Abstract
Background:
Ferroptosis is a newly identified type of iron-dependent cell death that characterized by an increase in intracellular iron ions, which disrupt the balance of the cellular lipid peroxidation system, causing lipid peroxidation and ultimately resulting in cell death. Interestingly, ferroptosis is modulated by hypoxia and plays a role in hypoxia-related diseases. Therefore, we performed a bibliometric review of the Web of Science Core Collection (WoSCC) database to investigate the link between ferroptosis and hypoxia from January 2013 to December 2023.
Method:
The core collection within the Web of Science bibliographic index was consulted to extract relevant articles and reviews. Data on publications, countries, institutions, authors, journals, citations, and keywords in the included studies were systematically analyzed using Microsoft Excel 2019 and CiteSpace 6.3.R1 software.
Result:
A comprehensive analysis and visualization of 472 research papers on ferroptosis under hypoxic conditions published between 2013 and 2023 revealed emerging research hotspots and trends. Initially, a scarcity of studies existed in this field. However, this was succeeded by a significant increase in research interest in subsequent years, culminating in a peak of 204 publications in 2023. Research in this field focused primarily on the Asian region. Notably, research hotspots include diseases related to hypoxia, treatment therapy and pathogenesis. Among the researchers in this field, Supuran emerged as the most prolific author. Wuhan University was the leading institution in terms of research output, and China was the most prolific country in this area of study. Among the top ten journals ranked by the number of publications, nine were classified as Q1, indicating the high level of credibility of these studies. The research conducted by Stockwell et al., featured in the journal “Cell,” currently has the most citations. Present scholarly pursuits are primarily focused on comprehending the mechanisms through which interventions affect hypoxia-related diseases through the ferroptosis pathway, as well as on probing and pinpointing prospective treatment targets.
Conclusion:
This study highlights key areas of interest and emerging trends in ferroptosis research in the presence of hypoxic conditions, thus providing valuable insights for future directions of exploration for the diagnosis and treatment of hypoxia-related diseases.
1 Introduction
Ferroptosis is a recently identified mechanism of cell death contingent on iron, which arises from the buildup of iron-catalyzed lipid peroxidation (Liang et al., 2022). Studies have indicated that the low molecular weight compound erastin triggers ferroptosis-specific cell death in tumor cells harboring the mutated oncogene RAS, this process is distinct from apoptosis, necrosis, and autophagy (Li et al., 2020a). The hallmarks of ferroptosis include the build-up of iron within cells and oxidation of lipids, which is predominantly linked to the accumulation of intracellular iron, exhaustion of glutathione (GSH), as well as the deactivation of GSH peroxidase 4 and escalation of lipid peroxidation (Ursini and Maiorino, 2020). A growing body of research has affirmed the association of ferroptosis with various signaling pathways and its involvement in the modulation of numerous diseases (Jiang et al., 2021; Stockwell et al., 2020).
Reduced oxygen availability in tissues, termed hypoxia, affects several cellular processes in diverse cell types and is linked to both normal physiological states and disease conditions, including altitude-induced effects, solid tumor growth, and organ ischemia (McClelland and Scott, 2019; Chen et al., 2022; Ow et al., 2018). Hypoxia modulates proteins involved in iron metabolism, thereby influencing iron levels and lipid peroxidation rate (Fuhrmann and Brune, 2022). Hypoxia-inducible factor-1, modulated by hypoxic conditions, facilitates increased iron absorption, which in turn affects susceptibility to ferroptosis (Gao et al., 2023). Hypoxia also controls ferroptosis through the Nrf2/HO-1 and p62/Keap1/Nrf2 signaling pathways (Cao et al., 2022; Li et al., 2020b). In addition, epigenetic alterations, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and methylation, contribute to the regulation of ferroptosis under hypoxic conditions (Yang et al., 2022; Dong et al., 2022; Liu et al., 2023). Accumulating evidence suggests that ferroptosis plays an important role in hypoxia-related diseases such as ischemia-reperfusion (I-R), ischemiastroke (IS), hypoxic tumors, Coronavirus disease-19 (Gao et al., 2023).
Bibliometrics uses mathematical and statistical techniques to collect data on publication rates, characteristics, and patterns in a specific field (Ahmad et al., 2020). Through the analyses of authors, co-occurrences, and citations, research trends and statuses in specific fields can be elucidated. Such research can help determine the productivity of researchers, internal collaboration, and distribution, as well as those of affiliated institutions and journals. Bibliometric studies can uncover focal points of research within a discipline and indicate potential avenues for future studies (Saeed et al., 2024; Agathokleous and Calabrese, 2024). Therefore, this study comprised a bibliometric review of the literature on ferroptosis under hypoxic conditions published between 2013 and 2023, thoroughly examining the prevailing research landscapes, focal areas, and emerging trends. The objective was to identify journal publications, collaborators, keywords, and research trends that can enhance our understanding of diseases, mechanisms, and treatments. Collectively, these findings provide valuable insights for future studies.
2 Material and methods
2.1 Data sources and search strategy
Web of Science was selected as the primary database for this study due to its comprehensive coverage of over 12,000 academic journals and its frequent usage by researchers. When compared to other databases such as Scopus, Medline, and PubMed, Web of Science provides the most comprehensive and reliable bibliometric analysis (Zhang et al., 2024; Jiang et al., 2023). So we determined that the Web of Science Core Collection (WoSCC) was the optimal database for bibliometric evaluation in our study. Consequently, we conducted a comprehensive literature search on ferroptosis under hypoxic conditions using the Science Citation Index Expanded function of the WoSCC database, spanning 2003–2023. The process of searching and downloading data for all documents was accomplished in a single day to avoid any bias that may have resulted from the regular updates of the database. The search criteria and methods we used were as follows: Title search equals (Ferroptosis and Hypoxia) AND Language equals (English), with a defined time frame from 1 January 2013 to 31 December 2023. To confirm that the collected publications were pertinent to the primary subject of our research, we scrutinized and documented each publication by examining its title and summary, excluded articles from low-quality or predatory journals. The exclusion criteria were as follows: (1) duplicate publications; (2) entries with missing information such as author names, journals, or publication dates; and literature types categorized as articles and reviews. An expansive flow diagram of the screening process is shown in Figure 1.
FIGURE 1

Flow diagram showing the screening process.
2.2 Data analysis and visualization
After screening the manuscripts, we extracted data from relevant publications, including titles, keywords, countries or regions, authors, institutions, journals, and the number of citations. Full records and cited references of the retrieved articles were downloaded from the WoSCC database. The data were then converted to TXT format and imported into CiteSpace 6.3.R1 for bibliometric analysis (Chen and Song, 2019).
2.3 Bibliometric analysis
We performed bibliometric analysis of several literature characteristics, including the number of publications, countries/regions, institutions, authors, journals, co-cited references, and keywords with the strongest citation bursts. Using CiteSpace, we constructed visual networks of various countries or regions and institutions based on collaborative or co-occurrence data. This tool facilitates the discovery of novel trends and developments in a research field, particularly in the analysis of literature citations and keywords. We used CiteSpace for co-citation analysis of keyword burst detection to identify the current status and hotspots of research on ferroptosis under hypoxic conditions and to predict future research directions in this field.
3 Results
3.1 Annual publication trends
Between 2013 and 2023, a total of 472 documents on ferroptosis associated with hypoxia were published. The concept of ferroptosis was first introduced by Dixon et al., in 2012, and research on ferroptosis began to emerge in 2013, including studies on ferroptosis under hypoxic conditions. As depicted in Figure 2, the publication count was modest and steady from 2013 to 2018, oscillating annually from one to six papers. Following this period, a notable increase in the number of publications was observed from 2019 to 2023, culminating in 204 articles in 2023. These results provide a promising outlook for the ongoing growth and advancement of this research field.
