographic characteristics of LTRs receiving vancomycin.
3.2 Pharmacokinetic outcome
According to Table 2, while the vancomycin concentrations measured through the HPLC method at intervals of 1 (trough1), 3 (intermediate), and 4 (trough2) were significantly higher in patients with vancomycin-induced nephrotoxicity, the vancomycin concentrations measured via CMIA method were statistically equal between the two groups at all intervals. In addition, among pharmacokinetic variables, T1/2, AUC12h, and AUC24h observed based on the HPLC method exhibited significant differences between LTR patients with and without vancomycin-induced nephrotoxicity, where patients with reduced kidney function had significantly longer T1/2 and also higher AUC12h, and AUC24h. In spite of that, no significant association was detected between any pharmacokinetic variables observed through the CMIA method and developing vancomycin-induced nephrotoxicity. Furthermore, calculated pharmacokinetic parameters indicated no significant correlation with the emergence of nephrotoxicity. The analysis also exposed a non-significant association between the observed and calculated pharmacokinetic outcomes in LTRs, underscoring the indispensability of TDM for ascertaining kinetic parameters and dosage modifications.

Table 2. Comparison of pharmacokinetic outcome in liver transpalnt recipients that had been administered vancomycin through HPLC and CMIA method (N = 34).
3.3 The correlation and association between HPLC and CMIA concentrations
Regarding low R square values shown in Table 3, there was no linear regression between concentrations obtained by HPLC and CMIA. Also, no significant correlation was detected between these two variables.
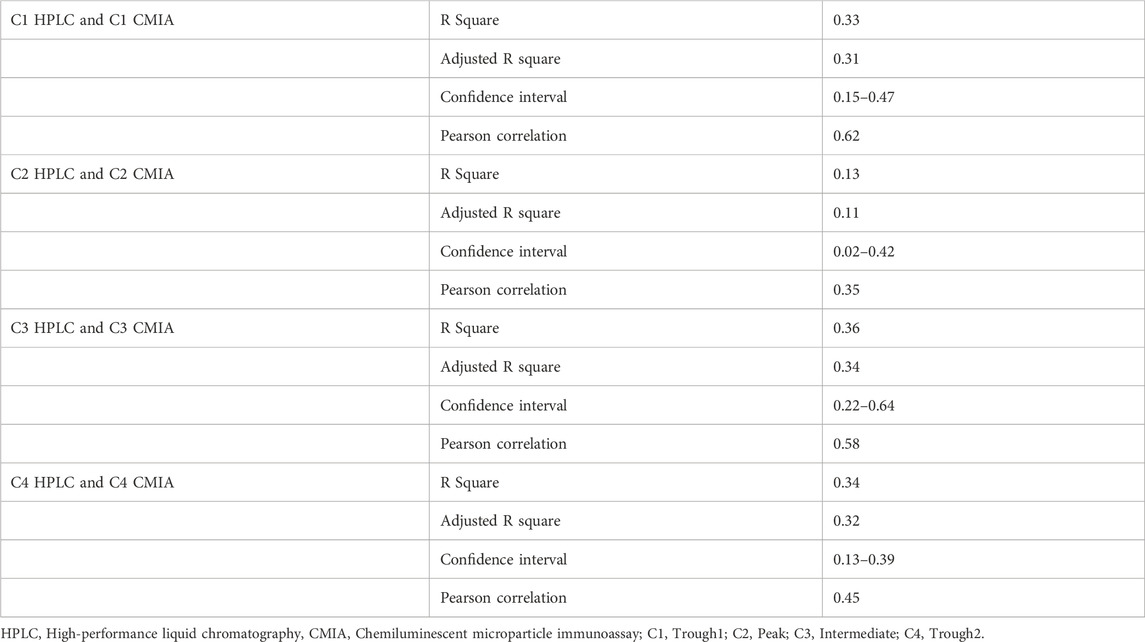
Table 3. The association between HPLC and CMIA concentrations among liver transpalnt recipients using linear regression. (N = 34).
