- 1Department of Hematology and Oncology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Oncology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Introduction: Primary liver cancer, characterized by an insidious onset, rapid progression, high degree of heterogeneity, difficulties in treatment, and a short survival time, poses a significant threat to human health. Jinlong capsule (JLC), an original drug developed in China, is a Chinese patent medicine used to treat liver cancer. Research has demonstrated the antitumor effects of JLC, attributed to its unique preparation process. When used in combination with modern treatment methods, JLC helps in preventing and treating liver cancer recurrence and metastasis, prolonging patient survival, increasing the tumor objective response rate, alleviating gastrointestinal adverse reactions, enhancing survival quality, regulating immune functions of the body, relieving clinical symptoms, and improving patient safety. This study provides a review of clinical and basic research results on JLC.
Methods: We performed literature searches in the Cochrane Library, Embase, PubMed, OVID Scopus, China Biology Medicine, China National Knowledge Infrastructure, VIP, and Wanfang databases for articles published from database inception to December 2023.
Results: Basic research has revealed that the effects of JLC include the inhibition of tumor growth in vivo and in vitro and immune modulation. The possible mechanisms include inhibiting tumor cell proliferation, promoting tumor cell apoptosis, inhibiting angiogenesis, and modulating cellular immunity.
Discussion: By attuning to the complex biological characteristics of liver cancer, harnessing the unique and unconventional advantages of traditional Chinese medicine, and focusing on clinical needs, we propose directions for future evidence-based research on using JLC in the prevention and treatment of liver cancer. This will contribute to the development of precision synergistic strategies that combine traditional Chinese medicine and modern medicine treatment methods.
1 Introduction
Primary liver cancer is the third leading cause of cancer-related death worldwide. A total of 865,269 were diagnosed worldwide in 2022 with a mortality of 757,948 (Bray et al., 2023). Primary liver cancer is currently the fourth most common malignant tumor and the second leading cause of death from tumors in China (Zhou et al., 2019). It includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, and combined hepatocellular-cholangiocarcinoma, with HCC accounting for 75%–85% of cases (Sung et al., 2021). In 2020, China recorded 410,038 new cases (accounting for 45.3% of new cases worldwide) of liver cancer and 391,152 liver cancer-related deaths (accounting for 47.1% of deaths worldwide) (World Health Organization, 2020). Approximately 70% of Chinese patients with HCC have middle-to late-stage disease at the time of initial consultation. In China, common treatment methods for liver cancer include hepatectomy (liver resection), liver transplantation, interventional therapy, radiotherapy, systemic therapy, and traditional Chinese medicine (TCM). In the current treatment landscape, the recurrence rate of primary liver cancer at 5 years after surgery is approximately 70% (Yang et al., 2019), and the 5-year overall survival (OS) of patients with liver cancer in China is merely 12.1% (Chinese Society of Liver Cancer, 2021). Therefore, early diagnosis and treatment, multidisciplinary collaboration, and combination therapy are crucial for improving survival and quality of life.
TCM has a long history and demonstrates unique advantages in the prevention and treatment of liver cancer, applicable at different stages of the disease. With the increasing depth of research on the biological characteristics of liver cancer, such as its high degree of heterogeneity and complex tumor microenvironment, the advantages of the holistic concept and multi-target synergy in TCM have become increasingly prominent and form a unique and important component of China’s comprehensive liver cancer prevention and treatment strategy. Jinlong capsule (JLC), an original drug produced from fresh medicinal animal materials by a unique process, is a Chinese patent drug used for the treatment of liver cancer. Since its market entry in 1998, JLC has been widely adopted in clinical practice. Clinical studies have reported that the use of JLC as monotherapy or in combination with modern treatment methods, such as surgery, transarterial chemoembolization (TACE), and radiofrequency ablation (RFA), provides definite advantages in preventing and treating cancer recurrence and metastasis, prolonging patient survival, increasing the tumor objective response rate (ORR), enhancing survival quality, and regulating immune functions of the body, with a favorable safety profile (Li, 2003; Ma et al., 2017; Yan et al., 2021). The use of JLC has been recommended in several Chinese guidelines and consensus statements, including the Chinese Society of Clinical Oncology guidelines on the diagnosis and treatment of primary liver cancer (2020 edition), the expert consensus on the reasonable use of Chinese patent drugs in standardized treatment of cancer pain (2021 edition), the guidelines on the diagnosis and treatment of primary liver cancer promulgated by the National Health Commission of the People’s Republic of China in 2022, and the guidelines for the diagnosis and treatment of integrated Chinese and Western medicine: primary liver cancer (2023 edition) (Chinese Society of Clinical Oncology, 2020; National Health Commission of the People’s Republic of China, 2022; Fan et al., 2021; caim.org, 2023).
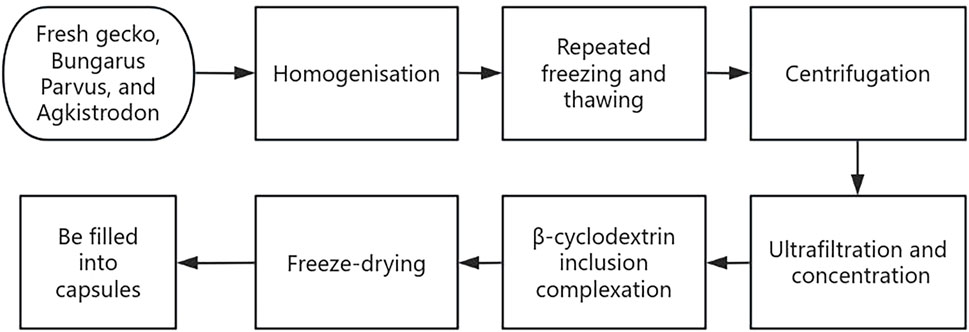
The clinical presentation of most patients with primary liver cancer includes fatigue, pain, abdominal distension, loss of appetite, irritability, and hepatosplenomegaly; all of which are symptoms of blood stasis and stagnation syndrome according to the TCM theory. Therefore, treatment should be performed in accordance with the principles of “blood stasis removal and static blood dispersion” and “depression relief and collateral clearing.” JLC was developed based on the TCM theory, is indicated for blood stasis and stagnation syndrome in primary liver cancer, and comprises fresh gecko, Bungarus Parvus, and Agkistrodon. Preparation of JLC involves the extraction, homogenization, repeated freezing and thawing, centrifugation, ultrafiltration, and freeze-drying of fresh medicinal animal materials using modern cryogenic and biochemical separation and extraction techniques (Figure 1). The drug is rich in amino acids, proteins, free monosaccharides, polysaccharides, and small molecules, with up to 98.6% of its components having a molecular weight of <10,000 Da. Its total amino acid and free amino acid contents are 1.5 and 2.6 times, respectively, that of other drugs prepared traditionally. Previous basic research has demonstrated that JLC can inhibit tumor angiogenesis and tumor cell vasculogenic mimicry. This coincides with the TCM theory that blood stasis removal and dispersion promote the normalization of human vasculature and inhibit the formation of twisted and disorganized tumor neo vasculature with coil-like dilations.
In the present study, by incorporating the understanding of the complex biological characteristics of liver cancer achieved by modern medical research with the progress in evidence-based research, we performed literature searches in the Cochrane Library, Embase, PubMed, OVID Scopus, China Biology Medicine, China National Knowledge Infrastructure, VIP, and Wanfang databases for articles published from database inception to December 2023. The Chinese subject headings used in the searches were “金龙胶囊” AND (“肝癌” OR “肝恶性肿瘤” OR “肝肿瘤”), and the English subject headings were “Jinlong capsule” AND (“Hepatic Neoplasms” OR “Cancer of Liver” OR “Liver Neoplasms” OR “Hepatic Cancers” OR “Liver Cancer”). By performing a review of the results of clinical and basic research on JLC and focusing on clinical needs, we aimed to identify approaches for harnessing the unique and unconventional advantages of the TCM and explore the development of precision synergistic strategies that combine the treatment methods of TCM and modern medicine.
2 Progress in clinical research on the use of JLC in the prevention and treatment of liver cancer
Surgery is the primary radical treatment method for liver cancer. However, in China, up to 70% of patients have middle-to late-stage liver cancer at their initial consultation. Therefore, the surgical resection rate among Chinese patients is extremely low. The recurrence rate of liver cancer after surgery is high, with a 5-year postoperative recurrence rate of up to 70%. Researchers have actively explored the effects of preoperative neoadjuvant therapy on the enhancement of the radical resection rate among patients with liver cancer. In 2023, the American Society of Clinical Oncology reported using preoperative neoadjuvant hepatic arterial infusion chemotherapy with the FOLFOX regimen in Barcelona Clinic Liver Cancer stage A/B patients with HCC beyond the Milan criteria. The results indicated that the median progression-free survival of patients in the treatment group was 22.7 months, which was longer than that of patients in the direct operation group (10.2 months) (Wei et al., 2023). The use of targeted therapy combined with immunotherapy as conversion therapy in liver cancer has also been investigated. A clinical study on the use of lenvatinib plus an anti-programmed cell death (PD)-1 agent in 56 patients with unresectable middle-to late-stage liver cancer reported a conversion success rate of 55.4% (Zhang et al., 2023). Other studies have examined the effects of postoperative adjuvant therapy on reducing the postoperative recurrence rate. Postoperative adjuvant therapies, such as FOLFOX+ hepatic arterial infusion chemotherapy and atezolizumab combined with bevacizumab, have been implemented in practice; however, there are no widely accepted adjuvant therapy regimens.