FIGURE 2
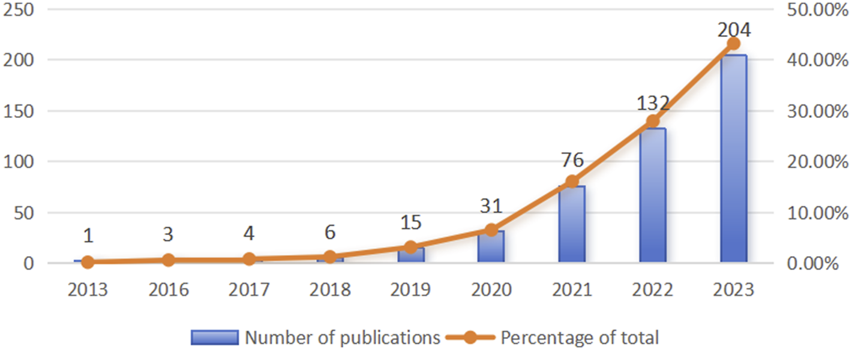
Publication count in the field of ferroptosis under hypoxic conditions from 2013 to 2023.
3.2 Analysis of geographical distribution of publications
As shown in Figure 3A, 42 countries participated in the studies analyzed, with six countries publishing more than ten studies each. China led the tally with the highest number of publications (n = 359), followed by the United States (n = 55), Japan (n = 17), and Germany (n = 17). CiteSpace analysis was also used to visualize country collaborations. As shown in Figure 3B, this network was composed of 42 nodes and 79 links, signifying collaborative ties among China, the United States, Germany, Japan, and Italy. Notably, as show in Table 1, China, the United States, and Germany were identified as the most pivotal participants based on their centrality scores (0.24, 0.21, and 0.17, respectively). The evaluation of both publication volume and centrality indices highlights the pivotal role of China, the United States, Germany, and Japan as leading forces in this research field.
FIGURE 3
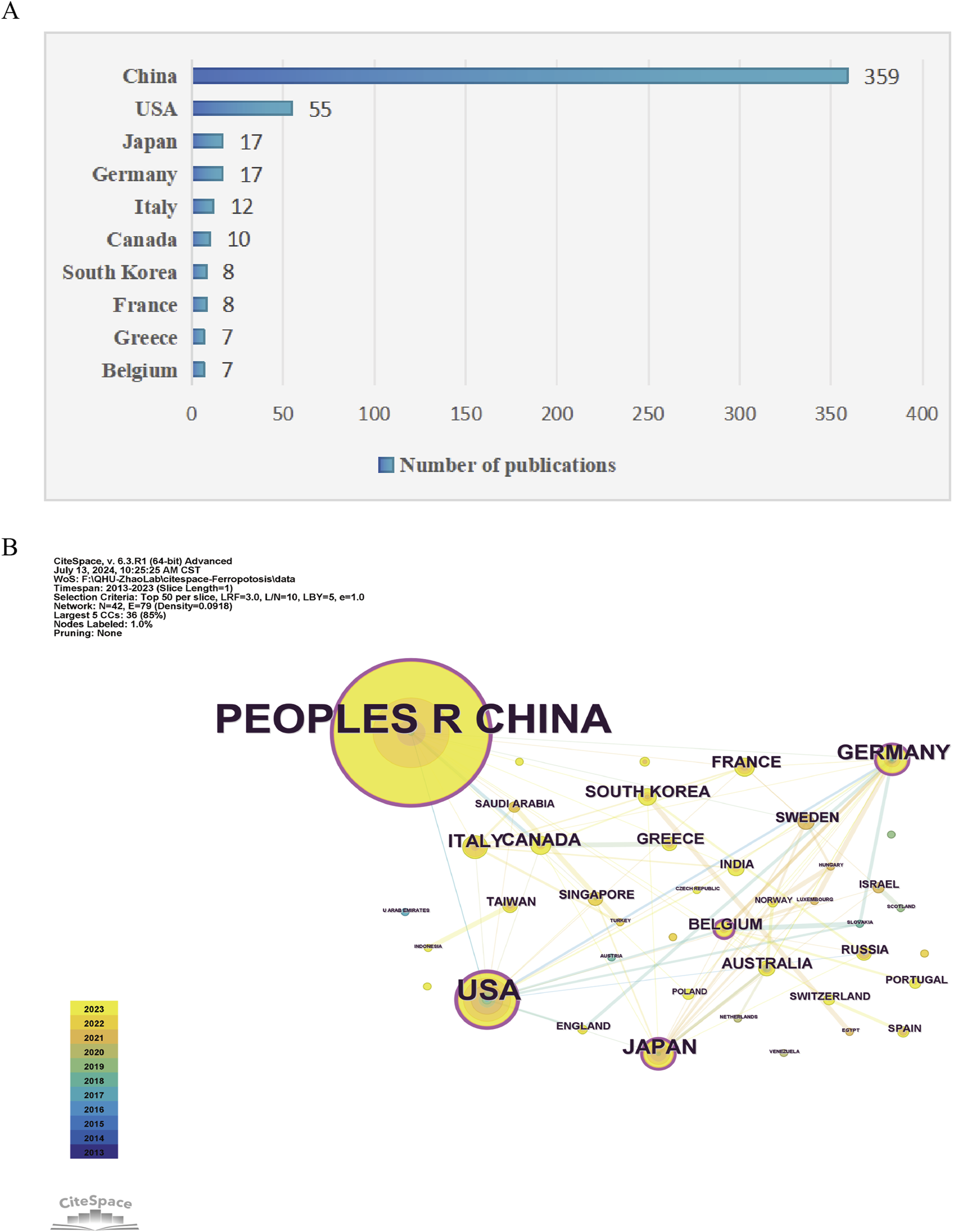
Analysis of geographical distribution of publications. (A) Top ten countries/regions with the most published research on ferroptosis under hypoxic conditions. (B) Visualization of cooperation among countries/regions where publications related to ferroptosis under hypoxic conditions were visualized using CiteSpace. The lines connecting countries/regions signify collaborative engagements. The largest cluster (green dots) included countries such as China and the United States, and cooperation was primarily focused on these.
TABLE 1
| Rank | Country | Count | Centrality | Institute (country) | Count | Centrality |
|---|---|---|---|---|---|---|
| 1 | CHINA | 359 | 0.24 | Wuhan University (China) | 23 | 0.04 |
| 2 | USA | 55 | 0.21 | Chinese Academy of Sciences (China) | 19 | 0.47 |
| 3 | GERMANY | 17 | 0.17 | Sun Yat Sen University (China) | 17 | 0.15 |
| 4 | JAPAN | 17 | 0.2 | Shanghai Jiao Tong University (China) | 15 | 0.11 |
| 5 | ITALY | 12 | 0.07 | Central South University (China) | 14 | 0.02 |
| 6 | C ANADA | 10 | 0.07 | Nanjing University (China) | 14 | 0.16 |
| 7 | FRANCE | 8 | 0.02 | Soochow University (China) | 13 | 0.04 |
| 8 | SOUTH KOREA | 8 | 0.07 | Fudan University (China) | 13 | 0.07 |
| 9 | GREECE | 7 | 0 | Southern Medical University (China) | 12 | 0.04 |
| 10 | AUSTRALIA | 7 | 0.07 | Anhui Medical University (China) | 11 | 0.05 |
Top 10 prolific countries and institutions in the field of ferroptosis under hypoxic conditions.
3.3 Visual analysis of institutions and authors
A total of 218 institutions were involved in the publication of research papers, with 38 of these contributing a minimum of five papers each. The top ten institutions each generated at least 11 papers each (Table 1). Wuhan University published the most articles at 23. The mapping of collaborative institutions, as depicted in Figure 4, revealed a network of 218 nodes interconnected by 525 links, signifying robust cooperative relationships. Wuhan University, Chinese Academy of Sciences, Sun Yat-sen University, Shanghai Jiao Tong University, and Central South University were identified as the most collaborative institutions, demonstrating extensive contributions and strong cooperative bonds among Chinese research institutions in this field. Notably, the Chinese Academy of Sciences had the highest ranking in terms of centrality.