3.4 Comparison of CMIA and HPLC concentrations in the studied population
The study investigated the vancomycin concentration profile over 12 h through blood sampling from 34 patients, using both the CMIA and HPLC methods (Figure 1). The results revealed significant differences between the two methods, particularly in peak concentration levels (Figure 2). As detailed in Table 4, the mean peak concentrations obtained via the CMIA and HPLC methods were 46.96 ± 23.20 mg/L and 22.94 ± 14.09 mg/L, respectively, with a p-value of <0.0001. Additionally, the trough concentrations also exhibited significant differences between the two methodologies (p-values: 0.02 and <0.000). The CMIA method reported higher first and second trough concentrations (17.55 ± 14.73 mg/L and 17.26 ± 13.40 mg/L, respectively) compared to the HPLC method (11.79 ± 7.7 mg/L and 9.03 ± 5.79 mg/L, respectively). Therefore, the study concluded that the CMIA method had low accuracy in measuring vancomycin concentrations and therefore recommended the use of TDM to ascertain kinetic parameters and dosage modifications.
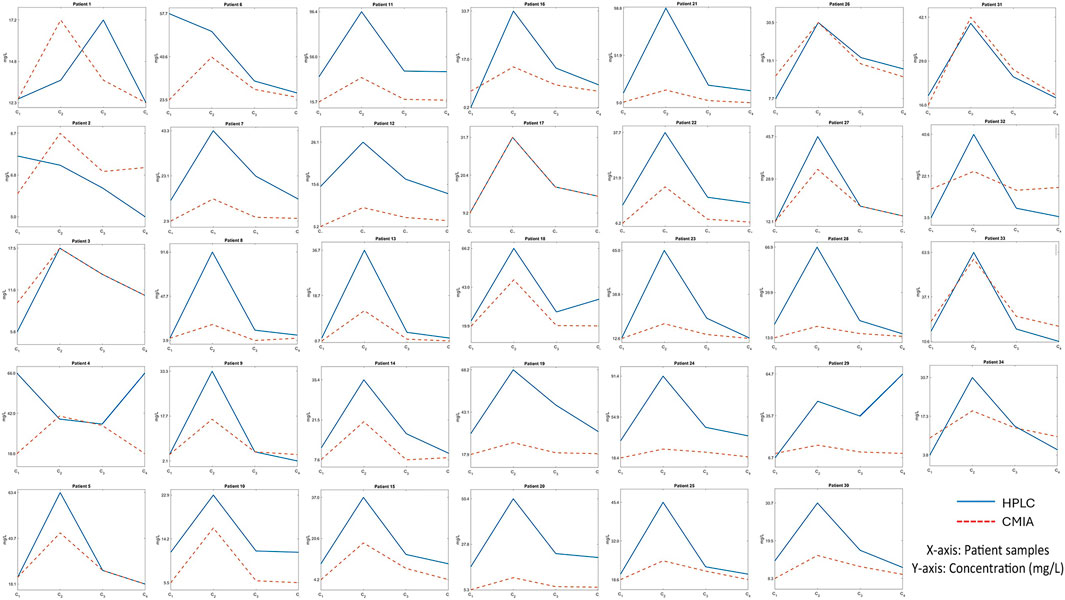
Figure 1. The vancomycin concentration curve in a 12-hour-period of blood sampling for 34 liver transplant recipients using both the CMIA and HPLC methods.

Figure 2. Comparison of different concentrations via HPLC and CMIA methods among 34 liver transplant recipients.

Table 4. The average concentrations and differences between HPLC and CMIA among liver transplant recipients (N = 34).
4 Discussion
Therapeutic vancomycin monitoring is essential for the optimization of its therapeutic effect and the avoidance of nephrotoxicity. Several methods have been developed for this purpose, including HPLC and CMIA. CMIA is a rapid, simple, automated, and inexpensive method that has been highly taken into consideration. However, the main concern of using this method can be the accuracy and precision of the measurement. This is the first study comparing HPLC and CMIA methods to assess vancomycin concentration in LTRs.
Currently, the existing guidelines do not provide specific dosing regimens for particular populations, such as LTRs. Therefore, it is essential to first calculate the Pharmacokinetic parameters within this population before determining the most optimal dosage.
None of the pharmacokinetic variables observed from the CMIA and HPLC concentrations have a significant relationship with the calculated variables in LTR and it emphasizes the need to use therapeutic monitoring.
The present study used an accurate, selective, and sensitive validated HPLC method (Ghasemiyeh et al., 2020) and Abbott® kit for vancomycin quantification. The limit of detection for HPLC and CMIA methods were 800 ng/mL and 3 μg/mL, respectively. So, HPLC provides a more sensitive assay for vancomycin.