For unresectable middle-to late-stage liver cancer, local interventional therapy and systemic medication therapy are commonly adopted treatments. Targeted therapy and immunotherapy further enhance the therapeutic effects based on traditional chemotherapy. Active systemic antitumor treatment can be considered for Child-Pugh class A or B patients (Child-Pugh score ≤7 points). Among the various targeted monotherapy regimens, sorafenib [approved by the Food and Drug Administration (FDA) and National Medical Products Administration (NMPA)] is the first to be used to treat HCC (Llovet et al., 2008; Cheng et al., 2009). A study showed that lenvatinib (approved by the FDA and NMPA) exhibited non-inferiority in the median OS (mOS) compared with sorafenib (13.6 months vs. 12.3 months, hazard ratio = 0.92, 95% confidence interval (CI): 0.79–1.06) (Kudo et al., 2018). Another study reported that donafenib (approved by the NMPA) significantly improved the mOS compared with sorafenib (12.1 months vs. 10.3 months, P = 0.0245) (Qin et al., 2021). Several phase 3 clinical studies have demonstrated that the combined targeted therapy and immunotherapy are superior to sorafenib monotherapy. The results of IMbrave150, a global phase 3 clinical trial, have indicated that atezolizumab plus bevacizumab (approved by the FDA and NMPA) significantly improved the mOS (19.2 months vs. 13.4 months, P < 0.001) (Finn et al., 2020; Finn et al., 2021). In the investigation of the therapeutic effects of immunotherapy alone, the global phase 3 trial HIMALAYA demonstrated for the first time that dual immunotherapy using tremelimumab plus durvalumab (STRIDE regimen, approved by the FDA) was superior to sorafenib. Patients of the STRIDE group showed a significant improvement in the mOS (16.43 months vs. 13.77 months, P = 0.0035) and a lower incidence rate of grade 3 and above treatment-related adverse events (25.8% vs. 36.9%) (Abou-Alfa et al., 2022). However, given that the regimens only benefit limited populations and are costly, the burden of liver cancer remains substantial in China and worldwide.
Therefore, raising the radical resection rate, reducing postoperative cancer occurrence, and enhancing the therapeutic effects in the treatment of middle-to late-stage liver cancer are crucial for achieving the goal of a 15% increase in the 5-year survival rate for all cancers stated in the Outline of the Health China 2030 Plan.
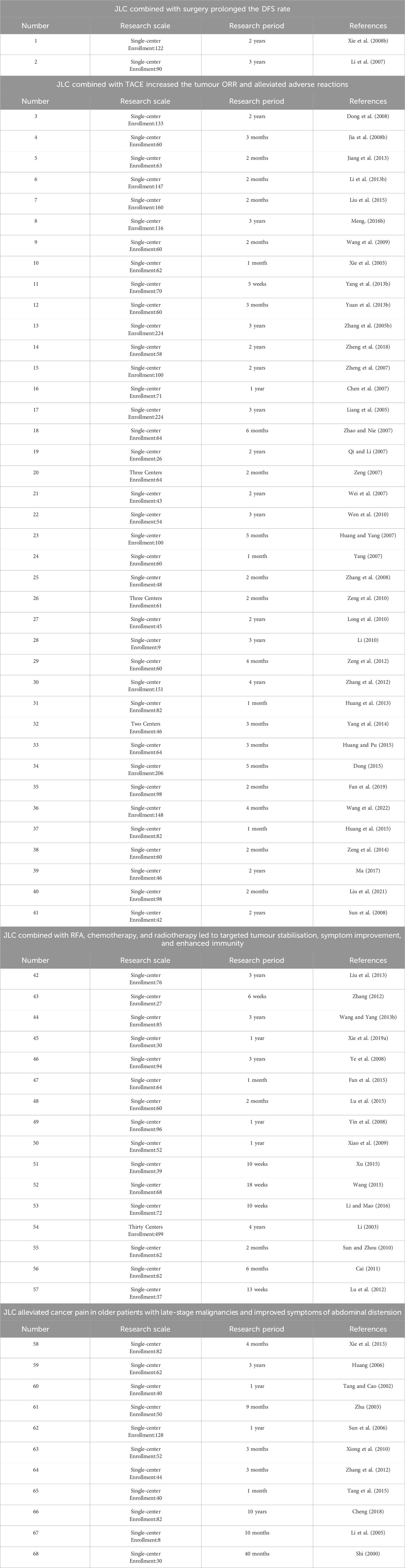
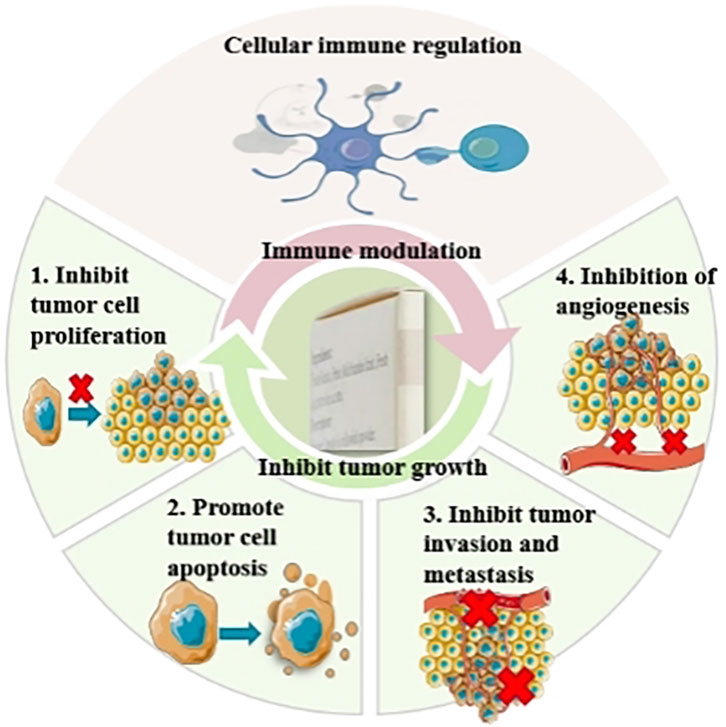
Clinical studies have indicated the wide use of JLC in various primary liver cancer treatment stages. A total of 68 pertinent clinical research articles were retrieved from the literature, and information on study size and study period of the clinical studies was summarized (Table 1). In which the effects of JLC (Figure 2) combined with surgery, TACE, ablation therapy, chemotherapy, radiotherapy, and targeted therapy were investigated, and the mechanism of action of JLC in anti-tumor was clarified (Figure 3). The main findings of these studies were as follows: (1) JLC combined with surgery prolonged disease-free survival (DFS); (2) JLC combined with TACE increased the tumor ORR and alleviated adverse reactions, such as gastrointestinal reactions; (3) JLC combined with ablation therapy reduced post-ablation liver injury, attenuated symptoms, and strengthened patient immunity, which demonstrated that JLC was a beneficial supplement to RFA; (4) JLC combined with radiotherapy led to an increase in the survival rate; (5) JLC combined with chemotherapy and targeted therapy enhanced the quality of life (QOL) of patients and reduced adverse reactions; and (6) JLC alleviated cancer pain in patients with liver cancer.
2.1 JLC combined with surgery prolonged the DFS rate
The postoperative recurrence rate of liver cancer is associated with the preoperative presence of microscopic, disseminated lesions or multicentric occurrence of the disease. Xie et al. (2008a) performed a study on 122 patients with primary liver cancer whose tumors could be completely resected (stage 1: 68 patients; stage 2: 54 patients). Patients in the treatment group received JLC at 1 g three times a day and were observed for 6 months, whereas the control group was administered fluorouracil (5-FU) starting from 4 weeks after surgery by intravenous infusion at a dose of 330 mg/m2 once every 4–6 weeks. The results indicated that the treatment group had a mOS of 15.0 months, which was significantly longer than that of the control group (10.7 months, P = 0.01), and exhibited an improvement in QOL.
2.2 JLC combined with TACE increased the tumor ORR and alleviated adverse reactions, such as gastrointestinal reactions
TACE is currently one of the most used non-surgical treatment methods in liver cancer. Research has shown that combining TACE with TCM enhanced patient survival and QOL while reducing the occurrence of adverse reactions, such as gastrointestinal reactions and myelosuppression (Xie et al., 2003; Zhang et al., 2005a; Dong et al., 2008; Jia et al., 2008a; Wang et al., 2009; Jiang et al., 2013; Li et al., 2013a; Yang et al., 2013a; Yuan et al., 2013a; Liu et al., 2015; Meng, 2016a; Zheng et al., 2018). A meta-analysis by Yan et al. (2021) that included 19 randomized controlled studies on the use of JLC combined with TACE for the treatment of primary liver cancer (1,740 cases) revealed that the combination therapy group had a higher ORR [Odds ratio (OR) = 2.23, 95% CI: (1.78–2.80), P < 0.001] and a higher Karnofsky Performance Status score [OR = 2.59, 95% CI: (1.86–3.60), P < 0.001]. Combination therapy with JLC and TACE also led to a reduction in adverse reactions, such as gastrointestinal reactions [OR = 0.43, 95% CI: (0.24–0.78), P = 0.005] and leukopenia [OR = 0.36, 95% CI: (0.27–0.49), P < 0.001] compared with the control group.