FIGURE 4
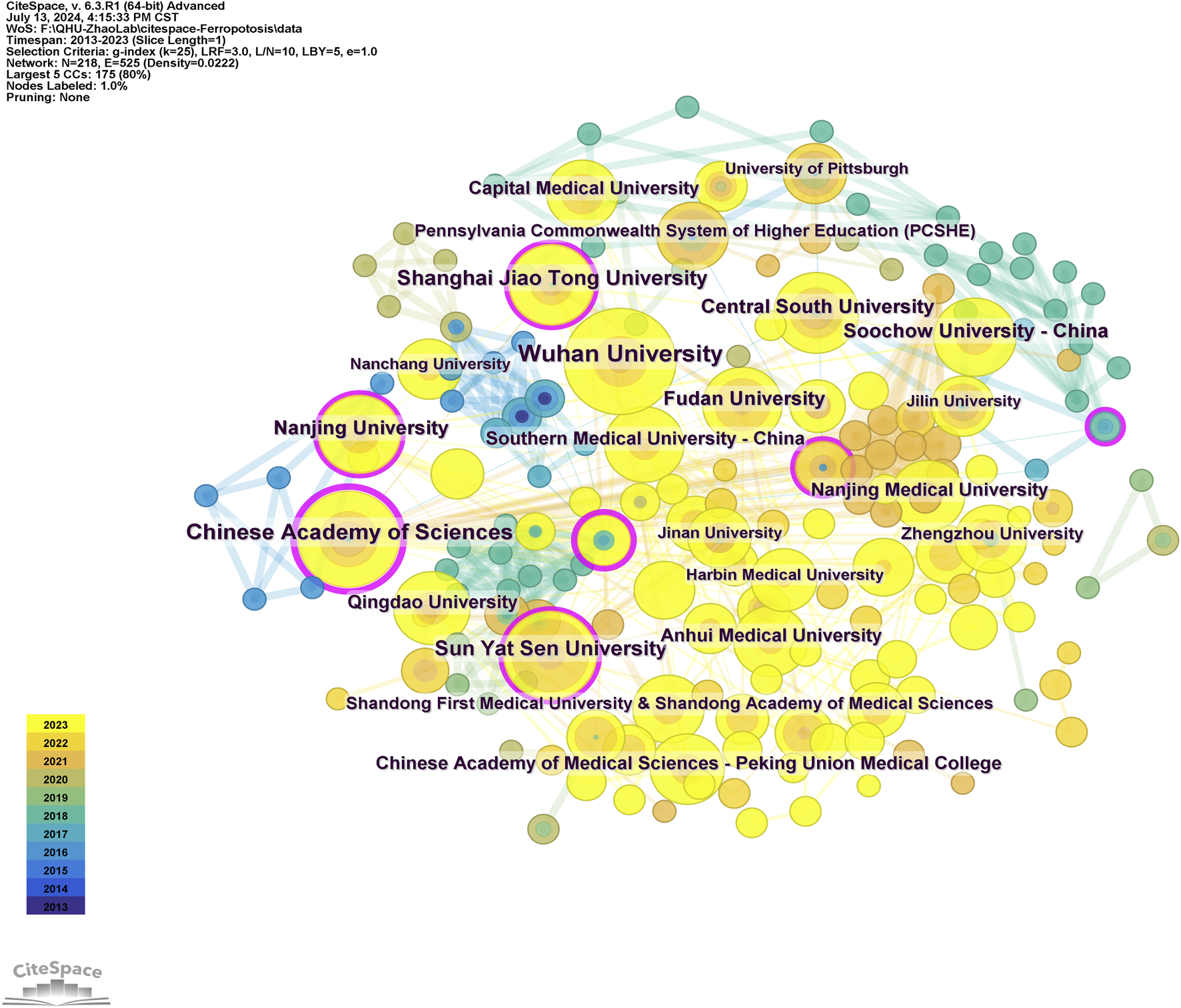
Robust cooperative relationships between institutions.
A total of 390 authors were engaged in research involving ferroptosis under hypoxic conditions. The eight most prolific authors along with their related information are presented in Table 2. The top eight authors collectively published 33 articles. Supuran was at the forefront with four articles, followed by Tang, Daolin, Pissas, Georgios, Kang, Rui, Beharier, Ofer, Eleftheriadis, Theodoros, Liakopoulos, Vassilios, Stefanidis, and Ioannis, each having published three articles. These distinguished authors were associated with six distinct research institutions.
TABLE 2
| Rank | Author | Institute | Count | Centrality |
|---|---|---|---|---|
| 1 | Supuran, ClaudiuT | Florence University | 4 | 0 |
| 2 | Tang, Daolin | Guangzhou Med Univ | 3 | 0 |
| 3 | Pissas, Georgios | Thessaly University | 3 | 0 |
| 4 | Kang, Rui | UT Southwestern Med Ctr | 3 | 0 |
| 5 | Beharier, Ofer | Pittsburgh University | 3 | 0 |
| 6 | Eleftheriadis, Theodoros | Thessaly University | 3 | 0 |
| 7 | Liakopoulos, Vassilios | Thessaly University | 3 | 0 |
| 8 | Stefanidis, Ioannis | Thessaly University | 3 | 0 |
Top eight authors with published research on ferroptosis under hypoxic conditions.
3.4 Visual analysis of journals
The dataset comprised 472 articles from 266 journals. The International Journal of Molecular Sciences had the maximum number of publications, totaling ten articles, followed by Biomedicine and Pharmacotherapy, European Journal of Pharmacology, Frontiers in Cell and Developmental Biology, and Frontiers in Oncology, each with nine articles. A group of 24 journals published more than five articles, totaling 161 articles. The ten leading journals, ranked by volume of publications, are presented in Table 3. Among the top ten journals, nine were classified as Q1, signifying a high level of academic credibility.
TABLE 3
| Rank | Journal | Count | IF (2023) | JCR (2023) |
|---|---|---|---|---|
| 1 | Intermational journal of molecular sciences | 10 | 6.208 | Q1 |
| 2 | Biomedicine and pharmacotherapy | 9 | 7.5 | Q1 |
| 3 | European journal of pharmacology | 9 | 5.0 | Q1 |
| 4 | Frontiers in cell and developmental biology | 9 | 5.5 | Q1 |
| 5 | Frontiers in oncology | 9 | 4.7 | Q2 |
| 6 | Acs applied materials and interfaces | 8 | 9.5 | Q1 |
| 7 | Free radical biology and medicine | 8 | 7.4 | Q1 |
| 8 | Cell death and disease | 7 | 9.0 | Q1 |
| 9 | Frontiers in pharmacology | 7 | 5.6 | Q1 |
| 10 | Redox biology | 7 | 11.4 | Q1 |
Top 10 journals in the field of ferroptosis under hypoxic conditions.
3.5 Analysis of the co-cited representative literature
Table 4 displays the top ten most-cited original articles on ferroptosis in hypoxia research: half for articles and half for reviews. The top ten cited articles were all published in Q1 journals, indicating that their IF and credibility were high. Moreover, the prominence of these studies, all of which were published between 2017 and 2021, highlights the fact that ferroptosis in hypoxia is an emerging research field. The top ten publications collectively received over 418 citations. The most frequently cited article was that of Stockwell et al., which appeared in the journal Cell, garnering 73 citations. This review elucidates the mechanisms underlying ferroptosis and their connection to other areas of biology and medicine regarding this emerging form of regulated cell death (Stockwell et al., 2017).
TABLE 4
| Rank | Cited number | Title | Type | Year | Journal | IF (2023) | JCR (2023) | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 73 | Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease |
Review | 2017 | Cell | 64.5 | Q1 | Stockwell et al. (2017) |
| 2 | 64 | Ferroptosis as a target for proection against cardiomyopathy | Article | 2019 | PNAS | 11.1 | Q1 | Fang et al. (2019) |
| 3 | 61 | Ferroptosis: mechanisms, biology and role in disease | Review | 2021 | Nat Rev Mol Cell Biol | 112.7 | Q1 | Jiang et al. (2021) |
| 4 | 56 | Ferroptosis: past, present and future | Review | 2020 | Cell Death Dis | 9.0 | Q1 | Li et al. (2020a) |
| 5 | 43 | FSP1 is a glutathione-independent ferroptosis suppressor | Article | 2019 | Nature | 64.8 | Q1 | Doll et al. (2019) |
| 6 | 40 | The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis | Article | 2019 | Nature | 64.8 | Q1 | Bersuker et al. (2019) |
| 7 | 38 | Broadening horizons: the role of ferroptosis in cancer | Review | 2021 | Nat Rev Clin Oncol | 78.8 | Q1 | Chen et al. (2021) |
| 8 | 37 | Ferroptosis: molecular mechanisms and health implications | Review | 2020 | Cell Res | 44.1 | Q1 | Tang et al. (2020) |
| 9 | 36 | ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition | Article | 2017 | Nat Chem Biol | 14.8 | Q1 | Doll et al. (2017) |
| 10 | 33 | Hypoxia inhibis frritinophagy, increasesmitochondrial fritin, and proectsfrom ferroptosis | Article | 2020 | Redox Biol | 11.4 | Q1 | Fuhrmann et al. (2020) |
Top 10 most cited original articles on ferroptosis in hypoxia research.