Our results showed that vancomycin concentration obtained by HPLC and CMIA presented no linear regression between the two methods and the HPLC method was considered more accurate, precise, and versatile for both pharmacokinetic analysis and distinguishing the concentration differences between the patients with and without vancomycin-induced nephrotoxicity, significantly in trough 1, intermediate, and trough 2 concentrations. Considering the comparison of CMIA and HPLC concentrations in the studied population, the concentration investigated by CMIA was higher than that by HPLC in almost all patients during the 12-hour sampling. The peak concentration indicated the greatest differences and had a 105% deviation on average. Fan et al. have also reported an overestimated concentration via CMIA compared to UPLC/MS among hemodialysis patients, possibly secondary to interference of various panels of serum samples (Fan et al., 2019). Furthermore, a case report study serendipitously discovered a falsely high level of vancomycin using immunoassay methods in a patient not actually receiving this medication, perhaps due to being affected by endogenous proteins cross-interaction (Tsoi et al., 2019). However, Scribel et al. reported no cross-reactivity via CMIA among patients on hemodialysis (Scribel et al., 2024). On the other hand, numerous studies have examined the link between vancomycin trough levels and the risk of nephrotoxicity, generally finding that higher trough levels correlate with increased nephrotoxicity rates. In this regard, Horey et al. observed a correlation between vancomycin trough levels and nephrotoxicity rates. They found that the rates of nephrotoxicity were 4.9%, 3.1%, 10.6%, 23.6%, and 81.8% for maximal troughs of 5–10 mg/L, 10.1–15 mg/L, 15.1–20 mg/L, 20.1–35 mg/L, and greater than 35 mg/L, respectively (Horey et al., 2012). Similarly, Lodise et al. revealed that patients with trough levels greater than 10 mg/mL significantly more experienced nephrotoxicity compared to trough levels less than 10 mg/mL (22% vs. 5.1%) (Lodise et al., 2009). Cano et al. also noted an increase in nephrotoxicity from 7% at levels below 10 mg/L to 34% at levels above 20 mg/L (Cano et al., 2012). Barriere et al. observed no renal adverse events in patients with trough levels below 10 mg/L, while those with levels above this threshold experienced increasing incidences of renal issues (3% for 10–15 mg/L and 17% for above 15 mg/L) (Barriere et al., 2014). The present study similarly indicated that LTR patients having developed vancomycin-induced nephrotoxicity had a significantly higher vancomycin concentration at trough 1 (C1), intermediate (C3), and trough 2 (C4) observed via HPLC, however, the CMIA method was not accurate enough to distinguish the significant differences between the two groups at any intervals. In addition, the data obtained by HPLC showed that trough concentrations higher than 10 mg/L were associated with an increased risk of acute kidney injury in LTRs, highlighting the importance of monitoring pharmacokinetic parameters in this population to reduce adverse effects.
LTRs may experience altered pharmacokinetic parameters compared to the normal population due to the post-transplant inflammation state, changes in plasma protein level, the presence of hepatorenal syndrome, postoperative complications, and polypharmacy (Morales Junior et al., 2023; Righi, 2018; De Simone et al., 2024). The mean Vd in our investigation measured by HPLC and CMIA were 1.1 ± 0.7 and 0.53 ± 1.08 L/kg, respectively. Vd obtained from CMIA was in the typical range of 0.4 L/kg to 1 L/kg previously observed in the normal populations, while the value obtained from HPLC was significantly higher than this range which was also noted by other studies (Zhang et al., 2020) Among the studied population, many inter-individual variations were observed in Vd values. In this regard, the lack of significant correlation between the calculated and observed Vd highlights the possible changes due to fluid retention caused by organ dysfunction, enhanced capillary permeability resulting from systemic inflammatory response syndrome (SIRS) in ICU admitted patients, and ascites presence as a third-space volume. Additionally, lower baseline albumin levels in liver transplant recipients can also contribute to an increase in volume of distribution (Vd), particularly for hydrophilic antibiotics such as vancomycin. This effect was also emphasized in ICU-admitted critically ill patients in the study of Polard et al. (1999), in pediatric LTRs in the study of Shoji et al. (2021), and in adults having undergone bone marrow transplantation in the study of