2.3 JLC combined with RFA, chemotherapy, and radiotherapy led to targeted tumor stabilization, improved symptoms, and enhanced immunity
Liu et al. (2013) performed observations of patients who underwent sequential treatment with JLC following RFA and compared them with patients who underwent RFA monotherapy. Treatment effectiveness was 53.84% in the observation group and 42.86% in the control group, with a statistically significant difference between the two groups (P < 0.05). Therefore, it is evident that JLC was capable of tumor stabilization, inhibiting tumor progression, improving survival quality, alleviating symptoms, strengthening patient immunity, and reducing post-RFA liver injury, thereby serving as a beneficial supplement to RFA. A study by Zhang (2012) involving 27 patients with lung metastases from primary liver cancer reported that the group that received chemotherapy with concomitant oral administration of JLC had greater short-term clinical benefit, a higher proportion of patients with reduced serum alpha-fetoprotein levels, better physical conditions, lower incidences of grade 3/4 leukopenia, and significantly higher CD4/CD8 count compared with patients who received chemotherapy alone. Wang and Yang (2013a) found that the 1- and 3-year OS of the combined three-dimensional conformal radiation therapy and JLC group were 74.4% (32/43) and 34.9% (15/43), which were higher than those of the radiation monotherapy group [66.7% (28/42) and 16.7% (7/42), P = 0.046]. Xie Y. L. et al. (2019) performed follow-up observations of patients who received combination therapy with apatinib (treatment group) and JLC and those who received apatinib alone (control group). At 3 months, 6 months, and 1 year after treatment, the treatment group showed improvements in alpha-fetoprotein, imaging measurements of tumor changes, and ascites compared with the control group. The treatment group also exhibited a better short-term QOL, lower incidence of adverse reactions, higher remission rate, and prolonged survival (P < 0.05).
2.4 JLC alleviated cancer pain in older patients with late-stage malignancies and improved abdominal distension symptoms
A study by Xie et al. (2013) on 82 older patients with late-stage malignancies showed that compared with the control group, treatment with JLC led to the alleviation of cancer pain and an improvement in the Karnofsky Performance Status score. Therefore, the 2021 edition of the expert consensus on the reasonable use of Chinese patent drugs in the standardized treatment of cancer pain recommends the use of JLC to relieve thoracic pain caused by cancers such as primary liver and pancreatic cancers. Huang (2006) performed clinical observations of 62 patients with late-stage liver cancer who received a combination of JLC and syndrome-based medication therapy, paying particular attention to symptoms such as thoracic pain and abdominal distension. The results indicated that the complete disappearance of symptoms occurred in 20 patients, and a partial improvement in the symptoms occurred in 30 patients, resulting in a high symptom improvement rate of 80.7%. This demonstrates that JLC, combined with syndrome-based medication therapy, can alleviate thoracic pain, abdominal distension, and other clinical symptoms associated with primary liver cancer.
3 Progress in basic research on the use of JLC in the prevention and treatment of liver cancer
Basic research on the use of JLC (Table 2) in the treatment of liver cancer has primarily focused on the effects of JLC on tumor growth inhibition and immune modulation. Potential mechanisms include inhibiting tumor cell proliferation, promoting tumor cell apoptosis, inhibiting tumor invasion and migration, inhibiting angiogenesis, and modulating cellular immunity.
3.1 Inhibition of tumor growth
An in vivo experiment was performed on nude mice inoculated with human liver carcinoma (HepG2) cells. The results indicated that the tumor inhibition rate was 37.6% after 4 weeks of drug administration. In another study that investigated the effect of JLC administration for 9 days on Kunming mice inoculated with the H22 mouse hepatoma cell line, the 37 g crude drug/kg dose group exhibited an average tumor inhibition rate of 44.4%. Xie Y. Z. et al. (2019) found that a high dose of JLC (1500 mg/kg) led to a tumor inhibition rate of 61.90% in nude mice receiving intracranial implantation of a U87-RFP glioma. Liu et al. (2001) performed interventions twice with JLC in a high metastatic tumor model of murine cervical carcinoma (U14) and reported that the inhibition rates of local tumor recurrence were 54.8% and 66.3% for the two interventions, while the inhibition rates of metastasis were 50% and 54%. In vitro experiments revealed that the main antitumor mechanisms of JLC were as follows.
3.1.1 Inhibition of tumor cell proliferation
Li et al. (2018) found that JLC significantly inhibited the proliferation rate of the MGC-803 and BGC-823 gastric cancer cell lines in a concentration- and time-dependent manner. A study by Li (2012) reported that JLC provided good inhibitory effects on the proliferation of the BxPC-3 human pancreatic cancer cell line. Lü et al. (2010) performed in vitro culture of lung adenocarcinoma cells and used the cell counting method to observe the influence of different concentrations of JLC on cell growth. The results showed that the growth curve of the drug administration group was less steep compared with the control group and was significantly positively correlated with drug dose. This demonstrates that JLC exerted a significant and dose-dependent inhibitory effect on the proliferation of lung adenocarcinoma cells.
3.1.2 Promotion of tumor cell apoptosis
Zhao et al. (2002) found that JLC induced apoptosis of human leukemia (HL)-60 cells mainly during the S phase of the cell cycle. HL-60 cells treated with three different dose levels of JLC exhibited apoptotic rates of 17.05%, 40.06%, and 53.45% at 72 h after treatment, with both early and late apoptosis observed in the cells. In contrast, the apoptosis rate of the control group was only 6.97%. Li et al. (2018) reported that JLC promoted apoptosis in MGC-803 and BGC-823 human gastric cancer cell lines and blocked cell cycle progression at the S and G2/M phases. An investigation of the pertinent mechanisms revealed that this effect was mainly associated with reducing the protein expression levels of Bcl-2 and surviving.
3.1.3 Inhibition of angiogenesis
Existing literature has reported that the mechanisms by which JLC inhibits tumor angiogenesis are mainly related to the expression of matrix metalloproteinases and vascular endothelial growth factor (VEGF). Using an animal model and molecular biology experiments, Li (2011) demonstrated that the protein expression levels of VEGF, matrix metalloproteinase-2, and matrix metalloproteinase-9 in mouse tumor tissue were significantly lowered after treatment with JLC. Liu et al. (2016) reported that high, moderate, and low doses of JLC interfered with angiogenesis and downregulated the mRNA and protein expression of Mig-7 in the HCT116 colon cancer cell line.
3.1.4 Inhibition of tumor cell invasion and migration
Shi et al. (2019) performed in vitro experiments with the A172 and U251 human glioma cell lines and found that JLC inhibited tumor cell invasion and migration. An increase in the JLC concentration led to a significant dose-dependent decrease in the number of cells involved in invasion and migration. Further investigation of the mechanisms of action revealed that JLC exerted inhibitory effects on tumor cell invasion and migration by inhibiting the expression of phosphorylated mammalian target of rapamycin and phosphorylated S6. Many genes are associated with the growth, invasion, and adhesion of tumor cells, among which vanin 1 exhibited the most significant upregulation. Huang et al. (2014) established an animal model of in situ brain glioma and discovered that the vanin 1 expression level of the low-dose JLC group was 48.34 times that of the model group. This result suggests that JLC effectively inhibited intracranial tumor growth in nude mice, with vanin 1 as a potential target.
3.2 Immune modulation
In an in vitro study by Xu et al. (2005), JLC increased CD3+ cells, CD4+ cells, and the CD4+/CD8+ ratio in immunosuppressed animals. This indicates that JLC significantly countered immunosuppression induced by intraperitoneal injection of cyclosporin A and promoted the recovery of immune function. In clinical studies investigating the effects of JLC combined with TACE and chemotherapy, the treatment groups exhibited an increase in the CD4+/CD8+ ratio, which was indicative of improved cellular immune function.
4 Discussion
According to TCM, the liver governs the unclogging and deflation of qi and emotions and favors free and unobstructed movement. The loss of the unclogging and deflation function in the liver will lead to stagnation of liver qi, which leads to blood stasis. Long-term blood stasis accumulates beneath the ribs, becomes toxic, and develops into cancerous tumors. Therefore, stasis-induced toxicity, toxicity-induced pathological changes, and stasis-toxicity intermingling are the core pathogenic mechanisms of liver cancer. In approximately 40% of patients with primary liver cancer, the edges of the tongue exhibit green, dark purple, linear, strip-like, spot-like, or irregularly shaped patches with ecchymoses and petechiae. These patches, which possess distinct borders and may be raised above the tongue surface, are known as “lines of ganyin” (Wang and Wang, 2014). They serve as rough indicators for the investigation of middle-to-late-stage primary liver cancer and can indicate disease severity and prognosis. Hui et al. (2023) systematically searched the literature on TCM syndrome differentiation of primary liver cancer and retrieved 90 articles (10,304 patients) that fulfilled the search criteria. The results revealed that the qi stagnation and blood stasis syndrome was the most common TCM syndrome among the patients (20.77%, 2,140 patients). Therefore, the use of the blood stasis syndrome as a key research component in exploring features that benefit populations can be considered. As of the end of 2023, 77 clinical studies on the application of JLC in other types of cancer have been published. The cancer types include stomach cancer, pancreatic cancer, lung cancer, breast cancer, colorectal cancer, nasopharyngeal carcinoma, cervical cancer, and brain glioma, and the effects include the prevention of recurrence and metastasis, reduction of toxicity and enhancement of effectiveness, and immune modulation (Huang et al., 2016; Li and Mao, 2016; Zou et al., 2016; Dong et al., 2018; Lai et al., 2021; Wen et al., 2021; Pei and Wang, 2022; Wu and Cheng, 2022). TCM treatment is performed based on syndrome differentiation, and the concept of treating different diseases with the same therapy if they belong to the same syndrome is also widely applied. JLC is used in treating multiple types of solid tumors, which is akin to the important role served by anti-angiogenesis therapy and immune checkpoint inhibitors in various cancers, such as stomach cancer, lung cancer, and liver cancer. Blood stasis syndrome is the common target among these treatment methods, suggesting the presence of common mechanisms in the prevention and treatment of multiple types of solid tumors by JLC. Therefore, further exploring the material basis and molecular phenotype characteristics underlying blood stasis syndrome may be worthwhile.