3.6 Keyword analysis
3.6.1 Keyword co-occurrence analysis
Keywords related to ferroptosis under hypoxic conditions were identified and analyzed using the CiteSpace software. The keywords were analyzed over the publication period of 2013–2023. The top five keywords were cell death (n = 104), hypoxia (n = 96), oxidative stress (n = 91), ferroptosis (n = 65), and expression (n = 62). Large centrality values represent more cooperation of one node with other nodes. Centrality analysis revealed that oxidative stress (n = 0.21), ferroptosis (n = 0.18), cell death (n = 0.17), hypoxia (n = 0.12), and expression (n = 0.11) exhibited high centrality and emerged as the most influential keywords.
3.6.2 Keyword cluster analysis
The keyword clustering graph illustrates the structural features of the clusters, highlighting their key nodes and essential connections (Wang et al., 2024). A network map of the keyword clusters was generated using the findings of the co-occurrence analysis. The network map has a rich clustering structure, and the structure is persuasive, as indicated by the modularity Q = 0.4557 > 0.3 and mean silhouette = 0.6916 > 0.5 of the keyword clustering map, both of which denote a well-defined and logical clustering framework that is highly reliable. As depicted in Figure 5, the top eight cluster tag groups (#0–7), which were scrutinized and refined using clustered keywords, encapsulated an overarching research paradigm for studies on ferroptosis under hypoxic conditions. Within these clusters, Cluster 0 (red) displayed topics on ischemia, Cluster 1 (orange) concentrated on photodynamic therapy, Cluster 2 (yellow) was concerned with myocardial infarction, and Cluster 3 (light green) focused on histone deacetylases. Cluster 4 was dedicated to hepatocellular carcinoma research, Cluster 5 (dark green) was centered on carbonic anhydrases, Cluster 6 (light blue) was centered on acute myeloid leukemia, and Cluster 7 (blue) was dedicated to amyotrophic lateral sclerosis.
FIGURE 5
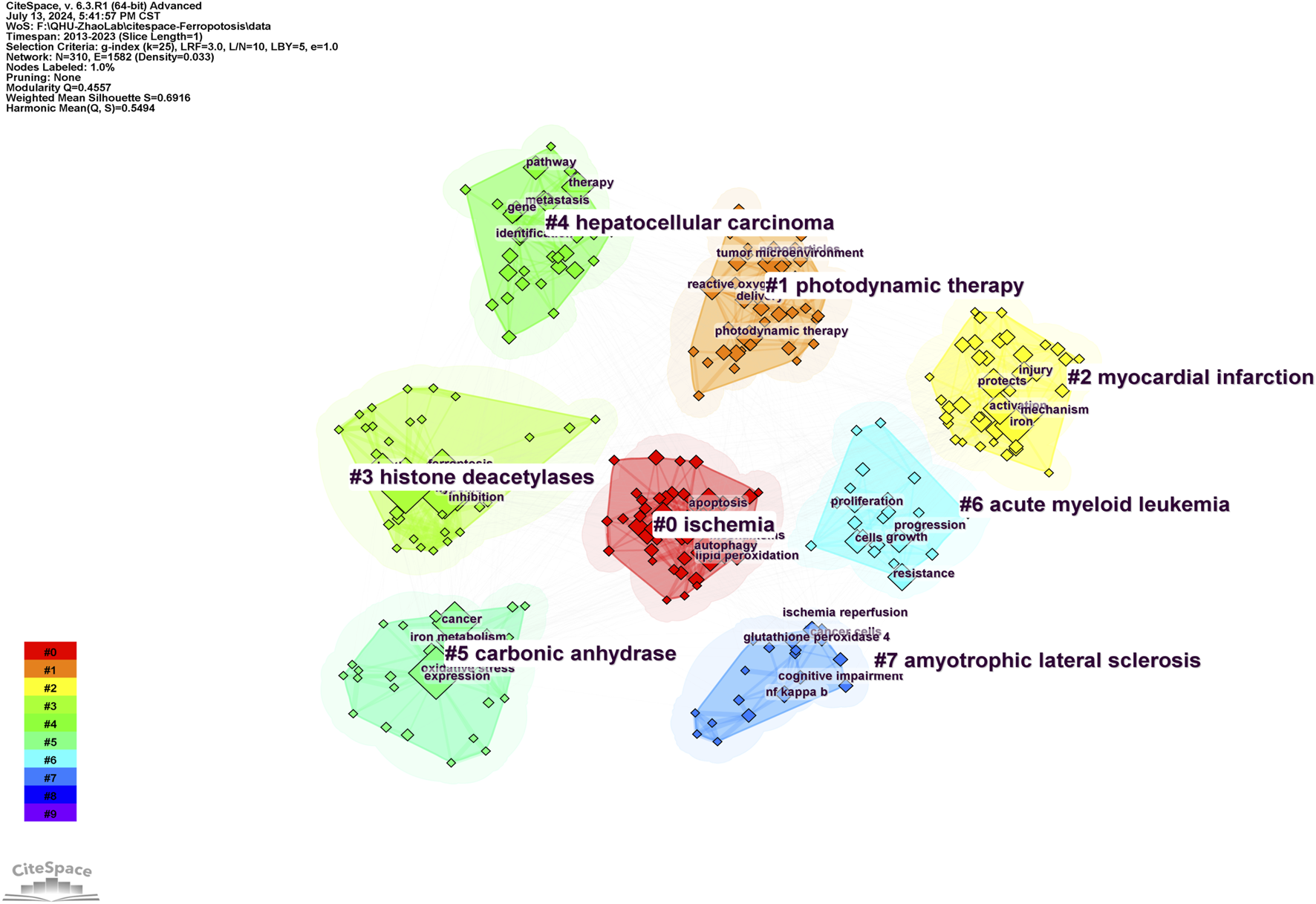
Keyword clustering graph for ferroptosis under hypoxic conditions.
3.6.3 Keyword citation burst analysis
Analysis of keyword citation bursts can reveal research trends over a defined period (Zhang et al., 2024). Using an analysis of keyword co-occurrence, a burst analysis of keyword citations was conducted to identify the top 25 most-cited terms, as shown in Figure 6. In this burst analysis, “Begin” and “End” indicate the time of the burst. “Strength” refers to the strength of a burst and represents credibility over time. By concentrating on terms with pronounced surge rates, trending subjects in this research field can be identified by researchers.