Taghizadeh Ghehi et al. (2013). Our findings demonstrated an increase in vancomycin clearance in LTRs, with a mean clearance of 4.91 ± 3.00 L/h and 2.93 ± 2.02 L/h obtained from HPLC and CMIA, respectively, compared to the general population’s average of 2.6 L/h. This elevation in clearance can be attributed to lower levels of albumin in LTRs, leading to higher unbound vancomycin and increased total clearance (both renal and non-renal) (Flannery et al., 2020; Rodvold et al., 1988), which are more manifest in data measured via the HPLC method in our study. Furthermore, the inflammatory condition prevalent in post-transplant recipients and also the use of diuretic drugs in such patients can exacerbate the renal excretion of antibiotics, leading to enhanced vancomycin clearance (Shimamoto et al., 2013). Moreover, we obtained a longer t1/2 for vancomycin in LTRs than normal population. Harada H et al. have also found that patients with hepatic impairment exhibit a prolonged vancomycin t1/2 (Harada et al., 1999). Additionally, there was a significant increase in t1/2 in LTRs with renal insufficiency compared to non-vancomycin-induced nephrotoxicity LTRs through HPLC measurement, while CMIA data demonstrated no differences. Cheung et al. study reported longer vancomycin half-life in patients with impaired renal function, as well (Cheung and DiPiro, 1986). Our study also revealed a lower k1/2 in LTRs than the normal population due to changes in both Clv and Vd values. In addition, our AUC24h and AUC12h values were below therapeutic levels reported in previous studies, denoting that higher loading doses may be necessary to achieve therapeutic AUC in LTRs. According to recent guidelines from ASHP, IDSA, and SIDP, the recommended AUC for effective treatment of MRSA infections is generally considered to be ≥400 mg·hr/L, with some studies suggesting that higher AUC values may be necessary for more severe infections, Nevertheless, there have been no reports on the optimal dosing required to ensure adequate drug exposure in LTRs (Rybak et al., 2020; Shoji et al., 2021). In a study by Alvarez et al. on critically ill patients admitted to ICU has been demonstrated that patients receiving a loading dose exhibited an AUC ranging between 600 and 700 mg.h/L (Álvarez et al., 2017). Holmes et al. conducted another study on patients with S.aureus bacteremia, indicating that 54% of patients with hepatic impairment or receiving immunosuppressive agents had an AUC greater than 400 mg.h/L (Holmes et al., 2013). Brown et al. also performed a study on patients with infective endocarditis and complex MRSA bacteremia, which demonstrated that the mortality rate was significantly lower in patients whose AUC/MIC values exceeded 400 mg.h/L (Brown et al., 2012). In our present study, we found that 41.2% of LTRs had an AUC above 400 mg.h/L, and failure to achieve the target AUC was associated with higher mortality rates. The average AUC was 383.3 ± 192.3 and 635.61 ± 301.60 via HPLC and CMIA measurement, respectively. When HPLC was used to assay AUC, LTR patients with higher values were significantly more susceptible to developing vancomycin-induced nephrotoxicity. Accordingly, the two points should be considered. First, HPLC is a more reliable test for vancomycin assessment than CMIA. Second, higher loading doses may be necessary for the LTRs to achieve therapeutic AUC levels. The pharmacokinetic alteration after liver transplantation indicates that standard dosing regimens may not achieve adequate therapeutic levels of the drug, necessitating tailored dosing protocols to ensure optimal drug exposure. Such adjustments are essential for the effective management of severe MRSA infections while also minimizing the risk of toxicity (Shoji et al., 2021). Prolonged exposure to suboptimal vancomycin levels can lead to the development of microbial resistance particularly in MRSA infections (Nyandoro et al., 2025), while higher vancomycin exposure particularly when AUC exceeds 550 mg.h/L is associated with nephrotoxicity in the treatment of MRSA bacteremia (Lodise et al., 2020; Chiu and Sarwal, 2023). Notably, our study highlighted that patients not reaching the AUC target had a higher mortality rate, emphasizing the need for optimized dosing strategies in this population. Clinicians should adopt an individualized approach to vancomycin dosing in LTRs through routine TDM, considering factors like renal function, concurrent medications, and nutritional status. Also, the potential overestimation of concentration using the CMIA method may lead to errors in drug dose adjustments and negatively impact therapeutic outcomes if this method is employed.