Given the clinical needs of liver cancer, which include the resolution of the issues of a low surgical resection rate and high postoperative recurrence rate, it is imperative that neoadjuvant and adjuvant regimens consisting of JLC are explored to enhance the pathological complete response rate and surgical resection conversion rate. In completed studies on the use of JLC as a postoperative adjuvant therapy, the endpoint was the median survival, and the control group had received chemotherapy with 5-FU. Currently, studies on postoperative adjuvant therapies for liver cancer are no longer solely focused on chemotherapy; they have further delved into the exploration of targeted therapy, anti-angiogenesis therapy, and immune therapy, with most study endpoints being relapse-free survival or DFS. Therefore, indicators such as relapse-free survival and DFS can be set as study endpoints in future research. For patients at high risk of recurrence, the combination of JLC with neoadjuvant therapy, TACE, lenvatinib, or atezolizumab can be investigated in studies on postoperative adjuvant therapies, with the aim of achieving better therapeutic effects based on current treatment methods. In addition, considering that the main functions of JLC are “blood stasis removal and static blood dispersion” and “depression relief and collateral clearing,” future clinical studies may also be focused on populations with blood stasis syndrome or the use of patients with blood stasis syndrome as a separate stratification factor for outcome analysis, to identify the true benefiting populations.
The progress of new drug development and clinical research has led to the emergence of various first-line treatment strategies for unresectable or metastatic HCC. Given the clinical needs related to the limited therapeutic effects of combination therapy on mid-to-late-stage liver cancer and the toxic side effects of therapies, the exploration of combination treatment modalities involving the use of JLC with TACE, chemotherapy, radiotherapy, targeted therapy, and RFA therapy will be of great significance for improving the outcomes of patients with liver cancer. Most previous studies on unresectable or metastatic HCC have merely investigated the combination of JLC with TACE. In these studies, an emphasis has also been placed on the short-term therapeutic effects, with the tumor ORR mainly adopted as the study endpoint and less attention given to the mOS. Therefore, OS may be the primary efficacy endpoint in future study designs. In a clinical study on icaritin, the benefiting population was defined as patients with tumor necrosis factor-alpha levels <2.5 pg/mL and interferon-gamma levels ≥7.0 pg/mL, suggesting that icaritin may be more beneficial for patients with HCC with concomitant hepatitis B virus and/or hepatitis C virus infections (Sun et al., 2021). Given that JLC is a dominant drug for blood stasis removal and static blood dispersion and that the incidence of the blood stasis syndrome in patients with concomitant viral hepatitis and cirrhosis is relatively high in clinical practice, it is possible that JLC would be more useful for patients with liver cancer and hepatitis or cirrhosis. The characteristics of immunological markers or cytokines in these populations should be studied further. In future studies, researchers may perform detailed investigations of these specific populations or include such populations as a stratification factor for analyses within large, comprehensive studies. Besides research on the use of JLC in postoperative adjuvant therapies, it is also worthwhile to conduct clinical studies related to the combined use of JLC with targeted drugs or immunotherapy to further enhance existing therapeutic effects.
The inhibition of tumor angiogenesis serves as the mechanism of action in most targeted drugs currently used for the treatment of liver cancer, including sorafenib, lenvatinib, and bevacizumab. The occurrence and development of liver cancer is closely associated with tumor angiogenesis. VEGF-mediated angiogenesis is a major driving factor for immune evasion in tumors. Upon binding with VEGF receptor-2 (VEGFR2), VEGF can promote endothelial cell proliferation and migration and induce vascular changes in liver cancer, thereby promoting liver cancer cell growth (Lan et al., 2024). PD-1 is a checkpoint molecule that is expressed on the surfaces of natural killer T cells, B cells, dendritic cells, monocytes/macrophages, CD4+ T cells, and CD8+ T cells. Interactions between PD-1 and its ligand, PD-L1, inhibit T cells' activation, proliferation, and effects. Under physiological conditions, PD-1, PD-L1, and PD-L2 activate T cells during peripheral tolerance. This leads to the inhibition of immune tissue damage caused by self-reactive T cells, thereby preventing autoimmunity. Under pathological conditions, activation of the PD-1/PD-L1 pathway can induce T cell deactivation, apoptosis, and exhaustion; modulate the differentiation of CD4+ T cells into Treg cells; and suppress immune responses. Solid tumors can hijack the PD-1/PD-L1 axis, induce immunosuppression, and evade T cell receptor recognition. It has been demonstrated that blockade of the PD-1/PD-L1 axis causes reversal of the immunosuppressive phenotype, activates the adaptive immune system, triggers tumor antigen recognition, activates cytotoxic CD4+ and CD8+ T cells, and enhances antitumor efficacy. Therefore, monoclonal antibodies targeting PD-1/PD-L1 can exert antitumor effects (Liu et al., 2023). Sintilimab and atezolizumab are PD-1/PD-L1 inhibitors commonly used in the clinical treatment of liver cancer. Good therapeutic effects have been achieved with the combined use of anti-angiogenic agents and PD-1/PD-L1 inhibitors in clinical treatment. This further demonstrates the complex biological characteristics of liver cancer and suggests that combined treatments with multiple targets may lead to better clinical therapeutic effects. JLC is characterized by low toxicity, multiple targets, and multiple pathways; however, its detailed mechanisms of action have not yet been fully elucidated as previous basic research lacks research methods and depth. For instance, the investigation of immune modulation has been largely limited to a single indicator, namely CD4+/CD8+. Tumor immunity encompasses many types of negative immune regulators released in the tumor microenvironment, including PD-1, lymphocyte-activation gene −3, and indoleamine 2,3-dioxygenase (Chen et al., 2024), which should be further investigated. Besides improving the tumor environment, JLC also exerts effects on tumor cells, which should not be underestimated. Tumor stem cells, characterized by self-renewal and an unlimited proliferative capacity, are highly associated with liver cancer recurrence and metastasis (Jiang et al., 2024; Chen et al., 2024). Therefore, the exploration of the mechanisms of action of JLC on tumor stem cells also serves as a basis for preventing recurrence and metastasis from the source.
Primary liver cancer possesses high heterogeneity and clinical complexity. Therefore, the prognosis of patients with liver cancer remains poor, and the current treatment landscape requires further improvement. JLC has been used in clinical practice for years and has demonstrated definite therapeutic effects. Previous clinical studies have reported that the combined use of JLC with modern medical therapies enables the synergistic enhancement of treatment effects, and basic research has indicated the manifestation of multi-target mechanisms of action by JLC, such as the inhibition of angiogenesis, immune modulation, and anti-inflammatory, antipyretic, and analgesic properties. These findings adequately demonstrate the multi-component, multi-target, and multi-pathway features of JLC, which is attuned to the complex biological characteristics of liver cancer. Therefore, the direction of future research should encompass the elucidation of the specific mechanisms of action of JLC and the exploration of ways to maximize its antitumor effects. Given the background of viral hepatitis and liver cirrhosis in most cases of liver cancer in China and the important role of the blood stasis syndrome in the occurrence of liver cancer, it is of utmost necessity to investigate the mechanisms of action of JLC, which possesses blood stasis removal and static blood dispersion functions. With the support of existing omics research and network pharmacology methodologies, elucidation of the material basis of the antitumor mechanisms of JLC has become highly possible. Based on definite mechanisms of action, clinical studies with a rigorous design and the effective and complementary combination of TCM and Western medicine approaches enable the targeting of benefiting populations and enhancement of clinical therapeutic effects.
The use of fresh medicinal ingredients for disease treatment is a prominent feature of TCM. JLC, an original drug produced from fresh medicinal animal materials by a unique process, is a Chinese patent drug used for the treatment of liver cancer that is attuned to the complex biological characteristics of liver cancer. It harnesses the unique and unconventional advantages of TCM and focuses on the clinical needs of liver cancer. Applying JLC in future research can further enhance evidence-based research on the TCM-based prevention and treatment of primary liver cancer, the development of precise synergistic strategies that combine TCM with modern medical treatment methods, and the improvement of treatment outcomes.