FIGURE 6
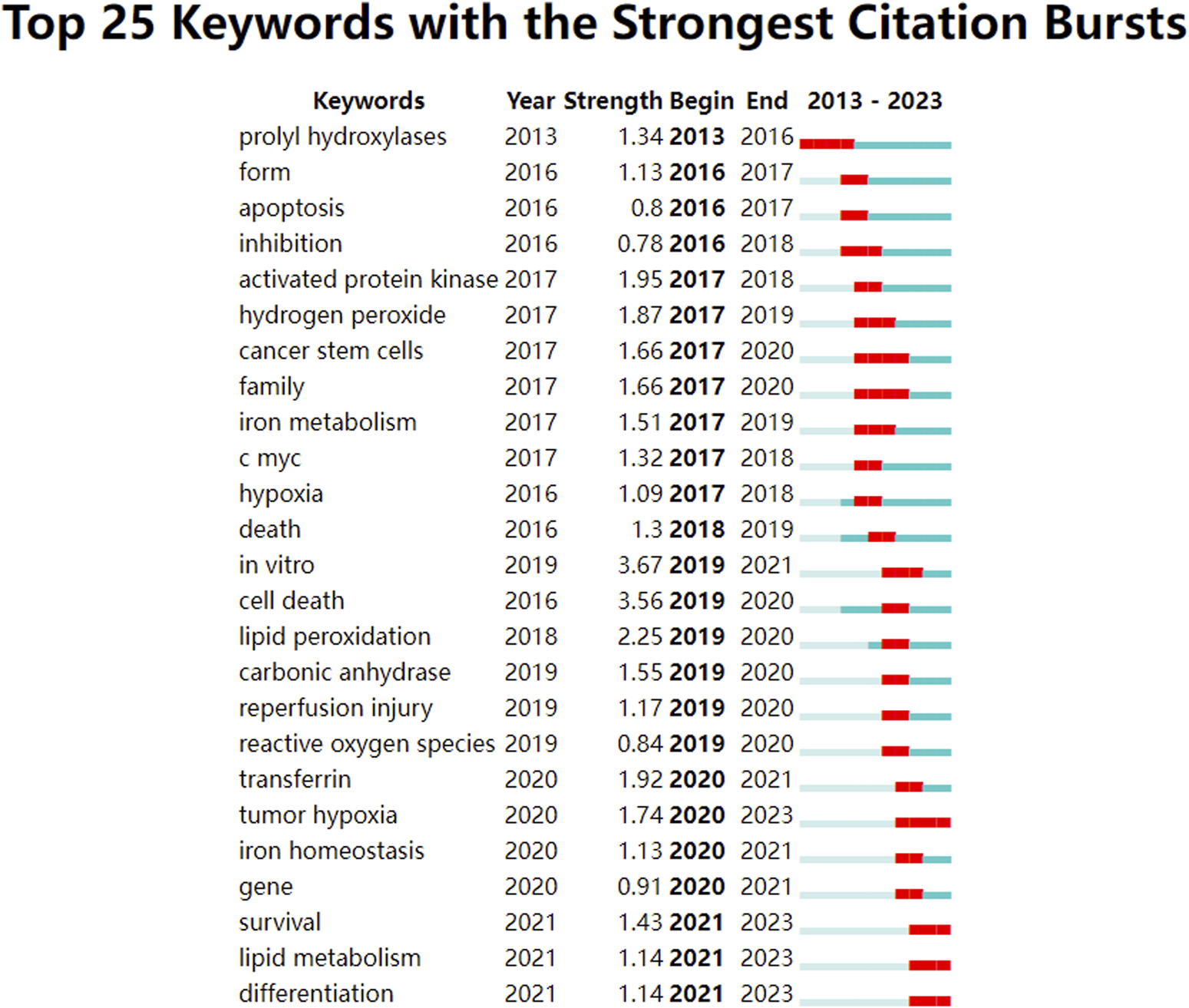
Keywords with intense citation bursts in the field of ferroptosis under hypoxic conditions.
The keyword citation burst analysis delineates that encompassing the years 2013–2017 spotlights terms such as prolyl hydroxylases, apoptosis, inhibition, activated protein kinase, iron metabolism, and c-myc, which predominantly address alterations in physiological processes and underlying molecular dynamics. From 2018 to 2020, terms such as lipid peroxidation, reperfusion injury, reactive oxygen species, transferrin, tumor hypoxia, iron homeostasis, and genes are focused in, thereby highlighting the repercussions of ferroptosis under hypoxic conditions. From 2021 to 2023, the terms survival, lipid metabolism, and differentiation are commonly observed, thus emphasizing the influence and underlying mechanisms of various diseases. The keywords from 2021 to 2023 remain relevant today and represent emerging trends and future research directions.
4 Discussion
4.1 General overview
Over the past 10 years, a significant increase in academic attention and investigative work on ferroptosis under hypoxic conditions can be noted, culminating in an expanding volume of studies annually. This quantitative literature review used CiteSpace to analyze and portray 472 papers on this subject to uncover critical research hotspots and trends. In 2012, a novel molecular entity, erastin, was shown to enhance peroxide accumulation during Fe2+ synthesis, leading to intracellular mitochondrial atrophy and increased membrane density. This unique mechanism results in a distinct form of cell death, known as ferroptosis (Dixon et al., 2012). Initial research on ferroptosis was limited. However, from 2018 to 2020, a marked increase in the volume of publications was recorded, signifying an expanded scope and depth of research. After 2020, a swift increase in this trend was noted. This pattern highlights the vast potential of ferroptosis under hypoxic conditions, which is garnering increasing interest from researchers. Therefore, this field of study is expected to continue to increase in popularity in coming years.
Within the ranking of the top ten countries contributing to the literature in this research field, nine were developed countries, with China being the sole developing country. However, China had the highest number of publications and its centrality score (0.24) was the highest, indicating benign connections with developed countries in the field.
An analysis of the institutional cooperation network revealed that institutions in China, such as Wuhan University, Chinese Academy of Sciences, Sun Yat Sen University, Shanghai Jiao Tong University, and Central South University, were leading in research on ferroptosis under hypoxic conditions. The top eight scholars listed in Table 2 were largely associated with developed countries. The research group of Supuran at the University of Florence focused primarily on the conception of groundbreaking treatment plans for malignant solid tumors, aiming to counteract the progression and drug resistance induced by hypoxic and acidic conditions within tumors. Their investigative work has revealed that the use of carbonic anhydrase inhibitors potentiates ferroptosis, leading to a significant reduction in tumor expansion (Nerella et al., 2022; Yusuf et al., 2022; Supuran, 2021). Tang et al. elucidated a pioneering molecular process underlying sorafenib resistance and proposed that MT-1G functions as an emergent modulator of ferroptosis in hepatocellular carcinoma cells (Sun et al., 2016). Furthermore, they systematically summarized the principal molecular underpinnings of ferroptosis; outlined the interactions between ferroptosis and cancer-related signaling pathways; and explored the possible therapeutic uses of ferroptosis in systemic therapy, radiotherapy, and immunotherapy (Chen et al., 2021; Tang et al., 2021).
Eleftheriadis was primarily committed to investigating the underlying processes of ischemia-reperfusion (I-R) damage and is pursuing efficacious treatment methods aimed at forestalling or mitigating I-R lesions. His research has shown that during I-R injury, periods of oxygen deprivation and subsequent reoxygenation lead to upregulation of the enzyme IDO, also known as indoleamine 2,3-dioxygenase 1. This upregulation triggers apoptosis through GCN2K and ferroptosis through the activation of aryl hydrocarbon receptor (Eleftheriadis et al., 2021a). Furthermore, he discovered that the aryl hydrocarbon receptor becomes active during the reoxygenation phase, causing the generation of reactive oxygen species, lipid peroxidation, and cell death through ferroptosis (Eleftheriadis et al., 2021b). Meanwhile, Myocardial Ischemia-Reperfusion Injury (MIRI) occupies an important position in ischemia-reperfusion (I-R) injury. Many studies have also discovered the relationship between ferroptosis and MIRI. MIRI is a type of cardiac dysfunction commonly seen in conditions like acute myocardial infarction and coronary heart disease (Xiang et al., 2024). During this process, hypoxia due to ischemia leads to calcium overload, which in turn causes mitochondrial dysfunction and subsequently triggers cardiomyocyte ferroptosis (Jiang et al., 2016). Elevated ROS levels promote ferroptosis by triggering the Fenton reaction, decreasing GPX4 and Nrf2 levels, and reducing HIF-1 due to hypoxia. Additionally, hypoxia impairs the mitochondrial electron transport chain (ETC.), leading to higher ROS production and inducing ferroptosis (Zhao et al., 2023). Moreover, over-activation of HIF due to hypoxia upregulates TfR (transferrin receptor) expression, causing iron overload during MIRI and ultimately leading to ferroptosis (Zhang et al., 2019). In summary, calcium overload, excessive ROS, and iron overload driven by HIF all contribute to the occurrence of ferroptosis. Therefore, ferroptosis is intrinsically linked to cardiomyocyte damage in MIRI under hypoxic conditions.