Furthermore, this study presents several limitations that must be acknowledged when interpreting the findings regarding therapeutic vancomycin monitoring in LTRs. Firstly, the sample size of our study was relatively small, which may limit the generalizability of the results. A larger sample size would provide more robust data and potentially reveal additional insights into the pharmacokinetics of vancomycin in this specific population. Secondly, this research was conducted at a single center, which may introduce biases related to local practices, patient demographics, and clinical protocols. The findings may not be applicable to other institutions with different patient populations or treatment approaches. Multi-center studies would be beneficial to validate our results across diverse settings. Additionally, the focus of this study was exclusively on liver transplant recipients, which restricts the applicability of the findings to other transplant populations, such as kidney or heart transplant patients. Variations in drug metabolism and pharmacodynamics among different transplant types could yield different therapeutic monitoring requirements. Lastly, while HPLC is a reliable method, it lacks the sensitivity of techniques such as LC-MS, which could provide more precise vancomycin measurements, particularly in patients with fluctuating drug concentrations.
5 Conclusion
In conclusion, this study highlighted the importance of selecting the appropriate analytical method for TDM of vancomycin in liver transplant recipients. Our findings suggested that HPLC was a more sensitive and reliable method compared to CMIA for both measuring vancomycin concentration and detecting variable changes in its pharmacokinetic profile. The use of HPLC may help clinicians make more informed decisions regarding dosing adjustments and ultimately improve patient outcomes. But, while this study provides valuable insights into therapeutic vancomycin monitoring, the aforementioned limitations should be considered when interpreting the results and their implications for clinical practice. Further research addressing these limitations is warranted to enhance our understanding and explore other potential benefits of using HPLC for vancomycin TDM in LTRs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1400.021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SA: Conceptualization, Formal analysis, Writing – original draft, Supervision, Validation. SSJ: Data curation, Formal analysis, Writing – review and editing. SM-S: Conceptualization, Methodology, Validation, Supervision, Writing – review and editing. PG: Data curation, Methodology, Writing – review and editing. BG: Writing – review and editing, Supervision, Validation. HN: Conceptualization, Writing – review and editing, Supervision. AV: Methodology, Writing – review and editing, Supervision. MS: Conceptualization, Writing – review and editing, Supervision, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aboelezz, A., Tesfamariam, N. S., Kharouba, M., Gligoric, T., and Mahmoud, S. H. (2025). The development and validation of a simple HPLC-UV method for the determination of vancomycin concentration in human plasma and application in critically ill patients. Molecules 30 (5), 1062. doi:10.3390/molecules30051062
Abu-Shandi, K. H. (2009). Determination of vancomycin in human plasma using high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 395 (2), 527–532. doi:10.1007/s00216-009-2948-9
Álvarez, O., Plaza-Plaza, J. C., Ramirez, M., Peralta, A., Amador, C. A., and Amador, R. (2017). Pharmacokinetic assessment of vancomycin loading dose in critically ill patients. Antimicrob. Agents Chemother. 61 (8), e002800–e002817. doi:10.1128/aac.00280-17
Barriere, S. L., Stryjewski, M. E., Corey, G. R., Genter, F. C., and Rubinstein, E. (2014). Effect of vancomycin serum trough levels on outcomes in patients with nosocomial pneumonia due to Staphylococcus aureus: a retrospective, post hoc, subgroup analysis of the Phase 3 ATTAIN studies. BMC Infect. Dis. 14, 183. doi:10.1186/1471-2334-14-183