5 Conclusion
Primary liver cancer, a heterogeneous and deadly malignancy, demands innovative therapies. JLC, a Chinese patent medicine, shows clinical benefits in prolonging survival, enhancing response rates, and modulating immunity when combined with modern treatments. Preclinical studies reveal mechanisms including tumor growth inhibition, angiogenesis suppression, and immune regulation. Future research should clarify its molecular pathways, design rigorous trials with long-term survival endpoints and precise population stratification to develop synergistic strategies integrating JLC with modern medicine, providing new directions for liver cancer prevention and treatment.
Author contributions
LL: Writing – original draft, Writing – review and editing. SR: Writing – original draft, Writing – review and editing. ZZ: Supervision, Writing – review and editing. HL: Conceptualization, Methodology, Supervision, Writing – review and editing. JL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Scientific and technological innovation project of China Academy of Chinese Medical Sciences (No. CI2021A05502).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
5-FU, fluorouracil; CI, confidence interval; DFS, disease-free survival; FDA, Food and Drug Administration; HCC, hepatocellular carcinoma; JLC, jinlong capsule; mOS, median OS; NMPA, National Medical Products Administration; OR, Odds ratio; ORR, objective response rate; OS, overall survival; PD, programmed cell death; QOL, quality of life; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TCM, traditional Chinese medicine; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor-2.
References
Abou-Alfa, G. K., Lau, G., Kudo, M., Chan, S. L., Kelley, R. K., Furuse, J., et al. (2022). Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 1, EVIDoa2100070. doi:10.1056/EVIDoa2100070
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2023). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-CANCER J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Cai, M. H. (2011). Clinical obervation of liver metastasis of colorectal cancer treated with combined chemotherapy with Jinlong Capsule. Sichuan Med. J. 32 (2), 227–229. doi:10.3969/j.issn.1004-0501.2011.02.036
caim.org (2023). Guidelines for the integrated treatment of primary liver cancer with Chinese and Western medicine. Available online at: http://www.caim.org.cn/info_content.jsp?id=10322.
Chen, D. P., Huang, C. X., Wu, C. Y., Wei, Y., and Ming, K. D. (2024). Advances in research on the interaction between the tumor immune microenvironment and treatment. Biomed. Transl. Res. 5, 46–57 + 66. doi:10.12287/j.issn.2096-8965.20240206
Chen, X. M., Ye, X., and Tian, R. H. (2007). “Efficacy observation of Jinlong capsule combined with hepatic arterial chemoembolization in advanced liver cancer,” in Proceedings of the 8th national conference on tumor interventional therapy, 473–475.
Cheng, A. L., Kang, Y. K., Chen, Z., Tsao, C. J., Qin, S., Kim, J. S., et al. (2009). Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34. doi:10.1016/S1470-2045(08)70285-7
Cheng, J. H. (2018). Therapeutic iideas and clinical practice of pure traditional Chinese medicine in the treatment of liver cancer. J. Jiangxi Univ. Traditional Chin. Med. 30 (4), 45–49.
Chinese Society of Clinical Oncology (CSCO). (2020). Guidelines for the diagnosis and treatment of primary liver cancer.
Chinese Society of Liver Cancer (2021). Expert consensus on multidisciplinary integrated treatment for liver cancer in China. J. Clin. Hepatobiliary Dis. 37, 278–285. doi:10.3969/j.issn.1001-5256.2021.02.008
Dong, H. T., Zhao, W., Lu, W. P., Chen, L. Z., Yin, Q. Z., Zhang, Y., et al. (2008). Clinical observation of Jinlong Capsule combined with hepatic artery interventional therapy for primary liver cancer in 133 cases. Chin. Clin. Oncol. 35, 378–380. doi:10.3969/j.issn.1000-8179.2008.07.006
Dong, Y. N., Bao, Y. L., and Wang, Y. Y. (2018). Observation of the efficacy of Jinlong Capsule combined with DP regimen in the treatment of advanced cervical cancer. Mod. Drugs Clin. Med. 33, 3250–3253.
Dong, Z. Y. (2015). Clinical study on the effect of Jinlong Capsule on HBV-DNA of hepatocellular carcinoma after TACE. Tianjin: Tianjin Medical University. doi:10.7666/d.D798481
Fan, B., Hou, L., Jia, L., Liu, D., Liu, F., and Guohui, L. (2021). Expert consensus on the rational use of traditional Chinese medicine in the standardized treatment of cancer pain. Chin. J. Pain Med. 27(01):9–17. doi:10.3969/j.issn.1006-9852.2021.01.003
Fan, S., Li, Q. Y., Zhou, Z. T., He, W. L., Wu, Z. G., and Zhong, P. (2019). Study on the efficacy of TACE combined with Jinlong capsule in the treatment of primary liver cancer. China Pract. Med. 14 (21), 42–44. doi:10.14163/j.cnki.11-5547/r.2019.21.022
Fan, X. W., Huang, W. K., Yang, S. F., and You, L. N. (2015). Clinical analysis to treatment to radiofrequency ablation united jinlong capsule to patients of heptocellular carcinoma. Cap. Food Med. 22 (12), 57–59.
Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2020). Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905. doi:10.1056/NEJMoa1915745
Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2021). IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 39 (3_Suppl. l), 267. doi:10.1200/JCO.2021.39.3_suppl.267
Huang, F. D., and Pu, J. (2015). Randomized controlled analysis of Jinlong capsule combined with hepatic arterial chemoembolization in the treatment of colorectal cancer liver metastasis. Mod. Dig. and Intervention 20 (3), 287–289. doi:10.3969/j.issn.1672-2159.2015.03.041
Huang, H., Cui, X. W., Yue, G. J., et al. (2014). Preliminary exploration on the anti-brain tumor efficacy and mechanism of compound Chinese medicine Jinlong Capsule. J. PLA Pharm. 30, 188–191 + 209. doi:10.3969/j.issn.1008-9926.2014.03.002
Huang, J. C. (2006). Clinical observation of Jinlong Capsule combined with pattern differentiation medication in the treatment of primary liver cancer in 62 cases. Med. Ind. Inf. 3 (21), 100–101. doi:10.3969/j.issn.1673-7210.2006.21.079
Huang, M., and Yang, Y. S. (2007). “Clinical summary of Jinlong capsule combined with hepatic arterial infusion chemotherapy in the treatment of colorectal cancer liver metastasis: 50 cases,” in Proceedings of the 8th national conference on tumor interventional therapy, 467–468.
Huang, W. K., Fan, X. W., Yang, S. F., You, L. N., Baikere, PAHAERDING., Liu, M., et al. (2013). Clinical observation of decreasing adverse reaction for Jinlong capsule after transcatheter arterial chemoembolization to patients with heptocellular carcinoma. Chin. Clin. Oncol. 40 (3), 157–160. doi:10.3969/j.issn.1000-8179.2013.03.009
Huang, W. K., Liu, D. Y., You, L. N., Yang, S. F., Liu, M., Gu, P., et al. (2015). Jinlong capsule decreases adverse reactions after transcatheter arterial chemoembolization (TACE) in patients with hepatocellular carcinoma. Oncol. Transl. Med. 1 (2), 87–91. doi:10.1007/s10330-014-1416-y
Huang, Z. C., Zeng, C. S., Guo, S. J., and Chen, X. M. (2016). Clinical observation of Jinlong Capsule combined with GP regimen in the treatment of advanced pancreatic cancer. J. Gannan Med. Univ. 36, 61–63. doi:10.3969/j.issn.1001-5779.2016.01.017
Hui, Y. Y., Xue, J. D., Gao, G. Y., et al. (2023). Literature research on traditional Chinese medicine syndrome types and syndrome factors in primary liver cancer. World Trad. Chin. Med. 18, 401–405. doi:10.3969/j.issn.1673-7202.2023.03.018
Jia, C., Wang, W. Y., and Kang, Y. (2008a). Clinical study of Jinlong Capsule combined with hepatic arterial infusion chemotherapy and embolization for liver cancer. Chin. J. Cancer Prev. Treat. 15, 1416–1418. doi:10.3969/j.issn.1673-5269.2008.18.017
Jia, C. H., Wang, W. Y., and Kang, Y. (2008b). Clinical studies on combination therapy of jinlong capsule and transarterial chemoembolization in treatment of primary hepatic carcinoma. Chin. J. Cancer Prev. Treat. 15 (18), 1416–1418. doi:10.3969/j.issn.1673-5269.2008.18.017
Jiang, C. Y., Cao, J. J., and Cheng, Q. (2013). Clinical evaluation of jinlong capsule combined with TACE for primary liver cancer. Med. Innovation China (26), 111–110. doi:10.3969/j.issn.1674-4985.2013.26.053
Jiang, J., Liu, X. T., and Zhang, B. (2024). The current status and future prospects of cancer stem cells: based on the analysis of the completed projects of the National Natural Science Foundation of China. Mod. Oncol. Med. 32, 2074–2079. doi:10.3969/j.issn.1672-4992.2024.11.022
Kudo, M., Finn, R. S., Qin, S., Han, K. H., Ikeda, K., Piscaglia, F., et al. (2018). Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173. doi:10.1016/S0140-6736(18)30207-1
Lai, B. Y., Lü, L. Y., Zhao, J., Chu, A. J., Wang, C. H., and Pei, X. H. (2021). A network Meta-analysis of 10 oral. Chin. Herb. Med. Chin. Pat. Med. Comb. Chemother. Treat. breast cancer. 52, 6609–6624. doi:10.7501/j.issn.0253-2670.2021.21.019
Lan, X. L., Huang, Y. N., Zhu, M. M., Ma, P., and Dong, M. (2024). Advances in research on the mechanism of anti-VEGF and its receptor-targeted drugs in the treatment of liver cancer. Chin. J. Clin. Pharmacol. Ther. 29, 707–714. doi:10.12092/j.issn.1009-2501.2024.05.013
Li, B., Zhao, L. X., Liu, Z. W., Li, L., Ma, L. B., Zhou, Z. X., et al. (2013a). Clinical analysis of Jinlong Capsule combined with interventional therapy for primary liver cancer in 150 cases. Chin. J. Hepatobiliary Surg. 19, 530–533. doi:10.3760/cma.j.issn.1007-8118.2013.07.012
Li, B., Zhao, L. X., Liu, Z. W., Li, L., Ma, L. B., Zhou, Z. X., et al. (2013b). Jinlong capsule combined with interventional theraphy for primary hepatocellular carcinomas:a clincial analysis on 150 patients. Chin. J. Hepatobiliary Surg. 19 (7), 530–533. doi:10.3760/cma.j.issn.1007-8118.2013.07.012
Li, D., Jin, F., Tao, L., Ni, T. Y., Wang, H. B., Feng, J., et al. (2018). Effect of Jinlong Capsule on the invasion and metastasis of gastric cancer cells MGC-803 and BGC-823. Chin. Exp. Formulae J. 24, 117–123. doi:10.13422/j.cnki.syfjx.20181922
Li, H., Zhang, B., and Yu, G. Y. (2007). Preventive effects of Jinlong capsule on recurrence and metastasis after resection of resectable liver cancer. Cap. Med. 24, 35–36. doi:10.3969/j.issn.1005-8257.2007.24.025
Li, J. (2003). Summary of post-marketing phase IV clinical trial of Jinlong capsule: 2660 cases. Chinese Annual of Clinical Oncology, 368–371.