The analysis of publication output and citations revealed that among the top ten journals with the most published articles. The International Journal of Molecular Sciences published the most articles in this field; however, it was cited less frequently. Free Radical Biology and Medicine and Redox Biology published fewer articles, but the relevant articles in these journals were highly cited. Free Radical Biology and Medicine published several highly cited articles related to hypoxia and ferroptosis in different diseases, including osteoporosis, acute kidney injury, and myocardial ischemia/reperfusion injury. The journal Redox Biology published diverse studies on the association between hypoxia and ferroptosis. An article in this journal reported that under conditions of oxygen deficiency, the process of ferritinophagy is suppressed, whereas the levels of mitochondrial ferritin (FTMT) are augmented and the onset of ferroptosis is averted. These findings facilitate our understanding of the regulatory mechanisms of FTMT under hypoxic conditions and indicate a connection between ferritinophagy and the susceptibility of macrophages to ferroptosis (Fuhrmann et al., 2020). Another study revealed that hypoxia-triggered HIF-1α significantly upregulates lncRNA-PMAN, thereby modulating ferroptosis through the stabilization of SLC7A11 mRNA under hypoxic conditions within GC cells and promotes the formation of peritoneal disseminated tumors in vitro (Lin et al., 2022). Furthermore, other prestigious journals such as ACS Applied Materials and Interfaces and Cell Death and Disease also published valuable contributions to the field of ferroptosis under hypoxia research.
4.2 Research hotspots, frontiers, and prospects
The literature citation analysis can effectively reveal research trends, assess academic impact, and construct knowledge structures and research networks. To ensure the accuracy and reliability of the analysis results, we have established a minimum citation threshold to maintain the focus on influential research. Articles with fewer than 25 citations were excluded from our analysis. Additionally, self-citations and citations from the same author are excluded. Our analysis revealed that the review “Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease” by Stockwell et al. published in Cell in 2017 received the highest number of citations. This review highlights the mechanisms underlying ferroptosis, its relevance to various fields of biology and medicine, and proposes methodologies and criteria for examining this novel mode of programmed cell death (Stockwell et al., 2017). The article ranking second in citation frequency, titled “Ferroptosis: A Protective Target Against Cardiomyopathy,” featured in the Proceedings of the National Academy of Sciences of the United States in 2019, disclosed that mitochondrial oxidative harm is a predominant factor in heart injury stemming from ferroptosis. Moreover, the use of ferrostatin-1 and iron chelators improved cardiac dysfunction triggered by both sudden and prolonged I-R episodes in murine models, highlighting the potential of ferroptosis-targeted therapies to safeguard against cardiomyopathy (Fang et al., 2019). The article ranking thrid in citation frequency, titled “Ferroptosis: mechanisms, biology and role in disease,” featured in Nat Rev Mol Cell Biol in 2021, it revealed the potential physiological functions of ferroptosis in tumor suppression and immune surveillance, and its pathological roles, including roles of ferroptosis in neurotoxicity and brain injuries. Refers to brain injuries, Ischemic Stroke (IS) occupies an extremely important position in brain injuries due to its high incidence and disability rate (Zhu et al., 2024). IS primarily results from focal cerebral ischemia and hypoxia due to the obstruction of cerebral blood flow (Xu et al., 2023). A study revealed that hypoxia caused by IS upregulates the expression of ferritin and TfR1, which enhances iron uptake by neurons, leading to increased intracellular iron levels and subsequently triggering ferroptosis (Lan et al., 2020). Additionally, the deficiency of ceruloplasmin under hypoxic conditions can disrupt iron metabolism and exacerbate oxidative damage, facilitating iron accumulation-induced ferroptosis (Ryan et al., 2018). It has been reported that elevated glutamate levels under hypoxia can induce ferroptosis by disrupting intracellular iron homeostasis and causing brain injury (Li et al., 2017). Moreover, Oxidative stress induced by hypoxia results in excessive ROS (reactive oxygen species) accumulation, which activates Nrf2 (nuclear factor erythroid 2-related factor 2), thereby increasing GSH, SLC7A11, and GPX4 (glutathione peroxidase 4) to protect cells from ferroptosis in IS(Kwak et al., 2002; Dodson et al., 2019). Meanwhile, the accumulation of glutamate inhibits cystine uptake via the suppression of system Xc−, leading to decreased glutathione (GSH) levels and promoting ferroptosis through the activation of ATF4-mediated ferroptotic genes in IS and increased glutamate under hypoxia also contributes to ROS production, which activates Nrf2 and inhibits ferroptosis (Speer et al., 2013; Shibata and Kobayashi, 2008). The highly cited articles that we have analyzed here had many promoting effects on subsequent research. These articles provide a comprehensive foundational framework, elucidate mechanistic pathways, identify regulatory factors and therapeutic targets, link ferroptosis to pathological conditions, standardize research methods, and highlight future research directions. They have facilitated a deeper understanding of this regulated form of cell death and its impact on human health and disease.
Keywords play a crucial role in identifying emerging trends and guiding future research. The keyword clustering graph shows that ischemia, photodynamic therapy, myocardial infarction, histone deacetylases, hepatocellular carcinoma, carbonic anhydrase, acute myeloid leukemia, and amyotrophic lateral sclerosis were popular topics in this field. Keywords with the strongest citation burst analysis visually represent changes in research focus within this field over a specified period and show both continuity and variability in research hotspots. The top ten emerging keywords in this field were “in vitro,” “cell death,” “lipid peroxidation,” “activated protein kinase,” “transferrin,” “hydrogen peroxide,” “tumor hypoxia,” “cancer stem cells,” “family,” and “carbonic anhydrase.” Notably, “in vitro” and “cell death” ranked first and second in terms of emergence strength (strength = 3.67 and 3.56, respectively). The keywords“in vitro”well reflects the important role of in vitro experiments in this field. Significant progress has been made in the study of ferroptosis under hypoxic conditions through in vitro experiments, revealing the complex relationship between hypoxia and ferroptosis and providing new insights for the treatment of related diseases. These studies include the regulatory mechanisms of hypoxia on ferroptosis, the inhibition of ferroptosis in vitro experiments, the cell type-specific differences in hypoxia-induced ferroptosis and the interplay between ferroptosis and hypoxia in the tumor microenvironment (Zheng et al., 2023; Li et al., 2022; Liu et al., 2024; Li et al., 2024). Future research will further elucidate the molecular mechanisms of ferroptosis under hypoxic conditions, particularly the interactions between the HIF signaling pathway and iron metabolism or lipid peroxidation. Utilizing in vitro cell models to study hypoxia-induced ferroptosis will facilitate the development of novel therapeutic strategies targeting ferroptosis, especially in the treatment of neurodegenerative diseases and cancer (Costa et al., 2023; Wang et al., 2021). Moreover, combining gene editing, metabolomics and cell biology techniques will deepen the understanding of the regulatory network of ferroptosis under hypoxic conditions, providing theoretical support for clinical applications. The keyword “cell death” reflects the complex interplay between ferroptosis and hypoxia in cell death mechanisms, emphasizing the coordinated regulation of multiple cell death pathways under hypoxic conditions. Under hypoxic conditions, cell death involves the interplay of multiple cell death pathways. Hypoxia-induced cell death is not only associated with ferroptosis but also closely related to other cell death pathways, such as apoptosis, necroptosis, and pyroptosis, these pathways interact in complex ways (Yang et al., 2021; Lenihan and Taylor, 2013). Notably, there is a significant interaction between ferroptosis and PANoptosis (a composite form of cell death that includes pyroptosis, apoptosis, and necroptosis). Studies have shown that hypoxia-induced cell death can be alleviated by the combined use of ferroptosis inhibitors and PANoptosis inhibitors, indicating that both pathways may be simultaneously activated under hypoxic conditions (Wu et al., 2025). Refers to the key word “lipid peroxidation”, It has been reported that hypoxia caused by COVID-19 can increase circulating ferritin levels, leading to iron deficiency, oxidative stress, and lipid peroxidation, which ultimately result in ferroptosis (Qiu et al., 2024). The keywords “tumor hypoxia,” “cancer stem cells” revealed the high correlation between ferroptosis and cancer. Increasing evidence indicates that cancer development is associated with ferroptosis, which is often suppressed in various cancers, including hepatocellular carcinoma, pancreatic cancer, and gastric cancer. Moreover, the connection between cancer and ferroptosis is closely linked to hypoxia. Many solid tumors exhibit hypoxic regions due to inadequate blood flow (Li et al., 2021). Studies have shown that hypoxia is related to the interplay between SLC7A11 and ferroptosis (Zhang et al., 2018; Badgley et al., 2020). Specifically, hypoxia upregulates HIF-1, which in turn increases ELAVL1 levels. ELAVL1 then binds to SLC7A11, enhancing its expression and thereby promoting cancer cell growth by inhibiting ferroptosis (Lin et al., 2022). Under hypoxic conditions, SLC7A11 is upregulated through the inhibition of METTL14, which reduces reactive oxygen species (ROS) and thereby suppresses ferroptosis, thus facilitating the progression of hepatocellular carcinoma (HCC) (Fan et al., 2021). Additionally, hypoxia enhances the activity of HIF-1 transcription, leading to increased TfR1 and DMT1 expression, which helps cervical cancer (CC) cells resist ferroptosis (Christova and Templeton, 2007). However, the mechanisms underlying the relationship between ferroptosis and cancer under hypoxia are still not fully understood and require further investigation.