Brown, J., Brown, K., and Forrest, A. (2012). Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob. Agents Chemother. 56 (2), 634–638. doi:10.1128/aac.05609-11
Cano, E. L., Haque, N. Z., Welch, V. L., Cely, C. M., Peyrani, P., Scerpella, E. G., et al. (2012). Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin. Ther. 34 (1), 149–157. doi:10.1016/j.clinthera.2011.12.013
Cao, Y., Yu, J., Chen, Y., Zhang, J., Wu, X., Zhang, Y., et al. (2014). Development and validation of a new ultra-performance liquid chromatographic method for vancomycin assay in serum and its application to therapeutic drug monitoring. Ther. Drug Monit. 36 (2), 175–181. doi:10.1097/FTD.0b013e3182a458bc
Chen, C. Y., Li, M. Y., Ma, L. Y., Zhai, X. Y., Luo, D. H., Zhou, Y., et al. (2020). Precision and accuracy of commercial assays for vancomycin therapeutic drug monitoring: evaluation based on external quality assessment scheme. J. Antimicrob. Chemother. 75 (8), 2110–2119. doi:10.1093/jac/dkaa150
Chen, M., Lee, C., Gnyra, M., and Wong, M. (2022). Vancomycin area under the curve/minimum inhibitory concentration and trough level concordance-evaluation on an urban health unit. Ther. Adv. Infect. Dis. 9, 20499361221140368. doi:10.1177/20499361221140368
Cheng, X., Ma, J., and Su, J. (2022). An overview of analytical methodologies for determination of vancomycin in human plasma. Molecules 27 (21), 7319. doi:10.3390/molecules27217319
Cheung, R. P., and DiPiro, J. T. (1986). Vancomycin: an update. Pharmacotherapy 6 (4), 153–169. doi:10.1002/j.1875-9114.1986.tb03471.x
Chiu, C. Y., and Sarwal, A. (2023). Evaluating the nephrotoxicity of area-under-the-curve-based dosing of vancomycin with concomitant antipseudomonal beta-lactam antibiotics: a systematic review and meta-analysis. Med. Kaunas. 59 (4), 691. doi:10.3390/medicina59040691
De Simone, P., Battistella, S., Lai, Q., Ducci, J., D'Arcangelo, F., Marchetti, P., et al. (2024). Immunosuppression for older liver transplant recipients. Transpl. Rev. Orl. 38 (1), 100817. doi:10.1016/j.trre.2023.100817
Fan, Y., Peng, X., Yu, J., Liang, X., Chen, Y., Liu, X., et al. (2019). An ultra-performance liquid chromatography-tandem mass spectrometry method to quantify vancomycin in human serum by minimizing the degradation product and matrix interference. Bioanalysis 11 (10), 941–955. doi:10.4155/bio-2018-0310
Filburn, B. H., Shull, V. H., Tempera, Y. M., and Dick, J. D. (1983). Evaluation of an automated fluorescence polarization immunoassay for vancomycin. Antimicrob. Agents Chemother. 24 (2), 216–220. doi:10.1128/aac.24.2.216
Flannery, A. H., Bissell, B. D., Bastin, M. T., Morris, P. E., and Neyra, J. A. (2020). Continuous versus intermittent infusion of vancomycin and the risk of acute kidney injury in critically ill adults: a systematic review and meta-analysis. Crit. Care Med. 48 (6), 912–918. doi:10.1097/ccm.0000000000004326
Ghasemiyeh, P., Vazin, A., Zand, F., Azadi, A., Karimzadeh, I., and Mohammadi-Samani, S. (2020). A simple and validated HPLC method for vancomycin assay in plasma samples: the necessity of TDM center development in Southern Iran. Res. Pharm. Sci. 15 (6), 529–540. doi:10.4103/1735-5362.301337
Ghehi, M. T., Rezaee, S., Hayatshahi, A., Hadjibabaie, M., Gholami, K., Javadi, M., et al. (2013). Vancomycin pharmacokinetic parameters in patients undergoing hematopoietic stem cell transplantation (HSCT). Int. J. Hematol. Oncol. Stem Cell Res. 7 (4), 1–9.
Guerrero Garduño, Ó., González-Esquivel, D. F., Escalante-Membrillo, C., Fernández, Á., Rojas-Tomé, I. S., Jung Cook, H., et al. (2016). Comparison of a high-performance liquid chromatography method for quantification of carbamazepine with chemiluminescent microparticle immunoassay. Biomed. Chromatogr. 30 (6), 933–937. doi:10.1002/bmc.3631
Hagihara, M., Sutherland, C., and Nicolau, D. P. (2013). Development of HPLC methods for the determination of vancomycin in human plasma, mouse serum and bronchoalveolar lavage fluid. J. Chromatogr. Sci. 51 (3), 201–207. doi:10.1093/chromsci/bms128