Li, J. (2011). Summary of post-marketing phase IV clinical trial of Jinlong capsule: 2660 cases. Chinese Annual of Clinical Oncology, 368–371.
Li, J., and Mao, C. F. (2016). Clinical observation on the treatment of colorectal cancer liver metastasis with Jinlong capsule. Chin. Remedies and Clin. 16 (9), 1322–1324. doi:10.11655/zgywylc2016.09.034
Li, J., Wang, S. H., Fan, X. J., Zhang, D. J., Shi, Y., Wang, Z. X., et al. (2005). Clinical study on the treatment of intermediate and advanced malignant tumors with Jinlong capsule combined with pattern differentiation medication: 40 cases. J. Fourth Mil. Med. Univ. 26 (18), 1667. doi:10.3321/j.issn:1000-2790.2005.18.035
Li, K. (2010). Application of Jinlong capsule in advanced malignant liver tumors. Med. Innovation China 7 (34), 189–190. doi:10.3969/j.issn.1674-4985.2010.34.126
Li, L. X., Ye, S. L., Wang, Y. H., Li, J. S., Sun, R. X., Xue, Q., et al. (2011). Jinlong Capsule exerts a suppressive effect on the metastatic potential of human hepatocellular carcinoma cells with high metastatic propensity. Chin. Hepatol. 16 (03), 240–241. doi:10.14000/j.cnki.issn.1008-1704.2011.03.001
Li, Y. Y. (2012). Effect of jinlong capsule on proliferation and apoptosis of human pancreatic cancer cells BXPC-3 [D]. Beijing University of Chinese Medicine.
Liang, T. J., Qin, C. Y., Zhang, C. Q., and Zhao, X. X. (2005). Clinical studies on the combination therapy with jinlong capsule and chemotherapy plus EmboliZation by hepatic artery CatheteriZation on primary hepatic carcinoma. Chin. Clin. Oncol. 32 (11), 641–643. doi:10.3969/j.issn.1000-8179.2005.11.013
Liu, J. P., Yuan, C. J., Yu, T., Liu, L., et al. (2016). Effect of Jinlong Capsule on vasculogenic mimicry and Mig-7 in colon cancer. Cancer Prev. Treat. Res. 43, 1059–1062. doi:10.3971/j.issn.1000-8578.2016.12.010
Liu, N. N., Feng, X. X., and Kang, T. (2021). Clinical analysis of Jinlong capsule combined with interventional therapy in the treatment of primary liver cancer. Pharm. Wkly. 30 (6), 114.
Liu, X. T., Liu, J. B., Zhang, Z. Q., and Liu, S. Y. (2013). Jinlong capsule clinical observation of radiofrequency ablation technique for the treatment of primary liver cancer. Cap. Med. 20 (10), 47–48. doi:10.3969/j.issn.1005-8257.2013.10.028
Liu, Y. C., Cheng, T. C., and Bian, Z. L. (2023). Progress and challenges in the combined use of immune checkpoint inhibitors for hepatocellular carcinoma. Chin. J. Immunol. 39, 860–864. doi:10.3969/j.issn.1000-484X.2023.04.033
Liu, Y. Q., Gao, J., Xue, K. X., Zhao, X. M., Gu, B., and Li, J. S. (2001). Inhibitory effect of Jinlong Capsule (JLC) on growth and spontaneous metastasis of mouse cervical cancer U14 tumor strain. Chin. J. New Drugs. 10, 823–825. doi:10.3321/j.issn:1003-3734.2001.11.008
Liu, Z. Y., Zhang, X. H., Zhang, X. S., Li, J. Y., and Ji, X. W. (2015). Efficacy observation of jinlong capsule combined with TACE in the treatment of primary liver cancer. Heilongjiang Med. Sci. Pharm. 38 (4), 75–76. doi:10.3969/j.issn.1008-0104.2015.04.037
Llovet, J. M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J. F., et al. (2008). Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390. doi:10.1056/NEJMoa0708857
Long, L., Xiang, H., Zhang, Z. M., Liu, J. S., and Fang, Z. Y. (2010). Jinlong capsule combined with transcatheter arterial chemoembolization (TACE) in the treatment of liver metastases: a clinical analysis of 23 cases. Hunan J. Traditional Chin. Med. 26 (6), 60–61. doi:10.3969/j.issn.1003-7705.2010.06.032
Lu, H. X., Yang, M. D., and Gao, J. (2012). Clinical observation of jinlong capsule combined with chemotherapy in treatment of liver metastases of gastric cancer. Chin. Clin. Oncol. 39 (24), 2108–2110. doi:10.3969/j.issn.1000-8179.2012.24.030
Lü, Y. F., Chen, Q. X., and Liang, D. (2010). Study on the effect of Jinlong Capsule on the growth of human lung adenocarcinoma A549 cells. Chin. Pract. Med. 5, 32–33. doi:10.14163/j.cnki.11-5547/r.2010.25.201
Lu, Z. Y., Cai, L. M., Liu, Z. H., Liu, L. B., and Zeng, C. S. (2015). Effects of jinlong capsule on immune index of the microwave ablation in the treatment of hepatocellular carcinoma. J. Gannan Med. Univ. 35 (4), 590–592. doi:10.3969/j.issn.1001-5779.2015.04.032
Ma, C. L. (2017). The regulatory effect of Jinlong Capsule combined with interventional therapy on the immune function of patients with primary liver cancer. Chin. J. ofHistochemistry Cytochem. 5, 405.
Ma, L., Wang, B., Long, Y., and Li, H. (2017). Effect of traditional Chinese medicine combined with Western therapy on primary hepatic carcinoma: a systematic review with meta-analysis. Front. Med. 11, 191–202. doi:10.1007/s11684-017-0512-0
Meng, P. (2016a). Efficacy and mechanism analysis of Jinlong Capsule combined with transcatheter arterial chemoembolization for primary liver cancer. J. Pract. Oncol. 31, 403–406. doi:10.3969/j.issn.1001-5930.2016.03.017
Meng, P. (2016b). Clinical efficacy and mechanism of jinlong capsule plus hepatic artery embolism for primary liver cancer. Pract. J. Cancer 31 (3), 403–406. doi:10.3969/j.issn.1001-5930.2016.03.017
National Health Commission of the People's Republic of China (2022). Guidelines for the diagnosis and treatment of primary liver cancer. Electron. J. Compr. Oncol. Ther. 8, 16–53. doi:10.12151/JMCM.2022.02-04
Pei, S., and Wang, Y. Z. (2022). Analysis of the effect of Jinlong Capsule combined with radiotherapy and chemotherapy in the treatment of non-small cell lung cancer. J. Chin. Metall. Ind. Med. 39, 460–461. doi:10.13586/j.cnki.yjyx1984.2022.04.007
Qi, Y. F., and Li, X. R. (2007). “Clinical observation on hepatic arterial chemoembolization combined with traditional Chinese medicine and Jinlong capsule in the treatment of liver cancer,” in Proceedings of the 8th national conference on tumor interventional therapy and the 1st China anti-cancer association conference on tumor interventional nursing and the symposium on new progress in tumor interventional therapy, 475–476.