Overall, this study used the CiteSpace 6.2.R7 software to visualize and analyze the literature on ferroptosis under hypoxic conditions within the WoSCC database. However, this study had several limitations. First, the analysis only included articles from WoSCC and ignored articles from other databases, such as CNKI and Scopus. In addition, because citations are expected to peak gradually 3–10 years after publication, the current analysis may not have highlighted recently published articles.
5 Conclusion
Research on ferroptosis under hypoxic conditions is currently in the early stages of development. Based on current global trends, a significant increase in publications and the number of researchers actively involved in this topic is expected. Notable advancements have been achieved in research on ferroptosis related to hypoxia-related diseases. Significant progress has been made in the study of ferroptosis in relation to hypoxia-related diseases. The focal point of this study was the Asian continent, with China emerging as the most prolific contributor to this area of study. The primary areas of interest were concentrated on deciphering the modulatory mechanisms of hypoxia-associated diseases through the ferroptosis pathway and identifying potential therapeutic targets.
Statements
Author contributions
YH: Writing–original draft, Writing–review & editing, Data curation, Software. MM: Software, Writing–review and editing, Resources. DY: Software, Writing–review and editing. WL: Software, Resources, Writing–review and editing. XZ: Resources, Software, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Agathokleous E. Calabrese E. J. (2024). Evolution of hormesis research: a bibliometric analysis. Arch. Toxicol.98, 577–578. 10.1007/s00204-023-03635-9
2
Ahmad P. Asif J. A. Alam M. K. Slots J. (2020). A bibliometric analysis of Periodontology 2000. Periodontol. 82, 286–297. 10.1111/prd.12328
3
Badgley M. A. Kremer D. M. Maurer H. C. Delgiorno K. E. Lee H. J. Purohit V. et al (2020). Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science368, 85–89. 10.1126/science.aaw9872
4
Cao G. Yang C. Jin Z. Wei H. Xin C. Zheng C. et al (2022). FNDC5/irisin reduces ferroptosis and improves mitochondrial dysfunction in hypoxic cardiomyocytes by Nrf2/HO-1 axis. Cell Biol. Int.46, 723–736. 10.1002/cbin.11763
5
Chen C. Song M. (2019). Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One14, e0223994. 10.1371/journal.pone.0223994
6
Chen G. Wu K. Li H. Xia D. He T. (2022). Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol.12, 961637. 10.3389/fonc.2022.961637
7
Chen X. Kang R. Kroemer G. Tang D. (2021). Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol.18, 280–296. 10.1038/s41571-020-00462-0
8
Christova T. Templeton D. M. (2007). Effect of hypoxia on the binding and subcellular distribution of iron regulatory proteins. Mol. Cell Biochem.301, 21–32. 10.1007/s11010-006-9393-2
9
Costa I. Barbosa D. J. Silva V. Benfeito S. Borges F. Remiao F. et al (2023). Research models to study ferroptosis's impact in neurodegenerative diseases. Pharmaceutics15, 1369. 10.3390/pharmaceutics15051369
10
Dixon S. J. Lemberg K. M. Lamprecht M. R. Skouta R. Zaitsev E. M. Gleason C. E. et al (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell149, 1060–1072. 10.1016/j.cell.2012.03.042
11
Dodson M. Castro-Portuguez R. Zhang D. D. (2019). NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol.23, 101107. 10.1016/j.redox.2019.101107
12
Dong B. Ding C. Xiang H. Zheng J. Li X. Xue W. et al (2022). USP7 accelerates FMR1-mediated ferroptosis by facilitating TBK1 ubiquitination and DNMT1 deubiquitination after renal ischemia-reperfusion injury. Inflamm. Res.71, 1519–1533. 10.1007/s00011-022-01648-1
13
Eleftheriadis T. Pissas G. Filippidis G. Liakopoulos V. Stefanidis I. (2021a). Reoxygenation induces reactive oxygen species production and ferroptosis in renal tubular epithelial cells by activating aryl hydrocarbon receptor. Mol. Med. Rep.23, 41. 10.3892/mmr.2020.11679
14
Eleftheriadis T. Pissas G. Golfinopoulos S. Liakopoulos V. Stefanidis I. (2021b). Role of indoleamine 2,3-dioxygenase in ischemia-reperfusion injury of renal tubular epithelial cells. Mol. Med. Rep.23, 472. 10.3892/mmr.2021.12111
15
Fang X. Wang H. Han D. Xie E. Yang X. Wei J. et al (2019). Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A.116, 2672–2680. 10.1073/pnas.1821022116
16
Fan Z. Yang G. Zhang W. Liu Q. Liu G. Liu P. et al (2021). Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2-dependent silencing of SLC7A11. J. Cell Mol. Med.25, 10197–10212. 10.1111/jcmm.16957
17
Fuhrmann D. C. Brune B. (2022). A graphical journey through iron metabolism, microRNAs, and hypoxia in ferroptosis. Redox Biol.54, 102365. 10.1016/j.redox.2022.102365
18
Fuhrmann D. C. Mondorf A. Beifuss J. Jung M. Brune B. (2020). Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol.36, 101670. 10.1016/j.redox.2020.101670
19
Gao X. Hu W. Qian D. Bai X. He H. Li L. et al (2023). The mechanisms of ferroptosis under hypoxia. Cell Mol. Neurobiol.43, 3329–3341. 10.1007/s10571-023-01388-8
20
Jiang Y. Q. Chang G. L. Wang Y. Zhang D. Y. Cao L. Liu J. (2016). Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell Physiol. Biochem.39, 407–421. 10.1159/000445634
21
Jiang S. Liu Y. Zheng H. Zhang L. Zhao H. Sang X. et al (2023). Evolutionary patterns and research frontiers in neoadjuvant immunotherapy: a bibliometric analysis. Int. J. Surg.109, 2774–2783. 10.1097/JS9.0000000000000492
22
Jiang X. Stockwell B. R. Conrad M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol.22, 266–282. 10.1038/s41580-020-00324-8
23
Kwak M. K. Itoh K. Yamamoto M. Kensler T. W. (2002). Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell Biol.22, 2883–2892. 10.1128/mcb.22.9.2883-2892.2002
24
Lan B. Ge J. W. Cheng S. W. Zheng X. L. Liao J. He C. et al (2020). Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J. Integr. Med.18, 344–350. 10.1016/j.joim.2020.01.008
25
Lenihan C. R. Taylor C. T. (2013). The impact of hypoxia on cell death pathways. Biochem. Soc. Trans.41, 657–663. 10.1042/BST20120345
26
Liang D. Minikes A. M. Jiang X. (2022). Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell82, 2215–2227. 10.1016/j.molcel.2022.03.022
27
Li D. Zhang Z. Wang L. (2024). Emerging role of tumor microenvironmental nutrients and metabolic molecules in ferroptosis: mechanisms and clinical implications. Biomed. Pharmacother.179, 117406. 10.1016/j.biopha.2024.117406
28
Li J. Cao F. Yin H. L. Huang Z. J. Lin Z. T. Mao N. et al (2020a). Ferroptosis: past, present and future. Cell Death Dis.11, 88. 10.1038/s41419-020-2298-2
29
Lin Z. Song J. Gao Y. Huang S. Dou R. Zhong P. et al (2022). Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol.52, 102312. 10.1016/j.redox.2022.102312
30
Li P. Lv X. Liu L. Peng M. Qin D. (2022). The role of ferroptosis-related molecules and significance of ferroptosis score in cervical cancer. J. Oncol.2022, 7835698. 10.1155/2022/7835698