Han, F. F., Liu, H. C., Hu, T., Li, P. F., Zhao, R., and An, Z. L. (2025). Development and validation of a rapid HPLC-MS/MS method for simultaneous determination of cyclosporine A and tacrolimus in whole blood for routine therapeutic drug monitoring in organ transplantation. Rapid Commun. Mass Spectrom. 39 (2), e9932. doi:10.1002/rcm.9932
Harada, H., Miyagawa, S., Kawasaki, S., Hayashi, K., Kitamura, H., Katsuyama, Y., et al. (1999). Study of the pharmacokinetics of vancomycin in patients with impaired liver function. J. Infect. Chemother. 5 (2), 104–107. doi:10.1007/s101560050018
Holmes, N. E., Turnidge, J. D., Munckhof, W. J., Robinson, J. O., Korman, T. M., O'Sullivan, M. V., et al. (2013). Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 57 (4), 1654–1663. doi:10.1128/aac.01485-12
Horey, A., Mergenhagen, K. A., and Mattappallil, A. (2012). The Relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran's population: a retrospective analysis. Ann. Pharmacother. 46 (11), 1477–1483. doi:10.1345/aph.1R158
Kaushik, A., Kest, H., Sood, M., Steussy, B. W., Thieman, C., and Gupta, S. (2024). Biofilm producing methicillin-resistant Staphylococcus aureus (MRSA) infections in humans: clinical implications and management. Pathogens 13 (1), 76. doi:10.3390/pathogens13010076
Lodise, T. P., Patel, N., Lomaestro, B. M., Rodvold, K. A., and Drusano, G. L. (2009). Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49 (4), 507–514. doi:10.1086/600884
Lodise, T. P., Rosenkranz, S. L., Finnemeyer, M., Evans, S., Sims, M., Zervos, M. J., et al. (2020). The emperor's new clothes: PRospective observational evaluation of the association between initial VancomycIn exposure and failure rates among ADult HospitalizEd patients with methicillin-resistant Staphylococcus aureus bloodstream infections (PROVIDE). Clin. Infect. Dis. 70 (8), 1536–1545. doi:10.1093/cid/ciz460
Matsumoto, K., Oda, K., Shoji, K., Hanai, Y., Takahashi, Y., Fujii, S., et al. (2022). Clinical practice guidelines for therapeutic drug monitoring of vancomycin in the framework of model-informed precision dosing: a consensus review by the Japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. Pharmaceutics 14 (3), 489. doi:10.3390/pharmaceutics14030489
Mei, S., Wang, J., Chen, D., Zhu, L., Zhao, M., Tian, X., et al. (2018). Simultaneous determination of cyclosporine and tacrolimus in human whole blood by ultra-high performance liquid chromatography tandem mass spectrometry and comparison with a chemiluminescence microparticle immunoassay. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1087-1088, 36–42. doi:10.1016/j.jchromb.2018.04.028
Morales Junior, R., Telles, J. P., Kwiatkowski, S. Y., Juodinis, V. D., de Souza, D. C., and Santos, S. (2023). Pharmacokinetic and pharmacodynamic considerations of antibiotics and antifungals in liver transplantation recipients. Liver Transpl. 29 (1), 91–102. doi:10.1002/lt.26517
Ningrum, V. D. A., Amalia, S. P., and Wibowo, A. (2024). Vancomycin bioanalysis for TDM services by using immunoassay and HPLC: a scoping review. Pharm. Educ. 24 (3), 197–203. doi:10.46542/pe.2024.243.197203
Nyandoro, V. O., Ismail, E. A., Tageldin, A., Gafar, M. A., Peters, X. Q., Mautsoe, R., et al. (2025). Potential of nanocarrier-mediated delivery of vancomycin for MRSA infections. Expert Opin. Drug Deliv. 22, 347–365. doi:10.1080/17425247.2025.2459756
Oyaert, M., Peersman, N., Kieffer, D., Deiteren, K., Smits, A., Allegaert, K., et al. (2015). Novel LC-MS/MS method for plasma vancomycin: comparison with immunoassays and clinical impact. Clin. Chim. Acta 441, 63–70. doi:10.1016/j.cca.2014.12.012
Pai, M. P., Neely, M., Rodvold, K. A., and Lodise, T. P. (2014). Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv. Drug Deliv. Rev. 77, 50–57. doi:10.1016/j.addr.2014.05.016
Polard, E., Le Bouquin, V., Le Corre, P., Kérebel, C., Trout, H., Feuillu, A., et al. (1999). Non steady state and steady state PKS Bayesian forecasting and vancomycin pharmacokinetics in ICU adult patients. Ther. Drug Monit. 21 (4), 395–403. doi:10.1097/00007691-199908000-00003
Righi, E. (2018). Management of bacterial and fungal infections in end stage liver disease and liver transplantation: current options and future directions. World J. Gastroenterol. 24 (38), 4311–4329. doi:10.3748/wjg.v24.i38.4311