Qin, S., Bi, F., Gu, S., Bai, Y., Chen, Z., Wang, Z., et al. (2021). Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II–III trial. J. Clin. Oncol. 39, 3002–3011. doi:10.1200/JCO.21.00163
Qu, Y. Y., Yue, G. J., Li, J. S., and Huang, H. (2014). The reversing and sensitizing effects of Jinlong Capsule on tumor cell lines resistant to paclitaxel and vincristine. Cancer Res. Prev. Treat. 41 (08), 884–887. doi:10.3971/j.issn.1000-8578.2014.08.006
Shi, H. Z. (2000). Clinical observation on the treatment of primary liver cancer with Jinlong capsule combined with pattern differentiation and treatment. Beijing J. Traditional Chin. Med. 19 (5), 30–32. doi:10.3969/j.issn.1674-1307.2000.05.013
Shi, J. R., Zhang, W. L., He, L., Kong, F., Pan, M., Guo, J., et al. (2019). Jinlong capsule inhibits migration and invasion in human glioblastoma cells via the modulation of mTOR/S6 signaling pathway. Drug Des. devel. Ther. 13, 1023–1032. doi:10.2147/DDDT.S195409
Sun, B. M., Luo, M., Wu, S. B., and Chen, X. X. (2008). Jinlong Capsule combined with transarterial chemoembolization in treatment of gastric cancer with liver metastasis. J. Chin. Integr. Med. 6 (9), 968–970. doi:10.3736/jcim20080919
Sun, H., and Zhou, M. C. (2010). Clinical obervation of Jinlong Capsule combined with Chemotherapy in treatment of liver metastasis of colorectal cancer. J. Hebei Med. Univ. 31 (7), 768–771. doi:10.3969/j.issn.1007-3205.2010.07.007
Sun, J. H., Yang, S. S., and Ma, Y. L. (2006). Effects of Jinlong capsule on the quality of life and survival period in patients with advanced liver cancer. Hubei J. Traditional Chin. Med. 28 (5), 34. doi:10.3969/j.issn.1000-0704.2006.05.018
Sun, Y., Qin, S., Li, W., Guo, Y., Zhang, Y., Meng, L., et al. (2021). A randomized, double-blinded, phase III study of icaritin versus huachashu as the first-line therapy in biomarker-enriched HBV-related advanced hepatocellular carcinoma with poor conditions: interim analysis result. J. Clin. Oncol. 39 (15_Suppl. l), 4077. doi:10.1200/JCO.2021.39.15_suppl.4077
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tang, W. H., She, Z. C., Ding, Z. X., Wang, G. J., and Tang, S. S. (2015). Effects of jinlong capsule on expression of thioredoxin reductase in patients with advanced primary liver cancer. J. Hubei Univ. Chin. Med. 17 (2), 35–36. doi:10.3969/j.issn.1008-987x.2015.02.011
Tang, Y. J., and Cao, F. (2002). “Clinical study on Jinlong capsule in the treatment of primary liver cancer,” in Proceedings of the 8th national symposium on integrated traditional Chinese and western medicine in Oncology, Beijing: China association of integrated traditional Chinese and western medicine, 303–305.
Wang, H. B., and Yang, J. Q. (2013a). Jinlong Capsule combined with three-dimensional conformal radiotherapy in the treatment of primary liver cancer. Chin. Clin. Oncol. 40, 784–787. doi:10.3969/j.issn.1000-8179.2013.13.009
Wang, H. B., and Yang, J. Q. (2013b). Combination therapy of Jinlong capsule and three-dimensional conformal radiotherapy for primary hepatocellular carcinoma. Chin. J. Clin. Oncol. (13), 784–787. doi:10.3969/j.issn.1000-8179.2013.13.009
Wang, J., Jia, J. W., Miao, J., and Guo, L. Y. (2022). Effect of Jinlong capsule combined with transcatheter arterial chemoembolization on medium and advanced liver cancer with blood stasis stagnation syndrome and its immunomodulatory effect. Shanxi Med. J. 51 (4), 367–370. doi:10.3969/j.issn.0253-9926.2022.04.002
Wang, X. H., Yang, J. Q., and Li, Y. H. (2009). Clinical observation on combination of Jinlong capsule and interventional therapy for primary liver cancer. Chin. J. Intergtative Mdeicine 29 (3), 273–274. doi:10.3321/j.issn:1003-5370.2009.03.025
Wang, Y. F. (2015). Clinical exploration of Jinlong capsule combined with chemotherapy in the treatment of gastric cancer liver metastasis. World Latest Med. Inf. 93, 59. doi:10.3969/j.issn.1671-3141.2015.93.031
Wang, Z. P., and Wang, C. S. (2014). Research progress on the relationship between Gan Ying Line and primary liver cancer. J. Southeast Univ. Med. Ed. . 33, 828–830. doi:10.3969/j.issn.1671-6264.2014.06.035
Wei, H., Liu, D. H., Wang, S. L., and Hua, Y. (2007). “Clinical observation on Jinlong capsule combined with interventional therapy in the treatment of primary liver cancer,” in Proceedings of the 8th national conference on tumor interventional therapy, 472–473.
Wei, W., Li, S. H., Zhao, R. C., Cheng, Y., Li, Q., Lu, L., et al. (2023). Neoadjuvant hepatic arterial infusion chemotherapy with FOLFOX could improve outcomes of resectable BCLC stage A/B hepatocellular carcinoma patients beyond Milan criteria: a multi-center, phase 3, randomized, controlled clinical trial. J. Clin. Oncol. 41 (16_Suppl.), 4023. doi:10.1200/JCO.2023.41.16_suppl.4023
Wen, M. Y., Sheng, Q., and Zhang, G. D. (2021). Effect of Jinlong Capsule combined with XELOX chemotherapy on the quality of life and cellular immune function of gastric cancer patients. New Chin. Med. 53, 152–155. doi:10.13457/j.cnki.jncm.2021.19.034
Wen, S. W., Dang, Z. J., and Yuan, T. W. (2010). Clinical evaluation of Jinlong capsule in improving the quality of life of hepatocellular carcinoma patients undergoing interventional therapy. Cap. Med. 17 (16), 55. doi:10.3969/j.issn.1005-8257.2010.16.038
World Health Organization (2020). Globocan. Available online at: http://gco.iarc.fr/today (Accessed June 05, 2023).
Wu, K. L., and Cheng, Q. (2022). Effect of Jinlong Capsule combined with radiotherapy and chemotherapy on T lymphocyte subsets and prognosis in patients undergoing brain glioma surgery. Explor. Ration. Drug Use China 19, 55–61. doi:10.3969/j.issn.2096-3327.2022.09.006
Xiao, Z. Y., Deng, J. H., Xiong, S. Z., Wang, C., and Tang, X. Y. (2009). Efficacy analysis of three-dimensional conformal radiotherapy combined with Jinlong capsule in the treatment of primary liver cancer: a clinical study of 52 cases. Cap. Med. 16 (16), 45–46. doi:10.3969/j.issn.1005-8257.2009.16.030
Xie, B., Tang, C., and Huang, J. (2008a). Preliminary clinical observation of the effect of Jinlong Capsule on recurrence and metastasis after liver cancer resection. Chin. J. Cancer Prev. Treat. 15, 1584–1586. doi:10.3969/j.issn.1673-5269.2008.20.018
Xie, B., Tang, C., and Huang, J. (2008b). Effect of jinlong capsule combined with hepatectomy on HCC intrahepatic metastasis. Chin. J. Cancer Prev. Treat. 15 (20), 1584–1586. doi:10.3969/j.issn.1673-5269.2008.20.018
Xie, R. L., Wang, M. Y., Shi, H. Q., Wu, L. Q., and Long, H. M. (2013). The impact of Jinlong Capsule on the quality of life and immune function in elderly patients with advanced malignant tumors. Chin. J. Pract. Med. 40 (20), 82–83. doi:10.3760/cma.j.issn.1674-4756.2013.20.034
Xie, Y. F., Tan, X. F., Ma, Q. H., and Liu, S. Z. (2003). Clinical observation on combination of Jinlong capsule and interventional therapy for primary liver cancer. Chin. J. Clin. Oncol. 30 (4), 297–298. doi:10.3969/j.issn.1000-8179.2003.04.022
Xie, Y. L., Li, Z. B., Zhang, J. H., Song, C. X., Ying, Y., and He, X. (2019a). Clinical study on the efficacy of apatinib combined with Jinlong Capsule in the treatment of advanced liver cancer. Drug Eval. 16, 22–23. doi:10.3969/j.issn.1672-2809.2019.16.011
Xie, Y. Z., Li, Y. Z., Zhu, H., Shi, Y. X., Jin, J., and Li, H. Q. (2019b). Effects of Jinlong Capsule and its polysaccharide and protein components on human glioma in situ mouse model. Chin. J. Comp. Med. 29, 55–60. doi:10.3969/j.issn.1671-7856.2019.08.009
Xiong, T. Q., Wang, Q. F., and Zhang, Y. (2010). Clinical analysis of Jinlong capsule in the treatment of primary liver cancer: 26 cases. J. Med. Theory Pract. 23 (1), 51–52. doi:10.3969/j.issn.1001-7585.2010.01.029
Xu, S. L., Wang, X. H., Zhang, Y. X., Zhao, D., Li, Y. M., Wang, J., et al. (2005). Effect of Jinlong Capsule on lymphocyte subsets in immunosuppressed mice. Chin. J. Basic Med. Trad. Chin. Med. 12, 908–909. doi:10.3969/j.issn.1006-3250.2005.12.013
Xu, Z. (2015). Clinical observation of jinlong capsule combined with chemotherapy in treatment of liver metastases of colon cancer. Cap. Food Med. 22, 73–74.