31
Li T. Jiang D. Wu K. (2020b). p62 promotes bladder cancer cell growth by activating KEAP1/NRF2-dependent antioxidative response. Cancer Sci.111, 1156–1164. 10.1111/cas.14321
32
Liu X. Q. Shi M. Z. Bai Y. T. Su X. L. Liu Y. M. Wu J. C. et al (2024). Hypoxia and ferroptosis. Cell Signal122, 111328. 10.1016/j.cellsig.2024.111328
33
Liu F. Jiang L. J. Zhang Y. X. Xu S. T. Liu S. L. Ye J. T. et al (2023). Inhibition of miR-214-3p attenuates ferroptosis in myocardial infarction via regulating ME2. Biochem. Biophys. Res. Commun.661, 64–74. 10.1016/j.bbrc.2023.04.031
34
Li Y. Yu P. Chang S. Y. Wu Q. Yu P. Xie C. et al (2017). Hypobaric hypoxia regulates brain iron homeostasis in rats. J. Cell Biochem.118, 1596–1605. 10.1002/jcb.25822
35
Li Y. Zhao L. Li X. F. (2021). Hypoxia and the tumor microenvironment. Technol. Cancer Res. Treat.20, 15330338211036304. 10.1177/15330338211036304
36
Mcclelland G. B. Scott G. R. (2019). Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu. Rev. Physiol.81, 561–583. 10.1146/annurev-physiol-021317-121527
37
Nerella S. G. Singh P. Arifuddin M. Supuran C. T. (2022). Anticancer carbonic anhydrase inhibitors: a patent and literature update 2018-2022. Expert Opin. Ther. Pat.32, 833–847. 10.1080/13543776.2022.2083502
38
Ow C. P. C. Ngo J. P. Ullah M. M. Barsha G. Meex R. C. Watt M. J. et al (2018). Absence of renal hypoxia in the subacute phase of severe renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol.315, F1358-F1369–F1369. 10.1152/ajprenal.00249.2018
39
Qiu B. Zandkarimi F. Saqi A. Castagna C. Tan H. Sekulic M. et al (2024). Fatal COVID-19 pulmonary disease involves ferroptosis. Nat. Commun.15, 3816. 10.1038/s41467-024-48055-0
40
Ryan F. Zarruk J. G. Losslein L. David S. (2018). Ceruloplasmin plays a neuroprotective role in cerebral ischemia. Front. Neurosci.12, 988. 10.3389/fnins.2018.00988
41
Saeed H. Martini N. D. Scahill S. (2024). Exploring telepharmacy: a bibliometric analysis of past research and future directions. Res. Soc. Adm. Pharm.20, 805–819. 10.1016/j.sapharm.2024.04.017
42
Shibata N. Kobayashi M. (2008). The role for oxidative stress in neurodegenerative diseases. Brain Nerve60, 157–170. 10.11477/mf.1416100224
43
Speer R. E. Karuppagounder S. S. Basso M. Sleiman S. F. Kumar A. Brand D. et al (2013). Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: from ferroptosis to stroke. Free Radic. Biol. Med.62, 26–36. 10.1016/j.freeradbiomed.2013.01.026
44
Stockwell B. R. Friedmann Angeli J. P. Bayir H. Bush A. I. Conrad M. Dixon S. J. et al (2017). Ferroptosis: a regulated cell death Nexus linking metabolism, redox biology, and disease. Cell171, 273–285. 10.1016/j.cell.2017.09.021
45
Stockwell B. R. Jiang X. Gu W. (2020). Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol.30, 478–490. 10.1016/j.tcb.2020.02.009
46
Sun X. Niu X. Chen R. He W. Chen D. Kang R. et al (2016). Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology64, 488–500. 10.1002/hep.28574
47
Supuran C. T. (2021). Carbonic anhydrase inhibitors: an update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin. Investig. Drugs30, 1197–1208. 10.1080/13543784.2021.2014813
48
Tang D. Chen X. Kang R. Kroemer G. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Res.31, 107–125. 10.1038/s41422-020-00441-1
49
Ursini F. Maiorino M. (2020). Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic. Biol. Med.152, 175–185. 10.1016/j.freeradbiomed.2020.02.027
50
Wang C. Zhang Y. Zhang Y. Li B. (2024). A bibliometric analysis of gastric cancer liver metastases: advances in mechanisms of occurrence and treatment options. Int. J. Surg.110, 2288–2299. 10.1097/JS9.0000000000001068
51
Wang X. Liu Z. Ma L. Yu H. (2021). Ferroptosis and its emerging role in tumor. Biophys. Rep.7, 280–294. 10.52601/bpr.2021.210010
52
Wu M. F. Peng X. Zhang M. C. Guo H. Xie H. T. (2025). Ferroptosis and PANoptosis under hypoxia pivoting on the crosstalk between DHODH and GPX4 in corneal epithelium. Free Radic. Biol. Med.228, 173–182. 10.1016/j.freeradbiomed.2024.12.050
53
Xiang Q. Yi X. Zhu X. H. Wei X. Jiang D. S. (2024). Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol. Metab.35, 219–234. 10.1016/j.tem.2023.10.010
54
Xu Y. Li K. Zhao Y. Zhou L. Liu Y. Zhao J. (2023). Role of ferroptosis in stroke. Cell Mol. Neurobiol.43, 205–222. 10.1007/s10571-022-01196-6
55
Yang H. Hu Y. Weng M. Liu X. Wan P. Hu Y. et al (2022). Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J. Adv. Res.37, 91–106. 10.1016/j.jare.2021.10.001
56
Yang J. Hu S. Bian Y. Yao J. Wang D. Liu X. et al (2021). Targeting cell death: pyroptosis, ferroptosis, apoptosis and necroptosis in osteoarthritis. Front. Cell Dev. Biol.9, 789948. 10.3389/fcell.2021.789948
57
Yusuf Z. S. Uysal T. K. Simsek E. Nocentini A. Osman S. M. Supuran C. T. et al (2022). The inhibitory effect of boric acid on hypoxia-regulated tumour-associated carbonic anhydrase IX. J. Enzyme Inhib. Med. Chem.37, 1340–1345. 10.1080/14756366.2022.2072837
58
Zhang L. Zheng H. Jiang S. T. Liu Y. G. Zhang T. Zhang J. W. et al (2024). Worldwide research trends on tumor burden and immunotherapy: a bibliometric analysis. Int. J. Surg.110, 1699–1710. 10.1097/JS9.0000000000001022
59
Zhang Y. Liu D. Hu H. Zhang P. Xie R. Cui W. (2019). HIF-1α/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed. Pharmacother.120, 109464. 10.1016/j.biopha.2019.109464
60
Zhang Y. Shi J. Liu X. Feng L. Gong Z. Koppula P. et al (2018). BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol.20, 1181–1192. 10.1038/s41556-018-0178-0
61
Zhao K. Chen X. Bian Y. Zhou Z. Wei X. Zhang J. (2023). Broadening horizons: the role of ferroptosis in myocardial ischemia-reperfusion injury. Naunyn Schmiedeb. Arch. Pharmacol.396, 2269–2286. 10.1007/s00210-023-02506-5
62
Zheng X. Liang Y. Zhang C. (2023). Ferroptosis regulated by hypoxia in cells. Cells12, 1050. 10.3390/cells12071050
63
Zhu W. He X. Huang D. Jiang Y. Hong W. Ke S. et al (2024). Global and regional burden of ischemic stroke disease from 1990 to 2021: an age-period-cohort analysis. Transl. Stroke Res. 10.1007/s12975-024-01319-9
Summary
Keywords
ferroptosis, hypoxia, bibliometric analysis, hotspot, citespace
Citation
Yu D, Hu Y, Ma M, Li W and Zhao X (2025) The landscape of research on ferroptosis under hypoxic conditions: a bibliometric analysis. Front. Pharmacol. 16:1519000. doi: 10.3389/fphar.2025.1519000
Received
29 October 2024
Accepted
04 March 2025
Published
26 March 2025
Volume
16 - 2025
Edited by
Jean Christopher Chamcheu, Louisiana State University, United States
Reviewed by
Jun Shang, Cincom System, Inc., United States
Lv Zhuangwei, Xinxiang Medical University, China
Tolulope Omolekan, Louisiana State University, United States
Updates
Copyright
© 2025 Yu, Hu, Ma, Li and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Zhao, xiaohuizhao@qhu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.