Rodvold, K. A., Blum, R. A., Fischer, J. H., Zokufa, H. Z., Rotschafer, J. C., Crossley, K. B., et al. (1988). Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob. Agents Chemother. 32 (6), 848–852. doi:10.1128/aac.32.6.848
Roy, A. K., Mc Gorrian, C., Treacy, C., Kavanaugh, E., Brennan, A., Mahon, N. G., et al. (2013). A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med. 3 (1), 26–37. doi:10.1159/000347037
Rybak, M. J., Le, J., Lodise, T. P., Levine, D. P., Bradley, J. S., Liu, C., et al. (2020). Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American society of health-system Pharmacists, the infectious diseases society of America, the pediatric infectious diseases society, and the society of infectious diseases Pharmacists. Am. J. Health-System Pharm. 77 (11), 835–864. doi:10.1093/ajhp/zxaa036
Scribel, L., Galiotto, A., Rodrigues, I. S., Hahn, R., Linden, R., and Zavascki, A. P. (2024). Comparison of vancomycin assays in patients undergoing hemodialysis. Braz J. Infect. Dis. 28 (5), 103869. doi:10.1016/j.bjid.2024.103869
Shafiekhani, M., Fatemi, S. A., Hosseini, P., Marhemati, F., Mohammadi, S., Sharifi, F., et al. (2023). Pharmacokinetic and pharmacodynamic considerations of novel antibiotic agents for pediatric infections: a narrative review. Surg. Infect. (Larchmt) 24 (8), 703–715. doi:10.1089/sur.2023.055
Shimamoto, Y., Fukuda, T., Tanaka, K., Komori, K., and Sadamitsu, D. (2013). Systemic inflammatory response syndrome criteria and vancomycin dose requirement in patients with sepsis. Intensive Care Med. 39 (7), 1247–1252. doi:10.1007/s00134-013-2909-9
Shoji, K., Saito, J., Nakagawa, H., Funaki, T., Fukuda, A., Sakamoto, S., et al. (2021). Population pharmacokinetics and dosing optimization of vancomycin in pediatric liver transplant recipients. Microbiol. Spectr. 9 (2), e0046021. doi:10.1128/Spectrum.00460-21
Tsoi, V., Bhayana, V., Bombassaro, A. M., Tirona, R. G., and Kittanakom, S. (2019). Falsely elevated vancomycin concentrations in a patient not receiving vancomycin. Pharmacotherapy 39 (7), 778–782. doi:10.1002/phar.2279
Ye, Z. K., Chen, Y. L., Chen, K., Zhang, X. L., Du, G. H., He, B., et al. (2016). Therapeutic drug monitoring of vancomycin: a guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. J. Antimicrob. Chemother. 71 (11), 3020–3025. doi:10.1093/jac/dkw254
Yeo, K. T., Traverse, W., and Horowitz, G. L. (1989). Clinical performance of the EMIT vancomycin assay. Clin. Chem. 35 (7), 1504–1507. doi:10.1093/clinchem/35.7.1504
Zhang, Y., Wang, T., Zhang, D., You, H., Dong, Y., Liu, Y., et al. (2020). Therapeutic drug monitoring coupled with bayesian forecasting could prevent vancomycin-associated nephrotoxicity in renal insufficiency patients: a prospective study and pharmacoeconomic analysis. Ther. Drug Monit. 42 (4), 600–609. doi:10.1097/ftd.0000000000000750
Keywords: chemiluminescent microparticle immunoassay, high-performance liquid chromatography, therapeutic drug monitoring, vancomycin, infectious disease
Citation: Azadi S, Jalali SS, Mohammadi-Samani S, Ghasemiyeh P, Geramizadeh B, Nikoupour H, Vazin A and Shafiekhani M (2025) Therapeutic vancomycin monitoring: a comparative analysis of high-performance liquid chromatography and chemiluminescent microparticle immunoassay methods in liver transplant recipients. Front. Pharmacol. 16:1516339. doi: 10.3389/fphar.2025.1516339
Received: 24 October 2024; Accepted: 09 May 2025;
Published: 03 June 2025.
Edited by:
Andy R. Eugene, Osawatomie State Hospital, United StatesCopyright © 2025 Azadi, Jalali, Mohammadi-Samani, Ghasemiyeh, Geramizadeh, Nikoupour, Vazin and Shafiekhani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mojtaba Shafiekhani, bW9qdGFiYXNoYWZpZWtoYW5pQGdtYWlsLmNvbQ==; Afsaneh Vazin, dmF6aW5hZkBnbWFpbC5jb20=
 Soha Azadi
Soha Azadi Seyed Soroush Jalali1,2
Seyed Soroush Jalali1,2 Soliman Mohammadi-Samani
Soliman Mohammadi-Samani Parisa Ghasemiyeh
Parisa Ghasemiyeh Hamed Nikoupour
Hamed Nikoupour Mojtaba Shafiekhani
Mojtaba Shafiekhani