Yan, K., Wang, J., Li, G., and He, J. M. (2021). Efficacy and safety of Jinlong Capsule combined with transcatheter arterial chemoembolization for primary liver cancer: a Meta-analysis. J. Trad. Chin. Oncol. 3, 95–102. doi:10.19811/j.cnki.ISSN2096-6628.2021.02.021
Yang, J. D., Hainaut, P., Gores, G. J., Amadou, A., Plymoth, A., and Roberts, L. R. (2019). A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16, 589–604. doi:10.1038/s41575-019-0186-y
Yang, J. Q. (2007). “Clinical observation on Jinlong capsule combined with interventional therapy in the treatment of primary liver cancer,” in Proceedings of the 8th national conference on tumor interventional therapy, 470–471.
Yang, P. Y., Sun, Y. Y., Zhang, Y. C., Zhang, X., Sun, B. X., and Jia, Y. J. (2013a). Clinical study of Jinlong Capsule pre-intervention in TACE for primary liver cancer. Chin. Clin. Oncol. 40, 45–49. doi:10.3969/j.issn.1000-8179.2013.01.012
Yang, P. Y., Sun, Y. Y., Zhang, Y. C., Zhang, X., Sun, B. X., and Jia, Y. J. . (2013b). Effectiveness of early intervention with Jin-long capsules and transarterial chemoembolization for the treatment of primary liver cancer. Chin. J. Clin. Oncol. 40 (1), 45–49. doi:10.3969/j.issn.1000-8179.2013.01.012
Yang, Y. T., Fang, X. Y., Song, H. Y., Cheng, X., Gong, L., Jin, Y. S., et al. (2014). Effect of combined Jinlong capsule and transcatheter arterial chemoembolization on hepatic metastasis of colonic carcinoma. Acad. J. Chin. PLA Med. Sch. 41 (3), 231–233. doi:10.3969/j.issn.2095-5227.2014.03.010
Ye, X., Ge, Z. M., Fei, X. B., Wang, S., Cheng, Y. Y., Chen, X. M., et al. (2008). Clinical study on HIFU combined with jinlong capsules in treating 54 cases of primary liver cancer. Chin. Clin. Oncol. 35 (7), 372–374. doi:10.3969/j.issn.1000-8179.2008.07.004
Yin, L. J., Zhao, G. H., Ding, T. G., and Peng, Z. X. (2008). Clinical study of Jinlong capsule combined with whole-body gamma knife in the treatment of primary liver cancer: 96 cases. Chin. Clin. Oncol. 35 (7), 381–382. doi:10.3969/j.issn.1000-8179.2008.07.007
Yuan, T. W., Wen, S. W., Dang, Z. J., Zhang, X. Q., Chang, J. P., and Xue, Y. Q. (2013a). Observation on the immunomodulatory effect of Jinlong Capsule combined with interventional therapy in patients with primary liver cancer. Chin. Clin. Oncol. 40, 1116–1118. doi:10.3969/j.issn.1000-8179.20130206
Yuan, T. W., Wen, S. W., Dang, Z. J., Zhang, X. Q., Chang, J. P., Xue, Y. Q., et al. (2013b). Regulation of immune functions by combined Jinlong capsule and interventional therapy in patients with primary liver cancer. Chin. J. Clin. Oncol. (18), 1116–1118. doi:10.3969/j.issn.1000-8179.20130206
Zeng, B. Z. (2007). The clinc observation of the influence of jinlong capsule cooperated with TACE on quality of life of patients with primary carcinoma of the liver. Henan University of Chinese Medicine. doi:10.7666/d.y1095277
Zeng, B. Z., Yang, Y. Q., and Han, X. W. (2010). Clinical study on the treatment of primary liver cancer with TACE combined with Jinlong capsule: 31 cases. Traditional Chin. Med. Res. 23 (8), 35–37. doi:10.3969/j.issn.1001-6910.2010.08.018
Zeng, C. S., Cai, L. M., Huang, Z. C., and Xu, Q. Y. (2014). The effect of Jinlong capsule on the immune function for intervened patients with primary liver cancer. Chinese-German J. Clin. Oncol. 13, 80–83. doi:10.1007/s10330-013-1278-8
Zeng, C. S., Cai, L. M., Li, J. W., Huang, Z. C., Xiao, Y. H., Zhang, C. Y., et al. (2012). Influence of jinlong capsule combined with transarterial chemo-embolization on quality of life of patients with primary liver cancer. Chin. Clin. Oncol. 39 (22), 1839–1842. doi:10.3969/j.issn.1000-8179.2012.22.036
Zhang, C. Q., Liang, T. J., and Yuan, M. B. (2005a). Observation of the efficacy of Jinlong Capsule combined with hepatic arterial chemoembolization for primary liver cancer. Beijing Med. 27, 357–359.
Zhang, C. Q., Liang, T. J., and Yuan, M. B. (2005b). Clinical studies of the combination therapy with Jinlong capsule and chemical therapy and embolization by hepatic artery catheterization on primary hepatic carcinoma. Beijing Med. J. 27 (6), 357–359. doi:10.3969/j.issn.0253-9713.2005.06.013
Zhang, H. J., Yang, J. J., Wang, W. X., Jiang, X., Mao, Y. J., Yang, Z. A., et al. (2008). Effects of Jinlong Capsule on expressions of interleukin-2 and soluble interleukin-2 receptor in patients with primary liver cancer after transarterial chemoembolization therapy. J. Chin. Integr. Med. 6 (9), 907–910. doi:10.3736/jcim20080906
Zhang, S. Z., Yang, M. D., and Gao, J. (2012). Clinical analysis of Jinlong capsule in the treatment of primary liver cancer: 22 cases. Cap. Med. 19 (20), 35–36. doi:10.3969/j.issn.1005-8257.2012.20.019
Zhang, W., Tong, S., Hu, B., Wan, T., Tang, H., Zhao, F., et al. (2023). Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J. Immunother. Cancer 11, e007366. doi:10.1136/jitc-2023-007366
Zhang, X. Q. (2012). Jinlong Capsule combined with chemotherapy for primary liver cancer with pulmonary metastasis: 14 cases. Jiangxi J. Traditional Chin. Med. 43 (5), 35–37. doi:10.3969/j.issn.0411-9584.2012.05.017
Zhang, X. Q., Guo, P., Dang, Z. J., and Wen, S. W. (2012). Jinlong capsules combined with interventional therapy for hepatocellular carcinomas: clinical observation. J. Interventional Radiology 21 (3), 249–251. doi:10.3969/j.issn.1008-794X.2012.03.020
Zhao, D. M., Shi, Y. J., Guan, D. C., Cao, X. H., Liu, S. B., and Wu, S. L. (2002). Jinlong Capsule inhibits the growth of HL-60 cells and induces apoptosis. Chin. Med. J. (Engl.). 06, 346–348 + 384. doi:10.3969/j.issn.1673-1727.2002.06.010
Zhao, H. B., and Nie, X. C. (2007). “Observation on the treatment of intermediate and advanced liver cancer with Jinlong capsule combined with hepatic arterial chemoembolization,” in Proceedings of the 8th national conference on tumor interventional therapy, 477–478.
Zheng, J. P., Shao, G. L., Chen, Y. T., and Yu, Y. P. (2007). “Trans-arterial chemoembolization combined with Jinlong capsule for primary liver cancer,” in Proceedings of the 8th national conference on tumor interventional therapy and the 1st China anti-cancer association conference on tumor interventional nursing and the symposium on new progress in tumor interventional therapy, 479—480.
Zheng, C., Zhang, R. S., Pan, Y., and Liu, H. L. (2018). The efficacy of Jinlong Capsule combined with interventional therapy for primary liver cancer and its effects on T lymphocyte subsets and tumor immune factors. Mod. and Intervention 23 (4), 506–509. doi:10.3969/j.issn.1672-2159.2018.04.023
Zhou, M., Wang, H., Zeng, X., Yin, P., Zhu, J., Chen, W., et al. (2019). Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 394, 1145–1158. doi:10.1016/S0140-6736(19)30427-1
Zhu, X. (2003). Clinical observation on the efficacy of Jinlong capsule in the treatment of advanced primary liver cancer. Mod. J. Integr. Traditional Chin. West. Med. 12 (16), 1739–1740. doi:10.3969/j.issn.1008-8849.2003.16.040
Keywords: primary liver cancer, Jinlong capsule, evidence-based research, precision synergistic strategy, prospective research
Citation: Lv L, Ren S, Zhang Z, Lin H and Liu J (2025) Clinical and basic research advances on Jinlong capsule for the prevention and treatment of liver cancer. Front. Pharmacol. 16:1522945. doi: 10.3389/fphar.2025.1522945
Received: 05 November 2024; Accepted: 19 May 2025;
Published: 09 June 2025.
Edited by:
Ashwell Rungano Ndhlala, University of Limpopo, South AfricaReviewed by:
Madan Kumar Perumal, Central Food Technological Research Institute (CSIR), IndiaXinhua Song, Capital Medical University, China
Copyright © 2025 Lv, Ren, Zhang, Lin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liu, ZHIubGl1amllQDE2My5jb20=; Hongsheng Lin, ZHJsaW5ob25nc2hlbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Liyuan Lv1†
Liyuan Lv1† Simeng Ren
Simeng Ren Zunyi Zhang
Zunyi Zhang Jie Liu
Jie Liu